Abstract
Our understanding of risk factors and interventions influencing outcomes from coronavirus disease 2019 (COVID-19) has continued to evolve, revealing advances emerging from hypotheses formed at the start of the pandemic. Epidemiologic studies have shown that asthma control, rather than a diagnosis of asthma, is a determinant of COVID-19 severity. Clinical outcomes in patients with primary immunodeficiencies, even in those with impaired cellular immunity, are variable. IL-6 has emerged as a reliable biomarker of COVID-19 severity, and large clinical trials have shown the potential for improving outcomes through inhibition of IL-6 signaling in some patients. Studies of genetic risk factors for severe COVID-19 have also revealed the importance of interferon homeostasis in the defense against severe acute respiratory syndrome coronavirus 2. Because COVID-19 vaccines constitute the primary tool for ending this pandemic, strategies have been developed to address potential allergic and immune-mediated reactions. Here, we discuss advances in our understanding of COVID-19 risk factors and outcomes within the context of allergic and immunologic mechanisms.
Key words: SARS-CoV-2, COVID-19, asthma, primary immunodeficiency, biologics
Abbreviations used: COVID-19, Coronavirus disease 2019; CVID, Common variable immunodeficiency; CXCL, CXC motif chemokine ligand; EUA, Emergency use authorization; HR, Hazard ratio; IFN, Interferon; JAK, Janus kinase; OR, Odds ratio; PEG, Polyethylene glycol; PID, Primary immunodeficiency; RCT, Randomized controlled trial; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TLR, Toll-like receptor
The enormity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic galvanized an unabated search for answers and interventions. This review focuses on coronavirus disease 2019 (COVID-19) outcomes relevant to allergic and/or immunologic disorders and mechanisms. These include clinical outcomes in patients with asthma and primary immunodeficiencies (PIDs), investigations of cytokines as predictors of COVID-19 severity, and comparisons of clinical trials using biologics to target cytokine-driven inflammation. We also discuss immunologically driven reactions to COVID-19 vaccines that did not emerge until the vaccines were available to the general public. We synthesize common themes and notable differences among studies, with the goal of highlighting immunologic mechanisms underlying clinical outcomes that have emerged in the past 2 years of the COVID-19 pandemic.
Clinical outcomes in patients with asthma
At the start of the COVID-19 pandemic, asthma was investigated as a risk factor for severe disease,1 given the known association between asthma and respiratory infections.2, 3, 4 Two early studies of patients from Wuhan, China, identified that asthma occurred in less than 1% of patients with COVID-19.5 , 6 Subsequent studies have supported these initial findings that a diagnosis of asthma is not correlated with severe disease,7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and multiple studies have even reported a lower risk.5 , 17, 18, 19, 20 The baseline severity of asthma does not seem to impact the likelihood of a positive SARS-CoV-2 antigen test result or the severity of infection.10 , 15 , 16 , 19 Sunjaya et al17 performed a meta-analysis of asthmatic patients with COVID-19 that included 57 studies, for a total of 587,280 patients. Compared with those without asthma, patients with asthma were found to have a 13% reduction in hospitalization with COVID-19 (95% CI, 0.77-0.99; P = .03); there was no significant difference in critical care admission, rates of mechanical ventilation, or mortality. Hussein et al14 also performed a meta-analysis of 107,983 patients and found that there was no differential risk of hospitalization rate, critical care admission, or development of acute respiratory distress syndrome between asthmatic and nonasthmatic patients; however, asthmatic patients had an increased rate of intubation (relative risk, 1.27; 95% CI, 1.02-1.58; P = .03). The authors note that the age group with the highest risk of intubation, spanning age 50 to 64 years, also had high rates of elevated body mass index and obesity, 2 additional known risk factors for severe COVID-19. Similarly, multiple studies have concluded that comorbidities in adults as well as baseline asthma control influence an individual’s risk for severe COVID-19.10 , 19 , 21, 22, 23
Studies also investigated the risk of COVID-19 associated with either allergic or TH2-low, nonallergic asthma. TH2-dependent gene signatures and elevated circulating levels of IL-13 have been found to be inversely proportional to the expression of the angiotensin-converting enzyme 2 receptor, which enables SARS-CoV-2 entry into cells,24 , 25 suggesting a mechanism for reduced viral load in patients with allergic asthma. A number of studies have found that individuals with nonallergic asthma had a higher risk of SARS-CoV-2 test positivity, hospitalization, need for intensive care, and/or death, as compared with TH2-high asthmatic patients.5 , 21 , 22 , 26 In patients with allergic asthma, eosinophilia before and during hospitalization with SARS-CoV-2 correlated with better outcomes, possibly due to antiviral properties of the eosinophils.1 , 16 , 27 , 28 In South Korea, Yang et al26 investigated the clinical implications of allergic disorders in the setting of COVID-19, including data from 219,959 patients. Of 7340 patients with positive SARS-CoV-2 test results, those with nonallergic asthma had a greater risk of severe clinical outcomes as compared with those with allergic asthma (odds ratio [OR], 4.09; 95% CI, 1.69-10.52; P < .05), including critical care admissions, intubation, or death.26 However, other studies found no significant differences in outcomes between those with atopic versus nonatopic asthma.1 , 19 These disparate results may arise from the differences in genetic background, microbiota, environmental exposures, and comorbidities, which lead to clinical variability within each asthma endotype.29 , 30
Although a diagnosis of asthma is not itself a risk factor for severe COVID-19, studies have suggested that poorly controlled asthma is associated with more severe disease. In a cohort of 24 patients requiring critical care for SARS-CoV-2 in February 2020, all 3 of the mechanically ventilated patients had received systemic glucocorticoids for asthma exacerbations within 1 week of admission.31 A retrospective study of 61,338 patients subsequently found that asthmatic patients who had used both bronchodilators and corticosteroids within 6 months of SARS-CoV-2 infection had higher risks of needing hospitalization, intensive respiratory support, and critical care (OR, 1.47-1.66; P < .05).32 Adir et al33 compared the outcomes of SARS-CoV-2 infections in a cohort of 80,602 adult asthmatic patients over 9 months, during which time 10.2% tested positive for SARS-CoV-2. Chronic steroid use and a higher number of filled steroid prescriptions within the preceding year were associated with an increased risk for severe disease, even after adjusting for comorbid conditions, age, sex, and smoking.33 Biologics, including inhibitors of IL-5/IL-5R (mepolizumab, reslizumab, and benralizumab), the IL-4Ra inhibitor dupilumab, and the IgE inhibitor omalizumab, did not increase the risk of moderate or severe COVID-19.33 Additional studies have similarly found that the use of biologics, in addition to short-term steroid use, did not increase the risk of a severe COVID-19.10 , 16 , 18 , 22 , 33 From a public health standpoint, metered-dose inhalers have been recommended over nebulized treatments to minimize the risk of aerosolizing highly infectious viral particles.34 , 35 Given the importance of maintaining asthma control, multiple centers have endorsed the use of telemedicine and mobile applications for clinical care.34 , 36 , 37 Equitable access to patients and families is important, because racial and ethnic minority populations have had higher rates of positive test results and hospitalizations for COVID-19.15 , 16 , 38
