Abstract
Background
Novel immunotherapeutic strategies targeting the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) axis are often administered when metastatic tumors show PD-L1 positivity, even in the setting of lung cancer brain metastasis (LCBM). However, biological differences exist between primary tumors and metastatic sites. The objective of this study was to analyze rates of PD-L1 receptor discordance between primary tumors and LCBM.
Methods
A systematic review of studies of biopsied or resected LCBM evaluating PD-L1 discordance published in the Medline database was performed using PRISMA guidelines. Weighted random effects models were used to calculate pooled estimates.
Results
Six full-text articles (n = 230 patients) with a median of 32 patients in each study (range: 24–73) reported PD-L1 receptor expression analyses of both primary lung tumors and brain metastases and met inclusion criteria. The pooled estimate for tumor cell (TC) PD-L1 receptor discordance between primary tumors and LCBM was 19% (95% confidence interval [CI]: 10–27%). For PD-L1 receptor expression in tumor-infiltrating lymphocytes (TIL), the weighted pooled estimate for discordance was 21% (95% CI: 8–44%). For primary versus LCBM, the positive rates by expression levels of <1%, 1–50%, and >50% were 52% (95% CI: 30–73%) versus 56% (95% CI: 34–76%), 30% (95% CI: 22–40%) versus 20% (95% CI: 10–35%), and 15% (95% CI: 6–36%) versus 22% (95% CI: 15–31%) (P = .425), respectively.
Conclusions
PD-L1 discordance occurs in ~20% of LCBM, with the greatest discordance in the 1–50% expression category. Although controversial, confirming discordance might be important for selection of immune checkpoint inhibitor therapy and in the analysis of patterns of failure after treatment.
Keywords: brain, discordance, lung cancer, metastasis, PD-L1, receptor
Key Points.
The pooled estimate for TC PD-L1 receptor discordance between primary tumors and LCBM was 19% (95% CI: 10–27%).
For PD-L1 receptor expression in TIL, the weighted pooled estimate for discordance was 21% (95% CI: 8–44%).
PD-L1 discordance occurs in ~20% of LCBM, with the greatest discordance in the 1–50% expression category
Importance of the Study.
Novel immunotherapeutic strategies targeting the PD-1/PD-L1 axis are often administered when metastatic tumors show PD-L1 positivity, even in the setting of LCBM. However, brain metastases from NSCLC demonstrate variable response rates to anti-PD-L1 therapy, and potentially discordant from patients derived clinical benefit from the therapy. Understanding the potential reasons may help in improving patient selection for these therapies and evaluating response to treatment. Growing evidence suggests that PD-L1 expression varies in response to the tumor microenvironment. A thorough examination of PD-L1 expression is necessary in order to determine the best treatment options. The objective of this study was to analyze rates of PD-L1 receptor discordance in both TC and TIL between primary tumors and LCBM.
Lung cancer accounts for nearly half of patients diagnosed with brain metastasis (BM) in adults. Resection, whole brain radiotherapy, and stereotactic radiosurgery remain the standard-of-care options.1 Increasingly, blood–brain barrier penetrating second- and third-generation receptor tyrosine kinase inhibitors targeting specific signaling pathways are being considered for up-front use, especially for small, asymptomatic brain metastases.2
Novel immunotherapeutic strategies, such as those targeting the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) axis, have shown promising results in patients with metastatic lung cancer and are often administered when tumors show PD-L1 positivity.3 These agents have been demonstrated to be effective in non-small cell lung cancer (NSCLC) in multiple studies; consequently, recent guidelines reflect the importance of selecting an immunotherapy treatment regimen based on the PD-L1 receptor status.4 However, brain metastases from NSCLC demonstrate variable response rates to anti-PD-L1 therapy, and potentially discordant from patients derived clinical benefit from the therapy.5 Understanding the potential reasons may help in improving patient selection for these therapies and evaluating response to treatment.
