Abstract
This paper investigates the composition of major, trace, and rare earth elements in 15 different species of wild edible mushrooms and the possible effect of urban pollution on elemental uptake. The collected mushrooms include different species from the green areas of the city, exposed to urban pollution, and from the forests, with limited anthropogenic influence. Through a comprehensive approach that included the analysis of 46 elements, an attempt was made to expand knowledge about element uptake by mushroom fruiting bodies. The results showed a wide variability in the composition of mushrooms, suggesting a number of factors influencing their element uptake capacity. The data obtained do not indicate significant exposure to anthropogenic influences, regardless of sampling location. While major elements’ levels appear to be influenced more by species-specific affinities, this is not true for trace elements, whose levels presumably reflect the geochemical characteristics of the sampling site. However, the risk assessment showed that consumption of excessive amounts of the mushrooms studied, both from urban areas and from forests, may have adverse health effects.
Keywords: mushrooms, trace elements, rare earth elements, urban soils, forest
1. Introduction
Mushrooms have been a part of the human diet for centuries. Nowadays, they are not only readily consumed, but they are also very popular in the category of healthy foods and delicacies. Beyond diets, mushrooms feature in some types of traditional medicine, but also as fractions in various medical supplements [1,2]. In Europe, they are also defined as “novel foods”, that is, foods that were not consumed to any significant extent before 1997.
Since food consumption is generally considered the most likely route of human exposure to heavy metals, the levels of individual metal species in mushrooms became of interest not only to the scientific community, but also to many regulatory agencies. Due to the increased intake of heavy metals and their persistence in the environment, these pollutants are among the most serious environmental problems. Their accumulation in soils as well as slow degradation processes enable their efficient transfer into living organisms, and consequently through the food chain to humans [3]. For this reason, the content of metals and metalloids in mushrooms, both wild and cultivated, should be continuously monitored.
Mushrooms are known to readily accumulate high concentrations of metals and metalloids, such as mercury, cadmium, lead, copper, arsenic, and radionuclides [4,5,6,7]. They take up elements from a substrate through an extensive mycelium, and their total content is influenced by both fungal and environmental factors [8,9,10]. In addition to species-specific affinity, the morphological part of the fruiting body, developmental stages, age of the mycelium, biochemical composition, interval between fructifications, and element accumulation in mushrooms is also influenced by environmental factors, such as the amount of organic matter, pH, temperature, water content, and element concentrations in the soil [9,11,12]. In urban areas, soils are additionally burdened by the influence of traffic and industrial pollution, which may lead to the accumulation of heavy metals and metalloids that are of particular importance for human health due to their toxic effects even at low concentrations, such as As, Cd, and Pb [3,13,14,15]. Mushrooms growing near pollution sources are generally avoided for consumption, and those from forests are considered safe, while the geochemical composition of the underlying soil is usually neglected as a potentially significant source of elements. This is particularly important when considering mushrooms growing in areas with naturally elevated concentrations of metals in the soil [12]. Consequently, elevated levels of metals and metalloids in mushrooms have been reported not only near industrial areas [16,17] and intensive traffic [9,10,13,16,18,19], but also in areas with naturally elevated metal concentrations due to the geological composition of the bedrock [12].
To date, numerous studies have been carried out on the content of individual elements in fruiting bodies from different areas [20], such as France [21], the Czech Republic [22], Poland [4,23,24,25], Slovakia [6], Spain [9,10,26], Portugal [16], Turkey [27,28,29,30], USA [31], China [32,33], Greece [18], Serbia [34,35], and Italy [36,37,38,39]. However, published results often differ significantly and usually include a narrow range of elements and/or mushroom species studied [20]. Studies on the metal content in mushrooms from Croatia are also sparse and mostly limited to several measured elements [40,41,42].
In this work, we report on the concentration of 46 elements, including macroelements, trace elements, and rare earth elements with Y (REY), determined in 15 edible wild mushroom species from urban and forest areas in northwestern Croatia. As this region is characterized by naturally elevated levels of aluminum (Al), bismuth (Bi), cobalt (Co), chromium (Cr), cesium (Cs), iron (Fe), molybdenum (Mo), nickel (Ni), lead (Pb), antimony (Sb), scandium (Sc), titanium (Ti), thallium (Tl), uranium (U), vanadium (V), zinc (Zn), and rare earth elements, including yttrium (REY) [12,43,44], the distribution of elements in edible mushroom species from the study area will provide new insights into metal uptake by mushroom fruiting bodies.
This comprehensive study aims to increase the knowledge on element distribution in different edible mushrooms, and particularly to investigate the importance of site-specific properties (soil geochemistry) versus the species-specific uptake of elements in the investigated mushrooms and the influence of urban pollution on element uptake by mushrooms. This was accomplished by (i) studying the distribution of elements in different mushrooms from specific locations, and (ii) comparing element content in mushrooms from urban and forest locations. To assess the health risks associated with the consumption of the wild mushrooms studied, the daily intake and hazard index for elements that may have a negative impact on human health were also calculated.
