Abstract
1,3-Oxazole chemicals are a unique class of five-membered monocyclic heteroarenes, containing a nitrogen atom and an oxygen. These alkaloids have attracted extensive attention from medicinal chemists and pharmacologists owing to their diverse arrays of chemical structures and biological activities, and a series of 1,3-oxazole derivatives has been developed into therapeutic agents (e.g., almoxatone, befloxatone, cabotegravir, delpazolid, fenpipalone, haloxazolam, inavolisib). A growing amount of evidence indicates that marine organisms are one of important sources of 1,3-oxazole-containing alkaloids. To improve our knowledge regarding these marine-derived substances, as many as 285 compounds are summarized in this review, which, for the first time, highlights their sources, structural features and biological properties, as well as their biosynthesis and chemical synthesis. Perspective for the future discovery of new 1,3-oxazole compounds from marine organisms is also provided.
Keywords: 1,3-oxazole; alkaloid; marine natural product; bioactive property; marine organism; marine sponge; symbiotic microorganism
1. Introduction
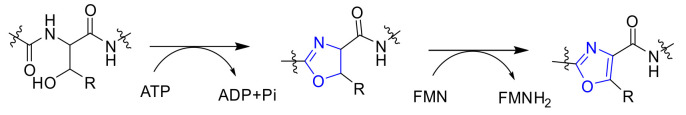
Natural products and their structural analogs are an invaluable source of inspiration in drug design and development. 1,3-Oxazole chemicals are a unique class of five-membered monocyclic heteroarenes, containing a nitrogen atom and an oxygen atom, and usually possess a 2,4- or 2,5-substitution pattern with diverse chemical structures. It is noteworthy that these alkaloids display a wide range of biological properties, such as antibacterial, antifungal, anti-inflammatory, antimalarial, antioxidant, antiviral, cytotoxic, insecticidal, repellent activity, and an inhibitory effect on kinase, showcasing great therapeutic potential [1,2]. A growing amount of evidence indicates that 1,3-oxazole heterocycle is a fundamental structural motif found in marine natural products, which is putatively biosynthesized by the cyclodehydration and dehydrogenation of two amino acids (Figure 1) [3].
Figure 1.
Proposed biosynthetic pathway for 1,3-oxazoles.
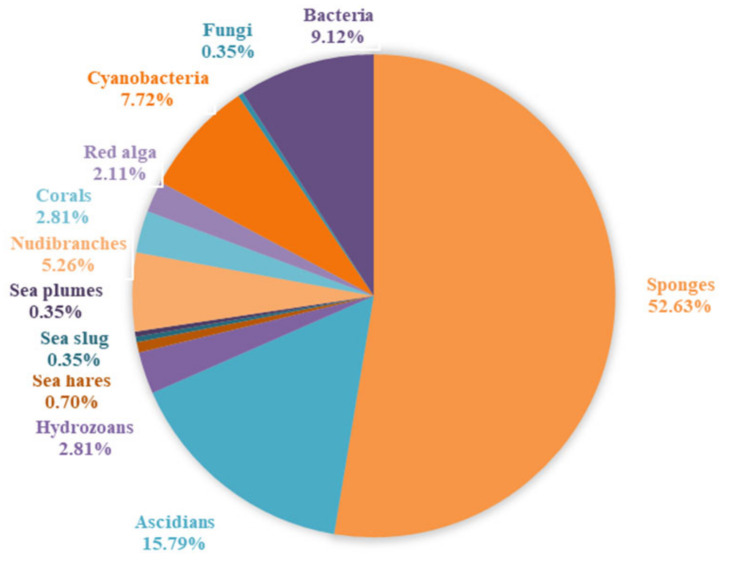
By an extensive and in-depth literature search using DNP (dictionary of natural products) and SciFinder tools, a total of 285 marine-derived 1,3-oxazole-containing compounds (1–285) were isolated and reported until 2020. Almost 80% of these substances were produced by animals (sponges, ascidian, nudibranch, hydrozoan, coral, sea hare and plume) followed by plants (cyanobacteria and red algae) and microorganisms (bacteria and fungi) (Figure 2). To better understand these marine-derived 1,3-oxazole substances and promote marine drugs, here, a systematic and comprehensive review is summarized for the first time. It highlights their biological sources, structural features and bioactivities, as well as biosynthesis and chemical synthesis by comparing with two recent review works, in which one mainly focuses on the biological activities of synthetic and natural oxazoles [4], and the other puts more emphasis on the sources, biological activities, structural features and chemical synthesis of natural oxazole-containing peptides [5]. On the basis of chemical structures, these 1,3-oxazole-based alkaloids are grouped into four major types including peptide, macrolide, polyketide and benzoxazole, and are respectively introduced herein. Detailed information for these natural products is supplied in the Supplementary Materials.
Figure 2.
Percentage distribution of 1,3-oxazole-containing alkaloids from marine organisms.
2. Peptides
2.1. Linear Peptides
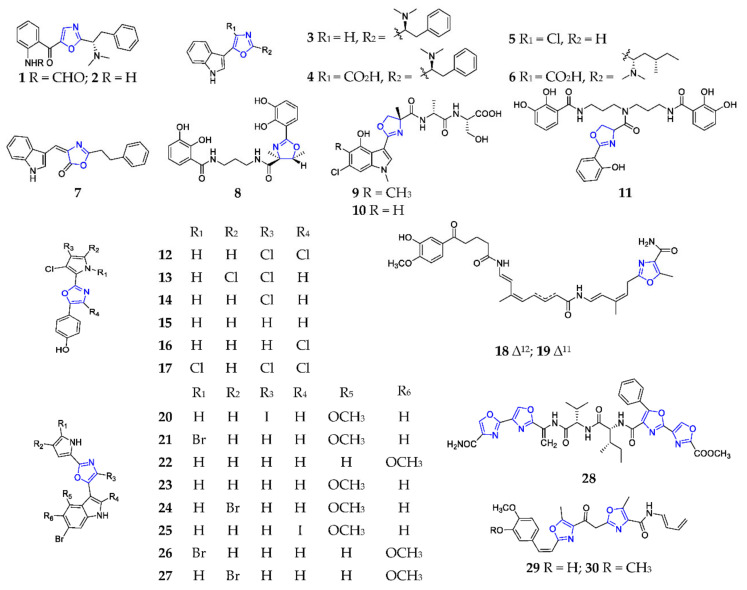
Marine algae and microorganisms are the major sources of linear peptides with the 1,3-oxazole motif. The chemical investigation of a red seaweed of Delesseriaceae from the coasts of north Dakar (Dakar, Senegal) successively led to the isolation of four new linear dipeptides almazoles A–D (1–4) (Scheme 1), in which each compound bears an unusual 2,5-disubstituted oxazole moiety [6,7,8]. Absolute structures of these algal metabolites were originally determined by biomimetic synthesis from L-tryptophan with N,N-dimethyl-L-phenylalanin, for which Robinson–Gabriel cyclization served as the key oxazole forming step [6]. Lately, almazole C (3) has been well prepared by a four-step synthesis, with a 32% overall yield through the Aza-Wittig reaction [9], while compound 4 was the first bioactive member of the almazole family, possessing potent antibacterial against Serratia marcescens and Salmonella typhi XLD [8], and its original structure was revised as 5-(3-indolyl)oxazole-4-carboxylic acid by Horne and his coworkers [10]. Streptochlorin (5) and martefragin A (6) are another two novel 5-(3-indolyl)oxazole-containing alkaloids, respectively produced by marine strain Streptomyces sp. 04DH110 from the east sea of Korea [11] and the red algae Martensia fragilis collected off the coast of Uozu (Uozu, Japan) [12]. In addition to potent fungicidal activity against Pythium, Botrytis cinerea, Septoria tritici, Pyricularia Oryzae, Fusarium culmorum and Rhizoctonia solani, compound 5 displayed a strong inhibitory effect on angiogenesis by blocking NF-κB signaling, as well as human leukemia and normal liver cell lines with IC50 values of 1.05 and 10.9 µg/mL, respectively [11,13,14]. It is noteworthy that one three-step synthesis of compound 5 had been readily achieved by sequential asmic-ester condensations and sulfanyl-Lithium exchange-trapping [15]. As a promising inhibitor of lipid peroxidation, compound 6 was firstly synthesized by Nishida and coworkers in 1998, and its absolute configuration was unambiguously determined as ′′S, ′′S [16]. Almazolone (7) had similar biogenetic features to compounds 1–4 and was purified from Senegal red alga Haraldiophyllum sp. [17].
Scheme 1.
Marine-derived 1,3-oxazole-containing linear peptides (1–30).
Co-culture of Serratia sp. and Shewanella sp. resulted in the production of serratiochelin A (8), which displayed antiproliferative activity against human melanoma cell lines and non-malignant lung fibroblasts, as well as antimicrobial activity toward Staphylococcus aureus [18]. Two new indole-containing peptides, JBIR-34 (9) and JBIR-35 (10), were produced by one symbiotic strain Streptomyces sp. Sp080513GE-23 from Haliclona sp., and exhibited a potent DPPH radical scavenging capability with IC50 values of 1.0 and 2.5 mM, respectively [19]. The unique 4-methyloxazoline moiety was formed by a α-methyl-L-serine from D-alanine and a 5,10-methylene-tetrahydrofolate by FmoH [20]. Nigribactin (11) was isolated as a new siderophore from marine Vibrio nigripulchritudo and was shown to enhance the expression of the spa encoding protein A [21]. Phorbazoles A–D (12–15) were a novel class of marine alkaloids, containing chlorinated pyrrole and 1,3-oxazole moieties from the Indo-Pacific sponge Phorbas aff. clathrata (Levi) [22]. Recently, the chemical preparation of phorbazole B (13) has been achieved by simple catalytic chlorination and iodization to protect the oxazole ring [23]. The first chemical analysis of the marine mollusc Aldisa andersoni, collected off Muttom coast, (Kanyakumari, India) afforded two new cytotoxic phorbazole analogs, 9-chloro-phorbazole D (16) and N1-methyl-phorbazole A (17), as well as phorbazoles A (2), B (13) and D (15) [24].
Two novel linear and achiral polyketide-peptides, ariakemicins A (18) and B (19), were obtained as an inseparable mixture from one marine gliding bacterium Rapidithrix sp., collected off the muddy land alongside the Ariake Inland Sea, and displayed selectively antimicrobial activities against Gram-positive bacteria (Brevibacterium sp., S. aureus, and Bacillus subtilis), and weak cytotoxicity against cancer cell lines A549 and BHK [25]. Breitfussins A–H (20–27) were the first marine natural products containing an indole-oxazole-pyrrole framework from hydrozoan Thuiaria breitfussi inhabits in the Arctic ocean, and were found to excellently inhibit PIM1 and DRAK1 kinases [26,27]. Furthermore, breitfussin C (22) strongly exhibited a cytotoxic effect on cancer cell lines (MCF-7, HT-29, MOLT-4, MV-4-11 and MRC-5). One concise total synthesis of the halogen-rich dipeptides 20 and 21 was created by Bayer and his coworkers in 2015, which consists of the palladium-catalyzed cross-coupling of indole and pyrrole on an oxazole core and selective lithiation/iodination of a common indole-oxazole fragment [28]. Mechercharmycin B (28) was a new linear peptide containing four 1,3-oxazole rings produced by the marine strain Thermoactinomyces sp. YM3-251 from Mecherchar (Palau) [29]. Siphonazole (29) and dimethoxy analog (30) were the first naturally occurring substances that incorporated oxazole subunits connected by a two-carbon tether from a marine microbe Herpetosiphon sp., and exhibited selective cytotoxicity to human breast cancer HTB-129 and acute T cell leukemia TIB-152 [30]. The first chemical synthesis of 29 was achieved in 2007 through the preparation of an oxazole ring using rhodium carbene, and the installation of the pentadienyl amino side-chain [31].
2.2. Monocyclic Peptides
2.2.1. Pentapeptides
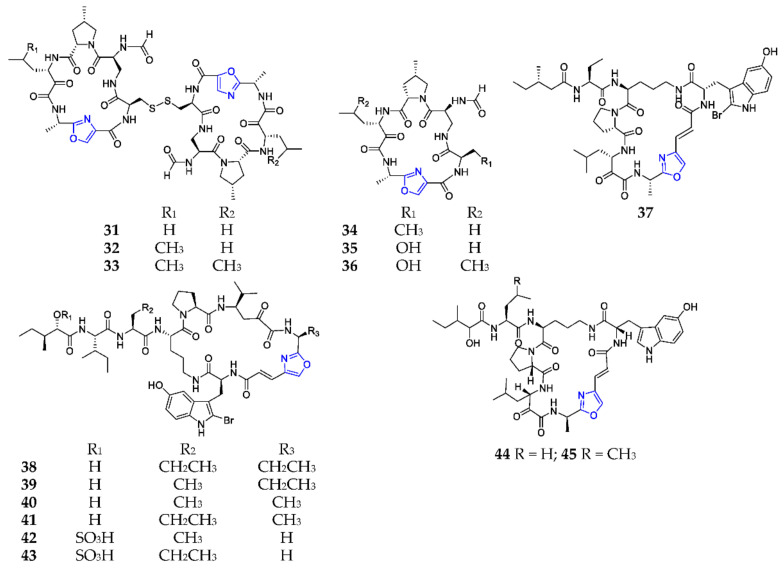
To the best of our knowledge, the marine sponge genus Theonella is the most common and important producer of 1,3-oxazole-containing pentapeptides. An inseparable mixture of three dimeric cyclic pentapeptides, nazumazoles A–C (31–33) (Scheme 2), was obtained from the aqueous alcoholic extract of Theonella swinhoei, collected from Hachijo Island (Tokyo, Japan), and exhibited a potent cytotoxicity against P388 murine leukemia cells with an IC50 value of 0.83 μM [32]. Subsequently, three new monomeric analogs, nazumazoles D–F (34–36), were purified from the same specimen and shown to remarkably inhibit chymotrypsin with IC50 values of 2, 3 and 10 μM, respectively [33]. All absolute configurations of the amino acid residues in compounds 31–36 were unambiguously determined by Marfey’s method. Seven 2-bromotryptophan-containing pentapeptides, orbiculamide A (37), keramamides B–E (38–41), M (42), and N (43) from an Okinawan Theonella sp., displayed, in vitro, a strongly cytotoxic effect on cell lines P388, LI210 and KB [34]. Discobahamins A (44) and B (45) were isolated from a deep-water marine sponge Discodermia sp. collected off Goulding Cay (Goulding Cay, Bahamas), and were shown to have weak antifungal activity against Candida albicans [35].
