Abstract
Cataracts are one of the most common eye diseases that can cause blindness. Discovering susceptibility factors in the proteome that contribute to cataract development would be helpful in gaining new insights in the molecular mechanisms of the cataract process. We used label-free nanoflow ultra-high-performance liquid chromatography–tandem mass spectrometry to compare aqueous humor protein expressions in cataract patients with different cataract risk factors such as diabetes mellitus (DM) and smoking and in controls (with cataract) without risk exposure. Eight patients with diabetes and who smoked (with double risk factors), five patients with diabetes and five patients who smoked (both with a single risk factor), and nine aged-matched cataract controls patients (non-risk exposure) were enrolled. In total, 136 aqueous humor proteins were identified, of which only alpha-2-Heremans–Schmid (HS)-glycoprotein was considered to be significantly risk-associated because it was differentially expressed in these three groups and exhibited increased expression with increasing risk factors. Significant changes in the aqueous humor level of alpha-2-HS-glycoprotein between DM and control samples and between smoking and control samples were confirmed using ELISA. The alpha-2-HS-glycoprotein, called fetuin-a, could be a potential aqueous biomarker associated with DM and smoking, which were cataract risk factors.
Keywords: aqueous humor, label free, cataract, risk factor, proteomics, alpha-2-HS-glycoprotein, fetuin-A
1. Introduction
In developed countries, cataracts are one of the most common causes of blindness [1]. They are classified by cause as age-related cataracts, pediatric cataracts, and cataracts secondary to other causes. As shown by many studies, age is the biggest risk factor [2,3]. Considering the location of opacification within the lens, cataracts are divided into three major types: nuclear, cortical, and posterior subcapsular cataracts. Cataract development can be caused by many other risk factors, including environmental factors and genetic changes [4]. Diabetes mellitus (DM), long-term use of corticosteroids, cigarette smoking, prolonged exposure to ultraviolet light, and alcohol abuse are well-known risk factors [2]. Cigarette smoking is a risk factor for nuclear and posterior subcapsular cataracts [4]. DM was identified as a common cause of posterior subcapsular and cortical cataracts [5,6]. Increased age is a risk factor for the development of all types of cataracts. Throughout life, a high myopia of over −6.0 D is associated with nuclear cataracts and posterior subcapsular cataracts [7]. Other causes of cataracts include mechanical trauma, chemical injury, electrical injury, radiation, and certain medications. However, the underlying cataractogenic mechanisms of cataract development are still not well documented, with many still being investigated. Proteomics analysis is an extensively used technique to discover changes in protein levels in tissues and cells. Recent proteomic studies in cataract disease of the human aqueous humor (AH) revealed multiple proteins of interest in patients [8,9,10,11,12]. Ji et al. [13] used isobaric tags for the relative and absolute quantitation (iTRAQ) methodology to compare AH protein profiles among high myopia, glaucoma, and vitrectomy surgery patients, and controls. They identified multiple candidate protein biomarkers associated with cataract development in each group. Furthermore, Kim et al. [14] analyzed the aqueous proteome from age-related macular degeneration (AMD) patients and non-AMD cataract controls to identify novel pathogenic proteins that are useful as potential clinical biomarkers. The differential expressions of three proteins were reported in the AH of AMD patients compared with those of cataract controls. Those studies used a good model that inspired a new idea for us of using proteomics to discuss different risk factors of cataract formation. To our knowledge, there has been no previous investigation of different cataract risk factors by comparing proteomic evidence. We used proteomics to discover the pathogenesis of different cataract risks and to possibly identify candidate biomarker proteins identified in patients predisposed to this condition. In this study, we employed Nanoflow ultra-high-performance liquid chromatography–tandem mass spectrometry (n-UPLC-MS/MS) to examine the protein compositions of aqueous solutions obtained from human cataract eyes of patients who had a single risk factor of either DM or cigarette smoking, those who had double risk factors of DM and cigarette smoking, and aged-matched cataract controls (with neither risk factor). This sensitive proteomics approach could help examine the underlying pathophysiology of cataract formation using relatively scarce amounts of aqueous samples, thereby favoring the methodological approach for this investigation. This study may reveal valuable insights into the molecular changes in the AH in the course of cataract pathogenesis.
2. Materials and Methods
2.1. Subjects
The study protocol was approved by the Medical Ethics and Institutional Review Board of Taoyuan General Hospital, Ministry of Health and Welfare (TYGH109009) (Taoyuan, Taiwan), and conducted as per the tenets of the Declaration of Helsinki. All study participants provided written informed consent before their enrollment, and the nature and possible consequences of the study were explained to them. Human AH samples from treatment-naive patients with a single risk factor (n = 10) of DM (n = 5) or cigarette smoking (n = 5), double risk factors (n = 8) of DM combined with cigarette smoking, and aged-matched cataract controls with neither risk factor (n = 9) were collected while patients were undergoing cataract surgery at Taoyuan General Hospital. The diagnostic criterion for cataracts was defined with a slit lamp with no other ocular diseases, trauma, or previous intraocular operation history. The presence of type 2 diabetes was defined as any one or more of the following: (1) having had a diagnosis of type 2 diabetes that was confirmed by a physician (ICD10: E11); (2) self-report of a diabetes diagnosis and use of hypoglycemic medications; (3) a fasting glucose level of ≥126 mg/dL; (4) a 2 h post-challenge plasma glucose level of ≥200 mg/dL. All subjects were included as cases of type 2 diabetes within a follow-up time of five years. A cigarette smoking history was obtained from all patients. Their cigarette consumption varied with a mean duration of more than 20 years. Data on control eyes were collected from senior cataract patients who were free from other ocular or systemic diseases. In these three groups, inclusion criteria were cataract patients aged older than 55 years. Exclusion criteria were a history of any systemic or ocular disorder or condition including ocular surgery, trauma, or disease. Best corrected visual acuity (BCVA) was measured as the logarithm of the minimum angle of resolution (logMAR).
2.2. AH Sample Collection
AH samples were obtained from patients during the implantation of phakic intraocular lenses. To avoid hemorrhaging and ocular surface contamination, a sample was collected using a 1 mL tuberculin syringe with a 30 gauge needle at the limbus before any other entry into the eye under a surgical microscope. Note that 50–100 μL of AH was collected from each patient by anterior chamber paracentesis. Undiluted AH samples were collected and stored at −80 °C within 24 h until preparation was initiated.
2.3. n-UPLC-MS/MS
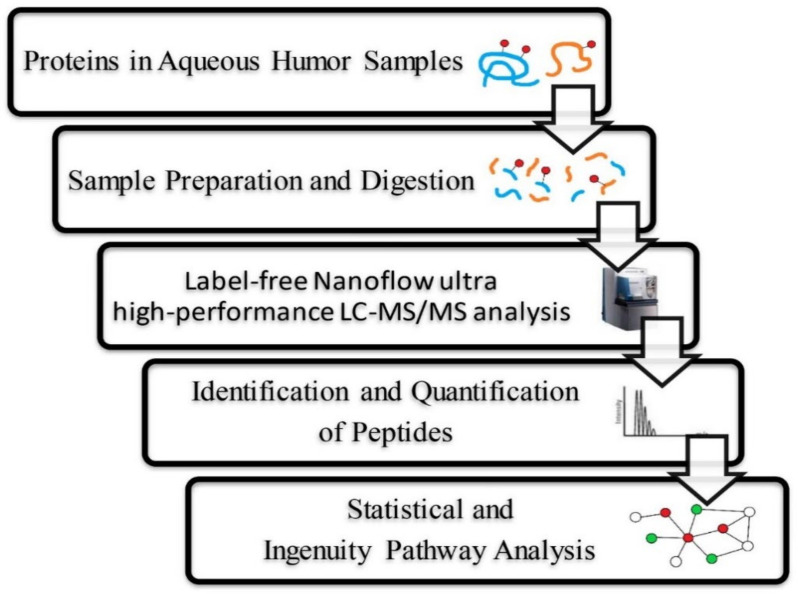
Protein concentrations of AH samples were determined by a dye-binding method based on the Bradford assay (Bio-Rad Laboratories, Richmond, CA, USA) (Table 1), and samples were further diluted in 1× phosphate-buffered saline (PBS) to a final concentration of 0.1 μg/μL. Samples were prepared as per the SMART digestion kit protocol from ThermoFisher Scientific (Waltham, MA, USA) and cleaned up using solid-phase extraction (SPE) plates from ThermoFisher. The resulting peptides collected from the filters were dried in a vacuum centrifuge and stored at −80 °C. Then, 50 μL of diluted AH samples was resuspended in 0.1% formic acid and analyzed by n-UPLC-MS/MS. Tryptic peptides were loaded into an LTQ-Orbitrap mass spectrometer with a nanoelectrospray ionization source (Thermo Electron, MA, USA) connected to a nanoACQUITY UPLC system (Waters, MA, USA). Peptide samples were separated on a 25 cm × 75 μm BEH130 C18 column (Waters) with a 0–95% segmented gradient of 3–40% B for 168 min, 40–95% B for 2 min, and 95% B for 10 min at a flow rate of 0.5 μL/min. Mobile phase A was 0.1% formic acid in water, while mobile phase B was 0.1% formic acid in acetonitrile. The mass spectrometer was set to the data-dependent acquisition method (isolation width: 1.5 Da). As per the data-dependent acquisition method, the first ten most intensively charged peptide ions were selected and fragmented using a collision-induced dissociation (CID) method (Figure 1).