2. Materials and Methods
2.1. Study Area
During the sampling campaign in October 2016, an attempt was made to collect different edible wild mushroom species from the urban and forest areas (Table 1); mushrooms of a particular species were collected from different locations, and different species were collected from each sampling location. Sampling was conducted in northwestern Croatia (Figure 1), in green areas within the capital city of Zagreb, and in rural parts of Karlovac County. The city of Zagreb has a population of approximately 800,000 people inhabiting an area of 641 km2. Green areas of the city where the mushrooms were collected include forested areas of the city parks: Dotrščina (2 km2; samples 1/7272–1/7275), Jelenovac (54 ha; samples 1/7258–1/7263), and Maksimir (316 ha; samples 1/7267–1/7269), as well as smaller green areas in residential areas of the city (samples 1/7256, 1/7293, and 1/7294). These mushrooms, although exposed to urban pollution due to their location in the capital, were not sampled in the immediate vicinity of roads, but in the central areas of city parks. Only samples 1/7293 and 1/7294 were collected in the immediate vicinity of a road with low traffic volume. The city of Zagreb, despite being a capital city with industries and dense traffic, had good air quality with low PM10 content at the time of sampling, as shown by the data collected by the Croatian Environmental Agency. The samples from Karlovac County (1/7284, 1/7288, 1/7291, and 1/7292) included various forest sites distributed throughout the district, far from roads and other pollution sources.
Table 1.
Investigated species of mushrooms, and their lifestyle and location information. Trophic mode (a saprotroph, b pathotroph/saprotroph or c symbiotroph).
| Taxon/Family | Locality | Habitat | Substrate | |
|---|---|---|---|---|
| Urban area, Zagreb | ||||
| 1/7256 b |
Pleurotus dryinus
Pleurotaceae |
Črnomerec | forest of Populus nigra, Acer campestre,
Sambucus nigra |
wood |
| 1/7258 a |
Infundibulicybe gibba
Tricholomataceae |
Jelenovac | forest of Quercus robur, Carpinus betulus,
Fagus sylvatica |
soil |
| 1/7259 a |
Lycoperdon excipuliforme
Agaricaceae |
Jelenovac | forest of Quercus robur, Carpinus betulus,
Fagus sylvatica |
soil |
| 1/7260 a |
Lycoperdon perlatum
Agaricaceae |
Jelenovac | forest of Quercus robur, Carpinus betulus,
Fagus sylvatica |
soil |
| 1/7262 a |
Paralepista flaccida
Tricholomataceae |
Jelenovac | forest of Quercus robur, Carpinus betulus,
Fagus sylvatica |
soil |
| 1/7263 a |
Psathyrella multipedata
Psathyrellaceae |
Jelenovac | forest of Quercus robur, Carpinus betulus,
Fagus sylvatica |
soil |
| 1/7267 a |
Lycoperdon perlatum
Agaricaceae |
Maksimir Park | forest of Quercus robur, Q. petraea,
Carpinus betulus |
soil |
| 1/7268 a |
Psathyrella piluliformis
Psathyrellaceae |
Maksimir Park | forest of Quercus robur, Q. petraea,
Carpinus betulus |
wood |
| 1/7269 a |
Macrolepiota procera
Agaricaceae |
Maksimir Park | forest of Quercus robur, Q. petraea,
Carpinus betulus |
soil |
| 1/7272 a |
Infundibulicybe gibba
Tricholomataceae |
Dotršćina Park | forest of Quercus petraea, Carpinus betulus, Fagus sylvatica |
soil |
| 1/7273 a |
Psathyrella piluliformis
Psathyrellaceae |
Dotršćina Park | forest of Quercus sp., Carpinus betulus | wood |
| 1/7274 a |
Coprinus comatus
Agaricaceae |
Dotršćina Park | forest of Quercus sp., Carpinus betulus | soil |
| 1/7275 a |
Hymenopellis radicata
Physalacriaceae |
Dotršćina Park | forest of Quercus sp., Carpinus betulus | wood |
| 1/7293 a |
Leucoagaricus leucothites
Agaricaceae |
Ruđer Bošković Institute | grassland | soil |
| 1/7294 b |
Agrocybe cylindracea
Strophariaceae |
Ruđer Bošković Institute | grassland, on old living tree of Populus nigra |
wood |
| Forest area, Karlovac County | ||||
| 1/7284 c |
Lactarius deterrimus
Russulaceae |
Dvorišće Ozaljsko | grassland, near planted Picea abies | soil |
| 1/7288 c |
Lactarius deterrimus
Russulaceae |
Vukova Gorica | grassland, near planted Picea abies | soil |
| 1/7291 a |
Lepista nuda
Tricholomataceae |
Vukova Gorica | forest of Quercus robur, Corylus avellana | soil |
| 1/7292 c |
Craterellus cornucopioides
Cantharellaceae |
Novaki Ozaljski | forest of Fagus sylvatica | soil |
Figure 1.
Map of the study area, its geographical position (A), and sampling area (B).
A total of 19 samples, representing 15 edible mushroom species from the phylum Basidiomycota, were collected and analyzed for total element concentration (Table 1). Photographs of the identified mushroom species and details of collection, morphological and molecular identification, and screening of bioactive compounds can be found elsewhere [45]. Basic information on the mushroom species and sampling is also presented here (Table 1) to be easily accessible for interpretation of the data from this study. All mushrooms used in this study are stored in the Croatian National Fungarium (CNF).
2.2. Sample Collection and Preparation
In the laboratory, soil particles were carefully removed from the fruiting bodies. Approximately 5 g of raw fruiting bodies from each sample was stored in a separate plastic bag at −82 °C. Prior to analyses, the frozen samples were freeze-dried (Finn-Aqua Lyovac GT 2) and pulverized with liquid nitrogen.
2.3. Multi-Element Analysis
Prior to multi-element analysis, subsamples (0.05 g) of the previously freeze-dried samples were subjected to digestion in the microwave oven (Multiwave ECO, Anton Paar, Graz, Austria) with 7 mL of HNO3 (65%, supra pur) and 0.1 mL of HF (48%, pro analysi) [46]. Digests were acidified with 2% (v/v) HNO3 (65%, supra pur), without further dilution, and indium (In, 1 µg L−1) was added as an internal standard.