Growing evidence suggests that PD-L1 expression varies in response to the tumor microenvironment.6 In one recent study, PD-L1 expression varied depending on metastatic locations and histological transformation.7 A thorough examination of PD-L1 expression is necessary in order to determine the best treatment options. The differential PD-L1 expression in primary lung tumors and corresponding BM has only been addressed in a few studies. While the optimum criteria for selecting immune checkpoint inhibitors are still being debated, the tumor cell (TC) PD-L1 expression in general predicts a higher likelihood of response.8 In addition, although most legacy studies have focused on the expression of PD-L1 in TC alone, emerging reports indicate the significance of PD-L1 expression in tumor-infiltrating lymphocytes (TIL) for predicting the response to anti-PD-1/PD-L1 inhibitors in breast cancer.9 Few studies have looked into the discordance of PD-L1 expression in TC and TIL between primary lung cancers and BM.10 Therefore, the objective of this meta-analysis was to analyze PD-L1 receptor discordance in both TC and TIL between the primary tumor and lung cancer brain metastasis (LCBM). To the best of our knowledge, this is the first meta-analysis assessing PD-L1 expression for both TC and TIL in primary lung cancer and associated BM.
Methods
Selection of Articles
All studies of biopsied or resected LCBM evaluating PD-L1 discordance that were published prior to June 2021 in the Medline database were systematically reviewed using PRISMA guidelines.11 MEDLINE (PubMed) and Cochrane electronic bibliographic databases were queried to identify appropriate published studies. Additional studies were included after review of the bibliographies of the selected articles. To achieve a thorough initial search, key words included “lung cancer” and “brain metastasis” combined with “programmed death ligand 1/PD-L1,” “receptor discordance,” and “receptor concordance.” Only full-text publications written in English were considered for further evaluation.
The initial inquiry yielded 269 publications which were screened by careful review of all pertinent details. All original articles of 15 adult patients and above directly reporting PD-L1 expression status in primary lung tumors compared to LCBM and receptor conversion/discordance were included in this analysis. Nonclinical papers, expert opinions, commentaries, studies without data on <15 patients, and reports that only compared receptor discordance between extracranial metastases and the primary tumor were excluded. Publications in other languages or available only in abstract form were not included. A thorough review of the references of the retrieved articles was also conducted. Duplicate studies were reviewed for new information, with the most recent report with the most patients being included in the final analysis. Supplementary Figure 1 depicts the search approach for this report as well as the study inclusion technique.
The year of publication, single center or multi-institutional study, duration of the study period, number of patients included, median age, sex (male/female), smoking status (smoker/never smoker), and histology (NSCLC/small cell lung cancer [SCLC]) were all abstracted for this analysis. The timing of BM (synchronous/metachronous) was documented, and chemotherapy, radiotherapy, and corticosteroid usage prior to BM surgery was noted.
These studies included the assay used for assessing the PD-L1 expression and the criteria for PD-L1 expression scoring. Various cutoff levels for PD-L1 expression in TC and TIL between primary tumors and LCBM, such as <1%, 1–50%, and >50%, were extracted. The PD-L1 expression concordance and discordance between primary tumors and LCBM in both TC and TIL was documented.
Outcome Measures and Statistical Analysis
PD-L1 expression status at diagnosis of the primary tumor and of LCBM was extracted for both TC and TIL. A trichotomized approach of scoring, using <1%, 1–50%, and >50% expression, was utilized. We defined PD-L1 concordance (c) as “concordance = [{number of patients expressing PD-L1 both in the primary tumor and also in the BM (sometimes also known as ‘positive concordance’, p) + number of patients not expressing PD-L1 both in the primary tumor and the BM (sometimes also known as negative concordance”, n)}/total number of patients evaluated for receptor expression in the primary and BM, t] × 100 (c = [(p+n)/t] × 100). Discordance (d) was defined as = 100- concordance (d = 100-c). Discordance would include the following scenarios: A. Primary expresses receptor, but the BM does not. B. Primary does not express the receptor, but the BM does.
R version 4.1.0 and R package metafor were used for statistical analyses.12 We used method proposed by DerSimonian and Laird to estimate variances and weighted random effects models to calculate pooled estimates.13 Due to the heterogeneity of studies included in the analysis, we used the random effects model, instead of the fixed effects model, when calculating pooled estimates.14 For identifying heterogeneity, we used I2 statistic; values of 0%, 25%, 50%, and 75% were inferred as absent, low, moderate, and high heterogeneity, respectively.15 For identification of publication bias, Funnel plots and the Egger test were used; a P value of <.05 indicated presence of publication bias. Finally, meta-regression analysis was used to detect associations between selected covariates and PD-L1 expression status.