Scheme 2.
Marine-derived 1,3-oxazole-containing monocyclic pentapeptides (31–45).
2.2.2. Hexapeptides
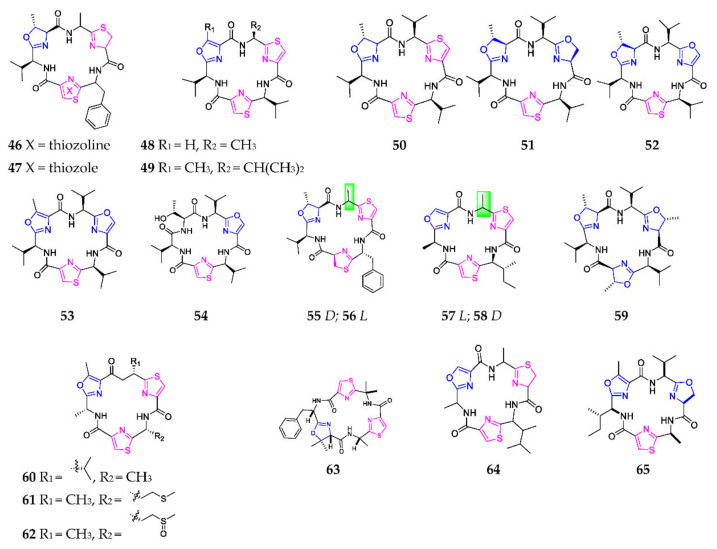
Ascidians of the genera Lissoclinum and Didemnum are prolific producers of cyclic hexapeptides with 1,3-oxazole moieties and thiazole, thiazoline or oxazoline rings. During the period from 1989 to 2017, a total of thirteen hexapeptides, bistratamides A–N (46–58) (Scheme 3), had been found in ascidian L. bistratum. The structure-activity relationship (SAR) analysis indicated that bistratamides containing two thiazole rings possessed stronger cytotoxic activity than others containing one thiazole ring and/or one oxazole ring [36,37,38,39,40]. Westiellamide (59) and dendroamides A-C (60–62) are new bistratamide analogs derived from blue-green algae. Compound 59 exhibited moderate cytotoxicity against KB and LoVo cell lines at 2 μg/mL and 60 showed excellent activity toward multidrug resistant tumors [41,42]. Chemical study of the extracts of ascidian Didemnum molle collected in Madagascar afforded didmolamide A (63), with a mild cytotoxic effect on tumor cell lines A549, HT29 and MEL28 [43]. Bertram and his coworkers firstly invented a novel method to synthesize bistratamides and didmolamides by mediating the coupling reaction of azole amino acids via FDPP-i-Pr2NEt [44].
Scheme 3.
Marine-derived 1,3-oxazole-containing monocyclic hexapeptides (46–65).
Dolastatins E (64) and I (65) were obtained from the Japanese sea hare Dokizbelh auriculariu, and displayed inconspicuous cytotoxic activity against HeLa S3 cells [45,46]. The former substance structurally consisted of one L-alanine, one D-alanine, one D-cysteine, one allo-D-isoleucine, one serine and one cysteine [47], and the later was formed by a N-Boc-L-valine and N-Boc-L-serine [48]. One cytotoxic 1,3-oxazole-containing hexapeptide, leucamide A (66) (Scheme 4), was produced by an Australian sponge Leucetta microraphis [49], and its uniquely mixed 4,2-bisheterocycle tandem pair provided antiviral activity through a specific interaction with DNA/RNA or other targets [50]. Furthermore, the construction of the 4,2-bisheterocycle tandem pair was successfully forged by a diethylaminosulfur trifluoride (DAST)-mediated cyclization of ꞵ-hydroxy amide in the first total synthesis [51].
Scheme 4.
Marine-derived 1,3-oxazole-containing monocyclic hexapeptides (66–81).
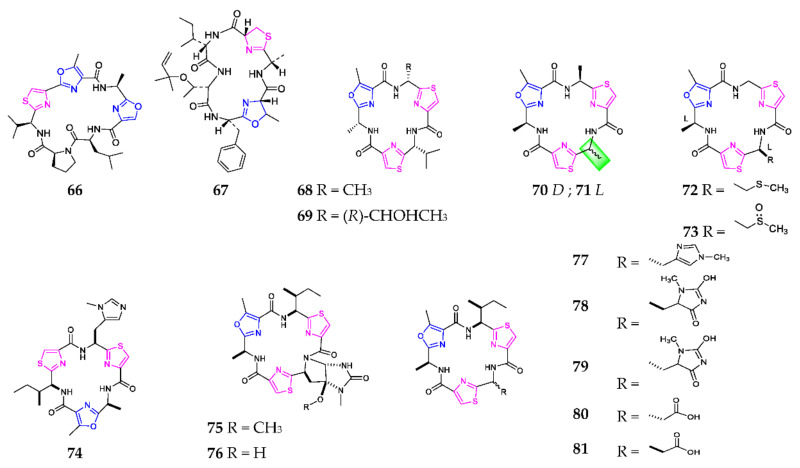
One novel cyclic hexapeptide, comoramide A (67), produced by the ascidian Didemnum molle and collected at Dzaoudzi Mayotte, was found to exhibit mild cytotoxicity against tumor cells A549, HT29 and MEL-28 [52]. Bioassay-guided fractionation of the marine cyanobacterium Oscillatoria sp., collected off Buenaventura Bay, led to the isolation of two antimalarial agents, venturamides A (68) and B (69), which were chemically synthesized using an efficient one-pot method with respective yields of 32.7% and 30.7% [53]. Tenuecyclamides A–D (70–73) were purified from the cyanobacterium N. spongiaeforme var. tenue, of which 70, 72 and 73 had an inhibitory effect on the sea urchin embryos’ division with an ED100 of 10.8, 9.0 and 19.1 µM, respectively [54]. The stereochemistry of 70 and 71 was established through the solid-phase assembly of heterocyclic amino acids in 2004 [55]. Microcyclamides are one class of cyclic hexapeptides formed by the ribosomal pathway [56]. Chemical study of the cyanobacterium Microcystis aeruginosa afforded eight cytotoxic microcyclamide derivatives (74–81) [57,58].
2.2.3. Heptapeptides
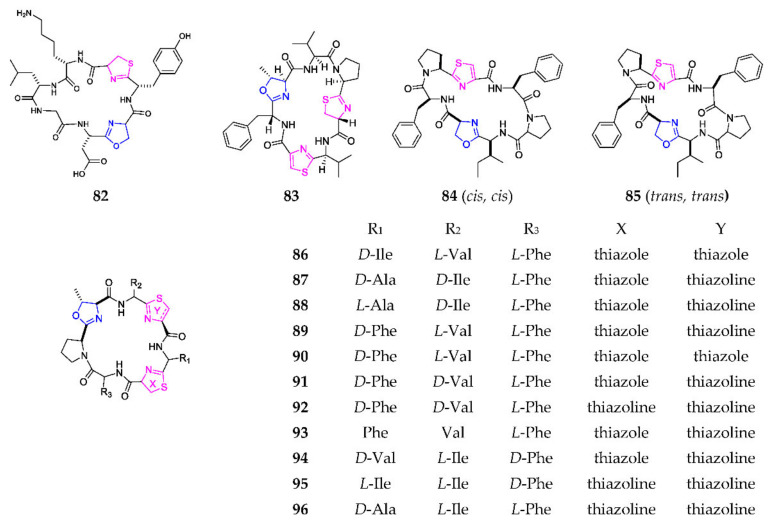
Using the heat shock method, one novel 1,3-oxazole-containing heptapeptide, cyanothecamide C (82) (Scheme 5), was produced by the marine cyanobacteria Cyanothece sp. PCC 7425 at 37° for 24 h [59]. Cyclodidemnamide (83) was derived from the marine ascidian Didemnum molle, collected on the Philippine Islands, and exhibited weak cytotoxicity towards human colon tumor cells [60]. Two isomers, (cis, cis)-ceratospongamide (84) and (trans, trans)-ceratospongamide (85), were obtained from the Indonesian red algae Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica, of which the latter possessed a potent anti-inflammatory effect with an ED50 value of 32 nM [61]. Chemical investigation of the ascidian Lissoclinum patella resulted in the sequential isolation of 11 cytotoxic heptapeptides, including lissoclinamides (86–95) and ulicyclamide (96) [62,63,64,65,66,67]. SAR analysis indicated that cytotoxic effects of 89, 90 and 92 were obviously reduced if the thiazoline ring was replaced by oxazoline, and that of 92 was significantly increased by replacing oxazoline in the macrocycle with thiazoline [68].
Scheme 5.
Marine-derived 1,3-oxazole-containing monocyclic heptapeptides (82–96).
2.2.4. Octapeptides
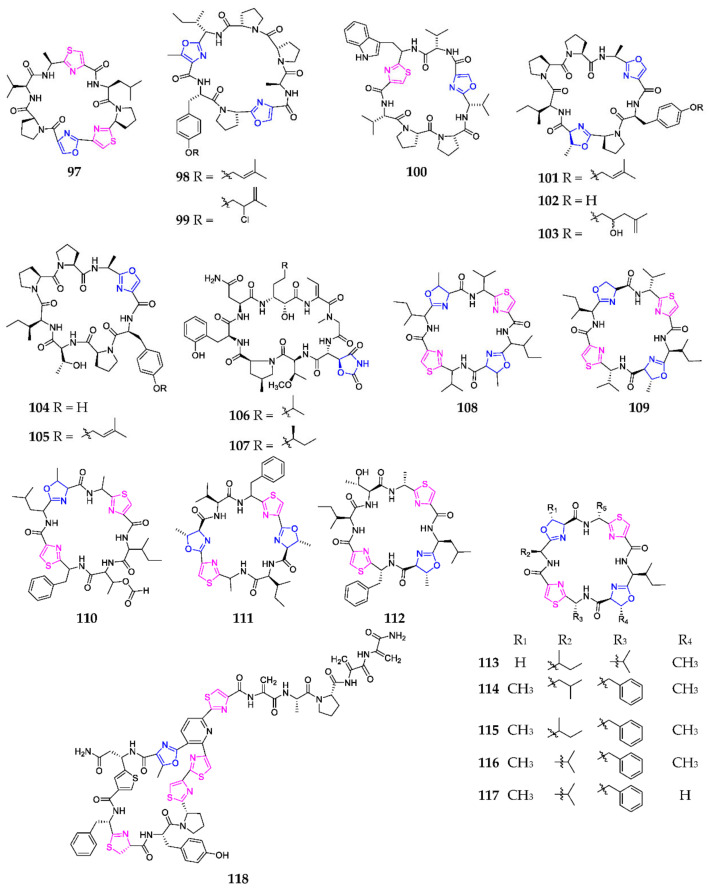
Sanguinamide B (97) (Scheme 6) was a modified macrocyclic octapeptide containing five L-amino acids, two thiazoles and one oxazole from the nudibranch Hexabranchus sanguineus [69]. The first total synthesis of 97 was achieved by Singh and coworkers in 2012 and determined its configuration as trans [70]. Myriastramides A–C (98–100) were new modified cyclic peptides purified from the Philippines marine sponge Myriastra clavosa [71]. Haliclonamides A–E (101–105), produced by the marine sponge Haliclona sp., displayed an antifouling effect on the blue mussel Mytilus edulis galloprovincialis [72,73]. Chemical analysis of the marine sponge T. swinhoei, collected on an isolated reef of the Solomon Islands, yielded two anti-inflammatory octapeptides, perthamides J (106) and K (107) [74]. Ascidiacyclamide (108), formed by the cyclization of [Ile-Oxz-D-Val-Tz-]2, was purified from an unidentified species of ascidian grown on Rodda Reef (Australia) and contains four five-membered heterocycles, where the number and position of oxazoline residues were shown to affect its cytotoxicity [75]. Prepatellamide A (109), prepatellamide B formate (110) and patellamides A–G (111–117) were successively obtained from the ascidian Lissoclinum patella, of which compounds 109, 113, 114 and 111 exhibited modest cytotoxicity against P388 murine leukemia cell lines [76,77,78,79,80,81,82,83,84]. Gene knockout studies indicated that the Pat cluster (pat A-G) was responsible for patellamide biosynthesis [85]. One anti-MRSA thiazolyl peptide, kocurin (118), produced by the marine-derived bacterium Kocuria palustris, collected in Florida Keys (Florida, FL, USA), structurally consists of L-Tyr, L-Pro, L-Ala, L-Asp, L-Phe, L-Cya, L-Pro (×2), L-Ala and L-Cys [86]. Biosynthetically, the formation of oxazole and thiazoline rings of 118 were catalyzed by KocE, and its serine residues were dehydrated by KocB and KocC, followed by the Aza–Diels–Alder reaction catalyzed by KocD, resulting in a generation of a 29-membered cycle [87].
Scheme 6.
Marine-derived 1,3-oxazole-containing monocyclic octapeptides (97–118).