The multi-element analysis was performed by High-Resolution Inductively Coupled Plasma Mass Spectrometry (HR-ICP-MS) using an Element 2 instrument (Thermo, Bremen, Germany). The instrument conditions and measurement parameters used in this work are described in the ref. [47]. Standards for multi-element analysis were prepared by appropriate dilution of a multi-element reference standard (Analytika, Prague, Czech Republic) containing Al, As, Be, Cd, Co, Cr, Cu, Fe, Li, Mn, Ni, Pb, Rb, Sr, and Ti, to which single-element standard solutions of Sn (Analytika, Prague, Czech Republic) and Sb (Analytika, Prague, Czech Republic) were added. For the analysis of REY, separate standards were prepared by appropriate dilution of a multi-elemental reference standard (Analytika, Prague, Czech Republic) containing Ce, Dy, Er, Eu, Gd, Ho, La, Lu, Nd, Pr, Sm, Tb, Tm, Y, and Yb.
The samples were analyzed for the total concentration of 46 elements (Al, As, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Gd, Ho, K, La, Li, Lu, Mg, Mn, Mo, Na, Nd, Ni, Pb, Pr, Rb, Sb, Sc, Se, Sn, Sm, Sr, Tb, Ti, Tl, Tm, U, V, Y, Yb, and Zn). All concentrations refer to dry matter.
2.4. Quality Control
Limits of detection (LOD) and limits of quantification (LOQ) were calculated as 3 and 10 times, respectively, the standard deviation of 10 consecutive measurements of analyte concentration in the procedural blank. Quality control of the analytical procedure was performed by simultaneous analysis of the blank sample and the certified reference material for Citrus leave (NCS ZC73018, China National Analysis Center for Iron and Steel). Good agreement was obtained between the analyzed and certified concentrations for all the elements measured, with recoveries ranging from 85% to 106%. Measurement precision was determined from five consecutive measurements in two Citrus leave CRM samples, and averaged 5%.
2.5. Health Risk Assessment
The assessment of non-carcinogenic health risks of heavy metals from mushroom consumption was performed according to the USEPA procedure described in the ref. [48]. The risk assessment included metals known to be harmful to human health, such as As, Ba, Be, Cd, Cu, Fe, Mn, Ni, Pb, Sb, Sr, You, and Zn. First, the intake factor, that is, the estimated daily intake, was calculated according to Equation (1):
| (1) |
where Cmetal is the metal concentration in dried mushrooms (µg kg−1), IR is the intake rate (in kg per person per day) corresponding to 0.03 kg of dried mushrooms [11], ED is the exposure duration (30 years for adults), EF is the exposure frequency (350 days per year−1), BW is the average body weight (70 kg for adults), and AT is the average exposure duration (10,500 days, calculated Cmeta as EF × ED).
Then, the target hazard quotient (THQ) of a given metal (Equation (2)) was calculated as the ratio between the estimated daily intake (EDI) and the reference dose (RfD). The RfD value is an estimate of the daily dose that is unlikely to pose a significant risk to human health over a lifetime [49]. This value is specific to the element being assessed and is defined in Integrated Risk Information System assessments (IRIS) under the USEPA programme.
| (2) |
Finally, the risk assessment related to the cumulative effect of several heavy metals present in the mushrooms was performed by calculating the overall hazard index as the sum of exposures scaled by the toxicity of each metal (Equation (3)),
| (3) |
where THQi is the target hazard quotient for a single metal.
2.6. Statistical Analysis
Data were statistically analyzed using STATISTICA 7.0 (StatSoft Inc., Tulsa, OK, USA). The Spearman rank correlation coefficient was used for correlation of investigated elements in studied mushrooms. Multivariate principal component analysis (PCA) was performed on the data matrix consisting of element concentrations. The significance level was set at p < 0.05.
3. Results
The concentrations of macro- and trace elements determined in the studied mushrooms, along with calculated LODs and LOQs, are presented in Table 2. Elements that showed the widest range of concentrations among different mushrooms were As, Co, Cs, Mo, Rb, and Tl, while Be, Cr, Fe, K, Li, Mg, Mn, Ni, Sn, and Zn were present in a narrow range. The measured elements appeared in the following order of abundance: K > Mg > Na > Ca > Rb > Zn > Fe > Al > Cu > Mn > Ti > Cd > Pb > As > Cs > Ba > Ni > Se > Sr > Mo > ∑REY > Cr > Co > V > Sn > Tl > Li > Sb > U > Bi > Be.
Table 2.
Concentrations (in mg kg−1, dry matter) of macro- and trace elements in the investigated mushrooms, including calculated LODs and LOQs (in mg kg−1), minimum (min), maximum (max), and average (avg) values.
