Abstract
The outbreaks of infectious diseases that spread across countries have generally existed for centuries. An example is the occurrence of the COVID-19 pandemic in 2020, which led to the loss of lives and economic depreciation. One of the essential ways of handling the spread of viruses is the discovery and administration of vaccines. However, the major challenges of vaccination programs are associated with the vaccine cold chain management and cold storage facilities. This paper discusses how vaccine cold chain management and cold storage technology can address the challenges of vaccination programs. Specifically, it examines different systems for preserving vaccines in either liquid or frozen form to help ensure that they are not damaged during distribution from manufacturing facilities. Furthermore, A vaccine is likely to provide very low efficacy when it is not properly stored. According to preliminary studies, the inability to store vaccine properly is partly due to the incompetency of many stakeholders, especially in technical matters. The novelty of this study is to thoroughly explore cold storage technology for a faster and more comprehensive vaccine distribution hence it is expected to be one of the reference and inspiration for stakeholders.
Keywords: Big data, COVID-19, Efficient energy, Health, Innovation, IoT, Passive cooling, Refrigeration, Thermal stability
1. Introduction
The occurrence and widespread of diseases, also known as epidemics and pandemics, have been recorded for centuries. Pandemic is detrimental to the economy, morals, culture, and human civilization (Boao Farum for Asia, 2021). The first recorded pandemic was smallpox in 1157 BC, with Pharaoh Rameses V of Egypt as one of the victims (Paul and Pal, 2020). This virus, spread to various parts of the world such as China from 25 to 49, Europe from the 11th to 13th centuries, and America from 1617 to 1619 (Thèves et al., 2016). In addition, other diseases have also occurred, such as Antonine (165 AD–180 AD), Justinian (mid-sixth century), black death (1347 AD–1400 AD), a different type of smallpox in the Former Yugoslavia (1972), Spanish Flu (1918 AD–1980 AD), HIV (1980 AD–1990s M), SARS Cov (2002–2003), H5N1 (2009–2010), Ebola (2014–2016), and Zika Virus (2015–2016) (Huremović, 2019). The most current pandemic in the world from 2019 until now is COVID-19 (WHO, 2021a).
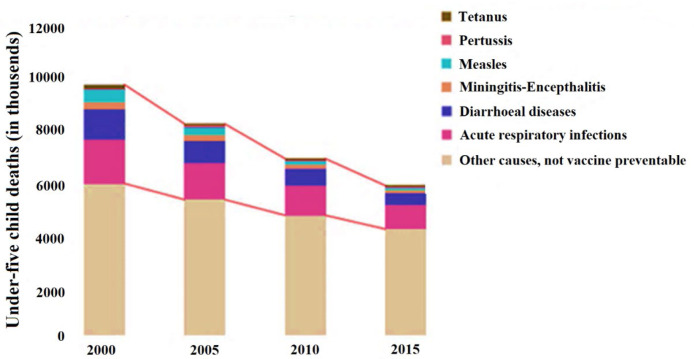
The rapid scientific findings of vaccines associated with these pandemics always help inhibit, prevent, and eradicate the incidence of disease transmission quickly and widely. According to the World Health Organization (WH0), the world successfully eliminated the smallpox epidemic in December 1979 (WHO, 2016a). The success attributed to this epidemic is partly dedicated to the great discovery of a vaccine by Edward Jenner in 1796 (WHO, 2016b) and developments by other scientists. However, the world is struggling to eradicate the most significant killer diseases such as tetanus, pertussis, meningitis-encephalitis, diarrhea, acute respiratory infections, and others that cannot be vaccinated. Fig. 1 shows the data on the efficacy of vaccines in reducing mortality from 2000–2015 (WHO, 2017a).
Fig. 1.
Data on the efficacy of vaccines in reducing mortality in 2000–2015 (WHO, 2017a).
From previous experience, it is understood that vaccination has succeeded in reducing the death rate due to pandemics and eliminating the existence of these viruses. Every year, millions of lives are saved through vaccination, which is widely recognized as the world’s most successful and cost-effective health intervention (WHO, 2019, Lloyd and Cheyne, 2017). Therefore, this program is one of the main priorities to stop the ongoing COVID-19 pandemic (Goralnick et al., 2021). In June 2021, WHO noted the establishment of 287 registered COVID-19 vaccines, with 102 at the clinical stage (WHO, 2021b). The sheer number of successful candidates at the clinical stage bodes well for progress in the ongoing efforts.
Significant logistical mode of distribution is needed in addition to providing a vaccine with high efficacy and without logistical challenges during distribution (Al-Qahtani and Alsafhi, 2021). This is because SARS-CoV-2 is very easy to infect and mutate (Dai et al., 2020). This is similar to the influenza virus, which is easily mutated hence it requires an annual update of vaccine strains (Navarro-Torné et al., 2019). Furthermore, the world’s population, which reached 7.7 billion in 2019 (United Nations, 2019), is certainly a challenge in terms of vaccine logistics. This is a consideration of the population size and operational, budgetary (Lim et al., 2019), institutional, analytical capacities, and geographical conditions (Aminah et al., 2021).
A panel meeting carried out by vaccination experts in Washington, DC, on May 4–5, 2012, agreed that the main challenge in vaccine delivery is the complexity in all areas of the process, which is often underestimated (Tan et al., 2014). Each vaccine has its characteristics however the existing ones need to be generally stored at a temperature range of −70 to 8 (WHO and UNICEF, 2021). Furthermore, cold storage is essential because vaccines are substances that quickly change their properties and efficacy when stored at other temperatures (Hanson et al., 2017, Kumru et al., 2014).
Therefore, this study aims to review the various kinds of recent study findings on cold storage technology and cold chain management. It also summarizes various criteria for packaging, storage, and distribution according to the standards issued by WHO (World Health Organization), vaccine manufacturer, CDC (Centers for Disease Control and Prevention), and FDA (Food and Drug Administration). Furthermore, this study is expected to facilitate authors, policymakers, field implementers, expedition/logistics businessmen, and technology developers (engineers) in implementing the vaccination program. It is very important because due to the need to improve the understanding level of stakeholders on vaccine management (Osei et al., 2019, Yakum et al., 2015, Mohammed et al., 2021b, Bogale et al., 2019, Thielmann et al., 2020, Dairo et al., 2016, Vangroenweghe, 2017, Thielmann et al., 2019b). Studies have shown numerous failures in vaccine management, especially in terms of temperature in storage units and standard for distribution (Thielmann et al., 2019b, Yauba et al., 2017). Therefore, success in understanding and adequately addressing supply chain issues can significantly reduce the impact of any vaccine (Lee and Haidari, 2017).
2. Methodology
2.1. Data collection
Data on the latest findings and reports related to vaccine cold storage and chain management were collected through a literature review. Each recent finding was grouped according to its respective subtopic by using the following literacy criteria as a reference:
-
•
Materials published from 2000 to 2021 on vaccine manufacturers were not limited to that year and are still relevant to current conditions.
-
•
Literature from reputable international journals or conference proceedings, guidelines, reports, and books issued by official government agencies for Health Affairs, WHO, and UNICEF, as well as manuals and reports issued by manufacturers.
-
•
Literature that discusses study topics on vaccine cold storage, cold chain, management, transport, temperature, and thermostability.
2.2. Data analysis and presentation
The analysis and presentation of the study data are divided into 2 chapters. The first is a storage specification challenge which contains general guidelines regarding packaging, storage unit, space, and distribution. These guidelines refer to manufacturers and official government agencies in charge of the health sector, both nationally and internationally. Therefore, the presentations in the latest findings and innovations of the second chapter do not violate the general guidelines that need to be adhered. The second chapter discusses vaccine cold storage technology and supply chain management. Furthermore, this chapter is divided into several sub-chapters to facilitate the understanding of the classification process.
3. Storage specification standard challenge
3.1. Packaging
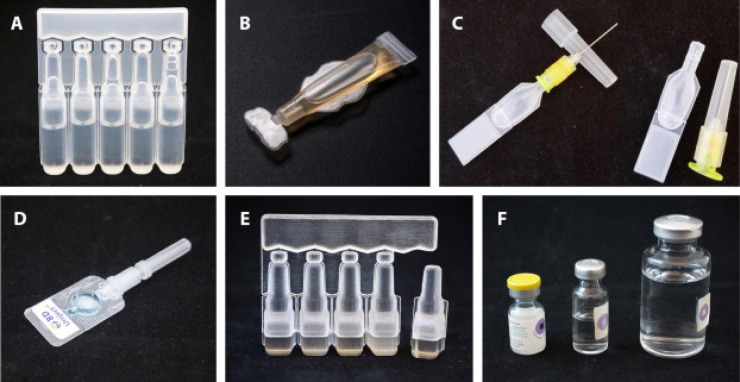
In general, packaging at the producer level is divided into 3, namely primary, secondary, and tertiary (Ortiz et al., 2020, Ramakanth et al., 2021). Manufacturers produce vaccines and diluents for reconstituted products in primary packaging, which are then stored in secondary containers in the form of cartons (Vaccine Presentation and Packaging Advisory Group, 2015, WHO et al., 2017, Taneja et al., 2018). The several types of primary packaging currently available are shown in Fig. 2. Furthermore, the secondary packaging is repackaged in a larger carton known as a “tertiary carton” (Ortiz et al., 2020, Vaccine Presentation and Packaging Advisory Group, 2015, Taneja et al., 2018) (see Fig. 3).
Fig. 2.
Types of primary packaging (A) Oral BFS MMD, (B) preformed polymer tube, (C) BFS CPAD, (D) preformed CPAD device, (E) parenteral BFS MMD ampule; and (F) glass vials (Sedita et al., 2018).
Fig. 3.
Cartons storage recommended by WHO, UNICEF, and EVM (WHO et al., 2017).
3.2. Storage unit
In general, vaccines need to be stored in cold temperature therefore it is imperative to make use of the cold storage method (Hatchett, 2017). The Rotavirus (ROTASIIL®), with a lifespan of 6 months at 37 °C–40 °C, increased to 30 months when stored below 25 °C (Naik et al., 2017) (see Fig. 4).
Fig. 4.
Vaccines arrangement of storage units recommended by the Public Health Unit of Canada (2021).
The selection of cold storage is very important considering the property and efficacy of vaccines are very sensitive to changes in temperature (Hatchett, 2017). Vaccines are stable enough to be used as drugs through efficient cold chain maintenance (manufacture, distribution, storage, and administration) (WHO and UNICEF, 2021, Kumru et al., 2014). The selection and management of cold storage operations are very important because vaccines are very sensitive when frozen (e.g., aluminum adjuvant vaccines) (Hanson et al., 2017, Kumru et al., 2014). Meanwhile, other types will lose their efficacy when temperature increases (e.g., live attenuated virus vaccines) (Kumru et al., 2014).
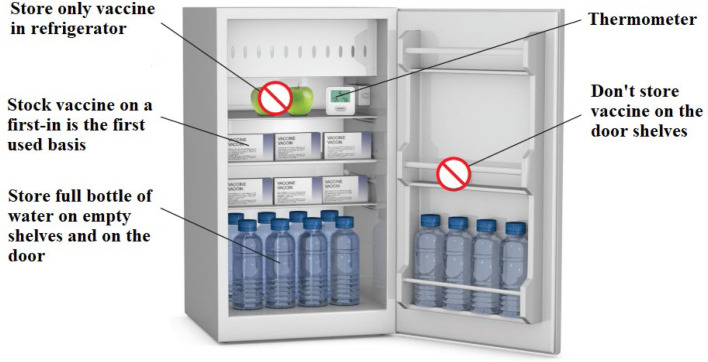
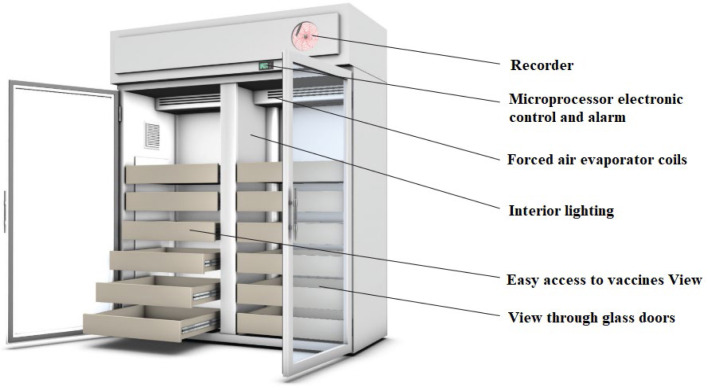
A total of 10 items consisting of the following details need to be fulfilled to achieve a good refrigerator management quality indicator (Thielmann et al., 2019a) (see Fig. 5, Fig. 6):
Fig. 5.
Refrigerator design used for vaccine storage recommended by the Public Health Unit of Canada (2021).
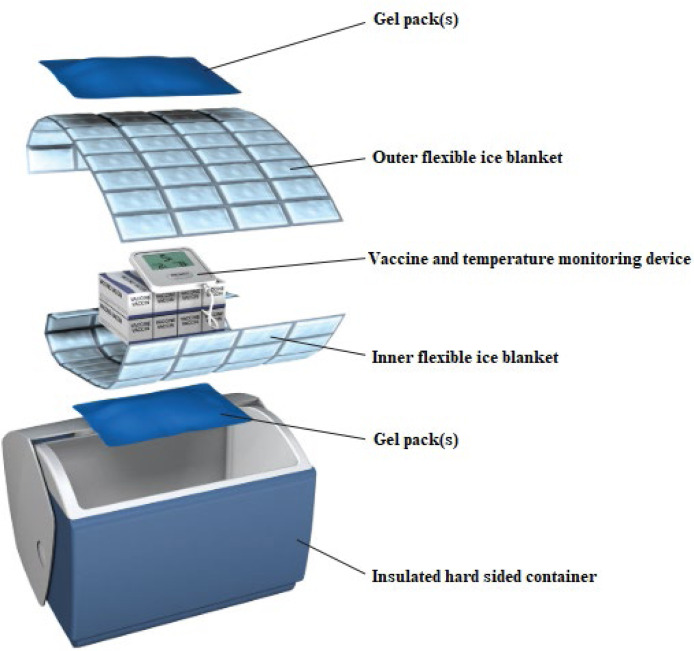
Fig. 6.
