Abstract
The low resection and high recurrence rates in hepatocellular carcinoma (HCC) are the major challenges to improving prognosis. Neoadjuvant and conversion therapies are underlying strategies to overcome these challenges. To date, no guideline or consensus has been published on the neoadjuvant and conversion therapies in HCC. Recent studies showed that neoadjuvant therapy for resectable HCC and conversion therapy for unresectable HCC are safe, feasible, and effective. Neoadjuvant and conversion therapies have the following advantages in treating HCC: R0 resection with sufficient volume of future liver remnant, relatively simple operation, and wide applicability. Therefore, it was necessary to conduct a widely accepted consensus among the experts in China who have extensive expertise and experience in treating HCC using neoadjuvant and conversion therapies, which is important to standardize the application of neoadjuvant and conversion therapies for the management of HCC. The strategies of neoadjuvant therapy include the selection of the eligible patients, therapy regimen, cycles, effect evaluations, and multidisciplinary treatment. The management of patients with insufficient volume of future liver remnant and patients who cannot achieve R0 resection is the key to the strategies of conversion therapy. Here, we present the resultant evidence- and experience-based consensus to guide the application of neoadjuvant and conversion therapies in clinical practice.
Keywords: Consensus, Hepatocellular carcinoma, Neoadjuvant therapy, Conversion therapy
Core Tip: Recent studies showed that neoadjuvant therapy for resectable hepatocellular carcinoma and conversion therapy for unresectable hepatocellular carcinoma are safe, feasible, and effective. It was necessary to conduct a widely accepted consensus among the experts in China who have extensive expertise and experience in treating hepatocellular carcinoma using neoadjuvant and conversion therapies.
INTRODUCTION
Significance of developing the consensus
Background: Liver cancer is associated with a high recurrence rate and a low resection rate, while neoadjuvant and conversion therapies are important to overcome such challenges.
Hepatectomy is the surgical resection (whole or partial removal) of the liver. Radical therapies, including liver transplantation, resection, and radiofrequency ablation, are highly appropriate for early-stage liver cancer patients [e.g., patients with stage Ia, Ib, and IIa according to the China Liver Cancer (CNLC) staging system], with a median survival of longer than 5 years[1]. Still, the short-term recurrence rate of liver cancer after surgery is relatively noticeable. The 5-year recurrence rate is as high as 70%, and the postoperative survival of the majority of patients is suboptimal[1,2]. About 64% of Chinese liver cancer patients are identified with stage CNLC-II or III [or equivalent to Barcelona Clinic Liver Cancer (BCLC) stage B or C] at the time of diagnosis, and surgical resection is not their first therapeutic option[1,3,4]. For patients with intermediate or advanced liver cancer, surgery following neoadjuvant or conversion therapy could result in higher treatment efficacy[1,5-7]. A previous study that analyzed the surgical data of 10966 patients with primary hepatocellular carcinoma showed that several patients with stages IIb-IIIa could still benefit from surgical resection, whereas the 5-year recurrence rate was as high as 80%[8] (Table 1). Therefore, how to reduce the postoperative recurrence rate and increase the radical resection rate of liver cancer is critical for improving patients’ prognoses. Therefore, neoadjuvant and conversion therapies for liver cancer have been developed to tackle such challenges, which could increase the disease-free survival (DFS) rate.
Table 1.
Analysis of surgical data of 10966 patients with primary hepatocellular carcinoma
| Stages and criteria | n (%) |
Overall survival
|
Tumor recurrence
|
||||||||
|
Median time (mo)
|
1-yr survival rate (%)
|
3-yr survival rate (%)
|
5-yr survival rate (%)
|
10-yr survival rate (%)
|
Median time (mo)
|
1-yr recurrence rate (%)
|
2-yr recurrence rate (%)
|
3-yr recurrence rate (%)
|
5-yr recurrence rate (%)
|
||
| First round of hepatectomy for HCC | 2592 | 79.4 | 87.2 | 71.0 | 59.1 | 40.4 | 36.1 | 31.8 | 43.5 | 9.9 | 60.7 |
| Stages Ia-IIIa | 2549 | 82.3 | 88.1 | 71.9 | 60.0 | 41.0 | 37.0 | 31.0 | 42.9 | 49.3 | 60.3 |
| Ia | 1175 | - | 96.5 | 87.5 | 77.2 | 55.9 | 67.2 | 15.0 | 27.7 | 34.1 | 45.9 |
| Ib | 635 | 79.4 | 88.2 | 73.5 | 62.5 | 37.0 | 34.1 | 32.4 | 43.7 | 51.8 | 63.4 |
| IIa | 205 | 43.5 | 89.8 | 56.0 | 40.8 | 27.2 | 15.7 | 45.7 | 59.8 | 65.5 | 84.3 |
| IIb | 119 | 38.0 | 83.6 | 55.3 | 37.4 | 23.2 | 10.0 | 55.9 | 67.7 | 74.0 | 80.1 |
| IIIa | 415 | 21.9 | 65.1 | 38.2 | 23.8 | 16.0 | 7.9 | 59.9 | 69.8 | 73.8 | 80.2 |
| IIIb | 43 | 8.7 | 34.1 | 15.8 | 0 | 0 | 3.9 | 77.1 | 77.1 | 82.8 | - |
Overall survival and tumor recurrence of patients with different stages of hepatocellular carcinoma after undergoing hepatectomy[8]. Stages and criteria; overall survival; tumor recurrence; n; median time (months); 1-yr survival rate (%); 3-yr survival rate (%); 5-yr survival rate (%); 10-yr survival rate (%); median time (months); 1-yr recurrence rate (%); 2-yr recurrence rate (%); 3-yr recurrence rate (%); 5-yr recurrence rate (%); first round of hepatectomy for hepatocellular carcinoma; stages Ia-IIIa. HCC: Hepatocellular carcinoma.
