Abstract
Manganese (Mn) is an important element; yet acute and/or chronic exposure to this metal has been linked to neurotoxicity and neurodegenerative illnesses such as Parkinson’s disease and others via an unknown mechanism. To better understand it, we exposed a human neuroblastoma cell model (SH-SY5Y) to two Mn chemical species, MnCl2 and Citrate of Mn(II) (0–2000 µM), followed by a cell viability assay, transcriptomics, and bioinformatics. Even though these cells have been chemically and genetically modified, which may limit the significance of our findings, we discovered that by using RA-differentiated cells instead of undifferentiated SH-SY5Y cell line, both chemical species induce a similar toxicity, potentially governed by disruption of protein metabolism, with some differences. The MnCl2 altered amino acid metabolism, which affects RNA metabolism and protein synthesis. Citrate of Mn(II), however, inhibited the E3 ubiquitin ligases–target protein degradation pathway, which can lead to the buildup of damaged/unfolded proteins, consistent with histone modification. Finally, we discovered that Mn(II)-induced cytotoxicity in RA-SH-SY5Y cells shared 84 percent of the pathways involved in neurodegenerative diseases.
Keywords: manganese speciation, SH-SY5Y, neurotoxicity, neurodegeneration, protein metabolism
1. Introduction
Manganese (Mn) is the twelfth most abundant element in the crust. Environmentally, it ranges from 1–200 g/L in fresh water to 410–6700 mg/kg (dry weight) in sediments [1]. Mn is a trace mineral that is found in low concentrations in legumes, pineapples, beans, nuts, tea, and cereals [2,3]. Mn is also a key cofactor for enzymes such as glutamine synthetase, pyruvate decarboxylase, serine/threonine protein phosphatase I, Mn-superoxide dismutase, and arginase [4]. Consequently, it is an essential element to maintain normal physiological development including the metabolism of lipid, protein, and carbohydrate; blood sugar regulation; bone formation; immunological response; reproduction; neurotransmitter synthesis and metabolism, as well as neuronal and glial function [3].
Local levels of Mn in the environment can be dramatically increased due to natural and human causes [5,6,7,8,9,10]. Additionally, it can be found as Mn(II), Mn(III), and Mn(IV) in aquatic systems owing to oxi-reductive processes [1,11,12,13,14,15]; these chemical species are environmentally and toxicologically relevant [10,16,17]. Occupational exposure [17] and consumption of contaminated well water [7] represent the most relevant means by which humans are exposed to Mn, with high risks for health [7,17,18,19,20], consistent with increasing evidence of developmental neurotoxicity due to oral parenteral nutrition [7,21].
Mn neurotoxicity, which is characterized by motor and sensory problems, known as manganism, as well as neuropsychiatric and cognitive impairments [3], is the most serious adverse consequence of this metal. Hypertonia with cogwheel stiffness, bradykinesia, “cock-gait”, fast postural tremor, and a tendency to stumble when walking backwards are all symptoms of parkinsonism [3]. These symptoms have been linked to an excess of Mn in the basal ganglia, especially the globus pallidus, subthalamic nucleus, substantia nigra, and striatum, which are involved in motor control and nonmotor functions [3,4]. However, additional brain areas, such as the cerebellum, red nucleus, pons, cortex, thalamus, and anterior horn of the spinal cord, may be altered by Mn exposure [14]. These cellular features, including Mn-induced mitochondrial dysfunction, inflammation, autophagy, overexpression of α-synuclein (αSyn) in vitro, and their aggregation in vivo in neurons and glial cells, have been linked to Parkinson’s disease (PD) [4]. These traits distinguish parkinsonism, which is characterized by the lack of Lewy bodies (another hallmark of PD). Surprisingly, dopaminergic neurons of the substantia nigra pars compacta are particularly destroyed following chronic or acute Mn exposure [3].
Furthermore, Mn toxicity has been connected to Huntington’s disease (HD), since cultured striatal cells surprisingly reduced the vulnerability of mutant expressing cells STHdhQ111/Q111 [22]. Furthermore, pre-manifest YAC128 transgenic mice, another model of HD, exposed to MnCl2 had a decreased response to transcriptional and protein alterations, whereas manifest YAC128 animals had a suppressed metabolic response, despite equivalent elevations in whole striatal Mn [23]. Mn has also been linked to Amyotrophic Lateral Sclerosis (ALS), since certain ALS patients have T1-weighted hyperintensity during MRI, a neuroradiological signal associated with Mn overload, as well as an increase in MnSOD levels in motor neurons and genetic variations of two melastatins, TRPM2 and 7. Whereas early study in macaques has suggested that chronic Mn treatment induces upregulation of amyloidlike protein 1 and diffuse amyloid-β plaques in the frontal cortex, perhaps implying a relationship between advanced-stage manganism and Alzheimer’s disease (AD) [4]. In addition, Mn exposure in dogs enhanced the expression of nuclear neuronal NF-B and iNOS, as well as changed blood–brain barrier (BBB) function, diffuse Aβ plaques, neurofibrillary tangles, and alteration of Mn-dependent antioxidant enzyme, as reported in nonhuman primates and humans [24]. All these alterations can be influenced by chemical fractionation and speciation, developmental stage [14,16,25], and cell type [14].
The cytotoxicity of chemical compounds of Mn and their mechanisms has been supported by several works in silico, in vitro, and in vivo. In this manner, both undifferentiated and differentiated human neuroblastoma cell models (SH-SY5Y) were employed to investigate the role of Mn in neurotoxicity [26,27,28]. However, some issues must be considered. For example, undifferentiated SH-SY5Y cells can have fluctuations in the cell cycle and are considered immature catecholaminergic neurons [29]. While retinoic acid (RA) synchronizes the cell cycle and generates a modest rate of proliferation, RA differentiates cell morphologically close to primary neurons and increases electrical excitability of the plasma membrane [29], which could leave axons more susceptible for chemical injury [30]. Furthermore, NoRA SH-SY5Y cells are disabled in ATP production [31] while RA induces survival of SH-SY5Y cells. Consequently, RA-differentiated cells are more resilient to toxins [29]. Altogether, the in vitro cell model should mimic the phenotypes and be sensitive to cellular alterations commonly verified in vivo and specifically in humans [30,31]. It this respect, it has been shown that RA differentiates human neuroblastoma SH-SY5Y cells, which generate a largely mature dopaminergic (DAergic)-like neurotransmitter phenotype found in vivo as well as other neurotransmitters in lower expression, such as noradrenaline, acetylcholine, glutamate, serotonin, and histamine [31]. This allows the study of PD, ALS, AD, and HD [31,32,33] together with the relationship between these illnesses and Mn-induced neurotoxicity [26,27,28], potentially governed by disturbance of protein synthesis [25,34,35].
Protein synthesis is an energy-intensive process that is highly controlled and tightly linked to other cellular activities such as the cell cycle and metabolic pathways [36,37]. Previous research with NoRA SH-SY5Y cells revealed that MnCl2 causes endoplasmic reticulum (ER) stress [38,39], accompanied by autophagy [38], and accumulation of parkin protein and its redistribution to aggregated Golgi complex [39]. Two other independent transcriptomics studies verified that MnCl2 induces cytotoxicity in SH-SY5Y cells by promoting mitophagy through BNIP3-mediated oxidative stress [40] and/or upregulation of apoptotic pathways, neuronal differentiation, and synaptic transmission [26]. However, they have not identified alteration in the ER–Golgi system, involved in protein metabolism before energy–mitochondrial dysfunction and cell death [28].
However, the research described above has not contemplated the significance of chemical speciation. It is well known that the MnCl2 (aqua-complex of Mn or Mn-free) can cross the brain–blood barrier easier than Citrate of Mn(II) or other species of Mn(II) or Mn(III) [41]. In cerebellar granule neurons, the MnCl2 was more bioconcentrated than Citrate of Mn(II), although both species displayed similar cytotoxicity, associated with energy–mitochondrial impairment [14]. However, this energetic dysfunction reduced the influx of the two species of Mn in rat brain [42]. In zebrafish embryos, after exposure to species of Mn(II) or Mn(III), the Mn appeared in fluids mainly as Mn-free, followed by Citrate of Mn(II), a species that induced more bioaccumulation of Mn and gene overexpression than MnCl2, but both species perturbed the calcium homeostasis and protein metabolism [16,35].
Finally, considering the issues stated above, we hypothesized that impairment of pathways linked to protein biosynthesis drives Mn-induced neurotoxicity and potentially neurodegeneration, which can be affected by chemical speciation. Thus, we aimed to develop a toxicogenomics study in the RA-differentiated SH-SY5Y-DAergic cell model, exposed to MnCl2 and Citrate of Mn(II). Using system biology approaches, we provided additional evidence that connects Mn-induced impairment of protein metabolism to Mn-neurotoxicity to neurodegenerative disorders (AD, ALS, HD, and PD).
