Abstract
Both human and mouse cells express an alternatively spliced variant of BRCA1, BRCA1-Δ11, which lacks exon 11 in its entirety, including putative nuclear localization signals. Consistent with this, BRCA1-Δ11 has been reported to reside in the cytoplasm, a localization that would ostensibly preclude it from playing a role in the nuclear processes in which its full-length counterpart has been implicated. Nevertheless, the finding that murine embryos bearing homozygous deletions of exon 11 survive longer than embryos that are homozygous for Brca1 null alleles suggests that exon 11-deleted isoforms may perform at least some of the functions of Brca1. We have analyzed both the full-length and the exon 11-deleted isoforms of the murine Brca1 protein. Our results demonstrate that full-length murine Brca1 is identical to human BRCA1 with respect to its cell cycle regulation, DNA damage-induced phosphorylation, nuclear localization, and association with Rad51. Surprisingly, we show that endogenous Brca1-Δ11 localizes to discrete nuclear foci indistinguishable from those found in wild-type cells, despite the fact that Brca1-Δ11 lacks previously defined nuclear localization signals. However, we further show that DNA damage-induced phosphorylation of Brca1-Δ11 is significantly reduced compared to full-length Brca1, and that gamma irradiation-induced Rad51 focus formation is impaired in cells in which only Brca1-Δ11 is expressed. Our results suggest that the increased viability of embryos bearing homozygous deletions of exon 11 may be due to expression of Brca1-Δ11 and suggest an explanation for the genomic instability that accompanies the loss of full-length Brca1.
Germ line mutations in BRCA1 predispose women to early-onset breast and ovarian cancers (18, 38). The BRCA1 gene is composed of 23 exons that encode a 1,863-amino-acid full-length protein, over half of which is encoded by an unusually large exon, exon 11, which is 3.4 kb in length. In addition to the full-length BRCA1 protein, p220BRCA1, human cells contain alternatively spliced variants referred to as BRCA1-Δ11 (referred to here as p97BRCA1) and BRCA1-Δ11b (referred to here as p110BRCA1), which lack all and most of exon 11, respectively (54, 58). These isoforms arise from in-frame splicing events and retain the highly conserved amino-terminal RING finger and carboxyl-terminal BRCT domains found in full-length BRCA1 but lack the nuclear localization signals previously identified in exon 11 (11, 54, 58). The abundant expression of p97BRCA1 and p110BRCA1 has been demonstrated in a variety of adult tissues, including the human mammary gland, in which transcripts encoding p110BRCA1 are expressed at levels comparable to those encoding p220BRCA1 (33, 54, 58).
The observation that human BRCA1 is phosphorylated in response to UV light, ionizing radiation, and other agents that damage DNA, and the identification of BRCA1-interacting proteins such as RAD51 and RAD50-Mre11-p95 complexes that colocalize with BRCA1 following DNA damage have suggested a role for BRCA1 in DNA repair (49, 55, 56). Subsequent experiments have confirmed this suggestion by demonstrating that human and mouse Brca1 are required for the repair of double-stranded DNA breaks (37, 51). BRCA1 has also been implicated in transcriptional regulation through the ability of its carboxyl-terminal domain to stimulate transcription in a variety of functional assays as well as by virtue of its demonstrated interaction with the nuclear proteins p53, pRB, CtIP, CBP/p300, ATF1, and RNA polymerase II holoenzyme complexes (2, 3, 10, 22, 26, 30, 35, 39, 40, 45–47, 63–65). In addition, the recent finding that BRCA1 is a component of a SWI/SNF-related complex suggests that BRCA1 may play a role in coordinating processes such as repair and transcription through the remodeling of chromatin (7).
Initial reports describing the subcellular localization of BRCA1 were highly controversial. BRCA1 has been reported by different groups to localize to the cytoplasm, to the nucleus, to cytoplasmic tube-like invaginations in the nucleus, or to be secreted (14, 28, 50; E. Coene, P. Van Oostveldt, K. Willems, J. van Emmelo, and C. R. De Potter, Letter, Nat. Genet. 16:122–124, 1997). These reports preceded experiments demonstrating functional roles for BRCA1 in DNA damage and transcription, each of which would have suggested that BRCA1 was likely to reside in the nucleus. Indeed, the subsequent observation that BRCA1 compartmentalizes to nuclear foci during S phase and undergoes a DNA damage-dependent dynamic redistribution served to focus efforts on experiments designed to identify a nuclear role for BRCA1 (48).
In contrast to BRCA1, the properties and functions of the exon 11-deleted isoforms of BRCA1 are largely unknown. Previous experiments suggesting that BRCA1-Δ11 is localized to the cytoplasm were based on transient transfection protocols (54). Transient transfection methods have also been used to suggest that the murine counterpart to p110BRCA1 is localized predominantly in the cytoplasm (4). However, the fact that similar approaches indicated a cytoplasmic localization for p220BRCA1 suggests that determining the localization of exon 11-deleted isoforms will require examination of their endogenous expression patterns (58). Inconclusive results have been obtained regarding the cellular localization of p110BRCA1; biochemical fractionation of transiently transfected cells has shown that p110BRCA1 is distributed equally between nuclear and cytoplasmic fractions, whereas immunofluorescence analysis of the same ectopically expressed protein was reported to yield exclusively cytoplasmic staining (58). These reports appear to be at odds with studies of endogenous BRCA1 proteins that use BRCA1 antibodies that recognize determinants shared by full-length BRCA1 and its isoforms, since these studies have generally failed to reveal the presence of BRCA1 proteins in the cytoplasm (50).
Notably, murine embryos bearing targeted mutations that selectively abolish expression of full-length Brca1, while leaving Brca1-Δ11 expression intact, survive significantly longer than mice bearing targeted mutations that abolish expression of both Brca1 and Brca1-Δ11 (18, 20, 23, 31, 32, 52, 61). This finding suggests that in mouse cells Brca1-Δ11 is able to partially compensate for the functions of full-length Brca1. Despite the decreased severity of their associated embryonic phenotype, embryonic cells derived from mice engineered to express only Brca1-Δ11 exhibit hypersensitivity to gamma irradiation, defective G2-M checkpoint function, centrosome amplification, and genomic instability (20, 52, 61). Furthermore, mice bearing mammary-specific deletions of exon 11 develop mammary adenocarcinomas with chromosomal instability (60). These data suggest that while Brca1-Δ11 may partially compensate for Brca1 function during embryogenesis, this naturally occurring isoform lacks the ability to maintain genomic stability and suppress tumorigenesis.
In this report, we demonstrate by biochemical fractionation and immunofluorescence that full-length and exon 11-deleted isoforms of murine Brca1 are cell cycle regulated and compartmentalize to nuclear foci during S phase. We show that in contrast to full-length Brca1, Brca1-Δ11 is not phosphorylated in response to DNA damage, is deficient in its ability to bind to Rad51, and is unable to promote the efficient formation of Rad51 foci. Taken together, these data suggest that Brca1-Δ11 may provide some of the functions of full-length Brca1 during murine embryogenesis but is unable to fully supplant the functions of full-length Brca1 in the response to DNA damage.
MATERIALS AND METHODS
Generation of antisera.
Regions encompassing amino acids 69 to 278 (mAb1), 809 to 1062 (mAb2), 995 to 1244 (mAb3), and 1365 to 1609 (mAb4) of the murine Brca1 cDNA were subcloned into pGEX-6P-1 (Pharmacia). Lysates from Escherichia coli transformed with these constructs were passed over a glutathione-Sepharose column, and recombinant Brca1 protein was cleaved from the glutathione S-transferase polypeptide with PreScission Protease according to the manufacturer's instructions (Pharmacia). Antisera to purified Brca1 polypeptides were raised in rabbits (Cocalico Biologicals) and were affinity purified according to published methods (24).