Clinical outcomes in patients with PIDs
In contrast to asthma, PIDs are rare diseases. Thus, aside from a few larger cohort studies, most outcome studies are limited to single case reports or small case series. Overall, the association of PID with severe outcomes from SARS-CoV-2 infection remains variable. In the largest international study of SARS-CoV-2 infections in 94 patients with PIDs, Meyts et al39 found that the overall case-fatality rate of 10% was concordant with the 1% to 20% case-fatality rate found in the global population at that time; furthermore, 37% of the patients with PIDs had mild or asymptomatic disease. Similarly, a study of 20 patients with PIDs from a multicenter study in Israel also did not find an increased risk of morbidity or mortality compared with the general population.40 However, the UK Primary Immunodeficiency Network reported a hospitalization rate of 53.3% and case-fatality ratio of 37.5% in a cohort of 60 PID patients with COVID-19, both of which exceed that of the reference population.41 Smaller studies in Turkey and Iran also identified higher case-fatality rates in patients with PIDs than for the general population in each study’s country.42 , 43 The variable association between PIDs and higher case-fatality rates arises from multiple factors. Primary antibody deficiencies were the most common PIDs in the international and UK studies, whereas combined immunodeficiencies were the most common PID subtype in the studies from Israel, Turkey, and Iran. Meyts et al39 identified similar rates of nonimmunologic COVID-19 risk factors, including preexisting cardiac, pulmonary, or renal disease, in the patients with PIDs compared with the general population; however, all of the patients with PIDs and additional comorbidities died. Shields et al41 showed greater prevalence of preexisting pulmonary disease (62.5% vs 16.7%) and liver disease (12.5% vs 3.1%) in hospitalized patients with PIDs compared with that in the general hospitalized population.41 The authors of these studies note that differences in patient adherence to social distances, the overall number of COVID-19 cases in each country, governmental policies, and access to medical resources also influence the outcomes of SARS-CoV-2 infection in immunologically vulnerable individuals.39, 40, 41, 42, 43
In the earliest report of COVID-19 outcomes in patients with humoral immunodeficiencies, 2 patients with agammaglobulinemia and absent B cells had milder disease not requiring any treatment, while 4 of 5 patients with common variable immunodeficiency (CVID) required mechanical ventilation and 1 died.44 In the study by Meyts et al,39 most patients (53 of 94) had primary antibody deficiency, including 29 with CVID. Of these, 17 (32%) were asymptomatic or had mild disease, whereas the remainder (68%) required hospitalization and 7 patients (13%) died. Shields et al41 reported higher mortality in the patients with CVID compared with those with unspecified primary antibody deficiency or X-linked agammaglobulinemia, but noted that individuals with CVID were older. A report of SARS-CoV-2 infection in a patient with CVID due to a loss-of-function variant in NFKB2 revealed how COVID-19 amplified the juxtaposition of immunodeficiency and hyperinflammation often seen in patients with CVID.45, 46, 47 The patient’s respiratory arrest, cardiac arrhythmias, cytokine storm, and transaminitis resolved after mechanical ventilation, remdesivir, intravenous immunoglobulin, convalescent plasma, and a single dose of tocilizumab.45 Conversely, other studies have noted mild disease in patients with CVID.40 , 48 The range of clinical outcomes thus parallels the incomplete penetrance associated with some genetically defined subtypes of CVID, such as NFKB1 or NFKB2 haploinsufficiency, as well as the wide range of immunodeficiency seen in patient cohorts with CVID.49 , 50
Because cellular immunity is critical for host defense against viral infections, multiple studies have characterized the severity of COVID-19 in patients with T-cell lymphopenia or dysfunction. Meyts et al39 identified 14 patients with combined immunodeficiency. Of these, 47% required hospitalization and an additional 20% required critical care.39 Cohort studies in which combined immunodeficiencies constituted the most common PID also had higher case-fatality rates than the general population.42 , 43 Patients with a disorder encompassing combined immunodeficiency and immune dysregulation, such as haploinsufficiency of cytotoxic T-lymphocyte–associated protein 4 or deficiency of LPS-responsive beige-like anchor protein, have been associated with asymptomatic as well as more severe disease.39 , 51 Notably, T-cell lymphopenia itself has not been universally associated with severe COVID-19, as indicated by a report of male twin newborns with complete 22q11.2 syndrome who developed only fevers and mild respiratory symptoms.52 Because both patients had a strong type I interferon (IFN)-stimulated genes signature attributed to congenital cytomegalovirus infections, the authors postulated that increased innate immune activation may have mitigated the severity of COVID-19 in these patients.52
The contributions of IFN signaling to immunity against SARS-CoV-2 have been underscored by patients with hemizygous loss-of-function Toll-like receptor (TLR)7 variants, leading to impaired type I IFN and type II IFN responses.53, 54, 55 Complementarily, multiple studies have identified autoantibodies against type I IFNs in patients with severe COVID-19.56, 57, 58 A study of 987 patients with severe COVID-19 pneumonia found that 10.2% had neutralizing IgG autoantibodies against IFN-ω, IFN-α, or both, suggesting a crucial role for type I IFNs in immunity against SARS-CoV-2.56 Plasmapheresis was effective treatment for a pediatric patient with autoimmune polyendocrine syndrome 1, autoantibodies to type I IFNs, and severe COVID-19.59 Lastly, enrichment of variants impairing TLR3- and IRF7-dependent type I IFN immunity has been identified in some,60 but not all,61 , 62 patient cohorts with severe COVID-19. As a mechanistic counterpoint to the association of severe COVID-19 with impaired IFN signaling, genetic variants increasing type I and II IFN signaling have been identified in children with multisystem inflammatory syndrome in children,63 , 64 a postinfectious pediatric complication of SARS-CoV-2 characterized by diffuse immune activation typically responsive to immunomodulatory doses of intravenous immunoglobulin and glucocorticoid treatment.65 Deleterious variants in genes known to restrain inflammation, specifically, SOCS1 (encoding suppressor of cytokine signaling 1), CYBB (encoding the beta subunit of cytochrome b), and XIAP (encoding X-linked inhibitor of apoptosis), were identified in a single-center cohort of 17 patients with multisystem inflammatory syndrome in children.63 , 64 Collectively, these studies show the importance of IFN homeostasis in the immune response to SARS-CoV-2 infection.
