Abstract
Pleuromutilin is a fungal diterpene natural product with antimicrobial properties, semisynthetic derivatives of which are used in veterinary and human medicine. The development of bacterial resistance to pleuromutilins is known to be very slow, which makes the tricyclic diterpene skeleton of pleuromutilin a very attractive starting structure for the development of new antibiotic derivatives that are unlikely to induce resistance. Here, we report the very first synthetic modifications of pleuromutilin and lefamulin at alkene position C19–C20, by two different photoinduced addition reactions, the radical thiol-ene coupling reaction, and the atom transfer radical additions (ATRAs) of perfluoroalkyl iodides. Pleuromutilin were modified with the addition of several alkyl- and aryl-thiols, thiol-containing amino acids and nucleoside and carbohydrate thiols, as well as perfluoroalkylated side chains. The antibacterial properties of the novel semisynthetic pleuromutilin derivatives were investigated on a panel of bacterial strains, including susceptible and multiresistant pathogens and normal flora members. We have identified some novel semisynthetic pleuromutilin and lefamulin derivatives with promising antimicrobial properties.
Keywords: pleuromutilin, lefamulin, synthesis, photoinitiated thiol-ene addition, atom transfer radical addition, perfluoroalkylated side chains, semisynthetic antibiotics, antibacterial effect, MRSA
1. Introduction
The development of novel semisynthetic antibiotics is essential for society, to protect human health from threatening antimicrobial infections and antibiotic resistance. It is estimated that by 2050, the number of deaths caused by antimicrobial resistant pathogens will be 10 million per year. The WHO have stated that “antibiotic resistance is putting the achievements of modern medicine at risk” [1]. This statement summarizes the difficulty of antibiotic developments in one sentence. Luckily, modern medicinal chemistry sometimes finds “forgotten” drugs for improvement; previously discovered drugs can find new therapeutic applications. Moreover, some synthetic modifications can enhance the antibacterial effect and/or break down antibiotic resistance.
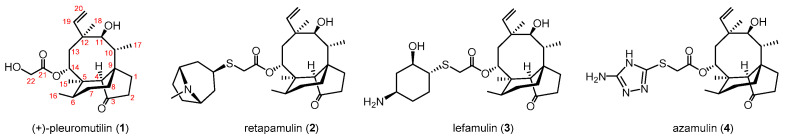
Pleuromutilin (1) was discovered in the middle of the 20th century in species of Clitopilus fungi, a secondary metabolite diterpene with a tricyclic skeleton. It exerts antibiotic activity against Gram-positive bacteria by acting as a peptidyl tranferase inhibitor [2] (Figure 1). Semisynthetic derivatives [3,4,5,6,7,8,9,10], bearing a basic substituent at the C14 glycolic ester moiety of the parent pleuromutilin, have been developed for human and veterinary use. Valnemulin and tiamulin have long been used in veterinary medicine [11], retamapulin (2) was introduced in 2007 for the treatment of bacterial skin infections [12], and lefamulin (3) [13,14] has very recently been approved for systemic use to treat community-acquired pneumonia (CAP) in adults. Azamulin (4), an azole derivative of pleuromutilin was also intended for human use; however, it did not progress beyond a Phase I trial, because it proved to be a strong and irreversible inhibitor of CYP3A, the most abundant isozyme of cytochrome P450 enzymes. The C22 modifications of these compounds broadened the antibacterial spectrum to include drug-resistant Gram-negative bacteria [8].
Figure 1.
Pleuromutilin derivatives approved or studied for human antibacterial therapies.
Although there are three known resistance mechanisms against these drugs [8] (mutations in ribosomal protein L3, nucleotide methylation by Cfr methyltransferase and efflux), the development of all types of resistance is slow, which is one of the most valuable features of pleuromutilins [3]. Semisynthetic pleuromutilin derivatives is a subject of heightened interest, because of the lack of cross-resistance with other antibiotic classes [3]. In addition, the COVID-19 pandemic has caused a high incidence of infections in the bloodstream during hospitalization, which places an urgent need for the development of antibiotics that are effective against nosocomial infections [15].
The mechanism of action [3,6,16] of pleuromutilin and its derivatives is based on the inhibition of bacterial protein synthesis by binding to the 50S ribosomal subunit at the peptidyl transferase center, and finally inhibiting the peptide bond formation.
Several novel semisynthetic pleuromutilin-moieties are known from the literature, mainly obtained by modifications at the glycolic ester residue (position C-22) [8]. These modifications have shifted the antibacterial effect of these drugs to a broader spectrum, and the activities against Gram-negative bacterial strains were enhanced. Recently, novel pleuromutilin derivatives with substituted thiadiazole [17] and triazole moieties [18] at position C-22 have been published as promising antibacterial agents.
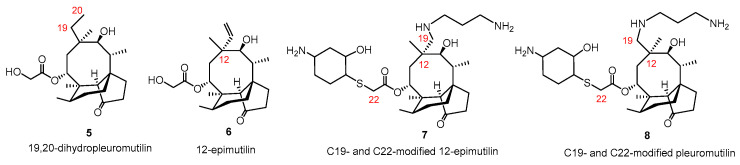
Some examples for modified pleuromutilin at position C-19 and C-20 are known, namely, 19,20-dihydropleuromutilin 5, as well as chain elongated derivatives (e.g., 7, 8) of pleuromutilin and its epimeric compound 12-epimutilin (6, Figure 2) [19]. The antibacterial evaluation of these compounds revealed that saturation of the C19 double bond had no influence on the antimicrobial effect, indicating that the C19–C20 alkene is not essential for activity [8]. Moreover, while the introduction of a basic side chain at the C19-C20 position of 12-epimutilin provided derivatives (e.g., 7) with activity against drug-resistant Gram-negative pathogens, the same modification of the parent pleuromutilin (8) led to a slight decrease in the antibacterial effect [18].
Figure 2.
Structural modifications of pleuromutilin and C12-epi-mutilin at position C19-C20.
While synthetic modifications of pleuromutilin at the C22 position have extensively been studied leading to the discovery of several clinical and advanced preclinical agents, modifications at other positions, including the C19–C20 alkene moiety, are quite unexplored [8]. We decided to perform a systematic study to investigate how lipophilic, hydrophilic, and perfluorous substituents at position C20 modify the antibacterial activity of the parent compound. Considering the literature data, we focused on the introduction of side chains of a non-basic character. We assumed that radical addition reactions are ideally suited to the diverse functionalization of pleuromutilin at the C20 position under mild conditions, and the most successful derivatizations could be applied in the case of lefamulin.
The photoinduced radical-mediated addition of thiols to terminal alkenes could provide an easy and efficient method for the regioselective introduction of either hydrophilic or lipophilic groups at position C20. Our previous works demonstrated the wide range and useful application of hydrothiolation [20,21,22,23,24] in the field of carbohydrate, nucleoside, and antibiotic chemistry. Moreover, the light-promoted radical addition reaction [25] of commercially available perfluoroalkyl iodides onto the C19-C20 double bond allows the introduction of fluorous side chains into the pleuromutilin skeleton. Importantly, perfluoroalkylated derivatives of teicoplanin and vancomycin have recently been synthesized [26] by our group, and the introduced perfluorous side chains having a dual hydrophobic and lipophobic character [27] have remarkably improved the antimicrobial properties of the parent glycopeptide antibiotics.
It is important to note that, to the best of our knowledge, only one example can be found for the modification of pleuromutilin by radical addition reactions at alkene position C19–C20. Bacqué and et. al. have modified the pleuromutilin by thermally induced radical additions of various xanthates [28]. Unfortunately, the biological activity of these pleuromutilin derivatives has not been reported.
2. Materials and Methods
2.1. General Methods
Optical rotations were measured at room temperature, with a Perkin-Elmer 241 automatic polarimeter (Perkin-Elmer, Waltham, MA, USA). TLC analysis was performed on Kieselgel 60 F254 (Merck KGaA, Darmstadt, Germany) silica gel plates with visualization, by immersion in a sulfuric-acid solution (5% in EtOH) followed by heating. Flash column chromatography was performed on silica gel 60 (Merck KGaA, Darmstadt, Germany) 0.040–0.063 mm. The organic solutions were dried over Na2SO4 and concentrated under vacuum. The melting points were measured by a Büchi B-540 melting-point apparatus (Merck KGaA, Darmstadt, Germany). The 1H (360, 400 and 500 MHz) and 13C NMR (90, 100.28, 125.76 MHz) spectra were recorded with Bruker DRX-360, DRX-400 and Bruker Avance II 500 spectrometers. Chemical shifts are referenced to Me4Si or DSS (0.00 ppm for 1H) and to solvent signals (CDCl3: 77.00 ppm, CD3OD: 49.15 ppm, DMSO-d6: 39.52 ppm for 13C). NMR spectra of all compounds are given as Supplementary Information. ESI-QTOF MS measurements were carried out on a maXis II UHR ESI-QTOF MS instrument (Bruker, Billerica, MA, USA), in positive ionization mode. The following parameters were applied for the electrospray ion source: capillary voltage: 3.5 kV; end plate offset: 500 V; nebulizer pressure: 0.8 bar; dry gas temperature: 200 °C; dry gas flow rate: 4.5 L/min. Constant background correction was applied for each spectrum; the background was recorded before each sample by injecting the blank sample matrix (solvent). Na-formate calibrant was injected after each sample, which enabled internal calibration during data evaluation. Mass spectra were recorded by OTOF Control version 4.1 (build: 3.5, Bruker, Billerica, MA, USA) and processed by Compass DataAnalysis version 4.4 (build: 200.55.2969, Bruker, Billerica, MA, USA). MALDI-TOF MS measurements were carried out with a Bruker Autoflex Speed mass spectrometer, equipped with a time-of-flight (TOF) mass analyzer. In all cases, 19 kV (ion source voltage 1) and 16.65 kV (ion source voltage 2) were used. For reflectron mode, 21 kV and 9.55 kV were applied as reflector voltage 1 and reflector voltage 2, respectively. A solid-phase laser, (355 nm, ≥100 μJ/pulse) operating at 500 Hz, was applied to produce laser desorption, and 3000 shots were summed. Additionally, 2,5-Dihydroxybenzoic acid (DHB) was used as matrix, and F3CCOONa as the cationising agent in DMF.
The photoinitiated reactions were carried out in a borosilicate vessel by irradiation, with an Hg-lamp giving maximum emission at 365 nm (See Figure S28).
For the in vitro MIC measurements, we used 12 different Gram-positive bacterial strains. Some of these strains were purchased from the American Type Culture Collection (ATCC), whereas others were clinical isolates. Our bacterial collection contained wild-typed-sensitive and also multiresistant strains. According to the EUCAST (European Committee on Antimicrobial Susceptibility Testing) reading guide for broth microdilution [29], compounds were two-fold serially diluted from 256 to 0.5 mg/L in Müller–Hinton broth. Then, 100 µL of each dilution was inoculated with 10 µL of 0.5 McFarland bacterial suspension. Incubation was performed at 37 °C for 24 h without shaking, and determination of MIC was made with the naked eye.
2.2. General Method for Photoinitiated Thiol-Ene Addition
To a solution of pleuromutilin (0.50 mmol) in the specified solvent (5 mL), thiol (1-2 equiv.) and 2,2-dimethoxy-2-phenylacetophenone (DPAP, 12 mg, 0.050 mmol) were added. The solution was irradiated with UV light at room temperature, or under cooling, for 1–3 × 15 min (the addition of 0.1 equiv. of DPAP and thiol (1-2 equiv.) were repeated before each irradiation cycle) at the given temperature. Then, the mixture was concentrated, and the residue was purified using column chromatography.
2.3. Synthesis
2.3.1. Compound 10a
Pleuromutilin (95 mg, 0.25 mmol) and thiol 9a (153 mg, 0.5 mol, 2 × 1 equiv.) were reacted in CH3CN at room temperature according to the general method, using two irradiating cycles. The crude product was purified by flash column chromatography (CH2Cl2/acetone 99/1) to result in compound 10a as white powder (140 mg, 81%). Rf: 0.31 (CH2Cl2/acetone 9/1), [α]24D +43.0 (c 0.1, MeOH), m.p. 192–193 °C. 1H NMR (400 MHz, Chloroform-d) δ 5.62 (d, J = 8.2 Hz, 1H), 5.24 − 5.20 (m, 1H), 5.12 (t, J = 9.9 Hz, 1H), 5.03 (dd, J = 10.0, 3.3 Hz, 1H), 4.55 (d, J = 9.7 Hz, 1H), 4.12 − 3.97 (m, 2H), 3.96 − 3.86 (m, 1H), 3.38 (d, J = 5.7 Hz, 1H), 3.20 (s, 1H), 2.60 (dt, J = 11.9, 6.0 Hz, 1H), 2.45 (td, J = 12.0, 5.0 Hz, 1H), 2.30 (t, J = 6.7 Hz, 1H), 2.11 (d, J = 1.4 Hz, 9H), 2.03 (s, 4H), 1.92 (s, 3H), 1.86 − 1.67 (m, 2H), 1.65 − 1.39 (m, 3H), 1.35 (s, 4H), 1.21 (d, J = 5.2 Hz, 1H), 1.16 (d, J = 6.4 Hz, 3H), 1.14 − 1.01 (m, 1H), 0.99 (s, 3H), 0.90 (d, J = 7.0 Hz, 3H), 0.63 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 216.9, 172.2, 170.6, 170.2, 170.1, 82.9, 75.9, 72.8, 72.3, 70.6, 69.5, 67.7, 61.3, 58.2, 45.5, 42.0, 41.8, 41.2, 36.5, 34.5, 34.4, 30.9, 30.1, 29.6, 26.8, 26.6, 24.9, 24.8, 20.9, 20.6, 16.5, 16.4, 14.7, 11. HRMS (ESI): m/z calcd. for C34H52NaO12S: 707.3077 [M+Na]+; found: 707.3070.