Cell culture, synchronization, and fractionation.
HC11 cells were grown in RPMI medium containing 10% bovine calf serum, 5 μg of insulin (Sigma) per ml, 10 ng of epidermal growth factor (Sigma) per ml, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Mouse embryo fibroblasts (MEFs) were grown in Dulbecco modified Eagle medium containing 15% fetal bovine serum supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. 293T cells were grown in Dulbecco modified Eagle medium containing 10% bovine calf serum supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. 293T transfections employed the calcium phosphate method. HC11 cells were transfected using Fugene-6 (Roche Molecular Biochemicals). HC11 cells and fibroblasts were serum starved at 75% confluency and refed with regular growth media 48 h later. Cellular fractionation was performed according to the manufacturer's instructions with the NE-PER kit (Pierce).
Northern analysis, immunoblotting, and immunoprecipitation.
Northern hybridization was performed as described previously using PCR-generated probes encompassing nucleotides 2541 to 3298 within exon 11 and nucleotides 4827 to 5354 within the carboxyl terminus of Brca1 (43). Cell lysates for immunoblotting were prepared in 50 mM Tris (pH 8.0), 120 mM NaCl, and 0.05% Nonidet P-40 with 100 μg of Pefabloc (Boehringer Mannheim Biochemicals) per ml, 20 μg of aprotinin per ml, 10 μg of leupeptin per ml, 0.1 mM β-glycerophosphate, 50 mM NaF, and 1 mM sodium orthovanadate. Samples were loaded onto sodium dodecyl sulfate–7% polyacrylamide gel electrophoresis (SDS–7% PAGE) gels with the exception of experiments designed to detect changes in the mobility of Brca1, for which 5 or 6% gels were run for extended periods. Wet transfer to nitrocellulose was performed overnight in a buffer containing 192 mM glycine, 25 mM Tris base, and 20% methanol. Membranes were blocked for 1 h in phosphate-buffered saline containing 5% nonfat dried milk and 0.5% Nonidet P-40. RAD51 Ab-1 (Calbiochem), RAD50 Clone 13 (Transduction Laboratories), and cyclin A H-432 (Santa Cruz Biotechnology) antibodies were each used at a 1:1,000 dilution in a blocking buffer for 1 h. β-Tubulin antibody N-357 (Amersham) was used at a 1:40,000 dilution. A peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody H+L (Jackson Immunoresearch) was used at a 1:3,000 dilution. Immunoprecipitations were performed for 1 h at 4°C. Rad51 antibodies Ab-1 (Oncogene Research) and I-20 and C-20 (Santa Cruz Biotechnology) and affinity-purified Brca1 antibodies were employed at 2 μg/ml. Immune complexes were precipitated with 20 μl of protein A Sepharose and were washed five times with lysis buffer prior to the addition of 1× Laemmli sample buffer.
Treatment with DNA-damaging agents and orthophosphate labeling.
Gamma irradiation was administered using a CIS bio international (IBL 437c) source. UV doses were administered using a Stratalinker (Stratagene). Hydroxyurea (HU; Sigma) was used at a final concentration of 1 mM. Cells were lysed 1 h following treatment with genotoxic agents. For in vivo labeling experiments, gamma-irradiated cells were incubated with 5 mCi of [32P]orthophosphate in serum-free medium immediately following dosing for 1 h prior to lysis and immunoprecipitation.
Immunofluorescence analysis.
Cells were fixed and permeabilized according to published protocols (48). Affinity-purified Brca1 antisera were used at a concentration of 2 μg/ml. RAD51 (Ab-1) antisera were used at a 1:1,000 dilution. Tetramethyl rhodamine isothiocyanate-conjugated secondary antibody (Jackson Immunoresearch) was used at a dilution of 1:250. All images were obtained by laser scanning confocal microscopy.
RESULTS
Characterization of mouse Brca1 antisera.
Immunoblotting analysis of HC11 murine mammary epithelial cell extracts using murine Brca1 antibodies mAb1, mAb2, mAb3, and mAb4 identified a specific band that migrated at a predicted molecular mass of 210 kDa and that was not recognized by preimmune sera (data not shown). To confirm that these antibodies recognize bona fide mouse Brca1, 293T cells were transfected with a mouse Brca1 cDNA and lysates were prepared for immunoblotting. These studies revealed that polyclonal antibodies mAb1, mAb2, mAb3, and mAb4 each recognize a specific band at the predicted molecular mass for mouse Brca1 in extracts of Brca1-transfected 293T cells. (Fig. 1A and data not shown).
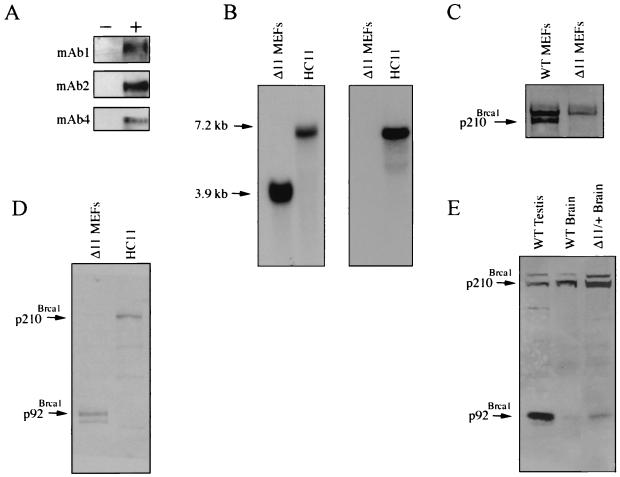
FIG. 1.
Detection of mouse Brca1 isoforms. (A) Immunoblot analysis demonstrating that mAb1, mAb2, and mAb4 recognize murine Brca1. Ten micrograms of either empty vector (pBKCMV) or pBKCMVBrca1 (mAb1) and pcDNA3.1 or pcDNA3.1-mBrca1 (mAb2 and mAb4) was introduced into 293T cells by calcium phosphate transfection. (−), empty vector; +, vector containing murine Brca1 cDNA. Cell extracts were prepared 48 h following transfection, and 50 μg of lysate was used for immunoblotting. Affinity-purified antibodies were employed at 1 μg/ml. (B) Northern analysis demonstrating the absence of full-length Brca1 transcripts in Brca1Δ11/Δ11 MEFs. Ten micrograms of poly(A) mRNA was loaded per lane. Probes encompassing exon 11-specific sequences (right panel; see Materials and Methods) and C-terminal sequences (left panel; see Materials and Methods) were derived by PCR amplification using the mouse Brca1 cDNA as a template. (C) Immunoblot analysis of p210Brca1 expression in wild-type and Brca1Δ11/Δ11 MEFs. Fifty micrograms of cell extract per lane was probed with affinity-purified mAb1 at 1 μg/ml. (D) mAb1 recognizes a predominant gene product of ∼92 kDa in Brca1Δ11/Δ11 MEFs and 210 kDa in HC11 cells. Thirty micrograms of extract was loaded per lane. (E) p92Brca1 is expressed in testis and brain of wild-type mice. One hundred fifty micrograms of lysate per sample was subjected to SDS-PAGE on an 8% acrylamide gel. Δ11/+Brain, tissue derived from a mouse heterozygous for the wild-type and exon 11-deleted alleles of Brca1.
