Abstract
An increasing world population, rising affluence, urbanization, and changing eating habits are all contributing to the diversification of protein production. Protein is a building block of life and is an essential part of a healthy diet, providing amino acids for growth and repair. The challenges and opportunities for production of protein-rich foods from animals (meat, dairy, and aquaculture), plant-based sources (pulses), and emerging protein sources (insects, yeast, and microalgae) are discussed against the backdrop of palatability, nutrition, and sustainability.
Keywords: protein, livestock, aquaculture, plant protein, alternative protein
Introduction
The global population is expected to grow to 9.7 billion by 2050. The population of the world is changing with rapid growth of the middle class, most of which is taking place in Asia. In fact, the world has reached a tipping point where more than half of it is now considered middle class or wealthier, with a majority living in urban areas. By 2030, it is estimated that this will increase to two-thirds. Current estimates predict that we will require ∼70% more food than is currently produced.1 As incomes rise, individuals are moving toward more energy-dense diets that often include more protein. The United Nations Sustainable Development Goal 12 “Responsible Consumption and Production” highlights the need to meet the food gap while maintaining planetary health. Moderating the intake of energy-dense and nutrient-poor discretionary foods should be a priority in strategies seeking to promote healthy and sustainable diets.2
Another challenge that we face in our current food system is a lack of diversity. Globally, we rely on a small range of foods, with 75% of the global food supply coming from only 12 plant and 5 animal species.3 Just three plant species (rice, maize, and wheat) make up nearly 60% of calories from plants in the entire human diet. This lack of diversity can negatively impact our health. While people in the middle class and beyond may be consuming sufficient calories, energy-dense diets contribute to obesity,4 and consuming from narrow diets does not necessarily provide enough vitamins and minerals, leading to undernourishment.5 There are a broad range of untapped foods offering superior nutrient density, and that can also impart unique flavors and textures for discerning consumers. Furthermore, accessing biodiversity via a deeper gene pool in our agricultural systems also builds resilience in crop and livestock systems against pests and diseases, climate change, and extreme weather, which, in turn, increases food security.
There is a mismatch between the current consumption patterns in different regions. For instance, consumption of red meat, poultry, and dairy in North America is notably high, while there is an unbalanced reliance on starchy vegetables in sub-Saharan Africa. One recommendation of the report is for consumption of lower amounts of animal-sourced protein in regions with high consumption complemented by increased protein intake derived from plant or alternative sources.2,5 However, only a small proportion of the increasing population has the opportunity to choose their diets. Full life cycle analyses are required to take a whole supply chain perspective on the sustainability of food products and explore different aspects that contribute to overall sustainability of individual components of a diet.6 Here, we examine how science and technology innovations address the environmental and nutrition impacts of various protein sources and identify further opportunities for traditional and emerging protein ecosystems (Figure 1).
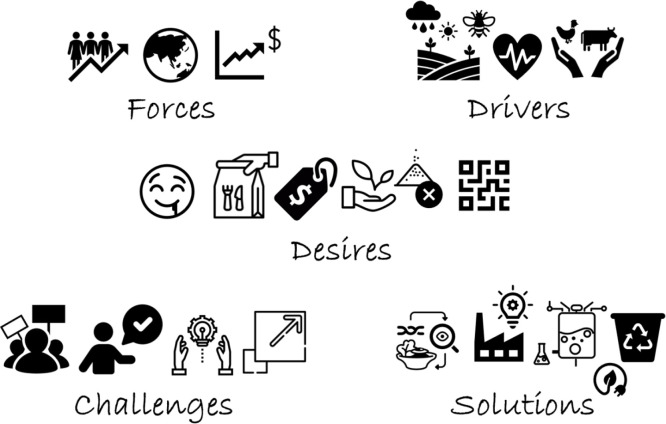
Figure 1.
External forces, such as population growth and dynamics in the face of climate change, are leading to consumer concerns over the impact of their diet on the planet, their own health, and animal welfare. Consumers are increasingly making discerning choices looking for tasty, convenient, affordable, natural, clean label, nutritious products with verifiable credentials. Protein industries face a range of challenges from social license and consumer acceptance to research translation/adoption to scaled production. To address the forces, drivers, and desires, sustainable solutions, such as nutritionally optimized foods, innovative food manufacturing, novel protein production systems, and co-product valorization and/or waste utilization must be implemented.
Animal Protein
Livestock-Derived Protein
Meat, eggs, and dairy offer compelling nutritional benefits, including proteins, fats (including omega-3 fatty acids), carbohydrates and micronutrients, such as a diverse range of minerals (iron, zinc) and vitamins. Undernutrition, including insufficient consumption of protein, remains a persistent problem in the developing world, with both the quantity and quality (bioavailability and digestibility) of protein presenting issues. Incorporation of livestock-derived protein in protein-deficient diets provides valuable outcomes, such as prevention of stunting, sarcopenia, osteoporosis, and anemia.