Neoadjuvant and conversion therapies need to be standardized for liver cancer treatment
Neoadjuvant and conversion therapies are already widely applied to treat colorectal cancer, but owing to several clinical and non-clinical barriers, they have not been globally popularized for the treatment of liver cancer[9-11]. To date, the principles and functions of neoadjuvant and conversion therapies have not been fully clarified, and there are still controversies regarding the selection of patients for receiving neoadjuvant or conversion therapy in clinical practice. In addition, variations in treatment targets have also resulted in differences in selecting an appropriate treatment method, treatment duration, and postoperative criteria for follow-up[12]. Several scholars have conducted preliminary studies on neoadjuvant and conversion therapies for liver cancer, while the lack of high-grade evidence and absence of universally acknowledged standard treatments have restricted the popularization of those therapies[2].
Primary objective and significance of developing the present consensus
The Chinese Expert Consensus on Conversion Therapy for Hepatocellular Carcinoma (2021 edition) published in June 2021 emphasized the broad application of conversion therapy. It provided a reliable basis for the clinical application of conversion therapy. In order to further elucidate the mechanisms of neoadjuvant and conversion therapies and to clarify the treatment targets for different groups of patients, our research group attempted to develop the Chinese Expert Consensus on Neoadjuvant and Conversion Therapies for Hepatocellular Carcinoma (2021 edition) to provide additional reliable suggestions for preoperative decision making according to the features of diagnosing and treating liver cancer in China and to standardize those therapeutic methods for their universal popularization.
CONCEPTS AND TARGETS OF NEOADJUVANT AND CONVERSION THERAPIES
According to the treatment targets, preoperative treatments for liver cancer include neoadjuvant therapy for resectable liver cancer and conversion therapy for unresectable liver cancer. These two treatments are distinguished by the achievement of R0 resection and are different from the aspects of study subjects, treatment targets, and treatment regimens.
Neoadjuvant therapy
Neoadjuvant therapy refers to the interventions including systemic or local treatments for liver cancer patients with technically resectable tumors [R0 resection, with sufficient volume of the future liver remnant (FLR)] and a high risk of recurrence, which could reduce the tumor size, eliminate the undetectable minimal lesions, and increase the negative surgical margins as early as possible, thereby attenuating the incidence of postoperative complications[12].
At present, only limited high-grade evidence for treating liver cancer via neoadjuvant therapy is available. Generally, in clinical practice, neoadjuvant therapy is not directly recommended for patients with CNLC stage Ia or Ib and some patients with CNLC stage IIa. However, because such patients are accompanied by a high risk of recurrence and require neoadjuvant therapy, the therapy can be performed in clinical trials after approval of the study protocol by ethic committees. There are substantial controversies regarding whether surgeries can be directly performed for patients with CNLC stage IIb or IIIa (i.e. the resection is technically applicable). The current recommendation is to benefit surgery after neoadjuvant therapy to reduce the postoperative recurrence rate.
Conversion therapy
Unresectable liver cancer refers to liver cancer that could not be safely resected, including intolerable liver functions, insufficient volume of FLR, and disability to ensure the negative surgical margins or zero residual lesion (i.e. achieving R0 resection).
Conversion therapy refers to the use of interventions to convert unresectable tumors into resectable ones, which includes the conversion of surgically unresectable lesions, such as insufficient volume of FLR, to resectable lesions, and it supports the conversion of R1 and R2 resection to R0 resection.
According to the Chinese Expert Consensus on Neoadjuvant and Conversion Therapies for Hepatocellular Carcinoma (2021 edition), existing evidence supports conversion therapy, which can be applied in clinical practice after a comprehensive evaluation of patients’ clinical conditions.
STRATEGIES FOR CONDUCTING NEOADJUVANT THERAPY
Eligible patients for neoadjuvant therapy
Neoadjuvant therapy aims to reduce cancer recurrence. According to the Chinese guidelines, neoadjuvant therapy can be performed for patients with initially resectable liver cancer (including CNLC stage Ia-IIIa/BCLC stage A, or beyond the BCLC criteria, while being still resectable) and for those patients who are at a high risk of postoperative recurrence. As the high-grade evidence for neoadjuvant liver cancer therapy is limited, neoadjuvant therapy is not recommended for patients with CNLC stage Ia or Ib and for some patients with stage IIa (i.e. R0 resection can be directly achieved). However, if the comprehensive evaluation of the patients’ clinical conditions (i.e. patients at high risk of postoperative recurrence and uncertainty for R0 resection) suggests that neoadjuvant therapy can be performed, the therapy is recommended to be conducted in clinical trials after ethical approval. For patients with technically resectable CNLC stage IIb or IIIa liver cancer, while those are at a high risk of recurrence, neoadjuvant therapy is recommended preoperatively to reduce the incidence of postoperative recurrence.
The documented high risk of liver cancer recurrence includes macroscopic cancer embolus, microvascular invasion, multiple tumors, satellite nodules, and lymph node metastases[13]. With the elevation of the CNLC stage, the risk of recurrence and metastasis after surgical resection also increases[2]. Therefore, multiple examinations should be carried out preoperatively to evaluate the risk of postoperative recurrence. For patients at a high risk of postoperative recurrence, neoadjuvant therapy could control the development of metastatic cancer lesions and improve patients’ prognoses with adequate resection margins.
Cycles of neoadjuvant therapy
During neoadjuvant therapy, the patients might not be eligible for surgery due to contraindications to surgery, such as disease progression, postoperative toxicity, and other severe adverse effects[2]. Therefore, the management of preoperative cycles of neoadjuvant therapy is critical to ensure that the treatment targets can be met within a limited period, thereby minimizing the “failure rate” of neoadjuvant therapy.