Insulated containers Recommendation from the Public Health Unit of Canada (2021).
-
1.
Type of refrigerator suitable for vaccine cold storage (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c),
-
2.
The thermometer allows digital minimum–maximum recording (NS Department of Health Wellness, 2014, Health Protection Scotland, 2017, Salisbury et al., 2013),
-
3.
The temperature checking uses a thermometer placed in the middle of the refrigerator (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017),
-
4.
The temperature log is seen near the refrigerator (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017, Salisbury et al., 2013),
-
5.
No vaccines are stored on door shelves (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017),
-
6.
No food and biomaterials are stored together in the same container with vaccines (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017, Salisbury et al., 2013),
-
7.
All vaccines are stored in original carton packs (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017, Salisbury et al., 2013),
-
8.
All vaccine boxes have no contact with outer walls (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017, Salisbury et al., 2013),
-
9.
All vaccines in bins, baskets, and shelves are placed separately (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017),
-
10.
There is no sticking together or sufficient space between vaccines (NS Department of Health Wellness, 2014, Australian Goverment Department of Helth, 2019, CDC, 2021c, Health Protection Scotland, 2017, Salisbury et al., 2013).
Storage units are divided into 4 types in accordance with temperature range, morning needs, and cold distribution specifications.
Based on a summary review by Kumru et al. (2014), the FDA-approved storage temperature is 2–8 °C (Kumru et al., 2014) with details shown in Table 1.
Table 1.
Cold storage type comparison (WHO, 2015a, ADB, 2022).
| Temperature range | Monitoring needs | Need approval for cold distribution specifications | |
|---|---|---|---|
| Traditional Cold Distribution Chain | 2 °C to 8 °C | Yes, monitoring the vaccine vial | No |
| Frozen Distribution Chain | −20 °C to 17 °C | Yes, monitoring the vial | No |
| Ultra-Cold Distribution Chain (Highly Frozen) | −80 °C to -70 °C | Yes, monitoring the vial | No |
| (Extended) Controlled Temperature Chain 2 | 2 °C to 8 °C initial storage and allows up to 40 °C for at least 3 days before use | Yes, monitoring the vial and peak temperature threshold indicator | Need to fulfill the requirements set by WHO |
FDA-approved viral vaccine storage temperatures range from 2-8 °C and frozen. Table 2 below is the summary result conducted by Kumru et al. (2014) with confirmed data from credible sources.
Table 2.
Examples of vaccine types and temperature parameters for bacterial vaccines based on CDC, FDA, WHO, and Manufacturer Kumru et al. (2014).
| Vaccine type | Product | Producer | Recommended temperature for storage | Freeze sensitive | Heat sensitive | Source |
|---|---|---|---|---|---|---|
| Live attenuated bacteria | ||||||
| BCG | TICE BCG | Organon | 2 to 8 °C | No | >9 days (54 °C) | Kumru et al. (2014) and FDA (2009c) |
| BCG Vaccine |
Organon |
48 days (23 °C) |
Kumru et al. (2014) and Fda and Cber (2009) |
|||
| Typhoid | Vivotif | Berna | 2 to 8 °C | No | >9 days (54 °C) | FDA, 2013, PaxVax USA, 2015 and Emergent BioSolutions UK (2018) |
| Conjugated polysaccharide carrier | ||||||
| Meningococcal | Menactra | Sanofi Pasteur | 2 to 8 °C | Yes | Unknown | FDA (2018a) |
| (ACYW Group) | Menveo | Novartis | 2 to 8 °C | Yes | Unknown | GlaxoSmithKline Inc. (2020) and CDC (2011) |
| Pneumococcal (7-valent) | Prevnar | Pfizer | 2 to 8 °C | Yes | Unknown | Kumru et al. (2014) |
| Pneumococcal (13-valent) | Prevnar-13 | Pfizer | 2 to 8 °C | Yes | 4 days (40 °C) | Kumru et al., 2014, Pfizer, 2021 |
| Pneumococcal (23-valent) | Pneumovax 23 |
Merck |
2 to 8 °C |
Yes |
Unknown |
FDA (2020c) |
| H. Influenzae | Hiberix | GSK | 2 to 8 °C | No | week (55 °C) | (60) |
| ActHIB Sanofi | Pasteur | 2 to 8 °C | Yes | Unknown | FDA (2019b) | |
| PedvaxHIB | Merck | 2 to 8 °C | Yes | Unknown | FDA (2020c) | |
| Subunit, purified bacterial antigen | ||||||
| Tetanus Toxoid | No trade names identified. | Sanofi Pasteur | 2 to 8 °C | Yes | Unknown | Kumru et al. (2014) |
| Tetanus Toxoid Adsorbed | No trade names identified. | Sanofi Pasteur | 2 to 8 °C | Yes | Unknown | Kumru et al. (2014) |
| Anthrax | Biothrax | Emergent | 2 to 8 °C | Yes | Unknown | FDA (2015a) |
| Typhoid | Typhim Vi | Sanofi Pasteur | 2 to8 °C | Yes | Unknown | FDA (2019b) |
| Meningococcal (Groups ACWY) | Menomune | Sanofi Pasteur | 2 to8 °C | Yes | 6 weeks (60 °C) | FDA (2016) |
| Combination vaccine | ||||||
| DTP with Hepatitis B | Pediarix |
GSK |
2 to 8 °C |
Yes |
Unknown |
FDA, 2010a, CDC, 2013 |
| DTP | Infanrix | GSK | 2 to 8 °C | Yes | Unknown | FDA (2008a) |
| Tripedia | Sanofi Pasteur and BIKEN | 2 to 8 °C | Yes | Unknown | FDA (2005) | |
| Daptacel |
Sanofi Pasteur |
2 to 8 °C |
Yes |
Unknown |
FDA (2004) |
|
| DTP with inactive d Polio | Kinrix |
Merck |
2 to 8 °C |
Yes |
Unknown |
FDA (2008b) |
| Haemophilus B and hepatitis B vaccine | Comvax |
Sanofi Pasteur |
2 to 8 °C |
Yes |
Unknown |
CDC (2015) |
| Diphtheria and Tetanus | No trade names identified. | Sanofi Pasteur | 2 to 8 °C | Yes | Unknown | Kumru et al. (2014) |
| DecaVac | Sanofi Pasteur | 2 to 8 °C | Yes | Unknown | Kumru et al. (2014) | |
| TeniVac | Sanofi Pasteur | 2 to 8 °C | Yes | Unknown | FDA (2019d) | |
| TDVAX |
Mass Biologics |
2 to8 °C |
Yes |
Unknown |
FDA (2018g) |
|
| Tetanus Toxoid | Adacel |
Sanofi Pasteur |
2 to 8 °C |
Yes |
Unknown |
FDA (2017b) |
| Diphtheria & Acellular pertussis | Boostrix |
GSK |
2 to 8 °C |
Yes |
Unknown |
FDA (2020b) |
| Diphtheria and tetanus, Acellular pertussis, Inactive polio, Haemophilus B |
Pentacel |
Sanofi Pasteur |
2 to 8 °C |
Yes |
Unknown |
FDA (2021d) |
| Meningococcus, Hib (CY-Hib Group) |
Menhibrix | GSK | 2 to 8 °C | No | Unknown | FDA (2012b) |
Furthermore, the Covid 19 vaccine is described in Table 3. (See Table 4.)
Table 3.
Examples of vaccine types and temperature parameters for FDA-approved viral vaccines Kumru et al. (2014).
| Vaccine type | Product | Producer | Recommended temperature for storage | Freeze sensitive | Heat sensitive | Source |
|---|---|---|---|---|---|---|
| Live attenuated virus | ||||||
| Varisela | Varivax | Merck | Frozen | No | 6 days (27 °C) | Cber and Fda (2020) and I. MERCK & CO. (2005) |
| Zoster | Zostavax | Merck | Frozen | No | Unknown | CDC (2020) and FDA (2018i) |
| Rotavirus | Rotarix |
GSK |
2–8 °C |
No (extreme melting temperature shock −20 °C to 42 °C) Naik et al. (2017). 40 °C (72 h) 20 °C and −80 °C (12 h) Asowata et al. (2019) |
<25 °C (36 months) 37 °C −40 °C (18 months) short term exposure up to 55 °C Naik et al. (2017) |
WHO (2017b) |
| RotaTeq | Merck | 2–8 °C | Unknown | Unknown | WHO (2021c) and Merck Canada Inc. (2018) | |
| Flumist |
MedImmune |
2–8 °C |
Yes |
Unknown |
FDA (2019a) |
|
| Chickenpox | ACAM2000 |
Sanofi Pasteur |
Frozen |
No |
Unknown |
Vangroenweghe (2017) and FDA (1998) |
| Yellow fever | YF-Vax | Sanofi Pasteur | 2–8 °C | Yes | Unknown | WHO (2012) and FDA (2019f) |
| Inactive virus | ||||||
| Hepatitis A | Vaqta | Merck | 2–8 °C | Yes | 3 months (28 °C) | FDA (2018h) |
| Havrix |
GSK |
2–8 °C |
Yes |
1–3 weeks (37 °C) |
(GSK (2017)) |
|
| Influenza | Fluarix | GSK | 2–8 °C | Yes | 12 weeks (20 °C) | GSK, 2013, GSK, 2021a |
| Flulaval | ID Biomed | 2–8 °C | Yes | Unknown | GSK (2021b) | |
| Agriflu | Novartis | 2–8 °C | Yes | Unknown | Seqirus Canada (2020) and FDA (2020a) | |
| Fluvirin | Novartis | 2–8 °C | Yes | Unknown | FDA (2017a) | |
| Fluzone | Sanofi Pasteur | 2–8 °C | Yes | Unknown | FDA (2021b) | |
| Flucelvax | Novartis | 2–8 °C | Yes | Unknown | FDA (2021c) | |
| Afluria | CSL Limited | 2–8 °C | Yes | Unknown | FDA (2019c) | |
| H5N1 | Sanofi Pasteur | 2–8 °C | Yes | Unknown | FDA (2007) | |
| H5N1 | ID Biomed | 2–8 °C | Yes | Unknown | FDA (2012a) | |
| 2009 (H1N1) | CSL Limited | 2–8 °C | Yes | Unknown | FDA (2009b) | |
| 2009 (H1N1) | MedImmune | For storage 2–8 °C (18 weeks) For distribution −25 °C ±5 °C (20 weeks) |
Yes | Unknown | Committee for Medicinal Products for Human Use (CHMP) (2016) | |
| 2009 (H1N1) |
Novartis |
2–8 °C |
Yes |
Unknown |
FDA (2009a) |
|
| Rabies | RabAvert | Novartis | 2–8 °C | No | Unknown | FDA (2018e) |
| Imovax | Sanofi Pasteur | 2–8 °C | Yes | Unknown | FDA (2019e) | |
| Nobivac Rabies |
Merck |
2–8 °C |
Yes |
25 °C (6 months) 30 C (3 months) Lankester et al. (2016) |
Blackmor et al. (2014) |
|
| Polio | IPOL |
Sanofi Pasteur |
2–8 °C |
Yes |
Unknown |
Pasteur (2008) |
| Japanese encephalitis | Ixiaro | Intercell | 2–8 °C | Yes | Unknown | FDA (2018b) |
| Recombinant vaccines | ||||||
| Hepatitis B | Engerix B | GSK | 2–8 °C | Yes | 72 h (25 °C) | GlaxoSmithKline Inc. (2019) |
| Recombivax HB |
Merck |
2–8 °C |
Yes |
Unknown |
FDA (2018f) |
|
| Human papilloma virus Influenza |
FDA (2015b) | |||||
| Gardasil | Merck | 2–8 °C | Yes | 3 months (45 °C) | European Medicines Agency (2012) | |
| Cervarix | GSK | 2–8 °C | Yes | 3 days (8–25 °C) | FDA (2021e) | |
| Combination vaccines | ||||||
| MMR | II | Merck | Frozen | No | 7 days (37 °C) | FDA (2018c) |
| DTP with Hepatitis B | Pediarix | GSK | 2–8 °C | Yes | Unknown | FDA (2010b) |
| Hepatitis A & B | Twinrix | GSK | 2–8 °C | Yes | 7 days (37 °C) | FDA (2001) |
| MMR with Varicella | ProQuad | Merck | Frozen | No | Unknown | FDA (2018d) |
Table 4.