2. Materials and Methods
2.1. Preparation of Manganese Species
Compounds of manganese (MnCl2 and Citrate of Mn(II) or Mn(II)Cit, 10 mM each) were prepared and characterized according to previous works of our group [14,16] and stored at 4 °C. For these experiments we used manganese(II) chloride tetrahydrate, MnCl2·4H2O (99.99% trace metals basis, Merck, São Paulo, SP, Brazil) and sodium citrate tribasic dihydrate (Cit; HOC(COONa)(CH2COONa)2·2H2O) (99.99% trace metals basis, Merck, São Paulo, SP, Brazil). The work solution was prepared the same day of cell exposure by diluting an aliquot to reach the desired final concentration, according to each experiment.
2.2. Human Neuroblastoma SH-SY5Y Cell Line Experimental Setup
Human neuroblastoma SH-SY5Y cells, a third generation subclone of SK-SN-SH cells, are a brain-derived catecholaminergic neuroblastoma cell line. SH-SY5Y cells differentiate into neuronlike cells and cease proliferating [43]. SH-SY5Y cells were grown in Dulbecco′s Modified Eagle′s Medium/Nutrient Mixture F-12 Ham (DMEM/F12 nutritional mixture (1:1), Merck, São Paulo, SP, Brazil) supplemented with 10% of Fetal Bovine Serum (FBS, Merck, São Paulo, SP, Brazil) and penicillin/streptomycin (50 IU/mL, Merck, São Paulo, SP, Brazil) in a humidified environment of 5% CO2 and 95% air at 37 °C. The medium was changed every 4–5 days. Serum was lowered to 2% FBS for all experimental conditions. Cells were differentiated for 7 days in the presence of 10 nM of trans-Retinoic Acid (trans-RA, Merck, São Paulo, SP, Brazil). Cultures were inspected under a microscope prior to treatment to determine differentiation. When 80 percent or more of the cells in a culture showed neurite outgrowth extensions >2–3 times longer than the cell’s body diameter, the culture was declared differentiated. In the presence or absence of the Mn species, the cells were seeded at a density of 5 × 104 cells per 100 mL in a 96-well plate format for cell viability assay and 6-well plates for array experiment.
2.3. Cell Viability Assay
Cell viability was determined in quintuplicate from three separate cell cultures using the MTT test, which measures the reduction of 3-[4,5-dimethythiazol-2-il]-2,5-diphenyl-tetrazolium bromide (MTT, Merck, São Paulo, SP, Brazil) by live cells. We conducted the assay according to Hernández et al. [14] after 1 day of treatment with MnCl2 or Citrate of Mn(II) from 0 to 2000 µM.
2.4. Transcriptomics
2.4.1. RNA Extraction and Purification
The RA-SH-SY5Y cells were treated for 24 h with 500 µM of MnCl2 or Mn(II)Cit. Then the cells were collected in RNAlater (Qiagen, Toronto, ON, Canada), and total RNA was extracted and purified from three different pools of cells using the miRNeasy mini kit (Qiagen, Toronto, ON, Canada), followed by a DNase I (Qiagen, Toronto, ON, Canada) treatment, as directed by the manufacturer. The amount of RNA was quantified using a spectrophotometer, and the quality was assessed using BioAnalyzer equipment (Agilent Technologies, Palo Alto, CA, USA). All RNA samples showed clear 18S and 28S rRNA peaks and demonstrated an RNA integrity number (RIN) level higher than 8.
2.4.2. Microarray Assays
Using an accessible and commercial human gene expression microarray kit, studies on differential gene expression were done in triplicates from three independent biological groups to discover the mode of action of each chemical species of manganese in RA-differentiated SH-SY5Y cells. The experiment was carried out in accordance with the manufacturer’s procedures for one-color microarray-based gene expression analysis (Agilent), which are accessible in: http://www.agilent.com/cs/library/usermanuals/Public/G4140-90040_GeneExpression_OneColor_6.9.pdf, accessed on 29 November 2021).
2.4.3. Validation of Toxicogenomics Results through Real-Time Reverse Transcription-PCR (qRT-PCR)
The qRT-PCR assay has been used for identification of gene alterations in previous studies [44,45]. Thus, we used this approach to verify our results about Microarray (item 2.2.2.2). Table 1 shows the primer sequences that were employed. To complete the qRT-PCR experiment, we extracted total RNA from each sample using the Qiagen RNA extraction kit, followed by cDNA production using the iScript cDNA synthesis kit with SYBR green supermix (Bio-Rad, Hercules, CA, USA), as directed by the manufacturer. qRT-PCR was used to quantify mRNA using Rotor-Gene RG-300 from Corbett research [46]. All investigations were carried out in triplicate by three different biological groups.
Table 1.
Genes selected for qRT-PCR analysis. Primer sequences and associated parameters are included.
| Gene Symbol | Gene Name | PL | Sequence (5′→3′) | TS | Length | Start | Stop | Tm | GC% | Self C. | Self 3′ C. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RPS29 | RPS29 ribosomal protein S29 | 135 | FP | ACACTGGCGGCACATATTGA | Plus | 20 | 49,585,986 | 49,586,005 | 60.04 | 50 | 4 | 2 |
| RP | GGTAGTAGCCGTCTGAGTGC | Minus | 20 | 49,586,120 | 49,586,101 | 59.9 | 60 | 4 | 3 | |||
| MT-CO1 | mitochondrially encoded cytochrome c oxidase I |
107 | FP | CCCCGATGCATACACCACAT | Plus | 20 | 7232 | 7251 | 60.18 | 55 | 6 | 2 |
| RP | TCGAAGCGAAGGCTTCTCAA | Minus | 20 | 7338 | 7319 | 59.68 | 50 | 7 | 3 | |||
| COX4I2 | cytochrome c oxidase subunit 4I2 |
118 | FP | GATGAACCGTCGCTCCAATG | Plus | 20 | 30,135,127 | 30,135,146 | 59.35 | 55 | 5 | 3 |
| RP | GATGAGGTGTTGCCACTCAC | Minus | 20 | 30,135,244 | 30,135,225 | 58.84 | 55 | 4 | 3 | |||
| MT-CYB | mitochondrially encoded cytochrome b |
134 | FP | ACCCCCTAGGAATCACCTCC | Plus | 20 | 15,366 | 15,385 | 60.03 | 60 | 6 | 1 |
| RP | GCCTAGGAGGTCTGGTGAGA | Minus | 20 | 15,499 | 15,480 | 60.03 | 60 | 6 | 2 | |||
| MT-ND4 | mitochondrially encoded NADH dehydrogenase 4 | 296 | FP | CCCCATCGCTGGGTCAATAG | Plus | 20 | 11,428 | 11,447 | 60.25 | 60 | 8 | 2 |
| RP | TAAGCCCGTGGGCGATTATG | Minus | 20 | 11,723 | 11,704 | 60.25 | 55 | 6 | 1 | |||
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase | 117 | FP | AAAGGGCCCTGACAACTCTTT | Plus | 21 | 6,538,069 | 6,538,089 | 59.78 | 47.62 | 8 | 3 |
| RP | GGTGGTCCAGGGGTCTTACT | Minus | 20 | 6,538,185 | 6,538,166 | 60.55 | 60 | 5 | 1 | |||
Product Length (PL), Forward primer (FP), Reverse primer (RP), Template Strand (TS), Self Complementarity (Self C.), Self 3′ Complementarity (Self 3′ C.).
2.5. Bioinformatics and Data Analysis
The results were presented as the mean ± SEM of at least three separate trials. Fitting sigmoidal curves (Hill slope) to concentration–response data of individual replicates and computing the mean of those replicates yielded the LC50. The D’Agostino and Pearson omnibus normality test verified that our data had a normal or statistical distribution. ANOVA (analysis of variance) and Bonferroni’s tests were employed to discover statistically significant differences. GraphPad Prism was used for fitting and statistical analysis (GraphPad 4.0 Software Inc., San Diego, CA, USA). The arrays were examined using Babelomics’ Gene Expression Pattern Analysis Suite (https://babelomics.bioinfo.cipf.es/, accessed on 13 May 2016) [47], which is an integrated web-based pipeline designed for the analysis of data generated in microarray studies. Normalization, grouping, differential gene expression, class prediction, and functional annotation are all included in the suite.
2.6. Prediction of Protein–Protein Interaction (PPI) and Gene Ontology (GO) Analysis
A PPI map is a heterogeneous network of proteins connected by interactions as edges. PPI and GO enrichment analyses were performed utilizing data from the current study, the String database (http://string-db.org, accessed on 30 November 2019) [48], and the Comparative Toxicogenomic Database—CTD (http://ctdbase.org/, accessed on 30 November 2019) [49]. Particularly, String discovers cellular pathways that are enriched in a target list of genes, proteins, or metabolites that are not approximated by the original omics data (using hypergeometric testing against either the entire genome or a user-supplied background gene list), allowing for the extraction of strong mechanistic information [48,50].
3. Results
Multiple genetic and environmental variables contribute to the onset and progression of Parkinson’s disease, which is related with the degradation of DAergic neurons in the substantia nigra pars compacta [31]. Indeed, epidemiological studies have connected the development of neurodegeneration to Mn toxicity [22,23,24,51,52]. This can be mediated by impairment of protein metabolism, according to findings in nonhuman models [25,34,35]. To test the validity of this hypothesis, we conducted an unbiased toxicogenomics study on Mn-induced acute neurotoxicity in the human model, RA-differentiated SH-SY5Y-DAergic cells.