2.2.5. Other Monocyclic Peptides
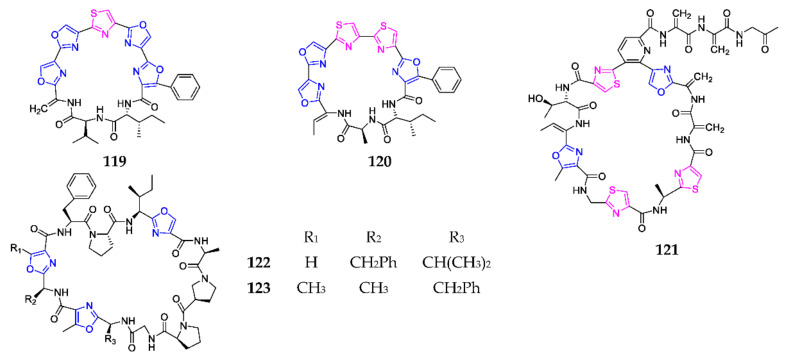
In addition to mechercharmycin B (28), one monocyclic nonapeptide, mechercharmycin A (119) (Scheme 7), was purified from the marine strain Thermoactinomyces sp. YM3-251 and shown to have excellent cytotoxicity toward A549 and Jurkat cells, with IC50 values of 40 and 46 nM, respectively [29]. Urukthapelstatin A (120) was detected in the fermentation broth of the marine strain Mechercharimyces asporophorigenens YM11-542, from Urukthapel Island (Palau), and displayed a broad spectrum of potential cytotoxicities [88]. By the macrocyclization of one macrothiolactone, and the azole formatted by the Aza-Wittig ring contraction, chemical synthesis of compound 120 was first achieved by Schwenk and coworkers [89]. Interestingly, the SAR study showed that the phenyl ring attached to the eastern oxazole, and the rigid lipophilic tripeptide section definitely affected its cytotoxicity [90]. One new thiopeptide TP-1161 (121) was isolated from one marine Nocardiopsis sp. and its biosynthetic gene cluster (BGC) was confirmed by targeted gene inactivation [91]. This substance exhibited no inhibitory effect on Gram-negative bacteria, but excellent antimicrobial activity against a panel of Gram-positive clinical isolates [92]. Wewakazole (122) and wewakazole B (123) were novel cyclic dodecapeptides produced by cyanobacteria Lyngbya majuscule from Papua New Guinea, and Moorea producens collected in the red sea, respectively [93]. Bioassay results suggested that compound 122 was active against non-small cell lung cancer H-460 cells (IC50 10.1 μM) and that 123 had a stronger cytotoxicity against H460 cells (IC50 1.0 μM) and MCF7 breast cancer cells (IC50 0.58 μM). The first total synthesis of 123 was achieved through the careful choice of amino acid-protecting groups and the construction of three different substituted oxazoles [94].
Scheme 7.
Marine-derived 1,3-oxazole-containing other monocyclic peptides (119–123).
2.3. Bicyclic Peptides
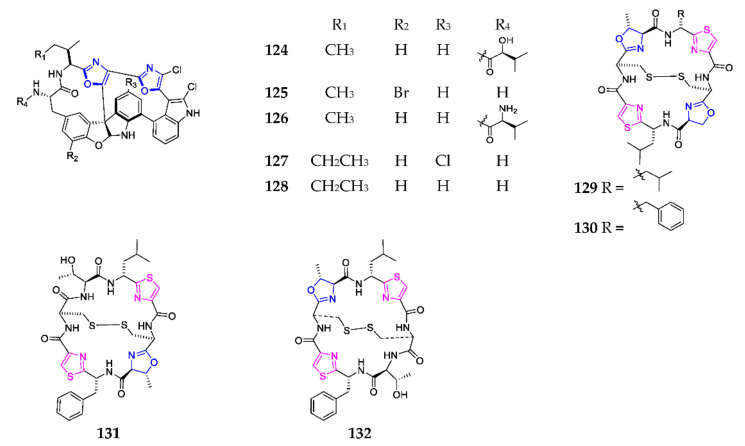
Chemical analysis of the ascidian Diazona angulate led to discovery of five new peptides with two 12-membered macrocycles connected by a quaternary carbon stereo center, substituted by triaryl groups in furan indole nuclei, diazonamides A-E (124–128) (Scheme 8). In particular, compound 124 displayed an extraordinary antitumor effect on a HCT-116 human colon carcinoma and B-16 murine melanoma cancer cell lines, with IC50 values less than 15 ng/mL [95,96], and was chemically synthesized by using the Witkop photocyclization reaction for the first time [97]. Lissoclinum patella is the prolific producer of the new sulfur-containing bicyclic peptides ulithiacyclamide (129), ulithiacyclamide B (130), ulithiacyclamide F (131) and ulithiacyclamide G (132) [67,76,98]. Biological evaluation showed that compounds 129 and 130 excellently inhibited the growth of the KB cell line with an IC50 of 35 and 17 ng/mL, respectively.
Scheme 8.
Marine-derived 1,3-oxazole-containing bicyclic peptides (124–132).
2.4. Depsipeptides
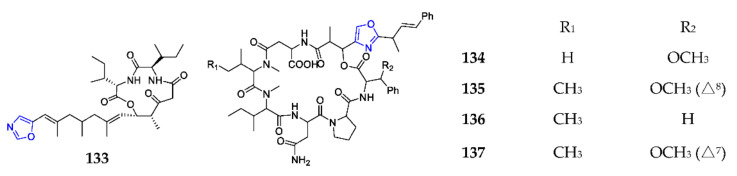
Taumycin A (133) was obtained from the Madagascan sponge Fascaplysinopsis sp. and found to inhibit the growth of the human UT-7 leukemic cell line at 1 µM [99]. Total synthesis study indicated that macrocyclization initiated by the thermal rearrangement of β-keto tert-butyl ester yielded 21% of 133 (Scheme 9) and determined the configuration of C-9 as S [100]. Four cyclic depsipeptides, discokiolides A–D (134–137), were characterized from the marine sponge Discodermia kiiensis and exhibited a broad spectrum of cytotoxic activities against P388, P388/ADM, B16-BL6, Lewis, Lu-99, HT-29 and CCD-19Lu with IC50 values ranged from 0.5 to 7.2 μg/mL [101].
Scheme 9.
Marine-derived 1,3-oxazole-containing depsipeptides (133–137).
3. Macrolides
3.1. Endocyclic Oxazoles
3.1.1. Mono-Oxazole Macrolides
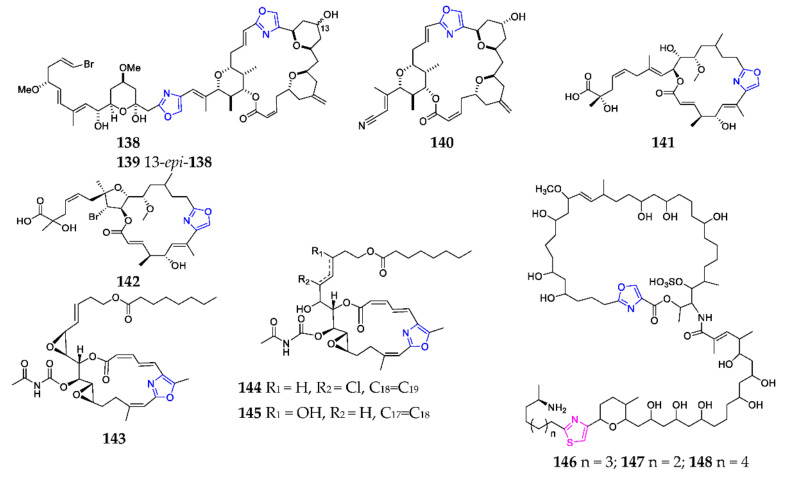
Until 2020, marine sponges were the only sources of macrolides containing a mono-oxazole motif. Two cytotoxic isomers, phorboxazoles A (138) (Scheme 10) and B (139), as well as the precursor (140) were detected in the Indian Ocean marine sponge Phorbas sp. [102,103]. Leiodolides A (141) and B (142) were the first members of a new class of mixed polyketide-nonribosomal peptide synthetase from the deep-water marine sponge Leiodermatium. They structurally possess a 19-membered ring and several unique functional groups, including a bromine substituent and an α-hydroxy-α-methyl carboxylic acid side-chain terminus. These substances had obvious cytotoxic effects against human colon cancer HCT-116 with IC50 values of 2.5 and 5.6 μM, respectively [104]. Chemical investigation of the Madagascan sponge Fascaplysinopsis sp. afforded three macrolides with bis-epoxide motif salarins 143–145, which showed pronounced inhibitory on human leukemia cell lines UT-7 [105,106]. Theonezolides A–C (146–148) from the Okinawan Theonella sp. were novel oxazole-containing macrolides that compose of two main fatty acid chains, including a 37-membered macrolide ring with long side chains connected by amide bonds. They exhibited cytotoxicity on a murine lymphoma L1210 and human epidermoid carcinoma KB cells and induced a platelet morphology change and aggregation in rabbits [107,108,109].
Scheme 10.
Marine-derived endocyclic mono-oxazole macrolides (138–148).
3.1.2. Dioxazole Macrolides
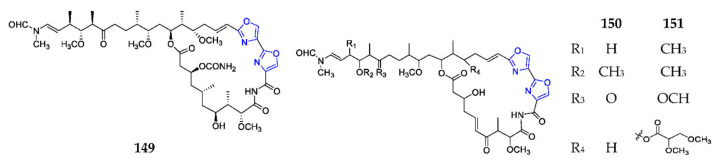
Three 25-membered macrolides consisted of two consecutive oxazole rings, kabiramide I (149) (Scheme 11), halishigamide B (150) and mycalolide D (151), were respectively isolated from the Thai sponge Pachastrissa nux, the Okinawan Halichondria sp., and the stony coral Tubastrea faulkneri, of which compound 150 had a weak cytotoxicity against L1210 and a modest antifungal effect on T. mentagrophytes, while 151 showed a modest general cytotoxicity with average LC50 values of 0.6 µM against the NCI’s 60-human tumor cell line [110,111,112].
Scheme 11.
Marine-derived endocyclic dioxazole macrolides (149–151).
3.1.3. Trioxazole Macrolides
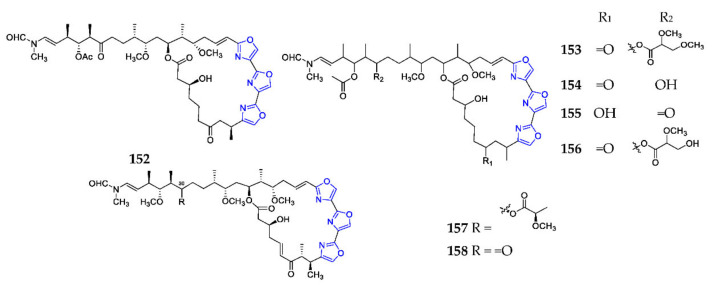
Trioxazole macrolides with extended aliphatic tails are an unprecedented class of natural products and have not been found in terrestrial organisms before [113]. Ulapualides A–E (152–156) (Scheme 12) were extracted from the nudibranch Hexabranchus sanguineus, and compounds 152 and 153 displayed an inhibitory effect on the L1210 leukemia cell with IC50 values ranged from 0.01 to 0.03 µg/mL [114]. Miuramides A (157) and B (158) of the Mycale sp. sponge collected from Miura Peninsula were shown to possess potent cytotoxicities against 3Y1 cells in the nM range [115].
Scheme 12.
Marine-derived endocyclic trioxazole macrolides (152–158).
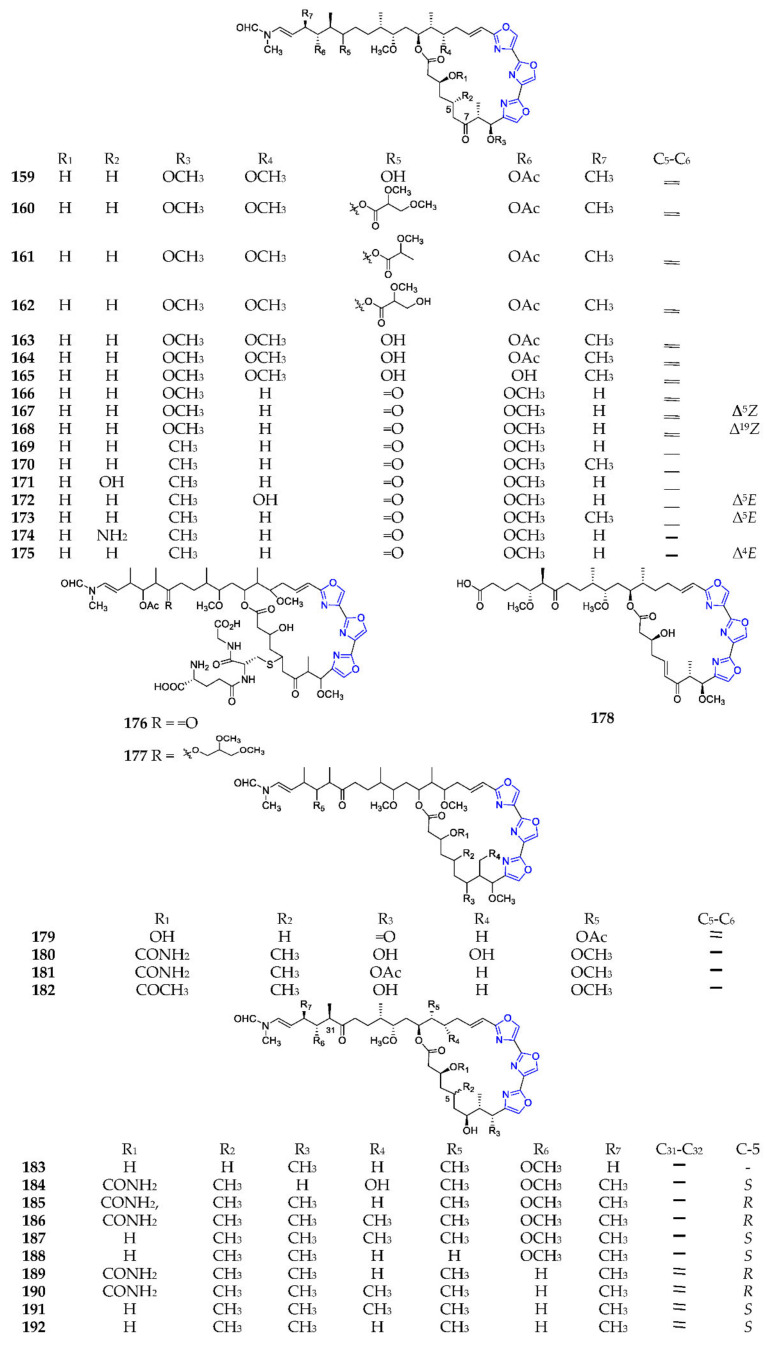
Mycalolides A–C (159–161) (Scheme 13) and their derivatives (162–165, 176, 177, 179) were obtained from a marine sponge of the genus Mycale, except mycalolide E (166) from the coral Tubastrea faulkneri, of which 176 and 177 possessed one sulfur atom connected to the glutathionyl group (i-Glu-Cys-Gly) at C-5 [116]. Bioassay revealed that these substances exhibited excellent cytotoxicities [117]. Phytochemical analysis of sponges Halichondria sp., Jaspis sp., Chondrosia corticata and nudibranch Hexabranchus sanguineus resulted in the discovery of thirteen halichondramides (166–181, 183, 184), which had significant antifungal and cytotoxic effects [111,118]. Kabiramides (180–182, 185–192) were discovered in the Hexabranchus egg masses and the sponge Pachastrissa nux, and displayed extraordinary cytotoxicities, and antiplasmodial and antifungal activities [119].