2.3.2. Compound 10b
Pleuromutilin (125 mg, 0.33 mmol) and thiol 9b (119 mg, 0.66 mmol, 2 × 1 equiv.) were reacted in EtOH at room temperature according to the general method, using two irradiating cycles. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 95/5) to result in compound 10b as white powder (121 mg, 66%). Rf: 0.31 (CH2Cl2/MeOH 9/1), [α]24D +12.22 (c 0.09, MeOH), m.p. 91–93 °C. 1H NMR (400 MHz, Methanol-d4) δ 5.78 (d, J = 8.2 Hz, 1H), 4.57 − 4.43 (m, 1H), 4.14 (d, J = 17.3 Hz, 1H), 4.02 (d, J = 17.1 Hz, 1H), 3.88 − 3.77 (m, 1H), 3.69 (d, J = 3.2 Hz, 1H), 3.62 − 3.51 (m, 2H), 3.47 (d, J = 5.8 Hz, 1H), 3.33 (t, J = 1.7 Hz, 1H), 2.65 (td, J = 12.3, 4.2 Hz, 1H), 2.52 (td, J = 12.6, 4.9 Hz, 1H), 2.38 (dd, J = 14.6, 4.6 Hz, 2H), 2.33 − 2.23 (m, 1H), 2.19 (t, J = 9.4 Hz, 2H), 2.10 (ddd, J = 17.7, 11.4, 5.3 Hz, 2H), 2.00 − 1.80 (m, 4H), 1.77 − 1.54 (m, 4H), 1.45 (s, 3H), 1.38 (d, J = 21.9 Hz, 1H), 1.30 (t, J = 5.9 Hz, 4H), 1.23 − 1.11 (m, 1H), 1.04 (d, J = 2.9 Hz, 3H), 0.97 (d, J = 6.9 Hz, 3H), 0.74 (d, J = 6.1 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 217.0 172.5, 85.3, 75.1, 74.9, 74.3, 71.9, 69.6, 69.0, 60.5, 57.9, 45.4, 41.7, 41.4, 41.0, 36.7, 34.8, 33.9, 30.1, 30.0, 26.7, 25.9, 24.7, 24.3, 15.8, 15.6, 13.9, 10.4. HRMS (ESI): m/z calcd for C28H46NaO9S: 581.2760 [M + Na]+; found: 581.2754.
2.3.3. Compound 10c
The reaction was carried out using the general method, starting from pleuromutilin (190 mg, 0.5 mmol) and thiol 9c (365 mg, 1.0 mmol, 2 × 1 equiv.) in CH3CN at room temperature, irradiating two times. The crude product was purified by flash column chromatography (CH2Cl2/acetone 95/5) to result in compound 10c as white powder (219 mg, 59%). Rf: 0.38 (CH2Cl2/acetone 9/1), [α]24D +107.0 (c 0.1, CHCl3), m.p. 127–130 °C. 1H NMR (400 MHz, Methanol-d4) δ 5.71 (d, J = 8.3 Hz, 1H), 5.48 (d, J = 1.5 Hz, 1H), 5.36 (dd, J = 3.2, 1.5 Hz, 1H), 5.31 (t, J = 9.9 Hz, 1H), 5.25 (dd, J = 10.1, 3.3 Hz, 1H), 4.46 (ddd, J = 9.6, 4.8, 2.4 Hz, 1H), 4.31 (dd, J = 12.3, 4.8 Hz, 1H), 4.15 (dd, J = 12.3, 2.5 Hz, 1H), 4.11 − 3.97 (m, 2H), 3.48 (d, J = 5.8 Hz, 1H), 2.72 − 2.48 (m, 2H), 2.36 (d, J = 7.1 Hz, 2H), 2.31 − 2.19 (m, 1H), 2.18 (d, J = 1.6 Hz, 5H), 2.08 (d, J = 5.8 Hz, 6H), 2.03 (dd, J = 9.9, 7.3 Hz, 2H), 1.99 (s, 1H), 1.97 − 1.79 (m, 4H), 1.76 − 1.54 (m, 4H), 1.45 (s, 3H), 1.30 (dd, J = 16.0, 6.9 Hz, 2H), 1.23 − 1.11 (m, 1H), 1.05 (s, 3H), 0.97 (d, J = 7.0 Hz, 3H), 0.75 (d, J = 6.3 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 217.0, 172.1, 171.0, 170.3, 170.2, 170.1, 81.5, 74.9, 70.9, 69.8, 69.0, 68.7, 66.1, 62.4, 60.6, 57.8, 45.4, 41.8, 41.7, 41.0, 36.6, 34.8, 33.9, 30.0, 29.7, 26.7, 26.0, 25.9, 24.3, 19.4, 19.4, 19.3, 19.2, 15.8, 13.9, 10.4. HRMS (ESI): m/z calcd. for C36H54NaO14S: 765.3132 [M + Na]+; found: 765.3125.
2.3.4. Compound 10d
The reaction was carried out using the general method, starting from pleuromutilin (190 mg, 0.5 mmol) and thiol 9d (364 mg, 1.0 mmol, 2 × 1 equiv.) in CH3CN at room temperature, irradiating two times. The crude product was purified by flash column chromatography (CH2Cl2/acetone 95/5) to result in compound 10d as white powder (255 mg, 69%). Rf: 0.11 (CH2Cl2/acetone 9/1), [α]24D +13.0 (c 0.1, CHCl3), m.p. 97–98 °C. 1H NMR (400 MHz, Chloroform-d) δ 6.79 (d, J = 9.3 Hz, 1H), 5.70 (d, J = 8.2 Hz, 1H), 5.41 − 5.24 (m, 1H), 5.13 (t, J = 9.7 Hz, 1H), 4.68 (d, J = 10.4 Hz, 1H), 4.25 (dd, J = 12.3, 4.7 Hz, 1H), 4.19 − 4.10 (m, 3H), 4.10 − 3.97 (m, 1H), 3.76 (ddd, J = 9.9, 4.6, 2.4 Hz, 1H), 3.42 (d, J = 5.7 Hz, 1H), 2.80 (td, J = 12.3, 4.3 Hz, 1H), 2.37 (tq, J = 12.8, 7.8, 6.2 Hz, 3H), 2.29 − 2.21 (m, 1H), 2.18 (s, 1H), 2.14 − 2.06 (m, 4H), 2.08 − 1.95 (m, 10H), 1.95 − 1.74 (m, 3H), 1.73 − 1.52 (m, 2H), 1.53 − 1.44 (m, 2H), 1.42 (s, 4H), 1.33 − 1.20 (m, 2H), 1.14 (td, J = 14.0, 4.5 Hz, 1H), 1.03 (s, 3H), 0.96 (d, J = 6.9 Hz, 3H), 0.70 (d, J = 7.0 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 217.0, 172.7, 171.1, 171.1, 170.9, 169.4, 86.1, 76.0, 75.8, 73.8, 69.6, 68.4, 62.5, 61.4, 58.2, 53.5, 45.5, 42.3, 41.8, 41.3, 36.5, 34.7, 34.4, 30.8, 30.2, 27.7, 26.9, 26.7, 24.8, 23.2, 20.8, 20.8, 20.7, 16.6, 14.7, 11.1. HRMS (ESI): m/z calcd. for C36H55NNaO13S: 764.3292 [M + Na]+; found: 764.3287.
2.3.5. Compound 10e
The reaction was carried out using the general method, starting from pleuromutilin (190 mg, 0.5 mmol) and thiol 9e (142 mg, 0.6 mmol, 1.2 equiv.) in CH3CN/MeOH 4/1 at room temperature, irradiating once. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 8/2) to result in compound 10e as white amorphous solid (290 mg, 94%). Rf: 0.42 (CH2Cl2/MeOH 8/2), [α]24D +3.85 (c 0.13, CHCl3). 1H NMR (400 MHz, DMSO-d6) δ 7.74 (d, J = 9.4 Hz, 1H), 5.55 (d, J = 8.2 Hz, 1H), 5.39 (t, J = 6.6 Hz, 1H), 5.12 − 4.86 (m, 2H), 4.56 (d, J = 6.0 Hz, 1H), 4.50 (t, J = 6.0 Hz, 1H), 4.37 (d, J = 10.3 Hz, 1H), 4.21 − 4.11 (m, 1H), 4.03 − 3.79 (m, 2H), 3.75 − 3.63 (m, 1H), 3.22 − 3.05 (m, 3H), 2.64 − 2.48 (m, 2H), 2.46 − 2.30 (m, 2H), 2.18 (td, J = 12.7, 11.2, 6.8 Hz, 2H), 2.06 (dt, J = 18.7, 9.2 Hz, 1H), 1.94 − 1.77 (m, 5H), 1.67 (ttd, J = 22.0, 11.7, 7.8 Hz, 3H), 1.55 − 1.39 (m, 2H), 1.38 − 1.18 (m, 6H), 1.18 − 0.95 (m, 2H), 0.92 (s, 3H), 0.84 (d, J = 6.9 Hz, 3H), 0.62 (d, J = 6.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 217.7, 172.1, 169.6, 86.0, 81.2, 75.9, 74.1, 70.9, 68.3, 61.8, 60.8, 57.7, 55.2, 49.0, 45.4, 42.3, 41.8, 41.2, 36.8, 35.1, 34.4, 31.1, 30.6, 27.3, 26.7, 24.8, 23.5, 16.7, 15.0, 12.0. HRMS (ESI): m/z calcd for C30H49NNaO10S: 638.2975 [M + Na]+; found: 638.2969.
2.3.6. Compound 10f
The reaction was carried out using the general method, starting from pleuromutilin (190 mg, 0.5 mmol) and thiol 9f (118 mg, 0.6 mmol, 1.2 equiv.) in MeOH at room temperature, irradiating once. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 8/2) and resulted in compound 10f as white amorphous solid (216 mg, 75%). Rf: 0.71 (CH2Cl2/MeOH 7/3), [α]24D= +17.8 (c 0.09, CHCl3). 1H NMR (400 MHz, DMSO-d6) δ 5.75 (d, J = 2.7 Hz, 1H), 5.55 (d, J = 8.1 Hz, 1H), 5.33 (s, 1H), 4.98 (d, J = 40.6 Hz, 2H), 4.59 (d, J = 5.7 Hz, 1H), 4.46 (s, 1H), 4.27 (dd, J = 9.6, 2.7 Hz, 1H), 3.90 (q, J = 17.0 Hz, 2H), 3.67 (d, J = 11.8 Hz, 1H), 3.15 (dd, J = 10.1, 6.6 Hz, 2H), 3.08 (d, J = 9.2 Hz, 1H), 2.96 (t, J = 9.3 Hz, 1H), 2.63 − 2.39 (m, 5H), 2.35 (s, 1H), 2.18 (t, J = 8.4 Hz, 2H), 2.15 − 2.00 (m, 1H), 2.00 − 1.79 (m, 2H), 1.80 − 1.56 (m, 2H), 1.58 − 1.40 (m, 2H), 1.38 − 1.20 (m, 6H), 1.17 − 0.97 (m, 1H), 0.94 (d, J = 2.7 Hz, 3H), 0.85 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 6.2 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 217.6, 172.0, 86.3, 81.1, 78.6, 74.0, 73.8, 70.5, 68.3, 61.8, 60.9, 57.7, 45.4, 42.6, 41.8, 41.2, 36.8, 35.1, 34.4, 31.6, 30.6, 27.3, 27.1, 25.9, 24.8, 16.7, 15.0, 12.0. HRMS (ESI): m/z calcd. for C28H46NaO10S: 597.2709 [M + Na]+; found: 597.2704.
2.3.7. Compound 10g
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9g (109 mg, 0.3 mmol, 1.2 equiv.) in EtOAc at room temperature, irradiating once. The crude product was purified by flash column chromatography (CH2Cl2/acetone 95/5) to result in compound 10g as white amorphous solid (114 mg, 61%). Rf: 0.33 (CH2Cl2/acetone 9/1), [α]24D +19.3 (c 0.14, CHCl3). 1H NMR (360 MHz, Chloroform-d) δ 5.66 (d, J = 8.3 Hz, 1H), 5.43 (d, J = 3.3 Hz, 1H), 5.36 − 5.18 (m, 3H), 5.12 (dd, J = 10.0, 3.3 Hz, 1H), 4.66 (d, J = 9.9 Hz, 1H), 4.16 − 3.97 (m, 4H), 3.42 (d, J = 5.6 Hz, 1H), 2.81 (t, J = 5.7 Hz, 1H), 2.57 (t, J = 8.5 Hz, 2H), 2.40 − 2.11 (m, 6H), 2.05 (s, 4H), 1.99 (s, 3H), 1.86 (ddd, J = 32.2, 16.3, 8.6 Hz, 5H), 1.70 − 1.43 (m, 6H), 1.42 (s, 3H), 1.32 − 1.22 (m, 1H), 1.21 − 1.02 (m, 5H), 0.97 (d, J = 6.9 Hz, 3H), 0.70 (d, J = 6.8 Hz, 3H). 13C NMR (91 MHz, Chloroform-d) δ 216.6, 172.3, 170.6, 170.2, 169.8, 84.1, 76.1, 74.0, 71.9, 69.7, 67.4, 67.3, 61.4, 61.3, 58.1, 45.4, 41.9, 41.8, 41.3, 36.4, 34.4, 34.3, 30.5, 30.0, 26.8, 25.5, 24.8, 20.9, 20.6, 16.6, 14.7, 10.9. HRMS (ESI): m/z calcd. for C36H54NaO14S: 765.3132 [M + Na]+; found: 765.3126.
