Abstract
Background
Spinal cord injury (SCI) leads to a loss of descending motor and sympathetic control below the level of injury (LOI), which ultimately results in chronically altered cardiovascular function and remodeling. While supervised, laboratory-based exercise training can generate cardiovascular adaptations in people with SCI, it is unknown whether behavioral community-based interventions effectively generate such adaptations for individuals with SCI.
Objective
Examine the effects of a tailored behavioral physical activity (PA) intervention on cardiac and vascular structure and function in individuals with SCI.
Methods
In this randomized controlled trial, 32 participants with SCI (18-65 years, SCI >1 year) were assigned to PA (8-week behavioral intervention) or control (CON) groups. At baseline and postintervention, measures of resting left ventricular (LV) structure and function, carotid intima-media thickness and pulse-wave velocity were assessed with ultrasound and tonometry.
Results
Twenty-eight participants completed the study (n = 14/group). Across the full study cohort there were no significant changes in indices of LV or vascular structure and function, despite notable improvements in peak power and oxygen uptake in the PA group. However, in a subanalysis for LOI, individuals in the PA group with LOIs below T6 had evidence of altered LV geometry (ie, increased LV internal diameter, reduced sphericity index and relative wall thickness; group × time P < 0.05 for all), which was not seen in individuals with higher LOIs at or above T6.
Conclusion
An 8-week behavioral PA intervention appears to promote adaptations in cardiac geometry more readily in individuals with lower level SCI than those with higher-level SCI.
Keywords: spinal cord injuries, exercise, intervention study, cardiovascular system, echocardiography
Introduction
Individuals with spinal cord injury (SCI) have functional, contextual, and health condition-related barriers to physical activity (PA) participation. 1 Given the large quantities of these barriers and their associated challenges, it is not surprising that people with SCI report some of the lowest PA levels compared with able-bodied individuals and other populations with chronic disease and disability. 2 Recently, PA guidelines have been developed for the SCI population based on findings from supervised exercise trials in controlled laboratory settings. 26 These guidelines recommend ≥20 minutes of moderate-to-vigorous aerobic activity twice weekly complimented with resistance exercise for fitness benefits, and ≥30 minutes of moderate-to-vigorous aerobic activity 3 times per week for cardiometabolic benefits. Behavioral interventions, or strategies that support self-management of PA, may be a pragmatic and effective way to address the low PA levels observed in the SCI population, and ultimately support those individuals toward achieving the recommended PA guidelines. 3 Such interventions in SCI have shown cardiorespiratory and metabolic benefits4,5; however, to our knowledge, no trial has yet evaluated the effects of a community-based behavioral PA intervention on cardiac and vascular structure and function in the SCI population.
The combined loss of motor control and descending sympathetic control below the level of SCI results in chronically altered cardiovascular function and remodeling of the heart and vasculature6,7 that are generally more pronounced with higher levels of injury (LOI).8,9 These cardiovascular consequences have important clinical relevance as SCI has been associated with ~3- to 4-fold increased odds of cardiovascular disease. 10 While the impacts of SCI on the vasculature are relatively well-established (eg, blood pressure dysregulation, 11 arterial stiffening, 12 and remodeling 13 ), the cardiac responses to SCI have only recently gained increasing attention. In a meta-analysis of echocardiographic data, our group has identified that left ventricular (LV) volumes and structural dimensions are smaller in SCI compared with able-bodied individuals, in addition to reduced LV mass and altered ejection and filling function. 6
Exercise training is a potent stimulus for physiological cardiovascular adaptations. However, studies examining the effects of increased PA in people with SCI most often apply cross-sectional designs comparing highly trained individuals (ie, national- or Paralympic-level athletes14-16), who are not representative of the wider SCI population, with nonathletes. Among longitudinal and case-control studies to date, few have examined echocardiographic measures of cardiac structure and function following a PA intervention.17-21 Findings are highly variable amongst those studies, and most have applied laboratory-based exercise modalities such as functional electrical stimulation (FES) exercise17,20,22 or bodyweight supported treadmill training 21 that are not widely available to individuals within their local communities.
Accordingly, the main aim of this study was to examine the effects of a tailored, community-based behavioral PA intervention (ProACTIVE SCI) on cardiovascular structure and function in individuals with SCI. While peak oxygen uptake and power output data from the ProACTIVE SCI trial are previously reported,23,24 the current study’s key outcomes included LV end-diastolic volume (EDV), stroke volume (SV), and pulse-wave velocity (PWV). It was hypothesized that LV volumes would increase and PWV would decrease following the 8-week behavioral PA intervention. Next, given that high-level SCI impairs descending sympathetic input to the heart and vasculature, 7 an exploratory subanalysis was performed to assess the influence of LOI on the cardiovascular responses to the PA intervention. Finally, we sought to elucidate predictors of cardiorespiratory fitness in individuals with SCI.
Methods
Study Participants and Ethical Approval
A total of 32 participants were recruited from the greater Vancouver, Canada area from May to July 2017. Ethical approval was granted by the University of British Columbia Clinical Research Ethics Board (H17-00559), and written informed consent was obtained from all participants. The study conformed to the Declaration of Helsinki.
Participants were included if they were living with chronic (>1 year) SCI, 18 to 65 years of age, cleared by a physician to exercise, and currently performing <150 minutes of moderate-to-vigorous PA/week (Canadian Physical Activity Guidelines for Aerobic Activity 25 ). Participants were excluded if they had an active stage 3 or 4 pressure ulcer, trauma, or surgery <3 months prior to the study, lacked proficiency in English that would affect their ability to follow instructions, and/or had any unstable medical/psychiatric condition that might prevent them from completing the study.
Prior Publications From the ProACTIVE SCI Trial
Primary manuscripts for the ProACTIVE intervention detail its development and report changes in weekly PA participation, peak oxygen uptake (VO2peak) and peak power (POpeak).23,24 This manuscript uniquely reports on resting cardiac and vascular measures, as well as changes in peak cardiorespiratory function (eg, peak oxygen pulse [O2pulsepeak], heart rate [HRpeak], and ventilation [VEpeak]) that are not previously reported from the ProACTIVE trial (Supplemental Table 2). VO2peak and POpeak data are included in the current article for the subgroup analysis based on LOI, and to assess the potential cardiac, vascular, and demographic predictors of cardiorespiratory fitness in individuals with SCI.
Study Design
Randomized Controlled Trial
Participants were initially matched by baseline self-reported leisure time PA levels and randomly assigned to either the physical activity (PA) intervention or control (CON) groups using a random numbers generator. Interventionists were not blinded to group assignment; however, all analyses of cardiovascular outcomes were performed blinded. The RCT followed the preregistered protocol at clinicaltrials.gov (identifier: NCT03111030). Individuals were informed verbally of their group assignment on completion of baseline measures.
