Abstract
Introduction
Extended interval (EI) dosing for immune checkpoint inhibitor (ICI) mono- or consolidation therapy initiated due to the COVID-19 pandemic led to a significant reduction in ICI-related site visits for patients with stage III and IV non–small cell lung cancer. Here we report the safety and efficacy compared to standard dose (SD) schedules.
Method
In this retrospective analysis, patients who received ICI mono- or consolidation therapy, or adjuvant ICI therapy were assessed. Safety and efficacy of EI dosing with data of SD schedules were compared.
Results
One hundred seventeen patients received EI dosing for ICI and 88 patients SD. Patient characteristics were comparable. We observed 237 adverse events in the EI dosing cohort versus 118 in the SD group (P= .02). Overall, there was no difference in the occurrence of grade ≥3 adverse events (EI dosing: 21/237 [8.9%]; SD group: 20/118 [17.0%], P = .42), except for the pembrolizumab EI dosing cohort. Of all patients who received an EI dosing schedule, however, only 8 (6.8%) were reduced to SD because of toxicity. In 5 (4.3%) patients ICI was permanently stopped because of severe toxicity compared to 11 (12.5%) discontinuations in the SD group. Short-term treatment interruption occurred with similar frequencies in both groups. Progression-free survival and overall survival were comparable in patients receiving pembrolizumab and in those receiving adjuvant durvalumab. Progression-free survival and OS were better in the EI dosing cohort of nivolumab.
Conclusion
EI dosing for ICI did not lead to an increase of clinically relevant toxicities resulting in dose reduction and/or treatment discontinuation. Efficacy of EI dosing of pembrolizumab and durvalumab were comparable to SD. Based on our safety and efficacy data EI dosing for ICI seems a safe and effective strategy.
Keywords: Checkpoint blockade, Immunotherapy, Dose adaptation, Coronavirus
Micro-Abstract
Aim: Retrospective analysis of the safety and efficacy of extended interval dosing (EI) ICI compared to standard dose (SD) schedules. Results: 117 patients received EI dosing and 88 SD. In the EI dosing cohort was no increase in toxicity leading to dose reduction and/or discontinuation of treatment. Furthermore, efficacy of EI dosing of pembrolizumab and durvalumab were comparable to SD. Based on our safety and efficacy data EI dosing for ICI seem a safe and effective strategy and should be continued also beyond the COVID-19 pandemic.
Introduction
The COVID-19 pandemic forced oncologists to cut down face-to-face patient contacts, thereby reducing the risk of exposure to the virus and reallocating resources to provide the necessary care for COVID-19 patients. As alternative for keeping up our oncology services for stage III and IV non–small cell lung cancer (NSCLC), extended interval (EI) dosing for immune checkpoint inhibitor (ICI) was used for mono- and consolidation therapy. However, the question arises whether EI dosing will have impact on the safety and efficacy of ICI. Awaiting data from randomized controlled trials (ClinicalTrials.gov NCT04295863), we performed a retrospective cohort study to assess the safety and efficacy of EI dosing for ICI and compared the results to standard dose (SD) schedules in a real-world NSCLC population.
Patients and Treatment
Between January 1, 2019 and June 1, 2021 all consecutive patients with stage III/IV NSCLC treated at the University Medical Center Groningen with mono-ICI, ICI + chemotherapy or adjuvant ICI were enrolled. Standard dosing (SD) was compared with EI schedules. EI dosing was introduced by March 1, 2020.
SD was defined as pembrolizumab mono- or consolidation therapy (the latter ± pemetrexed, after 4 cycles ICI + chemotherapy) every 3 weeks at a dose of 200 mg, nivolumab every 2 weeks at a dose of 240 mg and durvalumab every 2 weeks at a dose of 10 mg/kg. After at least 2 cycles of ICI standard dose (SD) without clinically relevant toxicity the dose was escalated (EI dosing) to pembrolizumab 400 mg every 6 weeks,1 nivolumab 480 mg every 4 weeks2 and durvalumab 1500 mg every 4 weeks.3 All patients already receiving ICI monotherapy on March 1, 2020 without clinically relevant toxicity were escalated to the EI dosing schedule. Otherwise, patients received the EI schedule after 2 cycles of standard treatment without clinical toxicity. Those receiving pembrolizumab-pemetrexed consolidation therapy were either continued on the combination, or after discontinuation of pemetrexed the pembrolizumab was escalated to the EI dosing schedule.