2.3.8. Compound 10h
The reaction was carried out using the general method, starting from pleuromutilin (48 mg, 0.125 mmol) and thiol 9h (191 mg, 0.375 mmol, 3 × 1 equiv.) in toluene/MeOH 1/1 at −40 °C, irradiating three times. The crude product was purified by flash column chromatography (CH2Cl2/acetone 9/1) to result in compound 10h as white amorphous solid (69 mg, 62%). Rf: 0.20 (CH2Cl2/acetone 8/2), [α]24D +46.7 (c 0.12, MeOH). 1H NMR (400 MHz, Chloroform-d) δ 5.59 (d, J = 8.1 Hz, 1H), 5.46 (d, J = 10.1 Hz, 1H), 5.31 (q, J = 4.9, 3.6 Hz, 2H), 4.95 − 4.76 (m, 1H), 4.37 − 4.22 (m, 1H), 4.16 − 3.94 (m, 4H), 3.94 − 3.66 (m, 4H), 3.38 (d, J = 5.5 Hz, 1H), 2.71 (dd, J = 12.7, 4.6 Hz, 1H), 2.62 (s, 1H), 2.57 (dd, J = 12.2, 4.1 Hz, 1H), 2.43 (td, J = 11.7, 5.2 Hz, 1H), 2.30 (dt, J = 10.0, 4.8 Hz, 1H), 2.22 − 2.06 (m, 6H), 2.02 (d, J = 1.8 Hz, 8H), 1.86 (s, 4H), 1.75 (dt, J = 9.8, 5.8 Hz, 2H), 1.68 − 1.50 (m, 2H), 1.50 − 1.42 (m, 1H), 1.41 (s, 4H), 1.38 − 1.29 (m, 1H), 1.24 (s, 5H), 1.10 (td, J = 14.1, 3.0 Hz, 1H), 1.03 (s, 3H), 0.91 (d, J = 6.8 Hz, 3H), 0.67 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 218.0, 172.4, 170.9, 170.8, 170.3, 170.2, 168.4, 83.3, 76.0, 73.9, 69.8, 69.7, 68.3, 67.2, 62.4, 61.3, 58.3, 53.8, 52.9, 49.3, 45.4, 41.7, 41.7, 41.4, 38.0, 36.5, 34.4, 30.1, 29.6, 29.3, 28.0, 26.8, 26.1, 24.8, 24.5, 23.1, 21.2, 20.9, 20.8, 16.6, 14.8, 11.0. HRMS (ESI): m/z calcd. for C42H63NNaO17S: 908.3714 [M+Na]+; found: 908.3709.
2.3.9. Compound 10i
The reaction was carried out using the general method, starting from pleuromutilin (190 mg, 0.5 mmol) and thiol 9i (390 mg, 1.5 mmol, 3 × 1 equiv.) in MeOH/DMF 9/1 at −80 °C, irradiating three times. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 95/5 to 9:1) to result in compound 10i as white amorphous solid (204 mg, 64%). Rf: 0.51 (CH2Cl2/MeOH 8/2), [α]24D +14.0 (c 0.1, MeOH). 1H NMR (400 MHz, Methanol-d4) δ 7.80 (d, J = 8.0 Hz, 1H), 5.88 (d, J = 4.8 Hz, 1H), 5.74 (t, J = 8.1 Hz, 2H), 4.23 (t, J = 4.7 Hz, 1H), 4.13 (q, J = 5.3, 4.6 Hz, 3H), 4.07 (s, 1H), 4.02 (s, 1H), 3.44 (d, J = 5.8 Hz, 1H), 3.35 (s, 1H), 3.31 (p, J = 1.6 Hz, 1H), 3.01 − 2.90 (m, 2H), 2.59 (td, J = 11.9, 4.9 Hz, 1H), 2.46 (td, J = 12.1, 5.4 Hz, 1H), 2.34 (qd, J = 11.6, 9.9, 4.3 Hz, 3H), 2.25 (dd, J = 10.3, 7.9 Hz, 1H), 2.14 (dt, J = 19.1, 9.3 Hz, 1H), 2.02 − 1.78 (m, 4H), 1.72 − 1.52 (m, 3H), 1.48 − 1.32 (m, 5H), 1.26 (d, J = 16.3 Hz, 2H), 1.20 − 1.09 (m, 1H), 1.01 (s, 3H), 0.95 (d, J = 6.9 Hz, 3H), 0.72 (d, J = 6.2 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 173.5, 166.1, 152.3, 142.7, 103.0, 90.9, 85.0, 76.3, 75.0, 73.4, 70.3, 61.9, 59.3, 48.9, 46.8, 43.3, 43.1, 42.3, 38.0, 36.2, 35.3, 35.1, 31.8, 31.4, 29.4, 28.1, 27.4, 25.7, 17.0, 15.3, 11.8. HRMS (ESI): m/z calcd. for C31H46N2NaO10S: 661.2873 [M + Na]+; found: 661.2765.
2.3.10. Compound 10j
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9j (39 mg, 35µL, 0.5 mmol, 2 equiv.) in CH3CN/MeOH 2/1 at −40 °C, irradiating once. The crude product was purified by flash column chromatography (n-hexane/acetone 8/2) to result in compound 10j as white powder (89 mg, 78%). Rf: 0.10 (n-hexane/acetone 7/3), [α]24D +10.3 (c 0.35, MeOH), m.p. 189 °C. 1H NMR (400 MHz, Methanol-d4) δ 5.75 (d, J = 8.3 Hz, 1H, H-14), 4.03 (q, J = 17.1 Hz, 2H, H-22ab), 3.67 (t, J = 6.9 Hz, 2H, H-24ab), 3.44 (d, J = 5.9 Hz, 1H, H-11), 2.74 (dt, J = 13.8, 6.9 Hz, 1H, H-23a), 2.66 (dt, J = 13.6, 6.9 Hz, 1H, H-23b), 2.48 (td, J = 12.2, 4.7 Hz, 1H, H-19a*), 2.44 − 2.34 (m, 2H, H-10, H-19b*), 2.33 (s, 1H, H-4), 2.26 (dd, J = 19.2, 10.7 Hz, 1H, H-2a), 2.20 − 2.08 (m, 1H, H-2b), 2.03 − 1.93 (m, 1H, H-20a*), 1.92 − 1.80 (m, 3H, H-8a, H-13a, H-20b*), 1.73 − 1.65 (m, 1H, H-1a), 1.64 − 1.53 (m, 2H, H-6, H-7a), 1.45 (dd, J = 8.3, 6.2 Hz, 1H, H-1b), 1.42 (s, 3H, H-15abc), 1.37 (d, J = 10.8 Hz, 1H, H-7b), 1.32 − 1.25 (m, 1H, H-13b), 1.20 − 1.10 (m, 1H, H-8b), 1.01 (s, 3H, H-18abc), 0.95 (d, J = 7.0 Hz, 3H, H-17abc), 0.72 (d, J = 6.1 Hz, 3H, H-16abc). * H-19a↔H-20a and H-19b↔H-20b are interchangeable. 13C NMR (101 MHz, Methanol-d4) δ 219.6 (1C, C-3), 173.5 (1C, C-21), 76.4 (1C, C-11), 70.3 (1C, C-14), 62.8, 61.9 (2C, C-22, C-24), 59.3 (1C, C-4), 46.8 (1C, C-9), 43.1 (1C, C-13), 43.1 (1C, C-12), 42.2 (1C, C-5), 38.1 (1C, C-6), 36.2 (1C, C-10), 35.3, 34.9 (2C, C-2, C-23), 31.6, 31.4, 28.2, 28.1 (4C, C-7, C-19, C-8, C-20), 27.4 (1C, C-18), 25.7 (1C, C-1), 17.0 (1C, C-16), 15.3 (1C, C-15), 11.8 (1C, C-17). HRMS (ESI): m/z calcd. for C20H40NaO6S: 479.2443 [M + Na]+; found: 479.2438.
2.3.11. Compound 10k
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9k (82 mg, 0.5 mmol, 2 × 1 equiv.) in MeOH at −80 °C, irradiating two times. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 9/1) to result in compound 10k as white-yellow powder (125 mg, 92%). Rf: 0.31 (CH2Cl2/MeOH 7/3), [α]24D +34.3 (c 0.14, MeOH), m.p. 73–74 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.85 (d, J = 6.2 Hz, 1H, NH), 5.56 (d, J = 7.8 Hz, 1H, H-14), 4.22 (s, 1H, H-24), 4.01 (d, J = 17.1 Hz, 1H, H-22a), 3.82 (d, J = 17.1 Hz, 1H, H-22b), 3.34 (d, J = 5.1 Hz, 1H, H-11), 2.91 (d, J = 9.7 Hz, 1H, H-23a), 2.74 (dd, J = 12.4, 5.8 Hz, 1H, H-23b), 2.46 − 2.25 (m, 3H, H-4, H-19ab*), 2.18 (t, J = 14.3 Hz, 2H, H-10, H-2a), 2.11 − 1.99 (m, 1H, H-2b), 1.86 (s, 3H, AcCH3), 1.81 (d, J = 8.0 Hz, 1H, H-13a), 1.64 (dd, J = 29.7, 9.9 Hz, 4H, H-8a, H-7a, H-20ab*), 1.44 (dd, J = 23.1, 9.1 Hz, 2H, H-6, H-1a), 1.32 (s, 3H, H-15abc), 1.29 − 1.22 (m, 2H, H-1b, H-7b), 1.09 (d, J = 16.0 Hz, 1H, H-13b), 1.00 (d, J = 12.7 Hz, 1H, H-8b), 0.91 (s, 3H, H-18abc), 0.82 (d, J = 6.6 Hz, 3H, H-17abc), 0.61 (d, J = 5.9 Hz, 3H, H-16abc). *H-19a↔H-20a, H-19b↔H-20b and C19↔20 are interchangeable signals. 13C NMR (101 MHz, DMSO-d6) δ 217.2 (1C, C-3), 172.3, 168.9 (3C, AcCO, COOH, C-21), 73.6 (1C, C-11), 67.7 (1C, C-14), 60.4 (1C, C-22), 57.3 (1C, C-4), 53.3 (1C, C-24), 55.0, 45.0, 41.4, 40.7 (4C, C-5, C-12, C-13, C-9), 36.4 (1C, C-6), 34.7 (1C, C-10), 34.5, 34.0 (2C, C-23, C-2), 30.2, 29.7 (2C, C-8, C-20*), 27.6 (1C, C-19*), 26.9 (1C, C-18), 26.7 (1C, C-1), 24.4 (1C, C-7), 22.8 (1C, AcCH3), 16.2 (1C, C-16), 14.56 (1C, C-15), 11.6 (1C, C-17). HRMS (MALDI): m/z calcd. for C27H43NNaO8S: 564.2607 [M + Na]+; found: 564.2616.
2.3.12. Compound 10l
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9l (172 mg, 0.5 mmol, 2 × 1 equiv.) in toluene/MeOH 1/1 at −40 °C, irradiating two times. The crude product was purified by flash column chromatography (toluene/MeOH 95/5) to result in compound 10l as yellow powder (169 mg, 93%). Rf: 0.11 (toluene/MeOH 8/2), [α]24D +15.3 (c 0.15, MeOH), m.p. 143–147 °C. 1H NMR (500 MHz, DMSO-d6) δ 7.89 (d, J = 7.6 Hz, 2H), 7.74 (t, J = 6.9 Hz, 2H), 7.42 (t, J = 7.5 Hz, 2H), 7.34 (t, J = 7.5 Hz, 2H), 5.60 (d, J = 8.0 Hz, 1H), 4.32 − 4.23 (m, 2H), 4.22 (q, J = 6.7, 6.1 Hz, 2H), 4.06 (d, J = 17.1 Hz, 1H), 3.82 (d, J = 17.1 Hz, 1H), 3.00 (dd, J = 13.9, 4.5 Hz, 1H), 2.79 (dd, J = 13.5, 6.8 Hz, 1H), 2.37 (d, J = 21.5 Hz, 3H), 2.24 − 2.14 (m, 2H), 2.12 − 1.96 (m, 1H), 1.83 (dd, J = 16.2, 8.0 Hz, 1H), 1.67 (ddt, J = 37.8, 21.7, 10.0 Hz, 4H), 1.49 (d, J = 7.9 Hz, 2H), 1.40 − 1.28 (m, 5H), 1.30 − 1.21 (m, 5H), 1.11 (d, J = 15.8 Hz, 2H), 0.92 (s, 3H), 0.89 − 0.80 (m, 3H), 0.63 (d, J = 5.7 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 217.2, 172.20, 155.5, 143.9, 140.7, 127.6, 127.1, 125.4, 125.3, 120.1, 73.6, 67.5, 65.6, 60.3, 57.3, 46.7, 45.0, 41.3, 40.6, 36.3, 34.6, 33.9, 31.1, 30.2, 29.8, 29.0, 27.6, 26.9, 26.6, 24.4, 16.1, 14.5, 11.5. HRMS (MALDI): m/z calcd. for C40H51NNaO9S: 744.3182 [M + Na]+; found: 744.3163.
2.3.13. Compound 10m
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9m (165 mg, 1.0 mmol, 2 × 2 equiv.) in MeOH at 0 °C, irradiating two times. The crude product was purified by flash column chromatography (CH2Cl2/MeOH 95/5) to result in compound 10m as white powder (58 mg, 42%). Rf: 0.69 (CH2Cl2/MeOH 7/3), [α]24D +25.0 (c 0.12, MeOH), m.p. 113–114 °C. 1H NMR (400 MHz, Methanol-d4) δ 5.77 (d, J = 8.3 Hz, 1H), 4.19 − 3.93 (m, 2H), 3.47 (d, J = 5.9 Hz, 1H), 3.33 (p, J = 1.6 Hz, 1H), 3.09 (ddd, J = 9.1, 7.0, 2.2 Hz, 2H), 3.02 − 2.84 (m, 2H), 2.58 (td, J = 12.0, 4.7 Hz, 1H), 2.50 − 2.32 (m, 3H), 2.33 − 2.23 (m, 1H), 2.18 (d, J = 2.5 Hz, 1H), 1.91 (tdd, J = 25.3, 12.9, 4.1 Hz, 4H), 1.78 − 1.54 (m, 4H), 1.44 (s, 4H), 1.33 (d, J = 16.5 Hz, 2H), 1.27 − 1.08 (m, 1H), 1.05 (s, 3H), 0.97 (d, J = 7.0 Hz, 3H), 0.74 (d, J = 6.1 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 217.0, 172.0, 75.0, 69.0, 60.5, 57.9, 51.9, 45.5, 42.3, 41.7, 40.9, 36.7, 34.8, 33.9, 30.1, 30.0, 27.2, 26.7, 26.5, 25.9, 24.3, 15.6, 13.9, 10.4. HRMS (ESI): m/z calcd. for C24H39Na2O8S2: 565.1882 [M + Na]+; found: 565.1876.
