Abstract
The caspase 8 homologue FLICE-inhibitory protein (cFLIP) is a potent negative regulator of death receptor-induced apoptosis. We found that cFLIP can be upregulated in some cell lines under critical involvement of the NF-κB pathway, but NF-κB activation was clearly not sufficient for cFLIP induction in all cell lines. Treatment of SV80 cells with the proteasome inhibitor N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal (MG-132) or geldanamycin, a drug interfering with tumor necrosis factor (TNF)-induced NF-κB activation, inhibited TNF-induced upregulation of cFLIP. Overexpression of a nondegradable IκBα mutant (IκBα-SR) or lack of IκB kinase γ expression completely prevented phorbol myristate acetate-induced upregulation of cFLIP mRNA in Jurkat cells. These data point to an important role for NF-κB in the regulation of the cFLIP gene. SV80 cells normally show resistance to TNF-related apoptosis-inducing ligand (TRAIL) and TNF, as apoptosis can be induced only in the presence of low concentrations of cycloheximide (CHX). However, overexpression of IκBα-SR rendered SV80 cells sensitive to TRAIL-induced apoptosis in the absence of CHX, and cFLIP expression was able to reverse the proapoptotic effect of NF-κB inhibition. Western blot analysis further revealed that cFLIP, but not TRAF1, A20, and cIAP2, expression levels rapidly decrease upon CHX treatment. In conclusion, these data suggest a key role for cFLIP in the antiapoptotic response of NF-κB activation.
Several years ago, p65/RelA knockout mice, which show an embryonic lethal phenotype due to extensive apoptosis of hepatocytes, gave a first clue that NF-κB may have an important role in the inhibition of apoptosis (3). Subsequent studies revealed that inhibition of NF-κB activation enhances the apoptotic effects of a variety of death inducers, like tumor necrosis factor (TNF), ionizing radiation, and chemotherapeutic agents (4, 25, 36, 39, 43), whereas pretreatment of cells with NF-κB inducers, like interleukin 1 (IL-1), can confer resistance against the induction of apoptosis (12, 19). On the other hand, NF-κB activation can be inhibited by caspase-generated cleavage products of components of the NF-κB signaling pathway that act as dominant-negative variants of their full-length parental forms (1, 16, 22, 24, 31). Hence, activation of the NF-κB pathway and induction of apoptosis inhibit each other. This leads to a rapid amplification of the particular signaling pathway that is initially dominant. This regulatory circuit facilitates a clear decision between life and death at the cellular level in cells that are exposed to a complex pattern of distinct and possibly counteracting stimuli.
In line with its antiapoptotic properties, NF-κB regulates several genes encoding proteins with antiapoptotic properties, such as A20 (33), cIAP2 (8), TRAF1 (35, 40), Bfl-1/A1 (20, 45), IEX-1L (44), and Bcl-xL (7). Although ectopic overexpression of one or a combination of these proteins may efficiently prevent apoptosis induced by TNF, ionizing radiation, or chemotherapeutic agents, it is still questionable whether and to what extent these molecules account for the antiapoptotic effects of NF-κB at physiological expression levels. In order to identify novel antiapoptotic proteins that are upregulated by NF-κB-inducing ligands, we analyzed the steady-state mRNA levels of about 65 known apoptosis-related genes in TNF- and IL-1-treated fibroblasts. In addition to the upregulation of already known targets like TRAF1, cIAP2, and A20, we found a strong induction of caspase 8 homologue FLICE-inhibitory protein (cFLIP) both at the mRNA and protein levels. Moreover, cFLIP expression was highly sensitive towards cycloheximide (CHX) treatment. Together, these data strongly argue for cFLIP as an important NF-κB-dependent regulator of death receptor-induced apoptosis.
MATERIALS AND METHODS
Materials.
Geldanamycin (GA) was supplied from Calbiochem (Bad Schwalbach, Germany). Rabbit polyclonal anti-TRAF1 H-125, rabbit polyclonal anti-cIAP1 H-83, and rabbit polyclonal anti-cIAP2 H-85 antibodies were purchased from Santa Cruz (Heidelberg, Germany). Anti-caspase 8 monoclonal antibody (MAb), anti-cellular cFLIP MAb N19, and anti-A20 MAb were gifts from Klaus Schulze-Osthoff (anti-caspase 8; University of Münster, Münster, Germany), Ingo Schmitz and Peter Krammer (N19; DKFZ Heidelberg, Heidelberg, Germany), and Claudius Vincenz (anti-A20; University of Michigan, Ann Arbor, Mich.). The Jurkat-FLIP clone and TRAIL-R1-Fc and TRAIL-R2-Fc used for immunization of rabbits were obtained from Pascal Schneider and Jurg Tschopp (University of Lausanne, Lausanne, Switzerland). Jurkat cell lines Jurkat-I-κBαM and Jurkat-I-κB(2N), which stably overexpress nondegradable IκB mutants, were provided by Douglas R. Green (La Jolla Institute for Allergy and Immunology, La Jolla, Calif.) and John Hiscott (McGill University, Montreal, Canada), respectively. The IκB kinase γ (IKKγ)-deficient Jurkat cell line was a gift from S.-C. Sun (Pennsylvania State University College of Medicine, Hershey, Pa.), and the expression plasmid pI-κBα-SR encoding a nondegradable mutant of IκBα was provided by Johannes Schmid (University of Vienna, Vienna, Austria).
Cell culture.
HeLa, HEK293, Daudi, and Jurkat cells were maintained in RPMI 1640 medium containing 5% (HeLa and HEK293), 10% (Jurkat), and 20% (Daudi) heat-inactivated fetal calf serum in a humidified 5.0% CO2 environment. SV80, CD40, MCF7, and KB cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum in a humidified 7.0% CO2 environment. The Kym-1 cell line was maintained in Click RPMI 1640 medium supplemented with 10% fetal calf serum. SV80 cells stably expressing cFLIP-green fluorescent protein (GFP) were regularly cultured for 1 week per month under selection of 500 μg of G418 (Gibco BRL, Karlsruhe, Germany) per ml.
Cell death assays.
