Abstract
The aim of our study was to assess the effect of 8 weeks of pulmonary rehabilitation (PR) in patients with pulmonary embolism (PE) during unsupervised PR (unSPRgroup) versus supervised PR (SPRgroup) on cardiopulmonary exercise testing (CPET) parameters, sleep quality, quality of life and cardiac biomarkers (NT-pro-BNP). Fourteen patients with PE (unSPRgroup, n = 7, vs. SPRgroup, n = 7) were included in our study (age, 50.7 ± 15.1 years; BMI, 30.0 ± 3.3 kg/m2). We recorded anthropometric characteristics and questionnaires (Quality of life (SF-36) and Pittsburg sleep quality index (PSQI)), we performed blood sampling for NT-pro-BNP measurement and underwent CPET until exhausting before and after the PR program. All patients were subjected to transthoracic echocardiography prior to PR. The SPRgroup differed in mean arterial pressure at rest before and after the PR program (87.6 ± 3.3 vs. 95.0 ± 5.5, respectively, p = 0.010). Patients showed increased levels of leg fatigue (rated after CPET) before and after PR (p = 0.043 for SPRgroup, p = 0.047 for unSPRgroup) while the two groups differed between each other (p = 0.006 for post PR score). Both groups showed increased levels in SF-36 scores (general health; p = 0.032 for SPRgroup, p = 0.010 for unSPRgroup; physical health; p = 0.009 for SPRgroup, p = 0.022 for unSPRgroup) and reduced levels in PSQI (cannot get to sleep within 30-min; p = 0.046 for SPRgroup, p = 0.007 for unSPRgroup; keep up enough enthusiasm to get things done; p = 0.005 for SPRgroup, p = 0.010 for unSPRgroup) following the PR program. The ΝT-pro-BNP was not significantly different before and after PR or between groups. PR may present a safe intervention in patients with PE. The PR results are similar in SPRgroup and unSPRgroup.
Keywords: exercise, pulmonary embolism, pulmonary rehabilitation
1. Introduction
Pulmonary embolism (PE) is an acute and potentially fatal condition in which embolic material, usually a thrombus originating from the deep veins of the legs, blocks the pulmonary circulation resulting in impaired blood flow that may lead to right ventricle dysfunction [1]. PE and deep vein thrombosis are considered to be two manifestations of venous thromboembolism (VTE), which represents the third most common cardiovascular disorder in industrialized countries [2]. PE is difficult to diagnose due to lack of specificity of symptoms and clinical presentation [3]. Patients with history of PE often exhibit functional limitations and decreased quality of life even years after the episode, a condition that is considered as a long-term complication of acute PE and termed “post-PE-syndrome” or “Chronic Thromboembolic Disease” [4].
PE in the setting of COVID-19 is a common complication, frequent in hospitalized patients [5], and is associated with its severity [6]. On the pathophysiological level, the relationship between PE and COVID-19 is bidirectional. Hypercoagulable states and endothelial injury may be induced via virus–host interactions, while subsequent PE may account for persistent hypoxia following the resolution of the acute syndrome [7]. The incidence of PE following COVID-19 varies according to the population studied, the severity of COVID, the thromboprophylaxis dose, the screening protocol for VE, etc. According to a recent meta-analysis, the overall incidence of PE in COVID-19 inpatients is approximately 17%, with increased incidence in patients admitted to ICU (27.9%) versus those hospitalized in wards (7.1%) [8].
The “post-PE-syndrome” is characterized by suboptimal cardiac function but not pulmonary hypertension, altered pulmonary artery flow dynamics, and impaired oxygenation at rest or at exercise, associated with symptoms such as dyspnea, reduced exercise tolerance, or worsening of quality of life, that cannot be explained otherwise [4]. The pathophysiology of the syndrome is poorly understood while its treatment is not specified to measures other than anticoagulation and supportive care. Recent guidelines have outlined that an efficient follow-up strategy after PE should include exercise rehabilitation, although studies addressing the effect of pulmonary rehabilitation programs in these patients are lacking [9]. Pulmonary rehabilitation (PR) includes a supervised program of exercise training and breathing techniques that also addresses issues of health education. PR represents a safe and effective intervention which improves health indicators and quality of life of patients with certain lung diseases such as chronic obstructive pulmonary disease or lung involvement due to other conditions [10]. European Respiratory Society Council and Executive Committee [10] underlies the need to establish specialized rehabilitation programs in order to enhance patient accessibility to this treatment intervention. According to World Health Organization [11], the health indicators related to metabolic profile, physical activity [12], and aerobic and anaerobic capacity are assessed within the cardio-pulmonary exercise testing (CPET). Briefly, CPET is a non-invasive measurement which provides an objective quantitative assessment of metabolic, pulmonary and cardiovascular responses during exercise [13]. Several biomarkers for PE diagnosis, risk stratification and/or risk of recurrence exist but most of them require further validation before being applied in clinical practice. Cardiac troponin T, N-terminal-pro hormone BNP (NT-pro-BNP) and heart-type fatty-acid binding protein, are markers of myocardial strain and injury, which have prognostic value in risk assessment strategies [4].
There is paucity of data concerning the possible role of PR programs in patients with PE. This lack of data extends to unsupervised PR (unSPRgroup), which represents a telemedicine approach that has gained impetus during the COVID-19 pandemic [14]. Telemedicine approaches, including virtual reality applications, have had previous successful implementations in the setting of pulmonary disease rehabilitation. [15]. Home-based unsupervised rehabilitation has been shown to be an effective alternative to formal regimens during the pandemic, ensuring that patients rehabilitation milestones remain on-track following hospitalization [16]. A study from our group has indicated that the efficacy of unsupervised PR in COVID-19 is tangible, and associated with improvements in redox homeostasis, sleep health and anthropometric indices [17]. Considering the overlap between COVID-19 and PE, these studies further demonstrate the rationale and relevance of unsupervised PR in PE.
Currently, there is no study prospectively addressing the efficacy and safety of PR in exercise limitation and quality of life following an episode of PE, despite current guide-lines that suggest that exercise rehabilitation is part of the follow up of these patient group [8]. The effectiveness of different programs of physical activity is not well established but some studies suggest that supervised versus self-selected programs might have similar results [18]. The types of exercise programs in patients with PE have not been addressed in the literature, to this end, we designed this study in order to investigate the effect of 8 weeks of PR in patients with history of an acute episode of PE. Additionally, we aimed to address the results of PR in exercise limitation and quality of life and examine possible differences among patients subjected to supervised versus unsupervised exercise.