2.3.14. Compound 10n
To the mixture of pleuromutilin (95 mg, 0.25 mmol) and thiol 9n (57 mg, 54 µL, 0.75 mmol, 3 equiv.) in EtOAc at room temperature, 0.1 equivalent of DPAP (2,2-dimethoxy-2-phenylacetophenone) (0.025 mmol, 6 mg) and 0.1 equivalent of MAP (4-methoxyacetophenone) (0.025mmol, 4 mg) were added. It was irradiated once for one hour. The crude product was purified by flash column chromatography (n-hexane/acetone 8/2) to result in compound 10n as white powder (91 mg, 80%). Rf: 0.36 (n-hexane/acetone 6/4), [α]24D +54.7 (c 0.15, MeOH), m.p. 188–189 °C. 1H NMR (400 MHz, Methanol-d4) δ 5.69 (d, J = 8.2 Hz, 1H), 4.14 − 3.87 (m, 2H), 3.44 (d, J = 6.1 Hz, 1H), 3.03 − 2.89 (m, 1H), 2.66 (td, J = 12.4, 5.0 Hz, 1H), 2.33 (d, J = 3.1 Hz, 2H), 2.30 (s, 4H), 2.28 − 2.17 (m, 1H), 2.18 − 2.05 (m, 1H), 1.97 − 1.77 (m, 5H), 1.73 − 1.51 (m, 3H), 1.42 (s, 4H), 1.31 (d, J = 16.4 Hz, 1H), 1.14 (td, J = 13.7, 4.4 Hz, 1H), 1.07 (s, 3H), 0.94 (d, J = 7.0 Hz, 3H), 0.71 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 217.0, 194.0, 168.0, 73.3, 67.3, 59.0, 56.4, 43.9, 40.2, 40.2, 39.6, 35.1, 33.4, 32.4, 28.6, 28.2, 27.6, 25.2, 24.2, 22.8, 14.1, 12.4, 8.9. HRMS (ESI): m/z calcd. for C24H38NaO6S: 477.2287 [M + Na]+; found: 477.2281.
2.3.15. Compound 10o
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9o (45 mg, 60 µL, 0.5 mmol, 2 × 1 equiv.) in CH3CN/MeOH 2/1 at −40 °C, irradiating two times. The crude product was purified by flash column chromatography (CH2Cl2/acetone 95/5) to result in compound 10o as white powder (97 mg, 82%). Rf: 0.56 (CH2Cl2/acetone 9/1), [α]24D +52.2 (c 0.18, CHCl3), m.p. 177–180 °C. 1H NMR (400 MHz, Chloroform-d) δ 5.65 (d, J = 8.3 Hz, 1H), 4.01 (q, J = 17.1 Hz, 2H), 3.38 (d, J = 5.8 Hz, 1H), 2.51 (dd, J = 7.9, 6.8 Hz, 2H), 2.41 (t, J = 8.3 Hz, 2H), 2.37 − 2.27 (m, 2H), 2.24 − 2.15 (m, 1H), 2.13 (s, 2H), 1.91 (t, J = 8.4 Hz, 2H), 1.83 − 1.68 (m, 2H), 1.62 − 1.41 (m, 7H), 1.36 (s, 5H), 1.30 − 1.18 (m, 2H), 1.08 (td, J = 13.8, 4.3 Hz, 1H), 0.99 (s, 3H), 0.92 (d, J = 7.0 Hz, 3H), 0.88 (t, J = 7.3 Hz, 3H), 0.64 (d, J = 6.8 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 217.1, 172.3, 76.0, 69.9, 61.3, 58.3, 45.5, 42.4, 41.8, 41.2, 36.5, 34.5, 34.4, 31.9, 31.8, 30.1, 30.1, 27.4, 26.8, 26.8, 24.8, 22.0, 16.5, 14.7, 13.7, 11.1. HRMS (ESI): m/z calcd. for C26H44NaO5S: 491.2807 [M + Na]+; found: 491.2801.
2.3.16. Compound 10p
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9p (73 mg, 87 µL, 0.5 mmol, 2 equiv.) in CH3CN/MeOH 2/1 at −40 °C, irradiating once. The crude product was purified by flash column chromatography (CH2Cl2/acetone 95/5) to result in compound 10p as white amorphous solid (96 mg, 73%). Rf: 0.66 (CH2Cl2/acetone 9/1), [α]24D +20.0 (c 0.04, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.68 (d, J = 8.3 Hz, 1H), 4.04 (qd, J = 17.0, 4.1 Hz, 2H), 3.41 (d, J = 5.8 Hz, 1H), 2.54 (t, J = 7.4 Hz, 2H), 2.45 (dt, J = 9.3, 3.0 Hz, 2H), 2.41 − 2.28 (m, 2H), 2.28 − 2.14 (m, 2H), 2.09 (d, J = 2.7 Hz, 1H), 1.94 (ddd, J = 9.9, 6.2, 2.4 Hz, 2H), 1.81 − 1.74 (m, 2H), 1.68 − 1.43 (m, 7H), 1.40 (s, 5H), 1.28 (dt, J = 10.0, 5.1 Hz, 10H), 1.11 (td, J = 13.8, 4.4 Hz, 1H), 1.03 (s, 3H), 0.96 (d, J = 7.0 Hz, 3H), 0.91 − 0.82 (m, 3H), 0.67 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 216.7, 172.3, 76.1, 70.0, 61.3, 58.3, 45.5, 42.4, 41.8, 41.2, 36.5, 34.5, 34.4, 32.3, 31.8, 30.1, 30.1, 29.8, 29.3, 29.2, 29.0, 27.4, 26.8, 26.8, 24.9, 22.6, 16.6, 14.7, 14.1, 11.1. HRMS (ESI): m/z calcd. for C30H52NaO5S: 547.3433 [M + Na]+; found: 547.3427.
2.3.17. Compound 10q
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9q (202 mg, 240 µL, 1.0 mmol, 2 × 2 equiv.) in EtOH at 0 °C, irradiating two times. The crude product was purified by flash column chromatography (CH2Cl2/acetone 95/5) to result in compound 10q as white amorphous solid (61 mg, 42%). Rf: 0.66 (CH2Cl2/acetone 9/1), [α]24D +47.4 (c 0.19, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.72 (d, J = 8.3 Hz, 1H), 4.06 (q, J = 17.0 Hz, 2H), 3.43 (d, J = 6.0 Hz, 1H), 2.57 (t, J = 7.4 Hz, 2H), 2.48 (td, J = 10.6, 9.7, 6.1 Hz, 2H), 2.40 (dd, J = 13.8, 6.1 Hz, 2H), 2.31 − 2.16 (m, 2H), 2.11 (s, 1H), 1.97 (dt, J = 10.8, 4.8 Hz, 2H), 1.82 (td, J = 15.5, 14.2, 5.8 Hz, 2H), 1.73 − 1.48 (m, 7H), 1.42 (d, J = 9.2 Hz, 5H), 1.28 (d, J = 5.3 Hz, 18H), 1.14 (td, J = 13.8, 4.3 Hz, 1H), 1.06 (s, 3H), 0.99 (d, J = 7.0 Hz, 3H), 0.89 (t, J = 6.6 Hz, 3H), 0.70 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 216.7, 172.3, 76.2, 70.1, 61.3, 58.3, 45.5, 42.5, 41.9, 41.2, 36.5, 34.5, 34.4, 32.4, 31.9, 30.1, 30.1, 29.8, 29.7, 29.6, 29.6, 29.3, 29.3, 29.0, 27.4, 26.8, 26.8, 24.9, 22.7, 16.5, 14.7, 14.1, 11.0. HRMS (ESI): m/z calcd. for C34H60NaO5S: 603.4059 [M + Na]+; found: 603.4052.
2.3.18. Compound 10r
The reaction was carried out using the general method, starting from pleuromutilin (95 mg, 0.25 mmol) and thiol 9r (186 mg, 177 µL, 1.5 mmol, 3 × 2 equiv.) in toluene at −40 °C, it was irradiated three times. The crude product was purified by flash column chromatography (CH2Cl2/acetone 97/3) to result in compound 10r as white powder (100 mg, 79%). Rf: 0.60 (CH2Cl2/acetone 9/1), [α]24D +67.1 (c 0.21, CHCl3), m.p. 150–151 °C. 1H NMR (400 MHz, Methanol-d4) δ 7.45 − 7.40 (m, 2H), 7.30 (td, J = 7.1, 6.1, 1.3 Hz, 2H), 7.25 − 7.19 (m, 1H), 5.74 (d, J = 8.3 Hz, 1H), 4.13 − 3.95 (m, 2H), 3.88 (d, J = 13.5 Hz, 1H), 3.74 (d, J = 13.5 Hz, 1H), 3.41 (d, J = 6.0 Hz, 1H), 2.37 (ddd, J = 9.7, 5.8, 2.5 Hz, 2H), 2.33 − 2.29 (m, 1H), 2.29 − 2.21 (m, 1H), 2.17 (s, 2H), 2.04 (ddd, J = 13.7, 10.3, 6.5 Hz, 1H), 1.95 − 1.77 (m, 4H), 1.64 (dt, J = 11.4, 6.7 Hz, 4H), 1.44 (s, 4H), 1.23 (d, J = 16.2 Hz, 1H), 1.15 (td, J = 13.4, 4.3 Hz, 1H), 0.98 (s, 3H), 0.83 (d, J = 6.9 Hz, 3H), 0.75 (d, J = 6.0 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 217.0, 171.9, 139.2, 128.7, 128.0, 126.4, 74.9, 68.9, 60.6, 57.9, 45.5, 42.5, 41.7, 40.8, 36.7, 35.1, 34.8, 33.9, 30.8, 30.0, 26.8, 26.3, 26.1, 24.4, 15.7, 14.0, 10.5. HRMS (ESI): m/z calcd. for C29H42NaO5S: 525.2651 [M + Na]+; found: 525.2643.
2.3.19. Compound 11g
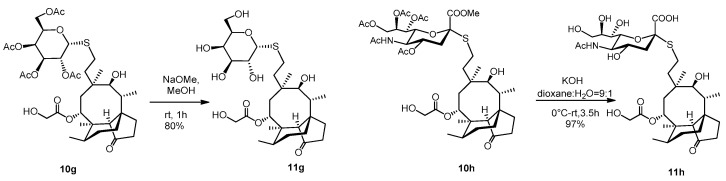
A catalytic amount of NaOMe (pH ~ 9) was added to a stirred solution of compound 10g (65 mg, 0.09 mmol) in dry MeOH (5 mL) and stirred for one hour at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered and evaporated. Then, the crude product was purified by flash column chromatography (CH2Cl2/MeOH 8/2) to result in compound 11g as white amorphous solid (42 mg, 80%). Rf: 0.50 (CH2Cl2/MeOH 8/2), [α]24D= +26.4 (c 0.14, MeOH). 1H NMR (400 MHz, Methanol-d4) δ 5.74 (d, J = 8.3 Hz, 1H), 5.51 (s, 1H), 4.53 − 4.45 (m, 1H), 4.05 (m, 2H), 3.89 (d, J = 0.9 Hz, 1H), 3.85 − 3.76 (m, 1H), 3.75 − 3.66 (m, 2H), 3.56 − 3.51 (m, 2H), 3.46 (d, J = 5.9 Hz, 1H), 3.37 (s, 1H), 2.63 (tq, J = 17.8, 6.4, 5.4 Hz, 2H), 2.42 − 2.05 (m, 5H), 2.02 − 1.78 (m, 3H), 1.76 − 1.53 (m, 3H), 1.52 − 1.28 (m, 7H), 1.22 − 1.11 (m, 1H), 1.05 (s, 3H), 0.96 (d, J = 7.0 Hz, 3H), 0.74 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 211.6, 173.5, 88.2, 80.4, 76.5, 76.2, 71.7, 70.7, 70.4, 63.1, 61.9, 59.3, 46.8, 43.1, 42.8, 42.3, 38.0, 36.1, 35.3, 31.7, 31.4, 28.1, 27.4, 26.9, 25.7, 17.0, 15.3, 11.7. HRMS (ESI): m/z calcd. for C28H46NaO10S [M + Na]+ 597.2709, found: 597.2704.
2.3.20. Compound 11h
To a stirred solution of compound 10h (65 mg, 0.073 mmol) in dioxane:water = 9:1 (2 mL) 0.2 M aqueous solution of KOH (1.83 mL, 0.36 mmol, 5 equiv.) was added, and the reaction mixture was stirred for half an hour at 0 °C, then for 3 h at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered and evaporated to obtain compound 11h as yellow amorphous solid (50 mg, 97%). Rf: 0.56 (CH3CN/H2O 9/1), [α]24D + 28.9 (c 0.09, MeOH). 1H NMR (400 MHz, DMSO-d6) δ 5.55 (d, J = 7.6 Hz, 1H, H-14), 4.09 (d, J = 5.7 Hz, 2H, H-22ab), 3.75 (dd, J = 19.5, 10.4 Hz, 4H), 3.63 − 3.56 (m, 2H), 3.51 (d, J = 9.7 Hz, 2H), 3.43 (d, J = 4.9 Hz, 1H, H-11), 3.27 (d, J = 1.2 Hz, 14H), 2.73 (dd, J = 12.4, 4.0 Hz, 1H), 2.65 − 2.56 (m, 1H), 2.47 (dd, J = 11.5, 3.7 Hz, 1H, H-19*a), 2.41 (s, 1H, H-19*b), 2.29 − 2.10 (m, 4H), 1.95 (s, 1H, H-20*a), 1.85 (s, 5H, H-20*b), 1.75 (d, J = 14.4 Hz, 1H, H-1a), 1.66 (d, J = 11.9 Hz, 2H), 1.59 − 1.50 (m, 1H), 1.49 − 1.39 (m, 4H, H-1b), 1.30 (s, 5H), 1.21 (dd, J = 29.0, 9.0 Hz, 6H), 1.09 − 0.97 (m, 2H), 0.91 (s, 3H, H18abc), 0.83 (d, J = 6.2 Hz, 3H, H-17abc), 0.60 (d, J = 5.3 Hz, 3H, H-16abc). * Interchangeable signals. 13C NMR (101 MHz, DMSO-d6) δ 174.3, 172.6, 171.1 (3C, C-1′, C-21, AcCO), 85.1 (1C, C-2′), 75.2, 73.9, 71.1, 69.2, 67.4, 66.9 53.6, 51.9 (8C, skeletal carbons, C-11, C-14), 63.4, 60.4, (2C, C-22, C-9′), 57.5 (1C, C-4), 45.0, 41.3, 40.8 (4C, C-12, C-13, C-5, C-9), 36.4 (1C, C-6), 34.0, 29.0, 27.4 (3C, C-19, C-20, C-8), 26.9 (1C, C-18), 25.5 (1C, C-1), 24.5 (1C, C-7), 22.3 (1C, AcCH3), 16.1 (1C, C-16), 14.6 (1C, C-15), 11.6 (1C, C-17). HRMS (MALDI): m/z calcd. for C33H53NNaO13S [M + Na]+ 726.3135, found: 726.3159.
