Abstract
Fungi are widely distributed in the terrestrial environment, freshwater, and marine habitat. Only approximately 100,000 of these have been classified although there are about 5.1 million characteristic fungi all over the world. These eukaryotic microbes produce specialized metabolites and participate in a variety of ecological functions, such as quorum detection, chemical defense, allelopathy, and maintenance of symbiosis. Fungi therefore remain an important resource for the screening and discovery of biologically active natural products. Sesquiterpenoids are arguably the richest natural products from plants and micro-organisms. The rearrangement of the 15 high-ductility carbons gave rise to a large number of different skeletons. At the same time, abundant structural variations lead to a diversification of biological activity. This review examines the isolation, structural determination, bioactivities, and synthesis of sesquiterpenoids that were specially produced by fungi over the past five years (2015–2020).
Keywords: sesquiterpenoids, fungus, structures, structural diversity, biological activity, synthesis
1. Introduction
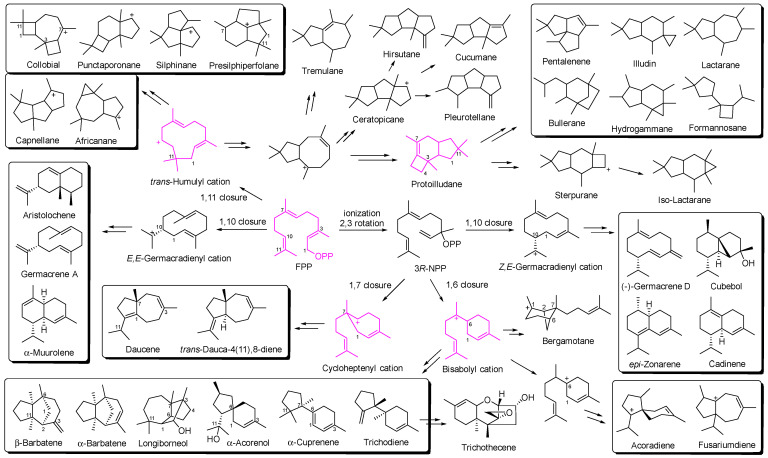
Fungi are undoubtedly important resources for natural products discovery. With the advancement of natural product research, the importance of its biological resources has been infinitely enlarged. In the giant natural product system of fungi, sesquiterpenes, due to their carbon skeletons and amounts, are the largest of all types. The C-15-hydrocarbon skeletal system of various sesquiterpenoids isolated from fungi, bacteria, and plants are synthesized from farnesyl pyrophosphate (FPP) under the catalysis of sesquiterpene synthases [1,2]. Sesquiterpene synthases catalyze different initial cyclization reactions to produce secondary or tertiary cyclic carbocation intermediates, which can then be further cyclized and reassembled until carbocation quenching at the active center, followed by the enzymatic release of the final sesquiterpenoid scaffold (Figure 1) [3]. A huge number of sesquiterpenoids were, consequently, produced [4,5,6]. Among various other resources, fungal species have an enormous contribution owing to their potential to carry out the bio-transformations and drug synthesis under environmentally acceptable conditions. For instance, hydroxymethylacylfulvene (HMAF) is a semisynthetic antitumor agent based on the naturally occurring illudin S occurring in the mushroom Omphalotus olearius [7]. It has been advanced into human clinical trials for the treatment of cancers [8,9]. Trichothecenes, a class of tricyclic sesquiterpenes produced by a wide variety of fungi, are toxic to animals and humans and frequently present in cereal crops. They have attracted much attention in the areas such as agriculture, food contamination, and health care [10,11,12,13].
Figure 1.
Cyclization of FPP by characterized fungal sesquiterpene synthases (Reference [3]).
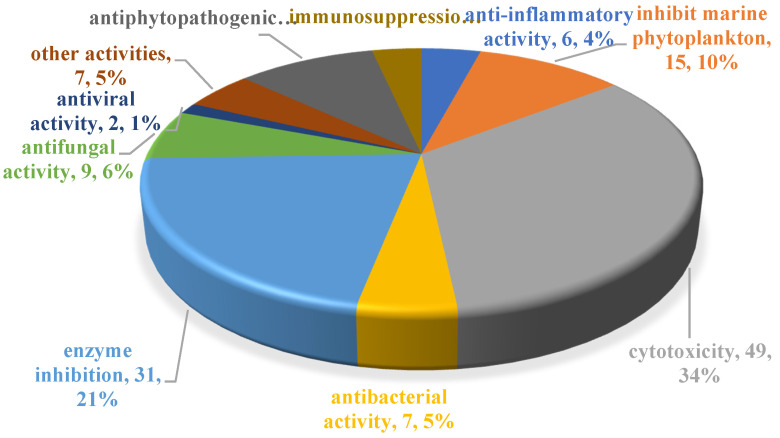
Our research group has been engaged in the study of the chemical composition of fungi for decades [14,15], while a large number of sesquiterpenoids have been reported [6]. It has been found that the vast majority of skeletons, such as alliacane, bergamotane, hirsutane, tremulane, etc., are specially produced by fungi. Many compounds displayed significant biological activities, and it is obvious that cytotoxic activity accounts for the largest proportion (Figure 2). In addition, with the development of synthetic biology, the biosynthesis of many fungal sesquiterpenoids has been figured out. This review gives an overview about the structures, biological activities, chemical synthesis and biosynthesis of sesquiterpenoids specially produced by fungi presented from 2015 to 2020.
Figure 2.
The proportion of one activity compared to the whole occurrence of activities of bioactive fungal sesquiterpenoids.
2. Composition and Bioactivities
2.1. Alliacane, Cadinene, Azulene, and Zierane
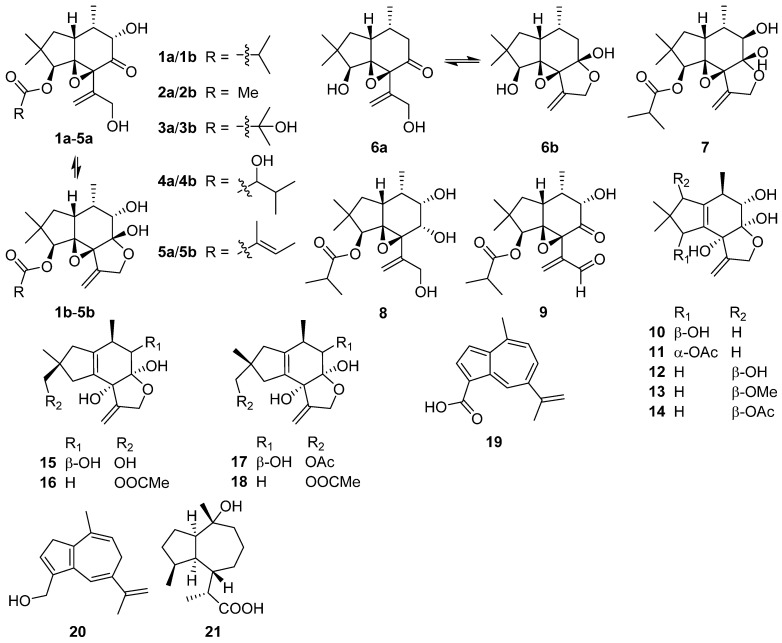
Nine alliacane sesquiterpenoids inonoalliacanes A–I 1a/1b–6a/6b–7–9 were isolated from the culture broth of the basidiomycete Inonotus sp. BCC 22670 [16]. Inonoalliacane A 1 exhibited moderate antibacterial activity against Bacillus cereus with a minimum inhibitory concentration (MIC) value of 25 µg/mL. Inonoalliacane B 2 showed antiviral activity against herpes simplex virus type 1 (HSV-1) with IC50 of 17 μg/mL.
Clitocybulols G–O 10–18, highly oxidized alliacane sesquiterpenoids, were isolated from the solid culture of the edible fungus Pleurotus cystidiosus [17]. Clitocybulols G 10 and L 15 showed weak inhibitory activity against protein tyrosine phosphatase-1B (PTP1B) with IC50 values of 49.5, 38.1 μM, respectively.
In the 1H NMR-guided fractionation of extracts from the edible mushroom Lactarius deliciosus, two new azulene-type sesquiterpenoids 19 and 20 were characterized [18]. Pestabacillin A 21 bearing a zierane-type sesquiterpene skeleton was isolated from the co-culture of the endophytic fungus Pestalotiopsis sp. with Bacillus subtilis [19]. Furthermore, the absolute configuration of 21 was confirmed by single-crystal X-ray diffraction analysis.
 |
2.2. Bergamotane, Spiroaminal, and Spiroaxane
Bergamotane sesquiterpenes bearing a bridged 6/4 bicyclic ring incorporated with an isopentyl unit, are naturally occurring in plants and fungi [20,21]. A new class of polyoxygenated bergamotanes with notable features inspired by a 6/4/5/5 tetracyclic ring system was very rare in nature and all examples of the polycyclic bergamotanes only derived from fungi [22,23,24,25].
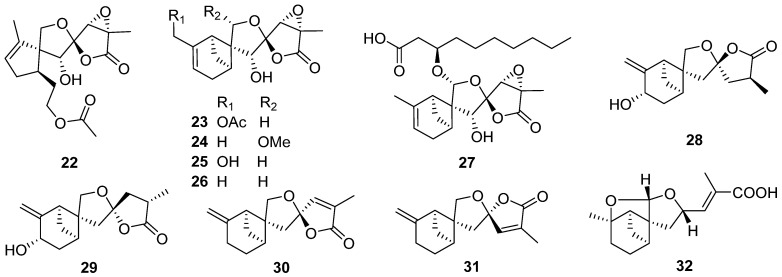 |
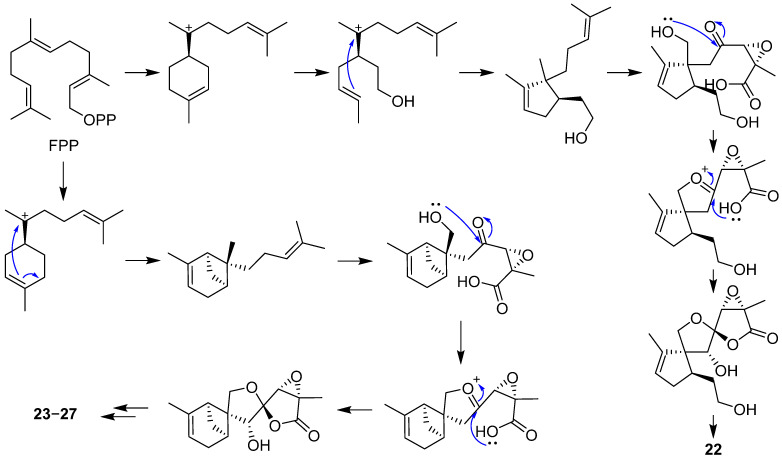
Purpurolide A 22, an unprecedented sesquiterpene lactone with a rarely encountered 5/5/5 spirocyclic skeleton, along with five new 6/4/5/5 tetracyclic sesquiterpene lactones (purpurolides B–F 23–27), was isolated from the cultures of the endophytic fungus Penicillium purpurogenum [26,27]. The structures and absolute configurations of 22–27 were established by spectroscopic analysis, a single-crystal X-ray diffraction, and calculations of the 13C NMR and ECD data. The plausible biosynthetic pathway of 22–27 is shown in Scheme 1. Compounds 22–27 showed significant inhibitory activity against pancreatic lipase with IC50 values of 1.22–7.88 μM.
Scheme 1.
Plausible biogenetic pathways for 22–27 (Reference [26]).
 |
Expansolides C 28 and D 29 were two new bergamotane sesquiterpene lactones isolated from the plant pathogenic fungus Penicillium expansum [28]. The epimeric mixture of expansolides C 28 and D 29 (in a ratio of 2:1 at the temperature of the bioassay) exhibited more potent α-glucosidase inhibitory activity (IC50 0.50 mM) as compared with the positive control acarbose (IC50 1.90 mM) in an in vitro bioassay.
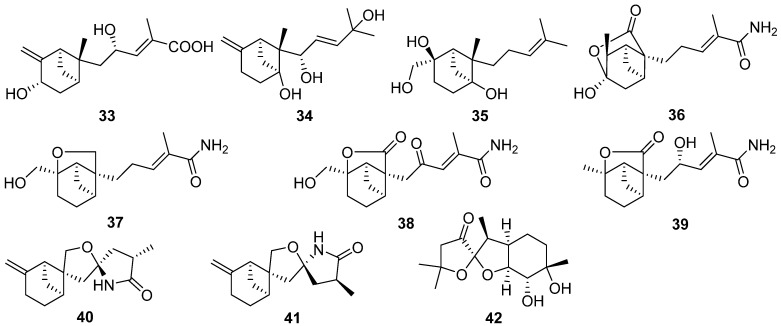
Donacinolides A 30 and B 31 and donacinoic acids A 32 and B 33, four new rare tetracyclic bergamotane-type sesquiterpenoids, were isolated from the mushroom-associated fungus Montagnula donacina [29]. Two new β-bergamotane sesquiterpenoids 34 and 35 were isolated from the marine-derived fungus Aspergillus fumigatus [30]. Brasilamides K–N 36–39 were isolated from the plant endophytic fungus Paraconiothynium Brasiliense [31].
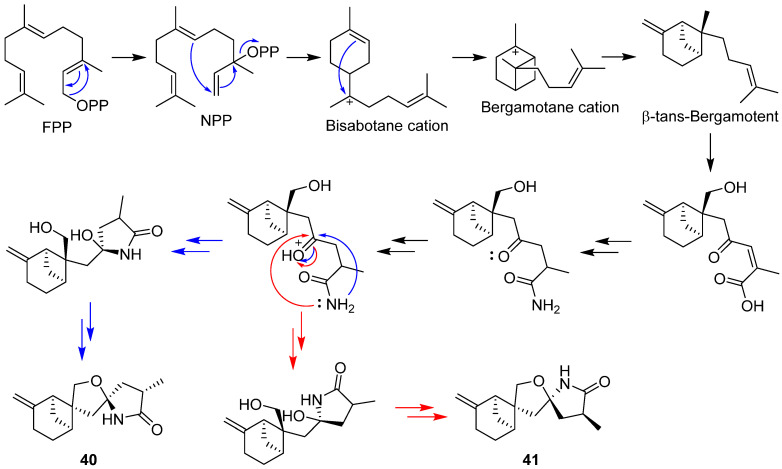
Sporulaminals A 40 and B 41, a pair of unusual epimeric spiroaminal derivatives bearing a 6/4/5/5 tetracyclic ring system derived from bergamotane sesquiterpenoid (Scheme 2), were isolated from a marine-derived fungus Paraconiothyrium sporulosum [32]. Pleurospiroketal F 42, a new perhydrobenzannulated 5,5-spiroketal sesquiterpene was isolated from solid-state fermentation of Pleurotus citrinopileatus, and the absolute configuration of 42 was determined by single-crystal X-ray diffraction analysis [33].
Scheme 2.
Plausible biosynthetic pathways of sporulaminals A 40 and B 41 (Reference [32]).
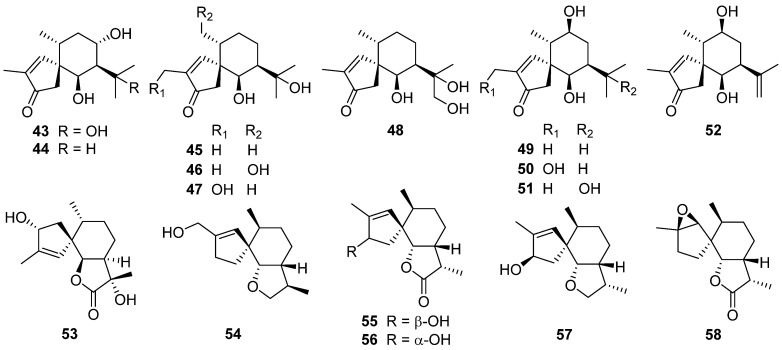
Flammuspirones A–J 43–52, ten spiroaxane sesquiterpenoids, were obtained from the edible mushroom Flammulina velutipes [34]. Flammuspirones A 43 and C 45 showed inhibition on HMG-CoA reductase with IC50 of 114.7 and 77.6 μM, respectively. Flammuspirones C–E 45–47 and H 50 showed inhibitory activity on DPP-4 with IC50 values in the range from 70.9 to 83.7 μM.
Talaminoid A 53 was obtained from the fungus Talaromyces minioluteus [35]. Talaminoid A 53 showed a significant suppressive effect on the production of nitric oxide (NO) on lipopolysaccharide (LPS) induced BV-2 cell, with IC50 of 5.79 μM. In addition, talaminoid A 53 exhibited significant anti-inflammatory activities against the production of TNF-α and IL-6. Further immunofluorescence experiments revealed the mechanism of action to be inhibitory the NF-κB-activated pathway. A new sesquiterpenoid 54 was isolated from the fungus Pholiota nameko [36]. Tramspiroins A–D 55–58 have been isolated from the cultures of Basidiomycete Trametes versicolor [37].
 |
2.3. Carotane, Cyclonerane, Cyclofarnesane, and Longifolene
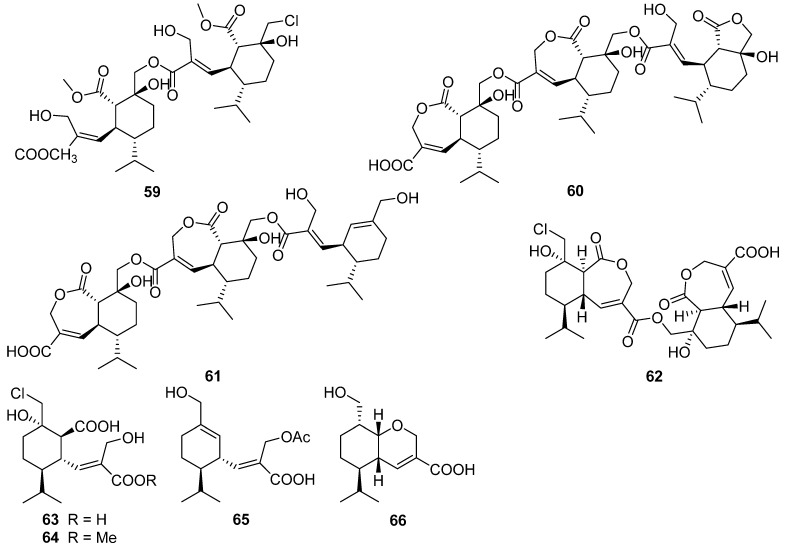
A new dimeric sesquiterpene divirensol H 59 and two exceptionally novel trimeric sesquiterpenes trivirensols A 60 and B 61 were purified from an endophytic fungus Trichoderma virens [38]. Divirensol H 59 showed significant activities against fungi Penicillium italicum, Fusarium oxysporum, Fusarium graminearum, Colletotrichum musae, and Colletotrictum gloeosporioides with MIC values of 6.25 to 25 μg/mL. Rhinomilisin A 62 and four new heptelidic acid derivatives, rhinomilisin B–E 63–66, were isolated from the endophytic fungus Rhinocladiella similis [39]. Rhinomilisins A 62 showed moderate cytotoxicity activity against the mouse lymphoma cell line L5178Y with an IC50 value of 5.0 μM.
 |
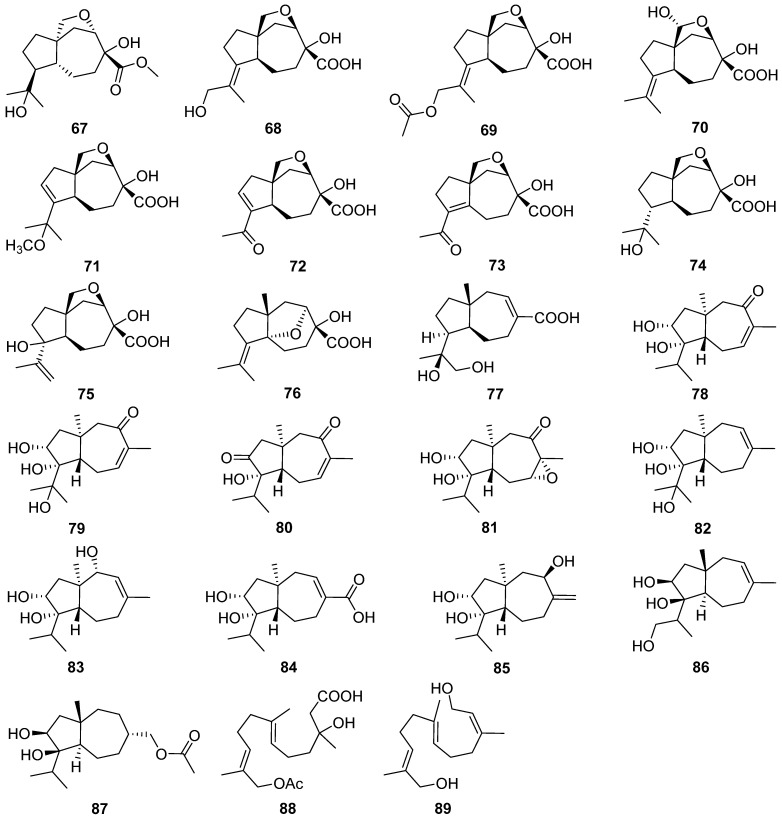 |
Peniterester 67, a new tricyclic sesquiterpene was isolated from the secondary metabolites of an artificial mutant Penicillium sp. T2-M20 [40]. Peniterester 67 showed significant activities against Bacillus subtilis, Escherichia coli, and Staphylococcus aureus in vitro with MICs of 8.0, 8.0, and 4.0 μg/mL, respectively.
