Abstract
Hypoxia-inducible factor 1 (HIF-1) is a transcriptional activator composed of HIF-1α and HIF-1β subunits. Several dozen HIF-1 targets are known, including the gene encoding vascular endothelial growth factor (VEGF). Under hypoxic conditions, HIF-1α expression increases as a result of decreased ubiquitination and degradation. The tumor suppressors VHL (von Hippel-Lindau protein) and p53 target HIF-1α for ubiquitination such that their inactivation in tumor cells increases the half-life of HIF-1α. Increased phosphatidylinositol 3-kinase (PI3K) and AKT or decreased PTEN activity in prostate cancer cells also increases HIF-1α expression by an undefined mechanism. In breast cancer, increased activity of the HER2 (also known as neu) receptor tyrosine kinase is associated with increased tumor grade, chemotherapy resistance, and decreased patient survival. HER2 has also been implicated as an inducer of VEGF expression. Here we demonstrate that HER2 signaling induced by overexpression in mouse 3T3 cells or heregulin stimulation of human MCF-7 breast cancer cells results in increased HIF-1α protein and VEGF mRNA expression that is dependent upon activity of PI3K, AKT (also known as protein kinase B), and the downstream kinase FRAP (FKBP-rapamycin-associated protein). In contrast to other inducers of HIF-1 expression, heregulin stimulation does not affect the half-life of HIF-1α but instead stimulates HIF-1α synthesis in a rapamycin-dependent manner. The 5′-untranslated region of HIF-1α mRNA directs heregulin-inducible expression of a heterologous protein. These data provide a molecular basis for VEGF induction and tumor angiogenesis by heregulin-HER2 signaling and establish a novel mechanism for the regulation of HIF-1α expression.
Angiogenesis is essential for tumorigenesis as well as metastasis (11, 16, 64), and vascular density is an important prognostic factor in breast cancer (19, 27, 58, 59). Vascular endothelial growth factor (VEGF) plays a major role in tumor angiogenesis (10), and its expression in breast cancer is inversely correlated with patient survival (29, 30). VEGF expression can be induced by exposure of tumor cells to hypoxia or growth factors and, in both cases, this expression is due in part to increased VEGF gene transcription that is mediated by hypoxia-inducible factor 1 (HIF-1) (6, 9, 12, 22, 44, 63, 65).
HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits (56, 57). Whereas HIF-1β is constitutively expressed, the expression and activity of the HIF-1α subunit are induced by exposure of cells to hypoxia or growth factors (reviewed in reference 49). HIF-1 activates the transcription of genes whose products are required for critical aspects of tumor progression including angiogenesis (plasminogen activator inhibitor 1 and VEGF), iron homeostasis (transferrin and transferrin receptor), and metabolic adaptation (glucose transporters and glycolytic enzymes), as well as several factors that affect tumor cell survival or proliferation (endothelin 1, inducible nitric oxide synthase, and insulin-like growth factor 2).
HIF-1α is overexpressed in primary and metastatic human tumors (1, 4, 5, 53, 62, 66). In breast cancer, HIF-1α overexpression can be detected in ductal carcinoma in situ but not in benign ductal hyperplasia (5), i.e., in early-stage cancer prior to invasion but concomitant with increased angiogenesis (15). HIF-1 activity is increased both by intratumoral hypoxia and by genetic alterations, including loss-of-function mutations in the tumor suppressor genes encoding p53, PTEN, and VHL (von Hippel-Lindau protein) as well as gain-of-function mutations in oncogenes that activate the phosphatidylinositol 3-kinase (PI3K), SRC, and mitogen-activated protein (MAP) kinase signal-transduction pathways (24, 34, 40, 41, 47, 48, 65, 66, 68). Loss or gain of HIF-1 activity is negatively and positively correlated, respectively, with tumor growth and angiogenesis in xenograft assays (6, 24, 28, 33, 40, 44, 45).
Among the genetic alterations identified in human breast cancer, one of the most important is the increased activity of the HER2 receptor tyrosine kinase encoded by the ERBB2 gene on chromosome 17q21, which occurs in approximately one-third of breast tumors and is associated with increased tumor grade, chemotherapy resistance, and decreased rates of patient survival (36, 43, 50, 51). Overexpression of HER2 transforms human mammary epithelial and mouse 3T3 cells and imparts resistance against the chemotherapeutic agents tamoxifen and Taxol (32, 39, 61). Treatment of breast cancer cells with a neutralizing antibody against HER2 results in a dose-dependent inhibition of VEGF expression (38). A humanized monoclonal antibody to HER2 inhibits breast cancer growth and has been approved for treatment of HER2-overexpressing tumors (35). Unlike other members of the epidermal growth factor receptor (EGFR) family, HER2 has tyrosine kinase activity in the absence of any known ligand. HER2 heterodimerizes with the EGFR family members HER3 and HER4, which bind the ligand heregulin (55). In breast cancer cells, heregulin activates AKT (also known as protein kinase B) via the PI3K pathway (31). HER2 overexpression is also associated with increased AKT activity (67). Recently, HER2 overexpression or heregulin stimulation has been shown to induce VEGF mRNA and protein expression in cancer cell lines (3, 60).
Because HIF-1 has been shown to lie downstream of EGFR and PI3K-AKT signaling and upstream of VEGF expression in tumor cells (9, 65, 68), we have analyzed HIF-1 activity in HER2-overexpressing 3T3 cells and heregulin-stimulated MCF-7 cells. Our results indicate that HIF-1 contributes to the induction of VEGF expression in these cells. Because hypoxia (52) and mutations in the tumor suppressor genes VHL (7, 54) and p53 (40) induce HIF-1 activity by inhibiting the ubiquitination and proteasomal degradation of HIF-1α (20, 26, 46), it was assumed that receptor tyrosine kinase signaling induced HIF-1α expression by the same mechanism. However, our results demonstrate that HER2 signaling induces HIF-1α protein synthesis rather than inhibiting its degradation, thus representing a novel mechanism for the regulation of HIF-1 and VEGF expression.
MATERIALS AND METHODS
Materials.
AG825, cycloheximide, LY294002, PD098059, and rapamycin were purchased from Calbiochem. Heregulin-α was from Oncogene Research Products and Sigma. Anti-HIF-1α monoclonal antibody H1α67 (66) was from Novus Biologicals, Inc. Anti-AKT, anti-phospho-AKT (phosphoserine-473), anti-p70 s6 kinase (anti-p70s6k), anti-phospho-p70s6k (phosphothreonine-389), and anti-4E-BP1 (phosphoserine-65) antibodies were from New England Biolabs. Plasmid pCMV5/AKT(K179M) was a gift from Tung Chan, Thomas Jefferson University. The rat HER2 cDNA in pcDNA3 and 3T3/NEU cells (ATCC CRL-1915) were gifts from Elizabeth Jaffee, Johns Hopkins Oncology Center. MCF-7 cells were a gift from Dominic Scudiero, Developmental Therapeutics Program, National Cancer Institute.