Table 1.
Demographic characteristics of enrolled patients with a single risk factor, those with Double risk factors, and cataract controls.
| Cataract Control | Single Risk | Double Risks | p Value # | |
|---|---|---|---|---|
| Gender | 0.003 | |||
| Female | 7 (77.8%) | 3 (30.0%) | 0 (0.0%) | |
| Male | 2 (22.2%) | 7 (70.0%) | 8 (100.0%) | |
| Protein (μg/μL) | 0.22 ± 0.06 | 0.36 ± 0.21 | 0.34 ± 0.11 | 0.049 |
| Age (years) | 74.00 ± 5.72 | 72.30 ± 10.14 | 69.38 ± 9.87 | 0.390 |
| VA (logMAR) | 0.41 ± 0.12 | 0.38 ± 0.14 | 0.27 ± 0.20 | 0.360 |
| AXL (mm) | 23.48 ± 0.59 | 24.02 ± 1.24 | 23.69 ± 0.95 | 0.552 |
| Smoking | 5 | |||
| Diabetes mellitus (DM) | 5 | |||
| Smoking + DM | 8 |
# By Fisher’s exact test, Wilcoxon test, or Kruskal–Wallis test.
Figure 1.
Label-free Nanoflow UHPLC-MS/MS analytical workflow for the proteomic analysis of human aqueous humor. Samples were digested using trypsin and were analyzed using an LTQ-Orbitrap DiscoveryTM hybrid mass spectrometer (Thermo Electron). Proteins were identified and quantified using the SEQUEST algorithm followed by analysis using Xcalibur 2.0 SR1 (Thermo Electron).
2.4. Protein Identification
Then, the acquired MS/MS raw data files were applied to search against a UniProt human protein database (containing 20,387 protein sequences; released on 9 April 2021; http://www.uniprot.org/ (accessed on 6 December 2021)) with PEAKS Studio 7.5 (Bioinformatic Solution, Ontario, CA, USA). The search settings of PEAKS Studio 7.5 combined with UniProt’s protein database were as follows: enzyme set to trypsin; up to two missing cut sites; precursor and fragment mass tolerances of 20 ppm and 0.8 Da, respectively; false discovery rate (FDR) of <1%, obtained from a search of the decoy database. Furthermore, based on a label-free quantitative analysis, each identified protein had to contain at least one unique peptide and protein quantification method. Moreover, spectral counts were normalized to the total identification spectrum of each biological sample.
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
An alpha-2-Heremans–Schmid (HS)-glycoprotein ELISA assay was performed to measure concentrations of AH samples among the single-risk group, double-risk group, and the age-matched cataract controls with a Human Alpha-2-HS-glycoprotein ELISA Kit (EH310RB, ThermoFisher Scientific), as per the manufacturer’s protocol.
2.6. Statistical Analysis
Clinical data were analyzed using Stata (vers. 16.1, StataCorp, College Station, TX, USA) to define the statistical significance between groups by a t-test or Chi-squared test, and p < 0.05 was considered to be statistically significant. Statistical analysis by Fisher’s exact test, Wilcoxon test, or Kruskal–Wallis test was used to confirm that there were no statistically significant differences in age among the single-risk group, double-risk group, and the age-matched cataract control group (Table 1).
Note: Single risk, patients with the DM or smoking risk factor; double risk, patients with both the DM and smoking risk factors; control, cataract patients with neither of these cataract risk factors; VA, visual acuity; AXL, axial length.
3. Results
Table 1 lists the demographic data of patients with a single risk factor, those with double risk factors, and the control group (with cataract). The mean age of single-risk-factor patients was 72.30 ± 10.14 years, for double-risk-factor patients was 69.38 ± 9.87 years, and for cataract control individuals was 74.00 ± 5.72 years. All patients had cataracts as revealed by a slit lamp examination. The mean protein concentrations were 0.36 ± 0.21 μg/μL in the single-risk-factor group, 0.34 ± 0.11μg/μL in the double-risk-factor group, and 0.22 ± 0.06 μg/μL in the cataract control group. There were statistical differences among total protein contents in these three groups (p = 0.049) but no statistical differences in age among these groups (p = 0.390). In total, 136 proteins were successfully identified by LC-ESI MS/MS in single-risk-factor, double-risk-factor, and cataract control AH samples (Table 2, Figure 2).
Table 2.
List of aqueous humor (AH) proteins identified by LC-ESI-MS/MS.
| Q9NQ66 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-1 | P0CG04 | Immunoglobulin lambda constant 1 |
| Q99460 | 26S proteasome non-ATPase regulatory subunit 1 | P01700 | Immunoglobulin lambda variable 1–47 |
| O95996 | Adenomatous polyposis coli protein 2 | P0DOX8 | Immunoglobulin lambda-1 light chain |
| P02768 | Albumin | B9A064 | Immunoglobulin lambda-like polypeptide 5 |
| P51648 | Aldehyde dehydrogenase family 3 member A2 | P24592 | Insulin-like growth factor-binding protein 6 |
| P02763 | Alpha-1-acid glycoprotein 1 | Q16270 | Insulin-like growth factor-binding protein 7 |
| P19652 | Alpha-1-acid glycoprotein 2 | Q14624 | Inter-alpha-trypsin inhibitor heavy chain H4 |
| P01011 | Alpha-1-antichymotrypsin | Q6UXX5 | Inter-alpha-trypsin inhibitor heavy chain H6 |
| P01009 | Alpha-1-antitrypsin | Q17R60 | Interphotoreceptor matrix proteoglycan 1 |
| P04217 | Alpha-1B-glycoprotein | Q9BZV3 | Interphotoreceptor matrix proteoglycan 2 |
| P02765 | Alpha-2-HS-glycoprotein | P01042 | Kininogen-1 |
| P01023 | Alpha-2-macroglobulin | P02750 | Leucine-rich alpha-2-glycoprotein |
| P02489 | Alpha-crystallin A chain | Q68G74 | LIM/homeobox protein Lhx8 |
| A0A140G945 | Alpha-crystallin A2 chain | P51884 | Lumican |
| P02511 | Alpha-crystallin B chain | P61626 | Lysozyme C |