It is recommended that the duration of neoadjuvant therapy should be 1.5-3 mo (no longer than 4 mo), and surgery should be performed as early as possible after the treatment targets have been met (regardless of the regression of lesions)[2,14]. An appropriate individualized treatment regimen can be selected according to the lesions’ locations, general conditions, and hepatic function reserve. Importantly, safe treatment methods are vital to avoid negative influences on the upcoming surgeries (Table 2).
Table 2.
Evidence of studies on the neoadjuvant therapy
|
Treatment regimen
|
Study design
|
Number of patients
|
Treatment cycle
|
Study outcomes
|
| Apatinib + camrelizumab[45] | Phase II | 20 | 6 wk | MPR: 29.4%. PCR: 5.9% |
| Cabozantinib + nivolumab[46] | Phase I | 15 | 8 wk | 12 patients received R0 resection, and MPR or PCR was found in 5 patients (41.7%) |
| Toripalimab ± lenvatinib | Phase Ib/II | 16 | 21-28 d | 3 patients (20%) with MPR |
| Ipilimumab + nivolumab[47] | Phase Ib | 7 | 6 wk | ORR of 20%; of the 5 patients with pathologically assessable tumors, 3 (60%) were found with pathological remission |
| Ipilimumab ± nivolumab[48] | Phase II | 30 | 6 wk | Pathological remission rate: 30% (8/27), MPR: 11% (3/27), PCR: 19% (5/27) |
MPR: Major pathological response; PCR: Pathological complete response; ORR: Objective remission rate.
Methods of neoadjuvant therapy
Interventional therapy: Transarterial chemoembolization (TACE): Various studies have shown that preoperative TACE does not improve the survival of patients with resectable liver cancer[15-17]. In a meta-analysis by Qi et al[18], 22 randomized and non-randomized studies were included. Their findings showed that preoperative TACE does not improve patient DFS or overall survival (OS). Subgroup analyses showed that the effects of surgery after neoadjuvant TACE were associated with the responses to TACE[18].
Hepatic arterial infusion chemotherapy (HAIC): An embolic agent is not used in the FOLFOX-based HAIC treatment. Thus, the treatment only induces relatively mild inflammatory responses, and certain advancements in the field of neoadjuvant therapy have been achieved[19]. In a Chinese phase III clinical trial, neoadjuvant HAIC was used to treat BCLC stage A/B liver cancer patients that exceeded the Milan criteria for liver transplantation, and it showed that the pathologically complete remission rate was 10.1% and objective remission rate was 63.6%, according to the Modified Response Evaluation Criteria in Solid Tumors criteria. Compared with the direct resection group (n = 100), the OS and progression-free survival in the neoadjuvant HAIC group (n = 99) both improved significantly (3-year OS rate: 63.5% vs 46.3%, P = 0.016; median progression-free survival: 14.1 mo vs 8.9 mo, P = 0.017), while the recurrence-free survival was not significantly different between the two groups[20] (grade of evidence: 2A). Another retrospective study showed that neoadjuvant HAIC reduced the risk of recurrence in patients at a high risk of hepatocellular carcinoma and improved patient survival rates. Compared with the control group, the 1-, 3-, and 5-year DFS rates (100%, 78.6%, and 78.6% vs 65.8%, 33.7%, and 26.6%, respectively, P = 0.003) and OS rate (100%, 100%, and 100% vs 91.7%, 77.8%, and 55.3%, respectively, P = 0.037) in the neoadjuvant HAIC group were significantly higher[21] (grade of evidence: 2B).
Radiotherapy: In a Chinese randomized controlled trial, the patients were randomized to hepatectomy plus neoadjuvant radiotherapy (n = 82) or hepatectomy alone (n = 82). The trial showed that for patients with resectable tumors accompanied with portal vein tumor thrombus, preoperative three-dimensional conformal radiotherapy could effectively improve the postoperative survival of the patients. Regarding patients in the hepatectomy plus neoadjuvant radiotherapy group, partial remission was achieved in 17 (20.7%) patients, and the 6-, 12-, 18-, and 24-mo OS rates were 89.0%, 75.2%, 43.9%, and 27.4% vs 81.7%, 43.1%, 16.7%, and 9.4% in the hepatectomy alone group, respectively (P < 0.001). In patients in the hepatectomy plus neoadjuvant radiotherapy group, the 6-, 12-, 18-, and 24-mo DFS rates were 56.9%, 33.0%, 20.3%, and 13.3% vs 42.1%, 14.9%, 5.0%, and 3.3% in the hepatectomy alone group, respectively (P < 0.001)[22] (grade of evidence: 2A).
In a Chinese retrospective study, 11920 patients (of whom 134 received neoadjuvant radiotherapy) were included. The adjusted 5-year OS rates in the neoadjuvant radiotherapy and surgery groups were 65.3% and 46.6%, respectively. The results of the adjusted Cox proportional-hazards regression analysis showed that neoadjuvant radiotherapy was significantly associated with longer OS (hazard ratio: 0.549; 95% confidence interval: 0.327-0.921; P = 0.023). In addition, the subgroup analysis showed that patients with N0 disease, alpha-fetoprotein-positive, and aged < 65-years-old could benefit more from the therapy[23] (grade of evidence: 2B).
Another Chinese retrospective study in patients with resectable liver cancer showed that neoadjuvant radiotherapy was associated with long-term patient survival[24] (grade of evidence: 2B).
Systemic therapy: The increasingly abundant systemic therapies have provided new ideas for neoadjuvant therapy of liver cancer. Several studies reported preliminary data regarding applying systemic therapy in neoadjuvant therapy. At the same time, the postoperative consequences of the treatment regimens need to be further verified by large-scale clinical studies.