Tissue culture.
NIH 3T3 cells were cultured in high-glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS), 1% glutamine, 1% pyruvate, 1% nonessential amino acids, and penicillin-streptomycin. 3T3/neu cells (21) were cultured in the same medium with the addition of 125 ng of methotrexate (Sigma)/ml. MCF-7 cells were cultured in high-glucose RPMI 1640 supplemented with 10% FBS and penicillin-streptomycin. Unless otherwise stated, cells were maintained at 37°C in a humidified 5% CO2–95% air incubator.
Immunoblot and electrophoretic mobility shift assays.
MCF-7 cells were serum starved for 20 h in medium lacking FBS and then exposed to FBS, kinase inhibitors, and/or heregulin for 6 h. 3T3 and 3T3/neu cells were exposed to inhibitors and/or hypoxia (1% O2–5% CO2–94% N2) for 6 h. Thirty or 100 μg of nuclear (57) or whole-cell (52) extracts, respectively, was fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and analyzed with anti-HIF-1α mouse monoclonal antibody H1α67 at a 1:1,000 dilution (66), affinity-purified anti-HIF-1β rabbit polyclonal antibodies at a 1:2,000 dilution (56), or anti-AKT, anti-phospho-AKT, anti-p70s6k, anti-phospho-p70s6k, and anti-phospho-4E-BP1 rabbit polyclonal antibodies at a 1:1,000 dilution. Horseradish peroxidase-conjugated rabbit anti-mouse and goat anti-rabbit secondary antibodies were used at a 1:2,500 dilution. The signal was developed with ECL reagents (Amersham). Electrophoretic mobility shift assays were performed on nuclear extracts as previously described (57).
Transfection assays.
Plasmid pSV-Renilla was constructed by replacing the HindIII/BglII fragment containing the thymidine kinase promoter of TK-Renilla (Promega) with the HindIII/BglII fragment containing the sivian virus 40 (SV40) promoter from pGL2-Promoter (Promega). A total of 4 × 104 3T3 cells were plated per well of a 24-well dish and 24 h later were transfected with 20 ng of pSV-Renilla; 200 ng of reporter p2.1, p2.4 (24), or pVEGF-KpnI (12); and 200 ng of expression vector pCMV5/AKT(K179M) encoding kinase-dead AKT (13), pcDNA3/HER2neu encoding rat HER2, or empty vector, in the presence of Fugene-6 (Boehringer Mannheim). Lysates were prepared 48 h after transfection. A total of 2 × 104 MCF-7 cells were plated per well and transfected with 10 ng of pSV-Renilla and 100 ng of pG5E1bLuc (plus 100 ng of pGalA or pGal0) (25) or 100 ng of p2.1 or p2.4, with Lipofectamine Plus (Life Technologies, Inc.). Twenty hours after transfection the cells were serum starved for 20 h and then treated with 10% FBS, 100 ng of heregulin-α/ml, or FBS plus heregulin for 24 h. 3T3 and MCF-7 cell lysates were analyzed with the Dual Luciferase Reporter Assay system (Promega). Relative luciferase expression was determined as the ratio of firefly to Renilla luciferase activity. Transfections were performed in triplicate, and the mean and standard error were calculated for each condition.
RNA blot-hybridization assays.
Cells were plated at 50% confluence and 24 h later were exposed to 1% O2 or heregulin for 16 h. Total RNA was isolated using TRIzol reagent (Life Technologies, Inc.), and 15-μg aliquots were fractionated by agarose gel electrophoresis, transferred to nitrocellulose membranes, and hybridized with a 32P-labeled human (GenBank accession number H95344; IMAGE clone 234423 [Research Genetics]) or rat (12) VEGF cDNA fragment or a PCR product encoding amino acids 330 to 528 of human HIF-1α that was amplified from cDNA clone pBluescriptSK-HIF-1α3.2-3 (56).
Pulse-chase assays.
A total of 2 × 106 MCF-7 cells were plated in a 10-cm dish, and 24 h later the cells were serum starved for 20 h. The cells were pretreated with 100 ng of heregulin-α/ml, 100 μM cobalt chloride, or 100 nM rapamycin for 30 min in methionine-free RPMI 1640. [35S]Met-Cys was added to a final concentration of 0.3 mCi/ml, and the cells were pulse-labeled for 20 to 40 min and then harvested. To chase, the cells were rinsed after pulse-labeling, incubated in the presence of media containing nonradioactive Met-Cys for 25 min in the presence or absence of heregulin, and then harvested. Whole-cell extracts were prepared with RIPA buffer (52). One milligram of extract was precleared with 60 μl of protein A-Sepharose for 1 h. Twenty microliters of anti-HIF-1α antibody H1α67 was added to the supernatant and rotated overnight at 4°C. Forty microliters of protein A-Sepharose was added, rotated for 2 h at 4°C, pelleted, and washed five times with 1 ml of RIPA buffer. An equal volume of 2× SDS loading buffer was added, and the samples were boiled and fractionated by SDS-polyacrylamide gel electrophoresis (52). The gel was dried and exposed to X-ray film.
HIF1A reporter assays.
Nucleotides −572 to +284 and −572 to +32 from the human HIF1A gene were amplified from plasmid PAC-RI (23) by PCR with Platinum Taq DNA Polymerase High Fidelity (Life Technologies) and ligated into pGL2-Basic (Promega). MCF-7 cells were seeded onto 24-well plates at 4 × 104 cells/well and the following day were exposed to 200 ng of pGL2/HIF1A(−572/+284) or pGL2/HIF1A(−572/+32) and 40 ng of pSV-Renilla for 3 h in the presence of Lipofectamine Plus (Life Technologies, Inc.). After 24 h, the cells were serum starved for 24 h, treated with 100 ng of heregulin-α/ml for 22 h, and harvested for firefly and Renilla luciferase activity with the Dual Luciferase Reporter system (Promega). Duplicate wells were washed with phosphate-buffered saline and lysed in TRIzol (Life Technologies), and total RNA was extracted. Three micrograms of total RNA from each well was used as a template for reverse transcription (RT) of cDNA with the Superscript Preamplification system (Life Technologies). Firefly and Renilla luciferase cDNA sequences were amplified by PCR (primer sequences and PCR conditions available upon request), and the products were analyzed by 1.5% agarose gel electrophoresis and ethidium bromide staining.
RESULTS
HIF-1α protein and VEGF mRNA levels increase in response to HER2 overexpression.