| Al | As | Ba | Be | Bi | Ca | Cd | Co | Cr | Cs | Cu | |
| LOD | 2 | 0.003 | 0.05 | 0.001 | 0.001 | 15 | 0.002 | 0.002 | 0.03 | 0.002 | 0.03 |
| LOQ | 6 | 0.01 | 0.15 | 0.003 | 0.003 | 45 | 0.006 | 0.006 | 0.1 | 0.006 | 0.1 |
| 1/7256 | 7.83 | 0.019 | 0.096 | <DL | <DL | 26 | 0.681 | 0.006 | 0.313 | 0.016 | 2.19 |
| 1/7258 | 30.9 | 0.318 | 1.599 | <DL | 0.001 | 171 | 0.379 | 0.026 | 0.265 | 0.124 | 46.6 |
| 1/7259 | 19.4 | 0.394 | 0.518 | <DL | 0.004 | 187 | 0.356 | 0.125 | 0.419 | 0.002 | 38.6 |
| 1/7260 | 17.0 | 0.542 | 0.484 | <DL | 0.003 | 164 | 0.313 | 0.171 | 0.378 | 0.003 | 84.3 |
| 1/7262 | 33.2 | 0.482 | 1.367 | <DL | 0.001 | 273 | 0.144 | 0.031 | 0.246 | 0.015 | 28.8 |
| 1/7263 | 23.5 | 3.684 | 1.126 | 0.001 | 0.003 | 374 | 0.365 | 0.100 | 0.221 | 0.124 | 78.0 |
| 1/7267 | 24.4 | 0.549 | 0.528 | <DL | 0.010 | 146 | 0.271 | 0.092 | 0.198 | 0.017 | 67.2 |
| 1/7268 | 99.3 | 0.087 | 1.725 | 0.001 | 0.002 | 216 | 0.396 | 0.084 | 0.305 | 0.333 | 11.3 |
| 1/7269 | 12.6 | 0.219 | 0.307 | <DL | 0.002 | 188 | 0.384 | 0.033 | 0.279 | 0.008 | 56.6 |
| 1/7272 | 27.6 | 0.661 | 0.979 | 0.002 | 0.001 | 262 | 0.216 | 0.018 | 0.306 | 0.037 | 52.4 |
| 1/7273 | 43.9 | 0.151 | 0.591 | 0.001 | 0.001 | 231 | 0.490 | 0.139 | 0.343 | 0.604 | 20.0 |
| 1/7274 | 22.9 | 0.489 | 0.580 | <DL | 0.001 | 545 | 0.486 | 0.058 | 0.608 | 0.006 | 42.3 |
| 1/7275 | 40.7 | 0.008 | 0.810 | <DL | <DL | 223 | 0.116 | 0.021 | 0.159 | 0.035 | 9.44 |
| 1/7284 | 91.0 | 0.639 | 1.453 | <DL | <DL | 276 | 0.593 | 0.069 | 0.268 | 0.167 | 16.5 |
| 1/7288 | 34.4 | 0.260 | 0.554 | <DL | 0.002 | 257 | 0.550 | 0.048 | 0.337 | 0.581 | 8.85 |
| 1/7291 | 26.1 | 0.593 | 0.512 | <DL | 0.001 | 255 | 0.177 | 0.125 | 0.223 | 0.043 | 41.0 |
| 1/7292 | 77.2 | 0.012 | 0.793 | 0.002 | 0.001 | 256 | 0.549 | 0.701 | 0.761 | 1.956 | 32.9 |
| 1/7293 | 13.0 | 0.310 | 0.294 | <DL | <DL | 144 | 4.547 | 0.043 | 0.278 | 0.482 | 26.2 |
| 1/7294 | 16.0 | <DL | 0.227 | <DL | <DL | 128 | 3.956 | 0.011 | 0.230 | 0.419 | 44.8 |
| avg | 34.8 | 0.52 | 0.77 | 0.001 | 0.002 | 227 | 0.79 | 0.10 | 0.32 | 0.26 | 37.3 |
| min | 7.83 | 0.01 | 0.10 | 0.001 | 0.001 | 25.6 | 0.12 | 0.01 | 0.16 | 0.002 | 2.19 |
| max | 99.3 | 3.68 | 1.73 | 0.002 | 0.010 | 545 | 4.55 | 0.70 | 0.76 | 1.96 | 84.3 |
| Fe | K | Li | Mg | Mn | Mo | Na | Ni | Pb | Rb | ||
| LOD | 3 | 15 | 0.002 | 6 | 0.25 | 0.005 | 3 | 0.03 | 0.03 | 0.03 | |
| LOQ | 10 | 45 | 0.006 | 18 | 0.75 | 0.015 | 10 | 0.1 | 0.1 | 0.1 | |
| 1/7256 | 11.0 | 42327 | 0.019 | 1706 | 5.58 | 0.018 | 77.2 | 0.325 | 0.072 | 14.7 | |
| 1/7258 | 95.7 | 25183 | 0.031 | 1086 | 21.5 | 0.557 | 60.4 | 0.559 | 0.135 | 10.3 | |
| 1/7259 | 52.5 | 17100 | 0.015 | 1194 | 13.6 | 0.332 | 70.3 | 0.611 | 1.11 | 5.48 | |
| 1/7260 | 103 | 22924 | 0.012 | 1597 | 27.7 | 0.355 | 36.1 | 0.742 | 0.509 | 2.59 | |
| 1/7262 | 49.4 | 41252 | 0.026 | 1395 | 31.4 | 0.812 | 53.5 | 0.363 | 0.228 | 2.69 | |
| 1/7263 | 49.0 | 65291 | 0.025 | 2014 | 11.6 | 0.488 | 829 | 0.547 | 0.352 | 24.6 | |
| 1/7267 | 79.7 | 17410 | 0.013 | 1520 | 38.2 | 0.375 | 41.6 | 0.472 | 0.868 | 26.5 | |
| 1/7268 | 87.9 | 66745 | 0.078 | 2067 | 22.9 | 0.015 | 932 | 0.327 | 0.261 | 289 | |
| 1/7269 | 60.6 | 33029 | 0.017 | 1300 | 11.5 | 0.409 | 49.5 | 0.358 | 0.437 | 10.1 | |