Vaccine types and temperature parameters for COVID-19 vaccines CDC (2021c) and ADB (2022).
| Vaccine Types | Product | Producer | Recommended Temperature for Storage | Freeze sensitive | Heat Sensitive | Source |
|---|---|---|---|---|---|---|
| mRNA | Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) | Pfizer-BioNTech | Very cold: −80 °C to −60 °C | No | Yes | CDC, 2021c, ADB, 2022, Ministry of Health, 2021, Santos et al., 2021 and Peters (2020) |
| mRNA | Moderna COVID-19 Vaccine (mRNA-1273) | Moderna, Inc. | • Frozen: −25 °C and −15 °C until the expiration date. •Cold: 2 °C to 8 °C lasts up to 30 days |
Yes (Can only be frozen once) |
Yes | CDC, 2021c, ADB, 2022, Ministry of Health, 2021, Santos et al., 2021 and Peters (2020) |
| Non-replicating viral vector | Janssen COVID-19 Vaccine | Johnson & Johnson | Cold: 2 °C to 8 °C lasts up to 30 days | Yes | Yes | Santos et al., 2021, Peters, 2020, CDC, 2021b and Yan et al. (2021) |
| Non-replicating viral vector | AZD1222 | Oxford/AstraZeneca | Cold: 2 °C to 8 °C | Yes | Yes | Ministry of Health, 2021, Santos et al., 2021 and Yan et al. (2021) |
| Recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 Spike (S) glycoprotein | Covishield | Serum Institute of India | Cold: 2 °C to 8 °C | Yes | Yes | Ministry of Health (2021) and Serum Institute of India (2021) |
| Non-replicating viral vector | Covaxin | Bharat Biotech | Cold: 2 °C to 8 °C | Yes | Yes | Santos et al., 2021, Peters, 2020 and Yan et al. (2021) |
| Inactivated | BBIBP-CorV | Sinopharm | Cold: 2 °C to 8 °C | Yes | Yes | Peters (2020) and Yan et al. (2021) |
| Inactivated 2 | CoronaVac | Sinovac | Cold: 2 °C to 8 °C | Yes | Yes | Peters (2020) and Yan et al. (2021) |
| Non-replicating viral vector | Ad5-nCoV | CansSino | Cold: 2 °C to 8 °C | Yes | Yes | Peters (2020) |
| Non-replicating viral vector | Spunik V | Gamaleya | Cold: 2 °C to 8 °C | Yes | Yes | Santos et al., 2021, Peters, 2020 and Yan et al. (2021) |
| Protein Subunit | Novavax | Novavax | Cold: 2 °C to 8 °C | Yes | Yes | Peters, 2020, Yan et al., 2021 and March (2020) |
3.3. Storage space
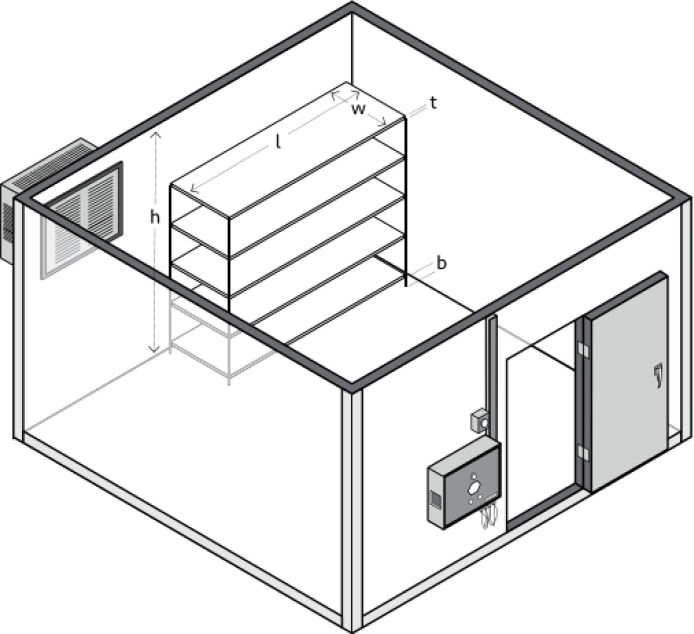
Storage units are ideally placed in a room with good ventilation leaving space between each unit, walls, and ceiling. Furthermore, it needs to be ensured that nothing is blocking the motor and engine cooling device, and when stacked, the cold storage needs to be level and firm with the unit underneath (Objio et al., 2021). A study showed that most cold storage is stored in a room with the best temperature between 20 °C and 25 °C (CDC, 2021c). WHO recommends that the deviation vector, walk-in cold room and walk-in freezer room is 0.67 (WHO et al., 2017). Moreover, the relative humidity for the storage space is less than 55% and the ambient humidity level is between 45%–75% (WHO, 2020) (see Fig. 7).
Fig. 7.
Vaccine storage space recommended by WHO, UNICEF, and EVM (WHO et al., 2017).
3.4. Distribution
Generally, distribution in each country is carried out in 3 levels (Yauba et al., 2017, Ortiz et al., 2020, Vaccine Presentation and Packaging Advisory Group, 2015). At the first level (national), the vaccine is packaged in a tertiary container, opened, and spilled at the second. Meanwhile, the vaccine is already in the primary package at the third level and distributed to public health services, clinics, and hospitals, as shown in Fig. 2. There are also several countries that implement a four-level distribution system, namely national, regional, district, and clinic (Lim et al., 2019, Haidari others, 2013).
Vaccines that are sent directly to health facilities with ready-to-use cold storage are the safest method (Yauba et al., 2017). However, this method is not always possible due to its cost. Therefore, it is preferable to transport the vaccine using a portable refrigerator with a temperature monitoring device (CDC, 2021c). In situations whereby a portable refrigerator is not available, qualified containers packaged with a Temperature Monitoring Device (TMD) can be used. Furthermore, it is important to transport only what is needed during transfer to clinics outside the area (CDC, 2021c).
Table 5 shows storage types based on distribution conditions.
Table 5.
Vaccine distribution system recommendations (CDC, 2021c).
| Storage type | Emergency delivery | Transportation for offsite clinic, public health center, or stock relocation |
|---|---|---|
| Portable Refrigerator or Freezer | Yes | Yes |
| Quality Containers and Packaging | Yes | Yes |
| Conditioned Water Bottle Transport System | Yes | No |
| Manufacturer’s Original Shipping Container | Yes (last option only) | No |
| Food/Beverage Cooler | No | No |
CDC and USA do not recommend the use of frozen gel due to its unit’s ability to freeze vaccines under certain conditions (CDC, 2021c). Many countries are still ignorant of this issue, which is also capable of eliminating its efficacy (Hanson et al., 2017). However, vaccines such as Varivax, Zostavax, II, and ProQuad can be stored in frozen conditions. However, while carrying out this process, adequate care is needed because even though this unit can freeze, it cannot last long. Therefore, using a monitoring system is very important to ensure that the quality is maintained (Yakum et al., 2015).
4. Latest inventions and innovations
4.1. Vaccine cold supply chain management
The success of a vaccination program is based not only on the percentage of vaccine effectiveness but also on cold supply chain management. This is because when this fails, the high effectiveness becomes wasted (Lee and Haidari, 2017, Omole et al., 2019). Several strategic plans need to be observed by stakeholders, as shown in Table 6.
Table 6.
Strategic plans for cold supply chain management.
This study also provides detailed discussions on the latest innovations in Cold Storage.
4.2. Vaccine cold storage technology
New cold storage technologies have the potential to address many of the supply chain challenges faced by countries in the world (Omole et al., 2019, UNICEF and WHO, 2016). These challenges are not only associated with the right processes needed for advanced technology to function properly, rather it also on its affordability for any country (Staruch et al., 2018). The following are some of the latest innovations related to this technology:
4.2.1. Monitoring
Real-time monitoring is necessary to ensure vaccines are stored at temperatures that comply with established standards throughout the supply chain (WHO, 2015b, Ashok et al., 2017). According to a study in Bangkok, not all refrigerators used to have the ability to maintain a stable temperature (Sooksriwong, 2017). Therefore, several innovations have been carried out on the use of cold chain monitoring as a prerequisite for ensuring the quality, efficacy, and safety of vaccination programs (Ateudjieu et al., 2013, WHO, 2011).
Ouzayd et al. 2018 created a temperature monitoring model on the vaccine cold chain using the Colored Petri Net (Ouzayd et al., 2018). This technology is able to monitor the physical parameters of cold chain performance in real-time. Furthermore, the decision-maker has the ability to simulate several scenarios to measure the efficiency of the system and propose numerous solutions. It is useful to determine the temperature stability of the vaccine because it is very risky to be at an inappropriate temperature during transportation (Kitamura et al., 2018, Billah et al., 2015).
Chaudhri, Borriello, and Anderson (2011) created mobile monitoring using a FoneAstra-based continuous system with an application study in Albania (Chaudhri et al., 2012). The technology offers convenience for vaccine logistics staff to diagnose critical problems in storage units. This is because the tool is capable of displaying detailed equipment temperatures in real-time using SMS. The use of IoT-based SMS was also developed by Hasanat et al. (2021), where the data storage base was combined with an SD card (Hasanat et al., 2021). In Laos, the Cold Chain Information System (CCIS) was also integrated with SMS to enable the easy submission of reports at the regency and public health center levels which cannot be accessed by the internet (Anderson others, 2014).
Nowadays, the 4.0 revolution technology has the potential to be used in vaccines cold chain management such as the Internet of Things (IoT) and big data cloud computing The information speed provided on this technology has the ability to increase its role in human affairs (Comes et al., 2018). Several aspects that need to be considered in IoT testing on cold chain management systems are rapid changes in environmental conditions such as humidity, vibration, and indoor signal blocking environments (Wu et al., 2020). After the 2010 earthquake in Haiti, the government implemented temperature monitoring devices (RTMDs) to address the problem of intermittent data gaps in its cold supply chain system (Cavallaro et al., 2018). The technology has the ability to identify typical temperature patterns that are consistent with the refrigerator door opening and closing, propane depletion, thermostat malfunction, and vaccine overstock.
Monteleone, Sampaio, and Maia (2017) proposed a concept that aims to overcome the drug Cold Chain challenges related to temperature monitoring (Monteleone et al., 2017). The concept offers the privilege of considering a complete Cold Chain from the perspective of the pharmaceutical and healthcare industries in one study. Meanwhile, Lorenc, Czuba, Szarata (2020) developed a predictive method to prevent disruptions related to temperature anomalies in the cold supply chain (Lorenc et al., 2021). Using Artificial Neural Network (ANN) technology, they predicted their ability to prevent more than 82% of disruptions in the cold chain. Furthermore, the model developed by Hasanat et al. (2021) and Poochaya and Widjaja (2018) provided a unique feature in which the system is capable of managing vaccine transport along with regular monitoring of temperature and humidity (Hasanat et al., 2020, Poochaya and Widjaja, 2018). Saritas et al. (2017) developed a temperature monitoring and control system with an Arduino system to overcome similar problems (Koklu et al., 2017).
Non-electronic monitoring methods, such as Vaccine Vial Monitors (VVM) is a label attached to the vaccine vial to warn of potential storage failure in the recommended temperature range with a certain color change (Ross et al., 2020). This technology is highly practical, and a shortage of energy is not a concern. However, the accuracy level of electronic Vaccine Vial Monitors (eVVM) is more accurate than VVM (Chen et al., 2021).
4.2.2. Temperature stabilization
Several conditions affect the stability of the storage temperature, such as the varying temperature conditions at each point, thereby making it difficult to determine the actual temperature. Therefore, vaccines are stored in a buffer to keep the range permitted by the manufacturer’s requirements. Furthermore, in practice, vaccines and thermometer probes should not be placed in drawers, on the floor, next to walls, indoors, and near the outlet of cold air from the freezer as the temperature is very likely to differ significantly from the inside of the storage unit and the vaccine (Long and Hayney, 2013). Therefore, the thermometer probe needs to be inserted into a glycol vial or another equivalent thermal buffer medium (Rusnack, 2018). Such thermal buffers are intended to reduce false alarms hence field personnel is determined with greater confidence that a warning is an event that requires action. Although this method theoretically promises accuracy, data reading errors often occur in practice. Therefore, to overcome this problem, Rusnack (2018) developed an algorithm for measuring temperature in vaccines to increase their accuracy (Rusnack, 2018). Furthermore, Clénet (2018) developed a multiple-month model of forced degradation with high accuracy results for the measurement of time and temperature profiles (Clénet, 2018).
Another cause of unstable storage temperature is the disruption of the energy source to activate the cooling machine. The problem associated with the availability of reliable electrical energy sources often occurs in rural and mobile areas (Wal et al., 2019). This problem is solved by installing an external battery, modifying the thermostat, and installing an automatic electrical energy storage system (Martin-de-Nicolas and McColloster, 2014). A monitoring system on the refrigerator is also important to overcome this problem (Bielenberg and Gasic, 2019, Hutten-Czapski, 2017). These methods are effective with low manufacturing costs because they can be carried out by modifying a conventional household refrigerator. It is imperative to note that the CDC does not recommend an unmodified refrigerator because of its low reliability in maintaining temperature (Leidner et al., 2020). The method of stabilizing the temperature can also be carried out using thermal ballast with a water-filled bottle (Chojnacky and Rodriguez, 2020).
Material selection in packaging is one of the essential factors in maintaining temperature stability. According to Ng et al. (2020), polystyrene foam boxes are effective when sealed with a minimum of 5 ice packs, while large cold boxes with polyethylene interior linings and polypropylene insulation were effective when sealed with a minimum of 3 (Ng et al., 2020). Furthermore, Zhao et al. (2020) developed a composite phase change cold storage material to stabilize the storage temperature (Zhao et al., 2020a).
