Abstract
Background
Vaccination is an important preventive health measure to protect against symptomatic and severe COVID-19. Impaired immunity secondary to an underlying malignancy or recent receipt of antineoplastic systemic therapies can result in less robust antibody titers following vaccination and possible risk of breakthrough infection. As clinical trials evaluating COVID-19 vaccines largely excluded patients with a history of cancer and those on active immunosuppression (including chemotherapy), limited evidence is available to inform the clinical efficacy of COVID-19 vaccination across the spectrum of patients with cancer.
Patients and methods
We describe the clinical features of patients with cancer who developed symptomatic COVID-19 following vaccination and compare weighted outcomes with those of contemporary unvaccinated patients, after adjustment for confounders, using data from the multi-institutional COVID-19 and Cancer Consortium (CCC19).
Results
Patients with cancer who develop COVID-19 following vaccination have substantial comorbidities and can present with severe and even lethal infection. Patients harboring hematologic malignancies are over-represented among vaccinated patients with cancer who develop symptomatic COVID-19.
Conclusions
Vaccination against COVID-19 remains an essential strategy in protecting vulnerable populations, including patients with cancer. Patients with cancer who develop breakthrough infection despite full vaccination, however, remain at risk of severe outcomes. A multilayered public health mitigation approach that includes vaccination of close contacts, boosters, social distancing, and mask-wearing should be continued for the foreseeable future.
Key words: COVID-19, vaccination, SARS-CoV-2, neoplasm, cancer
Introduction
The development of effective vaccines against COVID-19, the disease caused by SARS-CoV-2, has allowed widespread vaccination programs aimed at reducing symptomatic and severe COVID-19.1 , 2 The presence of underlying immunosuppression and receipt of recent systemic therapy for cancer have been associated with prolonged or severe infection and may reduce the efficacy of vaccination.3, 4, 5, 6, 7, 8 Lower seroconversion rates following the receipt of COVID-19 vaccines have been observed in patients with underlying malignancy compared with non-cancer controls, with more concerning findings seen in patients with hematologic malignancies compared with those with solid cancers.7 , 9
The clinical impact of these serological alterations is yet to be evaluated and the characteristics of breakthrough COVID-19 in vaccinated patients with cancer have not been reported, potentially due to low prevalence of SARS-CoV-2 during the time period of the published reports. Clinical trials evaluating COVID-19 vaccines report a high efficacy in the general population, with few-to-no severe breakthrough infections.1 , 2 As patients with a history of cancer and those on active immunosuppression (including chemotherapy) were largely excluded from these landmark studies, scant evidence informs the clinical efficacy of COVID-19 vaccination across the spectrum of patients with cancer. Given the predisposition of patients with cancer to suffer from severe outcomes in SARS-CoV-2 infection10 and considering their potential susceptibility to mount a less effective immune response following vaccination,11 better characterization of breakthrough COVID-19 following vaccination in this vulnerable population is needed.
Here, we sought to report the clinical attributes of patients with cancer who develop breakthrough SARS-CoV-2 infections and compare their weighted outcomes with those seen in a contemporary unvaccinated population, using data from the multi-institutional COVID-19 and Cancer Consortium (CCC19) registry.
Patients and methods
The large international CCC19 registry captures data on patients with a current or prior history of cancer who develop COVID-19 through a REDCap survey with methodology outlined previously.10 , 12 Deidentified data are collected using a comprehensive set of variables related to demographics, cancer status, anticancer therapies, SARS-CoV-2 infection, and COVID-19 vaccination. Data on COVID-19 vaccination were routinely collected on every newly entered case beginning with the first global approval in November 2020. Eligible cases included adult patients (>18 years of age) accrued from 1 November 2020 to 31 May 2021 with current or prior history of invasive cancer and laboratory-confirmed SARS-CoV-2 infection. Patients were excluded if vaccination status or timing was unknown or if the vaccine was administered after SARS-CoV-2 infection. We also excluded cases with poor data quality (score ≥5 using the previously defined metric).13
The primary endpoint was 30-day all-cause mortality among fully vaccinated patients compared with the unvaccinated population after Inverse Probability of Treatment Weighting (IPTW) to adjust for baseline clinical variables. Secondary endpoints included rates of intensive care unit (ICU) admission and/or mechanical ventilation (MV), and hospitalization rates in fully vaccinated, compared with unvaccinated patients after IPTW to adjust for baseline clinical variables.