Recommendations of the consensus
At present, the lack of high-grade evidence for neoadjuvant therapy is noteworthy. In addition, all patients will not benefit from neoadjuvant therapy[2]. In clinical practice, directly performing neoadjuvant therapy is not recommended for patients with early-stage liver cancer. Assessment of data by multidisciplinary treatment (MDT) is necessary to predict the risk of postoperative recurrence and metastasis and explore whether patients could benefit from neoadjuvant therapy[2]. When the main objective of neoadjuvant therapy is to reduce the risk of recurrence, the treatment cycles are strictly limited, and relatively safe treatment regimens with fewer adverse effects on the surgery are preferred.
STRATEGIES FOR CONDUCTING CONVERSION THERAPY
The main objective of conversion therapy is to eliminate the unresectable factors of liver cancer, thereby satisfying the surgical criteria for safely performing R0 resection.
Specifically, conversion therapy is characterized by active treatment regimens to convert risky surgeries into safe surgeries and change unresectable tumors into radically resectable tumors.
The cycles of conversion therapy could be longer than neoadjuvant therapy to meet the treatment targets, and the treatment duration is not strictly limited. If patients cannot tolerate the therapy or cannot alternatively undergo radical surgeries, the treatment regimen needs to be adjusted after a comprehensive evaluation.
Conversion therapy for patients with insufficient volume of FLR
Patients receiving conversion therapy for an insufficient volume of FLR: The conversion therapy for an insufficient volume of FLR aims to eliminate the unresectable factors from the surgical aspect. Therefore, the target group is the CNLC grade Ia-IIIb liver cancer patients with insufficient volume of FLR after radical therapy.
At present, the criteria for evaluating the functional hepatic reserve for safe resection are generally identical in various centers worldwide. The criteria are as follows: For patients with normal liver function (Child-Pugh class A), indocyanine green-R15 < 10%, and without liver cirrhosis, the FLR/standard liver volume (SLV) needs to be > 20%-30%; for patients accompanied with chronic liver diseases or hepatic parenchyma injuries (e.g., liver cirrhosis, severe fatty liver, and chemotherapy-induced liver injury), the FLR/SLV needs to be > 40%; for patients with liver dysfunction, a higher FLR is required (e.g., for patients with chronic liver diseases or cirrhosis and IGG-R15 of 10%-20%, the FLR/SLV needs to be > 50%)[1,4]. Patients who do not meet these criteria are considered with insufficient volume of FLR.
Considering the contraindications or complications, the treatments should be strictly limited to the following patients: Aged < 65-years-old, with normal liver function (Child-Pugh class A, indocyanine green-R15 < 10%), with insufficient volume of FLR (e.g., FLR/SLV < 30% for patients with normal liver and FLR/SLV < 40% for patients with chronic liver diseases or liver damages), in good general conditions, with good tolerability to surgery, without severe liver cirrhosis, severe fatty liver, or severe portal hypertension[1].
How to reach the target of conversion therapy for an insufficient volume of FLR: Insufficient volume of FLR after tumor resection can lead to an extremely high risk of postoperative liver failure[25]. Therefore, the conversion therapy for an insufficient volume of FLR should reach the following treatment targets: Using specific methods to promote the rapid increase of liver volume and convert the insufficient volume of FLR to a sufficient volume of FLR. After meeting the requirements of hepatectomy, the conversion therapy should convert the liver cancer from unresectable to safely resectable (i.e. converting a risky surgery to a safe surgery).
At present, the treatments for insufficient FLR volume include a two-stage hepatectomy procedure combined with portal vein embolization or portal vein ligation, a two-stage hepatectomy procedure combined with portal vein embolization and TACE/hepatic vein embolization, and associating liver partition and portal vein ligation for staged hepatectomy[4]. In addition, the insufficient FLR volume in some patients is caused by giant tumors, and a one-stage radical resection can substantially influence the FLR volume (e.g., possibly damaging blood vessels or bile ducts)[4]. For such patients, a non-surgical therapy (e.g., local therapy or systemic therapy plus local therapy) is effective in reducing tumor size, thereby decreasing the range of resection and increasing the FLR volume [for more details, please refer to section 4.2 (Conversion therapy for patients who are unable to achieve R0 resection)].
For detailed recommendations on selecting appropriate treatment strategies and perioperative management, please refer to the Chinese Expert Consensus on Conversion Therapy in Hepatocellular Carcinoma (2021 edition). The appropriate treatment methods should be selected according to the tumor type, status of local tumor progression, pathological finding of liver parenchyma, liver functional reserve, and patient tolerability to systemic surgery.
Conversion therapy for patients who are unable to achieve R0 resection
Patients receiving conversion therapy for being incapable of R0 resection: The target patients of the conversion treatment include patients who cannot initially achieve R0 resection due to the tumor burden. Evidence suggests that the efficacy of liver tumor resection in patients who cannot achieve an R0 resection is not significantly higher than non-surgical treatment[7,26-28]. For patients who cannot achieve R0 resection due to causes such as an extremely large tumor volume or blood vessel invasion, conversion therapy can be performed to provide a surgical opportunity for R0 resection, thereby improving long-term efficacy[27].
Reaching the target of conversion therapy for patients who cannot achieve R0 resection: The overall target is to decrease the tumor volume and tumor stage (i.e. reducing the volume and number of primary lesions and eliminating portal vein tumor thrombus and metastatic lesions), leading to radical resection[29,30].
The criteria of diagnosing resectable lesions after conversion therapy are as follows: Reduction of tumor volume or tumor stage, complete microvascular tumor thrombus necrosis, evaluation of complete/partial remission, and stable disease lasting for 3-4 mo (according to Modified Response Evaluation Criteria in Solid Tumors criteria)[1]. The details of different conditions are as follows: (1) Intrahepatic lesions: For patients with a giant tumor or a remarkable number of lesions, surgery is risky or is accompanied by surgical difficulties, and the target of a successful conversion is to reduce the number of lesions, ensuring negative surgical margins and decreasing surgical difficulty, according to the Modified Response Evaluation Criteria in Solid Tumors criteria. For patients with vascular invasion who cannot achieve R0 resection, the target of a successful conversion is spontaneous necrosis or regression of malignant tumors, complete microvascular tumor thrombus necrosis, and reduction of tumor stage (e.g., from CNLC stage IIIa to IIb); and (2) Extrahepatic metastasis: For patients with extrahepatic (mainly lung) metastasis (CNLB stage IIIb), the target of a successful conversion is to eliminate the metastatic lesions, reduce the tumor stage, and perform the surgical resection of the intrahepatic lesions.