2. Materials and Methods
2.1. Study Population
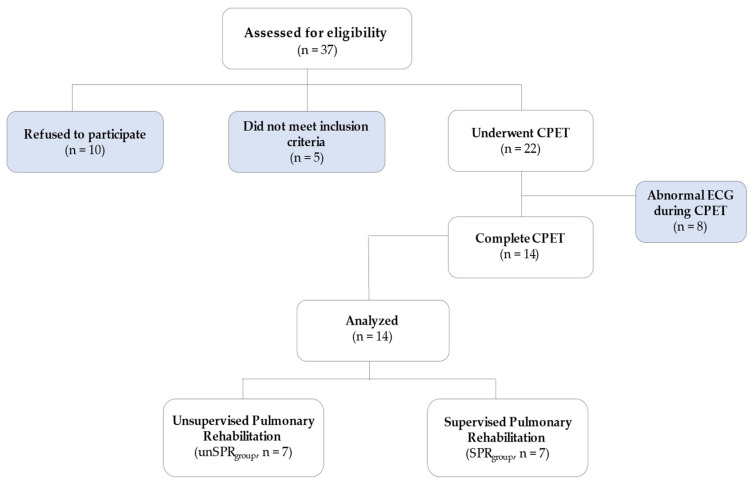
The present research is a pilot study. Patients with a history of PE were prospectively recruited from the PE outpatient clinic (Figure 1) between January 2017 to December 2018. The patients were randomly divided into two groups (using block randomization): unsupervised PR during telerehabilitation (unSPRgroup) and supervised PR (SPRgroup) in Pulmonary Rehabilitation Center (University of Thessaly). Some of these patients were present in a previous study and belong to the Proceedings of the 9th Conference of Biochemistry and Physiology of Exercise [19]. We included patients with PE diagnosis >6 months prior to enrollment and weekly exercise ≤100-min. Exclusion criteria included contraindications to the performance of CPET (i.e., recent acute myocardial infarction (3–5 days), unstable angina, uncontrolled arrhythmia causing hemodynamic instability, acute endocarditis, acute myocarditis or pericarditis, uncontrolled heart failure, lower extremity thrombosis, pregnancy, presence of severe comorbidity that may interfere with the results of the rehabilitation, i.e., COPD). The study was approved by the Institutional Ethics Committee of the corresponding institution. Verbal and written informed consent were obtained from all participants (No. of Ethical Committee: N° 2800, Scientific Council of University Hospital of Larissa).
Figure 1.
Study flow diagram.
2.2. Procedures
For all patients, we recorded the demographics and characteristics of PE episodes and all subjects underwent echocardiography. Prior to CPET, all participants answered the Pittsburgh Sleep Quality Index (PSQI) [20] and Quality of Life (SF-36) [21] questionnaires while we recorded anthropometric characteristics [22,23,24], pulmonary function parameters (FEV1: forced expiratory volume in 1st sec, FVC: forced vital capacity; Master Screen-CPX, VIASYS HealthCare, Hochberg, Germany) [25]. Blood sampling for NT-pro-BNP measurement was performed 30 min before CPET. BNP measurements were performed in complete blood samples with a commercial analyzer (Triage BNP test; BI-OSITE, San Diego, CA, USA). The same procedure was repeated after 8 weeks.
2.3. Echocardiography
All patients underwent echocardiographic study within 48 h of the CPET and PR program initiation. Two-dimensional echocardiography was performed, with the subjects resting in a left lateral decubitus position, using a Vivid BT08 (General Electric, Miami, FL, USA). Heart images were obtained in the standard parasternal long-axis and short-axis and apical four-and-two chamber planes. Wall thickness was measured from 2D short-axis views at end-diastole, with the greatest measurement within the left ventricular wall defined as the maximal wall thickness. M-mode echocardiograms derived from 2D images in the parasternal long axis were used for the measurement of end-diastolic and systolic dimensions according to the American Society of Echocardiography [26].
2.4. Cardiopulmonary Exercise Testing
CPET was performed on an electronic cycle ergometer (Ergoselect 100, Bitz, Germany) Master Screen-CPX and respiratory and cardiac parameters were recorded (VIASYS HealthCare, Höchberg, Germany). All patients, prior to testing, were familiarized with the test via a 2 min resting stage (1st stage); for a 3 min unloaded cycling as a warm-up (2nd stage); then, after the end of the maximal test (3rd stage), they performed a 5 min unloaded cycling for recovery (4th stage) purposes. In the 3rd stage the ramp work rate increased by 10–15 watts/min until exhaustion was reached. The work rate increment calculated using the Wasserman et al. [27] formula:
| Work Rate/min (ramp) = (VO2max − VO2unloaded)/100 |
| VO2max = (Height (cm) − Age (years)) × 20 (male) or × 14 (female) |
| VO2unloaded = 150 + (6 × Weight (kg)) |
During testing all patients were instructed to keep a steady speed of 60 ± 5 rpm throughout the four phases of testing. Each trial was terminated when the participant reached symptom-limited maximum exercise, which was confirmed by the presence of respiratory exchange ratio (RER) > 1.10, Heart Rate (HR) ≥ 80% of predicted HRmax, and/or plateau of oxygen consumption with increasing work load. Moreover, a 12-lead electrocardiogram (ECG) was also employed for HR monitoring, while a pulse oxymeter (MasterScreen, Höchberg, Germany) informed about oxygen saturation (SpO2). Blood pressure (cuff manometry, Mac, Japan) and Borg CR10 Scales (Leg Fatigue, Dyspnea) point were recorded every 2 min for all phases.
2.5. Pulmonary Rehabilitation Program
The PR program lasted 8 weeks with three sessions per week. The duration of each training session was about 70 min. All sessions were instructed to conduct the PR program either outdoors (walking) or in home (stretching, strength and breathing exercise) without supervision for patients of the unSPRgroup. Patients in the SPRgroup performed the PR program in the Laboratory of Pulmonary Rehabilitation of the University Hospital of Larissa. Each training session included a warm-up (unSPRgroup and SPRgroup: 5 min stretching exercises), the first main set (unSPRgroup: 40 min walking at 60% of VO2 calculated from heart rate vs. SPRgroup: 40 min intermittent exercise in cycle ergometer (30 s exercise at 70% of VO2max and 30 s resting)), the second main set of training (un-SPRgroup: 10 min (tele)breathing physiotherapy and 10 min multi-joint strength exercises vs. SPRgroup: 10 min respiratory physiotherapy and 10 min multi-joint strength exercises) and a recovery set (unSPRgroup and SPRgroup: 5 min stretching exercises). The set of exercises was analogue for both groups. Minor differences in the two PR programs exist in order to increase safety for injuries. The unSPRgroup performed exercise-PR unsupervised but according to instructions of a clinical exercise physiologist (VTS) that supervised the SPRgroup.
