Abstract
Dysfunctions in the endo-lysosomal system have been hypothesized to underlie neurodegeneration in major neurocognitive disorders due to Alzheimer’s disease (AD), Frontotemporal Lobar Degeneration (FTLD), and Lewy body disease (DLB). The aim of this study is to investigate whether these diseases share genetic variability in the endo-lysosomal pathway. In AD, DLB, and FTLD patients and in controls (948 subjects), we performed a targeted sequencing of the top 50 genes belonging to the endo-lysosomal pathway. Genetic analyses revealed (i) four previously reported disease-associated variants in the SORL1 (p.N1246K, p.N371T, p.D2065V) and DNAJC6 genes (p.M133L) in AD, FTLD, and DLB, extending the previous knowledge attesting SORL1 and DNAJC6 as AD- and PD-related genes, respectively; (ii) three predicted null variants in AD patients in the SORL1 (p.R985X in early onset familial AD, p.R1207X) and PPT1 (p.R48X in early onset familial AD) genes, where loss of function is a known disease mechanism. A single variant and gene burden analysis revealed some nominally significant results of potential interest for SORL1 and DNAJC6 genes. Our data highlight that genes controlling key endo-lysosomal processes (i.e., protein sorting/transport, clathrin-coated vesicle uncoating, lysosomal enzymatic activity regulation) might be involved in AD, FTLD and DLB pathogenesis, thus suggesting an etiological link behind these diseases.
Keywords: SORL1, DNAJC6, PPT1, endo-lysosomal genes, NGS, cross-disease, loss of function, multicarrier, allele dose effect
1. Introduction
Major neurocognitive disorders due to Alzheimer’s disease (AD), Frontotemporal Lobar Degeneration (FTLD), and Lewy body disease (DLB), are all characterized by abnormal protein accumulation [1,2]. AD is characterized by the deposition of beta-amyloid (Aβ) and phosphorylated tau peptides [3]. DLB, by alpha-synuclein deposits [4], and FTLD presents tau-, ubiquitin-, Fused-in-Sarcoma (FUS)-, and TAR DNA-binding protein 43 (TDP-43)-positive inclusions [5]. The pathogenesis of neurodegenerative disease was recently reviewed, describing AD as a mixed proteinopathy (amyloid and tau) frequently associated with other age-related co-pathologies, such as cerebrovascular lesions, Lewy and TDP-43 pathologies [6]. Moreover, it has been hypothesized that protein aggregates spread from neuron to neuron contributing to the progression of the disease [7]. Exosomes, a specific subtype of extracellular vesicle (EV) of endosomal origin, have been suggested as potential carriers of misfolded toxic proteins: Aβ and tau in AD [8] and alpha-synuclein in Parkinson’s disease (PD)/DLB [9].
The endo-lysosomal pathway is essential in maintaining protein homeostasis in the cell. Growing evidence suggests that endosomal and lysosomal dysfunctions, or dysregulation in protein trafficking, play an important role in neurodegeneration, leading to neurocognitive disorders [10]. Numerous human genetics studies support a critical role of endo-lysosomal dysfunction in AD and FTLD. In AD, mutations in presenilin 1 (the most common genetic cause of AD) may, besides altering amyloid processing, result in defective lysosomal acidification and proteolytic activity [11]. In FTLD, mutations in multiple genes related to lysosome and autophagy function have been described, including in GRN, C9orf72, SQSTM1/p62, UBQLN2, DCTN1, TBK1, OPTN, and VCP [12]. Specifically, a progranulin deficiency due to the presence of GRN pathogenic null mutations, one of the most common genetic causes of FTLD, leads to an upregulation of lysosomal genes as well as profound lysosomal defects [13]. Moreover, GRN null mutations cause a strong alteration of the release and composition of exosomes [14]. Of note, homozygous GRN null mutations cause adult-onset neuronal ceroid lipofuscinosis (NCL), a lysosomal storage disorder [15]. A pathogenic expansion in the C9orf72 gene, a common genetic cause of FTLD, also affects lysosomal function. In C9orf72 null mice, by mimicking the reduced C9orf72 expression observed in expansion carriers, a lysosomal enlargement and accumulation was reported [16]. Recently, it has been demonstrated that NDST3, a potent regulator of lysosomal functions, is downregulated in tissues and cells from FTD patients with C9orf72 haploinsufficiency [17]. The link between AD and FTLD and lysosomal genes was also suggested by genome-wide association studies (GWAS). Regarding AD, a pathway enrichment analysis of three large GWAS provided evidence that genetic variation within the endo-lysosomal system is associated with late-onset AD [18]. In FTLD, a large international GWAS revealed a genetic locus linked to the disease risk encompassing the RAB38 and cathepsin C (CTSC) genes, both involved in the lysosomal pathway [19]. In addition, recent large scale GWAS have provided insights into the genetic risk factors associated with PD, showing that the main contributors to PD etiology are, among others, the molecular processes underlying endo-lysosomal dysfunction [20].
Recently, we provided evidence that an alteration in EV release is common in AD, DLB, and FTLD. Specifically, we found a significant reduction in the plasma concentration of EVs and larger sized EVs in all patient groups: these EV parameters together can distinguish patients from controls with a strong sensitivity and specificity [21]. This study further supports the growing body of evidence that endo-lysosomal dysfunctions may be a converging mechanism in neurodegenerative diseases. The aim of this study is to investigate whether AD, DLB and FTLD share genetic variability in the genes involved in the endo-lysosomal pathway. To reach this goal, we performed, in a large group of AD, DLB and FTLD patients, a targeted deep sequencing of the top 50 genes belonging to the endo-lysosomal pathway. The top 50 genes were selected based on a high intolerance to variation and high expression in two or more brain regions (Tables S1 and S2). The large majority of the selected genes were described to have a potential role in AD, DLB/PD, FTLD pathogenesis, as reported in human genetics and/or human/mouse molecular studies (Table S3).
2. Results
2.1. Identification of Previously Reported Disease-Associated Variants and Predicted Null Variants
Targeted genetic screening for the presence of variants in the coding regions of 50 candidate endo-lysosomal genes was performed on a total of n = 697 patients (n = 282 AD, n = 114 DLB, n = 301 FTLD) and n = 251 controls (Table 1).
Table 1.
Demographic variables of patients and controls.
| AD (n = 282) |
DLB (n = 114) |
FTLD (n = 301) |
CTRL (n = 251) |
p Value | |
|---|---|---|---|---|---|
| Sex (% Female) | 61.3 | 43.9 | 46.8 | 47.4 | 0.0005 1 |
| Age, years | 67.0 ± 9.8 | 75.2 ± 7.6 | 67.2 ± 10.1 | 62.0 ± 9.4 | <0.0001 2 |
| Age at disease onset, years | 65.0 ± 9.6 | 72.5 ± 8.2 | 64.4 ± 10.4 | - | <0.0001 2 |
Kolmogorov–Smirnov test was used to evaluate normality. 1 Chi-square test; 2 Kruskal–Wallis test. The groups which differed from others (post hoc tests) are reported in bold. Mean ± standard deviation.
Thirty-one out of the fifty candidate endo-lysosomal genes were described to have a potential role in AD, DLB/PD, and FTLD pathogenesis, as reported in human genetics and human/mouse molecular studies. Specifically, out of the 31 genes, 12 genes were reported in the literature to present risk alleles or mutations associated with AD, DLB/PD and FTLD (Table S3). In the present study, we detected four previously reported disease-associated variants and three stop-gain heterozygous variants (Table 2, Table 3). Specifically, we found three previously reported variants in the sortilin-related receptor 1 (SORL1) gene: the SORL1 p.N1246K heterozygous variant, previously described as an AD risk factor [22,23], was found in a familial AD patient; the SORL1 p.N371T and p.D2065V heterozygous variants, previously described in two AD patients of North European ancestry [24], were found in an early onset AD and in an FTLD patient and in n = 4 AD, n = 2 DLB and n = 6 FTLD patients, respectively (Table 4). Moreover, in an FTLD and a DLB patient we found a variant in the DnaJ heat shock protein family (Hsp40) member C6/auxilin gene (DNAJC6 p.M133L), previously described in a sporadic early onset PD patient, even if of uncertain significance [25]. The effect of the variants on the protein stability in terms of the ∆∆G was assessed by the Mu-Pro and I-mutant 2.0 tools, and was available for three out of four of the known variants: of note, both computational tools converged in defining these variants as potentially deleterious based on their effect on protein stability.