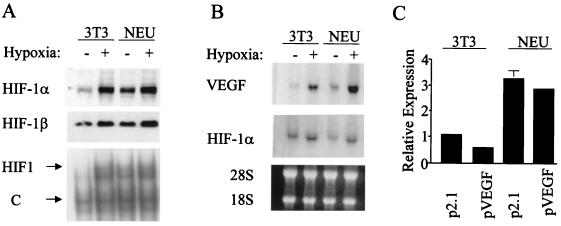
Stable transfectants of mouse NIH 3T3 cells expressing HER2 (21) and parental 3T3 cells were incubated under nonhypoxic and hypoxic conditions (20 and 1% O2, respectively), and nuclear extracts were prepared. The control 3T3 cells demonstrated modest levels of HIF-1α protein (Fig. 1A, top) and HIF-1 DNA-binding activity (Fig. 1A, bottom) under nonhypoxic conditions and a marked induction in response to hypoxia. The expression of HIF-1α protein and HIF-1 DNA-binding activity was significantly higher in the subclone overexpressing HER2 (hereafter referred to as 3T3/NEU cells) under nonhypoxic conditions. HER2 overexpression was also associated with increased VEGF mRNA expression (Fig. 1B, top). HER2 overexpression did not affect HIF-1α mRNA levels (Fig. 1B, middle), indicating that the increased expression of HIF-1α protein in 3T3/NEU cells was due to either increased synthesis or decreased degradation.
FIG. 1.
Effect of HER2 overexpression on HIF-1 activity and VEGF mRNA levels. (A) Analysis of HIF-1 expression and activity. Mouse NIH 3T3 and 3T3/neu (NEU) cells were incubated in complete media under hypoxic (1% O2) or nonhypoxic (20% O2) conditions for 6 h prior to nuclear extract preparation. Aliquots were subjected to immunoblot assay using anti-HIF-1α (top) or anti-HIF-1β (middle) antibodies. An electrophoretic mobility shift assay (bottom) was also performed which detected HIF-1 and constitutively expressed (C) DNA-binding activities. (B) Analysis of VEGF and HIF-1α RNA expression. Cells were incubated under hypoxic or nonhypoxic conditions for 16 h prior to total RNA preparation. Aliquots were fractionated by agarose gel electrophoresis and subjected to serial blot hybridization using rat VEGF (top) and human HIF-1α (middle) cDNA probes. The agarose gel was stained with ethidium bromide prior to transfer to demonstrate equivalent quantity and quality of RNA (28S and 18S rRNA species are indicated) in each lane (bottom). (C) Analysis of HIF-1 transcriptional activity. 3T3 and NEU cells were cotransfected with control reporter pSV-Renilla, containing Renilla luciferase coding sequences under the control of an SV40 promoter, and p2.1, which contains a 68-bp HRE from the human ENO1 gene inserted upstream of a minimal SV40 promoter and firefly luciferase coding sequences, or pVEGF, which contains a 2.7-kb human VEGF promoter fragment inserted upstream of firefly luciferase coding sequences. Luciferase activities were measured 48 h after transfection. For each condition, the ratio of firefly to Renilla luciferase was determined and normalized to the value obtained for 3T3 cells transfected with p2.1 (Relative Expression).
To determine whether the effect of HER2 on VEGF expression was mediated by HIF-1-dependent gene transcription, 3T3 and 3T3/NEU cells were transfected with reporter genes in which luciferase expression was driven either by the human VEGF promoter encompassing nucleotides −2274 to +379 relative to the transcription start site (pVEGF) or by a 68-bp HIF-1-dependent hypoxia-response element (HRE) cloned upstream of a basal SV40 promoter (p2.1). The expression of both reporters was increased approximately threefold in 3T3/NEU cells relative to that in 3T3 cells under nonhypoxic conditions (Fig. 1C). These results indicate that HER2 overexpression induces HIF-1-mediated VEGF gene transcription.
Heregulin stimulation of MCF-7 cells specifically induces HIF-1α protein expression.
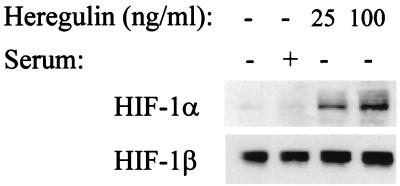
Heregulin stimulation of MCF-7 cells induces AKT activity via signaling of HER2-HER3 heterodimers (31). HIF-1α protein expression was induced in a dose-dependent manner by exposure of serum-starved MCF-7 cells to heregulin-α (Fig. 2). The induction of HIF-1α did not simply represent a nonspecific response of serum-starved cells to growth factor, since refeeding serum did not induce a comparable level of HIF-1α expression (Fig. 2, lane 2; see also Fig. 4B). VEGF mRNA expression was also induced by heregulin-α stimulation of MCF-7 cells (data not shown), similar to results recently reported for heregulin-β stimulation of MCF-7 and other cancer cell lines (3, 60).
FIG. 2.
Effect of heregulin stimulation on HIF-1α expression. Human MCF-7 breast cancer cells were serum starved for 20 h and then exposed to no treatment, 10% FBS (serum), or 25 to 100 ng of heregulin/ml for 6 h, prior to nuclear extract preparation and immunoblot assay using anti-HIF-1α (top) or anti-HIF-1β (bottom) antibodies.
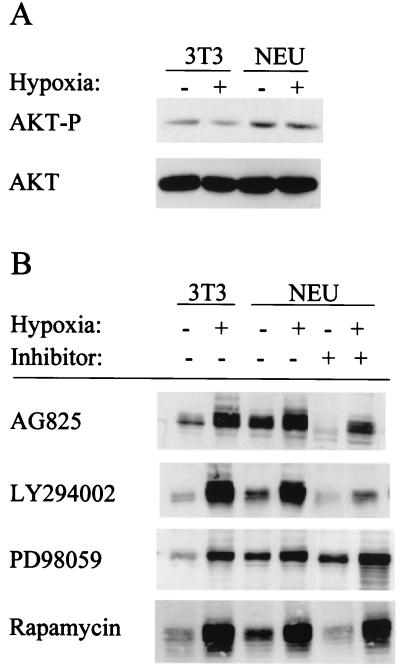
FIG. 4.
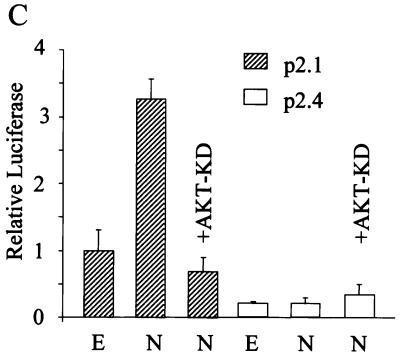
Involvement of PI3K and AKT in signaling from HER2 to HIF-1α. (A) Analysis of AKT activity. 3T3 and 3T3/NEU (NEU) cells were incubated under nonhypoxic or hypoxic conditions, and cell lysates were prepared. Aliquots were subjected to immunoblot assay with antibodies that recognize only phospho-AKT (top) or total AKT (bottom). (B) Effect of kinase inhibitors on HIF-1α expression. 3T3 and NEU cells were pretreated with no drug, 100 μM AG825, 100 μM LY294002, 100 μM PD098059, or 100 nM rapamycin for 30 min and incubated under nonhypoxic or hypoxic conditions for 6 h prior to HIF-1α immunoblot assay. (C) Effect of dominant-negative AKT on HER2-induced HIF-1 transcriptional activity. 3T3 cells were cotransfected with pSV-Renilla; wild-type p2.1 or mutant p2.4 reporter gene; and either empty vector (E) or expression vector encoding HER2 (N) in the presence or absence of expression vector encoding kinase-dead AKT (AKT-KD). Cell lysates were subjected to dual luciferase assays.