Wild-type or stably transfected SV80 cells (1.5 × 104 per well) were cultivated in 96-well microtiter plates overnight. Next day the reagents of interest were applied as indicated, and after an additional 6 h of culture, metabolic activity was measured by the MTT method. For transient GFP apoptosis assays, 5 × 106 cells were transfected with 6 μg of pEGFP (Clontech, Heidelberg, Germany) and with 24 μg of empty vector or pI-κB-SR by electroporation (250 V; capacity, 1,800 μF in Dulbecco's modified Eagle's medium). After splitting and 1 day, recovered cells were challenged with TNF or Flag-tagged TNF-related apoptosis-inducing ligand (TRAIL). The latter had been cross-linked with anti-Flag MAb M2 (Sigma, Deisenhofen, Germany) before treatment. After an additional 16 h, GFP-positive cells were analyzed for the percentage of cells showing morphological features of apoptosis.
Western blotting.
Cell lysates were prepared in radioimmunoprecipitation assay buffer supplemented with 0.1 volume of a protease inhibitor cocktail stock solution (Roche, Mannheim, Germany). Cell debris was removed by centrifugation at 10 000 × g for 10 min, and the protein concentrations were determined by the Bradford assay. Proteins (100 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose membranes by electroblotting, and nonspecific binding sites were blocked by incubation in Tris-buffered saline containing 0.05% Tween 20 and 3% (wt/vol) dry milk. Immunoblotting analyses were performed with the indicated antibodies. Immunocomplexes were visualized with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma) or horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma) and nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate as substrate.
RNase protection assay.
Cells (10 × 106 each of SV80, HeLa, HEK293, Kym-1, and KB; 30 × 106 each of Jurkat and Daudi) were treated as indicated. Total RNA was isolated with the RNA INSTAPURE kit (Eurogentech, Seraing, Belgium) according to the manufacturer's recommendations. The presence of transcripts of the indicated apoptosis-related genes as well as the internal controls L32 and GAPDH were analyzed using the hCK-3, hApo1c, hApo2, hApo3, hApo3b, hApo3c, hApo5, hApo5b, and hApo6 Multi-Probe template sets (PharMingen, Hamburg, Germany). Probe synthesis, hybridization, and RNase treatment were performed with the RiboQuant Multi-Probe RNase Protection Assay System (PharMingen) according to the manufacturer's recommendations. Finally, protected transcripts were resolved by electrophoresis on denaturing polyacrylamide gels (5%) and quantified on a PhosphorImager with the ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). To correct signals of protected transcripts for background intensities, the latter were determined for each individual lane in proximity to the respective mRNA signal and subtracted from the value of the protected transcript.
RESULTS AND DISCUSSION
IL-1, TNF, and a CD40-specific agonistic antibody induce a variety of apoptosis-related genes in SV80 cells, including IPL, TRAIL-R2, and cFLIP.
In the SV80-derived fibroblast cell line SV80-CD40, stably transfected with CD40, stimulation of tumor necrosis factor receptor 1 (TNF-R1) or of the TRAIL death receptors results in apoptosis only when protein synthesis is reduced, e.g., by CHX treatment (data not shown). Further, the induction of apoptosis in this cell line by TNF/CHX or TRAIL/CHX can be blocked by expression of one or more resistance-conferring proteins. In accordance with the well-known antiapoptotic properties of NF-κB, prestimulation of SV80-CD40 cells with NF-κB-inducers, like IL-1, TNF, or agonistic CD40-specific MAbs in the absence of CHX, protected against a subsequent apoptotic challenge with TNF/CHX or TRAIL/CHX (Fig. 1). To study the effects of IL-1, TNF, and agonistic CD40 antibody treatment on the transcription of apoptosis-related genes, we analyzed total RNA preparations from untreated as well as from IL-1-, TNF-, and agonistic CD40 antibody-stimulated SV80-CD40 cells. For this purpose we used the RNase protection analysis (RPA) technique with several template sets containing specific probes for a variety of apoptosis-related genes. In all cases L32 and glyceraldehyde-3-phosphate dehyrogenase (GAPDH) were included as internal controls. Upon treatment with the NF-κB-inducing ligands, we found a strong upregulation of TRAF1, cIAP1, and cIAP2 mRNA (Fig. 2A), coding for molecules that antagonize TNF-induced apoptosis in transient expression assays in concert with TRAF2 (40). A minor, barely detectable upregulation was also found for Bfl1/A1 (data not shown), a recently identified NF-κB-regulated member of the Bcl2 family also able to interfere with TNF- and chemotherapy-induced apoptosis (20, 45). Additional known NF-κB target genes which were found to be upregulated included Fas and transforming growth factor β2. However, besides these already known NF-κB-regulated genes, which mainly encode antiapoptotic molecules, we identified cFLIP (CLARP/casper/FLAME/I-FLICE/CASH/MRIT/Usurpin), an enzymatically inactive caspase 8 homologue (9, 10, 13–15, 29, 34), as a novel antiapoptotic gene, upregulated by IL-1, TNF, and CD40 (Fig. 2A and B). Two other novel target genes of these NF-κB inducers identified in this study were the imprinted gene IPL (TDAG51), known to couple T-cell receptor (TCR) signaling to Fas expression in activation-induced cell death (21, 27), and TRAIL-R2 (DR5/TRICK2/Killer), one of the two death domain-containing receptors for TRAIL (38). cFLIP has the capability to prevent death receptor-induced activation of the initiator caspases 8 and 10, thereby inhibiting apoptosis induction by all hitherto-known death receptors (9, 10, 13–15, 29, 34). Two splice forms of cFLIP have been described: a full-length 55-kDa form of cFLIP (cFLIP-L) containing two N-terminal death effector domains and a C-terminal caspase-like domain and an alternatively spliced short form (cFLIP-S) containing only the two death effector domains (9, 10, 13–15, 29, 34). Both splice forms are capable of inhibiting apoptosis, but the significance of the alternative splicing is not clear yet. The probe used in this study for RPA detects both cFLIP-L and cFLIP-S transcripts. In the RPAs shown in Fig. 2 and 3, the relative proportion of both splice forms is therefore not evident. However, Western blot analysis indicated that cFLIP-S, rather than cFLIP-L, is upregulated in SV80 (see Fig. 6A below) as well as in Jurkat cells (data not shown). Upregulation of cFLIP, cIAP1, cIAP2, and TRAF1 was verified at the protein level by Western blotting (see Fig. 6A below). Further, induction of Fas and TRAIL-R4 expression was confirmed by fluorescence-activated cell sorter (FACS) analysis (data not shown). Because of the prominent antiapoptotic properties that have been shown for both splice forms of cFLIP, we investigated the regulation of cFLIP-L/S in more detail.