Physical Activity Intervention
Detailed descriptions of the ProACTIVE SCI intervention and its co-development are provided elsewhere.23,24 The intervention was delivered by a single researcher (JM), an American College of Sports Medicine–certified inclusive fitness trainer with >7 years of experience training individuals with SCI. A 1-hour introductory session included assessments of baseline PA levels, activity preferences, barriers, and accessibility to PA. Together, the researcher and participant set PA goals toward achieving or exceeding the SCI PA guidelines, which recommend strength training of all major, functioning muscle groups ≥2 d/wk, plus ≥30 minutes aerobic activity ≥3 d/wk for cardiometabolic benefits. 26 Participants randomized to the PA intervention completed their introductory coaching session and 8 weekly PA coaching sessions (10-15 minutes each), while participants in the waitlist condition (CON) were scheduled to begin coaching sessions after their postintervention assessments for the study.
During each coaching session, the researcher and participant discussed PA progress and worked toward problem solving for issues related to education (eg, general PA information, self-regulatory strategies), referral (eg, peers, programs, organizations), or by revising their goals, PA program and/or action plan. Coaching sessions were delivered face-to-face at the research facility, over Skype, or when the former modes were unavailable, over the phone. PA was performed in the setting of their choosing (eg, home, gym, community center).
Assessments of Cardiovascular Outcomes and Peak Aerobic Fitness
Cardiovascular measures and peak exercise tests were performed at preintervention and repeated 9 weeks later at postintervention. Participants were instructed to take medications as normal, but refrain from exercise, caffeine, and alcohol on the day of testing, and avoid significant food or drink 4 hours prior to testing. During each testing session, participants were transferred to a flat medical bed and rested ≥10 minutes. Two participants who could not be transferred to the bed reclined their power wheelchairs to a near-supine position. Cardiac and vascular ultrasound and tonometry were sequentially performed, after which participants were transferred off the testing bed to complete a peak aerobic exercise test.
Specific Methodology
Participant Demographics and Medical Information
Demographic information including sex, age, completeness of injury, LOI, years postinjury, primary mode of mobility, highest level of education, ethnicity, and any current medications or medical complications were collected using an online form. When applicable, participant body mass was calculated using a wheelchair scale (PUA220A; Mettler) by subtracting chair mass from combined chair and body mass.
Transthoracic Echocardiography
All echocardiographic imaging and analyses were performed by a single, highly trained sonographer blinded to participant identifiers and condition. Images were acquired using a commercially available ultrasound system (Vivid 7, GE Healthcare) and 1.5- to 4-MHz phased-array transducer (M5S probe) and saved for offline analysis (EchoPAC v.113, GE Healthcare). The torso was positioned in a left-lateral orientation by placing a foam wedge under the right side-body. Two-dimensional B-mode and pulsed Doppler recordings were acquired at end-expiration for measures of LV structure and function in accordance with current guidelines, 28 as described previously. 29 Volumetric indices (ie, EDV, SV, end-systolic volume [ESV], ejection fraction [EF]) were measured using the modified Simpson’s biplane. LV length was averaged from measures in the apical 2- and 4-chambers at end-diastole. LV end-diastolic internal diameter (LVIDd), posterior wall thickness (PWT), and septal wall thickness (SWT) were measured in the parasternal long axis. Sphericity index, a ratio of the length-to-width geometry of the ventricle and an important determinant of LV function,30,31 was calculated as LV end-diastolic length/LVIDd. Relative wall thickness, the ratio of the LV wall thickness relative to its chamber diameter, which is often altered with physiological remodeling, was calculated as 2·PWT/LVIDd. LV mass was estimated as 1.04·[(SWT + LVIDd + PWT) 3 − LVIDd 3 ]·0.8 + 0.6 g. Images for speckle tracking analysis were acquired at 70–90 frames/s. All echo-derived data were averaged across 3 cardiac cycles.
Speckle-Tracking Analysis
Analyses of LV mechanics (ie, rotation, twist, strain) were performed with speckle tracking software (EchoPAC v.113, GE Healthcare) as detailed elsewhere. 29 Rotation and strain were measured across the entire myocardial region of interest. Data were time-aligned and transformed to 1200 points using cubic spline interpolation (2D Strain Analysis Tool), and peak values averaged across 3 cardiac cycles. Twist was calculated by subtracting time-aligned basal rotation from apical rotation. Tracking was inadequate for twist data in n = 5 individuals from PA and n = 2 from CON.
Vascular Ultrasound and Tonometry
Vascular imaging and tonometry were performed by a single experienced investigator. Common carotid artery (CCA) intima-media thickness (IMT) was assessed using 2-dimensional B-mode ultrasound (Vivid 7, GE Healthcare) and a 5- to 13-MHz linear-array transducer (12L probe). Images were captured in the anterolateral plane, 1 to 2 cm from the carotid bulb, across 5 cardiac cycles. Analyses were completed offline using commercial edge-detection software (EchoPAC v.113, GE Healthcare), and measures of distal wall IMT at end-diastole (R-wave) were averaged over 3 cardiac cycles. Mean CCA-IMT was calculated as the average of right- and left-sided measures. Data supporting the reliability of IMT measurements among individuals with SCI are described previously. 32
Measurements of carotid-femoral PWV were performed in accordance with current international guidelines. 33 Handheld tonometry (SPT-301; Millar Instruments) was applied sequentially at the left femoral and carotid arteries for the acquisition of arterial pressure waveforms. Brachial blood pressure was captured from the left arm with an automated cuff (Dinamap Carescape V100; GE Healthcare), and HR was continually recorded from three-lead electrocardiogram (ML123, ADInstruments). PWV was calculated as (0.8 × D) ÷ ∆t, where D is the distance between carotid and femoral sites and ∆t is the pulse transit time. 33 Arterial waveforms were bandpass filtered (2-30 Hz) and the minimum value of the filtered signal was used to identify the upstroke of the waveform. PWV was averaged across 20 consecutive cardiac cycles. Intraobserver test-retest reliability for PWV was ICC (intraclass correlation coefficient) = 0.923, similar to previous reports in SCI. 34
Peak Aerobic Exercise Test
Cardiorespiratory fitness was assessed during a graded exercise test on an electronically braked arm ergometer (Angio Rehab arm ergometer, Lode). Participants were instructed to maintain a cadence of ~50 rpm, and after an initial warm-up (0 W) power output was increased 10 W/min or 5 W/min for participants with paraplegia or tetraplegia, respectively, until volitional exhaustion (ie, unable to maintain ≥30 rpm 35 ). Breath-by-breath gases were collected using a metabolic gas analyzer (Quark CPET, Cosmed) and HR was monitored using a Polar Electro H1 sensor (Polar). Values for VO2peak, O2pulsepeak, volume of exhaled carbon dioxide (VCO2peak), HRpeak, VEpeak, breathing frequency (fRpeak), and tidal volume (VTpeak) were selected using 30-second rolling averages. Ratings of perceived exertion were collected in the final 10 seconds of each stage (Borg 6-20 scale). 27
Statistical Analyses and Power Calculation
All data are reported as mean ± standard deviation. Participant characteristics were compared between groups using independent-samples t tests for continuous variables and Pearson’s chi-square for categorical data. Cardiac and vascular data were assessed using analysis of covariance (ANCOVA) with main effects for time and group, and baseline VO2peak (mL/kg/min) as covariate to account for the influence of aerobic fitness on cardiovascular measures. 36 Cardiorespiratory data were assessed using a 2-way repeated-measures analysis of variance (ANOVA) with main effects for time (pre- vs postintervention period) and group (PA vs CON). Post hoc comparisons were performed using Tukey’s HSD (honest significant difference) test. A subanalysis for LOI was performed by splitting the study cohort into high (ie, at or above [≥] T6) and low LOI (ie, below [<] T6). The same ANCOVA analysis was performed for those LOI cohorts, and planned comparisons of baseline versus postintervention data were performed using Fisher’s LSD (least significant difference) method.