Scheme 13.
Marine-derived endocyclic trioxazole macrolides (159–192) (Cont.).
3.2. Exocyclic Oxazoles
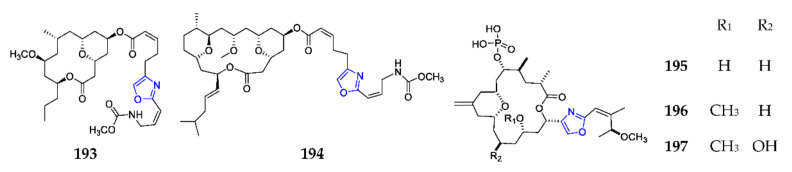
Neopeltolide (193) (Scheme 14) was one of the new exocyclic oxazole-containing macrolides from a deep-water sponge of the family Neopeltidae, and was found to have potential antifungal effects against C. albicans and inhibitory activities against A-549, NCI-ADR-RES and P388 cell lines with IC50 values of 1.2, 5.1 and 0.56 nM, respectively [120]. SAR analysis revealed that the substitution of the side chain and the stereochemistry of the macrolide carbon, especially C-11 and C-13, is vital for the overall biological property [121]. One doubly O-bridged 18-membered macrolide, leucascandrolide A (194), was detected in a calcareous sponge of a new genus Leucuscundra caveoluta from the Coral Sea [122]. Pharmacological study indicated that this metabolite was a novel inhibitor of cytochrome bc1 [123]. Enigmazoles 195–197, from the sponge Cinachyrella enigmatica, were a new structural family of marine phosphomacrolides with an 18-membered macrocyclic ring, which consists of an embedded 2,6-cis-substituted tetrahydropyran ring, a 2,4-disubstituted oxazole ring in the side chain, and a phosphate group. These substances can inhibit cells with either a wild-type or mutant c-Kit. However, their actual cellular targets are still unknown [124]. The first chemical synthesis of 195 and 196 required a tandem olefin cross-metathesis/intramolecular oxa-Michael addition reaction [125].
Scheme 14.
Marine-derived exocyclic oxazoles (193–197).
4. Polyketides
4.1. Mono-Oxazole Polyketides
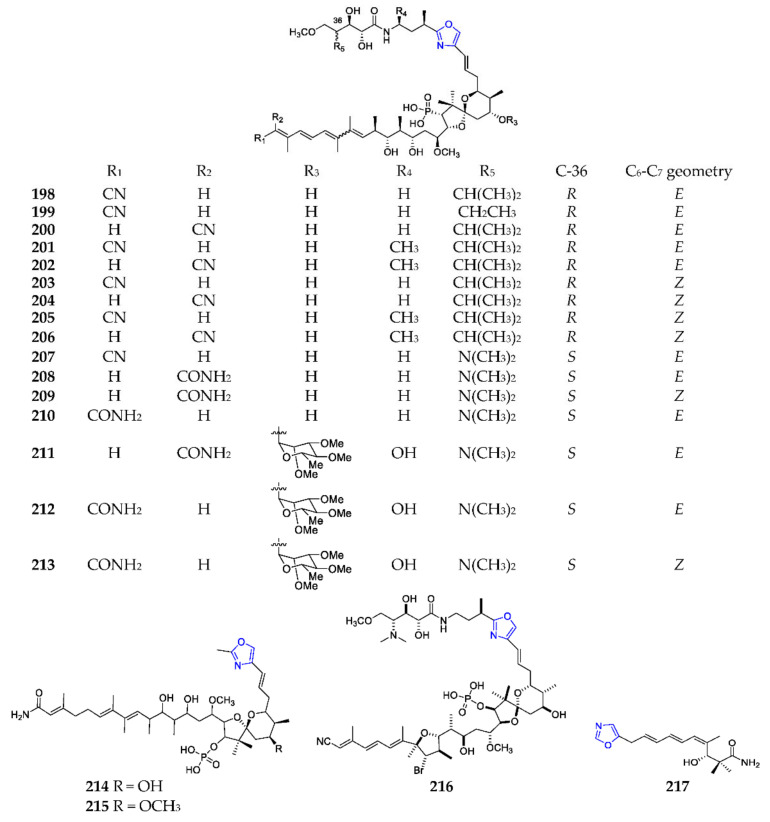
Calyculins (198–216) (Scheme 15) were novel polyketides containing a mono-oxazole and a phosphate group from the marine sponges Discodermia calyx, Lamellomorpha strongylat, Luffariella geometrica, Myriastra clavosa and Theonella swinhoei. These natural products exhibited extensive biological properties including cytotoxicity, antifungal activity and an inhibitory effect on protein phosphatases [126,127,128,129,130,131]. By OSMAC (one strain many compounds) strategy, inthomycin B (217) was produced by the marine sediment-derived Streptomyces YB104 and was found to have anti-oomycete, cytotoxic and herbicidal activities [132]. One concise method to synthesize 217 was developed by Webb and coworkers through the Stille coupling of a stannyl-diene with an oxazole vinyl iodide unit and a Kiyooka ketene acetal/amino acid-derived oxazaborolidine procedure as its cornerstones [133]. And the gene cluster (itm) responsible for biosynthesis of 217 was identified as a 95.3 kb trans-AT type I PKS system, of which the gene Itm15 is a cyclodehydrase to catalyze the formation of oxazole ring [132].
Scheme 15.
Marine-derived mono-oxazole polyketides (198–217).
4.2. Dioxazole Polyketides
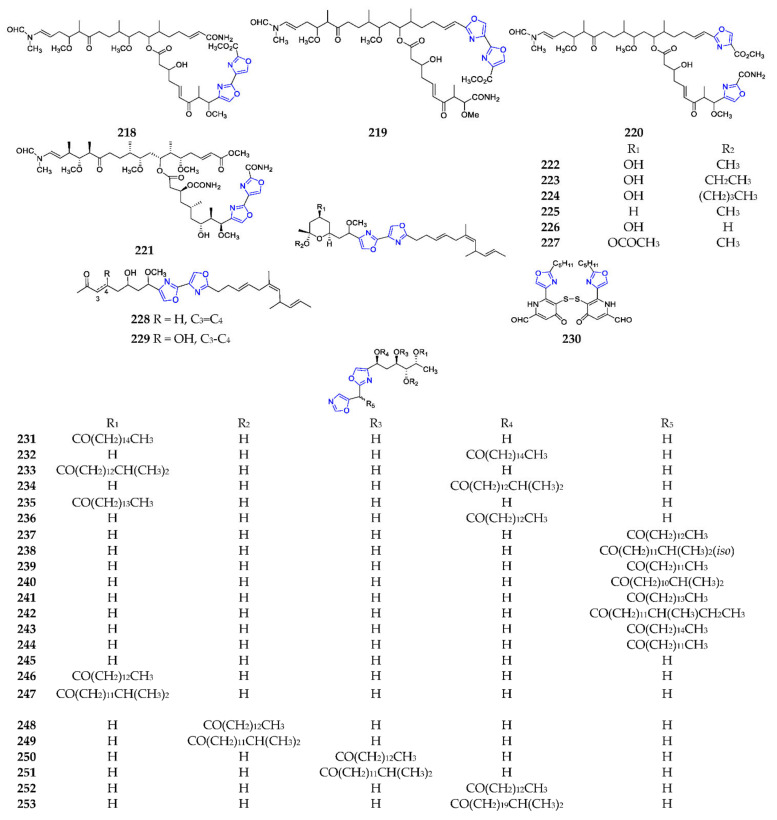
Four analogs of the trioxazole macrolide 169 linked with a methyl ester and a primary amide group, secohalichondramide (218) (Scheme 16), halishigamides C (219) and D (220), and kabiramide H (221), were isolated from marine sponges, including Chondrosia corticate, Halichondria sp. and Pachastrissa nux [110,111,134]. Metabolites 219 and 220 showed weak cytotoxicity against L1210 and KB cell lines and a modest antifungal activity against T. mentagrophytes [111]. By extensive chromatographic techniques, eight hennoxazoles (222–229) were isolated from the sponge Polyfibrospongia sp., of which 222 had peripheral analgesic activity equivalent to the positive control of indomethacin, and 228 exhibited the greatest cytotoxicity toward L1210 with an IC50 value of 2 µg/mL [135,136]. Antibiotic B-90063 (230) was a novel endothelin-converting enzyme (ECE) inhibitor from the marine strain Blastobacter sp. SANK 71894, collected off the coast of Ojika Peninsula [137]. Bengazoles 231–236 were antifungal agents obtained from the marine sponge Pachastrissa sp., collected at Musha Archipelago (Djibouti) [138]. An uncommon oxazole, bengazole A (237) from the sponge Jaspis sp., displayed remarkable ergosterol-dependent antifungal activity against C. albicans, which is equivalent to amphotericin B [139].
Scheme 16.
Marine-derived dioxazole polyketides (218–253).
5. Benzoxazoles
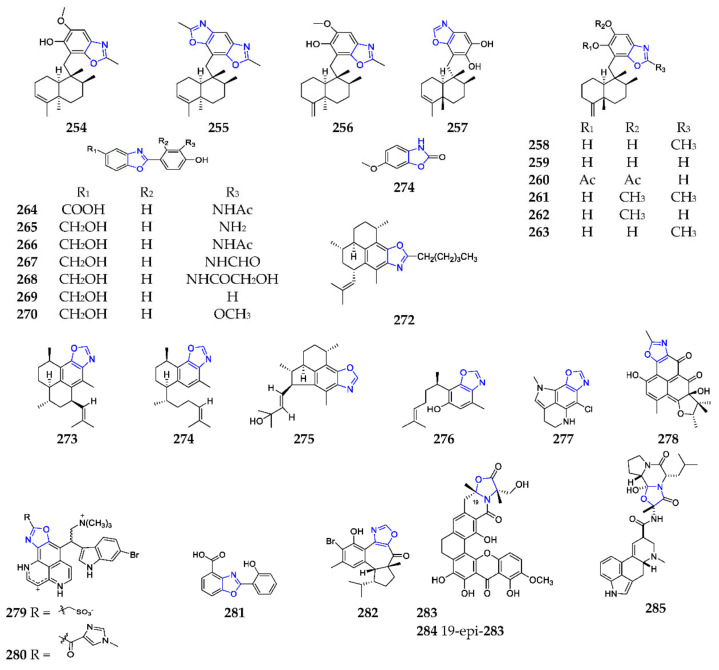
Benzoxazole-containing compounds are one important class of natural products with a potential application in pharmaceutical and agrochemical fields [140,141]. Ten sesquiterpenoid benzoxazoles (254–263) have been successively purified from marine sponges since 1995, including the Okinawan sponge of the family Spongiidae, the Smenospongia sp., the Hyrtios sp., and the Dactylospongia sp. [142,143,144,145]. A novel approach to totally synthesize these substances was developed through the cyclic closure of the N-(2-hydroxyphenyl)-formamide or -acetamide groups to obtain the desired dihydroxybenzoxazole substructure [146]. Chemical study of a halophilic strain Nocardiopsis lucentensis DSM 44048 yielded seven new benzoxazoles, nocarbenzoxazoles A–G (264–270) (Scheme 17), in which 270 had selective activity against HepG2 and HeLa cell lines with a IC50 of 3 and 1 μM, respectively [147]. Recently, a simple route to the synthesis of 269 and 270 was achieved by microwave-assisted construction of a benzoxazole skeleton [148]. One 6-methoxy-2(3H)-benzoxazolinone, coixol (271), was detected in the DCM-MeOH extract of the marine sponge Oceanapia sp., collected off Mandapam coast (India), and had extensive bioactivities, including an inhibitory effect on brine shrimp, anti-inflammation and anti-diabetes [149]. Bioassay-guided fractionation of an extract from the sea plum Pseudopterogorgia elisabethae led to the isolation of homopseudopteroxazole (272), pseudopteroxazole (273), seco-pseudopteroxazole (274) and ileabethoxazole (275) [150,151]. Oxazocurcuphenol (276) was isolated and characterized from the coral P. rigida, collected at Lighthouse Point on the Eleuthera island (Nassau, Bahamas) [152]. Biological tests suggested that 272 and 273–275 strongly inhibited the growth of Mycobacterium tuberculosis H37R0v at 12.5 µg/mL. Compounds 272 and 273 were chemically synthesized by a rapid one-pot method [153], and the total synthesis of 275 was accomplished using a new enantioselective approach [154].
Scheme 17.
Marine-derived benzoxazoles (254–285).
To the best of our knowledge, citharoxazole (277) was the first chlorinated oxazole-containing pyrrolo[4,3,2-de]quinoline from the Mediterranean deep-sea sponge Latrunculia (Biannulata) citharistae [155]. Culture of the marine fungus Penicillium sp. from a marine sediment resulted in the production of herqueioxazole (278), which was the first oxazole-containing phenalenone [156]. The marine sponge Suberites sp. was the unique source to produce nakijinamines C (279) and E (280) with a 1H-oxazolo[40,50:4,5]benzo[1,2,3-de] [1,6]naphthyridine ring system [157]. One new benzoxazole caboxamycin (281), from the deep-sea strain Streptomyces sp. NTK 937, was found to strongly inhibited phosphodiesterases [158]. Lately, one facile method for synthesis of this antibiotic has been developed via debenzylation and demethylation, under eco-friendly and simple reaction conditions [159], and one regulatory and nine structural genes involved in its biosynthesis have been confirmed through genetic manipulation [160]. Hamigeran M (282) was the first non-benzo-fused oxazole-containing terpenoid from the New Zealand marine sponge Hamigera tarangaensis, and exhibited the high efficacy against the HL-60 promyelocytic leukemia cell line with a mean IC50 value of 6.9 ± 0.4 μM [161]. Two novel anti-MRSA xanthones, citreamicins θA (283) and θB (284), were separated from one marine-derived strain of Streptomyces caelestis [162]. Ergosinine (285) was one ergot alkaloid possessing an oxazole ring from the sea slug Pleurobranchus forskalii, collected off Ishigaki Island (Ishigaki, Japan) and found to have an anti-adrenergic effect [163].