3.1. Manganese-Induced Toxicity and Differential Gene Expression in the RA-Differentiated SH-SY5Y-DAergic Cell Model
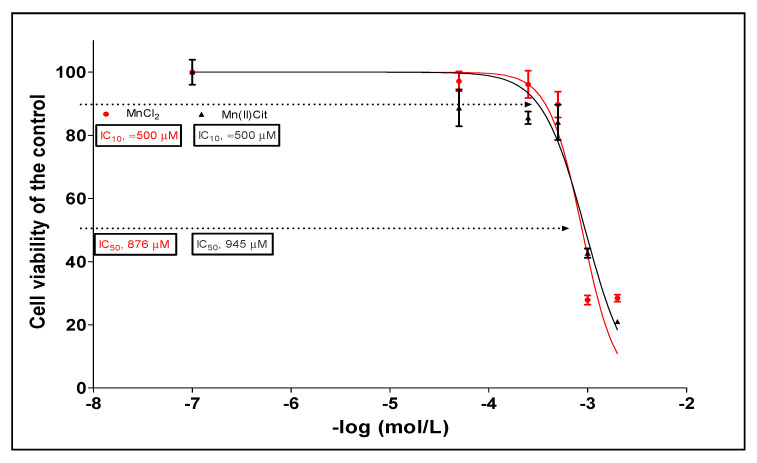
Through MTT assay we verified that both Mn species (MnCl2 and Mn(II)Cit) induced similar cytotoxicity, in a dose dependent manner, where approximately 900 and 500 µM induces 50% and 10% of cell death, respectively (Figure 1).
Figure 1.
Cytotoxicity induced by MnCl2 and Mn(II)Cit in RA-differentiated SH-SY5Y cells, after exposure for 24 h. Cell viability was quantified by MTT assay. The plot is representative of three biological replicates (mean ± sem); each experiment was made with five analytical replicates.
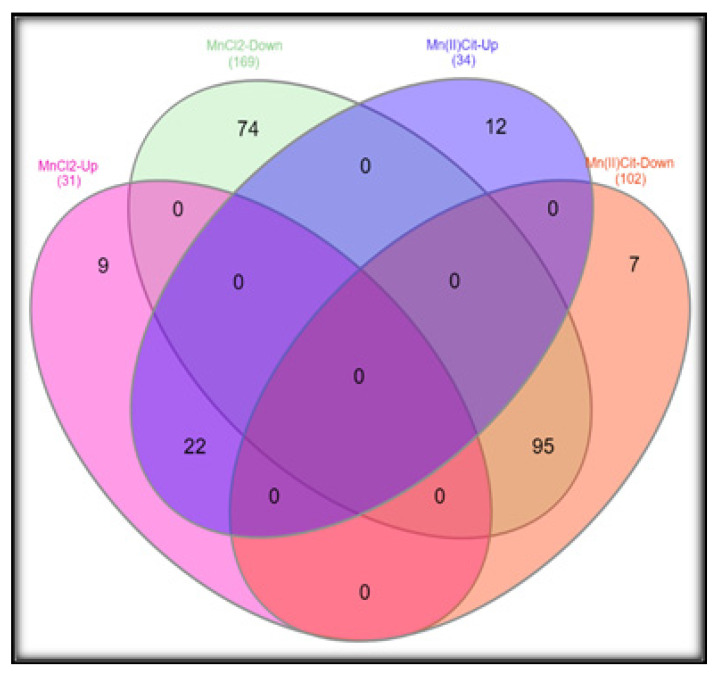
To find the mode of action of manganese, we designed a gene expression analysis in SH-SY5Y cells treated for 24 h to 500 µM of MnCl2 or Mn(II)Cit, which might reflect an environmental state of acute Mn exposure owing to water intake polluted with this metal [15,53,54]. In this way, we verified a significant (p < 0.05) impairment of 406 genes (Tables S1 and S2). There, we discovered 117 genes that are affected by both chemical species of manganese, which suppress rather than stimulate gene expression. In this case, MnCl2 reduced the expression of 169 genes (80%), while Mn(II)Cit decreased the expression of 102 genes (70%), Figure 2.
Figure 2.
Three microarray assays in SH-SY5Y after exposure to MnCl2 or Mn(II)Cit (0–500 µM) for 24 h yielded overlapping results of 117 genes differentially expressed and affected by both chemical species of Mn. Significant differences were estimated through two-way ANOVA, followed by false discovery rate and Bonferroni (p < 0.05).
3.2. Manganese-Induced Cross-Impairment of Several Pathways Associated with Neurotoxicity and Neurodegeneration
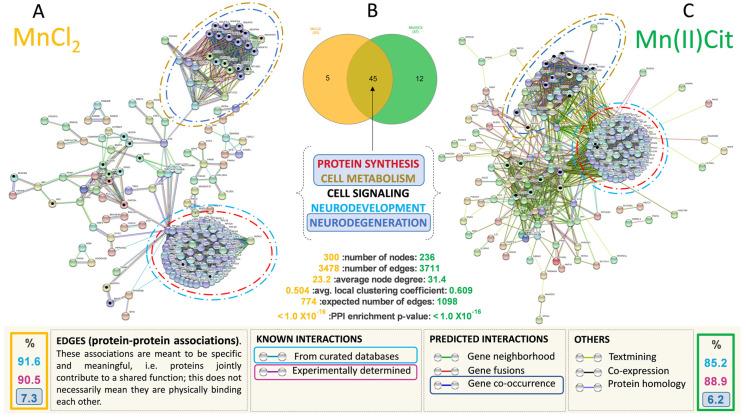
Additionally, based on all genes significantly affected under Mn stress, and using the String database [48], we used systems biology to create an enriched protein–protein interaction (PPI) map for each Mn species and an enriched gene ontology analysis. The String database studies a defined group of target proteins using physical interactions and functional relationships between proteins, and then expands the set by incorporating linked proteins to investigate toxicological pathways [48]. The network of functional Mn interactors was expanded to around 700 edges (proteins) (p-value 1.0 × 10−16), with approximately 85 percent of the new interactions experimentally validated [48]. A schematic representation of these interactors is shown in Figure 3A,C, including several processes/pathways potentially impaired by Mn (Table 2 and Table 3).
Figure 3.
(A,C) represent an inferred enriched protein–protein interactions (PPI) network for cells stressed with MnCl2 and Mn(II)Cit, respectively; p-value < 1.0 × 10−16. More than 85% of these interactions are derived experimentally. The predicted PPIs were calculated with high confidence score (0.9) and proteins/genes with no connections were removed. (B) Overlapping representation of 45 pathways that were affected by MnCl2 and Mn(II)Cit. These interactions can be categorized in Protein Synthesis, Cell Metabolism, Cell Signaling, Neurodevelopment, and Neurodegeneration groups.
Table 2.
Enriched cellular pathways influenced by MnCl2 in RA-differentiated SH-SY5Y cells, after gene ontology analysis using the String database.
| Cell Pathways | Term Description | Observed | Background | FDR |
|---|---|---|---|---|
| Gene Count | Gene Count | |||
| Protein Synthesis | Peptide chain elongation | 75 | 86 | 4.37 × 10−92 |
| Viral mRNA Translation | 75 | 86 | 4.37 × 10−92 | |
| SRP-dependent cotranslational protein targeting to membrane | 78 | 109 | 1.15 × 10−91 | |
| Eukaryotic Translation Termination | 75 | 90 | 1.22 × 10−91 | |
| Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) | 75 | 92 | 2.25 × 10−91 | |
| Formation of a pool of free 40S subunits | 75 | 98 | 5.96 × 10−90 | |
| L13a-mediated translational silencing of Ceruloplasmin expression | 75 | 107 | 5.93 × 10−88 | |
| GTP hydrolysis and joining of the 60S ribosomal subunit | 75 | 108 | 9.04 × 10−88 | |
| Ribosome | 76 | 130 | 3.15 × 10−84 | |
| Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) | 75 | 112 | 6.29 × 10−87 | |
| Major pathway of rRNA processing in the nucleolus and cytosol | 76 | 179 | 1.10 × 10−76 | |
| Metabolism of amino acids and derivatives | 77 | 354 | 2.98 × 10−59 | |
| Metabolism of RNA | 78 | 652 | 7.72 × 10−43 | |
| Formation of the ternary complex and subsequently the 43S complex | 31 | 49 | 1.27 × 10−34 | |
| Translation initiation complex formation | 31 | 55 | 1.85 × 10−33 | |
| Ribosomal scanning and start codon recognition | 31 | 55 | 1.85 × 10−33 | |
| Metabolism of proteins | 102 | 1948 | 1.12 × 10−27 | |
| Biosynthesis of amino acids | 9 | 72 | 5.98 × 10−05 | |
| Cell Metabolism | Selenocysteine synthesis | 75 | 90 | 1.22 × 10−91 |
| Selenoamino acid metabolism | 76 | 112 | 2.37 × 10−88 | |
| Metabolism | 137 | 2032 | 6.11 × 10−52 | |
| Oxidative phosphorylation | 26 | 131 | 9.48 × 10−18 | |
| The citric acid (TCA) cycle and respiratory electron transport | 28 | 173 | 7.83 × 10−18 | |
| Thermogenesis | 29 | 228 | 1.68 × 10−15 | |
| Complex I biogenesis | 17 | 55 | 8.42 × 10−15 | |
| Metabolic pathways | 50 | 1250 | 2.11 × 10−08 | |
| Glycolysis/Gluconeogenesis | 12 | 68 | 5.27 × 10−08 | |
| Fructose and mannose metabolism | 9 | 33 | 1.85 × 10−07 | |
| Metabolism of carbohydrates | 20 | 266 | 2.71 × 10−07 | |
| Carbon metabolism | 10 | 116 | 2.90 × 10−04 | |
| Cell Signaling | Retrograde endocannabinoid signaling | 18 | 148 | 2.25 × 10−09 |
| HIF-1 signaling pathway | 14 | 98 | 3.22 × 10−08 | |
| Negative regulation of MAPK pathway | 6 | 40 | 1.00 × 10−03 | |
| Neurodevelopment | Axon guidance | 79 | 541 | 3.00 × 10−49 |
| Neurodegeneration | Parkinson’s disease | 26 | 142 | 3.69 × 10−17 |
| Alzheimer’s disease | 20 | 168 | 3.22 × 10−10 | |
| Huntington’s disease | 19 | 193 | 1.57 × 10−08 |
False Discovery Rate—FDR.