Assessment of Safety and Efficacy
Safety and efficacy between both groups were compared. Adverse events (AE) were assessed by CTCAE 5.0. We report numbers of AEs at any moment during treatment [Total events] and AEs occurring in the escalation window [Escalation window]. In the EI dose cohort start of the escalation window is the actual moment of schedule adaptation. In the SD cohort, start of the escalation window was defined as the moment on which patients would have been escalated from SD to the extended dose interval.
Progression-free survival (PFS) was defined as the time from treatment start until first evidence of tumor progression or until death from any cause, whichever comes first. Overall survival (OS) was defined as the time from treatment start to death from any cause.
Statistics
The primary outcome of this analysis is safety. The difference of total AE frequency per dosing group (EI dosing vs. SD) overall and in the treatment groups (adjuvant durvalumab, nivolumab, pembrolizumab, pembrolizumab + chemotherapy) was assessed using the Mann-Whitney U test. Clinical outcome (explorative analysis) was evaluated at patient level by means of PFS and OS. The relationship between ICI dosing cohort and survival was explored by Kaplan–Meier survival plots and differences were assessed by using the log-rank test. Due to the planned schedule – escalation to EI dosing only after receiving two cycles of SD without early clinically relevant toxicity or early progression – a selection bias in terms of survival was introduced. To correct for this bias in the survival analysis, patients with early progression in the SD cohort were excluded from this analysis.
Results
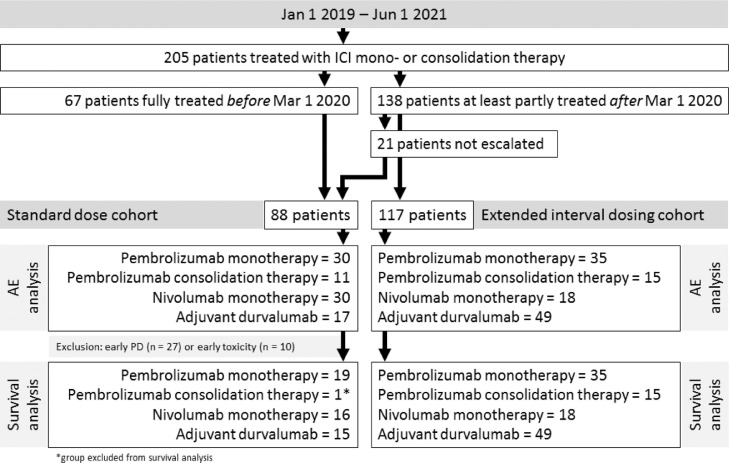
Two hundred five patients were included (Figure 1). Patient characteristics were similar between patients receiving SD (n = 88) and EI dosing (n = 117), except that in the SD cohort more patients were treated with nivolumab and less patients were treated with durvalumab (Table 1 ). From those receiving SD, 67 patients were fully treated before March 1, 2020. The remainder 21 patients were not escalated due to progression of disease before the escalation window (n = 8), early AEs (n = 10), or logistic reasons (n = 3).
Figure 1.
Consort diagram.
Table 1.