6. Conclusions
In recent decades, a tremendous number of 1,3-oxazole-containing alkaloids have been isolated and characterized from marine organisms, including marine invertebrates, nematodes, insects, vertebrates cyanobacteria, bacteria, and fungi. These substances possess unique chemical structures and exhibit a wide variety of biological properties. Many important marine-derived 1,3-oxazoles have great potential for the development of leading compounds in the search for new drugs and medicines. For example, mechercharmycin A (119) is a promising antitumor remedy, and (19Z)-halichondramide (168), neohalichondramide (175), and calyculin A–D (190, 200–202) have the potential to treat human leukemia cell line. Besides, plenty of these substances are potential antimicrobial agents, such as kocurin (118), TP-1161 (121) homopseudopteroxazole (272), and so on. However, (1) how to reduce cytotoxicity, (2) how to improve drug stability, and (3) how to make the drug work quickly and effectively in vivo still needs to be studied in further depth.
In recent years, however, the number of new 1,3-oxazole derivatives from marine organisms has greatly decreased, since almost all accessible macroorganisms have been collected and chemically analyzed. Simultaneously, marine microorganisms are shown to be one prolific and unexploited source of bioactive natural products, owing to their species richness and abundant secondary metabolite BGCs, especially symbiotic microbes of marine sponges, seaweeds, mangroves and tunicates [164,165,166,167]. In order to discover novel marine microbe-derived 1,3-oxazoles for new drug discovery, more efforts should be made to conduct strain isolation and chemical study using a combination of classical methods (e.g., cultivation and fermentation, bioassay-guided fractionation, structure elucidation) and advanced analytical techniques (e.g., metabolomics, higher-field NMR instruments, probe technology), genome mining and engineering and microbial cultivating systems (e.g., OSMAC approach) [168].
Abbreviations
| ADP | Adenosine diphosphate |
| Ala | Alanine |
| Anti-MRSA | Anti-methicillin-resistant Staphylococcus aureus |
| Asp | Aspartate |
| ATP | Adenosine triphospha |
| BGC | Biosynthetic gene cluster |
| Cys | Cysteine |
| DCM | Dichloromethane |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| ECE | Endothelin-converting enzyme |
| ED50 | Median effective dose |
| FMN | Flavin mononucleotide |
| FMNH2 | Flavin mononucleotide reduced |
| Glu | Glutamic acid |
| Gly | Glycine |
| IC50 | Half maximal inhibitory concentration |
| Ile | Isoleucine |
| LC50 | Median lethal concentration |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear magnetic resonance |
| NCI | National Cancer Institute |
| OSMAC | One strain many compounds |
| Phe | Phenylalanine |
| PKS | Polyketide synthase |
| Pro | Proline |
| SAR | Structure-activity relationship |
| S. aureu | Staphylococcus aureus |
| Tyr | Tyrosine |
| Val | Valine |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph14121274/s1. Detailed information for 1,3-oxazole-containing alkaloids (1–285) from marine organisms is available in Table S1.
Author Contributions
Conceptualization, H.Z.; Funding acquisition, H.W. and H.Z.; Project administration, H.Z.; Supervision, H.Z.; Writing—original draft, J.C., S.L., J.L.; Writing—review & editing, Y.Y. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Financial supports from the National Key R&D Program of China (2018YFC0311004), the National Natural Science Foundation of China (41776139) and the Fundamental Research Fund for the Provincial Universities of Zhejiang (China) (RF-C2019002) were greatly appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Detail information for all 1,3-oxazole-containing alkaloids described in this work is available at Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiacchio M.A., Lanza G., Chiacchio U., Giofrè S., Romeo R., Iannazzo D., Legnani L. Oxazole-based compounds as anticancer agents. Curr. Med. Chem. 2020;26:7337–7371. doi: 10.2174/0929867326666181203130402. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H.Z., Zhao Z.L., Zhou C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018;144:444–492. doi: 10.1016/j.ejmech.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y.X., Hou S.Y., Zhang M.Y., Wu Y.Y. Research progress in biosynthesis of five-membered heterocyclic rings in natural products. J. Microbiol. 2019;39:1–12. (In Chinese) [Google Scholar]
- 4.Kakkar S., Narasimhan B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019;13:1–24. doi: 10.1186/s13065-019-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mhlongo J.T., Brasil E., De La Torre B.G., Albericio F. Naturally occurring oxazole-containing peptides. Mar. Drugs. 2020;18:203. doi: 10.3390/md18040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N’Diaye I., Guella G., Chiasera G., Mancini I., Pietra F. Almazole A and almazole B, unusual marine alkaloids of an unidentified red seaweed of the family delesseriaceae from the coasts of Senegal. Tetrahedron Lett. 1994;35:4827–4830. doi: 10.1016/S0040-4039(00)76979-6. [DOI] [Google Scholar]
- 7.Guella G., Mancini I., Pietra F. Almazole C, a new indole alkaloid bearing an unusually 2,5-disubstituted oxazole moiety, and its putative biogenetic peptidic precursors, from a Senegalese delesseriacean seaweed. Helvetica Chimica Acta. 1994;77:1999–2006. doi: 10.1002/hlca.19940770726. [DOI] [Google Scholar]
- 8.N’Diaye I., Guella G., Mancini I., Pietra F. Almazole D. a new type of antibacterial 2,5-disubstituted oxazolic dipeptide from a red alga of the coast of Senegal. Tetrahedron Lett. 1996;37:3049–3050. doi: 10.1016/0040-4039(96)00466-2. [DOI] [Google Scholar]
- 9.Molina P., Fresneda P., Castañeda M., Blug M. Iminophosphorane-based preparation of 2,5-disubstituted oxazole derivatives: Synthesis of the marine alkaloid almazole C. Synlett. 2007;2007:324–326. doi: 10.1055/s-2007-967995. [DOI] [Google Scholar]
- 10.Miyake F., Hashimoto M., Tonsiengsom S., Yakushijin K., Horne D.A. Synthesis of 5-(3-indolyl)oxazole natural products. Structure revision of almazole D. Tetrahedron. 2010;66:4888–4893. doi: 10.1016/j.tet.2010.03.109. [DOI] [Google Scholar]
- 11.Shin H.J., Jeong H.S., Lee H.S., Park S.K., Kim H.M., Kwon H.J. Isolation and structure determination of streptochlorin, an antiproliferative agent from a marine-derived Streptomyces sp. 04DH110. J. Microbiol. Biotechnol. 2007;17:1403–1406. doi: 10.1007/s10295-007-0228-2. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S., Matsunaga T., Hasegawa C., Saito H., Fujita D., Kiuchi F., Tsuda Y. Martefragin A, a novel indole alkaloid isolated from red alga, inhibits lipid peroxidation. Chem. Pharm. Bull. 1998;46:1527–1529. doi: 10.1248/cpb.46.1527. [DOI] [PubMed] [Google Scholar]
- 13.Choi I.K., Shin H.J., Lee H.S., Kwon H.J. Streptochlorin, a marine natural product, inhibits NF-κB activation and suppresses angiogenesis in vitro. J. Microbiol. Biotechnol. 2007;17:1338–1343. [PubMed] [Google Scholar]
- 14.Zhang M.Z., Jia C.Y., Gu Y.C., Mulholland N., Turner S., Beattie D., Zhang W.H., Yang G.F., Clough J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017;126:669–674. doi: 10.1016/j.ejmech.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Mueller L.G., Jr., Chao A., Alwedi E., Natrajan M., Fleming F.F. Oxazole synthesis by sequential asmic-ester condensations and sulfanyl-lithium exchange-trapping. Org. Lett. 2021;23:1500–1503. doi: 10.1021/acs.orglett.1c00288. [DOI] [PubMed] [Google Scholar]
- 16.Nishida A., Fuwa M., Fujikawa Y., Nakahata E., Furuno A., Nakagawa M. First total synthesis of martefragin A, a potent inhibitor of lipid peroxidation isolated from sea alga. Tetrahedron Lett. 1998;39:5983–5986. doi: 10.1016/S0040-4039(98)01228-3. [DOI] [Google Scholar]
- 17.Guella G., N’Diaye I., Fofana M., Mancini I. Isolation, synthesis and photochemical properties of almazolone, a new indole alkaloid from a red alga of Senegal. Tetrahedron. 2006;62:1165–1170. doi: 10.1016/j.tet.2005.10.072. [DOI] [Google Scholar]
- 18.Schneider Y., Jenssen M., Isaksson J., Hansen K.O., Andersen J.H., Hansen E.H. Bioactivity of serratiochelin A, a sidero-phore isolated from a co-culture of Serratia sp. and Shewanella sp. Microorganisms. 2020;8:1042. doi: 10.3390/microorganisms8071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motohashi K., Takagi M., Shin-Ya K. Tetrapeptides possessing a unique skeleton, JBIR-34 and JBIR-35, isolated from a sponge-derived actinomycete, Streptomyces sp. Sp080513GE-23. J. Nat. Prod. 2010;73:226–228. doi: 10.1021/np900810r. [DOI] [PubMed] [Google Scholar]
- 20.Muliandi A., Katsuyama Y., Sone K., Izumikawa M., Moriya T., Hashimoto J., Kozone I., Takagi M., Shin-Ya K., Ohnishi Y. Biosynthesis of the 4-methyloxazoline-containing nonribosomal peptides, JBIR-34 and -35, in Streptomyces sp. Sp080513GE-23. Chem. Biol. 2014;21:923–934. doi: 10.1016/j.chembiol.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen A., Mansson M., Wietz M., Varming A.N., Phipps R.K., Larsen T.O., Gram L., Ingmer H. Nigribactin, a novel siderophore from Vibrio nigripulchritudo, modulates Staphylococcus aureus virulence gene expression. Mar. Drugs. 2012;10:2584–2595. doi: 10.3390/md10112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudi A., Stein Z., Green S., Goldberg I., Kashman Y., Benayahu Y., Schleyer M. Phorbazoles A-D, novel chlorinated phenylpyrrolyloxazoles from the marine sponge phorbas aff. clathrata. Tetrahedron Lett. 1994;35:2589–2592. doi: 10.1016/S0040-4039(00)77179-6. [DOI] [Google Scholar]
- 23.Guttormsen Y., Fairhurst M.E., Pandey S.K., Isaksson J., Haug B.E., Bayer A. Total synthesis of phorbazole B. Molecules. 2020;25:4848. doi: 10.3390/molecules25204848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuzzo G., Ciavatta M.L., Kiss R., Mathieu V., Leclercqz H., Manzo E., Villani G., Mollo E., Lefranc F., D’Souza L., et al. Chemistry of the nudibranch Aldisa andersoni: Structure and biological activity of phorbazole metabolites. Mar. Drugs. 2012;10:1799–1811. doi: 10.3390/md10081799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oku N., Adachi K., Matsuda S., Kasai H., Takatsuki A., Shizuri Y. Ariakemicins A and B, novel polyketide-peptide antibi-otics from a marine gliding bacterium of the genus Rapidithrix. Org. Lett. 2008;10:2481–2484. doi: 10.1021/ol8007292. [DOI] [PubMed] [Google Scholar]
- 26.Hansen K., Andersen J.H., Bayer A., Pandey S.K., Lorentzen M., Jørgensen K.B., Sydnes M.O., Guttormsen Y., Baumann M., Koch U., et al. Kinase chemodiversity from the arctic: The breitfussins. J. Med. Chem. 2019;62:10167–10181. doi: 10.1021/acs.jmedchem.9b01006. [DOI] [PubMed] [Google Scholar]
- 27.Hanssen K., Schuler B., Williams A., Demissie T.B., Hansen E., Andersen J.H., Svenson J., Blinov K., Repisky M., Mohn F., et al. A combined atomic force microscopy and computational approach for the structural elucidation of breitfussin A and B: highly modified halogenated dipeptides from Thuiaria breitfussi. Angew. Chem. Int. Ed. 2012;51:12238–12241. doi: 10.1002/anie.201203960. [DOI] [PubMed] [Google Scholar]
- 28.Pandey S.K., Guttormsen Y., Haug B.E., Hedberg C., Bayer A. A concise total synthesis of breitfussin A and B. Org. Lett. 2014;17:122–125. doi: 10.1021/ol503348n. [DOI] [PubMed] [Google Scholar]
- 29.Kanoh K., Matsuo Y., Adachi K., Imagawa H., Nishizawa M., Shizuri Y. Mechercharmycins A and B, cytotoxic substances from marine-derived Thermoactinomyces sp. YM3-251. J. Antibiot. 2005;58:289–292. doi: 10.1038/ja.2005.36. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Polishchuk E.A., Chen J., Ciufolini M.A. Development of an oxazole conjunctive reagent and application to the total synthesis of siphonazoles. J. Org. Chem. 2009;74:9140–9151. doi: 10.1021/jo9018705. [DOI] [PubMed] [Google Scholar]