Piltunines A–F 68–73 and penigrisacids A–D 74–77, ten new carotane sesquiterpenoids, were isolated from the marine-derived fungus Penicillium griseofulvum and Penicillium piltunense, respectively [41,42]. Penigrisacid D 75 showed a weak effect on ECA-109 tumor cells with an IC50 value of 28.7 µM [41]. Trichocarotins A–H 78–85, eight new carotane sesquiterpenes, were isolated from the culture of the fungus Trichoderma virens [43]. Trichocarotins C–E 80–82 and H 85 displayed potent inhibition against the four marine phytoplankton species (Chattonella marina, Heterosigma akashiwo, Karlodinium veneficum, and Prorocentrum donghaiense) tested, especially against C. marina with IC50 values ranging from 0.24 to 1.2 μg/mL.
Trichocaranes E 86 and F 87 were isolated from cultures of the insect pathogenic fungus Isaria fumosorosea [44]. Trichocaranes E 86 and F 87 showed potent cytotoxic activities against six tumor cell lines MDA, MCF-7, SKOV-3, Hela, A549, and HepG2 with IC50 values in a concentration range of 0.13–4.57 μg/mL. Two new carotane-type biogenetically related sesquiterpenes, aspterrics A 88 and B 89, were isolated from the deep-sea-derived fungus Aspergillus terreus [45].
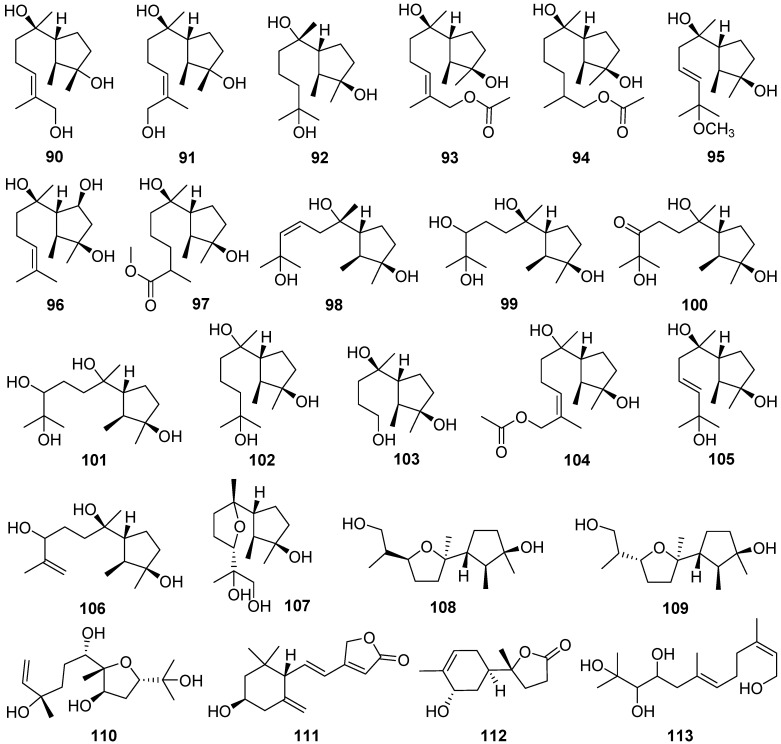 |
Two new cycloneranes 90 and 91 were isolated from the marine alga endophytic fungus Trichoderma citrinoviride [46]. The compound 90 had an inhibition to the marine phytoplankton species Karlodinium veneficum with an IC50 value of 8.1 μg/mL. Six new cycloneranes 92–97 were isolated from the fungus Trichoderma harzianum [47,48,49]. The three new ones 95–97 all exhibited growth inhibition of the four phytoplankton species (Chattonella marina, Heterosigma akashiwo, Karlodinium veneficum, and Prorocentrum donghaiense) with IC50 values ranging from 0.66 to 75 μg/mL [49].
Cyclonerotriol B 98 was isolated from the soil fungus Fusarium avenaceum [50]. Cyclonerodiol B 99 was isolated from the mangrove plant endophytic fungus Trichoderma sp. Xy24 [51]. Cyclonerodiol B 99 exhibited significant neural anti-inflammatory activity by inhibiting LPS-induced NO production in BV2 cells with the inhibitory rates of 75.0% at 0.1 μM, which are more potent than curcumin, positive control with the inhibitory rate of 21.1% at 0.1 μM.
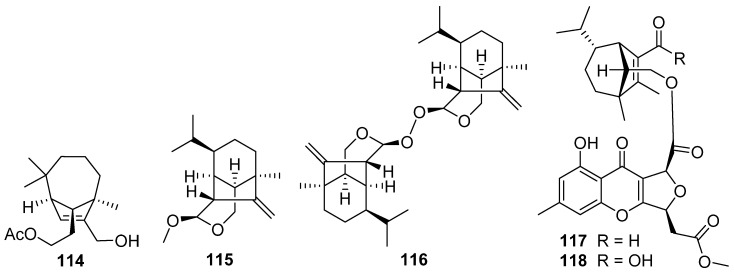 |
Ten new cycloneranes 100–109 were isolated from the algicolous endophytic fungus Trichoderma asperellum [52,53]. The seven new ones, 100–104, 108, and 109, all exhibited growth inhibition of the four phytoplankton species (Chattonella marina, Heterosigma akashiwo, Karlodinium veneficum, and Prorocentrum donghaiense) with IC50 values ranging from 2.4 to 76 μg/mL [52].
A new sesquiterpenoid 110 was isolated and identified from an endophytic fungus Umbelopsis dimorpha grown on host-plant Kadsura angustifolia and wheat bran [54]. Inonofarnesane 111, a new cyclofarnesane sesquiterpenoid, was isolated from cultures of the wood-rotting basidiomycete Inonotus sp. BCC 23706 [55].
One new norbisabolane sesquiterpenoid degradation, isopolisin B 112, was isolated from the fungus Pestalotiopsis heterocornis [56]. Koninginol D 113 as a new farnesane sesquiterpenoid was isolated from the endophytic fungus Trichoderma koningiopsis [57].
Bipolenin F 114, a new seco-longifolene sesquiterpenoid, and two new seco-sativene sesquiterpenoids, bipolenins D 115 and E 116, and two novel sesquiterpenoid-xanthone adducts, bipolenins I 117 and J 118, were obtained from cultures of potato endophytic fungus Bipolaris eleusines [58,59]. Bipolenins I 117 and J 118 exhibited potent inhibitory activity against the plant pathogens Alternaria solani with MIC values of 8 and 16 μg/mL, respectively [59].
 |
2.4. Cerapicane, Cucumane, Cuparene, Hirsutane, Isohirsutane, and Triquinane
Cuparane-type sesquiterpenoids of fungal origin possess a skeleton with a six-membered ring connected to a five-membered ring, of which the six-membered ring is always aromatic. Linear triquinane sesquiterpenoids have a basic skeleton 1H-cyclopenta[α]pentalene [60]. Many compounds displayed a wide range of biological activities, such as cytotoxic, antimicrobial, and anti-inflammatory activities. A review gives an overview about the isolation, structure, biological activities, and chemical synthesis of linear triquinane sesquiterpenoids [61].
 |
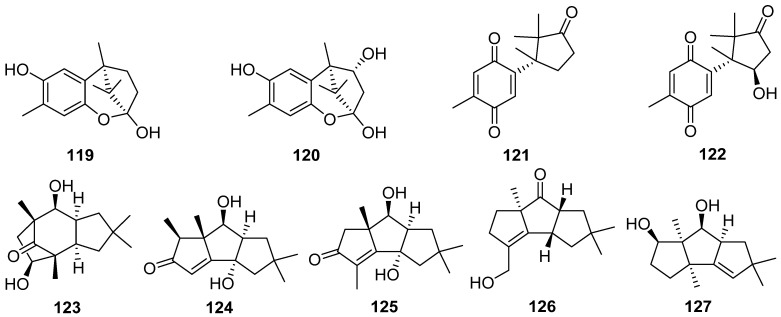
Enokipodins A–D 119–122, highly oxygenated cuparene-type sesquiterpenes were obtained from the fungi Flammulina rossica and Flammulina velutipes. In addition, enokipodins B 121 and D 122 are oxidized compounds of enokipodins A 119 and C 120, respectively [62].
One new cerapicane cerrenin A 123, and two new isohirsutane sesquiterpenoids cerrenins B 124 and C 125, were isolated from the broth extract of Cerrena sp. which was isolated from Pogostemon cablin [63]. Trefoliol C 126, one new cucumane sesquiterpenoid, was isolated from cultures of the basidiomycetes Tremella foliacea [64]. A new sesquiterpenoid 127 was isolated from the crude extract of Antrodiella albocinnamomea [65]. Two new hirsutane-type sesquiterpenoids, chondrosterins N 128 and O 129, were isolated from the marine fungus Chondrostereum sp. [66].
Ten new hirsutane-type sesquiterpenoids, sterhirsutins C–L 130–139, were isolated from the culture of Stereum hirsutum [67]. Sterhirsutins C 130 and D 131 possessed an unprecedented chemical skeleton with a 5/5/5/6/9/4 fused ring system, and the absolute configuration of sterhirsutin C 130 was assigned by single-crystal X-ray diffraction experiment. Sterhirsutin L 139 was the first sesquiterpene coupled with a xanthine moiety. Sterhirsutins C–L 130–139 showed cytotoxicity against K562 and HCT116 cell lines, and sterhirsutin K 138 induced autophagy in HeLa cells. Sterhirsutin G 133 inhibited the activation of the IFNβ promoter in Sendai virus-infected cells.
Cerrenins D 140 and E 141, two new triquinane-type sesquiterpenoids, were obtained from the endophytic fungus Cerrena sp. A593 [68]. Chondrosterins K–M 142–144 were isolated from the marine fungus Chondrostereum sp. [69]. Chondrosterins K–M 142–144 showed different degrees of cytotoxicities against various cancer cell lines (CNE1, CNE2, HONE1, SUNE1, A549, GLC82, and HL7702) in vitro, with IC50 values ranging from 12.03 to 58.83 µM.
 |
Antrodins A–E 145–149 were isolated from the fermentation of Antrodiella albocinnamomea [70]. Tremutin H 150 was isolated from cultures of the basidiomycetes Irpex lacteus [71]. The absolute configuration of 150 was determined by single-crystal X-ray diffraction analysis, and 150 shows a weak inhibitory effect on NO production with an IC50 value of 22.7 μM.
2.5. Eudesmanolide, Gymnomitrane, and Humulane
Humulane-type sesquiterpenoids are found rarely in nature. They have been recognized as being biogenetic precursors of many types of sesquiterpenoids [6]. The macrocyclic nature of members of the humulane group has proved to be troublesome for the determination of their absolute configurations.
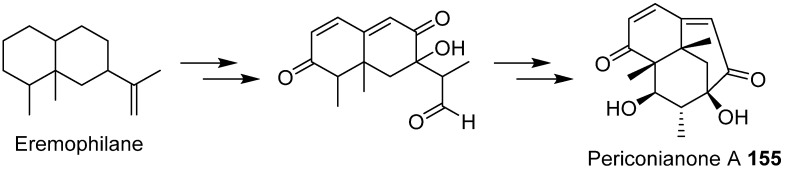
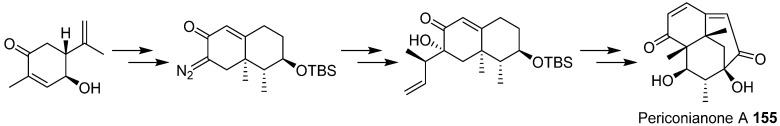
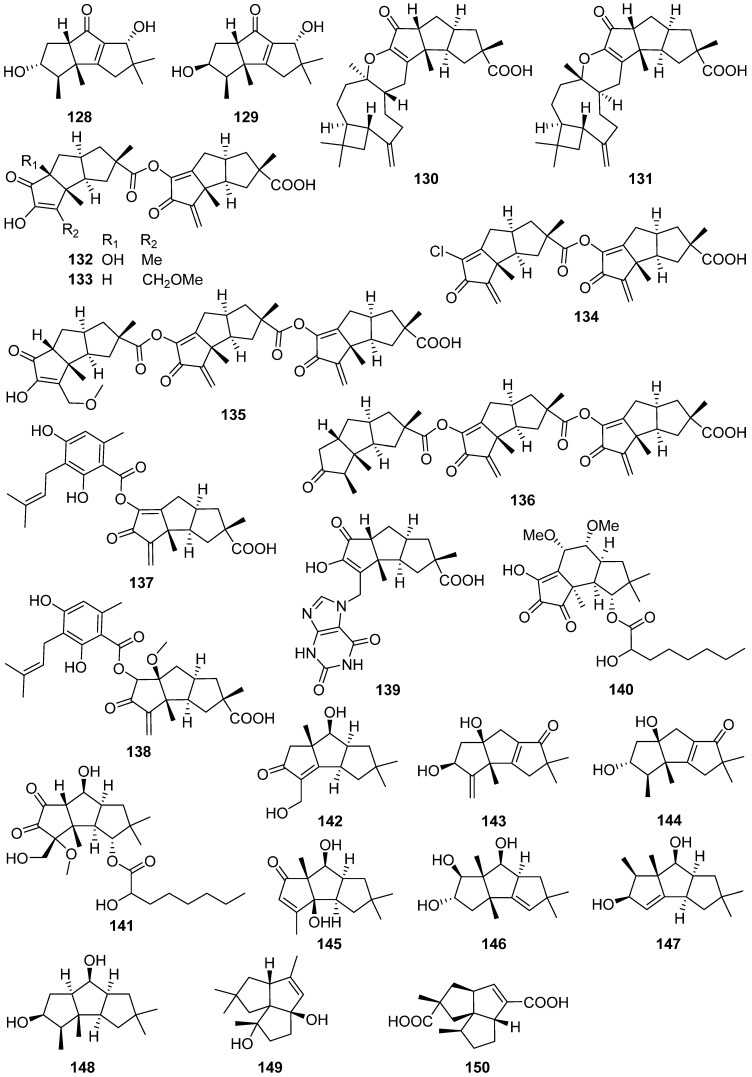
Four new 12,8-eudesmanolides 151–154 were isolated from a mangrove rhizosphere-derived fungus Eutypella sp. 1–15 [72]. Periconianone A 155, a polyoxygenated sesquiterpenoid with a new 6/6/6 tricarbocyclic skeleton, was isolated from the endophytic fungus Periconia sp., and the biosynthesis of the unusual six-membered carbonic ring of 155 was postulated to be formed through intramolecular aldol condensation (Scheme 3) [73]. The first enantioselective total synthesis of the periconianone A 155 based on a postulated biogenesis has been reported (Scheme 4) [74].
Scheme 3.
Hypothetical biosynthetic pathway of periconianone A 155 (Reference [73]).
Scheme 4.
Total synthesis of periconianone A 155 (Reference [74]).
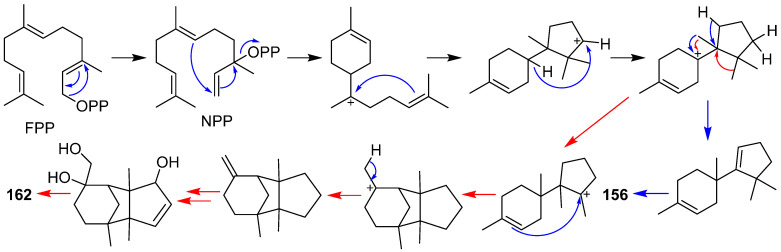
An unusual type sesquiterpene 156 possessed an unusual 14(7-6)-cuparane scaffold (Scheme 5), and six rarely-encountered gymnomitrane-type sesquiterpenoids 157–162, were isolated from the medicinal mushroom Ganoderma lingzhi [75]. A new gymnomitrane-type sesquiterpenoid 163 was isolated from the fruiting body of Ganoderma lucidum [76]. This compound 163 significantly inhibited the growth of epidermal growth factor receptor-tyrosine kinase inhibitor EGFR-TKI-resistant human lung cancer A549 and human prostate cancer PC3 cell lines. Antrodin F 164 was isolated from the fermentation of Antrodiella albocinnamomea [70].
Scheme 5.
Proposed biosynthetic pathway of 156 and 162 (Reference [75]).
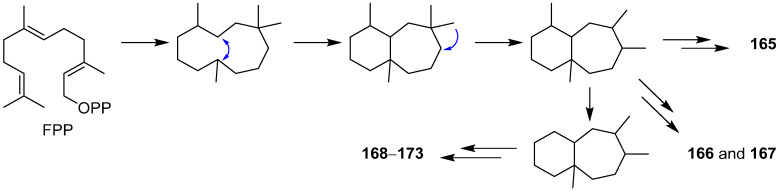
Nine new humulane-derived sesquiterpenoids, ochracenes A–I 165–173, were isolated from the Antarctic fungus Aspergillus ochraceopetaliformis [77]. A biogenetic pathway for them was given in Scheme 6. The two unprecedented 8,9-secocyclic sesquiterpenoids, ochracenes B 166 and C 167, exhibited inhibitory effects on LPS-induced NO release in RAW 264.7 mouse macrophage cell with IC50 values of 14.6 and 18.3 μM, respectively.
Scheme 6.
Postulated biogenetic pathway for ochracenes A–I 165–173 (Reference [77]).
 |
2.6. Illudane, Illudalane, Protoilludane, Marasmane, and Norilludane
A review offers a comprehensive description of the investigations that started with the discovery of illudins in 1950, led to HMAF clinical trials against various tumors as a single agent and in combination therapy beginning in 2002, and culminated in the past decade of advances in chemical synthesis and mechanisms of toxicity of AFs, including biotransformation processes, DNA alkylation products, unique influences of DNA repair capacities, and enzyme inhibition properties [9]. The 4/6/5 ring-fused protoilludane-type sesquiterpenoids are the precursors of many other sesquiterpenoids, representing the largest group of sesquiterpene metabolites of fungal origin.
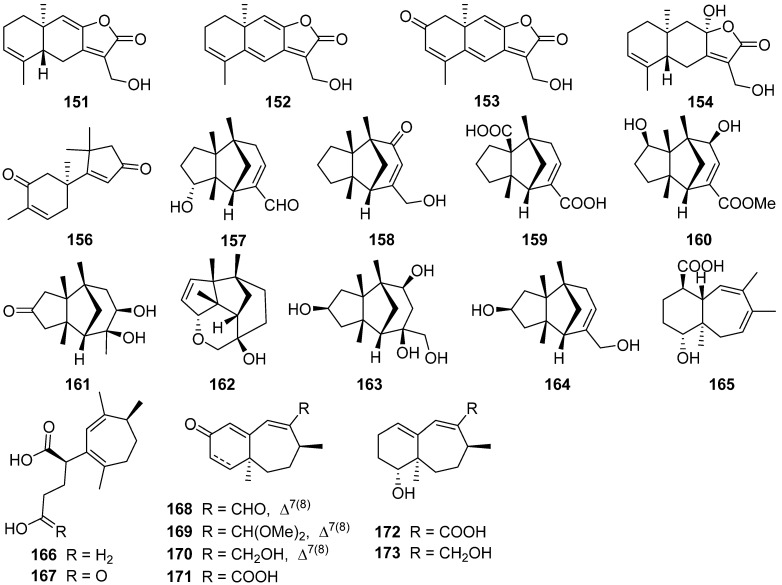
Phellinignin D 174 was isolated from the fungus Phellinus igniarius, which possessed a new carbon skeleton that might derive from an illudane framework by methyl shift and aromatization [78]. Phellinignin D 174 showed moderate cytotoxicities to three human cancer cell lines (HL-60, SMMC-7721, and SW480) with the IC50 values of 21.1, 12.3, and 13.9 µM, respectively.
Illudadienes A 175 and B 176 were obtained from the wood-decomposing fungus Granulobasidium vellereum [79]. Phellinuin J 177 and sulphureuine A 178 were isolated from cultures of Phellinus tuberculosus and Laetiporus sulphureus [80]. Agrocybins H–K 179–184 were obtained from the edible mushroom Agrocybe salicacola [81]. Craterellins D 185 and E 186 were isolated from cultures of Craterellus cornucopioides [82]. Illudalane derivative, granulolactone 187, and a 15-norilludane, granulodione 188, were isolated from an agar plate culture of Granulobasidium vellereum [83].
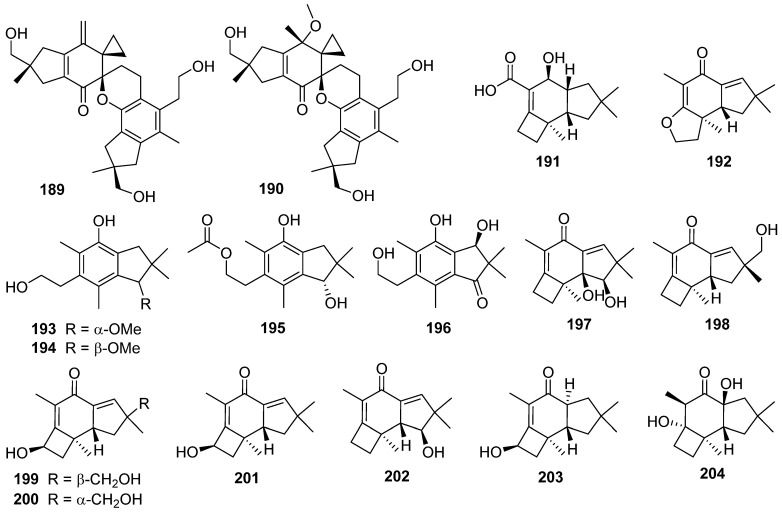 |
Two new disesquiterpenoid derivatives, bovistol B 189 and C 190, and a new protoilludane derivative, pasteurestin C 191, were isolated from the fermentation broth of the edible mushroom Cyclocybe aegerita [84]. Four illudalanes 192–195, an unusual 2,3-seco-protoilludane 196, and eight protoilludanes 197–204 were identified from the liquid culture of the endophytic fungus Phomopsis sp. TJ507A [85]. Phomophyllins A–G 196–202, and phomophyllin I 204 displayed β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitory activities ranging from 19.4% to 43.8% at the concentration of 40 μM.