With burgeoning populations demanding high-quality protein, livestock production will continue to increase7 and protein from livestock will remain an important part of the global food system. Livestock has the unique ability to “upcycle” human-inedible or low-value feed into highly nutritious protein,8 and it has been demonstrated in the developed world that ruminant livestock generates more high-value protein than it consumes, making a positive net contribution to global protein for human nutrition.9
In a mature industry, such as the livestock industry, the increase in protein will be met through innovations that enhance production efficiency and sustainability of production. The efficiency of livestock production has increased through technological innovations, such as on-animal and on-farm sensors and decision support tools that allow for the optimization of animal requirements and feed base. The advances in animal genetics and genomics are driving genetic improvement to maintain an efficient livestock population that enhances sustainability of production.
A key aspect of improving the sustainability of the ruminant livestock sector is the reduction of global human-induced greenhouse gas (GHG) emissions.10 Increases in production efficiency have seen the total emissions from livestock drop by 2.4% since the 1990s. Innovation in forages, feeding strategies, and supplements, such as inclusion of red macroalgae (seaweed) Asparagopsis spp., in cattle feed can reduce methane by 80%11 from rumen fermentation and results in meat and dairy products with improved environmental sustainability credentials.
Humanity has for thousands of years relied on the nutritional benefits and convenience of milk and milk products. From its beginnings, the dairy industry has built on this traditional knowledge and implemented and adopted the approach to design and manufacture high-quality and differentiated products from milk. This range of products includes a variety of fresh milk, yogurt, cheese, fat, butter, milk powder, protein powder, peptides, lactose, milk minerals, and others. Some of these are sold directly as consumer products, while others are incorporated as ingredients into processed foods, where they provide texture, color, taste, and nutrition. The industry has successfully implemented “zero waste” strategies, with especially larger manufacturers upcycling and value adding to all byproduct streams, exemplified by the many different whey protein products and ingredients developed from cheese whey that have become core ingredients for processed food manufacturers. The modern dairy industry has developed various strategies to remain relevant to enlightened consumers by developing dairy protein products meeting the nutrition needs of consumers at various life stages, with examples being various infant formulas and modified-fat milks fortified with calcium and vitamin D. The industry is also adapting to emerging consumer needs by responding with novel long-shelf life, portable, and more nutritional products. There are relatively few antinutritional components in dairy; however, milk allergy remains one of the dominant allergens, especially in children, and there is growing recognition of lactose intolerance in adults globally.
Approximately 20% of the meat from a beef carcase is high-value primal cuts, which are consumed as steaks, i.e., consumed as the unprocessed raw product. The other 80% of the meat is often sold as a commodity based on its fat content, i.e., chemical lean (CL), and used mainly for hamburgers in the developed world. With value addition to this 80%, there is an opportunity to achieve a significantly higher value for the carcase. This can be achieved by developing value-added meat products that meet the current and future needs of the consumers. With an aging population in the developed world, sarcopenia and dysphagia are prevalent. Red meat products that can be easily consumed by the elderly can make a significant difference to their health, wellbeing, and quality of life. Using technologies like high-pressure processing, clean label red meat products can be produced to meet the consumer needs for additive-free meat products. These days on-the-go foods are becoming more and more popular to cater for busy consumers, leading to the snackification trend. Currently, jerky is the only red-meat-based snack that fits the on-the-go criteria; hence, there is opportunity to develop high protein, health products using novel technologies and science to cater for this growing market. Additional value can be created from the remaining carcase through opportunities, such as conversion of animal byproducts into shelf-stable high-protein ingredients or value-added food products. Consumer acceptance of such products is not guaranteed, and neophobia is associated with some animal co-products.12 Other factors, including familiarity, level of naturalness, healthfulness, and state of the product/ingredient, all influence consumer acceptance. For example, broths can be made from bones and lower value meats from different animal species. Enzyme hydrolysis to obtain bioactive peptides13 that exert physiological effects, such as antihypertensive, antioxidant, antidiabetic, and antimicrobial activities, will find applications in the food industry and beyond, such as the pharmaceutical and cosmetic industries. An example is that of hydrolyzed collagen peptides that are available through health food stores and supermarkets, made popular by trends, such as the ketogenic diet, and as a cosmeceutical ingredient in personal care products targeting anti-aging.
Aquaculture-Derived Protein
The nutritional benefits of fish consumption have a positive link to increased food security and decreased poverty rates in developing countries, with over three billion people worldwide relying on fish as their primary source of animal protein.14 Fish provides high-value protein but also a wide range of essential micronutrients, including various vitamins (A, B, and D), minerals (including calcium, iodine, zinc, iron, and selenium), and long-chain polyunsaturated ω-3 (LCω3) fatty acids, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Despite the health benefits, seafood allergy is reported by 2–3% of the general population in the U.S.A., whereas seafood allergy is of high importance in Asia, where its prevalence is up to 3 times higher.