4.2.3. Passive cold devices
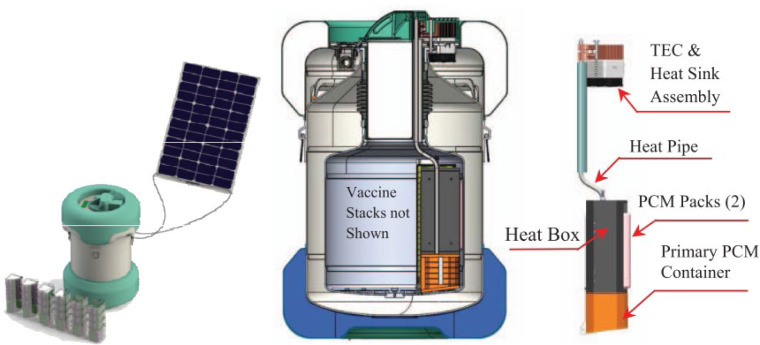
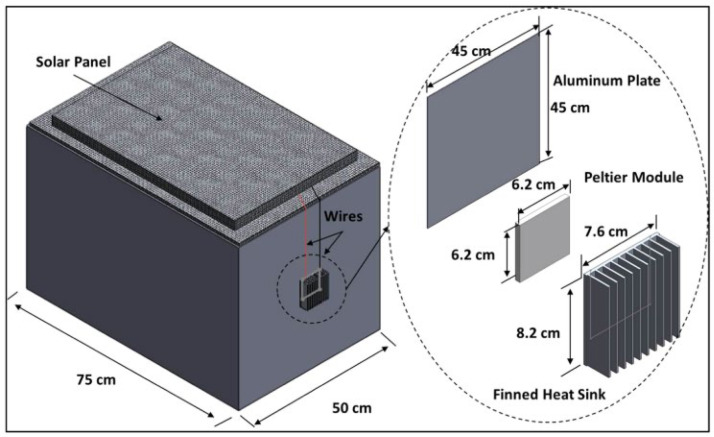
Large budgets are faced with the challenges of maintaining the temperature to match its requirements. According to several studies, the use of cooling technology integrated with passive cold storage devices (PCDs) reduces budgets (Norman et al., 2013, Saidi and Soudani, 2017, Chen et al., 2015, Lugelo et al., 2020). This technology generally integrates solar energy power generation systems (Alfariani and Pratama, 2018). With this concept, Buitendach, Jiya, and Gouws succeeded in creating a portable storage unit that tends to last up to 72 h (3 days) with a temperature control accuracy of 1 °C and a capacity of up to 250 vials (Buitendach et al., 2019). Al-Madhhachi and Al-Najideen (2020), with COMSOL Multiphysics technology, also succeeded in developing a storage unit by utilizing a lower solar panel power of 70 watts (Al-Madhhachi and Al-Najideen, 2021). Meanwhile, Satria, Jaenul, Gamayel (2021) developed a more practical concept by making a storage unit in the form of a backpack with solar cells mounted on the outside (Satria et al., 2021) (see Fig. 8, Fig. 9).
Fig. 8.
Vaccines Cold storage of using solar energy (Li et al., 2016).
Fig. 9.
Solar portable vaccine refrigerator by COMSOL Multiphysics (Al-Madhhachi and Al-Najideen, 2021).
Li et al. (2016) developed a storage technology that can last for 212 h by maintaining a temperature of 3 - 5 °C (Li et al., 2016). The technology adopts 1000 watt photovoltaic by combining Phase Change Material (PCM) and Passive Vaccine Storage Device (PVSD) technologies. PCM is one of the cold storage vaccine delivery methods that is currently a trend (Business and Research, 2021). Nie et al. (2021) used silica and graphene to improve the PCM nucleation process with an efficiency increase of 12.58% (Nie et al., 2021). Furthermore, Zhao (2020) using a mixed solution of tetradecane and lauryl alcohol as a base fluid managed to maintain the vaccine storage temperature at 2–8 °C (Zhao et al., 2020a). Meanwhile, Liu et al. (2021) used decyl alcohol and lauric acid as eutectic and managed to maintain the vaccine temperature also at 2–8 °C. (Liu et al., 2021). The PCM encapsulation technique in cold storage vaccines has the advantages of being easy to use, higher yields, and a little residual solvent (Magendran et al., 2019). Not only in the storage of vaccines and other medical interests (Li et al., 2019, Zhao et al., 2020b, Veerakumar and Sreekumar, 2016, Zhao et al., 2020c, Huo et al., 2018, Alva et al., 2017, Tariq et al., 2020, Ghoghaei others, 2020), The PCM use as a buffer for cold storage temperatures has been widely used as in agricultural products (Xu et al., 2018), food (Tas and Unal, 2021), air-conditioning system (Zheng et al., 2017, Xia et al., 2016) and buildings (Faraj et al., 2020). Considering the different capabilities of PCM and vaccine storage standards, modeling and simulation of PCM selection as needed has also been developed (Dao et al., 2016).
Passive cooling storage technology for not too cold temperatures was also developed. For instance, Vilibic-Cavlek et al. (2021) developed clay storage of Nobivac® Canine Rabies with a storage space of 26 °C and an ambient temperature of 42 °C (Lugelo et al., 2020). The vaccine was classified as thermotolerant due to its ability to survive at ambient temperature for months (Lankester et al., 2016). This method is an excellent solution because the raw materials are abundant, easy to make, and cheap (see Fig. 10).
Fig. 10.

Zeepot made of clay.
4.2.4. Computational Fluid Dynamics (CFD)
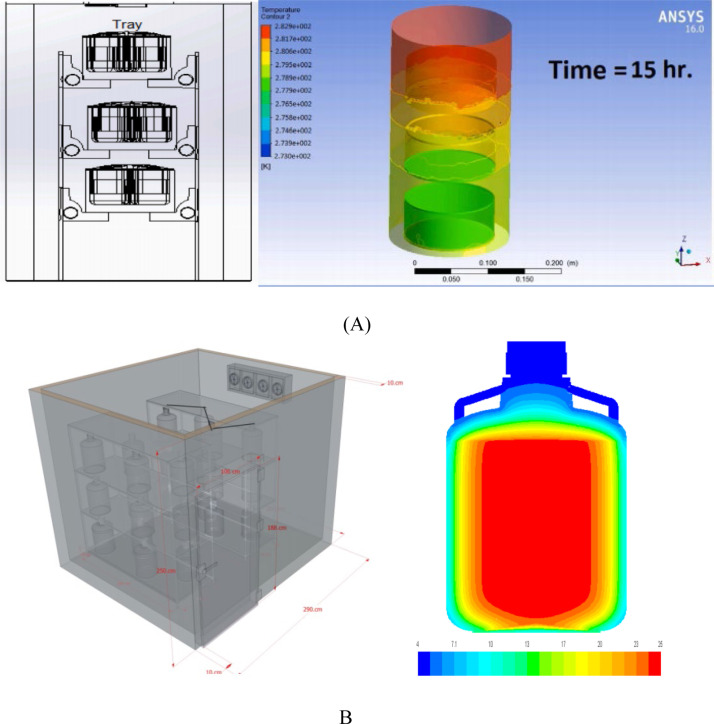
Devrani et al. (2021) developed vaccine storage with a semi-passive cooling rack system for delivery in rural areas (Devrani et al., 2021). The study used experimental and computational fluid dynamics (CFD) testing, geometric analysis, experimentally derived R values of insulation, and temperature monitoring. The results show that the innovation can improve the performance of increasing retention time from a certain temperature range by up to 17%. With a different arrangement model, Sularno, Soelami, and Bindar (2018) using CFD analysis found that the preparation of vaccine vials in cold storage can improve the performance (Sularno et al., 2018) (see Fig. 11).
Fig. 11.
Rack design and display of CFD tests (A) (Devrani et al., 2021) and B (Sularno et al., 2018).
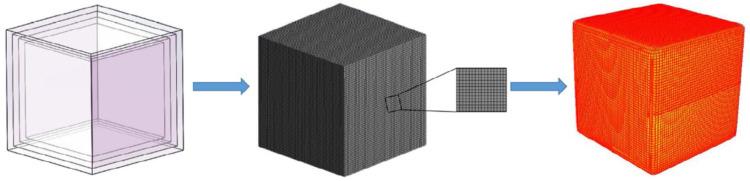
The use of numerical analysis and experimental studies was also carried out by Yin et al. (2020) to examine multi-level gradient heat transfer in vaccine storage boxes with certain material layers (Yin et al., 2020). The material tested is a composite with the constituents in the form of PCM (Phase Change Material), XPS (Extruded Polystyrene), and PU (Polyurethane). The test results show that the most optimal dual-layer board configuration is the XPS/PCM ratio of 1:3 (see Fig. 12).
Fig. 12.
The numerical simulation process (a) the simulative model, (b) integral mesh and partial display, (c) numerical temperature nephogram of incubator (Yin et al., 2020).
5. Summary
The vaccination program is a strategic step that needs to be taken by every policymaker to solve various problems associated with a pandemic. In practice, there are various kinds of problems faced, especially regarding cold supply chains. Therefore, stakeholders need to understand the challenges of storage specification standards set by manufacturers and authorized health agencies to avoid being misguided in carrying out their duties. This is very important because vaccines with very high efficacy will lose their efficacy if not handled properly. These challenges are related to packaging, storage, and space, as well as distribution. Therefore, to overcome this, it is necessary to carry out 12 strategic action plans as follows:
-
1.
Preparation of detailed operational standards
-
2.
Storage temperature according to manufacturer’s recommendations and standards
-
3.
Cold storage innovation
-
4.
Transportation innovation
-
5.
Thermostable vaccine innovation
-
6.
Collaboration between institutions and countries
-
7.
Monitoring
-
8.
Empowerment of local communities
-
9.
Increasing the number of vaccination staff
-
10.
Improving knowledge of vaccination staff
-
11.
Making an emergency plan
-
12.
Special attention to vulnerable groups
Furthermore, several innovation efforts by the previous researchers have been carried out related to cold storage technology which includes 3 main things. The first is monitoring based on Internet technology, SMS, IoT, big data cloud computing, Artificial Neural Network (ANN), Vaccine Vial Monitors (VVM), an electronic Vaccine Vial Monitors (eVVM). The second is temperature stabilization by using Phase Change Material (PCM), thermal buffer materials, measurement algorithms, mathematical modeling, installing energy backup batteries with automatic work, modifying the thermostat, using thermal ballast materials, and selecting heat insulation materials. Furthermore, the third is the development of passive cold storage devices through a portable system with solar energy and the use of clay as raw material for making refrigerators for thermotolerant vaccines. Semi-passive cold storage was also developed by placing vaccines on shelves. Further research related to vaccine storage temperature was also carried out using the CFD method to determine the design of the material layer and the arrangement of the vaccine placement in the storage container.
CRediT authorship contribution statement
Nugroho Agung Pambudi: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Visualization, Supervision, Project administration, Translate, Funding acquisition, Submission. Alfan Sarifudin: Conceptualization, Correspondence, Methodology, Validation, Investigation, Data Curation, Writing – original draft, Writing – review & editing, Visualization, Translate, Submission. Indra Mamad Gandidi: Validation, Review, Project administration, Funding acquisition. Rahmat Romadhon: Validation, Review, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to gratefully acknowledge Universitas Sebelas Maret, Indonesia [Grant Number 102.1/UN27.22/HK.07.00/ 2021], Program Penelitian Kolaborasi Indonesia (PPKI), LPPM Universitas Sebelas Maret, Ministry of Education and Culture of Indonesia.
Funding
This work was supported by the Program Penelitian Kolaborasi Indonesia (PPKI), LPPM Universitas Sebelas Maret, Indonesia, Ministry of Education and Culture of Indonesia .
References
- ADB Getting ready for the COVID-19 vaccine Rollout. ADB Br. 2022;7(166) doi: 10.22617/BRF210071-2. [DOI] [Google Scholar]
- Al-Madhhachi H., Al-Najideen M. Thermal, environmental, and cost analysis of effective solar portable vaccine refrigerator by COMSOL multiphysics. Heat Transf. 2021;50(1):179–195. doi: 10.1002/htj.21870. [DOI] [Google Scholar]
- Al-Qahtani W.S., Alsafhi F.A. A commentary on realities of developing covid-19 vaccines discussed through the global health safety perspective. Vaccines. 2021;9(3):1–7. doi: 10.3390/vaccines9030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano de Azevedo Guimarães E., Conceição de Oliveira V., Martins Oliveira M., da Fonseca Viegas S.M., Ferreira A.P., de Souza Dias F.C. Critical events in the maintenance of vaccine conservation. J. Nurs. UFPE/ Rev. Enferm. UFPE. 2018;12(6):1781–1789. [Online]. Available: https://periodicos.ufpe.br/revistas/revistaenfermagem/article/view/230909%0Ahttp://search.ebscohost.com/login.aspx?direct=true&AuthType=cookie,ip,shib&db=rzh&AN=130259136&site=ehost-live. [Google Scholar]
- Alfariani A.R., Pratama H. 2018. Eco-friendly solar-powered livestock vaccine storage with thermoelectric system, no. August; pp. 0–4. [DOI] [Google Scholar]
- Alva G., Huang X., Liu L., Fang G. Synthesis and characterization of microencapsulated myristic acid–palmitic acid eutectic mixture as phase change material for thermal energy storage. Appl. Energy. 2017;203:677–685. [Google Scholar]
- Aminah, S., Rahmat, S., Susilo, T., 2021. State capacity in implementing the Covid- 19 vaccination program in Indonesia. in: Advances in Social Science, Education and Humanities Research, Vol. 560, no. Acbleti 2020, pp. 67–72.
