Abstract
A 13-year-old patient developed severe shock due to administration of a Yersinia enterocolitica-contaminated red blood cell concentrate. Y. enterocolitica (serotype O:9, biotype II) was cultivated from the residual blood in the blood bag and from a stool sample of the blood donor. In the donor's plasma immunoglobulin M (IgM), IgA, and IgG antibodies against Yersinia outer proteins (YopM, -H, -D, and -E) were found. Since the donor remembered a short-lasting, mild diarrhea 14 days prior to blood donation, a transient attack of Yersinia enteritis may be associated with a longer than expected period of asymptomatic bacteremia that causes contamination of donor blood. Serological screening for IgM antibodies against Yersinia outer proteins might offer a way to reduce the risk of transfusion-associated Y. enterocolitica sepsis.
CASE REPORT
We report the case of a 13-year-old girl suffering from lung metastases of an osteoblastic sarcoma. She required chemotherapy and consecutive substitution of blood products. About 30 min after the beginning of the transfusion of a red blood cell concentrate (RBCC) she developed chills, abdominal pain, nausea, vomiting, pallor, tachypnea, dyspnea, and cough. The transfusion was stopped immediately, and the patient was admitted to the intensive care unit. Blood pressure was 95/60 torr, and heart rate was 170 beats/min. Arterial blood gas analysis showed hypoxemia (partial pressure of O2, 53 torr). Chest X-ray revealed pulmonary edema. Echocardiographically, the function of both ventricles was markedly impaired (acute deterioration of a preexisting adriamycin-induced cardiomyopathy). There was no evidence of acute intravascular hemolysis (normal haptoglobin and total bilirubin values). Red blood cell compatibility was reexamined immediately after the event and found to be inconspicuous. Half a day after the transfusion, a Gram-stained blood smear was prepared directly from the residual blood in the blood bag. It showed numerous gram-negative rods. Intravenous antibiotics (ceftazidime, gentamicin, and ampicillin) were started, and preexisting oral therapy with cotrimoxazole, polymyxin, and nystatin was continued. The patient's condition improved slowly, and she was discharged from the intensive care unit after 8 days.
Microbiological investigation.
Blood from the RBCC was plated on MacConkey, sheep blood (aerobe), and Schaedler (anaerobe) agar plates. After 1 day of incubation (at 37°C), small colonies that were identified as Yersinia enterocolitica by using a numeric profile based on 20 biochemical reactions (API 20E) were found. The fresh frozen plasma from the same donation showed no growth, as it had been stored in a frozen state. Twenty-five days after the blood donation, a stool specimen from the donor was examined by a cold-enrichment technique (10 days in phosphate-buffered saline at 4°C) (28), which also revealed Y. enterocolitica. Direct inoculation of the stool sample on cefsulodin-irgasan-novobiocin agar (28°C) (22) had been negative. The isolates from the blood bag and from the donor's stool were characterized as serotype O:9 (by agglutination with anti-Y. enterocolitica sera) and as biotype II (by using commercial tests with 20 and 49 biochemical reactions [API 20E and API 50CHE] and by additional testing of the indole reaction with a prolonged incubation of 4 days at 29°C). The antimicrobial susceptibility patterns of the isolates were in concordance with data reported in the literature (18). By genomic macrorestriction analysis using NotI digestion of chromosomal DNA and pulsed-field gel electrophoresis, identical restriction fragment patterns were obtained from both isolates (26).
