Abstract
Simple Summary
Malaria fever kills millions of people annually in the tropical and subtropical countries of Africa and Asia. Because there is no effective vaccine, malaria prevention is exclusively dependent on avoiding human-vector interaction. The interaction of Vitex negundo essential oil constituents with Anopheles gambiae Odorant Binding Proteins (OBP), as well as its compositional variation, repellent efficacy, and toxicity profile, are investigated in this work. The oils were subjected to GC-MS analysis, a mosquito behavioral test, OBP-ligand interactions, Anopheles species authentication, and toxicity profile. Docking protocol validation was achieved by redocking the co-crystallized ligands and root mean square deviation (RMSD) calculation. The oil yields and compositions are climate–soil dependent with ≈71.39% monoterpenes and ≈16.32% sesquiterpene. Optimal repellency is achieved at 15 min at ED50 0.08–0.48% v/v while the RMSD was estimated to be within 0.24–1.35 Å. Strong affinities, −6.4 to −5.4 kcal/mol, were demonstrated by α-pinene, citronellal, linalool, and myrcene for OBP1, OBP7, OBP4, and OBP. respectively. The hydrophobic interactions involve Leu17, Cys35, ALA52, Leu73, Leu76, Ala88, Met91, Lys93, Trp114, Phe123, and Leu124 receptors on α-helixes 1–7 within the binding cavities, and may block the olfactory receptors resulting in disorientation. α-pinene, linalool, and myrcene are safe and suitable for use in the development of green and innovative repellents because their ligand efficiency metrics, ADME/tox, and repellency screening are all within the threshold values.
Abstract
(1) Background: Malaria fever affects millions of people yearly in Africa and Asia’s tropical and subtropical areas. Because there is no effective vaccine, malaria prevention is solely dependent on avoiding human-vector interaction. (2) Aim: This study examines the interaction between the constituents of Vitex negundo essential oil and Anopheles gambiae Odorant Binding Proteins (OBP) as well as the compositional variation, repellent efficacy, and toxicity profile. (3) Methods: The oils were subjected to GC-MS and mosquito behavioral analysis. OBP–ligand interactions, Anopheles species authentication, and the toxicity profile were determined by molecular docking, PCR assay and in silico ADME/tox tool. Docking protocol validation was achieved by redocking the co-crystallized ligands into the protein binding pocket and root mean square deviation (RMSD) calculation. (4) Results: The oil yields and compositions are climate–soil dependent with ≈71.39% monoterpenes and ≈16.32% sesquiterpene. Optimal repellency is achieved at 15 min at ED50 0.08–0.48% v/v while the RMSD was estimated to be within 0.24–1.35 Å. Strong affinities were demonstrated by α-pinene (−6.4 kcal/mol), citronellal (−5.5 kcal/mol), linalool (−5.4 kcal/mol), and myrcene (−5.8 kcal/mol) for OBP1, OBP7, OBP4, and OBP; respectively. The hydrophobic interactions involve Leu17 (α-helix 1), Cys35 (α-helix 2), ALA52 (α-helix 3), Leu73, Leu76 (α-helix 4), Ala88, Met91, Lys93, Trp114 (α-helix 5), Phe123 (α-helix 6), and Leu124 (α-helix 7) receptors within the binding cavities, and may cause blocking of the olfactory receptors resulting in disorientation. (5) Conclusion: The ligand efficiency metrics, ADME/tox and repellency screening are within the threshold values; hence, α-pinene, linalool, and myrcene are safe and fit-to-use in the development of a green and novel repellent.
Keywords: mosquito, repellent, V. negundo, molecular docking, odorant binding proteins, ligand efficiency metric, essential oil, Anopheles gambiae
1. Introduction
Malaria has been and continues to be one of the world’s leading causes of mortality. Despite the fact that the disease has been eradicated in the United States, it remains a global health threat. Malaria affects an estimated 300 to 500 million people worldwide each year, resulting in 1.5 to 2.7 million fatalities. Sub-Saharan Africa, on the other hand, accounts for about 90% of all malaria cases worldwide [1], followed by the World Health Organization (WHO) South-East Asia Region (3.4%) and Eastern Mediterranean Region (2.1%). Nearly 85% of the worldwide malaria burden was carried by 19 nations in Sub-Saharan Africa and India. Nigeria (25%), the Democratic Republic of the Congo (12%), Uganda (5%), Côte d’Ivoire (4%), Mozambique (4%), and Niger (4%) accounted for more than half of all malaria cases globally [2]. In addition, one to two million people suffering from malaria die from the disease, the majority of whom are children under the age of five and pregnant women [3]. Unfortunately, 90% of malaria deaths occur in Sub-Saharan Africa, where approximately 3000 people die each year. The Nigerian situation is consistent with the global trend, as malaria is the leading cause of death among pregnant women in the country, accounting for one out of every ten deaths [4,5].
Malaria is caused by a species of Plasmodium which is vectored by the adult female Anopheles mosquito that thrives in hot and humid climates around the world [6]. The Anopheles gambiae complex consists of at least seven morphologically similar but six genetically and behaviorally distinct mosquito species from the genus Anopheles. The complex contains the most important malaria vectors in Sub-Saharan Africa, especially those that transmit Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, Plasmodium falciparum, and Plasmodium knowlesi [7]. A. gambiae is more than just a pest and a nuisance; it is also responsible for the spread of malaria and other deadly diseases across Africa.
Quinoline-based drugs were the mainstay of malaria treatment and prevention for decades. Unfortunately, the emergence of drug-resistant Plasmodium species as a result of mutation has rendered conventional therapeutic treatment ineffective [8,9]. WHO, on the other hand, has approved insecticides, fumigation, air shields, ultrasonic rays, pesticide spraying, insecticidal treated nets, and insecticidal-treated clothing as malaria prevention measures [10]. For a variety of reasons, insect repellents such as lotions, coils, mosquito-treated nets, and liquidators have limited efficiency [11,12]. However, consumers have recently become more interested in cheap plant-based commercial repellents, which are frequently considered as “safe” when compared to synthetic repellents; this is not always the case depending on the secondary metabolites present [13]. Plant-based metabolites have been shown to be an effective mosquito control alternative to synthetic insecticides or when used in conjunction with other pesticides as part of an integrated vector control strategy [14].
Several plant extracts have been reported to have mosquitocidal growth regulator activity, as well as insecticidal properties in the elimination of larval or adult mosquitos or as mosquito repellents to protect against mosquito bites [13,15,16]. Vitex negundo L. (Verbenaceae) is a well-known medicinal herb [17,18,19,20], which has been linked to a variety of pharmacological properties, including enzyme inhibition [21,22], antifeeding [23], larvicidal [24], and mosquito repellent activities [15].
Research on synthetic insect repellents has developed due to breakthroughs in our understanding of mosquito behavior and olfactory receptors [25], but there have been insufficient studies on the safety of plant-based mosquito repellents and their interaction with olfactory receptors. The olfactory system of insects is made up of a number of transmembrane odorant receptor proteins that are expressed in olfactory membrane neurons all throughout their bodies [26]. Odorant binding proteins (OBPs) are potent mosquito biosensors, according to Di Pietrantonio et al. [27], Sankaran et al. [28], and Possas-Abreu et al. [29]. Odorant-binding protein 1 (OBP1) (PDB ID 3N7H) and (OBP:PDB ID 2ERB), odorant-binding protein 7 (OBP7: PDB ID 3R1O), and odorant-binding protein 4 (OBP4: PDB ID 3Q8I) are the key olfactory proteins involved in signals for host recognition process, repellent alarm pheromone, and diphenyl ester-specific binding protein, respectively.
More systematic research is needed to better evaluate plant-based repellents and produce novel solutions that are safe for consumers. This study investigates the major constituents of V. negundo essential oil in order to establish repellent efficacy, predict their in-silico toxicity profile, and determine the interactions with Anopheles odorant binding proteins using a molecular docking-based technique.
2. Materials and Methods
2.1. Collection Sites and Identification of V. negundo Leaves
The leaves of V. negundo were harvested in September 2020 from six states of the North-Central Geopolitical Zone of Nigeria with the climatic condition and major soil type presented in Table 1. Samples of the leaves were identified at the Department of Medicinal Plant Research and Traditional Medicine, National Institute of Pharmaceutical Research and Development (NIPRD) Idu, Abuja and voucher specimens NIPRD/Hebarium/1101 were deposited.
Table 1.
Grid box information for the selection of the active pockets of the four odorant binding proteins.
| Centre | Dimension | |||||
|---|---|---|---|---|---|---|
| Proteins | Center_x | Center_y | Center_z | Size_x | Size_y | Size_z |
| 3N7H | 4.552872 | 15.28167 | −12.214 | 58.59585 | 78.51029 | 118.6278 |
| 3R1O | 4.1755 | −10.0047 | 18.80124 | 49.47825 | 50.92539 | 68.14412 |
| 3Q8I | 5.995551 | 1.440093 | 14.84848 | 49.47825 | 49.98114 | 46.37546 |
| 2ERB | 2.997585 | −0.91365 | −39.2475 | 42.39479 | 43.98579 | 64.02445 |
2.2. Leaf Processing and Extraction of Essential Oils
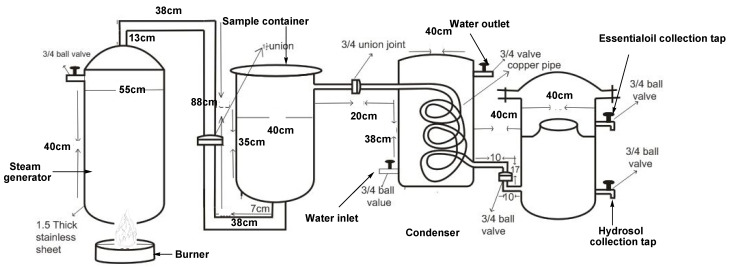
Collected fresh V. negundo leaves were washed with tap water and extracted within 12 h of collection using a 25 kg capacity fabricated Essential oil Distillation System (EDS) based on the steam distillation principle (Figure 1). The EDS steam generator was filled with 50 L of distilled water while the sample container was loaded to capacity and distilled over a period of 45 min. The distillate was recovered and separated in batches using a 2 L separatory funnel into essential oil and aqueous distillate (hydrosol), after which the essential oils were dried over anhydrous Na2SO4 and stored for further analysis. Finally, the oil yield was calculated relative to the fresh matter and the result presented as the mean ± standard deviation of triplicate extractions.
Figure 1.
Schematic of the Essential oil Distillation System (EDS).
2.3. GC-MS Profiling of the Essential Oils
The GC-MS analyses of the essential oils were performed with a Varian CP-3800 gas-chromatograph equipped with a HP-5 capillary column (30 mm × 0.25 mm; coating thickness 0.25 μm), carrier gas nitrogen at 1.2 mL/min, and a Varian Saturn 2000 ion trap mass detector. The oven temperature was programmed from 50 to 280 at 3 °C/min. Analytical conditions: injector and transfer line temperatures were 220 and 240 °C, respectively. Volume injected: 0.2 μL of 10% hexane solution, split ratio 1:30. Co-injection of the essential oil with a solution containing a similar series of C8–C22 n-alkanes yielded linear retention indices for all molecules. Retention indices were used to identify the individual components, which were then compared to compounds previously reported in the literature [30,31]. Further, the identification of the compounds was made using data of a computer library (Wiley 275L) connected to the GC-MS, Adams library (https://b-ok.cc/book/3506611/3b1f4f (accessed on 15 October 2021)), the NIST website (https://webbook.nist.gov/chemistry/ (accessed on 15 October 2021)) using RI values from comparable polarity columns, and/or the Mondello library (https://www.sisweb.com/software/wiley-ffnsc.htm (accessed on 15 October 2021)).
2.4. Sampling, Rearing, and Identification of Mosquitoes
In the months of March and April 2021, mosquito larvae were collected from selected localities in Kaduna metropolitan located between 10°33′ N of the equator and 07°27′ E of the Greenwich Meridian (Ungwan Gwari; 10°35.71′; 07°27.17′ Ungwan Romi 10°25.19′; 07°25.20′ Kamanzou 10°46.24′; 07°49.41′). The larvae were collected using a 7 cm diameter, 5 cm deep, and 30 cm long handle plastic standard dipper from a 0.12 m × 2.5 m deep temporary pool with grass vegetation. At the breeding sites, larvae identification and morphological classification were carried out. The absence of a siphon, the parallel swimming pattern on the water surface, and the morphology of the combs were used to sort larvae into the Anophelinae and Culicinae subfamilies under a compound microscope, compared to the Culex larva with a long siphon, lighter color, and “hairy” body, as well as the identification key of Gillies and Coetzee [32]. The immature larval stages were carefully transported in vials to the Insectary at the Biological Sciences laboratory, Kaduna State University, Nigeria. The larvae were then placed in an open plastic container 29 cm × 21 cm × 30 cm containing 1 L of ground water and allowed to acclimate for 2 h before being fed finely ground low-fat flour-baked food product [33].