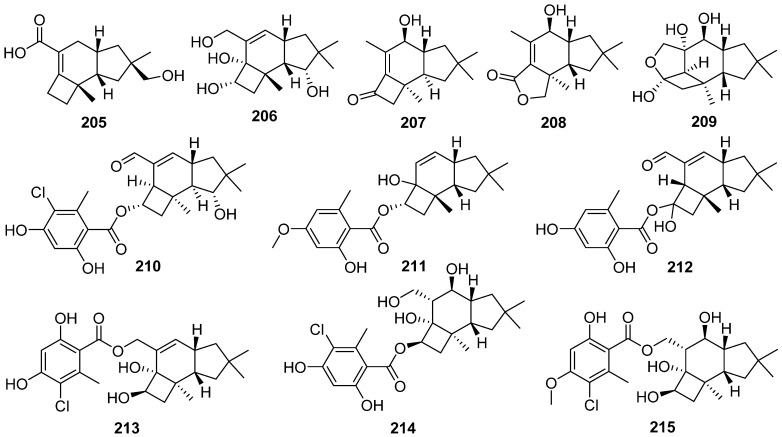
Epicoterpenes A–E 205–209, and armilliphatic A 210 were isolated from Armillaria sp. by co-culture with the endophytic fungus Epicoccum sp. associated with Gastrodia elata [86]. Epicoterpene D 208 was the first example of an ent-protoilludane sesquiterpenoid scaffold bearing a five-membered lactone. Two new protoilludane sesquiterpene aryl esters 211 and 212 were isolated from the mycelium of Armillaria mellea [87]. Compound 212 showed cytotoxic activity for HepG2 cells with an IC50 value of 18.03 μg/mL. Three new sesquiterpene aryl esters, melleolide N 213, Q 214, and R 215, were isolated from the EtOH extract of the mycelium of Armillaria mellea [88]. And 213–215 showed cytotoxicity to several human cancer cell lines.
 |
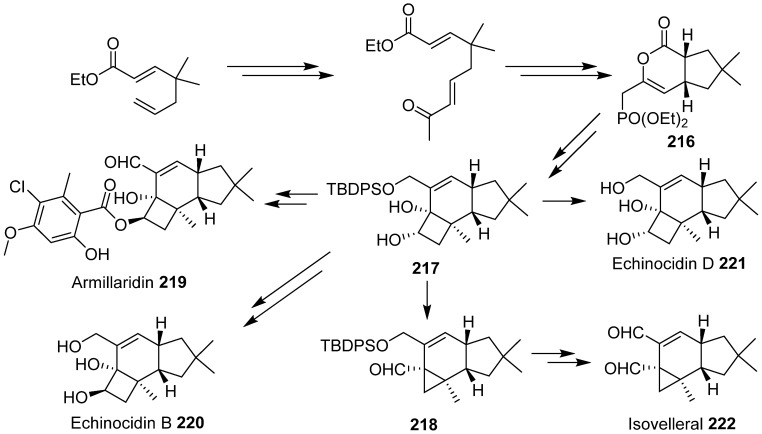
Unified total syntheses of marasmane, mellolide, and protoilludane sesquiterpenoids have been achieved through a key organocatalytic enantioselective annulation (Scheme 7) [89]. The elaboration of key bicyclic lactone 216 was the molecular springboard from which the first enantioselective total syntheses of protoilludanes echinocidin B 220 and echinocidin D 221, and the mellolide armillaridin 219, as well as the synthesis of the marasmane isovelleral 222, were accomplished. The vanadium(II)/zinc(II) reductive coupling yielded the final ring of the densely functionalized cis-fused carbocyclic core. Finally, the unexpected semi-Pinacol-type ring contraction to establish cyclopropyl aldehyde 218 from cyclobutanediol 217 was potentially biomimetic in origin.
Scheme 7.
Synthesis of armillaridin 219, echinocidins B 220 and D 221, and isovelleral 222 (Reference [89]).
 |
 |
2.7. Botryane and Seco-Probotryane
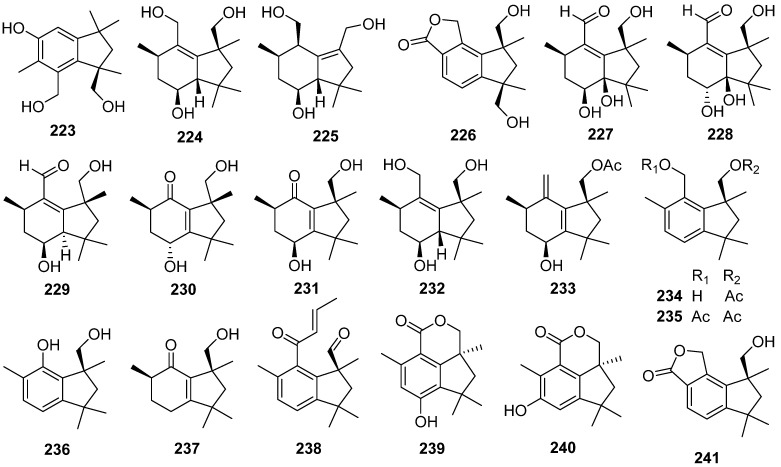
A botryane-type sesquiterpenoid 223 was identified from the liquid culture of the endophytic fungus Phomopsis sp. TJ507A [85]. Arthrinins E–G 224–226, three new sesquiterpenoids possessing non-isoprenoid botryane skeleton, were isolated from the endophytic fungus Arthrinium sp. HS66 [90]. Five new botryanes 227–231 were obtained from an endophytic fungus Nemania bipapillata [91]. Five new botryanes 232–236 were isolated from Trichoderma oligosporum [92]. Compounds 236 showed moderate cytotoxicity activity against K562 cells with an inhibitory rate of 45–60% at 6.25 µM (Taxol was used as a positive control with 60.3% inhibition at 2.0 µM).
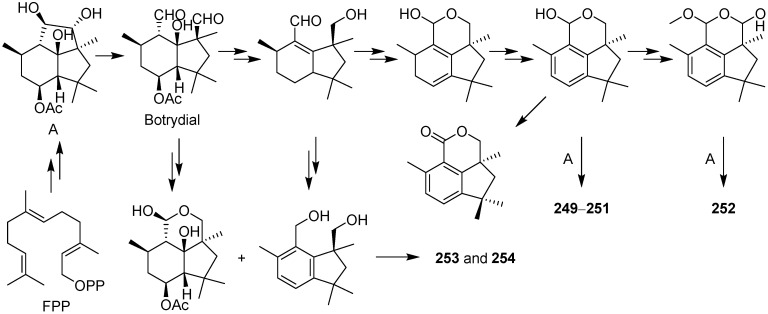
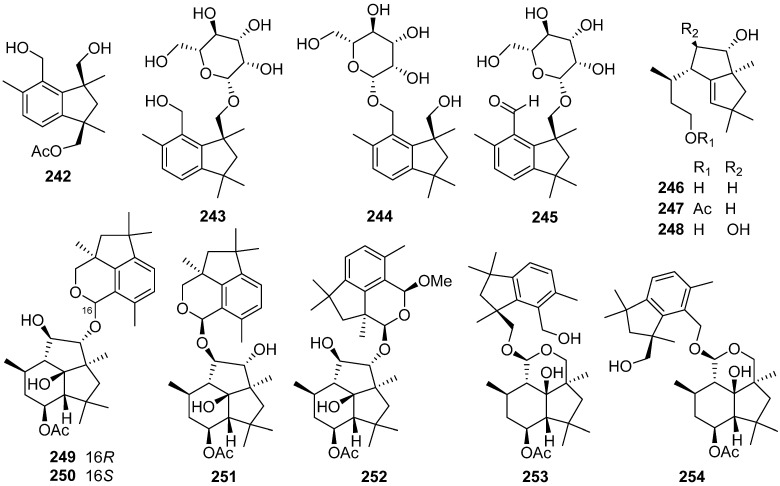
A new 10-norbotryane derivative 237 and three new botryanes 238–240 were isolated from the ascomycete Hypoxylon rickii [93,94]. Five new botryanes 241–245, along with 4,5-seco-Probotryenols A–C 246–248 derived from cleavage of the probotryane skeleton at C-4/C-5, were isolated from Stachybotrys bisbyi [95]. Six new heterodimeric botryane ethers, hypocriols A–F 249–254, were isolated from the insect-associated Hypocrea sp. EC1-35 [96]. A plausible biosynthetic pathway for 249–254 was given (Scheme 8). Hypocriols A–D 249–252 and F 254 showed significant activity against the HeLa cell, with IC50 values of 7.7, 3.1, 11.8, 3.8, and 4.6 μM, respectively. Hypocriol F 254 inhibited the proliferation of the HCT116 cell, showing an IC50 value of 2.7 μM.
Scheme 8.
Plausible biosynthetic pathways for hypocriols A–F 249–254 (Reference [96]).
 |
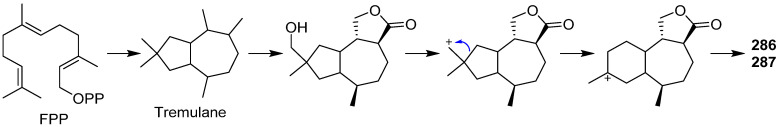
2.8. Tremulane, Sterpurane, Phlebiane, Merulane, and Irlactane
Tremulane-type sesquiterpenoids are a class of sesquiterpenoids with a 5/7-ringfused perhydroazulene carbon skeleton. The first example was isolated from the wood-decaying fungus Phellinus tremulae in 1993 [97]. The biosynthesis pathway was elucidated through a 13C-labeled feeding experiment revealed that tremulanes are derived from trans,trans-farnesyl pyrophosphate via humulene and a key step of methyl migration [98].
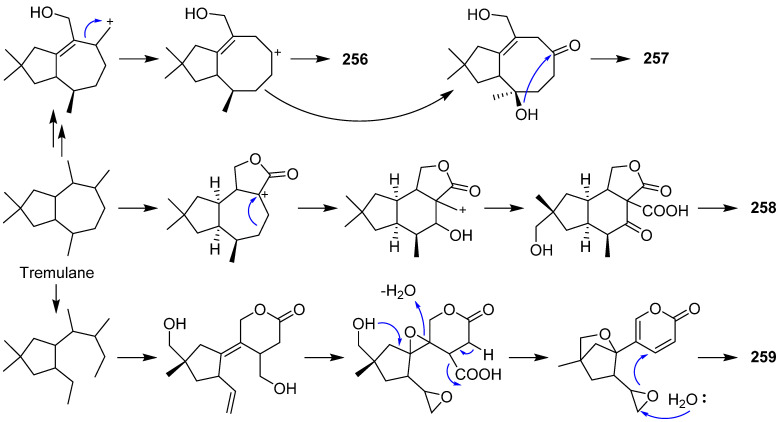
A new irlactane-type, irlactin K 255, was isolated from the fermentation broth of the medicinal fungus Irpex lacteus [99]. The absolute configuration of 255 was established by single-crystal X-ray diffraction analysis. Irlactin K 255 could be derived from the tremulane type sesquiterpene irlactin E via a ring rearrangement [100]. Conosiligins A–D 256–259, four ring-rearranged sesquiterpenoids, were isolated from cultures of the basidiomycete Conocybe siliginea [101]. Conosiligins A 256 and B 257 possessed a 5/8-fused ring system, while conosiligin C 258 has a 5/6-fused backbone conjugated with a γ-lactone. Conosiligin D 259 was a 5,6-seco tremulane derivative with the loss of a skeletal carbon, featuring a tetracyclic system involving a pyranone moiety (Scheme 9). Conosiligins C 258 and D 259 inhibited Con A-induced T cell proliferation with IC50 values of 12.3 and 6.6 μM, respectively.
Scheme 9.
Proposed biosynthetic pathway for conosiligins A–D 256–259 (Reference [101]).
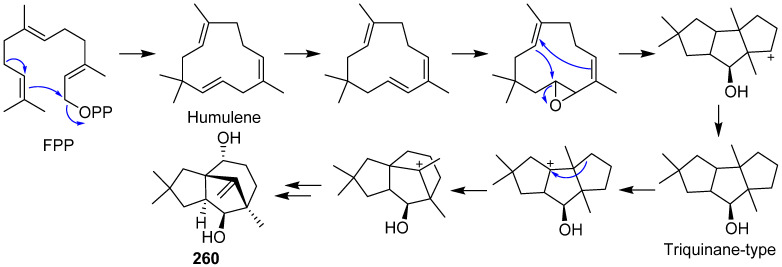
Antroalbocin A 260 possessing a bridged tricyclic system was isolated from cultures of the higher fungus Antrodiella albocinnamomea [102]. The structure with the absolute configuration was determined by extensive spectroscopic methods and single-crystal X-ray diffraction analysis and a plausible biosynthetic pathway for 260 was proposed (Scheme 10).
Scheme 10.
Proposed biosynthetic pathway for antroalbocin A 260 (Reference [102]).
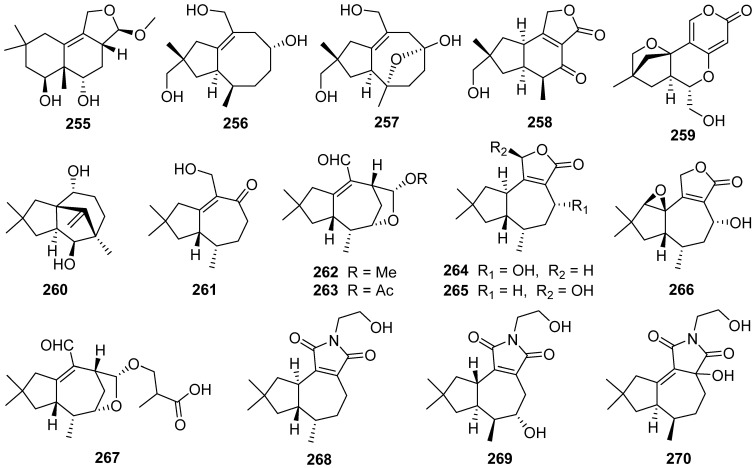
Twenty-two tremulanes, irlactins F–J 261–265, L–M 266–267, irlactam A 268, and irpexolactins A–N 269–282, were isolated from cultures of the medicinal fungus Irpex lacteus [99,103,104,105]. Irlactin I 264 exhibited moderate cytotoxicities on HL-60, SMMC-7721, A-549, MCF-7, and SW480 cells with IC50 values of 16.23, 20.40, 25.55, 19.05, and 18.58 μM, respectively [104].
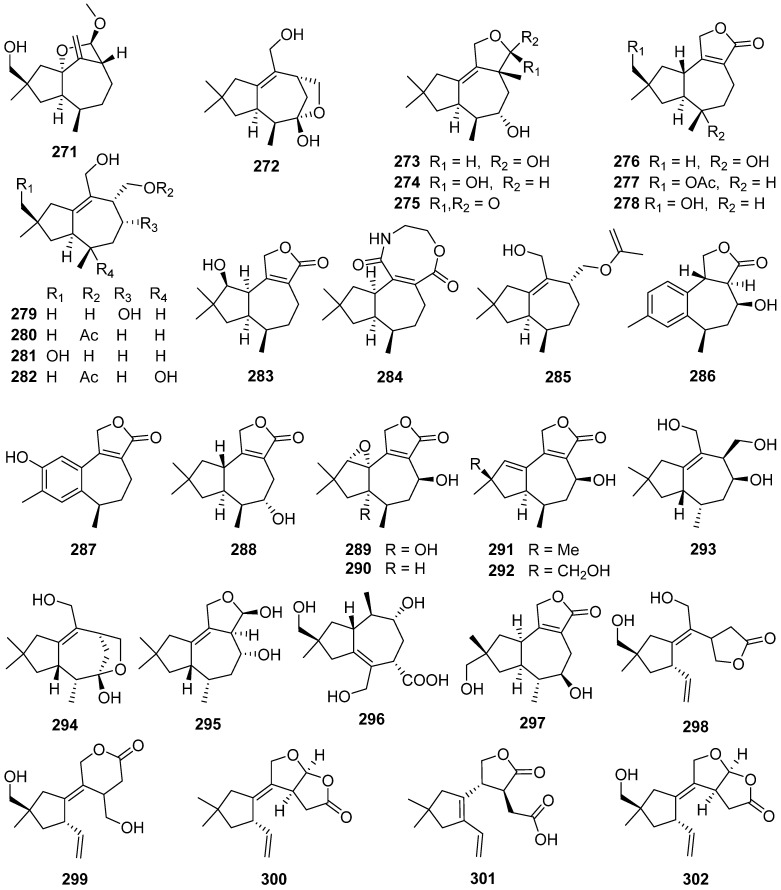 |
 |
Phellinignins A–C 283–285 were new tremulane sesquiterpenoids that have been isolated from Phellinus igniarius [78]. Phellinignins A 283 and B 284 showed certain cytotoxicities to three human cancer cell lines (HL-60, SMMC-7721, and SW480) with the IC50 values of 0.7–17.4 µM, respectively. Tremutins A–G 286–292 were isolated from cultures of the basidiomycetes Irpex lacteus [71]. Tremutins A 286 and B 287 possessed an unusual 6/7-fused ring system that might be derived from a tremulane framework (Scheme 11), 289 and 290 were the first tremulane examples with a 1,2-epoxy moiety to be reported. Tremutin A 286 inhibited the lipopolysaccharide (LPS)-induced proliferation of B lymphocyte cells with an IC50 value of 22.4 μM. Tremutin B 287 inhibited concanavalin A (Con A)-induced T cell proliferation and LPS-induced B lymphocyte cell proliferation with IC50 values of 16.7 and 13.6 μM, respectively.
Scheme 11.
Proposed biosynthetic pathway for tremutins A 286 and B 287 (Reference [71]).
Nigrosirpexin A 293 was produced by Nigrospora oryzae co-cultured with Irpex lacteus [106]. Two new tremulanes 294 and 295 were obtained from different cocultures of Nigrospora oryzae and Irpex lacteus in a solid medium [107]. 5-Demethyl conocenol C 294 showed antifungal activities against Didymella glomerate and Colletotrichum gloeosporioides with MICs of 1 and 8 μg/mL, respectively.
Davotremulanes A–D 296–299 were isolated from a plant-associated fungus X1-2 [108]. Davotremulanes A 296 and B 297 displayed selectively moderate activities to the A549 cell line with IC50 at 15.3, 25.2 μg/mL. A new tremulane sesquiterpenoid analogue 300 was isolated from the cultures of endophytic fungus Colletotrichum capsica [109]. Leptosphin B 301 was isolated from the endophytic fungus Leptosphaeria sp. XL026 [110]. Leptosphin B 301 showed moderate antibacterial activity against Bacillus cereus with a MIC value of 12.5 μg/mL.
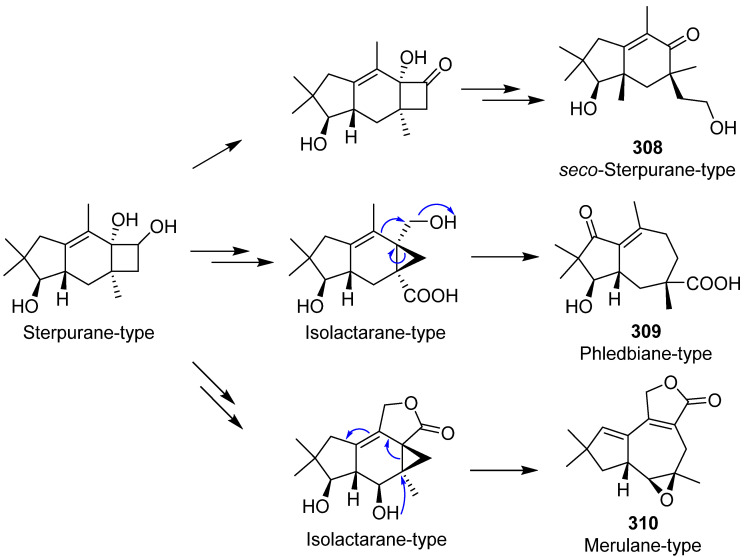
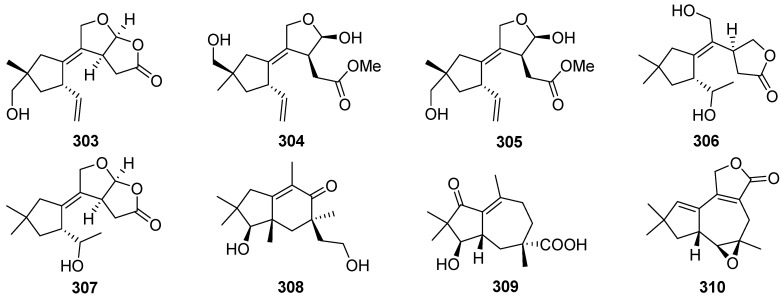
Six 5,6-seco-tremulane analogues 302–307 were isolated from the culture broth of the medicinal fungus Irpex lacteus [111]. Two sesquiterpenes with new carbon skeletons, seco-sterpurane 308 and phlebiane 309, and a novel merulane sesquiterpene 310 were isolated from cultures of the basidiomycete Phlebia tremellosa [112]. The plausible biogenetic pathways of 309 and 310 is shown in Scheme 12.
Scheme 12.
Plausible biogenetic pathways of 308–310 (Reference [112]).
2.9. Trichothecene, Merosesquiterpenoid, Norsesquiterpenoid, and Pyrone
Trichothecenes are a family of sesquiterpenoid mycotoxins produced by multiple genera of fungi, including plant and insect pathogens, and they are toxic to animals and humans and frequently detected in cereal crops [113]. Because of their diversity in structure and biological activity, trichothecenes are of concern in agriculture, food contamination, health care, and building protection.