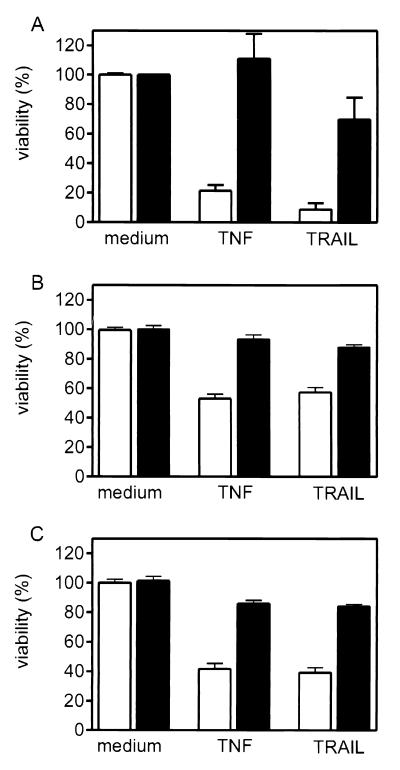
FIG. 1.
Impact of prestimulation with IL-1 (A), TNF (B), and the agonistic CD40-specific MAb G28.5 on TNF- and TRAIL-induced cytotoxicity. SV80-CD40 cells were cultivated in 96-well plates (15,000 cells/well) for 24 h and were then treated with IL-1 (10 ng/ml), TNF (10 ng/ml), and anti-CD40 MAb G28.5 (1 μg/ml) (A to C; solid bars) or remained untreated (A to C; empty bars). After 6 h the cells were washed twice with medium and challenged with TNF (50 ng/ml) or TRAIL-Flag (100 ng/ml) complexed with the anti-Flag MAb M2 (1 μg/ml) in the presence of 25 μg of CHX/ml for an additional 8 h. Cell viability was determined using the MTT assay.
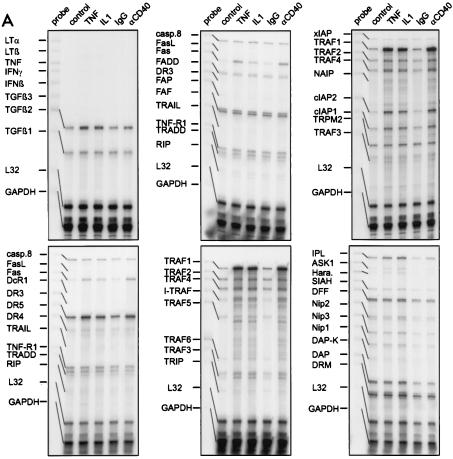
FIG. 2.
(A) RNase protection assay analysis of steady-state levels of mRNA of various apoptosis-related genes in SV80-CD40 cells which were stimulated with TNF (10 ng/ml), IL-1 (10 ng/ml), the agonistic CD40-specific MAb G28.5 (αCD40) (1 μg/ml), or control MAb (1 μg/ml) or remained untreated. Whole RNAs were isolated after treatment, and 10 μg of each RNA was analyzed with the hCK-3, hApo-1c, hApo-2, hApo-5, hApo-3, hApo-3c, hApo-5b, and hApo-6 Multi-Probe template sets to detect the indicated mRNAs. No cytokine-induced changes were observed with the Multi-Probe template sets hApo-1c, containing caspase-specific probes, and hApo-2, containing templates of bcl-2 family members (data not shown). L32 and GAPDH specific probes were included in each template set as internal controls. (B) SV80-CD40 cells were treated for the indicated times with IL-1 (10 ng/ml) or TNF (10 ng/ml), and Daudi and Jurkat cells were treated for 6 h with the agonistic CD40-specific MAb G28.5 (αCD40) (1 μg/ml) or P/I (I+P). con., control. Total RNAs were isolated, and 10 μg of each RNA was analyzed using RNase protection assays with the hApo-3b Multi-Probe template set, which contains, among others, probes for cFLIP and Fas. The position of the FLIP-specific band is indicated with an arrow.
FIG. 3.
The indicated cell lines were treated with TNF (10 ng/ml) for 6 h (+) or remained untreated (−). Total RNAs were isolated, and 10 μg of each RNA was analyzed as described above with the hApo-3b (A) and the hApo-5 (B) Multi-Probe template sets. The arrows indicate the positions of the bands specific for Fas, cFLIP, TRAF1, cIAP2, and cIAP1.
FIG. 6.
(A) Western blot analysis of cFLIP, caspase 8, TRAF1, and A20 expression levels in TNF- and IL-1-treated SV80-CD40 cells. SV80-CD40 cells (3 × 106) were incubated for 0, 7, and 11 h with TNF (10 ng/ml) or IL-1 (10 ng/ml). To determine the CHX sensitivity of the investigated proteins, cells were further treated for 11 h with TNF or IL-1 during the last 4 h that CHX (25 μg/ml) was added. Expression of cFLIP (FLIPS), caspase 8, TRAF1, cIAP1, cIAP2, and A20 was analyzed by Western blotting. Values on the left are in kilodaltons. (B) SV80 cells were pretreated with the indicated concentrations of CHX for 2 h and were subsequently challenged with TNF (10 ng/ml) for 6 h. Cells were analyzed with the hApo-3b Multi-Probe template set as described for Fig. 2.
CD40 plays an important role as a costimulatory molecule in B-cell activation. Stimulation of CD40 by CD40L on activated CD4+ T cells results in the upregulation of Fas in B cells, making these cells sensitive towards FasL in the absence of a proper B-cell receptor signal (30). In accordance with these data, Wang et al. (41) have recently shown an anti IgM-induced upregulation of cFLIP in B cells. In addition to that study, we found a significant upregulation of cFLIP in the Daudi B-cell line upon treatment with agonistic, CD40-specific MAbs (Fig. 2B). Moreover, treatment of the T-cell line Jurkat with ionomycin and phorbol myristate acetate (P/I), thus mimicking TCR stimulation, also induced transcription of cFLIP (Fig. 2B). These data are consistent with an antiapoptotic function of cFLIP during the early phase of T-cell activation, as discussed elsewhere (15).
TNF-induced upregulation of cFLIP is cell-type-specific.