To date, numerous explorative studies on conversion therapy have been performed, of which the treatment regimens include local therapy[31-36], systemic therapy[37], and combination therapy[38-42] (Table 3). Concerning the absence of a potent systemic treatment, TACE is the major method for conversion therapy[1]. Compared with traditional TACE, drug-eluting bead-TACE continuously releases chemotherapeutic drugs at a fixed dose with outstanding controllability, prolonging the treatment time between the cancer cells and the chemotherapeutic drugs as well as avoiding liver microcirculation injury. A Chinese cohort study on 32 patients with unresectable hepatocellular carcinoma showed that after drug-eluting bead-TACE treatment, the success rate of stage reduction was 59.4%; however, after subsequent radical therapy (surgery or ablation), the rate of complete remission was as high as 81.3%. The successful stage reduction using drug-eluting bead-TACE is associated with longer survival[43]. Several scholars demonstrated the effects of HAIC, selective internal radiation therapy, and radiotherapy for conversion therapy[1]. The rapid advances in systemic therapy also provide new ideas for conversion therapy. A great number of studies are currently available to support the application of systemic therapy plus local therapy in conversion therapy (Supplementary Tables 1 and 2). We recommend that a gradually progressing treatment strategy be performed according to the currently available treatment standards, taking both efficacy and safety of treatment into account. For detailed recommendations on selecting treatment methods and timing of surgical resection following conversion therapy, please refer to the Chinese Expert Consensus on Conversion Therapy in Hepatocellular Carcinoma (2021 edition)[1].
Table 3.
Evidence for systemic therapy plus local therapy in conversion therapy
|
Ref.
|
Treatment regimen
|
Number of patients, n (%)
|
Study results
|
| Zhang et al[37], 2020 | TKI: Lenvatinib. PD-1 antibody: Pembrolizumab/Sintilimab/Toripalimab | 33 | Success rate of conversion (imaging): 42.4%. Actual surgery rate following conversion: 30.3% |
| Li et al[40], 2017 | TACE + sorafenib | 142 | Second-stage resection rate following stage reduction: 14.8% |
| He et al[39], 2019 | HAIC + sorafenib | 125 | Surgical resection rate following conversion: 12.8% |
| He et al[41], 2018 | HAIC + sorafenib | 35 | Surgical resection rate following conversion: 14.3% |
| Zhang et al[42], 2021 | HAIC + TKI + PD-1 antibody (1, 7, and 17 patients used sorafenib, apatinib, and lenvatinib, respectively) | 25 | Surgical resection rate following conversion: 56.0%, 7 patients (28.0%) achieved pathologically complete remission |
| He et al[38], 2021 | Lenvatinib + toripalimab + HAIC | 71 | Surgical resection rate following conversion: 12.7% |
Grade of evidence in the guideline of Chinese Society of Clinical Oncology diagnosis and treatment. Feature of evidence; grade; level; sources; Chinese Society of Clinical Oncology degree of expert consensus; 1A: High, rigorous meta-analysis, large-scale randomized clinical study, unified consensus (supportive opinion: ≥ 80%); 1B: High, rigorous meta-analysis, large-scale randomized clinical study, generally unified consensus, with slight controversy (supportive opinion: 60%-80%); 2A: Slightly low, fair-quality meta-analysis, generally unified consensus, with slight controversy (supportive opinion: 60%-80%) or small-scale randomized clinical study, well-designed large-scale retrospective study, case-control study, unified consensus (supportive opinion: ≥ 80%); 2B: Slightly low, fair-quality meta-analysis, small-scale randomized clinical study, well-designed large-scale air-quality meta-analysis, generally unified consensus, with slight controversy (supportive opinion: 60%-80%) or small-scale randomized clinical study, well-designed large-scale retrospective study, case-control retrospective study, case-control study; generally unified consensus, with slight controversy (supportive opinion: 60%-80%); 3: Low, non-controlled single-arm clinical study, case report, expert opinion, no consensus, with low substantial controversy (supportive opinion: < 60%). TKI: Tyrosine kinase inhibitor; PD-1: Programmed cell death protein-1; TACE: Transarterial chemoembolization; HAIC: Hepatic arterial infusion chemotherapy.
NECESSITY OF MDT FOR NEOADJUVANT AND CONVERSION THERAPIES
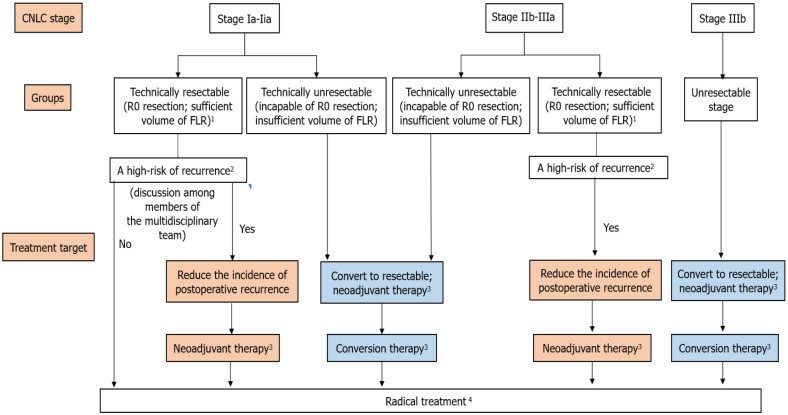
Due to the heterogeneity of liver cancer and the features of the multi-modal MDT, MDT is essential for neoadjuvant and conversion therapies. Therefore, it is important to form a relatively constant multidisciplinary team to make an individualized treatment decision for liver cancer patients. Before starting a neoadjuvant therapy, preoperative evaluation by a multidisciplinary team is required to predict the risk of postoperative recurrence and metastasis and to indicate whether patients can benefit from the neoadjuvant therapy. During the neoadjuvant and conversion therapies, the treatment methods and measurements, the timing of surgery after treatment, and management of adverse events during the treatment need to be discussed repeatedly among the multidisciplinary team members due to the advantages and disadvantages of different treatment regimens (Figure 1). After a treatment regimen is selected, discussion among the multidisciplinary team members should be regularly carried out, which could ensure the adjustment of treatment regimens according to the changes of disease conditions, enabling patients to benefit from the advantages of the treatment.