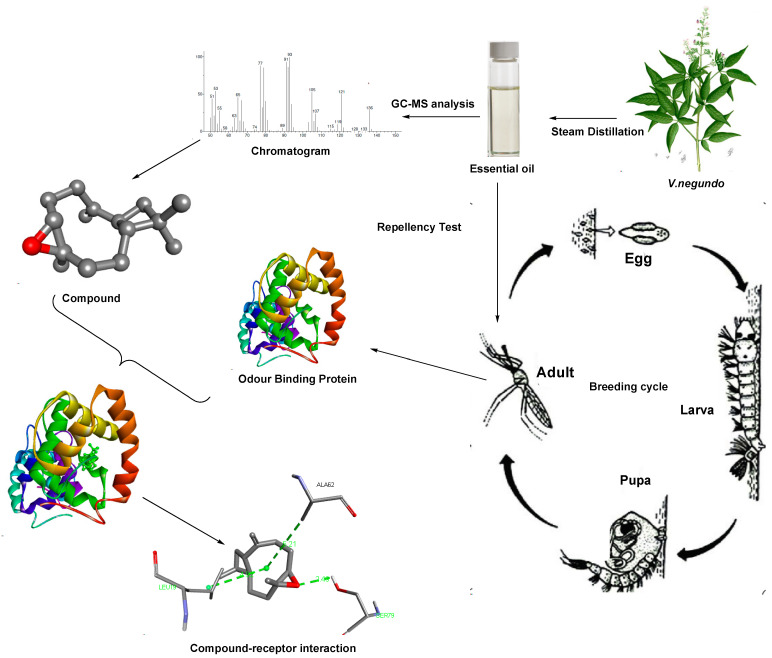
The larvae were batched in separate breeding containers and were reared to adults in separate 30 cm × 30 cm wooden made net chambers for three weeks under controlled optimum conditions of 25 °C, 65% relative humidity, and regulated light/dark (14/10 h) cycle. The emerged adults were identified morphologically using taxonomic characters such as the palps, proboscis, wing venation, and markings or tuffs on legs or abdomen as provided by the dichotomous keys employed by Coetzee and Gillies [34]. This was performed using the simple Olympus light microscope to genera and species level. The adults in the cages were fed a 10% sucrose solution after eclosion from their pupal cases and allowed to rest and mature for 2 to 3 days. Only newly emerged adult females A. gambiae were manually aspirated into a 200 mL perforated plastic container and allowed to rest for 1 hr before exposure to the essential oils (Figure 2).
Figure 2.
Graphical illustration of repellency and odorant binding protein efficiency using a molecular docking-based technique.
2.5. Anopheles Species Authentication: Genomic DNA Extraction and PCR Amplification
The emerged adult mosquitoes belonging to the A. gambiae (s.l) complex were subjected to PCR and genomic DNA assays designed for species, molecular form identification, and molecular analysis to fully authenticate the adult Anopheles species. The molecular analyses were conducted at the Centre for Biotechnology Research and Training, Ahmadu Bello University, Zaria. Genomic DNA (gDNA) was extracted from 20 Anopheles adult mosquitoes using the Quick-DNA™ Miniprep Plus Kit (D4069) product by ZYMO research company according to the protocol of the manufacturer.
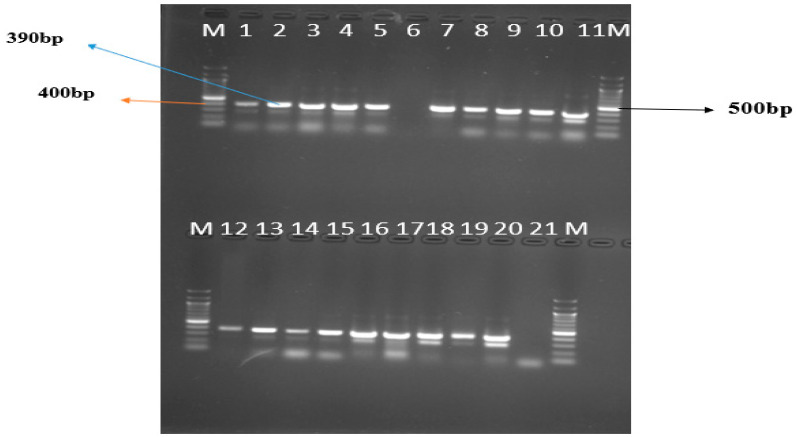
Genomic mosquito DNA (2 μL) was extracted and placed in a 0.2 mL thin-walled Eppendorf tube and 23 μL of the PCR reaction mixture containing species specific primers for A. gambiae (GA = 5′-CTGGTTTGGTCGGCACGTTT-3′), A. Arabiensis (AR = 5′-AAGTGTCCTTCTCCATCCTA-3′), and a unique primer for all species (UN = 5′-GTGTGCCCC TTCCTCGAT GT-3′) according to the protocols of Scott et al. [35] and Favia et al. [36]. Then, deoxynucleotide triphosphates, magnesium chloride, PCR buffer, distilled water, and recombinant Taq DNA polymerase were all added. Amplification was performed in an initial denaturation step at 94 °C for two minutes, then a 30-cycle denaturation at 94 °C for 30 s, annealing at 49 °C for 30 s and elongation at 68 °C for 5 min in a thermal cycler machine. The PCR amplicons ran on a 1.5% agarose gel electrophoresis gel tank and were viewed under the trans-illuminator UV light for the characteristic A. gambiae positive band sizes at 390 bp.
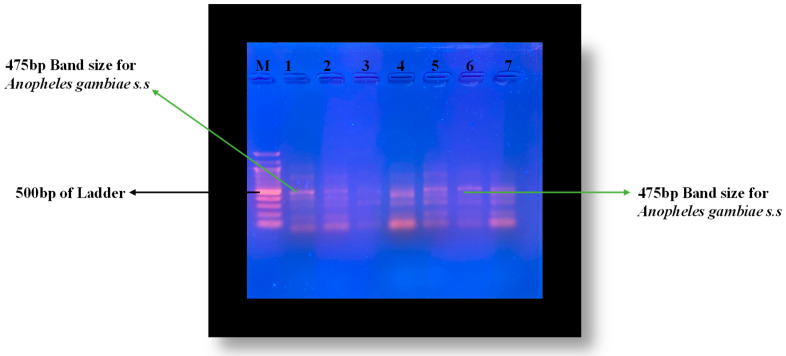
Again, from the samples that showed base pairs of 390 band sizes (A. gambiae s.l), seven out of a group of 20 were randomly selected and 1 μL picked and mixed in 24 μL of PCR reaction mixture containing species specific primers for A. gambiae s.s and coluzzi. Species identification of A. gambiae (s.l.) was performed by PCR according to Favia et al. [36]. The PCR conditions were 10 min at 94 °C as the initial step, followed by 30 cycles (94 °C for 30 s, 53 °C for 30 s, and 68 °C for 30 s). After the last cycle, the products were finally extended for 5 min at 68 °C. Primers used in the PCR were: R5 (5′-GCC AAT CCG AGC TGA TAG CGC-3′), R3 (5′-CGA ATT CTA GGG AGC TCC AG-3′), Mop int (5′-GCC CCT TCC TCG ATG GCA T-3′), and B/S int (5′-ACC AAG ATG GTT CGT TGC-3′). Amplified fragments were analyzed on a 1.5% agarose gel for the characteristic band size of 475 for A. gambiae s.s.
2.6. Mosquito Behavioral Study Repellency Y-Tube Olfactometer Test
This experiment used an olfactometer with a glass Y-tube of 1.6 cm diameter, 12-cm base, and two 40.62 cm arms at a 45° angle to one another [37]. V. negundo essential oil diluted with paraffin oil (analytical purity, Sigma-Aldrich) was added in a gradient sequence of 0.1, 0.25, 0.5, 0.75, and 1.0% v/v to a 5 g cotton ball, which was then mounted on the cotton ball holder of the test arm.
The cotton ball holder on the control arm of the glass Y-tube supported the negative control cotton ball (paraffin oil without the essential oil). At a rate of 180 mL/min, fresh air was provided by an electric air pump and filtered on a carbon and silicone base with air flowing into the respective arm of the Y-tube.
The essential oil was combined with the purified air and went through one arm of the Y-tube whereas purified air with no essential oil went through the opposite arm. Precisely, 100 female A. gambiae mosquitoes were discharged into the glass Y-tube, where behavioral responses were monitored and recorded for 1800 s [38]. In addition, to establish the mosquito behavioral activity and the degree of synergism between the pure constituents of the essential oil, selected commercially available pure constituents based on the absorption, distribution, metabolism, excretion, and toxicity (ADME/tox) and docking studies were evaluated. After each investigation, the Y-tubes were air cleaned with a stream of hot air (>60 °C), the cotton ball was removed, and the holder was cleaned. The olfactory test was repeated thrice. The repellent rate was calculated according to the following Equation (1) [39,40]:
| (1) |
The % mosquito repelled of 50% individuals (ED50) was estimated using the Probit analysis model in the IBM SPSS v.25 statistical software.
2.7. Target Protein Selection and Preparation
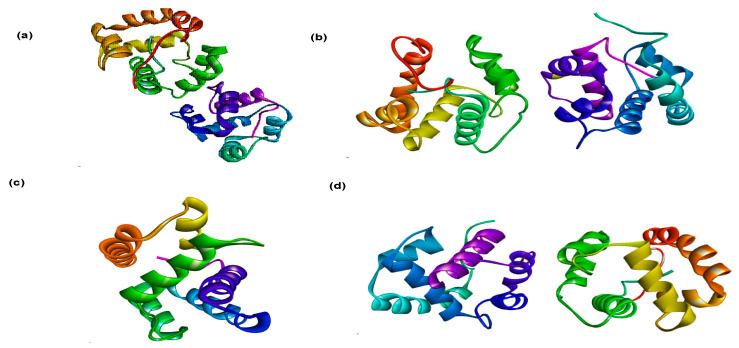
The odorant binding proteins (OBPs) were selected as a target based on their application as bio-recognition elements and biosensors for small ligands. The three-dimensional (3D) structures of four A. gambiae OBPs; OBP 1 (PDB ID 3N7H), OBP 7 (PDB ID 3R1O), OBP 4 (PDB ID 3Q8I), and OBP (PDB ID 2ERB) were retrieved from the Protein Data-bank (http://www.rcsb.org (accessed on 12 February 2021)) (Figure 3). The crystal structures of the of OBPs were processed by removing existing ligands and water molecules while missing hydrogen atoms were added according to the amino acid protonation state at pH 7.0 utilizing Autodock 4.2 (Molecular Graphics Laboratory, Scripps Research Institute, La Jolla, CA, USA). Thereafter, non-polar hydrogens were merged while polar hydrogens were added to each protein. The process was repeated for each protein and subsequently saved into a dockable format.
Figure 3.
3D structures of A. gambiae selected (a) OBP 1, (b) OBP 7, (c) OBP 4, and (d) OBP.
2.8. Ligands Preparation
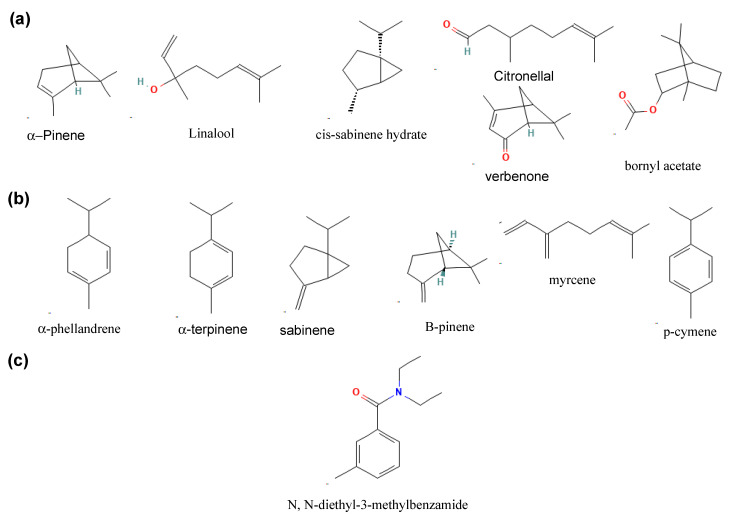
The 2D structures of the ligands subjected to docking investigation are presented in Figure 4. In this study, six ligands (α-pinene (PubChem CID 6654), linalool (PubChem CID 6549), cis-sabinene hydrate (PubChem CID 101629835), citronellal (PubChem CID 7794), verbenone (PubChem CID 29025), and bornyl acetate (PubChem CID 6448) identified through the GC-MS analysis in all essential oils irrespective of the collection location were retrieved from the PubChem database (www.pubchem.ncbi.nlm.nih.gov (accessed on 12 February 2021)) in the Structure Data Format (SDF) [41,42,43,44].
