Abstract
The aim of the present study was to assess applicability of metabolomics analysis of exudate from chicken breast muscle to explanation of differences in drip loss. The research was carried out on the skinless breast fillets sourced from 60 broiler carcasses (7-wk-old male Ross broilers). In the meat samples the pH value, color parameters, drip loss, chemical composition, and sensory quality were evaluated. After measuring, the samples were divided into 2 groups taking into consideration the volume of drip loss (low ≤2% and high >2% drip loss). The muscle juice samples were collected during 24 h muscle storage and metabolomic analysis was performed. The results showed that chickens with higher drip loss were characterized by heavier carcasses. The meat with higher drip loss proved to be more acid, lighter, less red, and more yellow with higher level of glucose as well as glycolytic potential. That meat was also characterized by lower cooking loss, protein content and worse overall sensory quality as well as oxidation of lipids. The metabolomics analyses have shown that in the group with higher drip loss from muscle tissue the increase of metabolism of energy transformations taking place in muscle tissue after slaughter was observed and that differences between groups are related to 11 metabolic pathways, mainly carbohydrate metabolism (glycolysis, gluconeogenesis, pentose phosphate pathway) adenine and adenosine salvage, adenosine nucleotides degradation, arsenate detoxification, methylglyoxal degradation. Finally, the results indicate that in the group with higher drip loss and with deeper glycolysis, more methylglyoxal (as a by-product of carbohydrate metabolism) is produced which may lead to changes of muscle proteins properties and contribute to an increase in drip loss.
Key words: poultry meat quality, drip loss, metabolomics, proteomics
INTRODUCTION
In the last 20 years the production and consumption of poultry meat have increased and it is expected that in a few coming years the poultry meat will become the main type of meat (40%) produced in the world. In 2020, the consumption of poultry meat in the world averaged around 15 kg per person per year (Statistical Yearbook of Agriculture, 2020). Poultry meat is willingly eaten by consumers due to its high nutritional value (high level of protein with its high biological value, low fat, balanced n-6 to n-3 PUFA ratio), high tenderness, relatively low price and easy culinary preparation as well as the absence of religious limitations to consume this kind of meat (Zampiga et al., 2018). This dynamic increase of production was possible due to intensive selection for growth rate and feed conversion rate (Kuttappan et al., 2017). The intensive selection resulted in changes of body composition (i.e., increasing breast yield and lowering carcass fatness) (Nadaf et al., 2007). However, these improvements have also led to the deterioration of meat quality. The results of multiple studies show that the quality of poultry meat has recently decreased, which could be observed due to the appearance of defects such as: PSE-like, white stripping (WS), wooden breast (WB), Oregon disease, spaghetti meat defect (SM), or increased drip loss (Nadaf et al., 2007; Kuttappan et al., 2017; Schilling et al., 2017; Zampiga et al., 2018). In order to explain such myopathies modern science, for instance genomics, proteomics, and metabolomics recently has been applied (Beauclercq et al., 2017; Kuttappan et al., 2017; Boerboom et al., 2018; Zampiga et al., 2018; Pampouille et al., 2019). The genomic research concerning WB and WS myopathies showed many differences in expression level of genes related to numerous metabolic pathways and consequently indicated that the selection for increased feed efficiency and growth rate might have changed expression and molecular pathways in breast muscle, resulting in an increased incidence and severity of muscle abnormalities (Zampiga et al., 2018; Pampouille et al., 2019). Additionally, that study gives a new perspective on myopathy diagnosis and applications of genetic markers for selection and meat quality improvements (Alnahhas et al., 2016; Beauclercq et al., 2017; Pampouille et al., 2019). Similar conclusions can be made based on proteomics and metabolomics approach (Abasht et al., 2016; Kuttappan et al., 2017; Boerboom et al., 2018). Final results show unique metabolic pathways and may identify potential biomarkers for diagnostic utilization and selection directions for applications in commercial breeding. Similar studies, according to myopathies of WS or WB, were made for analysis of the effect of ultimate pH on chicken meat quality (Alnahhas et al., 2016; Beauclercq et al., 2017). It must be underlined that higher drip loss like in PSE meat is very important parameter related to water holding capacity and technological as well as sensory quality (Garcia et al., 2010; Saelin et al., 2017; Kaić et al., 2021). The amount of excessive drip loss affects both qualitative and quantitative aspects of a muscle, and causes significant economic losses in the meat industry (Saelin et al., 2017). Therefore, research is being conducted on the determinants of drip loss and its limitation (Kaić et al., 2021). Drip loss effect on meat quality by applications of metabolomic analysis of exudate has not been studied from that perspective.
The aim of the present study was to assess applicability of metabolomics analysis of exudate from breast muscle to explanation of differences in drip loss. It is hypothesized that the analysis of metabolome of muscle juice can explain causes of increased drip loss from chicken meat and indicates specific markers for meat quality diagnosis.
MATERIALS AND METHODS
Material
The research was made on the skinless breast fillets sourced from 60 broiler carcasses (7-wk-old male Ross broilers, approximately 2.5 kg body weight) selected from a commercial broiler processing plant. The birds were stunned by electrical method in water bath with average value per bird 200 mA and 800 Hz during min. 4 s – in accordance with the European Union Council Regulations (EC) No 1099/2009. Then the broilers were bled, feathers removed and the broilers were gutted. Directly after removing the carcasses, they were cooled following immersion method. Carcasses were collected 20 min postmortem. Exactly 24 h postmortem Pectoralis major muscles were cut from the carcasses. After measuring pH24 the muscle samples were put into plastic bags, placed in a box with ice and transported to the laboratory for further analysis. Muscle exudate was collected after 24 h of samples storage at 4°C. After measuring, the samples were divided into 2 groups taking into consideration the volume of drip loss (low ≤2% and high >2% drip loss). The basis for dividing samples into 2 groups were considered on the basis of own experiences and literature data (Saelin et al., 2017; Kaić et al., 2021). The drip loss above 2% is considered as excessive and economically unfavorable (Gorsuch and Alvarado, 2010). Then for chemical analysis the samples were frozen at −-80°C and stored.
Methods
Technological and Sensory Quality of Meat
The pH value was measured 20 min after slaughter (pH1) and 24 h (pH24) after slaughter with a WTW 330i pH meter (Weilheim, Germany) with electrodes (SenTix SP number 103645), it was measured directly in meat (in triplicate).
Meat color was measured 48 h postmortem according to the CIE L*a*b* system using a CR310 Minolta Chroma meter (Osaka, Japan) with a D65 light source. Meat chops (4 × 4 cm) were cut and bloomed for 1 h at 4°C with no surface covering prior to color measurements (in triplicate).
The drip loss percentage was determined during 24 h of storing the meat samples (weighing approximately 100 g) according to Honikel (1987)). During that time, the samples were placed in plastic bags and kept at 4°C for the drip loss to appear. The drip loss was collected into Eppendorf vials.