Patients were categorized as fully vaccinated at the time of COVID-19 when two doses of vaccine had been administered and diagnosis of COVID-19 was recorded >4 weeks from first dose (BNT162b2),2 or when two doses of vaccine had been administered and a diagnosis of COVID-19 was recorded >6 weeks from the first dose (mRNA-1273),1 or in the event of a single dose of vaccination and a positive diagnosis >28 days post-vaccine (Ad.26.COV2.S).14 Patients who received at least one dose of vaccine and developed COVID-19 but did not meet the previous criteria were considered partially vaccinated. Unvaccinated patients were defined as having no known prior exposure to COVID-19 vaccination before COVID-19 diagnosis.
IPTW was used to adjust for differences in baseline clinical variables between fully vaccinated and unvaccinated patients (Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2021.12.006) using age (as a continuous variable truncated at 90 years due to Health Insurance Portability and Accountability Act requirements), biologic sex (female; male), race (non-Hispanic White; Hispanic; non-Hispanic Black; other), smoking status (current or former smoker; never smoker), Eastern Cooperative Oncology Group performance status (ECOG PS 0; 1; ≥2), baseline corticosteroid use [none; ≤10 mg/day prednisone dose equivalent (PDE); >10 mg/day PDE],15 lymphopenia [absolute lymphocyte count (ALC), ≤1000 versus >1000 per μl], modified Charlson comorbidity index (mCCI, 0; 1; ≥2; Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.12.006), cancer status (active and progressing versus not active and progressing), cancer type (solid organ tumor; hematologic neoplasm; both), and recent systemic anticancer therapy (any of cytotoxic chemotherapy, immunotherapy, targeted therapy, or endocrine therapy in the 3 months before COVID-19 diagnosis, or not).
The data dictionary used in the analysis, along with instructions on how to access to full CCC19 data dictionary and code to generate derived variables is found in Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.12.006. All data analyses were carried out using R 4.0.3 and the R packages Hmisc 4.4.2, MatchIt 4.2.0, ipw 1.0-11, survey 4.0, sandwich 3.0-1, and glmnet 4.1-1.
This study was exempt from Institutional Review Board (IRB) review (VUMC IRB#200467), was approved by IRBs at participating sites per respective institutional policy, and is registered on ClinicalTrials.gov (NCT04354701).
Results
Overall, we identified 1787 patients with cancer and COVID-19 who met inclusion criteria, of whom 1656 (97%) were unvaccinated, 77 (4%) partially vaccinated, and 54 (3%) fully vaccinated (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.12.006). Baseline clinical factors by vaccination status are shown in Table 1 .
Table 1.
Baseline clinical factors by baseline vaccination status
| Fully vaccinated N (%) |
Partially vaccinated N (%) |
Unvaccinated N (%) |
|
|---|---|---|---|
| Total patients (N = 1787) | 54 (3) | 77 (4) | 1656 (93) |
| Median age, years (IQR) | 65.5 (57.0-72.8) | 68.0 (58.0-78.0) | 64.0 (54.0-74.0) |
| Female sex | 35 (65) | 38 (49) | 903 (55) |
| Non-Hispanic White | 38 (70) | 58 (75) | 999 (60) |
| ECOG performance status ≥2 | 9 (17) | 14 (18) | 224 (14) |
| Modified Charlson comorbidity index ≥2 | 17 (31) | 26 (34) | 413 (25) |
| Current or former smoker | 26 (48) | 33 (43) | 732 (44) |
| Hematologic malignancy | 19 (35) | 18 (23) | 339 (20) |
| Cancer status active and progressing | 10 (19) | 17 (22) | 237 (14) |
| Systemic treatment within 3 months | 30 (56) | 31 (40) | 720 (43) |
| Baseline prednisone dose-equivalent >10 mg/day | 9 (17) | <5 (<6)c | 117 (7) |
| ALC ≤1000/μl (not drawn/not available N = 902) | 25 (46) | 26 (34) | 469 (28) |
| COVID-19 vaccinationa | — | — | — |
| Ad26.COV2.S (Johnson & Johnson) | 10 (19)b | 5 (6) | — |
| mRNA-1273 (Moderna) | 22 (29) | — | |
| BNT162b2 (Pfizer-BioNTech) | 44 (81) | 33 (43) | — |
| COVID-19 infection severity | — | — | — |
| 30-day mortality | 7 (13) | <5 (<6)c | 160 (10) |
| Admitted to intensive care | 10 (19) | 11 (14) | 215 (13) |
| Hospitalized | 35 (65) | 45 (58) | 834 (50) |
ALC, absolute lymphocyte count; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
Numbers for partially vaccinated add up to <100% as 17 patients were vaccinated but reported as other or unknown type.