Figure 4.
2D structures of the selected ligands (a) found in all essential oils irrespective of collection site, (b) with percentage composition ≥10%, and (c) N,N-diethyl-3-methylbenzamide (DEET).
An additional six major ligands with percentage composition ≥10% were also selected as potential repellent agents: α-phellandrene (PubChem CID 7460), α-terpinene (PubChem CID 7462), sabinene (PubChem CID 18818), β-pinene (PubChem CID 440967), myrcene (PubChem CID 31253), and p-cymene (PubChem CID 7463). N,N-diethyl-3-methylbenzamide (DEET) (PubChem CID 4284) was selected as a positive control in this study since it is widely used as a chemical repellent against a variety of insects [45,46] and has strong electrophysiological responses [47]. This is reinforced by DEET’s spatial repellence, acting as a “confusant” and “stimulus” to insects, interfering with odorant detection within the olfactory receptor neurons (ORNs) or odorant receptors (ORs), resulting in avoidance behavior [48].
2.9. Molecular Docking Studies
Molecular docking was carried out using PyRx-Python Prescription 0.8 software (Hangzhou, Zhejiang, China). The input file was in the form of PDB code of the receptor or PDB file format and the molecules were in PDB file format. The output file was a docking report. The docked image was viewed by “BIOVIA Discovery Studio Visualizer” software (Waltham, MA, USA) to review the interactions between ligands and proteins, and the length of the interaction along with amino acids. The ligands were imported into PyRx 0.8 through the OpenBabel plug-in tool for each docking phase, with the Universal Force Field (UFF) as the energy minimization parameter, and conjugate gradient descent as the optimization algorithm. Table 1 shows the coordinate of the active sites of the four A. gambiae odorant binding proteins as determined by the grid boxes utilized in the docking studies.
The inhibition constant and ligand efficiency metrics were quantitatively estimated using Equations (2)–(7) [49,50,51,52,53].
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
2.10. Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADME/tox) Investigation
The ADME/tox filtering analysis of all selected ligands for docking including the physiochemical were predicted using the ADMETlab 2.0 webserver (https://admetmesh.scbdd.com/service/evaluation/index (accessed on 15 October 2021)). The canonical SMILES of the ligands downloaded from the PubChem Database were used for the calculation of the ADME/tox parameters in default mode.
3. Results and Discussion
3.1. V. negundo Essential Oil Yields, Climate and Soil Type of the Six States
Table 2 indicates the general soil types, climatic conditions, temperature, and annual rainfall range of the collection sites, as reported in previous literature [54,55,56,57,58,59], and essential oil yield of the six states. Ferralsols and hydromorphic tropical soils were the predominant soil type of Kwara, while the soils of the Plateau are poorly drained sandy clay loam surfaces with clay subsurface. The Niger, Nasarawa, and Benue are dominated by sandy loamy soil types with a clay subsoil as well, except for Kogi soil which is richly sandy and loamy.
Table 2.
V. negundo essential oil yields (%), climatic per annum and soil types of the study sites.
| States | * Essential Oil Yield (%w/w) | Coordinate | Rainfall Range (mm) | Temp. Range (°C) | Major Soil Texture |
|---|---|---|---|---|---|
| Kwara | 0.13 ± 0.01 | 8°30 N 5°00′ E | 50.8–2413.3 | 30–35 | Ferralsols and hydromorphic tropical soil |
| Kogi | 0.03 ± 0.01 | 7°30 N 6°42′ E | 1200.0–1300.0 | 15–38 | Sandy loamy |
| Benue | 0.29 ± 0.02 | 7°20 N 8°45′ E | 100.0–200.0 | 21–37 | Sandy loamy with sandy clay in the subsoil |
| Nasarawa | 0.15 ± 0.04 | 8°32 N 8°18′ E | 100.1–308.9 | 14–38 | Sandy clay loam and clay loam to clay subsurface. |
| Plateau | 0.18 ± 0.02 | 9°10 N 9°45′ E | 1317.5–1460 | 12–22 | Deep, poorly to very poorly drained sandy clay loam surfaces over clay subsurface |
| Niger | 0.48 ± 0.10 | 10°00 N 6°00′ E | 59.9–274.2 | 14–38 | Loamy sand surface horizons over sandy clay to clay subsurface horizons |
* Values are presented as mean of triplicate determination.
The oil yields were generally high for all sampled states; however, V. negundo from Niger and Kogi produced the highest and lowest essential oil yields, respectively. Besides, there was no significant difference (p > 0.05) in the yields from Nasarawa, Plateau, and Kwara. In accordance with the findings of Tirillini et al., [60], the essential oil yield and composition were influenced by Ca2+ and K+ concentrations, percentage of organic matter, and temperature. The high yields in Niger, Nasarawa, Benue, Kwara, and Plateau may be attributable to their proximity to a region with a moderately low to low annual rainfall, low temperature, and predominant loamy soil with clay substructure with significant concentrations of K+, Na+, and Ca2+ [61]. However, the significantly high yield obtained in Niger is justifiably due to the low annual rainfall (59.9–274.2 mm) compared to other regions with the same soil substructure (Table 2). On the other hand, the low yield recorded in the Kogi sample may be directly linked to the lack of clay minerals in the soil.
3.2. Chemical Composition of V. negundo Essential Oils
Table 3 shows the results of the V. negundo essential oils GC-MS analysis collected from six states and the chromatograms are presented as Supplementary Figures S1–S6. The essential oil from the various collection sites showed compositional variation. Niger, Kwara, Benue, Plateau, Kogi, and Nasarawa oil, respectively, contained 16, 18, 30, 24, 15, and 28 identified compounds. Monoterpenes made up the majority of the constituents of the essential oils across all study states with about 74.65–96.23%, followed by about 0.75–16.32% sesquiterpene content. The rest were other compounds of about 3.55–10.88%. Essential oils from Niger, Kwara, and Kogi had more than 90.04% monoterpene while oils from Plateau, Nasarawa, and Benue demonstrated the highest sesquiterpene content (7.63–16.32%). Sesquiterpene content was found to be quite low in Niger and Kwara (0.75–1.23%), with sesquiterpene completely absent in the Kogi sample. The observed compounds are in consonant with the reports of Hebbalkar et al. [17], Huang et al. [62], and Kumar et al. [63].
Table 3.
Compositional variation in the essential oils of V. negundo from the six study locations.
| Plateau | Nasarawa | Niger | Benue | Kwara | Kogi | ||||
|---|---|---|---|---|---|---|---|---|---|
| RT | % | % | % | % | % | % | Compounds | RIExp | RILit |
| 4.711 | 0.69 | α-thuiene | 928 | 924 | |||||
| 5.750 | 40.2 | 39.83 | 20.09 | 27.94 | 28.76 | 16.01 | α-pinene | 934 | 931 |
| 6.789 | 0.81 | camphene | 946 | 943 | |||||
| 7.271 | 1.68 | 1.28 | 1.43 | sulcatone | 965 | 960 | |||
| 7.800 | 12.21 | 11.31 | 5.99 | 8.38 | 4.72 | sabinene | 983 | 975 | |
| 8.255 | 0.89 | 1.21 | 7.94 | 42.04 | β·pinene | 988 | 988 | ||
| 8.289 | 16.78 | myrcene | 994 | 993 | |||||
| 8.296 | 34.65 | 20.27 | α-phellandrene | 1005 | 1002 | ||||
| 8.384 | 8.10 | 1.44 | α·3-carene | 1012 | 1010 | ||||
| 8.391 | 20.36 | α-terpinene | 1020 | 1017 | |||||
| 8.615 | 8.57 | 16.47 | p-cymene | 1023 | 1022 | ||||
| 8.744 | 0.69 | 0.68 | β-phellandrene | 1025 | 1025 | ||||
| 8.805 | 0.86 | 0.65 | (E)-β-ocimene | 1032 | 1029 | ||||
| 8.812 | 1.04 | y-terpinene | 1047 | 1041 | |||||
| 9.060 | 9.192 | trans-sabinene hydrate | 1054 | 1054 | |||||
| 9.192 | 9.11 | 8.09 | 4.75 | 6.72 | 4.78 | Cis-linalool oxide | 1088 | 1086 | |
| 9.633 | 0.94 | 0.92 | 0.88 | trans-linalool oxide | 1098 | 1092 | |||
| 9.708 | 1.2 | 0.82 | terpinolene | 1100 | 1100 | ||||
| 9.776 | 1.12 | 1.38 | 0.77 | 1.16 | 0.79 | 0.67 | linalool | 1105 | 1102 |
| 9.871 | 1.31 | 1.35 | 0.74 | 1.03 | 0.67 | 0.6 | cis-sabinene hydrate | 1178 | 1174 |
| 10.47 | 4.03 | 2.64 | pelargonaldehyde | 1193 | 1186 | ||||
| 10.76 | 1.81 | camphor | 1318 | 1316 | |||||
| 11.01 | 1.39 | 2.01 | 0.59 | 2.06 | 1.79 | 1.25 | citronellal | 1395 | 1389 |
| 11.61 | 2.04 | 2.42 | 5.2 | 2.41 | borneol | 1414 | 1409 | ||
| 11.79 | 0.64 | terpinen-4-ol | 1425 | 1417 | |||||
| 11.96 | 1.05 | α-terpineol | 1445 | 1437 | |||||
| 12.07 | 1.89 | 1.00 | 2.85 | 1.52 | 2.84 | 0.12 | verbenone | 1456 | 1452 |
| 12.52 | 0.67 | n-decanal | 1459 | 1454 | |||||
| 12.61 | 1.86 | 1.07 | 1.72 | citronellol | 1466 | 1460 | |||
| 12.61 | 1.79 | 1.03 | 2.87 | geraniol | 1491 | 1489 | |||
| 13.01 | 5.11 | 3.95 | linalyl acetate | 1494 | 1492 | ||||
| 13.47 | 5.65 | 1.62 | 2.06 | 1.94 | 2.12 | 1.04 | bornyl acetate | 1499 | 1498 |
| 13.86 | 3.47 | 3.71 | 4-terpinenyl acetate | 1526 | 1522 | ||||
| 14.24 | 1.19 | 0.92 | 1.16 | 1.2 | 0.73 | α-terplnyl acetate | 1552 | 1548 | |
| 14.62 | 0.65 | α-cubebene | 1561 | 1561 | |||||
| 14.93 | 3.8 | 0.67 | 0.97 | 0.6 | α-ylangene | 1574 | 1574 | ||
| 15.56 | 2.23 | 1.23 | 0.82 | α-copaene | 1578 | 1576 | |||
| 15.99 | 1.09 | 0.94 | 0.63 | β-bourbonene | 1589 | 1582 | |||
| 16.14 | 1.96 | 0.7 | 0.67 | β-elemene | 1599 | 1592 | |||
| 16.56 | 1.04 | 0.75 | 0.77 | α-gurjunene | 1610 | 1608 | |||
| 16.68 | 1.71 | 1.65 | 0.64 | β-caryophyllene | 1637 | 1638 | |||
| 17.10 | 1.83 | 0.68 | trans·α·bergamotene | 1643 | 1649 | ||||
| 17.19 | 1.63 | α-guaiene | 1657 | 1652 | |||||
| 17.20 | 1.76 | α-humulene | 1660 | 1658 | |||||
| 17.67 | 1.59 | germacrene D | 1871 | 1880 | |||||
| 18.12 | 1.8 | 1.92 | 0.82 | β-selinene | 1889 | 1889 | |||
| 18.59 | 1.97 | 1.83 | 0.67 | ledene | 1891 | 1890 | |||
| 1.53 | 1.06 | 1.44 | 1.33 | 1.14 | 0.34 | Unknown | |||
| Monoterpenes | 76.5 | 74.65 | 90.04 | 80.16 | 94.08 | 96.232 | |||
| Sesquiterpenes | 16.32 | 14.09 | 0.75 | 7.63 | 1.23 | 0 | |||
| Others | 5.65 | 10.2 | 7.77 | 10.88 | 3.55 | 3.68 | |||
RT: Retention Time (min), RIExp: Experimental retention index, and RILit: Literature retention index.