| P06733 | Alpha-enolase | P01033 | Metalloproteinase inhibitor 1 |
| P03950 | Angiogenin | P05408 | Neuroendocrine protein 7B2 |
| P01019 | Angiotensinogen | P61916 | NPC intracellular cholesterol transporter 2 |
| P01008 | Antithrombin-III | Q9UBM4 | Opticin |
| P02647 | Apolipoprotein A-I | P10451 | Osteopontin |
| P02652 | Apolipoprotein A-II | Q9UQ90 | Paraplegin |
| P06727 | Apolipoprotein A-IV | P36955 | Pigment epithelium-derived factor |
| P05090 | Apolipoprotein D | Q15149 | Plectin |
| P02649 | Apolipoprotein E | P0CG47 | Polyubiquitin-B |
| P54253 | Ataxin-1 | P0CG48 | Polyubiquitin-C |
| P02749 | Beta-2-glycoprotein 1 | Q9ULS6 | Potassium voltage-gated channel subfamily S member 2 |
| P61769 | Beta-2-microglobulin | O94913 | Pre-mRNA cleavage complex 2 protein Pcf11 |
| P05813 | Beta-crystallin A3 | Q13395 | Probable methyltransferase TARBP1 |
| P53674 | Beta-crystallin B1 | A0A075B6H7 | Probable non-functional immunoglobulin kappa variable 3–7 |
| P43320 | Beta-crystallin B2 | O94823 | Probable phospholipid-transporting ATPase VB |
| P19022 | Cadherin-2 | Q9UHG2 | ProSAAS |
| P07339 | Cathepsin D | P41222 | Prostaglandin-H2 D-isomerase |
| Q8N163 | Cell cycle and apoptosis regulator protein 2 | Q92520 | Protein FAM3C |
| Q7Z7A1 | Centriolin | P05109 | Protein S100-A8 |
| P36222 | Chitinase-3-like protein 1 | Q9H6Z4 | Ran-binding protein 3 |
| Q9HAW4 | Claspin | P10745 | Retinol-binding protein 3 |
| O43809 | Cleavage and polyadenylation specificity factor subunit 5 | P02753 | Retinol-binding protein 4 |
| P10909 | Clusterin | P34096 | Ribonuclease 4 |
| P01024 | Complement C3 | P07998 | Ribonuclease pancreatic |
| P0C0L4 | Complement C4-A | Q5T481 | RNA-binding protein 20 |
| P0C0L5 | Complement C4-B | O75326 | Semaphorin-7A |
| P00751 | Complement factor B | P02787 | Serotransferrin |
| P00746 | Complement factor D | P00441 | Superoxide dismutase [Cu-Zn] |
| P05156 | Complement factor I | P05452 | Tetranectin |
| P01034 | Cystatin-C | Q8WZ42 | Titin |
| Q8WVS4 | Cytoplasmic dynein 2 intermediate chain 1 | O15050 | TPR and ankyrin repeat-containing protein 1 |
| Q96M86 | Dynein heavy chain domain-containing protein 1 | Q15582 | Transforming growth factor-beta-induced protein ig-h3 |
| P49792 | E3 SUMO-protein ligase RanBP2 | Q14956 | Transmembrane glycoprotein NMB |
| Q9HC35 | Echinoderm microtubule-associated protein-like 4 | P02766 | Transthyretin |
| Q13822 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 | P60174 | Triosephosphate isomerase |
| Q8TE68 | Epidermal growth factor receptor kinase substrate 8-like protein 1 | P35030 | Trypsin-3 |
| P02671 | Fibrinogen alpha chain | P62979 | Ubiquitin-40S ribosomal protein S27a |
| Q6ZV73 | FYVE, RhoGEF and PH domain-containing protein 6 | P62987 | Ubiquitin-60S ribosomal protein L40 |
| P07320 | Gamma-crystallin D | Q5THJ4 | Vacuolar protein sorting-associated protein 13D |
| P22914 | Gamma-crystallin S | P02774 | Vitamin D-binding protein |
| P06396 | Gelsolin | Q96PQ0 | VPS10 domain-containing receptor SorCS2 |
| P22352 | Glutathione peroxidase 3 | Q9P202 | Whirlin |
| Q14789 | Golgin subfamily B member 1 | P25311 | Zinc-alpha-2-glycoprotein |
| P00738 | Haptoglobin | P0CG04 | Immunoglobulin lambda constant 1 |
| P69905 | Hemoglobin subunit alpha | P01700 | Immunoglobulin lambda variable 1–47 |
| P68871 | Hemoglobin subunit beta | P0DOX8 | Immunoglobulin lambda-1 light chain |
| P02042 | Hemoglobin subunit delta | B9A064 | Immunoglobulin lambda-like polypeptide 5 |
| P02790 | Hemopexin | P24592 | Insulin-like growth factor-binding protein 6 |
| P62805 | Histone H4 | Q16270 | Insulin-like growth factor-binding protein 7 |
| P0DOX3 | Immunoglobulin delta heavy chain | Q14624 | Inter-alpha-trypsin inhibitor heavy chain H4 |
| P0DOX5 | Immunoglobulin gamma-1 heavy chain | Q6UXX5 | Inter-alpha-trypsin inhibitor heavy chain H6 |
| P01859 | Immunoglobulin heavy constant gamma 2 | Q17R60 | Interphotoreceptor matrix proteoglycan 1 |
| P01860 | Immunoglobulin heavy constant gamma 3 | Q9BZV3 | Interphotoreceptor matrix proteoglycan 2 |
| P01861 | Immunoglobulin heavy constant gamma 4 | P01042 | Kininogen-1 |
| P01780 | Immunoglobulin heavy variable 3–7 | P02750 | Leucine-rich alpha-2-glycoprotein |
| A0A0B4J1Y9 | Immunoglobulin heavy variable 3–72 | Q68G74 | LIM/homeobox protein Lhx8 |
| A0A0B4J1X5 | Immunoglobulin heavy variable 3–74 | P51884 | Lumican |
| A0A0J9YXX1 | Immunoglobulin heavy variable 5-10-1 | P61626 | Lysozyme C |
| A0A0B4J1U7 | Immunoglobulin heavy variable 6-1 | P01033 | Metalloproteinase inhibitor 1 |
| P01834 | Immunoglobulin kappa constant | P05408 | Neuroendocrine protein 7B2 |
| P0DOX7 | Immunoglobulin kappa light chain | P61916 | NPC intracellular cholesterol transporter 2 |
| P01624 | Immunoglobulin kappa variable 3–15 | Q9UBM4 | Opticin |
| P01619 | Immunoglobulin kappa variable 3–20 | P10451 | Osteopontin |
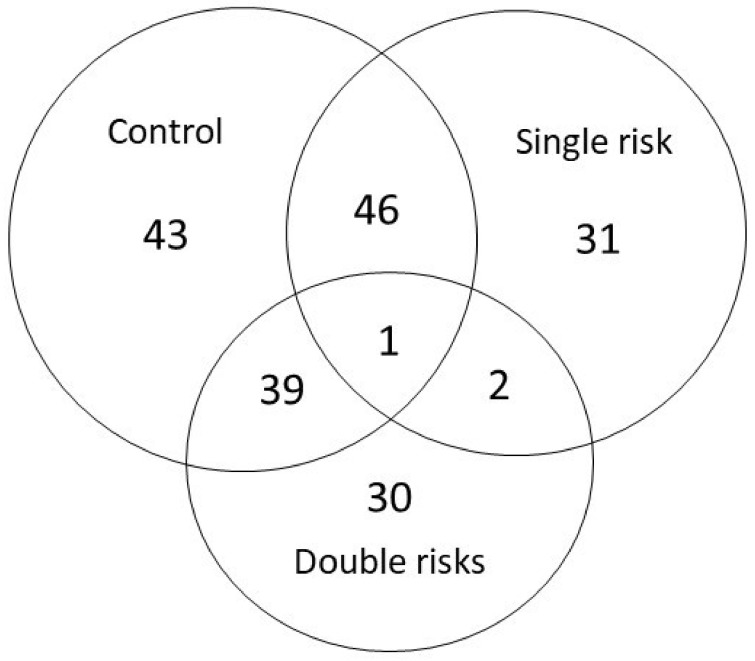
Figure 2.
Label-free Nanoflow UHPLC-MS/MS analytical workflow for the proteomic analysis of human aqueous humor. Samples were digested using trypsin and were analyzed using an LTQ-Orbitrap DiscoveryTM hybrid mass spectrometer (Thermo Electron). Proteins were identified and quantified using the SEQUEST algorithm followed by analysis using Xcalibur 2.0 SR1 (Thermo Electron). The intersection of each area represents the number of significant expression (p < 0.05) proteins between each groups. Only one protein was significantly deferentially expressed in each group.