Cytokines as predictors of COVID-19 outcomes
Characterizations of cytokines and chemokines are also relevant to clinical outcomes from SARS-CoV-2 infection in patients without underlying immune disorders.66 Most studies have shown an association between increased IL-6 levels and severe COVID-19.5 , 67, 68, 69, 70, 71, 72, 73 A 40-day longitudinal study of 1484 patients showed that elevated levels of IL-6, IL-8, and TNF-α independently predict mortality in patients with COVID-19.74 A retrospective analysis of 548 patients hospitalized with COVID-19 in China showed that an upward trend in circulating IL-6 levels during hospitalization was a risk factor for mortality (hazard ratio [HR], 2.63; 95% CI, 1.23-5.62).73 These results have been supported by additional observational studies and a meta-analysis of 10 studies showing that elevated IL-6 levels were associated with mortality,68 , 72 , 75 , 76 the need for increased respiratory support,67 , 69 , 74 and overall risk of severe disease.5 , 76, 77, 78, 79, 80 In addition to IL-6, multiple studies have highlighted the elevated levels of chemokines in plasma and bronchial lavage fluid from patients with severe COVID-19. These include chemokines important for leukocyte recruitment and adhesion (CXC motif chemokine ligand [CXCL]1, CXCL8, C-C motif ligand 2, C-C motif ligand 7, and C-C motif ligand 8), as well as the IFN-γ–associated chemokines (CXCL9 and CXCL10).70 , 77 , 81 , 82 Zaid et al81 also found higher levels of IL-6, CXCL1, and CXCL8 in bronchial lavage fluid from 45 patients with severe COVID-19 compared with 25 controls,81 concordant with the accumulation of proinflammatory monocyte-derived macrophages and neutrophils identified by single-cell RNA sequencing in the bronchial lavage fluid from patients with severe COVID-19.83 Longitudinal studies have shown prolonged elevation of IL-6 during the clinical course of severe COVID-19, further underscoring the hyperinflammatory nature of this disease.84 , 85
In contrast to the robust expression of IL-6 and inflammatory chemokines, studies have highlighted the variable expression of type I/II/III IFNs in patients with severe COVID-19. A study of 26 patients with severe COVID-19, none of whom received antiviral or immunomodulatory therapies, found that most patients exhibited increasing circulating concentrations of type I IFNs.84 Plasma levels of IFN-α peaked during the viral replication phase in the first 10 days of illness and subsequently declined.84 However, a minority (n = 5) of patients generated minimal circulating IFN-α throughout their hospitalization and required invasive ventilation and a longer duration of critical care.84 Other studies have similarly identified an association between reduced early levels of circulating type I IFNs and severe COVID-19.58 , 86 Mechanisms identified thus far include neutralizing autoantibodies against type I IFNs,56 , 57 , 59 as well as monogenic defects in genes important for the generation of a type I IFN response to viral infections, namely, TLR3, IRF7, and TLR7.53, 54, 55 , 60 Notably, studies have also shown that excessive type I IFN levels are associated with mortality in patients with severe COVID-19. A study of 201 patients identified distinct associations between disease severity, cytokine levels at admission, and mortality within 1 month of hospitalization.72 For patients with the most severe disease requiring extracorporeal membrane oxygenation, elevated levels of IL-6, TNF-α, IL-8, and IL-10 at admission were associated with increased mortality. For patients with COVID-19 requiring mechanical ventilation, but not extracorporeal membrane oxygenation, mortality was associated with higher levels of type I IFN, TNF-α, and IL-10 at admission.72 For patients with moderately severe COVID-19 who did not require mechanical ventilation, mortality was predominantly associated with elevated type I IFNs at admission.72 Increased levels of type I IFN in patients with moderately severe COVID-19 or those requiring mechanical ventilation were associated with higher viral loads.72 Collectively, these studies show that increased levels of specific cytokines are not universal biomarkers of severe COVID-19. Rather, aberrantly low or high levels of cytokines may reflect distinct pathways culminating in severe COVID-19, including genetic risk factors, acquired autoantibodies, or high viral loads.
Moderate to severe cases of COVID-19 are also associated with increased levels of circulating IL-10. IL-10 is an anti-inflammatory cytokine secreted by multiple cell types, including monocytes, regulatory T cells, effector TH1 and TH2 cells, and innate lymphoid cells.87 , 88 It inhibits antigen presentation and proinflammatory cytokine secretion from monocytes and dendritic cells by reducing MHC class II expression and restrains T-cell activation,87 , 88 in part through upregulating expression of the suppressor of cytokine signaling protein 3, which inhibits CD8+ T-cell proliferation.89 Many studies have identified an association between IL-10 levels and severe COVID-19,5 , 70 , 72 , 77 , 79 , 90 a finding also seen in patients and mouse models of other viral infections, such as influenza and respiratory syncytial virus.91 , 92 This reflects the counterregulatory role of IL-10 in restraining pathogen-induced immune cell activation.
Outcomes of studies using biologics to target cytokine signaling
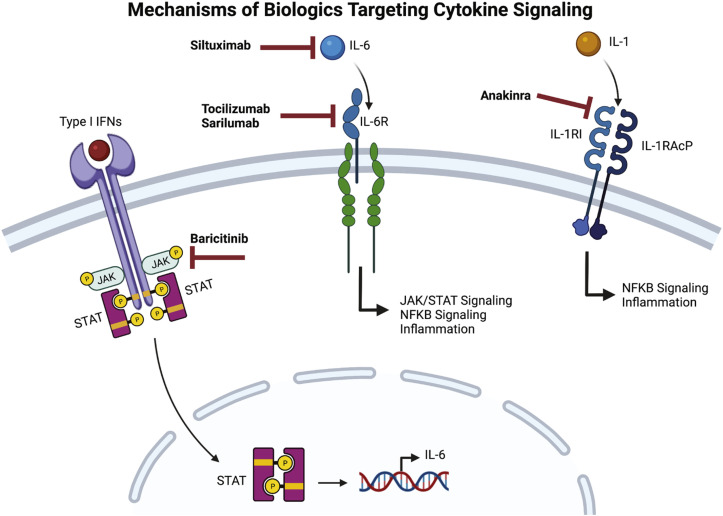
The hyperinflammatory state characteristic of moderate to severe COVID-19 prompted studies to determine the role of existing immunosuppressive therapies in the treatment of this disease. Because of their anti-inflammatory effects, glucocorticoids were investigated as therapeutic agents in the treatment of COVID-19. Based on results from several clinical trials, the National Institutes of Health treatment guidelines for COVID-19 now recommend the use of systemic corticosteroids in certain categories of hospitalized and nonhospitalized patients.93 In tandem, biologics were also investigated early in the pandemic as potential therapeutic agents. Here, we focus our discussion on biologics that are known to inhibit cytokine signaling and that have been investigated by multiple studies (Fig 1 ).
Fig 1.
Mechanisms of biologics targeting cytokine signaling in COVID-19. Baricitinib inhibits JAK1 and JAK2 signaling and is associated with reductions in circulating levels of IL-6. Siltuximab is an mAb to IL-6, whereas tocilizumab and sarilumab are mAbs against soluble and membrane-bound IL-6 receptors. Anakinra competitively binds the IL-1R1 receptor.