Next, we analyzed TNF-induced upregulation of cFLIP in various TNF-responsive cell lines. In five out of seven cell lines that all responded with robust NF-κB activation upon TNF treatment (data not shown), TNF induced a two- to fourfold upregulation of cFLIP mRNA (Fig. 3A; Table 1). Although the relative TNF-dependent upregulation of cFLIP mRNA was rather similar in these cell lines, the RNA levels of cFLIP with respect to the total RNA varied over a wide range (Table 1). For example, about 700 U of cFLIP mRNA was detectable in untreated SV80-CD40 cells, whereas the cFLIP mRNA levels ranged between 42 and 102 U in untreated Kym-1, MCF7, KB, and HeLa cells. Accordingly, induced levels of cFLIP mRNA varied between 1,716 U (SV80-CD40) and 146 U (MCF7) (Table 1). A cell-type-specific induction pattern was also found for the TNF-inducible antiapoptotic targets TRAF1, cIAP1,and cIAP2 (Fig. 3B; Table 1): for example, TNF upregulated TRAF1, cIAP1, and cIAP2 in KB cells, whereas in Jurkat and MCF7 cells only cIAP2 was induced (Fig. 3B; Table 1).
TABLE 1.
Cell-type-specific induction of antiapoptotic genes by TNF
| Gene | m-RNA intensity and expression ratio for indicated cell typea
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SV80
|
Kym-1
|
MCF7
|
HeLa
|
293
|
Jurkat
|
KB
|
||||||||
| Untreated | TNF | Untreated | TNF | Untreated | TNF | Untreated | TNF | Untreated | TNF | Untreated | TNF | Untreated | TNF | |
| cFLIP | 701 | 1,716 (3.3) | 102 | 183 (3.3) | 42 | 146 (3.9) | 75 | 174 (2.9) | 55 | 39 (0.7) | 73 | 46 (0.85) | 88 | 172 (2.1) |
| L32 | 1,523 | 1,142 (0.75) | 6,219 | 3,435 (0.55) | 3,008 | 2,707 (0.90) | 2,195 | 1,756 (0.8) | 2,139 | 2,601 (1.22) | 1,895 | 1,422 (0.75) | 1,607 | 1,509 (0.94) |
| TRAF1 | 143 | 2,763 (20) | ND | 13 (induced) | ND | ND (NI) | ND | 10 (induced) | ND | ND (NI) | ND | ND (NI) | ND | 40 (induced) |
| cIAP2 | 33 | 345 (11) | 3 | 11 (11) | 4 | 29 (8.4) | 2 | 24 (12) | ND | ND (NI) | 8 | 30 (5) | 11 | 127 (14) |
| cIAP1 | 82 | 238 (3) | 35 | 50 (4) | 16 | 13 (0.9) | 7 | 21 (3) | 21 | 36 (1.5) | 13 | 10 (1.1) | 24 | 98 (5) |
| L32 | 1,553 | 1,523 (0.98) | 575 | 188 (0.33) | 3,493 | 3,004 (0.86) | 2,173 | 2,135 (0.98) | 3,346 | 3,955 (1.18) | 1,128 | 832 (0.73) | 2,276 | 1,821 (0.8) |
mRNA intensities are already corrected for background levels. To calculate relative expression levels (parenthetical numbers in rows 1 and 3 to 5), the mRNA intensities of TNF-treated cells were divided by the mRNA intensities of untreated cells. To take into account differences in sample loadings, this ratio was finally corrected according to the ratio parenthetical numbers in rows 2 and 6 of the expression levels of the housekeeping L32 gene in treated and untreated groups. For example, TNF-induced cFLIP expression in SV80 cells was calculated as follows: 1,716/701 × 1,523/1,142 = 3.3. FLIP and TRAF1, cIAP1, and cIAP2 were analyzed with different template kits and corrected for the corresponding internal L32 control. ND, not determined. NI, not induced.
TNF-induced upregulation of cFLIP is dependent on NF-κB activation.
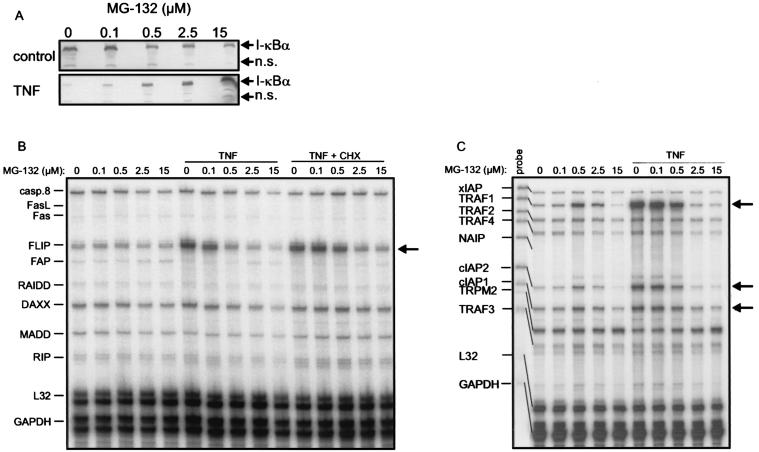
IL-1, TNF, P/I, and CD40L are potent activators of the NF-κB pathway. In fact, for TRAF1, cIAP2, A20, and Fas, which were all upregulated in our system, NF-κB-dependent transcription has already been shown (6, 8, 33, 35, 40). To verify a role for NF-κB in the cytokine-dependent upregulation of cFLIP in SV80-CD40 cells, we analyzed the impact of the proteasome inhibitor N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal (MG-132) on TNF-induced upregulation of cFLIP mRNA. As shown in Fig. 4, a MG-132 concentration of 2.5 μM was sufficient to completely prevent TNF-induced degradation of IκB (Fig. 4A) and upregulation of cFLIP mRNA (Fig. 4B). Notably, MG-132-dependent inhibition of the induction of cFLIP and the known NF-κB-regulated targets Fas, TRAF1, and cIAP2 could be observed with the same dose dependency (Fig. 4B and C). It has recently been shown that disruption of Hsp90 function by GA results in degradation of the receptor-interacting protein (RIP) (23), which is part of the TNF-R1 signaling complex and is essentially involved in TNF-induced NF-κB activation (18). To further substantiate an NF-κB-dependent mechanism of TNF-induced upregulation of cFLIP, we blocked Hsp90 function by treatment with GA for 14 h and analyzed the effects of this treatment on TNF-induced upregulation of cFLIP. As shown in Fig. 4D, GA pretreatment had a modest inhibitory effect on the constitutive cFLIP mRNA level. However, more important, GA pretreatment completely blocked TNF-induced upregulation of cFLIP (Fig. 4D). Together, these data argue for a NF-κB-dependent expression of cFLIP upon TNF treatment of SV80 cells.