Baseline Characteristics
| ICI Standard Dose(N = 88) | ICI extended Interval Dose(N = 117) | P Value | |
|---|---|---|---|
| Sex - no. (%) | |||
| Male | 53 (60.2%) | 69 (59.0%) | .97 |
| Female | 35 (39.8%) | 48 (41.0%) | |
| Age at start ICI - years | |||
| Mean (SD) | 66.3 (8.81) | 64.8 (10.0) | .28 |
| Median [Min, Max] | 67.0 [35.0, 82.0] | 66.0 [43.0, 87.0] | |
| ECOG performance-status - no. (%) | |||
| 0 | 32 (36.4%) | 54 (46.2%) | .21 |
| 1 | 47 (53.4%) | 57 (48.7%) | |
| 2 | 9 (10.2%) | 6 (5.1%) | |
| NSCLC subtype - no. (%) | |||
| Squamous cell carcinoma | 29 (33.0%) | 44 (37.6%) | .9 |
| Non-squamous cell carcinoma | 54 (61.4%) | 66 (56.4%) | |
| Mixed type (Sq/Nsq) | 1 (1.1%) | 1 (0.9%) | |
| NSCLC NOS | 4 (4.5%) | 6 (5.1%) | |
| Mutation - no. (%) | |||
| No mutation | 53 (60.2%) | 86 (73.5%) | .26 |
| BRAF | 3 (3.4%) | 3 (2.6%) | |
| MET | 0 (0%) | 1 (0.9%) | |
| KRAS | 29 (33.0%) | 24 (20.5%) | |
| Other | 3 (3.4%) | 3 (2.6%) | |
| PD-L1 - no. (%) | |||
| 0 | 31 (35.2%) | 24 (20.5%) | .17 |
| 1-49% | 17 (19.3%) | 27 (23.1%) | |
| ≥ 50% | 33 (37.5%) | 43 (36.8%) | |
| Missing | 7 (8.0%) | 23 (19.7%) | |
| Treatment | |||
| Pembrolizumab mono | 30 (34.1%) | 35 (29.9%) | .0013 |
| Pembrolizumab + chemo | 11 (12.5%) | 15 (12.8%) | |
| Nivolumab mono | 30 (34.1%) | 18 (15.4%) | |
| Durvalumab adjuvant | 17 (19.3%) | 49 (41.9%) | |
| Line of treatment | |||
| 1st line | 41 (46.6%) | 48 (41.0%) | .0025 |
| 2nd line | 29 (33.0%) | 19 (16.2%) | |
| 3rd line | 1 (1.1%) | 1 (0.9%) | |
| Adjuvant | 17 (19.3%) | 49 (41.9%) |
* P-value ≤ .05 considered statistically significant. N, number of patients.
We observed a total of 237 AEs in the EI dosing cohort versus 118 in the SD group (P= .02; Table 1). Of these events, 21/237 (8.9%) and 20/118 (17.0%), respectively, were CTCAE grade 3 or higher (P= .42). Of all events, 46.4% in the EI dosing cohort and 58.5% in the SD cohort occurred in the escalation window. Only in the pembrolizumab EI dosing cohort, more AEs were observed compared to SD (P= .02), which however did not result in an increased number of grade ≥3 events (Table 2) or events leading to treatment interruption or discontinuation (Table 3). Of the 117 patients receiving EI dosing schedule, only 8 (6.8%) patients were reduced to SD because of toxicity, and in 5 patients (4.3%) ICI was permanently stopped because of toxicity, compared to 11 (12.5%) in the SD group. Short-term treatment interruption occurred with similar frequencies in both groups (15.4% vs. 13.6%).
Table 2.
Adverse Events Overall and Per Treatment Group
| Standard Dose a |
Extended Interval Dosing a |
P Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Event |
Total Events |
Escalation Windowb |
Total Events |
Escalation Window |
Total Events |
Escalation Window |
||||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any grade/Grade ≥3 | Any grade/Grade ≥3 | |
| Overall | n = 88 | n = 51 | n = 117 | n = 117 | ||||||