Results
We identified 6 full-text articles (n = 230 patients) that met inclusion criteria with a median of 32 (range: 24–73) patients in each study (Table 1). All patients had at least one intracranial lesion biopsy or excision, which was compared to the primary tumor. There was no evidence of publication bias (P > .05) across the included reports (Supplementary Figure 2). All of the studies included were retrospective and should be considered hypothesis-generating evidence. Four of 6 studies were single-institution reports and 2 were multi-institutional.
Table 1.
Primary Lung Cancer and Brain Metastases PD-L1 Study Details and Patient Characteristics
| Author | Year | Institution | Years | Evidence Quality | N | Median Age | Sex | Smoking status | Histology | BM Timing | CT prior Lung Sx | RT prior Lung Sx | Steroid prior BM Sx | CT prior BM Sx | RT prior BM Sx | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Smoker | Never smoker | NSCLC | SCLC | Synchronous | Metachronous | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |||||||
| Mansfield et al.16 | 2016 | Single-center | 1994-2015 | Low | 73 | 61 | 40 | 33 | 58 | 15 | 56 | 17 | 8 | 5 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Bherghoff et al. 17 | 2016 | Single-center | 1990-2010 | Low | 32 | 58 | NA | NA | NA | NA | 0 | 32 | NA | NA | NA | NA | NA | NA | NA | NA | 15 | 17 | 3 | 29 |
| Takamori et al. 18 | 2018 | Multi-center | 2005-2016 | Low | 15 | 64 | 21 | 11 | 23 | 9 | 30 | 2 | NA | NA | 3 | 12 | NA | NA | NA | NA | NA | NA | 13 | 2 |
| Zhou et al. 19 | 2018 | Single-center | 2006-2014 | Low | 25 | 57 | 18 | 7 | 7 | 5 | NA | NA | 11 | 14 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Teglasi et al. 20 | 2019 | Single-center | NA | Low | 61 | 60 | 30 | 31 | 56 | 5 | NA | NA | NA | NA | 4 | 57 | 2 | 59 | 37 | 15 | 32 | 29 | 5 | 56 |
| Batur et al. 10 | 2019 | Multi-center | NA | Low | 24 | NA | 20 | 4 | NA | NA | 22 | 2 | 24 | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
BM, brain metastasis; CT, chemotherapy; N, number; NA, not available; NSCLC, non-small cell lung cancer; RT, radiotherapy; SCLC, small cell lung cancer; Sx, surgery.
The included literature did not report key patient features, demographics, or treatment information in a uniform or consistent manner for all patients. Across all studies, 60% of patients were male with a median age of 60 years (range: 57–64). The majority of patients (81%) were smokers, with 67% NSCLC and 33% SCLC. The BM interval across studies was recorded as synchronous in 43 patients, metachronous in 19 patients, and unknown/not reported in 168 patients. Only one study reported steroid usage (37 patients) prior to BM surgery. The most commonly used assay across studies was the Dako assay for PD-L1 expression in primary tumor and LCBM (Table 2).
Table 2.
