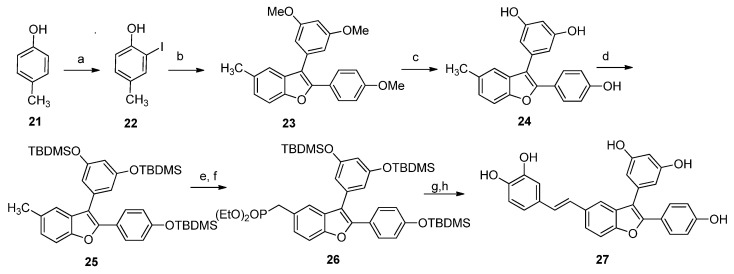
Scheme 4.
Reagents and conditions: (a) (i) p-TsOH∙H2O, ACN, rt, 10 min; (ii) NIS, rt, overnight, 97%; (b) (i) 4-ethynianisole, PdCl2(PPh3)2∙DCM, CuI, THF/TEA 1:3, rt, MW, 30 min, (ii) 3,5-dimethoxy-1-iodobenzene, ACN, 100 °C, MW, 25 min, 48%; (c) BBr3 1 M DCM, DCM, −78 °C to rt, overnight, 90%; (d) TBDMSCl, imidazole, DCE, 60 °C, 8 h, 86%; (e) NBS, AIBN, CCl4, reflux, 8 h, 37%; (f) P(OEt)3, 130 °C, overnight, 84%; (g) 4-bis((tert-butyldimethylsilyl)oxy)benzaldehyde, NaH, THF, 0 °C to rt, 24 h, 52%; (h) TBAF, THF, 0 °C to rt, 2 h, 60%.