- Anderson others R. Supporting immunization programs with improved vaccine cold chain information systems. Proc. 4th IEEE Glob. Humanit. Technol. Conf; GHTC 2014; 2014. pp. 215–222. [DOI] [Google Scholar]
- Arsenault C., Harper S., Nandi A., Rodríguez J.M.M., Hansen P.M., Johri M. An equity dashboard to monitor vaccination coverage. Bull. World Health Organ. 2017;95(2):128. doi: 10.2471/BLT.16.178079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgary A., Najafabadi M.M., Karsseboom R., Wu J. A drive-through simulation tool for mass vaccination during COVID-19 pandemic. Healthc. 2020;8(4):469. doi: 10.3390/HEALTHCARE8040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok A., Brison M., LeTallec Y. Improving cold chain systems: Challenges and solutions. Vaccine. 2017;35(17):2217–2223. doi: 10.1016/j.vaccine.2016.08.045. [DOI] [PubMed] [Google Scholar]
- Asowata O.E., Ashiru O.T., Willem Sturm A., Moodley P. Stability of a monovalent rotavirus vaccine after exposure to different temperatures observed in KwaZulu-natal. South Afr. Afr. Health Sci. 2019;19(2):1993–1999. doi: 10.4314/ahs.v19i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateudjieu J., Kenfack B., Nkontchou B.W., Demanou M. Program on immunization and cold chain monitoring: The status in eight health districts in Cameroon. BMC Res. Notes. 2013;6(1) doi: 10.1186/1756-0500-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Goverment Department of Helth . third ed. Commonwealth of Australia as represented by the Department of Health; Canberra: 2019. National vaccine storage guidelines Strive for 5. [Google Scholar]
- Azimi T., Franzel L., Probst N. Seizing market shaping opportunities for vaccine cold chain equipment. Vaccine. 2017;35(17):2260–2264. doi: 10.1016/j.vaccine.2016.12.073. [DOI] [PubMed] [Google Scholar]
- Babenko-Mould Y., Ferguson K., Riddell T., Hancock M., Atthill S. Influence of simulated and actual community vaccination clinics on student empowerment and self-efficacy for public health nursing competencies. Public Health Nurs. 2015;32(3):277–283. doi: 10.1111/PHN.12151. [DOI] [PubMed] [Google Scholar]
- Bailey L.C., Barrett N.R., Thorne M., Ford F.M., Elizabeth W., Psevdos G. Successful drive-thru point-of-distribution influenza vaccination program for veterans affairs medical center employees. Am. J. Infect. Control. 2020;48(8):S31. doi: 10.1016/J.AJIC.2020.06.201. [DOI] [Google Scholar]
- Bielenberg D.G., Gasic K. Controlled-temperature treatments with low-cost, off-the-shelf equipment for bud or seed forcing experiments. HortScience. 2019;54(4):766–768. doi: 10.21273/HORTSCI13649-18. [DOI] [Google Scholar]
- Billah M.M., et al. Cold-chain adaptability during introduction of inactivated polio vaccine in Bangladesh. J. Infect. Dis. 2015;216(1):S114–S121. doi: 10.1093/infdis/jiw591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset K.A., Paterson P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: A systematic review. Vaccine. 2018;36(20):2751–2759. doi: 10.1016/j.vaccine.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Blackmor Carina, et al. Florida Department of Health, Division of Control and Health Protection; Florida: 2014. Rabies Prevention and Control in Florida, 2014. [Google Scholar]
- Boao Farum for Asia . Boao Farum for Asia; Beijing: 2021. Sustainable Development: Asia and the Word Annual Report. [Google Scholar]
- Bogale H.A., Amhare A.F., Bogale A.A. Assessment of factors affecting vaccine cold chain management practice in public health institutions in east Gojam zone of Amhara region. BMC Publ. Health. 2019;19(1):1–6. doi: 10.1186/s12889-019-7786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.T., et al. The benefits of redesigning Benin’s vaccine supply chain. Vaccine. 2014;32(32):4097–4103. doi: 10.1016/j.vaccine.2014.04.090. [DOI] [PubMed] [Google Scholar]
- Buitendach H., Jiya I.N., Gouws R. Solar powered peltier cooling storage for vaccines in rural areas, indones. J. Electr. Eng. Comput. Sci. 2019;17(1):36–46. doi: 10.11591/ijeecs.v17.i1.pp36-46. [DOI] [Google Scholar]
- Bulula N., Mwiru D.P., Swalehe O., Thomas Mori A. Vaccine storage and distribution between expanded program on immunization and medical store department in Tanzania: a cost-minimization analysis. Vaccine. 2020;38(51):8130–8135. doi: 10.1016/j.vaccine.2020.10.088. [DOI] [PubMed] [Google Scholar]
- Business G., Research M. Characteristics, challenges, and opportunities of vaccine cold chain. Int. J. 2021;13(3) [Google Scholar]
- Cavallaro K.F., et al. Demonstration of the use of remote temperature monitoring devices in vaccine refrigerators in haiti. Publ. Health Rep. 2018;133(1):39–44. doi: 10.1177/0033354917742119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cber and Fda . 2020. Package insert - Varivax (Refrigerator) [Online]. Available: www.vaers.hhs.gov. (Accessed 20 July 2021) [Google Scholar]
- CDC . 2011. Selected biologicals Diphtheria Toxoid-, Tetanus Toxoid-and acellular Pertussis-containing. [Online]. Available: https://ldh.la.gov/assets/oph/Center-PHCH/Center-PH/immunizations/vaccine-storage-handling.pdf. (Accessed 25 June 2021) [Google Scholar]
- CDC . 2013. Pediarix vaccine questions and answers for healthcare providers CDC. https://www.cdc.gov/vaccines/vpd/hepb/hcp/faqs-hcp-pediarix.html. (Accessed 25 June 2021) [Google Scholar]
- CDC,, 2015. Haemophilus influenzae type B. in: Epidemiology and Prevention of Vaccine-Preventable Diseases.
- CDC . 2020. Zostavax storage and handling. https://www.cdc.gov/vaccines/vpd/shingles/hcp/zostavax/storage-handling.html. (Accessed 20 July 2021) [Google Scholar]
- CDC . 2021. Janssen COVID-19 vaccine (Johnson & Johnson) storage and handling summary; pp. 1–2. [Online]. Available: https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/downloads/janssen-storage-handling-summary.pdf. [Google Scholar]
- CDC . 2021. Vaccine storage and handling toolkit. [Online]. Available: https://www.cdc.gov/vaccines/imz-managers/awardee-imz-websites.html. (Accessed 16 June 2021) [Google Scholar]
- Chambers B.M. 2021. Response to hosangadi and others current state of mass vaccination preparedness and operational challenges in the United States, 2018–2019, 19 (s1) pp. S102–S103. [DOI] [PubMed] [Google Scholar]
- Chandra D., Kumar D. Identifying key performance indicators of vaccine supply chain for sustainable development of mission Indradhanush: A structural equation modeling approach. Omega. 2020;101 doi: 10.1016/j.omega.2020.102258. [DOI] [Google Scholar]
- Chaudhri R., Borriello G., Anderson R. Pervasive computing technologies to monitor vaccine cold chains in developing countries. IEEE Pervasive Comput. 2012;11(3):26–33. [Google Scholar]
- Chen D., Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev. Vaccin. 2014;8(5):547–557. doi: 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- Chen X., Liu J., Yao Q., Chen X. Evaluation on monitoring effect of the electronic vaccine vial monitor label. J. Biomed. Eng. 2021;38(1):154–160. doi: 10.7507/1001-5515.202011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.I., Norman B.A., Rajgopal J., Lee B.Y. Passive cold devices for vaccine supply chains. Ann. Oper. Res. 2015;230(1):87–104. doi: 10.1007/s10479-013-1502-5. [DOI] [Google Scholar]
- Chen D., et al. Thermostable formulations of a hepatitis B vaccine and a meningitis A polysaccharide conjugate vaccine produced by a spray drying method. Vaccine. 2010;28(31):5093–5099. doi: 10.1016/j.vaccine.2010.04.112. [DOI] [PubMed] [Google Scholar]
- Chojnacky M., Rodriguez A.L. Effect of thermal ballast loading on temperature stability of domestic refrigerators used for vaccine storage. PLoS One. 2020;15(7):1–18. doi: 10.1371/journal.pone.0235777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clénet D. Accurate prediction of vaccine stability under real storage conditions and during temperature excursions. Eur. J. Pharm. Biopharm. 2018;125(2017):76–84. doi: 10.1016/j.ejpb.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Comes T., Sandvik K. Bergtora, de Walle B. Van. Cold chains, interrupted: the use of technology and information for decisions that keep humanitarian vaccines cool. Humanit. Logist. Supply Chain Manag. 2018;8(1):49–69. doi: 10.1108/JHLSCM-03-2017-0006. [DOI] [Google Scholar]
- Committee for Medicinal Products for Human Use (CHMP) Eropean Medicines Agensy Science Medicine Health; London: 2016. Assessment report Pandemic influenza vaccine H5N1 MedImmune, Vol. 44. [Google Scholar]
- Dai H., Han J., Lichtfouse E. Who is running faster, the virus or the vaccine? Environ. Chem. Lett. 2020;18(6):1761–1766. doi: 10.1007/s10311-020-01110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairo D.M., Osizimete O.E., Dairo D.M., Osizimete O.E. Factors affecting vaccine handling and storage practices among immunization service providers in Ibadan, Oyo state, Nigeria. Afr. Health Sci. 2016;16(2):576–583. doi: 10.4314/ahs.v16i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao V.-D., Jin I.-K., Hur H., Choi H.-S. Comparison between water and N-tetradecane as insulation materials through modeling and simulation of heat transfer in packaging box for vaccine shipping. Clean Technol. 2016;22(1):45–52. doi: 10.7464/KSCT.2016.22.1.045. [DOI] [Google Scholar]
- Das M.K., Arora N.K., Mathew T., Vyas B., Sindhu M., Yadav A. Temperature integrity and exposure to freezing temperature during vaccine transfer under the universal immunization program in three states of India. Indian J. Public Health. 2019;63(2):139–142. doi: 10.4103/ijph.IJPH_123_18. [DOI] [PubMed] [Google Scholar]
- Dasaklis T.K., Rachaniotis N., Pappis C. 2016. Emergency supply chain management for controlling a smallpox outbreak: the case for regional mass vaccination, 4 (1) pp. 27–40. [DOI] [Google Scholar]
- Devrani S., Tiwari R., Khan N., Sankar K., Patil S., Sridhar K. Enhancing the insulation capability of a vaccine carrier box: An engineering approach. J. Energy Storage. 2021;36(2020) doi: 10.1016/j.est.2020.102182. [DOI] [Google Scholar]
- Dutta T., Agley J., Meyerson B.E., Barnes P.A., Sherwood-Laughlin C., Nicholson-Crotty J. Perceived enablers and barriers of community engagement for vaccination in India: Using socioecological analysis. PLoS One. 2021;16(6) doi: 10.1371/JOURNAL.PONE.0253318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emergent BioSolutions UK . 2018. Vivotif - summary of product characteristics (SmPC) - (emc) https://www.medicines.org.uk/emc/medicine/30294#gref. (Accessed 25 June 2021) [Google Scholar]
- European Medicines Agency . 2012. Cervarix, INN-Human papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed) [Online]. Available: https://www.ema.europa.eu/en/documents/product-information/cervarix-epar-product-information_en.pdf. (Accessed 21 July 2021) [Google Scholar]
- Excler J.L., Privor-Dumm L., Kim J.H. Supply and delivery of vaccines for global health. Curr. Opin. Immunol. 2021;71:13–20. doi: 10.1016/J.COI.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj K., Khaled M., Faraj J., Hachem F., Castelain C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020;119 doi: 10.1016/J.RSER.2019.109579. [DOI] [Google Scholar]
- FDA ACAM2000, (smallpox (vaccinia) vaccine, live) Interactions. 1998;50(July):1–25. [Online]. Available: http://pi.lilly.com/us/zyprexa-pi.pdf. [Google Scholar]
- FDA . 2001. TWINRIX [hepatitis A & hepatitis B (Recombinant) vaccine] suspension for intramuscular injection package insert. [Online]. Available: https://www.fda.gov/media/74182/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2004. Sanofi pasteur full prescribing information 253 – DAPTACEL. [Online]. Available: https://www.fda.gov/files/vaccines%2C blood %26 biologics/published/Package-Insert---DAPTACEL.pdf (Accessed 25 June 2021) [Google Scholar]
- FDA . 2005. Diphtheria and Tetanus DTaPToxoids and AcellularPertussis vaccine AdsorbedTripedia. [Google Scholar]
- FDA . 2007. Sanofi pasteur - h5n1 influenza virus vaccine. [Online]. Available: https://www.fda.gov/media/74534/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2008. Package insert - INFANRIX. [Online]. Available: https://www.fda.gov/media/75157/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2008. Package insert - kinrix. [Online]. Available: https://www.fda.gov/media/80128/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2009. Influenza a (H1N1) 2009 monovalent vaccine, novartis vaccines and diagnostics limited. [Online]. Available: https://www.fda.gov/media/77422/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2009. Package insert influenza A (H1N1) 2009 monovalent vaccine highlights of prescribing information. [Online]. Available: https://www.fda.gov/media/77464/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2009. TICE BCG FDA. https://www.fda.gov/vaccines-blood-biologics/vaccines/tice-bcg. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2010. Package insert - PEDIARIX. [Online]. Available: https://www.fda.gov/media/79830/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2010. PEDIARIX [Diphtheria and Tetanus toxoids and acellular pertussis adsorbed, Hepatitis B (Recombinant) and inactivated poliovirus vaccine] [Online]. Available: https://www.fda.gov/media/79830/download. [Google Scholar]