Numbers for AD26.COV2.S and mRNA-1273 are combined as n < 5 for one vaccination type, requiring masking per standard CCC19 protocol.
Numbers and percentages are masked for small cell counts per standard CCC19 protocol.
Median age of fully vaccinated patients was 65.5 years [interquartile range (IQR) 57.0-72.8 years], 35 (65%) were female and 38 (70%) were non-Hispanic White. A total of 19 (35%) had hematologic malignancies and 17 (31%) had an mCCI of ≥2. Before IPTW was carried out, more patients in the fully vaccinated cohort relative to the unvaccinated cohort had an underlying hematologic malignancy (35% versus 20%), were female (65% versus 55%), non-Hispanic White (70% versus 60%), received baseline prednisone or equivalent >10 mg/day (17% versus 7%), had ALC <1000/μl (46% versus 28%), or received systemic therapy within the prior 3 months (56% versus 43%).
Among the 54 fully vaccinated patients who developed COVID-19, 35 (65%) were hospitalized, 10 (19%) were admitted to ICU or required MV, and 7 (13%) died within 30 days. Comparable rates were observed in the unvaccinated group (Table 1).
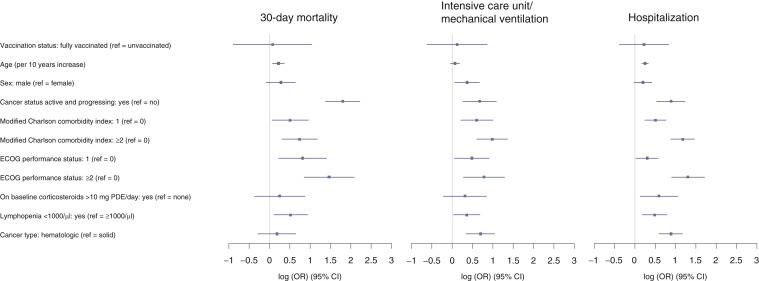
Following IPTW (Table 2 and Figure 1 ) there was no statistical difference in 30-day mortality between the fully vaccinated patients compared with the unvaccinated cohort, adjusted odds ratio (AOR) 1.08, 95% confidence interval (CI): 0.41-2.82. Increased 30-day mortality was associated with lymphopenia (AOR 1.68, 95% CI: 1.11-2.55), the presence of comorbid conditions (mCCI of 1 versus 0: AOR 1.66, 95% CI: 1.07-2.59 and mCCI ≥2 versus 0: AOR 2.10, 95% CI: 1.36-3.24), worse PS (ECOG PS 1 versus 0: AOR 2.26, 95% CI: 1.25-4.06 or ECOG PS ≥2 versus 0: AOR 4.34, 95% CI: 2.35-8.02), and baseline cancer status (active and progressing versus not active and progressing, AOR 6.07, 95% CI: 4.00-9.19).
Table 2.