To determine if mAb1 could specifically recognize endogenous Brca1, extracts from wild-type MEFs and from MEFs derived from mice harboring a germ line deletion of the exon 11 region of Brca1 were analyzed by immunoblotting (52). Brca1Δ11/Δ11 MEFs express an isoform of Brca1 analogous to the naturally occurring human BRCA1 variant encoding p97BRCA1. Northern analysis was performed using a probe encompassing nucleotides 4827 to 5354 that was predicted to recognize both the full-length and exon 11-deleted Brca1 transcripts. As expected, a 3.9-kb transcript was detected in Brca1Δ11/Δ11 cells whereas a 7.2-kb transcript was detected in cells that express p210Brca1 (Fig. 1B, left panel). A similar analysis performed with a probe encompassing nucleotides 2541 to 3298 within exon 11 detected only the full-length Brca1 transcript (Fig. 1B, right panel). Accordingly, immunoblotting of extracts prepared from wild-type MEFs revealed the presence of p210Brca1 whereas extracts prepared from Brca1Δ11/Δ11 MEFs did not, confirming that the 210-kDa polypeptide recognized by mAb1 is indeed Brca1 (Fig. 1C). To determine if the putative protein encoded by the exon 11-deleted transcript is detectable in extracts derived from Brca1Δ11/Δ11 MEFs immunoblotting was performed. A major band of the predicted molecular mass, referred to here as p92Brca1, was recognized by mAb1 antisera (Fig. 1D). This Brca1 isoform was also detected in embryonic brain extracts prepared from embryos heterozygous for the exon 11-deleted allele of Brca1, as well as in extracts of testis and brain derived from wild-type mice. These findings demonstrate that p92Brca1 is a naturally occurring isoform of Brca1 (Fig. 1E).
Full-length murine p210Brca1 and p92Brca1 are cell cycle regulated.
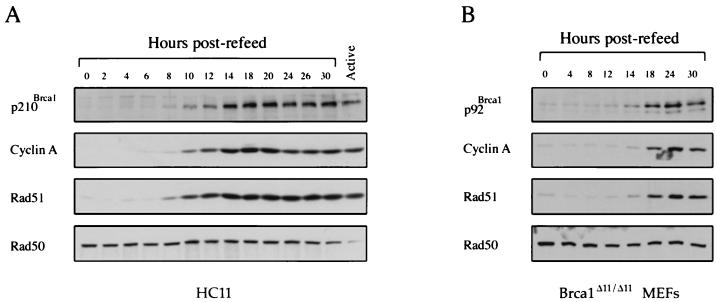
Previous experiments have shown that murine Brca1 mRNA expression is regulated in a cell cycle-dependent manner with maximal levels of Brca1 occurring during the S phase of the cell cycle (43). Human BRCA1 mRNA and protein share this cell cycle-dependent pattern of expression consistent with a conserved S phase-specific function for the human and mouse Brca1 proteins (13, 21, 44, 57). To determine if the protein expression pattern of Brca1 parallels that of its mRNA, synchronization experiments were performed using HC11 murine mammary epithelial cells. As shown in Fig. 2A, the mouse Brca1 protein, p210Brca1, is undetectable in serum-starved cells and becomes apparent when cells have progressed into the G1 phase of the cell cycle approximately 8 h following addition of serum-containing media. Parallel experiments performed on Brca1Δ11/Δ11 fibroblasts demonstrated that the cell cycle-dependent expression pattern of p92Brca1 closely mimics that of p210Brca1. Moreover, the temporal profile of p92Brca1 and p210Brca1 expression is similar to that of Rad51 and cyclin A, but contrasts with that of Rad50, which is expressed at relatively constant levels throughout the cell cycle.
FIG. 2.
Mouse p210Brca1 and p92Brca1 are cell cycle regulated. (A) Immunoblot analysis of cell cycle regulation of p210Brca1, cyclin A, and Rad51 in serum-starved HC11 cells. Active, exponentially growing cells. (B) Immunoblot analysis of cell cycle regulation of p92Brca1, cyclin A, and Rad51 in serum-starved MEFs homozygous for the targeted deletion of exon 11. Cells were starved as described in Materials and Methods. Cells stimulated to reenter the cell cycle by refeeding were harvested at the time points indicated. Cell extracts were prepared as described in Materials and Methods, and 10 μg of lysate was loaded per lane. Antibodies mAb1 and mAb2 revealed identical results in HC11 cells, whereas only mAb1 recognized a cell cycle-regulated band in Brca1Δ11/Δ11 MEFs (data not shown).
Phosphorylation of p92Brca1 is not detected in response to DNA damage.
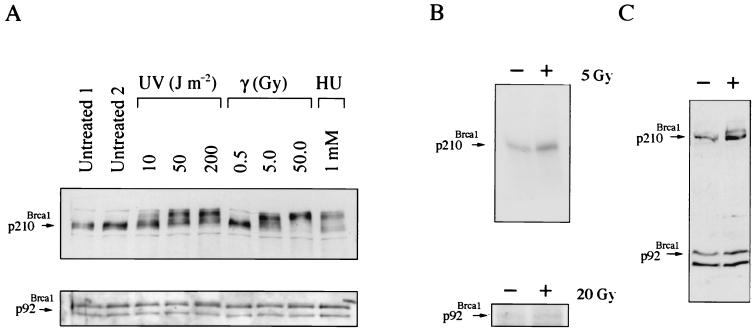
The phosphorylation of human p220BRCA1 following treatment of cells with DNA-damaging agents was an early indication that human BRCA1 is involved in a DNA damage response pathway. Similarly, immunoblotting analysis of lysates generated from HC11 cells 1 h following treatment with UV, gamma radiation, or HU revealed a dose-dependent shift in the migration of full-length murine Brca1 by SDS-PAGE (Fig. 3A, top panel). A complete shift of p210Brca1 similar to that described for human BRCA1 occurred in cells treated with 50 Gy.
FIG. 3.
p210Brca1 but not p92Brca1 undergoes a shift in response to DNA damage. (A) Immunoblot analysis of p210Brca1 and p92Brca1 in cells treated with UV, gamma irradiation, or HU. p210Brca1 exhibits a dose-dependent shift in response to UV and gamma irradiation. HC11 cells (top panel) or MEFs that express only p92Brca1 (bottom panel) were subjected to identical treatments with UV, gamma irradiation, or HU. Twenty micrograms of lysate was loaded per lane and immunoblotted with antibody mAb1. (B) Analysis of p210Brca1 phosphorylation in [32P]orthophosphate-labeled HC11 cells treated with gamma irradiation (upper panel). Immediately following irradiation, HC11 cells were incubated with 5 mCi of [32P]orthophosphate for 1 h. Three milligrams of cell extract was used for immunoprecipitation with 10 μl of the immunoglobulin G fraction of mAB1 antibody. The resolution of this assay was not sufficient to detect a mobility shift of phosphorylated products. Brca1Δ11/Δ11 MEFs irradiated with 20 Gy received identical treatment (lower panel). (C) Immunoblot analysis of p210Brca1 and p92Brca1 in HC11 cells treated with 200 J m−2 UV. p210Brca1 and not p92Brca1 exhibits a dose-dependent shift.
[32P]orthophosphate labeling of HC11 cells following treatment with 5 Gy demonstrated an increase in p210Brca1 labeling consistent with the supposition that, similar to human BRCA1, the observed mobility shift is due to phosphorylation (Fig. 3B, top panel). Exposure of cells to [32P]orthophosphate has previously been shown to cause an increase in phosphorylation of human BRCA1 (55). Therefore, the basal levels of phosphorylation observed in unirradiated HC11 cells may be due either to the activation of a DNA damage response pathway by 32P itself or to cell cycle-dependent phosphorylation of Brca1. Significantly, a shift in p92Brca1 was not observed in response to identical treatments with DNA-damaging agents, suggesting that this isoform may not be phosphorylated under these conditions (Fig. 3A, bottom panel). This possibility was confirmed by [32P]orthophosphate-labeling experiments in which increased phosphorylation of p92Brca1 was not detected following treatment of cells with 20 to 50 Gy (Fig. 3B, bottom panel, and data not shown).