Table 3.
Enriched cellular pathways influenced by Mn(II)Cit in RA-differentiated SH-SY5Y cells, after gene ontology analysis using String-db.
| Cell Pathways | Term Description | Observed | Background | FDR |
|---|---|---|---|---|
| Gene Count | Gene Count | |||
| Protein Synthesis | Peptide chain elongation | 76 | 86 | 7.69 × 10−103 |
| Viral mRNA Translation | 76 | 86 | 7.69 × 10−103 | |
| Eukaryotic Translation Termination | 76 | 90 | 2.96 × 10−102 | |
| Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) | 76 | 92 | 4.89 × 10−102 | |
| Formation of a pool of free 40S subunits | 76 | 98 | 1.34 × 10−100 | |
| L13a-mediated translational silencing of Ceruloplasmin expression | 76 | 107 | 1.58 × 10−98 | |
| GTP hydrolysis and joining of the 60S ribosomal subunit | 76 | 108 | 2.37 × 10−98 | |
| SRP-dependent cotranslational protein targeting to membrane | 76 | 109 | 3.59 × 10−98 | |
| Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) | 76 | 112 | 1.52 × 10−97 | |
| Ribosome | 77 | 130 | 6.14 × 10−95 | |
| Formation of the ternary complex and subsequently the 43S complex | 31 | 49 | 5.38 × 10−38 | |
| Translation initiation complex formation | 31 | 55 | 7.97 × 10−37 | |
| Ribosomal scanning and start codon recognition | 31 | 55 | 7.97 × 10−37 | |
| Metabolism of proteins | 88 | 1948 | 2.38 × 10−27 | |
| Biosynthesis of amino acids | 8 | 72 | 6.26 × 10−05 | |
| Senescence-Associated Secretory Phenotype (SASP) | 6 | 78 | 6.50 × 10−03 | |
| Amyloid fiber formation | 6 | 78 | 6.50 × 10−03 | |
| E3 ubiquitin ligases ubiquitinate target proteins | 5 | 53 | 7.80 × 10−03 | |
| Cell Metabolism | Selenocysteine synthesis | 76 | 90 | 2.96 × 10−102 |
| Oxidative phosphorylation | 26 | 131 | 2.08 × 10−20 | |
| Thermogenesis | 28 | 228 | 2.41 × 10−17 | |
| Metabolic pathways | 45 | 1250 | 1.71 × 10−09 | |
| Glycolysis/Gluconeogenesis | 12 | 68 | 3.66 × 10−09 | |
| Carbon metabolism | 10 | 116 | 3.67 × 10−05 | |
| Pentose phosphate pathway | 5 | 30 | 6.60 × 10−04 | |
| Starch and sucrose metabolism | 5 | 33 | 9.30 × 10−04 | |
| Galactose metabolism | 4 | 31 | 8.10 × 10−03 | |
| Endocrine resistance | 6 | 95 | 1.26 × 10−02 | |
| Cell Signaling | Retrograde endocannabinoid signaling | 16 | 148 | 2.85 × 10−09 |
| HIF-1 signaling pathway | 12 | 98 | 1.42 × 10−07 | |
| Negative regulation of MAPK pathway | 5 | 40 | 2.50 × 10−03 | |
| Neurodevelopment | Axon guidance | 78 | 541 | 5.39 × 10−57 |
| Neurodegeneration | Parkinson’s disease | 26 | 142 | 8.36 × 10−20 |
| Alzheimer’s disease | 20 | 168 | 3.71 × 10−12 | |
| Huntington’s disease | 19 | 193 | 2.88 × 10−10 |
False Discovery Rate—FDR.
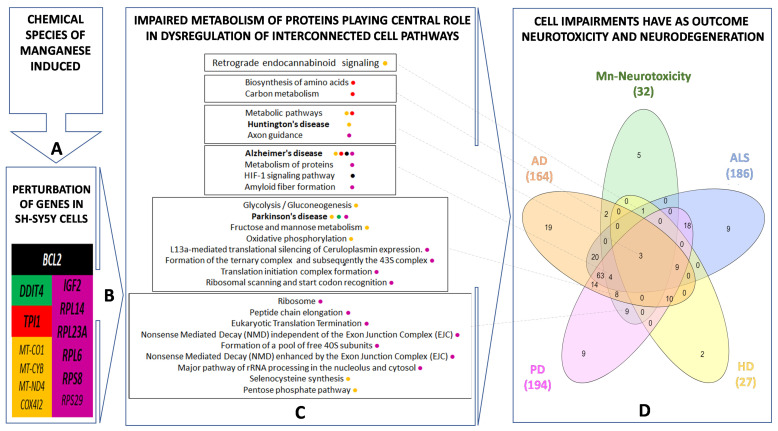
We identified that almost 50% of the pathways affected by Mn are directly linked to protein biosynthesis, including ribosomes, translation initiation, and termination. Additionally, we verified that MnCl2-induced impairment of protein metabolism involves alteration of the metabolism of amino acids. The Mn(II)Cit appears to affect the E3 ubiquitin ligases–target protein degradation pathway, which can lead to damaged/unfolded protein accumulation. This is followed by pathways associated with cell metabolism, especially energy metabolism (~30%) and cell signaling pathways (6%), Table 2 and Table 3. Although the chemical speciation has influenced the Mn-induced toxicity, we found that both species, MnCl2 and Mn(II)Cit shared impaired pathways (Figure 3B), which are similar to molecular changes linked with neurodegenerative illnesses such as AD, HD, and PD (Figure 3A,C and Table 2 and Table 3).
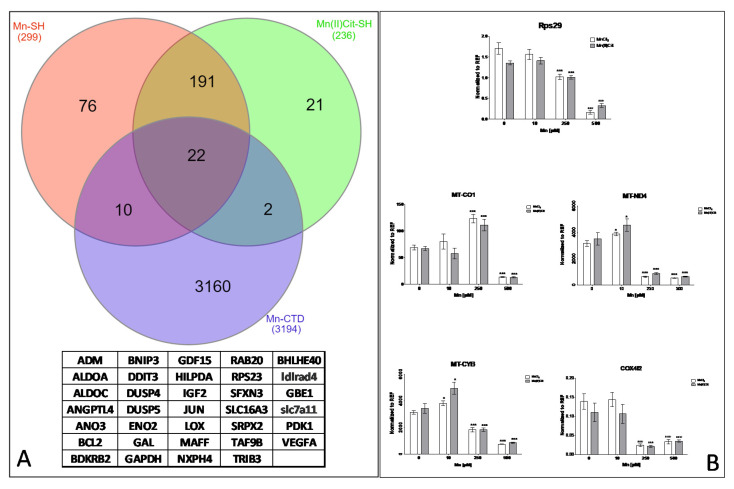
Furthermore, through an additional analysis using CTD [49], among the genes that were directly affected by Mn species (Tables S1 and S2) and the genes/proteins added by String (Figure 3A,C), we identified 34 curated genes (Figure 4A), previously affected by chemical species of Mn, including MnCl2. Again, these genes are involved in the pathways inferred in this study (Table 2 and Table 3). Moreover, we confirmed using qRT-PCR analysis that both MnCl2 and Mn(II)Cit disrupted the expression of the genes RPS29, MT-CO1, MT-ND4, MT-CYB, and COX4I2, which are associated with protein synthesis, energy metabolism, and neurodegeneration [49] (Figure 4B).
Figure 4.
(A) The enriched set of proteins identified using PPI data, including genes identified by transcriptomics analysis and added by String were analyzed using the comparative toxicogenomic data. A set of 34 curated genes were identified that previously were affected by MnCl2 or other Mn compounds. (B) Representative genes associated with protein synthesis, cell metabolism, and neurodegeneration were analyzed by qRT-PCR analysis (mean ± sem, n = 3). Statistical differences were verified by two-way ANOVA, followed by Bonferroni post test, (*, p < 0.05; ***, p < 0.001).