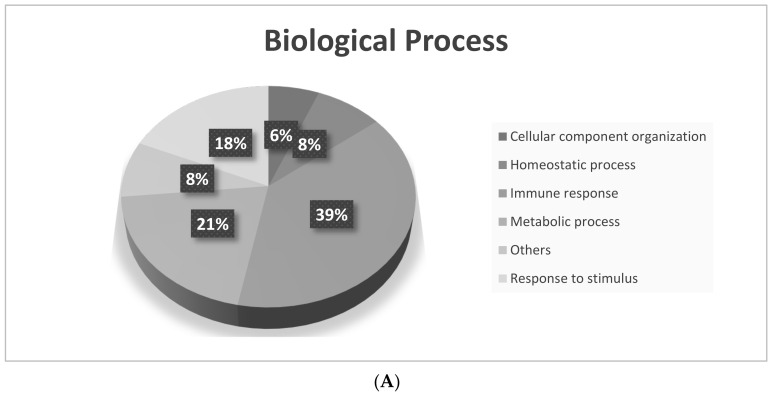
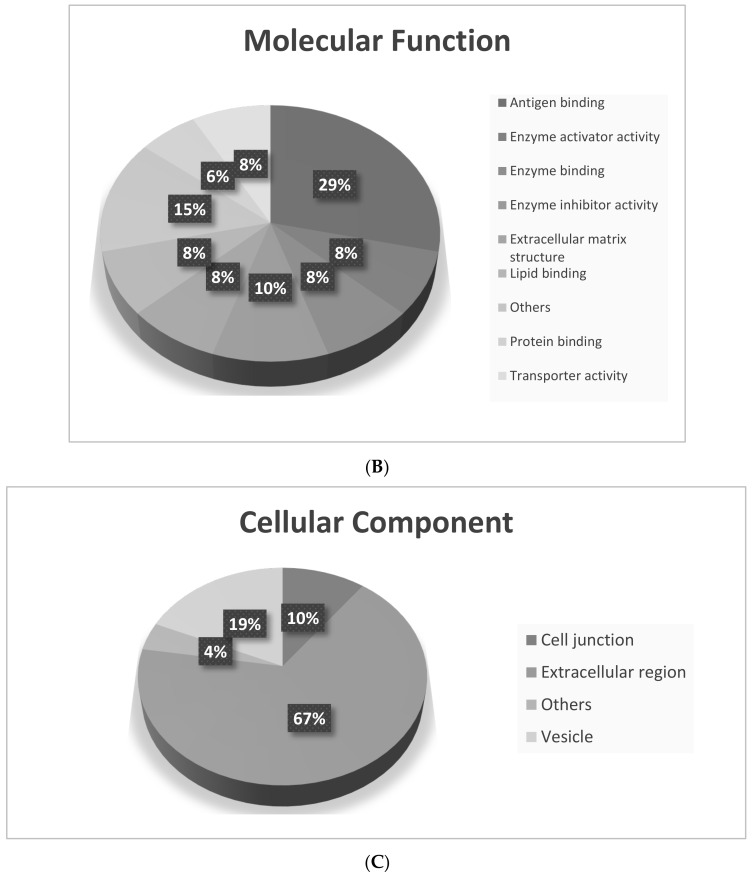
Comparing the single-risk group to the cataract control group, 125 proteins were found, which included 42 proteins that were present at higher expression levels and 83 proteins that were present at lower expression levels in the single-risk group. In the double-risk group, as compared to the cataract control group, 124 proteins were disclosed, among which 39 proteins had higher expression levels and 85 proteins had lower expression levels in the double-risk group. To understand the biological meaning of the changes of protein expression observed in different risk factor groups, differentially expressed proteins were analyzed for “molecular functions”, “biological processes”, and “cellular components” by GO annotations. Our results demonstrated that differentially expressed proteins in the three groups had different molecular functions, biological processes, and cellular components (Figure 3). The major biological processes of these proteins were biological regulation, including immune responses, metabolic processes, and responses to stimuli of the AH (Figure 3A). The major molecular functions of AH proteins enriched among single-risk and double-risk patients were antigen binding and enzyme inhibitor activity (Figure 3B). As per cellular component terms of the GO, most significant AH proteins were categorized as extracellular region proteins (Figure 3C). Then, we used Ingenuity Pathway Analysis (IPA, Qiagen) to show canonical pathways that are potentially involved in the pathogenesis of cataracts under the risks of diabetes and smoking. Table 3 lists pathways associated with AH proteins from single-risk patients, double-risk patents, and the cataract controls.
Figure 3.
Gene ontology (GO) analysis of differentially expressed proteins of the aqueous humor (AH) in the cataract control, single-risk, and double-risk groups. We compared identified AH proteins from the three groups: (A) biological processes; (B) molecular functions; (C) cellular components.
Table 3.
Pathway analysis of aqueous humor (AH) proteins using IPA tools.
| Canonical Pathways | Overlap of Proteins in the Single-Risk and Cataract Control Groups | Overlap of Proteins in the Double-Risk and Cataract Control Groups | Overlap of Proteins in the Single- and Double-Risk Groups |
|---|---|---|---|
| LXR/RXR Activation | 12 | 10 | 1 |
| FXR/RXR Activation | 12 | 10 | 1 |
| Acute-Phase Response Signaling | 11 | 11 | 1 |
| Clathrin-mediated Endocytosis Signaling | 12 | ||
| Atherosclerosis Signaling | 7 | ||
| Primary Immunodeficiency Signaling | 5 | ||
| IL-15 Signaling | 9 | 1 | |
| B Cell Receptor Signaling | 1 |
Single risk, patients with the DM or smoking risk factor; double risk, patients with both the DM and smoking risk factors; control, cataract patients with neither of these cataract risk factors.
The top canonical pathways, including LXR/RXR activation, FXR/RXR activation, and acute-phase response signaling, demonstrated significant associations with AH proteins. Statistical analysis was performed on these 136 proteins. In total, 47 proteins exhibited statistically significant changes in content in the group with a single risk factor compared to the cataract control group (Table 4).
Table 4.
List of selected potential biomarker candidates.
| Protein-ID | Protein Name | Cataract Control (Spc) |
Single (Spc) | Multiple of Change (Spc) | Cataract Control (Spc) | Double (Spc) | Multiple of Change (Spc) |
|---|---|---|---|---|---|---|---|
| Q99460 | 26S proteasome non-ATPase regulatory subunit 1 | 0.76 ± 1.18 | 2.99 ± 0.91 | 3.93 | 0.76 ± 1.18 | 2.95 ± 1.90 | 3.88 |
| P02763 | Alpha-1-acid glycoprotein 1 | 3.26 ± 3.45 | 0.00 ± 0.00 | 0 | 3.26 ± 3.45 | 0.00 ± 0.00 | 0 |
| P19652 | Alpha-1-acid glycoprotein 2 | 2.06 ± 1.89 | 0.00 ± 0.00 | 0 | 2.06 ± 1.89 | 0.00 ± 0.00 | 0 |
| P01011 | Alpha-1-antichymotrypsin | 2.87 ± 2.07 | 0.32 ± 0.52 | 0.11 | 2.87 ± 2.07 | 0.26 ± 0.74 | 0.09 |
| P02765 | Alpha-2-HS-glycoprotein | 0.00 ± 0.00 | 2.14 ± 1.72 | −100 | 0.00 ± 0.00 | 4.30 ± 2.08 | −100 |
| P02647 | Apolipoprotein A-I | 3.88 ± 4.11 | 10.49 ± 2.19 | 2.68 | 3.88 ± 4.11 | 9.41 ± 6.49 | 2.43 |
| P02652 | Apolipoprotein A-II | 0.09 ± 0.26 | 2.09 ± 1.33 | 23.22 | 0.09 ± 0.26 | 2.26 ± 1.52 | 25.11 |
| P02749 | Beta-2-glycoprotein 1 | 1.90 ± 1.49 | 0.09 ± 0.27 | 0.05 | 1.90 ± 1.49 | 0.33 ± 0.63 | 0.17 |
| P36222 | Chitinase-3-like protein 1 | 5.39 ± 2.93 | 1.15 ± 1.87 | 0.21 | 5.39 ± 2.93 | 0.71 ± 0.88 | 0.13 |
| Q13822 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 | 3.63 ± 3.78 | 0.11 ± 0.34 | 0.03 | 3.63 ± 3.78 | 0.14 ± 0.41 | 0.04 |
| P22352 | Glutathione peroxidase 3 | 1.15 ± 1.23 | 0.00 ± 0.00 | 0 | 1.15 ± 1.23 | 0.00 ± 0.00 | 0 |
| Q14789 | Golgin subfamily B member 1 | 0.54 ± 0.71 | 0.00 ± 0.00 | 0 | 0.54 ± 0.71 | 0.00 ± 0.00 | 0 |
| P02790 | Hemopexin | 21.12 ± 8.44 | 1.56 ± 1.62 | 0.07 | 21.12 ± 8.44 | 2.67 ± 3.40 | 0.13 |
