Abstract
Molnupiravir (MK-4482) is an investigational antiviral agent that is under development for the treatment of COVID-19. Given the potential high demand and urgency for this compound, it was critical to develop a short and sustainable synthesis from simple raw materials that would minimize the time needed to manufacture and supply molnupiravir. The route reported here is enabled through the invention of a novel biocatalytic cascade featuring an engineered ribosyl-1-kinase and uridine phosphorylase. These engineered enzymes were deployed with a pyruvate-oxidase-enabled phosphate recycling strategy. Compared to the initial route, this synthesis of molnupiravir is 70% shorter and approximately 7-fold higher yielding. Looking forward, the biocatalytic approach to molnupiravir outlined here is anticipated to have broad applications for streamlining the synthesis of nucleosides in general.
Short abstract
The conversion of simple reagents into the 1st antiviral for COVID-19 was realized through the use of a multienzyme cascade made possible by the development of an efficient phosphate catalytic cycle.
Introduction
The ongoing COVID-19 pandemic caused by SARS-CoV-2 has been devastating to the global population, with over 4 million deaths and infection rates continuing to rise around the world.1 While the rapid development of effective vaccines against COVID-19 offers a long-term control strategy, the pandemic has shone a spotlight on the lack of readily available and easily distributed antiviral agents that can be used to counter novel viruses. Moreover, the likelihood that SARS-CoV-2 will mutate to escape acquired immunity is a growing concern,2,3 and in the near-term, the treatment and prevention of an extremely high burden of existing COVID-19 infections remains a substantial challenge worldwide. As highlighted with the supply of vaccines for the prevention of COVID-19, manufacturing and raw material constraints can severely limit the number of patients that receive rapid access to life-saving treatments.4 Thus, equally important to the identification of effective antiviral agents for COVID-19 is the development of efficient and rapid synthetic routes that minimize the time from simple and readily available commodity reagents to medicines.
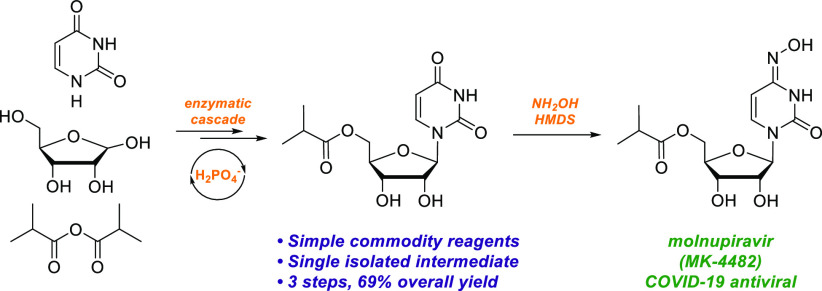
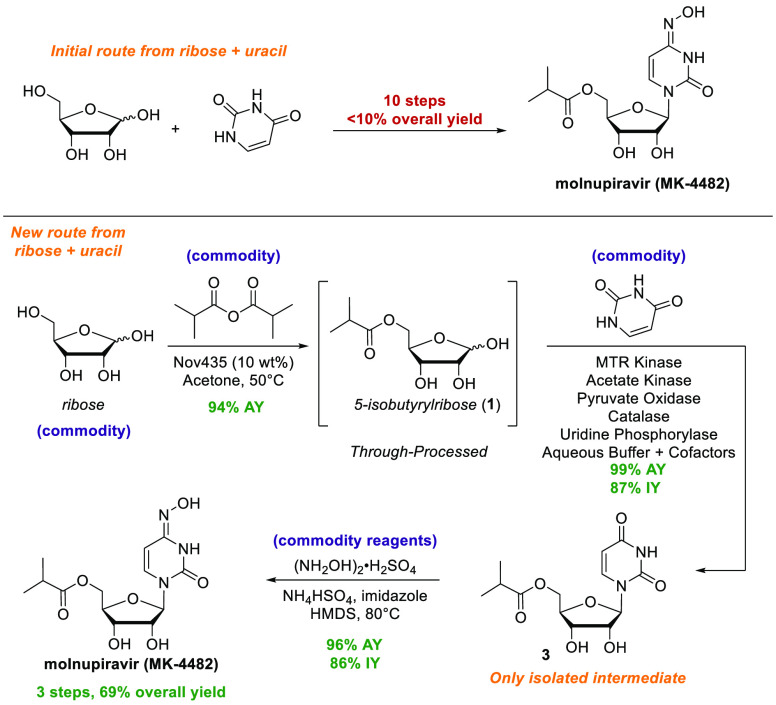
Molnupiravir (MK-4482, formerly EIDD-2801) is an orally dosed direct-acting antiviral agent that has been demonstrated to prevent COVID-19 in animal models.5−7 Recently reported phase 3 clinical data have demonstrated that molnupiravir reduced the risk of hospitalization or death by approximately 50% compared with placebo.8 Molnupiravir is currently supplied in five steps from uridine, which itself is synthesized from ribose and uracil in