Since the inability to detect a change in the phosphorylation status of p92Brca1 following DNA damage could be due to altered kinase signaling in Brca1Δ11/Δ11 fibroblasts rather than to properties specific to p92Brca1, a p92Brca1 expression vector was transiently transfected into HC11 cells to determine whether a shift in p92Brca1 could be detected. Although a shift in p210Brca1 was detected in response to treatment with DNA-damaging agents, a shift in p92Brca1 in the same cells was not detected (Fig. 3C). These findings suggest that the inability to detect p92 phosphorylation in Brca1Δ11/Δ11 fibroblasts in response to DNA damage is likely to be intrinsic to this exon 11-deleted isoform.
Murine p210Brca1 and p92Brca1 localize to nuclear foci.
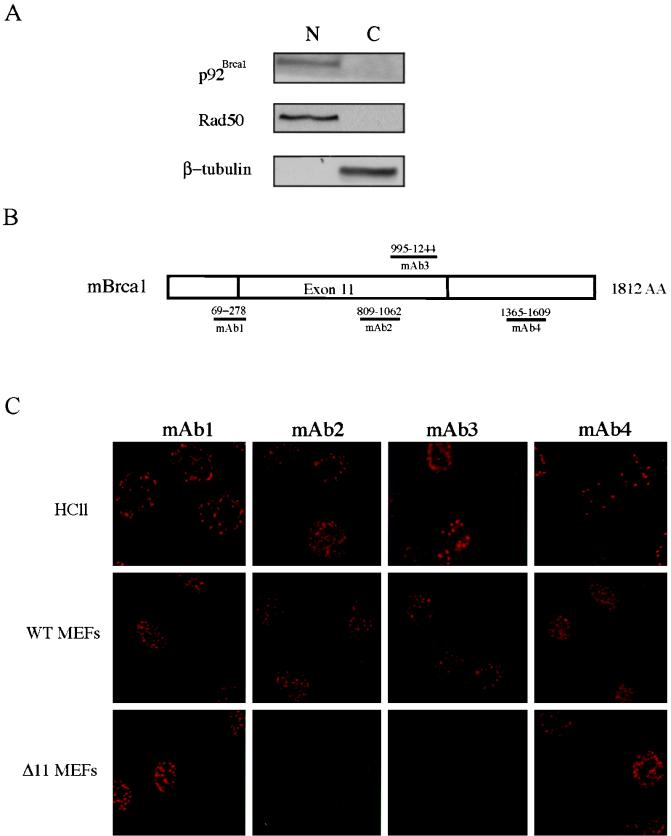
In order to determine if the lack of phosphorylation of p92Brca1 is due to aberrant subcellular localization, biochemical fractionation of exponentially growing Brca1Δ11/Δ11 fibroblasts was performed and nuclear and cytoplasmic fractions were analyzed by immunoblot analysis. To confirm the purity of these fractions, blots were probed with antisera specific for either β-tubulin or RAD50 as controls for cytoplasmic or nuclear proteins, respectively. Surprisingly, these studies revealed that p92Brca1 was present in the nuclear fraction (Fig. 4A). In order to confirm these results and to establish the subnuclear localization of p210Brca1 and p92Brca1, immunofluorescence analysis (IF) was performed (Fig. 4C) since localization to nuclear foci during S phase is a cardinal feature of human BRCA1 (48). Four independent antisera (mAb1-mAb4) raised against murine Brca1 revealed that p210Brca1 localizes to nuclear foci during S phase in both mammary epithelial cell and wild-type MEFs. Strikingly, when similar experiments were performed on Brca1Δ11/Δ11 fibroblasts using antisera directed against epitopes outside of exon 11, distinct nuclear foci that were indistinguishable from those observed in HC11 mammary epithelial cells and wild-type MEFs were observed (Fig. 4C). Since Brca1Δ11/Δ11 MEFs do not express p210Brca1, we reasoned that any specific signal would be due to p92Brca1. Consistent with this supposition, nuclear foci were not detected following IF using the exon 11-specific antisera mAb2 and mAb3. Notably, no signal was observed in the cytoplasm of HC11, wild-type MEFs, or Brca1Δ11/Δ11 MEFs using any of the above antisera.
FIG. 4.
Localization of p210Brca1 and p92Brca1 to nuclear foci. (A) Western analysis of biochemical fractionation of Brca1Δ11/Δ11 MEFs. Equal volumes of nuclear and cytoplasmic extract were loaded per lane. Antibodies were used as described in Materials and Methods. (B) Schematic of murine Brca1 cDNA indicating regions against which antisera were raised. Numbers above the lines represent amino acid coordinates. (C) Immunofluorescence analysis of Brca1 subcellular localization. HC11 cells, wild-type MEFs, and Brca1Δ11/Δ11 MEFs were grown on microscope slides as described in Materials and Methods. Following permeabilization, S phase cells were incubated with affinity-purified Brca1 antibodies at a concentration of 1 μg/ml.
Association of Rad51 with p92Brca1 and Rad51 focus formation are compromised in Brca1Δ11/Δ11 cells.
The exon 11 region of human BRCA1 protein has been shown to be required for binding to RAD51. This observation suggested the possibility that p92Brca1 may not associate with Rad51 in Brca1Δ11/Δ11 cells. To address this question, p92Brca1 was immunoprecipitated from extracts of Brca1Δ11/Δ11 MEFs and analyzed by Western blotting with Rad51. Immunoblotting analysis revealed that Rad51 was detected in extracts derived from HC11 cells in which mAb1, mAb3, or mAb4 had been used to immunoprecipitate p210Brca1 (Fig. 5). In contrast, Rad51 was not detected in extracts derived from Brca1Δ11/Δ11 MEFs that had been subjected to immunoprecipitation with the same anti-Brca1 antisera. In reciprocal coimmunoprecipitation experiments, p210Brca1 was detected in HC11 extracts immunoprecipitated with Rad51 antisera. However, it was not possible to determine if p92Brca1 was present in Rad51 immunoprecipitates due to the presence of a cross-reacting band that comigrated with p92Brca1 (data not shown).
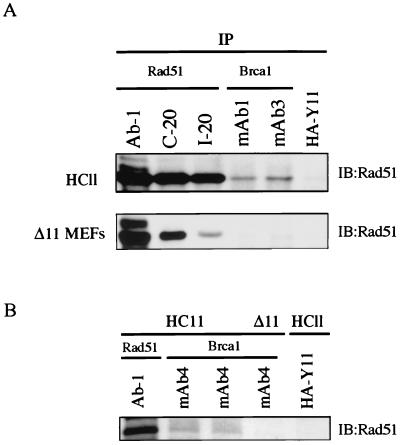
FIG. 5.
Rad51 association with p92Brca1 is not detected in Brca1Δ11/Δ11 MEFs. (A) Extracts generated from cycling HC11 and Brca1Δ11/Δ11 MEFs were prepared as described in Materials and Methods. One milligram of extract was used per sample for immunoprecipitation with 2 μg of antibody. mAb1 and mAb3 were affinity purified. Rad51 antibody Ab-1 was used at 1:1,000 for Western analysis. The cross-reacting faint band observed with mAb3 in Brca1Δ11/Δ11 MEFs does not comigrate with Rad51. (B) Seven milligrams of extract was used to detect association of p210Brca1 with Rad51. One quarter of the extract immunoprecipitated with Rad51 Ab-1 is represented in lane 1. Immunoprecipitation of p92Brca1 from Brca1Δ11/Δ11 MEFs with affinity purified mAb4 does not reveal detectable Rad51 protein.