| P0DOX5 | Immunoglobulin gamma-1 heavy chain | 34.76 ± 6.08 | 10.24 ± 4.37 | 0.29 | 34.76 ± 6.08 | 10.58 ± 5.89 | 0.3 |
| P01859 | Immunoglobulin heavy constant gamma 2 | 21.29 ± 3.52 | 5.29 ± 3.57 | 0.25 | 21.29 ± 3.52 | 6.27 ± 4.97 | 0.3 |
| P01860 | Immunoglobulin heavy constant gamma 3 | 22.01 ± 4.99 | 6.75 ± 3.30 | 0.31 | 22.01 ± 4.99 | 6.98 ± 4.25 | 0.32 |
| P01861 | Immunoglobulin heavy constant gamma 4 | 15.02 ± 3.42 | 4.14 ± 2.88 | 0.28 | 15.02 ± 3.42 | 4.92 ± 2.49 | 0.33 |
| P01780 | Immunoglobulin heavy variable 3–7 | 2.46 ± 2.13 | 0.00 ± 0.00 | 0 | 2.46 ± 2.13 | 0.25 ± 0.72 | 0.1 |
| A0A0B4J1Y9 | Immunoglobulin heavy variable 3–72 | 1.67 ± 1.09 | 0.00 ± 0.00 | 0 | 1.67 ± 1.09 | 0.13 ± 0.36 | 0.08 |
| A0A0B4J1X5 | Immunoglobulin heavy variable 3–74 | 2.08 ± 1.99 | 0.00 ± 0.00 | 0 | 2.08 ± 1.99 | 0.25 ± 0.72 | 0.12 |
| A0A0B4J1U7 | Immunoglobulin heavy variable 6–1 | 1.16 ± 1.28 | 0.09 ± 0.27 | 0.08 | 1.16 ± 1.28 | 0.00 ± 0.00 | 0 |
| P01834 | Immunoglobulin kappa constant | 16.50 ± 5.02 | 2.75 ± 2.24 | 0.17 | 16.50 ± 5.02 | 2.94 ± 2.81 | 0.18 |
| P0DOX7 | Immunoglobulin kappa light chain | 12.23 ± 3.03 | 2.75 ± 2.24 | 0.23 | 12.23 ± 3.03 | 2.94 ± 2.81 | 0.24 |
| P0CG04 | Immunoglobulin lambda constant 1 | 4.74 ± 1.71 | 2.29 ± 1.75 | 0.48 | 4.74 ± 1.71 | 1.66 ± 1.20 | 0.35 |
| P0DOX8 | Immunoglobulin lambda-1 light chain | 4.74 ± 1.71 | 2.29 ± 1.75 | 0.48 | 4.74 ± 1.71 | 1.66 ± 1.20 | 0.35 |
| B9A064 | Immunoglobulin lambda-like polypeptide 5 | 4.74 ± 1.71 | 2.29 ± 1.75 | 0.48 | 4.74 ± 1.71 | 1.66 ± 1.20 | 0.35 |
| Q16270 | Insulin-like growth factor-binding protein 7 | 3.52 ± 1.34 | 1.83 ± 1.03 | 0.52 | 3.52 ± 1.34 | 1.09 ± 1.28 | 0.31 |
| P01033 | Metalloproteinase inhibitor 1 | 0.78 ± 0.80 | 0.00 ± 0.00 | 0 | 0.78 ± 0.80 | 0.00 ± 0.00 | 0 |
| P61916 | NPC intracellular cholesterol transporter 2 | 1.05 ± 0.89 | 0.00 ± 0.00 | 0 | 1.05 ± 0.89 | 0.20 ± 0.58 | 0.19 |
| Q92520 | Protein FAM3C | 1.50 ± 1.23 | 0.00 ± 0.00 | 0 | 1.50 ± 1.23 | 0.00 ± 0.00 | 0 |
| P02753 | Retinol-binding protein 4 | 2.09 ± 0.97 | 0.71 ± 1.30 | 0.34 | 2.09 ± 0.97 | 0.86 ± 0.96 | 0.41 |
| O75326 | Semaphorin-7A | 0.98 ± 1.59 | 0.00 ± 0.00 | 0 | 0.98 ± 1.59 | 0.00 ± 0.00 | 0 |
| P02787 | Serotransferrin | 74.79 ± 23.85 | 31.40 ± 9.50 | 0.42 | 74.79 ± 23.85 | 30.22 ± 9.85 | 0.4 |
| P00441 | Superoxide dismutase [Cu-Zn] | 2.93 ± 1.87 | 0.19 ± 0.41 | 0.06 | 2.93 ± 1.87 | 0.25 ± 0.72 | 0.09 |
| P05452 | Tetranectin | 2.53 ± 1.41 | 0.00 ± 0.00 | 0 | 2.53 ± 1.41 | 0.00 ± 0.00 | 0 |
| P25311 | Zinc-alpha-2-glycoprotein | 8.92 ± 2.57 | 0.00 ± 0.00 | 0 | 8.92 ± 2.57 | 0.52 ± 1.12 | 0.06 |
| P06727 | Apolipoprotein A-IV | 0.11 ± 0.32 | 5.51 ± 4.11 | 50.09 | |||
| P02649 | Apolipoprotein E | 1.04 ± 1.80 | 3.92 ± 2.74 | 3.77 | |||
| O43809 | Cleavage and polyadenylation specificity factor subunit 5 | 0.98 ± 0.68 | 0.21 ± 0.68 | 0.21 | |||
| P01619 | Immunoglobulin kappa variable 3–20 | 1.06 ± 1.34 | 0.00 ± 0.00 | 0 | |||
| P24592 | Insulin-like growth factor-binding protein 6 | 1.88 ± 1.31 | 0.23 ± 0.72 | 0.12 | |||
| Q9UBM4 | Opticin | 0.09 ± 0.26 | 0.64 ± 0.74 | 7.11 | |||
| P0CG47 | Polyubiquitin-B | 1.54 ± 1.41 | 0.10 ± 0.33 | 0.06 | |||
| P0CG48 | Polyubiquitin-C | 1.54 ± 1.41 | 0.10 ± 0.33 | 0.06 | |||
| Q9ULS6 | Potassium voltage-gated channel subfamily S member 2 | 0.11 ± 0.32 | 0.65 ± 0.75 | 5.91 | |||
| P62979 | Ubiquitin-40S ribosomal protein S27a | 1.54 ± 1.41 | 0.10 ± 0.33 | 0.06 | |||
| P62987 | Ubiquitin-60S ribosomal protein L40 | 1.54 ± 1.41 | 0.10 ± 0.33 | 0.06 | |||
| P61769 | Beta-2-microglobulin | 5.22 ± 2.45 | 2.02 ± 1.83 | 0.39 | |||
| P0C0L4 | Complement C4-A | 0.41 ± 0.82 | 2.16 ± 2.53 | 5.27 | |||
| P0C0L5 | Complement C4-B | 0.41 ± 0.82 | 2.16 ± 2.53 | 5.27 | |||
| P41222 | Prostaglandin-H2 D-isomerase | 11.39 ± 1.97 | 8.00 ± 1.65 | 0.71 |
Single risk, patients with the DM or smoking risk factor; double risk, patients with both the DM and smoking risk factors; control, cataract patients with neither of these cataract risk factors; Spc, spectral count.
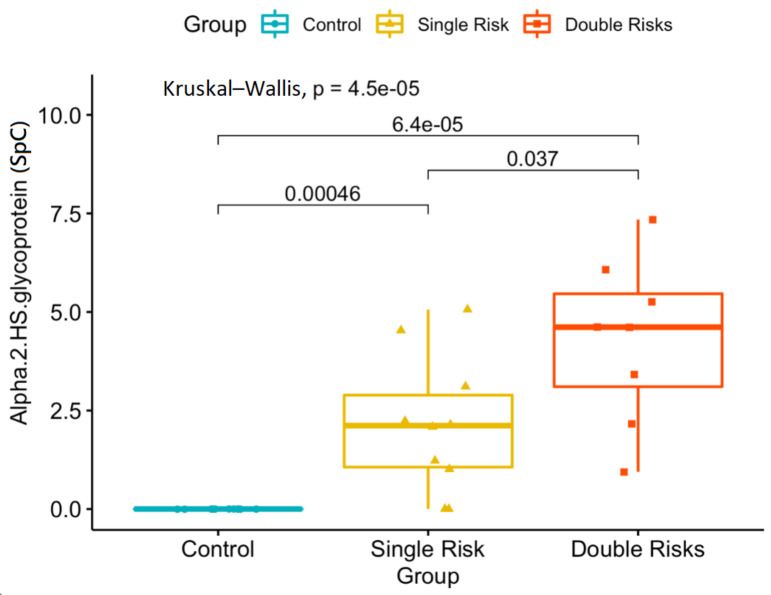
In a comparison of the double-risk-factor group with the cataract control group, 40 proteins were statistically significantly (p < 0.05) expressed (Table 4). Among the 51 proteins that were significantly changed, 10 proteins were increased in the single- or double-risk groups, including 26S proteasome non-ATPase regulatory subunit 1, alpha-2-HS-glycoprotein, apolipoprotein A-I, apolipoprotein A-II, apolipoprotein A-IV, apolipoprotein E, opticin, potassium voltage-gated channel subfamily S member 2, complement C4-A, and complement C4-B. Another 41 proteins exhibited decreased expression in the single- or double-risk groups compared to cataract controls (Table 4). In particular, alpha-2-HS-glycoprotein was the only one that presented a significant change among all three of the groups (cataract control vs. single: p = 0.00338; cataract control vs. double: p = 0.00062; single vs. double: p = 0.03309), which demonstrated an increasing trend with increase in risk (Figure 4).