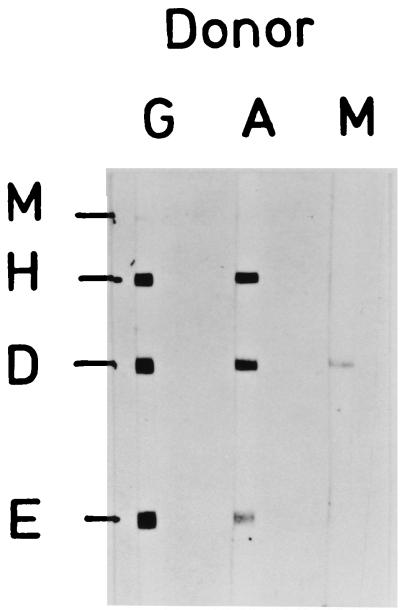
The donor's plasma (from the time of blood donation) contained agglutinating antibodies against Y. enterocolitica O-antigen O:9 at a titer of 1:160 as measured by the Widal method (normal level, ≤1:80). In the immunoblot analysis we found antibodies of the immunoglobulin M (IgM) class against YopD and antibodies of the IgA class and of the IgG class against YopM (weak), YopH, YopD, and YopE (13, 15, 17) (Fig. 1).
FIG. 1.
Immunoblot analysis of donor plasma (dilution, 1:200) for antibodies of the IgG (lane G), IgA (lane A), and IgM (lane M) classes against the Yops YopE, -D, -H, and -M.
The RBCC (buffy coat reduced, suspended in the additive solution SAG-M) causing the adverse transfusion reaction had been stored for 14 days in the refrigerator and had stayed at room temperature for some hours before transfusion. The donor was apparently healthy at the time of donation. After the event he was questioned again. He then remembered a very mild and short-lasting diarrhea without any other symptoms about 14 days before blood donation. This had not reduced his fitness for work so that he had forgotten it in the meantime.
The first case of transfusion-associated sepsis caused by Y. enterocolitica was reported from The Netherlands in 1975 (4). Since that time more than 35 further cases from all over the world have been reported (16). Autologous as well as homologous blood products were found to be contaminated with Y. enterocolitica (12); C. Richards, J. Kolins, and C. D. Trindade, Letter, JAMA 268:1541–1542, 1992. Most reports concerned red blood cell products (whole blood or RBCC) (16). Between April 1987 and November 1996, 20 cases of transfusion-associated Y. enterocolitica sepsis (12 fatal) were reported in the United States (6, 7). The true incidence of this complication of blood transfusion is not known, because the report of nonfatal cases was not obligatory. Although transfusion-associated Y. enterocolitica sepsis seems to be a rare event, it has received attention for its high fatality rate among the reported patients.
From the results of our microbiological investigation we conclude that Yersinia contamination of the blood donation was the result of a transient bacteremia of the donor, who suffered from mild enteric yersiniosis 2 weeks before blood donation. There are only a few reports on coisolation of Y. enterocolitica from donor blood and feces that suggest this way of infection, but our study adds a further case in which identical strains were isolated from the blood bag and the stool sample. Obviously, Y. enterocolitica is endowed with properties enabling the pathogen to cause symptomless transient low-level bacteremia during reconvalescence (25). Recently, the persistence of Yersinia antigens in peripheral white blood cells from patients with Y. enterocolitica infection for up to 4 years has been demonstrated (10).
Several approaches, such as reducing the storage time (1, 8) or the storage temperature of RBCCs (3), asking donors about gastrointestinal illness in the last weeks before blood donation (11), and inspecting the blood in the bags for color change a short time before the release of the units (19), have been suggested for preventing transfusion-associated Y. enterocolitica sepsis but have not yet been generally accepted. Removal of Y. enterocolitica from donated blood can be achieved by leukocyte filtration (5, 9, 20, 29). However, large numbers of bacteria can exceed the capacity of this method to prevent bacterial growth during the storage of RBCCs (5, 29). Testing for antibodies against Y. enterocolitica O antigens in donor serum by the bacterial agglutination method (23) or by an enzyme-linked immunosorbent assay A. J. Morris and D. G. Woodfield, Letter, Transfusion 38:1122, 1998 has not been recommended, either because of an unacceptably high rate of positive results for healthy donors (23) or because of an apparent lack of antibody response in some patients Morris and Woodfield, Letter.