Adherence to the program of unSPRgroup was determined via phone calls per week. Each call focused on whether the patients were able to follow the instructions and perform them, troubleshooting and reporting the physiological parameters.
2.6. Statistical Analysis
The Kolmogorov–Smirnov test was utilized to assess normality of distribution of values. A comparison of one group of individuals against themselves (pre-and-post the PR) was performed with the Wilcoxon signed-rank test according to variable distribution. A comparison between the two patient groups was performed with the use Mann–Whitney U-test according to variable distribution. Data are presented as absolute numbers, percentages or mean values and standard deviation (mean ± SD). For all the statistical analyses the statistical package SPSS 15 (SPSS Inc., Chicago, IL, USA) was used. The level of significance was set at p < 0.05.
3. Results
Out of the 37 individuals who were assessed for eligibility, 14 were included in the study (Figure 1). Table 1 and Table 2 presents demographical, clinical and echocardiography results of the study subjects. Briefly, the SPRgroup vs. the unSPRgroup did not differ significantly in terms of age (49.6 ± 15.4 vs. 51.9 ± 16.0, respectively), gender distribution (males 85.71% vs. 71.42%, respectively) and smoking status (smokers: 28.6% vs. 42.9%, respectively). Similarly, SPRgroup vs. the unSPRgroup had similar BMI (29.8 ± 3.9 vs. 30.1 ± 2.9, kg/m2, respectively), baseline physical activity (61.7 ± 24.7 vs. 70.0 ± 14.1, respectively) (Table 1). The two groups did not differ at baseline in terms of cardiovascular comorbidities, blood pressure measurements and spirometry results (Table 1). Mean Pulmonary Severity Index (PESI) score of the study participants was 52.48 ± 48.23. All patients were hospitalized during the acute period. Mean PESI score was not significantly different between SPRgroup and unSPRgroup (103.25 ± 50.90 vs. 100.33 ± 49.13, p > 0.05). Ejection fraction was within the normal range in both SPRgroup and unSPRgroup (59.2 ± 2.0 vs. 59.4 ± 1.3, respectively) (Table 2). Echocardiographic signs of right heart dysfunction (i.e., end-diastolic right ventricular diameter in four chambers view, right ventricular systolic pressure, right atrial area, tricuspid annular plane systolic excursion) were not significantly different between the two study groups (Table 2).
Table 1.
Demographical, clinical and spirometry results of the study population.
| SPRgroup | UnSPRgroup | p Value | |
|---|---|---|---|
| Age, years | 49.6 ± 15.4 | 51.9 ± 16.0 | 0.790 |
| Gender, M/F | 6/1 | 5/2 | 0.552 |
| Smokers, % | 28.6 | 42.9 | 0.611 |
| Body mass index, kg/m2 | 29.8 ± 3.9 | 30.1 ± 2.9 | 0.884 |
| Body surface area, m2 | 2.0 ± 0.5 | 2.2 ± 0.3 | 0.537 |
| Lean body mass, % | 60.9 ± 9.0 | 63.6 ± 6.3 | 0.531 |
| Total body water, L | 44.2 ± 8.9 | 44.7 ± 7.0 | 0.904 |
| Alcohol drinking, ml/month | 85.0 ± 13.7 | 83.3 ± 14.4 | 0.875 |
| Physical Activity, min/week | 61.7 ± 24.7 | 70.0 ± 14.1 | 0.703 |
| Systolic blood pressure, mmHg | 113.6 ± 13.8 | 117.1 ± 9.9 | 0.588 |
| Diastolic blood pressure, mmHg | 71.4 ± 6.3 | 72.9 ± 2.7 | 0.589 |
| Prevalence of CVD and diabetes, % | 42.9 | 57.1 | 0.626 |
| Prior VTE event, Y/N | 1/6 | 1/6 | 1.000 |
| Provoked event, Y/N | 3/4 | 6/1 | 0.109 |
| Under anticoagulant therapy, Y/N | 6/1 | 4/3 | 0.271 |
| MRC dyspnea scale, 0/I/II | 3/4/0 | 2/4/1 | 0.690 |
| FEV1, % of predicted | 101.0 ± 8.0 | 96.9 ± 10.2 | 0.434 |
| FVC, % of predicted | 96.8 ± 7.7 | 90.9 ± 11.6 | 0.280 |
Abbreviations: CDV, cardiovascular disease; FEV1, forced expiratory volume in 1st sec; FVC, forced vital capacity; M/F, male/female; VTE, venous thromboembolism event; Y/N, yes/no.
Table 2.
Echocardiographic characteristics of the study population.
| SPRgroup | UnSPRgroup | p Value | |
|---|---|---|---|
| Ejection fraction, % | 59.2 ± 2.0 | 59.4 ± 1.3 | 0.832 |
| End-diastolic RV diameter (4CH), cm | 3.6 ± 0.1 | 3.6 ± 0.5 | 0.763 |
| RVSP, mmHg | 23.3 ± 4.1 | 24.2 ± 5.8 | 0.777 |
| RA area, cm2 | 15.3 ± 2.5 | 15.2 ± 3.1 | 0.952 |
| TAPSE, mm | 20.5 ± 7.8 | 22.3 ± 4.3 | 0.626 |
Abbreviations: RA, right atrial; RV, right ventricle; RVS, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Respiratory parameters and CPET results, before and after the PR for both groups, are presented in Table 3. The SPRgroup differed in mean arterial pressure (MAP) at rest before and after the PR program (87.6 ± 3.3 vs. 95.0 ± 5.5, respectively, p = 0.010). MAP levels did not differ significantly before and after the PR program in the unSPRgroup (85.5 ± 8.5 vs. 88.6 ± 9.2, respectively). We observed an increasing trend in PETO2 in the SPRgroup after the PR program vs. at baseline that did not reach statistical significance (115.0 ± 7.0 vs. 108.7 ± 3.9, respectively, p = 0.059) (Table 3). Patients showed differences in leg fatigue before and after PR (SPRgroup: 2.6 ± 1.4 vs. 3.6 ± 1.3, respectively, p = 0.043 and unSPRgroup: 1.1 ± 0.7 vs. 1.7 ± 0.8, respectively, p = 0.047). Leg fatigue following the PR program was significantly higher in SPRgroup when compared to unSPRgroup (3.6 ± 1.3 vs. 1.7 ± 0.8, respectively, p = 0.006).
