Figure 6.
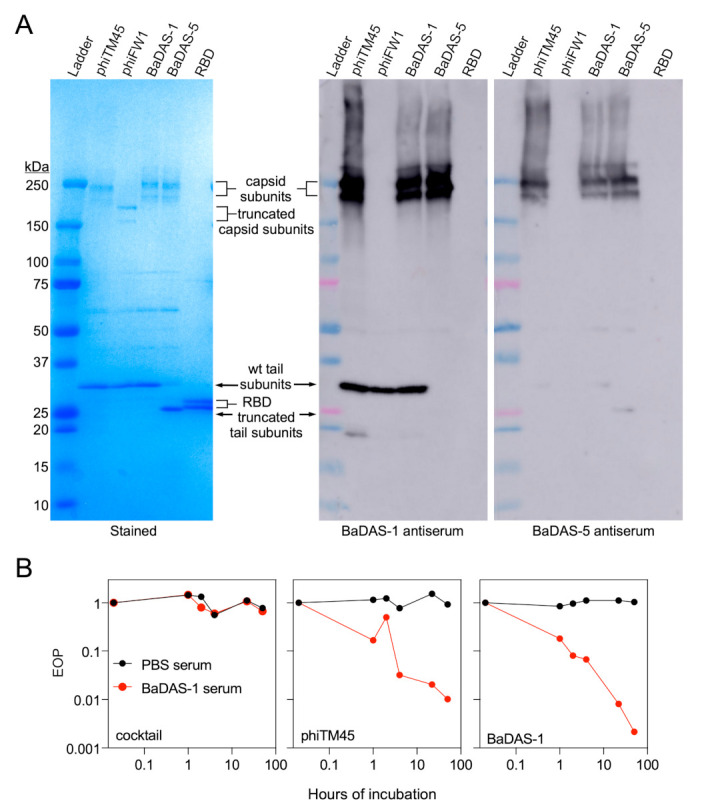
Native C-terminal extensions of Bxb1 capsid and tail proteins are immunodominant. (A) Coomassie-stained gel (left) and Western blots (right) of wild type phiTM45 and mutants phiFW1, BaDAS-1, and BaDAS-5, as well as recombinant SARS-CoV-2 RBD. When probed with serum from a mouse immunized with BaDAS-1, strong binding to wild type tail proteins is observed. In contrast, serum from a mouse immunized with the shaved-tail derivative, BaDAS-5, produces little to no binding to tail proteins. Robust binding to the capsid protein complexes of phiTM45, BaDAS-1 and BaDAS-5 is observed for both sera, while neither sera binds to the truncated capsid proteins of phiFW1. No binding to the SARS-CoV-2 RBD is observed from either sera, despite the presence of significant spike-binding antibodies as assayed by ELISA; this is due to the substantially stronger immune response to phage structural components compared to the display SARS-CoV-2 antigens. Western blots probing response to RBD only are shown in Figure S2. (B) A cocktail of unrelated phages, wild type phiTM45, and BaDAS-1 were incubated with sera from mice immunized with PBS or BaDAS-1 and, after the specified duration of incubation, titered. While the cocktail of unrelated phages were unaffected by this treatment, both phiTM45 and BaDAS-1 were substantially neutralized by the BaDAS-1 antiserum (but unaffected by the mock infection serum).