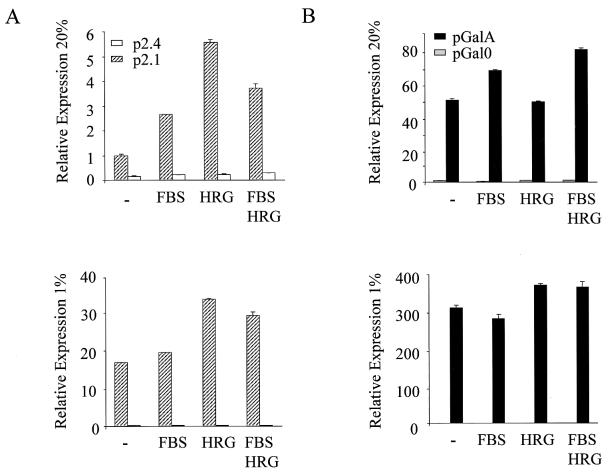
Treatment of serum-starved MCF-7 cells with heregulin stimulated transcription of the HRE-containing p2.1 reporter gene fivefold under nonhypoxic conditions, and this effect was dependent upon the presence of an intact HIF-1 binding site in the reporter (Fig. 3A). Heregulin also stimulated reporter gene expression twofold under hypoxic conditions (Fig. 3A). The degree of reporter gene expression in response to hypoxia (17-fold) was considerably greater than in response to heregulin stimulation (Fig. 3A). Treatment of serum-starved cells with FBS resulted in an even more modest stimulation of reporter gene expression under nonhypoxic conditions (<3-fold) and had no effect under hypoxic conditions.
FIG. 3.
Effect of heregulin stimulation on HIF-1 transcriptional activity. Transfected cells were serum starved and exposed to no growth factor (−), 10% FBS, heregulin (HRG) or FBS and HRG for 24 h, under either nonhypoxic (20% O2) (top panels) or hypoxic (1% O2) (bottom panels) culture conditions, prior to preparation of cell lysates for dual luciferase assays. (A) Analysis of HIF-1-mediated reporter gene transcription. MCF-7 cells were cotransfected with pSV-Renilla and p2.1 or p2.4, which contain a wild-type and mutated HRE, respectively. The ratio of firefly to Renilla luciferase expression was determined and normalized to the value obtained from nonhypoxic cells transfected with p2.1 (Relative Expression). (B) Analysis of HIF-1α transactivation domain function. MCF-7 cells were transfected with: pSV-Renilla; pG5E1bLuc, which contains five copies of a GAL4 DNA-binding site upstream of the Elb promoter and firefly luciferase coding sequences; and either pGal0 or pGalA, which encodes the GAL4 DNA-binding domain either alone or fused to HIF-1α amino acids 531 to 826, respectively. The ratio of firefly to Renilla luciferase expression was determined and normalized to the value obtained from nonhypoxic cells transfected with pGal0 (Relative Expression).
Previous studies have demonstrated that a fusion protein (GalA) consisting of the DNA-binding domain of the yeast protein GAL4 fused to HIF-1α amino acids 531 to 826 activates transcription of a luciferase reporter gene containing five GAL4 binding sites upstream of a basal promoter due to the presence of two transactivation domains, TAD-N (residues 531 to 575) and TAD-C (residues 786 to 826) (25). Furthermore, reporter gene transactivation is mediated by GalA in a hypoxia-inducible manner, as demonstrated in Fig. 3B. In contrast, heregulin stimulation of MCF-7 cells did not affect transactivation mediated by GalA under nonhypoxic or hypoxic conditions (Fig. 3B). FBS, in either the presence or absence of heregulin, also did not stimulate reporter gene transactivation mediated by GalA. Taken together, the results presented in Fig. 1 to 3 demonstrate that, like hypoxia, heregulin-HER2 signaling induces expression of HIF-1α protein and HIF-1 DNA-binding activity. HER2 signaling under nonhypoxic conditions induces more modest expression of VEGF mRNA or a HIF-1-dependent reporter gene because whereas hypoxia also induces the HIF-1α transactivation domain function, heregulin does not. However, HER2 signaling also amplifies HIF-1-mediated gene transcription under hypoxic conditions.
HER2- and heregulin-induced HIF-1α expression requires PI3K, AKT, and FRAP activity.
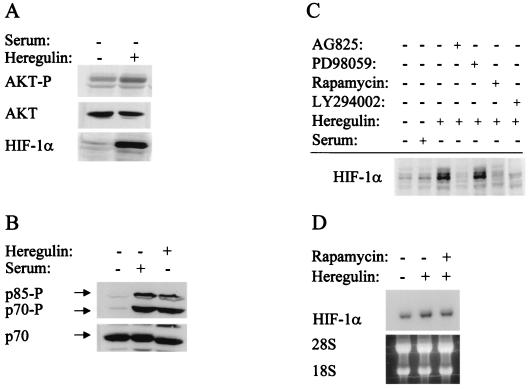
AKT activity, as measured by phosphorylation at Ser-473, was increased in HER2-overexpressing 3T3 cells (Fig. 4A) as previously reported (67). Hypoxia did not induce AKT activity in 3T3 or 3T3/NEU cells, in agreement with previous studies of prostate cancer cells (65). To determine whether activity of the PI3K-AKT pathway was required for HER2 signaling to HIF-1α, 3T3/NEU cells were treated with AG825, LY294002, or rapamycin, which inhibits the kinase activity of HER2, PI3K, FKBP-rapamycin-associated protein (FRAP; also known as a mammalian target of rapamycin), respectively. Treatment with AG825, LY294002, or rapamycin was associated with decreased HIF-1α expression, particularly under nonhypoxic conditions (Fig. 4B, compare lanes 3 and 5). In contrast to the effects of these inhibitors, PD098059, at concentrations that effectively inhibit the MAP kinase kinase MEK1, did not alter HIF-1α expression in 3T3/NEU cells. Expression of the HIF-1-dependent reporter gene p2.1 was stimulated by cotransfection of a HER2 expression vector in 3T3 cells, and this effect was blocked by cotransfection of a vector encoding a dominant-negative (kinase-dead) form of AKT (Fig. 4C). Neither the stimulatory effect of HER2 nor the inhibitory effect of kinase-dead AKT on reporter gene transcription was observed when the reporter (p2.4) contained an HRE that was mutated to prevent HIF-1 binding.