Muscle glucose (mmol/L) was set with an Accu-Check Active glucometer (Accu-Check Sensor Comfort, Roche, Germany), reactive strips were applied for the quantitative determination of glucose with the apparatus in the value range between 0.6 and 33.3 mmol/L. The results were obtained after placing a drop of 20 μL of drip loss on a reactive strip. Lactic acid (mmol/L) was established with the strip method using Accutrend lactate type 3012522 (Roche, Mannheim, Germany). The samples were diluted with distilled water to reach the adequate lactate concentration. The test results were also obtained 60 s after placing a drop on a reactive strip. The measurements were made twice. Muscle glycolytic potential (GP) was calculated according to the formula proposed by Monin and Sellier (1985) summing glucose and lactic acid and expressed as mmol lactic acid of fresh muscle tissue.
The cooking loss of meat was determined based on weight method.
Meat sensory quality was evaluated after heat treatment (75°C using thermometer TP-151-125-2-SPEC, Poland) with Quantitative Descriptive Analysis (ISO 13299, 2016) and an unstructured, linear graphical scale of 100 mm, which was later converted to numerical values (0–10 conventional units: c.u.). Sensory evaluation was conducted by the trained 10-person assessing panel, which had 4 to 16 yr practice in the field of sensory evaluation, good knowledge of used methodology and meat products sensory characteristics. Each evaluation was made twice (2 sessions).
Chemical Composition
The chemical determination was performed in an accredited laboratory of chemical analyses. All the methods were validated prior to their routine use. Fat content was obtained by the Soxhlet method (ISO 1444, 2000) with ether extraction. The individual sample, as raw meat, was about 5 g. Analysis of the samples was performed in triplicate. Protein content was established according to ISO standard: ISO 1871, 2009 (The Kjeldahl method; FOSS Tecator 1035 Analyzer). The individual sample was 0.2 to 0.4 g. The sample was mineralized in sulfuric acid with addition of Kjeldahl tabs as catalyst. The content of nitrogen was obtained by titration with HCl. Each sample was analyzed in triplicate.
Lipid Oxidation
The level of lipid oxidation in raw meat was evaluated using the TBA method – based on the content of malondialdehyde (MDA) (Shahidi, 1990). Two g of minced meat and 5 cm3 10% trichloric acid (TCA) were put into a centrifuge tube. The mixture was subjected to intensive mixing. Next, 5 cm3 0.02 M 2-tiobarbituric acid (TBA) solution was added to the mixture and again the content of the tube was mixed. Afterward, the tubes were centrifuged (4,000 rpm). After centrifuging, the content of the tubes was filtered into glass tubes. The tubes were covered with plastic foil and put in a boiling water bath for 35 min in order to develop the color. Simultaneously, the reagent test was prepared. The content of the tubes was cooled in cold water. After cooling, the absorbance was measured in solutions at a wavelength of λ = 532 nm in a spectrophotometer (Thermo Scientific, Waltham, MA, Genesys 20). The results were expressed as the content of MDA in meat [mg/kg]. Each sample of meat was evaluated in duplicate. The level of lipid oxidation was measured in meat 45 d after slaughter.
The Metabolomics Analysis
The 10 samples from each group were taken for metabolomics analyses. The selected samples reflected the mean values for the entire group.
Formic acid LC-MS grade was purchased from Sigma-Aldrich (St. Louis, MO). UHPLC-MS grade methanol (MeOH) was purchased from Chem-Solve S.Witko (Lodz, Poland). Ultra high purity water was prepared by system R5 UV Hydrolab (Wislina, Poland).
Extracts Samples for Metabolomics Analysis
Prior to UPLC-MS analysis, the extracts samples (50 µL) were diluted with prechilled methanol/ultra high purity water 1: 1 (1,500 µL), vortex mixed (10 min), and centrifuged at 13,000 rpm for 15 min at 4°C. The supernatants were subjected to the UPLC Q-TOF/MS system for analysis. A pooled “quality control” (QC) sample was prepared by mixing equal aliquots (20 μL) from all extract samples for the optimization of the chromatographic and TOF/MS conditions. The analysis was performed using a Waters Acquity Ultra Performance LC system (Waters Corp., Milford, MA) connected to a Synapt G2Si Q-TOF mass spectrometer (Waters MS Technologies, Manchester, UK) equipped with an electrospray (ESI) source (Waters, UK). An ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters, Ireland) was used with a ACQUITY UPLC BEH C18, 1.7 μm, VanGuard Pre-Column 3/Pk, 2.1 × 5 mm (Waters, Ireland). The injection volume was 5 μL and the separation was performed at 0.4 mL/min and 50°C. The gradient mobile phase was a mixture of 0.1% formic acid in water (A) and in methanol (B). The gradient elution of B was performed as follows: 0 to 2 min, 1% B; 2 to 6 min, 1 to 25% B; 6.0 to 10 min, 25 to 80% B; 10 to 12 min, 80 to 90% B; 12 to 21 min, 90 to 99.9% B; 21 to 23 min, 99.9% B; 23 to 24 min 99 to 1% B; 24 to 26 min 1% B. The electrospray ionization (ESI) source was operated in the positive and negative modes. The profile data from m/z 50 to 1200 were recorded. Nitrogen gas was used as the cone and desolvation gas. The desolvation gas flow was set at 900 L h−1 at a temperature of 350°C while the cone gas was set at 50 L h−1 and the source temperature was set at 120°C. In the positive ion mode, the capillary voltage was 3.2 kV and in the negative ion mode, the capillary voltage was 2.4 kV. All of the data were acquired using the lock spray to ensure accuracy and reproducibility. Leucine enkephalin was used as the lockmass at a scan time 0.1 s, interval 30 s and mass window ± 0.5 Da. MSE method was used for the data collection with the low collision energy (6 eV) and the high collision energy ramp (20–50 eV). Prior to the analysis, the QC sample ran 7 times first to test the stability of the instrument. During the analytical run the QC sample was injected after every 5 experimental samples to monitor the system consistency.
Statistical Analysis
Data of metabolomics analysis were analyzed by freely available interactive XCMS Online https://xcmsonline.scripps.edu. The raw data files were processed for chromatogram alignment, retention-time correction, peak detection, metabolite feature annotation, statistical comparison, and putative identification. The default XCMS parameter set for UPLC – High Res (Waters) with G2S MS was used with feature detection (method CentWave, maximal tolerated m/z deviation in consecutive scans 15 ppm, minimum peak width 2 s, maximum peak width 25 s), retention time correction (method obiwarp, step size for profile generation 0.5 m/z), alignment (allowable retention time deviations 2 s, width of overlapping m/z slices to use for creating peak density chromatograms and grouping peaks across samples 0.01), statistics (unpaired non-parametric Mann-Whitney test, P-value threshold 0.05, fold change threshold 1.5, normalization – median fold change), annotation (ppm error 5, m/z absolute error 0.015), identification (tolerance for database search 10 ppm, pathway deviation 5 ppm). Integration of METLIN and MUMMICHOG to XCMS Online allowed for putative identification of metabolites.