Primary Lung Cancer and Brain Metastasis PD-L1 Expression and Discordance Status
| Author | Year | N | Assay name | Criteria for PD-L1 expression measurement | PDL1 expression in TC (Lung) | PDL1 expression in TIL (Lung) | PDL1 in lung | PDL1 expression in TC (BM) | PDL1 expression in TIL (BM) | PDL1 in BM | Concordance in Lung/BM PDL1 TC (n [%]) | Discordance in Lung/BM PDL1 TC (n [%]) | Concordance in Lung/BM PDL1 TIL (n [%]) | Discordance in Lung/BM PDL1 TIL (n [%]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1 to 50 | >50 | <1 | 1 to 50 | >50 | Positive | Negative | <1 | 1 to 50 | >50 | <1 | 1 to 50 | >50 | Positive | Negative | |||||||||
| Mansfield et al.16 | 2016 | 73 | Leica Bond RX stainer | PDLI in >5% | NA | NA | NA | NA | NA | NA | 32 | 41 | NA | NA | NA | NA | NA | NA | 24 | 49 | 63 (86%) | 10 (14%) | 54 (74%) | 19 (26%) |
| Bherghoff et al. 17 | 2016 | 32 | Dako assay/ IHC | PDLI in >5% | NA | NA | NA | NA | NA | NA | 24 | 8 | NA | NA | NA | NA | NA | NA | 11 | 21 | 22 (70%) | 10 (30%) | NA | NA |
| Takamori et al. 18 | 2018 | 15 | Dako assay/ IHC | PDLI in >5% | 10 | 3 | 2 | NA | NA | NA | 4 | 11 | 11 | 1 | 3 | NA | NA | NA | 3 | 12 | 11 (75%) | 4 (25%) | NA | NA |
| Zhou et al. 19 | 2018 | 25 | Shuwen Biotech Co | PDLI in >5% | NA | NA | NA | NA | NA | NA | 17 | 8 | NA | NA | NA | NA | NA | NA | 16 | 9 | 18 (72%) | 7 (28%) | 15 (60%) | 10 (40%) |
| Teglasi et al. 20 | 2019 | 61 | Ventana | PDL1 for Tumor Cell 1%, 5%, and 50% | 39 | 18 | 4 | 34 | 20 | 6 | 61 | 0 | 40 | 10 | 11 | 38 | 19 | 4 | 61 | 0 | NA | NA | NA | NA |
| Batur et al. 10 | 2019 | 24 | Dako assay/ IHC | PDL1 for Tumor Cell 1%, 5%, and 50% | 6 | 9 | 9 | 6 | 13 | 5 | 18 | 6 | 7 | 9 | 8 | 6 | 16 | 2 | 17 | 7 | 23 (98%) | 1 (2%) | 23 (98%) | 1 (2%) |
BM, brain metastasis; IHC, immunohistochemistry; N, number; NA, not available; PD-L1, programmed death-ligand 1; TC, tumor cell; TIL, tumor-infiltrating lymphocytes.
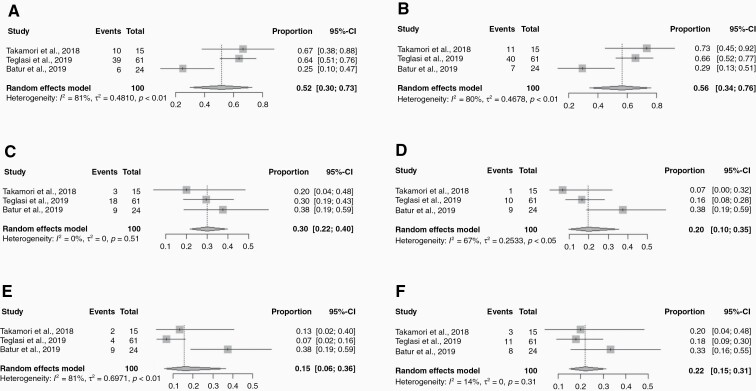
The pooled estimate for overall PD-L1 receptor concordance in TC between primary and LCBM was 81% (95% CI: 70–88%) (Figure 1A). The PD-L1 receptor positivity rate varied when analyzed by various expression levels. For <1%, 1–50%, and >50% PD-L1 expression, the positivity rates for primary tumors versus BM were 52% (95% confidence interval [CI]: 30–73%) versus 56% (95% CI: 34–76%), 30% (95% CI: 22–40%) versus 20% (95% CI: 10–35%), and 15% (95% CI: 6–36%) versus 22% (95% CI: 15–31%) (P = .425), respectively (Figure 2). The overall pooled estimate for PD-L1 positivity in the primary lung tumor was 59% (95% CI: 42–74%), with a negative rate of 41% (95% CI: 26–58%). In LCBM, the PD-L1 positivity rate was 64% (95% CI: 24–91%) and negative in 36% (95% CI: 9–76%). The overall pooled estimate for overall PD-L1 discordance between primary and LCBM was 19% (95% CI: 10–27%) (Figure 1B).
Figure 1.