five steps. Alternative routes to synthesize molnupiravir from other nucleosides such as cytidine have been reported.9−13 This strategy of converting natural nucleosides to non-natural nucleosides is a common approach yet often requires several inefficient steps and protecting group manipulations. Even where concise semisynthetic routes from nucleosides have been established,9−12 it is nevertheless true that natural nucleosides themselves are prepared in multiple steps from more simple building blocks, such as ribose, thus incurring additional lead times. We therefore sought to identify the most direct synthesis of molnupiravir from ribose (Figure 1), and provide a platform to streamline the synthesis of future non-natural nucleosides.
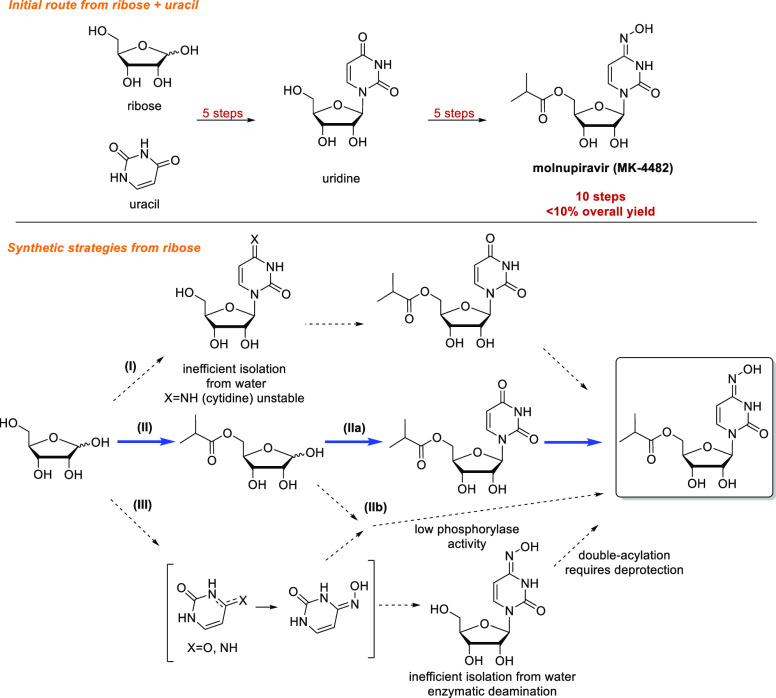
Figure 1.
Original route to molnupiravir and alternative synthetic strategies considered. Blue highlights indicate chosen route.
Results and Discussion
The synthesis of molnupiravir from ribose requires, at minimum, three transformations (Figure 1): (a) acylation of the primary alcohol, (b) nucleobase installation, and (c) installation of the oxime. In theory, these steps could be performed in any order; however, the ease of isolation and the stability of intermediates was a critical consideration when exploring these alternative sequences. Known biocatalytic and biosynthetic nucleoside pathways from ribose would allow for a cascade using wild-type enzymes to synthesize uridine and perhaps cytidine or N–OH cytidine14−17 (Figure 1, routes I and III). However, preliminary studies revealed that cytidine and N–OH cytidine suffered enzymatic deamination in the presence of E. coli host-cell enzymes, while phosphorylated ribose likewise was unstable due to dephopshorylation in the presence of E. coli host-cell enzymes. A further and critical consideration is related to isolation: although molnupiravir itself has reasonable organic solubility and could therefore be crystallized from organic solvent, uridine, cytidine, and N–OH cytidine (routes I and III) are extremely water-soluble and could not be efficiently recovered in high yield from enzymatic reactions. In turn, 5′-acylation of nucleosides or of ribose is known,18,19 but these reactions are run in organic solvents, which rendered biocatalytic synthesis of uridine or cytidine in water followed by 5′-acylation impractical. Finally, acylation of N–OH cytidine readily led to overacylation at the oxime, which would require subsequent deprotection. Based on these considerations, the envisioned ideal synthesis would begin with 5-acylation of ribose (Figure 1, route II). We hypothesized that this order of reactions would protect ribose from subsequent catabolism as well as facilitate isolation of the product formed after nucleobase installation.