Data characterizing technological quality, chemical composition and sensory quality of meat were developed using Student t test by Statistica ver. 13 software (TIBCO Software Inc. (2017). Statistica [data analysis software system], version 13. http://statistica.io). The samples were divided into 2 groups with different natural drip loss (≤2% and >2%).
RESULTS AND DISCUSSION
Meat Quality
Results presented in Table 1 show that the groups with different drip loss differ significantly as for ultimate pH, color parameters, glucose and glycolytic potential, drip loss, cooking loss, protein content, and overall sensory quality. The meat with higher drip loss proved to be more acid, lighter, less red, and more yellow with higher level of glucose as well as glycolytic potential (Table 1). That meat was also characterized by lower cooking loss, protein content and lower overall sensory quality (Table 1). The values presented in Table 1, in relation to pH, color parameters, glycolytic potential or drip loss, were within the ranges consistent with the data presented in the works of Ylä-Ajos et al. (2007), Bihan-Duval et al. (2008), Sibut et al. (2008), Zhuang et al. (2013), Alnahhas et al. (2016), Bowker et al. (2016) and Beauclercq et al. (2017). The relationship between natural drip loss and other meat quality traits has been reported in many research studies. Bowker et al. (2016) showed that meat with higher drip loss was characterized by higher cooking loss in broiler breast fillets. The negative relationship between ultimate pH and drip loss in poultry meat was shown in studies of Bihan-Duval et al. (2008), Sibut et al. (2008), Beauclercq et al. (2017) and Jlali et al. (2012). These studies also showed significant relationship between drip loss and glycolytic potential. The results presented in Table 1 show that the group with higher drip loss was characterized by lower ultimate pH and higher GP and differences in chemical composition. Numerous studies showed significant differences in drip loss and color parameters, cooking loss and protein content between normal and defective chicken meat (Mudalal et al., 2014; Bowker et al., 2016; Tasoniero et al., 2016; Cai et al., 2018). Research by Ylä-Ajos et al. (2007) and Sibut et al. (2008) also showed that lower ultimate pH was associated with higher GP. Moreover, Schilling et al. (2017) indicated significant relationship between more intensive glycolytic changes and decreasing meat quality, for example color, drip loss, or cooking loss. Sibut et al. (2008) argue that adenosine monophosphate-activated protein kinase plays an important role in controlling the regulation of muscle metabolism in chickens, especially with regard to the level of glycogen and the rate of postmortem glycolysis. Significant differences in GP, ultimate pH, lightness of meat and drip loss were reported between 2 chicken lines with lower and higher ultimate pH by Beauclercq et al. (2017) and these results are in agreement with the results presented in Table 1.
Table 1.
Characteristics of meat quality of groups with different level of drip loss.
| Item | Low drip loss | High drip loss | SEM |
|---|---|---|---|
| Weight of cold carcass (kg) | 1.79A | 2.32B | 0.10 |
| pH1 | 6.76 | 6.73 | 0.03 |
| pH24 | 6.03A | 5.85B | 0.03 |
| Color parameters: L* | 52.55A | 56.96B | 0.70 |
| a* | −2.43a | −1.97b | 0.10 |
| b* | 4.26A | 5.58B | 0.26 |
| Glucose (mmol/l) | 33.00a | 41.00b | 1.93 |
| Lactate (mmol/l) | 70.13 | 74.63 | 2.26 |
| Glycolytic potential (mmol/l) | 136.13a | 156.63b | 4.71 |
| Drip loss (%) | 1.20A | 2.22B | 0.16 |
| Cooking loss (%) | 29.65A | 32.79B | 1.65 |
| Protein (%) | 24.44A | 21.98B | 0.32 |
| Intramuscular fat (%) | 1.51 | 1.49 | 0.06 |
| Overall sensory quality (0–10 conventional units) |
7.79a | 7.12b | 0.27 |
pH1 measured 20 min after slaughter and pH24 measured 24 h after slaughter, L*,a*,b*-measured 48 h after slaughter.
Means marked with different small letters differ significantly at Pα < 0.05.
Means marked with different capital letters differ significantly at Pα < 0.01.
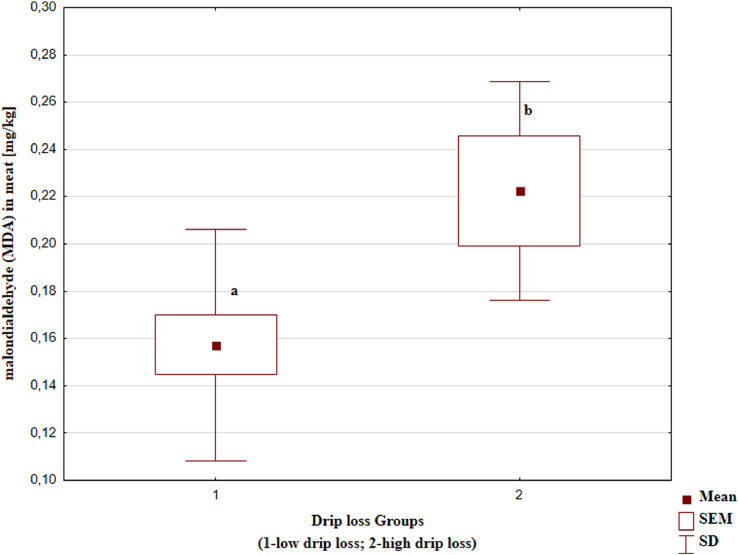
The results presented on Figure 1 also showed significant differences between groups in oxidation process during storage. The meat of chicken with higher drip loss showed higher malondialdehyde content, which is an indicator of the lipid oxidation processes in the meat. Alnahhas et al. (2016) studies also showed higher lipids oxidation in meat of chickens with lower ultimate pH and higher drip loss. Domínguez et al. (2019) report that many studies have shown higher lipid oxidation in meat with a lower ultimate pH. This could be related to the higher iron solubility in low pH and that heme-protein oxidation is favored at reduced pH. There is extensive evidence that this metal has an important role in lipid peroxidation as a primary initiator and catalyst (Amaral et al., 2018). An additional factor enhancing lipid oxidative processes may be intensive metabolic changes taking place in the muscle tissue after slaughter. The data in Table 3 show that intense oxidation and reduction processes are taking place there. Especially those related to degradation of methylglyoxal or arsenate detoxification. They contain glutathione, which is an antioxidant in many metabolic pathways. Glutathione can produce a reduction of hydroxyl, peroxy, or alkoxy radicals to hydroperoxides and glutathione disulphide. However, during this reaction O2●- can be produced, therefore, if this reactive species is not removed, the antioxidant activity of glutathione may be minimal (Domínguez et al., 2019). Additionally, the higher oxidations of lipids in group with higher drip loss may be the result of a higher content of glucose or other saccharides (fructose and pentose) and methylglyoxal, which favor the formation of advanced-glycation end products (AGEs) and by binding with their receptors of AGEs (RAGEs), can promote oxidative stress (Tables 1 and 3; Figure 2) (Shen et al., 2020). As it is stated above the meat with higher drip loss and lower pH could appear as more susceptible to oxidation process. Similar conclusion was presented in Beauclercq et al. (2017) studies that showed increased oxidative stress response in chicken meat with higher pHu in comparison to lower pHu. Research by Sundekilde et al. (2017) also indicates that the antioxidant defense system and inhibition of AGEs in defective chicken meat are lowered.