- FDA . 2012. Influenza A (H5N1) virus monovalent vaccine, adjuvanted emulsion for intramuscular injection. [Online]. Available: https://www.fda.gov/media/87479/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2012. MENHIBRIX (meningococcal groups C and Y and haemophilus b tetanus toxoid conjugate vaccine) [Online]. Available: https://www.fda.gov/media/83688/download. (Accessed 25 June 2021) [DOI] [PubMed] [Google Scholar]
- FDA . 2013. Vivotif package insert USA-updated 2013-increase of upper specification limit vivotif® Typhoid vaccine live oral Ty21a. [Google Scholar]
- FDA . 2015. BioThrax ® (Anthrax Vaccine Adsorbed) emergent biosolutions. [Online]. Available: https://www.fda.gov/media/71954/download (Accessed 25 June 2021) [Google Scholar]
- FDA . 2015. GARDASIL® [Human papillomavirus quadrivalent (Types 6, 11, 16, and 18) vaccine, recombinant] [Online]. Available: https://www.fda.gov/files/vaccines, blood & biologics/published/Package-Insert---Gardasil.pdf. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2016. Sanofi pasteur 14 march 2016, v0.4 276 Menomune® – A/C/Y/W-135. [Online]. Available: https://www.fda.gov/media/83562/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2017. FLUVIRIN® (influenza virus vaccine) [Online]. Available: https://www.fda.gov/files/vaccines%2C blood %26 biologics/published/Package-Insert---Fluvirin.pdf. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2017. Sanofi pasteur 306 - Adacel. [Online]. Available: https://www.fda.gov/files/vaccines%2C blood %26 biologics/published/Package-Insert---Adacel.pdf (Accessed 25 June 2021) [Google Scholar]
- FDA . 2018. Menactra ® LE7186 highlights of prescribing information these highlights do not include all the information needed to use Menactra ® safely and effectively. see full prescribing information for menactra vaccine. [Online]. Available: https://ldh.la.gov/assets/oph/Center-PHCH/Center-PH/immunizations/vaccine-storage-handling.pdf. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2018. Package insert - IXIARO (Japanese encephalitis vaccine, inactivated, adsorbed) [Online]. Available: https://www.fda.gov/media/75777/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2018. Package insert - M-M-R® II (Measles, Mumps, and Rubella virus vaccine live) [Online]. Available: https://www.fda.gov/media/75191/download. (Accessed 21 July 2021.) [Google Scholar]
- FDA . 2018. Package insert - refrigerator-stable formulation - ProQuad. [Online]. Available: https://www.fda.gov/media/75195/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2018. RabAvert rabies vaccine rabies vaccine for human use. [Online]. Available: https://www.fda.gov/files/vaccines%2C blood %26 biologics/published/Package-Insert---RabAvert.pdf( Accessed 21 July 2021) [Google Scholar]
- FDA . 2018. RECOMBIVAX HB® hepatitis B vaccine (recombinant) suspension for intramuscular injection. [Online]. Available: https://www.fda.gov/files/vaccines%2C blood %26 biologics/published/package-insert-recombivax-hb.pdf (Accessed 21 July 2021) [Google Scholar]
- FDA . 2018. Tetanus and diphtheria toxoids adsorbed TDVAX. [Online]. Available: https://www.fda.gov/media/76430/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2018. VAQTA® (Hepatitis A Vaccine, Inactivated) [Online]. Available: www.vaers.hhs.gov. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2018. ZOSTAVAX (zoster vaccine live) refrigerated package insert. [Online]. Available: www.vaers.hhs.gov. (Accessed 20 July 2021) [Google Scholar]
- FDA . 2019. FLUMIST® QUADRIVALENT. [Google Scholar]
- FDA . 2019. Package insert - ActHIB. [Online]. Available: https://www.fda.gov/media/74395/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2019. Package insert - AFLURIA. [Online]. Available: https://www.fda.gov/media/81559/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2019. Package insert - TENIVAC. [Online]. Available: https://www.fda.gov/media/76610/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2019. Rabies vaccine imovax ® rabies. [Online]. Available: https://www.fda.gov/media/75709/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2019. Yellow fever vaccine YF-VAX®. [Online]. Available: https://www.fda.gov/media/76015/download. [Google Scholar]
- FDA . 2020. AGRIFLU, influenza virus vaccine. [Online]. Available: https://www.fda.gov/media/134739/download. [Google Scholar]
- FDA . 2020. Package insert - BOOSTRIX. [Online]. Available: https://www.fda.gov/media/124002/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2020. Package insert - PNEUMOVAX 23. [Online]. Available: https://www.fda.gov/media/80547/download (Accessed 25 June 2021) [Google Scholar]
- FDA . 2021. Fluzone quadrivalent (influenza vaccine) [Online]. Available: https://www.fda.gov/media/119856/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2021. Package insert - flucelvax quadrivalent. [Online]. Available: https://www.fda.gov/media/115862/download. (Accessed 21 July 2021) [Google Scholar]
- FDA . 2021. Package insert - pentacel. [Online]. Available: https://www.fda.gov/media/74385/download. (Accessed 25 June 2021) [Google Scholar]
- FDA . 2021. Package insert- flublok quadrivalent. [Online]. Available: https://www.fda.gov/media/123144/download ( Accessed 21 July 2021) [Google Scholar]
- Fda and Cber . 2009. BCG live package insert. [Google Scholar]
- Findley S.E., et al. Effectiveness of a community coalition for improving child vaccination rates in new york city. Am. J. Public Health. 2011;98(11):1959–1962. doi: 10.2105/AJPH.2007.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuady A., Nuraini N., Sukandar K.K., Lestari B.W. Targeted vaccine allocation could increase the covid-19 vaccine benefits amidst its lack of availability: A mathematical modeling study in indonesia. Vaccines. 2021;9(5) doi: 10.3390/vaccines9050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganczak M., Gil K., Korzeń M., Bazydło M. Coverage and influencing determinants of influenza vaccination in elderly patients in a country with a poor vaccination implementation. Int. J. Environ. Res. Publ. Heal. 2017;14(6):665. doi: 10.3390/IJERPH14060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabadu S., Panda M., Ranjan S., Nanda S. Assessment of vaccine storage practices in 2 districts of eastern India -Using global assessment tool. Int. J. Heal. Clin. Res. 2020;3(11):62–66. [Google Scholar]
- Gharpure R., et al. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long-term care program — United States 2020–2021. Morb. Mortal. Wkly. Rep. 2021;70(5):178. doi: 10.15585/mmwr.mm7005e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoghaei others M.S. A review on the applications of micro-/nano-encapsulated phase change material slurry in heat transfer and thermal storage systems. J. Therm. Anal. Calorim. 2020;145(2):245–268. doi: 10.1007/S10973-020-09697-6. [DOI] [Google Scholar]
- GlaxoSmithKline Inc. 2019. Product monograph including patient medication information ENGERIX-B Hepatitis B vaccine (recombinant) [Online]. Available: https://ca.gsk.com/media/590068/engerix-b.pdf. [Google Scholar]
- GlaxoSmithKline Inc. 2020. MENVEO meningococcal (Groups A, C, W-135 and Y) oligosaccharide CRM197 conjugate vaccine powder and solution for injection active immunizing agent ATC code J07AH08. [Online]. Available: https://ca.gsk.com/media/1213533/menveo.pdf. (Accessed 25 June 2021) [Google Scholar]
- Goralnick E., Kaufmann C., Gawande A.A. Mass-vaccination sites — An essential innovation to curb the Covid-19 pandemic. N. Engl. J. Med. 2021;384(18):1–3. doi: 10.1056/NEJMp2102535. [DOI] [PubMed] [Google Scholar]
- GSK . 2013. Fluarix tetra product information. [Online]. Available: https://au.gsk.com/media/416636/fluarix-tetra_pi_007.pdf. [Google Scholar]
- GSK . 2017. HAVRIX ®1440 and HAVRIX® junior product information. [Online]. Available: https://au.gsk.com/media/367267/havrix_pi_006_approved.pdf. (Accessed 21 Jul 2021) [Google Scholar]
- GSK . 2021. FLUARIX QUADRIVALENT (influenza vaccine) injectable suspension, for intramuscular use 2021-2022 formula. [Online]. Available: www.vaers.hhs.gov. (Accessed 21 July 2021) [Google Scholar]
- GSK . 2021. Flulaval quadrivalent (Influenza vaccine) injectable suspension, for intramuscular use 2021-2022 formula. [Online]. Available: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Flulaval_Quadrivalent/pdf/FLULAVAL-QUADRIVALENT.PDF ( Accessed 21 July 2021) [Google Scholar]
- Haidari others L.A. Augmenting transport versus increasing cold storage to improve vaccine supply chains. PLoS One. 2013;8(5):1–7. doi: 10.1371/journal.pone.0064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C.M., George A.M., Sawadogo A., Schreiber B. Is freezing in the vaccine cold chain an ongoing issue? A literature review. Vaccine. 2017;35(17):2127–2133. doi: 10.1016/j.vaccine.2016.09.070. [DOI] [PubMed] [Google Scholar]
- Hasanat R.T., Arifur Rahman M.D., Mansoor N., Mohammed N., Rahman M.S., Rasheduzzaman M. An IoT based real-time data-centric monitoring system for vaccine cold chain. 2020 IEEE East-West Des. Test Symp; EWDTS 2020 - Proc; 2020. [DOI] [Google Scholar]
- Hasanat R.T., Mansoor N., Mohammed N., Rahman M.S., Rasheduzzaman M. Development of a monitoring system and power management for an IoT based vaccine carrier. J. Phys. Conf. Ser. 2021;1755(1) doi: 10.1088/1742-6596/1755/1/012023. [DOI] [Google Scholar]
- Hassett K.J., Meinerz N.M., Semmelmann F., Cousins M.C., Garcea R.L., Randolph T.W. Development of a highly thermostable, adjuvanted human Papillomavirus vaccine. Eur. J. Pharm. Biopharm. 2015;94(1):220–228. doi: 10.1016/J.EJPB.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchett R. The medicines refrigerator and the importance of the cold chain in the safe storage of medicines. Nurs. Stand. 2017;32(6):53–63. doi: 10.7748/ns.2017.e10960. [DOI] [PubMed] [Google Scholar]
- Health Protection Scotland . third ed. Health Protection Scotland Meridian; Glasgow: 2017. Guidance on Vaccine Storage and Handling. [Google Scholar]
- Heyerdahl others L.W. Innovative vaccine delivery strategies in response to a cholera outbreak in the challenging context of Lake Chilwa, A rapid qualitative assessment. Vaccine. 2018;36(44):6491–6496. doi: 10.1016/j.vaccine.2017.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X., et al. Chitosan composite microencapsulated comb-like polymeric phase change material via coacervation microencapsulation. Carbohydr. Polym. 2018;200:602–610. doi: 10.1016/j.carbpol.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Huremović D. Psychiatry of Pandemics. Springer Nature; Switzerland, AG: 2019. Brief history of pandemics pandemi; pp. 7–35. [Google Scholar]
- Hutten-Czapski P. From beer brewing to vaccine stability. Can. Fam. Physician. 2017;63(7) [PMC free article] [PubMed] [Google Scholar]
- I. MERCK & CO. 2005. VARIVAX ® [Varicella Virus Vaccine Live (Oka/Merck)] refrigerator-stable formulation. [Google Scholar]
- Karp C.L., et al. Evaluating the value proposition for improving vaccine thermostability to increase vaccine impact in low and middle-income countries. Vaccine. 2015;33(30):3471–3479. doi: 10.1016/j.vaccine.2015.05.071. [DOI] [PubMed] [Google Scholar]
- Kartoglu U., Milstien J. 2014. Tools and approaches to ensure quality of vaccines throughout the cold chain; pp. 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., et al. Assessment of temperatures in the vaccine cold chain in two provinces in Lao people’s democratic Republic: A cross-sectional pilot study. BMC Res. Notes. 2018;11(1):1–6. doi: 10.1186/s13104-018-3362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koklu M., Ozkan I.A., Saritas M.M. A novel embedded system based on cold box design for the cold chain. Int. J. Appl. Math. Electron. Comput. 2017;5(4):67–70. doi: 10.18100/ijamec.2017436077. [DOI] [Google Scholar]
- Kristensen D., Giersing B., Hickling J., Kazi F., Scarna T., Kahn A. 2020. Since 2020 elsevier has created a ?covid-19 resource centre with free information in english and mandarin on the novel coronavirus COVID- 19. the COVID-19 resource centre is hosted on elsevier connect, the company ’ s public news and information, no. January. [Google Scholar]
- Kristensen D.D., Lorenson T., Bartholomew K., Villadiego S. Can thermostable vaccines help address cold-chain challenges? Results from stakeholder interviews in six low- and middle-income countries. Vaccine. 2016;34(7):899–904. doi: 10.1016/j.vaccine.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G., Gupta S. Assessment of cold chain equipments and their management in government health facilities in a district of Delhi: A cross-sectional descriptive study. Indian J. Public Health. 2020;64(1):22–26. doi: 10.4103/ijph.IJPH_457_18. [DOI] [PubMed] [Google Scholar]