AKT activity was also increased by heregulin stimulation of serum-starved MCF-7 cells as demonstrated by an increase in AKT phosphorylation at Ser-473 (Fig. 5A, top). The induction of HIF-1α protein expression by heregulin (Fig. 5A, bottom) occurred without any change in HIF-1α mRNA expression (Fig. 5D), indicating an effect on HIF-1α protein synthesis or stability. Treatment of serum-starved MCF-7 cells with serum or heregulin induced phosphorylation of p70s6k, a downstream target of FRAP (Fig. 5B). The translational regulatory protein 4E-BP1 (also known as PHAS1), another downstream target of FRAP, showed a similar pattern of phosphorylation (data not shown). Heregulin-induced expression of HIF-1α was blocked by AG825, LY294002, and rapamycin, but not by PD098059 (Fig. 5C). Rapamycin blocked heregulin-induced HIF-1α protein expression without altering HIF-1α mRNA expression (Fig. 5D). These results demonstrate that increased levels of HIF-1α protein in response to HER2 overexpression or heregulin stimulation are dependent upon HER2, PI3K, AKT, and FRAP kinase activity. Furthermore, the modest levels of AKT activity in 3T3/NEU cells and the modest levels of heregulin-α to which MCF-7 cells were exposed suggest that the effects on HIF-1α expression demonstrated in these studies are physiologically relevant.
FIG. 5.
Involvement of the PI3K-AKT-FRAP pathway in heregulin-induced HIF-1α expression. (A) Stimulation of AKT activity and HIF-1α expression by heregulin. Serum-starved MCF-7 cells were exposed to vehicle or heregulin for 6 h and analyzed for phospho-AKT (top), total AKT (middle), or HIF-1α protein (bottom) by immunoblot assay. (B) Effect of serum and heregulin stimulation on S6 kinase activity. Serum-starved cells were treated with serum or heregulin for 6 h prior to immunoblot assay with antibodies specific for phosphorylated p70s6k and its p85 isoform (top) or total p70s6k protein (bottom). (C) Effect of kinase inhibitors on heregulin-stimulated HIF-1α expression. Serum-starved cells were pretreated for 30 min with vehicle or inhibitor (AG825, PD98059, rapamycin, or LY294002) and then exposed to no treatment, 10% FBS (Serum), or 100 ng of heregulin/ml for 6 h prior to HIF-1α immunoblot assay of whole-cell lysates. (D) Analysis of HIF-1α mRNA expression. MCF-7 cells were serum starved and exposed to heregulin in the absence or presence of rapamycin for 6 h. Total RNA was isolated, and blot hybridization was performed with a HIF-1α cDNA probe (top). A photograph of the ethidium bromide-stained gel demonstrates equal loading of RNA as determined by the intensity of the 18S and 28S rRNA bands (bottom).
HER2 overexpression and heregulin stimulation induce HIF-1α protein synthesis, not stability.
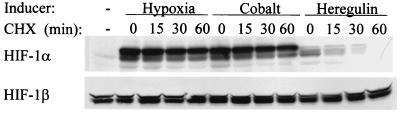
Although the PI3K-AKT-FRAP pathway was previously shown to regulate HIF-1α levels in human prostate cancer cells (65), the molecular basis for this effect was not delineated. In cells that are subjected to hypoxia, or in which p53 or VHL function has been lost, HIF-1α expression is induced as a result of decreased ubiquitin-dependent proteasomal degradation (7, 40, 52, 54). To investigate whether a similar mechanism was activated by HER2-PI3K-AKT-FRAP signaling, the kinetics of HIF-1α decay in MCF-7 cells treated with cycloheximide to block protein synthesis were determined. In cells exposed to hypoxia or cobalt chloride, HIF-1α levels remained constant over 60 min despite the lack of ongoing protein synthesis (Fig. 6, top), which is consistent with the known effects of these stimuli on inhibiting the ubiquitination and degradation of HIF-1α (20, 26, 34, 40, 46, 52). These results were in marked contrast to heregulin-treated cells, in which HIF-1α protein was completely degraded within 60 min after cycloheximide addition. The effects of hypoxia, CoCl2, and heregulin were specific for HIF-1α, as HIF-1β levels remained constant under all conditions (Fig. 6, bottom). Inhibition of protein synthesis by exposure of MCF-7 cells to 50 μg of anisomycin/ml, a protein synthesis inhibitor with a different mechanism of action, also resulted in rapid decay of HIF-1α levels in heregulin- but not in cobalt-treated cells (data not shown). These results indicate that heregulin stimulation does not inhibit HIF-1α degradation to the same degree as hypoxia or CoCl2 treatment.
FIG. 6.
Effect of hypoxia, cobalt, and heregulin stimulation on HIF-1α stability. HIF-1α expression was induced by exposure of MCF-7 cells to 1% O2 (Hypoxia), 100 μM CoCl2 (Cobalt), or 100 ng of heregulin/ml for 6 h. Cycloheximide (CHX) was added to a final concentration of 100 μM, and the cells were harvested after being incubated for the indicated time in the presence of CHX and the inducer. Nuclear extracts were analyzed for the expression of HIF-1α (top) and HIF-1β (bottom) by immunoblot assay.
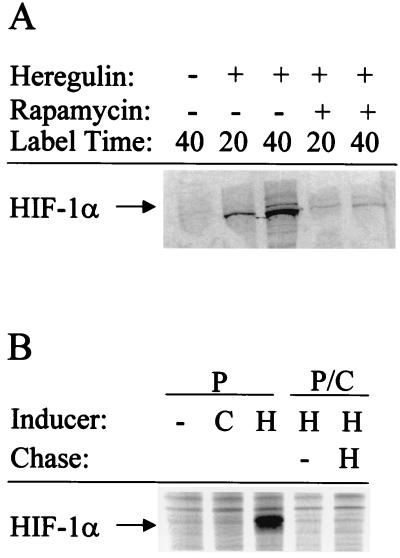
To analyze the rate of HIF-1α synthesis, serum-starved MCF-7 cells were pretreated with heregulin in the presence or absence of rapamycin for 30 min and then pulse-labeled with [35S]Met-Cys for 20 or 40 min, followed by immunoprecipitation of HIF-1α (Fig. 7A). In contrast to control serum-starved cells (Fig. 7A, lane 1), 35S-labeled HIF-1α was clearly demonstrated in heregulin-treated cells (lanes 2 and 3), whereas in the presence of rapamycin, the amount of labeled HIF-1α protein was significantly diminished (lanes 4 and 5). To compare the effects of heregulin and CoCl2 on HIF-1α synthesis, serum-starved MCF-7 cells were pretreated with heregulin or CoCl2 for 30 min and then pulsed with [35S]Met-Cys for 40 min (Fig. 7B). 35S-labeled HIF-1α was detectable in heregulin- but not in CoCl2-treated cells (Fig. 7B, compare lanes 2 and 3), demonstrating that heregulin stimulates HIF-1α synthesis, whereas CoCl2 has no effect on synthesis but instead inhibits HIF-1α degradation (as shown in Fig. 6). To analyze the effect of heregulin on HIF-1α protein stability, cells were pulsed with [35S]Met-Cys for 40 min in the presence of heregulin, and then the radioactivity was chased with unlabeled Met-Cys for 25 min in either the presence or the absence of heregulin (Fig. 7B). There was no detectable 35S-labeled HIF-1α in either case (lanes 4 and 5), indicating that heregulin treatment does not prevent HIF-1α degradation. Thus, both the cycloheximide addition and pulse-chase experiments provide no evidence for increased HIF-1α stability in heregulin-treated MCF-7 cells, whereas pulse-labeling studies demonstrate that heregulin stimulation increases the rate of HIF-1α synthesis.