Figure 1.
The content of malondialdehyde (MDA) in the meat of chickens with different drip loss. a.b – means marked with different letters differ significantly at Pα < 0.05.
Table 3.
Significant differences in metabolites detected in natural drip loss in group with higher drip loss group in relations to lower drip loss group.
| Metabolic pathways | Higher level | Lower level |
|---|---|---|
| Glycolysis | β-D-fructofuranose 6-phosphate pyruvate |
1.3-bisphospho-D-glycerate D-glyceraldehyde 3-phosphate dihydroxyacetone phosphate phosphoenolopyruvate |
| Methylglyoxal degradation I | (R)-lactate glutathione methylglyoxal pyruvate |
- |
| Gluconeogenesis | β-D-fructofuranose 6-phosphate pyruvate |
1.3-bisphospho-D-glycerate D-glyceraldehyde 3-phosphate dihydroxyacetone phosphate phosphoenolopyruvate |
| Adenine and Adenosine salvage III | adenosine inosine |
α-D-ribose-1-phosphate IMP |
| Arsenate detoxification I (glutaredoxin) | dihydrolipoate glutathione glutathione disulfide |
- |
| Pyrimidine ribonucleosides degradation | cytidine uridine |
α-D-ribose-1-phosphate |
| Adenine and adenosine salvage I | adenosine AMP |
α-D-ribose-1-phosphate |
| Pentose phosphate pathway | β-D-fructofuranose 6-phosphate D-erythrose 4-phosphate |
D-glyceraldehyde 3-phosphate D-ribulose 5-phosphate D-xylulose 5-phosphate |
| Methylglyoxal degradation VI | (R)-lactate methylglyoxal pyruvate |
- |
| Adenosine nucleotides degradation | adenosine AMP inosine |
α-D-ribose-1-phosphate |
| Sucrose degradation | β-D-fructofuranose 1-phosphate D-glyceraldehyde |
D-glyceraldehyde 3-phosphate dihydroxyacetone phosphate |
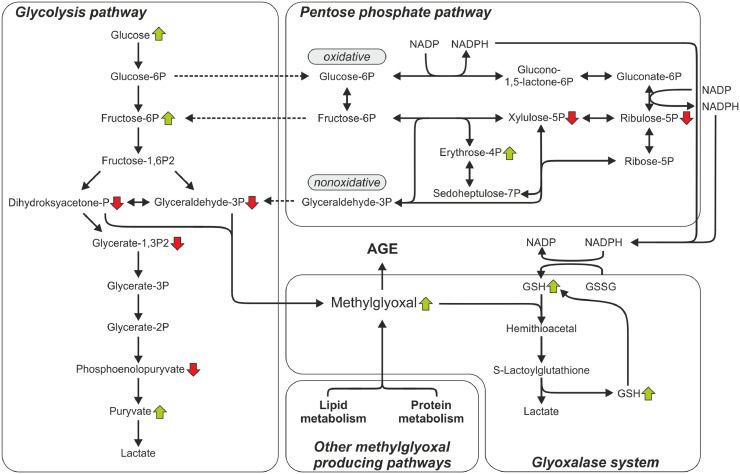
Figure 2.
The results of metabolomics analysis of carbohydrate metabolism in relations to production and elimination as well as the effect of methylglyoxal. ↑, level higher in group with higher drip loss in comparison to group with lover drip loss; ↓, level lower in group with higher drip loss in comparison to group with lover drip loss; NADP , Nicotinamide adenine dinucleotide phosphate; NADPH, is the reduced form of NADP; GSH, reduced glutathione; GSSG, oxidized glutathione; AGE, advanced glycation end products.
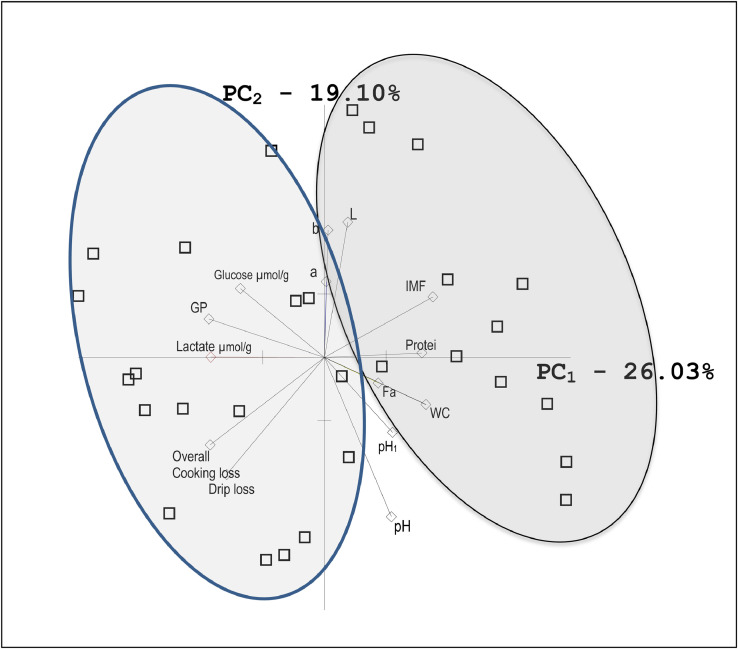
The similarities and differences in the characteristics of the studied meat traits and samples are presented using Principal Component Analysis plot on the Figure 3. The analysis based on all the examined meat quality traits clearly divided the tested samples into two groups. It should also be mentioned that chickens with higher drip loss were characterized by heavier carcasses (Table 1). It indicates that they grew faster as the age of the studied chickens was the same. Nadaf et al. (2007) also showed in their studies that the fast-growing chicken line was characterized by higher drip loss (although the difference was not statistically significant), lighter meat, less red and yellow, with lower ultimate pH. Additionally, Sibut et al. (2008) showed that meat from heavier carcasses was characterized by higher GP, lower pHu, lighter meat with higher drip loss. These results could be partly explained by the results of the study of Le Bihan-Duval et al. (2008) that showed significantly negative correlations between breast muscle mass and lightness (−0.55), drip loss (−0.65), thawing-cooking loss (−0.80), and Warner Bratzler shear force (−0.60) while body and breast muscle weights appeared to be significantly related to fiber size (0.69–0.76) and significantly negative with muscle GP (−0.58) and pHu (0.84). It is also worth mentioning that Alnahhas et al. (2016) showed a higher incidence of defective white striped meat (WS) in chickens with higher body weight and high muscle gain. They also showed that the relationship between the WS defect and pH indicated a possible relationship between the ability of muscle to store energy as carbohydrate and its likelihood of developing WS. Our results may indicate a similar relationship with respect to drip loss.