This synthetic route relied on several unknown transformations. In particular, biocatalytic nucleoside synthesis using a 5-esterified sugar is unprecedented, as nucleoside salvage pathways and de novo nucleoside biosynthesis both require a 5-phosphate group.20 In this case, we would need to identify a kinase capable of the diastereoselective phosphorylation of 5-isobutyryl ribose (1) to directly give the corresponding 1-phosphate. We would then need to identify a nucleoside phosphorylase capable of reacting with a non-natural substrate containing a 5-isobutyryl group to install either uracil or N–OH cytosine (Figure 1, IIa or IIb, respectively). Furthermore, we would need to identify conditions to efficiently install the N–OH moiety on either the free nucleobase or 5′-isobutyryl uridine. In addition to these synthetic challenges, we also needed to develop a viable large-scale process that could supply metric tons of molnupiravir within only a few months. With these goals in mind, we initiated screening efforts to identify suitable enzymes to catalyze the necessary transformations.
Our efforts began with the identification of an appropriate nucleoside phosphorylase. Nucleoside phosphorylases catalyze equilibration between sugar 1-phosphates and the nucleoside,21 affording the opportunity to test the desired reaction in the reverse direction starting from the nucleosides. We constructed a diverse panel of nucleoside phosphorylase enzymes and screened for the phosphorolysis of either molnupiravir or 3 to generate the 1-phosphate (2) and the corresponding nucleobase. While only trace activity could be observed in the phosphorolysis of molnupiravir itself, we identified E. coli uridine phosphorylase (UP; URP-003 from Codexis) as the most active enzyme on 3, providing nearly 40% cleavage in high-throughput reactions (Figure S1).
Having proof of concept for glycosidic bond formation with uridine phosphorylase, we then needed to identify a method to directly synthesize the key ribosyl-1-phosphate (2) from 1, the latter of which we prepared in over 90% yield using an immobilized lipase (Figures S2 and S3). We recognized that direct 1-phosphorylation of 5-S-methylthioribose (MTR) occurs as part of the methionine salvage pathway and is catalyzed by MTR kinases (Figure S4).22,23 Although MTR kinases do not naturally participate in nucleoside biosynthesis and have no reported biocatalytic applications, we hypothesized that an MTR kinase could generate the desired ribosyl-1-phosphate intermediate. A panel of MTR kinase enzymes was produced by heterologous expression in E. coli. The MTR kinases were then screened in a cascade reaction with E. coli uridine phosphorylase for the production of 3. Through these efforts, we identified the MTR kinase from Klebsiella spp. as a suitable enzyme, albeit with modest activity that would require significant improvement to enable a viable process to molnupiravir.
An evolution campaign targeted the MTR kinase for improved activity on the desired non-natural substrate. The initial round of evolution screened single-site-saturation variant libraries for increased activity in the cascade synthesis of 3 under kinase-limited conditions. From this screen, numerous beneficial mutations were identified (Tables S1 and S2, Figures S5 and S6). The best single mutant (L79H) was then used as the starting point for a combinatorial library containing other beneficial mutations that had been identified in round 1 (Tables S1 and S2). This screen led to identification of an improved quadruple mutant (V63P, T173S, F177I, I230L). An additional combinatorial library that contained remaining beneficial single mutations from round 1 was then screened, leading to the identification of an improved sextuple mutant (H10D, C65A, E68P, A168G, A244V, R384T) that was judged suitable for the process. This optimized MTR kinase could provide >90% conversion in cascade reactions using less than 1 wt % enzyme, and its activity was improved more than 100-fold from the starting enzyme.