Forest plots of concordance and discordance rates of PD-L1 expression in tumor cell (TC) and tumor-infiltrating lymphocytes (TIL) for primary lung tumor and brain metastasis (BM); (A) Concordance between PD-L1 expression in TC for primary lung tumor and BM; (B) Discordance between PD-L1 expression in TC for primary lung tumor and BM; (C) Concordance between PD-L1 expression in TIL for primary lung tumor and BM; (D) Discordance between PD-L1 expression in TIL for primary lung tumor and BM. The square boxes correspond to proportions from each study and the size of the box corresponds to the weight of each study, and horizontal line represents 95% confidence interval (CI). The diamonds are pooled estimates of the outcomes with 95% CI.
Figure 2.
Forest plots of various cutoff levels of PD-L1 expression in tumor cells (TC) for primary lung tumor and brain metastasis (BM): (A) Lung PD-L1 <1%; (B) BM PD-L1 <1%; (C) Lung PD-L1 1–50%; (D) BM PD-L1 1–50%; (E) Lung PD-L1 >50%; (F) BM PD-L1 >50%. The square boxes correspond to proportions from each study and the size of the box corresponds to the weight of each study, and horizontal line represents 95% confidence interval (CI). The diamonds are pooled estimates of the outcomes with 95% CI.
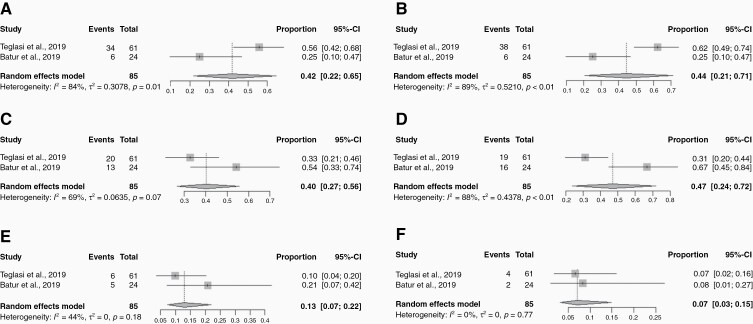
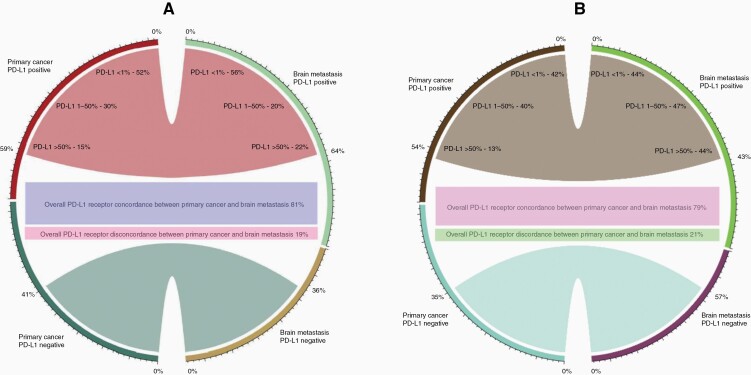
For PD-L1 TIL receptor positivity, the weighted pooled concordance estimate was 79% (95% CI: 56–92%) (Figure 1C). The PD-L1 receptor positivity rate varied when analyzed by various expression levels. For <1%, 1–50%, and >50% PD-L1 expression in TILs, the positivity rates for primary tumors versus BM were 42% (95% CI: 22–65%) versus 44% (95% CI: 21–71%), 40% (95% CI: 27–56%) versus 47% (95% CI: 24–72%), and 13% (95% CI: 7–22%) versus 7% (95% CI: 3–15%) (P = .042) (Figure 3). The weighted pooled estimate for overall PD-L1 discordance between primary and LCBM TILs was 21% (95% CI: 8–44%) (Figure 1D). Figure 4 illustrates a chordial representation of the PD-L1 expression between primary tumors and LCBM in TCs and TILs. Meta-regression analysis showed that patient factors such as age, sex, smoking status, and histology were not associated with PD-L1 receptor discordance.
Figure 3.