Figure 3.
Principal component analysis plot of the similarities and differences in the characteristics of the studied meat traits and samples. Abbreviations: WCC, weight of cold carcass; IMF, intramuscular fat; the ellipse on the right side of the axis groups the samples with the higher drip loss, the ellipse on the left side of the axis groups the samples with the lower drip loss.
Metabolomics Results
In order to have a better understanding of the changes occurring in the muscle tissue after slaughter in chickens, metabolomic studies of metabolites present in drip loss were applied and the results of metabolic pathway analysis are presented in Table 2. Żelechowska et al. (2012) and Di Luca et al. (2013) stated that muscle exudate provided valuable information about the pathways and processes underlying the post mortem ageing period. During the conversion of muscle to meet the shrinkage of myofibrils and as its consequence reducing the space available to hold the myowater, disintegration of cellular membranes during the development of rigor mortis as well as formation of drip channels (gaps) gives possibility to the water leakage together with solubilized components (Pearce et al., 2011). For this reason muscle juice obtained during aging reflects meat composition and provides valuable information about the pathways and processes occurring in this tissue after slaughter (Di Luca et al., 2013). Di Luca et al. (2013) and Przybylski et al. (2016) confirm that muscle exudate reflects the changes occurring in meat during aging and its composition.
Table 2.
Significant differences between groups in pathways analysis with XCMS online and MUMMICHOG.
| Metabolic pathways | Overlap size | Pathway size | P-value |
|---|---|---|---|
| Glycolysis | 6 | 7 | 0.00023 |
| Methylglyoxal degradation I | 4 | 4 | 0.00035 |
| Gluconeogenesis | 6 | 9 | 0.00039 |
| Adenine and adenosine salvage III | 4 | 5 | 0.00064 |
| Arsenate detoxification I (glutaredoxin) | 3 | 3 | 0.00098 |
| Pyrimidine ribonucleosides degradation | 3 | 3 | 0.00098 |
| Adenine and adenosine salvage I | 3 | 3 | 0.00098 |
| Pentose phosphate pathway (nonoxidative branch) | 4 | 6 | 0.00133 |
| Methylglyoxal degradation VI | 3 | 4 | 0.00279 |
| Adenosine nucleotides degradation | 4 | 7 | 0.00289 |
| Sucrose degradation | 4 | 8 | 0.00621 |
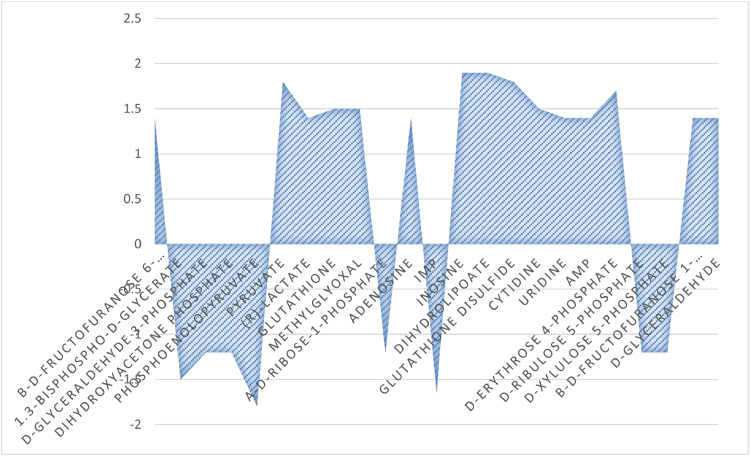
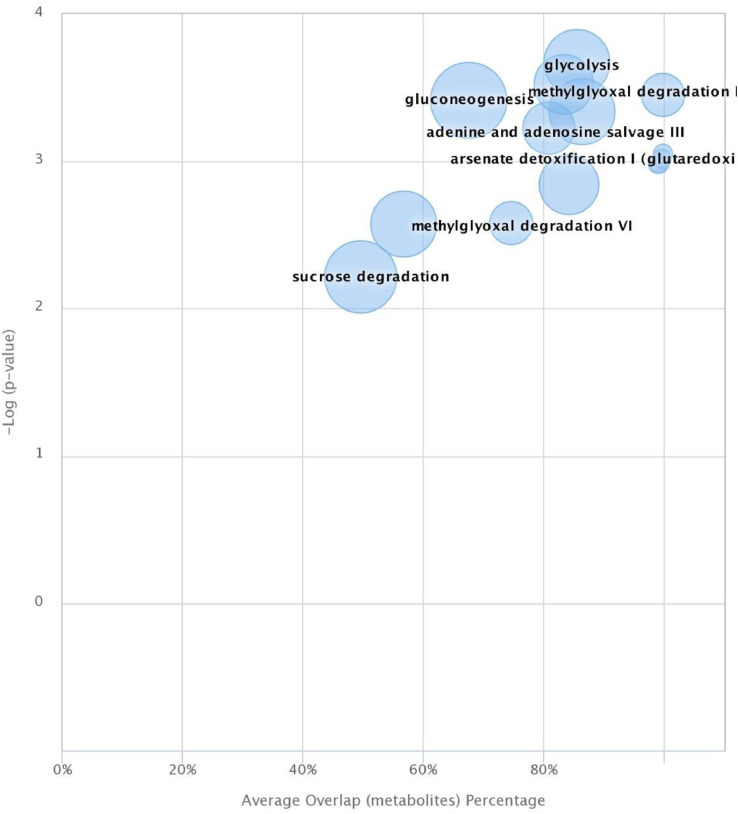
Analysis of Table 2 as well as Figure 4, Figure 5 showed significant differences between the studied groups in 11 metabolic pathways, such as: glycolysis, gluconeogenesis, sucrose degradation, adenine and adenosine salvage, adenosine nucleotides degradation, methylglyoxal degradation, penthose phosphate pathway, pyrimidine ribonucleosides degradation, arsenate detoxification. Welzenbach et al. (2016) also showed differences between meat characterized by different drip loss in 10 metabolic pathways, including glycolysis and gluconeogenesis, methylglyoxal degradation, sphingolipid metabolism and pyruvate metabolism. The presented research in metabolomics also confirm significant differences in gluconeogenesis and glycolysis metabolites between chickens with breast muscle myopathies (WS or WB) or with different ultimate pH in comparison to normal meat (Abasht et al., 2016; Beauclercq et al., 2016, 2017; Pampouille et al., 2019) while others tend to show more differences in many other different metabolic process (Boerboom et al., 2018; Pampouille et al., 2019). Moreover, Beauclercq et al. (2016, 2017) reported higher amount of glucose in the serum and of glycogen, glucose-6-phosphate and fructose 1,6-bisphosphate in muscle of chicken with lower pHu, which is similar to the results presented in Table 3 and Figure 2. In turn, Xing et al. (2020) in metabolomic studies on the conditions of the defect Wooden Breast using the technique 1H NMR spectra of muscle exudate showed eleven metabolites including amino acids, nucleotides and organic acid as the most influential metabolites affected by this myopathy.