Table 2.
List of previously reported disease-associated variants identified in AD, DLB and FTLD.
| Gene | AA Change | Variant Type | dbSNP | ∆∆G Mu-Pro and I-Mutant |
Diagnostic Group (Number of Carriers) | Previously Identified Diseases | |
|---|---|---|---|---|---|---|---|
| SORL1 | p.N1246K | non synonymous | rs1699102 | −0.39288546 | −1.30 | AD (1) | AD [22,23] |
| p.N371T | non synonymous | rs150609294 | −1.2548181 | −0.66 | AD (1); FTLD (1) | AD [24] | |
| p.D2065V | non synonymous | rs140327834 | −0.28006864 | −0.57 | AD (4); DLB (2); FTLD (6) | AD [24] | |
| DNAJC6 | p.M133L | non synonymous | rs61757223 | n.a. | n.a. | AD (1); DLB (1) | PD [25] |
AA, amino acid; dbSNP, database of single nucleotide polymorphism; ΔΔG, protein stability free energy change.
Table 3.
Predicted null variant in genes where LOF is a known mechanism of the disease.
| Gene | AA Change | Variant Type | dbSNP | Diagnostic Group (Number of Carriers) | Previously Associated Diseases (LOF Mechanism) |
|---|---|---|---|---|---|
| PPT1 | p.R48X | LOF | - | AD (1) | NCL [30,31] |
| SORL1 | p.R985X | LOF | rs372188860 | AD (1) | AD [26,27,28,29] |
| p.R1207X | LOF | rs774626685 | AD (1) |
AA, amino acid; LOF, loss of function variant; dbSNP, database of single nucleotide polymorphism; NCL, neuronal ceroid lipofuscinosis.
Table 4.
List and clinical characteristics of previously reported disease-associated variants and predicted null variants.
| Gene | Function * | AA Change | Variant Type | Diagnostic Group | Disease Onset | Family History | Association with Neurodegenerative Diseases in the Literature |
|---|---|---|---|---|---|---|---|
| SORL1 | Intracellular protein sorting/transport | N1246K | non synonymous | AD | 72 | F | AD—Genetic and molecular studies in humans [22,23,24,32] FTLD—Genetic studies in humans [33] PD—Genetic studies in humans [34] |
| N371T | non synonymous | AD | 53 | U | |||
| FTLD (bvFTD) | 72 | U | |||||
| D2065V | non synonymous | AD | 60 | F | |||
| AD | 61 | F | |||||
| AD | 50 | AS | |||||
| AD | 66 | F | |||||
| DLB | 74 | AS | |||||
| DLB | 68 | AS | |||||
| FTLD (bvFTD) | 63 | AS | |||||
| FTLD (bvFTD) | 75 | F | |||||
| FTLD | 62 | U | |||||
| FTLD (PPA) | 68 | F | |||||
| FTLD (bvFTD) | 76 | F | |||||
| FTLD (PPA) | 71 | AS | |||||
| R985X | LOF | AD | 46 | F | |||
| R1207X | LOF | AD | 68 | U | |||
| DNAJC6 | Uncoating of clathrin-coated vesicles | M133L | non synonymous | AD | 65 | U | PD—Genetic studies in humans [25,35,36] |
| DLB | 67 | U | |||||
| PPT1 | Catabolism of lipid-modified proteins during lysosomal degradation | R48X | LOF | AD | 61 | F | AD—Molecular studies in animal models [37] FTLD—Molecular studies in animal models [38] |
AA, amino acid; LOF, loss of function; bvFTD, behavioral variant FTD; PPA, primary progressive aphasia; F, positive family history; AS, apparently sporadic; U, unknown; * For a complete list of gene functions reported by Gene Ontology see Table S2.
In addition to these variants, we found three stop-gain heterozygous variants in SORL1 and palmitoyl-protein thioesterase 1 (PPT1) genes that are predicted to cause a loss of function (LOF) due to haploinsufficiency (Table 3); for both genes, LOF is a known mechanism of the disease. Specifically, loss of function mutations of SORL1 have been described in AD [26,27,28,29]. The LOF SORL1 R985X carrier was an early onset (46 years) familial AD patient and the LOF SORL1 R1207X carrier was a 68-year-old AD patient with an unknown family history. Similarly, we found a stop-gain variant in PPT1 (p.R48X) in an early onset (61 years) familial AD patient; PPT1 loss of function mutations have been previously demonstrated to cause an adult form of neuronal ceroid lipofuscinosis (NCL) [30,31].
Considering all the carriers of these variants, the majority of patients (70%) had a disease onset of ≤65 years and/or a positive family history (Table 4). All the previously described disease-associated variants and LOF variants detected in our patients were absent in the controls, and three of the previously described variants were found to be cross-disease variants. Specifically, the SORL1 p.D2065V variant was detected in all three diseases (AD, DLB, FTLD) and was present both in the Italian and Belgian patients; the SORL1 p.N371T variant was found in AD and FTLD; the very rare DNAJC6 p.M133L variant was found in AD and DLB.
2.2. Association Analyses of the Endo-Lysosomal Pathway Genes Involved in Neurodegenerative Diseases and Other Neurological Disorders
Considering all the rare variants detected in the selected 50 genes, including the previously reported variants and the LOF variants, we performed a single variant association analysis in the three diagnostic groups separately (AD, DLB, FTLD vs. CTRL) as well as in all the patients as a whole group (AD + DLB + FTLD vs. CTRL). We found five variants associated with the investigated diseases (Table 5). All these variants were absent in the controls. The effect of the variants on protein stability in terms of the ∆∆G was available for four variants and was assessed by the Mu-Pro or I-mutant 2.0 tool. Both computational tools converged in defining all variants as potentially deleterious based on their effect on protein stability. None of these enrichments were significant after multiple test correction, but we observed some nominally significant results of potential interest, considering the known role of SORL1 in AD [22,23,24,32]. Specifically, the previously AD-associated SORL1 p.D2065V variant was nominally associated with disease, when considering all the patients (p = 0.031) as well as in the DLB (p = 0.048) and FTLD (p = 0.021) groups, separately.
Table 5.
Single variant analysis.
| Gene | Variant | gnomAD_NFE | CADD | Poly Phen2 | ∆∆GMu-Pro and I-Mutant | Diagnostic Group | p Value | MAP | p Value Fdr | p Value Bonf. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SORL1 | p.D2065V | 0.00416 a | 28.5 | D | −0.28 | −0.57 | AD + DLB + FTLD | 0.031 | <0.001 | 0.611 | 1 |
| DLB | 0.048 | 0.048 | 0.618 | 1 | |||||||
| FTLD | 0.021 | 0.004 | 0.656 | 1 | |||||||
| AGRN | p.V554M | 0.0065 a | 25.8 | D | −0.52 | 0.03 | DLB | 0.048 | 0.048 | 0.618 | 1 |
| NEU1 | p.R397W | 0.00004617 b | 26.8 | D | −0.95 | −0.76 | DLB | 0.048 | 0.048 | 0.618 | 1 |
| TOM1 | p.V67A | 0.00007169 b | 26.5 | D | −1.165 | −2.26 | DLB | 0.048 | 0.048 | 0.618 | 1 |
| ABCA2 | p.H1449P | 0 c | 24.3 | D | n.a. | n.a. | DLB | 0.048 | 0.048 | 0.618 | 1 |
gnomAD_NFE, genome aggregation database non-Finnish European; a, 0.001 < gnomAD_NFE ≤ 0.01; b, 0 < gnomAD_NFE ≤ 0.001; c, gnomAD_NFE = 0; CADD, combined annotation dependent depletion; PolyPhen2, polymorphism phenotyping v2; D, damaging; P, potentially damaging; ΔΔG, protein stability free energy change; MAP, minimum achievable p-value; Fdr, false discovery rate corrected; Bonf., Bonferroni corrected. Previously reported variants are underlined in bold.