FIG. 4.
(A) SV80-CD40 cells were treated with the indicated concentrations of MG-132 or remained untreated. Cells were stimulated with TNF (10 ng/ml) for 20 min, and cellular IκB contents were compared by Western blotting. n.s., nonspecific. (B and C) SV80-CD40 cells were treated with the indicated concentrations of MG-132 for 7 h. In addition, the various groups were cotreated with TNF (10 ng/ml) or with TNF and CHX (25 μg/ml) for the last 6 h of MG-132 incubation. Total RNAs were isolated, and 10 μg of each RNA was analyzed using RNase protection assays with the hApo-3b (B) and the hApo-5 (C) Multi-Probe template sets. (D) SV80-CD40 cells were treated with the indicated concentrations of GA for 14 h. Cells were then challenged further with TNF (10 ng/ml) for 6 h or remained without additional treatment. Cell lysates were analyzed as described for panels B and C. Arrows indicate the positions of the bands specific for FLIP (B and D) and TRAF1, cIAP2, and cIAP1 (C).
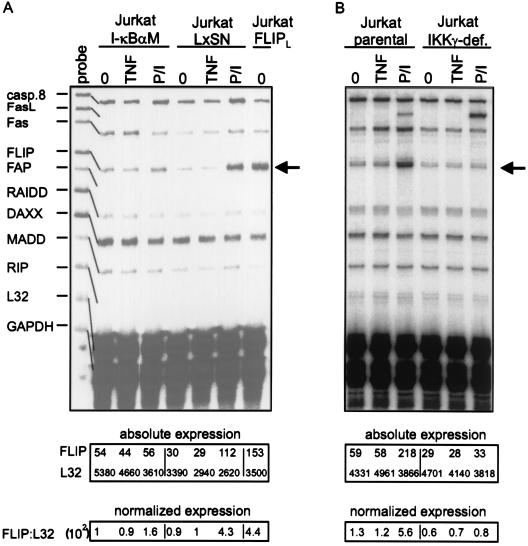
To verify the possible involvement of NF-κB activation in P/I-induced upregulation of cFLIP, we took advantage of Jurkat cell lines stably overexpressing IκB mutants [I-κBαM, I-κB(2N)] that efficiently block NF-κB activation (2, 17). P/I-induced cFLIP upregulation was almost completely inhibited in I-κBαM-expressing (Fig. 5A, left panel) as well as in I-κB(2N)-expressing (data not shown) Jurkat cells, compared to the respective vector-transfected control cells. Moreover, in a Jurkat clone deficient for IKKγ (NEMO) (11), an essential component of the NF-κB-inducing IKK complex, P/I-induced upregulation of cFLIP was completely abrogated (Fig. 5B, right panel). Thus, activation of NF-κB is essentially involved in P/I-induced transcription of cFLIP. Notably, the amounts of cFLIP mRNA induced upon P/I treatment were comparable to the expression level observed in a Jurkat clone stably transfected with cFLIP-L (Fig. 5A, rightmost two lanes), which is protected against TRAIL- and Fas-induced apoptosis (data not shown). The mRNA levels of TRAF1, TRAF2, cIAP1, and cIAP2 were unchanged in the Jurkat FLIP clone (data not shown), indicating that selective upregulation of cFLIP to a “physiological” extent is sufficient to confer resistance against death receptor-induced apoptosis.
FIG. 5.
(A and B) Jurkat-I-κBαM cells and the corresponding parental cell line transfected with empty vector (Jurkat-LxSN) (A) or IKKγ-deficient Jurkat cells together with the parental control cell line (B) were treated with TNF (10 ng/ml) or P/I for 6 h or remained untreated (0). Ten micrograms of total RNA of each sample was analyzed using RNase protection assay analysis with the hApo-5 Multi-Probe template set. FLIPL, Jurkat clone stably transfected with cFLIP-L. Absolute expression and normalized expression are given in arbitrary units.
cFLIP, but not TRAF1, A20, and cIAP2, is rapidly downregulated by CHX in SV80 cells.
The induction of the antiapoptotic molecules cFLIP, TRAF1, cIAP1, and cIAP2 upon treatment of SV80-CD40 cells with the NF-κB-inducers TNF, IL-1, and the CD40-specific MAb G28.5 (Fig. 2) correlates well with the ability of these agonists to prevent apoptosis by a subsequent challenge with CHX/TRAIL or CHX/TNF. However, it is unclear whether all of these molecules are necessary to establish the protected status of cells or whether a single factor may have a dominant role. As induction of apoptosis with TNF and TRAIL in SV80-CD40 cells critically depends on the presence of CHX, we investigated the impact of this metabolic inhibitor on the protein expression levels of the antiapoptotic factors mentioned above. Analysis of expression of the A20 zinc finger protein was also performed, because this protein is also known as a NF-κB-inducible factor with antiapoptotic properties (33) but was not included in RPA template sets used for Fig. 2. Cells were treated with TNF or IL-1 for 7 h to induce the expression of these factors and were then cultured for an additional 4 h in the presence or absence of CHX. Finally, cell lysates were analyzed by Western blotting. As shown in Fig. 6A, there was no significant effect of CHX treatment on the expression of cIAP1, cIAP2, TRAF1, A20, and the cFLIP-related molecule caspase 8. However, cFLIP-S, the short splice form of cFLIP, was undetectable after CHX treatment (Fig. 6A). Moreover, cFLIP-L, the long splice form of cFLIP, was hardly detectable after TNF or IL-1 treatment but vanished upon CHX treatment too (data not shown). CHX inhibits protein synthesis. In accordance with this, we found that after CHX treatment, cFLIP protein completely disappeared (Fig. 6A), whereas constitutive and induced mRNA levels of cFLIP did not decrease but even increased somewhat (Fig. 6B). While the half-life of cFLIP-L and -S protein was unusually short compared to those of other proteins, the half-life of cFLIP mRNA was comparable to those of several other species, including caspase 8 mRNA (data not shown).
The antiapoptotic status of SV80-CD40 cells induced by TNF, IL-1, or G28.5 pretreatment was of a transient nature, as TNF/CHX or TRAIL/CHX treatment induced delayed apoptosis (5 to 8 h) in these pretreated cells (data not shown). Ongoing apoptosis correlated well with the reduction in the expression levels of cFLIP by CHX treatment, suggesting that cFLIP plays a dominant role in conferring antiapoptotic status on SV80-CD40 cells.