| 1/7272 | 76.1 | 27369 | 0.016 | 959 | 36.4 | 0.606 | 47.9 | 0.663 | 0.128 | 40.1 | |
| 1/7273 | 67.4 | 80836 | 0.037 | 2093 | 25.6 | 0.016 | 653 | 0.247 | 0.406 | 185 | |
| 1/7274 | 57.0 | 49527 | 0.013 | 1639 | 14.5 | 0.164 | 835 | 0.181 | 0.161 | 27.9 | |
| 1/7275 | 32.9 | 38559 | 0.027 | 1060 | 21.3 | 0.045 | 410 | 0.187 | 0.122 | 27.3 | |
| 1/7284 | 62.7 | 34991 | 0.079 | 1248 | 8.13 | 0.007 | 60.6 | 0.508 | 0.180 | 144 | |
| 1/7288 | 29.7 | 25886 | 0.021 | 1153 | 4.93 | 0.010 | 35.9 | 0.505 | 0.187 | 191 | |
| 1/7291 | 48.0 | 31622 | 0.010 | 1031 | 37.6 | 1.145 | 72.5 | 0.432 | 0.535 | 37.6 | |
| 1/7292 | 77.3 | 46135 | 0.074 | 939 | 25.1 | 0.103 | 74.1 | 1.595 | 4.019 | 299 | |
| 1/7293 | 23.9 | 60912 | 0.025 | 1876 | 7.26 | 0.235 | 1011 | 0.162 | 0.299 | 46.8 | |
| 1/7294 | 22.1 | 43277 | 0.012 | 1694 | 5.87 | 0.050 | 25.2 | 0.259 | 0.234 | 133 | |
| avg | 57.1 | 40546 | 0.029 | 1451 | 19.5 | 0.302 | 283 | 0.476 | 0.539 | 79.8 | |
| min | 11.0 | 17100 | 0.010 | 939 | 4.93 | 0.007 | 25.2 | 0.162 | 0.072 | 2.59 | |
| max | 103 | 80836 | 0.079 | 2093 | 38.2 | 1.145 | 1011 | 1.595 | 4.019 | 299 | |
| Sb | Sc | Se | Sn | Sr | Ti | Tl | U | V | Zn | ||
| LOD | 0.002 | 0.002 | 0.02 | 0.003 | 0.5 | 0.5 | 0.001 | 0.001 | 0.002 | 1.5 | |
| LOQ | 0.006 | 0.006 | 0.06 | 0.01 | 1.5 | 1.5 | 0.003 | 0.003 | 0.006 | 4.5 | |
| 1/7256 | 0.002 | <DL | <DL | 0.095 | 0.052 | 0.52 | <DL | <DL | 0.011 | 17.3 | |
| 1/7258 | 0.015 | <DL | 0.144 | 0.094 | 0.386 | 2.89 | 0.001 | <DL | 0.153 | 74.2 | |
| 1/7259 | 0.006 | 0.099 | 0.725 | 0.195 | 0.394 | 1.15 | <DL | 0.010 | 0.094 | 143 | |
| 1/7260 | 0.006 | 0.047 | 0.788 | 0.140 | 0.391 | 0.65 | 0.001 | 0.005 | 0.081 | 109 | |
| 1/7262 | 0.013 | 0.031 | 0.842 | 0.156 | 0.734 | 2.16 | 0.005 | 0.004 | 0.131 | 100 | |
| 1/7263 | 0.068 | <DL | 1.593 | 0.118 | 1.198 | 2.29 | 0.003 | 0.004 | 0.083 | 97.6 | |
| 1/7267 | 0.004 | <DL | 0.679 | 0.086 | 0.282 | 1.20 | <DL | <DL | 0.068 | 127 | |
| 1/7268 | 0.012 | 0.012 | <DL | 0.085 | 0.966 | 9.70 | 0.006 | 0.002 | 0.174 | 89.4 | |
| 1/7269 | 0.005 | 0.026 | 0.387 | 0.107 | 0.309 | 1.91 | 0.003 | <DL | 0.052 | 72.8 | |
| 1/7272 | 0.007 | 0.011 | 0.254 | 0.116 | 0.609 | 1.69 | 0.001 | 0.001 | 0.111 | 66.9 | |
| 1/7273 | 0.003 | 0.012 | <DL | 0.117 | 0.497 | 3.51 | 0.011 | 0.001 | 0.083 | 69.8 | |
| 1/7274 | 0.011 | 0.004 | 0.386 | 0.083 | 0.756 | 1.57 | 0.003 | 0.003 | 0.054 | 58.3 | |
| 1/7275 | 0.004 | <DL | <DL | 0.102 | 0.428 | 3.58 | 0.001 | <DL | 0.067 | 45.8 | |
| 1/7284 | 0.002 | 0.024 | 0.414 | 0.068 | 0.734 | 8.65 | 0.052 | 0.008 | 0.138 | 133 | |
| 1/7288 | 0.003 | 0.013 | 0.318 | 0.122 | 0.479 | 2.73 | 0.129 | 0.003 | 0.038 | 103 | |
| 1/7291 | 0.004 | <DL | 0.205 | 0.057 | 0.318 | 1.18 | 0.004 | <DL | 0.136 | 58.3 | |
| 1/7292 | 0.004 | 0.094 | <DL | 0.114 | 0.543 | 7.76 | 0.002 | 0.005 | 0.150 | 64.2 | |
| 1/7293 | 0.035 | <DL | 1.49 | 0.066 | 0.277 | 0.93 | 0.007 | 0.001 | 0.018 | 75.3 | |
| 1/7294 | 0.003 | <DL | <DL | 0.069 | 0.488 | 1.51 | 0.007 | 0.001 | 0.025 | 91.5 | |
| avg | 0.011 | 0.034 | 0.633 | 0.105 | 0.518 | 2.93 | 0.012 | 0.003 | 0.088 | 84.1 | |
| min | 0.002 | 0.004 | 0.144 | 0.057 | 0.052 | 0.52 | 0.001 | 0.001 | 0.011 | 17.3 | |
| max | 0.068 | 0.099 | 1.593 | 0.195 | 1.198 | 9.70 | 0.129 | 0.010 | 0.174 | 143 |
DL—detection limits.