FIG. 7.
Metabolic labeling experiments. (A) Pulse-labeling of MCF-7 cells. Serum-starved cells were pretreated with no drug, heregulin, or heregulin plus rapamycin for 30 min in Met-free medium. [35S]Met-Cys was added, and the cells were incubated for 20 or 40 min prior to preparation of cell lysates and immunoprecipitation of HIF-1α. (B) Pulse-chase. Serum-starved MCF-7 cells were pretreated with 100 μM cobalt chloride (C) or 100 ng of heregulin/ml (H) for 30 min in Met-free medium. [35S]Met-Cys was added, and then the cells were incubated for 40 min and either harvested directly for analysis of pulse-labeling (P) or rinsed and incubated in medium containing unlabeled Met-Cys for 25 min in the presence or absence of heregulin for pulse-chase analysis (P/C) of immunoprecipitated HIF-1α.
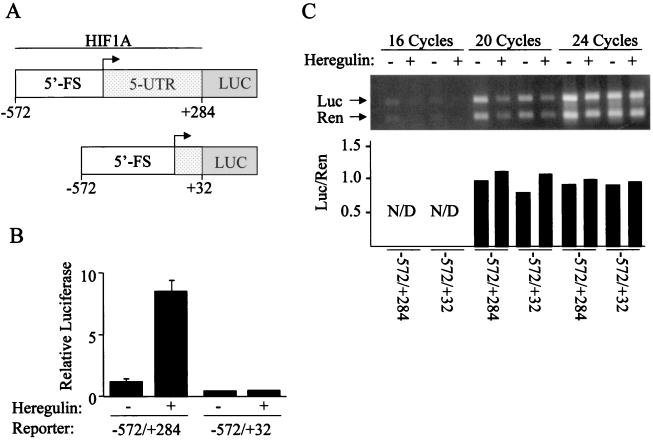
To determine whether the 5′ untranslated region (5′-UTR) of HIF-1α mRNA mediates the induction of protein synthesis by heregulin, two reporter plasmids were constructed in which 5′-UTR and 5′-flanking sequences (5′-FS) from the HIF1A gene (23) were inserted 5′ to firefly luciferase coding sequences (Fig. 8A). Compared to the activity of Renilla luciferase encoded by the cotransfected pSV-Renilla reporter, the activity of firefly luciferase encoded by the reporter containing 572 bp of 5′-FS and the complete 284-bp 5′-UTR increased >8-fold in heregulin-treated cells (Fig. 8B). However, in cells transfected with a reporter in which all but 32 bp of the 5′-UTR were removed, the response to heregulin treatment was lost. RT-PCR analysis revealed that expression of mRNA encoding firefly and Renilla luciferase was constant under all four experimental conditions (Fig. 8C). Thus, the 5′-UTR of HIF-1α mRNA mediates heregulin-induced expression of a heterologous protein at the translational level.
FIG. 8.
Effect of HIF1A 5′-UTR on luciferase expression. (A) MCF-7 cells were cotransfected with pSV-Renilla and firefly luciferase (LUC) reporter genes containing 572 bp of 5′-FS from the human HIF1A gene, followed by either 284 or 32 bp of the 5′-UTR. (B) Transfected cells were exposed to heregulin or vehicle for 24 h, and the ratio of firefly luciferase to Renilla (Relative Luciferase) activity was determined. The mean and standard deviation for each condition are shown based upon three independent transfections. (C) Expression of Renilla (Ren) and firefly luciferase (Luc) mRNA in transfected cells was determined by RT-PCR. The ratio of Renilla to firefly luciferase expression was determined by densitometry for the 20- and 24-cycle PCR but was not determined (N/D) for the 16-cycle PCR due to the low levels of product.
DISCUSSION
Clinical and experimental data indicate that increased HER2 activity is an important step in breast cancer progression that impacts negatively on patient survival (36). HER2 signaling provides increased resistance against apoptosis (induced by adverse conditions in the tumor microenvironment or chemotherapy) that is mediated by the PI3K-AKT pathway (31, 67). Another important consequence of HER2 signaling is increased VEGF expression (38, 60). We have demonstrated that HER2 signaling in nonhypoxic cells induces transcriptional activation of the VEGF gene by HIF-1 that is dependent upon PI3K and AKT activity (Fig. 9). Furthermore, activity of the downstream kinase FRAP is also required for HIF-1α expression under nonhypoxic conditions. The clinical relevance of these results is underscored by the recent demonstration that HIF-1α overexpression is significantly associated with HER2 and VEGF expression and with microvascular density in human ductal carcinoma in situ and invasive breast cancer (5).
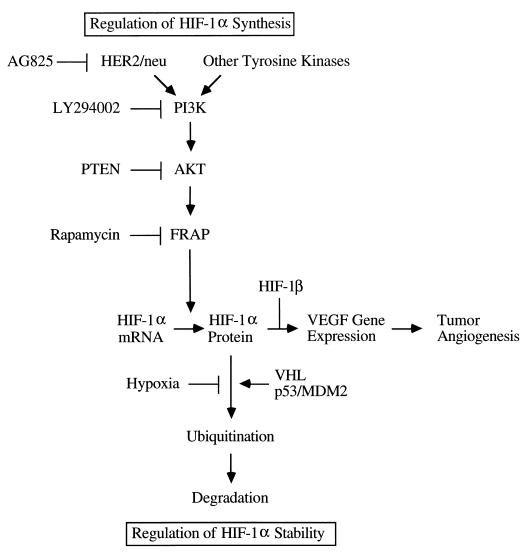
FIG. 9.
Dual mechanisms for induction of HIF-1α protein and VEGF mRNA expression. Signal transduction from HER2 (and possibly other tyrosine kinases such as EGFR and V-SRC) to PI3K, AKT, and FRAP increases the rate of HIF-1α synthesis, whereas hypoxia and loss of VHL or p53 activity decrease the rate of HIF-1α degradation by reducing its ubiquitination (VHL and p53-recruited MDM2 are ubiquitin-protein ligases).