Figure 1.
Algorithm of neoadjuvant and conversion therapies for liver cancer. 1Technically resectable criteria: R0 resection, sufficient volume of the future liver remnant, Child-Pugh class A/B (some patients)[1]. 2Comprehensive evaluation by preoperative imaging examination findings and serum levels of biomarkers should be performed to assess the risk of postoperative recurrence[44]. Postoperative recurrence risk factors: (1) For patients with stage Ia, Ib, and IIa liver cancer, unclear tumor boundary, closely adjacent of the tumor to blood vessels, and highly suspicious residual tumors are among the high-risk factors of recurrence; (2) For patients with stage IIb-IIIa liver cancer, the high-risk factors of recurrence include the number of tumors ≥ 3, tumor diameter > 5 cm, satellite nodules, macroscopic cancer emboli, microvascular invasion-positive, lymph node metastasis, invasion of adjacent organs, and high alpha-fetoprotein level before surgery[13,44]; and (3) Other recurrence factors include liver diseases (e.g., viral hepatitis and liver cirrhosis)[13]. 3(1) Stage Ia-IIa: Neoadjuvant therapy is not recommended in clinical practice; if the multidisciplinary treatment clarifies the high risk of postoperative recurrence, neoadjuvant therapy can be performed in clinical trials after ethical approval; (2) Stage IIb-IIIa: Radical treatment following neoadjuvant therapy is recommended for patients with technically resectable liver cancer and high risk of recurrence, aiming to reduce the postoperative recurrence; and (3) For patients with unresectable liver cancer who are incapable of R0 resection or insufficient volume of future liver remnant (FLR), conversion therapy can be conducted to eliminate unresectable hepatic tumors. 4Radical therapies, including liver transplantation, resection, and radiofrequency ablation, are highly appropriate for early-stage liver cancer patients. Liver transplantation: Patients who meet the Milan criteria or the University of California San Francisco (UCSF) criteria after preoperative treatment can be treated with liver transplantation. (1) Milan criteria: Diameter of a single tumor ≤ 5 cm, the number of tumors ≤ 3, in which the diameter of the largest tumor was ≤ 3 cm, and without large blood vessel or lymph node invasion; and (2) The University of California San Francisco criteria: Diameter of a single tumor is ≤ 6.5 cm, the number of tumors ≤ 3, in which the diameter of the largest tumor was ≤ 4.5 cm, and the sum of diameters of all tumors was ≤ 8.0 cm, without a large blood vessel or lymph node invasion[7]. Radiofrequency ablation: Patients reached China Liver Cancer (CNLC) stage Ia or Ib (e.g., a single tumor, the diameter of tumor ≤ 5 cm; or with 2-3 tumors, in which the largest diameter was ≤ 3 cm) after preoperative therapy or without blood vessel, bile duct, and adjacent organ invasion, without distal metastasis, and liver function of Child-Pugh class A/B could be treated by radiofrequency ablation, which can also achieve the effects of radical treatment[7].
FUTURE PROSPECTS
Characteristics of patients requiring neoadjuvant therapy or conversion therapy
The recommended treatment methods are as follows: (1) Surgical resection: It should be performed directly if the patients meet the criteria for R0 resection; (2) Conversion therapy: If patients are eligible for R0 resection according to the defined criteria, conversion therapy can be conducted to eliminate the unresectable hepatic tumors; and (3) Neoadjuvant therapy: Owing to the lack of evidence for neoadjuvant therapy, it should be performed in clinical practice; alternatively, patients can receive immunotherapy, neoadjuvant HAIC therapy, or radiotherapy based on sufficient evidence from discussion performed among members of the multidisciplinary team. Patients’ clinical characteristics are worthy of further investigation by experienced clinicians.
CONCLUSION
Neoadjuvant therapy and conversion therapy are important strategies for the preoperative treatment of intermediate or advanced liver cancer. Consensus on conversion therapy has already been achieved, while further evidence for neoadjuvant therapy needs to be presented. With the development of treatment methods, many high-quality clinical trials are in progress, providing further evidence-based support for neoadjuvant therapy. Based on the consensus, Chinese scholars’ explorations will scientifically support theories and methods for preoperative liver cancer treatment, thereby improving Chinese liver cancer patient OS.
Footnotes
Conflict-of-interest statement: The author has nothing to disclose.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 16, 2021
First decision: October 16, 2021
Article in press: December 8, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carey I, Thiam A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
Contributor Information
Hai-Tao Zhao, Department of Hepatobiliary Surgery, Peking Union Medical College Hospital, Beijing 100021, China.
Jian-Qiang Cai, Department of Hepatobiliary Surgery, Cancer Hospital Chinese Academy of Medical Sciences, Beijing 100021, China. caijianqiang2021@163.com.