Lastly, our findings suggest that chemical species of Mn induce cytotoxicity in the RA-differentiated SH-SY5Y-DAergic cell model (Figure 1 and Figure 5A) through alterations of the expression in a notable number of genes (Tables S1 and S2 and Figure 5B), because of which numerous pathways are harmed (Figure 3 and Figure 5C). There, disruption of protein metabolism, especially protein synthesis, appears to be a key event for Mn-induced cell perturbation (Figure 5C) responsible for Mn-induced neurotoxicity, which may result in neurodegeneration (Figure 5D).
Figure 5.
Comparative toxicogenomics analysis for Mn-induced cytotoxicity in SH-SY5Y. Exposure of SH-SYSY cells to different chemical species of Mn (A) results in disruption of genes shown in (B). This can lead to impairment of protein metabolism that plays a central role in the dysregulation of several pathways such as cell signaling; cell metabolism, including energy pathway; Alzheimer’s Disease (AD); Huntington’s Disease (HD) and Parkinson’s Disease (PD) (C). Overlapping pathways among Mn-induced neurotoxicity and Amyotrophic Lateral Sclerosis (ALS), AD, HD, and PD is shown in (D).
4. Discussion
In this work, we found that the 876 µM of MnCl2 or 945 µM of Mn(II)Cit decreased by 50% the viability of RA-differentiated SH-SY5Y-DAergic cells. These concentrations are higher than found in studies with NoRA-SH-SY5Y cells, where 800 µM [55] and approximately 600 µM [40] of MnCl2, respectively, induced 50% of cytotoxicity after exposure for 24 h. It is known that RA-differentiated SH-SY5Y cells show activated survival pathways, including Nrf2 (nuclear factor erythroid 2-related factor 2) or Akt (serine-threonine protein kinase) signaling [29], while the NoRA-differentiated cells are ATP-deficient compared to the RA-differentiated cells [31]. This could justify why NoRa SH-SY5Y cells are more susceptible for Mn treatment, as observed by Kemsheh and Oblitey [56]. On the other hand, the RA-differentiated SH-SY5Y-DAergic cell model shows increased dopamine and other neurotransmitters, which allowed the screening of Mn-induced neurotoxicity and their association with neurodegeneration [31,32,33,57], after exposure for 500 µM of both Mn-species, a concentration that induced approximately 10% of cell death and was used in previous studies with NoRA-differentiated cells [58]. In this toxicologic context, the CTD have systematized approximately 140 Mn compounds associated with 379 pathways [49], where the top fifty are dominated by eleven cellular processes, including cell signaling, diseases, immune system, metabolism, hemostasis, metabolism of proteins, apoptosis, endocrine resistance, cellular responses to stress, and neurodevelopment. These processes are potentially connected to 1522 diseases, of which 21 are associated with the nervous system [49]. Other functional and omics studies [25,26,27,28,34,35] have identified these potential mechanisms of Mn-induced neurotoxicity, reviewed by Tinkov et al. [52]. In line with this, using microarray analysis and systems biology approaches, we verified a connection between Mn-induced acute cytotoxicity in the RA-differentiated SH-SY5Y-DAergic cell model and impairment of several pathways, including protein synthesis (18 processes), cell metabolism (10 processes), cell signaling (3 processes), neurodevelopment (1 process), and neurodegeneration (3 processes) (Table 2 and Table 3).
Presynaptic local protein synthesis, the process by which mRNAs are translated in axons and terminals, is well shown to be mediated by retrograde endocannabinoid (eCB) transmission [59]. The eCBs are lipids that are mobilized by postsynaptic action and travel backward across the synapse to bind presynaptic Gi/o-coupled type 1 cannabinoid (CB1) receptors, inhibiting neurotransmitter release. CB1 activation, in turn, boosts protein synthesis via the mTOR pathway [59]. Indeed, we identified impairment of retrograde endocannabinoid signaling, which can contribute to the disruption of axon guidance, after exposure to Mn (Table 2 and Table 3). Furthermore, it has been proposed that chemical stresses can either upregulate or downregulate the endocannabinoid system. This can disrupt synaptic transmission and brain circuit functions [60], leading to neurodegenerative illnesses such as AD, ALS, HD, and PD [61,62], as well as activate other signaling systems [60] such as hypoxia-inducible factor 1 (HIF-1), whose activation contributes to recovery of synaptic functions [63]. However, depending on the chemical speciation and the biological model investigated, HIF-1 can be inactivated due to downregulation of the BCL2 gene in response to Mn stress. Mn deficiency reduces the production of BCL2 mRNA and protein [64]. Manganese (III)-tetrakis(4-benzoic acid)porphyrin has been shown in studies with isolated human endothelial cells to inhibit the decrease of protein BCL2 expression [65]. Exposure to maneb and mancozeb, both Mn-based pesticides, results in increased expression of BCL2 mRNA and BCL2 protein abundance [66,67].
In agreement with our findings (Figure 3 and Figure 5, Table 2 and Table 3), researchers discovered altered genes related to neurogenesis, neurodevelopment, synaptic transmission, and apoptosis after exposing NoRa-differentiated SH-SY5Y cells to 100 µM MnCl2 for 30 days, which may be linked to neurodegeneration [26]. However, this work did not identify that these alterations can be preceded by impaired protein metabolism. Indeed, before changes in the mitochondrial–energy pathway and neurotransmission system, Fernandes et al. discovered increased abundance of the genes BET1 (Golgi vesicular membrane–trafficking protein), ADAM10 (ADAM metallopeptidase domain 10), and ARFGAP3 (ADP-ribosylation factor GTPase-activating protein 3) [28]. Accordingly, we confirmed that 500 µM of Mn(II)Cit or MnCl2 caused neurotoxicity in RA-differentiated SH-SY5Y-DAergic cells by disrupting approximately 50% of protein metabolism-related pathways, followed by energy dysfunction and other related molecular cell changes (Table 2 and Table 3). This is consistent with previous observations that MnCl2-induced neurotoxicity in NoRA SH-SY5Y was caused by changes in the ER–Golgi complex [38,39]. Hernández’s group’s research with a variety of models ranging from yeast to mammals, different Mn-species, and different exposure times has also suggested that impaired protein metabolism is a key event in Mn-induced neurotoxicity [25,34,35].
Although RA-differentiated SH-SY5Y can have altered axons [29,30], certainly Mn induces disturbance of axonal functions potentially linked to protein synthesis impairment [68]. Studies with primary cerebellar granule neurons exposed for MnCl2 identified potential axonal alteration due to hypokalemia and overexpression of STX1A, implicated in the production of presynaptic local proteins that regulate neurotransmitter release [25]. Here, in RA-differentiated SH-SY5Y exposed for Mn, we identified significant alterations of inferred and curated genes such as IGF2, RPL14, RPL23A, RPL6, RPS8, and RPS29 (Figure 2B and Figure 4D), transcripts hit revealed using the String database [48] and CTD [49]. These genes are involved in an enriched set of pathways linked to translation, post-translation modifications, and protein degradation (Figure 3A,C, Table 2 and Table 3), that can be perturbed by disruption of energy pathways [36,37] as well. Here, we found that the species of Mn can increase or arrest the expression of genes such as MT-CO1, MT-CYB, MT-ND4, and COX4I2 (Figure 4B), which are associated with glycolysis and glycogenesis, and oxidative phosphorylation, along with neurodegenerative diseases such as AD, HD, and PD [49]. Concomitantly, we identified impairment of TPI1 gene, which is associated with one-carbon metabolism pathway, biosynthesis of amino acid, and AD [49]. Furthermore, disruption of amino acid biosynthesis has been linked to the alteration of translation efficiency [69], similar to that observed in yeast exposed to MnCl2 for 24 h, which showed decreased β-galactosidase reporter expression, heavy polysomes fractions, and the expression of NOP1 and NSR1 genes, associated with ribosome biogenesis [34]. Altogether, the RPL14 gene impaired by Mn(II) is a marker of PD, whose alteration has been detected already, after domestic exposure to maneb (manganese(2+);N-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate) [70]. We have discovered maneb-impaired protein metabolism in cerebellar granule neurons [25]. Another study identified Mn-induced ER-stress and increased phosphorylation of translation initiation factor eIF2α in SH-SY5Y cells [70]. Collectively, these findings confirm that the network among protein metabolism, energy, cell development, and metabolic pathways [36,37] may be disrupted by chemical species of Mn [34,35,71,72,73,74,75].