Major components (≥10%) such as α-phellandrene were found in Niger (34.65%) and Kwara (20.27%), sabinene in Plateau (12.21%) and Nasarawa (11.31%), β-pinene (42.04%) and p-cymene (16.47%) in Kogi, and myrcene (16.78%) in Benue. As a result, the predominant monoterpenes (≥10%) present in V. negundo essential oil throughout the six states were α-phellandrene (20.27–34.65%), sabinene (11.31–12.21%), β-pinene (42.04%), p-cymene (16.47%), and myrcene (16.78%). In the chemotaxonomical classification of V. negundo, unique compounds such as α-pinene, linalool, cis-sabinene hydrate, citronellal, verbenone, and bornyl acetate found in all samples irrespective of the collection site could be used in the fingerprint of the essential oil. Several factors, including growth conditions, temperature, altitude, soil type, agricultural methods and practices, developmental stage, plant part extracted, and harvesting period, are strong factors that determine the presence and absence of certain terpenes, according to Moghaddam and Mehdizadeh [64]. Few monoterpenes and sesquiterpenes reported by Issa et al. [65] and Khokra et al. [31] were noticeably absent across all six states; however, compounds such as p-cymene [66], α-terpinolene [67], and citronellal [68] with reported potent insecticidal properties were present in the oils.
3.3. PCR Confirmation of Anopheles Gambiae s.s
The PCR amplicons viewed under the trans-illuminator UV light showed positive band sizes of 390 bp characteristic for A. gambiae while band size of 315 indicates A. arabiensis (Figure 5).
Figure 5.
Lane M is the 100 bp marker, Lanes 1–20 are randomly selected Anopheles samples. Lane 21 = negative sample. Distinguishing band size: A. gambiae s.l at 390 bp; A. arabiensis 315 bp.
After conditioning the PCR, samples 1 to 7 of randomly picked A. gambiae s.l showed DNA band sizes of 475 bp, authenticating the species to be A. gambiae s.s (Figure 6)
Figure 6.
Agarose gel 1.5% for distinguishing A. gambiae s.s and coluzzi after PCR with primers (R3, R5, B/Sint and MoPint).
3.4. Mosquito Behavioural Study
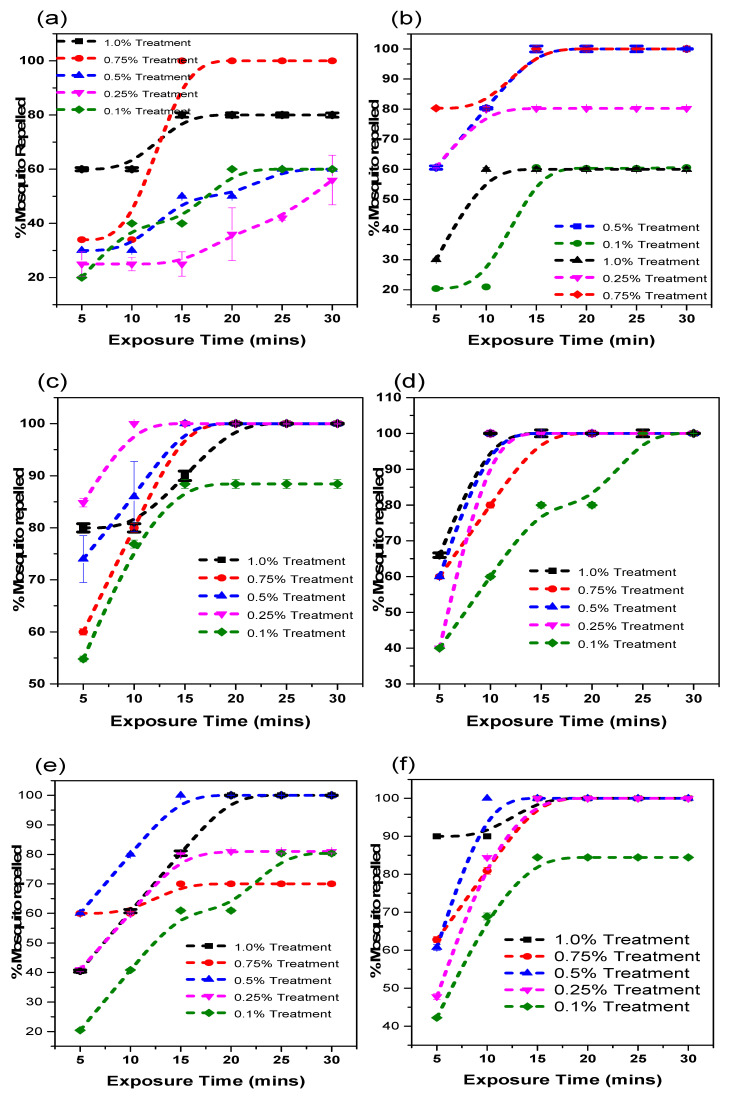
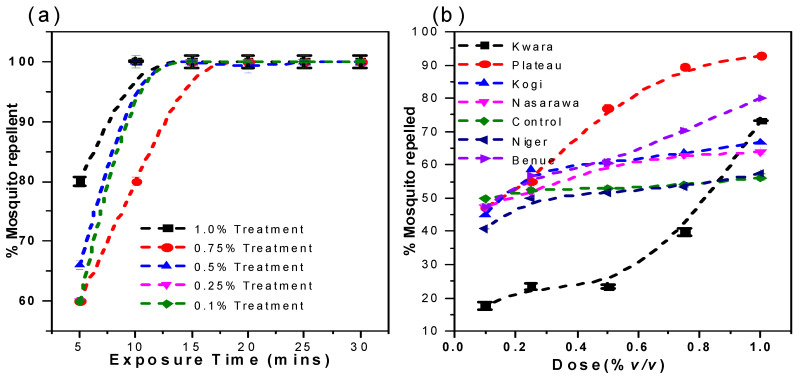
The exposure of adult female A. gambiae to the essential oil from all six states and N,N-diethyl-3-methylbenzamide (DEET) for a period of 30 min at doses ranging from 0.1–1% v/v was investigated and reported in Figure 7 and Figure 8.
Figure 7.
V. negundo essential oil obtained from (a) Kogi, (b) Benue, (c) Niger, (d) Nasarawa, (e) Plateau, and (f) Kwara, % mosquito repellency per 5 min at a 30 min exposure periods.
Figure 8.
(a) % mosquito repellency every 5 min at an exposure time of 30 min to N, N-diethyl-3-methylbenzamide (DEET) and (b) Dose response mosquito repellency study of V. negundo essential oils obtained from the six states.
There was an increase in the number of mosquitos repelled with time with an optimal % repellency activity attained at approximately 15 min. All the essential oil samples showed a significant increase in the percentage of mosquitoes repelled within the period of investigation with no significant difference in the percentage of mosquitoes repelled (p > 0.05) between the essential oils and the N,N-diethyl-3-methylbenzamide. As the doses increased, the repellency activity increased to a concentration where there are no observable changes in activity. However, the optimal concentration varies from state to state as a result of its compositional variation (Table 3). Essential oils from Niger, Kwara, Plateau and Nasarawa showed optimal repellency at a concentration of 0.5% v/v while Niger, Benue, and Kogi oil samples showed an optimal effect at 0.75% v/v. On the contrary, DEET showed no significant difference (p < 0.05) in repellency activity as the concentration changed. This inference is in consonance with the studies of Cárdenas-Ortega et al. [69] and Senthil-Nathan [70], which emphasize the slight variation in the repellency activities of samples due to the presence and percentage composition of unique compounds.
3.5. Effective Dose of the Essential Oils from the North-Central Geopolitical Zone
Using the Probit analysis model, the effective dose (ED50) that would repel 50% of the mosquito population is presented in Table 4. The ED50 of the oils and positive control are in the order of DEET > Kwara > Niger > Plateau and Nasarawa > Benue > Kogi. There is a significant different (p < 0.05) in the repellency of the oils from Kwara, Niger, Plateau, and Nasarawa compared to Benue and Kogi. The repellency property of the oils showed a composition–concentration dependent activity, which is not in variance with the result obtained in the mosquito behavioral investigation (Figure 7 and Figure 8). However, DEET showed very potent repellency at ED50 of 0.01%v/v compared to the oils from all states. This observation is due to the variation in the composition.
Table 4.
Effective does (%v/v) of essential oil from the six states and N,N-diethyl-3-methylbenzamide.
| Essential Oil Location | Effective Dose (%v/v) | R-Square Values |
|---|---|---|
| Nasarawa State | 0.14 | 0.8976 |
| Benue State | 0.48 | 0.8995 |
| Kwara State | 0.08 | 0.8254 |
| Plateau State | 0.14 | 0.9778 |
| Niger State | 0.11 | 0.9415 |
| Kogi State | 0.87 | 0.8268 |
| DEET | 0.01 | 0.8942 |
| Petrolatum (Negative control) | - | - |
DEET: N,N-diethyl-3-methylbenzamide.
3.6. Validation of Molecular Docking Protocol
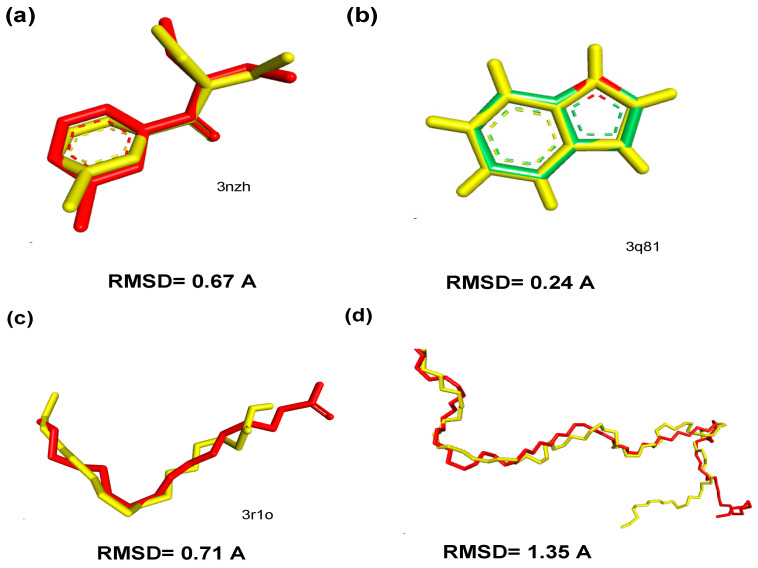
According to the literature, a validated protocol must have a RMSD value < 2.0 in the binding mode prediction, when superimposed on the crystallographic pose of the ligand [71,72]. To establish that the conformation of the interaction between co-crystallized ligands and OBPs can be replicated in silico to validate our docking method, the co-crystallized ligands were redocked in the protein binding pocket and the root mean square deviation (RMSD) data were used to evaluate the fitness of each redocked pose. Figure 9 illustrates the poses estimated in relation to the deposited PDB complexes, with the RMSD of 0.67 Å, 0.24 Å, 0.71 Å, and 1.35 Å for OBP1, OBP 7, OBP 4, and OBP; respectively.
Figure 9.
Crystallographic complexes (in red) overlapping with estimated poses (in yellow): (a) OBP 1 (PDB 3N7H), (b) OBP 4 (PDB 3Q8I), (c) OBP 7 (PDB 3R1O), and (d) OBP (PDB 2ERB).
3.7. Molecular Docking
The binding energies and inhibition constants of the proteins with the selected ligands are reported in Table 5.
Table 5.