Linear mixed effects modelling was performed to elucidate predictive factors of VO2peak in individuals with SCI. Left ventricular EDV, SV, cardiac output (Q), LVIDd, LVlength, LVmass, IMT, and PWV, as well as age, sex, LOI, VEpeak, and HRpeak were assessed as fixed factors. Time was included as a repeated factor, and participant as a random factor. Fixed factors that did not significantly contribute to the model were excluded from further iterations. Significance was set a priori at α = .05. All statistical analyses were performed using SPSS Statistics (version 27.0, IBM Corp).
Power and Sample Size Calculations
This study reports cardiovascular outcomes from the ProACTIVE SCI RCT wherein leisure time PA was the primary outcome of interest. 23 The sample size calculation for that study was based on a previous behavioral PA intervention for individuals with SCI. 37 With a power of 0.80 and α = .05, n = 30 individuals were required to detect the large-sized effect reported in a comparable study 37 (d = 0.96).
With regard to the primary cardiovascular outcomes of the current report, previous studies have reported altered EDV (+9 mL, SD = 9 mL) 17 and PWV (−0.5 cm/s, SD = 0.3 cm/s) 38 in individuals with SCI following PA interventions. With a power of 0.80 and α = .05, we required 14 individuals to detect these effects in the PA group. As such, we were sufficiently powered with n = 14 per group to detect significant alterations to our primary cardiovascular outcomes following the PA intervention.
Results
Participant Demographics
Twenty-eight participants completed the study (n = 14 per group). Two individuals had complete C4 injuries, which precluded them from completing a VO2peak test. Preintervention self-reported PA and accelerometer data are reported previously 23 and were not different between PA and CON groups. There were no between-group differences in age, mass, proportions of male and female participants, or injury demographics (Table 1). Both groups had similar proportions of individuals with high LOI (ie, SCI ≥T6), traumatic SCI as well as neurological completeness of injury. Additional details related to injury characteristics are provided in Supplemental Table 1.
Table 1.
Participant Demographics and Injury Characteristics. a
| Demographic | Physical activity intervention (PA), n = 14 | Control (CON), n = 14 | P |
|---|---|---|---|
| Age, y, mean (SD) | 45.8 (13.6) | 45.6 (10.5) | .96 |
| Body mass, kg, mean (SD) | 74.1 (22.8) | 77.7 (15.5) | .64 |
| Sex, male/female, n | 9/5 | 8/6 | 0.70 |
| Characteristics of SCI | |||
| Time post-injury, y, mean (SD) | 14.7 (13.9) | 18.1 (10.9) | .47 |
| SCI ≥T6 and AIS A, n (%) | 7 (60) | 6 (57) | .70 |
| SCI ≥T6 and AIS B-D, n (%) | 1 (36) | 3 (43) | .28 |
| SCI <T6, n (%) | 6 (57) | 5 (50) | .70 |
| Traumatic SCI, n (%) | 11 (79) | 11 (79) | 1.00 |
| Primary mode of transportation, manual chair/power chair b , n | 9/3 | 6/5 | .52 |
Abbreviations: SCI, spinal cord injury; AIS A, American Spinal Injury Association Impairment Scale: a classification of A indicates a complete injury with no motor or sensory function below level of injury; a classification of B-D indicates an incomplete injury; ≥T6, at or above the sixth thoracic level.
Demographics are provided for participants in the physical activity intervention (PA) and control (CON) groups.
Other primary modes of transportation include no aid (n = 1) and scooter (n = 1) in the PA group, primarily walking (n = 2) and canes/walking poles (n = 1) in CON. See Supplemental Table 1 for detailed individual injury characteristics.
Resting Cardiovascular Structure and Function Pre- and Postintervention
There were no significant group, time, or interaction effects for LV volumes (ie, EDV, SV; Figure 1A and B), hemodynamics (ie, EF, Q; Figure 1C and D), or LV geometry (Figure 1E and F), despite significant improvements in cardiorespiratory fitness in the PA group (eg, VO2peak and POpeak; see Supplemental Table 2 for cardiorespiratory data). Among diastolic measures, only E′ (P = 0.008) and A′ (P = 0.025) were lower at postintervention in the PA group though not different from CON (Supplemental Table 3). There were no effects for LV twist mechanics, although untwisting velocity was elevated in the PA group compared with CON at baseline (P = 0.014) but not postintervention. Neither PWT nor CCA-IMT were different between groups or altered over time (Figure 1G and H). There were no significant effects for blood pressure (Supplemental Table 4).
Figure 1.