Analysis of the antiapoptotic potential of cFLIP in stably transfected SV80 cells.
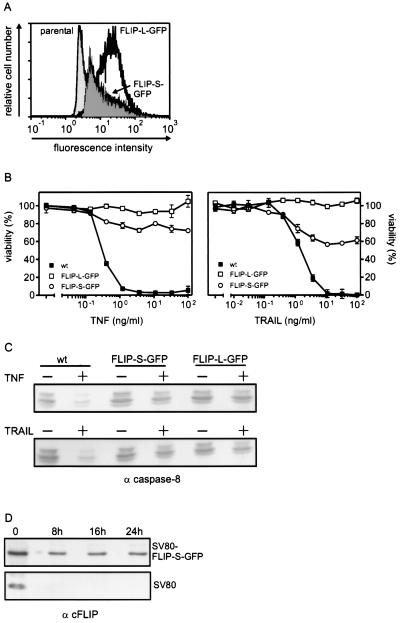
It has been shown that the antiapoptotic action of NF-κB counteracting TNF-induced cell death can be mimicked by simultaneous overexpression of TRAF1, TRAF2, cIAP1, and cIAP2. While coexpression of these molecules leads to a blockade of caspase 8 activation, each of these proteins was at best partially protective when overexpressed individually (40). Taking into account that cFLIP, a highly potent negative regulator of death receptors, is also induced by NF-κB, we considered the possibility that cFLIP has a more central role in the antiapoptotic NF-κB response than does the TRAF/IAP complex. We therefore analyzed the antiapoptotic status of SV80 cells stably overexpressing cFLIP-L and cFLIP-S with a C-terminally linked GFP tag (Fig. 7A). The SV80 FLIP-L–GFP transfectants were completely resistant to TNF- and TRAIL-induced apoptosis, and the SV80 FLIP-S–GFP transfectants showed a largely reduced sensitivity to these death-inducing ligands (Fig. 7B). The residual sensitivity of cFLIP-S–GFP-transfected cells correlated with the overexpression of cFLIP-S–GFP, which is weaker than that of cFLIP-L–GFP (Fig. 7A). Moreover, no indication of processing of procaspase 8 (Fig. 7C) and procaspase 3 (data not shown) was found in cFLIP-L–GFP as well as in cFLIP-S–GFP transfectants. Even after a prolonged TNF/TRAIL challenge (24 h) in the presence of CHX, the cFLIP-L– and cFLIP-S–GFP transfectants remained fully viable (data not shown). This correlated with the fact that the expression level of cFLIP-S–GFP was not affected or only modestly affected by CHX treatment, whereas in parental SV80 cells, endogenous cFLIP-L and -S vanished completely after 8 h of incubation with CHX (Fig. 7D). The insensitivity of FLIP-L– and FLIP-S–GFP expression against CHX treatment may be caused by the GFP tag and/or may reflect the strong transcriptional activity of the cytomegalovirus promoter controlling the cFLIP-L– and cFLIP-S–GFP cDNA. To rule out that selection (G418 and/or FACS sorting) of the cFLIP-L– and cFLIPS–GFP-expressing cells had in parallel led to the expansion of transfectants with downregulated death receptors, we determined the capability of TNF and TRAIL to induce NF-κB-dependent genes in the cFLIP-L– and cFLIP-S–GFP-expressing cells. As shown in Fig. 7E, TNF-induced upregulation of TRAF1 and cIAP1 was indistinguishable among SV80 FLIP-L–GFP, SV80 FLIP-S–GFP, and parental SV80 cells. We have recently shown that TRAIL is also able to activate NF-κB in the presence of CHX, provided that the concomitantly induced apoptotic process is blocked by z-VAD-fmk (26, 37). Under these conditions upregulation of NF-κB-dependent genes was also found in SV80 FLIP-S–GFP and SV80 FLIP-L–GFP cells upon TRAIL challenge. However, the level of induction was somewhat, but significantly, lower in the cFLIP-L–GFP transfectants than in the cFLIP-S–GFP transfectants and the parental control. To clarify whether this reflects partial inhibition of TRAIL-induced NF-κB activation by cFLIP-L as described elsewhere (37) or whether clones with reduced TRAIL responsiveness had been accumulated during selection of the transfectants will require refined analysis in the future. Similar results were obtained by analyses of SV80 transfectants expressing non-GFP-tagged forms of cFLIP-L and cFLIP-S (data not shown).
FIG. 7.
(A) FACS analysis of SV80 transfectants stably expressing FLIP-L–GFP or FLIP-S–GFP and mock-transfected SV80 cells. (B) Cells described for panel A were cultivated in 96-well plates (15,000 cells/well) for 24 h and were then treated with the indicated concentrations of TNF or TRAIL-Flag complexed with the anti-Flag MAb M2 (1 μg/ml) in the presence of 50 μg of CHX/ml for an additional 16 h. Cell viability was determined using the MTT assay. wt, wild type. (C) Cells described for panel A were challenged with TNF (10 ng/ml) and with cross-linked TRAIL-Flag in the presence of 50 μg of CHX/ml or remained untreated. Cells were lysed, and proteins were then separated by SDS-PAGE and transferred to nitrocellulose. The presence of the nonprocessed caspase 8 isoforms p53 and p55 was determined by Western blot analyses. wt, wild type; −, absence of TNF or TRAIL; +, presence of TNF or TRAIL. (D) SV80 and SV80 FLIP-S–GFP cells were incubated for the indicated times with CHX (25 μg/ml). Proteins (70 μg per lane) were then separated by SDS-PAGE and transferred to nitrocellulose, and the expression of endogenous cFLIP in SV80 cells and of cFLIP-S–GFP in the transfectants was detected on the same blot with the anti-FLIP MAb N19 and an alkaline-conjugated secondary antibody. (E) RNase protection assay analysis of various members of the TRAF and IAP protein families in SV80, SV80 FLIP-S–GFP, and SV80 FLIP-L–GFP. Cells were treated with the indicated combinations of TNF (20 ng/ml), agonistic anti-TRAIL-R2 antisera (αTR2) (1 μg/ml), z-VAD-fmk (Z) (20 μM), and CHX (C) (25 μg/ml) for 6 h. 0, untreated. Total RNAs were isolated after treatment, and 10 μg of each RNA was analyzed with the hApo-5 Multi-Probe template set to detect the indicated mRNAs. Absolute expression and normalized expression are given in arbitrary units. Relative expression levels were calculated as described in Materials and Methods. Arrows indicate the positions of the bands specific for TRAF1 and cIAP2.