Aquaculture, the controlled cultivation of aquatic organisms, leads to increased food security by providing employment and income generation for local communities, in particular, in Asia, Africa, and South America. In 2018, an estimated 60 million people were engaged in the primary sector of fisheries (∼40 million) and aquaculture (∼20 million).
With a growing population and increased awareness of the health benefits of seafood, the average world fish consumption is growing and at a high of 20 kg per individual per annum. This demand for fish now far surpasses the sustainable yield of the ocean. Aquaculture is a major contributor to protein supply by supplying more than half of the total fish production. The “Blue Revolution” began only 60 years ago through the development of large-scale industrial farming of fish, shellfish, and seaweed, and such rapid growth has attracted criticism about environmental sustainability. There are several challenges to be addressed to allow for the successful expansion of aquaculture, including the reliance on finite marine resources in aquaculture feeds (aquafeeds), environmental degradation and reduced water quality, disease, and a lack of governance and regulation in production.
The high inclusion rates of wild fish resources in aquafeeds, particularly for carnivorous fish, such as marine fish and shrimp, are considered unsustainable.15 Historically, aquafeeds have relied heavily on fishmeal and fish oil, which come from wild harvested pelagic fish catches. Fishmeal is a limited natural resource (∼5 million tonnes per annum) and an excellent source of protein and essential nutrients for fish. The derived fish oil is also in high demand as a result of its high LCω3 content. Over the years, market forces have reallocated the use of those finite strategic resources, with the aquafeed industry using three-quarters of all fishmeal and fish oil harvested.
Through ongoing nutrition research into requirements for digestible energy, amino acids and other micronutrients as well as the rigorous evaluation of alternative ingredients, fishmeal and fish oil inclusion rates have declined for the main commercial fish and crustacean species. The major alternative protein and oil sources have come from plants (soybean, corn, canola, lupin, and wheat meal) and animal-processing byproducts (poultry meal, blood meal, feather meal, and meat and bone meal). There has also been an increasing use of fish waste or fish-processing byproducts, which currently accounts for about 35% of fishmeal used in aquafeed. These alternative protein sources are becoming an important source of nutrients as part of a growing circular economy, and advances in processing have improved their bioavailability. One of the biggest challenges is current restrictions placed by many countries on the use of animal waste as protein sources. The availability and price of plant ingredients are also dependent upon external factors, such as freshwater availability, and could ultimately compete with direct human consumption. In this context and as a result of the increasing price of fishmeal, a greater range of alternative protein sources has emerged particularly in recent times from microbial processes as well as insects.
Those advances have had a positive impact on the ratio of “fish-in/fish-out”, which is approaching 1 or is less than 1 for most of the major fed aquaculture species today.16 For example, the culture of many fish species, such as tilapia, catfish, milkfish, and carp (40% of global production), yields 10 times more fish than it uses.16 The salmon industry is today a net producer of fish protein, which demonstrates that even carnivorous high-value species can be more sustainably cultured. The next nutritional challenge for those high-value fatty fish is to retain sufficient LCω3 as fish oil inclusion is reduced. In addition to alternative oil use to spare LCω3 from fish oil, two emerging technologies are now commercially available to directly replace fish oil: microalgal biomass and genetically modified (GM)-canola oils rich in DHA.17 While great progress has been made in the replacement and reduction of fishmeal and oil in aquafeeds, fishmeal is still used at relatively high inclusion levels in shrimp feeds today. Microbial technology can assist with removal of fishery products from feed. For example, Novacq is a prawn feed developed by Commonwealth Scientific and Industrial Research Organisation (CSIRO) that enhances prawn growth (on average 20–40% faster), improves prawn health, and decreases reliance on wild fish products in prawn diets.18 As another emerging alternative to fishmeal, food waste could provide an affordable and readily accessible nutrient source for aquaculture. Food waste represents a diverse source of micronutrients, including vitamins, minerals, and lesser understood nutrients, like nucleotides and organic acids, which may be beneficial for some aquatic animals. Repurposing food waste is not trivial because of the highly variable composition, making consistency a challenge, and the ultimate success will depend upon the nutrient profile and bioavailability.