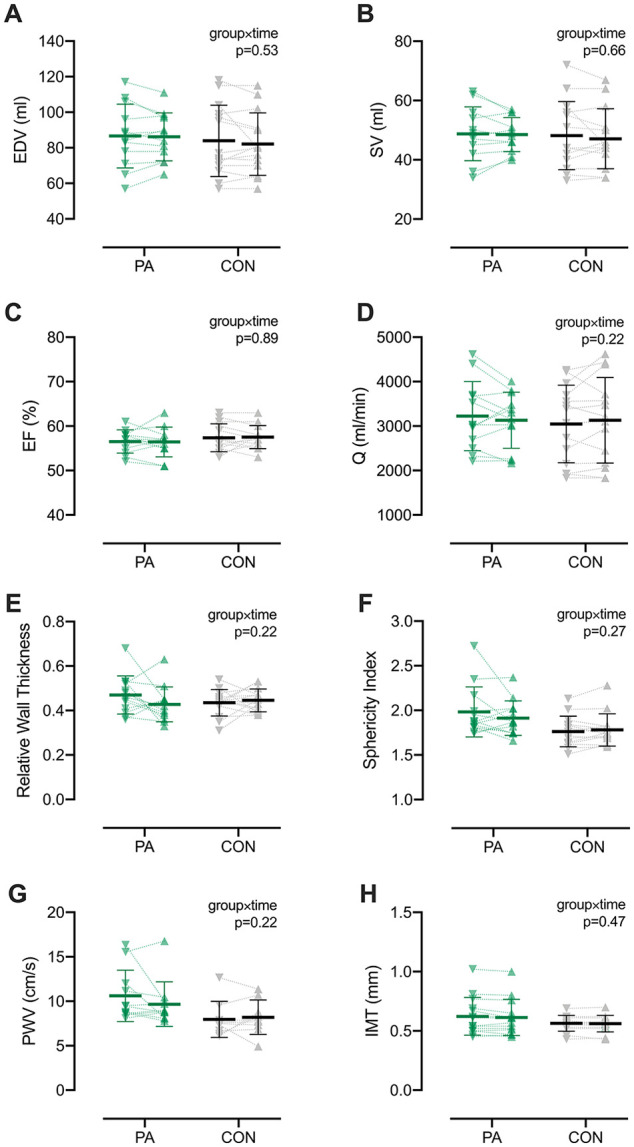
Resting cardiac and vascular measures at baseline (∇) and following the 8-week intervention period (∆). Bars represent means ± SD. Individual data are shown for participants in the physical activity (PA; green symbols) and control (CON; gray symbols). There were no main or interaction effects observed for echocardiographic measures of left ventricular end-diastolic volume (EDV; A), stroke volume (SV; B), ejection fraction (EF; C), cardiac output (Q; D), relative wall thickness (E), or sphericity index (F), despite significant improvements in cardiorespiratory fitness for the PA group (Supplemental Table 2). Likewise, vascular measures of pulse-wave velocity (PWV, G) and intima media thickness (IMT; H) were unchanged following the 8-week intervention period. n = 13 for both PA and CON. Additional cardiac and vascular data and statistics are found in Supplemental Tables 3 and 4.
Subanalysis for Level of Injury
No significant changes in cardiovascular structure or function were detected at the end of the intervention period in the high-LOI cohort; however, in low-LOI participants, significant interactions indicated that the PA group had increases to LVIDd (group × time P = .027) alongside reductions to sphericity index (group × time P = .049) and relative wall thickness (group × time P = .046; Figure 2A-C). There were otherwise no significant effects detected in the high- or low-LOI cohorts for measures of LV mechanics, Doppler velocities, IMT, PWV, or blood pressure. Both the high- and low-LOI cohorts had significant group × time interactions for relative VO2peak (≥T6 P = .002, <T6 P = .006; Figure 2D) and POpeak (P = .01 for both LOI cohorts; Supplemental Table 5). The increases to self-reported total PA (P = .99) and moderate-to-vigorous PA (P = .30) were not different between the high- and low-LOI PA cohorts.
Figure 2.
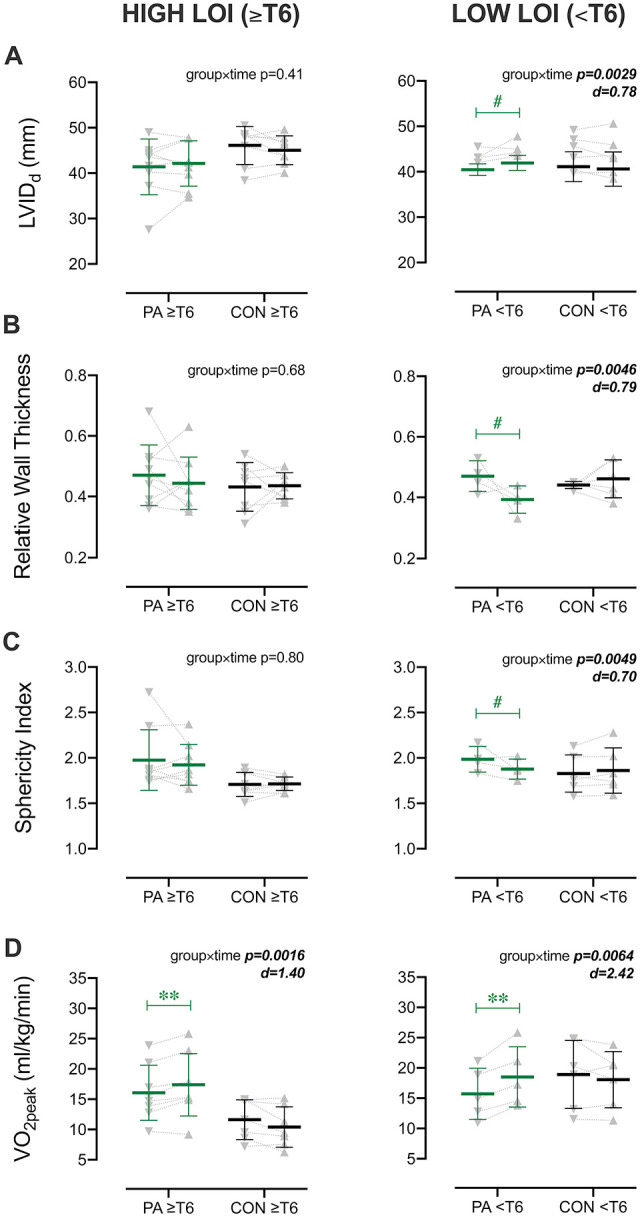
Subanalysis for level of injury (LOI) of cardiorespiratory and left ventricular (LV) measures at baseline and postintervention. Cohorts with higher LOI (at or above [≥] T6) and lower LOI (below [<] T6) are shown in left- and right-hand panels, respectively. Bars represent means ± SD. Individual data are shown at baseline (∇) and postintervention period (∆) for participants in the physical activity (PA; green symbols) and control (CON; gray symbols) groups. In contrast to the high-LOI cohort, only the low-LOI cohort had significant group × time interactions for LV end-diastolic internal diameter (LVIDd; A), relative wall thickness (B), and sphericity index (C). Both cohorts, however, had significant improvements in peak oxygen uptake (VO2peak; D). *P < .05, **P < .01, #P ≤ .08 vs baseline.