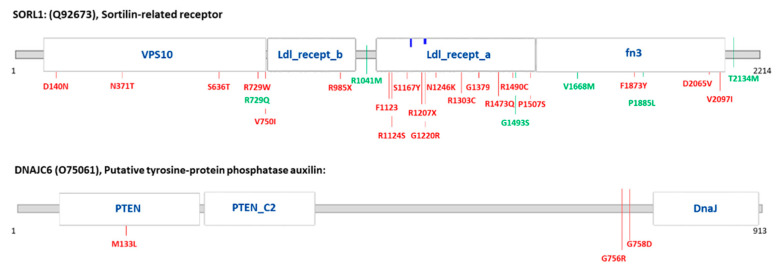
In order to explore whether rare variants were enriched in specific genes, we performed a gene burden analysis and found a nominally significant burden of variants in four genes: SORL1, DNAJC6, NEU1 and AP2A2 (Table 6). Once again, even if none of these enrichments were significant after multiple test correction, we observed some nominally significant results of potential interest, both in SORL1 which was consistently reported to be associated with AD, and in the DNAJC6 gene, consistently reported to be associated with PD [25,35,36]. The SORL1 gene showed a nominally significant burden of variants in the all patients group (p value skat = 0.038) and in FTLD patients (p.burden = 0.025 and p.skato = 0.016), and for DNAJC6 there was a variant burden in DLB patients (p.burden = 0.048 and p.skato = 0.048). The localization of variants within the protein sequence and functional domains is reported in Figure 1. In the SORL1 encoded protein (Sortilin-related receptor 1), all patient-specific variants (including the two stop-gain variants) were located in the VPS10, Low-density lipoprotein (LDL) receptor class a (LDL_recept_a), LDL receptor class b (LDL_recept_b) and Fibronectin type III (fn3) domains. Specifically, (i) the known p.N371T, p.N1246K and p.D2065V variants were located in the VPS10, LDL_recept_a and fn3 domains, respectively; (ii) the LOF p.R985X and p.R1207X variants were located in the LDL_recept_b and LDL_recept_a domains, respectively. In the DNAJC6 encoded protein (auxilin) the p.M133L known variant was located in the Tensin-type phosphatase (PTEN) domain, while two additional variants detected in patients were not located in a functional domain.
Table 6.
Gene burden analysis.
| Gene | Diagnostic Group | Number of Variants | Number of Carriers | p Value Burden | p Value Skato | p Value Skat | p Value Fdr | p Value Bonf. |
|---|---|---|---|---|---|---|---|---|
| SORL1 | AD + DLB + FTLD | 26 | 40 | 0.127 | 0.076 | 0.038 | 0.961 | 1 |
| FTLD | 19 | 26 | 0.025 | 0.016 | 0.017 | 0.481 | 0.963 | |
| DNAJC6 | DLB | 2 | 2 | 0.048 | 0.048 | 0.048 | 0.776 | 1 |
| NEU1 | DLB | 1 | 2 | 0.048 | 0.048 | 0.048 | 0.776 | 1 |
| AP2A2 | AD + DLB + FTLD | 7 | 18 | 0.037 | 0.058 | 0.392 | 0.522 | 1 |
| AD | 4 | 8 | 0.043 | 0.090 | 0.364 | 0.622 | 1 | |
| FTLD | 5 | 9 | 0.022 | 0.080 | 0.374 | 0.481 | 0.858 |
Fdr, false discovery rate corrected; Bonf., Bonferroni corrected.
Figure 1.
Localization of variants present in the genes of interest. Variant localization in the protein sequence, according to the Elaspic and Interpro webserver, and the relative functional domains involved. Variants present in the patient group are shown in red; Variants present in the control group are shown in green. VPS10: VPS10 domain; Ldl_recept_a: LDL receptor class a; Ldl_recept_b: LDL receptor class b; fn3: Fibronectin type 3 domain; PTEN: PTEN domain; PTEN_C2: C2 domain of PTEN tumor-suppressor protein; Dnaj: DnaJ domain.
2.3. Multiple Variant Carriers in the Endo-Lysosomal Pathway
Considering previously reported disease associated variants (Table 2) and predicted LOF variants (Table 3), we described four patients carrying an additional rare/very rare variant which was of unknown significance and absent in the controls (Table 7). Specifically (i) the known SORL1 p.D2065V variant was found along with a rare variant in GGA3 in a familial FTLD patient with a disease onset at 68 years; (ii) the LOF SORL1 p.R1207X was found along with a missense variant also in SORL1 (p.D140N) in an AD patient with a disease onset at 68 years and an unknown family history; (iii) the known DNAJC6 p.M133L variant was found along with a rare variant in AGRN (p.A897V) in an AD patient with a disease onset at 65 years and an unknown family history; (iv) the LOF PPT1 p.R48X variant was found along with a rare variant of GNPTG (p.R66Q) in an early onset familial AD patient. In addition, as shown in Table 7, considering the patients with a disease onset of ≤65 years and/or a positive family history, we described 14 more patients carrying two or three rare/very/ultrarare variants which were of unknown significance, and absent in controls. Among these, for example, were three patients carrying the ultrarare ABCA2 p.H1449P variant (found to be nominally associated with disease but this did not survive after multiple test correction) and one early onset (49 years) apparently sporadic FTLD patient who was carrying two compound heterozygous variants in the vacuolar protein sorting-associated protein 52 homolog gene (VPS52 p.Y508C, p.R578W).
Table 7.
List and clinical characteristics of multiple variant carriers.
| Patient | Diagnostic Group | FTLD Subtype | Disease Onset | FH | Sex | Study Group | Variants | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | FTLD | PPA | 68 | F | M | Belgium | SORL1 p.D2065V a | GGA3 p.K99R a |
- |
| 2 | AD | - | 68 | U | M | Belgium | SORL1 p.R1207X b | SORL1 p.D140N b | - |
| 3 | AD | - | 65 | U | M | Belgium | DNAJC6 p.M133L b | AGRN p.A897V a | - |
| 4 | AD | - | 61 | F | F | Belgium | PPT1 p.R48X b | GNPTG p.R66Q b | - |
| 5 | FTLD | PPA | 57 | F (low) * |
F | Italy | SORL1 p.S1167Y b | ABCA2 p.H1449P c | GPC1 p.R90W b |
| 6 | FTLD | PPA | 63 | AS | F | Italy | SORL1 p.R729W b | CTSA p.P330A b |
GGA3 p.P235L b |
| 7 | FTLD | bvFTD | 82 | F (low) * |
M | Italy | SORL1 V2097I a |
ABCA2 S1378F b | - |
| 8 | FTLD | - | 66 | F | M | Belgium | SORL1 p.S636T a | VPS39 p.V473M b | |
| 9 | FTLD | - | 49 | AS | F | Belgium | VPS52 p.Y508C b | VPS52 p.R578W b | - |
| 10 | FTLD | bvFTD + IBM | 70 | F | M | Belgium | AGRN p.R956H b | CD81 p.G129R b |
HGS p.L525V b |
| 11 | FTLD | PPA | 50 | F | M | Italy | ABCA2 p.H1449P c | ATP6V0D1 c.C817-2A c | - |
| 12 | FTLD | bvFTD | 59 | F (medium) * |
M | Italy | ABCA2 p.H1449P c | GGA2 p.L83I b |
- |
| 13 | FTLD | PPA | 60 | F (high) * |
M | Italy | AGRN p.V1691M b | ATP6V0D1 c.C817-2A c | - |
| 14 | FTLD | PPA | 54 | F (high) * |
M | Italy | DNM2 p.R318W b | ATP6V0D1 c.C817-2A c | - |
| 15 | DLB | - | 70 | F | M | Italy | GGA2 p.S39W c | GGA3 p.P40R b |
- |
| 16 | FTLD | bvFTD | 65 | F | M | Belgium | GGA2 p.R105G b | VPS39 p.F573L b |
- |
| 17 | FTLD | bvFTD | 39 | AS | F | Belgium | GNPTG p.R186W c | MGRN1 p.P67L c |
- |
| 18 | DLB | - | 78 | F | M | Belgium | NEU1 p.R397W b | TOM1 p.V67A b |
- |
bvFTD, behavioral variant FTD; PPA, primary progressive aphasia; IBM, inclusion body myopathy; FH, family history; F, positive family history; AS, apparently sporadic; U, unknown; * Fostinelli et al., 2018; a, 0.001 < gnomAD_NFE ≤ 0.01; b, 0 < gnomAD_NFE ≤ 0.001; c, gnomAD_NFE = 0. Previously reported variants and LOF variants are in underlined bold text.