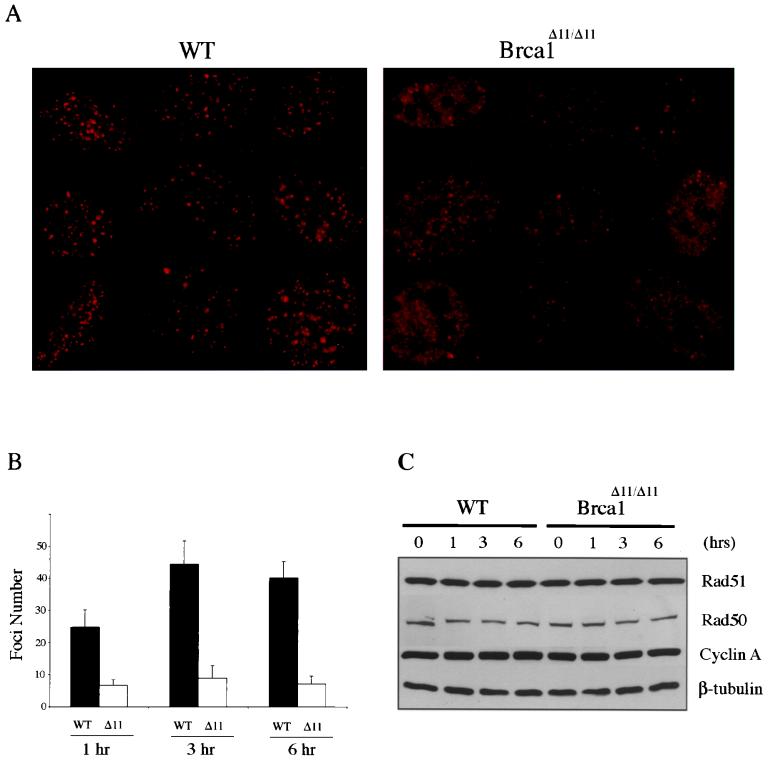
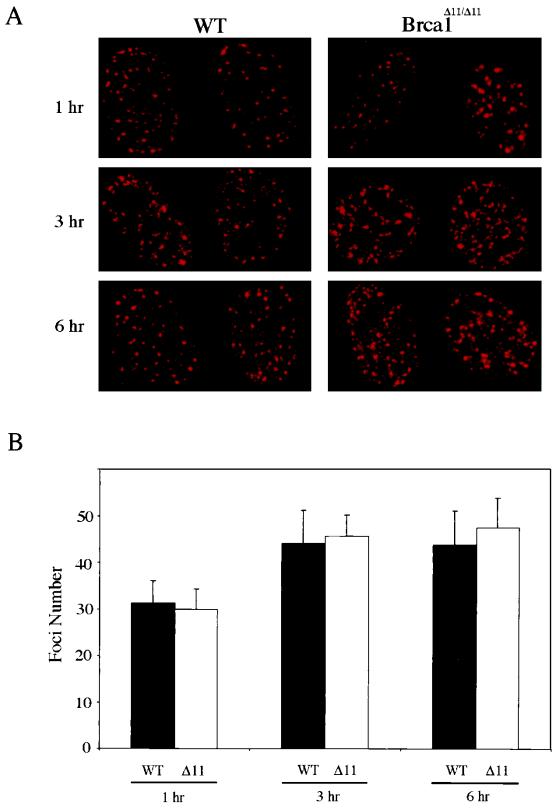
Rad51 has been shown to localize to subnuclear foci following treatment of cells with agents that induce double-stranded breaks (42). The apparent lack of association of p92Brca1 with Rad51 prompted us to examine Rad51 focus formation in wild-type and Brca1Δ11/Δ11 MEFs. At 1, 3, and 6 h following irradiation with 10 Gy, Rad51 focus formation was assessed in cycling wild-type and Brca1Δ11/Δ11 MEFs by IF (Fig. 6). At 1 h, an average of 25 Rad51 foci were detected in wild-type cells treated with 10 Gy, whereas an average of only 6 foci per cell were detected following similar treatment in Brca1Δ11/Δ11 cells (Fig. 6B). In order to determine if Rad51 focus formation in Brca1Δ11/Δ11 MEFs was simply delayed rather than deficient, foci were also assessed 3 and 6 h following irradiation. The numbers of Rad51 foci in wild-type cells increased to 44 and 40 per cell at 3 and 6 h postirradiation, respectively, whereas Brca1Δ11/Δ11 MEFs averaged only 8 and 7 foci, respectively, at these same time points. Furthermore, impaired Rad51 formation was not due to decreased levels of Rad51 in Brca1Δ11/Δ11 MEFs, as demonstrated by Western analysis of extracts from cells that had been treated in a manner identical to that used for immunofluorescence (Fig. 6C). Notably, the absence of p210Brca1 does not affect the previously demonstrated S phase-dependent expression of Rad51, suggesting that the inability to form foci is not due to aberrant cell cycle expression of Rad51 in Brca1Δ11/Δ11 cells (Fig. 2B) (19, 53, 62). As a control, the number of Brca1 foci was determined in wild-type and Brca1Δ11/Δ11 MEFs. This analysis revealed no significant differences in the numbers of Brca1 foci present in wild-type versus Brca1Δ11/Δ11 MEFs at 1, 3, or 6 h following irradiation with 10 Gy (Fig. 7). In aggregate, these data suggest that p92Brca1 has a diminished ability to associate with Rad51 and that Rad51 focus formation is impaired in Brca1Δ11/Δ11 MEFs.
FIG. 6.
Impaired Rad51 foci formation in Brca1Δ11/Δ11 MEFs. (A) Representative Rad51 immunostained nuclei from wild-type and Brca1Δ11/Δ11 MEFs 3 h following irradiation with 10 Gy. Cells were prepared for immunofluorescence using Rad51 antibody Ab-1 as described in Materials and Methods. Foci counts were obtained by visual inspection of 50 nuclei. (B) Graph depicting numbers of foci per nucleus following irradiation with 10 Gy at 1 h (P value = 9.1 × 10−17), 3 h (P value = 8.3 × 10−51), and 6 h (P value = 1.5 × 10−25). (C) Rad51 levels do not change in response to irradiation in wild-type and Brca1Δ11/Δ11 MEFs. At the time points indicated following irradiation with 10 Gy, extracts were prepared and analyzed by immunoblotting as described in Materials and Methods.
FIG. 7.
Brca1 Foci are present in irradiated Brca1Δ11/Δ11 MEFs. (A) Representative nuclei immunostained with mAb1. Cells were prepared for immunofluorescence as described in Materials and Methods. Foci counts were obtained by visual inspection of 10 to 15 nuclei. (B) Graph depicting numbers of foci per nucleus following irradiation with 10 Gy at 1 h (P value = 0.54), 3 h (P value = 0.55), and 6 h (P value = 0.24).
DISCUSSION
While human BRCA1 has been extensively characterized, little is currently known about its murine counterpart. In fact, the mouse Brca1 protein shares only 58% sequence identity to human BRCA1, a finding that has contributed to the suggestion that these proteins may have different functions (1, 8). In this report, we characterize mouse Brca1 proteins and demonstrate that multiple features of the regulation, localization, and interactions of the mouse and human Brca1 proteins are conserved. Similar to its human ortholog, mouse Brca1 is cell cycle regulated and localizes to nuclear foci during S phase. In addition, mouse Brca1 is phosphorylated in a dose-dependent manner in response to genotoxic agents suggesting that in human and murine cells there exists a similar kinase(s) that is upstream of Brca1 in a DNA damage response pathway. Like human BRCA1, murine Brca1 also forms a complex with Rad51 consistent with experiments demonstrating that mouse Brca1 functions in the repair of double-stranded breaks by homologous recombination (37). In aggregate, these data further validate the use of mouse models to study BRCA1 function in human cells.