While the production of protein alternatives through a growing circular economy will be critical to support the rapid and sustainable growth of the aquaculture industry, other environmental impact challenges will require advances in production systems. There are a multitude of culture systems operating today ranging from extensive systems with limited energy and feed inputs to superintensive systems, such as indoor recirculatory systems (RAS), as well as multi-species approaches, such as polyculture, aquaponics, and integrated multitrophic aquaculture (IMTA). All have their advantages and disadvantages in terms of production efficiency, waste mitigation, management complexity, species compatibility, and capital and running costs.16 Culture intensification can improve efficiency in terms of production volume per unit of land and sea use and, in some situations, can drastically reduce water use, improve food conversion efficiency, and enable waste bioremediation. This can occur using once again microbial processes, such as, for example, biofloc systems for shrimp, but also growing plants with nutrients from freshwater fish species in aquaponics, remediating nutrient discharge of fish sea-cages with seaweeds (as part of IMTA), or employing technically advanced mechanical and biofilter conversion processes in RAS. Clear opportunities exist in reducing the environmental burden of aquaculture through thorough life cycle assessment studies19 to evaluate trade-offs in systems depending upon the desired target species and location. Farm-level certification is setting new governance for sustainability, but even the two largest certification groups, the Aquaculture Stewardship Council (ASC) and the Global Aquaculture Alliance Best Aquaculture Practice (GAA-BAP) standards, only cover 3% of global production.16 There is an ongoing need for the entire aquaculture industry to embrace certification frameworks and refine best practice criteria as new feeds and production system designs become adopted toward a greener industry.
Plant Protein
Plant sources offer compelling nutrients, like fiber and short-chain ω-3 fatty acids. Soy, wheat, and to a lesser extent pea and potato remain the dominant plant-based protein sources. In the near term, soy will likely remain the mainstay because of its desirable food processing qualities (e.g., texture, flavor, and appearance) and the nutrient qualities. Soy has a near complete amino acid profile and a similar protein digestibility-corrected amino acid score (PDCAAS) to beef and dairy, indicating that they have similar human nutritional attributes. However, innovative approaches that enable other high protein pulses to be integrated into packaged food and beverages are being developed. As the sources of plant protein diversify, attention needs to be paid to ensure consumers receive a complete profile of essential amino acids because some plant protein sources lack certain essential amino acids needed by our bodies.
Reports, such as the Knorr/World Wide Fund for Nature’s 50 Future Foods, have identified several underused plant crops, such as fava beans and mung beans, or orphan crops, such as bambara groundnut, that have great potential as sources of protein in our diets. As an example, bambara groundnuts have gained interest among many sustainable food experts because they can grow in challenging environments, even in highly acidic soils, and boast an impressive nutrient profile, being considered a “complete food” because of the balance of macronutrients accompanied by the amino acid and fatty acid content.20 However, production of these crops from an agronomic perspective and the opportunity to improve them through breeding programs that reduce the impacts of biotic/abiotic stresses or increase adaptation have not yet been implemented.
Enhanced productivity alone will not see widescale adoption globally of underused and orphan crops. Work must also focus on enhancing sensory properties (color and taste) and/or removal of antinutritional components that are undesirable.21 Advancements in processing technology allow many of these constraints to be overcome21 and may also allow for higher value uses of current low-value high-protein extracts, such as the meal left after extraction of oil from canola (rapeseed). Ingredients from plant sources may be fractionated as individual macronutrients (proteins, carbohydrates, and fats) or be used as complex whole materials (beans, peas, and mushrooms). Plant-based ingredients differ in their physicochemical properties, structural elements, and functionality compared to those found in animal products. Plant proteins often have poorer solubility, foaming, emulsifying, and gelling properties, which can limit their use in food products.
Some ingredients can be used whole (e.g., mushrooms/mycoprotein), but in other cases, they are deconstructed by fractionation into protein ingredients (e.g., concentrates and isolates) before being reconstructed into intermediary goods (e.g., texturized vegetable protein) or finished products (e.g., meat mimetics). Plant-derived biopolymers are being used to form meat-like structures using mechanical processing devices, such as extruders, and the technology has been well-reviewed.22 It should be noted that many plant-based foods are highly refined or processed and contain additives that are used to impart functionality. Consequently, there is a need for more research on the development of processed plant-based foods that contain fewer ingredients and/or involve less processing.
Plant protein production is often called out as a sustainable means to meet future protein demand, but there are pros and cons to the production system. For instance, monoculture farming, the growing of a single crop in a field at a given time, is a topic of debate. Monoculture farming can offer improvements in productivity and efficiency, especially when combined with AgTech, such as the use of near/remote monitoring and smart decision-making tools. There are also challenges or concerns, such as higher use of pesticides and/or fertilizers, increased water use, and impacts on soils (degradation and fertility loss). Additionally, monoculture farming can negatively impact biological diversity and pollinators within agricultural ecosystems. In this way, crop rotation, effective/smart fertilizer application, and generation of varieties with improved water-use efficiency and biotic stress tolerance are critical considerations.
Additional research priorities include improving the protein quality and quantity of seed crops (e.g., amaranth, buckwheat, and quinoa) and legumes (e.g., lupin, chickpeas, fava beans, and lentils) that are considered underused in our food systems. There will also be a need to develop new varieties suited to different climatic regions and/or soils (adaptation) and to improve crop management (agronomy). Subsequently, technological innovations will lead to new plant-based and protein-rich ingredients to be used to fortify products, such as bakery, pasta, breakfast cereals, and snacks, or as foods, such as meat or dairy alternatives. The creation of novel plant-based foods is also being held back by a lack of large-scale manufacturing processes to convert plant-based ingredients into desirable end products. Finally, many of the plant feedstocks contain significant (∼60–85%) quantities of non-protein dry matter, most of which is edible and offers opportunities as co-product development as food or feed. Technologies to enhance efficiencies of conversion of these byproduct streams to functional and nutritional food ingredients are needed.