Results of regression analysis following truncated inverse probability of treatment weighting
| 30-Day mortality AOR (95% CI) | Intensive care unit/mechanical ventilation AOR (95% CI) | Hospitalization AOR (95% CI) | |
|---|---|---|---|
| Vaccination status (ref = unvaccinated) | — | — | — |
| Fully vaccinated | 1.08 (0.41-2.82) | 1.13 (0.54-2.37) | 1.25 (0.68-2.30) |
| Age (per 10 years increase) | 1.25 (1.08-1.44) | 1.07 (0.96-1.20) | 1.28 (1.18-1.39) |
| Sex (ref = female) | — | — | — |
| Male | 1.32 (0.92-1.90) | 1.44 (1.06-1.95) | 1.22 (0.98-1.52) |
| Cancer status active and progressing (ref = not active and progressing) | — | — | — |
| Yes | 6.07 (4.00-9.19) | 1.96 (1.30-2.96) | 2.42 (1.71-3.43) |
| Modified Charlson comorbidity index (ref = 0) | — | — | — |
| 1 | 1.66 (1.07-2.59) | 1.83 (1.24-2.71) | 1.65 (1.27-2.15) |
| ≥2 | 2.10 (1.36-3.24) | 2.67 (1.83-3.90) | 2.42 (1.71-3.43) |
| ECOG performance status (ref = 0) | — | — | — |
| 1 | 2.26 (1.25-4.06) | 1.62 (1.05-2.48) | 1.35 (1.02-1.78) |
| ≥2 | 4.34 (2.35-8.02) | 2.19 (1.32-3.64) | 3.68 (2.46-5.53) |
| On baseline corticosteroids >10 mg PDE/day (ref = none) | — | — | — |
| Yes | 1.28 (0.69-2.39) | 1.37 (0.81-2.31) | 1.81 (1.13-2.88) |
| Lymphopenia <1000/µl (ref = ≥1000/µl) | — | — | — |
| Yes | 1.68 (1.11-2.55) | 1.43 (1.03-1.97) | 1.62 (1.20-2.20) |
| Cancer type (ref = solid) | — | — | — |
| Hematologic | 1.20 (0.75-1.91) | 2.00 (1.41-2.83) | 2.42 (1.82-3.22) |
AOR, multivariable adjusted odds ratio; CI, confidence interval; PDE, prednisone dose-equivalent; ref, reference.
Figure 1.
Forest plot showing results of regression analysis following truncated Inverse Probability of Treatment Weighting by clinical outcomes.
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; PDE, prednisone dose-equivalent.
There were no significant differences in ICU/MV or hospitalization rates between the vaccinated patients compared with the unvaccinated cohort after adjustment (AOR 1.13, 95% CI: 0.54-2.37 and AOR 1.25, 95% CI: 0.68-2.30, respectively). In both vaccinated and unvaccinated patients, higher ICU/MV and hospitalization rates were identified in patients with lymphopenia (AOR 1.43, 95% CI: 1.03-1.97 and AOR 1.62, 95% CI: 1.20-2.20, respectively), the presence of comorbid conditions (mCCI of ≥2 versus 0: AOR 2.67, 95% CI: 1.83-3.90, and AOR 2.42, 95% CI: 1.71-3.43, respectively; mCCI of 1 versus 0: AOR 1.83, 95% CI: 1.24-2.71, and AOR 1.65, 95% CI: 1.27-2.15, respectively), poor ECOG PS (ECOG PS ≥2 versus 0: AOR 2.19, 95% CI: 1.32-3.64, and AOR 3.68, 95% CI: 2.46-5.53, respectively; ECOG PS 1 versus 0: AOR 1.62, 95% CI: 1.05-2.48, and AOR 1.35, 95% CI: 1.02-1.78, respectively) and hematologic as opposed to solid cancers (AOR 2.00, 95% CI: 1.41-2.83, and AOR 2.42, 95% CI: 1.82-3.22, respectively).
Secondary outcome regressions and sensitivity analyses are described in Supplementary Methods and Supplementary Tables S4-S8, available at https://doi.org/10.1016/j.annonc.2021.12.006.