Molecular docking results for the interaction between the selected ligands and the odorant binding proteins.
| OBP 1 | OBP 7 | OBP 4 | OBP | |||||
|---|---|---|---|---|---|---|---|---|
| Compounds | BE (kcal/mol) | Ki (mM) | BE (kcal/mol) | Ki (mM) | BE (kcal/mol) | Ki (mM) | BE (kcal/mol) | Ki (mM) |
| α-pinene | −6.4 | 0.0201 | −6.7 | 0.0121 | −5.8 | 0.0554 | −6.2 | 0.0282 |
| linalool | −6.9 | 0.0086 | −5.6 | 0.0777 | −5.4 | 0.1089 | −6.2 | 0.0282 |
| cis-sabinene hydrate | −7.2 | 0.0052 | −6.1 | 0.0334 | - | - | −6.0 | 0.0395 |
| citronellal | −6.5 | 0.0169 | −5.5 | 0.1528 | - | - | −6.1 | 0.0334 |
| verbenone | −7.8 | 0.0019 | −7.1 | 0.0062 | −6.1 | 0.0334 | −6.3 | 0.0238 |
| bornyl acetate | −7.5 | 0.0031 | −7.1 | 0.0062 | - | - | −6.6 | 0.0052 |
| α-phellandrene | −7.3 | 0.0044 | −7.1 | 0.0062 | - | - | −6.8 | 0.0102 |
| α-terpinene | −7.3 | 0.0044 | −7.1 | 0.0062 | - | - | −6.8 | 0.0102 |
| sabinene | −6.7 | 0.0121 | −6.8 | 0.0102 | - | - | −6.9 | 0.0086 |
| β-pinene | −6.7 | 0.0121 | −6.2 | 0.0062 | −5.9 | 0.0468 | −6.1 | 0.0334 |
| myrcene | −6.4 | 0.0201 | −6.2 | 0.0062 | - | - | −5.8 | 0.0554 |
| p-cymene | −7.1 | 0.0062 | −7.1 | 0.0062 | - | - | −6.7 | 0.0121 |
BE = Binding energy, Ki = Inhibition constant, and - = No interaction between ligands.
All the selected ligands demonstrated multiplicity of binding properties and varying degrees of interaction within the active pockets of the proteins with the exception of OBP 4 which only has affinity for α-pinene, linalool, verbenone, and β-pinene. In Table 5, α-pinene and myrcene (−6.4 kcal/mol), citronellal (−5.5 kcal/mol), linalool (−5.4 kcal/mol), and myrcene (−5.8 kcal/mol) demonstrated the strongest affinities for OBP1, OBP7, OBP4, and OBP, respectively. As a result, myrcene has been identified as an inhibitor of the main olfactory proteins involved in the signals for host recognition processes (OBP1 and OBP), which aid in the collection and transport of hydrophobic odorants into and through the fluid. Repellent alarm pheromone and diphenyl ester-specific binding proteins were inhibited by citronellal (0.1528 mM) and linalool (0.1089 mM), respectively. According to Bohacek et al. [73] and Hughes et al. [74], a molecule with a low Ki value in the micromolar range but less than 10−4 mM qualifies as a lead. The estimated Ki values of α-pinene and myrcene, citronellal, linalool, and myrcene against the four targeted OBPs are within the predicted range which qualifies them as a lead mosquito repellent agent. By implication, the strong binding energies and inhibition constants observed for α-pinene and myrcene, citronellal, linalool, and myrcene when compared to other investigated ligands may be suggestive of functional blocking of the olfactory receptor coreceptor, activation of specific odorant receptors (ORs), inhibition of specific ORs responding to attractants, and/or modulation of multiple ORs causing olfactory confusion, according to Tsitoura et al. [46] and Degennaro et al. [75].
3.8. Target OBPs Amino Acid–Ligand Interactions
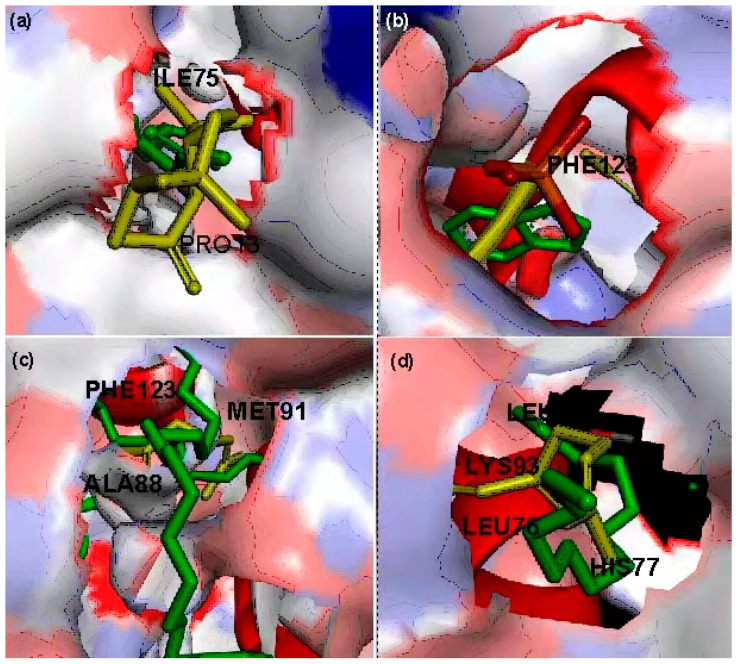
Figure 10 illustrates the 3D images of the active pockets of the four selected OBPs while Table 6 reports the list of the active pockets of the four selected OBPs from A. gambiae.
Figure 10.
3D images of the active pockets of (a) OBP 7, (b) OBP 1, (c) OBP, and (d) OBP 4 showing the native ligand (green) and the experimental ligand (yellow).
Table 6.
Active pockets on the four selected odorant binding proteins of A. gambiae.
| Proteins | Active Pockets |
|---|---|
| OBP 1 | ALA62, LEU73, LEU76, SER79, HIS85, ALA88, MET89, GLY92, LYS93, ARG94, TRP114, PHE123; |
| OBP 7 | PRO13, LEU17, CYS35, ILE75, PHE120, LEU124 |
| OBP 4 | THR2, GLN5, HIS29, LYS33, ALA52 |
| OBP | GLU14, ALA18, LEU58, ALA62, SER79, MET84, ALA88, MET89, MET91, ARG94, GLN116, PHE123 |
The structures of OBP, OBP1, and OBP7 are made up of two monomers, with OBP and OBP1 each having six α-helices and OBP7 having seven α-helices, with the odorant binding pocket placed in the center of a hydrophobic tunnel that runs through the dimeric interface. However, OBP4 is made up of a single monomer. In this investigation, the active pockets of odorant binding proteins were identified by removing the ligands that were previously linked to the receptors before targeting these cavities. The experimental ligands all docked at the same pockets as the native ligands, validating the docking protocol adopted in this study.
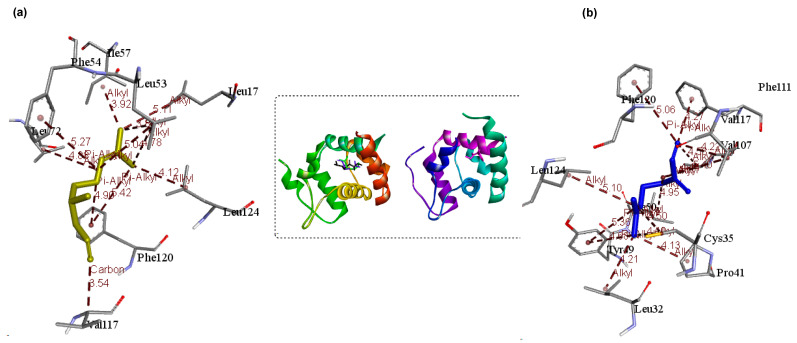
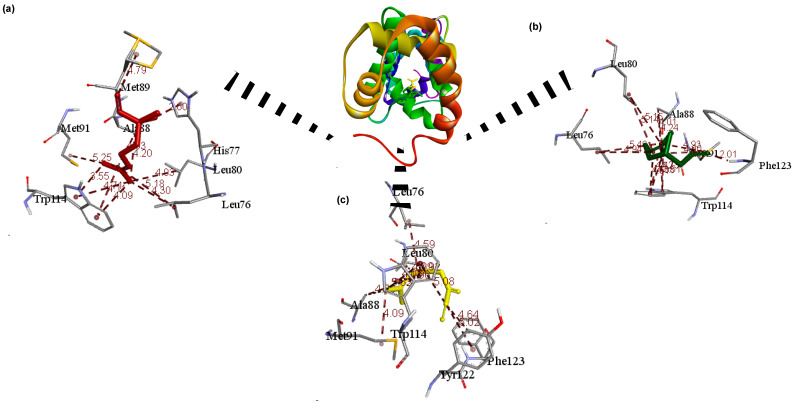
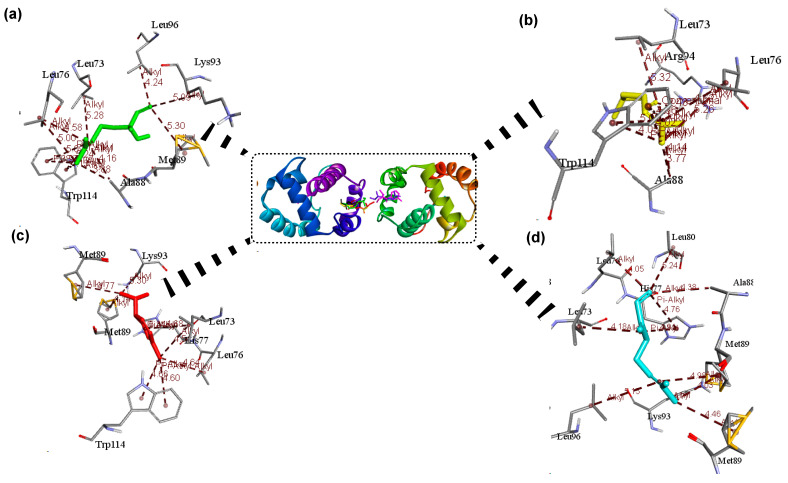
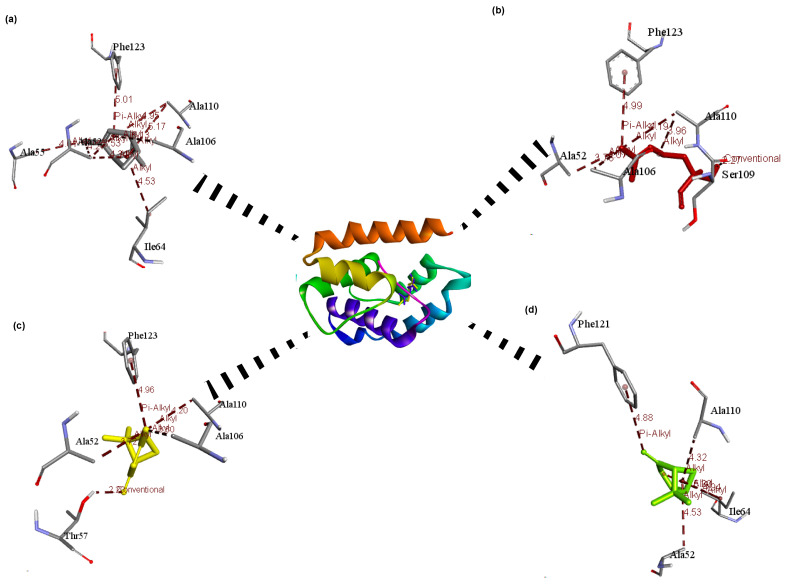
Figure 11, Figure 12, Figure 13 and Figure 14 depict the 3D interaction between OBPs and ligands, whereas Table 7 provides the active residues and ligand interaction types. In general, the proteins interact with their ligands primarily through hydrophobic interactions such as π-alkyl and alkyl interactions (Figure 11, Figure 12, Figure 13 and Figure 14). The interaction of the ligands revealed that they bind to at least one receptor in the pocket cavity of the OBPs. The OBPs demonstrated varying affinity for certain ligands as well as variation in the number of residues involved in the interactions. The crystallographic structure of OBP7 was favorably bound to citronellal (−5.5 kcal/mol) and myrcene (−6.2 kcal/mol) through residues in the binding cavity; Leu17 (α-helix 1), Phe120, Leu124 (α-helix 7). and Cys35 (α-helix 2) (Figure 11). Similarly, linalool (−6.2 kcal/mol), citronellal (−6.1 kcal/mol), and myrcene (−5.8 kcal/mol) favorably interacted with OBP through residues Ala88, Met91 (α-helix 5), and Phe123 (α-helix 6) (Figure 12). In the case of OBP1 linalool (−6.2 kcal/mol), citronellal (−6.1 kcal/mol), α-phellandrene, and myrcene (−5.8 kcal/mol) Leu73, Leu76(α-helix 4), Ala88, Met89, Lys93(α-helix 5), Trp114 (α-helix 5) (Figure 13) while OBP4 favorably interacted with α-pinene, linalool, verbenone, and β-pinene through ALA52 (α-helix 3) at a binding energy of ≈ −6.2 kcal/mol (Figure 14).