An example of a new venture science model for transforming food systems is that of v2food, an Australian producer of plant-based meat substitutes that is a joint venture between CSIRO and Competitive Foods Australia. v2food was established in response to the strong consumer demand for high-quality plant-based convenient products and employed new technology for producing texturized protein-based ingredients and flavor systems incorporating high-protein non-GM crops that deliver neutral flavor profiles (without the beany flavor).
Marine Protein Sources: Algae/Seaweed
Algae can be broadly divided into microalgae and macroalgae (seaweed). Algae reproduce rapidly and have a higher productivity compared to conventional crops. Algae have been part of the human diet for thousands of years and provide a wide range of nutrients for health and wellbeing, including vitamins, minerals, dietary fiber, and protein. In Mexico, Aztecs used cultures of Arthrospiramaxima (spirulina) to prepare a highly nutritious green cake.
Microalgae and seaweed are denoted as carbon negative because they absorb dissolved carbon dioxide directly from the sea (along with nitrogen and phosphorus). Capitalizing on algae for its protein content for human food applications will require scaled production and offers the benefit of a relatively low physical footprint per kilogram of protein23 because algae can be cultivated in closed loop production systems or bioreactors (microalgae) using recycled water and carbon dioxide produced by other industrial activities or in seawater and recycled water (macroalgae/seaweed). It has been estimated that single cell protein sources, such as microalgae, hold the potential to meet up to 18% of conventional crop-based animal feed protein demand by 2050.23
Much of the growth in algal protein production is expected to come from the two main freshwater species in the market, filamentous cyanobacterium Arthrospira platensis (spirulina) and unicellular green alga Chlorella. These are recognized as high-quality protein sources containing up to 70% dry weight protein and all essential amino acids (albeit lower for cysteine and lysine), but the lower bioavailability (in comparison to traditional protein sources) partially limits their nutritional value. Algae are noted to be rich in minerals, like calcium, iron, and copper, as well as ω-3 oils and are also one of the few non-animal sources of vitamin B12,23 important for vegetarians and vegans, with a single portion of Ulva lactuca (sea lettuce) providing the recommended intake for adults. However, their food applications (quantity and type of food/beverages) are limited by the strong pigments and flavor that they impart.
To further support adoption of algae as a protein-rich ingredient in commonly consumed beverages and foods, it is critical that alternate algae strains with specific attributes are identified and/or further develop technological approaches that further improve protein quality, bioavailability, and organoleptic properties of algae proteins. For instance, algae strains, such as Nannochloropsis sp. or Scenedesmus sp., possess a lysine content above 6.6%,24 akin to that found in animal proteins. Another is to use other plant proteins that have complementary profiles of essential amino acids and use a blending approach, such as combining algae protein with potato, soy, and/or pea proteins that are higher in specific amino acids.25
The protein within algal cells is often carbohydrate-bound or trapped by the algal cell wall (dietary fibers) that limit the availability of proteins to digestive enzymes. The phenolic compounds, which also vary markedly between algae species, have also been shown to react with amino acids to form insoluble compounds, lowering protein digestibility. If high-protein algae strains that are low in polysaccharides and phenolics cannot be identified, processing methodologies (enzymatic, temperature, pressure, etc.) that break down the anionic cell wall and remove antinutrients are needed to enhance the protein digestibility of algal proteins.26
The pigments (e.g., carotenes, chlorophylls, and phycobiliproteins) and flavors of whole algae and protein concentrations currently limit the amounts that can be added to a broad range of meals and food products. Because these pigments and flavors differ markedly between algae species and strains, identification of those strains that have desired color characteristics that match the food product can be capitalized upon. Likewise, a lower glutamate, alanine, and glycine content will result in algae that impose less distinctive algae and umami flavors.27 Other components, such as polyphenols (e.g., catechins, flavonols, and phlorotannins) can also impart bitter flavors and, although undesirable, can impart health benefits. As previously noted, processing technologies to purify the proteins or remove color and flavor can prevent development of these undesirable flavors.
A broad range of startups exist, such as Singapore-based Sophie’s Bionutrients that are cultivating algae in bioreactors to yield pure protein and U.S.-based Triton Algae Innovations that is delivering both protein ingredients and seafood alternatives. There is also strong interest in algae for their “green” credentials from multinationals focusing on sustainable ingredient sourcing.