Figure 4.
Proteomics analysis revealed significant concentration changes in the alpha-2-HS-glycoprotein (SpC, spectral count) among the three groups. Single risk, patients with the diabetes mellitus (DM) or smoking risk factor; double risk, patients with both the DM and smoking risk factors; control, cataract patients with neither of these cataract risk factors.
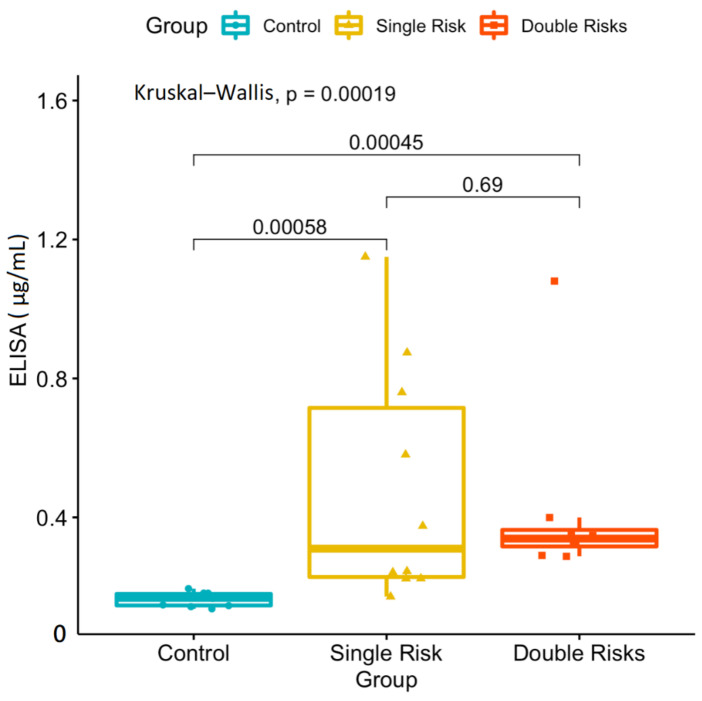
Furthermore, we performed an ELISA analysis to determine the concentration of alpha-2-HS-glycoprotein. Compared to the cataract control group, the average concentration of alpha-2-HS-glycoprotein was significantly higher in single-risk-factor group (0.43 μg/mL) patients (0.16 μg/mL) (p = 0.002) (Figure 5).
Figure 5.
ELISA analysis of significant concentration (μg/mL) changes of the alpha-2-HS-glycoprotein between risk factor and cataract control groups. However, there was no significant concentration change between the single- and double-risk-factor groups. Single risk, patients with the diabetes mellitus (DM) or smoking risk factor; double risk, patients with both the DM and smoking risk factors; control, cataract patients with neither of these cataract risk factors.
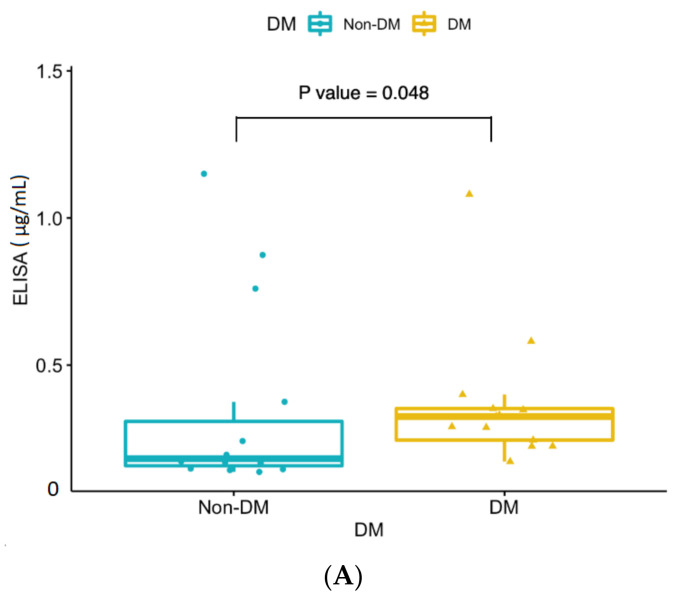
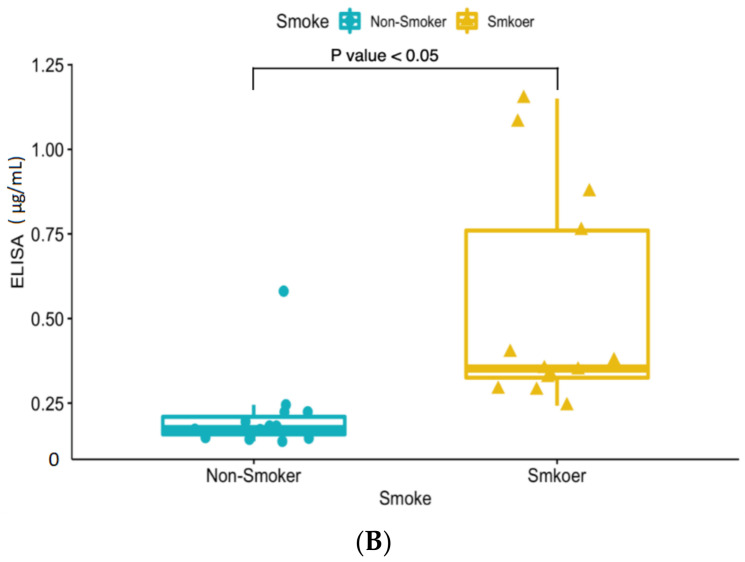
Furthermore, the average concentration significantly increased in double-risk-factor group (0.43 μg/mL) patients compared to the cataract control group (0.16 μg/mL) (p < 0.001) (Figure 5). The ELISA analysis revealed significant concentration changes between the risk factor and cataract control groups. However, there was no significant concentration change between the single- and double-risk-factor groups. A subgroup analysis was performed to confirm that DM and smoking risk factors significantly influenced the ELISA concentration compared to the cataract control group (Figure 6).
Figure 6.
(A) ELISA analysis of significant concentration (μg/mL) changes in the alpha-2-HS-glycoprotein between the diabetes mellitus (DM) groups and cataract control group. (B) ELISA analysis of significant concentration (μg/mL) changes in the alpha-2-HS-glycoprotein between the smoking groups and cataract control group. DM group (n = 13): DM single-risk patients (n = 5) + double-risk patients (n = 8); Non-DM group (n = 14): smoking single-risk patients (n = 5) + cataract control group (n = 9); Smokers (n = 13): smoking single-risk patients (n = 5) + double-risk patients (n = 8); Non-smokers (n = 14): DM single-risk patients (n = 5) + cataract control group (n = 9).