Discussion
To our knowledge, this is the first study to evaluate the clinical characteristics and outcomes of patients with cancer who experience breakthrough infection following COVID-19 vaccination. Vaccination has been widely effective at reducing the severity of SARS-CoV-2 infection, but the protection afforded by this preventive modality can be incomplete, and despite the high efficacy rates identified in clinical trials,1 , 2 some patients (such as the ones with cancer) remain at risk of developing symptomatic COVID-19, with potentially severe adverse outcomes. Vaccine effectiveness has been studied extensively in the general population as well as in patients with cancer and appears to wane over time. Based on the accumulation of knowledge to date, it appears that patients with hematologic malignancy are less likely to mount an effective immune response.16 The enrichment of hematologic malignancies (35% versus 20%) in this cohort is consistent with evidence that these patients may have a blunted serologic response to vaccination secondary to disease or therapy,17 , 18 compounding their probability to develop more severe COVID-19 outcomes relative to patients with other types of tumors.19
The described vaccinated cohort may be subject to ascertainment bias as a result of selective reporting, if reporting sites did not attain complete coverage of all eligible cases. Furthermore, it is possible that the vaccination exposure was not captured in some of the ostensibly unvaccinated patients. Neither post-vaccination titers nor T-cell-mediated immunity metrics are routinely checked in clinical care and were not captured in this study, leaving open the possibility that at least some of the patients manifesting COVID-19 did not have adequate immunity. Nevertheless, any infection in a fully vaccinated person meets the Centers for Disease Control and Prevention (CDC) definition of breakthrough infection (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html). Notably, boosters were not yet available during the timeframe of this study, and are not part of the definitions of fully vaccinated used in the original trials, which we adopted.1 , 2 , 14 The mortality and ICU/MV rates of 13% and 19%, respectively, recorded in the fully vaccinated group, with no significant differences compared with the unvaccinated group, represent a considerable residual risk. Additionally, this contrasts with a substantially lower rate of severe outcomes previously identified in vaccinated healthy individuals as opposed to matched unvaccinated controls,20 outlining the potential vulnerability of patients with cancer.
Lymphopenia, which has a strong association with severe SARS-CoV-2 infection,5 was present in 46% of fully vaccinated patients and 28% in the unvaccinated patients. This finding supports the notion that lymphopenic patients with cancer are at high risk for severe disease, even after vaccination.21 It has been previously shown that lymphocyte-depleted patients such as those receiving anti-CD20 monoclonal antibodies or chimeric antigen receptor T-cell (CAR-T-cell) treatment have a much weaker serological response to COVID-19 vaccines.22 Our results appear to confirm the clinical relevance of such previous observations.
Alternatively, more severe infection with resultant lymphopenia may have been present at diagnosis in vaccinated patients, potentially due to a lower suspicion of SARS-CoV-2 infection following full vaccination, resulting in a delayed presentation of vaccinated patients with cancer after COVID-19 symptom onset.
Some differences are apparent in the baseline characteristics of the fully vaccinated cohort relative to the unvaccinated population and can partially explain differences in clinical outcomes, even after adjustment. A higher prevalence of active and progressive cancer (19% versus 14%) and of recent receipt of systemic anticancer therapy (56% versus 43%) was seen in the fully vaccinated population relative to unvaccinated patients, with both being previously identified risk factors for adverse COVID-19 outcomes.5 , 10 The number of affected patients in this study is too small to make any definitive conclusions about specific types of anticancer therapy that might be associated with breakthrough infection.
Chronic corticosteroid use (≥10 mg/day PDE), which is associated with higher odds of hospitalization from COVID-19 viral respiratory illness in a cohort of patients with autoimmune conditions,23 also appeared to be more prevalent at baseline in the fully vaccinated cohort compared with the unvaccinated patients (24% versus 14%).
Following regression analysis, the association between advancing age, active and progressing cancer, ECOG PS ≥2, mCCI ≥2, and lymphopenia with mortality suggests that these established prognostic factors for COVID-19 outcomes remain relevant in defining those patients who may still be at risk of severe outcomes following completion of vaccination.
Limitations of this study include the dependence on clinically annotated data and the reliance on time intervals (instead of exact dates) to capture vaccine administration relative to COVID-19 diagnosis. Given the timeframe of this analysis, it is unlikely that any patients had infection >6 months out from vaccination, such that waning immunity is not likely a major factor; future studies will need to consider this as well as receipt of boosters. The true population prevalence of vaccinated individuals during the time period is unknown and likely changed substantially, as at-risk populations were variably prioritized before vaccines became more generally available in spring 2021. As such, the rate of serious illness or mortality from COVID-19 among the total population of vaccinated patients with cancer is unknown, as vaccination was widely administered and likely protected the majority from serious illness. Strengths include the high-quality data with a robust quality assurance process, along with a comprehensive list of clinical, demographic, and laboratory variables.