- Kumru O.S., Joshi S.B., Smith D.E., Middaugh C.R., Prusik T., Volkin D.B. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–259. doi: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Lankester F.J., et al. Thermotolerance of an inactivated rabies vaccine for dogs. Vaccine. 2016;34(46):5504–5511. doi: 10.1016/j.vaccine.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Lee B.Y., Haidari L.A. The importance of vaccine supply chains to everyone in the vaccine world. Vaccine. 2017;35(35):4475–4479. doi: 10.1016/j.vaccine.2017.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.Y., et al. The impact of making vaccines thermostable in niger’s vaccine supply chain. Vaccine. 2012;30(38):5637–5643. doi: 10.1016/j.vaccine.2012.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.Y., et al. Economic impact of thermostable vaccines. Vaccine. 2017;35(23):3135–3142. doi: 10.1016/j.vaccine.2017.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidner A.J., Fisun H., Trimble S., Lucas P., Noblit C., Stevenson J.M. Evaluation of temperature stability among different types and grades of vaccine storage units: Data from continuous temperature monitoring devices. Vaccine. 2020;38(14):3008–3014. doi: 10.1016/j.vaccine.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.M., Friend M., Miller A., Stone S. 2016. A SDD and PCM solution for vaccine storage and outreach; pp. 2–9. [Google Scholar]
- Li Y., Zhang X., Munyalo J.M., Tian Z., Ji J. Preparation and thermophysical properties of low temperature composite phase change material octanoic-lauric acid/expanded graphite. J. Mol. Liq. 2019;277:577–583. [Google Scholar]
- Lim J., Norman B.A., Rajgopal J. Redesign of vaccine distribution networks. Int. Trans. Oper. Res. 2019:1–26. doi: 10.1111/itor.12758. [DOI] [Google Scholar]
- Liu L., et al. Development of low-temperature eutectic phase change material with expanded graphite for vaccine cold chain logistics. Renew. Energy. 2021;179:2348–2358. [Google Scholar]
- Lloyd J., Cheyne J. The origins of the vaccine cold chain and a glimpse of the future. Vaccine. 2017;35(17):2115–2120. doi: 10.1016/j.vaccine.2016.11.097. [DOI] [PubMed] [Google Scholar]
- Lloyd J., Lydon P., Ouhichi R., Zaffran M. Reducing the loss of vaccines from accidental freezing in the cold chain: The experience of continuous temperature monitoring in Tunisia. Vaccine. 2015;33(7):902–907. doi: 10.1016/j.vaccine.2014.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long A.J., Hayney M.S. Best practices essential for storage and temperature monitoring of refrigerated vaccines. J. Am. Pharm. Assoc. 2013;53(6):660–661. doi: 10.1331/JAPhA.2013.13537. [DOI] [PubMed] [Google Scholar]
- Lorenc A., Czuba M., Szarata J. Big data analytics and anomaly prediction in the cold chain to supply chain resilience. FME Trans. 2021;49(2):315–326. doi: 10.5937/fme2102315l. [DOI] [Google Scholar]
- Lugelo A., Hampson K., Bigambo M., Kazwala R., Lankester F. Controlling human rabies: The development of an effective, inexpensive and locally made passive cooling device for storing thermotolerant animal rabies vaccines. Trop. Med. Infect. Dis. 2020;5(3) doi: 10.3390/tropicalmed5030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig N., Tommasi M. 2020. COVID-19 and social protection of poor and vulnerable groups in Latin America: a conceptual framework. [Online]. Available: https://repositorio.cepal.org/handle/11362/46939 ( Accessed 28 July 2021) [Google Scholar]
- Magendran S.S., et al. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Struct. Nano-Objects. 2019;20 [Google Scholar]
- Maglasang P.L.F., Butalid M.L.C., Pastoril M.F., Pratama A.N.W., Tan E.Y. A cross-sectional survey on cold chain management of vaccines in Cebu. Philippines, Pharm. Pract. (Granada) 2018;16(2):1–6. doi: 10.18549/PharmPract.2018.02.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood M.S., Siddique F., Hussain I., Ahmad S.I., Rafique A. Thermostable vaccines for newcastle disease: a review. Worlds. Poult. Sci. J. 2014;70(4):829–838. doi: 10.1017/S0043933914000889. [DOI] [Google Scholar]
- March U. 2020. Operation warp speed contracts for COVID-19 vaccines and ancillary vaccination materials; pp. 11–14. [Google Scholar]
- Marsot M., Durand B., Ben Hammouda W., Ammar H.H., Zrelli M., Khorchani R. Evaluation of human resources needed and comparison with human resources available to implement emergency vaccination in case of foot and mouth disease outbreaks in Tunisia. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-de-Nicolas A., McColloster P. Vaccine refrigerator regulator with data logger & back-up power supply. Procedia Vaccinol. 2014;8:89–93. doi: 10.1016/j.provac.2014.07.014. [DOI] [Google Scholar]
- Mathew J.L., Mittal S.K. Vaccination inequities in India: Current status and the way forward. Am. J. Prev. Med. 2021;60(1):S4–S10. doi: 10.1016/j.amepre.2020.10.005. [DOI] [PubMed] [Google Scholar]
- Medaglini D., De Azero M.R., Leroy O., Bietrix F., Denoel P. Innovation partnership for a roadmap on vaccines in europe (IPROVE): A vision for the vaccines of tomorrow. Vaccine. 2018;36(9):1136–1145. doi: 10.1016/j.vaccine.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Mendhe others H. Cold chain maintenance in rajnandgaon and bilaspur districts of chhattisgarh: A process evaluation. J. Fam. Med. Prim. Care. 2018;7(6):1510–1514. doi: 10.4103/jfmpc.jfmpc_128_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck Canada Inc. 2018. Product monograph RotaTeq® rotavirus vaccine, live, oral, pentavalent; pp. 1–30. [Online]. Available: https://www.merck.ca/static/pdf/ROTATEQ-PM_E.pdf. [Google Scholar]
- Michael O.T., et al. The use of UAV/Drones in the optimization of Nigeria vaccine supply chain. Int. J. Sci. Eng. Res. 2019;10(10):1273–1279. [Online]. Available: https://www.ijser.org/onlineResearchPaperViewer.aspx?The-Use-of-UAV-Drones-in-the-Optimization-of-Nigeria-Vaccine-Supply-Chain.pdf. [Google Scholar]
- Ministry of Health . 2021. COVID-19: vaccine storage and handling guidance highlights of changes; pp. 1–48. [Online]. Available: https://www.canada.ca/en/health-canada/services/drugs-health- [Google Scholar]
- Mistilis M.J., Bommarius A.S., Prausnitz M.R. Development of a thermostable microneedle patch for influenza vaccination. J. Pharm. Sci. 2015;104(2):740–749. doi: 10.1002/JPS.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed S.A., Workneh B.D., Haile M. Practical experience of vaccinators and vaccine handlers in vaccine cold chain management : A phenomenological study. Ethiop. J. Heal. Dev. 2021;35(1):29–37. [Google Scholar]
- Mohammed S.A., Workneh B.D., Kahissay M.H. Knowledge, attitude and practice of vaccinators and vaccine handlers on vaccine cold chain management in public health facilities, Ethiopia: Cross-sectional study. PLoS One. 2021;16(2):1–12. doi: 10.1371/journal.pone.0247459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone S., Sampaio M., Maia R.F. A novel deployment of smart cold chain system using 2G-RFID-sys temperature monitoring in medicine cold chain based on internet of things. Proc- 2017 IEEE Int. Conf. Serv. Oper. Logist. Informatics, vol. 2017; SOLI 2017; 2017. pp. 205–210. [DOI] [Google Scholar]
- Murhekar M.V., et al. Frequent exposure to suboptimal temperatures in vaccine cold-chain system in India: results of temperature monitoring in 10 states. Bull. World Health Organ. 2013;91(12):906–913. doi: 10.2471/blt.13.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S.P., et al. Stability of heat stable, live attenuated Rotavirus vaccine (rotasiil®) Vaccine. 2017;35(22):2962–2969. doi: 10.1016/j.vaccine.2017.04.025. [DOI] [PubMed] [Google Scholar]
- Navarro-Torné L., Hanrahan A., Kerstiens F., Aguar B., Matthiessen P. Public health – Driven research and innovation for next-generation influenza vaccines, European Union. Emerg. Infect. Diseases. 2019;25(2):e1–e8. doi: 10.3201/eid2502.180359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazirul M., Sarker I., Jie Z. Social security for vulnerable groups in Bangladesh on government perspective: Contribution of research leader. J. Publ. Policy Adm. 2017;1(1):1–9. doi: 10.11648/j.jppa.20170101.11. [DOI] [Google Scholar]
- Nelson C.M., Wibisono H., Purwanto H., Mansyur I., Moniaga V., Widjaya A. Hepatitis B vaccine freezing in the Indonesian cold chain: Evidence and solutions. Bull. World Health Organ. 2004;82(2):99–105. doi: 10.1590/S0042-96862004000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., et al. Monitoring temperatures in the vaccine cold chain in bolivia. Vaccine. 2007;25(3):433–437. doi: 10.1016/j.vaccine.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Ng C.Z., et al. Cold chain time-and temperature-controlled transport of vaccines: A simulated experimental study. Clin. Exp. Vaccine Res. 2020;9(1):8–14. doi: 10.7774/cevr.2020.9.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie B., et al. Thermal performance enhancement of a phase change material (PCM) based portable box for cold chain applications. J. Energy Storage. 2021;40 doi: 10.1016/J.EST.2021.102707. [DOI] [Google Scholar]
- Norman B.A., et al. A passive cold storage device economic model to evaluate selected immunization location scenarios. Vaccine. 2013;31(45):5232–5238. doi: 10.1016/j.vaccine.2013.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NS Department of Health Wellness . NS Department of Helath and Wellness; 2014. Vaccine Storage and Handling Guidelines for Immunzation Providers; pp. 1–14. [Google Scholar]
- Objio Tina, Morelli Valerie, Trimble Sean. 2021. Pinkbook vaccine storage and handling epidemiology of VPDs CDC. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-storage.html. (Accessed 19 November 2021) [Google Scholar]
- Omole T.M., et al. The challenges of Nigeria vaccine supply chain, a community of practice perspective. Int. J. Res. Sci. Innov. 2019;VI(Iii):151–157. [Google Scholar]
- O’Neill J., Newall F., Antolovich G., Lima S., Danchin M. 2019. Vaccination in people with disability: a review, 16 (1) pp. 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J.R., et al. The potential effects of deploying SARS-Cov-2 vaccines on cold storage capacity and immunization workload in countries of the WHO African Region.pdf. Vaccine. 2020;39:2165–2176. doi: 10.1016/j.vaccine.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei E., Ibrahim M., Kofi Amenuvegbe G. Effective vaccine management: The case of a rural district in Ghana. Adv. Prev. Med. 2019;2019:1–8. doi: 10.1155/2019/5287287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzayd F., Mansouri H., Tamir M., Chiheb R., Benhouma Z. Monitoring vaccine cold chain model with coloured Petri net. Int. J. Adv. Comput. Sci. Appl. 2018;9(5):433–438. doi: 10.14569/IJACSA.2018.090556. [DOI] [Google Scholar]
- Pagliusi S., Hayman B., Jarrett S. Vaccines for a healthy future: 21st dcvmn annual general meeting 2020report. Vaccine. 2021;39(18):2479–2488. doi: 10.1016/j.vaccine.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteur S. 2008. IPOL inactivated poliomyelitis vaccine (VERO) [Online]. Available: http://products.sanofi.com.au/vaccines/IPOL_NZ_PI.pdf. [Google Scholar]
- Paul R., Pal J. A brief history of pandemics. J. Indian Med. Assoc. 2020;118(05) [Google Scholar]
- PaxVax USA . 2015. Vivotif ® TYPHOID vaccine live oral attenuated TY21A active immunizing agent a package of vivotif ® contains a single foil blister with 4 enteric-coated capsules (each containing one dose of lyophilized bacteria) for oral administration. https://pdf.hres.ca/dpd_pm/00029121.PDF. (Accessed 25 June 2021) [Google Scholar]
- Peters T. 2020. Understanding the cold-chain challenge for Covid-19 vaccination. [DOI] [Google Scholar]
- Pfizer . 2021. PREVNAR® 13 how supplied/storage and handling (pneumococcal 13-valent conjugate vaccine - diphtheria CRM197 protein) pfizer medical information - US. https://www.pfizermedicalinformation.com/en-us/prevnar-13/storage-handling. (Accessed 25 June 2021) [Google Scholar]
- Poochaya S., Widjaja D. Enhancing vaccine refrigerator temperature reporting system using iot technology. Suranaree J. Sci. Technol. 2018;25(3):225–234. [Google Scholar]
- Popova O., De Palacios P.I. Reaching more children with vaccines in developing countries: Key challenges of innovation and delivery. Curr. Med. Res. Opin. 2016;32(1):177–181. doi: 10.1185/03007995.2015.1108910. [DOI] [PubMed] [Google Scholar]
- Porth J.M., Wagner A.L., Moyer C.A., Mutua M.K., Boulton M.L. Women’s empowerment and child vaccination in Kenya: The modifying role of wealth. Am. J. Prev. Med. 2021;60(1):S87–S97. doi: 10.1016/j.amepre.2020.08.015. [DOI] [PubMed] [Google Scholar]
- Public Health Unit of Canada . 2021. Vaccine storage and handling guidelines. Ontario. [Google Scholar]
- Ramakanth D., Singh S., Maji P.K., Lee Y.S., Gaikwad K.K. Advanced packaging for distribution and storage of COVID-19 vaccines: a review. Environ. Chem. Lett. 2021 doi: 10.1007/s10311-021-01256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J., Franzel L., Maire D. Innovations in cold chain equipment for immunization supply chains. Vaccine. 2017;35(17):2252–2259. doi: 10.1016/j.vaccine.2016.11.094. [DOI] [PubMed] [Google Scholar]
- Rogers B., Dennison K., Adepoju N., Dowd S., Uedoi K. 2010. Vaccine cold chain: Part 1. Proper handling and storage of vaccine, 58 (9) pp. 337–346. [DOI] [PubMed] [Google Scholar]