Forest plots of various cutoff levels of PD-L1 expression in tumor-infiltrating lymphocytes for primary lung tumor and brain metastasis (BM): (A) Lung PD-L1 <1%; (B) BM PD-L1 <1%; (C) Lung PD-L1 1–50%; (D) BM PD-L1 1–50%; (E) Lung PD-L1 >50%; (F) BM PD-L1 >50%. The square boxes correspond to proportions from each study and the size of the box corresponds to the weight of each study, and horizontal line represents 95% confidence interval (CI). The diamonds are pooled estimates of the outcomes with 95% CI.
Figure 4.
Chordial diagram illustrating the PD-L1 expression between primary tumor and brain metastasis; (A) tumor cells, (B) tumor-infiltrating lymphocytes.
Discussion
BM occur frequently in NSCLC patients; approximately 10% of NSCLC patients have BM at the time of diagnosis, and 30% develop intracranial relapse through the course of their disease.21 The FDA recently approved pembrolizumab, nivolumab, atezolizumab, and nivolumab plus ipilimumab as first-line treatment for metastatic NSCLC, and these treatments have shown to be efficacious and tolerable. Various immune checkpoint inhibitors and their approved indications are summarized in Supplementary Table 1. In a recent autopsy study, Suda et al. reported that PD-L1 expression heterogeneity was dependent on the site of the metastatic lesion.7
Although immune checkpoint inhibitors are now widely used in the treatment of lung cancer,22 several clinical trials still exclude patients with BM.23 PD-L1 is a predictive biomarker for immune checkpoint treatment response.24 However, the selection criteria for anti PD-1/PD-L1 therapy are still contentious, their predictive value in the context of LCBM is mostly unknown. PD-L1 expression in TCs and TILs could potentially affect the response to anti PD-1/PD-L1 therapy. We note that PD-L1 expression frequently changed between primary and LCBM, with discordance in TC and TIL reported in 19% and 21% of patients, for a total pooled discordance rate of approximately 20%. If a strong relationship between PD-L1 expression in BM and treatment response is established, this biomarker could become crucial.
Differences in antibodies and IHC platforms for PD-L1 have generated concerns regarding the comparability and diagnostic utility of these assays.25 SP142, SP263, 28-8, and 22C3 are the 4 FDA-approved antibodies for PD-L1 immunohistochemistry (IHC).26 The SP142 assay was found to be an outlier in most PD-L1 IHC comparative studies, as it stained less TCs than the other 3 assays. However, TIL staining for PD-L1 varied between all the 4 assays.26 The biomarker evaluation positivity thresholds also varied between studies and could be a cause of variation in the subgroup analysis. Different IHC scoring methods, such as combined positive score, tumor proportion score, and immune cell proportion score, which are widely used for IHC assessment of immunological checkpoints, could also potentially explain the variability between studies.27 The PD-L1 discordance was more common in studies using immune cell proportion score, which could be related to changes in micro-environmental immune infiltration between primary and metastatic tumors.28
Pembrolizumab, an anti-PD-1 drug, was approved as first-line treatment for NSCLC patients with ≥50% TC PD-L1 expression.29 The 5% cutoff threshold was used in a number of CheckMate studies,30 with more recent studies adopting a PD-L1 expression of 1% or greater and less than 1% cutoff level.31 We observed that the greatest discordance in PD-L1 expression between primary and LCBM was noted in the 1–50% PD-L1 expression category for TC (30% vs 20%) and in the >50% PD-L1expression category for TIL (13% vs 7%). It is worthwhile to be mindful of this discrepancy, particularly when selecting an immune checkpoint inhibitor therapy, as it may aid to analyze potential failure trends in LCBM patients. Although the outcomes of concordant or discordant brain metastasis cases were not uniformly described, the clinical significance and relevance of PDL-1 expression discordance remains of key interest. In select studies, outcomes were compared among patient subgroups. For example, Takamori et al. reported that the PD-L1-positive BM group had a substantially shorter brain-specific disease-free survival than the PD-L1-negative BM group (P < .05).18 However, the OS did not differ significantly between the PD-L1 positive and negative BM groups (P = .33). According to Berghoff et al., patients with PD-L1 expression on TILs for BM had a better survival prognosis (29 vs 6 months; P = .002).17 Furthermore, the presence of PD-L1 in more than 5% of viable tumor cells showed no correlation with survival. None of the studies included in the meta-analysis reported response and outcomes between discordant and nondiscordant cases, which could be taken into account in future studies.