Trichoderminol 311 was isolated from the filamentous fungus Trichoderma albolutescens [114]. Trichobreols A–E 312–316 were isolated from the marine-derived fungus Trichoderma cf. brevicompactum [115,116]. Trichobreols A–E 312–316 inhibited the growth of two yeast-like fungi, Candida albicans, and Cryptococcus neoformans, with a range of MIC values of 1.6 to 50 μg/mL [115,116]. Three new macrocyclic trichothecenes, miophytocen D 317, roridin F 318, and satratoxin I 319, were isolated from a deadly poisonous mushroom Podostroma cornu-damae [117]. Satratoxin I 319 showed cytotoxic potency to etoposide against four human breast cancer cell lines (Bt549, HCC70, and MDA-MB-231), with IC50 values of 1.8, 7.7, and 3.6 μM, respectively.
Epiroridin acid 320, verrucarins Y 321 and Z 322, and dihydromyrothecine C 323, four new macrocyclic trichothecenes, were isolated from the endophytic fungus Myrothecium roridum [118,119,120,121]. The cytotoxic mechanisms result showed that the epiroridin acid 320 induced the apoptosis of cancer cell HepG-2 via activation of caspase-9 and caspase-3, up-regulation of bax gene expression, down-regulation of bcl-2 gene expression, and disruption of the mitochondrial membrane potential of the HepG-2 cell [118].
Chartarenes A–D 324–327 were isolated from the sponge-derived fungus Stachybotrys chartarum [122]. Chartarenes A–D 324–327 exerted potent or selective inhibition against a panel of tumor cell lines including HCT-116, HepG2, BGC-823, NCI-H1650, and A2780, with IC50 values ranging from 0.68 to 10 µM. In addition, chartarenes B 326, C 327, and D 324 showed potent inhibition against tumor-related kinases FGFR3, IGF1R, PDGFRb, and TRKB, with IC50 values ranging from 0.1 to 12.9 µM.
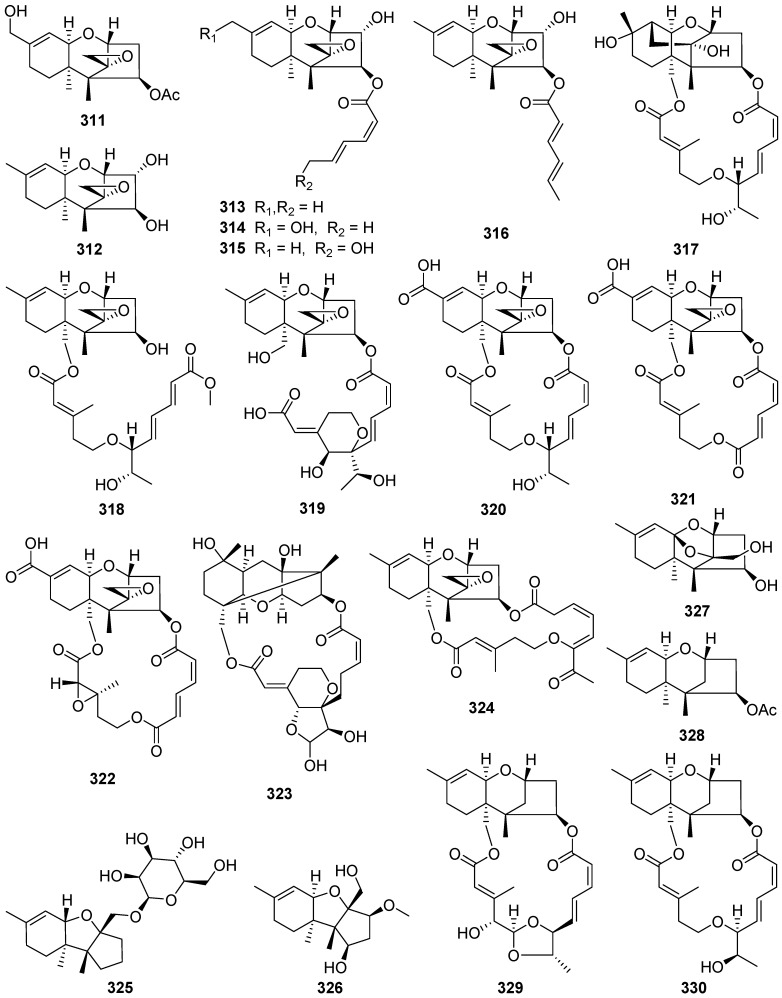 |
12-Deoxytrichodermin 328, 12-deoxyroridin J 329, and 12-deoxyepiisororidin E 330 were isolated from the fungus Calcarisporium arbuscular, and Trichoderma sp., respectively [123,124]. The structure-activity relationship investigation of 328–330 with other known natural trichothecenes against a human colon cancer cell line (COLO201) and filamentous fungus Cochliobolus miyabeanus revealed that the 12-epoxide functionality is essential for the antifungal activity [124].
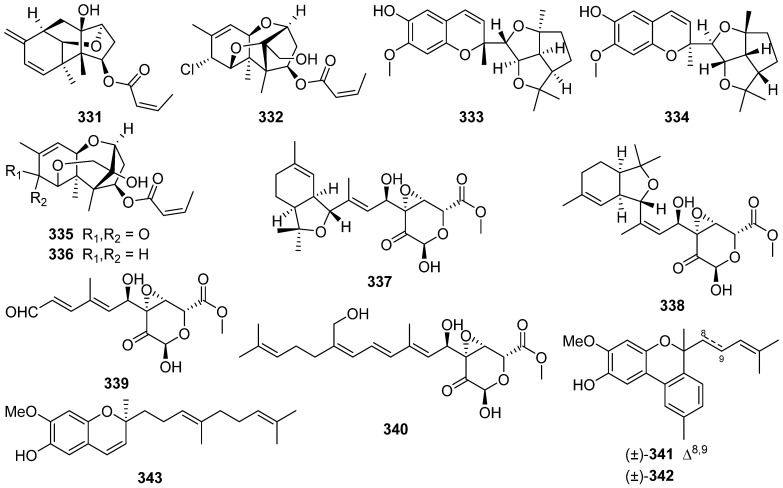 |
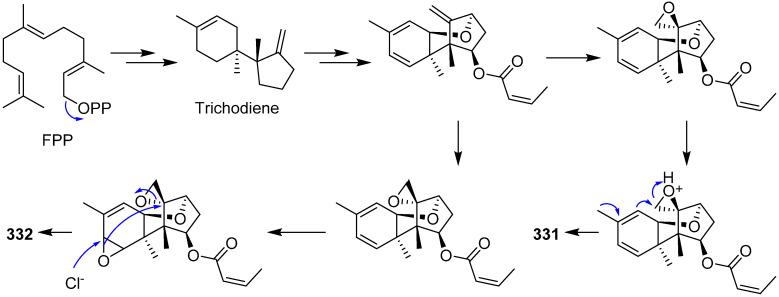
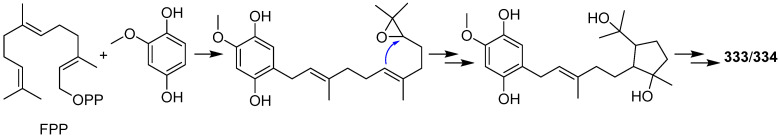
Four novel trichothecene sesquiterpenoids possessing new ring systems, trichothecrotocins A 331 and B 332, trichothecrotocins K 335 and L 336, and a merosesquiterpenoid racemate possesses a novel 6/6–5/5/5 fused ring system, (±)-trichothecrotocin C (333 and 334), and seven new merosesquiterpenoids, trichothecrotocins D–J 337–343, were obtained from potato endophytic fungus Trichothecium crotocinigenum by bioguided isolation (Scheme 13 and Scheme 14) [125,126]. Compounds 337–340 were rare meroterpenoids featuring a seco-phenyl group, while 337 and 338 possessed a novel 6–6/5 fused ring system. Compounds 331–335, and 337–340 showed antiphytopathogenic activities with MIC values of 8–128 μg/mL [125,126].
Scheme 13.
Proposed biosynthetic pathway for 331 and 332 (Reference [126]).
Scheme 14.
Proposed biosynthetic pathway for 333 and 334 (Reference [125]).
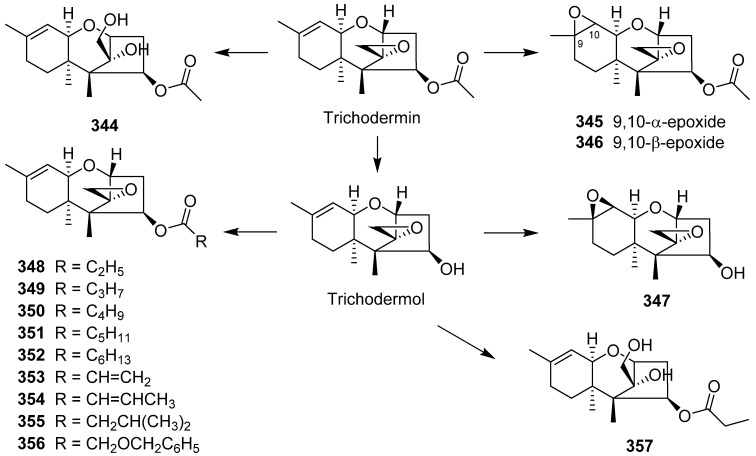
The semisynthesis of several trichodermin and trichodermol derivatives has been developed (Scheme 15) [127]. Some derivatives with a short chain at the C-4 position displayed selective antimicrobial activity against Candida albicans and they showed MIC values similar to those displayed by trichodermin. It was important to highlight the cytotoxic selectivity observed for compounds 350, 354, and 356, which presented average IC50 values of 2 µg/mL and were cytotoxic against tumorigenic cell line MCF-7 (breast carcinoma) and not against Fa2N4 (non-tumoral immortalized human hepatocytes).
Scheme 15.
Chemical transformations were carried out on trichodermin and trichodermol for the preparation of compounds 344–357 (Reference [127]).
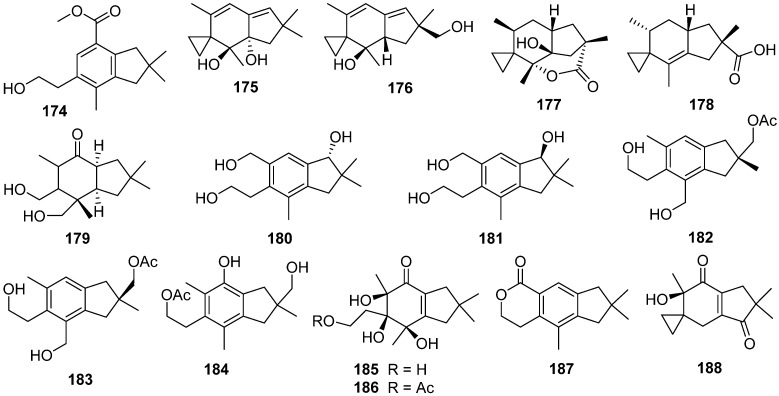
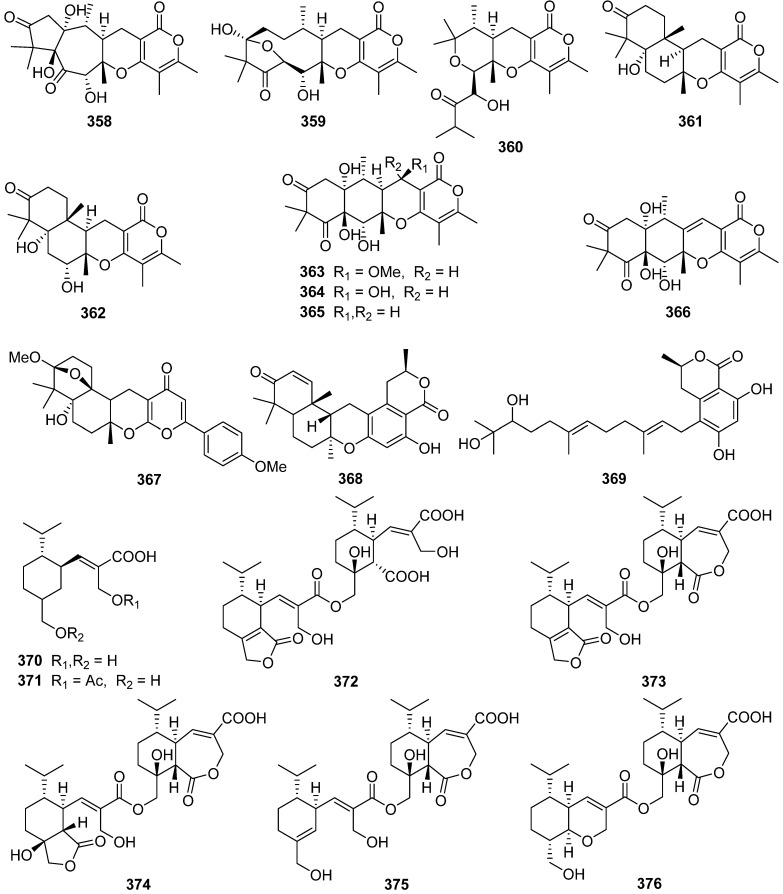
Three novel highly oxygenated α-pyrone merosesquiterpenoids, emerones A–C 358–360, have been obtained from the fungus Emericella sp. XL029 [128]. Structurally, emerone A 358 possessed an unprecedented 5/7 bicyclic ring architecture, emerone B 359 had an unusual substituted 10-membered ring, and emerone C 360 had an undescribed norsesquiterpene skeleton. Ochraceopone F 361, a new α-pyrone merosesquiterpenoid possessing an angular tetracyclic carbon skeleton, was isolated from the marine fungus Aspergillus flocculosus [129].
Five new highly oxygenated α-pyrone merosesquiterpenoids, ochraceopones A–E 362–366, were isolated from an Antarctic soil-derived fungus Aspergillus ochraceopetaliformis [130]. Ochraceopones A–D 363–366 were the first examples of α-pyrone merosesquiterpenoids possessing a linear tetracyclic carbon skeleton. Ochraceopone A 363 exhibited antiviral activities against the H3N2 influenza virus with IC50 values of 12.2 μM. Yaminterritrem C 367 was isolated from a deep-sea-derived fungus Penicillium chrysogenum [131]. Verruculides A 368 and B 369 were isolated from a culture broth of the Indonesian ascidian-derived Penicillium verruculosum [132]. Verruculide A 368 inhibited the activity of PTP1B with an IC50 value of 8.4 μM.
 |
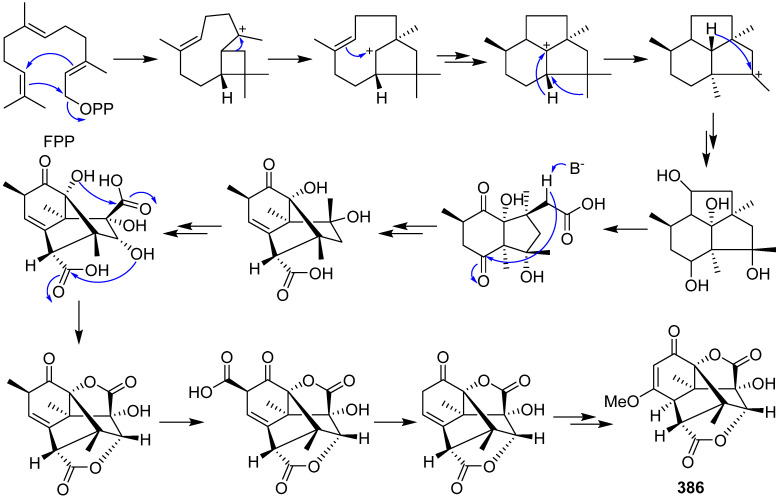
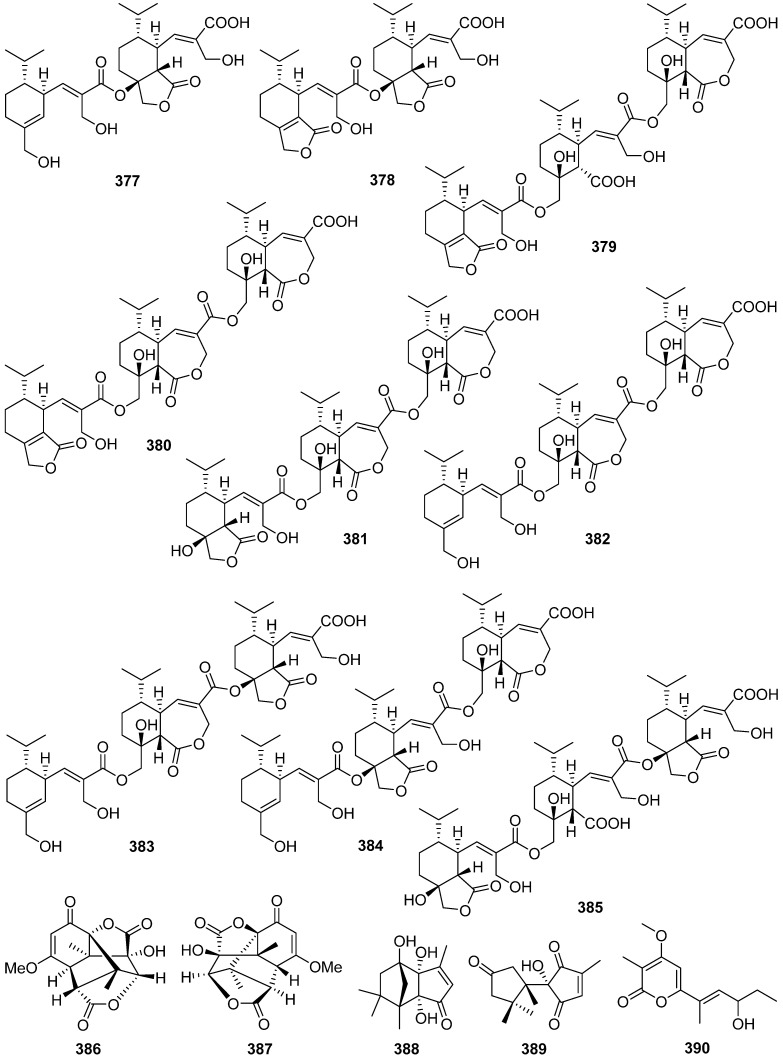
Two new sesquiterpenes 370 and 371 and seven new dimeric norsesquiterpene congeners, divirensols A–G 372–378, along with seven new firstin-class trimeric sesquiterpenes, trivirensols A–G 379–385, were obtained from the Australian termite nest-derived fungus Trichoderma virens [133,134]. A pair of rare naturally enantiomeric norsesquiterpenoids, (±)-preuisolactone A (386 and 387) featuring an unprecedented tricyclo[4.4.01,6.02,8]decane carbon scaffold were isolated from Preussia isomera (plausible biosynthetic pathway shown in Scheme 16) [135]. (±)-Preuisolactone A (386 and 387) exhibited remarkable antibacterial activity against Micrococcus luteus with a MIC value of 10.2 μM.
Scheme 16.
Proposed biosynthetic pathway for (+)-preuisolactone A 386 (Reference [135]).
 |
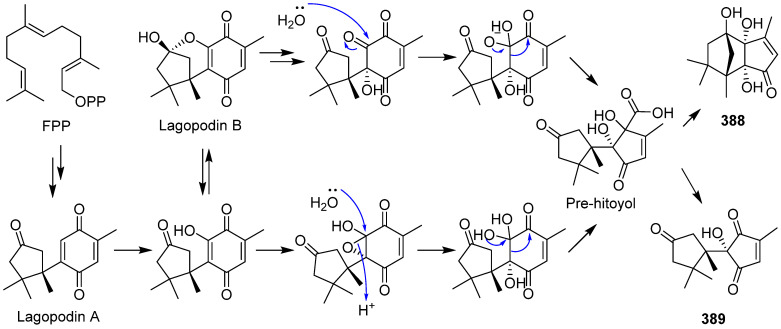
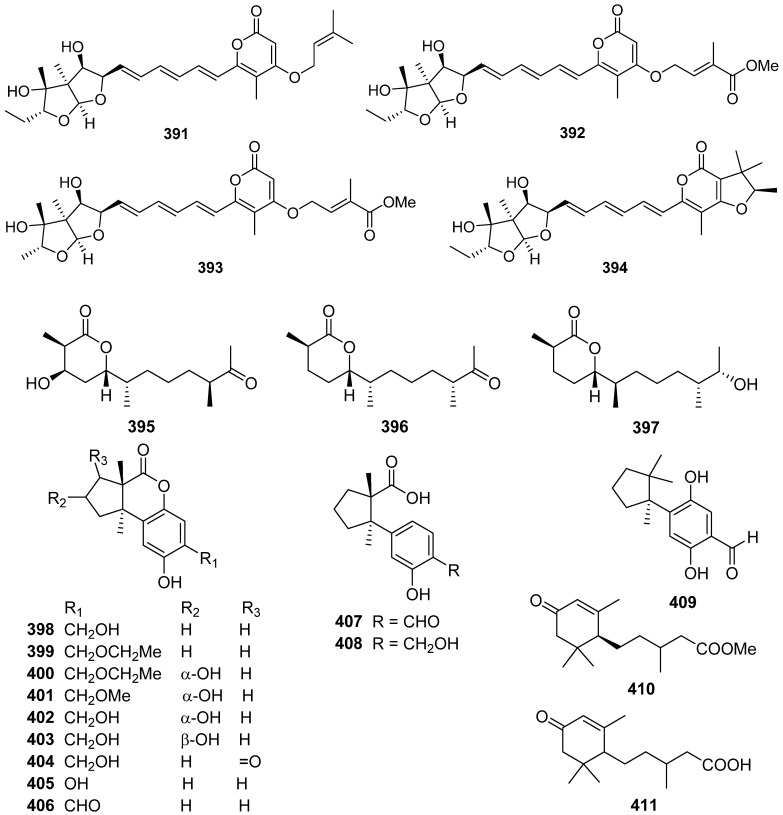
Hitoyol A 388, an unprecedented norsesquiterpenoid with an exo-tricyclo[5.2.1.02,6]decane skeleton, along with a novel skeletal hitoyol B 389 containing 4-cyclopentene-1,3dione, was isolated from the fungus Coprinopsis cinerea [136]. Hitoyol A 388 was possibly biosynthesized through decarboxylation-induced cyclization of lagopodin B, a known cuparene-type sesquiterpenoid (Scheme 17). Hitoyol B 389 showed weak antimalarial activity against Plasmodium falciparum with an IC50 of 59 μM.