2.3.21. Compound 11l
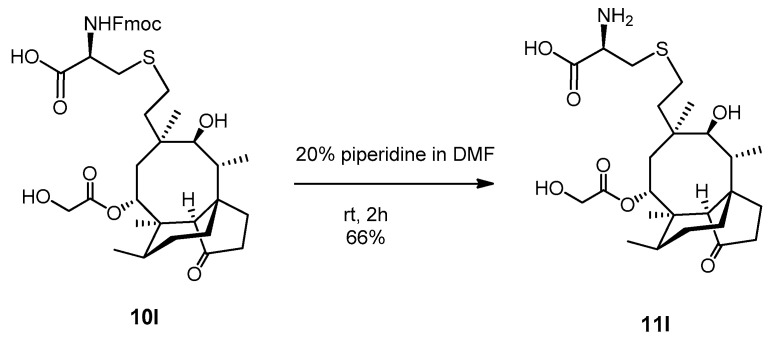
Compound 10l (100 mg, 0.138 mmol) was dissolved in 20% piperidine solution in N,N-dimethylformamide (5 mL) and stirred for two hours at room temperature. The reaction mixture was evaporated, and the crude product was purified by flash column chromatography (CH3CN/H2O 9/1) to result in compound 11l as yellow powder (46 mg, 66%). Rf: 0.10 (CH3CN/H2O 9/1), [α]24D +41.7 (c 0.06, MeOH), m.p. 197–199 °C. 1H NMR (400 MHz, Methanol-d4) δ 5.69 (t, J = 8.6 Hz, 1H), 4.22 (dd, J = 25.1, 17.2 Hz, 1H), 4.04 (dd, J = 17.2, 13.4 Hz, 1H), 3.79 (dt, J = 10.9, 5.2 Hz, 1H), 3.47 (t, J = 5.0 Hz, 1H), 3.37 (s, 2H), 3.33 (p, J = 1.7 Hz, 1H), 3.22 (dd, J = 15.2, 4.8 Hz, 1H), 3.19 − 3.07 (m, 1H), 2.52 (tq, J = 10.8, 5.2, 4.8 Hz, 2H), 2.41 − 2.31 (m, 2H), 2.31 − 2.24 (m, 1H), 2.17 (q, J = 9.6 Hz, 1H), 2.13 − 1.98 (m, 1H), 1.98 − 1.48 (m, 5H), 1.45 (d, J = 3.7 Hz, 5H), 1.39 − 1.27 (m, 2H), 1.17 (td, J = 13.8, 4.3 Hz, 1H), 1.03 (d, J = 5.0 Hz, 3H), 0.95 (dd, J = 7.1, 1.8 Hz, 3H), 0.74 (dd, J = 7.0, 2.0 Hz, 3H). 13C NMR (101 MHz, Methanol-d4) δ 217.0, 190.0, 173.3, 74.9, 69.3, 60.5, 57.8, 53.8, 45.4, 41.6, 41.0, 36.6, 34.6, 33.9, 32.8, 30.0, 28.4, 28.1, 27.2, 26.8, 25.8, 24.2, 15.8, 13.9, 10.3. HRMS (ESI): m/z calcd. for C25H41NNaO7S [M + Na]+ 522.2501, found: 522.2496.
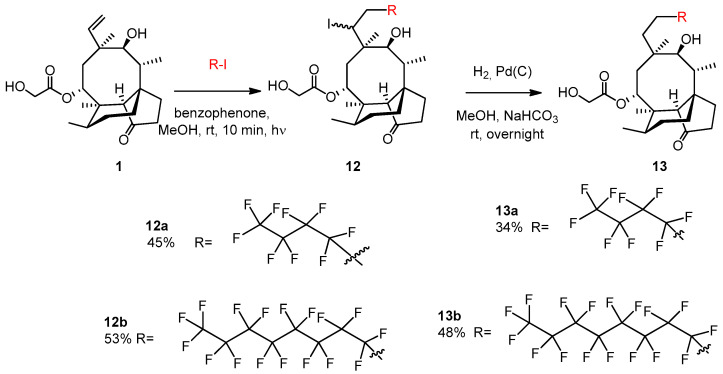
2.3.22. Compound 13a
Nonafluoro-1-iodobutane (103 μL, 0.6 mmol, 1.2 equiv.) and benzophenone (5 mg, 0.023 mmol) were added to the solution of pleuromutilin 1 (189 mg, 0.5 mmol) in methanol (5 mL). Argon gas was bubbled through the solution, and then irradiation occurred with UV light for 10 min. The solvent was evaporated, and the product was purified by flash column chromatography (CH2Cl2/acetone 95:5) to yield 12a (163 mg, 45%) as a colorless liquid. Rf = 0.51 (CH2Cl2/MeOH 95:5). To the solution of 12a (130 mg, 0.18 mmol), methanol (5 mL), 10% palladium on activated charcoal (25 mg) and NaHCO3 (53 mg, 2.5 equiv.) were added. The reaction mixture was stirred overnight under H2 atmosphere, then filtered through a pad of Celite, and the solvent was evaporated. The residue was dissolved in dichloromethane (50 mL) and the solution was washed with distilled water (10 mL) two times, and dried over anhydrous Na2SO4. The solvent was then evaporated in vacuum. The product was purified by flash column chromatography (CH2Cl2/acetone 97:3 then 95:5) to yield 13a (36 mg, 34%) as a colorless liquid. Rf = 0.25 (CH2Cl2/acetone 97:3), [α]24D +11.8 (c 0.22, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.63 (d, J = 8.2 Hz, 1H, H-14), 4.77 (dd, J = 9.3, 6.5 Hz, 1H), 4.20 − 4.12 (m, 1H, H-22a), 4.06 (dd, J = 18.3, 5.8 Hz, 1H, H-22b), 3.62 (s, 1H, H-11), 3.21 (d, J = 6.0 Hz, 1H), 2.62 (t, J = 5.3 Hz, 1H), 2.44 − 2.37 (m, 1H), 2.24 (dd, J = 17.1, 8.2 Hz, 3H), 2.16 (s, 1H, H-2b), 1.92 (s, 3H), 1.82 (d, J = 16.2 Hz, 2H), 1.77 − 1.70 (m, 2H), 1.64 (dd, J = 16.2, 8.3 Hz, 1H), 1.51 (t, J = 8.6 Hz, 2H), 1.44 (s, 3H, H-15abc), 1.18 (d, J = 6.8 Hz, 1H), 1.16 − 1.09 (m, 1H), 1.05 (s, 3H, H-18abc), 0.94 (d, J = 7.0 Hz, 3H, H-17abc), 0.69 (t, J = 6.0 Hz, 3H, H-16abc). 13C NMR (101 MHz, Chloroform-d) δ 174.9 (1C, C-21), 74.7, 71.1 (2C, C-11, C-14), 63.3, 61.5 (1C, C-22), 58.6, 46.4, 45.8, 42.0, 40.8 (4C, C-5, C-12, C-13, C-9), 36.3, 34.9 (2C, C-6, C-10), 34.4, 34.2 (2C, C-2, C-23), 29.8, 27.1 (2C, C-7, C-19), 24.7, 20.5, 17.0 (1C, C-16), 14.6 (1C, C-15), 10.5 (1C, C-17). HRMS (ESI): m/z calcd. for C26H35F9KO5 [M + K]+ 637.2188, found: 637.2182.
2.3.23. Compound 13b
Heptadecafluoro-1-iodooctane (0.160 mL, 327 mg, 0.6 mmol, 1.2 equiv.) and benzophenone (5 mg, 0.023 mmol) were added to the solution of pleuromutilin 1 (0.189 g, 0.5 mmol) in methanol (5 mL). Argon gas was bubbled through the solution and then irradiation occurred with UV light for 10 min. The solvent was evaporated, and the product was purified by flash column chromatography (CH2Cl2/acetone 97:3) to yield 12b (247 mg, 53%) as a colorless liquid. Rf = 0.55 (CH2Cl2/acetone 95:5). To the solution of 12b (0.120 g, 0.25 mmol) in methanol (5 mL) 10% palladium on activated charcoal (25 mg) and NaHCO3 (53 mg, 2.5 equiv.) were added. The reaction mixture was stirred overnight under H2 atmosphere, then filtered through a pad of Celite, and the solvent was evaporated. The residue was dissolved in dichloromethane (50 mL) and the solution was washed with distilled water (10 mL) two times, and dried with anhydrous Na2SO4. Then, the solvent was evaporated in vacuum. The product was purified by flash column chromatography (CH2Cl2/acetone 97:3) to yield 13b (49 mg, 48%) as a colorless liquid. Rf=0.22 (CH2Cl2/acetone 95:5), [α]24D +4.71 (c 0.34, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.64 (d, J = 8.2 Hz, 1H), 4.78 (dd, J = 9.0, 4.9 Hz, 1H), 4.20 − 4.00 (m, 2H), 3.62 (d, J = 4.0 Hz, 1H), 3.19 (s, 1H), 2.59 (s, 7H), 2.47 − 2.36 (m, 1H), 2.24 (dd, J = 17.1, 8.4 Hz, 3H), 1.88 (s, 1H), 1.82 (d, J = 16.2 Hz, 2H), 1.78 − 1.59 (m, 4H), 1.51 (dd, J = 11.5, 5.9 Hz, 2H), 1.44 (s, 3H, H-15abc), 1.17 (s, 1H), 1.16 − 1.09 (m, 1H), 1.05 (s, 3H, H-18abc), 0.94 (d, J = 7.0 Hz, 3H, H-17abc), 0.70 (d, J = 7.0 Hz, 3H, H-16abc). 13C NMR (101 MHz, Chloroform-d) δ 174.9 (1C, C-21), 74.8, 71.1 (2C, C-11, C-14), 63.4, 61.6 (1C, C-22), 58.6 (1C, C-4), 46.4, 45.9, 42.0, 40.9 (4C, C-5, C-12, C-13, C-9), 36.3, 34.4, 34.2, 29.9, 27.1, 24.7, 20.5, 16.9 (1C, C-16), 14.7 (1C, C-15), 10.5 (1C, C-17). HRMS (ESI): m/z calcd. for C30H35F17KO5 [M + K]+ 837.2060, found: 837.2054.
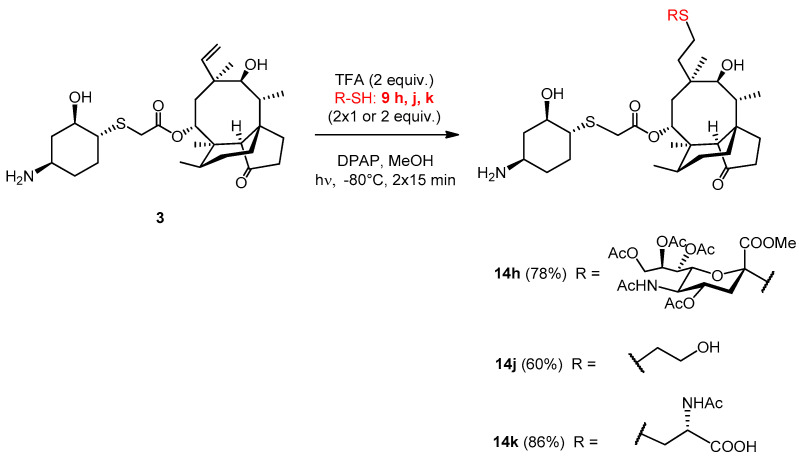
2.3.24. Compound 14h
The reaction was carried out using the general method, starting from lefamulin (51 mg, 0.1 mmol), trifluoroacetic acid (15 µL, 0.2 mmol, 2 equiv.) and thiol 9h (203 mg, 0.4 mmol, 2 × 2 equiv.) in methanol at −80 °C. It was irradiated two times. The reaction mixture was co-evaporated with toluene three times and the crude product was purified by flash column chromatography (CH2Cl2/MeOH 85/15) to result in compound 14h as white amorphous solid (79 mg, 78%). Rf: 0.56 (CH2Cl2/MeOH 7/3), [α]24D +24.4 (c 0.09, DMSO). 1H NMR (400 MHz, DMSO-d6) δ 7.77 (d, J = 6.5 Hz, 1H, NH), 5.42 (d, J = 6.8 Hz, 1H, H-14), 5.20 (d, J = 2.3 Hz, 1H, H-8″), 5.16 (d, J = 7.1 Hz, 1H), 4.69 (s, 1H, H-4″), 4.65 (s, 1H, OH), 4.21 (d, J = 12.1 Hz, 1H, H-9″a), 4.04 (dd, J = 11.6, 4.5 Hz, 1H, H-9″b), 3.84 (s, 1H, H-5″), 3.80 (d, J = 1.6 Hz, 1H), 3.54 (d, J = 14.5 Hz, 1H), 3.42 (s, 5H), 3.33 (d, J = 12.8 Hz, 3H, H-11, H-2′), 3.02 (s, 1H, H-4′), 2.64 (d, J = 8.7 Hz, 1H, H-3″a), 2.56 (s, 1H, H-1′), 2.51 (d, J = 1.4 Hz, 2H), 2.35 (s, 1H, H-4), 2.16 (d, J = 10.9 Hz, 2H, H-5′a, H-10), 2.07 (d, J = 1.7 Hz, 3H, AcCH3), 2.01 (s, 3H, AcCH3), 1.96 (s, 3H, AcCH3), 1.92 (d, J = 1.7 Hz, 3H, AcCH3), 1.83 (d, J = 16.5 Hz, 1H, H-13a), 1.75 (d, J = 12.3 Hz, 1H, H-3″b), 1.65 (s, 5H, AcCH3, H-7a, H-8a), 1.49 (s, 2H, H-6), 1.37 (s, 3H, H-15abc), 1.23 (s, 6H, H-7b), 1.13 (d, J = 16.1 Hz, 2H, H-13b), 1.08 − 0.95 (m, 2H, H-8b), 0.90 (s, 3H, H-18abc), 0.80 (d, J = 5.4 Hz, 3H, H-17abc), 0.64 (d, J = 5.7 Hz, 3H, H-16abc). 13C NMR (101 MHz, DMSO-d6) δ 217.1 (1C, C-3), 170.1, 169.7, 169.4, 169.3, 169.3, 169.1, 168.4 (7C, 7×CO), 83.1 (1C, C-2″), 73.7, 73.3, 72.3, 67.3 (4C, C-11, C-2′, C-7″, C-6″), 69.6 (1C, C-4″), 68.9 (1C, C-14), 68.4 (1C, C-8″), 61.8 (1C, C-9″), 57.2 (1C, C-4), 54.9, 53.0 (1C, COOCH3), 49.7 (1C, C-1′), 47.8 (1C, C-5″), 47.2 (1C, C-4′), 44.9 (1C, C-9), 41.2, 40.6 (3C, C-13, C-12, C-5), 36.4 (1C, C-6), 34.4 (1C, C-10), 33.9 (1C, C-5′), 33.7 (1C, C-22), 30.1 (1C, C-8), 29.8, 29.6, 28.2, 26.8, 23.7 (6C, C-19, C-20, C-3′, C-6′, C-1, C-2), 26.2 (1C, C-18), 24.3 (1C, C-7), 22.6 (1C, NAcCH3), 20.9, 20.7, 20.6, 20.6 (4C, 4xOAcCH3), 16.8 (1C, C-16), 14.6 (1C, C-15), 11.5 (1C, C-17). HRMS (ESI): m/z calcd. for C48H75N2O17S2 [M + H]+ 1015.4507, found: 1015.4502.