Lung cancer is frequently treated with various systemic therapy and radiation therapy; therefore, it is crucial to examine if the BM TC PD-L1 positivity changes are consequential to exposure to various therapies. Studies have shown that the PD-L1 conversion rate is higher in metachronous compared to synchronous metastases, which could possibly be related to therapeutic selection pressure.20 Takamori et al. reported that smoking status and BM irradiation were both linked with PD-L1 positivity rates in the BM.18 Several in vivo and in vitro preclinical studies have shown that radiation up-regulates PD-L1 expression in BM.32 Furthermore, experimental studies have revealed that irradiation facilitates CD8+ T-cell recruitment, which is associated with intratumoral PD-L1 expression.33,34 In this analysis, we did not find an association between patient factors such as age, sex, smoking status, histology, and prior treatment with chemotherapy, radiotherapy, and corticosteroid usage with PD-L1 discordance.
The current study has several limitations. Because the included studies were retrospective, some inherent methodologic bias was unavoidable. Second, due to the small number of patients involved in some of the reports, the pooled analysis of conversion rates demonstrates significant heterogeneity. Third, most studies did not adequately document systemic therapy and radiation therapy details for each individual, nor did they investigate the effect of these treatments on PD-L1 conversion. Fourth, the reported studies contained few patients overall and therefore the overall sample size of this study appears low. Fifth, the response rates and outcomes between discordant and nondiscordant cases were not reported in the studies. For further validation, high-quality studies with prospective designs, large sample sizes, and detailed reporting of patient and tumor biology characteristics are required in the future.
Conclusion
PD-L1 status discordance in TCs and TILs occurs in approximately 20% of LCBM, with the greatest discordance in the 1–50% expression category in TC between primary and LCBM. Assessing PD-L1 expression in both the primary tumor and the LCBM is highly recommended for providing an informed clinical decision on immune checkpoint treatment.
Supplementary Material
Acknowledgment
The authors also wish to thank Lisa Rosen for her writing assistance presented in Society for Neuro-Oncology Virtual Conference on Brain Metastases, August 19, 2021, held in association with the AANS/CNS Section on Tumors.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authorship Statement. Conception and design: R.T., M.R., and R.K. Analysis: M.R. Critical review of manuscript: R.T., M.R., H.A., M.T., M.H., Y.O., M.W.M.D., M.S.A., M.P.M., and R.K.
Conflict of interest statement. H.A.: Consulting for Novocure. M.T.: Institutional research funding from Blue Earth Diagnostics Ltd. M.H.: Honorarium from Accuray, Inc. Proton Collaborative Group Executive Committee Institutional Representative and Voting Member, Miami Cancer Institute (unpaid). Grant Funding: Live Like Bella Pediatric Cancer Research Initiative, Florida Department of Health Grant 8LA04. Y.O.: No conflicts of interest; Trial Support (BMS, Novocure), DSMC (GammaTile, Actuate, Oncoceutics/Chimerix), Advisory Board (Novocure, Abbvie), and Consulting (Abbvie). M.W.M.: Consulting for Deinde Medical and Stryker Corporation. M.S.A.: Receipt of grants/research supports: Astrazeneca, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Mimivax, Merck. Receipt of honoraria or consultation fees: Bayer, Novocure, Kiyatec, Insightec, GSK, Nuvation, Cellularity, Apollomics, SDP Oncology, Prelude, Janssen. Stock shareholder: Doctible, Mimivax, Cytodyn, MedInnovate Advisors LLC. M.P.M.: Consulting for Karyopharm, Sapience, Zap, Mevion, Xoft. Board of Directors: Oncoceutics. R.K.: Honoraria from Accuray Inc., Elekta AB, Viewray Inc., Novocure Inc., Elsevier Inc, and Brainlab. Institutional research funding from Medtronic Inc., Blue Earth Diagnostics Ltd., Novocure Inc., GT Medical Technologies, Astrazeneca, Exelixis, Viewray Inc, and Brainlab. R.T. and M.R. declared no conflict of interest.