3. Discussion
Neurodegenerative diseases are characterized by abnormal intracellular protein inclusions or extracellular protein aggregates [1]. There is strong interest in understanding the common molecular mechanisms that contribute to neurodegenerative disorders, and thus, in exploring the etiological link behind these brain diseases [39].
Alterations in the endo-lysosomal system, leading to the failure of proper protein trafficking and degradation, have been hypothesized to underlie neuronal dysfunction in these diseases. Emerging data argue for an interdependence between the production of exosomes (a specific subtype of EVs of endosomal origin) and endosomal pathway integrity in the brain [40]. We recently described that an alteration in the release of EVs is common across AD, FTLD, and DLB and that plasma EV parameters (EV concentration/size) can distinguish patients from controls with strong sensitivity and specificity [21]. Evidence from monogenic diseases and experimental models suggest that autophagy and the endo-lysosomal system may be mechanistically involved in the neurodegenerative processes leading to AD and FTLD [41]. Genetic variation within the endo-lysosomal system is associated with a late-onset AD risk, as demonstrated by the pathway enrichment analysis of three large GWAS: of note, this aggregate genetic association was unique for the autophagic and endo-lysosomal system, and in the same study, an association signal was also observed in PD [18].
Herein, we investigated genetic variability in the genes involved in the endo-lysosomal pathway in major neurocognitive disorders. In a large group consisting of AD, DLB, FTLD and CTRL subjects (948 in total), we performed targeted deep sequencing of the top 50 genes belonging to the endo-lysosomal pathway, with prioritization based on a high intolerance to variation and high brain expression (Tables S1–S3). Specifically, since we were looking for cross-disease genetic variants, we selected genes highly expressed in at least two brain regions. Genetic analyses revealed, in our patients’ dataset, four previously described variants in SORL1 and DNAJC6 genes. The SORL1 p.N1246K variant, previously described as an AD risk factor [22,23], was found in a familial AD patient; the SORL1 p.N371T and the SORL1 p.D2065V variants, previously described in two unrelated AD patients of North European ancestry [24], were found in our dataset in AD, DLB and FTLD patients. SORL1 p.N371T was found both in early onset AD and FTLD, while SORL1 p.D2065V was found in several patients from all three diagnostic groups (four AD, two DLB and six FTLD patients). The DNAJC6 p.M133L variant was found in an AD patient and a DLB patient. This variant was previously described in a sporadic early onset PD patient [25], and even if of uncertain significance, our data also suggest a role of this variant in AD and DLB, an alpha-synuclein associated disease like PD. In addition to known variants, predicted null variants in SORL1 and PPT1 were found in familial early onset AD cases (SORL1 p.R985X, and PPT1 p.R48X) and in an AD patient with an unknown family history (SORL1 p.R1207X). Loss of function variants of SORL1 have already been described in AD [26,27,28,29] and were proposed to cause AD by inducing defects in the endolysosome-autophagy network [42]. PPT1 loss of function variants, in the homozygous or compound heterozygous state, were demonstrated to cause neuronal ceroid lipofuscinosis, an inherited, progressive neurodegenerative disease [30,43].
Interestingly, one of the most common worldwide progranulin loss of function mutations is associated with FTLD in the heterozygous state and with NCL in the homozygous state [15,44]. Similarly, we cannot exclude strictly different clinico-pathological phenotypes (AD versus NCL) also determined by the PPT1 mutation dosage.
Protein palmitoylation is an important process to regulate the physiological function of the brain. A number of studies have reported that defects in the palmitoylation step or in the enzymes for palmitoylation/depalmitoylation are associated with several neurological disorders including AD [45]. Since APP palmitoylation seems to enhance the amyloidogenic pathway, the loss of a depalmitoylating enzyme such as PPT1 might result in an increased amyloid production; this hypothesis is line with the observed clinical phenotype of the carrier, an early onset familial AD patient. Since LOF is a known mechanism of disease for both genes, the evidence that these variants are pathogenic is strong [46].
Single variant association tests further supported a role of the SORL1 p.D2065V variant as a genetic determinant/risk factor for AD, DLB and FTLD. The gene burden analysis showed a nominally significant burden of potential interest in SORL1 in the all patients group and in FTLD patients, and in DNAJC6 in the DLB group. Regarding SORL1, the majority of the variants found in patients were located in the VPS10 protein domain, the Low-density LDL_recept_a/b domains and the fibronectin type III domain. Of note, a recent meta-analysis of burden tests at the protein domain level of SORL1 missense variants showed a significant association of the VPS10, LDL_recept_a and fibronectin type III domains with AD [47]. The LDL_recept_a domain was demonstrated to have a critical role for the function of SORL1 in AD, as it is involved in amyloid precursor protein (APP) binding and APP retrograde endosome transport to the TGN [48]. The VPS10 domain is involved in Aβ peptides binding and targeting to the lysosomes and variants in this domain have been described which impair lysosomal sorting of Aβ and cause familial AD [27], and the fibronectin type III domain interacts with APP [49]. More importantly, we also provided evidence of an involvement of this gene in FTLD and DLB as we described cross-disease variants, specifically, (i) a previously known AD variant in FTLD and AD patients, and (ii) a previously known AD variant in FTLD, DLB and AD patients, that was found to be a nominally significant associated variant. Regarding the DNAJC6 encoded protein auxilin, only the p.M133L variant was located in a functional domain, and specifically, in the PTEN domain, which is important for the recruitment of auxilin onto clathrin-coated vesicles. Auxilin has a well-established role in clathrin uncoating [50]. Since endocytosis and clathrin-uncoating defects at synapses were demonstrated in auxilin knockout mice, a specialized role for this protein in the clathrin-dependent recycling of synaptic vesicles at synapses was suggested [51]. Of note, a splicing variant affecting the PTEN domain was associated with juvenile parkinsonism [52]. The present study also suggests the possible involvement of this gene in DLB and AD. Interestingly, some of the patients carrying known variants and predicted LOF variants also carried additional rare/very rare variants of still unknown significance (but not present in controls) in endo-lysosomal genes. In addition, considering the patients with early onset disease and a positive family history, we described 14 more cases carrying two or three rare/very rare/ultrarare variants of still unknown significance (but not present in controls). Among these, (i) a Belgian patient with a very early onset FTLD (49 years) carrying two potentially damaging compound heterozygous variants in VPS52, a subunit of the Golgi-associated retrograde protein complex (interacting with the PD-associated LRRK2) which is involved in retrograde transport of early and late endosomes to the Golgi [53]; (ii) three patients carrying the ultrarare ABCA2 p.H1449P variant, which was found to be nominally associated with disease, but this did not survive after multiple test correction.
There are limitations in the present study: (i) This being a pilot study, further validation in larger groups is needed. Such large studies will, on one side give a definitive answer on the role played by SORL1, DNAJC6, and PPT1 in neurocognitive disorders and, on the other side, unveil the potential of the AP2A2, ABCA2, NEU1, TOM1, and AGRN genes in contributing to the disease. (ii) Disease segregation studies on families for variants with the strongest evidence of pathogenicity are needed. (iii) We explored only a portion of endo-lysosomal genes and thus a more comprehensive genetic screening (including all genes belonging to this pathway) as well functional studies on identified variants could be of interest.
Based on our data and the literature data on the role played by the endo-lysosomal system in neurocognitive disorders, epigenetic studies evaluating the complex interplay of genetic and environmental factors are warranted to better explore the etiological link behind these diseases. The final goal is to develop new strategies for the development of innovative therapeutic approaches targeting the endo-lysosomal pathway and taking advantage of the current knowledge in this field [54,55,56].