IL-6 signaling
mAbs targeting the IL-6/IL-6R axis are used to treat cytokine release syndrome caused by chimeric antigen receptor T cells as well as macrophage activation syndrome.94 Although studies have noted the lower levels of IL-6 in patients with severe COVID-19 compared with those with cytokine release syndromes,94 IL-6 has been studied as a possible target for the treatment of COVID-19 due to the multiple mechanisms by which elevated levels of IL-6 lead to immune dysregulation. These include upregulation of suppressor of cytokine signaling protein 3, downregulation of IFN-γ expression by CD8+ T cells and natural killer cells, and reduced expression of granzyme B and perforin in natural killer cells.95 In a study of patients hospitalized for moderate to severe COVID-19, serum IL-6 levels inversely correlated with the frequency of granzyme A–expressing natural killer cells.96 Inhibitors of IL-6 signaling include siltuximab, which inhibits IL-6, as well as tocilizumab and sarilumab, both of which inhibit IL-6RA. The binding affinity of sarilumab is approximately 20-fold higher than that of tocilizumab to the IL-6 receptor-α subunit.97
As the oldest biologic inhibitor of IL-6 signaling, tocilizumab has been the most extensively studied in patients with COVID-19. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial is the largest randomized controlled trial (RCT) to date of tocilizumab in patients hospitalized for COVID-19, in which 2022 patients received tocilizumab in addition to standard care and 2094 patients received standard care. By day 28, those treated with tocilizumab had 0.85 times the rate of death compared with those treated with standard care alone (95% CI, 0.76-0.94; P = .0028), manifested as a mortality rate of 31% in the tocilizumab group compared with 35% mortality in the standard care group.98 The Evaluating Minority Patients with Actemra (EMPACTA) trial enrolled participants from racial and ethnic minority populations with a high prevalence of COVID-19: 56% Hispanic or Latino, 14.9% Black, 12.7% American Indian or Alaska Native, 12.7% non-Hispanic White, and 3.7% of other or unknown race and/or ethnicity.99 This study, in which 249 patients were randomized to tocilizumab and 128 patients to placebo, found that a significantly reduced percentage of those receiving tocilizumab required mechanical ventilation: 12% of the tocilizumab group required mechanical ventilation (95% CI, 8.5-16.9), compared with 19.3% of the placebo group (95% CI, 13.3-27.4).99 However, mortality at day 28 did not significantly differ between the 2 groups.99 In contrast, the Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA) RCT of 294 patients treated with tocilizumab and 144 with placebo found no difference in clinical status or mortality by day 28.100 In addition to the aforementioned differences in racial and ethnic backgrounds of the study groups, these studies differed in the proportions of patients with moderate, severe, and critical COVID-19 at the time of enrollment, with COVACTA having the highest percentage of patients requiring mechanical ventilation (38.4% compared with 14% in RECOVERY).98 , 100 Patients in the EMPACTA trial had the least severe COVID-19, because this study excluded any patient who required either noninvasive or invasive ventilatory support.99 Given the differences in findings from these and other RCTs, a meta-analysis of 10,930 hospitalized patients with COVID-19 from 27 international RCTs was performed.101 Patients who received tocilizumab had a lower rate of invasive mechanical ventilation or death, compared with standard of care or placebo (OR, 0.74; 95% CI, 0.66-0.82) and less mortality at day 28 (OR, 0.77; 95% CI, 0.68-0.87).101 No studies showed significantly increased adverse safety events with tocilizumab treatment in patients with COVID-19.101 Consequently, the Food and Drug Administration granted emergency use authorization (EUA) for tocilizumab use in patients aged 2 years or older who are hospitalized for COVID-19 requiring systemic corticosteroids and any level of respiratory support.102
Although tocilizumab has been the most extensively studied inhibitor of IL-6 signaling in patients with COVID-19, investigators have also studied the effect of sarilumab and siltuximab on COVID-19. The Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) RCT of 803 patients (353 to tocilizumab, 48 to sarilumab, and 402 to control) found that patients treated with either tocilizumab or sarilumab had more organ support–free days (median adjusted OR of 1.64 for tocilizumab, with a 95% credible interval of 1.25-2.14, and 1.76 for sarilumab, with a 95% credible interval of 1.17-2.91) compared with those receiving standard care.103 In addition, survival at 90 days was improved in patients who received either therapeutic (HR, 1.61; 95% credible interval, 1.25-2.08).103 In contrast, the Sarilumab COVID-19 Global Study Group found no significant differences in clinical improvement scores or survival after sarilumab treatment.104 This double-blind, international RCT of 419 patients hospitalized with COVID-19 comprised 159 patients randomized to 200 mg of sarilumab, 173 patients to 400 mg sarilumab, and 84 patients randomized to placebo; each patient received 1 dose, with the option of a second dose using the study’s benefit-risk assessment protocol.104 Notably, sample size and disease severity differed between this study and the REMAP-CAP study. The Sarilumab COVID-19 Global Study Group excluded all patients with dysfunction in 2 or more organ systems and enrolled 332 patients to sarilumab, compared with 48 in the REMAP-CAP study. Siltuximab is the least studied of biologic inhibiting IL-6 signaling, as a multicenter RCT of siltuximab for patients requiring ventilatory support for acute COVID-19 is ongoing. The SISCO (Siltuximab in Severe COVID-19) observational, control cohort study of 30 patients in the siltuximab group and 188 patients in the standard care group showed that siltuximab use improved ventilation status and increased survival.105 Collectively, these studies have shown the potential for clinical benefit with anti–IL-6/IL-6R blockade in some patients hospitalized with COVID-19, without an increase in adverse events.
Janus kinase inhibitors
In targeting members of the Janus kinase (JAK) family (JAK1, JAK2, JAK3, and TYK2), JAK inhibitors reduce immune cell activation and cytokine secretion and are used to treat inflammatory conditions, including graft versus host disease, rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, and myelofibrosis.106 These drugs differ in their specificity: upadacitinib is a selective JAK1 inhibitor, fedratinib targets JAK2, ruxolitinib and baricitinib inhibit JAK1 and JAK2, and tofacitinib inhibits JAK1, JAK2, and JAK3.106 , 107 Adverse reactions common to all JAK inhibitors include increased infections, cytopenias, and transaminitis. In addition, long-term usage of tofacitinib, baricitinib, and upadacitinib has been associated with an increased risk of malignancy.106 Baricitinib is the only JAK inhibitor with Food and Drug Administration EUA for the treatment of COVID-19. The ACTT-2 (Adaptive COVID-19 Treatment Trial 2) double-blind, placebo-controlled trial was composed of 515 patients treated with remdesivir and baricitinib and 518 patients treated with remdesivir and placebo. The baricitinib-remdesivir group had a median of 7 days until recovery, compared with 8 days for the placebo-remdesivir group (HR, 1.15; 95% CI, 1.00-1.31; P = .047), with a lower rate of secondary infections and serious adverse events.108 There were no significant differences in mortality between the 2 groups.108 In contrast, the Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER) double-blind placebo-controlled study of 764 patients with baricitinib and 761 patients with placebo showed no differences in survival, progression to ventilatory support, or adverse events between the 2 groups.109 Of note, only 19% of the patients in the COV-BARRIER received remdesivir, in contrast to 100% of the patients in ACTT-2. Other observational and meta-analysis studies showed that the use of baricitinib was associated with reductions in circulating levels of IL-6, monocyte chemotactic protein 3, IL-10, CXCL10, and IFN-γ,70 decreased mortality,110 , 111 and decreased need for mechanical ventilation.110 Initially, the Food and Drug Administration issued an EUA for the use of bariticinib only in combination with remdesivir for the treatment of COVID-19, but subsequently expanded this EUA to include patients hospitalized with COVID-19 aged 2 years or older who require any form of respiratory support. In 1 RCT, improvement with ruxolitinib use was limited in significance to faster improvement on lung computed tomography and recovery from lymphopenia,112 whereas a meta-analysis that included 2 additional clinical trials concluded that ruxolitinib use reduces the risk of death (relative risk, 0.33; 95% CI, 0.13-0.88; P = .0.3).110
IL-1 antagonists
Inhibitors of IL-1 signaling are used for treating hemophagocytic lymphohistiocytosis and macrophage activation syndrome as well as monogenic autoinflammatory disorders.113, 114, 115, 116, 117, 118 Of the 3 available IL-1β antagonists, anakinra, canakinumab, and rilonacept, anakinra, which competitively inhibits the IL-1 type I receptor, has been the most extensively studied in patients with COVID-19. Several observational studies early in the pandemic suggested that treatment with anakinra reduced mortality, without any increase in secondary infections or other adverse events.119, 120, 121 Based on these outcomes, the suPAR-guided Anakinra treatment for Validation of the risk and Early management of SRF by COVID-19 (SAVE-MORE) double-blind, placebo-controlled clinical trial randomized 405 patients to anakinra and 189 patients to placebo.122 Those who received anakinra had reduced risk of worse clinical status at day 28 (adjusted proportional odds of 0.36; 95% CI, 0.26-0.5) and lower mortality (HR, 0.45; P = .045).122 Similarly, a meta-analysis of 1185 patients from 9 randomized controlled, comparative, and observational studies found reduced mortality in those treated with anakinra compared with placebo (adjusted OR, 0.32; 95% CI, 0.20-0.51).123 Additional RCTs are needed to determine whether treatment with anakinra benefits patients with COVID-19.