Table 3.
Pulmonary function parameters and cardiopulmonary exercise testing results between groups before and after the pulmonary rehabilitation program. Continuous variables are presented as mean ± standard deviation.
| SPRgroup | UnSPRgroup | p Value between Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-PR | p Value | Baseline | Post-PR | p Value | PRpre | PRpost | |
| Resting | ||||||||
| VO2, mL/min | 330.6 ± 91.6 | 349.0 ± 120.8 | 0.735 | 336.3 ± 84.5 | 312.0 ± 53.9 | 0.533 | 0.905 | 0.473 |
| VCO2, mL/min | 257.1 ± 67.1 | 240.6 ± 101.8 | 0.095 | 267.9 ± 102.7 | 236.1 ± 32.9 | 0.452 | 0.821 | 0.095 |
| PETCO2, mmHg | 29.9 ± 3.7 | 29.5 ± 2.4 | 0.832 | 27.2 ± 4.1 | 27.1 ± 3.6 | 0.952 | 0.227 | 0.159 |
| PETO2, mmHg | 110.0 ± 5.1 | 114.0 ± 4.2 | 0.132 | 113.8 ± 7.7 | 112.5 ± 5.4 | 0.736 | 0.305 | 0.568 |
| HR, bpm | 81.0 ± 18.2 | 82.6 ± 18.2 | 0.875 | 78.7 ± 10.1 | 73.9 ± 9.6 | 0.374 | 0.776 | 0.285 |
| MAP, mmHg | 87.6 ± 3.3 | 95.0 ± 5.5 | 0.010 | 85.5 ± 8.5 | 88.6 ± 9.2 | 0.516 | 0.549 | 0.133 |
| Maximal effort | ||||||||
| VO2, mL/min | 1559.1 ± 372.8 | 1579.3 ± 430.7 | 0.927 | 1946.0 ± 640.2 | 1896.1 ± 390.0 | 0.863 | 0.192 | 0.175 |
| VCO2, mL/min | 1536.6 ± 440.8 | 1525.5 ± 497.6 | 0.966 | 1954.1 ± 672.1 | 1791.0 ± 453.9 | 0.604 | 0.194 | 0.318 |
| PETCO2, mmHg | 37.5 ± 4.1 | 36.7 ± 3.5 | 0.710. | 35.5 ± 4.6 | 35.6 ± 4.4 | 0.970 | 0.416 | 0.615 |
| PETO2, mmHg | 108.7 ± 3.9 | 115.0 ± 7.0 | 0.059 | 112.8 ± 3.8 | 115.9 ± 4.8 | 0.199 | 0.079 | 0.788 |
| VE/MVV, % | 44.1 ± 10.8 | 48.1 ± 10.5 | 0.494 | 53.3 ± 6.3 | 53.5 ± 12.7 | 0.977 | 0.076 | 0.408 |
| VE/VCO2 | 28.6 ± 3.0 | 27.8 ± 3.0 | 0.279 | 28.5 ± 2.5 | 28.2 ± 3.3 | 0.786 | 0.357 | 0.679 |
| HR, bpm | 133.3 ± 18.6 | 133.0 ± 14.2 | 0.975 | 149.0 ± 13.3 | 138.4 ± 12.9 | 0.158 | 0.094 | 0.470 |
| MAP, mmHg | 119.5 ± 18.8 | 117.2 ± 10.6 | 0.786 | 123.3 ± 12.5 | 121.4 ± 9.4 | 0.757 | 0.660 | 0.438 |
| Leg fatigue, Borg Scale | 2.6 ± 1.4 | 3.6 ± 1.3 | 0.043 | 1.1 ± 0.7 | 1.7 ± 0.8 | 0.047 | 0.062 | 0.006 |
| Dyspnea, Borg Scale | 1.4 ± 1.0 | 2.0 ± 1.2 | 0.337 | 0.9 ± 1.1 | 1.3 ± 0.8 | 0.403 | 0.317 | 0.196 |
Abbreviations: HR, heart rate; MAP, mean arterial pressure; MVV, maximum voluntary volume; PETCO2, end-tidal carbon dioxide pressure; PETO2, end-tidal oxygen pressure; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
Both patient groups showed statistically significant differences before and after PR in the Quality of Life and Sleep Quality questionnaires. At baseline no differences were observed in both groups for all the subscales of SF-36 (Table 4). After the PR program, we observed that both groups had a higher score in the SF-36 parameters “physical health” and “general health” versus to their baseline values. In more detail, “physical health” increased significantly after the PR program when compared to baseline in the SPRgroup (92.9 ± 8.1 vs. 71.1 ± 10.7, respectively, p = 0.009) and the unSPRgroup (93.6 ± 6.9 vs. 76.4 ± 15.7, respectively, p = 0.022). “General health” score increased following PR vs. before in the SPRgroup (81.4 ± 16.8 vs. 63.6 ± 9.9, respectively, p = 0.032) and the unSPRgroup (72.1 ± 8.6 vs. 51.4 ± 15.7, respectively, p = 0.010). The others parameters of SF-36 were not different before and after PR period (Table 4).
Table 4.
Quality of life and sleep quality results between groups before and after pulmonary rehabilitation. Continuous variables are presented as mean ± standard deviation.