In parallel, we also evolved the UP for improved activity in the synthesis of 3, screening single-site-saturation variant libraries followed by the recombination of beneficial mutations in subsequent rounds. The initial round of evolution on UP screened the reverse reaction for improved phosphorolysis of 3 and led to identification of a single mutant (E91I) with >3-fold-improved activity. The E91I single mutant was then used as the starting point for an additional site-saturation library (Tables S3, S4 round 3) that yielded a 1.9-fold-improved mutant (A252M). Other beneficial single mutants identified in rounds 1 and 2 were then tested in a combinatorial library in round 3, yielding a 2×-improved sextuple mutant (K14E, V35L, H58W, A200R, I239L, T244S). This variant was carried forward to a further site-saturation library in round 4, which yielded a 1.3×-improved single mutant (L20M). The final round then recombined beneficial single mutations, leading to the final variant, which was 2.4-fold improved and contained a further 10 mutations (V17S, D40A, K51N, P111G, L132G, L137M, H190R, K192Q, A200E, T248V). The final variant could provide full conversion at 1 wt % and was more than 80-fold improved over the wild-type enzyme (Figures S7–S10).
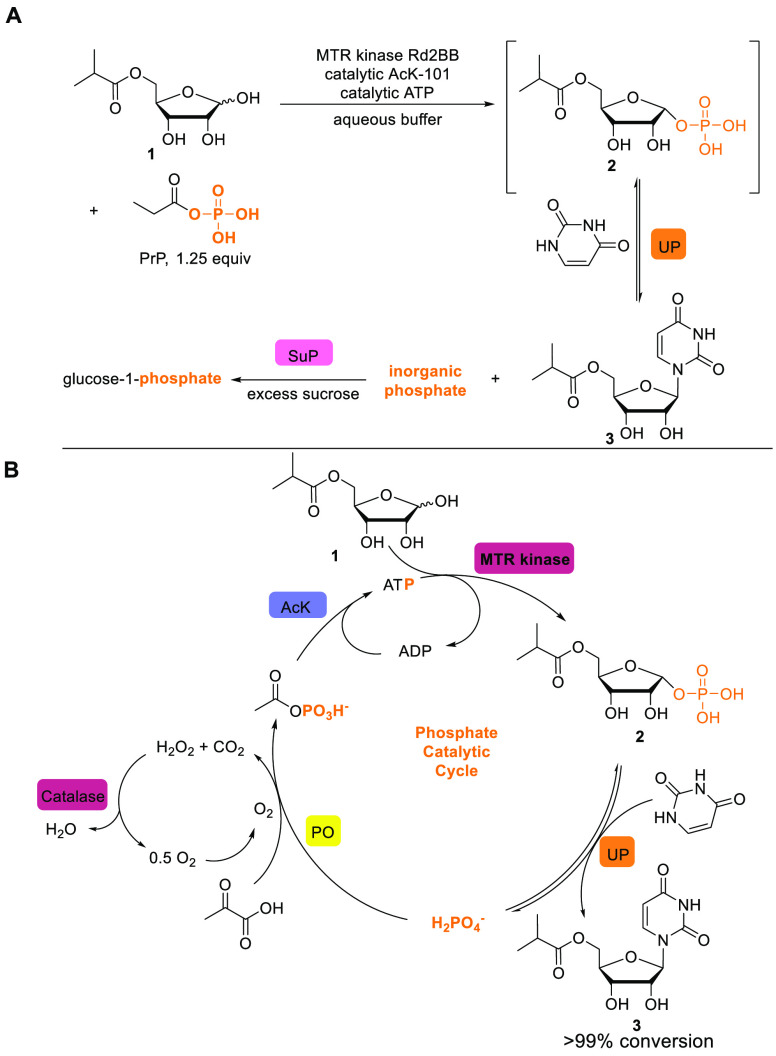
With optimized enzymes in hand, we began the development of a scalable cascade reaction from 1 to 3. As we examined the potential to scale this chemistry, we recognized two critical and intertwined issues: first, enzymatic phosphorylation using ATP generally requires an excess of a independently synthesized phosphoryl donor, such as phosphoenol pyruvate or acetyl phosphate, to be added in order to recycle ATP. Furthermore, in the nucleobase incorporation step, an equivalent of phosphate is generated, which needs to be sequestered to drive the equilibrium toward product formation. We previously employed the addition of sucrose and sucrose phosphorylase to address the equilibrium issue in nucleoside synthesis,14 and a similar approach works here as well, using propionyl phosphate instead of acetyl phosphate (Figure 2A). However, this approach requires the addition of excess sucrose and limits the reaction concentration due to the large amount of sucrose and the high ionic strength due to stoichiometric phosphate and byproduct propionate
Figure 2.