2.3.25. Compound 14j
The reaction was carried out using the general method, starting from lefamulin (51 mg, 0.1 mmol), trifluoroacetic acid (15.3 µL, 0.2 mmol, 2 equiv.) and thiol 9j (31 mg, 28 µL, 0.4 mmol, 2 × 2 equiv.) in methanol at −80 °C. It was irradiated two times. The reaction mixture was co-evaporated with toluene three times and the crude product was purified by flash column chromatography (CH3CN/H2O 9/1) to result in compound 14j as white-yellow amorphous solid (35 mg, 60%). Rf: 0.19 (CH3CN/H2O 9/1), [α]24D +50.0 (c 0.07, H2O). 1H NMR (400 MHz, DMSO-d6) δ 5.51 (d, J = 7.6 Hz, 1H, H-14), 4.61 (s, 1H, OH), 3.63 − 3.46 (m, 3H, H-24ab), 3.40 − 3.29 (m, 3H, H-11, H-2′), 2.98 (s, 1H, H-4′), 2.57 (t, J = 7.0 Hz, 2H, H-23ab), 2.51 (s, 2H), 2.37 (t, J = 13.0 Hz, 3H, H-4), 2.23 − 2.02 (m, 3H, H-10, H-3′a), 1.96 (s, 3H), 1.91 − 1.78 (m, 2H, H-13a, H-5′a), 1.67 (t, J = 16.9 Hz, 3H, H-7a, H-8a), 1.49 (d, J = 5.9 Hz, 1H, H-6), 1.42 (d, J = 15.5 Hz, 1H), 1.37 (s, 3H, H-15abc), 1.33 − 1.18 (m, 7H, H-7b, H-3′a, H-5′b, H-6′ab), 1.12 (d, J = 16.0 Hz, 1H, H-13b), 1.07 − 0.98 (m, 1H, H-8b), 0.93 (s, 3H, H-18abc), 0.83 (d, J = 6.3 Hz, 3H, H-17abc), 0.64 (d, J = 6.2 Hz, 3H, H-16abc). 13C NMR (101 MHz, DMSO-d6) δ 217.1 (1C, C-3), 169.3 (1C, C-21), 73.5, 72.3 (2C, C-11, C-2′), 68.7 (1C, C-14), 61.0 (1C, C-24), 57.2 (1C, C-4), 49.5 (1C, C-1′), 47.3 (1C, C-4′), 45.0, 41.3, 40.6, 40.3 (4C, C-5, C-13, C-12, C-9), 36.4 (1C, C-6), 34.6 (1C, C-10), 33.9, 33.5, 30.5, 30.1, 28.3, 27.0, 26.7 (8C, C-3′, C-5′, C-6′, C-1, C-2, C-19, C-20, C-23), 26.9 (1C, C-18), 24.3 (1C, C-7), 16.5 (1C, C-16), 14.6 (1C, C-15), 11.5 (1C, C-17). HRMS (ESI): m/z calcd. for C30H52NO6S2 [M + H]+ 586.3236, found: 586.3230.
2.3.26. Compound 14k
The reaction was carried out using the general method, starting from lefamulin (50.8 mg, 0.1 mmol), trifluoroacetic acid (15.3 µL, 0.2 mmol, 2 equiv.) and thiol 9k (33 mg, 0.2 mmol, 2 × 1 equiv.) in methanol at −80 °C. It was irradiated two times. The reaction mixture was evaporated with added toluene three times and the crude product was purified by flash column chromatography (CH3CN/H2O 9/1) resulted in compound 14k as white-yellow amorphous solid (57.4 mg, 86%). Rf: 0.46 (CH3CN/H2O 85/15), [α]24D +36.0 (c 0.2, H2O). 1H NMR (400 MHz, DMSO-d6) δ 7.42 (d, J = 7.1 Hz, 1H, NH), 5.50 (d, J = 8.0 Hz, 1H, H-14), 4.57 (d, J = 4.4 Hz, 1H, OH), 4.08 (dd, J = 12.1, 5.4 Hz, 1H, H-24), 3.68 (d, J = 16.2 Hz, 2H, H-22a), 3.35 (dd, J = 21.8, 11.0 Hz, 2H, 10H, H-2′, H-11), 3.19 − 3.13 (m, 2H, H-22b), 2.95 (dt, J = 19.3, 9.7 Hz, 2H, H-23a, H-4′), 2.74 − 2.60 (m, 2H, H-23b, H-1′), 2.53 − 2.49 (m, 2H), 2.42 − 2.32 (m, 2H, H-4), 2.32 − 2.22 (m, 1H), 2.18 (dd, J = 12.1, 6.1 Hz, 2H, H-10, H-5′a), 2.03 (ddd, J = 21.3, 17.6, 5.7 Hz, 4H, H-5′b), 1.84 (s, 3H, AcCH3), 1.79 (dd, J = 13.9, 9.4 Hz, 3H, H-13a), 1.72 − 1.60 (m, 6H, H-7a, H-8a), 1.48 (dd, J = 11.9, 9.0 Hz, 1H, H-6), 1.41 (d, J = 13.3 Hz, 1H, H-6′a), 1.33 (s, 3H, H-15abc), 1.31 − 1.23 (m, 3H, H-7b), 1.18 (dd, J = 9.7, 6.4 Hz, 2H, H-13b), 1.07 − 0.97 (m, 1H, H-8b), 0.91 (s, 3H, H-18abc), 0.83 (d, J = 6.8 Hz, 3H, H-17abc), 0.60 (d, J = 6.6 Hz, 3H, H-16abc). 13C NMR (101 MHz, DMSO-d6) δ 217.3 (1C, C-3), 173.0 (1C, C-21), 169.0, 168.4 (2C, AcCO, COOH), 73.6 (2C, C-2′, C-11), 72.8, 68.5 (1C, C-14), 57.2 (1C, C-4), 53.9 (1C, C-24), 47.4, 47.2 (1C, C-4′, C-1′), 41.2 (1C, C-13), 45.0, 40.7 (3C, C-5, C-12, C-9), 36.4 (1C, C-6), 35.9 (1C, C-23), 34.5 (1C, C-10), 34.0 (1C, C-5′), 33.0 (1C, C-22), 30.1 (1C, C-8), 31.1, 29.2, 28.2, 27.7 (4C, C-19, C-20, C-3′, C-6′), 26.8 (1C, C-18), 24.3 (1C, C-7), 22.9 (1C, AcCH3), 16.4 (1C, C-16), 14.6 (1C, C-15), 11.5 (1C, C-17). HRMS (MALDI): m/z calcd. for C33H54N2NaO8S2 [M + Na]+ 693.3219, found: 693.3219.
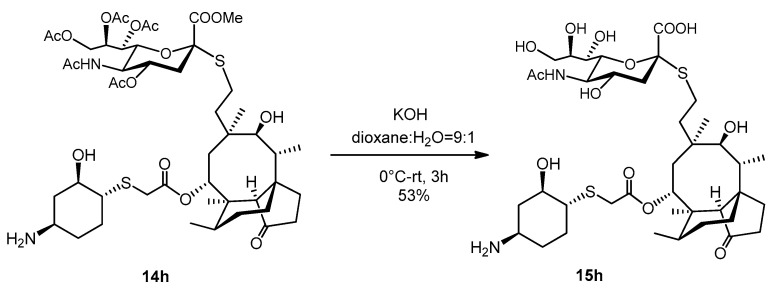
2.3.27. Compound 15h
To a stirred solution of compound 14h (58 mg, 0.06 mmol) in dioxane: water = 9:1 (2 mL) and 1.5mL of 0.2 M aqueous solution of KOH (1.5 mL, 0.3 mmol, 5 equiv.) was added (pH = 12). The reaction mixture was stirred for half an hour at 0 °C, then stirred for 3 h at room temperature. The reaction mixture was neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered, evaporated, and purified by flash column chromatography (CH3CN/H2O 9/1) to obtain compound 15h as white amorphous solid (25 mg, 53%). Rf: 0.4 (CH3CN/H2O 8/2), [α]24D -3.33 (c 0.06, DMSO). 1H NMR (400 MHz, DMSO-d6) δ 8.05 (d, J = 7.9 Hz, 1H, NH), 5.49 (d, J = 7.8 Hz, 1H, H-14), 5.17 (s, 1H, H-8″), 4.87 (s, 1H), 4.69 (s, 1H, H-4″), 4.48 (d, J = 5.5 Hz, 1H), 4.08 (s, 1H), 3.60 (t, J = 14.9 Hz, 4H, H-5″, H-9″a, H-11, H-2′), 3.26 (s, 1H, H-9″b), 3.16 (s, 2H), 2.98 (s, 1H, H-4′), 2.89 (s, 1H), 2.74 (s, H), 2.69 (dd, J = 8.6, 3.6 Hz, 1H, H-22a), 2.63 (d, J = 12.8 Hz, 1H, H-1′), 2.56 (d, J = 6.7 Hz, 1H, H-22b), 2.35 (s, 1H, H-4), 2.21 − 2.08 (m, 3H, H-5′a, H-10), 2.03 (d, J = 9.2 Hz, 3H, H-5′b), 1.89 (s, 3H), 1.77 (t, J = 16.3 Hz, 4H, H-13a), 1.63 (dd, J = 21.1, 9.8 Hz, 3H, H-7a, H-8a), 1.48 (dd, J = 21.3, 10.7 Hz, 5H, H-6), 1.33 (s, 3H), 1.24 (s, 3H, H-7b), 1.19 (s, 3H, H-13b), 1.03 (dd, J = 16.5, 4.5 Hz, 2H, H-8b), 0.94 (s, 3H, H-18abc), 0.83 (d, J = 6.5 Hz, 3H, H-17abc), 0.61 (d, J = 6.3 Hz, 3H, H-16abc). 13C NMR (101 MHz, DMSO-d6) δ 217.2 (1C, C-3), 172.4, 172.2, 168.5 (3C, AcCO, C-1″, C-21), 84.8 (1C, C-2″), 75.2, 73.5, 72.0, 71.2 (3C, C-11, C-2′), 68.8, 68.6 (2C, C-8″, C-14), 67.7, 63.2 (1C, C-9″), 57.2 (1C, C-4), 54.9, 52.8, 49.8 (1C, C-1′), 47.3, 47.1 (1C, C-4′), 44.9 (1C, C-9), 42.3, 41.2, 40.9 (3C, C-12, C-13, C-5), 36.4 (1C, C-6), 35.8, 34.4 (1C, C-10), 34.0 (1C, C-5′), 33.0 (1C, C-22), 31.1, 28.9, 30.1 (1C, C-8), 26.8 (1C, C-18), 24.4 (1C, C-7), 24.2 (1C, C-22), 22.5 (1C, AcCH3), 16.4 (1C, C-, 6), 14.6 (1C, C-15), 11.4 (1C, C-17). HRMS (ESI): m/z calcd. for C39H64N2NaO13S2 [M + Na]+ 855.3748, found: 855.3746.
3. Results and Discussion
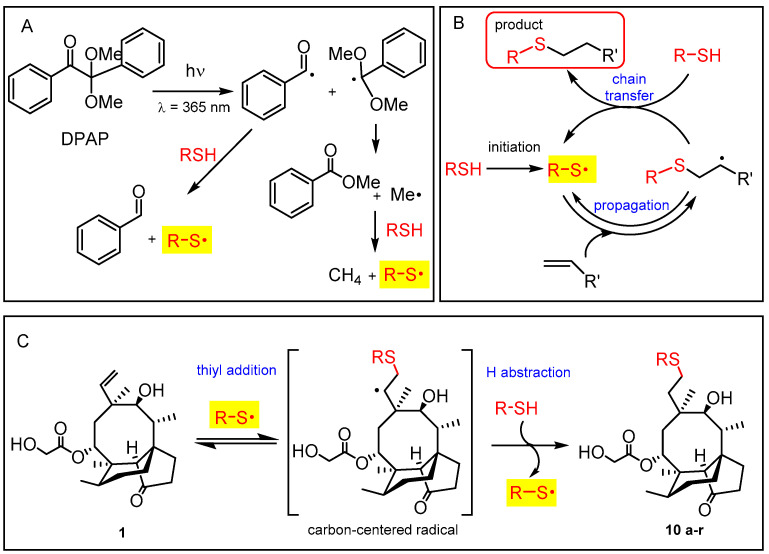
Firstly, the pleuromutilin skeleton was modified by the photoinitiated radical addition of a wide range of thiols to the C-C double bond. Hydrothiolation of terminal alkenes is known to occur by a free free-radical chain mechanism (Scheme 1) [20,21,22,23]. It is known from previous studies that the addition of thiols to terminal alkenes results in the novel C-S bond with complete regioselectivity, as the reaction goes under anti-Markovnikov-rule [30].