| SPRgroup | UnSPRgroup | p Value between Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-PR | p Value | Baseline | Post-PR | p Value | Baseline | Post-PR | ||
| Quality of life (SF-36) | Physical Health | 71.1 ± 10.7 | 92.9 ± 8.1 | 0.009 | 76.4 ± 15.7 | 93.6 ± 6.9 | 0.022 | 0.923 | 0.862 |
| Physical Functioning | 78.6 ± 26.7 | 92.9 ± 18.9 | 0.271 | 75.0 ± 25.0 | 76.4 ± 25.3 | 0.917 | 0.801 | 0.194 | |
| Body Pain | 82.5 ± 18.0 | 87.5 ± 13.4 | 0.567 | 80.0 ± 17.0 | 83.9 ± 13.8 | 0.643 | 0.794 | 0.631 | |
| General Health | 63.6 ± 9.9 | 81.4 ± 16.8 | 0.032 | 51.4 ± 15.7 | 72.1 ± 8.6 | 0.010 | 0.109 | 0.217 | |
| Vitality | 67.9 ± 16.0 | 77.1 ± 13.2 | 0.260 | 60.7 ± 13.7 | 68.6 ± 8.0 | 0.214 | 0.387 | 0.167 | |
| Social Role Functioning | 75.0 ± 20.4 | 92.9 ± 12.2 | 0.070 | 85.7 ± 18.3 | 85.4 ± 14.1 | 0.968 | 0.321 | 0.308 | |
| Emotional Role Functioning | 85.7 ± 26.2 | 98.6 ± 2.4 | 0.175 | 85.7 ± 26.2 | 90.5 ± 16.2 | 0.690 | 0.989 | 0.147 | |
| Mental Health | 73.6 ± 24.0 | 78.3 ± 19.6 | 0.635 | 77.1 ± 17.5 | 81.1 ± 12.2 | 0.629 | 0.692 | 0.749 | |
| Sleep quality (PSQI) | Cannot get to sleep within 30 min | 2.9 ± 0.4 | 1.8 ± 0.5 | 0.046 | 2.6 ± 0.5 | 2.0 ± 0.1 | 0.007 | 0.779 | 0.025 |
| Wake up in the middle of the night or early morning | 1.4 ± 0.3 | 1.4 ± 0.4 | 0.968 | 1.3 ± 0.1 | 1.3 ± 0.2 | 0.878 | 0.613 | 0.694 | |
| Have to get up to use the bathroom | 1.1 ± 0.1 | 1.0 ± 0.5 | 0.317 | 1.1 ± 0.4 | 0.9 ± 0.7 | 0.986 | 0.955 | 0.613 | |
| Cannot breathe comfortably | 1.2 ± 0.3 | 1.1 ± 0.4 | 0.867 | 1.1 ± 0.3 | 1.1 ± 0.6 | 0.831 | 0.986 | 0.978 | |
| Cough or snore loudly | 1.0 ± 0.2 | 0.9 ± 0.6 | 0.317 | 0.9 ± 0.3 | 0.8 ± 0.4 | 0.326 | 0.694 | 0.281 | |
| Feel too cold | 1.1 ± 0.3 | 1.0 ± 0.6 | 0.325 | 1.0 ± 0.4 | 0.9 ± 0.7 | 0.679 | 0.732 | 0.796 | |
| Feel too hot | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.371 | 1.1 ± 0.2 | 0.9 ± 0.3 | 0.317 | 0.698 | 0.789 | |
| Had bad dreams | 1.2 ± 0.2 | 1.1 ± 0.1 | 0.157 | 1.1 ± 0.3 | 1.0 ± 0.2 | 0.175 | 0.121 | 0.779 | |
| Have pain | 0.8 ± 0.1 | 0.7 ± 0.4 | 0.152 | 0.8 ± 0.2 | 0.6 ± 0.8 | 0.336 | 0.956 | 0.232 | |
| …taken medicine to help you sleep | - | - | / | - | - | / | / | / | |
| …trouble staying awake (driving, eating meals, or social activity) | 0.6 ± 0.3 | 0.6 ± 0.5 | 0.317 | 0.5 ± 0.1 | 0.5 ± 0.6 | 0.307 | 0.463 | 0.397 | |
| …keep up enough enthusiasm to get things done? | 1.9 ± 0.4 | 1.0 ± 0.2 | 0.005 | 2.5 ± 0.5 | 1.3 ± 0.4 | 0.010 | 0.779 | 0.029 | |
| …sleep quality overall | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.157 | 0.2 ± 0.2 | 0.1 ± 0.4 | 0.317 | 0.294 | 0.281 | |
Sleep quality as assessed by PSQI showed differences in both groups before and after PR in parameter “cannot get to sleep within 30 min”, (Table 4) and “keep up enough enthusiasm to get things done” (Table 4). The PSQI score decreased after PR vs. at baseline (unSPRgroup: 5.7 ± 1.4 vs. 3.9 ± 1.8, respectively, p = 0.035; SPRgroup: 6.6 ± 1.8 vs. 4.1 ± 1.8, respectively, p = 0.026) compared to the period before PR. The other parameters of PSQI were not different before and after PR period but presented an increasing trend in both groups that did not reach statistical significance.
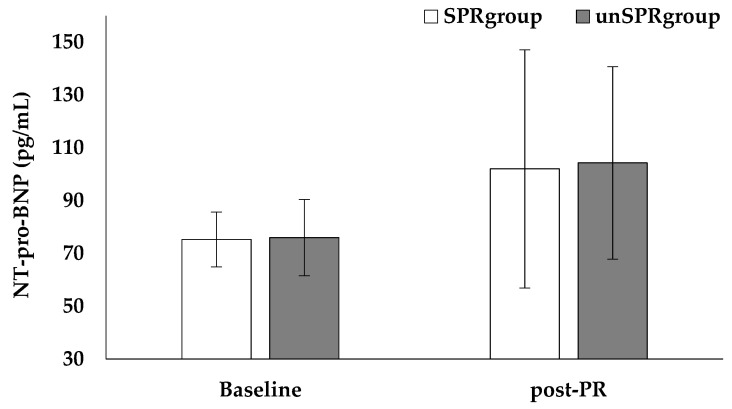
The ΝT-pro-BNP levels were not significantly different before and after PR (SPRgroup: 75.3 ± 10.4 vs. 102.0 ± 45.1 pg/mL, respectively, p = 0.147; unSPRgroup: 76.0 ± 14.4 vs. 104.3 ± 36.5 pg/mL, respectively, p = 0.116, Figure 2) or between groups (Baseline, SPRgroup: 75.3 ± 10.4 vs. unSPRgroup: 76.0 ± 14.4, pg/mL, p = 0.917; post-PR, SPRgroup: 102.0 ± 45.1 vs. unSPRgroup: 104.3 ± 36.5, pg/mL, p = 0.919, Figure 2).
Figure 2.
NT-pro-BNP results between groups before and after pulmonary rehabilitation. The bars correspond to the mean value and the outliers correspond to standard deviation.
4. Discussion
The aim of this pilot study was to investigate the effect of 8 weeks of PR in patients with PE. Patients underwent PR program either by SPRgroup in a Pulmonary Rehabilitation Center or by unSPRgroup during telerehabilitation and groups were compared to each other. We observed differences in MAP at rest before and after the PR program in the SPRgroup while both groups showed differences in leg fatigue before and after PR as well as compared to each other. Importantly, the two group of patients showed differences in parameters of quality of life and sleep quality before and after the PR. We did not observe major differences when SPRgroup was compared to unSPRgroup with the exception of reduced leg fatigue reported by the SPRgroup and the parameter “keep up enough enthusiasm to get things done” in PSQI which was in favor of the unSPRgroup. However, due to the small number of patients included, no definite conclusions can be drawn concerning differences in the effectiveness of each approach.