The most surprising result of the present study is the novel finding that activation of the PI3K-AKT-FRAP pathway by heregulin stimulation of MCF-7 human breast cancer cells does not affect HIF-1α stability but instead dramatically increases the rate of HIF-1α protein synthesis, as determined by three independent experimental approaches involving cycloheximide addition, pulse-chase labeling, and reporter gene transfection assays. The effect of heregulin-HER2 signaling is therefore similar to the forced expression of recombinant HIF-1α in transient transfection experiments (12) in which VHL becomes limiting (54), resulting in failure to degrade all of the HIF-1α that is expressed under nonhypoxic conditions. In contrast, previous studies have demonstrated that hypoxia and loss of p53 or VHL activity affect HIF-1α protein stability via altered ubiquitination (Fig. 9). Whereas hypoxia increases both the stability of HIF-1α protein and its specific transcriptional activity (25), heregulin-HER2 signaling induces HIF-1α protein synthesis, such that the combination of HER2 overexpression and hypoxia has a synergistic effect on VEGF gene expression (Fig. 1B).
Data from cycloheximide-addition experiments suggest that activation of the PI3K-AKT-FRAP pathway by other receptor and nonreceptor tyrosine kinases, including EGFR and V-SRC, also induces HIF-1α protein synthesis (K. Chiles, E. Laughner, P. Taghavi, and G. L. Semenza, unpublished data), although this conclusion will need to be confirmed by pulse-chase analyses. The PI3K-AKT-FRAP pathway may also be activated by physiological stimulation of normal cells, such as the induction of HIF-1α expression in vascular smooth muscle cells exposed to angiotensin II, platelet-derived growth factor BB, or thrombin (42). Thus, stimulation of HIF-1α synthesis by the PI3K-AKT-FRAP pathway is likely to represent a major mechanism for induction of HIF-1 and its downstream target genes in a variety of physiological and pathophysiological conditions.
The pulse-chase studies demonstrate a requirement for FRAP activity, as rapamycin markedly inhibited heregulin-induced HIF-1α protein synthesis (Fig. 7A). How does FRAP regulate the rate of HIF-1α synthesis? One possible mechanism involves the phosphorylation of 4E-BP1 by FRAP (14, 17, 37). The eukaryotic translation initiation factor 4F (eIF-4F) performs the rate-limiting function of recruiting the 40S ribosomal subunit to mRNA, with the eIF-4E subunit binding directly to the 5′ cap structure. 4E-BP1 binds eIF-4E and inhibits its activity. Phosphorylation of 4E-BP1 by FRAP decreases its ability to bind eIF-4E. Thus, FRAP activity positively regulates translation. The other major downstream targets of FRAP are the p70 and p85 kinases, which phosphorylate the S6 protein of the 40S ribosomal subunit. S6 kinases have been shown to control the translation of mRNAs that containing polypyrimidine tracts within their 5′-UTR (8). The HIF-1α 5′-UTR contains tracts of 8, 9, and 17 pyrimidines downstream of nucleotide +32 (23). However, 4E-BP1, p70s6k, and p85s6k were highly phosphorylated in MCF-7 cells exposed to either serum or heregulin, whereas only heregulin markedly induced HIF-1α expression (Fig. 5). Thus, further studies are required to determine whether phosphorylation of 4E-BP1 or S6 kinases is necessary for HIF-1α induction. Phosphorylation of the translation initiation factor eIF-2α has recently been shown to control stress-induced protein synthesis (18), but the PI3K-AKT-FRAP pathway has not been implicated in this process.
Recent studies have revealed that a consequence of dysregulated expression of multiple tumor suppressor proteins and signal transduction pathways is an increase in HIF-1 transcriptional activity that occurs via three different molecular mechanisms. First, loss of p53 or VHL increases HIF-1α protein expression by interfering with its ubiquitination and proteasomal degradation (7, 34, 40, 54). Second, RAF/MEK/extracellular signal-regulated kinase signaling stimulates transcription of HIF-1-dependent target genes but does not increase HIF-1α expression, suggesting a direct effect on transactivation (41). Third, PI3K-AKT-FRAP signaling increases the rate of HIF-1α synthesis, as demonstrated in this study. The consequences of activating signal transduction pathways may be cell type specific, since treatment of mouse embryo fibroblasts with the organomercurial compound mersalyl induces HIF-1α protein expression via a signaling pathway that requires MAP kinase activity (2). In addition to genetic alterations involving oncogene and tumor suppressor gene products, HIF-1α protein stability and transcriptional activity are also induced by intratumoral hypoxia which, as in the case of HER2 overexpression, is associated with poor clinical outcome (reviewed in reference 49). The molecular data indicating that multiple genetic and physiological stimuli induce HIF-1 in human cancers are consistent with immunohistochemical data indicating that HIF-1α overexpression occurs frequently in breast and other common human cancers (5, 53, 66) and correlates with tumor grade and vascularization (5, 62) and patient survival (1, 4). Thus, HER2 overexpression does not activate HIF-1-dependent gene transcription in isolation but rather in combination with other tumor-specific genetic and physiological alterations. Taken together, the clinical and molecular studies suggest that increased HIF-1α expression may contribute to tumor progression by mediating angiogenesis, metabolic adaptation, and other aspects of invasion and metastasis that define the lethal cancer phenotype.
ACKNOWLEDGMENTS
We are grateful to Tung Chan, Elizabeth Jaffee, and Dominic Scudiero for providing cell lines and plasmids.
This work was supported by grants to G.L.S. from the Children's Brain Tumor Foundation and the National Institutes of Health (R01-DK39869 and R01-HL55338).
E.L. and P.T. contributed equally to this work.