Vaccines
The mechanisms and efficacy of COVID-19 vaccines in the general population have been extensively reviewed elsewhere.124, 125, 126 Therefore, this section will address topics specific to the patient populations with immunologic or allergic diseases.
Vaccine responses in patients with PIDs
At the time of this study, studies have begun to investigate the response to COVID-19 vaccines in patients with PIDs. An early study assessing cellular and humoral immunity following 2 doses of the Pfizer-BioNTech vaccine in 22 patients with PID showed that 81% (excluding 4 patients with X-linked agammaglobulinemia) generated antispike antibodies; 73% of the cohorts generated polyfunctional T-cell responses to spike peptide stimulation, although only 2 patients with cellular defects were studied.127 A larger study of 81 patients with PIDs showed that 85.1% of patients generated detectable antispike antibodies; lower rates of seroconversion were associated with prior use of rituximab, T- or B-cell lymphopenia, and a history of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy requiring chronic immunosuppressive therapy.128 As increasing numbers of patients with immunodeficiency begin to receive a third booster dose, future studies are needed to determine the real-world impact of additional vaccine doses in mitigating severe COVID-19 in these patients.
Allergic reactions to COVID-19 vaccines
The phase 3 clinical trials of COVID-19 vaccines showed no significant difference in the incidence of anaphylaxis between those who received placebo and vaccine.129 As of the time of this publication, the rates of anaphylaxis to COVID-19 mRNA vaccines have been found to be comparable to those of other vaccines.130 A study of 64,900 health care workers reported that higher rates of allergic reactions occurred in females and patients with a previous history of atopy or other allergies within 30 minutes of the dose.131 Vaccine administration has not been associated with exacerbation of mastocytosis.132 , 133 Allergic reactions to COVID-19 vaccines were initially attributed to polyethylene glycol (PEG) and polysorbate 80, 2 hydrophilic polymers used as excipients in the mRNA-based COVID-19 vaccines as well as other medical and cosmetic products.134 , 135 Approximately 4 cases of PEG-associated anaphylaxis occur in the United States annually.134 Skin testing for PEG or polysorbate 80 was initially suggested as a risk-stratification strategy for those with suspected allergic reactions to the vaccines.129 , 134 , 135 However, positive skin testing result to PEG or polysorbate and/or a history of immediate reaction to the first COVID-19 vaccination were not found to be predictive of allergic reactions to the second dose.135 , 136 Thus, empiric skin testing with PEG, polysorbate, or SARS-CoV-2 vaccines is not recommended, except for patients in whom anaphylaxis to the first dose is strongly suspected.129 In these cases, allergy consultation to determine graded dosing or switching vaccine types was recommended.129 An observation period of 30 minutes (compared with 15 minutes) after the second dose was suggested for those who developed a severe allergic reaction to their first dose,135 but this is not routinely recommended because it does not change outcomes.129 Oral glucocorticoids are not recommended as premedication for patients at risk for allergic reactions due to the potential for reduced immunity to the vaccine.129
In addition to anaphylaxis, both immediate and delayed-type cutaneous reactions were reported.137, 138, 139, 140 Immediate cutaneous reactions included rashes, hives, or angioedema, and were not found to recur in a cohort of 49,197 vaccinated health care workers.137 More severe, but less common cutaneous reactions include delayed subepidermal blistering eruptions developing a median of 1 week after either the first or second vaccination dose.140 The eruptions lasted a median of 3 weeks; treatments used include topical or systemic glucocorticoids, doxycycline, and nicotinamide.140 In a series of 13 patients with subepidermal blistering eruptions, 5 developed the eruption after the first dose; of these, 3 patients received the second dose, which was tolerated without any recurrence of blistering.140 It has been suggested that reactions after the first dose of the COVID-19 vaccine could also indicate non-IgE mechanisms, including direct complement or mast cell activation from the lipid nanoparticle or the RNA itself.141 , 142 Vasovagal or anxiety-related responses are also possible and can often be confused with anaphylaxis.142
Vaccine-induced immune thrombotic thrombocytopenia is rare, a nonallergic, immune-mediated adverse event associated with 2 adenoviral vector-based vaccines, the ChAdOx1 and Ad26.COV2.S vaccines.143 , 144 The symptoms of atypical thrombosis developed within 2 weeks of the first dose in patients who were found to have autoantibodies against platelet factor 4.143 Anti–platelet factor 4 antibodies are most commonly seen as an adverse reaction to heparin, but the patients with vaccine-induced immune thrombotic thrombocytopenia had no history of receiving heparin.145 , 146 The mechanisms underlying this phenomenon remain unknown, because studies have not identified a correlation between the development of anti–SARS-CoV-2 antibodies and anti–platelet factor 4 antibodies.143 , 147 Because vaccine-induced immune thrombotic thrombocytopenia is potentially fatal, this is an absolute contraindication for revaccination with the ChAdOx1 vaccine, but the vaccine remains available to the general population due to the rarity of this adverse event.
Conclusions
Collectively, these studies highlight advances that have been achieved in mitigating the risks of severe COVID-19 (Table I ). For patient with asthma, these studies have revealed the contributions of asthma control, rather than merely the diagnosis of asthma, in influencing COVID-19 severity. For patients with PIDs, unpredictable clinical outcomes have underscored the importance of COVID-19 vaccination, further supported by investigations showing the generation of spike-specific antibodies and T cells in most patients with PIDs studied thus far. The identification of genetic variants either impairing IFN signaling in a subgroup of patients with severe COVID-19 or increasing IFN signaling in those with multisystem inflammatory syndrome in children has highlighted the importance of a balanced immune response to SARS-CoV-2 infection. Concordant with this, clinical outcomes can be improved in some patients with severe COVID-19 through inhibition of mediators downstream of IFN signaling, such as IL-6 and JAK signaling. Future studies delineating the mechanistic nuances governing the immunobiology of SARS-CoV-2 infections will enable the development of interventions for better clinical outcomes.
Table I.
Key advances in 2021 in the knowledge of COVID-19 clinical outcomes
| Topic | Lessons learned |
|---|---|
| Asthma and COVID-195,7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,21, 22, 23,31, 32, 33 |
|
| PIDs and COVID-1941, 42, 43,45, 46, 47, 48, 49, 50, 51, 52,127,128 |
|
| The role of cytokines in COVID-195,58,67, 68, 69, 70, 71, 72, 73, 74,76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86,94,98,101,103, 104, 105,108,110,111 |
|
| COVID-19 vaccines142 |
|
Acknowledgments
We thank Dr Raif S. Geha for his insightful input and guidance during the writing of this manuscript.