Scheme 17.
Plausible biosynthetic pathway for hitoyol A 388 and hitoyol B 389 (Reference [136]).
An α-pyrone 9-hydroxyxylarone 390 was isolated from a culture broth of endophytic fungus Xylaria sp. NC1214 [137]. Four new polyenic α-pyrone mycotoxins, avertoxins A–D 391–394, were obtained from an endophytic fungus Aspergillus versicolor [138]. Avertoxins B 392 and C 393 showed activity against human tumor HCT116 and HeLa cell lines with an IC50 value of 10 μM. And avertoxin B 392 was an active inhibitor against human acetylcholinesterase with the IC50 value of 14.9 μM.
 |
2.10. Other Types
Three new sesquiterpenoids, chermesiterpenoids A–C 395–397, were isolated and identified from the marine red algal-derived fungus Penicillium chermesinum [139]. Chermesiterpenoid B 396 showed antimicrobial activities against the aquatic pathogens Vibrio anguillarum, Vibrio parahaemolyticus, Micrococcus luteus, and human pathogen Escherichia coli with minimum inhibitory concentration (MIC) values of 0.5, 16, 64, and 64 µg/mL, respectively. Similarly, chermesiterpenoid C 397 showed activities against the aquatic pathogens V. anguillarum, V. parahaemolyticus, and M. luteus with MIC values of 1, 32, and 64 µg/mL, respectively. Chermesiterpenoids A–C 395–397 exhibited activity against the plant pathogenic fungus Colletottichum gloeosporioides with MIC values of 64, 32, and 16 µg/mL, respectively.
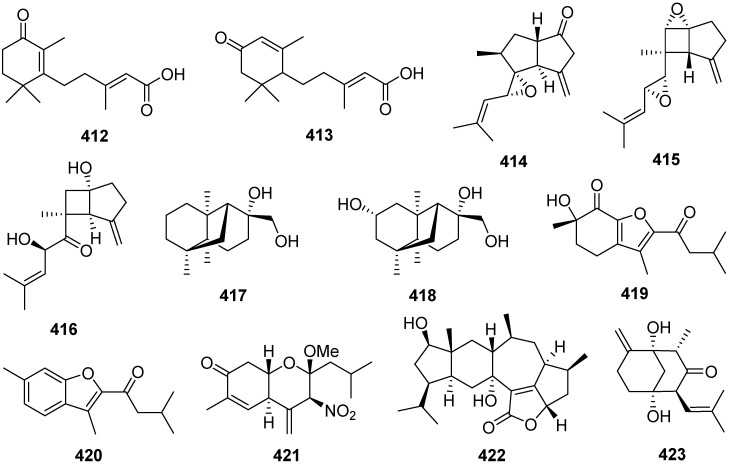 |
Fomitopins A–L 398–409 were isolated via bioassay-guided purification from the bracket fungus Fomitopsis pinicola [140]. Fomitopin K 408 exhibited the most potent anti-inflammatory activity with IC50 of 0.81 μM for inhibition of superoxide anion generation and IC50 of 0.74 μM for inhibition of elastase release. Fomitopins J 407 and L 409 also exhibited moderate inhibition of superoxide anion generation with IC50 of 1.66 and 1.72 μM, respectively.
1-Methoxypestabacillin B 410 was obtained from the solid cultures of a mangrove endophytic fungus Diaporthe sp. SCSIO 41011 [141]. Pestabacillin B 411 was isolated from the co-culture of the endophytic fungus Pestalotiopsis sp. with Bacillus subtilis [19]. Two new abscisic acid-type sesquiterpenes 412 and 413 were isolated from the fermentation extract of Amycolatopsis alba [142]. Pseudapenes A–C 414–416 possessing unique carbon skeletons were isolated from the marine-derived fungus Pseudallescheria apiosperma [143].
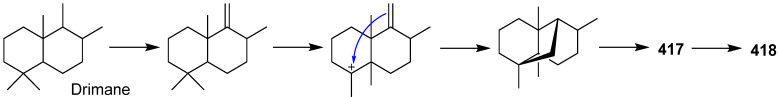
Emericellins A 417 and B 418, representing a new type of sesquiterpenoid with an unprecedented tricyclo[1,2,4,4]hendecane scaffold (Scheme 18), were isolated from the liquid cultures of an endophytic fungus Emericella sp. associated with the leaves of Panax notoginseng [144]. Emericellins A 417 and B 418 displayed moderate activities against three fungal strains (Verticillium dahliae Kleb, Helminthosporium maydis, and Botryosphaeria dothidea) and three bacterial strains (Bacillus subtilis, Bacillus cereus, and Escherichia coli) with MIC values of 25–50 μg/mL.
Scheme 18.
The proposed formation of 417 and 418 from the drimane-type sesquiterpenoid skeleton (Reference [144]).
Stereumenes A–C 419–421 were isolated and identified from the fungus Stereum sp. [145]. Stereumene B 420 showed weak nematicidal activity against Caenorhabditis elegans, which killed 41.1% of C. elegans at 200 mg/L in 24 h. Sesteralterin 422 was obtained from the culture extract of an Alternaria alternata strain isolated from the surface of the marine red alga Lomentaria hakodatensis [146]. Colletotrichine A 423 was obtained from the endophyte fungus Colletotrichum gloeosporioides [147].
Four novel mixed terpenes, stereumamides A–D 424–427, which were sesquiterpenes combined with α-amino acids to form quaternary ammonium hybrids, were isolated from the mycelium of mushroom Stereum hirsutum [148]. Stereumamides A 424 and D 427 showed antibacterial activity against Escherichia coli, Staphylococcus aureus, and Salmonella typhimurium, with MIC values of 12.5–25.0 μg/mL.
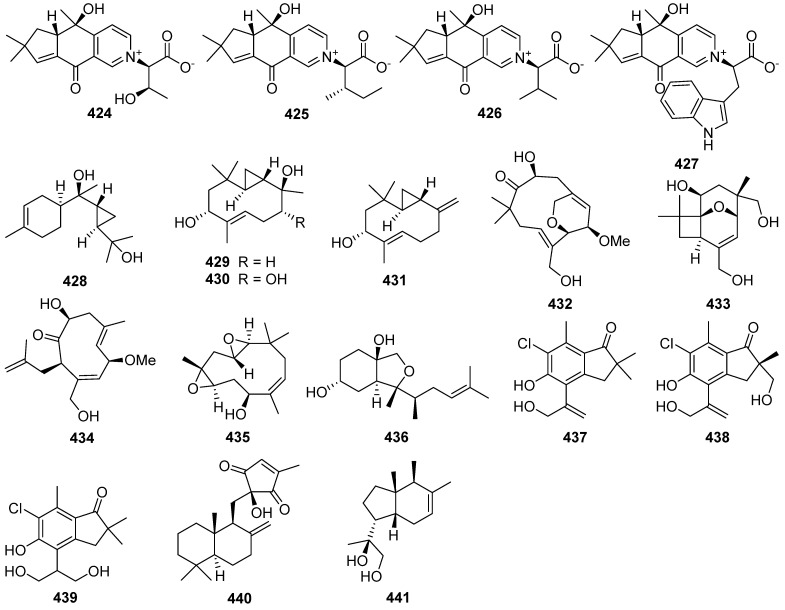 |
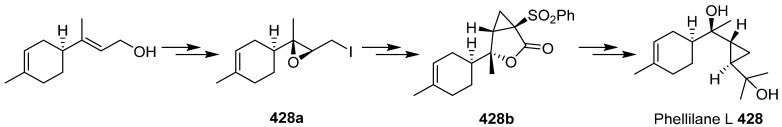
Phellilane L 428, a new cyclopropane-containing sesquiterpenoid, was isolated from the medicinal mushroom Phellinus linteus [149]. The first asymmetric, protecting group-free total synthesis of the sesquiterpenoid phellilane L 428, featuring a highly stereoselective one-pot synthesis involving intermolecular alkylation/cyclization/lactonization on epoxyiodide 428a to construct the key cyclopropane-γ-lactone intermediate 428b has been reported (Scheme 19) [149].
Scheme 19.
Total synthesis of phellilane L 428 (Reference [149]).
Hypocoprins A–C 429–431 have a distinctive ring system consisting of fused cyclopropane and cyclodecene units were isolated from the Coprophilous fungus Hypocopra rostrate [150]. Pestaloporonins A–C 432–434, three new sesquiterpenoids related to the caryophyllene-derived punctaporonins, were isolated from cultures of a fungicolous isolate of Pestalotiopsis sp. MYC-709 [151]. Among them, pestaloporonins A 432 and B 433 contained new bicyclic and tricyclic ring systems, respectively, and the absolute configuration of 432 was confirmed by single-crystal X-ray crystallographic analysis.
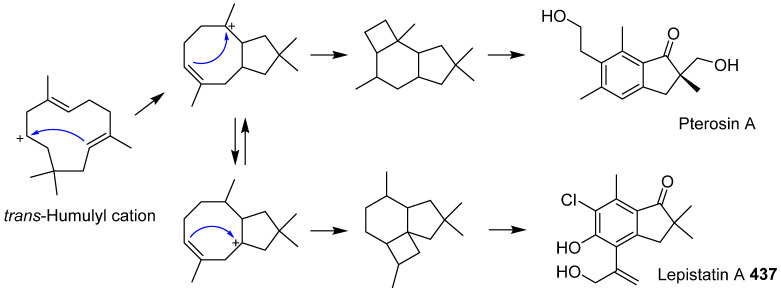
Phomanoxide 435 was isolated from the solid substrate fermentation cultures of the fungus Phoma sp. [152]. Colletotrichine B 436 was produced by the fungal Colletotrichum gloeosporioides [153]. Three new chlorinated sesquiterpenes, lepistatins A–C 437–439, were isolated from the culture broth of Basidiomycete Lepista sordida [154]. The structures of lepistatins A–C 437–439 feature the indanone core structure but differ from other indanone-containing sesquiterpenes of fungal origin by the alkyl substitution pattern. This indicates that lepistatins A–C 437–439 probably possessed a new sesquiterpene scaffold derived from the common precursor, trans-humulyl cation, by an alternative cyclization (Scheme 20).
Scheme 20.
Plausible biosynthetic pathway for lepistatin A 437 and pterosin A (Reference [154]).
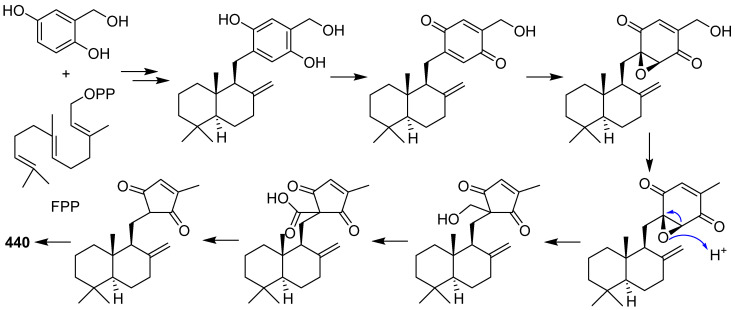
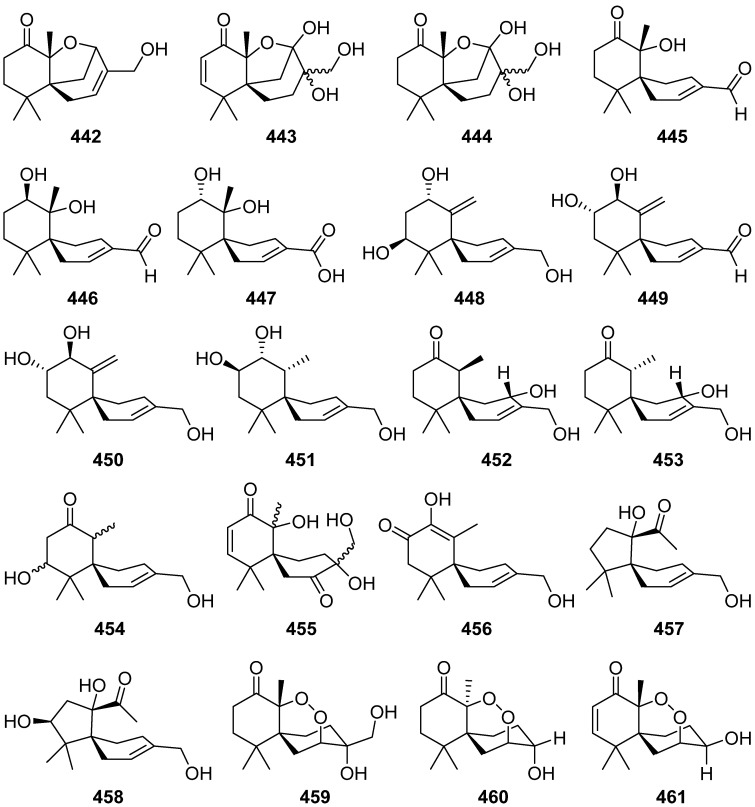
A novel sesquiterpene methylcyclopentenedione, penicilliumin B 440, was obtained from a deep sea-derived fungus Penicillium sp. F00120 [155]. Penicilliumin B 440, presenting the first example with the sesquiterpene cyclopentenedione skeleton as natural products (Scheme 21), was structurally determined by analysis of the NMR and MS spectroscopic data, while the absolute configurations were assigned by single-crystal X-ray experiments. Penicilliumin B 440 with low toxicity showed significant potential to inhibit the kidney fibrogenic action in vitro by a mechanism dependent on disruption of oxidative stress. Seiricardine D 441 was a new bicyclic sesquiterpene obtained from the endophytic fungus Cytospora sp. [156]. Twenty new sesquiterpenes (442–461) were isolated from the endophytic fungus Pseudolagarobasidium acaciico [157]. Among them, compounds 459 and 460 displayed cytotoxicity against several cancer and normal cell lines.
Scheme 21.
Plausible biosynthetic pathway of penicilliumin B 440 (Reference [155]).
 |
3. Biosynthesis
3.1. Asperterpenoid A
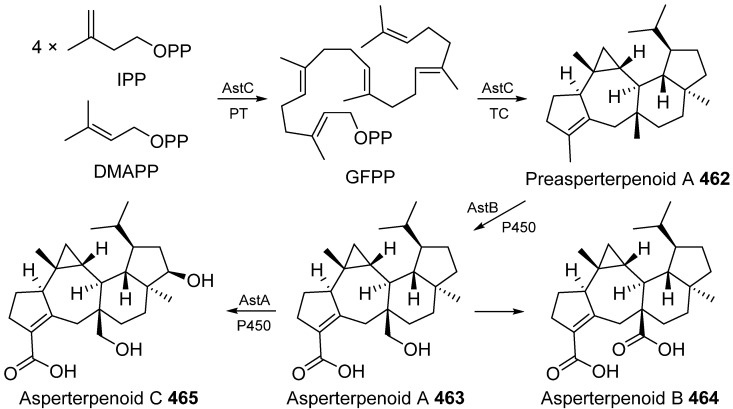
A putative three-gene cluster for asperterpenoid A was identified [158]. Stepwise reconstitution of this gene cluster in Aspergillus oryzae reveals that astC encodes a sesterterpene cyclase to synthesize preasperterpenoid A 462, which was dually oxidized by a P450 enzyme AstB to give asperterpenoid A 463 along with a minor product asperterpenoid B 464, and asperterpenoid A 463 was further oxidized by another P450 enzyme AstA to afford a new sesterterpenoid asperterpenoid C 465 (Scheme 22). Asperterpenoids A 463 and B 464 exhibit potent inhibitory activity against Mycobacterium tuberculosis protein tyrosine phosphatase B with IC50 values of 3–6 μM.
Scheme 22.
Complete biosynthetic pathway of asperterpenoids A–C 463–465 (Reference [158]).
3.2. Cuparene
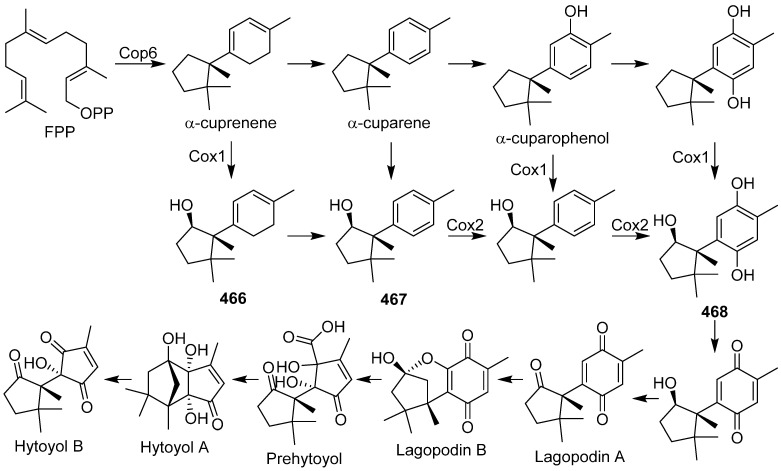
Use of the ku70-deficient strain of Coprinopsis cinerea enabled confirmation within the native context of the central role the sesquiterpene synthase Cop6 plays in lagopodin biosynthesis [159]. Furthermore, yeast in vivo bioconversion and in vitro assays of two cytochrome P450 monooxygenases Cox1 and Cox2 allowed elucidation of the network of oxidation steps that build structural complexity onto the α-cuprenene framework during the biosynthesis of lagopodins (Scheme 23). Three new compounds 466–468 were identified as intermediates formed by the redox enzymes.
Scheme 23.
The proposed biosynthetic pathway of lagopodins and hytoyols (Reference [159]).
3.3. Fusariumdiene and Fusagramineol
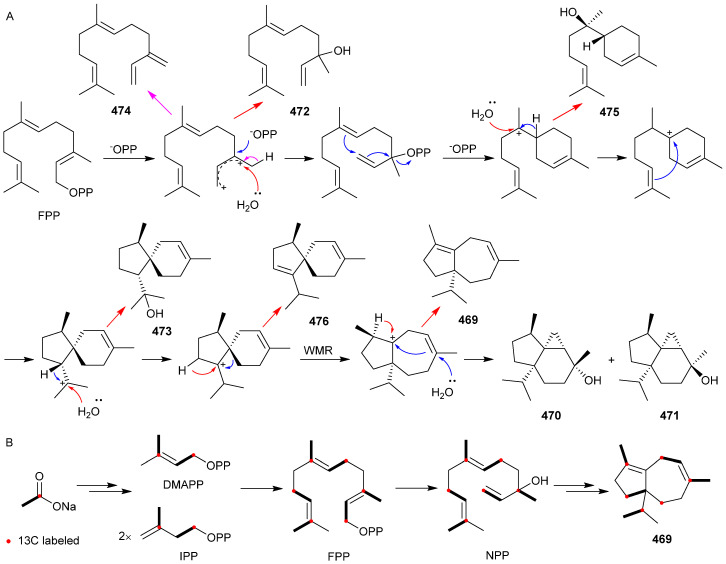
The novel sesquiterpenes fusariumdiene 469, epi-fusagramineol 470, and fusagramineol 471 with 5/7 bicyclic and 5/6/3 tricyclic ring systems, respectively, as well as five known sesquiterpenes 472–476 have been produced by exploiting the potential power of sesquiterpene synthase FgJ03939 from Fusarium graminearum in a farnesyl diphosphate-overexpressing Saccharomyces cerevisiae chassis (Scheme 24) [160].
Scheme 24.
(A) Proposed mechanisms for the enzymatic cyclization of FPP to compounds 469–476; (B) Summary of feeding experiments with [1-13C,2H3] sodium acetate (Reference [160]).
3.4. Hirsutenoid
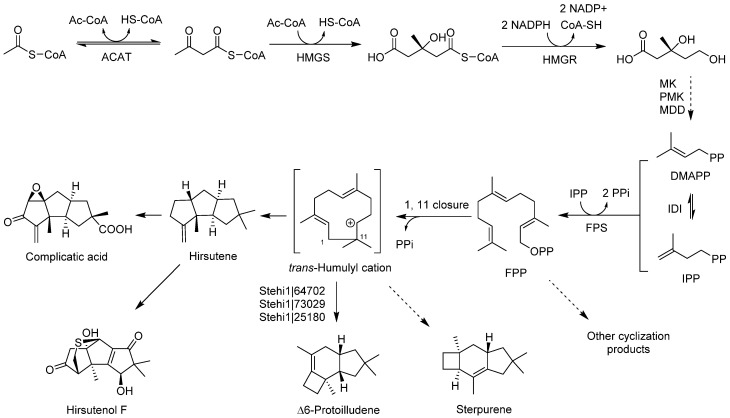
The identification and successful cloning of the previously elusive hirsutene synthase from the wood-rotting mushroom Stereum hirsutum provide the biosynthetic pathways of hirsutane-type sesquiterpenoids (Scheme 25) [161]. The hirsutene synthase, as an unexpected fusion protein of a sesquiterpene synthase (STS) with a C-terminal 3-hydroxy-3-methylglutaryl-coenzyme A (3-hydroxy-3-methylglutaryl-CoA) synthase (HMGS) domain, was part of a biosynthetic gene cluster that includes P450s and oxidases that were expressed and could be cloned from cDNA.
Scheme 25.
Overview of pathways to trans-humulyl cation-derived sesquiterpenoids in S. hirsutum (Reference [161]).