- 31.Linder J., Moody C.J. The total synthesis of siphonazole, a structurally unusual bis-oxazole natural product. Chem. Commun. 2007:1508–1509. doi: 10.1039/b618160k. [DOI] [PubMed] [Google Scholar]
- 32.Fukuhara K., Takada K., Okada S., Matsunaga S. Nazumazoles A–C, cyclic pentapeptides dimerized through a disulfide bond from the marine sponge Theonella swinhoei. Org. Lett. 2015;17:2646–2648. doi: 10.1021/acs.orglett.5b01020. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara K., Takada K., Okada S., Matsunaga S. Nazumazoles D–F, cyclic pentapeptides that inhibit chymotrypsin, from the marine sponge Theonella swinhoei. J. Nat. Prod. 2016;79:1694–1697. doi: 10.1021/acs.jnatprod.6b00261. [DOI] [PubMed] [Google Scholar]
- 34.Fusetani N., Sugawara T., Matsunaga S., Hirota H. Orbiculamide A: A novel cytotoxic cyclic peptide from a marine sponge Theonella sp. J. Am. Chem. Soc. 1991;113:7811–7812. doi: 10.1021/ja00020a080. [DOI] [Google Scholar]
- 35.Gunasekera S.P., Pomponi S.A., McCarthy P. Discobahamins A and B, new peptides from the Bahamian deep water marine sponge Discodermia sp. J. Nat. Prod. 1994;57:79–83. doi: 10.1021/np50103a011. [DOI] [PubMed] [Google Scholar]
- 36.Degnan B.M., Hawkins C.J., Lavin M.F., McCaffrey E.J., Parry D.L., Watters D.J. Novel cytotoxic compounds from the ascidian Lissoclinum bistratum. J. Med. Chem. 1989;32:1354–1359. doi: 10.1021/jm00126a035. [DOI] [PubMed] [Google Scholar]
- 37.Perez L.J., Faulkner D.J. Bistratamides E−J, modified cyclic hexapeptides from the Philippines ascidian Lissoclinum bistratum. J. Nat. Prod. 2003;66:247–250. doi: 10.1021/np0204601. [DOI] [PubMed] [Google Scholar]
- 38.Biard J.F., Roussakis C., Kornprobst J.M., Gouiffes-Barbin D., Verbist J.F., Cotelle P., Foster M., Ireland C.M., Debitus C. Bistramides A, B, C, D, and K: A new class of bioactive cyclic polyethers from Lissoclinum bistratum. J. Nat. Prod. 1994;57:1336–1345. doi: 10.1021/np50112a002. [DOI] [PubMed] [Google Scholar]
- 39.Foster M.P., Concepcion G.P., Caraan G.B., Ireland C.M. Bistratamides C and D. Two new oxazole-containing cyclic hex-apeptides isolated from a Philippine Lissoclinum bistratum ascidian. J. Org. Chem. 1992;57:6671–6675. doi: 10.1021/jo00050a063. [DOI] [Google Scholar]
- 40.Urda C., Fernández R., Rodríguez J., Pérez M., Jiménez C., Cuevas C. Bistratamides M and N, oxazole-thiazole containing cyclic hexapeptides isolated from Lissoclinum bistratum interaction of Zinc (II) with bistratamide K. Mar. Drugs. 2017;15:209. doi: 10.3390/md15070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prinsep M.R., Moore R.E., Levine I.A., Patterson G.M.L. Westiellamide, a bistratamide-related cyclic peptide from the Blue-Green Alga Westiellopsis prolifica. J. Nat. Prod. 1992;55:140–142. doi: 10.1021/np50079a022. [DOI] [PubMed] [Google Scholar]
- 42.Ogino J., Moore R.E., Patterson G.M., Smith C.D. Dendroamides, new cyclic hexapeptides from a blue-green alga. Multi-drug-resistance reversing activity of dendroamide A. J. Nat. Prod. 1996;59:581–586. doi: 10.1021/np960178s. [DOI] [PubMed] [Google Scholar]
- 43.Rudi A., Chill L., Aknin M., Kashman Y. Didmolamide A and B, two new cyclic hexapeptides from the marine ascidian Didemnum molle. J. Nat. Prod. 2003;66:575–577. doi: 10.1021/np020531w. [DOI] [PubMed] [Google Scholar]
- 44.Bertram A., Maulucci N., New O.M., Mohd Nor S.M., Pattenden G. Synthesis of libraries of thiazole, oxazole and imidazole-based cyclic peptides from azole-based amino acids. A new synthetic approach to bistratamides and didmolamides. Org. Biomol. Chem. 2007;5:1541–1553. doi: 10.1039/b701999h. [DOI] [PubMed] [Google Scholar]
- 45.Ojika M., Nemoto T., Nakamura M., Yamada K. Dolastatin E, a new cyclic hexapeptide isolated from the sea hare Dolabella auricularia. Tetrahedron Lett. 1995;36:5057–5058. doi: 10.1016/00404-0399(50)0922Y-. [DOI] [Google Scholar]
- 46.Sone H., Kigoshi H., Yamada K. Isolation and stereostructure of dolastatin I, a cytotoxic cyclic hexapeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron. 1997;53:8149–8154. doi: 10.1016/S0040-4020(97)00504-8. [DOI] [Google Scholar]
- 47.Nakamura M., Shibata T., Nakane K., Nemoto T., Ojika M., Yamada K. Stereochemistry and total synthesis of dolastatin E. Tetrahedron Lett. 1995;36:5059–5062. doi: 10.1016/00404-0399(50)0923Z-. [DOI] [Google Scholar]
- 48.Kigoshi H., Yamada S. Synthesis of dolastatin I, a cytotoxic cyclic hexapeptide from the sea hare Dolabella auricularia. Tetrahedron. 1999;55:12301–12308. doi: 10.1016/S0040-4020(99)00729-2. [DOI] [Google Scholar]
- 49.Kehraus S., König G.M., Wright A.D., Woerheide G. Leucamide A: a new cytotoxic heptapeptide from the Australian sponge Leucetta microraphis. J. Org. Chem. 2002;67:4989–4992. doi: 10.1021/jo020058r. [DOI] [PubMed] [Google Scholar]
- 50.Wang W.L., Yao D.Y., Gu M., Fan M.Z., Li J.Y., Xing Y.C., Nan F.J. Synthesis and biological evaluation of novel bisheterocycle-containing compounds as potential anti-influenza virus agents. Bioorg. Med. Chem. Lett. 2005;15:5284–5287. doi: 10.1016/j.bmcl.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 51.Wang W., Nan F. First total synthesis of leucamide A. J. Org. Chem. 2003;68:1636–1639. doi: 10.1021/jo026799+. [DOI] [PubMed] [Google Scholar]
- 52.Rudi A., Aknin M., Gaydou E.M., Kashman Y. Four new cytotoxic cyclic hexa- and heptapeptides from the marine ascidian Didemnum molle. Tetrahedron. 1998;54:13203–13210. doi: 10.1016/S0040-4020(98)00801-1. [DOI] [Google Scholar]
- 53.Liu Y., He P., Zhang Y., Zhang X., Liu J., Du Y. One-pot enantiomeric synthesis of thiazole-containing amino acids: Total synthesis of venturamides A and B. J. Org. Chem. 2018;83:3897–3905. doi: 10.1021/acs.joc.8b00244. [DOI] [PubMed] [Google Scholar]
- 54.Banker R., Carmeli S. Tenuecyclamides A−D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J. Nat. Prod. 1998;61:1248–1251. doi: 10.1021/np980138j. [DOI] [PubMed] [Google Scholar]
- 55.You S.L., Deechongkit S., Kelly J.W. Solid-phase synthesis and stereochemical assignments of tenuecyclamides A-D employing heterocyclic amino acids derived from commercially available fmoc α-amino acids. Org. Lett. 2004;6:2627–2630. doi: 10.1021/ol049020m. [DOI] [PubMed] [Google Scholar]
- 56.Ziemert N., Ishida K., Quillardet P., Bouchier C., Hertweck C., deMarsac N.T., Dittmann E. Microcyclamide biosynthesis in two strains of Microcystis aeruginosa: From structure to genes and vice versa. Appl. Environ. Microbiol. 2008;74:1791–1797. doi: 10.1128/AEM.02392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raveh A., Moshe S., Evron Z., Flescher E., Carmeli S. Novel thiazole and oxazole containing cyclic hexapeptides from a waterbloom of the cyanobacterium Microcystis sp. Tetrahedron. 2010;66:2705–2712. doi: 10.1016/j.tet.2010.02.008. [DOI] [Google Scholar]
- 58.Ishida K., Nakagawa H., Murakami M. Microcyclamide, a cytotoxic cyclic hexapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 2000;63:1315–1317. doi: 10.1021/np000159p. [DOI] [PubMed] [Google Scholar]
- 59.Houssen W., Koehnke J., Zollman D., Vendome J., Raab A., Smith M., Naismith J., Jaspars M. The discovery of new cyanobactins from Cyanothece PCC 7425 defines a new signature for processing of patellamides. ChemBioChem. 2012;13:2683–2689. doi: 10.1002/cbic.201200661. [DOI] [PubMed] [Google Scholar]
- 60.Toske S.G., Fenical W. Cyclodidemnamide: a new cyclic heptapeptide from the marine ascidian Didemnum Molle. Tetrahedron Lett. 1995;36:8355–8358. doi: 10.1016/0040-4039(95)01806-S. [DOI] [Google Scholar]
- 61.Tan L.T., Williamson R.T., Gerwick W.H., Watts K.S., McGough K., Jacobs R. cis,cis- and trans,trans-ceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 2000;65:419–425. doi: 10.1021/jo991165x. [DOI] [PubMed] [Google Scholar]
- 62.Wasylyk J.M., Biskupiak J.E., Costello C.E., Ireland C.M. Cyclic peptide structures from the tunicate Ceratodictyon spongiosum by FAB mass spectrometry. J. Org. Chem. 1983;48:4445–4449. doi: 10.1021/jo00172a001. [DOI] [Google Scholar]
- 63.Degnan B.M., Hawkins C.J., Lavin M.F., McCaffrey E.J., Parry D.L., Brenk A.L.V.D., Watters D.J. New cyclic peptides with cytotoxic activity from the ascidian Lissoclinum patella. J. Med. Chem. 1989;32:1349–1354. doi: 10.1021/jm00126a034. [DOI] [PubMed] [Google Scholar]
- 64.Schmitz F.J., Ksebati M.B., Chang J.S., Wang J.L., Hossain M.B., Van der Helm D., Engel M.H., Serban A., Silfer J.A. Cyclic peptides from the ascidian Lissoclinum patella: Conformational analysis of patellamide D by X-ray analysis and molecular modeling. J. Org. Chem. 1989;54:3463–3472. doi: 10.1021/jo00275a036. [DOI] [Google Scholar]
- 65.Morris L.A., Bosch J.K.V.D., Versluis K., Thompson G.S., Jaspars M. Structure determination and MSn analysis of two new lissoclinamides isolated from the Indo–Pacific ascidian Lissoclinum patella: NOE restrained molecular dynamics confirms the absolute stereochemistry derived by degradative methods. Tetrahedron. 2000;56:8345–8353. doi: 10.1016/S0040-4020(00)00746-8. [DOI] [Google Scholar]
- 66.Hawkins C.J., Lavin M.F., Marshall K.A., van den Brenk A.L., Watters D.J. Structure-activity relationships of the lissoclinaides: Cytotoxic cyclic peptides from the ascidian Lissoclinum patella. J. Med. Chem. 1990;33:1634–1638. doi: 10.1021/jm00168a016. [DOI] [PubMed] [Google Scholar]
- 67.Ireland C., Scheuer P.J. Ulicyclamide and ulithiacyclamide, two new small peptides from a marine tunicate. J. Am. Chem. Soc. 1980;102:5688–5691. doi: 10.1021/ja00537a053. [DOI] [Google Scholar]
- 68.Wipf P., Fritch P.C., Geib S.J., Sefler A.M. Conformational studies and structure-activity analysis of lissoclinamide 7 and related cyclopeptide alkaloids. J. Am. Chem. Soc. 1998;120:4105–4112. doi: 10.1021/ja973580h. [DOI] [Google Scholar]
- 69.Dalisay D.S., Rogers E.W., Edison A., Molinski T.F. Structure elucidation at the nanomole scale. 1. Trisoxazole macrolides and thiazole-containing cyclic peptides from the nudibranch Hexabranchus sanguineus. J. Nat. Prod. 2009;72:732–738. doi: 10.1021/np8007649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh E.K., Ramsey D.M., McAlpine S.R. Total synthesis of trans,trans-sanguinamide B and conformational isomers. Org. Lett. 2012;14:1198–1201. doi: 10.1021/ol203290n. [DOI] [PubMed] [Google Scholar]
- 71.Erickson K.L., Gustafson K.R., Milanowski D.J., Pannell L.K., Klose J.R., Boyd M.R. Myriastramides A–C, new modified cyclic peptides from the Philippines marine sponge Myriastra clavosa. Tetrahedron. 2003;59:10231–10238. doi: 10.1016/j.tet.2003.10.060. [DOI] [Google Scholar]
- 72.Guan L.L., Sera Y., Adachi K., Nishida F., Shizuri Y. Isolation and evaluation of nonsiderophore cyclic peptides from ma-rine sponges. Biochem. Biophys. Res. Commun. 2001;283:976–981. doi: 10.1006/bbrc.2001.4890. [DOI] [PubMed] [Google Scholar]
- 73.Sera Y., Adachi K., Fujii K., Shizuri Y. Isolation of haliclonamides: New peptides as antifouling substances from a marine sponge species, Haliclona. Mar. Biotechnol. 2002;4:441–446. doi: 10.1007/s10126-001-0082-6. [DOI] [PubMed] [Google Scholar]
- 74.Festa C., De Marino S., D’Auria M.V., Monti M.C., Bucci M., Vellecco V., Debitus C., Zampella A. Anti-inflammatory cyclopeptides from the marine sponge Theonella swinhoei. Tetrahedron. 2012;68:2851–2857. doi: 10.1016/j.tet.2012.01.097. [DOI] [Google Scholar]
- 75.Asano A., Yamada T., Taniguchi T., Sasaki M., Yoza K., Doi M. Ascidiacyclamides containing oxazoline and thiazole motifs assume square conformations and show high cytotoxicity. J. Pept. Sci. 2018;24:3120. doi: 10.1002/psc.3120. [DOI] [PubMed] [Google Scholar]
- 76.Fu X., Do T., Schmitz F.J., Andrusevich V., Engel M.H. New cyclic peptides from the ascidian Lissoclinum patella. J. Nat. Prod. 1998;61:1547–1551. doi: 10.1021/np9802872. [DOI] [PubMed] [Google Scholar]