| All events, n (% of all) | 118 | 20 (16.9) | 69 | 11 (15.9) | 237 | 21 (8.9) | 110 | 12 (10.9) | .02*/.42 | .003*/.1 |
| Adjuvant durvalumab | n = 17 | n = 15 c | n = 49 | n = 49 | ||||||
| All events, n (% of all) | 26 | 3 (11.5) | 19 | 2 (10.5) | 79 | 6 (7.6) | 46 | 5 (10.9) | .75/.58 | .11/.74 |
| Skin | 8 (30.8) | 1 (33) | 6 (31.6) | - | 24 (30.4) | 1 (17) | 8 (17.4) | - | ||
| Fatigue | 5 (19.2) | - | 3 (15.8) | - | 23 (29.1) | - | 15 (32.6) | - | ||
| Endocrinopathy | 3 (11.5) | - | 2 (10.2) | - | 10 (12.7) | - | 7 (15.2) | - | ||
| Gastrointestinal | 3 (11.5) | 2 (67) | 3 (15.8) | 2 (100) | 7 (8.9) | 2 (33) | 5 (10.9) | 2 (40) | ||
| Musculoskeletal | 3 (11.5) | - | 1 (5.3) | - | 7 (8.9) | - | 5 (10.9) | - | ||
| Pneumonitis | 3 (11.5) | - | 3 (15.8) | - | 4 (5.1) | 3 (50) | 4 (8.7) | 3 (60) | ||
| Hepatitis | - | - | - | 2 (2.5) | - | 1 (2.2) | - | |||
| Sicca syndrome | 1 (3.8) | - | 1 (5.3) | - | - | - | - | - | ||
| Ocular | - | - | - | - | 1 (1.3) | - | - | - | ||
| Nephritis | - | - | - | - | 1 (1.3) | - | 1 (2.2) | - | ||
| Pembrolizumab mono | n = 30 | n = 19 c | n = 35 | n = 35 | ||||||
| All events, n (% of all) | 39 | 10 (25.6) | 23 | 6 (26.1) | 80 | 7 (8.8) | 28 | 5 (17.9) | .02*/.36 | .01*/.14 |
| Skin | 12 (30.8) | 3 (30) | 6 (26.1) | 2 (33.3) | 33 (41.3) | 2 (28.6) | 7 (25) | 1 (20) | ||
| Fatigue | 5 (12.8) | - | 3 (13) | - | 11 (13.8) | - | 4 (14.3) | - | ||
| Endocrinopathy | 9 (23.1) | - | 5 (21.7) | - | 16 (20.0) | 1 (14.3) | 5 (17.9) | 1 (20) | ||
| Gastrointestinal | 4 (10.3) | 4 (40) | 2 (8.7) | 2 (33.3) | 6 (7.5) | 1 (14.3) | 5 (17.9) | 1 (20) | ||
| Musculoskeletal | 3 (7.7) | - | 3 (13) | - | 5 (6.3) | 1 (14.3) | - | - | ||
| Pneumonitis | 3 (7.7) | 2 (20) | 2 (8.7) | 1 (16.7) | 3 (3.7) | 1 (14.3) | 3 (10.7) | 1 (20) | ||
| Hepatitis | 3 (7.7) | 1 (10) | 2 (8.7) | 1 (16.7) | 2 (2.5) | 1 (14.3) | 2 (7.1) | 1 (20) | ||
| Sicca syndrome | - | - | - | - | 2 (2.5) | - | - | - | ||
| Ocular | - | - | - | - | 1 (1.3) | - | 1 (3.6) | - | ||
| Infusion related reaction | - | - | - | - | 1 (1.3) | - | 1 (3.6) | - | ||
| Nivolumab mono | n = 30 | n = 16 c | n = 18 | n = 18 | ||||||
| All events, n (% of all) | 33 | 5 (15.1) | 25 | 3 (12) | 50 | 6 (12) | 18 | 1 (5.6) | .08/.12 | .13/.46 |
| Skin | 5 (15.2) | - | 4 (16) | - | 15 (30) | 1 (16.7) | 3 (16.7) | - | ||
| Fatigue | 6 (18.2) | - | 3 (12) | - | 11 (22) | - | 4 (22.2) | - | ||
| Endocrinopathy | 3 (9.1) | - | 3 (12) | - | 7 (14) | 1 (16.7) | 3 (16.7) | 1 (100) | ||
| Gastrointestinal | 11 (33.3) | 2 (40) | 10 (40) | 2 (66.7) | 10 (20) | 1 (16.7) | 6 (33.3) | - | ||
| Musculoskeletal | 2 (6.1) | 1 (20) | 2 (8) | 1 (33.3) | 2 (4) | - | - | - | ||
| Pneumonitis | 3 (9.1) | 1 (20) | 1 (4) | - | 1 (2) | - | 1 (5.6) | - | ||
| Hepatitis | - | - | - | - | 2 (4) | 1 (16.7) | 1 (5.6) | - | ||
| Sicca syndrome | - | - | - | - | 1 (2) | 1 (16.7) | - | - | ||
| Ocular | 1 (3) | - | 1 (4) | - | - | - | - | - | ||
| Infusion related reaction | 1 (3) | 1 (20) | - | - | 1 (2) | 1 (16.7) | - | - | ||
| Neurological | 1 (3) | - | 1 (4) | - | - | - | - | - | ||