References
- 1.Sun H, Xie Q, Jia W, Zhao M, Liu X, Bi X, Li G, Bai X, Ji Y, Xu L, Wang Z, Zhu X. Chinese expert consensus on translational therapies for liver cancer (2021 Edition) Zhongguo Shi Yong Waike Zazhi . 2021;41:618–632. [Google Scholar]
- 2.Xiao Y, Guo L, Zhou J. Advances in neoadjuvant therapy for primary liver cancer. Fubu Waike. 2021;34:1–3+9. [Google Scholar]
- 3.Zhang ZF, Luo YJ, Lu Q, Dai SX, Sha WH. Conversion therapy and suitable timing for subsequent salvage surgery for initially unresectable hepatocellular carcinoma: What is new? World J Clin Cases. 2018;6:259–273. doi: 10.12998/wjcc.v6.i9.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Peng Y, Wang Z. Surgical treatment controversies and consensus for remnant liver cancer. Zhongguo Shi Yong Waike Zazhi. 2018;38:126–132. [Google Scholar]
- 5.Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, Aikata H, Nagata Y, Chayama K, Ohdan H. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg. 2017;44:223–228. doi: 10.1016/j.ijsu.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 6.Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han KH, Choi JS. Downstaging with Localized Concurrent Chemoradiotherapy Can Identify Optimal Surgical Candidates in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Ann Surg Oncol. 2018;25:3308–3315. doi: 10.1245/s10434-018-6653-9. [DOI] [PubMed] [Google Scholar]
- 7.Code for diagnosis and treatment of primary liver cancer (2019 Edition) Chuan Ran Bing Xinxi. 2020;33:481–500. [Google Scholar]
- 8.Xia Y, Zhang F, Li X, Kong L, Zhang H, Li D, Cheng F, Pu L, Zhang C, Qian X, Wang P, Wang K, Wu Z, Lv L, Rao J, Wu X, Yao A, Shao W, Fan Y, You W, Dai X, Qin J, Li M, Zhu Q, Wang X. Surgical treatment of primary liver cancer: an analysis of 10 966 cases. Zhonghua Waike Zazhi . 2021;59:E003–E003. doi: 10.3760/cma.j.cn112139-20201110-00791. [DOI] [PubMed] [Google Scholar]
- 9.Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704–3721. doi: 10.3748/wjg.v25.i28.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Zhang L, Xu Y, Lu X, Zhao H, Yang H, Sang X. Neoadjuvant therapy and immunotherapy strategies for hepatocellular carcinoma. Am J Cancer Res. 2020;10:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 11.Chinese guidelines for the diagnosis and comprehensive treatment of liver metastases from colorectal cancer (2020 Edition) Linchuang Gan Dan Zazhi. 2021;37:543–553. [Google Scholar]
- 12.Yuan S, Zhou W. Advances and hotspots for integrative therapies for primary liver cancer. Zhonghua Xiaohua Waike Zazhi. 2021;20:163–170. [Google Scholar]
- 13.Wen T, Jin C, Facciorusso A, Donadon M, Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, Du S, Jia C, Xu F, Shi J, Sun J, Zhu P, Nara S, Millis JM MDT of West China Hospital*. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7:353–371. doi: 10.21037/hbsn.2018.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su YY, Li CC, Lin YJ, Hsu C. Adjuvant vs Neoadjuvant Immunotherapy for Hepatocellular Carcinoma: Clinical and Immunologic Perspectives. Semin Liver Dis. 2021;41:263–276. doi: 10.1055/s-0041-1730949. [DOI] [PubMed] [Google Scholar]
- 15.Si T, Chen Y, Ma D, Gong X, Yang K, Guan R, Peng C. Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma in Asia area: a meta-analysis of random controlled trials. Scand J Gastroenterol. 2016;51:1512–1519. doi: 10.1080/00365521.2016.1216588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jianyong L, Jinjing Z, Wentao W, Lunan Y, Qiao Z, Bo L, Tianfu W, Minqing X, Jiaying Y, Yongang W. Preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: a single center analysis. Ann Hepatol. 2014;13:394–402. [PubMed] [Google Scholar]
- 17.Shi HY, Wang SN, Wang SC, Chuang SC, Chen CM, Lee KT. Preoperative transarterial chemoembolization and resection for hepatocellular carcinoma: a nationwide Taiwan database analysis of long-term outcome predictors. J Surg Oncol. 2014;109:487–493. doi: 10.1002/jso.23521. [DOI] [PubMed] [Google Scholar]
- 18.Qi X, Liu L, Wang D, Li H, Su C, Guo X. Hepatic resection alone vs in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:36838–36859. doi: 10.18632/oncotarget.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association LcSCoCa. Hepatic arterial infusion chemotherapy for hepatocellular carcinoma Chinese expert consensus (2021 Edition) Zhonghua Xiaohua Waike Zazhi. 2021;20:754–759. [Google Scholar]
- 20.Li SH, Zhong C, Li Q, Zou JW, Wang QX, Shang CZ. Neoadjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of resectable BCLC stage A/B hepatocellular carcinoma patients beyond Milan criteria: An interim analysis of a multi-center, phase 3, randomized, controlled clinical trial. J Clin Oncol . 2021 [Google Scholar]
- 21.Tsutsui R, Nagamatsu H, Itano O, Deguchi A, Tsutsumi T, Hiraki M, ea Neoadjuvant hepatic arterial infusion chemotherapy for resectable hepatocellular carcinomas. Hepatoma Resear. 2018;4:13. [Google Scholar]
- 22.Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol. 2019;37:2141–2151. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo ZW, Che X, Cai JQ, Zhao H, Jin J, Tang Y. Neoadjuvant radiotherapy to improve overall survival in resectable hepatocellular carcinoma. J Clin Oncol. 2021 [Google Scholar]
- 24.Lin H, Li X, Liu Y, Hu Y. Neoadjuvant radiotherapy provided survival benefit compared to adjuvant radiotherapy for hepatocellular carcinoma. ANZ J Surg. 2018;88:E718–E724. doi: 10.1111/ans.14387. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Xiao Y, Li H. Primary modality and evaluation of conversion therapy for initial unresectable liver cancer. Zhongguo Shiyong Waike Zazhi . 2021;41:280–284. [Google Scholar]