The results of the measurement of REY (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y), along with calculated LODs and LOQs, are shown in Table 3. The description of REY distribution in the samples was based on geochemical classification into light (LREE—La, Ce, Pr, Nd, Sm, Eu, Gd) and heavy (HREE—Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y). The concentrations of REY in all the analyzed samples ranged over three orders of magnitude, from below the detection limit (Eu, Gd, Tb, Ho, Er, Tm, and Lu in some samples) to 0.137 mg kg−1 (Ce), with ΣREY ranging from 0.031 mg kg−1 to 0.452 mg kg−1 (Table 3). Among them, Ce was present in the highest concentrations in all samples and accounted for between 19% and 36% of the total REY, while Eu, Gd, Tb, Ho, Tm, and Lu had the lowest values. In general, the mushrooms showed great variability in terms of REY concentrations, with an RSD of up to 120% and ΣREY variability of up to one order of magnitude. The highest REY values were measured in L. deterrimus (1/7284, 0.452 mg kg−1), while the lowest were observed in P. dryinus (1/7256, 0.031 mg kg−1). LREEs were found to be more abundant in the majority of the samples studied, with LREE/HREE ratios ranging from 0.7 to 3.8 and the average being 1.9. The content of LREEs accounted for 41.9–79.3% of the total REYs in the mushrooms studied.
Table 3.
Concentration of REY (in mg kg−1, dry matter) in the investigated mushrooms, including calculated LODs and LOQs (in mg kg−1), minimum (min), maximum (max), and average (avg) values.
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | ∑REY | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| LOQ | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | |
| 1/7256 | 0.003 | 0.006 | 0.001 | 0.002 | 0.001 | <DL | <DL | <DL | 0.009 | <DL | <DL | <DL | 0.007 | <DL | 0.002 | 0.031 |
| 1/7258 | 0.012 | 0.028 | 0.003 | 0.012 | 0.003 | 0.001 | <DL | <DL | 0.011 | 0.001 | 0.001 | <DL | 0.008 | <DL | 0.012 | 0.092 |
| 1/7259 | 0.005 | 0.009 | 0.001 | 0.004 | 0.001 | 0.000 | <DL | <DL | 0.010 | <DL | 0.001 | <DL | 0.008 | <DL | 0.004 | 0.043 |
| 1/7260 | 0.006 | 0.010 | 0.001 | 0.005 | 0.001 | 0.001 | <DL | <DL | 0.011 | <DL | 0.001 | <DL | 0.008 | <DL | 0.007 | 0.051 |
| 1/7262 | 0.016 | 0.031 | 0.004 | 0.016 | 0.003 | 0.001 | <DL | <DL | 0.011 | 0.001 | 0.001 | <DL | 0.009 | <DL | 0.013 | 0.094 |
| 1/7263 | 0.021 | 0.039 | 0.006 | 0.022 | 0.006 | 0.001 | 0.002 | 0.001 | 0.013 | 0.001 | 0.002 | <DL | 0.008 | <DL | 0.024 | 0.146 |
| 1/7267 | 0.009 | 0.017 | 0.002 | 0.007 | 0.001 | 0.001 | <DL | <DL | 0.011 | <DL | <DL | <DL | 0.008 | <DL | 0.004 | 0.060 |
| 1/7268 | 0.053 | 0.115 | 0.013 | 0.057 | 0.010 | 0.002 | <DL | 0.001 | 0.016 | 0.002 | 0.003 | 0.001 | 0.010 | 0.001 | 0.032 | 0.316 |
| 1/7269 | 0.005 | 0.012 | 0.001 | 0.003 | 0.001 | <DL | <DL | <DL | 0.010 | <DL | <DL | <DL | 0.007 | <DL | 0.006 | 0.045 |
| 1/7272 | 0.015 | 0.026 | 0.003 | 0.012 | 0.002 | 0.001 | <DL | <DL | 0.011 | <DL | 0.001 | <DL | 0.008 | <DL | 0.009 | 0.088 |
| 1/7273 | 0.019 | 0.035 | 0.004 | 0.014 | 0.003 | 0.001 | <DL | <DL | 0.011 | 0.001 | 0.001 | <DL | 0.008 | <DL | 0.012 | 0.109 |
| 1/7274 | 0.010 | 0.019 | 0.003 | 0.010 | 0.003 | 0.001 | <DL | <DL | 0.010 | <DL | 0.001 | <DL | 0.007 | <DL | 0.006 | 0.070 |
| 1/7275 | 0.024 | 0.051 | 0.006 | 0.026 | 0.005 | 0.001 | <DL | <DL | 0.011 | 0.000 | 0.001 | <DL | 0.008 | <DL | 0.011 | 0.144 |
| 1/7284 | 0.093 | 0.137 | 0.017 | 0.063 | 0.011 | 0.002 | <DL | 0.002 | 0.020 | 0.003 | 0.006 | 0.001 | 0.014 | 0.001 | 0.082 | 0.452 |
| 1/7288 | 0.028 | 0.031 | 0.004 | 0.014 | 0.003 | 0.001 | <DL | 0.001 | 0.013 | 0.001 | 0.003 | <DL | 0.007 | <DL | 0.040 | 0.146 |
| 1/7291 | 0.016 | 0.024 | 0.003 | 0.014 | 0.003 | 0.001 | <DL | <DL | 0.008 | <DL | 0.001 | <DL | 0.006 | <DL | 0.009 | 0.085 |
| 1/7292 | 0.050 | 0.080 | 0.008 | 0.034 | 0.007 | 0.001 | <DL | 0.001 | 0.013 | 0.001 | 0.004 | 0.001 | 0.009 | 0.001 | 0.029 | 0.239 |
| 1/7293 | 0.008 | 0.013 | 0.001 | 0.006 | 0.002 | <DL | <DL | <DL | 0.010 | <DL | <DL | <DL | 0.007 | <DL | 0.004 | 0.051 |
| 1/7294 | 0.010 | 0.019 | 0.002 | 0.008 | 0.002 | <DL | <DL | <DL | 0.010 | <DL | 0.001 | <DL | 0.007 | <DL | 0.005 | 0.064 |
| avg | 0.021 | 0.037 | 0.004 | 0.017 | 0.004 | 0.001 | 0.002 | 0.001 | 0.012 | 0.001 | 0.002 | 0.001 | 0.008 | 0.001 | 0.016 | 0.122 |
| min | 0.003 | 0.006 | 0.001 | 0.002 | 0.001 | <DL | <DL | <DL | 0.008 | <DL | <DL | <DL | 0.006 | <DL | 0.002 | 0.031 |
| max | 0.093 | 0.137 | 0.017 | 0.063 | 0.011 | 0.002 | 0.002 | 0.002 | 0.02 | 0.003 | 0.006 | 0.001 | 0.014 | 0.001 | 0.082 | 0.452 |
DL—detection limits.