(A) Stoichiometric phosphoryl donor using phosphate sequestration strategy. (B) Phosphate recycling strategy using pyruvate oxidase. See the Supporting Information for additional details. AcK: acetate kinase; ATP: adenosine 5′-triphosphate; ADP: adenosine 5′-diphosphate; MTR: 5-S-methylthioribose; UP: uridine phosphorylase; PO: pyruvate oxidase; SuP: sucrose phosphorylase.
All of these issues could be circumvented if the use of a superstoichiometric phosphoryl donor could be avoided. To that end, we conjectured that the enzyme pyruvate oxidase (PO),24 which catalyzes the formation of acetyl phosphate from pyruvic acid and inorganic phosphate, could be used to generate acetyl phosphate in situ (Figure 2B). Since inorganic phosphate is the byproduct of the glycosylation, this strategy might enable transient formation and subsequent consumption of inorganic phosphate, avoiding both the need for a stoichiometric phosphoryl donor such as acetyl phosphate and the need for a separate phosphate sequestration step.
The use of PO for ATP regeneration has been applied to improve in vitro protein synthesis25 but has not been applied in biocatalysis. We realized the fully enzymatic cascade to 3 by employing PO and acetate kinase (AcK) for ATP regeneration, MTR kinase for the phosphorylation of 1, UP for uracil incorporation, and catalase to decompose hydrogen peroxide formed by PO. In total, this novel biocatalytic cascade can be run at high concentrations (>80 g/L) of 1, forms the product in quantitative yield, and allows for facile isolation of 3 in >99.5% purity through extraction with 2-MeTHF and subsequent crystallization (Figure S11). Beyond the practical advantages of using this novel catalytic phosphate approach on scale, we are further developing this as a general approach to the synthesis of nucleoside derivatives.
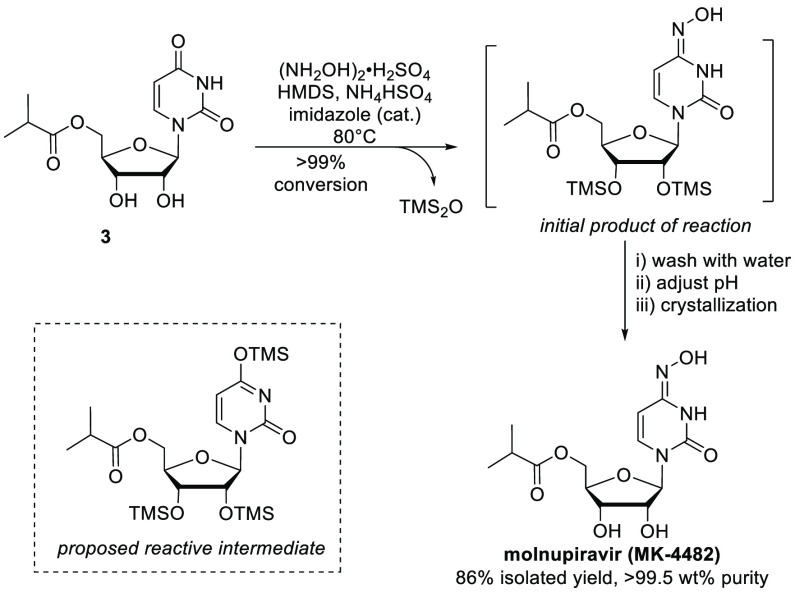
Completion of the synthesis of molnupiravir requires conversion of the amidic carbonyl in the uracil ring to the corresponding oxime. Initial investigation into this transformation found that high-energy activating agents, such as diethyl chlorophosphate, could be used to selectively activate the carbonyl group, and subsequent reaction with hydroxylamine served to form molnupiravir. Despite the success of this approach, the use of these types of reagents was not pursued, as they are classified as highly toxic and hazardous and are not in line with the goal of developing a safe and sustainable process. We anticipated that simple trimethylsilyl (TMS) reagents could promote the desired transformation through activation of the uracil ring, with the reaction ultimately driven by the formation of innocuous hexamethyldisiloxane (TMS2O).26 Furthermore, we hypothesized that the alcohols present in 3 would react to form TMS ethers and enable the isolation of high-purity molnupiravir (vide infra). While TMSCl is inexpensive and readily available, it carries corrosivity risks and handling concerns; thus, we focused solely on reactions that utilize hexamethyldisilazane (HMDS) as a mild dehydrating agent that is readily available on metric-ton scales.