Another possibility of serological testing is the detection of antibodies against Yersinia outer proteins (Yops) (13). Yops are clearly associated with virulence properties of pathogenic strains (24). They are borne by the virulence plasmid pYV, which is present in all human pathogenic Yersinia species and serotypes (15). In contrast to testing for antibodies to O antigens (2), screening for antibodies to Yops is not restricted to single serotypes (21). Antibodies of the IgM class—especially those directed against YopD—may indicate an acute infection (27). A preliminary study with a small number of healthy blood donors had shown a specificity of 97% for immunoblot analysis for IgM antibodies against Yops (14). Further studies are required to evaluate the suitability of the detection of IgM antibodies against Yops for reducing the risk of transfusion-associated Y. enterocolitica sepsis.
REFERENCES
- 1.Arduino M J, Bland L A, Tipple M A, Aguero S M, Favero M S, Jarvis W R. Growth and endotoxin production of Yersinia enterocolitica and Enterobacter agglomerans in packed erythrocytes. J Clin Microbiol. 1989;27:1483–1485. doi: 10.1128/jcm.27.7.1483-1485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottone E J, Sheehan D J. Yersinia enterocolitica: guidelines for serologic diagnosis of human infections. Rev Infect Dis. 1983;5:898–906. doi: 10.1093/clinids/5.5.898. [DOI] [PubMed] [Google Scholar]
- 3.Bradley R M, Gander R M, Patel S K, Kaplan H S. Inhibitory effect of 0°C storage on the proliferation of Yersinia enterocolitica in donated blood. Transfusion. 1997;37:691–695. doi: 10.1046/j.1537-2995.1997.37797369443.x. [DOI] [PubMed] [Google Scholar]
- 4.Bruining M, De Wilde-Beekhuizen C C M. A case of contamination of donor blood by Yersinia enterocolitica type 9. Medikon. 1975;4:30–31. [Google Scholar]
- 5.Buchholz D H, AuBuchon J P, Snyder E L, Kandler R, Edberg S, Piscitelli V, Pickard C, Napychank P. Removal of Yersinia enterocolitica from AS-1 red cells. Transfusion. 1992;32:667–672. doi: 10.1046/j.1537-2995.1992.32792391043.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Update: Yersinia enterocolitica bacteremia and endotoxin shock associated with red blood cell transfusions—United States, 1991. Morb Mortal Wkly Rep. 1991;40:176–178. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Red blood cell transfusions contaminated with Yersinia enterocolitica—United States, 1991–1996, and initiation of a national study to detect bacteria-associated transfusion reactions. JAMA. 1997;278:196–197. [PubMed] [Google Scholar]
- 8.Franzin L, Gioannini P. Growth of Yersinia species in artificially contaminated blood bags. Transfusion. 1992;32:673–676. doi: 10.1046/j.1537-2995.1992.32792391044.x. [DOI] [PubMed] [Google Scholar]
- 9.Gong J, Högman C F, Hambraeus A, Johansson C S, Eriksson L. Transfusion-transmitted Yersinia enterocolitica infection. Vox Sang. 1993;65:42–46. doi: 10.1111/j.1423-0410.1993.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 10.Granfors K, Merilahti-Palo R, Luukkainen R, Möttönen T, Lahesmaa R, Probst P, Märker-Hermann E, Toivanen P. Persistence of Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O:3 infection with or without reactive arthritis. Arthritis Rheum. 1998;41:855–862. doi: 10.1002/1529-0131(199805)41:5<855::AID-ART12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Grossman B J, Kollins P, Lau P M, Perreten J L, Bowman R J, Malcolm S, Palko W M. Screening blood donors for gastrointestinal illness: a strategy to eliminate carriers of Yersinia enterocolitica. Transfusion. 1991;31:500–501. doi: 10.1046/j.1537-2995.1991.31691306245.x. [DOI] [PubMed] [Google Scholar]
- 12.Haditsch M, Binder L, Gabriel C, Müller-Uri P, Watschinger R, Mittermayer H. Yersinia enterocolitica septicemia in autologous blood transfusion. Transfusion. 1994;34:907–909. doi: 10.1046/j.1537-2995.1994.341095026979.x. [DOI] [PubMed] [Google Scholar]