REFERENCES
- 1.Aebersold D M, Burri P, Beer K T, Laissue J, Djonov V, Greiner R H, Semenza G L. Expression of hypoxia-inducible factor-1a: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- 2.Agani F, Semenza G L. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol. 1998;54:749–754. doi: 10.1124/mol.54.5.749. [DOI] [PubMed] [Google Scholar]
- 3.Bagheri-Yarmand R, Vadlamudi R K, Wang R A, Mendelsohn J, Kumar R. Vascular endothelial growth factor upregulation via p21-activated kinase-1 signaling regulates heregulin-β1-mediated angiogenesis. J Biol Chem. 2000;275:39451–39457. doi: 10.1074/jbc.M006150200. [DOI] [PubMed] [Google Scholar]
- 4.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1α is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- 5.Bos R, Zhong H, Hanrahan C F, Mommers E C M, Semenza G L, Pinedo H M, Abeloff M D, Simons J W, van Diest P J, van der Wall E. Levels of hypoxia-inducible factor 1α during breast carcinogenesis. J Natl Cancer Inst. 2000;93:309–314. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Dor Y, Herbert J-M, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch C J, Ratcliffe P, Moons L, Jain R K, Collen D, Keshet E. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation, and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 7.Cockman M E, Masson N, Mole D R, Jaakkola P, Chang G W, Clifford S C, Maher E R, Pugh C W, Ratcliffe P J, Maxwell P H. Hypoxia-inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 8.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 9.Feldser D, Agani F, Iyer N V, Pak B, Ferreira G, Semenza G L. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 10.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 11.Fidler I J, Ellis L M. The implication of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 12.Forsythe J A, Jiang B-H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 14.Gingras A-C, Gygi S P, Raught B, Polakiewicz R D, Abraham R T, Hoekstra M F, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidi A J, Fischer L, Harris J R, Schnitt S J. Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1994;86:614–619. doi: 10.1093/jnci/86.8.614. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 17.Hara K, Yonezawa K, Kozlowski M T, Sugimoto T, Andrabi K, Weng Q-P, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 18.Harding H P, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 19.Horak E R, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris A L. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antiobodies, as an indicator of node metastases and survival in breast cancer. Lancet. 1992;340:1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- 20.Huang L E, Gu J, Schau M, Bunn H F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung M C, Schechter A L, Chevray P Y, Stern D F, Weinberg R A. Molecular cloning of the neu gene: absence of gross structural alteration in oncogenic alleles. Proc Natl Acad Sci USA. 1986;83:261–264. doi: 10.1073/pnas.83.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer N V, Kotch L E, Agani F, Leung S W, Laughner E, Wenger R H, Gassmann M, Gearhart J D, Lawler A M, Yu A Y, Semenza G L. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer N V, Leung S W, Semenza G L. The human hypoxia-inducible factor 1α gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. doi: 10.1006/geno.1998.5416. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B-H, Agani F, Passaniti A, Semenza G L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328–5335. [PubMed] [Google Scholar]
- 25.Jiang B-H, Zheng J Z, Leung S W, Roe R, Semenza G L. Transactivation and inhibitory domains of hypoxia-inducible factor 1α: modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 26.Kallio P J, Wilson W J, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 27.Kern F G, Lippman M E. The role of angiogenic growth factors in breast cancer progression. Cancer Metastasis Rev. 1996;15:213–219. doi: 10.1007/BF00437474. [DOI] [PubMed] [Google Scholar]
- 28.Kung A L, Wang S, Klco J M, Kaelin W G, Livingston D M. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 29.Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18:1423–1431. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 30.Linderholm B, Tavelin B, Grankvist K, Henriksson R. Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J Clin Oncol. 1998;16:3121–3128. doi: 10.1200/JCO.1998.16.9.3121. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Li J, Roth R A. Heregulin regulation of Akt/protein kinase B in breast cancer cells. Biochem Biophys Res Commun. 1999;261:897–903. doi: 10.1006/bbrc.1999.1144. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, el-Ashry D, Chen D, Ding I Y, Kern F G. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 33.Maxwell P H, Dachs G U, Gleadle J M, Nicholls L G, Harris A L, Stratford I J, Hankinson O, Pugh C W, Ratcliffe P J. Hypoxia-inducible factor 1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxwell P H, Wiesener M S, Chang G-W, Clifford S C, Vaux E C, Cockman M E, Wykoff C C, Pugh C W, Maher E R, Ratcliffe P J. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 35.Pegram M D, Lipton A, Hayes D F, Weber B L, Baselga J M, Tripathy D, Baly D, Baughman S A, Twaddell T, Glaspy J A, Slamon D J. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2 monoclonal antibody plus cisplatin in patients with HER2-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 36.Pegram M D, Pauletti G, Slamon D J. HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 1998;52:65–77. doi: 10.1023/a:1006111117877. [DOI] [PubMed] [Google Scholar]
- 37.Peterson R T, Desai B N, Hardwick J S, Schreiber S L. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin-associated protein. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petit A M, Rak J, Hung M C, Rockwell P, Goldstein N, Fendly B, Kerbel R S. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce J H, Arnstein P, DiMarco E, Artrip J, Kraus M H, Lonardo F, Di Fiore P P, Aaronson S A. Oncogenic potential of erbB-2 in human mammary epithelial cells. Oncogene. 1991;6:1189–1194. [PubMed] [Google Scholar]
- 40.Ravi R, Mookerjee B, Bhujwalla Z M, Sutter C H, Artemov D, Zeng Q, Dillehay L E, Madan A, Semenza G L, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 41.Richard D E, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1α (HIF-1α) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 42.Richard D E, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia inducible factor 1α in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 43.Rilke F, Colnaghi M I, Cascinelli N, Andreola S, Baldini M T, Bufalino R, Della Porta G, Menard S, Pierotti M A, Testori A. Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer. 1991;49:44–49. doi: 10.1002/ijc.2910490109. [DOI] [PubMed] [Google Scholar]
- 44.Ryan H E, Lo J, Johnson R S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan H E, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit J M, Johnson R S. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 46.Salceda S, Caro J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions: its stabilization by hypoxia depends upon redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 47.Salnikow K, An W G, Melillo G, Blagosklonny M V, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999;20:1819–1823. doi: 10.1093/carcin/20.9.1819. [DOI] [PubMed] [Google Scholar]
- 48.Salnikow K, Costa M, Figg W D, Blagosklonny M V. Hyperinducibility of hypoxia-responsive genes without p53/p21-dependent checkpoint in aggressive prostate cancer. Cancer Res. 2000;60:5630–5634. [PubMed] [Google Scholar]
- 49.Semenza G L. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 50.Slamon D J, Clark G M, Wong S G, Levin W J, Ullrich A, McGuire W L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 51.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J A, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 52.Sutter C H, Laughner E, Semenza G L. Hypoxia-inducible factor 1α protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci USA. 2000;97:4748–4753. doi: 10.1073/pnas.080072497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talks K L, Turley H, Gatter K C, Maxwell P H, Pugh C W, Ratcliffe P J, Harris A L. The expression and distribution of the hypoxia-inducible transcription factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzahar E, Yarden Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. Biochim Biophys Acta. 1998;1377:M25–M37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 56.Wang G L, Jiang B-H, Rue E A, Semenza G L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang G L, Semenza G L. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 58.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred E N, Moore D H, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 59.Weidner N, Semple J P, Welch J R, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 60.Yen L, You X-L, Al Moustafa A-E, Batist G, Hynes N E, Mader S, Meloche S, Alaoui-Jamali M A. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. 2000;19:3460–3469. doi: 10.1038/sj.onc.1203685. [DOI] [PubMed] [Google Scholar]
- 61.Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell T J, Hung M C. Overexpression of ErbB2 blocks taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 62.Zagzag D, Zhong H, Scalzitti J M, Laughner E, Simons J W, Semenza G L. Expression of hypoxia-inducible factor 1α in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2806–2818. [PubMed] [Google Scholar]
- 63.Zelzer E, Levy Y, Kahana C, Shilo B-Z, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zetter B R. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 65.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu M-M, Simons J W, Semenza G L. Modulation of HIF-1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 66.Zhong H, De Marzo A M, Laughner E, Lim M, Hilton D A, Zagzag D, Buechler P, Isaacs W B, Semenza G L, Simons J W. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 67.Zhou B P, Hu M C T, Miller S A, Yu Z, Xia, Lin S Y, Hung M C. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-κB pathway. J Biol Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 68.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk A R, Ryan H E, Johnson R S, Jefferson A B, Stokoe D, Giaccia A J. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]