4. Discussion
4.1. Toxic Elements (Pb, Cd, As)
The non-essential metals Pb, Cd, and As show adverse effects on human health even at low concentrations. They readily accumulate in living organisms and interfere with metabolic processes and biological functions. In the investigated mushrooms, with a few exceptions, they were mostly present in low concentrations. A comparison of measured element concentrations with those from other studies in the region is shown in Table 4.
Table 4.
Concentration of macro- and trace elements in different mushrooms from the region (expressed in mg/kg or * g/kg). If not otherwise specified, the results refer to the fruiting body.
| Croatia | Serbia | Italy | |||||
|---|---|---|---|---|---|---|---|
| Element | Edible (Caps and Stipes) [40,41,42] |
Agaricus sp., Trichaptum biforme [12] |
M. procera (Caps and Stipes) [34,35] |
Edible [50] | Boletaceae (Edible) [51] | Different Fungal Species [38] | Morchella Group [37] |
| Al | 623–925 | 29–5664 | 54.2–396 | 27.7–608 | 0.05–0.33 | ||
| As | 0.19–3.64 | 0.01–3.40 | 0.03–1.66 | 0.10–11.6 | <DL–0.23 | ||
| Ba | 19.2–72.4 | 0.20–46 | 1.20–8.7 | 0.33–4.06 | |||
| Be | 0.02–0.41 | <DL | |||||
| Bi | 0.04–0.08 | 0.02–1.40 | <DL | ||||
| Cd | 0.60–3.23 | 0.02–0.18 | 0.04–43.5 | 0.27–2.93 | 0.68–2.94 | 0.16–101 | 0.18–21.5 |
| Co | 0.17–2.92 | <DL–12.0 | 0.07–0.72 | 0.06–0.28 | |||
| Cr | 0.77–3.85 | 1.32–13.19 | 0.20–13.8 | 3.25–10.8 | 0.16–1.34 | 0.26–2.18 | |
| Cs | 0.15–1.18 | 0.05–0.48 | |||||
| Cu | 7.41–78.2 | 6.67–32.4 | 29–304 | 12.2–73.6 | 4.66–34.3 | 10.1–63 | |
| Fe | 49.3–154 | 365–4905 | 30–4018 | 45.9–319 | 24.4–515 | 35–517 | |
| Li | 0.49–7.57 | 0.21–1.27 | 0.03–0.67 | ||||
| Mn | 18.9–248 | 7.6–367 | 9.0–35.5 | 2.91–23.8 | 7.5–46.3 | ||
| Mo | 0.16–0.43 | 1.14–2.3 | 0.2–0.45 | ||||
| Ni | 2.02–4.10 | 1.02–7.16 | 0.09–11.7 | 2.24–5.04 | 0.40–1.69 | 0.6–2.33 | |
| Pb | 0.48–1.91 | 1.29–5.73 | <DL–14.3 | 6.32–9.8 | 0.29–10.6 | 0.58–10.6 | <DL–1.09 |
| Rb | 2.28–12.6 | 23.74–500 | 2.55–36.6 | ||||
| Sb | 0.05–0.28 | 4.9–26.1 | 0.01–0.05 | ||||
| Sc | |||||||
| Se | 0.17–3.3 | 0.04–2.32 | 0.2–94.4 | <DL–0.49 | |||
| Sn | 0.14–0.61 | <DL | |||||
| Sr | 7.98–21.7 | 0.08–29.9 | 1.15–4.34 | 0.50–4.71 | 0.8–5.8 | ||
| Ti | 59.02–647 | 1.2–156 | 6.31–18.8 | ||||
| Tl | 0.01–0.17 | 0.002–0.13 | |||||
| You | 0.04–0.46 | 0.001–0.02 | |||||
| V | 1.34–15.7 | 0.06–1.26 | |||||
| Zn | 41.99–90.56 | 21.3–59.1 | 27.3–535 | 38.7–117 | 17.6–301.6 | 99.7–259 | |
| Ca * | 0.02–4.43 | 0.55–2.12 | 0.11–2.33 | 0.28–3.51 | |||
| K * | 11–120 | 11.3–41.8 | 10.1–21.2 | 25.6–> 43 | |||
| Mg * | 0.69–3.4 | 0.29–1.12 | 0.79–2.64 | ||||
| Na * | 11–1900 | 0.41–0.93 | 39.8–916 | 0.1–0.83 | |||
The content of lead in the studied mushrooms ranged from 0.072 mg kg−1 in P. dryinus (1/7256) to 1.11 mg kg−1 in L. excipuliforme (1/7259) (Table 2). The exception was C. cornucopioides (1/7292) with the determined concentration of 4.02 mg kg−1. These values are within the usual range of concentrations of Pb in wild mushrooms from uncontaminated sites (<5 mg kg−1; [52]). Pb concentrations in certain species (A. cylindracea, M. procera, L. deterrimus, C. comatus, L. nuda, L. leucothites, and L. perlatum) were sometimes significantly lower compared to available data from other studies in the region [35,40,41,42] (Table 4) and in other areas [10,19,40,53,54,55].