In our study, we analyzed the aqueous protein contents of the AH samples of single-risk and double-risk patients and a control group (with cataract) using label-free n-UPLC-MS/MS quantitation. We reported that in cataract patients with different risk profiles, 51 AH proteins were significantly changed compared to cataract controls. The alpha-2-HS-glycoprotein was significantly differently expressed between risk groups and cataract controls and could be a potential aqueous protein marker for detecting smoking and DM cataract risk factors. The increased levels of total protein concentrations were reported in the AH, which provides a possible marker to monitor the AH of cataract risk exposure. Note that additional studies exploring the roles of this protein in the development or the pathogenesis molecular pathway of cataracts would be beneficial. To our knowledge, this is the first study to analyze how cataract risk factors influenced the AH in the development of cataract disease. We reported that only one protein had significantly changed, which was the alpha-2-HS-glycoprotein; its expression increased in the presence of risk factors. Alpha-2-HS-glycoprotein, known as fetuin-A, was reported to be a systemic inhibitor of precipitation of basic calcium phosphate, thereby preventing unwanted calcification [15] and influencing the mineral phase of bone [16]. The alpha-2-HS-glycoprotein is synthesized in the liver, electively concentrated in the bone matrix, and secreted in plasma. The dysfunction of the gene represented by this entry is associated with alopecia-mental retardation syndrome [17]. There was previous evidence demonstrating that the alpha-2-HS-glycoprotein was present in the rabbit AH following two different cataract surgery incision procedures [18]; furthermore, there were significant decreases in the AH of 5-year-old buphthalmic rabbits [19] but not in the 2-year-old group, demonstrating that alpha-2-HS-glycoprotein alters with pathologic changes in DM, anterior lens capsule, and the angular meshwork. In humans, it was shown to be an inhibitor of transforming growth factor (TGF)-β2 [20], a protein that shows increased expression in the trabecular meshwork (TM) in open-angle glaucoma causing extracellular matrix (ECM) deposition in the human TM [21]. The alpha-2-HS-glycoprotein inhibits bone morphogenetic proteins that are changed in the TM in open-angle glaucoma [22]. This evidence suggests the potential interactions of the alpha-2-HS-glycoprotein with multiple proteins that are important in open-angle glaucoma. However, there is scarce evidence demonstrating a relationship between the alpha-2-HS-glycoprotein and cataract disease in human beings to date. Interestingly, the serum levels of alpha-2-HS-glycoprotein, called fetuin-A, are known to be highly associated with DM in humans. Initially, it was discussed in the context of preventing glucose toxicity in early 2002 [23,24]. Then, in the past two decades, the alpha-2-HS-glycoprotein was linked to insulin resistance, obesity, and cardiovascular diseases [25,26,27,28,29,30,31]. Guo et al. and Roshanzamir et al. revealed evidence using meta-analyses that higher serum alpha-2-HS-glycoprotein levels are associated with increased risk of type 2 DM [32,33]. All these previous studies reported the correlation of alpha-2-HS-glycoprotein levels in urine [34] or serum [35] with diabetes. Yuksel et al. performed a serum and AH alpha-2-HS-glycoprotein (fetuin-A) level comparison in pseudoexfoliation syndrome (PEXS) patients [36]. They found significantly increased alpha-2-HS-glycoprotein levels in the AH of patients with PEXS, but no correlation between the AH and serum levels of alpha-2-HS-glycoprotein between the groups. They suggested that the increase in alpha-2-HS-glycoprotein levels in the AH was due to disruption of the blood–aqueous barrier because of the hypoperfusion and anterior chamber hypoxia in PEXS. Thus, until now there was scarce evidence to prove that the serum level of alpha-2-HS-glycoprotein was associated with that in AH. However, our results are the first to report that human aqueous levels of the alpha-2-HS-glycoprotein are associated with diabetes risk factors for cataract formation. The ELISA confirmation of aqueous alpha-2-HS-glycoprotein levels confirmed these results. In certain diabetic patients, we provide a novel way of thinking about changes in alpha-2-HS-glycoprotein levels in the circulation and in the aqueous fluid. We suggest that the alpha-2-HS-glycoprotein could be an aqueous-specific marker of cataract risk, which is highly associated with diabetes. The alpha-2-HS-glycoprotein is known as an immune-reactive protein that was determined to be smoking- and age-associated with the development of head and neck cancers. The consistent association of chronic smoking shows an immune reactivity status that changes the serum levels of alpha-2-HS-glycoprotein in head and neck cancer patients [37]. Marechal et al. demonstrated a negative correlation between serum fetuin-A levels and a history of smoking, in which fetuin-A levels were determined by a common haplotype of the AHSG gene, low plasma cholesterol, and a history of smoking in renal transplant recipients [38]. They considered that it might reflect consequences of tobacco smoking on liver function, physical activity, or weight loss, which increased aortic calcification and risk of cardiovascular events in renal transplant recipients. These previous studies support our result that the alpha-2-HS-glycoprotein may be associated with the smoking habit. We considered that the alpha-2-HS-glycoprotein could be an aqueous-specific marker of cataract risks that is highly associated with smoking. However, multiple limitations of this study should be reported. First, only eight to ten samples in each group were investigated, and future large-scale studies could help confirm our results. The small sample numbers may be attributed to ELISA, which could not validate the proportional results of aqueous alpha-2-HS-glycoprotein levels in the three groups. Second, only a small amount of AH could be obtained because of anatomical features, which limited our ability to conduct subsequent validation assays. Third, the development of multiplex immunoassays can be improved. Finally, we can only provide the results of proteomic and ELISA data correlated with smoking and DM risk factors. The exact pathway by which the alpha-2-HS-glycoprotein is involved in cataract pathogenesis remains unclear. More future investigations of molecular pathways are required to discuss how and why the proteomics data varied with smoking and DM, and finally to supply better knowledge of cataracts for the whole of humanity. More studies are also required to analyze the alpha-2-HS-glycoprotein levels in AH of non-diabetic cataract patients, along with further serum and AH comparison analyses of cataract patients with diabetes. In conclusion, our results are from a pioneering exploration of the protein profile for the risk factors involved in cataracts. Cataracts form because of a complicated pathological process involving several proteins that participate in immune reactions and metabolic processes that were identified in AH using a proteomics analysis. The alpha-2-HS-glycoprotein, called fetuin-a, could be a potential aqueous biomarker associated with DM and smoking, which are cataract risk factors. Additional studies are required to complete the analysis and to understand the functions of these cataract-specific proteins, which could provide significant information for the diagnosis, clinical treatment, and prognosis of cataracts.