Predictors of VO2peak
Using linear mixed modeling, cardiac and vascular variables of interest were initially included in the model (see Statistical Analyses and Power Calculation section for details). Due to significant multicollinearity, EDV, SV, and ESV were not input simultaneously. For predictors of absolute VO2peak, only EDV and EF significantly contributed to the model (F = 6.17, P = .004; Model 1, Table 2). Participant demographics (body mass, age, LOI, sex) were thereafter input to the model, whereby biological sex (F = 14.07, P < .001) and LOI (F = 10.44, P = .001) were the strongest predictors of VO2peak (F = 15.7, P < .001, Model 2), while EDV (P = .87) and EF (P = .11) became negligible. Inclusion of SV, Q, LVIDd, LVlength, LVmass, IMT or PWV did not improve the model fit. Finally, with the addition of peak cardiorespiratory measures, HRpeak significantly contributed to the model (F = 7.16, P = .011, Model 3) while VEpeak did not.
Table 2.
Cardiorespiratory and Demographic Predictors of VO2peak Derived From Linear Mixed Modeling for the Complete Study Cohort.a
| Final corrected model |
Estimates of fixed effects |
||||||
|---|---|---|---|---|---|---|---|
| Intercept | F | P | Variable | Coefficient | F | P | |
| VO2peak (mL/min) | |||||||
| Model 1 | 1785 | 6.17 | .004 | EDV | 8.3 | 7.55 | .009 |
| EF | −23.7 | 4.04 | .05 | ||||
| Model 2 | 1125 | 15.7 | <0.001 | Sex | M = 382.1 F = 0 |
14.07 | <.001 |
| LOI | H = −370.3 L = 0 |
10.44 | 0.001 | ||||
| Model 3 | 460 | 8.54 | <.001 | Sex | M = 382.3 F = 0 |
13.60 | .001 |
| LOI | H = −262.4 L = 0 |
6.84 | .012 | ||||
| HRpeak | 4.6 | 7.16 | .011 | ||||
| VO2peak (mL/kg/min) | |||||||
| Model 4 | 17.5 | 3.64 | .062 | LOI | H = −3.50 L = 0 |
3.64 | .062 |
| Model 5 | 9.8 | 5.29 | .009 | LOI | H = −3.45 L = 0 |
4.27 | .045 |
| HRpeak | 0.061 | 5.47 | .024 | ||||
Abbreviations: EDV, end-diastolic volume; EF, ejection fraction; LOI, level of injury; F: female; M:male; H, high-level injury (≥T6); L, low-level injury (<T6); HRpeak: peak heart rate during aerobic exercise test.
Models for absolute and relative VO2peak derived utilizing generalized linear mixed model, including “time” as a repeated effect and “participant” as a random effect. See Statistical Analyses and Power Calculation section for detailed modeling approach.
The same mixed modelling approach was applied for predictors of relative VO2peak (mL/kg/min). No resting cardiac or vascular variables were significant predictors; however, when demographics were included, LOI appeared to contribute to the model (F = 3.64, P = .062, Model 4). Finally, with the addition of peak cardiorespiratory variables, HRpeak and LOI together were predictive of relative VO2peak (F = 5.29, P = .009, Model 5).
Additional Cardiorespiratory Analyses
Further to the previously reported improvements in POpeak and VO2peak23,24 (Supplemental Table 2 for statistics), additional cardiorespiratory analyses found significant group × time interactions for VEpeak (P = .004) and respiratory frequency (fRpeak, P = .0043), both of which tended to be augmented at postintervention in the PA group (P = 0.06 for both). VTpeak was otherwise unchanged at postintervention. HRpeak was unaltered, however O2pulsepeak was augmented in the PA group at post-intervention (Supplemental Table 2). There were no effects for respiratory exchange ratio, end-tidal PCO2, or ratings of perceived exertion, nor were there significant changes in body mass in the PA (−0.11 ± 4.9 kg; P = .94) or CON (+0.77 ± 3.5 kg; P = .44) groups.
Discussion
This study is the first to report on the effects of a community-based behavioral PA intervention on resting cardiac and vascular structure and function among people with SCI. In contrast to published interventions applying lab-based exercise modalities (eg, FES) and cross-sectional studies of highly trained athletes with SCI, the RCT employed community-based exercise in a sample representative of the wider SCI population. Following the 8-week ProACTIVE intervention period, resting cardiac and vascular measures were largely unaltered in the PA group, in contrast to our hypothesis. Interestingly, however, our subanalysis indicated that individuals with lower LOI (ie, <T6) may be more likely to alter LV geometry in response to a PA intervention. Finally, by combining this trial’s cardiac, vascular, aerobic fitness and demographic data, we have highlighted that EDV and EF, as well as LOI and HRpeak are important predictors of cardiorespiratory fitness in individuals with SCI.
Cardiac and Vascular Adaptations in SCI: A Role for Level of SCI?
Although cardiac and vascular outcomes were not significantly altered in the PA group, the subanalysis of LOI provides evidence of LV remodeling in those with lower-level injuries. Specifically, significant interaction effects indicated alterations to LV geometry (ie, sphericity index and relative wall thickness) in those with injuries below T6, but not in individuals with SCI at or above T6. In the low LOI cohort, the lowered sphericity index and relative wall thickness resulted from a widening of LVIDd rather than from increased LV length or wall thickness. Such alterations to LV geometry are characteristic of eccentric remodeling and may have resulted from increased preload following the PA intervention. Cardiac preload is chronically lowered in individuals with SCI, and contributes to smaller LV dimensions and volumes in comparison with able-bodied individuals. 6 Lowered LV preload in SCI is hypothesized to result, in part, from reductions to total blood volume associated with paralysis and a loss of descending sympathetic control below the LOI that impairs venous return and causes blood to pool in the capacitance vessels.9,39 In the current study, those with lower LOI achieved greater exercise intensities (ie, VO2peak and POpeak) than those with higher LOI, which may have facilitated blood volume expansion, 40 improved venous return and increased preload to ultimately alter LV geometry.