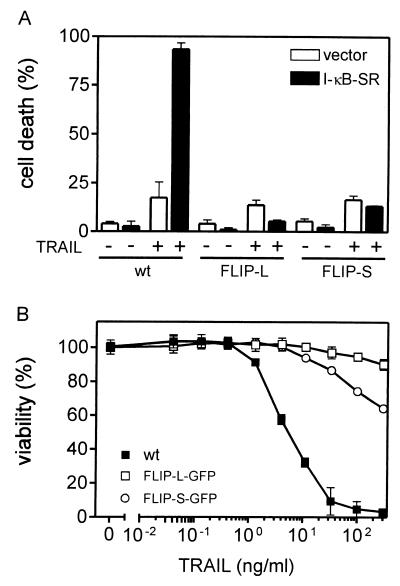
The antiapoptotic status of SV80 cells induced by IL-1 or TNF is accompanied by upregulation of TRAF1 (Fig. 3B and 6A), cIAP2 (Fig. 3B and 6A), A20 (Fig. 6A), and cFLIP (Fig. 3A and 6A). However, only cFLIP expression was rapidly decreased upon CHX treatment (Fig. 6A), leading to reversion of the antiapoptotic status of IL-1/TNF-treated cells. As already discussed above for Jurkat cells, this again argues for cFLIP, in particular cFLIP-S, as the major mediator of the antiapoptotic NF-κB response. We consistently found that transient overexpression of a nondegradable mutant of IκB (of IκB-SR), which inhibits TNF- and TRAIL-induced NF-κB activation, rendered SV80 wild-type cells but not cFLIP-L- or cFLIP-S-expressing cells sensitive to TNF or TRAIL in the absence of CHX (Fig. 8A). Similarly, treatment of SV80 wild-type cells but not of cFLIP-S– and cFLIP-L–GFP-expressing cells with MG-132 also allowed apoptosis induction by TNF or TRAIL in the absence of CHX (Fig. 8B).
FIG. 8.
(A) Transient expression of IκB-SR renders SV80 cells, but not FLIP-L- or FLIP-S-expressing cells, sensitive to TRAIL in the absence of CHX. SV80, SV80 FLIP-L, and SV80 FLIP-S cells were transfected with pEGFP along with empty vector or pIκB-SR encoding a nondegradable form of IκB and were split. After 1 day of recovery they were challenged with TRAIL-Flag complexed with the anti-Flag MAb M2 (1 μg/ml) for an additional 16 h or remained untreated. Finally GFP-positive cells were analyzed for the percentage of cells with morphological features of apoptosis. −, absence of TRAIL; +, presence of TRAIL. (B) Cells were cultivated in 96-well plates (15,000 cells/well) for 24 h and were then treated 1 h with MG-132 (10 μM). Subsequently, the indicated concentrations of cross-linked TRAIL-Flag were added for an additional 16 h. Cell viability was determined using the MTT assay. wt, wild type.
Thus, the antiapoptotic TRAF1/TRAF2/cIAP1/cIAP2 complex mentioned above may exert its protective functions under more specialized conditions. Indeed, several data from the literature support this theory: the TRAF1/TRAF2/cIAP1/cIAP2 complex inhibits TNF-induced caspase 8 processing, suggesting that this complex may act at the level of the receptor signaling complex (40). Hence, the TRAF components of the complex may be responsible for the recruitment of the complex, whereas the IAP components mediate caspase 8 inhibition. Indeed, it has been shown that cIAP1 and -2 directly associate with and inhibit caspases 3 and 7 but not caspase 8 (32). Consequently, the TRAF1/TRAF2/cIAP1/cIAP2 complex and its recruitment into the receptor signaling complex of TNF-R1 could allow caspase 8 and cIAPs to interact. As the TRAF proteins are not or at least no major binding partners of Fas, TRAIL-R1, and TRAIL-R2, this complex should predominantly interfere with TNF-induced apoptosis. We have recently found that the 50% effective dose (ED50) of gene induction by TNF is about 500 times lower than its ED50 for the induction of apoptosis, whereas the dose response analysis of TRAIL and FasL revealed no differences for these responses (37). This dominance of the gene-inducing pathway over the apoptosis-inducing pathway in the case of TNF is again in good agreement with the existence of a TNF-R1-selective antiapoptotic mechanism distinct from cFLIP induction. Moreover, researchers have shown that degradation/depletion of TRAF2 leads to a drastic enhancement of TNF-R1- but not Fas- and TRAIL-R-induced apoptosis (5, 28, 42). Again, this is in good agreement with the existence of a TRAF2-dependent, TNF-R1-selective antiapoptotic mechanism distinct from cFLIP induction.
ACKNOWLEDGMENTS
We are grateful to S.-C. Sun for the IKKγ/NEMO-deficient Jurkat cells. We thank P. Schneider and J. Tschopp for TRAIL-R1/2-Fc, Jurkat-FLIP cells, and TRAIL. We are grateful to J. Hiscott and D. R. Green for Jurkat-I-κBα(2N) and Jurkat-I-κBαM, respectively. We thank I. Schmitz and P. Krammer, K. S. Schulze-Osthoff, and C. Vinzenc for antibodies against cFLIP, caspase 8, and A20, respectively.
This work was supported by Deutsche Forschungsgemeinschaft grant Wa 1025/3–1 and Sonderforschungsbereich 495 project A5.