4.1. Cardiopulmonary Exercise Testing
CPET provides an objective and quantitative measure of metabolic, pulmonary and cardiovascular responses during exercise which is both non-invasive and safe and can serve as an independent predictor of long-term outcomes [9]. Previous studies have documented reductions in VO2max at various single time points following the PE event [28]. Kahn et al. [29] observed persistent exercise limitations 1-year after PE. Our results showed significant differences in MAP (at rest) in the SPRgroup before and after PR while both groups showed differences in leg fatigue pre-and-post PR. Additionally, we demonstrated differences in leg fatigue between groups (SPRgroup versus unSPRgroup). CPET may be limited by leg fatigue that may underlie muscle weakness and leg effort [30]. Most patients tolerate only minimal leg discomfort before stopping the measurement while average healthy individuals may tolerate a greater degree of discomfort, suggesting the subjectiveness of the intensity of leg effort [30]. Leg fatigue may be associated with muscle metabolism impairment and probably increased peripheral oxygen uptake [31]. These findings are of significant clinical relevance and represent the standard of care for the management of patients with cardiovascular diseases such as pulmonary hypertension [32]. According to Kwan et al. [33], the use of exercise training programs of cardiac rehabilitation may benefit patients with a history of acute PE in a manner similar to that of patients suffering acute coronary syndromes.
4.2. Quality of Life and Psychological Aspects
Previous studies have shown that PR may have beneficial effects in patients with cardiovascular diseases, in terms of improved functional capacity and quality of life [34]. Quality of life has become an important outcome aspect of medical care. Patients with PE may have reduced chronic functional capacity for many years after the event and that may be the main determinant of impaired quality of life [34]. Our results showed difference before and after PR in quality of life, as assessed by the SF-36 questionnaire, in parameters such as “general health” and “physical health”. Reduced functional capacity may relate to persistent dyspnea, while physical activity and exertion were the most common behavior changes in patients. In our study, patients with PE during rehabilitation performed combination exercise and respiratory physiotherapy. This combination may relate to behavior change in patients following PR. Although not statistically significant, we observed a trend for improvement in exercise capacity which may be attributed to improved muscle function and desensitization to dyspnea. Desensitization to dyspnea is often considered a mechanism to explain benefit in the rehabilitation of patients with respiratory diseases and these altered perceptions of dyspnea even without associated physiological changes [34]. It should be noted that the interventions via PR/uns-PR also affect several psychological components. PR for chronic lung diseases has been shown to reduce anxiety and depression in these patients [35]. Telemedicine approaches, either as simple as a follow-up via phone [36] or as intricate as virtual reality [15] have also been shown to confer the same beneficial effect in both anxiety and depression experienced by these patients. A limitation in our study, however, is that we did not specifically assess these parameters, and therefore cannot comment on how rehabilitation may have affected them in this patient population.
4.3. Sleep Quality
Sleep disorder breathing and PE are a major health issue in industrialized countries. PE patients have a 2–4 times greater risk of suffering from moderate and severe obstructive sleep apnea syndrome (OSAS) [37]. Previous studies suggest that intermittent hypoxia and fragmentation of sleep may result in blood hypercoagulability, endothelial dysfunction and venous stasis. According to García-Ortega et al. [38], patients with acute PE have increased risk of coexisting moderate and severe OSAS when compared with controls. A polysomnography study showed a lower degree of oxygen saturation in PE patients and higher risk of PE in patients with isolated OSAS while the high hypoxic burden may be related to PE prevalence [39]. Patients with acute PE are at an increased risk of coexisting sleep disorders [38]. Our results showed a difference in sleep quality according to the PSQI questionnaire, in parameters such as “cannot get to sleep within 30-min” and “keep up enough enthusiasm to get things done” following the PR program. According to Stavrou et al. [40,41], the exercise, in patients with sleep disorders, may reduce the apnea-hypopnea index and improve the sleep quality while the daily physical activity may have a protective role in disease progression.
4.4. Implication of Rehabilitation Program
Data concerning rehabilitation after an acute episode of PE are sparse, while the literature lacks data concerning pulmonary rehabilitation program following PE recovery. Many patients have persistent symptoms months after an acute episode of PE and some of those suffer from post-PE syndrome, characterized by exercise limitation and suboptimal right heart function [4]. These patients may benefit the most from PR programs; however, data on this population are lacking. Rehabilitation program may be safe in the PE population in terms of death and serious events [42]. Exercise training after VTE showed differences in VO2max in previous studies with 3-month intervention duration [43]. To our knowledge, this is the first study addressing the effectiveness and feasibility of an unsupervised PR in patients with a PE. The results of our study may help to establish a specialized rehabilitation program with potential important benefits and provide data on the safety, which seems to be a valuable alternative during the pandemic, of exercise programs in pulmonary embolism patient. Moreover, PR could be a highly valuable tool for promoting exercise and symptom recovery following pulmonary embolism and a novel approach concerning the treatment of persistently symptomatic patients with PE may arise.
4.5. Biomarkers
Several biomarkers have been implicated in the diagnosis and risk stratification of PE. Systemic biomarkers of myocardial injury and ventricular dysfunction have been extensively used in everyday clinical practice for the initial risk assessment of patients with acute PE [8]. High levels of BNP may help in the identification of subjects with PE and higher risk of in-hospital complications and death [44]. Additionally, NT-pro-BNP has emerged as a potential biomarker of early diagnosis of Chronic Thromboembolic Pulmonary Hypertension [45]. However, little is known about NT-pro-BNP levels in patients following an acute PE episode, as well as its possible association with exercise limitation. We did not observe differences in NT-pro-BNP levels among patients before and after PR program. We acknowledge that the strength of our results is limited due to the small number of patients included and therefore we suggest that further studies are warranted in order to exert any definite conclusions.
5. Conclusions
In conclusion, pulmonary rehabilitation has beneficial effects on quality of life and sleep quality in patients with pulmonary embolism. It also shows an improving tendency of indicators related to physical capacity. There were no major differences between SPRgroup and unSPRgroup (except reduced leg fatigue in the SPRgroup and improvement in PSQI parameter “keep up enough enthusiasm to get things done” in the unSPRgroup). Uns-PR may be a feasible alternative to supervised regimens in the pandemic era, considering its relevance to PE and the increased prevalence of PE due to COVID-19. A larger trial is needed to extend these observations and provide evidences for the long-term effects of pulmonary rehabilitation and confirm the findings.