Our data highlight that genes controlling key endo-lysosomal processes such as intracellular protein sorting/transport, the uncoating of clathrin-coated vesicles and the regulation of enzymatic activity in lysosomes, might be involved in AD, DLB and FTLD pathogenesis. Altogether our data further confirm the key role of the endo-lysosomal pathway in these diseases and suggest the existence of cross-diseases mechanisms involved in major neurocognitive disorders. Our data strongly support a critical role for SORL1 in AD and related diseases and highlight SORL1 as a potential therapeutic target for drug development.
4. Materials and Methods
4.1. Participants
This retrospective study was carried out on DNA from a total of n = 697 patients (n = 282 AD, n = 114 DLB, n = 301 FTLD) and n = 251 subjects with normal cognitive function (CTRL) (Table 1). Clinical diagnosis for probable AD, DLB and FTLD was made according to international guidelines [57,58,59,60,61,62]. The family history was determined by a family history questionnaire and FTLD pedigrees were classified as previously described [63,64,65]. DNA samples were available from the biological banks of the IRCCS Fatebenefratelli Brescia and IRCCS Besta Milan (Italian cohort), from the Neurodegenerative Brain Diseases Human Biobank of the VIB Center for Molecular Neurology, Antwerp, Belgium (Belgian patient cohort [66,67]), and from Project MinE (Belgian control cohort, [68]). Written informed consent was obtained from all subjects. The study protocol was approved by the local ethics committee (Prot. N. 111/2017).
4.2. Gene Selection
The candidate genes were selected employing web resources: KEGG pathway, Gene Ontology (GO), Gene Set Enrichment Analysis (GSEA), and Reactome for selecting genes belonging to the endo-lysosomal pathway. The Genotype-Tissue Expression (GTEx) dataset for gene selection was based on brain expression and the Residual Variation Intolerance Score (RVIS) score was used as a ranking method to prioritize genes with a high intolerance to variation (more negative values express an increasing intolerance to mutations). A list of 314 genes was obtained including genes belonging to pathways connected to lysosomes, endosomes and endo-lysosomes. Genes were then filtered according to brain tissue expression. For each gene, in each region we calculated if it fell above the 3rd quartile of expression for that specific tissue and we filtered out any genes that were not highly expressed in at least two brain regions. Applying this strategy, we selected 93 genes. Fifty genes were further selected from this list, with a priority given to genes with the lowest RVIS score (Tables S1 and S2).
4.3. Genetic Analyses
The entire coding regions of the 50 candidate genes were analyzed by amplicon-based target enrichment and Next-Generation Sequencing (NGS) of the exons and exon-intron boundaries on a Illumina® MiSeq platform (Illumina, San Diego, CA, USA). NGS analysis was performed on n = 65 AD; n = 102 DLB, n = 58 FTLD and n = 75 CTRL samples. The quality assessment of gDNA was performed on a 0.8% agarose gel and gDNA was quantified with a Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). A total of 200 ng of gDNA was used for library preparation with a Nextera Flex for Enrichment kit (Illumina, Inc., USA). gDNA was tagmented, amplified and purified with AMPure XP Beads (Beckman Coulter, Inc., Brea, CA, USA). The size, quality and quantity of libraries was assessed with a High Sensitivity DNA kit on a Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA, USA). A 12 pM sample of the pooled library was loaded on a MiSeq reagent cartridge v3 and sequenced on an Illumina MiSeq platform. Whole exome sequencing (WES) was performed on a NextSeq 500 platform (Illumina, USA) on n = 217 AD, n = 12 DLB, n = 243 FTLD samples. The exons were captured by a SeqCap® EZ Human Exome Probes v3.0 (Roche, Basel, Switzerland) kit with a paired-end read length of 250 bp. Whole genome sequencing (WGS) was performed on a HiSeq X platform (Illumina, USA) on n = 176 CTRL samples as described before [69]. Sanger sequencing was performed on a SeqStudio Genetic Analyzer (Applied Biosystems, Waltham, MA, USA) and on an AB3730 DNA analyzer (Life Technologies, Waltham, MA, USA) to confirm selected variants with a low coverage. The chromatograms were viewed through a CLC Main Workbench 20.0.4 (QIAGEN, Copenhagen, Denmark).
4.4. Variant Annotation, Filtering and Bioinformatics
Sequence reads from amplicon-based target enrichment were processed for quality-control purposes with the FastQC tool before alignment to the hg19 human reference sequence using Spliced Transcripts Alignment to a Reference (STAR), (The National Human Genome Research Institute, Bethesda, MD, USA) software. Duplicated reads were removed with Picard tools. Local realignment, recalibration, and variant calling were performed with the Genome Analysis Tool Kit (GATK) [70]. Variants with a QUAL < 20 and QualByDepth < 3 were excluded from the analysis. WES reads were aligned to the reference genome GRCh37 using a Burrows-Wheler Aligner (BWA) implemented using in house Genomecomb software [71] and variants were called using the GATK Haplotype Caller. WGS reads were aligned to the GRCh37 genome using Illumina’s (Illumina, San Diego, CA, USA) standard iSAAC aligner and variants were called using the iSAAC variant caller. Variant mapping to the 50 endo-lysosomal pathway genes were extracted from the WES and WGS datasets according to the coordinates of the BED files of the endo-lysosomal gene panel. WGS data were processed for quality control as described before [69]. Variants with a coverage < 20, genotype quality (GQ) < 99 and an allelic ratio >3 were excluded from the WES data. WES and WGS data were annotated using in house Genomecomb software [71]. Gene coordinates and transcripts were annotated using the RefSeq genes. Detected variants were filtered, including all “non synonymous” SNVs and premature stop codons (stop-gain, essential splice, site, frameshift indels), all variants with a Combined Annotation Dependent Depletion (CADD) score > 20, all variants annotated as damaging (D) or potentially damaging (P) according to Polymorphism Phenotyping v2 (PolyPhen2_HDIV) and with a frequency in the non-Finnish European exomes cohort reported in the Genome Aggregation Database v2.1.1. (gnomAD_NFE) < 0.01. Localization of the variants within the protein sequence was performed with Elaspic [72] and Interpro [73]. The prediction of the effect on protein stability of single amino acid substitutions was performed using two different computational tools, I-mutant 2.0 (https://folding.biofold.org/i-mutant/i-mutant2.0.html, accessed on 10 August 2021 [74]) and Mu-Pro (http://mupro.proteomics.ics.uci.edu, accessed on 10 August 2021 [75]). The change in the Gibbs free energy (ΔΔG) between wild-type and mutant protein was calculated. For both computational tools, a ΔΔG < 0 indicates a decreased protein stability upon substitution.
4.5. Statistical Analyses
For testing associations between rare variants and phenotypes, burden tests focusing on the cumulative effects of rare variants in genetic regions was adopted [76]. In addition, to overcome the potential loss of power when the assumption of causal and same direction effects is violated, a test which builds upon the kernel machine regression framework (sequence kernel association test—SKAT) was applied. Finally, the optimal unified test (SKAT-O), obtained as an optimal linear combination of the burden test and SKAT, was used to maximize the power [77]. For single variant tests, the efficient resampling method (ER) for a score statistic was chosen and the minimum achievable p-value (MAP) was provided; while, for multiple variant tests, a hybrid method, based on the total minor allele count (MAC), the number of individuals with minor alleles (m) and the degree of case-control imbalance, was adopted [77]. In addition, multiple testing false discovery rate and Bonferroni corrections were applied.
Acknowledgments
The authors thank the personnel of the NBD Human Biobank, the Diagnostic Screening Facility, the Neuromics Support Facility and the VIB-UAntwerp Center for Molecular Neurology. We further thank the members of the Belgian Neurology Consortium (BELNEU) for their recruitment and clinical phenotyping of patients to the study populations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222413633/s1.