REFERENCES
- 1.Barkett M, Xue D, Horvitz H R, Gilmore T D. Phosphorylation of IkappaB-alpha inhibits its cleavage by caspase CPP32 in vitro. J Biol Chem. 1997;272:29419–29422. doi: 10.1074/jbc.272.47.29419. [DOI] [PubMed] [Google Scholar]
- 2.Beauparlant P, Kwon H, Clarke M, Lin R, Sonenberg N, Wainberg M, Hiscott J. Transdominant mutants of IκBα block Tat-tumor necrosis factor synergistic activation of human immunodeficiency virus type 1 gene expression and virus multiplication. J Virol. 1996;70:5777–5785. doi: 10.1128/jvi.70.9.5777-5785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Chan F K-M, Lenardo M J. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Chan H, Bartos D P, Owen-Schaub L B. Activation-dependent transcriptional regulation of the human fas promoter requires NF-κB p50–p65 recruitment. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Edelstein L C, Gelinas C. The Rel/NF-κB family directly activates expression of the apoptosis inhibtor Bcl-xL. Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Z-L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goltsev Y V, Kovalenko A V, Arnold E, Varfolomeev E E, Brodianskii V M, Wallach D. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- 10.Han D K, Chaudhary P M, Wright M E, Friedman C, Trask B J, Riedel R T, Baskin D G, Schwartz S M, Hood L. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc Natl Acad Sci USA. 1997;94:11333–11338. doi: 10.1073/pnas.94.21.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harhaj E W, Good L, Xiao G, Uhlik M, Cvijic M E, Rivera-Walsh I, Sun S-C. Somatic mutagenesis studies of NF-kappa B signaling in human T cells: evidence for an essential role of IKK gamma in NF-kappa B activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene. 2000;19:1448–1456. doi: 10.1038/sj.onc.1203445. [DOI] [PubMed] [Google Scholar]
- 12.Hess S, Gottfried E, Smola H, Grunwald U, Schuchmann M, Engelmann H. CD40 induces resistance to TNF-mediated apoptosis in a fibroblast cell line. Eur J Immunol. 1998;28:3594–3604. doi: 10.1002/(SICI)1521-4141(199811)28:11<3594::AID-IMMU3594>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Vincenz C, Ni J, Gentz R, Dixit V M. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 14.Inohara N, Koseki T, Hu Y, Chen S, Nunez G. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc Natl Acad Sci USA. 1997;94:10717–10722. doi: 10.1073/pnas.94.20.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J-L, Schroter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 16.Irmler M, Martinon F, Holler N, Steiner V, Ruegg C, Wajant H, Tschopp J. Caspase-induced inactivation of the anti-apoptotic TRAF1 during Fas ligand-mediated apoptosis. FEBS Lett. 2000;468:129–133. doi: 10.1016/s0014-5793(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 17.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 18.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 19.Kothny-Wilkes G, Kulms D, Pöppelmann B, Luger T A, Kubin M, Schwarz T. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 1998;273:29247–29253. doi: 10.1074/jbc.273.44.29247. [DOI] [PubMed] [Google Scholar]
- 20.Lee H H, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-κB-mediated upregulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M P, Feinberg A P. Genomic imprinting of a human apoptosis gene homologue, TSSC3. Cancer Res. 1998;58:1052–1056. [PubMed] [Google Scholar]
- 22.Levkau B, Scatena M, Giachelli C M, Ross R R, Raines E W. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-kappa B loop. Nat Cell Biol. 1999;1:227–233. doi: 10.1038/12050. [DOI] [PubMed] [Google Scholar]
- 23.Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, Liu Z G. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000;275:10519–10526. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Devin A, Rodriguez Y, Liu Z. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z G, Hsu H L, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 26.Mühlenbeck F, Schneider P, Bodmer J-L, Schwenzer R, Hauser A, Schubert, Scheurich P, Moosmayer D, Tschopp J, Wajant H. The tumor necrosis factor-related apoptosis-inducing ligand receptors TRAIL-R1 and TRAIL-R2 have distinct cross-linking requirements for initiation of apoptosis and are non-redundant in JNK activation. J Biol Chem. 2000;275:32208–32213. doi: 10.1074/jbc.M000482200. [DOI] [PubMed] [Google Scholar]
- 27.Park C G, Lee S Y, Kandala G, Lee S Y, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;4:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- 28.Pimentel-Muinos F X, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- 29.Rasper D M, Vaillancourt J P, Hadano S, Houtzager V M, Seiden I, Keen S L, Tawa P, Xanthoudakis S, Nasir J, Martindale D, Koop B F, Peterson E P, Thornberry N A, Huang J, MacPherson D P, Black S C, Hornung F, Lenardo M J, Hayden M R, Roy S, Nicholson D W. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5:271–288. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- 30.Rathmell J C, Townsend S E, Xu J C, Flavell R A, Goodnow C C. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 31.Reuther J Y, Baldwin A S. Apoptosis promotes a caspase-induced amino-terminal truncation of IκBα that functions as a stable inhibitor of NF-κB. J Biol Chem. 1999;274:20664–20670. doi: 10.1074/jbc.274.29.20664. [DOI] [PubMed] [Google Scholar]
- 32.Roy N, Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarma V, Lin Z, Clark L, Rust B M, Tewari M, Noelle R J, Dixit V M. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc finger protein that inhibits apoptosis. J Biol Chem. 1995;270:12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasula S M, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce C M, Litwack G, Tomaselli K J, Armstrong R C, Alnemri E S. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- 35.Schwenzer R, Siemienski K, Liptay S, Schubert G, Peters N, Scheurich P, Schmid R, Wajant H. The human tumor necrosis factor (TNF) receptor-associated factor 1 gene (TRAF1) is up-regulated by cytokines of the TNF ligand family and modulates TNF-induced activation of NF-kappaB and c-Jun N-terminal kinase. J Biol Chem. 1999;274:19368–19374. doi: 10.1074/jbc.274.27.19368. [DOI] [PubMed] [Google Scholar]
- 36.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 37.Wajant H, Haas E, Schwenzer R, Mühlenbeck F, Kreuz S, Schubert G, Grell M, Smith C, Scheurich P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- 38.Walczak H, Krammer P H. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 39.Wang C Y, Mayo M W, Baldwin A S. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 40.Wang C-Y, Mayo M V, Korneluk R G, Goeddel D V, Baldwin A S. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and cIAP1 and cIAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Lobito A A, Shen F, Hornung F, Winoto A, Lenardo M J. Inhibition of Fas-mediated apoptosis by the B cell antigen receptor through c-FLIP. Eur J Immunol. 2000;30:155–163. doi: 10.1002/1521-4141(200001)30:1<155::AID-IMMU155>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.Weiss T, Grell M, Siemienski K, Dürkop H, Pfizenmaier K, Scheurich P, Wajant H. TNFR80-dependent enhancement of TNFR60-induced cell death is mediated by TRAF2 and is specific for TNFR60. J Immunol. 1998;161:3136–3142. [PubMed] [Google Scholar]
- 43.Wu M, Lee H Y, Bellas R E, Schauer S L, Arsura M, Katz D, Fitzgerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB /Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 44.Wu M X, Ao Z, Prasad K V S, Wu R, Schlossman S F. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 45.Zong W-X, Edelstein L C, Chen C, Bash J, Gélinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]