3.5. Koraidiol
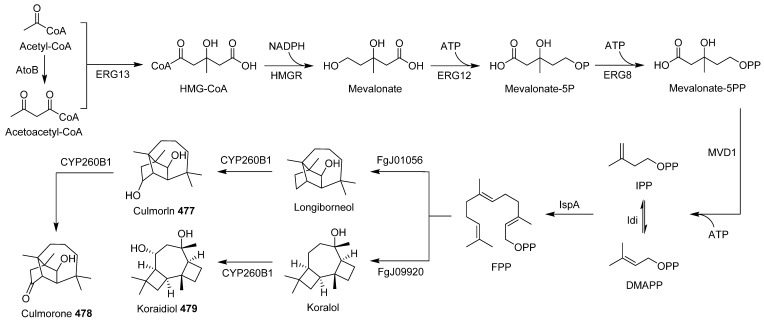
Two known oxygenated sesquiterpenoid products, culmorin 477 and culmorone 478, and a new compound, koraidiol 479, were successfully generated and characterized by a combinatorial biosynthesis approach which was utilized by the combination of a promiscuous myxobacterial P450 (CYP260B1) with two sesquiterpene cyclases (FgJ01056, FgJ09920) of filamentous fungi Fusarium graminearum (Scheme 26) [162].
Scheme 26.
Biosynthesis pathways of culmorin 477, culmorone 478, and koraidiol 479 in E. coli (Reference [162]).
3.6. Protoilludenes
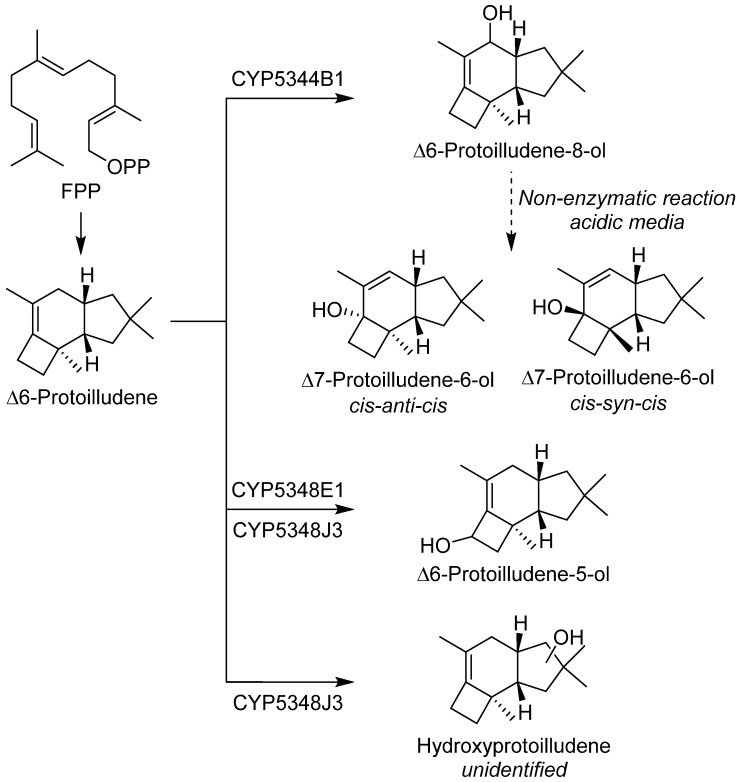
Sixteen sesquiterpene synthases genes as full-length cDNAs have been isolated by using RT-PCR, and heterologous expression revealed that the sesquiterpene synthases could produce a series of sesquiterpene scaffolds with distinct metabolic profiles (Scheme 27) [163].
Scheme 27.
Reaction pathways of protoilludene metabolism by PpSTS-08 and PpCYPs (Reference [163]).
3.7. Trichothecenes
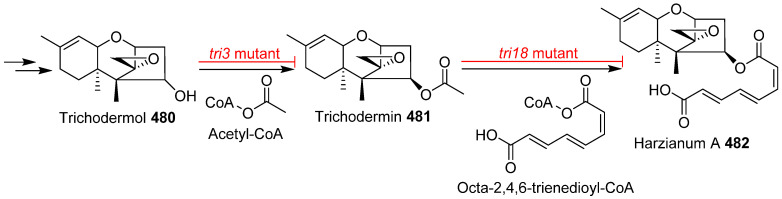
The acyltransferase-encoding gene tri18-encoded acyltransferase (TRI18) and a previously characterized acyltransferase (TRI3) were required in the saprotroph Trichoderma arundinaceum for conversion of the trichothecene biosynthetic intermediate trichodermol 480 to harzianum A 482, an antifungal trichothecene analog with an octa-2,4,6-trienedioyl acyl group [164]. Previous studies indicate that tri18 may not be necessary for the biosynthesis of harzianum A 482 because all catalytic activities required for its formation can be accounted for by activities of enzymes (TRI5, TRI4, TRI22, TRI17, and TRI3) encoded by other tri genes [165,166]. Further analysis proposed that TRI3 catalyzes trichothecene 4-O-acetylation, and subsequently, TRI18 catalyzes replacement of the resulting acetyl group with octa-2,4,6-trienedioyl to form harzianum A 482 (Scheme 28) [164].
Scheme 28.
Biosynthesis pathways of trichodermol 480 to harzianum A 482. The symbol ⊥ indicates that the pathway is partially or completely blocked at the step indicated in the tri3 and tri18 mutants (Reference [164]).
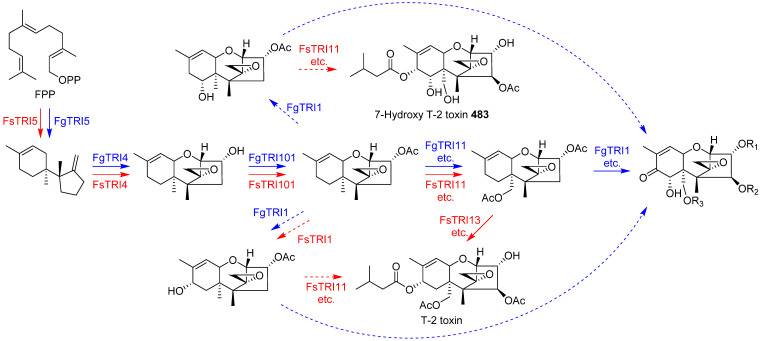
An artificial metabolic route to an unnatural trichothecene was designed by taking advantage of the broad substrate specificities of the T-2 toxin biosynthetic enzymes of Fusarium sporotrichioides [167]. By feeding 7-hydroxyisotrichodermin, a shunt pathway metabolite of F. graminearum, to a trichodiene synthase-deficient mutant of F. sporotrichioides, 7-hydroxy T-2 toxin 483 was obtained as the final metabolite (Scheme 29). The toxicity of 7-hydroxy T-2 toxin 483 was 10 times lower than that of T-2 toxin in HL-60 cells.
Scheme 29.
Biosynthetic approach to generate 7-hydroxy T-2 toxin 483. Red arrows indicate the metabolic pathway of F. sporotrichioides, whereas blue arrows indicate that of F. graminearum. Solid arrows indicate the main pathway, and dotted arrows indicate the shunt pathway (Reference [167]).
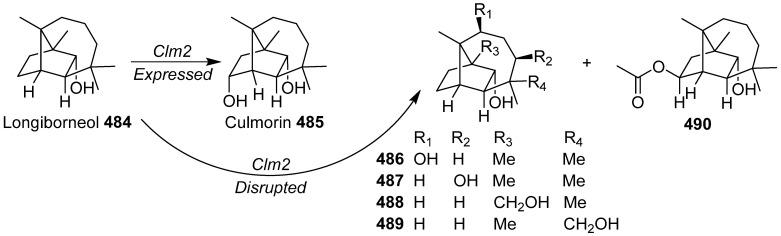
The candidate gene, Clm2, a second structural gene required for culmorin biosynthesis in the plant pathogen Fusarium graminearum, encodes a regio- and stereoselective cytochrome P450 monooxygenase for C-11 of longiborneol 484 (Scheme 30) [168]. Clm2 gene disruptants were grown in liquid culture and assessed for culmorin production via HPLC-evaporative light scattering detection. The analysis indicated a complete loss of culmorin 485 from the liquid culture of the ΔClm2 mutants. Culmorin production resumed in a ΔClm2 complementation experiment. A detailed analysis of the secondary metabolites extracted from the largescale liquid culture of disruptant ΔClm2D20 revealed five new natural products: 486–490. The structures of the new compounds were elucidated by a combination of HRMS, 1D and 2D NMR, and single-crystal X-ray crystallography analysis.
Scheme 30.
Biosynthetic pathways of 484–490 (Reference [168]).
4. Conclusions and Future Prospects
Natural products, in particular bioactive molecules as precursor pharmaceutical compounds, have attracted particular attention in the field of health promotion and drug discovery and development. Compared with other sources, fungal species play a decisive role in bio-transformations and drug synthesis owing to their wide varieties, easy cultivation, diverse chemical compositions, and distinct biological activities. This process has been accelerated by considerable advances in microbial genome research and in understanding the structure of genes and their corresponding products. Genome mining-based natural products discovery programs mainly use the most identifiable terpene synthases and prenyltransferases to locate and quickly identify new terpenoids. In the last five years, nearly 500 new sesquiterpenes, including about 20 new skeletons were identified from fungi. These sesquiterpenoids exhibit various biological activities, such as anti-tumor, anti-viral, anti-microbial, anti-inflammatory, etc. These efforts have clearly led to a global promotion of discovery and characterization of fungal terpenoids and offer optimism for the future of fungal terpenoid discovery.
This review summarized the isolation, chemical structures, plausible biosynthetic pathways, bioactivity, chemical synthesis, and biosynthesis of 490 recent sesquiterpenoids. This could be a useful reference for modern researchers studying this category of compounds.
Author Contributions
Q.D. and F.-L.Z. contributed equally to this paper. Conceptualization, T.F.; resources, Q.D. and F.-L.Z.; discussion of the contents, Q.D., F.-L.Z. and T.F.; writing—original draft preparation, Q.D. and F.-L.Z.; writing—review and editing, and editing, T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (grant number 81872762) and the Research Fund of Central University, South-Central University for Nationalities (grant number CZP21001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Christianson D.W. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol. 2008;12:141–150. doi: 10.1016/j.cbpa.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minami A., Ozaki T., Liu C., Oikawa H. Cyclopentane-forming di/sesterterpene synthases: Widely distributed enzymes in bacteria, fungi, and plants. Nat. Prod. Rep. 2018;35:1330–1346. doi: 10.1039/C8NP00026C. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt-Dannert C. In: Biosynthesis of Terpenoid Natural Products in Fungi. Advances in Biochemical Engineering-Biotechnology. Schrader J., Bohlmann J., editors. Volume 148. Springer; New York, NY, USA: 2015. pp. 19–61. [DOI] [PubMed] [Google Scholar]
- 4.Li D., Wang K.W. Natural new sesquiterpenes: Structural diversity and bioactivity. Curr. Org. Chem. 2016;20:994–1042. doi: 10.2174/1385272819666151008014405. [DOI] [Google Scholar]
- 5.Fraga B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012;29:1334–1366. doi: 10.1039/c2np20074k. [DOI] [PubMed] [Google Scholar]
- 6.Chen H.P., Liu J.K. Secondary metabolites from higher fungi. Prog. Chem. Org. Nat. Prod. 2017;106:1–201. doi: 10.1007/978-3-319-59542-9_1. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez Del Val A., Platas G., Arenal F., Orihuela J.C., Garcia M., Hernandez P., Royo I., De Pedro N., Silver L.L., Young K., et al. Novel illudins from Coprinopsis episcopalis (syn. Coprinus episcopalis), and the distribution of illudin-like compounds among filamentous fungi. Mycol. Res. 2003;107:1201–1209. doi: 10.1017/S0953756203008487. [DOI] [PubMed] [Google Scholar]
- 8.Alexandre J., Raymond E., Kaci M.O., Brain E.C., Lokiec F., Kahatt C., Faivre S., Yovine A., Goldwasser F., Smith S.L., et al. Phase I and pharmacokinetic study of irofulven administered weekly or biweekly in advanced solid tumor patients. Clin. Cancer Res. 2004;10:3377–3385. doi: 10.1158/1078-0432.CCR-03-0349. [DOI] [PubMed] [Google Scholar]
- 9.Tanasova M., Sturla S.J. Chemistry and biology of acylfulvenes: Sesquiterpene-derived antitumor agents. Chem. Rev. 2012;112:3578–3610. doi: 10.1021/cr2001367. [DOI] [PubMed] [Google Scholar]
- 10.McMullen M., Jones R., Gallenberg D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 11.Eriksen G.S., Pettersson H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004;114:205–239. doi: 10.1016/j.anifeedsci.2003.08.008. [DOI] [Google Scholar]
- 12.Qinghua W., Vlastimil D., Kami K., Zonghui Y. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013;14:641–660. doi: 10.2174/1389200211314060002. [DOI] [PubMed] [Google Scholar]
- 13.Pascari X., Maul R., Kemmlein S., Marin S., Sanchis V. The fate of several trichothecenes and zearalenone during roasting and enzymatic treatment of cereal flour applied in cereal-based infant food production. Food Control. 2020;114:107245. doi: 10.1016/j.foodcont.2020.107245. [DOI] [Google Scholar]
- 14.Zhou Z.Y., Liu J.K. Pigments of fungi (macromycetes) Nat. Prod. Rep. 2010;27:1531–1570. doi: 10.1039/c004593d. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M.Y., Feng T., Liu J.K. N-Containing compounds of macromycetes. Nat. Prod. Rep. 2011;28:783–808. doi: 10.1039/c0np00006j. [DOI] [PubMed] [Google Scholar]
- 16.Isaka M., Sappan M., Supothina S., Srichomthong K., Komwijit S., Boonpratuang T. Alliacane sesquiterpenoids from submerged cultures of the basidiomycete Inonotus sp. BCC 22670. Phytochemistry. 2017;136:175–181. doi: 10.1016/j.phytochem.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Tao Q.Q., Ma K., Bao L., Wang K., Han J.J., Zhang J.X., Huang C.Y., Liu H.W. New sesquiterpenoids from the edible mushroom Pleurotus cystidiosus and their inhibitory activity against alpha-glucosidase and PTP1B. Fitoterapia. 2016;111:29–35. doi: 10.1016/j.fitote.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Tala M.F., Qin J.C., Ndongo J.T., Laatsch H. New azulene-type sesquiterpenoids from the fruiting bodies of Lactarius deliciosus. Nat. Prod. Bioprospect. 2017;7:269–273. doi: 10.1007/s13659-017-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S., Dai H.F., Heering C., Janiak C., Lin W.H., Liu Z., Proksch P. Inducing new secondary metabolites through co-cultivation of the fungus Pestalotiopsis sp. with the bacterium Bacillus subtilis. Tetrahedron Lett. 2017;58:257–261. doi: 10.1016/j.tetlet.2016.12.026. [DOI] [Google Scholar]
- 20.Cane D.E. Enzymic formation of sesquiterpenes. Chem. Rev. 1990;90:1089–1103. doi: 10.1021/cr00105a002. [DOI] [Google Scholar]
- 21.Fraga B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013;30:1226–1264. doi: 10.1039/c3np70047j. [DOI] [PubMed] [Google Scholar]
- 22.Massias M., Rebuffat S., Molho L., Chiaroni A., Riche C., Bodo B. Expansolides A and B: Tetracyclic sesquiterpene lactones from Penicillium expansum. J. Am. Chem. Soc. 1990;112:8112–8115. doi: 10.1021/ja00178a039. [DOI] [Google Scholar]
- 23.Oh H., Gloer J.B., Shearer C.A. Massarinolins A-C: New bioactive sesquiterpenoids from the aquatic fungus Massarina tunicata. J. Nat. Prod. 1999;62:497–501. doi: 10.1021/np980447+. [DOI] [PubMed] [Google Scholar]
- 24.Che Y., Gloer J.B., Koster B., Malloch D. Decipinin A and decipienolides A and B: New bioactive metabolites from the coprophilous fungus Podospora decipiens. J. Nat. Prod. 2002;65:916–919. doi: 10.1021/np010575p. [DOI] [PubMed] [Google Scholar]
- 25.MacíAs F.A., Varela R.M., Simonet A.M., Cutler H.G., Cutler S.J., Hill R.A. Absolute configuration of bioactive expansolides A and B from Aspergillus fumigatus Fresenius. Tetrahedron Lett. 2003;44:941–943. doi: 10.1016/S0040-4039(02)02778-8. [DOI] [Google Scholar]
- 26.Wang Y.N., Xia G.Y., Wang L.Y., Ge G.B., Zhang H.W., Zhang J.F., Wu Y.Z., Lin S. Purpurolide A, 5/5/5 spirocyclic sesquiterpene lactone in nature from the endophytic fungus Penicillium purpurogenum. Org. Lett. 2018;20:7341–7344. doi: 10.1021/acs.orglett.8b03323. [DOI] [PubMed] [Google Scholar]
- 27.Xia G.Y., Wang L.Y., Zhang J.F., Wu Y.Z., Ge G.B., Wang Y.N., Lin P.C., Lin S. Three new polyoxygenated bergamotanes from the endophytic fungus Penicillium purpurogenum IMM 003 and their inhibitory activity against pancreatic lipase. Chin. J. Nat. Med. 2020;18:75–80. doi: 10.1016/S1875-5364(20)30007-8. [DOI] [PubMed] [Google Scholar]
- 28.Ying Y.M., Fang C.A., Yao F.Q., Yu Y., Shen Y., Hou Z.N., Wang Z., Zhang W., Shan W.G., Zhan Z.J. Bergamotane sesquiterpenes with α-glucosidase inhibitory activity from the plant pathogenic fungus Penicillium expansum. Chem. Biodivers. 2017;14:e1600184. doi: 10.1002/cbdv.201600184. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z.Z., Zhao K., Chen H.P., Bai X., Zhang L., Liu J.K. Terpenoids from the mushroom-associated fungus Montagnula donacina. Phytochemistry. 2018;147:21–29. doi: 10.1016/j.phytochem.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Li D.H., Li Z.L., Sun Y.J., Hua H.M., Liu T., Bai J. Terpenoids from the marine-derived fungus Aspergillus fumigatus YK-7. Molecules. 2016;21:31. doi: 10.3390/molecules21010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z., Ren F.X., Che Y.S., Liu G., Liu L. New bergamotane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Molecules. 2015;20:14611–14620. doi: 10.3390/molecules200814611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L.H., Feng B.M., Chen G., Li S.G., Sun Y., Wu H.H., Bai J., Hua H.M., Wang H.F., Pei Y.H. Sporulaminals A and B: A pair of unusual epimeric spiroaminal derivatives from a marine-derived fungus Paraconiothyrium sporulosum YK-03. RSC Adv. 2016;6:42361–42366. doi: 10.1039/C6RA01401A. [DOI] [Google Scholar]
- 33.Tao Q.Q., Ma K., Bao L., Wang K., Han J.J., Wang W.Z., Zhang J.X., Huang C.Y., Liu H.W. Sesquiterpenoids with PTP1B inhibitory activity and cytotoxicity from the edible mushroom Pleurotus citrinopileatus. Planta Med. 2016;82:639–644. doi: 10.1055/s-0041-111629. [DOI] [PubMed] [Google Scholar]
- 34.Tao Q.Q., Ma K., Yang Y.L., Wang K., Chen B.S., Huang Y., Han J.J., Bao L., Liu X.B., Yang Z.L., et al. Bioactive sesquiterpenes from the edible mushroom Flammulina velutipes and their biosynthetic pathway confirmed by genome analysis and chemical evidence. J. Org. Chem. 2016;81:9867–9877. doi: 10.1021/acs.joc.6b01971. [DOI] [PubMed] [Google Scholar]
- 35.Chen C.M., Sun W.G., Liu X.R., Wei M.S., Liang Y., Wang J.P., Zhu H.C., Zhang Y.H. Anti-inflammatory spiroaxane and drimane sesquiterpenoids from Talaromyces minioluteus (Penicillium minioluteum) Bioorg. Chem. 2019;91:103166. doi: 10.1016/j.bioorg.2019.103166. [DOI] [PubMed] [Google Scholar]
- 36.Yang X.Y., Niu W.R., Li R.T., Cui X.M., Liu J.K. Two new sesquiterpenes from cultures of the higher fungus Pholiota nameko. Nat. Prod. Res. 2018;33:1992–1996. doi: 10.1080/14786419.2018.1483921. [DOI] [PubMed] [Google Scholar]
- 37.Wang S.R., Zhang L., Chen H.P., Li Z.H., Dong Z.J., Wei K., Liu J.K. Four new spiroaxane sesquiterpenes and one new rosenonolactone derivative from cultures of Basidiomycete Trametes versicolor. Fitoterapia. 2015;105:127–131. doi: 10.1016/j.fitote.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Hu Z.B., Tao Y.W., Tao X.Y., Su Q.H., Cai J.C., Qin C., Ding W.J., Li C.Y. Sesquiterpenes with phytopathogenic fungi inhibitory activities from fungus Trichoderma virens from Litchi chinensis Sonn. J. Agric. Food Chem. 2019;67:10646–10652. doi: 10.1021/acs.jafc.9b04053. [DOI] [PubMed] [Google Scholar]
- 39.Liu S., Zhao Y.P., Heering C., Janiak C., Muller W.E.G., Akone S.H., Liu Z., Proksch P. Sesquiterpenoids from the endophytic fungus Rhinocladiella similis. J. Nat. Prod. 2019;82:1055–1062. doi: 10.1021/acs.jnatprod.8b00938. [DOI] [PubMed] [Google Scholar]
- 40.Duan R.T., Yang R.N., Li H.T., Tang L.H., Liu T., Yang Y.B., Zhou H., Ding Z.T. Peniterester, a carotane-type antibacterial sesquiterpene from an artificial mutant Penicillium sp. T2-M20. Fitoterapia. 2020;140:104422. doi: 10.1016/j.fitote.2019.104422. [DOI] [PubMed] [Google Scholar]
- 41.Xing C.P., Xie C.L., Xia J.M., Liu Q.M., Lin W.X., Ye D.Z., Liu G.M., Yang X.W. Penigrisacids A-D, four new sesquiterpenes from the deep-sea-derived Penicillium griseofulvum. Mar. Drugs. 2019;17:507. doi: 10.3390/md17090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afiyatullov S.S., Zhuravleva O.I., Antonov A.S., Leshchenko E.V., Pivkin M.V., Khudyakova Y.V., Denisenko V.A., Pislyagin E.A., Kim N.Y., Berdyshev D.V., et al. Piltunines A-F from the marine-derived fungus Penicillium piltunense KMM 4668. Mar. Drugs. 2019;17:647. doi: 10.3390/md17110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Z.Z., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg. Chem. 2018;81:319–325. doi: 10.1016/j.bioorg.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J., Liu S.S., Yuan W.Y., Wei J.J., Zhao Y.X., Luo D.Q. Carotane-type sesquiterpenes from cultures of the insect pathogenic fungus Isaria fumosorosea. J. Asian Nat. Prod. Res. 2017;21:234–240. doi: 10.1080/10286020.2017.1410143. [DOI] [PubMed] [Google Scholar]
- 45.Li Y.L., Liu W., Xu W., Zeng X., Cheng Z.B., Li Q. Aspterrics A and B, new sesquiterpenes from deep sea-derived fungus Aspergillus terreus YPGA10. Rec. Nat. Prod. 2020;14:18–22. doi: 10.25135/rnp.131.19.04.1247. [DOI] [Google Scholar]