Acknowledgments
The authors appreciate the friendly cooperation of patients with pulmonary embolism.
Author Contributions
V.T.S. and F.M. conceived and designed the experiments; V.T.S., F.B. and E.K. performed the cardiopulmonary exercises testing; V.T.S. analyzed the findings of cardiopulmonary exercises testing; V.T.S. and M.G. performed the pulmonary rehabilitation program; V.T.S., F.B., D.G.R. and A.K. contributed to sample collection; V.T.S. and F.M. performed the experiments and analyzed the data; V.T.S. and M.G. performed the pulmonary rehabilitation programs; K.T. and F.T. performed the echocardiography examinations and analyzed the echocadiographic findings; V.T.S., G.D.V. and F.M. wrote the paper; V.T.S., K.I.G., F.M. and Z.D. edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (No. of Ethical Committee: N° 2800, Scientific Council of University Hospital of Larissa).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study and written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lavorini F., Di Bello V., De Rimini M.L., Lucignani G., Marconi L., Palareti G., Pesavento R., Prisco D., Santini M., Sverzellati N., et al. Diagnosis and treatment of pulmonary embolism: A multidisciplinary approach. Multidiscip. Respir. Med. 2013;8:75. doi: 10.1186/2049-6958-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douma R.A., Kamphuisen P., Büller H.R. Acute pulmonary embolism. Part 1: Epidemiology and diagnosis. Nat. Rev. Cardiol. 2010;7:585–596. doi: 10.1038/nrcardio.2010.106. [DOI] [PubMed] [Google Scholar]
- 3.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003;7:107. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 4.Klok F., van der Hulle T., Exter P.D., Lankeit M., Huisman M., Konstantinides S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28:221–226. doi: 10.1016/j.blre.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.García-Ortega A., Oscullo G., Calvillo P., López-Reyes R., Méndez R., Gómez-Olivas J.D., Bekki A., Fonfría C., Trilles-Olaso L., Zaldívar E., et al. Incidence, risk factors, and thrombotic load of pulmonary embolism in patients hospitalized for COVID-19 infection. J. Infect. 2021;82:261–269. doi: 10.1016/j.jinf.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roncon L., Zuin M., Barco S., Valerio L., Zuliani G., Zonzin P., Konstantinides S.V. Incidence of acute pulmonary embo-lism in COVID-19 patients: Systematic review and meta-analysis. Eur. J. Intern. Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poor H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest. 2021;160:1471–1480. doi: 10.1016/j.chest.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., Le Mao R., Rodriguez C., Hunt B.J., Monreal M., et al. Incidence of VTE and Bleeding among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., Huisman M.V., Humbert M., Jennings C.S., Jiménez D., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur. Heart J. 2019;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 10.Grünig E., Eichstaedt C., Barberà J.-A., Benjamin N., Blanco I., Bossone E., Cittadini A., Coghlan G., Corris P., D’Alto M., et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur. Respir. J. 2018;53:1800332. doi: 10.1183/13993003.00332-2018. [DOI] [PubMed] [Google Scholar]
- 11.WHO Global Reference List of 100 Core Health Indicators (Plus Health-Related SDGs) [(accessed on 1 November 2021)]. WHO/HIS/IER/GPM/2018. Available online: https://apps.who.int/iris/handle/10665/259951.
- 12.ACSM . Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer Health; Philadelphia, PA, USA: 2018. [Google Scholar]
- 13.Boutou A.K., Pitsiou G.G., Argyropoulou P. Non-invasive markers of pulmonary hypertension in interstitial lung disease: Is cardiopulmonary exercise testing the Holy Grail? Respirology. 2014;19:621–622. doi: 10.1111/resp.12321. [DOI] [PubMed] [Google Scholar]
- 14.Werneke M.W., Deutscher D., Grigsby D., Tucker A.C., Mioduski E.J., Hayes D. Telerehabilitation during the COVID-19 Pandemic in Outpatient Rehabilitation Settings: A Descriptive Study. Phys. Ther. 2021;101:110. doi: 10.1093/ptj/pzab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkowski S., Szczegielniak J., Szczepańska-Gieracha J. Evaluation of the Efficacy of Immersive Virtual Reality Therapy as a Method Supporting Pulmonary Rehabilitation: A Randomized Controlled Trial. J. Clin. Med. 2021;10:352. doi: 10.3390/jcm10020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Xia W., Zhan C., Liu S., Yin Z., Wang J., Chong Y., Zheng C., Fang X., Cheng W., et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): A randomised controlled trial. Thorax. 2021;26:thoraxjnl-2021-217382. doi: 10.1136/thoraxjnl-2021-217382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stavrou V.T., Tourlakopoulos K.N., Vavougios G.D., Papayianni E., Kiribesi K., Maggoutas S., Nikolaidis K., Fradelos E.C., Dimeas I., Daniil Z., et al. Eight Weeks Unsupervised Pulmonary Rehabilitation in Previously Hospitalized of SARS-CoV-2 Infection. J. Pers. Med. 2021;11:806. doi: 10.3390/jpm11080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzuca P., Montesi L., Mazzoni G., Grazzi G., Micheli M.M., Piergiovanni S., Pazzini V., Forlani G., Latessa P.M., Marchesini G. Supervised vs. self-selected physical activity for individuals with diabetes and obesity: The Lifestyle Gym program. Intern. Emerg. Med. 2016;12:45–52. doi: 10.1007/s11739-016-1506-7. [DOI] [PubMed] [Google Scholar]
- 19.Stavrou V., Griziotis M., Raptis D., Bardaka F., Karetsi E., Kiritsis A., Daniil Z., Tsarouhas K., Triposkiadis F., Gour-goulianis K.I., et al. Eight Weeks of Pulmonary Rehabilitation in Patients with Pulmonary Embolism: A Preliminary Report. Proceedings. 2019;25:37. doi: 10.3390/proceedings2019025037. [DOI] [Google Scholar]
- 20.Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Ngo-Metzger Q., Sorkin D.H., Mangione C.M., Gandek B., Hays R.D. Evaluating the SF-36 Health Survey (Version 2) in Older Vietnamese Americans. J. Aging Health. 2008;20:420–436. doi: 10.1177/0898264308315855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosteller R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987;317:1098. doi: 10.1056/nejm198710223171717. [DOI] [PubMed] [Google Scholar]