Contrary to the transcriptomics studies conducted by Gandhi et al. [26] and Fernandes et al. [28], which considered only MnCl2, we verify that chemical speciation is important for Mn-induced impairment of gene expression and protein metabolism on RA-differentiated SH-SY5Y cells. For example, the MnCl2-induced alteration of the metabolism of amino (AA) acids, which influences RNA metabolism and protein synthesis [76], similar to that identified in yeast exposed for MnCl2 [34]. It has been demonstrated that amino acids are a regulator of late endosomes/lysosomes anterograde transport, which operate as mRNA translation platforms to produce new proteins necessary to support mitochondria function in axons growth, which is sensible to energy stress [77]. Indeed, we verified cross-talk alterations of the metabolism of AAs, proteins, cell energy, and axon guidance in response to cytotoxic concentrations of Mn. A metabolomics study with NoRA SH-SY5Y cells exposed to noncytotoxic concentrations of MnCl2 revealed a positive association between Mn exposure and glutamate and N-acetylglutamate semialdehyde, and a negative relationship with other AAs such as leucine/isoleucine, 4-imidazoleacetate, histidine, arginine, and valine. As a result, neurotransmitter-related metabolites such as GABA, adrenochrome, N4-acetylaminobutanal, N-methyl salsolinol, and dopamine sulfate were dramatically altered [27]. This corroborates the importance of the AAs for local protein synthesis, during neurodevelopment, which can be impaired by Mn.
Although RA can be anti-proteasome inhibitor in SH-SY5Y cells [33], we deduced that Mn(II)Cit induces neurotoxicity associated with impairment of E3 ubiquitin ligases–target protein degradation pathway, which is involved in the proteasome’s identification of substrates and proteins for degradation [78]. This can lead to damaged/unfolded protein accumulation, consistent with altered expression of the genes HIST1H2BB, HIST1H2BH, and HIST1H2BO (Figure 3A,C). Histone modification is linked to the creation of amyloid fibers (Table 3) and, as a result, the development of neurodegenerative diseases [79]. Other research has shown that Mn-induced neurotoxicity can target the ubiquitin system. For example, in cultured astrocytes treated with MnCl2, SNAT3 protein degradation and Gln homeostasis disruption occur via the ubiquitin-mediated proteolytic mechanism [80]. Additionally, in cerebellar granule cells stressed with MnCl2 or maneb, the Mn induces impairment of protein metabolism, involving dysregulation of the ubiquitin system as well [25]. However, the ubiquitin system was not altered in dopaminergic cells (SH-SY5Y and CATH.a), with impaired ER–Golgi complex, under the effect of Mn [39].
5. Conclusions
Lastly, we identified some advantages to using the RA-differentiated cells instead of the undifferentiated SH-SY5Y cell line to study Mn-induced neurotoxicity in humans. These cells are chemically and genetically modified; consequently, it cannot be considered normal [31], which might restrict the experiment’s outcomes in a variety of ways. Our findings, however, are equivalent to those obtained with primary culture of mouse cerebellar granule neurons. At the same time, we revealed that RA-differentiated SH-SY5Y cells respond differently to distinct chemical species of Mn, which was not considered in previous studies with NoRA-differentiated SH-SY5Y cells. Indeed, Mn causes disruption of cross-talk networks of pathways in the RA-differentiated SH-SY5Y-DAergic cells, which may be mediated by protein metabolism disturbance, evidently influenced by chemical speciation, for example, the MnCl2-altered amino acid metabolism, which affects RNA metabolism and protein synthesis. Mn(II)Cit altered the E3 ubiquitin ligases–target protein degradation pathway, potentially leading to the accumulation of damaged/unfolded proteins, which is consistent with histone modification. These findings support the relevance of chemical speciation in understanding the process behind Mn-induced neurotoxicity and neurodegeneration, which appears to be conserved from yeast [34], to zebrafish [35], to mammals [25,28]. A functional analysis of cross-species translation in the presence of Mn could either confirm or refute our findings.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxics9120348/s1, Table S1: Genes differentially expressed in RA-differentiated SH-SY5Y cells after exposure to MnCl2 are listed below. Table S2: Genes differentially expressed in RA-differentiated SH-SY5Y cells after exposure to MnCl2 are listed below.
Author Contributions
R.B.H. was responsible for the principal idea of this study, coexecutor of all designed experiments, and coordinators of the grants that supported this work. J.K. together with M.v.H. and J.P. participated of the design, execution, and data analysis of microarrays assays. N.C.d.S.-P. participated in data analysis. H.M. participated directly in the experiments on gene expression and data analysis. A.G. and D.B. participated actively in the execution of bioinformatic approaches and data analysis. All authors wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation, FAPESP, grant numbers 19/27840-5, 16/50483-6, and 15/24207-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howe P.D., Malcolm H.M., Dobson S. Manganese and Its Compounds: Environmental Aspects. World Health Organization & International Programme on Chemical Safety; Geneva, Switzerland: 2004. [Google Scholar]
- 2.Luo X.G., Li S.F., Lu L., Liu B., Kuang X., Shao G.Z., Yu S.X. Gene Expression of Manganese-Containing Superoxide Dismutase as a Biomarker of Manganese Bioavailability for Manganese Sources in Broilers. Poult. Sci. 2007;86:888–894. doi: 10.1093/ps/86.5.888. [DOI] [PubMed] [Google Scholar]
- 3.Kwakye G., Paoliello M., Mukhopadhyay S., Bowman A., Aschner M. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int. J. Environ. Res. Public Health. 2015;12:7519–7540. doi: 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman A.B., Kwakye G.F., Herrero E., Aschner M. Journal of Trace Elements in Medicine and Biology Role of Manganese in Neurodegenerative Diseases. J. Trace Elem. Med. Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Šarić M., Lucchini R. In: Handbook on the Toxicology of Metals. 3rd ed. Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T., editors. Academic Press Inc.; Burlington, MA, USA: 2007. pp. 645–674. [Google Scholar]
- 6.Hafeman D., Factor-Litvak P., Cheng Z., van Geen A., Ahsan H. Association between Manganese Exposure through Drinking Water and Infant Mortality in Bangladesh. Environ. Health Perspect. 2007;115:1107–1112. doi: 10.1289/ehp.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljung K., Vahter M. Time to Re-Evaluate the Guideline Value for Manganese in Drinking Water? Environ. Health Perspect. 2007;115:1533–1538. doi: 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordão C.P., Pereira J.L., Jham G.N., Bellato C.R. Distribution of Heavy Metals in Environmental Samples Near Smelters and Mining Areas in Brazil. Environ. Technol. 1999;20:489–498. doi: 10.1080/09593332008616844. [DOI] [Google Scholar]
- 9.Avnimelech Y., Kochba M. Evaluation of Nitrogen Uptake and Excretion by Tilapia in Bio Floc Tanks, Using 15N Tracing. Aquaculture. 2009;287:163–168. doi: 10.1016/j.aquaculture.2008.10.009. [DOI] [Google Scholar]
- 10.Bonne Hernández R., Oliveira E., Espósito B.P. Distribution and Behavior of Manganese in the Alto Do Paranapanema Basin. J. Environ. Monit. 2009;11:1236. doi: 10.1039/b822579f. [DOI] [PubMed] [Google Scholar]
- 11.Templeton D.M., Ariese F., Cornelis R., Danielsson L.-G., Muntau H., van Leeuwen H.P., Lobinski R. Guidelines for Terms Related to Chemical Speciation and Fractionation of Elements. Definitions, Structural Aspects, and Methodological Approaches (IUPAC Recommendations 2000) Pure Appl. Chem. 2000;72:1453–1470. doi: 10.1351/pac200072081453. [DOI] [Google Scholar]
- 12.Stueben B.L., Cantrelle B., Sneddon J., Beck J.N. Manganese K-Edge XANES Studies of Mn Speciation in Lac Des Allemands as a Function of Depth. Microchem. J. 2004;76:113–120. doi: 10.1016/j.microc.2003.11.011. [DOI] [Google Scholar]
- 13.Kenneth Klewicki J., Morgan J.J. Kinetic Behavior of Mn(III) Complexes of Pyrophosphate, EDTA, and Citrate. Environ. Sci. Technol. 1998;32:2916–2922. doi: 10.1021/es980308e. [DOI] [Google Scholar]
- 14.Hernández R.B., Farina M., Espósito B.P., Souza-Pinto N.C., Barbosa F., Suñol C. Mechanisms of Manganese-Induced Neurotoxicity in Primary Neuronal Cultures: The Role of Manganese Speciation and Cell Type. Toxicol. Sci. 2011;124:414–423. doi: 10.1093/toxsci/kfr234. [DOI] [PubMed] [Google Scholar]