Figure 11.
3D interactions showing the selected ligands: (a) citronellal, and (b) myrcene with the most interaction at the active sites of the OBP 7.
Figure 12.
3D interactions showing the selected ligands: (a) linalool, (b) citronellal, and (c) myrcene with the most interaction at the active sites of the OBP.
Figure 13.
3D interactions showing the selected ligands: (a) linalool, (b) citronellal, (c) α-phellandrene, and (d) myrcene with the most interaction at the active sites of the OBP1.
Figure 14.
3D interactions showing the selected ligands: (a) α-pinene, (b) linalool, (c) verbenone, and (d) β-pinene with the most interaction at the active sites of the OBP4.
Table 7.
The number and type of bonds for the OBD–ligand complexes.
| Interacting Amino Acids in the Active Pockets | ||||
|---|---|---|---|---|
| Ligands | OBP 1 | OBP 7 | OBP | OBP 4 |
| α-pinene | Leu76, Trp114, Phe123 | Phe120, Leu124 | Ala88, Met89 | Ala52 |
| linalool | Leu73, Leu76, Ala88, Met89, Lys93, Trp114 | Cys35, Phe120 | Ala88, Met91, Met 89 | Ala52 |
| cis-sabinene hydrate | Leu73, Ala88 | Phe120 | Phe123 | Nil |
| citronellal | Leu73, Leu76, Ala88, Arg94, Trp114 | Leu17, Phe120, Leu124 | Ala88, Met91, Phe123 | Nil |
| verbenone | Met89, Lys93, Arg94, | Phe120 | Phe123 | Ala52 |
| bornyl acetate | Trp114, Phe123 | Cys35, Phe120 | Phe123 | Nil |
| α-phellandrene | Leu73, Leu76, Met89, Lys93, Trp114 | Phe120 | Ala88 | Nil |
| α-terpinene | Leu73, Met89, Lys93 | Phe120 | ALA88 | Nil |
| sabinene | Leu73, Ala88, Trp114 | Cys35, Phe120 | Met89, Met91 | Nil |
| β-pinene | Leu73, Ala88, Met89, Lys93 | Cys35 | Met91, PHE123 | Ala52 |
| myrcene | Leu73, Leu76, Ala88, Met89, Lys93 | Cys35, Phe120, Leu124 | Ala88, Met91, Phe123 | Nil |
| p-cymene | Leu73, Leu76,Ala88, Trp114 | Phe120 | Ala88, Met91 | Nil |
Interestingly, all major ligand interactions with the OBP, OBP1, OBP4, and OBP7 involve similar residues (Table 7) but differ in the number of interactions as well as distance (Figure 11, Figure 12, Figure 13 and Figure 14). The observed OBP–linalool/citronellal interaction with Ala88 and Met91 involves the 3,7-dimethyl groups of as well as a π-alkyl of the 6-enal interaction on Met 89 at 4.79 Å and on Phe 123 at 2.01 Å; accordingly. OBP-Myrcene complex was formed at the active cavity around Met91 (4.09 Å), Phe123 (4.02 Å), and Ala88 (4.22 Å) (Figure 12). OBP 7 inhibitions were as a result of the following interactions: citronellal: (alkyl, 5.11 Å, Leu17), (pi-alkyl, 4.90 Å, Phe120), (alkyl, 4.20 Å, Leu124), myrcene: (alkyl, 4.13 Å, Csy35), (pi-alkyl, 5.00 Å, Phe120), (alkyl, 5.10 Å, Leu124) (Figure 11). In the case of OBP 4 the inhibitions due to α-pinene (4.11 Å), linalool (3.57 Å), verbenone (3.12 Å), and β-pinene (4.53 Å) were focused at the Ala52 due to alkyl interaction (Figure 14). Consequently, these strong ligand–OBP interactions may result in a functional mutation causing inhibition.
The mechanisms of interaction between the various ligands differ and will most likely result in a variety of activities ranging from functional blocking of the olfactory receptor coreceptor due to repression of Leu73 in OBP1, inhibition of specific ORs responding to attractants, and/or modulation of multiple Ors causing disorientation, as reported by Murphy et al. [76]. A strong affinity of OBP7 for citronellal and myrcene, according to Sun et al. [77], could create disturbance in the insect’s chemical information decoding potential. These rare interactions of α-pinene, linalool, verbenone, and β-pinene with OBP4 are strongly associated with their spatial orientation of the dialkyl and π-alkyl groups; with the likelihood of blocking the olfactory receptor coreceptor and modulate OBP4, resulting in the susceptibility of A. gambiae to these molecules in the repellent.
3.9. Efficiency Metrics of Selected Ligands
Supplementary Tables S1–S4 show the ligand efficiency metrics of the selected ligands, which were calculated using Equations (3)–(7). Ligand Efficiency (LE), Ligand Lipophilic Efficiency (LLE), and Fit Quality (FQ) are expected to have threshold values of 0.3, 3, and 0.8 for a molecule to be classified as a hit quantitatively [78]. During lead discovery, the Ligand Efficiency Lipophilic Price (LELP) is estimated to be between −10 and 10 [79]. The ligand efficiency metrics against the four OBPs are within the criteria, qualifying them as a possible odorant binding protein repellent lead.
3.10. In Silico ADMET Properties of the Ligands against the Odorant Binding Proteins
3.10.1. ADMET Properties
The ADMET properties of all selected ligands were carried out to determine the molecules as safe potential OBP inhibitors and the results are presented in Tables S5–S16. Even though cis-sabinene hydrate, citronellal, sabinene, and verbenone failed the human oral bioavailability test (values were 0.7–1.0), this pharmacokinetic parameter is less of a concern regarding skin sensitization and eye irritation for dermally applied products such as repellent lotion or aerosols. In this investigation the empirical decision for skin sensitization and eye irritation tests for linalool, cis-sabinene hydrate, citronellal, sabinene, verbenone, α-terpinene, bornyl acetate, β-pinene, and α-phellandrene are >0.8, which is within the rejection zone because such molecules could induce allergic contact dermatitis, cornea, and conjunctiva tissue damage [80]. Furthermore, citronellal and α-phellandrene have been discovered to be respiratory and human hepatotoxicants, respectively, with high morbidity and mortality potential [81]. The plasma protein binding and blood-brain barrier penetration of cis-sabinene hydrate, α-phellandrene, and α-terpinene were found to be greater than 90%, indicating that these compounds have a low therapeutic index [82]. The metabolic profile of the ligands indicated that they are all either substrates or inhibitors of human cytochrome P450 based on chemical biotransformation reactions [83].
3.10.2. In-silico Environmental Toxicity
To estimate the environmental impact of the essential oil, and the bioconcentration factor (BCF), the concentration of the selected ligands in water in mg/L that causes 50% growth inhibition of Tetrahymena pyriformis after 48 h (IGC50), 50% of fathead minnow to die after 96 h (LC50), and 50% of Daphnia magna to die after 48 h (LC50DM) were evaluated. The result of the analysis is presented in Table 8.
Table 8.
In-silico environmental toxicity profile of the selected ligands.
| Ligands | BCF (L/kg) | IGC50 ((mg/L)/(1000 ∗ MW)) | LC50 ((mg/L)/(1000 ∗ MW)) | LC50DM ((mg/L)/(1000 ∗ MW)) |
|---|---|---|---|---|
| α-pinene | 2.986 | 4.327 | 5.287 | 5.948 |
| linalool | 1.347 | 2.192 | 3.547 | 5.056 |
| cis-sabinene hydrate | 2.745 | 3.547 | 3.657 | 4.233 |
| citronellal | 1.233 | 3.174 | 4.168 | 5.454 |
| verbenone | 0.553 | 3.166 | 3.989 | 4.187 |
| bornyl acetate | 2.166 | 3.737 | 4.334 | 4.720 |
| α-phellandrene | 2.360 | 3.080 | 3.674 | 4.176 |
| α-terpinene | 2.246 | 3.064 | 4.331 | 4.538 |
| sabinene | 2.874 | 3.776 | 4.337 | 4.400 |
| β-pinene | 3.003 | 4.675 | 5.624 | 5.587 |
| myrcene | 2.021 | 4.471 | 5.331 | 5.450 |
| p-cymene | 2.874 | 3.776 | 4.337 | 4.400 |
Bioconcentration factors range from 0.553–3.003 L/kg, reflecting the very low potential for the ligands to bioaccumulate in the environment. Citronellal, verbenone (with high human oral bioavailability) (Tables S10 and S12), linalool, and myrcene demonstrated the lowest BCF compared to β-pinene, p-cymene, sabinene, α-pinene, and cis-sabinene hydrate (Table 8). The IGC50, LC50, and LC50DM of the selected molecules are 3.080–4.675 (mg/L)/(1000 ∗ MW), 3.547–5.624 (mg/L)/(1000 ∗ MW), and 4.176–5.948 (mg/L)/(1000 ∗ MW), respectively. All studied ligands demonstrated very low ecotoxicological profiles against Tetrahymena pyriformis after 48 h, fathead minnow after 96 h, and Daphnia magna after 48 h [84].
3.11. Repellence Study of the Pure Selected Ligands
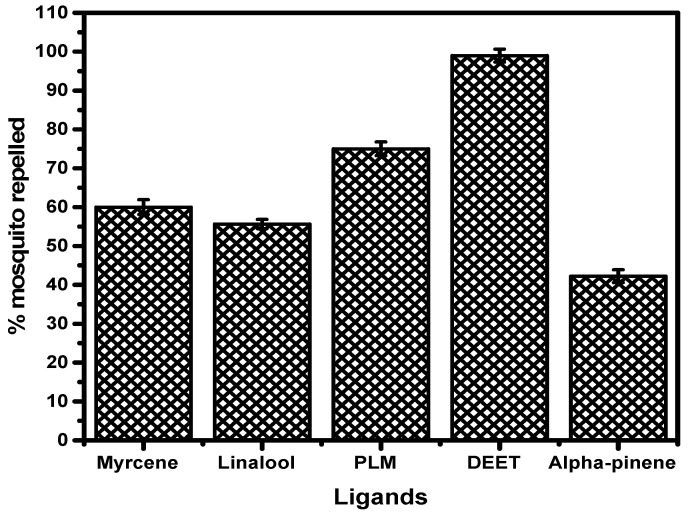
Pure α-pinene, linalool, and myrcene could be employed as a safe active ingredient in the development of a new mosquito repellent, according to ADME/tox and docking studies. Exactly 1.0% v/v of pure α-pinene, linalool, myrcene, equivalent mixture of α-pinene, linalool, myrcene (PLM), and N-diethyl-3-methylbenzamide (DEET) were evaluated for repellent activities and reported after exposure for 30 min (Figure 15).
Figure 15.
Percentage mosquito repellence test of commercially available main ligands, PLM, and DEET after exposure for 30 min.
The pure linalool and myrcene demonstrated above 50% mosquito repellency activity except for α-pinene. This repellence activity is comparatively lower than the observed activity of the essential oil after 30 min exposure (Figure 7). However, there is a significant difference in the mosquito repellence activity of DEET (100%) compared to the pure ligands (42–60%). Despite the strong interactions of the ligands with the OBPs the observed repellency activity of the essential oil is significantly synergistic in nature. The synergistic activity was validated by comparing the repellence activity of PLM (73%) with DEET (100%) after 30 min of exposure.
4. Conclusions
The essential oils of V. negundo from six states in the North Central Geopolitical Zone were found to have composition-dependent mosquito repellent efficacy against A. gambiae. In comparison to the other states, the essential oil derived from Niger, Kwara Plateau, and Nasarawa demonstrated substantial repellency with an ED50 of 0.14–0.08% v/v. α-phellandrene, sabinene, β-pinene, p-cymene, and myrcene were the most common terpenes found in the essential oil throughout the six states. Regardless of the collection site, all essential oils contained α-pinene, linalool, cis-sabinene hydrate, citronellal, verbenone, and bornyl acetate. Linalool, α-pinene, verbenone, β-pinene, myrcene, and citronellal had the strongest affinity for OBPs, while -pinene, citronellal, linalool, and myrcene inhibited strongly by creating hydrophobic interactions at the binding pocket. The LE, LLE, FQ, and LELP values were all within the predicted ranges, indicating that the ligands are quantitatively hit and so qualify as a potential odorant binding protein repellent lead. Linalool, cis-sabinene hydrate, citronellal, sabinene, verbenone, α-terpinene, bornyl acetate, α-pinene, and α-phellandrene all had a low ecotoxicological profile, while linalool, cis-sabinene hydrate, citronellal, sabinene, verbenone, and α-terpinene did not. According to ADME/tox and docking results, α-pinene, linalool, and myrcene could be used as safe active components in the development of an environmentally friendly new mosquito repellent. Commercial standards of α-pinene, linalool, and myrcene were themselves active in mosquito repellent assays, and a mixture containing these compounds in equivalent proportions was found to be as significantly active as DEET, suggestive of a synergistic activity itself. Docking showed that these ligands bind to OBPs and may play an important role in blocking the olfactory receptor (ORs) coreceptor and inhibition of specific ORs causing disorientation and confusion in A. gambiae.