Footnotes
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. R01DK130465 to J.C.) and the National Institute of Allergy and Infectious Diseases (grant no. R01AI139633-04S1 to J.C.; grant no. T32AI007512 to B.L. and A.A.N.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Skevaki C., Karsonova A., Karaulov A., Xie M., Renz H. Asthma-associated risk for COVID-19 development. J Allergy Clin Immunol. 2020;146:1295–1301. doi: 10.1016/j.jaci.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raita Y., Camargo C.A., Bochkov Y.A., Celedón J.C., Gern J.E., Mansbach J.M., et al. Integrated-omics endotyping of infants with rhinovirus bronchiolitis and risk of childhood asthma. J Allergy Clin Immunol. 2021;147:2108–2117. doi: 10.1016/j.jaci.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padayachee Y., Faiez T.S., Singanayagam A., Mallia P., Johnston S.L. Asthma and viruses: a focus on rhinoviruses and SARS-CoV-2. J Allergy Clin Immunol. 2021;147:1648–1651. doi: 10.1016/j.jaci.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller R.L., Grayson M.H., Strothman K. Advances in asthma: new understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J Allergy Clin Immunol. 2021;148:1430–1441. doi: 10.1016/j.jaci.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 7.Lovinsky-Desir S., Deshpande D.R., De A., Murray L., Stingone J.A., Chan A., et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146:1027–1034.e4. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caminati M., Lombardi C., Micheletto C., Roca E., Bigni B., Furci F., et al. Asthmatic patients in COVID-19 outbreak: few cases despite many cases. J Allergy Clin Immunol. 2020;146:541–542. doi: 10.1016/j.jaci.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler M.W., O’Reilly A., Dunican E.M., Mallon P., Feeney E.R., Keane M.P., et al. Prevalence of comorbid asthma in COVID-19 patients. J Allergy Clin Immunol. 2020;146:334–335. doi: 10.1016/j.jaci.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Foer D., Bates D.W., Boyce J.A., Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020;146:808–812. doi: 10.1016/j.jaci.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirumbolo S., Bjørklund G. The bimodal SARS-CoV-2 outbreak in Italy as an effect of environmental and allergic causes. J Allergy Clin Immunol. 2020;146:331–332. doi: 10.1016/j.jaci.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Chen J., Chen W., Liu L., Dong M., Ji J., et al. Does asthma increase the mortality of patients with COVID-19? A systematic review and meta-analysis. Int Arch Allergy Immunol. 2021;182:76–82. doi: 10.1159/000510953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S.C., Son K.J., Han C.H., Jung J.Y., Park S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19) Sci Rep. 2020;10:21805. doi: 10.1038/s41598-020-77791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussein M.H., Elshazli R.M., Attia A.S., Nguyen T.P., Aboueisha M., Munshi R., et al. Asthma and COVID-19; different entities, same outcome: a meta-analysis of 107,983 patients. J Asthma. 2021:1–8. doi: 10.1080/02770903.2021.1881970. [DOI] [PubMed] [Google Scholar]
- 15.Margolis R.H.F., Patel S.J., Sheehan W.J., Simpson J.N., Kachroo N., Bahar B., et al. Association between pediatric asthma and positive tests for SARS-CoV-2 in the District of Columbia. J Allergy Clin Immunol Pract. 2021;9:3490–3493. doi: 10.1016/j.jaip.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;9:1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunjaya A.P., Allida S.M., Di Tanna G.L., Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2021:1–14. doi: 10.1080/02770903.2021.1888116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson L.B., Wang L., Fu X., Wallace Z.S., Long A.A., Zhang Y., et al. COVID-19 severity in asthma patients: a multi-center matched cohort study. J Asthma. 2021:1–14. doi: 10.1080/02770903.2020.1857396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberca R.W., Yendo T., Aoki V., Sato M.N. Asthmatic patients and COVID-19: different disease course? Allergy. 2021;76:963–965. doi: 10.1111/all.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146:327–329.e4. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izquierdo J.L., Almonacid C., González Y., Del Rio-Bermudez C., Ancochea J., Cárdenas R., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57 doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoki K., Chakraborty A., Sur S. Molecular mechanisms and epidemiology of COVID-19 from an allergist’s perspective. J Allergy Clin Immunol. 2020;146:285–299. doi: 10.1016/j.jaci.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradding P., Richardson M., Hinks T.S.C., Howarth P.H., Choy D.F., Arron J.R., et al. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma—implications for COVID-19. J Allergy Clin Immunol. 2020;146:208–211. doi: 10.1016/j.jaci.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camiolo M., Gauthier M., Kaminski N., Ray A., Wenzel S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146:315–324.e7. doi: 10.1016/j.jaci.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J.M., Koh H.Y., Moon S.Y., Yoo I.K., Ha E.K., You S., et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estirado A.D., Ibarra G.Z., Skrabski F., Martínez F.J.D.C., Pedro J.D., Sanz M.P.S., et al. COVID-19, severe asthma and biologicals targeting type 2 inflammation: results in a third-level hospital in Madrid, Spain. J Allergy Clin Immunol. 2021;147:AB40. [Google Scholar]
- 28.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang H.H.F., Teo S.M., Sly P.D., Holt P.G., Inouye M. The intersect of genetics, environment, and microbiota in asthma—perspectives and challenges. J Allergy Clin Immunol. 2021;147:781–793. doi: 10.1016/j.jaci.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Kuruvilla M.E., Lee F.E.-H., Lee G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–233. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang B.Z., Chen Z., Sidell M.A., Eckel S.P., Martinez M.P., Lurmann F., et al. Asthma disease status, COPD, and COVID-19 severity in a large multiethnic population. J Allergy Clin Immunol Pract. 2021;9:3621–3628.e2. doi: 10.1016/j.jaip.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adir Y., Humbert M., Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real-world evidence. J Allergy Clin Immunol. 2021;148:361–367.e13. doi: 10.1016/j.jaci.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaker M.S., Oppenheimer J., Grayson M., Stukus D., Hartog N., Hsieh E.W.Y., et al. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8:1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amirav I., Newhouse M.T. COVID-19: time to embrace MDI+ valved-holding chambers. J Allergy Clin Immunol. 2020;146:331. doi: 10.1016/j.jaci.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malipiero G., Paoletti G., Puggioni F., Racca F., Ferri S., Marsala A., et al. An academic allergy unit during COVID-19 pandemic in Italy. J Allergy Clin Immunol. 2020;146:227. doi: 10.1016/j.jaci.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Searing D.A., Dutmer C.M., Fleischer D.M., Shaker M.S., Oppenheimer J., Grayson M.H., et al. A phased approach to resuming suspended allergy/immunology clinical services. J Allergy Clin Immunol Pract. 2020;8:2125–2134. doi: 10.1016/j.jaip.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendes N.F., Jara C.P., Mansour E., Araújo E.P., Velloso L.A. Asthma and COVID-19: a systematic review. Allergy Asthma Clin Immunol. 2021;17:5. doi: 10.1186/s13223-020-00509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus N., Frizinsky S., Hagin D., Ovadia A., Hanna S., Farkash M., et al. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shields A.M., Burns S.O., Savic S., Richter A.G., Anantharachagan A., Arumugakani G., et al. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147:870–875.e1. doi: 10.1016/j.jaci.2020.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esenboga S., Ocak M., Akarsu A., Bildik H.N., Cagdas D., Iskit A.T., et al. COVID-19 in patients with primary immunodeficiency. J Clin Immunol. 2021;41:1515–1522. doi: 10.1007/s10875-021-01065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delavari S., Abolhassani H., Abolnezhadian F., Babaha F., Iranparast S., Ahanchian H., et al. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J Clin Immunol. 2021;41:345–355. doi: 10.1007/s10875-020-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I., et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham R.S., Marshall J.M., Kuehn H.S., Rueda C.M., Gibbs A., Guider W., et al. Severe SARS-CoV-2 disease in the context of a NF-κB2 loss-of-function pathogenic variant. J Allergy Clin Immunol. 2021;147:532–544.e1. doi: 10.1016/j.jaci.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abyazi ML, Bell KA, Gyimesi G, Baker TS, Byun M, Ko HM, et al. Convergence of cytokine dysregulation and antibody deficiency in common variable immunodeficiency with inflammatory complications [published online ahead of print June 17, 2021]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2021.06.008. [DOI] [PMC free article] [PubMed]