- 15.Queiroz H.M., Ying S.C., Abernathy M., Barcellos D., Gabriel F.A., Otero X.L., Nóbrega G.N., Bernardino A.F., Ferreira T.O. Manganese: The Overlooked Contaminant in the World Largest Mine Tailings Dam Collapse. Environ. Int. 2021;146:106284. doi: 10.1016/j.envint.2020.106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández R.B., Nishita M.I., Espósito B.P., Scholz S., Michalke B. The Role of Chemical Speciation, Chemical Fractionation and Calcium Disruption in Manganese-Induced Developmental Toxicity in Zebrafish (Danio Rerio) Embryos. J. Trace Elem. Med. Biol. 2015;32:209–217. doi: 10.1016/j.jtemb.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Mehrifar Y., Bahrami M., Sidabadi E., Pirami H. The Effects of Occupational Exposure to Manganese Fume on Neurobehavioral and Neurocognitive Functions: An Analytical Cross-Sectional Study among Welders. EXCLI J. 2020;19:372–386. doi: 10.17179/excli2019-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neves-Silva P., Heller L. Rompimento Da Barragem Em Brumadinho e o Acesso à Água Das Comunidades Atingidas: Um Caso de Direitos Humanos. Ciência Cult. 2020;72:47–50. doi: 10.21800/2317-66602020000200013. [DOI] [Google Scholar]
- 19.De Azevedo D.C.B., de Araujo Toledo G., Cohen S.C., Kligerman D.C., de Oliveira Cardoso T.A. Desastre de Brumadinho: Contribuições Para Políticas Públicas e Gestão Do Saneamento Em Períodos Emergenciais. Saúde Debate. 2020;44:221–233. doi: 10.1590/0103-1104202012416. [DOI] [Google Scholar]
- 20.De Freitas C.M., Barcellos C., Asmus C.I.R.F., da Silva M.A., Xavier D.R. Da Samarco Em Mariana à Vale Em Brumadinho: Desastres Em Barragens de Mineração e Saúde Coletiva. Cad. Saúde Pública. 2019;35:1–7. doi: 10.1590/0102-311x00052519. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell E.J., Frisbie S.H., Roudeau S., Carmona A., Ortega R. How Much Manganese Is Safe for Infants? A Review of the Scientific Basis of Intake Guidelines and Regulations Relevant to the Manganese Content of Infant Formulas. J. Trace Elem. Med. Biol. 2021;65:126710. doi: 10.1016/j.jtemb.2020.126710. [DOI] [PubMed] [Google Scholar]
- 22.Williams B.B., Kwakye G.F., Wegrzynowicz M., Li D., Aschner M., Erikson K.M., Bowman A.B. Altered Manganese Homeostasis and Manganese Toxicity in a Huntington’s Disease Striatal Cell Model Are Not Explained by Defects in the Iron Transport System. Toxicol. Sci. 2010;117:169–179. doi: 10.1093/toxsci/kfq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfalzer A.C., Wilcox J.M., Codreanu S.G., Totten M., Bichell T.J.V., Halbesma T., Umashanker P., Yang K.L., Parmalee N.L., Sherrod S.D., et al. Huntington’s Disease Genotype Suppresses Global Manganese-Responsive Processes in Pre-Manifest and Manifest YAC128 Mice. Metallomics. 2020;12:1118–1130. doi: 10.1039/D0MT00081G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins A.C., Morcillo P., Ijomone O.M., Venkataramani V., Harrison F.E., Lee E., Bowman A.B., Aschner M. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int. J. Environ. Res. Public Health. 2019;16:3546. doi: 10.3390/ijerph16193546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández R.B., Carrascal M., Abian J., Michalke B., Farina M., Gonzalez Y., Iyirhiaro G., Moteshareie H., Burnside D., Golshani A., et al. Manganese-Induced Neurotoxicity in Cerebellar Granule Neurons Due to Perturbation of Cell Network Pathways with Potential Implications for Neurodegenerative Disorders. Metallomics. 2020;12:1656–1678. doi: 10.1039/D0MT00085J. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi D., Sivanesan S., Kannan K. Manganese-Induced Neurotoxicity and Alterations in Gene Expression in Human Neuroblastoma SH-SY5Y Cells. Biol. Trace Elem. Res. 2018;183:245–253. doi: 10.1007/s12011-017-1153-5. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes J., Chandler J.D., Liu K.H., Uppal K., Hao L., Hu X., Go Y.-M., Jones D.P. Metabolomic Responses to Manganese Dose in SH-SY5Y Human Neuroblastoma Cells. Toxicol. Sci. 2019;169:84–94. doi: 10.1093/toxsci/kfz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes J., Chandler J.D., Lili L.N., Uppal K., Hu X., Hao L., Go Y.-M., Jones D.P. Transcriptome Analysis Reveals Distinct Responses to Physiologic versus Toxic Manganese Exposure in Human Neuroblastoma Cells. Front. Genet. 2019;10:676. doi: 10.3389/fgene.2019.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalevich J., Langford D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2013;1078:9–21. doi: 10.1007/978-1-62703-640-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altamirano M., Coates C.W., Grundfest H. Mechanisms of direct and neural excitability in electroplaques of electric eel. J. Gen. Physiol. 1955;38:319–360. doi: 10.1085/jgp.38.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korecka J.A., van Kesteren R.E., Blaas E., Spitzer S.O., Kamstra J.H., Smit A.B., Swaab D.F., Verhaagen J., Bossers K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE. 2013;8:e63862. doi: 10.1371/journal.pone.0063862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlachetzki J.C.M., Saliba S.W., de Oliveira A.C.P. Studying Neurodegenerative Diseases in Culture Models. Rev. Bras. Psiquiatr. 2013;35((Suppl. 2)):S92–S100. doi: 10.1590/1516-4446-2013-1159. [DOI] [PubMed] [Google Scholar]
- 33.Xicoy H., Wieringa B., Martens G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017;12:10. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández R.B., Moteshareie H., Burnside D., McKay B., Golshani A. Manganese-Induced Cellular Disturbance in the Baker’s Yeast, Saccharomyces Cerevisiae with Putative Implications in Neuronal Dysfunction. Sci. Rep. 2019;9:6563. doi: 10.1038/s41598-019-42907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonne Hernández R., Moteshareie H., Golshani A. Manganese-Induced Disruption of Cross-Talking Pathways in Danio Rerio (Zebrafish) Is Potentially Linked to Toxicity and Neurodegeneration. EC Pharmacol. Toxicol. 2019;7:175–187. [Google Scholar]
- 36.Warner J.R. The Economics of Ribosome Biosynthesis in Yeast. Trends Biochem. Sci. 1999;24:437–440. doi: 10.1016/S0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 37.Thomson E., Ferreira-Cerca S., Hurt E. Eukaryotic Ribosome Biogenesis at a Glance. J. Cell Sci. 2013;126:4815–4821. doi: 10.1242/jcs.111948. [DOI] [PubMed] [Google Scholar]
- 38.Liu C., Yan D., Wang C., Ma Z., Deng Y., Liu W., Xu B. Manganese Activates Autophagy to Alleviate Endoplasmic Reticulum Stress–Induced Apoptosis via PERK Pathway. J. Cell. Mol. Med. 2020;24:328–341. doi: 10.1111/jcmm.14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higashi Y., Asanuma M., Miyazaki I., Hattori N., Mizuno Y., Ogawa N. Parkin Attenuates Manganese-Induced Dopaminergic Cell Death. J. Neurochem. 2004;89:1490–1497. doi: 10.1111/j.1471-4159.2004.02445.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y., Wen Q., Huang J., Luo M., Xiao Y., Mo R., Wang J. Manganese (II) Chloride Leads to Dopaminergic Neurotoxicity by Promoting Mitophagy through BNIP3-Mediated Oxidative Stress in SH-SY5Y Cells. Cell. Mol. Biol. Lett. 2021;26:23. doi: 10.1186/s11658-021-00267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabin O., Hegedus L., Bourre J.-M., Smith Q.R. Rapid Brain Uptake of Manganese(II) Across the Blood-Brain Barrier. J. Neurochem. 2006;61:509–517. doi: 10.1111/j.1471-4159.1993.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 42.Yokel R.A. Manganese Flux Across the Blood–Brain Barrier. NeuroMol. Med. 2009;11:297–310. doi: 10.1007/s12017-009-8101-2. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson A.P., Schneider J.A., Nelson B.C., Atha D.H., Jain A., Soliman K.F.A., Aschner M., Mazzio E., Reams R.R. Manganese-Induced Oxidative DNA Damage in Neuronal SH-SY5Y Cells: Attenuation of Thymine Base Lesions by Glutathione and N-Acetylcysteine. Toxicol. Lett. 2013;218:299–307. doi: 10.1016/j.toxlet.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traverso M., Malnati M., Minetti C., Regis S., Tedeschi S., Pedemonte M., Bruno C., Biassoni R., Zara F. Multiplex Real-Time PCR for Detection of Deletions and Duplications in Dystrophin Gene. Biochem. Biophys. Res. Commun. 2006;339:145–150. doi: 10.1016/j.bbrc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Jouannic J., Stieltjes N., Costa J., Girodon E. Quantitative Real-Time PCR Assay for Rapid Identification of Deletion Carriers in Hemophilia. Clin. Chem. 2004;50:1269–1270. doi: 10.1373/clinchem.2004.031609. [DOI] [PubMed] [Google Scholar]