In contrast to the low LOI cohort, participants with high LOI did not have notable structural or functional alterations in the heart or arterial system, despite their improvements in aerobic fitness. These observations suggest that increased exercise intensities and/or muscular recruitment may be required to generate significant cardiovascular adaptations in high-level SCI. To date, only studies that have applied FES to the lower limbs have demonstrated altered cardiac function and remodeling in individuals with high-level SCI.17,22 Likewise, most longitudinal studies reporting altered arterial structure, function, or PWV have applied FES in laboratory-based PA interventions.41-43 Lower-body FES exercise may generate such cardiovascular adaptations via increased loading to the heart and vasculature (eg, preload, wall stress, shear), either by generating a lower-limb muscle pump with increased venous return, 17 augmenting arterial blood flow to the paralyzed limbs, 44 or facilitating blood volume expansion. 39 Therefore, exercise modalities that augment cardiovascular loading either acutely during the exercise bout or chronically over the PA intervention may be necessary to alter cardiac or vascular outcomes in individuals with high-level SCI.
Although resting cardiac and vascular measures were largely unaltered in the PA group, we cannot preclude potential improvements to cardiovascular function during exercise. Similar to the current study, Milia et al 18 reported improved VO2peak in an SCI cohort without detectable changes in resting cardiac measures following 12 months’ arm-ergometry training. However, they did observe enhanced cardiovascular responses (eg, HR, blood pressure, cardiac output) during metaboreflex activation. Davis et al 45 also reported enhanced SV responses to isometric and submaximal exercise following 16 weeks’ arm-ergometer training, despite unaltered resting hemodynamics. It is therefore possible that the ProACTIVE intervention could have improved cardiac “reserve” or the cardiovascular responses to acute physiological stressors like exercise in the current study. Though we were unable to perform echocardiography during arm-crank exercise, the PA group had increased peak O2pulse at postintervention. Given that O2pulse is a validated and reliable measure of SV during exercise in able-bodied individuals and SCI, 46 it is possible that the PA intervention facilitated improved cardiac responses to exercise in the current study. Future research should consider assessing the cardiovascular responses to physiological stressors, where possible, to better understand the potential benefits of PA interventions in SCI.
The improvements in VO2peak and exercise performance following the 8-week ProACTIVE intervention could otherwise be attributable, in part, to improvements in muscular strength or pulmonary function. Peripheral factors including skeletal muscle function are believed to represent a major limitation for VO2peak in nonathletic individuals with SCI,40,47 and increased skeletal muscle strength has been linked to improved VO2peak in individuals with paraplegia and tetraplegia.48,49 SCI can additionally paralyze the expiratory muscles and accessory inspiratory muscles, thereby limiting pressure generation with increasing ventilatory demand. 47 Indeed, efforts to improve pulmonary function, including abdominal binding 50 and short-term respiratory muscle training51,52 have supported improvements to VO2peak and POpeak in highly trained tetraplegic individuals, even when PA levels are unchanged. In the current study, improved VEpeak in the PA group (P = 0.06) may have supported the increased aerobic performance to some degree. Ultimately, any long-term improvements to peripheral factors or pulmonary function could allow individuals with SCI to achieve greater exercise intensities and generate significant cardiovascular adaptations beyond the time period observed in the current study.
Regulators and Predictors of Cardiorespiratory Fitness in SCI
In an effort to elucidate the potential cardiac and vascular factors that regulate VO2peak in SCI, our mixed model analyses revealed resting LV EDV and EF as significant predictors of absolute VO2peak. This is a novel observation amongst the SCI literature to date, and indicates that larger LV volumes may support greater systolic “reserve” during aerobic exercise in SCI. While an inverse association between EF and VO2peak may appear at odds with the known relationship between systolic function and VO2peak, it is important to note that EF spanned a “healthy” and normal range (ie, EF = 51%-63%) in the current cohort. 28 If participants with EFs lower than the healthy range were included, an inverse relationship would not be expected. When demographic and peak exercise data were included in the model, LOI, sex and HRpeak replaced EDV and EF as predictors of absolute VO2peak, presumably due to the influences of LOI and biological sex on LV volumes and ejection function.9,53 As predictors of VO2peak, LOI was not surprising given that higher-level injuries are characterized by lower maximal oxygen uptake and HRpeak 47 ; however, biological sex until now has not been considered in the context of SCI despite being a well-known independent predictor of maximal aerobic capacity in able-bodied males and females. 54 These modeling data therefore highlight important links between LV volumes, LOI and biological sex, which ultimately play a role in determining aerobic exercise capacity among individuals with SCI.
Considerations and Future Directions
While it is possible that a longer intervention (>8 weeks) could allow for significant cardiovascular adaptations in the PA group, such a role for prolonged PA is not necessarily indicated by the current literature in SCI. Some studies comparing highly trained versus untrained individuals with SCI suggest an effect of long-term PA on cardiac and vascular measures,16,55 though others often do not show differences between these groups.14,15,40 Alternatively, the inability to activate the sympathetic nervous system and/or lower-limb motor function during exercise may preclude an effective stimulus for measurable cardiovascular effects, given the growing evidence that sympathetic activation may be necessary for cardiac and vascular adaptation.56,57 To specifically address the important role of the autonomic system, future studies should consider the influence of “autonomic completeness” on the cardiac and vascular adaptations to PA interventions in individuals with SCI.
When mixed-model analysis was applied for relative VO2peak (mL/kg/min), none of the measured cardiac or vascular outcomes were significant predictors. Though the inclusion of allometrically-scaled cardiac and vascular data would have been more appropriate for modeling relative VO2peak, validated methods to calculate either body surface area or fat-free mass in individuals with SCI do not currently exist, 40 precluding our ability to include scaled data in this model. It is critical that future work consider assessing approaches for allometric scaling (eg, “height” or body length) to allow for improved data analysis and interpretation among cardiovascular research in SCI.
Conclusions
In contrast to traditional laboratory-based, fully-supervised exercise training interventions, this study uniquely reports on the first RCT of a community-based behavioral PA intervention that assesses cardiac and vascular outcomes among individuals with SCI. The individualized ProACTIVE intervention appears to promote adaptations in cardiac geometry more readily in individuals with lower-level injuries than those with higher-level SCI.
Supplemental Material
Supplemental material, sj-pdf-1-nnr-10.1177_15459683211017504 for Effects of a Tailored Physical Activity Intervention on Cardiovascular Structure and Function in Individuals With Spinal Cord Injury by Alexandra M. Williams, Jasmin K. Ma, Kathleen A. Martin Ginis and Christopher R. West in Neurorehabilitation and Neural Repair
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ontario Neurotrauma Foundation/Rick Hansen Institute (2015-RHI-PEPA-998). AMW is supported by MSFHR, Rick Hansen Institute and ICORD. JM is supported by MSFHR and The Arthritis Society. KMG holds the Reichwald Family Southern Medical Program Chair in Chronic Disease Prevention. CRW is supported by an Investigator Award from MSFHR and senior personnel award from Heart and Stroke Foundation of Canada. Research in the laboratory of CRW is supported by the Canadian Foundation for Innovation and British Columbia Knowledge Development Fund.