Scheme 1.
Generation of thiyl radical by the cleavable photoinitiator DPAP upon UV irradiation (A); free-radical chain process of hydrothiolation of alkenes (B); sequential thiyl-addition and hydrogen abstraction steps during the thiol-ene coupling reaction of pleuromutilin (C).
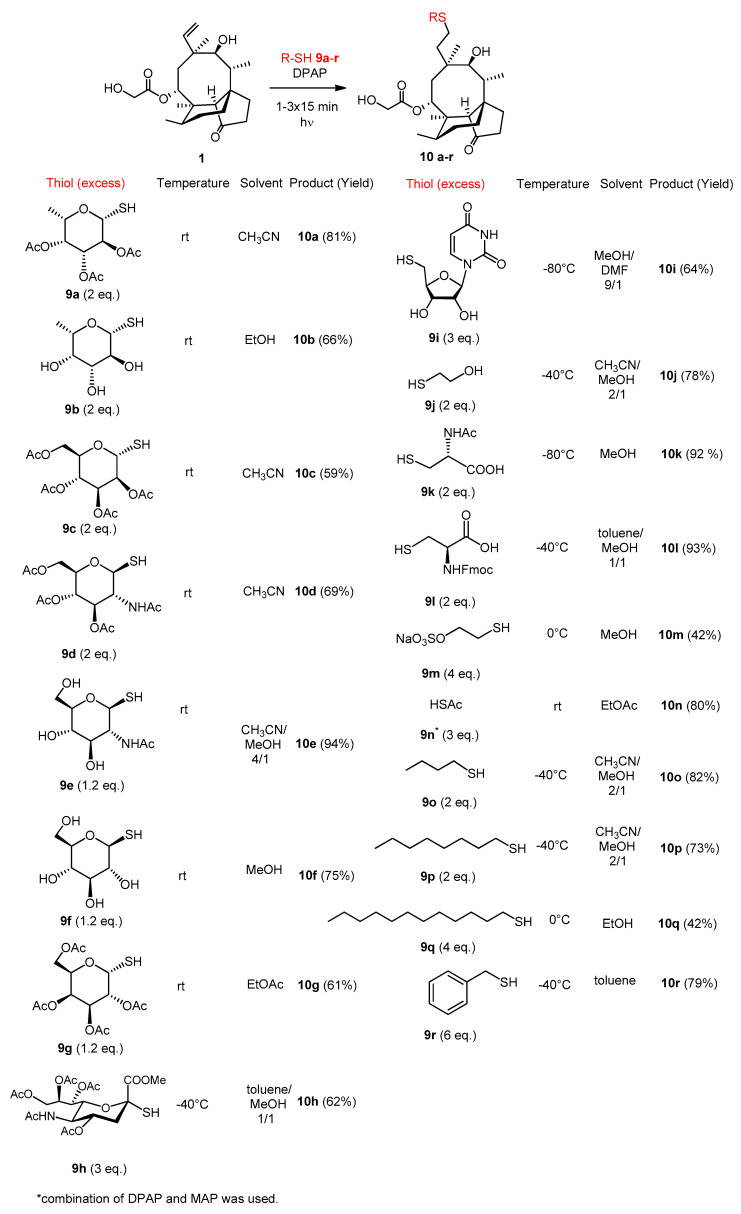
For the introduction of hydrophilic substituents to position C20 per-O-acetylated or unprotected glycosyl thiols (α-l-fuco- 9a and 9b, α-d-manno- 9c, β-d-GlcNAc- 9d and 9e, β-d-gluco- 9f and β-d-galactopyranose 9g, and 5-NAc-α-d-neuraminic acid 9h), 5′-thio-thymidine 9i, 2-mercaptoethanol 9j, N-acetyl-l-cysteine 9k, N-Fmoc-l-cysteine 9l, Mesna 9m and thioacetic acid 9n were used. Alkyl thiols (n-butyl 9o, n-octyl 9p, n-dodecyl 9q) and benzyl mercaptan (9r) were applied for the lipophilic modifications. The thiol-ene reactions were performed under the previously established conditions [19,20,21,22,23] using DPAP (2,2-dimethoxy-2-phenylacetophenone) as photoinitiator and irradiation with UV light at 365 nm (Scheme 2). We have demonstrated that for several short irradiation cycles, adding a new dose of initiator (which is consumed in the initiation step) to the reaction mixture before each cycle, is more effective than a longer-term continuous irradiation. Thus, if the conversion was not satisfactory after 15 min., the reaction mixture was subjected to UV-irradiation for further 1–2 × 15 min. The thiol-ene coupling is tolerant to water and oxygen and is compatible with any solvent [24,31,32,33]. Therefore, we performed the reactions without any caution to exclude air or moisture, and selected different solvents on the basis of the solubility of the reactants.
Scheme 2.
Hydrothiolation of pleuromutilin at position C19–C20 with thiols 9a–9r.
The desired compounds 10a–10r were isolated with moderate (42%) to excellent (94%) yields and full regioselectivity. Recently, we have demonstrated that the reaction temperature influences the efficacy of the photoinitiated thiol-ene reactions in a unique way. Cooling promotes, whereas heating inhibits, the thiol-ene couplings [20,21,22,24,31,32,33]. Cooling exerts the beneficial effect by increasing the half-life of the carbon centered radical intermediate, thus shifting the equilibrium of the reversible step toward product formation, and increasing the yield [20,21,23]. Therefore, when a low conversion was observed at room temperature, the reaction was repeated by cooling, in the temperature range of 0 °C to −80 °C. The experimental set-up of the low-temperature photoinitiated thiol-ene coupling reactions is shown in Supplementary Materials (Figure S28). In the case of compound 9n, only moderate yield was achieved by the standard DPAP-catalyzed addition reaction. In this case, a synergistic photoinitiator combination of MAP (4′-methoxyacetophenone) and DPAP was used, based on Scalan’s work on the successful addition of acyl thiols to alkenes [34]. In each case, the most effective conditions used are presented. The 13C NMR measurements confirmed the formation of the new C-S bond, namely, the signals of the C=C double bond disappeared (absence of signals around 140 and 115 ppm) and the characteristic peak of the new -CH2-S group appeared at ~30 ppm in the carbon spectra of the products.
The C14 glycolate side chain is essential for the antibacterial activity [8] that had to be taken into account when performing basic deprotection of the ester protecting groups of 10g and 10h. Zemplén-deacetylation of compound 10g with a catalytic amount of NaOMe proceeded with excellent yield, without affecting the glycolate ester bond. Moreover, the core antibiotic survived the KOH-mediated removal of the methyl ester group of the sialic acid derivative 10h (Scheme 3).
Scheme 3.
Successful deacetylation of the galactose-modified pleuromutilin 10g and hydrolysis of the sialic-acid derivative 10h by KOH.
The Fmoc group of the cysteine-derivative 10l was removed using piperidine, resulting in compound 11l with a yield of 66% (Scheme 4).
Scheme 4.
Deprotection of compound 10l.
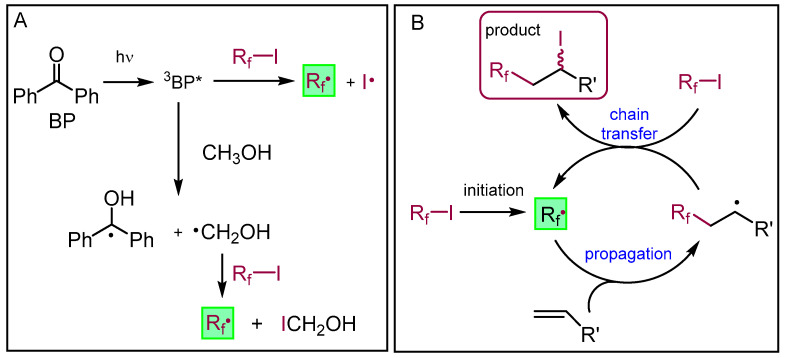
Finally, we modified the pleuromutilin by the additional reaction of perfluoroalkyl iodide derivatives on the C19–C20 alkene. The atom-transfer radical addition of iodoperfluoralkanes (RfI) onto olefins can be very effectively activated by organocatalysts, under irradiation with visible or UV light [25,35]. A key step of the mechanism is the initiation involving radical Rf generation, which then enters the radical propagation chain and adds to the alkene (Scheme 5). In the radical chain process, the addition of Rf occurs to the terminal carbon in a regioselective way, producing a carbon centered radical which stabilizes by the abstraction of an iodine, to give the iodoperfluoralkylated product in the form of a diastereoisomeric mixture (Scheme 5B). We performed the reactions in MeOH using benzophenone (BP) as the catalyst under irradiation with UV-light at 365 nm. Under these conditions, the generation of the Rf radical mainly occurs through the reaction of MeOH with the excited state BP, producing a CH2OH radical which then abstracts an iodine from RfI. The Rf radical could also be generated through a direct energy transfer from the BP triplet excited state (3BP*) to RfI. The direct photolysis of RfI upon irradiation at 365 nm represents a minor pathway of initiation (Scheme 5A) [25].
Scheme 5.
(A) Generation of the Rf· radical from perfluoroalkyl iodide with benzophenone in MeOH under UV irradiation (3BP*: benzophenone triplet); (B) mechanism of ATRA of perfluoroalkyl iodide onto terminal alkenes.
The light-promoted atom-transfer radical addition of the commercially available perfluorobutyl iodide and perfluorooctyl iodide onto pleuromutilin was conducted in MeOH, at room temperature, using a BP catalyst under argon. The reaction resulted in the expected iodoperfluooralkylated 12a and 12b as mixtures of diastereoisomers in moderate yields. A subsequent catalytic hydrogenation of the C19-iodo derivatives gave the final products 13a and 13b with 34% and 48% yields, respectively (Scheme 6).
Scheme 6.
Modification of pleuromutilin with perfluoroalkylated side-chains.
We have examined the antibacterial properties of the novel semisynthetic compounds on a panel of bacteria (Table 1). The 2-hydroxyethyl (10j), N-acetyl-l-cysteine (10k) and deprotected sialic acid (11h) derivatives were found to be potent antimicrobial compounds against biofilm-producing S. epidermidis and were as active against Enterococcus faecium VanA isolated from drain as the parent pleuromutilin. Moreover, these compounds showed excellent antibacterial properties against MRSA strain. Compound 10k provided excellent activity against Bacillus subtilis. Unfortunately, the other semisynthetic derivatives proved to be inactive, and neither the fluorophilic nor lipophilic modifications resulted in effective compounds (MIC > 256 μg/mL).
Table 1.
Antibacterial effect of semisynthetic pleuromutilins based on Minimal Inhibitory Concentration [28] of active compounds (MIC: mg/L) (ATCC: American Type Culture Collection, MRSA/MSSA: methicillin resistant/sensitive S. aureus, VanA and VanB: vancomycin resistant phenotypes.)
| Bacterial Strains | 1 | 10j | 10k | 11h | 3 | 14j | 14k | 15h |
|---|---|---|---|---|---|---|---|---|
| Bacillus subtilis ATCC 6633 | 16 | 32 | 4 | 32 | 0.5 | 16 | 256 | 512 |
| Staphylococcus aureus MSSA ATCC 29213 | 4 | 16 | 8 | 16 | 0.5 | 16 | 512 | 512 |
| Staphylococcus aureus MRSA ATCC 33591 | 2 | 4 | 4 | 4 | 0.5 | 32 | 512 | 256 |
| Staphylococcus epidermidis ATCC 35984 biofilm | 1 | 2 | 0.5 | 2 | 0.5 | 16 | 512 | 128 |
| Staphylococcus epidermidis mecA | 4 | 16 | 8 | 16 | 0.5 | 16 | 512 | 256 |
| Enterococcus faecalis ATCC 29212 | 64 | 256 | 32 | 256 | 64 | 64 | 512 | 512 |
| Enterococcus faecalis 15376 VanA | 128 | 256 | 64 | 128 | 1 | 16 | 512 | 256 |
| Enterococcus faecium VanA 38276 urine | 128 | 128 | 128 | 128 | 1 | 2 | 256 | 256 |
| Enterococcus faecium VanA 25192 bloodculture | 128 | 128 | 128 | 128 | 2 | 2 | 512 | 256 |
| Enterococcus faecium VanA 3452 drain | 2 | 2 | 2 | 2 | 1 | 2 | 256 | 512 |
| Enterococcus faecium VanA 24581 wound | 128 | 128 | 128 | 128 | 1 | 1 | 512 | 512 |
| Enterococcus faecalis ATCC 51299 VanB | 128 | 256 | 32 | 256 | 64 | 64 | 256 | 256 |
Based on the promising antibacterial activity of pleuromutilin derivatives 10j, 10k and 11h, modification of lefamulin by the thiol-ene coupling reaction was also performed. Lefamulin 3 was reacted with 2-mercaptoethanol 9j, N-acetyl-l-cysteine 9k, and the 5-NAc-2-thio-α-d-neuraminic acid derivative 9h (Scheme 7). In order to avoid deprotonation of the thiol reactants by the basic primary amino group, lefamulin was first converted into trifluoroacetate salt by treatment of trifluoroacetic acid, and the additional reactions were carried out under cooling, with excess amounts of thiols. Finally, lefamulin derivatives were isolated with good yields.
Scheme 7.
Hydrothiolation of lefamulin at position C19–C20.
The acetyl protecting groups and methyl ester group of the sialic acid derivative 14h were removed by KOH to result in the sialic acid modified lefamulin 15h (Scheme 8).
Scheme 8.
Deprotection of thiosialylated lefamulin derivative 14h.