Exploration of reaction conditions with HMDS, acidic promoters, and various hydroxylamine reagents was conducted and provided proof of concept for the formation of molnupiravir. Our initial hit utilized sulfolane as a cosolvent and triflic acid as the acidic promoter at 80 °C (Table S5). More practical conditions were identified that utilized HMDS as the sole reaction solvent, with mildly acidic bisulfate salts (Na, K, NH4) to promote the reaction. Finally, the addition of a catalytic quantity of imidazole led to a dramatic increase on the reaction rate and conversion, likely through the in situ formation of TMS-imidazole as a silylation catalyst.26−29
After further development of the reaction conditions, conversion of 3 to molnupiravir in the presence of HMDS was shown to occur in nearly quantitative yield, without the generation of any significant side-products (Figure 3). As anticipated, the initial product of the reaction is the bis-TMS derivative of molnupiravir. This in situ silylation allows for facile removal of the inorganic reagents and byproducts via aqueous washes without any loss of product. Molnupiravir itself is highly water-soluble, and the removal of inorganic salts would be difficult if not for the transient masking of the alcohols as TMS ethers. Following removal of the inorganic species, the TMS groups were easily cleaved by adjusting the pH, after which the product could be crystallized, completing our novel three-step synthesis of molnupiravir with a single isolated intermediate. Furthermore, the use of HMDS as a mild dehydrating agent for heterocycle functionalization more broadly, avoiding highly toxic reagents, is likely to be of general interest and is the focus of ongoing work.
Figure 3.
Completion of the synthesis of molnupiravir
Conclusions
In summary, we have developed an efficient and sustainable synthesis of molnupiravir (Figure 4) that utilizes only simple raw materials and enzymes, thus enabling the rapid supply of molnupiravir on large scale. The three-step route from ribose has only a single isolated intermediate, uses two enzymatic steps, including a novel cascade of engineered enzymes that incorporates an innovative phosphate recycling system, and a mild dehydration reaction that provides molnupiravir in high yield and purity. Compared to the initial route, the new route is 70% shorter, approximately 7-fold higher in overall yield, and enabled through the development and investment in novel chemistry. Notably, the enzymes were discovered and evolved, and the reactions developed and implemented on large scale in only 6 months. These results not only offer new strategies for the synthesis of nucleoside-based therapeutics but also highlight the growing capability to discover and implement novel enzymes on manufacturing scale in rapid fashion.
Figure 4.
Summary of initial and new routes to molnupiravir from ribose and uracil. AY: Assay yield at the end of reaction, as determined using a calibrated UPLC instrument. IY: Isolated yields of >99.5% purity material. For additional details, see the Supporting Information.
Acknowledgments
We thank our colleagues Rebecca Ruck, L.-C. Campeau, Guy Humphrey, and Kevin Campos for their support and helpful feedback. We thank Cecilia Bottecchia, Kevin Stone, Jerry Taylor, Eric Sirota, Ivan Lee, Dan Lehnherr, Shane Grosser, Keith Mattern, Yang Cao, Marc Poirier, Gilmar Brito, Jacob Waldman, Lushi Tan, Rachel Bade, Nastaran Salehi Marzijarani, and Gao Shang for helpful discussions and experimental support. We thank Jiaxuan Yan, Joseph Gouker, Heather Wang, Jackson Hall, Rebecca Arvary, Jameson Bothe, and Sherry Song for analytical support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00608.