- 46.Samanfar B., Shostak K., Moteshareie H., Hajikarimlou M., Shaikho S., Omidi K., Hooshyar M., Burnside D., Márquez I.G., Kazmirchuk T., et al. The Sensitivity of the Yeast, Saccharomyces Cerevisiae, to Acetic Acid Is Influenced by DOM34 and RPL36A. PeerJ. 2017;2017:e4037. doi: 10.7717/peerj.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alonso R., Salavert F., Garcia-Garcia F., Carbonell-Caballero J., Bleda M., Garcia-Alonso L., Sanchis-Juan A., Perez-Gil D., Marin-Garcia P., Sanchez R., et al. Babelomics 5.0: Functional Interpretation for New Generations of Genomic Data. Nucleic Acids Res. 2015;43:W117–W121. doi: 10.1093/nar/gkv384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., et al. The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis A.P., Grondin C.J., Johnson R.J., Sciaky D., King B.L., McMorran R., Wiegers J., Wiegers T.C., Mattingly C.J. The Comparative Toxicogenomics Database: Update 2017. Nucleic Acids Res. 2017;45:D972–D978. doi: 10.1093/nar/gkw838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., Wadi L., Meyer M., Wong J., Xu C., et al. Pathway Enrichment Analysis and Visualization of Omics Data Using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019;14:482–517. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarale P., Daiwile A.P., Sivanesan S., Stöger R., Bafana A., Naoghare P.K., Parmar D., Chakrabarti T., Krishnamurthi K. Manganese Exposure: Linking down-Regulation of MiRNA-7 and MiRNA-433 with α-Synuclein Overexpression and Risk of Idiopathic Parkinson’s Disease. Toxicol. In Vitro. 2018;46:94–101. doi: 10.1016/j.tiv.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Tinkov A.A., Paoliello M.M.B., Mazilina A.N., Skalny A.V., Martins A.C., Voskresenskaya O.N., Aaseth J., Santamaria A., Notova S.V., Tsatsakis A., et al. Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update. Int. J. Mol. Sci. 2021;22:4646. doi: 10.3390/ijms22094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dos Santos Vergilio C., Lacerda D., de Oliveira B.C.V., Sartori E., Campos G.M., de Souza Pereira A.L., de Aguiar D.B., da Silva Souza T., de Almeida M.G., Thompson F., et al. Metal Concentrations and Biological Effects from One of the Largest Mining Disasters in the World (Brumadinho, Minas Gerais, Brazil) Sci. Rep. 2020;10:5936. doi: 10.1038/s41598-020-62700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson F., de Oliveira B.C., Cordeiro M.C., Masi B.P., Rangel T.P., Paz P., Freitas T., Lopes G., Silva B.S., Cabral A.S., et al. Severe Impacts of the Brumadinho Dam Failure (Minas Gerais, Brazil) on the Water Quality of the Paraopeba River. Sci. Total Environ. 2020;705:135914. doi: 10.1016/j.scitotenv.2019.135914. [DOI] [PubMed] [Google Scholar]
- 55.Maddirala Y., Tobwala S., Ercal N. N-Acetylcysteineamide Protects against Manganese-Induced Toxicity in SHSY5Y Cell Line. Brain Res. 2015;1608:157–166. doi: 10.1016/j.brainres.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Kemsheh M., Oblitey R. All Zyzzogeton Presentations. Volume 15 Augsburg University; Minneapolis, MN, USA: 2020. Manganese Toxicity on Cultured SH-SY5Y Cells. [Google Scholar]
- 57.Lehmkuhl E.M., Zarnescu D.C. Lost in Translation: Evidence for Protein Synthesis Deficits in ALS/FTD and Related Neurodegenerative Diseases. Adv Neurobiol. 2018:283–301. doi: 10.1007/978-3-319-89689-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Sun L., Cai T., Zhang Y., Lv S., Wang Y., Ye L. α-Synuclein Overexpression during Manganese-Induced Apoptosis in SH-SY5Y Neuroblastoma Cells. Brain Res. Bull. 2010;81:428–433. doi: 10.1016/j.brainresbull.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Younts T.J., Monday H.R., Dudok B., Klein M.E., Jordan B.A., Katona I., Castillo P.E. Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron. 2016;92:479–492. doi: 10.1016/j.neuron.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kano M. Control of Synaptic Function by Endocannabinoid-Mediated Retrograde Signaling. Proc. Jpn. Acad. Ser. B. 2014;90:235–250. doi: 10.2183/pjab.90.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoeckli E.T. Understanding Axon Guidance: Are We Nearly There Yet? Development. 2018;145:dev151415. doi: 10.1242/dev.151415. [DOI] [PubMed] [Google Scholar]
- 62.Liu X.-A., Rizzo V., Puthanveettil S. Pathologies of Axonal Transport in Neurodegenerative Diseases. Transl. Neurosci. 2012;3:355–372. doi: 10.2478/s13380-012-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho Y., Shin J.E., Ewan E.E., Oh Y.M., Pita-Thomas W., Cavalli V. Activating Injury-Responsive Genes with Hypoxia Enhances Axon Regeneration through Neuronal HIF-1α. Neuron. 2015;88:720–734. doi: 10.1016/j.neuron.2015.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou C., Wang Y., Liu J., Wang C., Long J. Neurodegenerative Disease Related Proteins Have Negative Effects on SNARE-Mediated Membrane Fusion in Pathological Confirmation. Front. Mol. Neurosci. 2017;10:66. doi: 10.3389/fnmol.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corgnali M., Piconi L., Ihnat M., Ceriello A. Evaluation of Gliclazide Ability to Attenuate the Hyperglycaemic ‘Memory’ Induced by High Glucose in Isolated Human Endothelial Cells. Diabetes Metab. Res. Rev. 2008;24:301–309. doi: 10.1002/dmrr.804. [DOI] [PubMed] [Google Scholar]
- 66.Fei Q., Ethell D.W. Maneb Potentiates Paraquat Neurotoxicity by Inducing Key Bcl-2 Family Members. J. Neurochem. 2008;105:2091–2097. doi: 10.1111/j.1471-4159.2008.05293.x. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava A.K., Mishra S., Ali W., Shukla Y. Protective Effects of Lupeol against Mancozeb-Induced Genotoxicity in Cultured Human Lymphocytes. Phytomedicine. 2016;23:714–724. doi: 10.1016/j.phymed.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Kim E., Jung H. Local Protein Synthesis in Neuronal Axons: Why and How We Study. BMB Rep. 2015;48:139–146. doi: 10.5483/BMBRep.2015.48.3.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu X.P., Yang Y., Ma B.G. Amino Acid Flux from Metabolic Network Benefits Protein Translation: The Role of Resource Availability. Sci. Rep. 2015;5:11113. doi: 10.1038/srep11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seo Y.A., Li Y., Wessling-Resnick M. Iron Depletion Increases Manganese Uptake and Potentiates Apoptosis through ER Stress. NeuroToxicol. 2013;38:67–73. doi: 10.1016/j.neuro.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Putrament A., Baranowska H., Ejchart A., Prazmo W. Manganese Mutagenesis in Yeast. A Practical Application of Manganese for the Induction of Mitochondrial Antibiotic-Resistant Mutations. J. Gen. Microbiol. 1975;90:265–270. doi: 10.1099/00221287-90-2-265. [DOI] [PubMed] [Google Scholar]
- 72.Putrament A., Baranowska H., Ejchart A., Jachymczyk W. Manganese Mutagenesis in Yeast-VI. Mn2+ Uptake, MitDNA Replication and ER Induction. Comparison with Other Divalent Cations. MGG Mol. Gen. Genet. 1977;151:69–76. doi: 10.1007/BF00446914. [DOI] [PubMed] [Google Scholar]
- 73.Donaldson S.G., Fox O.F., Kishore G.S., Carubelli R. Effect of Manganese Ions on the Interaction between Ribosomes and Endoplasmic Reticulum Membranes Isolated from Rat Liver. Biosci. Rep. 1981;1:727–731. doi: 10.1007/BF01116471. [DOI] [PubMed] [Google Scholar]
- 74.Dambach M., Sandoval M., Updegrove T.B., Anantharaman V., Aravind L., Waters L.S., Storz G. The Ubiquitous YybP-YkoY Riboswitch Is a Manganese-Responsive Regulatory Element. Mol. Cell. 2015;57:1099–1109. doi: 10.1016/j.molcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bray M.S., Lenz T.K., Haynes J.W., Bowman J.C., Petrov A.S., Reddi A.R., Hud N.V., Williams L.D., Glass J.B. Multiple Prebiotic Metals Mediate Translation. Proc. Natl. Acad. Sci. USA. 2018;115:12164–12169. doi: 10.1073/pnas.1803636115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee C.-D., Tu B.P. Metabolic Influences on RNA Biology and Translation. Crit. Rev. Biochem. Mol. Biol. 2017;52:176–184. doi: 10.1080/10409238.2017.1283294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cioni J.-M., Lin J.Q., Holtermann A.V., Koppers M., Jakobs M.A.H., Azizi A., Turner-Bridger B., Shigeoka T., Franze K., Harris W.A., et al. Late Endosomes Act as MRNA Translation Platforms and Sustain Mitochondria in Axons. Cell. 2019;176:56–72. doi: 10.1016/j.cell.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giasson B.I., Lee V.M.-Y. Are Ubiquitination Pathways Central to Parkinson’s Disease? Cell. 2003;114:1–8. doi: 10.1016/S0092-8674(03)00509-9. [DOI] [PubMed] [Google Scholar]
- 79.Agbas A. Neurochemistry. IntechOpen; London, UK: 2018. Trends of Protein Aggregation in Neurodegenerative Diseases. [DOI] [Google Scholar]
- 80.Sidoryk-Wȩgrzynowicz M., Lee E.-S., Ni M., Aschner M. Manganese-Induced Downregulation of Astroglial Glutamine Transporter SNAT3 Involves Ubiquitin-Mediated Proteolytic System. Glia. 2010;58:1905–1912. doi: 10.1002/glia.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.