- 77.Fu X., Su J.Y., Zeng L.M. Prepatellamide A, a new cyclic peptide from the ascidian Lissoclinum patella. Sci. China Ser. B. 2000;43:643–648. doi: 10.1007/BF02969512. [DOI] [Google Scholar]
- 78.Sesin D.F., Gaskell S.J., Ireland C.M. The chemistry of Lissoclinum patella. Bull. Soc. Chim. Belq. 1986;95:853–867. doi: 10.1002/bscb.19860950911. [DOI] [Google Scholar]
- 79.Nam S.J., Kauffman C., Jensen P.R., Moore C.E., Rheingold A.L., Fenical W. Actinobenzoquinoline and actinophenanthrolines A–C, unprecedented alkaloids from a marine actinobacterium. Org. Lett. 2015;17:3240–3243. doi: 10.1021/acs.orglett.5b01387. [DOI] [PubMed] [Google Scholar]
- 80.In Y., Doi M., Inoue M., Ishida T., Hamada Y., Shioiri T. Patellamide A, a cytotoxic cyclic peptide from the ascidian Lissoclinum patella. Acta Crystallogr. C. 1994;50:432–434. doi: 10.1107/S010827019300811X. [DOI] [PubMed] [Google Scholar]
- 81.Ireland C.M., Durso A.R., Newman R.A., Hacker M.P. Antineoplastic cyclic peptides from the marine tunicate Lissoclinum patella. J. Org. Chem. 1982;47:1807–1811. doi: 10.1021/jo00349a002. [DOI] [Google Scholar]
- 82.Williams A.B., Jacobs R.S. A marine natural product, patellamide D, reverses multidrug resistance in a human leukemic cell line. Cancer Lett. 1993;71:97–102. doi: 10.1016/0304-3835(93)90103-G. [DOI] [PubMed] [Google Scholar]
- 83.McDonald L.A., Ireland C.M. Patellamide E: a new cyclic peptide from the ascidian Lissoclinum patella. J. Nat. Prod. 1992;55:376–379. doi: 10.1021/np50081a016. [DOI] [PubMed] [Google Scholar]
- 84.Rashid M.A., Gustafson K.R., Cardellina J.H., Boyd M.R. Patellamide F, a new cytotoxic cyclic peptide from the colonial ascidian Lissoclinum patella. J. Nat. Prod. 1995;58:594–597. doi: 10.1021/np50118a020. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt E.W., Nelson J.T., Rasko D.A., Sudek S., Eisen J.A., Haygood M.G., Ravel J. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc. Natl. Acad. Sci. USA. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin J., Sousa T.D.S., Crespo G., Palomo S., González I., Tormo J.R., De La Cruz M., Anderson M., Hill R.T., Vicente F., et al. Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar. Drugs. 2013;11:387–398. doi: 10.3390/md11020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linares-Otoya L., Linares-Otoya V., Armas-Mantilla L., Blanco C., Crüsemann M., Ganoza-Yupanqui M.L., Campos-Florián J.V., König G.M., Schäberle T.F. Identification and heterologous expression of the kocurin biosynthetic gene cluster. Microbiology. 2017;163:1409–1414. doi: 10.1099/mic.0.000538. [DOI] [PubMed] [Google Scholar]
- 88.Matsuo Y., Kanoh K., Yamori T., Kasai H., Katsuta A., Adachi K., Shin-Ya K., Shizuri Y. Urukthapelstatin A, a novel cytotoxic substance from marine-derived Mechercharimyces asporophorigenens YM11-542. I. Fermentation, isolation and biological activities. J. Antibiot. 2007;60:251–255. doi: 10.1038/ja.2007.30. [DOI] [PubMed] [Google Scholar]
- 89.Schwenk S., Ronco C., Oberheide A., Arndt H.D. Biomimetic synthesis of urukthapelstatin A by Aza-Wittig ring contraction. Eur. J. Org. Chem. 2016;2016:4795–4799. doi: 10.1002/ejoc.201600994. [DOI] [Google Scholar]
- 90.Oberheide A., Schwenk S., Ronco C., Semmrau L.M., Görls H., Arndt H.-D. Synthesis, structure, and cytotoxicity of urukthapelstatin A polyazole cyclopeptide analogs. Eur. J. Org. Chem. 2019;2019:4320–4326. doi: 10.1002/ejoc.201900206. [DOI] [Google Scholar]
- 91.Engelhardt K., Degnes K.F., Zotchev S.B. Isolation and characterization of the gene cluster for biosynthesis of the thiopeptide antibiotic TP-1161. Appl. Environ. Microbiol. 2010;76:7093–7101. doi: 10.1128/AEM.01442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engelhardt K., Degnes K.F., Kemmler M., Bredholt H., Fjærvik E., Klinkenberg G., Sletta H., Ellingsen T.E., Zotchev S.B. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl. Environ. Microbiol. 2010;76:4969–4976. doi: 10.1128/AEM.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nogle L.M., Marquez A.B.L., Gerwick W.H. Wewakazole, a novel cyclic dodecapeptide from a Papua new guinea Lyngbya majuscula. Org. Lett. 2002;5:3–6. doi: 10.1021/ol026811k. [DOI] [PubMed] [Google Scholar]
- 94.Long B., Zhang J., Tang X., Wu Z. Total synthesis of wewakazole B. Org. Biomol. Chem. 2016;14:9712–9715. doi: 10.1039/C6OB01783E. [DOI] [PubMed] [Google Scholar]
- 95.Lindquist N., Fenical W., Van Duyne G.D., Clardy J. Isolation and structure determination of diazonamides A and B, unusual cytotoxic metabolites from the marine ascidian Diazona chinensis. J. Am. Chem. Soc. 1991;113:2303–2304. doi: 10.1021/ja00006a060. [DOI] [Google Scholar]
- 96.Fernández R., Martín M.J., Rodríguez-Acebes R., Reyes F., Francesch A., Cuevas C. Diazonamides C–E, new cytotoxic metabolites from the ascidian Diazona sp. Tetrahedron Lett. 2008;49:2283–2285. doi: 10.1016/j.tetlet.2008.02.012. [DOI] [Google Scholar]
- 97.Nicolaou K.C., Chen D.Y.-K., Huang X., Ling T., Bella M., Snyder S.A. Chemistry and biology of diazonamide A: First total synthesis and confirmation of the true structure. J. Am. Chem. Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i. [DOI] [PubMed] [Google Scholar]
- 98.Williams D.E., Moore R.E., Paul V.J. The structure of ulithiacyclamide B. Antitumor evaluation of cyclic peptides and macrolides from Ceratodictyon spongiosum. J. Nat. Prod. 1989;52:732–739. doi: 10.1021/np50064a011. [DOI] [PubMed] [Google Scholar]
- 99.Komatsu N., Nakauch H., Miwa A., Ishihara T., Eguchi M., Moroi M., Okada M., Sato Y., Wada H., Yawata Y., et al. Establishment and characterization of a human leukemic cell line with megakaryocytic features: Dependency on granulocyte-macrophage colony-stimulating factor, interleukin 3, or erythropoietin for growth and survival. Cancer Res. 1991;51:341–348. doi: 10.1093/ckj/sfs143. [DOI] [PubMed] [Google Scholar]
- 100.Degruyter J.N., Maio W.A. The taumycin A macrocycle: Asymmetric total synthesis and revision of relative stereochemistry. Org. Lett. 2014;16:5196–5199. doi: 10.1021/ol5025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tada H., Tozyo T., Terui Y., Hayashi F. Discokiolides. Cytotoxic cyclic depsipeptides from the marine sponge Discodermia kiiensis. Chem. Lett. 1992;21:431–434. doi: 10.1246/cl.1992.431. [DOI] [Google Scholar]
- 102.Molinski T.F. Absolute configuration of phorboxazoles A and B from the marine sponge, Phorbas sp. 2. C43 and complete stereochemistry. Tetrahedron Lett. 1996;37:7879–7880. doi: 10.1016/0040-4039(96)01804-7. [DOI] [Google Scholar]
- 103.Dalisay D.S., Molinski T.F. Structure elucidation at the nanomole scale. 2. Hemi-phorboxazole A from Phorbas sp. Org. Lett. 2009;11:1967–1970. doi: 10.1021/ol9004189. [DOI] [PubMed] [Google Scholar]
- 104.Sandler J.S., Colin P.L., Kelly M., Fenical W. Cytotoxic macrolides from a new species of the deep-water marine sponge Leiodermatium. J. Org. Chem. 2006;71:7245–7251. doi: 10.1021/jo060958y. [DOI] [PubMed] [Google Scholar]
- 105.Bishara A., Rudi A., Aknin M., Neumann D., Ben-Califa N., Kashman Y. Salarin C, a new cytotoxic sponge-derived nitrogenous macrolide. Tetrahedron Lett. 2008;49:4355–4358. doi: 10.1016/j.tetlet.2008.05.038. [DOI] [Google Scholar]
- 106.Bishara A., Rudi A., Aknin M., Neumann D., Ben-Califa N., Kashman Y. Salarins D–J, seven new nitrogenous macrolides from the Madagascar sponge Fascaplysinopsis sp. Tetrahedron. 2010;66:4339–4345. doi: 10.1016/j.tet.2010.04.035. [DOI] [Google Scholar]
- 107.Kobayashi J., Kondo K., Ishibashi M., Walchli M.R., Nakamura T. Theonezolide A: A novel polyketide natural product from the Okinawan marine sponge Theonella sp. J. Am. Chem. Soc. 1993;115:6661–6665. doi: 10.1021/ja00068a024. [DOI] [Google Scholar]
- 108.Kondo K., Ishibashi M., Kobayashi J. Isolation and structures of theonezolides B and C from the Okinawan marine sponge Theonella sp. Tetrahedron. 1994;50:8355–8362. doi: 10.1016/S0040-4020(01)85558-7. [DOI] [Google Scholar]
- 109.Rho M.C., Park Y.H., Sasaki S., Ishibashi M., Kondo K., Kobayashi J., Ohizumi Y. The mode of rabbit platelet shape change and aggregation induced by theonezolide-A, a novel polyketide macrolide, isolated from the Okinawan marine sponge Theonella sp. Can. J. Physiol. Pharmacol. 1996;74:193–199. doi: 10.1139/y95-235. [DOI] [PubMed] [Google Scholar]
- 110.Petchprayoon C., Asato Y., Higa T. Four new kabiramides from the Thai sponge, Pachastrissa nux. Heterocycles. 2006;69:447. doi: 10.1002/chin.200714225. [DOI] [Google Scholar]
- 111.Kobayashi J., Tsuda M., Fuse H., Sasaki T., Mikami Y. Halishigamides A−D, new cytotoxic oxazole-containing metabolites from Okinawan sponge Halichondria sp. J. Nat. Prod. 1997;60:150–154. doi: 10.1021/np960558d. [DOI] [Google Scholar]
- 112.Rashid M.A., Gustafson K.R., Cardellina J.H., Boyd M.R. Mycalolides D and E, new cytotoxic macrolides from a collection of the stony coral Tubastrea faulkneri. J. Nat. Prod. 1995;58:1120–1125. doi: 10.1021/np50121a025. [DOI] [PubMed] [Google Scholar]
- 113.Klenchin V.A., Allingham J.S., King R., Tanaka J., Marriott G., Rayment I. Trisoxazole macrolide toxins mimic the binding of actin-capping proteins to actin. Nat. Struct. Biol. 2003;10:1058–1063. doi: 10.1038/nsb1006. [DOI] [PubMed] [Google Scholar]
- 114.Roesener J.A., Scheuer P.J. Ulapualide A and B, extraordinary antitumor macrolides from nudibranch eggmasses. J. Am. Chem. Soc. 1986;108:846–847. doi: 10.1021/ja00264a052. [DOI] [Google Scholar]
- 115.Suo R., Takada K., Kohtsuka H., Ise Y., Okada S., Matsunaga S. Miuramides A and B, trisoxazole macrolides from a Mycale sp. marine sponge that induce a protrusion phenotype in cultured mammalian cells. J. Nat. Prod. 2018;81:1108–1112. doi: 10.1021/acs.jnatprod.8b00101. [DOI] [PubMed] [Google Scholar]
- 116.Matsunaga S., Liu P., Celatka C.A., Panek J.S., Fusetani N. Relative and absolute stereochemistry of mycalolides, bioactive macrolides from the marine sponge Mycale magellanica. J. Am. Chem. Soc. 1999;121:5605–5606. doi: 10.1021/ja990817w. [DOI] [Google Scholar]
- 117.Fusetani N., Yasumuro K., Matsunaga S., Hashimoto K. Mycalolides A-C, hybrid macrolides of ulapualides and halichon-dramide, from a sponge of the genus Mycale. Tetrahedron Lett. 1989;30:2809–2812. doi: 10.1016/S0040-4039(00)99131-7. [DOI] [Google Scholar]
- 118.Kobayashi J., Murata O., Shigemori H., Sasaki T. Jaspisamides A-C, new cytotoxic macrolides from the Okinawan sponge Jaspis sp. J. Nat. Prod. 1993;56:787–791. doi: 10.1021/np50095a021. [DOI] [PubMed] [Google Scholar]
- 119.Sirirak T., Kittiwisut S., Janma C., Yuenyongsawad S., Suwanborirux K., Plubrukarn A. Kabiramides J and K, trisoxazole macrolides from the sponge Pachastrissa nux. J. Nat. Prod. 2011;74:1288–1292. doi: 10.1021/np100886y. [DOI] [PubMed] [Google Scholar]
- 120.Youngsaye W., Lowe J.T., Pohlki F., Ralifo P., Panek J.S. Total synthesis and stereochemical reassignment of (+)-neopeltolide. Angew. Chem. Int. Ed. 2007;46:9211–9214. doi: 10.1002/anie.200704122. [DOI] [PubMed] [Google Scholar]
- 121.Custar D.W., Zabawa T.P., Hines J., Crews C.M., Scheidt K.A. Total synthesis and structure-activity investigation of the marine natural product neopeltolide. J. Am. Chem. Soc. 2009;131:12406–12414. doi: 10.1021/ja904604x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.D’Ambrosio M., Guerriero A., Debitus C., Pietra F. Leucascandrolide A, a new type of macrolide: The first powerfully bio-active metabolite of calcareous sponges (Leucascandra caveolata, a new genus from the coral sea) Helv. Chim. Acta. 1996;79:51–60. doi: 10.1002/hlca.19960790107. [DOI] [Google Scholar]