Experimental details, methods, and characterization data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ accessed Oct 12th, 2021.
- Wibmer C. K.; Ayres F.; Hermanus T.; Madzivhandila M.; Kgagudi P.; Lambson B. E.; Vermeulen M.; van den Berg K.; Rossouw T.; Boswell M.; Ueckermann V.; Meiring S.; von Gottberg A.; Cohen C.; Morris L.; Bhiman J. N.; Moore P. L.. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. BioRxiv 2021. [DOI] [PubMed]
- Harvey W. T.; Carabelli A. M.; Jackson B.; Gupta R. K.; Thomson E. M.; Harrison E. M.; Ludden C.; Reeve R.; Rambaut A.; Peacock S. J.; Robertson D. L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409. 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://covid.cdc.gov/covid-data-tracker/#vaccinations.
- Cox R. M.; Wolf J. D.; Plemper R. K. Nat. Microbiol. 2021, 6, 11–18. 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P.; Sims A.C.; Zhou S.; Graham R.L.; Pruijssers A.J.; Agostini M.L.; Leist S.R.; Schäfer A.; Dinnon K.H.; Stevens L.J.; Chappell J.D.; Lu X.; Hughes T.M.; George A.S.; Hill C.S.; Montgomery S.A.; Brown A.J.; Bluemling G.R.; Natchus M.G.; Saindane M.; Kolykhalov A.A.; Painter G.; Harcourt J.; Tamin A.; Thornburg N.J.; Swanstrom R.; Denison M.R.; Baric R.S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A.; Gralinski L. E.; Johnson C. E.; Yao W.; Kovarova M.; Dinnon K. H.; Liu H.; Madden V. J.; Krzystek J. M.; De C.; White K. K.; Gully K.; Schäfer A.; zaman T.; Leist S. R.; Grant P. O.; Bluemling G. R.; Kolykhalov A. A.; Natchus M. G.; Askin F. B.; Painter G.; Browne E. P.; Jones C. D.; Pickles R. J.; Baric R. S.; Garcia J. V. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451. 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50% Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study; Press Release from Merck & Co., Inc.; Oct 1, 2021.
- Painter G. R.; Bluemling G. R.; Natchus M. G.; Guthrie D.. N4-Hydroxycytidine and Derivatives and Anti-Viral Uses Related Thereto. WO2019113462, 2018.
- Painter G. R.; Perryman D.; Bluemling G. R.. 4'-Halogen Containing Nucleotide and Nucleoside Therapeutic Compositions and Uses Related Thereto. WO2019173602, 2019.
- Vasudevan N.; Ahlqvist G. P.; McGeough C. P.; Paymode D. J.; Cardoso F. S. P.; Lucas T.; Dietz J.-P.; Opatz T.; Jamison T. F.; Gupton F. B.; Snead D. A concise route to MK-4482 (EIDD-2801) from cytidine. Chem. Commun. 2020, 56, 13363–13364. 10.1039/D0CC05944G. [DOI] [PubMed] [Google Scholar]
- Gopalsamuthiram V.; Williams C.; Noble J.; Jamison T.F.; Gupton F.B.; Snead D.R. A Concise Route to MK-4482 (EIDD-2801) from Cytidine: Part 2. Synlett 2021, 32, 326. 10.1055/a-1275-2848. [DOI] [Google Scholar]
- Ahlqvist G. P.; McGeough C. P.; Senanayake C.; Armstrong J. D.; Yadaw A.; Roy S.; Ahmad S.; Snead D. R.; Jamison T. F. Progress Toward a Large-Scale Synthesis of Molnupiravir (MK-4482, EIDD-2801) from Cytidine. ACS Omega 2021, 6, 10396. 10.1021/acsomega.1c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman M. A.; Fryszkowska A.; Alvizo O.; Borra-Garske M.; Campos K. R.; Canada K.; Devine P. N.; Duan D.; Forstater J. H.; Grosser S. T.; Halsey H. M.; Hughes G. J.; Jo J.; Joyce L. A.; Kolev J. N.; Liang J.; Maloney K. M.; Mann B. F.; Marshall N. M.; McLaughlin M.; Moore J. C.; Murphy G. S.; Nawrat C. C.; Nazor J.; Novick S.; Patel N. R.; Rodriguez-Granillo A.; Robaire S. A.; Sherer E. C.; Truppo M. D.; Whittaker A. M.; Verma D.; Xiao L.; Xu Y.; Yang H. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259. 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lucas J. Multienzymatic synthesis of nucleic acid derivatives: a general perspective. Appl. Microbiol. Biotechnol. 2015, 99, 4615–4627. 10.1007/s00253-015-6642-x. [DOI] [PubMed] [Google Scholar]