| Pembro + chemo | n = 11 | n = 1 c | n = 15 | n = 15 | ||||||
| All events, n (% of all) | 20 | 2 (10) | 2 | - | 28 | 2 (7.1) | 18 | 1 (5.6) | .87/.84 | N.a./N.a. |
| Skin | 4 (20) | - | - | - | 6 (21.4) | - | 4 (22.2) | - | ||
| Fatigue | 6 (30) | - | 1 (50) | - | 5 (17.9) | - | 4 (22.2) | - | ||
| Endocrinopathy | 2 (10) | - | - | - | 8 (28.6) | - | 4 (22.2) | - | ||
| Gastrointestinal | 3 (15) | - | - | - | 3 (10.7) | - | 2 (11.1) | - | ||
| Musculoskeletal | 1 (5) | - | - | - | 1 (3.6) | - | 1 (5.6) | - | ||
| Pneumonitis | 1 (5) | - | - | - | 1 (3.6) | 1 (50) | - | - | ||
| Hepatitis | 1 (5) | 1 (50) | - | - | 2 (7.1) | 1 (50) | 1 (5.6) | 1 (100) | ||
| Sicca syndrome | 1 (5) | - | 1 (50) | - | 2 (7.1) | - | 2 (11.1) | - | ||
| Cardiovascular | 1 (5) | 1 (50) | - | - | - | - | - | - | ||
Adverse events were assessed in the standard dose cohort (SD) and the EI dosing cohort (EI).
Start of the escalation window is defined as the moment in which patients were escalated from standard (SD) to the extended dose interval due to the COVID-19 pandemic: 4 weeks after start of treatment with adjuvant durvalumab, or 6 weeks after start of pembrolizumab mono- or consolidation therapy or nivolumab monotherapy.
Decreased case number due to drop out of patients before entering the escalation window as result of early ICI-related adverse events and/or early progression of disease.
P-value ≤ .05 considered statistically significant. N.a., not assessed. N, number of patients
Table 3.
Treatment Adjustments Due to Adverse Events
| Standard dose n (%*) | Extended interval dosing n (%*) | |
|---|---|---|
| Total number of treatment adjustments | 23 (26.1) | 31 (26.5) |
| - Treatment reduced to single dose | - | 8 (6.8) |
| • By treatment schedule | ||
| Chemotherapy + ICI | - | - |
| Pembrolizumab monotherapy | - | 4 |
| Nivolumab monotherapy | - | 2 |
| Durvalumab adjuvant | - | 2 |
| • By PD-L1 expression | ||
| PD-L1 ≥ 50% | - | 4 |
| PD-L1 < 50% | - | 1 |
| PD-L1 not assessed | - | 2 |
| Treatment interrupted | 12 (13.6) | 18 (15.4) |
| • By treatment schedule | ||
| Chemotherapy + ICI | 3 | 4c |
| Pembrolizumab monotherapy | 5a | 10d |
| Nivolumab monotherapy | 4b | 3 |
| Durvalumab adjuvant | - | 1e |
| • By PD-L1 expression | ||
| PD-L1 ≥ 50% | 5 | 12 |
| PD-L1 < 50% | 7 | 5 |
| PD-L1 not assessed | - | 1 |
| Treatment discontinued | 11 (12.5) | 5 (4.3) |
| • By treatment schedule | ||
| Chemotherapy + ICI | 1 | - |
| Pembrolizumab monotherapy | 5a | - |
| Nivolumab monotherapy | - | 2 |
| Durvalumab adjuvant | 5 | 3 |
| • By PD-L1 expression | ||
| PD-L1 ≥ 50% | 5 | 1 |
| PD-L1 < 50% | 3 | 3 |
| PD-L1 not assessed | 3 | 1 |
Percent of all patients in the standard dose (nSD = 88) and the EI dosing cohort (nEI = 117). Each cohort includes patients receiving pembrolizumab monotherapy (nSD = 30 and nEI = 35), pembrolizumab consolidation therapy (nSD = 11 and nEI = 15), nivolumab monotherapy (nSD = 30 and nEI = 18), and adjuvant durvalumab (nSD = 17 and nEI = 49).