Author Contributions
L.B. and A.L. contributed equally to this paper. R.G., L.B., C.K. and J.v.d.Z.: conceptualization. A.L., C.K., S.B., R.N., C.S., C.B., S.F., R.Z., M.C. (Marcella Catania), M.M., P.V.D., G.D.F., G.B. and C.V.B.: data acquisition. A.L., M.C. (Matteo Carrara), C.K., S.B., C.F., M.M. and J.v.d.Z.: data analysis. L.B. and R.G.: data interpretation. L.B., A.L., C.K., C.V.B., J.v.d.Z. and R.G.: writing—original draft preparation. A.L., C.K., S.B., C.F., R.N. and C.S.: writing—tables and figures preparation. L.B., A.L., C.K., M.C. (Matteo Carrara), S.B., C.F., R.N., C.S., C.B., S.F., R.Z., M.C. (Marcella Catania), M.M., P.V.D., G.D.F., G.B., C.V.B., J.v.d.Z. and R.G.: writing—review and editing. R.G., C.V.B. and J.v.d.Z.: supervision. R.G. and C.V.B.: funding acquisition. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Italy, under the aegis of the EU Joint Programme—Neurodegenerative Disease Research (JPND), grant number PATHWAYS-200-059, and by the Italian Ministry of Health, Italy, Ricerca Corrente. The research of the Neurodegenerative Brain Diseases group of the VIB-UAntwerp Center for Molecular Neurology was in part supported by the Flemish Government initiated Methusalem Excellence Program and the Flanders Impulse Program on Networks for Dementia Research. Project MinE Belgium was supported by a grant from IWT and by the Belgian ALS Liga.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethics committee (Prot. N. 111/2017).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jellinger K.A. Neuropathological Aspects of Alzheimer Disease, Parkinson Disease and Frontotemporal Dementia. Neurodegener Dis. 2008;5:118–121. doi: 10.1159/000113679. [DOI] [PubMed] [Google Scholar]
- 2.Soto C., Estrada L.D. Protein Misfolding and Neurodegeneration. Arch. Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 3.Brion J.P. Neurofibrillary Tangles and Alzheimer’s Disease. Eur. Neurol. 1998;40:130–140. doi: 10.1159/000007969. [DOI] [PubMed] [Google Scholar]
- 4.Beyer K., Domingo-Sàbat M., Ariza A. Molecular Pathology of Lewy Body Diseases. Int. J. Mol. Sci. 2009;10:724–745. doi: 10.3390/ijms10030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann M., Mackenzie I.R.A. Review: Neuropathology of Non-Tau Frontotemporal Lobar Degeneration. Neuropathol. Appl. Neurobiol. 2019;45:19–40. doi: 10.1111/nan.12526. [DOI] [PubMed] [Google Scholar]
- 6.Jellinger K.A. Neuropathological Assessment of the Alzheimer Spectrum. J. Neural Transm. 2020;127:1229–1256. doi: 10.1007/s00702-020-02232-9. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s Diseases: The Prion Concept in Relation to Assembled Aβ, Tau, and A-Synuclein. Science. 2015;349:1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 8.Rajendran L., Bali J., Barr M.M., Court F.A., Krämer-Albers E.M., Picou F., Raposo G., van der Vos K.E., van Niel G., Wang J., et al. Emerging Roles of Extracellular Vesicles in the Nervous System. J. Neurosci. 2014;34:15482–15489. doi: 10.1523/JNEUROSCI.3258-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Erviti L., Seow Y., Schapira A.H., Gardiner C., Sargent I.L., Wood M.J., Cooper J.M. Lysosomal Dysfunction Increases Exosome-Mediated Alpha-Synuclein Release and Transmission. Neurobiol. Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y.B., Dammer E.B., Ren R.J., Wang G. The Endosomal-Lysosomal System: From Acidification and Cargo Sorting to Neurodegeneration. Transl. Neurodegener. 2015;4:18. doi: 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G., et al. Lysosomal Proteolysis and Autophagy Require Presenilin 1 and are Disrupted by Alzheimer-Related PS1 Mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casterton R.L., Hunt R.J., Fanto M. Pathomechanism Heterogeneity in the Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Disease Spectrum: Providing Focus through the Lens of Autophagy. J. Mol. Biol. 2020;432:2692–2713. doi: 10.1016/j.jmb.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Lui H., Zhang J., Makinson S.R., Cahill M.K., Kelley K.W., Huang H.Y., Shang Y., Oldham M.C., Martens L.H., Gao F., et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia Via Complement Activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benussi L., Ciani M., Tonoli E., Morbin M., Palamara L., Albani D., Fusco F., Forloni G., Glionna M., Baco M., et al. Loss of Exosomes in Progranulin-Associated Frontotemporal Dementia. Neurobiol. Aging. 2016;40:41–49. doi: 10.1016/j.neurobiolaging.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Smith K.R., Damiano J., Franceschetti S., Carpenter S., Canafoglia L., Morbin M., Rossi G., Pareyson D., Mole S.E., Staropoli J.F., et al. Strikingly Different Clinicopathological Phenotypes Determined by Progranulin-Mutation Dosage. Am. J. Hum. Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Rourke J.G., Bogdanik L., Yáñez A., Lall D., Wolf A.J., Muhammad A.K., Ho R., Carmona S., Vit J.P., Zarrow J., et al. C9orf72 is Required for Proper Macrophage and Microglial Function in Mice. Science. 2016;351:1324–1329. doi: 10.1126/science.aaf1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q., Liu M., Liu Y., Hwang R.D., Zhang T., Wang J. NDST3 Deacetylates A-Tubulin and Suppresses V-ATPase Assembly and Lysosomal Acidification. EMBO J. 2021;40:e107204. doi: 10.15252/embj.2020107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao S., Casey A.E., Sargeant T.J., Mäkinen V.P. Genetic Variation within Endolysosomal System is Associated with Late-Onset Alzheimer’s Disease. Brain. 2018;141:2711–2720. doi: 10.1093/brain/awy197. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari R., Hernandez D.G., Nalls M.A., Rohrer J.D., Ramasamy A., Kwok J.B., Dobson-Stone C., Brooks W.S., Schofield P.R., Halliday G.M., et al. Frontotemporal Dementia and its Subtypes: A Genome-Wide Association Study. Lancet Neurol. 2014;13:686–699. doi: 10.1016/S1474-4422(14)70065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall A., Bandres-Ciga S., Diez-Fairen M., Quinn J.P., Billingsley K.J. Genetic Risk Profiling in Parkinson’s Disease and Utilizing Genetics to Gain Insight into Disease-Related Biological Pathways. Int. J. Mol. Sci. 2020;21:7332. doi: 10.3390/ijms21197332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longobardi A., Benussi L., Nicsanu R., Bellini S., Ferrari C., Saraceno C., Zanardini R., Catania M., Di Fede G., Squitti R., et al. Plasma Extracellular Vesicle Size and Concentration are Altered in Alzheimer’s Disease, Dementia with Lewy Bodies, and Frontotemporal Dementia. Front. Cell. Dev. Biol. 2021;9:667369. doi: 10.3389/fcell.2021.667369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caglayan S., Bauerfeind A., Schmidt V., Carlo A.S., Prabakaran T., Hübner N., Willnow T.E. Identification of Alzheimer Disease Risk Genotype that Predicts Efficiency of SORL1 Expression in the Brain. Arch. Neurol. 2012;69:373–379. doi: 10.1001/archneurol.2011.788. [DOI] [PubMed] [Google Scholar]