ORCID iDs: Alexandra M. Williams  https://orcid.org/0000-0003-1571-9168
https://orcid.org/0000-0003-1571-9168
Christopher R. West  https://orcid.org/0000-0002-8618-5995
https://orcid.org/0000-0002-8618-5995
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
References
- 1.Fekete C, Rauch A. Correlates and determinants of physical activity in persons with spinal cord injury: a review using the International Classification of Functioning, Disability and Health as reference framework. Disabil Health J. 2012;5:140-150. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg-Emons RJ, Bussmann JB, Stam HJ. Accelerometry-based activity spectrum in persons with chronic physical conditions. Arch Phys Med Rehabil. 2010;91:1856-1861. [DOI] [PubMed] [Google Scholar]
- 3.Ma JK, Ginis KAM. A meta-analysis of physical activity interventions in people with physical disabilities: content, characteristics, and effects on behaviour. Psychol Sport Exerc. 2018;37:262-273. [Google Scholar]
- 4.Gorgey AS, Witt O, O’Brien L, et al. Mitochondrial health and muscle plasticity after spinal cord injury. Eur J Appl Physiol. 2019;119:315-331. [DOI] [PubMed] [Google Scholar]
- 5.Nooijen CF, Stam HJ, Sluis T, Valent L, Twisk J, van den Berg-Emons RJ. A behavioral intervention promoting physical activity in people with subacute spinal cord injury: secondary effects on health, social participation and quality of life. Clin Rehabil. 2017;31:772-780. [DOI] [PubMed] [Google Scholar]
- 6.Williams AM, Gee CM, Voss C, West CR. Cardiac consequences of spinal cord injury: systematic review and meta-analysis. Heart. 2019;105:217-225. [DOI] [PubMed] [Google Scholar]
- 7.Squair JW, DeVeau KM, Harman KA, et al. Spinal cord injury causes systolic dysfunction and cardiomyocyte atrophy. J Neurotrauma. 2018;35:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West CR, Mills P, Krassioukov AV. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord. 2012;50:484-492. [DOI] [PubMed] [Google Scholar]
- 9.Kessler KM, Pina I, Green B, et al. Cardiovascular findings in quadriplegic and paraplegic patients and in normal subjects. Am J Cardiol. 1986;58:525-530. [DOI] [PubMed] [Google Scholar]
- 10.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013;81:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankel HL, Michaelis LS, Golding DR, Beral V. The blood pressure in paraplegia. I. Paraplegia. 1972;10:193-200. [DOI] [PubMed] [Google Scholar]
- 12.Phillips AA, Cote AT, Bredin SSD, Krassioukov AV, Warburton DER. Aortic stiffness increased in spinal cord injury when matched for physical activity. Med Sci Sports Exerc. 2012;44:2065-2070. [DOI] [PubMed] [Google Scholar]
- 13.de Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT. Rapid and extensive arterial adaptations after spinal cord injury. Arch Phys Med Rehabil. 2006;87:688-696. [DOI] [PubMed] [Google Scholar]
- 14.Currie KD, West CR, Stöhr EJ, Krassioukov AV. Left ventricular mechanics in untrained and trained males with tetraplegia. J Neurotrauma. 2017;34:591-598. [DOI] [PubMed] [Google Scholar]
- 15.Gates PE, Campbell IG, George KP. Absence of training-specific cardiac adaptation in paraplegic athletes. Med Sci Sports Exerc. 2002;34:1699-1704. [DOI] [PubMed] [Google Scholar]
- 16.Huonker M, Schmid A, Sorichter S. Cardiovascular differences between sedentary and wheelchair-trained subjects with paraplegia. Med Sci Sports Exerc. 1998;30:609-613. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons RS, Stock CG, Andrews BJ, Gall A, Shave RE. The effect of FES-rowing training on cardiac structure and function: pilot studies in people with spinal cord injury. Spinal Cord. 2016;54:822-829. [DOI] [PubMed] [Google Scholar]
- 18.Milia R, Roberto S, Marongiu E, et al. Improvement in hemodynamic responses to metaboreflex activation after one year of training in spinal cord injured humans. Biomed Res Int. 2014;2014:893468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash MS, Bilsker MS, Kearney HM, Ramirez JN, Applegate B, Green BA. Effects of electrically-stimulated exercise and passive motion on echocardiographically-derived wall motion and cardiodynamic function in tetraplegic persons. Paraplegia. 1995;33:80-89. [DOI] [PubMed] [Google Scholar]
- 20.Phillips CA, Danopulos D, Kezdi P, Hendershot D. Muscular, respiratory and cardiovascular responses of quadriplegic persons to an F. E. S. bicycle ergometer conditioning program. Int J Rehabil Res. 1989;12:147-158. [DOI] [PubMed] [Google Scholar]
- 21.Turiel M, Sitia S, Cicala S, et al. Robotic treadmill training improves cardiovascular function in spinal cord injury patients. Int J Cardiol. 2011;149:323-329. [DOI] [PubMed] [Google Scholar]
- 22.Nash MS, Bilsker S, Marcillo AE, et al. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia. 1991;29:590-599. [DOI] [PubMed] [Google Scholar]
- 23.Ma JK, West CR, Martin Ginis KA. The effects of a patient and provider co-developed, behavioral physical activity intervention on physical activity, psychosocial predictors, and fitness in individuals with spinal cord injury: a randomized controlled trial. Sports Med. 2019;49:1117-1131. [DOI] [PubMed] [Google Scholar]
- 24.Ma JK, Cheifetz O, Todd KR, et al. Co-development of a physiotherapist-delivered physical activity intervention for adults with spinal cord injury. Spinal Cord. 2020;58:778-786. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay MS, Warburton DER, Janssen I, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011;36:36-46;47-58. [DOI] [PubMed] [Google Scholar]
- 26.Hoekstra F, McBride CB, Borisoff J, et al. Translating the international scientific spinal cord injury exercise guidelines into community and clinical practice guidelines: a Canadian evidence-informed resource. Spinal Cord. 2020;58:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Scheer JW, Hutchinson MJ, Paulson T, Martin Ginis KA, Goosey-Tolfrey VL. Reliability and validity of subjective measures of aerobic intensity in adults with spinal cord injury: a systematic review. PM R. 2018;10:194-207. [DOI] [PubMed] [Google Scholar]
- 28.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [DOI] [PubMed] [Google Scholar]