Acknowledgments
We appreciate the Vaal University of Technology, South Africa, Kaduna State University, Kaduna State, Nigeria and Bingham University, Karu, Nasarawa State, Nigeria for providing the necessary environment for this collaborative investigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects12121061/s1: Figure S1: V. negundo essential oil from Benue State, Figure S2: V. negundo essential oil from Kogi State, Figure S3: V. negundo essential oil from Kwara State, Figure S4: V. negundo essential oil from Nasarawa State, Figure S5: V. negundo essential oil from Niger State and Figure S6: V. negundo essential oil from Plateau State. Table S1: Ligand efficiency metrics of the ligands on interaction with odorant binding protein 1 (PDB ID 3N7H), Table S2: Ligand efficiency metrics of the ligands on interaction with odorant binding protein 7 (PDB ID 3R1O), Table S3: Ligand efficiency metrics of the ligands on interaction with odorant binding protein 4 (PDB ID 3Q8I), Table S4: Ligand efficiency metrics of the ligands on interaction with odorant binding protein (PDB ID 2ERB), Table S5: ADME, Physiochemical, Toxicity, and Environmental Toxicity of Myrcene, Table S6: ADME, Physiochemical, Toxicity, and Environmental Toxicity of α-pinene, Table S7: ADME, Physiochemical, Toxicity, and Environmental Toxicity of β-Pinene, Table S8: ADME, Physiochemical, Toxicity, and Environmental Toxicity of linalool, Table S9: ADME, Physiochemical, Toxicity, and Environmental Toxicity of cis-sabinene hydrate, Table S10: ADME, Physiochemical, Toxicity, and Environmental Toxicity of citronellal, Table S11: ADME, Physiochemical, Toxicity, and Environmental Toxicity of α-terpinene, Table S12: ADME, Physiochemical, Toxicity, and Environmental Toxicity of verbenone, Table S13: ADME, Physiochemical, Toxicity, and Environmental Toxicity of bornyl acetate, Table S14: ADME, Physiochemical, Toxicity, and Environmental Toxicity of α-phellandrene, Table S15: ADME, Physiochemical, Toxicity, and Environmental Toxicity of sabinene and Table S16: ADME, Physiochemical, Toxicity, and Environmental Toxicity of p-cymene
Author Contributions
Conceptualization, B.J.O., Z.L. and F.M.; Data curation, Z.L. and F.M.; Formal analysis, B.J.O. and Y.C.H.; Funding acquisition, B.J.O.; Investigation, B.J.O., Z.L., F.M. and Y.C.H.; Methodology, B.J.O., Z.L., F.M. and Y.C.H.; Project administration, Z.L.; Resources, B.J.O., Z.L. and F.M.; Software, B.J.O. and F.M.; Supervision, B.J.O., Z.L. and F.M.; Validation, B.J.O., Z.L., F.M. and Y.C.H.; Visualization, B.J.O., Z.L. and F.M.; Writing—original draft, B.J.O. and Y.C.H.; Writing—review and editing, B.J.O. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tertiary Education Trust Fund, grant number TETFund/DR&D/CE/NRF/UNI/CC/22 and The APC was funded by Tertiary Education Trust Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support this study are available within the manuscript.
Conflicts of Interest
The authors have declared that there is no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mattingly P.F. Anopheles gambiae B. [(accessed on 5 September 2021)]. Available online: http://didaktorika.gr/eadd/handle/10442/7428.
- 2.World Malaria Report. [(accessed on 25 June 2021)]. The “World Malaria Report 2019” at a Glance. Available online: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019.
- 3.Di Gennaro F., Marotta C., Locantore P., Pizzol D., Putoto G. Malaria and COVID-19: Common and Different Findings. Trop. Med. Infect. Dis. 2020;5:141. doi: 10.3390/tropicalmed5030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agomo C.O., Oyibo W.A., Anorlu R.I., Agomo P.U. Prevalence of malaria in pregnant women in Lagos, South-West Nigeria. Korean J. Parasitol. 2009;47:179–183. doi: 10.3347/kjp.2009.47.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okoroiwu H.U., Uchendu K.I., Essien R.A. Causes of morbidity and mortality among patients admitted in a tertiary hospital in southern Nigeria: A 6 year evaluation. PLoS ONE. 2020;15:e0237313. doi: 10.1371/journal.pone.0237313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drugs I., Arrow K.J., Panosian C., Gelband H. Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance. National Academies Press (US); Washington, DC, USA: 2004. The Parasite, the Mosquito, and the Disease. [PubMed] [Google Scholar]
- 7.Coluzzi M., Sabatini A., Petrarca V., Di Deco M.A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- 8.White N.J. Antimalarial drug resistance. J. Clin. Investig. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse E.G., Korsik M., Todd M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019;18:1–21. doi: 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC CDC—Malaria—Malaria Worldwide-How Can Malaria Cases and Deaths Be Reduced?—Vaccines. [(accessed on 5 September 2021)]; Available online: http://www.cdc.gov/malaria/malaria_worldwide/reduction/vaccine.html.
- 11.Sharma V.P. Health Hazards of Mosquito Repellents and Safe Alternatives. [(accessed on 5 September 2021)]. Available online: https://www.researchgate.net/publication/237353181_Health_hazards_of_mosquito_repellents_and_safe_alternatives.
- 12.Hogarh J.N., Antwi-Agyei P., Obiri-Danso K. Application of mosquito repellent coils and associated self-reported health issues in Ghana. Malar. J. 2016;15:61. doi: 10.1186/s12936-016-1126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz J.H. Chemical and plant-based insect repellents: Efficacy, safety, and toxicity. Wilderness Environ. Med. 2016;27:153–163. doi: 10.1016/j.wem.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Lee M.Y. Essential Oils as Repellents against Arthropods. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/6860271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choochote W., Chaithong U., Kamsuk K., Jitpakdi A., Tippawangkosol P., Tuetun B., Champakaew D., Pitasawat B. Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia. 2007;78:359–364. doi: 10.1016/j.fitote.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Maia M.F., Moore S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011;10:S11. doi: 10.1186/1475-2875-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebbalkar D.S., Hebbalkar G.D., Sharma R.N., Joshi V.S., Bhat V.S. Mosquito repellent activity of oils from Vitex negundo Linn. leaves. Indian J. Med. Res.-Sect. A Infect. Dis. 1992;95:200–203. [PubMed] [Google Scholar]
- 18.Kheder D.A., Al-Habib O.A.M., Gilardoni G., Vidari G. Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects. Molecules. 2020;25:804. doi: 10.3390/molecules25040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A., Niranjan U.S., Rao M.M., Padhi M.M., Babu R. A review of the important chemical constituents and medicinal uses of Vitex genus. Asian J. Tradit. Med. 2011;6:54–60. [Google Scholar]
- 20.Zheng C.J., Li H.Q., Ren S.C., Xu C.L., Rahman K., Qin L.P., Sun Y.H. Phytochemical and pharmacological profile of Vitex negundo. Phyther. Res. 2015;29:633–647. doi: 10.1002/ptr.5303. [DOI] [PubMed] [Google Scholar]
- 21.Jagetia G.C., Baliga M.S. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. J. Med. Food. 2004;7:343–348. doi: 10.1089/jmf.2004.7.343. [DOI] [PubMed] [Google Scholar]
- 22.Alam M.I., Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J. Ethnopharmacol. 2003;86:75–80. doi: 10.1016/S0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 23.Chandramu C., Manohar R.D., Krupadanam D.G.L., Dashavantha R.V. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phyther. Res. 2003;17:129–134. doi: 10.1002/ptr.1088. [DOI] [PubMed] [Google Scholar]
- 24.Abd Aziz N.A., Hasham R., Sarmidi M.R., Suhaimi S.H., Idris M.K.H. A review on extraction techniques and therapeutic value of polar bioactives from Asian medicinal herbs: Case study on Orthosiphon aristatus, Eurycoma longifolia and Andrographis paniculata. Saudi Pharm. J. 2021;29:143–165. doi: 10.1016/j.jsps.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paluch G., Bartholomay L., Coats J. Mosquito repellents: A review of chemical structure diversity and olfaction. Pest Manag. Sci. 2010;66:1155. doi: 10.1002/ps.2027. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W., Peng W., Zhu C., Zhang Q., Saccone G., Zhang H. Identification and expression profile analysis of odorant binding proteins in the oriental fruit fly Bactrocera dorsalis. Int. J. Mol. Sci. 2013;14:14936–14949. doi: 10.3390/ijms140714936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pietrantonio F., Benetti M., Cannata D., Varriale A., D’Auria S., Palla-Papavlu A., Serra P., Verona E. Surface acoustic wave biosensor based on odorant binding proteins deposited by laser induced forward transfer; Proceedings of the IEEE International Ultrasonics Symposium; IUS, Prague, Czech Republic. 21–25 July 2013; pp. 2144–2147. [Google Scholar]
- 28.Sankaran S., Panigrahi S., Mallik S. Olfactory receptor based piezoelectric biosensors for detection of alcohols related to food safety applications. Sens. Actuators B Chem. 2011;155:8–18. doi: 10.1016/j.snb.2010.08.003. [DOI] [Google Scholar]
- 29.Possas-Abreu M., Rousseau L., Ghassemi F., Lissorgues G., Habchi M., Scorsone E., Cal K., Persaud K. Biomimetic diamond MEMS sensors based on odorant-binding proteins: Sensors validation through an autonomous electronic system; Proceedings of the ISOEN 2017—ISOCS/IEEE International Symposium on Olfaction and Electronic Nose; Montreal, QC, Canada. 28–31 May 2017; Piscataway, NJ, USA: Institute of Electrical and Electronics Engineers Inc.; 2017. [Google Scholar]
- 30.Padalia R.C., Verma R.S., Chauhan A., Chanotiya C.S., Thul S. Phytochemical diversity in essential oil of Vitex negundo L. populations from India. Rec. Nat. Prod. 2016;10:452–464. [Google Scholar]
- 31.Khokra S., Prakash O., Jain S., Aneja K., Dhingra Y. Essential oil composition and antibacterial studies of Vitex negundo Linn. extracts. Indian J. Pharm. Sci. 2008;70:522–526. doi: 10.4103/0250-474X.44610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillies M.T., Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) Volume 55 Johannesburg Publications of the South African Institute for Medical Research; Johannesburg, South Africa: 1987. [Google Scholar]
- 33.WHO . WHO|Vector Resistance to Pesticides. World Health Organization; Geneve, Switzerland: 2016. (Technical Report Series N°818). [Google Scholar]
- 34.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar. J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott J.A., Brogdon W.G., Collins F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 36.Favia G., Lanfrancotti A., Spanos L., Sidén-Kiamos I., Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol. Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 37.Achee D.N.L., Grieco J.P., Sarah Moore D.U.B. Guidelines for Efficacy Testing of Spatial Repellents. World Health Organization; Geneve, Switzerland: 2013. pp. 5–7, 14–15, 28–30, 41–48. [Google Scholar]
- 38.Costantini C., Birkett M.A., Gibson G., Ziesmann J., Sagnon N., Mohammed H.A., Coluzzi M., Pickett J.A. Electroantennogram and behavioural responses of the malaria vector Anopheles gambiae to human-specific sweat components. Med. Vet. Entomol. 2001;15:259–266. doi: 10.1046/j.0269-283x.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- 39.Badolo A., Ilboudo-Sanogo E., Ouédraogo A.P., Costantini C. Evaluation of the sensitivity of Aedes aegypti and Anopheles gambiae complex mosquitoes to two insect repellents: DEET and KBR 3023. Trop. Med. Int. Health. 2004;9:330–334. doi: 10.1111/j.1365-3156.2004.01206.x. [DOI] [PubMed] [Google Scholar]