The antibacterial properties of the novel semisynthetic lefamulin compounds were tested on the same panel of bacteria (Table 1) than in the case of pleuromutilin. The 2-hydroxyethyl (14j) derivative was found to show potent antimicrobial activity against VanA resistant Enterococcus faecium strains isolated from urine, blood culture, drain and wound. Although the 50S ribosomal subunit occurs only in prokaryotes, antibiotics that inhibit protein synthesis by binding to the 50S subunit—as pleuromutilin derivates do—may be severely toxic to eukaryotes [36]. Therefore, it is essential to perform in vitro and in vivo toxicological studies of the active derivatives in the future.
4. Conclusions
The modification of pleuromutilin with the addition of a series of thiols provided the development of three novel semisynthetic antibiotics effective against B. subtilis, sensitive and resistant staphylococci and resistant enterococci having vanA and vanB genes. The 2-mercaptoethanol, N-Ac-l-cystein and 2-thio-sialic acid addition could improve the antimicrobial activity of the pleuromutilin. However, neither further hydrophilic (carbohydrate side-chains) nor fluorophilic and alkyl- and aryl-type lipophilic modification resulted in effective compounds. The successful modifications were introduced to lefamulin, using photoinitiated hydrotiolation, which resulted in an ethylthio-containing novel semisynthetic lefamulin, with excellent activity against VanA resistant Enterococcus faecium strains. Considering the simple and efficient way to synthesize semisynthetic mutilins by the thiol-ene coupling reaction, we can conclude that we found effective semisynthetic mutilin-type antibiotics with promising antimicrobial effects.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/article/10.3390/pharmaceutics13122028/s1, Figure S1: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 10a. Figure S2: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10b. Figure S3: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10c. Figure S4: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 10d. Figure S5: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 10e. Figure S6: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 10f. Figure S7: 1H and 13C NMR spectrum (360 MHz, CDCl3) of compound 10g. Figure S8: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 10h. Figure S9: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10i. Figure S10: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10j. Figure S11: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 10k. Figure S12: 1H and 13C NMR spectrum (500 MHz, MeOD) of compound 10l. Figure S13: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10m. Figure S14: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10n. Figure S15: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 10o. Figure S16: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 10p. Figure S17: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 10q. Figure S18: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 10r. Figure S19: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 11g. Figure S20: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 11h. Figure S21: 1H and 13C NMR spectrum (400 MHz, MeOD) of compound 11l. Figure S22: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 13a. Figure S23: 1H and 13C NMR spectrum (400 MHz, CDCl3) of compound 13b. Figure S24: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 14h. Figure S25: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 14j. Figure S26: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 14k. Figure S27: 1H and 13C NMR spectrum (400 MHz, DMSO) of compound 15h. Figure S28: The experimental setup for carrying out hydrothiolation reactions at low temperature. Figure S29: ESI-MS spectrum of compound 10j. Figure S30: MALDI-MS spectrum of compound 10k. Figure S31: Calibrated MALDI-MS spectrum of compound 10k. Figure S32: MALDI-MS spectrum of compound 11h. Figure S33: Calibrated MALDI-MS spectrum of compound 11h. Figure S34: ESI-MS spectrum of compound 14j. Figure S35: MALDI-MS spectrum of compound 14k. Figure S36: Calibrated MALDI-MS spectrum of compound 14k. Figure S37: ESI-MS spectrum of compound 15h.
Author Contributions
Conceptualization, P.H., A.B. and M.C.; investigation, S.T.L., D.P., E.R., T.T., N.D., M.B., I.B., E.O. and M.M.; writing—original draft preparation, A.B. and M.C.; writing—review and editing, P.H., A.B., E.O. and M.C.; supervision, P.H., A.B. and M.C.; funding acquisition, M.C. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The synthetic work was supported by the National Research and Development and Innovation Office of Hungary (K119509, M.Cs. and K132870, A.B.) and the EU and co-financed by the European Regional Development Fund under the projects GINOP-2.3.2-15-2016-00008 and GINOP-2.3.4-15-2020-00008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. 2019. [(accessed on 18 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
- 2.Kavanagh F., Hervey A., Robbins W.J. Antibiotic substances from basidiomycetes: VIII. Pleurotus multilus (Fr.) Sacc. and Pleurotus passeckerianus Pilat. Proc. Natl. Acad. Sci. USA. 1951;37:570. doi: 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paukner S., Riedl R. Pleuromutilins: Potent drugs for resistant bugs—Mode of action and resistance. Cold Spring Harb. Perspect. Med. 2017;7:a027110. doi: 10.1101/cshperspect.a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang R., Wang J., Guo W., Liang J. Efficient antibacterial agents: A review of the synthesis, biological evaluation and mechanism of pleuromutilin derivatives. Curr. Top. Med. Chem. 2013;13:3013–3025. doi: 10.2174/15680266113136660217. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y.-Z., Liu Y.-H., Chen J.-X. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini Rev. Med. Chem. 2012;12:53–61. doi: 10.2174/138955712798868968. [DOI] [PubMed] [Google Scholar]
- 6.Eyal Z., Matzov D., Krupkin M., Paukner S., Riedl R., Rozenberg H., Zimmerman E., Bashan A., Yonath A. A novel pleuromutilin antibacterial compound, its binding mode and selectivity mechanism. Sci. Rep. 2016;6:39004. doi: 10.1038/srep39004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown P., Dawson M.J. Progress in Medicinal Chemistry. Elsevier; Amsterdam, The Netherlands: 2015. A perspective on the next generation of antibacterial agents derived by manipulation of natural products; pp. 135–184. [DOI] [PubMed] [Google Scholar]
- 8.Goethe O., Heuer A., Ma X., Wang Z., Herzon S.B. Antibacterial properties and clinical potential of pleuromutilins. Nat. Prod. Rep. 2019;36:220–247. doi: 10.1039/C8NP00042E. [DOI] [PubMed] [Google Scholar]
- 9.Egger H., Reinshagen H. New pleuromutilin derivatives with enhanced antimicrobial activity II. Structure-activity correlations. J. Antibiot. 1976;29:923–927. doi: 10.7164/antibiotics.29.923. [DOI] [PubMed] [Google Scholar]
- 10.Alberti F., Khairudin K., Venegas E.R., Davies J.A., Hayes P.M., Willis C.L., Bailey A.M., Foster G.D. Heterologous expression reveals the biosynthesis of the antibiotic pleuromutilin and generates bioactive semi-synthetic derivatives. Nat. Commun. 2017;8:1–9. doi: 10.1038/s41467-017-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Duijkeren E., Greko C., Pringle M., Baptiste K.E., Catry B., Jukes H., Moreno M.A., Pomba M.C.M.F., Pyörälä S., Rantala M. Pleuromutilins: Use in food-producing animals in the European Union, development of resistance and impact on human and animal health. J. Antimicr. Chemother. 2014;69:2022–2031. doi: 10.1093/jac/dku123. [DOI] [PubMed] [Google Scholar]
- 12.Daum R.S., Kar S., Kirkpatrick P. Retapamulin. Nat. Rev. Drug Disc. 2007;6:865–866. doi: 10.1038/nrd2442. [DOI] [Google Scholar]
- 13.Veve M.P., Wagner J.L. Lefamulin: Review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38:935–946. doi: 10.1002/phar.2166. [DOI] [PubMed] [Google Scholar]
- 14.Mercuro N.J., Veve M.P. Clinical Utility of Lefamulin: If Not Now, When? Curr. Infect. Dis. Rep. 2020;22:1–13. doi: 10.1007/s11908-020-00732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacobbe D.R., Battaglini D., Ball L., Brunetti I., Bruzzone B., Codda G., Crea F., De Maria A., Dentone C., Di Biagio A. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020;50:e13319. doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgin L.A., Högenauer G. The Mode of Action of Pleuromutilin Derivatives: Effect on Cell-Free Polypeptide Synthesis. Eur. J. Biochem. 1974;47:527–533. doi: 10.1111/j.1432-1033.1974.tb03721.x. [DOI] [PubMed] [Google Scholar]
- 17.Shang R., Pu X., Xu X., Xin Z., Zhang C., Guo W., Liu Y., Liang J. Synthesis and biological activities of novel pleuromutilin derivatives with a substituted thiadiazole moiety as potent drug-resistant bacteria inhibitors. J. Med. Chem. 2014;57:5664–5678. doi: 10.1021/jm500374c. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z., Li K., Zhang G.-Y., Tang Y.-Z., Jin Z. Design, synthesis and biological activities of novel pleuromutilin derivatives with a substituted triazole moiety as potent antibacterial agents. Eur. J. Med. Chem. 2020;204:112604. doi: 10.1016/j.ejmech.2020.112604. [DOI] [PubMed] [Google Scholar]
- 19.Thirring K., Riedl W.H.R., Kollmann H., Ivezic Z., Schoenfeld W.W.S., Paukner S., Strickmann D. 12-epi-pleuromutilins. WO2015110481 A1. World Patent. 2015 July 30;
- 20.Bege M., Bereczki I., Herczeg M., Kicsák M., Eszenyi D., Herczegh P., Borbás A. A low-temperature, photoinduced thiol–ene click reaction: A mild and efficient method for the synthesis of sugar-modified nucleosides. Org. Biol. Chem. 2017;15:9226–9233. doi: 10.1039/C7OB02184D. [DOI] [PubMed] [Google Scholar]
- 21.Borbás A. Photoinitiated Thiol-ene Reactions of Enoses: A Powerful Tool for Stereoselective Synthesis of Glycomimetics with Challenging Glycosidic Linkages. Chem. Eur. J. 2020;26:6090–6101. doi: 10.1002/chem.201905408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eszenyi D., Kelemen V., Balogh F., Bege M., Csávás M., Herczegh P., Borbás A. Promotion of a Reaction by Cooling: Stereoselective 1, 2-cis-α-Thioglycoconjugation by Thiol-Ene Coupling at −80 °C. Chem. Eur. J. 2018;24:4532–4536. doi: 10.1002/chem.201800668. [DOI] [PubMed] [Google Scholar]
- 23.Lázár L., Csávás M., Herczeg M., Herczegh P., Borbás A. Synthesis of S-linked glycoconjugates and S-disaccharides by thiol–ene coupling reaction of enoses. Org. Lett. 2012;14:4650–4653. doi: 10.1021/ol302098u. [DOI] [PubMed] [Google Scholar]
- 24.Kelemen V., Bege M., Eszenyi D., Debreczeni N., Bényei A., Stürzer T., Herczegh P., Borbás A. Stereoselective Thioconjugation by Photoinduced Thiol-ene Coupling Reactions of Hexo-and Pentopyranosyl D-and L-Glycals at Low-Temperature—Reactivity and Stereoselectivity Study. Chem. Eur. J. 2019;25:14477. doi: 10.1002/chem.201903859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beniazza R., Atkinson R., Absalon C., Castet F., Denisov S.A., McClenaghan N.D., Lastécouères D., Vincent J.M. Benzophenone vs. Copper/Benzophenone in Light-Promoted Atom Transfer Radical Additions (ATRAs): Highly Effective Iodoperfluoroalkylation of Alkenes/Alkynes and Mechanistic Studies. Adv. Synth. Cat. 2016;358:2949–2961. doi: 10.1002/adsc.201600501. [DOI] [Google Scholar]
- 26.Bereczki I., Magdolna Csávás D., Szűcs Z., Rőth E., Batta G., Ostorházi E., Naesens L., Borbás A., Herczegh P. Synthesis of antiviral perfluoroalkyl derivatives of teicoplanin and vancomycin. ChemMedChem. 2020;15:1661. doi: 10.1002/cmdc.202000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffries B., Wang Z., Felstead H.R., Le Questel J.-Y., Scott J.S., Chiarparin E., Graton J., Linclau B. Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs. J. Med. Chem. 2020;63:1002–1031. doi: 10.1021/acs.jmedchem.9b01172. [DOI] [PubMed] [Google Scholar]
- 28.Bacqué E., Pautrat F., Zard S.Z. A flexible strategy for the divergent modification of pleuromutilin. Chem. Comm. 2002;20:2312–2313. doi: 10.1039/B206568A. [DOI] [PubMed] [Google Scholar]
- 29.EUCAST. 2021. [(accessed on 18 November 2021)]. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_3.0_2021.pdf.
- 30.Sinha A.K., Equbal D. Thiol−Ene Reaction: Synthetic Aspects and Mechanistic Studies of an Anti-Markovnikov-Selective Hydrothiolation of Olefins. Asian J. Org. Chem. 2019;8:32–47. doi: 10.1002/ajoc.201800639. [DOI] [Google Scholar]
- 31.Csávás M., Eszenyi D., Mező E., Lázár L., Debreczeni N., Tóth M., Somsák L., Borbás A. Stereoselective Synthesis of Carbon-Sulfur-Bridged Glycomimetics by Photoinitiated Thiol-Ene Coupling Reactions. Int. J. Mol. Sci. 2020;21:573. doi: 10.3390/ijms21020573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bege M., Bereczki I., Molnár D.J., Kicsák M., Pénzes-Daku K., Bereczky Z., Ferenc G., Kovács L., Herczegh P., Borbás A. Synthesis and oligomerization of cysteinyl nucleosides. Org. Biomol. Chem. 2020;18:8161–8178. doi: 10.1039/D0OB01890B. [DOI] [PubMed] [Google Scholar]
- 33.Kelemen V., Csávás M., Hotzi J., Herczeg M., Rathi B., Herczegh P., Jain N., Borbás A. Photoinitiated Thiol− Ene Reactions of Various 2, 3-Unsaturated O-, C-, S- and N-Glycosides–Scope and Limitations Study. Chem. Asian J. 2020;15:876–891. doi: 10.1002/asia.201901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCourt R.O., Scanlan E.M. A Sequential Acyl Thiol–Ene and Thiolactonization Approach for the Synthesis of δ-Thiolactones. Org. Lett. 2019;21:3460–3464. doi: 10.1021/acs.orglett.9b01271. [DOI] [PubMed] [Google Scholar]
- 35.Arceo E., Montroni E., Melchiorre P. Photo-Organocatalysis of Atom-Transfer Radical Additions to Alkenes. Angew. Chem. Int. Ed. 2014;53:12064–12068. doi: 10.1002/anie.201406450. [DOI] [PubMed] [Google Scholar]
- 36.Dalhoff A. Selective toxicity of antibacterial agents—Still a valid concept or do we miss chances and ignore risks? Infection. 2021;49:29–56. doi: 10.1007/s15010-020-01536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and Supplementary Materials.