- 46.Liu X.H., Hou X.L., Song Y.P., Wang B.G., Ji N.Y. Cyclonerane sesquiterpenes and an isocoumarin derivative from the marine-alga-endophytic fungus Trichoderma citrinoviride A-WH-20-3. Fitoterapia. 2020;141:104469. doi: 10.1016/j.fitote.2020.104469. [DOI] [PubMed] [Google Scholar]
- 47.Shi T., Shao C.L., Liu Y., Zhao D.L., Cao F., Fu X.M., Yu J.Y., Wu J.S., Zhang Z.K., Wang C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020;11:572. doi: 10.3389/fmicb.2020.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang S.T., Wang Y.J., Ma X.Y., Yin X.L., Ji N.Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1-4. Nat. Prod. Res. 2019;33:3127–3133. doi: 10.1080/14786419.2018.1522314. [DOI] [PubMed] [Google Scholar]
- 49.Song Y.P., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018;81:2553–2559. doi: 10.1021/acs.jnatprod.8b00714. [DOI] [PubMed] [Google Scholar]
- 50.Jiang C.X., Li J., Zhang J.M., Jin X.J., Yu B., Fang J.G., Wu Q.X. Isolation, identification, and activity evaluation of chemical constituents from soil fungus Fusarium avenaceum SF-1502 and endophytic fungus Fusarium proliferatum AF-04. J. Agric. Food Chem. 2019;67:1839–1846. doi: 10.1021/acs.jafc.8b05576. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M., Zhao J.L., Liu J.M., Chen R.D., Xie K.B., Chen D.W., Feng K.P., Zhang D., Dai J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017;19:651–658. doi: 10.1080/10286020.2016.1251908. [DOI] [PubMed] [Google Scholar]
- 52.Song Y.P., Miao F.P., Liu X.H., Yin X.L., Ji N.Y. Cyclonerane derivatives from the algicolous endophytic fungus Trichoderma asperellum A-YMD-9-2. Mar. Drugs. 2019;17:252. doi: 10.3390/md17050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Y.P., Liu X.H., Shi Z.Z., Miao F.P., Fang S.T., Ji N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry. 2018;152:45–52. doi: 10.1016/j.phytochem.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Qin D., Wang L., Han M.J., Wang J.Q., Song H.C., Yen X., Duan X.X., Dong J.Y. Effects of an endophytic fungus Umbelopsis dimorpha on the secondary metabolites of host-plant Kadsura angustifolia. Front. Microbiol. 2018;9:2845. doi: 10.3389/fmicb.2018.02845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isaka M., Yangchum A., Supothina S., Boonpratuang T., Choeyklin R., Kongsaeree P., Prabpai S. Aromadendrane and cyclofarnesane sesquiterpenoids from cultures of the basidiomycete Inonotus sp. BCC 23706. Phytochemistry. 2015;118:94–101. doi: 10.1016/j.phytochem.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Lei H., Lin X.P., Han L., Ma J., Ma Q.J., Zhong J.L., Liu Y.H., Sun T.M., Wang J.H., Huang X.S. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar. Drugs. 2017;15:69. doi: 10.3390/md15030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S.C., Li H.H., Chen Y.C., Li S.N., Xu J.L., Guo H., Liu Z.M., Zhu S., Liu H.X., Zhang W.M. Three new diterpenes and two new sesquiterpenoids from the endophytic fungus Trichoderma koningiopsis A729. Bioorg. Chem. 2019;86:368–374. doi: 10.1016/j.bioorg.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Yang M.S., Cai X.Y., He Y.Y., Lu M.Y., Liu S., Wang W.X., Li Z.H., Ai H.L., Feng T. Seco-sativene and seco-longifolene sesquiterpenoids from cultures of endophytic fungus Bipolaris eleusines. Nat. Prod. Bioprospect. 2017;7:147–150. doi: 10.1007/s13659-016-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He J., Yang M.S., Wang W.X., Li Z.H., Elkhateeb W.A.M., Wen T.C., Ai H.L., Feng T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019;9:128–131. doi: 10.1039/C8RA09861A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Bideau F., Kousara M., Chen L., Wei L., Dumas F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017;117:6110–6159. doi: 10.1021/acs.chemrev.6b00502. [DOI] [PubMed] [Google Scholar]
- 61.Qiu Y., Lan W.J., Li H.J., Chen L.P. Linear triquinane sesquiterpenoids: Their isolation, structures, biological activities, and chemical synthesis. Molecules. 2018;23:2095. doi: 10.3390/molecules23092095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tabuchi A., Fukushima-Sakuno E., Osaki-Oka K., Futamura Y., Motoyama T., Osada H., Ishikawa N.K., Nagasawa E., Tokimoto K. Productivity and bioactivity of enokipodins A-D of Flammulina rossica and Flammulina velutipes. Biosci. Biotechnol. Biochem. 2020;84:876–886. doi: 10.1080/09168451.2020.1714421. [DOI] [PubMed] [Google Scholar]
- 63.Liu H.X., Tan H.B., Chen K., Chen Y.C., Li S.N., Li H.H., Zhang W.M. Cerrenins A-C, cerapicane and isohirsutane sesquiterpenoids from the endophytic fungus Cerrena sp. Fitoterapia. 2018;129:173–178. doi: 10.1016/j.fitote.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Ding J.H., Li Z.H., Wei K., Dong Z.J., Ding Z.H., Feng T., Liu J.K. Two new sesquiterpenoids from cultures of the basidiomycete Tremella foliacea. J. Asian Nat. Prod. Res. 2016;18:46–50. doi: 10.1080/10286020.2015.1055256. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z.M., Wang S.L. Two new compounds from cultures of the basidiomycete Antrodiella albocinnamomea. Nat. Prod. Res. 2015;29:1985–1989. doi: 10.1080/14786419.2015.1017493. [DOI] [PubMed] [Google Scholar]
- 66.Huang L., Lan W.J., Li H.J. Two new hirsutane-type sesquiterpenoids chondrosterins N and O from the marine fungus Chondrostereum sp. Nat. Prod. Res. 2018;32:1578–1582. doi: 10.1080/14786419.2017.1389935. [DOI] [PubMed] [Google Scholar]
- 67.Qi Q.Y., Ren J.W., Sun L.W., He L.W., Bao L., Yue W., Sun Q.M., Yao Y.J., Yin W.B., Liu H.W. Stucturally diverse sesquiterpenes produced by a Chinese Tibet fungus Stereum hirsutum and their cytotoxic and immunosuppressant activities. Org. Lett. 2015;17:3098–3101. doi: 10.1021/acs.orglett.5b01356. [DOI] [PubMed] [Google Scholar]
- 68.Liu H.X., Tan H.B., Chen Y.C., Li S.N., Li H.H., Zhang W.M. Cytotoxic triquinane-type sesquiterpenoids from the endophytic fungus Cerrena sp. A593. Nat. Prod. Res. 2019;34:2430–2436. doi: 10.1080/14786419.2018.1539977. [DOI] [PubMed] [Google Scholar]
- 69.Huang L., Lan W.J., Deng R., Feng G.K., Xu Q.Y., Hu Z.Y., Zhu X.F., Li H.J. Additional new cytotoxic triquinane-type sesquiterpenoids chondrosterins K-M from the marine fungus Chondrostereum sp. Mar. Drugs. 2016;14:157. doi: 10.3390/md14090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z.M., Chen H.P., Wang F., Li Z.H., Feng T., Liu J.K. New triquinane and gymnomitrane sesquiterpenes from fermentation of the basidiomycete Antrodiella albocinnamomea. Fitoterapia. 2015;102:61–66. doi: 10.1016/j.fitote.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Wang M., Du J.X., Yang H.X., Dai Q., Liu Y.P., He J., Wang Y., Li Z.H., Feng T., Liu J.K. Sesquiterpenoids from cultures of the basidiomycetes Irpex lacteus. J. Nat. Prod. 2020;83:1524–1531. doi: 10.1021/acs.jnatprod.9b01177. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y.Z., Wang Y., Wu A.A., Zhang L., Hu Z.Y., Huang H.Y., Xu Q.Y., Deng X.M. New 12,8-eudesmanolides from Eutypella sp. 1–15. J. Antibiot. 2017;70:1029–1032. doi: 10.1038/ja.2017.89. [DOI] [PubMed] [Google Scholar]
- 73.Zhang D.W., Ge H.L., Zou J.H., Tao X.Y., Chen R.D., Dai J.G. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 2014;16:1410–1413. doi: 10.1021/ol500197x. [DOI] [PubMed] [Google Scholar]
- 74.Liffert R., Linden A., Gademann K. Total synthesis of the sesquiterpenoid periconianone A based on a postulated biogenesis. J. Am. Chem. Soc. 2017;139:16096–16099. doi: 10.1021/jacs.7b10053. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Z.Z., Liang X.B., Feng W.S., Wu Y., Zhi Y.L., Xue G.M., Chen H.P., Liu J.K. Unusual constituents from the medicinal mushroom Ganoderma lingzhi. RSC Adv. 2019;9:36931–36939. doi: 10.1039/C9RA08566A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binh P.T., Descoutures D., Dang N.H., Nguyen N.P., Dat N.T. A new cytotoxic gymnomitrane sesquiterpene from Ganoderma lucidum fruiting bodies. Nat. Prod. Commun. 2015;10:1911–1912. doi: 10.1177/1934578X1501001125. [DOI] [PubMed] [Google Scholar]
- 77.Wang J.F., He W.J., Kong F.D., Tian X.P., Wang P., Zhou X.J., Liu Y.H. Ochracenes A-I, humulane-derived sesquiterpenoids from the antarctic fungus Aspergillus ochraceopetaliformis. J. Nat. Prod. 2017;80:1725–1733. doi: 10.1021/acs.jnatprod.6b00810. [DOI] [PubMed] [Google Scholar]
- 78.Wu P.F., Ding R., Tan R., Liu J., Hu E.M., Li C.Y., Liang G.Y., Yi P. Sesquiterpenes from cultures of the fungus Phellinus igniarius and their cytotoxicities. Fitoterapia. 2020;140:104415. doi: 10.1016/j.fitote.2019.104415. [DOI] [PubMed] [Google Scholar]
- 79.Nord C., Menkis A., Broberg A. Cytotoxic illudane sesquiterpenes from the fungus Granulobasidium vellereum (Ellis and Cragin) Jülich. J. Nat. Prod. 2015;78:2559–2564. doi: 10.1021/acs.jnatprod.5b00500. [DOI] [PubMed] [Google Scholar]
- 80.He J.B., Tao J., Miao X.S., Feng Y.P., Bu W., Dong Z.J., Li Z.H., Feng T., Liu J.K. Two new illudin type sesquiterpenoids from cultures of Phellinus tuberculosus and Laetiporus sulphureus. J. Asian Nat. Prod. Res. 2015;17:1054–1058. doi: 10.1080/10286020.2015.1040774. [DOI] [PubMed] [Google Scholar]
- 81.Dai Q., Zhang F.L., Du J.X., Li Z.H., Feng T., Liu J.K. Illudane sesquiterpenoids from edible mushroom Agrocybe salicacola and their bioactivities. ACS Omega. 2020;5:21961–21967. doi: 10.1021/acsomega.0c03314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo H., Diao Q.P., Zhang B., Feng T. Two new illudane sesquiterpenoids and one new menthane monoterpene from cultures of Craterellus cornucopioides. J. Asian Nat. Prod. Res. 2019;21:123–128. doi: 10.1080/10286020.2017.1394293. [DOI] [PubMed] [Google Scholar]
- 83.Kokubun T., Scott-Brown A., Kite G.C., Simmonds M.S.J. Protoilludane, illudane, illudalane, and norilludane sesquiterpenoids from Granulobasidium vellereum. J. Nat. Prod. 2016;79:1698–1701. doi: 10.1021/acs.jnatprod.6b00325. [DOI] [PubMed] [Google Scholar]
- 84.Surup F., Hennicke F., Sella N., Stroot M., Bernecker S., Pfutze S., Stadler M., Ruhl M. New terpenoids from the fermentation broth of the edible mushroom Cyclocybe aegerita. Beilstein J. Org. Chem. 2019;15:1000–1007. doi: 10.3762/bjoc.15.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie S.S., Wu Y., Qiao Y.B., Guo Y., Wang J.P., Hu Z.X., Zhang Q., Li X.N., Huang J.F., Zhou Q., et al. Protoilludane, illudalane, and botryane sesquiterpenoids from the endophytic fungus Phomopsis sp. TJ507A. J. Nat. Prod. 2018;81:1311–1320. doi: 10.1021/acs.jnatprod.7b00889. [DOI] [PubMed] [Google Scholar]
- 86.Li H.T., Tang L.H., Liu T., Yang R.N., Yang Y.B., Zhou H., Ding Z.T. Protoilludane-type sesquiterpenoids from Armillaria sp. by co-culture with the endophytic fungus Epicoccum sp. associated with Gastrodia elata. Bioorg. Chem. 2020;95:103503. doi: 10.1016/j.bioorg.2019.103503. [DOI] [PubMed] [Google Scholar]
- 87.Li Z.J., Wang Y.C., Jiang B., Li W.L., Zheng L.H., Yang X.G., Bao Y.L., Sun L.G., Huang Y.X., Li Y.X. Structure, cytotoxic activity and mechanism of protoilludane sesquiterpene aryl esters from the mycelium of Armillaria mellea. J. Ethnopharmacol. 2016;184:119–127. doi: 10.1016/j.jep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 88.Chen C.C., Kuo Y.H., Cheng J.J., Sung P.J., Ni C.L., Chen C.C., Shen C.C. Three new sesquiterpene aryl esters from the mycelium of Armillaria mellea. Molecules. 2015;20:9994–10003. doi: 10.3390/molecules20069994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hovey M.T., Cohen D.T., Walden D.M., Cheong P.H.Y., Scheidt K.A. A carbene catalysis strategy for the synthesis of protoilludane natural products. Angew. Chem. Int. Ed. 2017;56:9864–9867. doi: 10.1002/anie.201705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su X.Z., Tang J.W., Hu K., Li X.N., Sun H.D., Puno P.T. Arthrinins E-G, three botryane sesquiterpenoids from the plant endophytic fungus Arthrinium sp. HS66. Nat. Prod. Bioprospect. 2020;10:201–207. doi: 10.1007/s13659-020-00248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Medina R.P., Araujo A.R., Batista J.M., Jr., Cardoso C.L., Seidl C., Vilela A.F.L., Domingos H.V., Costa-Lotufo L.V., Andersen R.J., Silva D.H.S. Botryane terpenoids produced by Nemania bipapillata, an endophytic fungus isolated from red alga Asparagopsis taxiformis-Falkenbergia stage. Sci. Rep. 2019;9:12318. doi: 10.1038/s41598-019-48655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen B.S., Li E.W., Liu L., Liao M.F., Zhu Z.X., Zhuang W.Y., Bao L., Liu H.W. Botryane sesquiterpenoids, cyclopentadepsipeptides, xanthones, and trichothecenes from Trichoderma oligosporum. Planta Med. 2018;84:1055–1063. doi: 10.1055/a-0593-6030. [DOI] [PubMed] [Google Scholar]
- 93.Kuhnert E., Surup F., Wiebach V., Bernecker S., Stadler M. Botryane, noreudesmane and abietane terpenoids from the ascomycete Hypoxylon rickii. Phytochemistry. 2015;117:116–122. doi: 10.1016/j.phytochem.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Wiebach V., Surup F., Kuhnert E., Stadler M. Rickicaryophyllane A, a caryophyllane from the ascomyceteous fungus Hypoxylon rickii and a 10-norbotryane congener. Nat. Prod. Commun. 2016;11:909–912. doi: 10.1177/1934578X1601100711. [DOI] [PubMed] [Google Scholar]
- 95.Bao Y.R., Chen G.D., Gao H., He R.R., Wu Y.H., Li X.X., Hu D., Wang C.X., Liu X.Z., Li Y., et al. 4,5-seco-Probotryenols A-C, a new type of sesquiterpenoids from Stachybotrys bisbyi. RSC Adv. 2015;5:46252–46259. doi: 10.1039/C5RA07122D. [DOI] [Google Scholar]
- 96.Ren F.X., Zhu S.M., Wang B., Li L., Liu X.Z., Su R.B., Che Y.S. Hypocriols A-F, heterodimeric botryane ethers from Hypocrea sp., an insect-associated fungus. J. Nat. Prod. 2016;79:1848–1856. doi: 10.1021/acs.jnatprod.6b00394. [DOI] [PubMed] [Google Scholar]
- 97.Ayer W.A., Cruz E.R. The tremulanes, a new group of sesquiterpenes from the aspen rotting fungus Phellinus tremulae. J. Org. Chem. 1993;58:7529–7534. doi: 10.1021/jo00078a035. [DOI] [Google Scholar]
- 98.Ayer W.A., Cruz E.R. 2-Carbomethoxyoxepin: 1-carbomethoxybenzene 1,2-oxide and the biosynthesis of methyl salicylate in Phellinus tremulae. J. Nat. Prod. 1995;58:622–624. doi: 10.1021/np50118a026. [DOI] [Google Scholar]
- 99.Chen H.P., Ji X., Li Z.H., Feng T., Liu J.K. Irlactane and tremulane sesquiterpenes from the cultures of the medicinal fungus Irpex lacteus HFG1102. Nat. Prod. Bioprospect. 2020;10:89–100. doi: 10.1007/s13659-020-00239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding J.H., Feng T., Cui B.K., Wei K., Li Z.H., Liu J.K. Novel sesquiterpenoids from cultures of the basidiomycete Irpex lacteus. Tetrahedron Lett. 2013;54:2651–2654. doi: 10.1016/j.tetlet.2013.03.038. [DOI] [Google Scholar]
- 101.He J., Pu C.J., Wang M., Li Z.H., Feng T., Zhao D.K., Liu J.K. Conosiligins A–D, ring-rearranged tremulane sesquiterpenoids from Conocybe siliginea. J. Nat. Prod. 2020;83:2743–2748. doi: 10.1021/acs.jnatprod.0c00681. [DOI] [PubMed] [Google Scholar]
- 102.Li W., He J., Feng T., Yang H.X., Ai H.L., Li Z.H., Liu J.K. Antroalbocin A, an antibacterial sesquiterpenoid from higher fungus Antrodiella albocinnamomea. Org. Lett. 2018;20:8019–8021. doi: 10.1021/acs.orglett.8b03595. [DOI] [PubMed] [Google Scholar]
- 103.Ding J.H., Li Z.H., Feng T., Liu J.K. Two new sesquiterpenes from cultures of the fungus Irpex lacteus. J. Asian Nat. Prod. Res. 2020;23:348–352. doi: 10.1080/10286020.2020.1737857. [DOI] [PubMed] [Google Scholar]
- 104.Ding J.H., Li Z.H., Feng T., Liu J.K. Tremulane sesquiterpenes from cultures of the basidiomycete Irpex lacteus. Fitoterapia. 2018;125:245–248. doi: 10.1016/j.fitote.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Ding J.H., Li Z.H., Feng T., Liu J.K. A new tremulane sesquiterpenoid from the fungus Irpex lacteus. Nat. Prod. Res. 2019;33:316–320. doi: 10.1080/14786419.2018.1448816. [DOI] [PubMed] [Google Scholar]
- 106.Zhou Q.Y., Yang X.Q., Zhang Z.X., Wang B.Y., Hu M., Yang Y.B., Zhou H., Ding Z.T. New azaphilones and tremulane sesquiterpene from endophytic Nigrospora oryzae cocultured with Irpex lacteus. Fitoterapia. 2018;130:26–30. doi: 10.1016/j.fitote.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 107.Wu Y.M., Zhou Q.Y., Yang X.Q., Luo Y.J., Qian J.J., Liu S.X., Yang Y.B., Ding Z.T. Induction of antiphytopathogenic metabolite and squalene production and phytotoxin elimination by adjustment of the mode of fermentation in cocultures of phytopathogenic Nigrospora oryzae and Irpex lacteus. J. Agric. Food Chem. 2019;67:11877–11882. doi: 10.1021/acs.jafc.9b04209. [DOI] [PubMed] [Google Scholar]
- 108.Guo Z.Y., Li X.S., Zhang L., Feng Z.W., Deng Z.S., He H.B., Zou K. Cytotoxic tremulanes and 5,6-secotremulanes, four new sesquiterpenoids from a plant-associated fungus X1-2. Nat. Prod. Res. 2016;30:2582–2589. doi: 10.1080/14786419.2015.1135140. [DOI] [PubMed] [Google Scholar]
- 109.Wang F.Q., Ma H.R., Hu Z.X., Jiang J.C., Zhu H., Cheng L., Yang Q.W., Zhang H., Zhang G., Zhang Y.H. Secondary metabolites from Colletotrichum capsici, an endophytic fungus derived from Siegesbeckia pubescens Makino. Nat. Prod. Res. 2017;31:1849–1854. doi: 10.1080/14786419.2016.1261346. [DOI] [PubMed] [Google Scholar]
- 110.Chen H.Y., Liu T.K., Shi Q., Yang X.L. Sesquiterpenoids and diterpenes with antimicrobial activity from Leptosphaeria sp. XL026, an endophytic fungus in Panax notoginseng. Fitoterapia. 2019;137:104243. doi: 10.1016/j.fitote.2019.104243. [DOI] [PubMed] [Google Scholar]