Figure 4.
Fold changes of metabolites in drip loss of chicken meat with higher drip loss in relation to lower drip loss.
Figure 5.
Pathway cloud plot for control (low drip loss) vs. high drip loss. Plot focuses on P-value < 0.01 illustrating 11 dysregulated pathways. Each circle presents overlapping metabolite. Pathways are plotted as a function of pathway significance versus average metabolic pathway overlap. The radius of each circle represents the number of metabolites relative to the number of metabolites represented by other circles. Significantly dysregulated pathways appear in the upper right-hand quadrant of the plot.
Metabolomic studies showed significant differences between groups characterized by differentiated drip loss in the metabolism of adenosine, inosine, and AMP (Table 3). Significant differences in the level of muscle AMP were also shown in study of Beauclercq et al. (2016) between groups with different level of glycogen and ultimate pH. Wang et al. (2020) also showed higher level of glucose and inosine in breast extracts of myodegenerated muscles. Warner et al. (2015) in metabolomic studies showed significant correlation between changes in ATP, ADP, AMP, IMP, and inosine levels and the initial decrease in pH values. According to Zhang et al. (2017) in broilers stressed by transport or heat, the muscle ATP concentration as well as ATP:ADP ratio significantly decreased while simultaneously AMP concentration and AMP/ATP ratio increased and that was accompanied by the acceleration of glycolysis. In turn, Muroya et al. (2014) in studies on the metabolomic profile of glycolytic and oxidative muscles showed significant differences in the levels of adenosine, adenine and inosine; these authors attributed these differences to the diverse 5′-nucleotidase activity (NT5C, EC 3.1.3.5). AMP and IMP contents have been shown to be related to meat palatability (umami taste). In turn higher IMP and hypoxanthine content is associated with meat flavor and bitter taste. This issue may be slightly confirmed in the results of own research. The group with higher drip loss and lower level of IMP (Table 3) was characterized by lower overall sensory meat quality (Table 1). It must be pointed that overall quality is highly correlated with tenderness, juiciness, and palatability. The results presented in Table 3 indicate a higher content of adenosine and inosine in the group with a higher drip loss that is, until IMP resources, which are responsible for the aroma and flavor of meat. Abasht et al. (2016) in metabolic studies on wooden breast defects observed a reduced level of IMP, ADP, and AMP in defective meat.
Research showed that in the group with increased drip loss also the increase of metabolites associated with the transformation of arsenate detoxification in the body was observed (Table 3). It indicates disorders of the homeostasis of the body, in particular antioxidative protection. Additionally, higher level of glutathione showed in metabolomic analysis (Table 3) in this study indicated increased activity of the second level of antioxidant defence (Surai et al., 2019). The results of research presented in Table 3 also showed higher level of methylglyoxal (MG), which is formed from dihydroxyacetone phosphate (DHAP) or glyceraldehyde-3-phosphate (GAP) that came from glycolysis pathway as well as from metabolism of lipids and proteins, an important metabolite at a cross-road between several metabolic pathways (Figure 2) (Ouali et al., 2013; Allaman et al., 2015). It is estimated that 0.1 to 0.4% of the glycolytic flux results in MG production and this metabolite is highly reactive and its half-life is short in a biological environment and therefore, at the time and site of production local concentrations may by significantly higher. High levels of MG occur when the concentrations of their precursors are elevated, such as in hyperglycemia, impaired glucose utilization and triosephosphate isomerase deficiency (Allaman et al., 2015). Higher level of methylglyoxal in drip loss from chicken meat in group with higher exudate of muscle juice corresponds to a higher glucose and glycolytic potential content in this group (Tables 1 and 3). The fact that a significant amount of glucose is converted into methylglyoxal (as a by-product) during glycolysis is confirmed by the results presented in Table 1. Despite significant differences between the groups in the glucose content, no significant differences as for the level of lactic acid were obtained. As a significant proportion of glucose is metabolized to MG. Baldi et al. (2018) argues that the differences in glycolysis in poultry myopathies are related to different glucose utilization rather than glucose availability. Another source of substrates for the production of methylglyoxal is the penthose phosphate pathway providing glyceraldehyde-3P (Figure 2). As previously mentioned, higher levels of methylglyoxal increase the formation of AGEs and contribute to an increase in oxidative stress, which may result in the oxidation of lipids and proteins (Shen et al., 2020). According to Huff-Lonergan and Lonergan (2005) oxidation of myofibrillar proteins occurred in postmortem muscle during aging reduces the functionality of proteins. The summary of the results presented in Figure 1 showed that the second phase of glycolysis results in large amounts of methylglyoxal as a by-product. It triggers the defense and detoxification mechanism of methylglyoxal generating the need for NADPH and thus activates the penthose phosphate pathway (PPP) more (Stincone et al., 2015). About 10 to 30% of glucose is converted in the pentose-phosphate cycle, and even more depending on the tissue (Malinowska, 1999). There are 2 phases, oxidative and nonoxidative. The dominant type of activity depends on the metabolic state of the cell. In the situation of changes taking place in the muscle tissue after slaughter, when the demand for ATP and (as shown by the data obtained in Table 3, Figure 2) the demand for NADPH increases (the need to defend and neutralize methylglyoxal), the oxidative phase prevails and significant amounts of glyceraldehyde-3-phosphate, which feed the glycolysis process (Stincone et al., 2015) is produced. As it was mentioned earlier, MG is one of the most potent glycating agents that are very harmful. MG readily reacts with lipids, nucleic acids, and proteins to form AGEs. It causes changes in the function of proteins that also affect cells by interacting with specific AGE receptors (RAGE ). This process triggers inflammation and plays an important role in various pathophysiological mechanisms (Allaman et al., 2015). For defense, cells possess various MG detoxification mechanisms, such as the glyoxalase, aldose reductase, aldehyde dehydrogenase, and carbonyl reductase pathways (Ouali et al., 2013). The glyoxalase system is the major detoxification system for MG in eukaryotic cells (Figure 2). It plays a major role in cellular defense against glycation and oxidative stress. MG is neutralized by two sequential enzymatic reactions catalyzed by glyoxase-1 (Glo-1) and glyoxase-2 (Glo-2), using glutathione as a cofactor (Figure 2). A large increase in MG levels or low Glo-2 activity may result in the accumulation of S-D-lactoylglutathione leading to reduced GSH availability for other cellular processes, such as defense against oxidative stress (Allaman et al., 2015). The results of research indicate a significant intensification of these processes, as higher levels of MG and GSH were found in the group with higher drip loss and, at the same time, higher glucose level. The higher level of lipid oxidation found in this group (Figure 1) and the above results indicate that in the group with higher drip loss and more intense glycolysis, more MG is produced, which may lead to glycation and changes in muscle proteins. It can alter their properties and contribute to an increase in drip loss. This hypothesis is confirmed by the studies by Traore et al. (2012), who found a higher level of protein oxidation in meat with higher drip loss. The obtained results allow to present the hypothesis that the increased natural drip loss is not only the result of the glycolysis process influencing the denaturation of muscle proteins, but also the influence of side metabolites, such as MG, on changing the properties of muscle proteins. These results shed new light on how to explain the causes of increased exudate in muscle tissue characterized by high glycolytic potential, high glucose content, and low ultimate pH.