Food Waste and Insects
There is an imbalance between food loss and waste, wherein regions like Australia or North America greater loss is observed at the consumer end of the supply chain. In contrast, very little food is wasted in sub-Saharan Africa or Southeast Asia, but in these regions, food is lost at the production end. To address the food waste issue, insects, like black soldier fly, are being used to upcycle food waste into protein for feed, pet food, or even human food. Insects are highly efficient at food conversion and, in parallel, can produce frass that is used as a valuable fertilizer; thus, such systems are a considerable contributor to a circular economy. The efficiency of insects to convert feed into edible food is multifactorial; insects have fast growth rates, are rich in protein, produce many offspring, have low water consumption, negligible levels of GHG emissions, and are often consumed whole, thus eliminating waste.28
Insects are of high nutritional value,28 being rich in protein and several essential amino acids, with values for protein digestibility equivalent or slightly lower (species dependent) than that of protein derived from egg or beef. The nutritional content of insects can vary greatly by species, stage of growth, and feed. For instance, adult mealworms are a source of iron, iodine, manganese, magnesium, and zinc, while larvae are rich in B-group vitamins. Edible crickets are a rich source of macronutrients, protein (up to 70%), lipids (7–25%), and carbohydrates, as well as micronutrients, such as vitamins. Balanced direct comparisons between different protein sources and insects revealed that their digestibility and nutrient quality showed that insects were similar and suitable for both livestock feeds and human consumption.29
Entomophagy or the practice of eating insects has been a part of human history for millennia, and it plays a significant role in cultural and some religious practices around the world. In fact, the consumption of locusts, winged locusts, crickets, and grasshoppers is encouraged in the Book of Leviticus from the Old Testament. Looking further back in history, Australian Aborigines practiced entomophagy as a sustainable source of food, consuming witjuti grubs, bardi grubs, bogong moths, and honey ants. A diverse range of insect species (>2000) is consumed daily by around 2 billion people from >100 countries. Despite their long history of consumption in some regions, insects have not entered the mainstream in Western society. In developed countries, consumer acceptance is limiting the adoption and, thereby, the impact of a switch from traditional protein sources. Crickets, mealworms, and black soldier fly are now being farmed in both large and small operations, providing new ingredients used to fortify foods. The inclusion of insect-derived protein in powdered form may remove some of the barriers to consumer adoption.
There are, however, legal or regulatory barriers that need to be overcome to see the full potential of insects as food realized. The recent approval of mealworm for human consumption by the European Food Safety Authority may pave the way for these less common foods to be used as snacks, ingredients, or perhaps even center of plate options. Companies focused on mealworm production include French-based Ynsect, while Dutch-based Protix and Australian-based Goterra harness the feed conversion efficiency of black soldier fly.
Many insect species are eaten around the world with little evidence of ill effect, suggesting they are safe to eat.21 While the benefits of entomophagy are many, one of the largest barriers is food safety. Classed as chemical, biological, or allergenic, many of these food safety concerns are indicative of the maturity of insect-derived products for feed and food ingredients. These concerns will likely be resolved as they have for many foods that overtime have now become commercially produced and accepted by consumers. Potential microbiological and chemical health risks will depend upon production, harvesting, and processing techniques and require assessment in parallel with implementation of hygiene practices in the entire edible insect value chain.29 Further research into the potential hazards of farming insects fed on food waste is required to shore up the potential of this otherwise cost-effective solution to waste valorization. Another area requiring further research is the potential accumulation of toxins or the introduction of allergens in food systems, wherein cricket proteins exhibit cross-reactivity with those that trigger shellfish allergy.
Protein from Fermentation
Fermentation has a long history in food use, either for the whole-cell conversion of ingredients into end products with unique properties (e.g., yogurt, bread, and beer) or as nutritional microbial biomass in and by itself (e.g., mycoprotein as produced under the brand Quorn or Fy from Nature’s Fynd). Precision fermentation (PF) as a third category is a more recent iteration of an established technology. This technology is a convergence of genetic engineering and synthetic biology and is being harnessed to synthesize compounds that would otherwise be expensive and/or complicated to harvest. The two most well-known examples of high-value materials derived from precision fermentation are insulin (used in the treatment of diabetes) and chymosin, the key component of rennet (an enzyme used during cheesemaking) that was traditionally obtained from calf stomachs.30 In fact, by 2006, fermentation-derived chymosin occupied as much as 80% of the global market share for rennet.30 However, as the costs associated with precision fermentation decline, the compounds that this technology can produce will start to reach cost-competitiveness with a wider range of traditional materials.