- Ross J.C., Saidu Y., Nzuobontane D., Voukings M.Z., Embrey S.R. Application of the remaining vaccine vial monitor life calculation to field temperature monitoring data to improve visibility into cold chain equipment performance. Vaccine. 2020;38(48):7683–7687. doi: 10.1016/j.vaccine.2020.09.078. [DOI] [PubMed] [Google Scholar]
- Rusnack M.R. Improving vaccine safety by using an algorithmic model as a replacement for a physical thermal buffer. Inov. Pharm. 2018;9(1):10. doi: 10.24926/iip.v9i1.962. [DOI] [Google Scholar]
- Saboo others S. Optimized formulation of a thermostable spray-dried virus-like particle vaccine against human papillomavirus. Mol. Pharm. 2016;13(5):1646–1655. doi: 10.1021/acs.molpharmaceut.6b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi I., Soudani D. 2017. Design and realization of a vaccine refrigerator powered by photovoltaic energy, no. October. [Google Scholar]
- Salisbury D., Ramsay M., Noakes K. third ed. The Stationery Office under licence from the Department of Health; London: 2013. Immunisation Against Infectious Disease. [Google Scholar]
- Santos A.F., Gaspar P.D., de Souza H.J.L. Refrigeration of COVID-19 vaccines: Ideal storage characteristics, energy efficiency and environmental impacts of various vaccine options. Energies. 2021;14(7):1849. doi: 10.3390/en14071849. [DOI] [Google Scholar]
- Saraswati L.D., Ginandjar P., Budiyono Martini, Udiyono A., Kairul Vaccines cold chain monitoring: A cross sectional study at three district in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018;116(1) doi: 10.1088/1755-1315/116/1/012082. [DOI] [Google Scholar]
- Sarley D., et al. Transforming vaccines supply chains in Nigeria. Vaccine. 2017;35(17):2167–2174. doi: 10.1016/j.vaccine.2016.11.068. [DOI] [PubMed] [Google Scholar]
- Satria M.H., Jaenul A., Gamayel A. 2021. Design of solar powered vaccine backpack, vol. 7; pp. 590–594. [Google Scholar]
- Sedita J., et al. Cost of goods sold and total cost of delivery for oral and parenteral vaccine packaging formats. Vaccine. 2018;36(12):1700–1709. doi: 10.1016/j.vaccine.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seqirus Canada . 2020. AGRIFLU® Influenza vaccine, surface antigen, inactivated. [Online]. Available: https://www.seqirus.ca/-/media/seqirus-canada/docs-en/2020-agriflu-product-monograph--revised-23oct2020.pdf. [Google Scholar]
- Serum Institute of India . 2021. COVISHIELD FAQs - serum institute of India. https://www.seruminstitute.com/health_faq_covishield.php. (Accessed 06 August 2021) [Google Scholar]
- Sheikh A.B., Pal S., Javed N., Shekhar R. COVID-19 vaccination in developing nations: Challenges and opportunities for innovation. Infect. Dis. Rep. 2021;13(2):429–436. doi: 10.3390/IDR13020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SN D., D K. Closing the global immunization gap: delivery of lifesaving vaccines through innovation and technology. Pediatr. Rev. 2014;35(7):e32–40. doi: 10.1542/PIR.35-7-E32. [DOI] [PubMed] [Google Scholar]
- Sooksriwong C. 2017. Capability of refrigerators to control the cold chain temperature for vaccine storage, no. december. [Google Scholar]
- Staruch R.M.T., Beverly A., Sarfo-Annin J.K., Rowbotham S. Calling for the next WHO global health initiative: The use of disruptive innovation to meet the health care needs of displaced populations. J. Glob. Health. 2018;8(1):8–11. doi: 10.7189/jogh.08.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sularno A., Soelami F., Bindar Y. Experimental and numerical investigation of cooling performance of a cold storage in a pharmaceutical industry. J. Phys. Conf. Ser. 2018;1090(1) doi: 10.1088/1742-6596/1090/1/012012. [DOI] [Google Scholar]
- Swift M.D., et al. Emergency preparedness in the workplace: The flulapalooza model for mass vaccination. Am. J. Public Health. 2017;107(s2):S168–S176. doi: 10.2105/AJPH.2017.303953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L.J., et al. From refrigerator to arm: Issues in vaccination delivery. Vaccine. 2014;32(21):2389–2393. doi: 10.1016/j.vaccine.2014.02.045. [DOI] [PubMed] [Google Scholar]
- Taneja N., Deb P.K., Maheshwari R., Tekade R.K. Package types for different dosage forms. Dosage Form Des. Parameters. 2018;2:553–590. doi: 10.1016/B978-0-12-814421-3.00016-6. [DOI] [Google Scholar]
- Tanner A.R., Dorey R.B., Brendish N.J., Clark T.W. Influenza vaccination: protecting the most vulnerable. Eur. Respir. Rev. 2021;30(159):1–9. doi: 10.1183/16000617.0258-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq S.L., Ali H.M., Akram M.A., Janjua M.M., Ahmadlouydarab M. Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 2020;176 [Google Scholar]
- Tas C.E., Unal H. Thermally buffering polyethylene/halloysite/phase change material nanocomposite packaging films for cold storage of foods. J. Food Eng. 2021;292:2018. [Google Scholar]
- Terna G.Z., et al. Thermostable vaccines in the optimization of African vaccine supply chain, the perspective of the Nigerian health supply chain professionals. Glob. Sci. Journals. 2019;7(11):1377–1386. [Google Scholar]
- Thèves C., Crubézy E., Biagini P. History of smallpox and its spread in human populations. Microbiol. Spectr. 2016;4(4) doi: 10.1128/microbiolspec.poh-0004-2014. [DOI] [PubMed] [Google Scholar]
- Thielmann A., Puth M.T., Kersting C., Porz J., Weltermann B. Vaccine cold chain in general practices: A prospective study in 75 refrigerators (Keep Cool study) PLoS One. 2019;14(11):1–13. doi: 10.1371/journal.pone.0224972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielmann A., Puth M.T., Weltermann B. Visual inspection of vaccine storage conditions in general practices: A study of 75 vaccine refrigerators. PLoS One. 2019;14(12):1–13. doi: 10.1371/journal.pone.0225764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielmann A., Puth M.T., Weltermann B. Improving knowledge on vaccine storage management in general practices: Learning effectiveness of an online-based program. Vaccine. 2020;38(47):7551–7557. doi: 10.1016/j.vaccine.2020.09.049. [DOI] [PubMed] [Google Scholar]
- Tu T.D., Van Phuc K., Dinh N.T.K., Quoc D.N., Spradbrow P.B. Vietnamese trials with a thermostable newcastle disease vaccine (strain I2) in experimental and village chickens. Prev. Vet. Med. 1998;34(2–3):205–214. doi: 10.1016/S0167-5877(97)00065-2. [DOI] [PubMed] [Google Scholar]
- UNICEF and WHO . 2016. Achieving immunization targets with the comprehensive effective vaccine management ( EVM ) framework, WHO/UNICEF Jt. statement, vol. 2014; pp. 1–5. [Online]. Available: http://www.who.int/immunization/programmes_systems/supply_chain/EVM-JS_final.pdf. [Google Scholar]
- United Nations . 2019. World Population Prospects 2019. no. 141. [Google Scholar]
- Vaccine Presentation and Packaging Advisory Group . WHO; 2015. Preferred Product Profile for Vaccines; pp. 1–31. [Online]. Available: http://www.who.int/immunization/policy/committees/VPPAG_Generic_PPP_and_Workplan.pdf. [Google Scholar]
- Vangroenweghe F. Good vaccination practice: It all starts with a good vaccine storage temperature. Porc. Heal. Manag. 2017;3:1–7. doi: 10.1186/s40813-017-0071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerakumar C., Sreekumar A. Phase change material based cold thermal energy storage: Materials, techniques and applications – a review. Int. J. Refrig. 2016;67:271–289. [Google Scholar]
- Wal P., Wadhwa S., Wal A. Current practices in insulin and vaccine storage. Pharmacophore. 2019;10(3):70–81. [Google Scholar]
- WHO . 2011. Study protocol for temperature monitoring in the vaccine cold chain study protocol for temperature monitoring in the vaccine cold chain immunization, vaccines and biologicals; p. 42. [Google Scholar]
- WHO,, 2012. Vaccine-preventable diseases and vaccines-2017 update. in: International travel and health, pp. 1–63.
- WHO . WHO Press; China: 2015. Immunization in Practice a Practical Guide for Health Staff. [Google Scholar]
- WHO . WHO Vaccine Management Handbook Module VMH-E2. World Health Organization; 2015. How to monitor temperatures in the vaccine supply chain; p. 31. [Google Scholar]
- WHO . 2016. Smallpox. https://www.who.int/news-room/q-a-detail/smallpox. (Accessed 09 June 2021) [Google Scholar]
- WHO . 2016. Smallpox vaccines. https://www.who.int/news-room/feature-stories/detail/smallpox-vaccines. (Accessed 09 June 2021) [Google Scholar]
- WHO,, 2017a. The power of vaccines: still not fully utilized. in: Ten Years in Public Health 2007-2017, pp. 82–90.
- WHO . 2017. Statement on Rotarix® and vaccine vial monitor (VVM) compliance. [Online]. Available: https://www.who.int/immunization_standards/vaccine_quality/Rotarix_VVM_Statement-2017.pdf. [Google Scholar]
- WHO . 2019. Immunization coverage, fact sheet. https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage. (Accessed 21 June 2021) [Google Scholar]
- WHO . WHO; 2020. Humidity Control for Vaccine Refrigerators, no. August; pp. 1–11. [Online]. Available: https://extranet.who.int/pqweb/sites/default/files/documents/E003 TPP for Humidity Control.pdf. [Google Scholar]
- WHO . World Health Organization; 2021. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals. [Google Scholar]
- WHO . 2021. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. (Accessed 09 June 2021) [Google Scholar]
- WHO . 2021. Rotavirus vaccine attributes and storage conditions. [Online]. Available: https://www.merck.ca/static/pdf/ROTATEQ-PM_E.pdf. [Google Scholar]
- WHO, UNICEF . WHO and UNICEF; 2021. COVID-19 Vaccination : Supply and Logistics Guidance. [Google Scholar]
- WHO, UNICEF, EVM . WHO and UNICEF; Switzerland: 2017. WHO Vaccine Management Handbook Module VMH-E3-01.1 how to Calculate Vaccine Volumes and Cold Chain Capacity Requirements, no. March. [Google Scholar]
- Wikatama M.F.S., Arzaki M., Rusmawati Y. Verifying vaccine supply chain system in Indonesia using linear-time temporal logic. 2018 6th Int. Conf. Inf. Commun. Technol; ICoICT 2018; 2018. pp. 245–253. [DOI] [Google Scholar]
- Wirkas T., Toikilik S., Miller N., Morgan C., Clements C.J. A vaccine cold chain freezing study in PNG highlights technology needs for hot climate countries. Vaccine. 2007;25(4):691–697. doi: 10.1016/j.vaccine.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Woldemichael B., Bekele D., Esmael A. Cold chain status and knowledge of vaccine providers at primary health care of units bale zone, southeast ethiopia: Cross-sectional study. Immunome Res. 2018;14(1) doi: 10.4172/1745-7580.1000152. [DOI] [Google Scholar]
- Wu W., Zhao F., Ma C., Huang G.Q. Experimental investigation of a real-time monitoring system for cold chain logistics. IEEE Int. Conf. Autom. Sci. Eng. 2020;2020(Augus):1201–1206. doi: 10.1109/CASE48305.2020.9216739. [DOI] [Google Scholar]
- Xia M., Yuan Y., Zhao X., Cao X., Tang Z. Cold storage condensation heat recovery system with a novel composite phase change material. Appl. Energy. 2016;175:259–268. [Google Scholar]
- Xu X., Zhang X., Liu S. Experimental study on cold storage box with nanocomposite phase change material and vacuum insulation panel. Int. J. Energy Res. 2018;42(14):4429–4438. doi: 10.1002/ER.4187. [DOI] [Google Scholar]
- Yakum M.N., Ateudjieu J., Walter E.A., Watcho P. Vaccine storage and cold chain monitoring in the north west region of Cameroon: A cross sectional study. BMC Res. Notes. 2015;8(1):1–7. doi: 10.1186/s13104-015-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., et al. The COVID-19 vaccines: Recent development, challenges and prospects. Vaccines. 2021;9(4):0–16. doi: 10.3390/vaccines9040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauba S., et al. Temperature monitoring in the vaccine cold chain in Cameroon. J. Vaccines Vaccin. 2017;09(01) doi: 10.4172/2157-7560.1000384. [DOI] [Google Scholar]
- Yin H., et al. Experimental and numerical study on thermal protection by silica aerogel based phase change composite. Energy Rep. 2020;6:1788–1797. doi: 10.1016/j.egyr.2020.06.026. [DOI] [Google Scholar]
- Zaffran M., et al. The imperative for stronger vaccine supply and logistics systems. Vaccine. 2013;31(2):B73–B80. doi: 10.1016/j.vaccine.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang N.N., et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182(5):1271–1283.e16. doi: 10.1016/J.CELL.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang X., Xu X., Zhang S. Development of composite phase change cold storage material and its application in vaccine cold storage equipment. J. Energy Storage. 2020;30(February) doi: 10.1016/j.est.2020.101455. [DOI] [Google Scholar]
- Zhao Y., Zhang X., Xu X., Zhang S. Research progress of phase change cold storage materials used in cold chain transportation and their different cold storage packaging structures. J. Mol. Liq. 2020;319 [Google Scholar]
- Zhao J., et al. Recyclable low-temperature phase change microcapsules for cold storage. J. Colloid Interface Sci. 2020;564:286–295. doi: 10.1016/J.JCIS.2019.12.037. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang W., Liang F. A review about phase change material cold storage system applied to solar-powered air-conditioning system. Adv. Mech. Eng. 2017;9(6):2017. doi: 10.1177/1687814017705844. [DOI] [Google Scholar]