CONCLUSIONS
The results show that poultry meat with different level of drip loss differs significantly in many quality traits: ultimate pH, color parameters, glucose and glycolytic potential level, cooking loss, protein content and sensory quality. The meat with higher drip loss proved to be more acid, lighter, less red, and more yellow with higher level of glucose as well as glycolytic potential. That meat was also characterized by lower cooking loss, protein content and lower overall sensory quality as well as oxidation of lipids. The results also showed that chickens with higher drip loss were characterized by heavier carcasses. The metabolomics analyses has shown that in the group with higher drip loss from muscle tissue the increase of metabolism of energy transformations taking place in muscle tissue after slaughter was observed and that differences between groups are related to 11 metabolic pathways, mainly carbohydrate metabolism (glycolysis, gluconeogenesis, pentose phosphate pathway) adenine and adenosine salvage, adenosine nucleotides degradation, arsenate detoxification, and methylglyoxal degradation. Finally, the results indicate that in the group with higher drip loss and with deeper glycolysis, more methylglyoxal (as a by-product of carbohydrate metabolism) is produced, which may lead to changes of muscle proteins properties and contribute to an increase in drip loss.
ACKNOWLEDGMENTS
Research was financed by Polish Ministry of Science and Higher Education within funds of Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS), for scientific research.
DISCLOSURES
There are no conflicts of interest.
REFERENCES
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 2016;11:4. doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I., Bélanger M., Magistretti P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015;9:1–12. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnahhas N., Berri C., Chabault M., Chartrin P., Boulay M., Bourin M.C., Le Bihan-Duval E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genetics. 2016;17:61. doi: 10.1186/s12863-016-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral A.B., da Silva M.V., Lannes S.C.S. Lipid oxidation in meat: mechanisms and protective factors – a review. Food Sci. Technol. Campinas. 2018;38(Suppl. 1):1–15. [Google Scholar]
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Beauclercq S., Hennequet-Antier C., Praud C., Godet E., Collin A., Tesseraud S., Métayer-Coustard S., Bourin M., Moroldo M., Martins F., Lagarrigue S., Le Bihan-Duval E., Berri C. Muscle transcriptome analysis reveals molecular pathways and biomarkers involved in extreme ultimate pH and meat defect occurrence in chicken. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-06511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauclercq S., Nadal-Desbarats L., Hennequet-Antier C., Collin A., Tesseraud S., Bourin M., Bihan-Duval E.Le, Berri C. Serum and muscle metabolomics for the prediction of ultimate pH, a key factor for chicken-meat quality. J. Proteome Res. 2016;15:1168–1178. doi: 10.1021/acs.jproteome.5b01050. [DOI] [PubMed] [Google Scholar]
- Boerboom G., Kempen van T., Navarro-Villa A., Pérez-Bonilla A. Unraveling the cause of white striping in broilers using metabolomics. Poult. Sci. 2018;97:3977–3986. doi: 10.3382/ps/pey266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker B., Gamble G., Zhuang H. Exudate protein composition and meat tenderness of broiler breast fillets. Poult. Sci. 2016;95:133–137. doi: 10.3382/ps/pev312. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y.L., Nair M.N., Suman S.P., Beach C.M., Guyton M., Schilling M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Di Luca A., Elia G., Hamill R., Mullen A.M. 2D DIGE proteomic analysis of early post mortem muscle exudate highlights the importance of the stress response for improved water-holding capacity of fresh pork meat. Proteomics. 2013;13:1528–1544. doi: 10.1002/pmic.201200145. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Pateiro M., Gagaoua M., Barba F.J., Zhang W., Lorenzo J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 2019;8:429. doi: 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R.G., Freitas L.W.de, Schwingel A.W., Farias R.M., Caldara F.R., Gabriel A.M.A., Graciano J.D., Komiyama C.M., Almeida Paz I.C.L. Incidence and physical properties of PSE chicken meat in a commercial processing plant. Braz. J. Poult. Sci. 2010;12:233–237. [Google Scholar]
- Gorsuch V., Alvarado C.Z. Postrigor tumble marination strategies for improving color and water holding capacity in normal and pale broiler breast fillets. Poult. Sci. 2010;89:1002–1008. doi: 10.3382/ps.2009-00023. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. The water binding of meat. Fleischwirtschaft. 1987;67:1098–1102. [Google Scholar]
- Huff-Lonergan E., Lonergan S. Mechanism of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- ISO 1871. 2009. Food and feed products – general guidelines for the determination of nitrogen by the Kjeldahl method. ISO, Geneva, Switzerland.
- ISO 1444. 2000. Meat and meat products. Free fat contents determination. Polish Committee for Standardization, Warsaw, Poland.
- ISO 13299. 2016. Sensory Analysis. Methodology. General guidance for establishing a sensory profile. ISO, Geneva, Switzerland.