With the emergence of the B.1.617.2 (delta) variant, which displays a higher transmissibility than previous strains of the virus,24 and for which available vaccines appear to show decreased neutralization and efficacy with a higher rate of breakthrough cases,25, 26, 27 the current findings underscore the need to maintain the implementation of public health measures required to control infection spread and protect vulnerable populations. Delta and subsequent variants will continue to raise the possibility of immune escape from the first generation of vaccines. Additional research is needed to further categorize the patients who remain at risk of symptomatic COVID-19 following vaccination, and test strategies that may reduce this risk. Based on experience in patients with prior organ transplantation,28 the strategy of administering a third primary series vaccination dose to increase antibody titers is one option recently suggested by the CDC to be considered for immunosuppressed patients, including patients with cancer on systemic anticancer therapies (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html). Further correlation of antibody responses to clinical benefit will be needed to validate the use of these strategies, as well as boosters and emerging strategies such as ‘mix and match’, where a different booster vaccine is administered from the primary vaccination course.29
Overall, vaccination remains an invaluable strategy in protecting vulnerable populations, including patients with cancer, against COVID-19. Patients with cancer who develop breakthrough infection despite full vaccination, however, remain at risk of severe outcomes and should not be neglected. A mitigation approach that includes vaccination of close contacts, boosters, social distancing, and mask-wearing in public should be continued for the foreseeable future.
Acknowledgements
We thank all members of the CCC19 steering committee: Toni K. Choueiri, Narjust Duma, Dimitrios Farmakiotis, Petros Grivas, Gilberto de Lima Lopes Jr, Corrie A. Painter, Solange Peters, Brian I. Rini, Dimpy P. Shah, Michael A. Thompson, and Jeremy L. Warner, for their invaluable guidance of the CCC19 consortium. We thank Donna R. Rivera and Jill Barnholtz-Sloan for careful review of the manuscript.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (NCI/NIH), United States [grant numbers T32 CA236621, P30 CA046592]. TKC is supported in part by the Dana-Farber/Harvard Cancer Centre Kidney SPORE [grant numbers P50 CA101942, P30 CA006516], both from NCI/NIH; the Kohlberg Chair at Harvard Medical School, United States and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber Cancer Institute, United States (no grant numbers). NCI/NIH [grant number P30 CA068485]; National Center for Advancing Translational Sciences, United States (NCATS, NIH [grant number UL1 TR000445] (to SM, CYH, YS, JLW). NCI/NIH [grant number U01 CA231840] (to JLW). NCATS/NIH, United States [grant number UL1 TR001873] and Young Investigator Award from the Prostate Cancer Foundation, United States (to JEH). NCI/NIH [grant number P30 CA023100] (to RRM). REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research, United States grant support [grant number UL1 TR000445 from NCATS/NIH].
Disclosure
ALS reports non-financial support from Astellas, non-financial support from Pfizer, outside the submitted work. CL reports research support from Genentech/imCORE, outside the submitted work. ZB reports non-financial support from Bristol Myers Squibb, grants from Genentech/imCORE, personal fees from UpToDate, outside the submitted work. CYH reports personal fees from NashBio, outside the submitted work. SAB reports personal fees from Exelixis, personal fees from Seattle Genetics, personal fees from Pfizer, personal fees from Bristol Myers Squibb, outside the submitted work. CRF reports research grants from the Merck Foundation and National Comprehensive Cancer Network (NCCN)/Pfizer, outside the submitted work. EAG reports personal fees and other from Alexion Pharmaceuticals, personal fees and non-financial support from Novartis Pharmaceuticals, personal fees, non-financial support and other from Astex/Otsuka Pharmaceuticals, other from Apellis Pharmaceuticals, personal fees, non-financial support, and other from Celgene/Bristol Myers Squibb, grants and personal fees from AbbVie/Genentech, other from Celldex Therapeutics, personal fees from Boston Biomedical, outside the submitted work. JEH reports research funding paid to her institution from Dendreon Pharmaceuticals LLC, research funding paid to her institution from Regeneron Pharmaceuticals, personal fees from Genzyme, personal fees from Seagen, outside the submitted work. RRM reports grants and personal fees from Bayer, grants from Pfizer, grants from Tempus, personal fees from AVEO, personal fees from Caris, personal fees from Bristol Myers Squib, personal fees from Exelixis, personal fees from Janssen, personal fees from Novartis, personal fees from Pfizer, personal fees from Sanofi, personal fees from Tempus, personal fees from Dendreon, personal fees from Vividion, personal fees from AstraZeneca, personal fees from Calithera, personal fees from Merck, outside the submitted work. OAP reports personal fees from International Consulting Associates, Inc., outside the submitted work. EMW-B reports personal fees from Astellas, personal fees from AVEO Oncology, personal fees from Bristol Myers Squibb, other from Exelixis, grants from Pfizer Global Medical Grants, other from Nektar, other from Immunomedics, outside the submitted work. SM reports personal fees from National Geographic, outside the submitted work. DF reports a grant from Merck to study COVID-19 in immunocompromised patients, outside of the submitted work. YS reports personal fees from Novartis, personal fees from Roche, personal fees from Pfizer, personal fees from Janssen, personal fees from Eisai, personal fees from AstraZeneca, outside of the submitted work. JLW reports personal fees from Westat, personal fees from Roche, personal fees from Melax Tech, personal fees from Flatiron Health, other from HemOnc.org LLC (ownership), outside the submitted work. TKC reports institutional and personal, paid and unpaid support for research, advisory boards, consultancy, and honoraria from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVIA, Ipsen, Kanaph, Lilly, Merck, Nikang, Novartis, Pfizer, Roche, Sanofi/Aventis, Takeda, Tempest, UpToDate, CME events (Peerview, OncLive and others), outside the submitted work. All other authors have declared no conflicts of interest.