One patient had two occurrences of the same toxicity on pembrolizumab monotherapy (hepatitis).
One patient had two occurrences of same toxicity on nivolumab monotherapy (colitis).
Therapy was interrupted in one patient receiving chemotherapy-ICI combination (hepatitis and endocrinopathy).
Therapy was interrupted in two patients treated with pembrolizumab monotherapy, patient 1: pneumonitis and endocrinopathy; patient 2: two occurrences of skin toxicity.
Patient with two different toxicities: fatigue leading to dose reduction and skin toxicity leading to treatment interruption.
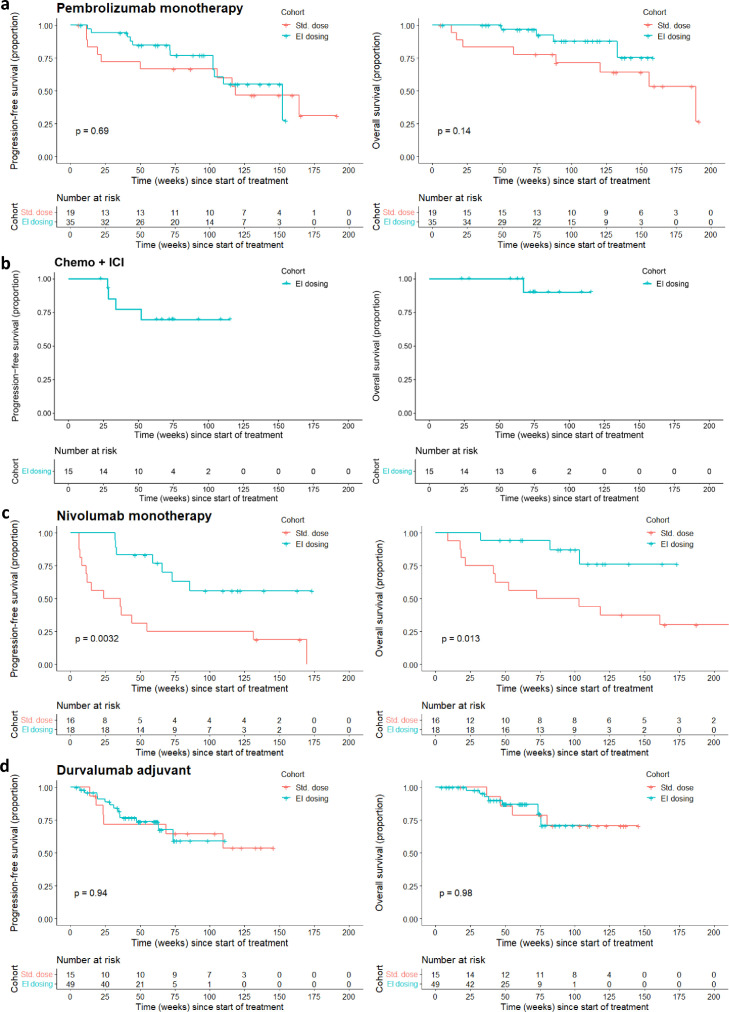
PFS and OS were comparable between both dosing groups (Figure 2 ) in patients receiving pembrolizumab monotherapy and in patients receiving adjuvant durvalumab (Pembrolizumab, median follow-up 93.7 weeks [range, 6.1-191.4], EI dosing cohort: median PFS 152 weeks [95% CI, 102 to ∞] and median OS not reached, SD cohort: median PFS 118 weeks [95% CI 50 to ∞] and median OS 189 weeks [95% CI, 121 to ∞]. Durvalumab, median follow-up 54.6 weeks [range, 4.4-145.7], median PFS and OS not reached in both cohorts).
Figure 2.
PFS and OS according to ICI treatment schedule (red, Standard dose cohort; blue, Extended interval dosing cohort; two-sided log-rank test). For the survival analysis, patients with ICI-related toxicity and/or PD before start of the escalation window were excluded from the standard dose group. (a) Patients treated with pembrolizumab monotherapy (n = 54). (b) Patients treated with chemo-ICI combination therapy (n = 15; EI dosing cohort only). (c) Patients treated with nivolumab monotherapy (n = 34). (d) Patients treated with durvalumab (n = 64).