- Jlali M., Gigaud V., Métayer-Coustard S., Sellier N., Tesseraud S., Bihan-Duval E.Le, Berri C. Modulation of glycogen and breast meat processing ability by nutrition in chickens: effect of crude protein level in 2 chicken genotypes. J. Anim. Sci. 2012;90:447–455. doi: 10.2527/jas.2011-4405. [DOI] [PubMed] [Google Scholar]
- Kaić A., Janeječić Z., Žanetić A., Ugarkocić N.K., Potočnik K. EZ-drip loss assessment in chicken breast meat using different sample areas, fiber orientation, and measurement intervals. Animals. 2021;11:1095. doi: 10.3390/ani11041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Bottje W., Ramnathan R., Hartson S.D., Coon C.N., Kong B.W., Owens C.M., Vazquez-Añon. Hargis B.M. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poult. Sci. 2017;96:2992–2999. doi: 10.3382/ps/pex069. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Debut M., Berri C.M., Sellier N., Santé-Lhoutellier V., Jégo Y., Beaumont C. Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genetics. 2008;9:53. doi: 10.1186/1471-2156-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska M. SGGW Publishing House; Warsaw, Poland: 1999. Animal Biochemistry. [Google Scholar]
- Monin G., Sellier P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: the case of the Hampshire breed. Meat Sci. 1985;13:49–63. doi: 10.1016/S0309-1740(85)80004-8. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Babini E., Cavani C., Petracci M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poult. Sci. 2014;93:2108–2116. doi: 10.3382/ps.2014-03911. [DOI] [PubMed] [Google Scholar]
- Muroya S., Oe M., Nakajima I., Ojima K., Chikuni K. CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles. Meat Sci. 2014;98:726–735. doi: 10.1016/j.meatsci.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Nadaf J., Gilbert H., Pitel F., Beri C.M., Feve K., Beaumont C., Duclos M.J., Vignal A., Porter T.E., Simon J., Aggrey S.E., Cogburn L.A., Le Bihan-Duval E. Identification of QTL controlling meat quality traits in an F2 cross between two chicken lines selected for either low or high growth rate. BMC Genomics. 2007;8:155. doi: 10.1186/1471-2164-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouali A., Gagaoua M., Boudida Y., Becila S., Boudjellal A., Herrera-Mendez C.H., Sentandreu M.A. Biomarkers of meat tenderness: present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013;95:854–870. doi: 10.1016/j.meatsci.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Pampouille E., Hennequet-Antier C., Praud C., Juanchich A., Brionne A., Godet E., Bordeau T., Fagnoul F., Le Bihan-Duval E., Berri C. Differential expression and coexpression gene network analyses reveal molecular mechanisms and candidate biomarkers involved in breast muscle myopathies in chicken. Sci. Rep. 2019;9:14905. doi: 10.1038/s41598-019-51521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce K.L., Rosenvold K., Andersen H.J., Hopkins D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes – a review. Meat Sci. 2011;89:111–124. doi: 10.1016/j.meatsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Przybylski W., Sionek B., Jaworska D., Santé-Lhoutellier V. The application of biosensors for drip loss analysis and glycolytic potential evaluation. Meat Sci. 2016;117:7–11. doi: 10.1016/j.meatsci.2016.02.025. [DOI] [PubMed] [Google Scholar]
- Saelin S., Wattanachant S., Youravong W. Evaluation of water holding capacity in broiler breast meat by electrical conductivity. I. Food Res. J. 2017;24:2593–2598. [Google Scholar]
- Schilling M.W., Suman S.P., Zhang X., Nair M.N., Desai M.A., Cai K., Ciaramella M.A., Allen P.J. Proteomic approach to characterize biochemistry of meat quality defects. Meat Sci. 2017;132:131–138. doi: 10.1016/j.meatsci.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Shen C.Y., Lu C.H., Wu C.H., Li K.J., Kuo Y.M., Hsieh S.C., Yu C.L. The development of Maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related disease. Molecules. 2020;25:5591. doi: 10.3390/molecules25235591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi, F. 1990. The 2-tiobarbituric acid (TBA) methodology for the evaluation of warmed-over flavour and rancidity in meat products. 36th ICoMST, Havana, Cuba.
- Sibut V., Bihan-Duval E.Le, Tesseraud S., Godet E., Bordeau T., Cailleau-Audouin E., Chartrin P., Duclos J.M., Berri C. Adenosine monophosphate-activated protein kinase involved in variations muscle glycogen and breast meat quality between lean and fat chickens. J. Anim. Sci. 2008;86:2888–2896. doi: 10.2527/jas.2008-1062. [DOI] [PubMed] [Google Scholar]
- Stincone A., Prigione A., Cramer T., Wamelink M.M.C., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.M., Krüger A., Alam M.T., Keller M.A., Breitenbach M., Brindle K.M., Rabinovitz J.D., Raiser M. The return of metabolism: biochemistry and physiology of the penthose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistical Yearbook of Agriculture. Central Statistical Office, Warsaw, Poland, 2020.
- Sundekilde U.K., Rasmussen M.K., Young J.F., Bertram H.C. High resolution magic angle spinning NMR spectroscopy reveals that pectoralis muscle dystrophy in chicken is associated with reduced muscle content of anserine and carnosine. Food Chem. 2017;217:151–154. doi: 10.1016/j.foodchem.2016.08.104. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasoniero G., Cullere M., Cecchinato M., Puolanne E., Dalle Zotte A. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by white striping and wooden breast myopathies. Poult. Sci. 2016;96:2707–2714. doi: 10.3382/ps/pew215. [DOI] [PubMed] [Google Scholar]
- Traore S., Aubry L., Gatellier P., Przybylski W., Jaworska D., Kajak-Siemaszko K., Santé-Lhoutellier V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012;90:917–924. doi: 10.1016/j.meatsci.2011.11.033. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang Y., Pan D., He J., Cao J., Wang H., Ertbjerg P. Metabolite profile based on 1H NMR of broiler chicken breasts affected by wooden breast myodegeneration. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125852. [DOI] [PubMed] [Google Scholar]
- Warner R.D., Jacob R.H., Rosenvold K., Rochfort S., Trenerry C., Plozza T., McDonagh M .B. Altered post-mortem metabolism identified in very fast chilled lamb M. longissimus thoracis et lumborum using metabolomic analysis. Meat Sci. 2015;108:155–164. doi: 10.1016/j.meatsci.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Welzenbach J., Neuhoff C., Heidt H., Cinar M.U., Looft C., Schellander K., Tholen E., Groβe-Brinkhaus C. Integrative analysis of metabolomic, proteomic and genomic data to reveal functional pathways and candidate genes for drip loss in pigs. Int. J. Mol. Sci. 2016;17:1426. doi: 10.3390/ijms17091426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Zhao X., Xu X., Li J., Zhang L., Gao F. Physiochemical properties, protein and metabolite profiles of muscle exudate of chicken meat affected by wooden breast myopathy. Food Chem. 2020;316 doi: 10.1016/j.foodchem.2020.126271. [DOI] [PubMed] [Google Scholar]
- Ylä-Ajos M., Ruusunen M., Puolanne E. Glycogen debranching enzyme and some other factors relating to post-mortem pH decrease in poultry muscles. J. Sci. Food Agric. 2007;87:394–398. [Google Scholar]
- Zampiga M., Flees J., Meluzzi A., Dridi S., Sirri F. Applications of omics technologies for a deeper insight into quali-quantitative production tratis in broiler chickens: a review. J. Anim. Sci. Biotechnol. 2018;9:61. doi: 10.1186/s40104-018-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żelechowska E., Przybylski W., Jaworska D., Santé-Loutellier V. Technological and sensory pork quality in relation to muscle and drip loss protein profiles. Eur. Food Res. Technol. 2012;234:883–894. [Google Scholar]
- Zhang L., Wang X., Li J., Zhu X., Gao F., Zhou G. Creatine monohydrate enhances energy status and reduces glycolysis via inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers. J. Agric. Food Chem. 2017;65:6991–6999. doi: 10.1021/acs.jafc.7b02740. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Bowker B.C., Buhr R.J., Bourassa D.V., Kiepper B.H. Effects of broiler carcass scalding and chilling methods on quality of early-deboned breast fillets. Poult. Sci. 2013;92:1393–1399. doi: 10.3382/ps.2012-02814. [DOI] [PubMed] [Google Scholar]