- 29.Williams AM, Shave RE, Stembridge M, Eves ND. Females have greater left ventricular twist mechanics than males during acute reductions to preload. Am J Physiol Heart Circ Physiol. 2016;311:H76-H84. [DOI] [PubMed] [Google Scholar]
- 30.van Dalen BM, Kauer F, Vletter WB, et al. Influence of cardiac shape on left ventricular twist. J Appl Physiol (1985). 2010;108:146-151. [DOI] [PubMed] [Google Scholar]
- 31.Choi HF, Rademakers FE, Claus P. Left-ventricular shape determines intramyocardial mechanical heterogeneity. Am J Physiol Heart Circ Physiol. 2011;301:H2351-H2361. [DOI] [PubMed] [Google Scholar]
- 32.Stoner L, Credeur D, Dolbow DR, Gater DR. Vascular health toolbox for spinal cord injury: recommendations for clinical practice. Atherosclerosis. 2015;243:373-382. [DOI] [PubMed] [Google Scholar]
- 33.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445-448. [DOI] [PubMed] [Google Scholar]
- 34.Currie KD, Hubli M, Krassioukov AV. Applanation tonometry: a reliable technique to assess aortic pulse wave velocity in spinal cord injury. Spinal Cord. 2014;52:272-275. [DOI] [PubMed] [Google Scholar]
- 35.Claydon VE, Hol AT, Eng JJ, Krassioukov AV. Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Arch Phys Med Rehabil. 2006;87:1106-1114. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latimer-Cheung AE, Arbour-Nicitopoulos KP, Brawley LR, et al. Developing physical activity interventions for adults with spinal cord injury. Part 2: motivational counseling and peer-mediated interventions for people intending to be active. Rehabil Psychol. 2013;58:307-315. [DOI] [PubMed] [Google Scholar]
- 38.Tordi N, Mourot L, Chapuis A, Parratte B, Regnard J. Effects of a primary rehabilitation programme on arterial vascular adaptations in an individual with paraplegia. Ann Phys Rehabil Med. 2009;52:66-73. [DOI] [PubMed] [Google Scholar]
- 39.Houtman S, Oeseburg B, Hopman MT. Blood volume and hemoglobin after spinal cord injury. Am J Phys Med Rehabil. 2000;79:260-265. [DOI] [PubMed] [Google Scholar]
- 40.Schumacher YO, Ruthardt S, Schmidt M, Ahlgrim C, Roecker K, Pottgiesser T. Total haemoglobin mass but not cardiac volume adapts to long-term endurance exercise in highly trained spinal cord injured athletes. Eur J Appl Physiol. 2009;105:779-785. [DOI] [PubMed] [Google Scholar]
- 41.Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001;82:832-839. [DOI] [PubMed] [Google Scholar]
- 42.Hopman MTE, Groothuis JT, Flendrie M, Gerrits KHL, Houtman S. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol (1985). 2002;93:1966-1972. [DOI] [PubMed] [Google Scholar]
- 43.Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord. 2007;45:49-56. [DOI] [PubMed] [Google Scholar]
- 44.Olive JL, Slade JM, Dudley GA, McCully KK. Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol (1985). 2003;94:701-708. [DOI] [PubMed] [Google Scholar]
- 45.Davis GM, Shephard RJ, Leenen FHH. Cardiac effects of short term arm crank training in paraplegics: echocardiographic evidence. Eur J Appl Physiol Occup Physiol. 1987;56:90-96. [DOI] [PubMed] [Google Scholar]
- 46.Bernardi M, Guerra E, Rodio A, et al. Assessment of exercise stroke volume and its prediction from oxygen pulse in paralympic athletes with locomotor impairments: cardiac long-term adaptations are possible. Front Physiol. 2020;10:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopman MT, Dueck C, Monroe M, Philips WT, Skinner JS. Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med. 1998;19:98-103. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs PL, Nash MS, Rusinowski JW. Circuit training provides cardiorespiratory and strength benefits in persons with paraplegia. Med Sci Sports Exerc. 2001;33:711-717. [DOI] [PubMed] [Google Scholar]
- 49.Kim DI, Lee H, Lee BS, Kim J, Jeon JY. Effects of a 6-week indoor hand-bike exercise program on health and fitness levels in people with spinal cord injury: a randomized controlled trial study. Arch Phys Med Rehabil. 2015;96:2033-2040.e1. [DOI] [PubMed] [Google Scholar]
- 50.West CR, Campbell IG, Shave RE, Romer LM. Effects of abdominal binding on cardiorespiratory function in cervical spinal cord injury. Respir Physiol Neurobiol. 2012;180:275-282. [DOI] [PubMed] [Google Scholar]
- 51.Gee CM, Williams AM, Sheel AW, Eves ND, West CR. Respiratory muscle training in athletes with cervical spinal cord injury: effects on cardiopulmonary function and exercise capacity. J Physiol. 2019;597:3673-3685. [DOI] [PubMed] [Google Scholar]
- 52.West CR, Taylor BJ, Campbell IG, Romer LM. Effects of inspiratory muscle training on exercise responses in Paralympic athletes with cervical spinal cord injury. Scand J Med Sci Sports. 2014;24:764-772. [DOI] [PubMed] [Google Scholar]
- 53.Chung AK, Das SR, Leonard D, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113:1597-1604. [DOI] [PubMed] [Google Scholar]
- 54.Cureton K, Bishop P, Hutchinson P, Newland H, Vickery S, Zwiren L. Sex difference in maximal oxygen uptake. Effect of equating haemoglobin concentration. Eur J Appl Physiol Occup Physiol. 1986;54:656-660. [DOI] [PubMed] [Google Scholar]
- 55.De Rossi G, Matos-Souza JR, Costa E, Silva ADEA, et al. Physical activity and improved diastolic function in spinal cord-injured subjects. Med Sci Sports Exerc. 2014;46:887-892. [DOI] [PubMed] [Google Scholar]
- 56.Harkema SJ, Legg Ditterline B, Wang S, et al. Epidural spinal cord stimulation training and sustained recovery of cardiovascular function in individuals with chronic cervical spinal cord injury. JAMA Neurol. 2018;75:1569-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legg Ditterline BE, Wade S, Ugiliweneza B, et al. Beneficial cardiac structural and functional adaptations after lumbosacral spinal cord epidural stimulation and task-specific interventions: a pilot study. Front Neurosci. 2020;14:554018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-nnr-10.1177_15459683211017504 for Effects of a Tailored Physical Activity Intervention on Cardiovascular Structure and Function in Individuals With Spinal Cord Injury by Alexandra M. Williams, Jasmin K. Ma, Kathleen A. Martin Ginis and Christopher R. West in Neurorehabilitation and Neural Repair