PFS and OS were better in the EI dosing cohort of nivolumab (median follow-up 98.6 weeks [range, 8.9-219.9], EI dosing cohort: median PFS and median OS not reached, SD cohort: median PFS 29.8 weeks [95% CI, 11.1 to ∞] and median OS 87.8 weeks [95% CI, 41.7 to ∞]). Patients in the EI dosing cohort of nivolumab had been escalated after a median of 17 cycles (range, 5-52). The comparison of survival could not be performed in the chemo-ICI combination group due to low case numbers of the SD cohort (n = 1) after patient selection (median follow-up 74.0 weeks [range, 23.0-115.4], EI dosing cohort: median PFS and median OS not reached).
Discussion
In this retrospective single-center cohort study, we compared safety and efficacy of EI dosing for ICI mono- or consolidation treatment during the COVID-19 pandemic with data of SD schedules in patients with stage III and IV NSCLC.
Low grade AEs were observed more frequently only in the pembrolizumab EI cohort compared to the SD cohort. After dose escalation, however, we did not observe an increase in clinically relevant toxicity leading to treatment interruption and/or discontinuation compared to the SD cohort.
Efficacy of pembrolizumab and durvalumab were comparable between both groups, whereas a better survival of EI dosing for nivolumab was suggested by our data. The apparent increased survival of this cohort, however, can be explained by a shift of patients to other centers in the Netherlands throughout the years 2019-2021. As a consequence, mainly long-term responders were escalated during the COVID-19 pandemic in our center, skewing PFS and OS in favor of the EI dosing cohort. The selection bias and the reporting bias of especially low-grade AEs are the biggest limitations of this retrospective analysis. In addition we could not include enough patients to properly power the explorative PFS and OS analysis.
Until now, only limited data about EI dosing for ICI in NSCLC is available. One small observational study in 32 NSCLC patients receiving either SD or EI dose of durvalumab reported comparable rates of ICI related AEs and survival in both dose cohorts.4 Data of a randomized controlled trial assessing nivolumab or pembrolizumab EI dosing in locally advanced or metastatic cancers is expected in 2025 (ClinicalTrials.gov NCT04295863). EI dosing for nivolumab in metastatic melanoma, on the contrary, is common practice.5
To our knowledge this is the first comprehensive analysis of safety and efficacy of EI dosing for pembrolizumab mono- or consolidation therapy, nivolumab monotherapy and adjuvant durvalumab in stage III and stage IV NSCLC patients. Based on our data these schedules seem a safe and effective strategy not only to decrease the number of visits during and after the COVID-19 pandemic.
Clinical practice points
-
•
Until now, only limited data about extended interval (EI) dosing for ICI in NSCLC is available. One small observational study in 32 NSCLC patients receiving either standard dose or EI dose of durvalumab reports comparable rates of ICI related AE and survival in both dose cohorts.
-
•
Our study in 117 patients shows extended interval dosing did not lead to an increase in clinically relevant toxicity, no increase of patients needing dose reduction and no increased frequency of treatment discontinuations.
-
•
Efficacy of pembrolizumab monotherapy and adjuvant durvalumab were comparable in both groups. Based on our study these schedules seem a safe and effective strategy.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Lala M., Li TR, de Alwis DP, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68–75. doi: 10.1016/j.ejca.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X., Shen J, Ivaturi V, et al. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann Oncol. 2020;31:302–309. doi: 10.1016/j.annonc.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L., Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 4.Mac S., Hui R.L., Nishimura Kris, et al. Evaluation of the safety and effectiveness of switching from standard to extended interval dosing for durvalumab in unresectable stage III non-small cell lung cancer. J Clin Oncol. 2021;39:e20501. [Google Scholar]
- 5.Swetter S.M., Thompson J.A., Albertini M.R, et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Canc Netw. 2021;19:364–376. doi: 10.6004/jnccn.2021.0018. [DOI] [PubMed] [Google Scholar]