- 40.Barasa S.S., Ndiege I.O., Lwande W., Hassanali A. Repellent activities of stereoisomers of p-menthane-3,8-diols against Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 2002;39:736–741. doi: 10.1603/0022-2585-39.5.736. [DOI] [PubMed] [Google Scholar]
- 41.Aguiar R.W.S., Dos Santos S.F., Da Silva Morgado F., Ascencio S.D., De Mendonça Lopes M., Viana K.F., Didonet J., Ribeiro B.M. Insecticidal and repellent activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex quinquefasciatus. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0116765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y., Qiao H., Ling Y., Yang S., Rui C., Pelosi P., Yang X. New analogues of (E)-β-farnesene with insecticidal activity and binding affinity to aphid odorant-binding proteins. J. Agric. Food Chem. 2011;59:2456–2461. doi: 10.1021/jf104712c. [DOI] [PubMed] [Google Scholar]
- 43.Da Silva R.C.S., Milet-Pinheiro P., Da Silva P.C.B., Da Silva A.G., Da Silva M.V., Do Amaral Ferraz Navarro D.M., Da Silva N.H. (E)-Caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of commiphora leptophloeos leaf oil. PLoS ONE. 2015;10:e0144586. doi: 10.1371/journal.pone.0144586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu P., Liu X.C., Dong H.W., Liu Z.L., Du S.S., Deng Z.W. Chemical composition and insecticidal activity of the essential oil of illicium pachyphyllum fruits against two grain storage insects. Molecules. 2012;17:14870–14881. doi: 10.3390/molecules171214870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G., Carey A.F., Carlson J.R., Zwiebel L.J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsitoura P., Koussis K., Iatrou K. Inhibition of Anopheles gambiae odorant receptor function by mosquito repellents. J. Biol. Chem. 2015;290:7961–7972. doi: 10.1074/jbc.M114.632299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu P., Choo Y.M., De La Rosa A., Leal W.S. Mosquito odorant receptor for DEET and methyl jasmonate. Proc. Natl. Acad. Sci. USA. 2014;111:16592–16597. doi: 10.1073/pnas.1417244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohbot J.D., Dickens J.C. Odorant receptor modulation: Ternary paradigm for mode of action of insect repellents. Neuropharmacology. 2012;62:2086–2095. doi: 10.1016/j.neuropharm.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds C.H., Bembenek S.D., Tounge B.A. The role of molecular size in ligand efficiency. Bioorg. Med. Chem. Lett. 2007;17:4258–4261. doi: 10.1016/j.bmcl.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins A.L., Keserü G.M., Leeson P.D., Rees D.C., Reynolds C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014;13:105–121. doi: 10.1038/nrd4163. [DOI] [PubMed] [Google Scholar]
- 51.Hopkins A.L., Groom C.R., Alex A. Ligand efficiency: A useful metric for lead selection. Drug Discov. Today. 2004;9:430–431. doi: 10.1016/S1359-6446(04)03069-7. [DOI] [PubMed] [Google Scholar]
- 52.Edwards M.P., Price D.A. Role of Physicochemical Properties and Ligand Lipophilicity Efficiency in Addressing Drug Safety Risks. Volume 45 Academic Press; Cambridge, MA, USA: 2010. [Google Scholar]
- 53.Murray C.W., Erlanson D.A., Hopkins A.L., Keseru G.M., Leeson P.D., Rees D.C., Reynolds C.H., Richmond N.J. Validity of Ligand Efficiency Metrics. ACS Med. Chem. Lett. 2014;5:616–618. doi: 10.1021/ml500146d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawal B., Lawal A., Noma S., Singh A., Adeboye M., Odofin A. Properties, classification and agricultural potentials of the soils of lower Oshin river floodplains in Kwara State, Nigeria. Niger. J. Technol. Res. 2013;7 doi: 10.4314/njtr.v7i3.88839. [DOI] [Google Scholar]
- 55.Akpan-Idiok A.U., Ukabiala M.E., Amhakhian O.S. Characterization and classification of river Benue floodplain soils in Bassa Local Government Area of Kogi State, Nigeria. Int. J. Soil Sci. 2013;8:32–46. doi: 10.3923/ijss.2013.32.46. [DOI] [Google Scholar]
- 56.Adaikwu A.O., Ali A., Agber P. Characterization and Classification of Soils from Selected Areas in Benue State Southern Guinea Savanna of Nigeria. Asian J. Plant Soil Sci. 2017;2:17–24. [Google Scholar]
- 57.Abubakar M.A., Zega Z.M., Jayeoba O.J. Major characteristics and classification of soils in duduguru, obi lga of Nasarawa State, Nigeria. Agric. Res. J. 2019;56:417. doi: 10.5958/2395-146X.2019.00067.X. [DOI] [Google Scholar]
- 58.Idoga S., Azagaku D.E. Characterization and classification of soils of Janta area, Plateau State of Nigeria. Niger. J. Soil Sci. 2006;15:116–122. [Google Scholar]
- 59.Njinga R.L., Moyo M.N., Abdulmaliq S.Y. Analysis of Essential Elements for Plants Growth Using Instrumental Neutron Activation Analysis. Int. J. Agron. 2013;2013:156520. doi: 10.1155/2013/156520. [DOI] [Google Scholar]
- 60.Tirillini B., Pellegrino R.M., Chessa M., Pintore G. Chemical composition of Thymus serrulatus Hochst. ex Benth. essential oils from Ethiopia: A statistical approach. Nat. Prod. Commun. 2008;3:2069–2074. doi: 10.1177/1934578X0800301224. [DOI] [Google Scholar]
- 61.Claridge G.G.C. The Clay Mineralogy and Chemistry of Some Soils from the Ross Dependency, Antarctica. N. Z. J. Geol. Geophys. 1965;8:186–220. doi: 10.1080/00288306.1965.10428107. [DOI] [Google Scholar]
- 62.Huang H.C., Chang T.Y., Chang L.Z., Wang H.F., Yih K.H., Hsieh W.Y., Chang T.M. Inhibition of melanogenesis Versus antioxidant properties of essential oil extracted from leaves of Vitex negundo linn and chemical composition analysis by GC-MS. Molecules. 2012;17:3902–3916. doi: 10.3390/molecules17043902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S.V., Kumar R.A., Mani P., Bastin T.M.M.J., Ravikumar G. Mosquito larvicidal, oviposition deterrent and repellent properties of Vitex negundo L. extracts against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus. J. Pharm. Res. 2011;4:2060–2063. [Google Scholar]
- 64.Moghaddam M., Mehdizadeh L. Soft Chemistry and Food Fermentation. Elsevier; Amsterdam, The Netherlands: 2017. Chemistry of Essential Oils and Factors Influencing Their Constituents; pp. 379–419. [Google Scholar]
- 65.Issa M., Chandel S., Pal Singh H., Rani Batish D., Kumar Kohli R., Singh Yadav S., Kumari A. Appraisal of phytotoxic, cytotoxic and genotoxic potential of essential oil of a medicinal plant Vitex negundo. Ind. Crops Prod. 2020;145:112083. doi: 10.1016/j.indcrop.2019.112083. [DOI] [Google Scholar]
- 66.Bonjardim L.R., Cunha E.S., Guimarães A.G., Santana M.F., Oliveira M.G.B., Serafini M.R., Araújo A.A.S., Antoniolli Â.R., Cavalcanti S.C.H., Santos M.R.V., et al. Evaluation of the anti-inflammatory and antinociceptive properties of p-Cymene in mice. Z. Naturforsch.-Sect. C J. Biosci. 2012;67:15–21. doi: 10.1515/znc-2012-1-203. [DOI] [PubMed] [Google Scholar]
- 67.Conti B., Benelli G., Flamini G., Cioni P.L., Profeti R., Ceccarini L., Macchia M., Canale A. Larvicidal and repellent activity of Hyptis suaveolens (Lamiaceae) essential oil against the mosquito Aedes albopictus Skuse (Diptera: Culicidae) Parasitol. Res. 2012;110:2013–2021. doi: 10.1007/s00436-011-2730-8. [DOI] [PubMed] [Google Scholar]
- 68.Kim J.-K., Kang C.-S., Lee J.-K., Kim Y.-R., Han H.-Y., Yun H.K. Evaluation of Repellency Effect of Two Natural Aroma Mosquito Repellent Compounds, Citronella and Citronellal. Entomol. Res. 2005;35:117–120. doi: 10.1111/j.1748-5967.2005.tb00146.x. [DOI] [Google Scholar]
- 69.Cárdenas-Ortega N., González-Chávez M., Figueroa-Brito R., Flores-Macías A., Romo-Asunción D., Martínez-González D., Pérez-Moreno V., Ramos-López M. Composition of the Essential Oil of Salvia ballotiflora (Lamiaceae) and Its Insecticidal Activity. Molecules. 2015;20:8048–8059. doi: 10.3390/molecules20058048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senthil-Nathan S. A Review of Resistance Mechanisms of Synthetic Insecticides and Botanicals, Phytochemicals, and Essential Oils as Alternative Larvicidal Agents against Mosquitoes. Front. Physiol. 2020;10:1591. doi: 10.3389/fphys.2019.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castro A.L.G., Cruz J.N., Sodré D.F., Correa-Barbosa J., Azonsivo R., de Oliveira M.S., de Sousa Siqueira J.E., da Rocha Galucio N.C., de Oliveira Bahia M., Burbano R.M.R., et al. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated from Eleutherine plicata herb. Using bioassays and in silico approaches. Arab. J. Chem. 2021;14:103084. doi: 10.1016/j.arabjc.2021.103084. [DOI] [Google Scholar]
- 72.Araújo P.H.F., Ramos R.S., da Cruz J.N., Silva S.G., Ferreira E.F.B., de Lima L.R., Macêdo W.J.C., Espejo-Román J.M., Campos J.M., Santos C.B.R. Identification of potential COX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules. 2020;25:4183. doi: 10.3390/molecules25184183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes J.P., Rees S.S., Kalindjian S.B., Philpott K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohacek R.S., McMartin C., Guida W.C. The art and practice of structure-based drug design: A molecular modeling perspective. Med. Res. Rev. 1996;16:3–50. doi: 10.1002/(SICI)1098-1128(199601)16:1<3::AID-MED1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 75.Degennaro M., McBride C.S., Seeholzer L., Nakagawa T., Dennis E.J., Goldman C., Jasinskiene N., James A.A., Vosshall L.B. Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy E.J., Booth J.C., Davrazou F., Port A.M., Jones D.N.M. Interactions of Anopheles gambiae odorant-binding proteins with a human-derived repellent: Implications for the mode of action of N,N-diethyl-3-methylbenzamide (DEET) J. Biol. Chem. 2013;288:4475–4485. doi: 10.1074/jbc.M112.436386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Y.F., de Biasio F., Qiao H.L., Iovinella I., Yang S.X., Ling Y., Riviello L., Battaglia D., Falabella P., Yang X.L., et al. Two odorant-binding proteins mediate the behavioural response of aphids to the alarm pheromone (e)-ß-farnesene and structural analogues. PLoS ONE. 2012;7:e32759. doi: 10.1371/journal.pone.0032759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schultes S., De Graaf C., Haaksma E.E.J., De Esch I.J.P., Leurs R., Krämer O. Ligand efficiency as a guide in fragment hit selection and optimization. Drug Discov. Today Technol. 2010;7:e157–e162. doi: 10.1016/j.ddtec.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Keserü G.M., Makara G.M. The influence of lead discovery strategies on the properties of drug candidates. Nat. Rev. Drug Discov. 2009;8:203–212. doi: 10.1038/nrd2796. [DOI] [PubMed] [Google Scholar]
- 80.Jones K.C. International programme on chemical safety (IPCS) environmental health criteria. Environ. Pollut. 1994;84:203. doi: 10.1016/0269-7491(94)90105-8. [DOI] [Google Scholar]
- 81.Rossi S.E., Erasmus J.J., McAdams H.P., Sporn T.A., Goodman P.C. Pulmonary drug toxicity: Radiologic and pathologic manifestations. Radiographics. 2000;20:1245–1259. doi: 10.1148/radiographics.20.5.g00se081245. [DOI] [PubMed] [Google Scholar]
- 82.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esteves F., Rueff J., Kranendonk M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics. 2021;11:94–114. doi: 10.3390/jox11030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sohoni P., Tyler C.R., Hurd K., Gaunter J., Hetheridge M., Williams T., Woods C., Evans M., Toy R., Gargas M., et al. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas) Environ. Sci. Technol. 2001;35:2917–2925. doi: 10.1021/es000198n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support this study are available within the manuscript.