- 23.Rogaeva E., Meng Y., Lee J.H., Gu Y., Kawarai T., Zou F., Katayama T., Baldwin C.T., Cheng R., Hasegawa H., et al. The Neuronal Sortilin-Related Receptor SORL1 is Genetically Associated with Alzheimer Disease. Nat. Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vardarajan B.N., Zhang Y., Lee J.H., Cheng R., Bohm C., Ghani M., Reitz C., Reyes-Dumeyer D., Shen Y., Rogaeva E., et al. Coding Mutations in SORL1 and Alzheimer Disease. Ann. Neurol. 2015;77:215–227. doi: 10.1002/ana.24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olgiati S., Quadri M., Fang M., Rood J.P., Saute J.A., Chien H.F., Bouwkamp C.G., Graafland J., Minneboo M., Breedveld G.J., et al. DNAJC6 Mutations Associated with Early-Onset Parkinson’s Disease. Ann. Neurol. 2016;79:244–256. doi: 10.1002/ana.24553. [DOI] [PubMed] [Google Scholar]
- 26.Le Guennec K., Tubeuf H., Hannequin D., Wallon D., Quenez O., Rousseau S., Richard A.C., Deleuze J.F., Boland A., Frebourg T., et al. Biallelic Loss of Function of SORL1 in an Early Onset Alzheimer’s Disease Patient. J. Alzheimers Dis. 2018;62:821–831. doi: 10.3233/JAD-170981. [DOI] [PubMed] [Google Scholar]
- 27.Caglayan S., Takagi-Niidome S., Liao F., Carlo A.S., Schmidt V., Burgert T., Kitago Y., Füchtbauer E.M., Füchtbauer A., Holtzman D.M., et al. Lysosomal Sorting of Amyloid-Β by the SORLA Receptor is Impaired by a Familial Alzheimer’s Disease Mutation. Sci. Transl. Med. 2014;6:223ra20. doi: 10.1126/scitranslmed.3007747. [DOI] [PubMed] [Google Scholar]
- 28.Pottier C., Hannequin D., Coutant S., Rovelet-Lecrux A., Wallon D., Rousseau S., Legallic S., Paquet C., Bombois S., Pariente J., et al. High Frequency of Potentially Pathogenic SORL1 Mutations in Autosomal Dominant Early-Onset Alzheimer Disease. Mol. Psychiatry. 2012;17:875–879. doi: 10.1038/mp.2012.15. [DOI] [PubMed] [Google Scholar]
- 29.Verheijen J., Van den Bossche T., van der Zee J., Engelborghs S., Sanchez-Valle R., Lladó A., Graff C., Thonberg H., Pastor P., Ortega-Cubero S., et al. A Comprehensive Study of the Genetic Impact of Rare Variants in SORL1 in European Early-Onset Alzheimer’s Disease. Acta Neuropathol. 2016;132:213–224. doi: 10.1007/s00401-016-1566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann S.L., Das A.K., Yi W., Lu J.Y., Wisniewski K.E. Genotype-Phenotype Correlations in Neuronal Ceroid Lipofuscinosis due to Palmitoyl-Protein Thioesterase Deficiency. Mol. Genet. Metab. 1999;66:234–239. doi: 10.1006/mgme.1999.2803. [DOI] [PubMed] [Google Scholar]
- 31.van Diggelen O.P., Thobois S., Tilikete C., Zabot M.T., Keulemans J.L., van Bunderen P.A., Taschner P.E., Losekoot M., Voznyi Y.V. Adult Neuronal Ceroid Lipofuscinosis with Palmitoyl-Protein Thioesterase Deficiency: First Adult-Onset Patients of a Childhood Disease. Ann. Neurol. 2001;50:269–272. doi: 10.1002/ana.1103. [DOI] [PubMed] [Google Scholar]
- 32.Chou C.T., Liao Y.C., Lee W.J., Wang S.J., Fuh J.L. SORL1 Gene, Plasma Biomarkers, and the Risk of Alzheimer’s Disease for the Han Chinese Population in Taiwan. Alzheimers Res. Ther. 2016;8:53. doi: 10.1186/s13195-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciani M., Bonvicini C., Scassellati C., Carrara M., Maj C., Fostinelli S., Binetti G., Ghidoni R., Benussi L. The Missing Heritability of Sporadic Frontotemporal Dementia: New Insights from Rare Variants in Neurodegenerative Candidate Genes. Int. J. Mol. Sci. 2019;20:3903. doi: 10.3390/ijms20163903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiromerisiou G., Bourinaris T., Houlden H., Lewis P.A., Senkevich K., Hammer M., Federoff M., Khan A., Spanaki C., Hadjigeorgiou G.M., et al. SORL1 Mutation in a Greek Family with Parkinson’s Disease and Dementia. Ann. Clin. Transl. Neurol. 2021;8:1961–1969. doi: 10.1002/acn3.51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gialluisi A., Reccia M.G., Modugno N., Nutile T., Lombardi A., Di Giovannantonio L.G., Pietracupa S., Ruggiero D., Scala S., Gambardella S., et al. Identification of Sixteen Novel Candidate Genes for Late Onset Parkinson’s Disease. Mol. Neurodegener. 2021;16:35. doi: 10.1186/s13024-021-00455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Köroğlu Ç., Baysal L., Cetinkaya M., Karasoy H., Tolun A. DNAJC6 is Responsible for Juvenile Parkinsonism with Phenotypic Variability. Parkinsonism Relat. Disord. 2013;19:320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Aladeokin A.C., Akiyama T., Kimura A., Kimura Y., Takahashi-Jitsuki A., Nakamura H., Makihara H., Masukawa D., Nakabayashi J., Hirano H., et al. Network-Guided Analysis of Hippocampal Proteome Identifies Novel Proteins that Colocalize with Aβ in a Mice Model of Early-Stage Alzheimer’s Disease. Neurobiol. Dis. 2019;132:104603. doi: 10.1016/j.nbd.2019.104603. [DOI] [PubMed] [Google Scholar]
- 38.Huang M., Modeste E., Dammer E., Merino P., Taylor G., Duong D.M., Deng Q., Holler C.J., Gearing M., Dickson D., et al. Network Analysis of the Progranulin-Deficient Mouse Brain Proteome Reveals Pathogenic Mechanisms Shared in Human Frontotemporal Dementia Caused by GRN Mutations. Acta Neuropathol. Commun. 2020;8:163. doi: 10.1186/s40478-020-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka M., Toldi J., Vécsei L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020;21:2431. doi: 10.3390/ijms21072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathews P.M., Levy E. Exosome Production is Key to Neuronal Endosomal Pathway Integrity in Neurodegenerative Diseases. Front. Neurosci. 2019;13:1347. doi: 10.3389/fnins.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C., Telpoukhovskaia M.A., Bahr B.A., Chen X., Gan L. Endo-Lysosomal Dysfunction: A Converging Mechanism in Neurodegenerative Diseases. Curr. Opin. Neurobiol. 2018;48:52–58. doi: 10.1016/j.conb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Hung C., Tuck E., Stubbs V., van der Lee S.J., Aalfs C., van Spaendonk R., Scheltens P., Hardy J., Holstege H., Livesey F.J. SORL1 Deficiency in Human Excitatory Neurons Causes APP-Dependent Defects in the Endolysosome-Autophagy Network. Cell. Rep. 2021;35:109259. doi: 10.1016/j.celrep.2021.109259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das A.K., Becerra C.H., Yi W., Lu J.Y., Siakotos A.N., Wisniewski K.E., Hofmann S.L. Molecular genetics of palmitoyl-protein thioesterase deficiency in the U.S. J. Clin. Invest. 1998;102:361–370. doi: 10.1172/JCI3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benussi L., Ghidoni R., Pegoiani E., Moretti D.V., Zanetti O., Binetti G. Progranulin Leu271LeufsX10 is One of the most Common FTLD and CBS Associated Mutations Worldwide. Neurobiol. Dis. 2009;33:379–385. doi: 10.1016/j.nbd.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Cho E., Park M. Palmitoylation in Alzheimer’s Disease and Other Neurodegenerative Diseases. Pharmacol. Res. 2016;111:133–151. doi: 10.1016/j.phrs.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campion D., Charbonnier C., Nicolas G. SORL1 Genetic Variants and Alzheimer Disease Risk: A Literature Review and Meta-Analysis of Sequencing Data. Acta Neuropathol. 2019;138:173–186. doi: 10.1007/s00401-019-01991-4. [DOI] [PubMed] [Google Scholar]
- 48.Andersen O.M., Schmidt V., Spoelgen R., Gliemann J., Behlke J., Galatis D., McKinstry W.J., Parker M.W., Masters C.L., Hyman B.T., et al. Molecular Dissection of the Interaction between Amyloid Precursor Protein and its Neuronal Trafficking Receptor SorLA/LR11. Biochemistry. 2006;45:2618–2628. doi: 10.1021/bi052120v. [DOI] [PubMed] [Google Scholar]