- 13.Heesemann J, Eggers C, Schröder J, Laufs R. Serological diagnosis of yersiniosis by the immunoblot technique using plasmid-encoded antigens of Yersinia enterocolitica. In: Simon C, Wilkinson P, editors. Diagnosis of infectious diseases—new aspects. Stuttgart, Germany: Schattauer; 1986. pp. 79–88. [Google Scholar]
- 14.Heesemann J, Eggers C, Schröder J. Serological diagnosis of yersiniosis by immunoblot technique using virulence-associated antigen of enteropathogenic Yersiniae. Contrib Microbiol Immunol. 1987;9:285–289. [PubMed] [Google Scholar]
- 15.Heesemann J, Gross U, Schmidt N, Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia species grown in calcium-deficient media. Infect Immun. 1986;54:561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Högman C F, Engstrand L. Factors affecting growth of Yersinia enterocolitica in cellular blood products. Transfus Med Rev. 1996;10:259–275. doi: 10.1016/s0887-7963(96)80002-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoogkamp-Korstanje J A A, de Koning J, Heesemann J. Persistence of Yersinia enterocolitica in man. Infection. 1988;16:81–85. doi: 10.1007/BF01644307. [DOI] [PubMed] [Google Scholar]
- 18.Kanazawa Y, Ikemura K, Kuramata T. Drug susceptibility of Yersinia enterocolitica and Yersinia pseudotuberculosis. Contrib Microbiol Immunol. 1987;9:127–135. [PubMed] [Google Scholar]
- 19.Kim D M, Brecher M E, Bland L A, Estes T J, Carmen R A, Nelson E J. Visual identification of bacterially contaminated red cells. Transfusion. 1992;32:221–225. doi: 10.1046/j.1537-2995.1992.32392213804.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim D M, Brecher M E, Bland L A, Estes T J, McAllister S K, Aguero S M, Carmen R A, Nelson E J. Prestorage removal of Yersinia enterocolitica from red cells with white cell-reduction filters. Transfusion. 1992;32:658–662. doi: 10.1046/j.1537-2995.1992.32792391041.x. [DOI] [PubMed] [Google Scholar]
- 21.Mäki-Ikola O, Heesemann J, Lahesmaa R, Toivanen A, Granfors K. Combined use of released proteins and lipopolysaccharide in enzyme-linked immunosorbent assay for serologic screening of Yersinia infections. J Infect Dis. 1991;163:409–412. doi: 10.1093/infdis/163.2.409. [DOI] [PubMed] [Google Scholar]
- 22.Nash P, Krenz M M. Culture media. In: Balows A, Hausler W J, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. p. 1237. [Google Scholar]
- 23.Prentice M. Transfusing Yersinia enterocolitica. Br Med J. 1992;305:663–664. doi: 10.1136/bmj.305.6855.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Köhler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saken E, Roggenkamp A, Aleksic S, Heesemann J. Characterisation of pathogenic Yersinia enterocolitica serogroups by pulsed-field gel electrophoresis of genomic NotI restriction fragments. J Med Microbiol. 1994;41:329–338. doi: 10.1099/00222615-41-5-329. [DOI] [PubMed] [Google Scholar]
- 27.Stahlberg T H, Heesemann J, Granforrs K, Toivanen A. Immunoblot analysis of IgM, IgG and IgA responses to plasmid encoded released proteins of Yersinia enterocolitica in patients with and without Yersinia triggered reactive arthritis. Ann Rheum Dis. 1989;48:577–581. doi: 10.1136/ard.48.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Noyen R, Selderslaghs R, Wauters G, Vandepitte J. Optimal recovery of Yersinia enterocolitica O:3 and O:9 from stools of patients with intestinal disorders. Contrib Microbiol Immunol. 1987;9:272–278. [PubMed] [Google Scholar]
- 29.Wenz B, Burns E R, Freundlich L F. Prevention of growth of Yersinia enterocolitica in blood by polyester fiber filtration. Transfusion. 1992;32:663–666. doi: 10.1046/j.1537-2995.1992.32792391042.x. [DOI] [PubMed] [Google Scholar]