References
- 1. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. [DOI] [PubMed] [Google Scholar]
- 2. Soffietti R, Ahluwalia M, Lin N, Rudà R. Management of brain metastases according to molecular subtypes. Nat Rev Neurol. 2020;16(10):557–574. [DOI] [PubMed] [Google Scholar]
- 3. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 Expression in lung cancer. J Thorac Oncol. 2016;11(7):964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanna NH, Schneider BJ, Temin S, et al. . Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38(14):1608–1632. [DOI] [PubMed] [Google Scholar]
- 5. Téglási V, Reiniger L, Fábián K, et al. . Evaluating the significance of density, localization, and PD-1/PD-L1 immunopositivity of mononuclear cells in the clinical course of lung adenocarcinoma patients with brain metastasis. Neuro Oncol. 2017;19(8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer. 2016;99:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suda K, Murakami I, Yu H, et al. . Heterogeneity in immune marker expression after acquisition of resistance to EGFR kinase inhibitors: analysis of a case with small cell lung cancer transformation. J Thorac Oncol. 2017;12(6):1015–1020. [DOI] [PubMed] [Google Scholar]
- 8. Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2(9):1217–1222. [DOI] [PubMed] [Google Scholar]
- 9. Kim A, Lee SJ, Kim YK, et al. . Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep. 2017;7(1):11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Batur S, Dulger O, Durak S, et al. . Concordance of PD-L1 expression and CD8+ TIL intensity between NSCLC and synchronous brain metastases. Bosn J Basic Med Sci. 2020;20(3):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 14. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 16. Mansfield AS, Aubry MC, Moser JC, et al. . Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berghoff AS, Ricken G, Wilhelm D, et al. . Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J Neurooncol. 2016;130(1):19–29. [DOI] [PubMed] [Google Scholar]
- 18. Takamori S, Toyokawa G, Okamoto I, et al. . Clinical significance of PD-L1 expression in brain metastases from non-small cell lung cancer. Anticancer Res. 2018;38(1):553–557. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Gong Z, Jia Q, Wu Y, Yang ZZ, Zhu B. Programmed death ligand 1 expression and CD8+ tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498(4):751–757. [DOI] [PubMed] [Google Scholar]
- 20. Téglási V, Pipek O, Lózsa R, et al. . PD-L1 expression of lung cancer cells, unlike infiltrating immune cells, is stable and unaffected by therapy during brain metastasis. Clin Lung Cancer. 2019;20(5):363–369.e2. [DOI] [PubMed] [Google Scholar]
- 21. Palmer JD, Trifiletti DM, Gondi V, et al. . Multidisciplinary patient-centered management of brain metastases and future directions. Neurooncol Adv. 2020;2(1):vdaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist. 2017;22(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu Y, Zeng D, Ou Q, et al. . Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open. 2019;2(7):e196879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chae YK, Pan A, Davis AA, et al. . Biomarkers for PD-1/PD-L1 blockade therapy in non-small-cell lung cancer: is PD-L1 expression a good marker for patient selection? Clin Lung Cancer. 2016;17(5):350–361. [DOI] [PubMed] [Google Scholar]
- 25. Büttner R, Gosney JR, Skov BG, et al. . Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35(34):3867–3876. [DOI] [PubMed] [Google Scholar]
- 26. Rimm DL, Han G, Taube JM, et al. . A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3(8):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirsch FR, McElhinny A, Stanforth D, et al. . PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222. [DOI] [PubMed] [Google Scholar]
- 28. Lotfinejad P, Kazemi T, Mokhtarzadeh A, et al. . PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020;259:118297. [DOI] [PubMed] [Google Scholar]
- 29. Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. 2017;6(2):196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators . First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ready N, Hellmann MD, Awad MM, et al. . First-line Nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong X, Li X, Jiang T, et al. . Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1085–1097. [DOI] [PubMed] [Google Scholar]
- 33. Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother. 2014;63(3):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen J, Cao Y, Markelc B, Kaeppler J, Vermeer JA, Muschel RJ. Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J Clin Invest. 2019;129(10):4224–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.