We have analyzed the expression of a naturally occurring Brca1 isoform in fibroblasts derived from mouse embryos in which the exon 11 region of Brca1 has been specifically deleted. Strikingly, we have found that p92Brca1 is localized to nuclear foci. This finding is consistent with our biochemical fractionation studies revealing that endogenous p92Brca1 is present in the nucleus, as well as with previous findings that in human cells anti-BRCA1 antibodies do not appear to detect cytoplasmic BRCA1 staining, despite the fact that p97BRCA1 and p110BRCA1 would otherwise be expected to be found in the cytoplasm. Our finding that exon 11-deleted isoforms of Brca1 are also present in the nucleus raises for the first time the possibility that this isoform may partially compensate for mutations affecting Brca1 and may possess additional nuclear functions that are as of yet unrecognized.
Notably, our findings contrast with the cytoplasmic localization previously reported for human p97BRCA1 and p110BRCA1, each of which lacks the nuclear localization sequences reportedly required for nuclear transport of p220BRCA1 (54). Nevertheless, the reported partial nuclear localization of human p110BRCA1 suggests that sequences other than the canonical BRCA1 nuclear localization sequences can be utilized for transport into the nucleus or that exon 11-deleted isoforms of BRCA1 can be transported to the nucleus via binding to other nuclear proteins (15, 25, 36). Such cryptic nuclear localization sequences may also be responsible for the nuclear localization of p92Brca1. Alternatively, the difference in localization between the mouse and human isoforms may be due to cell type-specific differences, to species-specific differences, or to the nature of the assays employed for these studies. We favor the last hypothesis. Whereas studies in human cells determined the subcellular localization of exogenously expressed p97BRCA1 and p110BRCA1 using transient transfection assays, we have determined the localization of the endogenous Brca1 proteins. In this regard, previous reports have shown that the high levels of expression characteristic of transient transfection experiments may lead to mislocalization of BRCA1 to the cytoplasm (58). Nevertheless, we cannot rule out the possibilities that p92Brca1 may localize to the cytoplasm in cell types other than those examined here or that human and mouse exon 11-deleted isoforms may localize differently.
Significantly, p210Brca1 displays a mobility shift indicative of phosphorylation in response to DNA damage, whereas p92Brca1 does not. Consistent with this, 32P-labeling experiments failed to reveal a significant increase in phosphate incorporation in p92Brca1 in response to gamma irradiation, suggesting that the inability to detect a shift is not due to a conformation of p92Brca1 that precludes altered mobility by SDS-PAGE. Diminished phosphorylation is also not due to defects in the activities of kinases that converge on Brca1 since a DNA damage-induced mobility shift in p92Brca1 is not detected in HC11 cells in which p210Brca1 does undergo a shift. Several kinases involved in cell cycle checkpoint control including ATM, Cds1, and ATR have been demonstrated to phosphorylate human BRCA1 in vivo in response to DNA-damaging agents (12, 16, 29, 56). The observation that a putative Cds1 phosphorylation site present in mouse Brca1 is located within exon 11 suggests that p92Brca1 may not be a target of Cds1. Moreover, a shift in the mobility of Cds1 protein by SDS-PAGE, which has been shown to correlate with kinase activation, occurs in both wild-type MEFs and Brca1Δ11/Δ11 MEFs following irradiation, suggesting that the absence of p92Brca1 phosphorylation is not the result of an inactive Cds1 kinase (data not shown) (6, 9, 34). In addition to an impaired response to gamma irradiation, we were not able to detect a shift in p92Brca1 in response to HU or UV. Putative phosphorylation sites for ATR and ATM are present within exon 11 of Brca1 and may explain, in part, the inability to detect phosphorylation of p92Brca1. Alternatively, ATM or ATR phosphorylation of Brca1 in response to DNA damage could be dependent on initial phosphorylation of serine 988 by Cds1 or the exon 11 region of Brca1 may be required for binding to these kinases which may in turn be required for Brca1 phosphorylation (12).
Our inability to detect a stable association between p92Brca1 and Rad51 in Brca1Δ11/Δ11 MEFs is consistent with results demonstrating that RAD51 binds to the exon 11 region of human BRCA1 (49). We now provide evidence that this interaction may be required for the efficient formation of Rad51 foci in response to gamma irradiation, a finding that is consistent with evidence that Rad51 foci are reduced in embryonic stem cells harboring a similar Brca1 mutation (5). These data suggest that the inability to localize Rad51 may compromise the capacity of these cells to repair double-stranded breaks, thereby contributing to the defective G2/M checkpoint observed in response to ionizing radiation in these cells (61). In human cells, the relocalization of RAD51 foci to sites of DNA damage has been shown to follow the formation of BRCA1 foci (41). Our results suggest that in spite of the presence of p92Brca1 foci in Brca1Δ11/Δ11 MEFs, Rad51 focus formation is impaired, suggesting that the exon 11 region is required for proper recruitment of Rad51. Nevertheless, while we have confirmed the previously reported association between p210Brca1 and Rad51, this interaction is nonstoichiometric and requires a substantial amount of extract to visualize. This is consistent with the observation that the interaction of human BRCA1 with RAD51 is indirect (49). In this regard, the demonstration that BRG-1, a component of the SWI/SNF complex, interacts directly with human BRCA1 through the exon 11 region is intriguing in that it suggests a model in which the chromatin-remodeling function of BRCA1 may be associated with its ability to mediate the proper assembly of RAD51 (7).
Despite the shared properties of p210Brca1 and p92Brca1, which suggest that exon 11-deleted isoforms may have nuclear functions, mouse knockout models clearly indicate that significant functional differences exist between full-length and exon 11-deleted isoforms of Brca1. Foremost, mice engineered to express only p92Brca1 are not viable, and embryonic cells derived from these mice demonstrate hypersensitivity to gamma irradiation, defective G2-M checkpoint function, centrosome amplification, and genomic instability (20, 52, 61). Moreover, cre-mediated excision of exon 11 of Brca1 in epithelial cells of the murine mammary gland leads to abnormal ductal morphogenesis and tumor formation (60). These experiments demonstrate that the exon 11 region is critical for normal Brca1 function. In this context, our data suggest that the inability of p92Brca1 to provide G2-M checkpoint function, maintain genomic stability, and suppress tumorigenesis is not due to an inability of p92Brca1 to be transported to the nucleus, to localize to nuclear foci, or to be cell cycle regulated but rather may be related to the inability of p92Brca1 to associate with Rad51 or other proteins such as BRG-1 and Rad50 (66). As such, our data suggesting that p92Brca1 is not phosphorylated in response to DNA damage imply that the signal transduction pathways activated by the replication checkpoint and by lesions caused by UV irradiation do not converge on the p92Brca1 protein. Accordingly, deletion of exon 11 of BRCA1 appears to impair its DNA damage-dependent phosphorylation, which may in turn affect the localization to nuclear foci or the function of BRCA1-interacting proteins such as BARD1, BRCA2, or the RAD50/MRE11/NBS complex.