Author Contributions
Conceptualization, W.-C.C. and C.-W.C.; methodology, C.-C.L. and C.-W.C.; software, C.-C.L. and C.-H.L.; validation, W.-C.C., C.-W.C. and S.-H.C.; formal analysis, C.-H.L.; investigation, W.-C.C.; writing—original draft preparation, W.-C.C.; writing—review and editing, C.-W.C.; visualization, C.-C.L.; supervision, C.-W.C.; funding acquisition, W.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Taoyuan General Hospital, Ministry of Health and Welfare (TYGH109009 and 30 April 2020) (Taoyuan, Taiwan).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rao G.N., Khanna R., Payal A. The global burden of cataract. Curr. Opin. Ophthalmol. 2011;22:4–9. doi: 10.1097/ICU.0b013e3283414fc8. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y.C., Wilkins M., Kim T., Malyugin B., Mehta J.S. Cataracts. Lancet. 2017;390:600–612. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- 3.Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 4.Mukesh B.N., Le A., Dimitrov P.N., Ahmed S., Taylor H.R., McCarty C.A. Development of cataract and associated risk factors: The Visual Impairment Project. Arch. Ophthalmol. 2006;124:79–85. doi: 10.1001/archopht.124.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Foster P.J., Wong T.Y., Machin D., Johnson G.J., Seah S.K.L. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the Chinese population of Singapore: The Tanjong Pagar Survey. Br. J. Ophthalmol. 2003;87:1112–1120. doi: 10.1136/bjo.87.9.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker C., Schneider C., Aballéa S., Bailey C., Bourne R., Jick S., Meier C. Cataract in patients with diabetes mellitus-incidence rates in the UK and risk factors. Eye. 2018;32:1028–1035. doi: 10.1038/s41433-017-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan C.W., Boey P.Y., Cheng C.Y., Saw S.M., Tay W.T., Wang J.J., Tan A.G., Mitchell P., Wong T.Y. Myopia, axial length, and age-related cataract: The Singapore Malay eye study. Investig. Ophthalmol. Vis. Sci. 2013;54:4498–4502. doi: 10.1167/iovs.13-12271. [DOI] [PubMed] [Google Scholar]
- 8.Kyselova Z. Mass spectrometry-based proteomics approaches applied in cataract research. Mass. Spectrom. Rev. 2011;30:1173–1184. doi: 10.1002/mas.20317. [DOI] [PubMed] [Google Scholar]
- 9.Truscott R.J., Friedrich M.G. Old proteins and the Achilles heel of mass spectrometry. The role of proteomics in the etiology of human cataract. Proteom. Clin. Appl. 2014;8:195–203. doi: 10.1002/prca.201300044. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B.N., Wu X., Dai Y., Qi B., Fan C., Huang Y. Proteomic analysis of aqueous humor from cataract patients with retinitis pigmentosa. J. Cell. Physiol. 2021;236:2659–2668. doi: 10.1002/jcp.30031. [DOI] [PubMed] [Google Scholar]
- 11.Bennett K.L., Funk M., Tschernutter M., Breitwieser F.P., Planyavsky M., Mohien C.U., Müller A., Trajanoski Z., Colinge J., Giulio S.F., et al. Proteomic analysis of human cataract aqueous humour: Comparison of one-dimensional gel LCMS with two-dimensional LCMS of unlabelled and iTRAQ(R)-labelled specimens. J. Proteom. 2011;74:151–166. doi: 10.1016/j.jprot.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Schey K.L., Wang Z., Friedrich M.G., Garland D.L., Truscott R.J.W. Spatiotemporal changes in the human lens proteome: Critical insights into long-lived proteins. Prog. Retin. Eye Res. 2020;76:1008–1902. doi: 10.1016/j.preteyeres.2019.100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y., Rong X., Ye H., Zhang K., Lu Y. Proteomic analysis of aqueous humor proteins associated with cataract development. Clin. Biochem. 2015;48:1304–1309. doi: 10.1016/j.clinbiochem.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim T.W., Kang J.W., Ahn J., Lee E.K., Cho K.C., Han B.N.R., Hong N.Y., Park J., Kim K.P. Proteomic analysis of the aqueous humor in age-related macular degeneration (AMD) patients. J. Proteome Res. 2012;11:4034–4043. doi: 10.1021/pr300080s. [DOI] [PubMed] [Google Scholar]
- 15.Heiss A., Chesne A.D., Denecke B., Grötzinger J., Yamamoto K., Renné T., Dechent W.J. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 2003;278:1333–1341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 16.Lee C.C., Bowman B.H., Yang F.M. Human alpha 2-HS-glycoprotein: The A and B chains with a connecting sequence are encoded by a single mRNA transcript. Proc. Natl. Acad. Sci. USA. 1987;84:4403–4407. doi: 10.1073/pnas.84.13.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reza Sailani M., Jahanbani F., Nasiri J., Behnam M., Salehi M., Sedghi M., Hoseinzadeh M., Takahashi S., Zia A., Gruber J., et al. Association of AHSG with alopecia and mental retardation (APMR) syndrome. Hum. Genet. 2017;136:287–296. doi: 10.1007/s00439-016-1756-5. [DOI] [PubMed] [Google Scholar]
- 18.Stastna M., Behrens A., McDonnell P.J., Jennifer E.V.E. Analysis of protein composition of rabbit aqueous humor following two different cataract surgery incision procedures using 2-DE and LC-MS/MS. Proteome Sci. 2011;9:8. doi: 10.1186/1477-5956-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edward D.P., Bouhenni R. Anterior segment alterations and comparative aqueous humor proteomics in the buphthalmic rabbit (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2011;109:66–114. [PMC free article] [PubMed] [Google Scholar]
- 20.Szweras M., Liu D., Partridge E.A., Pawling J., Sukhu B., Clokie C., Dechent W.J., Tenenbaum H.C., Swallow C.J., Grynpas M.D., et al. Alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 21.Wordinger R.J., Fleenor D.L., Hellberg P.E., Pang I.H., Tovar T.O., Zode G.S., Fuller J.A., Clark A.F. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: Implications for glaucoma. Investig. Ophthalmol. Vis. Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 22.Umulis D., O’Connor M.B., Blair S.S. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnaud P., Kalabay L. Alpha2-HS glycoprotein: A protein in search of a function. Diabetes Metab. Res. Rev. 2002;18:311–314. doi: 10.1002/dmrr.315. [DOI] [PubMed] [Google Scholar]
- 24.Ren J., Davidoff A.J. Alpha2-Heremans Schmid glycoprotein, a putative inhibitor of tyrosine kinase, prevents glucose toxicity associated with cardiomyocyte dysfunction. Diabetes Metab. Res. Rev. 2002;18:305–310. doi: 10.1002/dmrr.299. [DOI] [PubMed] [Google Scholar]
- 25.Trepanowski J.F., Mey J., Varady K.A. Fetuin-A: A novel link between obesity and related complications. Int. J. Obes. 2015;39:734–741. doi: 10.1038/ijo.2014.203. [DOI] [PubMed] [Google Scholar]
- 26.Jung T.W., Yoo H.J., Choi K.M. Implication of hepatokines in metabolic disorders and cardiovascular diseases. BBA Clin. 2016;5:108–113. doi: 10.1016/j.bbacli.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dabrowska A.M., Stanislaw Tarach J.S., Duma B.W., Duma D. Fetuin-A (AHSG) and its usefulness in clinical practice. Review of the literature. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2015;159:352–359. doi: 10.5507/bp.2015.018. [DOI] [PubMed] [Google Scholar]
- 28.Singh M., Sharma P.K., Garg V.K., Mondal S.C., Singh A.K., Kumar N. Role of fetuin-A in atherosclerosis associated with diabetic patients. J. Pharm. Pharmacol. 2012;64:1703–1708. doi: 10.1111/j.2042-7158.2012.01561.x. [DOI] [PubMed] [Google Scholar]
- 29.Rasul S., Wagner L., Kautzky-Willer A. Fetuin-A and angiopoietins in obesity and type 2 diabetes mellitus. Endocrine. 2012;42:496–505. doi: 10.1007/s12020-012-9754-4. [DOI] [PubMed] [Google Scholar]
- 30.Mori K., Emoto M., Inaba M. Fetuin-A and the cardiovascular system. Adv. Clin. Chem. 2012;56:175–195. doi: 10.1016/b978-0-12-394317-0.00010-8. [DOI] [PubMed] [Google Scholar]
- 31.Horshuns’ka M., Karachentsev I.L., Kravchun N.O., Ĭensen E., Leshchenko Z.A., Hladkykh O.I., Krasova N.S., Tyzhnenko T.V., Opaleĭko I.A., Poltorak V.V. Biological role of fetuin A and its potential importance for prediction of cardiovascular risk in patients with type 2 diabetes mellitus. Ukr. Biokhim. Zh. 2013;85:10–21. [PubMed] [Google Scholar]
- 32.Guo V.Y., Cao B., Cai C., Cheng K.K.Y., Cheung B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018;55:87–98. doi: 10.1007/s00592-017-1068-9. [DOI] [PubMed] [Google Scholar]
- 33.Roshanzamir F., Miraghajani M., Rouhani M.H., Mansourian M., Ghiasvand R., Safavi S.M. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: Systematic review and meta-analysis of observational studies. J. Endocrinol. Investig. 2018;41:33–47. doi: 10.1007/s40618-017-0697-8. [DOI] [PubMed] [Google Scholar]
- 34.Inoue K., Wada J., Eguchi J., Nakatsuka A., Teshigawara S., Murakami K., Ogawa D., Takahiro T. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS ONE. 2013;8:e77118. doi: 10.1371/journal.pone.0077118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou H.Y., Yang Y.C., Wu H.T., Wu J.S., Lu F.H., Chang C.J. Serum fetuin-A concentrations are elevated in subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes. Clin. Endocrinol. 2011;75:450–455. doi: 10.1111/j.1365-2265.2011.04070.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuksel N., Takmaz T., Turkcu U.O., Ergin M., Altinkaynak H., Bilgihan A. Serum and Aqueous Humor Levels of Fetuin-A in Pseudoexfoliation Syndrome. Curr. Eye Res. 2017;42:1378–1381. doi: 10.1080/02713683.2017.1324629. [DOI] [PubMed] [Google Scholar]
- 37.Wolf G.T., Chretien P.B., Weiss J.F., Edwards B.K., Spiegel H.E. Effects of smoking and age on serum levels of immune reactive proteins. Otolaryngol. Head Neck Surg. 1982;90:319–326. [PubMed] [Google Scholar]
- 38.Marechal C., Schlieper G., Nguyen P., Krüger T., Coche E., Robert A., Floege J., Goffin E., Jadoul M., Devuyst O. Serum fetuin-A levels are associated with vascular calcifications and predict cardiovascular events in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2011;6:974–985. doi: 10.2215/CJN.06150710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.