Since these early successes, significant reductions in the cost of both reading and writing genetic information now allow for rapid reprogramming of microorganisms as “cellular factories” (or chassis) with increasing complexity to produce a whole suite of specific food (protein) ingredients in a cost-efficient and sustainable manner. While previous access to Nature’s almost boundless palette of molecules that can be used to furnish flavors, textures, and aromas was limited as a result of scale and sustainability constraints, the first wave of PF companies is paving the way toward new food products that address the concerns and desires of consumers. The iron-containing soy leghemoglobin is produced via PF by Impossible Foods to give its plant-based Impossible burger the unique color and taste of meat and has recently obtained FSANZ regulatory approval (Application A1186) for the use as a PF-derived ingredient. Further examples of startups built on PF technology include Perfect Day (casein and whey dairy proteins), Eden Brew (PF milk and dairy), Change Foods (cheese), and IndieBio accelerator graduate Clara Foods (now EVERY Company, egg white protein), while new synthetic biology players on the horizon, such as Nourish Ingredients (animal lipid flavors) and Motif Foodworks, are developing flavor alternatives to further improve the overall quality of alternative protein products.
Consumers could soon be able to access products made with bespoke ingredients that can deliver improved sensory and functional attributes that were previously not accessible or could only be sourced at low levels. Food fermentation companies attracted a total of US$435 million in venture capital investment during the first 7 months of 2020 as reported by the Good Food Institute. A majority of the food fermentation startups in 2020 are active in the PF space, while Motif Foodworks and Perfect Day are leading the way by raising US$200–300 million during their latest funding series in 2020–2021.
Although there is clearly a major market-driven interest in novel fermentation technologies for the manufacture of ingredients for the alternative protein sector, several challenges remain for this new market. Most PF startups are still at an early stage, and current fermentation infrastructure to operate at scale is severely limited, highlighting the urgent need for investment in larger scale fermentation and downstream processing facilities. The involvement of techno-economic analyses is needed early in the process to assess overall economic viability during scale-up and to identify critical factors that determine the cost of goods. Regulatory approval frameworks with regard to synthetic biology and PF are being revised in many jurisdictions to keep up with technological innovations, such as genome editing. Consumer perceptions related to novel PF-derived food products and appropriate labeling will require social sciences to ensure continued trust and transparency. Future research focus areas to improve the cost effectiveness of PF processes include improved genetic componentry and artificial-intelligence-informed metabolic modeling to achieve higher metabolite yields and engineering novel platform production strains that can grow on alternative and cost-effective feedstocks other than refined sugar, such as various (food) waste streams and even CO2 (e.g., Air Protein). Improvements in productivity on a volumetric basis, i.e., how much product can be made over a given period, will, in turn, improve capital utilization and lower unit costs. Innovation in bioreactor redesign tailored for food-grade fermentation is required, and these systems will need to be powered by renewable energy resources to deliver their full impact potential. Minimizing downstream purification/processing and maximizing value extraction from left-over microbial biomass through the delivery of co-products during downstream processing will improve the overall techno-economic feasibility of PF technology.
A Way Forward
Within the shifting landscape of the protein industry, science, technology, and innovation will play a crucial role in enhancing protein production. Adoption and scale-up of technology will require strategic partnerships spanning industry, government, and academia. Increasing productivity, accessing biodiversity, and implementing advanced manufacturing, biotechnology approaches as well as data/digital science will act as accelerators toward a more sustainable future. Several of the protein ecosystems are facing increasing pressure around stewardship of edible and non-edible byproducts, and there are opportunities through material flow, life cycle, and techno-economic analyses to identify pathways that ensure long-term sustainability as the protein ecosystems grow to address the protein gap created by population growth. As technology is implemented and matures, greater focus on protein affordability is required to address not only protein quality but also ensure food security, moving beyond addressing the desires of high affluence consumers to providing accessible nutrition to the world. Feeding the growing population of the world will only be possible by providing additive solutions, including co-product utilization, waste minimization, and implementing new protein technologies to complement traditional industries. This requires that all sectors work together to build a more sustainable food system than can be achieved by working separately.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.1c05989.
Nutrition information and references related to protein sources (Table 1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Food and Agriculture Organization of the United Nations (FAO) . How to Feed the World in 2050: High-Level Expert Forum; FAO: Rome, Italy, Oct 12–13, 2009.
- Ridoutt B. G.; Baird D.; Hendrie G. A. Diets within planetary boundaries: What is the potential of dietary change alone?. Sustainable Production and Consumption 2021, 28, 802–810. 10.1016/j.spc.2021.07.009. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) . Women: Users, Preservers and Managers of Agrobiodiversity; FAO: Rome, Italy, 1999; http://www.fao.org/3/y5609e/y5609e02.htm.