- 26.Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, Zhang YJ, Meng Y, Yang YF, Cong WM, Wu MC, Lau WY, Cheng SQ. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine (Baltimore) 2016;95:e3015. doi: 10.1097/MD.0000000000003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuan H. Translational resection for unresectable and moderately advanced HCC. Fubu Waike . 2021;34:85–87. [Google Scholar]
- 28.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang B, Li L. Surgical strategies and perspectives for downstaging in advanced and middle-stage HCC. Zhongguo Aizheng Fangzhi Zazhi . 2019;11:359–362. [Google Scholar]
- 30.Yuan X. Downstaging transformation therapy for middle - and advanced stage primary liver cancer. Linchuang Gan Dan Bing Zazhi . 2020;36:267–271. [Google Scholar]
- 31.Orlacchio A, Chegai F, Merolla S, Francioso S, Giudice CD, Angelico M, Tisone G, Simonetti G. Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: Strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J Hepatol. 2015;7:1694–1700. doi: 10.4254/wjh.v7.i12.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, Zhang Z, Zheng L, Deng H, Li S, Xie Q, Guo R, Shi M, Xu L, Cai X, Wu P, Zhao M. Hepatic arterial infusion of oxaliplatin plus fluorouracil/Leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69:60–69. doi: 10.1016/j.jhep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, Zhou J, Qiu SJ, Lu JZ. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674–678. doi: 10.1159/000018676. [DOI] [PubMed] [Google Scholar]
- 34.Goto Y, Hisaka T, Sakai H, Takagi K, Fukutomi S, Akagi Y, Okuda K. Salvage Surgery for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Downstaged by Hepatic Arterial Infusion Chemotherapy. Anticancer Res. 2020;40:4773–4777. doi: 10.21873/anticanres.14479. [DOI] [PubMed] [Google Scholar]
- 35.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shui Y, Yu W, Ren X, Guo Y, Xu J, Ma T, Zhang B, Wu J, Li Q, Hu Q, Shen L, Bai X, Liang T, Wei Q. Stereotactic body radiotherapy based treatment for hepatocellular carcinoma with extensive portal vein tumor thrombosis. Radiat Oncol. 2018;13:188. doi: 10.1186/s13014-018-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang WW, Hu BY, Han J, Wang HG, Wang ZB, Ye HY. A real-world study of PD-1 inhibitors combined with TKIs for HCC with major vascular invasion as the conversion therapy: A prospective, non-randomized, open-label cohort study. Ann Oncol. 2020:31: S1307. [Google Scholar]
- 38.He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, Chen MS, Guo RP, Li QJ, Shi M. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi: 10.1177/17588359211002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Zhou J. Initial report of a two-stage resection for hepatocellular carcinoma following downstaging with transarterial chemoembolization and sorafenib. Fubu Waike . 2017;30:295–298+301. [Google Scholar]
- 41.He MK, Zou RH, Li QJ, Zhou ZG, Shen JX, Zhang YF, Yu ZS, Xu L, Shi M. Phase II Study of Sorafenib Combined with Concurrent Hepatic Arterial Infusion of Oxaliplatin, 5-Fluorouracil and Leucovorin for Unresectable Hepatocellular Carcinoma with Major Portal Vein Thrombosis. Cardiovasc Intervent Radiol. 2018;41:734–743. doi: 10.1007/s00270-017-1874-z. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Zhang JL, Zhang XH, Mu H, Yu G, Xing WG. Triple combination therapy comprising angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma. J Clin Oncol . 2021:39. doi: 10.3389/fonc.2021.729764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai L, Li H, Guo J, Zhao W, Duan Y, Hou X, Cheng L, Du H, Shao X, Diao Z, Li C. Drug-eluting bead transarterial chemoembolization is an effective downstaging option for subsequent radical treatments in patients with hepatocellular carcinoma: A cohort study. Clin Res Hepatol Gastroenterol. 2021;45:101535. doi: 10.1016/j.clinre.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Chinese expert consensus on perioperative management of liver resection for HCC (2021 Edition) Zhonghua Yixue Xinxi Daobao. 2021;36:10–10. [Google Scholar]
- 45.Xia Y, Wang P, Pu L, Qian X, Cheng F, Wang K. Preliminary efficacy and safety of perioperative treatment of camrelizumab combined with apatinib in resectable hepatocellular carcinoma (HCC): A prospective phase II study. J Clin Oncol . 2021:39. [Google Scholar]
- 46.Yarchoan M, Zhu Q, Durham JN, Gross N, Charmsaz S, Leatherman JM. Feasibility and efficacy of neoadjuvant cabozantinib and nivolumab in patients with borderline resectable or locally advanced hepatocellular carcinoma (HCC) J Clin Oncol . 2021:39. [Google Scholar]
- 47.Pinato DJ, Cortellini A, Sukumaran A, Cole T, Pai M, Habib N, Spalding D, Sodergren MH, Martinez M, Dhillon T, Tait P, Thomas R, Ward C, Kocher H, Yip V, Slater S, Sharma R. PRIME-HCC: phase Ib study of neoadjuvant ipilimumab and nivolumab prior to liver resection for hepatocellular carcinoma. BMC Cancer. 2021;21:301. doi: 10.1186/s12885-021-08033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaseb AO, Cao HST, Mohamed YI, Qayyum A, Vence LM, Blando JM. Final results of a randomized, open label, perioperative phase II study evaluating nivolumab alone or nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol . 2020:38. [Google Scholar]