- Mikhailopulo I. A.; Miroshnikov A. I. Naturae New trends in nucleoside biotechnology. Acta 2010, 2, 36–58. 10.32607/20758251-2010-2-2-36-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham W. R.; Starbird C. A.; Panosian T. D.; Nannemann D. P.; Iverson T. M.; Bachmann B. O. Bioretrosynthetic construction of a didanosine biosynthetic pathway. Nat. Chem. Biol. 2014, 10, 392–399. 10.1038/nchembio.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris F.; Gotor V. A useful and versatile procedure for the acylation of nucleosides through an enzymic reaction. J. Org. Chem. 1993, 58, 653–660. 10.1021/jo00055a018. [DOI] [Google Scholar]
- Pulido R.; Ortiz F. L.; Gotor V. Enzymatic regioselective acylation of hexoses and pentoses using oxime esters. J. Chem. Soc., Perkin Trans. 1 1992, 2891–2898. 10.1039/p19920002891. [DOI] [Google Scholar]
- Fernandez-Lucas J. Multienzymatic synthesis of nucleic acid derivatives: a general perspective. Appl. Microbiol. Biotechnol. 2015, 99, 4615. 10.1007/s00253-015-6642-x. [DOI] [PubMed] [Google Scholar]
- Tozzi M. G.; Camici M.; Mascia L.; Sgarrella F.; Ipata P. L. Pentose phosphates in nucleoside interconversion and catabolism. FEBS J. 2006, 273, 1089–1101. 10.1111/j.1742-4658.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- Ferro A. J.; Barrett A.; Shapiro S. K. 5-Methylthioribose kinase. A new enzyme involved in the formation of methionine from 5-methylthioribose. J. Biol. Chem. 1978, 253, 6021–6025. 10.1016/S0021-9258(17)34573-8. [DOI] [PubMed] [Google Scholar]
- Albers E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′-methylthioadenosine. IUBMB Life 2009, 61, 1132–1142. 10.1002/iub.278. [DOI] [PubMed] [Google Scholar]
- Sedewitz B.; Schleifer K. H.; Gotz F. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J. Bacteriol. 1984, 160, 273–278. 10.1128/jb.160.1.273-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. M.; Swartz J. R. Prolonging cell-free protein synthesis with a novel ATP regeneration system. Biotechnol. Bioeng. 1999, 66, 180–188. . [DOI] [PubMed] [Google Scholar]
- Nomura M.; Sato T.; Washinosu M.; Tanaka M.; Asao T.; Shuto S.; Matsuda A. Nucleosides and nucleotides. Part 212: Practical large-scale synthesis of 1-(3-C-ethynyl-β-d-ribo-pentofuranosyl)cytosine (ECyd), a potent antitumor nucleoside. Isobutyryloxy group as an efficient anomeric leaving group in the Vorbrüggen glycosylation reaction. Tetrahedron 2002, 58, 1279–1288. 10.1016/S0040-4020(01)01249-2. [DOI] [Google Scholar]
- Birkofer L.; Ritter A. The use of silylation in organic syntheses. Angew. Chem., Int. Ed. Engl. 1965, 4, 417. 10.1002/anie.196504171. [DOI] [Google Scholar]
- Fleming I. Tilden Lecture. Some uses of silicon compounds in organic synthesis. Chem. Soc. Rev. 1981, 10, 83. 10.1039/cs9811000083. [DOI] [Google Scholar]
- Lalonde M.; Chan T. H. Use of organosilicon reagents as protective groups in organic synthesis. Synthesis 1985, 1985, 817. 10.1055/s-1985-31361. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.