The naturally occurring expression of p92Brca1 during murine embryogenesis and in adult tissues suggests that exon 11-deleted isoforms may function in a variety of tissues. Moreover, in spite of a defect in gamma irradiation-induced Rad51 focus formation in cells lacking full-length Brca1, analysis of Brca1Δ11/Δ11 embryos suggests that p92Brca1 partially compensates for the lack of full-length Brca1 during murine embryogenesis. The most striking evidence for this conclusion is the postnatal survival of targeted mouse lines in which only the p92Brca1 protein is predicted to be expressed (17). Presumably this is due to interactions outside the exon 11 region. In this regard, several proteins including BARD1, CtIP, and BAP have been shown to interact with human BRCA1 through the amino- and carboxyl-terminal regions of the protein (27, 30, 59, 63). In addition to these functions, however, it is interesting to speculate that p92Brca1 may also have functions that are distinct from those of p210Brca1.
ACKNOWLEDGMENTS
We thank members of the Chodosh laboratory and Prakash K. Rao for helpful discussions and critical reading of the manuscript. Confocal microscopy was made possible by the help of James F. Sanzo and Irina Chernysh of the Biomedical Imaging Core Facility at the University of Pennsylvania Medical Center.
This research was supported by NIH grants CA71513 and CA78410 from the National Cancer Institute, and U. S. Army Breast Cancer Research Program grants DAMD17-98-1-8230 (L.J.H.), DAMD17-96-1-6111 (S.R.M.), DAMD17-00-1-0403 (C.J.S.), DAMD17-98-1-8226, and DAMD17-96-1-6113.
REFERENCES
- 1.Abbott D W, Thompson M E, Robinson-Benion C, Tomlinson G, Jensen R A, Holt J T. BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J Biol Chem. 1999;274:18808–18812. doi: 10.1074/jbc.274.26.18808. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S F, Schlegel B P, Nakajima T, Wolpin E S, Parvin J D. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat Genet. 1998;19:254–256. doi: 10.1038/930. [DOI] [PubMed] [Google Scholar]
- 3.Aprelikova O, Fang B, Meissner E, Cotter S, Campbell M, Kuthiala A, Bessho M, Jensen R, Liu E. BRCA1-associated growth arrest is RB-dependent. Proc Natl Acad Sci USA. 1999;96:11866–11871. doi: 10.1073/pnas.96.21.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachelier R, Dalla Venezia N, Mazoyer S, Frappart L, Lenoir G M, Vincent A. Differential expression and subcellular localization of murine BRCA1 and BRCA1-delta11 isoforms in murine and human cell lines. Int J Cancer. 2000;88:519–524. doi: 10.1002/1097-0215(20001115)88:4<519::aid-ijc2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya A, Ear U S, Koller B H, Weichselbaum R R, Bishop D K. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 6.Blasina A, de Weyer I V, Laus M C, Luyten W H, Parker A E, McGowan C H. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 7.Bochar D A, Wang L, Beniya H, Kinev A, Xue Y, Lane W S, Wang W, Kashanchi F, Shiekhattar R. BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell. 2000;102:257–265. doi: 10.1016/s0092-8674(00)00030-1. [DOI] [PubMed] [Google Scholar]
- 8.Boyd J. BRCA1: more than a hereditary breast cancer gene? Nat Genet. 1995;9:335–336. doi: 10.1038/ng0495-335. [DOI] [PubMed] [Google Scholar]
- 9.Brown A L, Lee C H, Schwarz J K, Mitiku N, Piwnica-Worms H, Chung J H. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai Y L, Cui J, Shao N, Shyam E, Reddy P, Rao V N. The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter. Oncogene. 1999;18:263–268. doi: 10.1038/sj.onc.1202323. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-F, Li S, Chen Y, Chen P-L, Sharp Z, Lee W-H. The nuclear localization sequences of the BRCA1 protein interact with the importin-α subunit of the nuclear transport signal receptor. J Biol Chem. 1996;271:32863–32868. doi: 10.1074/jbc.271.51.32863. [DOI] [PubMed] [Google Scholar]
- 12.Chen J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60:5037–5039. [PubMed] [Google Scholar]
- 13.Chen Y, Farmer A, Chen C-F, Jones D, Chen P-L, Lee W-H. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996;56:3168–3172. [PubMed] [Google Scholar]
- 14.Chen Y M, Chen C F, Riley D J, Allred D C, Chen P L, Von Hoff D, Osborne C, Lee W-H. Aberrant subcellular localization of BRCA1 in breast cancer. Science. 1995;270:789–791. doi: 10.1126/science.270.5237.789. [DOI] [PubMed] [Google Scholar]
- 15.Christophe D, Christophe-Hobertus C, Pichon B. Nuclear targeting of proteins: how many different signals? Cell Signal. 2000;12:337–341. doi: 10.1016/s0898-6568(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 16.Cortez D, Wang Y, Qin J, Elledge S. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 17.Cressman V, Backlund D, Avrutsdaya A, Leadon S, Godfrey V, Koller B. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol Cell Biol. 1999;19:7061–7075. doi: 10.1128/mcb.19.10.7061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 18.Easton D, Steele L, Fields P, Ormiston W, Averill D, Daly P, McManus R, Neuhausen S, Ford D, Wooster R, Cannon-Albright L, Stratton M, Goldgar D. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12–13. Am J Hum Genet. 1997;61:120–128. doi: 10.1086/513891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flygare J, Benson F, Hellgren D. Expression of the human RAD51 gene during the cell cycle in primary human peripheral blood lymphocytes. Biochim Biophys Acta. 1996;1312:231–236. doi: 10.1016/0167-4889(96)00040-7. [DOI] [PubMed] [Google Scholar]
- 20.Gowen L C, Johnson B L, Latour A M, Sulik K K, Koller B. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat Genet. 1996;12:191–194. doi: 10.1038/ng0296-191. [DOI] [PubMed] [Google Scholar]
- 21.Gudas J, Li T, Nguyen H, Jensen D, Rauscher F I, Cowan K. Cell cycle regulation of BRCA1 messenger RNA in human breast epithelial cells. Cell Growth Differ. 1996;7:717–723. [PubMed] [Google Scholar]
- 22.Haile D T, Parvin J D. Activation of transcription in vitro by the BRCA1 carboxyl-terminal domain. J Biol Chem. 1999;274:2113–2117. doi: 10.1074/jbc.274.4.2113. [DOI] [PubMed] [Google Scholar]
- 23.Hakem R, de la Pompa J, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, Firpo E, Hui C, Roberts J, Rossant J, Mak T. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1024. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Using antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 25.Hood J K, Silver P A. In or out? Regulating nuclear transport. Curr Opin Cell Biol. 1999;11:241–247. doi: 10.1016/s0955-0674(99)80032-5. [DOI] [PubMed] [Google Scholar]
- 26.Houvras Y, Benezra M, Zhang H, Manfredi J J, Weber B L, Licht J D. BRCA1 physically and functionally interacts with the ATF1 transcription factor. J Biol Chem. 2000;275:36230–36237. doi: 10.1074/jbc.M002539200. [DOI] [PubMed] [Google Scholar]
- 27.Jensen D E, Proctor M, Marquis S T, Gardner H P, Ha S I, Chodosh L A, Ishov A M, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz D C, Wilkinson K D, Maul G G, Barlev N, Berger S L, Prendergast G C, Rauscher F J., III BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 28.Jensen R, Thompson M, Jetton T, Szabo C, van der Meer R, Helou B, Tronick S, Page D, King M-C, Holt J. BRCA1 is secreted and exhibits properties of a granin. Nat Genet. 1996;12:303–308. doi: 10.1038/ng0396-303. [DOI] [PubMed] [Google Scholar]