- 49.Noda Y., Kuzuya A., Tanigawa K., Araki M., Kawai R., Ma B., Sasakura Y., Maesako M., Tashiro Y., Miyamoto M., et al. Fibronectin Type III Domain-Containing Protein 5 Interacts with APP and Decreases Amyloid Β Production in Alzheimer’s Disease. Mol. Brain. 2018;11:61. doi: 10.1186/s13041-018-0401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisenberg E., Greene L.E. Multiple Roles of Auxilin and hsc70 in Clathrin-Mediated Endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 51.Yim Y.I., Sun T., Wu L.G., Raimondi A., De Camilli P., Eisenberg E., Greene L.E. Endocytosis and Clathrin-Uncoating Defects at Synapses of Auxilin Knockout Mice. Proc. Natl. Acad. Sci. USA. 2010;107:4412–4417. doi: 10.1073/pnas.1000738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edvardson S., Cinnamon Y., Ta-Shma A., Shaag A., Yim Y.I., Zenvirt S., Jalas C., Lesage S., Brice A., Taraboulos A., et al. A Deleterious Mutation in DNAJC6 Encoding the Neuronal-Specific Clathrin-Uncoating Co-Chaperone Auxilin, is Associated with Juvenile Parkinsonism. PLoS ONE. 2012;7:e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beilina A., Bonet-Ponce L., Kumaran R., Kordich J.J., Ishida M., Mamais A., Kaganovich A., Saez-Atienzar S., Gershlick D.C., Roosen D.A., et al. The Parkinson’s Disease Protein LRRK2 Interacts with the GARP Complex to Promote Retrograde Transport to the Trans-Golgi Network. Cell. Rep. 2020;31:107614. doi: 10.1016/j.celrep.2020.107614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarouchlioti C., Parfitt D.A., Li W., Gittings L.M., Cheetham M.E. DNAJ Proteins in Neurodegeneration: Essential and Protective Factors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20160534. doi: 10.1098/rstb.2016.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alabi S.B., Crews C.M. Major Advances in Targeted Protein Degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 2021;296:100647. doi: 10.1016/j.jbc.2021.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pei J., Wang G., Feng L., Zhang J., Jiang T., Sun Q., Ouyang L. Targeting Lysosomal Degradation Pathways: New Strategies and Techniques for Drug Discovery. J. Med. Chem. 2021;64:3493–3507. doi: 10.1021/acs.jmedchem.0c01689. [DOI] [PubMed] [Google Scholar]
- 57.Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., et al. Classification of Primary Progressive Aphasia and its Variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKeith I.G., Boeve B.F., Dickson D.W., Halliday G., Taylor J.P., Weintraub D., Aarsland D., Galvin J., Attems J., Ballard C.G., et al. Diagnosis and Management of Dementia with Lewy Bodies: Fourth Consensus Report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group Under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 60.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The Diagnosis of Dementia due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diagnostic and Statistical Manual of Mental Disorders: DSM-5™. 5th ed. American Psychiatric Publishing, Inc; Arlington, VA, USA: 2013. pp. 1–947. [DOI] [Google Scholar]
- 63.Farrer L.A., Myers R.H., Connor L., Cupples L.A., Growdon J.H. Segregation Analysis Reveals Evidence of a Major Gene for Alzheimer Disease. Am. J. Hum. Genet. 1991;48:1026–1033. [PMC free article] [PubMed] [Google Scholar]
- 64.Fostinelli S., Ciani M., Zanardini R., Zanetti O., Binetti G., Ghidoni R., Benussi L. The Heritability of Frontotemporal Lobar Degeneration: Validation of Pedigree Classification Criteria in a Northern Italy Cohort. J. Alzheimers Dis. 2018;61:753–760. doi: 10.3233/JAD-170661. [DOI] [PubMed] [Google Scholar]
- 65.Goldman J.S., Farmer J.M., Wood E.M., Johnson J.K., Boxer A., Neuhaus J., Lomen-Hoerth C., Wilhelmsen K.C., Lee V.M., Grossman M., et al. Comparison of Family Histories in FTLD Subtypes and Related Tauopathies. Neurology. 2005;65:1817–1819. doi: 10.1212/01.wnl.0000187068.92184.63. [DOI] [PubMed] [Google Scholar]
- 66.Cacace R., Heeman B., Van Mossevelde S., De Roeck A., Hoogmartens J., De Rijk P., Gossye H., De Vos K., De Coster W., Strazisar M., et al. Loss of DPP6 in Neurodegenerative Dementia: A Genetic Player in the Dysfunction of Neuronal Excitability. Acta Neuropathol. 2019;137:901–918. doi: 10.1007/s00401-019-01976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smolders S., Philtjens S., Crosiers D., Sieben A., Hens E., Heeman B., Van Mossevelde S., Pals P., Asselbergh B., Dos Santos Dias R., et al. Contribution of Rare Homozygous and Compound Heterozygous VPS13C Missense Mutations to Dementia with Lewy Bodies and Parkinson’s Disease. Acta Neuropathol. Commun. 2021;9:25. doi: 10.1186/s40478-021-01121-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Rheenen W., Pulit S.L., Dekker A.M., Al Khleifat A., Brands W.J., Iacoangeli A., Kenna K.P., Kavak E., Kooyman M., McLaughlin R.L., et al. Project MinE: Study Design and Pilot Analyses of a Large-Scale Whole-Genome Sequencing Study in Amyotrophic Lateral Sclerosis. Eur. J. Hum. Genet. 2018;26:1537–1546. doi: 10.1038/s41431-018-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Spek R.A.A., van Rheenen W., Pulit S.L., Kenna K.P., van den Berg L.H., Veldink J.H., Project MinE ALS Sequencing Consortium The Project MinE Databrowser: Bringing Large-Scale Whole-Genome Sequencing in ALS to Researchers and the Public. Amyotroph Lateral Scler. Front. Degener. 2019;20:432–440. doi: 10.1080/21678421.2019.1606244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing Next-Generation DNA Sequencing Data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reumers J., De Rijk P., Zhao H., Liekens A., Smeets D., Cleary J., Van Loo P., Van Den Bossche M., Catthoor K., Sabbe B., et al. Optimized Filtering Reduces the Error Rate in Detecting Genomic Variants by Short-Read Sequencing. Nat. Biotechnol. 2011;30:61–68. doi: 10.1038/nbt.2053. [DOI] [PubMed] [Google Scholar]
- 72.Witvliet D.K., Strokach A., Giraldo-Forero A.F., Teyra J., Colak R., Kim P.M. ELASPIC Web-Server: Proteome-Wide Structure-Based Prediction of Mutation Effects on Protein Stability and Binding Affinity. Bioinformatics. 2016;32:1589–1591. doi: 10.1093/bioinformatics/btw031. [DOI] [PubMed] [Google Scholar]
- 73.Blum M., Chang H.Y., Chuguransky S., Grego T., Kandasaamy S., Mitchell A., Nuka G., Paysan-Lafosse T., Qureshi M., Raj S., et al. The InterPro Protein Families and Domains Database: 20 Years On. Nucleic Acids Res. 2021;49:D344–D354. doi: 10.1093/nar/gkaa977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capriotti E., Fariselli P., Casadio R. I-Mutant2.0: Predicting Stability Changes upon Mutation from the Protein Sequence or Structure. Nucleic Acids Res. 2005;33:W306–W310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng J., Randall A., Baldi P. Prediction of Protein Stability Changes for Single-Site Mutations using Support Vector Machines. Proteins. 2006;62:1125–1132. doi: 10.1002/prot.20810. [DOI] [PubMed] [Google Scholar]
- 76.Lee S., Emond M.J., Bamshad M.J., Barnes K.C., Rieder M.J., Nickerson D.A., NHLBI GO Exome Sequencing Project—ESP Lung Project Team. Christiani D.C., Wurfel M.M., Lin X. Optimal Unified Approach for Rare-Variant Association Testing with Application to Small-Sample Case-Control Whole-Exome Sequencing Studies. Am. J. Hum. Genet. 2012;91:224–237. doi: 10.1016/j.ajhg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S., Fuchsberger C., Kim S., Scott L. An Efficient Resampling Method for Calibrating Single and Gene-Based Rare Variant Association Analysis in Case-Control Studies. Biostatistics. 2016;17:1–15. doi: 10.1093/biostatistics/kxv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon request.