- 23.Watson P.E., Watson I.D., Batt R.D. Total body water volumes for adult males and females estimated from simple anthro-pometric measurements. Am. J. Clin. Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Hume R. Prediction of lean body mass from height and weight. J. Clin. Pathol. 1966;19:389–391. doi: 10.1136/jcp.19.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., Crapo R., Enright P., Van Der Grinten C.P.M., Gustafsson P., et al. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell C., Rahko P.S., Blauwet L.A., Canaday B., Finstuen J.A., Foster M.C., Horton K., Ogunyankin K.O., Palma R.A., Velazquez E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Wasserman K., Hansen J.E., Sue D.Y., Stringer W.W., Whipp B. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 4th ed. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2004. [Google Scholar]
- 28.Albaghdadi M.S., Dudzinski D.M., Giordano N., Kabrhel C., Ghoshhajra B., Jaff M.R., Weinberg I., Baggish A. Cardiopulmonary Exercise Testing in Patients Following Massive and Submassive Pulmonary Embolism. J. Am. Hear. Assoc. 2018;7:e006841. doi: 10.1161/JAHA.117.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn S.R., Akaberi A., Granton J.T., Anderson D.R., Wells P.S., Rodger M.A., Solymoss S., Kovacs M.J., Rudski L., Shimony A., et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: Results of the ELOPE cohort study. Am. J. Med. 2017;130:990.e9–990.e21. doi: 10.1016/j.amjmed.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Stavrou V., Boutou A.K., Vavougios G.D., Pastaka C., Gourgoulianis K.I., Koutedakis Y., Daniil Z., Karetsi E. The use of cardiopulmonary exercise testing in identifying the presence of obstructive sleep apnea syndrome in patients with compatible symptomatology. Respir. Physiol. Neurobiol. 2019;262:26–31. doi: 10.1016/j.resp.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Vanuxem D., Badier M., Guillot C., Delpierre S., Jahjah F., Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997;91:551–557. doi: 10.1016/S0954-6111(97)90089-5. [DOI] [PubMed] [Google Scholar]
- 32.Mereles D., Ehlken N., Kreuscher S., Ghofrani S., Hoeper M., Halank M., Meyer F.J., Karger G., Buss J., Juenger J., et al. Exercise and Respiratory Training Improve Exercise Capacity and Quality of Life in Patients with Severe Chronic Pulmonary Hypertension. Circulation. 2006;114:1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 33.Kwan G., Balady G.J. Cardiac rehabilitation 2012: Advancing the field through emerging science. Circulation. 2012;125:369–373. doi: 10.1161/CIRCULATIONAHA.112.093310. [DOI] [PubMed] [Google Scholar]
- 34.Tavoly M., Utne K.K., Jelsness-Jørgensen L.-P., Wik H.S., Klok A.F., Sandset P.M., Ghanima W. Health-related quality of life after pulmonary embolism: A cross-sectional study. BMJ Open. 2016;6:e013086. doi: 10.1136/bmjopen-2016-013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paz-Díaz H., Montes de Oca M., López J.M., Celli B.R. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am. J. Phys. Med. Rehabil. 2007;86:30–36. doi: 10.1097/PHM.0b013e31802b8eca. [DOI] [PubMed] [Google Scholar]
- 36.Kord Z., Fereidouni Z., Mirzaee M.S., Alizadeh Z., Behnammoghadam M., Rezaei M., Abdi N., Delfani F., Zaj P. Telenursing home care and COVID-19: A qualitative study. BMJ Support. Palliat. Care. 2021;29:bmjspcare-2021-003001. doi: 10.1136/bmjspcare-2021-003001. [DOI] [PubMed] [Google Scholar]
- 37.Cooper C.B. Desensitization to dyspnea in COPD with specificity for exercise training mode. Int. J. Chronic Obstr. Pulm. Dis. 2008;4:33–43. doi: 10.2147/COPD.S3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Ortega A., Mañas E., López-Reyes R., Selma M.J., García-Sánchez A., Oscullo G., Jiménez D., Martínez-García M. Ángel Obstructive sleep apnoea and venous thromboembolism: Pathophysiological links and clinical implications. Eur. Respir. J. 2019;53:1800893. doi: 10.1183/13993003.00893-2018. [DOI] [PubMed] [Google Scholar]
- 39.Xie J., Li F., Wu X., Hou W. Prevalence of pulmonary embolism in patients with obstructive sleep apnea and chronic ob-structive pulmonary disease: The overlap syndrome. Heart Lung. 2019;48:261–265. doi: 10.1016/j.hrtlng.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Stavrou V., Bardaka F., Karetsi E., Seitanidis G., Daniil Z., Gourgoulianis K.I. The effect of physical strain on breeders patients with obstructive sleep apnea syndrome. Respir. Physiol. Neurobiol. 2019;260:137–139. doi: 10.1016/j.resp.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Stavrou V., Karetsi E., Daniil Z., Gourgoulianis I.K. Four Weeks Exercise in Obstructive Sleep Apnea Syndrome Patient with Type 2 Diabetes Mellitus and without Continuous Positive Airway Pressure Treatment: A Case Report. Sleep Med. Res. 2019;10:54–57. doi: 10.17241/smr.2019.00374. [DOI] [Google Scholar]
- 42.Amoury M., Noack F., Kleeberg K., Stoevesandt D., Lehnigk B., Bethge S., Heinze V., Schlitt A. Prognosis of patients with pulmonary embolism after rehabilitation. Vasc. Health Risk Manag. 2018;14:183–187. doi: 10.2147/VHRM.S158815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakoski S.G., Savage P.D., Berkman A., Penalosa L., Crocker A., Ades P.A., Kahn S.R., Cushman M. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: A randomized clinical trial. J. Thromb. Haemost. 2015;13:1238–1244. doi: 10.1111/jth.12989. [DOI] [PubMed] [Google Scholar]
- 44.Klok E., Mos I.C.M., Huisman M.V. Brain-Type Natriuretic Peptide Levels in the Prediction of Adverse Outcome in Patients with Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2008;178:425–430. doi: 10.1164/rccm.200803-459OC. [DOI] [PubMed] [Google Scholar]
- 45.Dentali F., Donadini M., Gianni M., Bertolini A., Lonn E., Venco A., Cattozzo G., Ageno W. Brain natriuretic peptide as a preclinical marker of chronic pulmonary hypertension in patients with pulmonary embolism. Intern. Emerg. Med. 2009;4:123–128. doi: 10.1007/s11739-009-0231-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.