- 123.Ulanovskaya O.A., Janjic J., Suzuki M., Sabharwal S.S., Schumacker P.T., Kron S.J., Kozmin S.A. Synthesis enables identification of the cellular target of leucascandrolide A and neopeltolide. Nat. Chem. Biol. 2008;4:418–424. doi: 10.1038/nchembio.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oku N., Takada K., Fuller R.W., Wilson J.A., Peach M.L., Pannell L.K., McMahon J.B., Gustafson K.R. Isolation, structural elucidation, and absolute stereochemistry of enigmazole A, a cytotoxic phosphomacrolide from the Papua new guinea marine sponge Cinachyrella enigmatica. J. Am. Chem. Soc. 2010;132:10278–10285. doi: 10.1021/ja1016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sakurai K., Sakamoto K., Sasaki M., Fuwa H. Unified total synthesis of (-)-enigmazole A and (-)-15-O-Methylenigmazole A. Chem. Asian. J. 2020;15:3494–3502. doi: 10.1002/asia.202001015. [DOI] [PubMed] [Google Scholar]
- 126.Kato Y., Fusetani N., Matsunaga S., Hashimoto K., Fujita S., Furuya T. Bioactive marine metabolites. Part 16. Calyculin A. A novel antitumor metabolite from the marine sponge Discodermia calyx. J. Am. Chem. Soc. 1986;108:2780–2781. doi: 10.1021/ja00270a061. [DOI] [Google Scholar]
- 127.Matsunaga S., Wakimoto T., Fusetani N. Isolation of four new calyculins from the marine sponge Discodermia calyx. J. Org. Chem. 1997;62:9388. doi: 10.1021/jo9740251. [DOI] [PubMed] [Google Scholar]
- 128.Matsunaga S., Fujiki H., Sakata D., Fusetani N. ChemInform Abstract: Calyculins E, F, G, and H, Additional inhibitors of protein phosphatases 1 and 2A, from the marine sponge Discodermia calyx. Tetrahedron. 2010;22:2999–3006. doi: 10.1002/chin.199128245. [DOI] [Google Scholar]
- 129.Kehraus S., König G.M., Wright A.D. A new cytotoxic calyculinamide derivative, geometricin A, from the Australian sponge Luffariella geometrica. J. Nat. Prod. 2002;65:1056–1058. doi: 10.1021/np010544u. [DOI] [PubMed] [Google Scholar]
- 130.Matsunaga S., Wakimoto T., Fusetani N., Suganuma M. Isolation of dephosphonocalyculin a from the marine sponge, Discodermia calyx. Tetrahedron Lett. 1997;38:3763–3764. doi: 10.1016/S0040-4039(97)00748-X. [DOI] [Google Scholar]
- 131.Fu X., Schmitz F.J., Kelly-Borges M., McCready A.T.L., Holmes C.F.B. Clavosines A−C from the marine sponge Myriastra clavosa: Potent cytotoxins and inhibitors of protein phosphatases 1 and 2A. J. Org. Chem. 1998;63:7957–7963. doi: 10.1021/jo981249q. [DOI] [Google Scholar]
- 132.Hou S.Y., Zhang M.Y., Wang H.D., Zhang Y.X. Characterization of the biosynthesis gene cluster and oxazole ring formation enzyme for inthomycins in Streptomyces sp. strain SYP-A7193. Appl. Environ. Microbiol. 2020;86:1–44. doi: 10.1128/AEM.01388-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Webb M.R., Donald C., Taylor R.J.K. A general route to the Streptomyces-derived inthomycin family: the first synthesis of (+)-inthomycin B. Tetrahedron Lett. 2006;47:549–552. doi: 10.1016/j.tetlet.2005.11.042. [DOI] [Google Scholar]
- 134.Shin J., Lee H.S., Kim J.-Y., Shin H.J., Ahn J.W., Paul V.J. New macrolides from the sponge Chondrosia corticata. J. Nat. Prod. 2004;67:1889–1892. doi: 10.1021/np040124f. [DOI] [PubMed] [Google Scholar]
- 135.Higa T., Tanaka J.I., Kitamura A., Koyama T., Takahashia M., Uchida T. Bioactive compounds from marine sponges. Pure Appl. Chem. 1994;66:2227–2230. doi: 10.1351/pac199466102227. [DOI] [Google Scholar]
- 136.Ichiba T., Yoshida W.Y., Scheuer P.J., Higa T., Gravalos D.G. Hennoxazoles, bioactive bisoxazoles from a marine sponge. J. Am. Chem. Soc. 1991;113:3173–3174. doi: 10.1021/ja00008a056. [DOI] [Google Scholar]
- 137.Takaishi S., Tuchiya N., Sato A., Negishi T., Takamatsu Y., Matsushita Y., Watanabe T., Iijima Y., Haruyama H., Kinoshita T., et al. B-90063, a novel endothelin converting enzyme inhibitor isolated from a new marine bacterium, Blastobacter sp. SANK 71894. J. Antibiot. 1998;51:805–815. doi: 10.7164/antibiotics.51.805. [DOI] [PubMed] [Google Scholar]
- 138.Fernández R., Dherbomez M., Letourneux Y., Nabil M., Verbist A.J.F., Biard§ J.F. Antifungal metabolites from the marine sponge Pachastrissa sp.: New bengamide and bengazole derivatives. J. Nat. Prod. 1999;62:678–680. doi: 10.1021/np980330l. [DOI] [PubMed] [Google Scholar]
- 139.Mulder R.J., Shafer C.M., Dalisay D.S., Molinski T.F. Synthesis and structure–activity relationships of bengazole A analogs. Bioorganic Med. Chem. Lett. 2009;19:2928–2930. doi: 10.1016/j.bmcl.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao B.B., Guo H.J., Liu Y., Luo X.Y., Yang S.X., Wang Y.T., Leng X., Mo C.F., Zou Q. K313, a novel benzoxazole derivative, exhibits anti-inflammatory properties via inhibiting GSK3β activity in LPS-induced RAW264.7 macrophages. J. Cell Biochem. 2018;119:5382–5390. doi: 10.1002/jcb.26685. [DOI] [PubMed] [Google Scholar]
- 141.Demmer C.S., Bunch L. Benzoxazoles and oxazolopyridines in medicinal chemistry studies. Eur. J. Med. Chem. 2015;97:778–785. doi: 10.1016/j.ejmech.2014.11.064. [DOI] [PubMed] [Google Scholar]
- 142.Hwang I.H., Oh J., Zhou W., Park S., Kim J.H., Chittiboyina A., Ferreira D., Song G.Y., Oh S., Na M., et al. Cytotoxic activity of rearranged drimane meroterpenoids against colon cancer cells via down-regulation of β-catenin expression. J. Nat. Prod. 2015;78:453–461. doi: 10.1021/np500843m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J., Mu F.R., Jiao W.-H., Huang J., Hong L.L., Yang F., Xu Y., Wang S.P., Sun F., Lin H.W. Meroterpenoids with protein tyrosine phosphatase 1B inhibitory activity from a Hyrtios sp. marine sponge. J. Nat. Prod. 2017;80:2509–2514. doi: 10.1021/acs.jnatprod.7b00435. [DOI] [PubMed] [Google Scholar]
- 144.Daletos G., deVoogd N.J., Müller W.E.G., Wray V., Lin W., Feger D., Kubbutat M., Aly A.H., Proksch P. Cytotoxic and protein kinase inhibiting nakijiquinones and nakijiquinols from the sponge Dactylospongia metachromia. J. Nat. Prod. 2014;77:218–226. doi: 10.1021/np400633m. [DOI] [PubMed] [Google Scholar]
- 145.Ovenden S.P.B., Nielson J.L., Liptrot C.H., Willis R.H., Tapiolas D.M., Wright A.D., Motti C.A. Sesquiterpene benzoxazoles and sesquiterpene quinones from the marine sponge Dactylospongia elegans. J. Nat. Prod. 2011;74:65–6810. doi: 10.1021/np100669p. [DOI] [PubMed] [Google Scholar]
- 146.Takeda Y., Nakai K., Narita K., Katoh T. A novel approach to sesquiterpenoid benzoxazole synthesis from marine sponges: Nakijinols A, B and E–G. Org. Biomol. Chem. 2018;16:3639–3647. doi: 10.1039/C8OB00721G. [DOI] [PubMed] [Google Scholar]
- 147.Sun M., Zhang X., Hao H., Li W., Lu C. Nocarbenzoxazoles A-G, benzoxazoles produced by halophilic Nocardiopsis lucen-tensis DSM 44048. J. Nat. Prod. 2015;78:2123–2127. doi: 10.1021/acs.jnatprod.5b00031. [DOI] [PubMed] [Google Scholar]
- 148.Kim T., Lee S.A., Noh T., Choi P., Choi S.J., Song B.G., Kim Y., Park Y.T., Huh G., Kim Y.J., et al. Synthesis, structure revision, and cytotoxicity of nocarbenzoxazole G. J. Nat. Prod. 2019;82:1325–1330. doi: 10.1021/acs.jnatprod.9b00072. [DOI] [PubMed] [Google Scholar]
- 149.Venkateswarlu Y., Reddy N.S., Ramesh P., Rao J.V. Coixol: A bioactive principle from a marine sponge Oceanapia sp. Biochem. Syst. Ecol. 1999;27:519–520. doi: 10.1016/S0305-1978(98)00117-3. [DOI] [Google Scholar]
- 150.Rodríguez I.I., Rodríguez A.D. Homopseudopteroxazole, a new antimycobacterial diterpene alkaloid from Pseudopterogorgia elisabethae. J. Nat. Prod. 2003;66:855–857. doi: 10.1021/np030052c. [DOI] [PubMed] [Google Scholar]
- 151.Rodriguez A.D., Ramıirez C., Rodriguez I.I., Gonzalez E. Novel antimycobacterial benzoxazole alkaloids, from the west Indian sea whip Pseudopterogorgia elisabethae. Org. Lett. 1999;1:527–530. doi: 10.1021/ol9907116. [DOI] [PubMed] [Google Scholar]
- 152.Georgantea P., Ioannou E., Evain-Bana E., Bagrel D., Martinet N., Vagias C., Roussis V. Sesquiterpenes with inhibitory activity against CDC25 phosphatases from the soft coral Pseudopterogorgia rigida. Tetrahedron. 2016;72:3262–3269. doi: 10.1016/j.tet.2016.04.059. [DOI] [Google Scholar]
- 153.McCulloch M.W.B., Berrue F., Haltli B., Kerr R.G. One-Pot syntheses of pseudopteroxazoles from pseudopterosins: A rapid route to non-natural congeners with improved antimicrobial activity. J. Nat. Prod. 2011;74:2250–2256. doi: 10.1021/np2006555. [DOI] [PubMed] [Google Scholar]
- 154.Zhang X., Fang X., Xu M., Lei Y., Wu Z., Hu X. Enantioselective total synthesis of pseudopteroxazole and ileabethoxazole. Angew. Chem. Int. Ed. 2019;58:7845–7849. doi: 10.1002/anie.201901651. [DOI] [PubMed] [Google Scholar]
- 155.Genta-Jouve G., Francezon N., Puissant A., Auberger P., Vacelet J., Pérez T., Fontana A., Al Mourabit A., Thomas O.P. Structure elucidation of the new citharoxazole from the Mediterranean deep-sea sponge Latrunculia (Biannulata) citharistae. Magn. Reson. Chem. 2011;49:533–536. doi: 10.1002/mrc.2772. [DOI] [PubMed] [Google Scholar]
- 156.Julianti E., Lee J.H., Liao L., Park W., Park S., Oh D.C., Oh K.B., Shin J. New polyaromatic metabolites from a marine-derived fungus Penicillium sp. Org. Lett. 2013;15:1286–1289. doi: 10.1021/ol4002174. [DOI] [PubMed] [Google Scholar]
- 157.Takahashi Y., Kubota T., Shibazaki A., Gonoi T., Fromont J., Kobayashi J. Nakijinamines C-E, new heteroaromatic alkaloids from the sponge Suberites species. Org. Lett. 2011;13:3016–3019. doi: 10.1021/ol2008473. [DOI] [PubMed] [Google Scholar]
- 158.Hohmann C., Schneider K., Bruntner C., Irran E., Nicholson G., Bull A.T., Jones A.L., Brown R., Stach J.E.M., Goodfellow M., et al. Caboxamycin, a new antibiotic of the benzoxazole family produced by the deep-sea strain Streptomyces sp. NTK 937. J. Antibiot. 2009;62:99–104. doi: 10.1038/ja.2008.24. [DOI] [PubMed] [Google Scholar]
- 159.Tagawa Y., Koba H., Tomoike K., Sumoto K. Synthesis of caboxamycin and its derivatives using eco-friendly oxidation. Heterocycles. 2011;83:867. doi: 10.3987/COM-11-12138. [DOI] [Google Scholar]
- 160.Losada A.A., Cano-Prieto C., Garcia-Salcedo R., Brana A.F., Mendez C., Salas J.A., Olano C. Caboxamycin biosynthesis pathway and identification of novel benzoxazoles produced by cross-talk in Streptomyces sp. NTK 937. Microb. Biotechnol. 2017;10:873–885. doi: 10.1111/1751-7915.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dattelbaum J.D., Singh A.J., Field J.J., Miller J.H., Northcote P.T. The nitrogenous hamigerans: Unusual amino acid-derivatized aromatic diterpenoid metabolites from the New Zealand marine sponge Hamigera tarangaensis. J. Org. Chem. 2015;80:304–312. doi: 10.1021/jo502370b. [DOI] [PubMed] [Google Scholar]
- 162.Liu L.L., Xu Y., Han Z., Li Y.X., Lu L., Lai P.Y., Zhong J.L., Guo X.R., Zhang X.X., Qian P.Y. Four new antibacterial xanthones from the marine-derived actinomycetes Streptomyces caelestis. Mar. Drugs. 2012;10:2571–2583. doi: 10.3390/md10112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Susic D., Djordjevic N., Kentera D. Hemodynamic effects of two ergot derivatives in the conscious spontaneously hyper-tensive rat. Pharmacology. 1984;29:215–223. doi: 10.1159/000138016. [DOI] [PubMed] [Google Scholar]
- 164.Zhang H., Zhao Z., Wang H. Cytotoxic natural products from marine sponge-derived microorganisms. Mar. Drugs. 2017;15:68. doi: 10.3390/md15030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kamat S., Kumari M., Taritla S., Jayabaskaran C. Endophytic fungi of marine alga from Konkan coast, India—A rich source of bioactive material. Front. Mar. Sci. 2020;7:1–16. doi: 10.3389/fmars.2020.00031. [DOI] [Google Scholar]
- 166.Chen S., Cai R., Liu Z., Cui H., She Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2021 doi: 10.1039/D1NP00041A. [DOI] [PubMed] [Google Scholar]
- 167.Ramesh C., Tulasi B.R., Raju M., Thakur N., Dufosse L. Marine natural products from tunicates and their associated mi-crobes. Mar. Drugs. 2021;19:308. doi: 10.3390/md19060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Atanasov A.G., Zotchev S.B., Dirsch V.M., the International Natural Product Sciences Taskforce. Supuran C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detail information for all 1,3-oxazole-containing alkaloids described in this work is available at Table S1.