- 29.Lee J S, Collins K M, Brown A L, Lee C H, Chung J H. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Chen P L, Subramanian T, Chinnadurai G, Tomlinson G, Osborne C K, Sharp Z D, Lee W H. Binding of CtIP to the BRCT repeats of BRCA1 involved in the transcription regulation of p21 is disrupted upon DNA damage. J Biol Chem. 1999;274:11334–11338. doi: 10.1074/jbc.274.16.11334. [DOI] [PubMed] [Google Scholar]
- 31.Liu C-Y, Flesken-Nikitin A, Li S, Zeng Y, Lee W-H. Inactivation of the mouse Brca1 gene leads to failure in the morphogenesis of the egg cylinder in early postimplantation development. Genes Dev. 1996;10:1835–1843. doi: 10.1101/gad.10.14.1835. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig T, Chapman D L, Papaioannou V E, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 33.Magdinier F, Dalla Venezia N, Lenoir G M, Frappart L, Dante R. BRCA1 expression during prenatal development of the human mammary gland. Oncogene. 1999;18:4039–4043. doi: 10.1038/sj.onc.1202780. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro A, August A, Hanafusa H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc Natl Acad Sci USA. 1996;93:13595–13599. doi: 10.1073/pnas.93.24.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moroianu J. Nuclear import and export pathways. J Cell Biochem. 1999;32–33(Suppl):76–83. doi: 10.1002/(sici)1097-4644(1999)75:32+<76::aid-jcb10>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 37.Moynahan M, Chiu J, Koller B, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 38.Narod S A, Ford D, Devilee P, Barkardottir R B, Lynch H T, Smith S A, Ponder B A, Weber B L, Garber J E, Birch J M, et al. An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:254–264. [PMC free article] [PubMed] [Google Scholar]
- 39.Neish A S, Anderson S F, Schlegel B P, Wei W, Parvin J D. Factors associated with the mammalian RNA polymerase II holoenzyme. Nucleic Acids Res. 1998;26:847–853. doi: 10.1093/nar/26.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouchi T, Lee S W, Ouchi M, Aaronson S A, Horvath C M. Collaboration of signal transducer and activator of transcription 1 (STAT1) and BRCA1 in differential regulation of IFN-gamma target genes. Proc Natl Acad Sci USA. 2000;97:5208–5213. doi: 10.1073/pnas.080469697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paull T T, Rogakou E P, Yamazaki V, Kirchgessner C U, Gellert M, Bonner W M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 42.Raderschall E, Golub E I, Haaf T. Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc Natl Acad Sci USA. 1999;96:1921–1926. doi: 10.1073/pnas.96.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajan J V, Wang M, Marquis S T, Chodosh L A. Brca2 is coordinately regulated with Brca1 during proliferation and differentiation in mammary epithelial cells. Proc Natl Acad Sci USA. 1996;93:13078–13083. doi: 10.1073/pnas.93.23.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruffner H, Verma I. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci USA. 1997;94:7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlegel B P, Green V J, Ladias J A, Parvin J D. BRCA1 interaction with RNA polymerase II reveals a role for hRPB2 and hRPB10alpha in activated transcription. Proc Natl Acad Sci USA. 2000;97:3148–3153. doi: 10.1073/pnas.070452397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scully R, Anderson S, Chao D, Wei W, Ye L, Young R, Livingston D, Parvin J. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1997;94:5605–5610. doi: 10.1073/pnas.94.11.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scully R, Anderson S F, Chao D M, Wei W, Ye L, Young R A, Livingston D M, Parvin J D. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1997;94:5605–5610. doi: 10.1073/pnas.94.11.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scully R, Chen J, Ochs R, Keegan K, Hoekstra M, Feunteun J, Livingston D. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 49.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston D. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 50.Scully R, Ganesan S, Brown M, De Caprio J A, Cannistra S, Feunteun J, Schnitt S, Livingston D M. Location of BRCA1 in human breast and ovarian cancer cells. Science. 1996;272:123–125. doi: 10.1126/science.272.5258.123. [DOI] [PubMed] [Google Scholar]
- 51.Scully R, Ganesan S, Vlasakova K, Chen J, Socolovsky M, Livingston D. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol Cell. 1999;4:1093–1099. doi: 10.1016/s1097-2765(00)80238-5. [DOI] [PubMed] [Google Scholar]
- 52.Shen S X, Weaver Z, Xu X, Li C, Weinstein M, Chen L, Guan X Y, Ried T, Deng C X. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17:3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- 53.Tashiro S, Kotomura N, Shinohara A, Tanaka K, Ueda K, Kamada N. S phase specific formation of the human Rad51 protein nuclear foci in lymphocytes. Oncogene. 1996;12:2165–2170. [PubMed] [Google Scholar]
- 54.Thakur S, Zhang H, Peng Y, Le H, Carroll B, Ward T, Yao J, Farid L, Couch F, Wilson R, Weber B. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997;17:444–452. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas J, Smith M, Tonkinson J, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–809. [PubMed] [Google Scholar]
- 56.Tibbetts R S, Cortez D, Brumbaugh K M, Scully R, Livingston D, Elledge S J, Abraham R T. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaughn J, Davis P, Jarboe M, Huper G, Evans A, Wiseman R, Berchuck A, Iglehart J, Futreal P, Marks J. BRCA1 expression is induced before DNA synthesis in both normal and tumor-derived breast cells. Cell Growth Differ. 1996;7:711–715. [PubMed] [Google Scholar]
- 58.Wilson C, Payton M, Elliott G, Buass F, Cajulis E, Grosshans D, Ramos L, Reese D, Slamon D, Calzone F. Differential subcellular localization, expression and biological toxicity of BRCA1 and the splice variant BRCA1-delta11b. Oncogene. 1997;14:1–16. doi: 10.1038/sj.onc.1200924. [DOI] [PubMed] [Google Scholar]
- 59.Wu L C, Wang Z W, Tsan J T, Spillman M A, Phung A, Xu X L, Yang M C, Hwang L Y, Bowcock A M, Baer R. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 60.Xu X, Wagner K U, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng C X. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Weaver Z, Linke S P, Li C, Gotay J, Wang X W, Harris C C, Ried T, Deng C X. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto A, Taki T, Yagi H, Habu T, Yoshida K, Yoshimura Y, Yamamoto K, Matsushiro A, Nishimune Y, Morita T. Cell cycle-dependent expression of the mouse Rad51 gene in proliferating cells. Mol Gen Genet. 1996;251:1–12. doi: 10.1007/BF02174338. [DOI] [PubMed] [Google Scholar]
- 63.Yu X, Wu L C, Bowcock A M, Aronheim A, Baer R. The C-terminal (BRCT) domains of BRCA1 interact in vivo with CtIP, a protein implicated in the CtBP pathway of transcriptional repression. J Biol Chem. 1998;273:25388–25392. doi: 10.1074/jbc.273.39.25388. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Somasundaram K, Peng Y, Tian H, Bi D, Weber B L, El-Deiry W S. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16:1713–1721. doi: 10.1038/sj.onc.1201932. [DOI] [PubMed] [Google Scholar]
- 65.Zheng L, Pan H, Li S, Flesken-Nikitin A, Chen P, Boyer T G, Lee W. Sequence-specific transcriptional corepressor function for BRCA1 through a novel zinc finger protein, ZBRK1. Mol Cell. 2000;6:757–768. doi: 10.1016/s1097-2765(00)00075-7. [DOI] [PubMed] [Google Scholar]
- 66.Zhong Q, Chen C F, Li S, Chen Y, Wang C C, Xiao J, Chen P L, Sharp Z D, Lee W H. Association of BRCA1 with the hRad50-hMre11–p95 complex and the DNA damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]