- 111.Chen H.P., Zhao Z.Z., Li Z.H., Feng T., Liu J.K. Seco-tremulane sesquiterpenoids from the cultures of the medicinal fungus Irpex lacteus HFG1102. Nat. Prod. Bioprospect. 2018;8:113–119. doi: 10.1007/s13659-018-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakashima K., Tomida J., Hirai T., Kawamura Y., Inoue M. Sesquiterpenes with new carbon skeletons from the basidiomycete Phlebia tremellosa. J. Nat. Med. 2019;73:480–486. doi: 10.1007/s11418-019-01286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu M.Z., Cen Y.F., Ye W., Li S.N., Zhang W.M. Recent advances on macrocyclic trichothecenes, their bioactivities and biosynthetic pathway. Toxins. 2020;12:417. doi: 10.3390/toxins12060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ryu S.M., Lee H.M., Song E.G., Seo Y.H., Lee J., Guo Y., Kim B.S., Kim J.J., Hong J.S., Ryu K.H., et al. Antiviral activities of trichothecenes isolated from Trichoderma albolutescens against pepper mottle virus. J. Agric. Food Chem. 2017;65:4273–4279. doi: 10.1021/acs.jafc.7b01028. [DOI] [PubMed] [Google Scholar]
- 115.Yamazaki H., Yagi A., Takahashi O., Yamaguchi Y., Saito A., Namikoshi M., Uchida R. Antifungal trichothecene sesquiterpenes obtained from the culture broth of marine-derived Trichoderma cf. brevicompactum and their structure-activity relationship. Bioorg. Med. Chem. Lett. 2020;30:127375. doi: 10.1016/j.bmcl.2020.127375. [DOI] [PubMed] [Google Scholar]
- 116.Yamazaki H., Takahashi O., Kirikoshi R., Yagi A., Ogasawara T., Bunya Y., Rotinsulu H., Uchida R., Namikoshi M. Epipolythiodiketopiperazine and trichothecene derivatives from the NaI-containing fermentation of marine-derived Trichoderma cf. brevicompactum. J. Antibiot. 2020;73:559–567. doi: 10.1038/s41429-020-0314-5. [DOI] [PubMed] [Google Scholar]
- 117.Lee S.R., Seok S., Ryoo R., Choi S.U., Kim K.H. Macrocyclic trichothecene mycotoxins from a deadly poisonous mushroom, Podostroma cornu-damae. J. Nat. Prod. 2019;82:122–128. doi: 10.1021/acs.jnatprod.8b00823. [DOI] [PubMed] [Google Scholar]
- 118.Ye W., Chen Y.C., Li H.H., Zhang W.M., Liu H.X., Sun Z.H., Liu T.M., Li S.N. Two trichothecene mycotoxins from Myrothecium roridum induce apoptosis of HepG-2 cells via caspase activation and disruption of mitochondrial membrane potential. Molecules. 2016;21:781. doi: 10.3390/molecules21060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu H.X., Liu W.Z., Chen Y.C., Sun Z.H., Tan Y.Z., Li H.H., Zhang W.M. Cytotoxic trichothecene macrolides from the endophyte fungus Myrothecium roridum. J. Asian Nat. Prod. Res. 2016;18:684–689. doi: 10.1080/10286020.2015.1134505. [DOI] [PubMed] [Google Scholar]
- 120.Shen L., Zhu L., Tan Q.W., Wan D., Xie J., Peng J.N. New cytotoxic trichothecene macrolide epimers from endophytic Myrothecium roridum IFB-E012. J. Antibiot. 2016;69:652–655. doi: 10.1038/ja.2016.86. [DOI] [PubMed] [Google Scholar]
- 121.Mondol M.A., Surovy M.Z., Islam M.T., Schuffler A., Laatsch H. Macrocyclic trichothecenes from Myrothecium roridum strain M10 with motility inhibitory and zoosporicidal activities against Phytophthora nicotianae. J. Agric. Food Chem. 2015;63:8777–8786. doi: 10.1021/acs.jafc.5b02366. [DOI] [PubMed] [Google Scholar]
- 122.Li Y., Liu D., Cheng Z.B., Proksch P., Lin W.H. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017;7:7259–7267. doi: 10.1039/C6RA26956G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsumoto M., Tanaka S., Tonouchi A., Hashimoto M. 12-Deoxyroridin J and 12-deoxyepiisororidin E from Calcarisporium arbuscular. Tetrahedron Lett. 2018;59:1992–1995. doi: 10.1016/j.tetlet.2018.03.089. [DOI] [Google Scholar]
- 124.Matsumoto M., Nishiyama M., Maeda H., Tonouchi A., Konno K., Hashimoto M. Structure-activity relationships of trichothecenes against COLO201 cells and Cochliobolus miyabeanus: The role of 12-epoxide and macrocyclic moieties. Bioorg. Med. Chem. Lett. 2019;29:982–985. doi: 10.1016/j.bmcl.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 125.Yang H.X., Ai H.L., Feng T., Wang W.X., Wu B., Zheng Y.S., Sun H., He J., Li Z.H., Liu J.K. Trichothecrotocins A-C, antiphytopathogenic agents from potato endophytic fungus Trichothecium crotocinigenum. Org. Lett. 2018;20:8069–8072. doi: 10.1021/acs.orglett.8b03735. [DOI] [PubMed] [Google Scholar]
- 126.Yang H.X., He J., Zhang F.L., Zhang X.D., Li Z.H., Feng T., Ai H.L., Liu J.K. Trichothecrotocins D–L, antifungal agents from a potato-associated Trichothecium crotocinigenum. J. Nat. Prod. 2020;83:2756–2763. doi: 10.1021/acs.jnatprod.0c00695. [DOI] [PubMed] [Google Scholar]
- 127.Barua J.E., de la Cruz M., de Pedro N., Cautain B., Hermosa R., Cardoza R.E., Gutierrez S., Monte E., Vicente F., Collado I.G. Synthesis of trichodermin derivatives and their antimicrobial and cytotoxic activities. Molecules. 2019;24:3811. doi: 10.3390/molecules24203811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen H.Y., Liu T.K., Yang J., Yang X.L. Emerones A-C: Three novel merosesquiterpenoids with unprecedented skeletons from Emericella sp. XL029. Org. Biomol. Chem. 2019;17:8450–8455. doi: 10.1039/C9OB01788G. [DOI] [PubMed] [Google Scholar]
- 129.Shin H.J., Choi B.K., Trinh P.T.H., Lee H.S., Kang J.S., Van T.T.T., Lee H.S., Lee J.S., Lee Y.J., Lee J. Suppression of RANKL-induced osteoclastogenesis by the metabolites from the marine fungus Aspergillus flocculosus isolated from a sponge Stylissa sp. Mar. Drugs. 2018;16:14. doi: 10.3390/md16010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J.F., Wei X.Y., Qin X.C., Tian X.P., Liao L., Li K.M., Zhou X.F., Yang X.W., Wang F.Z., Zhang T.Y., et al. Antiviral merosesquiterpenoids produced by the antarctic fungus Aspergillus ochraceopetaliformis SCSIO 05702. J. Nat. Prod. 2016;79:59–65. doi: 10.1021/acs.jnatprod.5b00650. [DOI] [PubMed] [Google Scholar]
- 131.Chen S.T., Wang J.F., Wang Z., Lin X.P., Zhao B.X., Kaliaperumal K., Liao X.J., Tu Z.C., Li J.L., Xu S.H., et al. Structurally diverse secondary metabolites from a deep-sea-derived fungus Penicillium chrysogenum SCSIO 41001 and their biological evaluation. Fitoterapia. 2017;117:71–78. doi: 10.1016/j.fitote.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 132.Yamazaki H., Nakayama W., Takahashi O., Kirikoshi R., Izumikawa Y., Iwasaki K., Toraiwa K., Ukai K., Rotinsulu H., Wewengkang D.S., et al. Verruculides A and B, two new protein tyrosine phosphatase 1B inhibitors from an Indonesian ascidian-derived Penicillium verruculosum. Bioorg. Med. Chem. Lett. 2015;25:3087–3090. doi: 10.1016/j.bmcl.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 133.Jiao W.H., Dewapriya P., Mohamed O., Khalil Z.G., Salim A.A., Lin H.W., Capon R.J. Divirensols: Sesquiterpene dimers from the Australian termite nest-derived fungus Trichoderma virens CMB-TN16. J. Nat. Prod. 2019;82:87–95. doi: 10.1021/acs.jnatprod.8b00746. [DOI] [PubMed] [Google Scholar]
- 134.Jiao W.H., Salim A.A., Khalil Z.G., Dewapriya P., Lin H.W., Butler M.S., Capon R.J. Trivirensols: Selectively bacteriostatic sesquiterpene trimers from the Australian termite nest-derived fungus Trichoderma virens CMB-TN16. J. Nat. Prod. 2019;82:3165–3175. doi: 10.1021/acs.jnatprod.9b00760. [DOI] [PubMed] [Google Scholar]
- 135.Xu L.L., Chen H.L., Hai P., Gao Y., Xie C.D., Yang X.L., Abe I. (+)- and (−)-Preuisolactone a: A pair of caged norsesquiterpenoidal enantiomers with a tricyclo[4.4.01,6.02,8]decane carbon skeleton from the endophytic fungus Preussia isomera. Org. Lett. 2019;21:1078–1081. doi: 10.1021/acs.orglett.8b04123. [DOI] [PubMed] [Google Scholar]
- 136.Otaka J., Hashizume D., Masumoto Y., Muranaka A., Uchiyama M., Koshino H., Futamura Y., Osada H. Hitoyol A and B, two norsesquiterpenoids from the basidiomycete Coprinopsis cinerea. Org. Lett. 2017;19:4030–4033. doi: 10.1021/acs.orglett.7b01784. [DOI] [PubMed] [Google Scholar]
- 137.Wei H., Xu Y.M., Espinosa-Artiles P., Liu M.X., Luo J.G., U’Ren J.M., Arnold A.E., Gunatilaka A.A. Sesquiterpenes and other constituents of Xylaria sp. NC1214, a fungal endophyte of the moss Hypnum sp. Phytochemistry. 2015;118:102–108. doi: 10.1016/j.phytochem.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang M.Z., Sun M.W., Hao H.L., Lu C.H. Avertoxins A-D, prenyl asteltoxin derivatives from Aspergillus versicolor Y10, an endophytic fungus of Huperzia serrata. J. Nat. Prod. 2015;78:3067–3070. doi: 10.1021/acs.jnatprod.5b00600. [DOI] [PubMed] [Google Scholar]
- 139.Hu X.Y., Li X.M., Yang S.Q., Liu H., Meng L.H., Wang B.G. Three new sesquiterpenoids from the algal-derived fungus Penicillium chermesinum EN-480. Mar. Drugs. 2020;18:194. doi: 10.3390/md18040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tai S.H., Kuo P.C., Hung C.C., Lin Y.H., Hwang T.L., Lam S.H., Kuo D.H., Wu J.B., Hung H.Y., Wu T.S. Bioassay-guided purification of sesquiterpenoids from the fruiting bodies of Fomitopsis pinicola and their anti-inflammatory activity. RSC Adv. 2019;9:34184–34195. doi: 10.1039/C9RA05899K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Luo X.W., Chen C.M., Li K.L., Lin X.P., Gao C.H., Zhou X.F., Liu Y.H. Sesquiterpenoids and meroterpenoids from a mangrove derived fungus Diaporthe sp. SCSIO 41011. Nat. Prod. Res. 2019;35:282–288. doi: 10.1080/14786419.2019.1627355. [DOI] [PubMed] [Google Scholar]
- 142.Li X.M., Li X.M., Lu C.H. Abscisic acid-type sesquiterpenes and ansamycins from Amycolatopsis alba DSM 44262. J. Asian Nat. Prod. Res. 2017;19:946–953. doi: 10.1080/10286020.2017.1285909. [DOI] [PubMed] [Google Scholar]
- 143.Li H.J., Jiang C.W., Xu M.Y., Yan D.F., Xu J., Lan W.J. Pseudapenes A-C, sesquiterpenoids from the marine-derived fungus Pseudallescheria apiosperma F52-1. Tetrahedron Lett. 2019;60:150953. doi: 10.1016/j.tetlet.2019.150953. [DOI] [Google Scholar]
- 144.Pang X.J., Zhang S.B., Xian P.J., Wu X., Yang D.F., Fu H.Y., Yang X.L. Emericellins A and B: Two sesquiterpenoids with an unprecedented tricyclo [4,4,2,1] hendecane scaffold from the liquid cultures of endophytic fungus Emericella sp. XL 029. Fitoterapia. 2018;131:55–58. doi: 10.1016/j.fitote.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 145.Yan J.M., Wang X., Tian M.Q., Liu C.M., Zhang K.Q., Li G.H. Chemical constituents from the fungus Stereum sp. YMF1.04183. Phytochem. Lett. 2017;22:6–8. doi: 10.1016/j.phytol.2017.08.001. [DOI] [Google Scholar]
- 146.Shi Z.Z., Miao F.P., Fang S.T., Liu X.H., Yin X.L., Ji N.Y. Sesteralterin and tricycloalterfurenes A–D: Terpenes with rarely occurring frameworks from the marine-alga-epiphytic fungus Alternaria alternata k21-1. J. Nat. Prod. 2017;80:2524–2529. doi: 10.1021/acs.jnatprod.7b00478. [DOI] [PubMed] [Google Scholar]
- 147.Chen X.W., Yang Z.D., Sun J.H., Song T.T., Zhu B.Y., Zhao J.W. Colletotrichine A, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2018;32:880–884. doi: 10.1080/14786419.2017.1365071. [DOI] [PubMed] [Google Scholar]
- 148.Duan Y.C., Feng J., Bai N., Li G.H., Zhang K.Q., Zhao P.J. Four novel antibacterial sesquiterpene-α-amino acid quaternary ammonium hybrids from the mycelium of mushroom Stereum hirsutum. Fitoterapia. 2018;128:213–217. doi: 10.1016/j.fitote.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 149.Ota K., Yamazaki I., Saigoku T., Fukui M., Miyata T., Kamaike K., Shirahata T., Mizuno F., Asada Y., Hirotani M., et al. Phellilane L, sesquiterpene metabolite of Phellinus linteus: Isolation, structure elucidation, and asymmetric total synthesis. J. Org. Chem. 2017;82:12377–12385. doi: 10.1021/acs.joc.7b02141. [DOI] [PubMed] [Google Scholar]
- 150.Jayanetti D.R., Yue Q., Bills G.F., Gloer J.B. Hypocoprins A-C: New sesquiterpenoids from the coprophilous fungus Hypocopra rostrata. J. Nat. Prod. 2015;78:396–401. doi: 10.1021/np5007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hwang I.H., Swenson D.C., Gloer J.B., Wicklow D.T. Pestaloporonins: Caryophyllene-derived sesquiterpenoids from a fungicolous isolate of Pestalotiopsis sp. Org. Lett. 2015;17:4284–4287. doi: 10.1021/acs.orglett.5b02080. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J.Y., Liu L., Wang B., Zhang Y., Wang L.L., Liu X.Z., Che Y.S. Phomanolides A and B from the fungus Phoma sp.: Meroterpenoids derived from a putative tropolonic sesquiterpene via hetero-diels-alder reactions. J. Nat. Prod. 2015;78:3058–3066. doi: 10.1021/acs.jnatprod.5b00969. [DOI] [PubMed] [Google Scholar]
- 153.Chen X.W., Yang Z.D., Li X.F., Sun J.H., Yang L.J., Zhang X.G. Colletotrichine B, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2019;33:108–112. doi: 10.1080/14786419.2018.1437437. [DOI] [PubMed] [Google Scholar]
- 154.Kang H.S., Ji S.A., Park S.H., Kim J.P. Lepistatins A-C, chlorinated sesquiterpenes from the cultured basidiomycete Lepista sordida. Phytochemistry. 2017;143:111–114. doi: 10.1016/j.phytochem.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 155.Lin X.P., Wu Q.Y., Yu Y.Y., Liang Z., Liu Y.H., Zhou L.L., Tang L., Zhou X.F. Penicilliumin B, a novel sesquiterpene methylcyclopentenedione from a deep sea-derived Penicillium strain with renoprotective activities. Sci. Rep. 2017;7:10757. doi: 10.1038/s41598-017-11007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Deng Q., Li G., Sun M.Y., Yang X.B., Xu J. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal. Nat. Prod. Res. 2018;34:1404–1408. doi: 10.1080/14786419.2018.1512993. [DOI] [PubMed] [Google Scholar]
- 157.Wibowo M., Prachyawarakorn V., Aree T., Mahidol C., Ruchirawat S., Kittakoop P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Phytochemistry. 2016;122:126–138. doi: 10.1016/j.phytochem.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 158.Huang J.H., Lv J.M., Wang Q.Z., Zou J., Lu Y.J., Wang Q.L., Chen D.N., Yao X.S., Gao H., Hu D. Biosynthesis of an anti-tuberculosis sesterterpenoid asperterpenoid A. Org. Biomol. Chem. 2019;17:248–251. doi: 10.1039/C8OB02832J. [DOI] [PubMed] [Google Scholar]
- 159.Masuya T., Tsunematsu Y., Hirayama Y., Sato M., Noguchi H., Nakazawa T., Watanabe K. Biosynthesis of lagopodins in mushroom involves a complex network of oxidation reactions. Org. Biomol. Chem. 2019;17:234–239. doi: 10.1039/C8OB02814A. [DOI] [PubMed] [Google Scholar]
- 160.Bian G.K., Hou A.W., Yuan Y.J., Hu B., Cheng S., Ye Z.L., Di Y.T., Deng Z.X., Liu T.G. Metabolic engineering-based rapid characterization of a sesquiterpene cyclase and the skeletons of fusariumdiene and fusagramineol from Fusarium graminearum. Org. Lett. 2018;20:1626–1629. doi: 10.1021/acs.orglett.8b00366. [DOI] [PubMed] [Google Scholar]
- 161.Flynn C.M., Schmidt-Dannert C. Sesquiterpene synthase-3-hydroxy-3-methylglutaryl coenzyme A synthase fusion protein responsible for hirsutene biosynthesis in Stereum hirsutum. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yuan Y.J., Litzenburger M., Cheng S., Bian G.K., Hu B., Yan P., Cai Y.S., Deng Z.X., Bernhardt R., Liu T.G. Sesquiterpenoids produced by combining two sesquiterpene cyclases with promiscuous myxobacterial CYP260B1. ChemBioChem. 2019;20:677–682. doi: 10.1002/cbic.201800670. [DOI] [PubMed] [Google Scholar]
- 163.Ichinose H., Kitaoka T. Insight into metabolic diversity of the brown-rot basidiomycete Postia placenta responsible for sesquiterpene biosynthesis: Semi-comprehensive screening of cytochrome P450 monooxygenase involved in protoilludene metabolism. Microb. Biotechnol. 2018;11:952–965. doi: 10.1111/1751-7915.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lindo L., McCormick S.P., Cardoza R.E., Busman M., Alexander N.J., Proctor R.H., Gutierrez S. Requirement of two acyltransferases for 4-O-acylation during biosynthesis of harzianum A, an antifungal trichothecene produced by Trichoderma arundinaceum. J. Agric. Food Chem. 2019;67:723–734. doi: 10.1021/acs.jafc.8b05564. [DOI] [PubMed] [Google Scholar]
- 165.Cardoza R.E., Malmierca M.G., Hermosa M.R., Alexander N.J., McCormick S.P., Proctor R.H., Tijerino A.M., Rumbero A., Monte E., Gutiérrez S. Identification of loci and functional characterization of trichothecene biosynthesis genes in filamentous fungi of the genus Trichoderma. Appl. Environ. Microbiol. 2011;77:4867–4877. doi: 10.1128/AEM.00595-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Proctor R.H., McCormick S.P., Kim H.-S., Cardoza R.E., Stanley A.M., Lindo L., Kelly A., Brown D.W., Lee T., Vaughan M.M., et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018;14:e1006946. doi: 10.1371/journal.ppat.1006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kamata K., Sato H., Maeda K., Furihata K., Aikawa S., Adachi K., Tanaka A., Tokai T., Nakajima Y., Yoshida Y., et al. Exploring an artificial metabolic route in Fusarium sporotrichioides: Production and characterization of 7-hydroxy T-2 toxin. J. Nat. Prod. 2018;81:1041–1044. doi: 10.1021/acs.jnatprod.7b00398. [DOI] [PubMed] [Google Scholar]
- 168.Bahadoor A., Schneiderman D., Gemmill L., Bosnich W., Blackwell B., Melanson J.E., McRae G., Harris L.J. Hydroxylation of longiborneol by a Clm2-encoded CYP450 monooxygenase to produce culmorin in Fusarium graminearum. J. Nat. Prod. 2016;79:81–88. doi: 10.1021/acs.jnatprod.5b00676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.