Contributor Information
COVID-19 and Cancer Consortium:
T.K. Choueiri, N. Duma, D. Farmakiotis, P. Grivas, G. de Lima Lopes, Jr., C.A. Painter, S. Peters, B.I. Rini, D.P. Shah, M.A. Thompson, and J.L. Warner
Supplementary data
References
- 1.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi B., Choudhary M.C., Regan J., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akalin E., Azzi Y., Bartash R., et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and cancer consortium. Ann Oncol. 2021;32(6):787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshen-Lago T., Waldhorn I., Holland R., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reischig T., Kacer M., Vlas T., et al. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am J Transplant. 2021 doi: 10.1111/ajt.16902. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakkar A., Pradhan K., Jindal S., et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2(4):392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinstein S.M., Steinharter J.A., Warner J., Rini B.I., Peters S., Choueiri T.K. The COVID-19 and cancer consortium: a collaborative effort to understand the effects of COVID-19 on patients with cancer. Cancer Cell. 2020;37(6):738–741. doi: 10.1016/j.ccell.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abidi M., Aboulafia D.M., Accordino M.K., et al. A systematic framework to rapidly Obtain data on patients with cancer and COVID-19: CCC19 governance, protocol, and quality assurance. Cancer Cell. 2020;38(6):761–766. doi: 10.1016/j.ccell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon W.G., Kezouh A., Bernatsky S., Suissa S. The influence of systemic glucocorticoid therapy upon the risk of non-serious infection in older patients with rheumatoid arthritis: a nested case–control study. Ann Rheum Dis. 2011;70(6):956–960. doi: 10.1136/ard.2010.144741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkrief A., Wu J.T., Jani C., et al. Learning through a pandemic: the current state of knowledge on COVID-19 and cancer. Cancer Discov. 2021 doi: 10.1158/2159-8290.CD-21-1368. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herishanu Y., Avivi I., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry C., Luttwak E., Balaban R., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijenthira A., Gong I.Y., Fox T.A., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt A.A., Nafady-Hego H., Chemaitelly H., et al. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination. Int J Infect Dis. 2021;110:353–358. doi: 10.1016/j.ijid.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derosa L., Melenotte C., Griscelli F., et al. The immuno-oncological challenge of COVID-19. Nat Cancer. 2020;1:946–964. doi: 10.1038/s43018-020-00122-3. [DOI] [PubMed] [Google Scholar]
- 22.Ollila T.A., Lu S., Masel R., et al. Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol. 2021;7(11):1714–1716. doi: 10.1001/jamaoncol.2021.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Deng A, Li K, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 delta variant. 2021. Preprint. https://www.medrxiv.org/content/10.1101/2021.07.07.21260122v2. [DOI] [PMC free article] [PubMed]
- 25.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 26.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen P.A., Olsen R.J., Long S.W., et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am J Pathol. 2021 doi: 10.1016/j.ajpath.2021.10.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juno J.A., Wheatley A.K. Boosting immunity to COVID-19 vaccines. Nat Med. 2021;27:1874–1875. doi: 10.1038/s41591-021-01560-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.