- Rouhani M. H.; Haghighatdoost F.; Surkan P. J.; Azadbakht L. Associations between dietary energy density and obesity: A systematic review and meta-analysis of observational studies. Nutrition 2016, 32, 1037–1047. 10.1016/j.nut.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Aiking H.; de Boer J. The next protein transition. Trends Food Sci. Technol. 2020, 105, 515–522. 10.1016/j.tifs.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridoutt B.; Baird D.; Hendrie G. A. Diets within Environmental Limits: The Climate Impact of Current and Recommended Australian Diets. Nutrients 2021, 13, 1122. 10.3390/nu13041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D.; Clark M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- Mottet A.; de Haan C.; Falcucci A.; Tempio G.; Opio C.; Gerber P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Global Food Security 2017, 14, 1–8. 10.1016/j.gfs.2017.01.001. [DOI] [Google Scholar]
- Thomas D.T.; Beletse Y.G.; Dominik S.; Lehnert S.A. Net protein contribution and enteric methane production of pasture and grain-finished beef cattle supply chains. Animal 2021, 15, 100392. 10.1016/j.animal.2021.100392. [DOI] [PubMed] [Google Scholar]
- Gerber P.; Steinfeld H.; Henderson B.; Mottet A.; Opio C.; Dijkman J.; Falcucci A.; Tempio G.Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013.
- Roque B. M.; Venegas M.; Kinley R. D.; de Nys R.; Duarte T. L.; Yang X.; Kebreab E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80% in beef steers. PLoS One 2021, 16, e0247820. 10.1371/journal.pone.0247820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchion M.; McCarthy M.; O’Callaghan J. Transforming Beef By-products into Valuable Ingredients: Which Spell/Recipe to Use?. Front. Nutr. 2016, 3, 53. 10.3389/fnut.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldrá F.; Mora L.; Reig M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. 10.1016/j.meatsci.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) . The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2012; http://www.fao.org/3/i2727e/i2727e.pdf.
- Naylor R.; Goldburg R.; Primavera J.; Kautsky N.; Beveridge M. C. M.; Clay J.; Folke C.; Lubchenco J.; Mooney H.; Troell M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- Naylor R. L.; Hardy R. W.; Buschmann A. H.; Bush S. R.; Cao L.; Klinger D. H.; Little D. C.; Lubchenco J.; Shumway S. E.; Troell M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. 10.1038/s41586-021-03308-6. [DOI] [PubMed] [Google Scholar]
- Petrie J. R.; Zhou X.-R.; Leonforte A.; McAllister J.; Shrestha P.; Kennedy Y.; Belide S.; Buzza G.; Gororo N.; Gao W.; Lester G.; Mansour M. P.; Mulder R. J.; Liu Q.; Tian L.; Silva C.; Cogan N. O. I.; Nichols P. D.; Green A. G.; de Feyter R.; Devine M. D.; Singh S. Development of a Brassica napus (Canola) Crop Containing Fish Oil-Like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727. 10.3389/fpls.2020.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross B.; Tabrett S.; Irvin S.; Wade N.; Anderson M.; Blyth D.; Smith D.; Coman G.; Preston N. An analysis of the effect of diet and genotype on protein and energy utilization by the black tiger shrimp, Penaeus monodon—Why do genetically selected shrimp grow faster?. Aquacult. Nutr. 2013, 19, 128–138. 10.1111/j.1365-2095.2012.00941.x. [DOI] [Google Scholar]
- Ghamkhar R.; Boxman S. E.; Main K. L.; Zhang Q.; Trotz M. A.; Hicks A. Life cycle assessment of aquaculture systems: Does burden shifting occur with an increase in production intensity?. Aquacultural Engineering 2021, 92, 102130. 10.1016/j.aquaeng.2020.102130. [DOI] [Google Scholar]
- Majola N. G.; Gerrano A. S.; Shimelis H. Bambara Groundnut (Vigna subterranea [L.] Verdc.) Production, Utilisation and Genetic Improvement in Sub-Saharan Africa. Agronomy 2021, 11, 1345. 10.3390/agronomy11071345. [DOI] [Google Scholar]
- Van der Spiegel M.; Noordam M. Y.; van der Fels-Klerx H. J. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. 10.1111/1541-4337.12032. [DOI] [PubMed] [Google Scholar]
- McClements D. J.; Grossmann L. A brief review of the science behind the design of healthy and sustainable plant-based foods. npj Sci. Food 2021, 5, 17. 10.1038/s41538-021-00099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporgno M. P.; Mathys A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. 10.3389/fnut.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M.; Welladsen H. M.; Mangott A.; Li Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS One 2015, 10, e0118985. 10.1371/journal.pone.0118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman L.; Nommensen P.; Pennings B.; Laus M. C. Comprehensive overview of the quality of plant- And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. 10.1002/fsn3.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S. U.; Tiwari B. K.; O’Donnell C. P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. 10.1021/jf400819p. [DOI] [PubMed] [Google Scholar]
- Pulz O.; Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- van Huis A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. 10.1146/annurev-ento-120811-153704. [DOI] [PubMed] [Google Scholar]
- Imathiu S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. 10.1016/j.nfs.2019.11.002. [DOI] [Google Scholar]
- Johnson M. E.; Lucey J. A. Major technological advances and trends in cheese. J. Dairy Sci. 2006, 89, 1174–1178. 10.3168/jds.S0022-0302(06)72186-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.