- 47.Romberg N., Le Coz C., Glauzy S., Schickel J.-N., Trofa M., Nolan B.E., et al. CVID patients with autoimmune cytopenias exhibit hyperplastic yet inefficient germinal center responses. J Allergy Clin Immunol. 2019;143:258–265. doi: 10.1016/j.jaci.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen B., Rubinstein R., Gans M.D., Deng L., Rubinstein A., Eisenberg R. COVID-19 infection in 10 common variable immunodeficiency patients in New York City. J Allergy Clin Immunol Pract. 2021;9:504–507.e1. doi: 10.1016/j.jaip.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wehr C., Gennery A.R., Lindemans C., Schulz A., Hoenig M., Marks R., et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135:988–997.e6. doi: 10.1016/j.jaci.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 50.Gathmann B., Mahlaoui N., CEREDIH. Gérard L., Oksenhendler E., Warnatz K., et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 51.Tangye S.G., Bucciol G., Meyts I. Mechanisms underlying host defense and disease pathology in response to severe acute respiratory syndrome (SARS)-CoV2 infection: insights from inborn errors of immunity. Curr Opin Allergy Clin Immunol. 2021;21:515–524. doi: 10.1097/ACI.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 52.Colmenero-Velázquez A., Esteso G., del Rosal T., Calvo Apalategui A., Reyburn H., López-Granados E. Marked changes in innate immunity associated with a mild course of COVID-19 in identical twins with athymia and absent circulating T cells. J Allergy Clin Immunol. 2021;147:567–568. doi: 10.1016/j.jaci.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solanich X., Vargas-Parra G., van der Made C.I., Simons A., Schuurs-Hoeijmakers J., Antolí A., et al. Genetic screening for TLR7 variants in young and previously healthy men with severe COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Renkilaraj M.R.L.M., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med. 2021;218 doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez J., Mommert M., Mouton W., Pizzorno A., Brengel-Pesce K., Mezidi M., et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J Exp Med. 2021;218 doi: 10.1084/jem.20211211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemarquis A., Campbell T., Aranda-Guillén M., Hennings V., Brodin P., Kämpe O., et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J Allergy Clin Immunol. 2021;148:96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q., Bastard P., Liu Z., Pen J.L., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Povysil G., Butler-Laporte G., Shang N., Wang C., Khan A., Alaamery M., et al. Rare loss-of-function variants in type I IFN immunity genes are not associated with severe COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI147834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Backman J.D., Li A.H., Marcketta A., Sun D., Mbatchou J., Kessler M.D., et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 2021;599:628–634. doi: 10.1038/s41586-021-04103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee P.Y., Platt C.D., Weeks S., Grace R.F., Maher G., Gauthier K., et al. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J Allergy Clin Immunol. 2020;146:1194–1200.e1. doi: 10.1016/j.jaci.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chou J., Platt C.D., Habiballah S., Nguyen A.A., Elkins M., Weeks S., et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C) J Allergy Clin Immunol. 2021;148:732–738.e1. doi: 10.1016/j.jaci.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son M.B.F., Murray N., Friedman K., Young C.C., Newhams M.M., Feldstein L.R., et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146:518–534.e1. doi: 10.1016/j.jaci.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Utrero-Rico A., Ruiz-Hornillos J., González-Cuadrado C., Rita C.G., Almoguera B., Minguez P., et al. IL-6–based mortality prediction model for COVID-19: validation and update in multicenter and second wave cohorts. J Allergy Clin Immunol. 2021;147:1652–1661.e1. doi: 10.1016/j.jaci.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galván-Román J.M., Rodríguez-García S.C., Roy-Vallejo E., Marcos-Jiménez A., Sánchez-Alonso S., Fernández-Díaz C., et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol. 2021;147:72–80.e8. doi: 10.1016/j.jaci.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sims J.T., Krishnan V., Chang C.-Y., Engle S.M., Casalini G., Rodgers G.H., et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2021;147:107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Singer M., Brahier T., Ngai M., Wright J., Weckman A.M., Erice C., et al. COVID-19 risk stratification algorithms based on sTREM-1 and IL-6 in emergency department. J Allergy Clin Immunol. 2021;147:99–106.e4. doi: 10.1016/j.jaci.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorgham K., Quentric P., Gökkaya M., Marot S., Parizot C., Sauce D., et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J Allergy Clin Immunol. 2021;147:2098–2107. doi: 10.1016/j.jaci.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen R., Sang L., Jiang M., Yang Z., Jia N., Fu W., et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146:89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laguna-Goya R., Utrero-Rico A., Talayero P., Lasa-Lazaro M., Ramirez-Fernandez A., Naranjo L., et al. IL-6–based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:799–807.e9. doi: 10.1016/j.jaci.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Awasthi S., Wagner T., Venkatakrishnan A.J., Puranik A., Hurchik M., Agarwal V., et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021;7:55. doi: 10.1038/s41420-021-00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y., Shen C., Li J., Yuan J., Wei J., Huang F., et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146:119–127.e4. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu Y., Wang D., Chen C., Lu W., Liu H., Lv T., et al. PaO2/FiO2 and IL-6 are risk factors of mortality for intensive care COVID-19 patients. Sci Rep. 2021;11:7334. doi: 10.1038/s41598-021-86676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:e2141. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zaid Y., Doré É., Dubuc I., Archambault A.-S., Flamand O., Laviolette M., et al. Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19. J Allergy Clin Immunol. 2021;148:368–380.e3. doi: 10.1016/j.jaci.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perreau M., Suffiotti M., Marques-Vidal P., Wiedemann A., Levy Y., Laouénan C., et al. The cytokines HGF and CXCL13 predict the severity and the mortality in COVID-19 patients. Nat Commun. 2021;12:4888. doi: 10.1038/s41467-021-25191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 84.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.-C., Perret M., et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. 2020;146:206–208.e2. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Commins S., Steinke J.W., Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 88.Sun H., Wu Y., Zhang Y., Ni B. IL-10-producing ILCs: molecular mechanisms and disease relevance. Front Immunol. 2021;12:979. doi: 10.3389/fimmu.2021.650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rottenberg M., Carow B. SOCS3, a major regulator of infection and inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y., Zhang C., Huang F., Yang Y., Wang F., Yuan J., et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev. 2020;7:1003–1011. doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rojas J.M., Avia M., Martín V., Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. 2017;2017 doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forbester J.L., Humphreys I.R. Genetic influences on viral-induced cytokine responses in the lung. Mucosal Immunol. 2021;14:14–25. doi: 10.1038/s41385-020-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.COVID-19 Treatment Guidelines Information on COVID-19 treatment, prevention and research. https://www.covid19treatmentguidelines.nih.gov Available at:
- 94.Vanderbeke L., Van Mol P., Van Herck Y., De Smet F., Humblet-Baron S., Martinod K., et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. 2021;12:4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ochayon D.E., Waggoner S.N. The effect of unconventional cytokine combinations on NK-cell responses to viral infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.645850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130:4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu C., Rafique A., Potocky T., Paccaly A., Nolain P., Lu Q., et al. Differential binding of sarilumab and tocilizumab to IL-6Rα and effects of receptor occupancy on clinical parameters. J Clin Pharmacol. 2021;61:714–724. doi: 10.1002/jcph.1795. [DOI] [PubMed] [Google Scholar]
- 98.Abani O., Abbas A., Abbas F., Abbas M., Abbasi S., Abbass H., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Domingo P., Mur I., Mateo G.M., Gutierrez M. del M., Pomar V., et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.U.S. Food and Drug Administration Coronavirus (COVID-19) Update: FDA authorizes drug for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-treatment-covid-19 Available at:
- 103.The REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lescure F.-X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gritti G., Raimondi F., Bottazzi B., Ripamonti D., Riva I., Landi F., et al. Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19. Leukemia. 2021;35:2710–2714. doi: 10.1038/s41375-021-01299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Damsky W., Peterson D., Ramseier J., Al-Bawardy B., Chun H., Proctor D., et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814–826. doi: 10.1016/j.jaci.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 107.Mullally A., Hood J., Harrison C., Mesa R. Fedratinib in myelofibrosis. Blood Adv. 2020;4:1792–1800. doi: 10.1182/bloodadvances.2019000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marconi V.C., Ramanan A.V., de Bono S., Kartman C.E., Krishnan V., Liao R., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen C., Wang J., Li H., Yuan L., Gale R.P., Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35:2616–2620. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stebbing J., Sánchez Nievas G., Falcone M., Youhanna S., Richardson P., Ottaviani S., et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–146.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoffman H.M. Therapy of autoinflammatory syndromes. J Allergy Clin Immunol. 2009;124:1129–1140. doi: 10.1016/j.jaci.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boisson B., Laplantine E., Prando C., Giliani S., Israelsson E., Xu Z., et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gernez Y., de Jesus A.A., Alsaleem H., Macaubas C., Roy A., Lovell D., et al. Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1β inhibition. J Allergy Clin Immunol. 2019;144:1122–1125.e6. doi: 10.1016/j.jaci.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Koning H.D., Schalkwijk J., van der Meer J.W.M., Simon A. Successful canakinumab treatment identifies IL-1β as a pivotal mediator in Schnitzler syndrome. J Allergy Clin Immunol. 2011;128:1352–1354. doi: 10.1016/j.jaci.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 117.Goldbach-Mansky R., Kastner D.L. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–1151. doi: 10.1016/j.jaci.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ryan J.G., de Koning H.D., Beck L.A., Booty M.G., Kastner D.L., Simon A. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission. J Allergy Clin Immunol. 2008;121:260–262. doi: 10.1016/j.jaci.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 119.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pontali E., Volpi S., Signori A., Antonucci G., Castellaneta M., Buzzi D., et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J Allergy Clin Immunol. 2021;147:1217–1225. doi: 10.1016/j.jaci.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bozzi G., Mangioni D., Minoia F., Aliberti S., Grasselli G., Barbetta L., et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: an observational cohort study. J Allergy Clin Immunol. 2021;147:561–566.e4. doi: 10.1016/j.jaci.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kyriazopoulou E., Panagopoulos P., Metallidis S., Dalekos G.N., Poulakou G., Gatselis N., et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. eLife. 2021;10 doi: 10.7554/eLife.66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kyriazopoulou E., Huet T., Cavalli G., Gori A., Kyprianou M., Pickkers P., et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3:e690–e697. doi: 10.1016/S2665-9913(21)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharif N., Alzahrani K.J., Ahmed S.N., Dey S.K. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vacc. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Centers for Disease Control and Prevention ACIP GRADE evidence tables for vaccine recommendations. 2021. https://www.cdc.gov/vaccines/acip/recs/grade/table-refs.html Available at:
- 127.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Delmonte O.M., Bergerson J.R.E., Burbelo P.D., Durkee-Shock J.R., Dobbs K., Bosticardo M., et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148:1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greenhawt M., Abrams E.M., Shaker M., Chu D.K., Khan D., Akin C., et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9:3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maltezou HC, Anastassopoulou C, Hatziantoniou S, Poland GA, Tsakris A. Anaphylaxis rates associated with COVID-19 vaccines are comparable to those of other vaccines [published online ahead or print November 27, 2021]. Vaccine. https://doi.org/10.1016/j.vaccine.2021.11.066. [DOI] [PMC free article] [PubMed]
- 131.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rama T.A., Moreira A., Castells M. mRNA COVID-19 vaccine is well tolerated in patients with cutaneous and systemic mastocytosis with mast cell activation symptoms and anaphylaxis. J Allergy Clin Immunol. 2021;147:877–878. doi: 10.1016/j.jaci.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valent P., Akin C., Bonadonna P., Brockow K., Niedoszytko M., Nedoszytko B., et al. Risk and management of patients with mastocytosis and MCAS in the SARS-CoV-2 (COVID-19) pandemic: expert opinions. J Allergy Clin Immunol. 2020;146:300–306. doi: 10.1016/j.jaci.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bruusgaard-Mouritsen MA, Jensen BM, Poulsen LK, Duus Johansen J, Garvey LH. Optimizing investigation of suspected allergy to polyethylene glycols [published online ahead or print May 28, 2021]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2021.05.020. [DOI] [PubMed]
- 135.Wolfson A.R., Robinson L.B., Li L., McMahon A.E., Cogan A.S., Fu X., et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9:3308–3320.e3. doi: 10.1016/j.jaip.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krantz M.S., Kwah J.H., Stone C.A., Phillips E.J., Ortega G., Banerji A., et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robinson L.B., Fu X., Hashimoto D., Wickner P., Shenoy E.S., Landman A.B., et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol. 2021;157:1000. doi: 10.1001/jamadermatol.2021.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157:716. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tomayko M.M., Damsky W., Fathy R., McMahon D.E., Turner N., Valentin M.N., et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750–751. doi: 10.1016/j.jaci.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Risma K.A., Edwards K.M., Hummell D.S., Little F.F., Norton A.E., Stallings A., et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147:2075–2082.e2. doi: 10.1016/j.jaci.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J. Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arepally G.M., Ortel T.L. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021;138:293–298. doi: 10.1182/blood.2021012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelton J.G., Arnold D.M., Nazy I. Lessons from vaccine-induced immune thrombotic thrombocytopenia. Nat Rev Immunol. 2021;21:753–755. doi: 10.1038/s41577-021-00642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tiede A., Sachs U.J., Czwalinna A., Werwitzke S., Bikker R., Krauss J.K., et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138:350–353. doi: 10.1182/blood.2021011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uzun G., Althaus K., Bakchoul T. No correlation between anti-PF4 and anti–SARS-CoV-2 antibodies after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385:1334–1336. doi: 10.1056/NEJMc2111305. [DOI] [PMC free article] [PubMed] [Google Scholar]