Uptake from contaminated soil, where it is mostly deposited by atmospheric processes, is considered the main source of Pb in wild mushrooms [19]. Although its use in gasoline has been banned, traffic is still the main source of Pb in urban soils due to its accumulated amounts [15], and recent studies have demonstrated a direct influence of traffic on Pb concentrations [16,19].
The narrow range of Pb concentrations in this study suggests a limited influence of traffic on Pb content in the studied mushrooms. This is also supported by the low content in the species C. comatus (1/7274), L. perlatum (1/7260, 1/7267), and L. nuda (1/7291), which readily accumulate Pb and are considered good indicators of traffic pollution [9,52]. However, it should be noted that mushrooms were mostly sampled from larger green areas within the city, while in studies where traffic pollution caused elevated Pb levels in mushrooms, samples were collected closer to roads (up to 50 m), which may have contributed to pollution levels [9,13,16,56].
Schlecht and Säumel [19] found that although traffic pollution undoubtedly affected Pb levels in mushrooms, high Pb concentrations were also determined in mushrooms from areas where low urban pollution is expected, while low concentrations were found in mushrooms from potentially polluted areas [17]. Although the Pb concentrations determined in this study do not exceed the usual level in mushrooms, it can be observed that the highest concentration was determined in C. cornucopioides (1/7292) sampled in a forest far from any significant source of pollution, such as the impact of the industry or traffic (Table 2), implying that Pb uptake in this case is mainly determined by the geochemistry of the underlying soil. This is supported by the fact that this species also had elevated or maximal concentrations of lithogenic elements (Al, Be, Cr, Cs, Li, Ni, Rb, REY, Ti, and V; Table 2). However, genetic factors appeared to predominate in Pb uptake by L. deterrimus, as similar Pb concentrations were obtained in the two samples from distant sites (1/7284 and 1/7288).
Cadmium concentrations in the investigated mushrooms varied from 0.116 mg kg−1 in H. radicata (1/7275) to 0.681 mg kg−1 in P. dryinus (1/7256), with the exception of L. leucothites (1/7293) and A. cylindracea (1/7294), for which much higher values (4.55 mg kg−1 and 3.96 mg kg−1, respectively) were obtained (Table 2). The values obtained are in agreement with previous studies from the region [40,41,42,50,51] (Table 4) and are within the accepted range of values for wild mushrooms (0.5–5 mg kg−1; [52]). The values obtained for certain species (C. comatus, L. nuda, L. perlatum, M. procera) are similar to those previously determined [40,53]. However, higher [33,54,57,58], as well as lower [27,53,55] concentrations have also been reported.
According to Melgar et al. [58], bioaccumulation of Cd by mushrooms is species-dependent, and the age of the mycelium and the period between fructifications are suggested as important factors in determining the metal content. However, higher Cd levels in plants, like Pb, are mostly associated with urban contamination [59]. Schlecht and Säumel [19] found that traffic pollution affected Cd levels only in some mushroom species, highlighting site-specific characteristics as a determining factor for uptake. This is supported by the results of our study, as the two mushroom species (L. leucothites (1/7293) and A. cylindracea (1/7294)) with higher Cd levels (Table 2) were sampled at the same site, near a road in the capital city of Zagreb (Table 1), and thus were exposed to the influence of nearby traffic. Considering that these two species have different lifestyles, that is, grow on different substrates (L. leucothites in soil and A. cylindracea on wood), but both have significantly elevated Cd levels, it seems that the sampling location, such as soil properties and/or urban pollution, had a predominant influence on the uptake of Cd by these mushrooms. A positive correlation between Cd and typical lithophilic elements, such as Cs and Rb (r = 0.53 and 0.51, respectively, p < 0.05; Table S1), confirms that soil geochemistry is an important factor in Cd uptake.
Arsenic concentrations were below 0.7 mg kg−1 (Table 2), with the exception of P. multipedata (1/7263), in which 3.68 mg kg−1 was determined. Other mushrooms from the same sampling area (Jelenovac; 1/7258, 1/7259, 1/7260, 1/7262) or the same family (Psathyrellaceae; 1/7268 and 1/7273) had significantly lower values, indicating species-specific affinities as the main source of the elevated As content. However, P. multipedata was not recognized as an As accumulator in previous studies, and this maximum is outside the accepted range for As concentration for mushrooms from uncontaminated areas (<1 mg kg−1, [52]).
Vetter [60] found that the level of As in a given species is the result of several factors, both environmental and genetic, with some species having an accumulative affinity for As, regardless of their habitat. In this study, lower As levels were found in mushrooms growing on wood (1/7256, 1/7268, 1/7273, 1/7275, 1/7294), supporting the work of Falandysz and Rizal [61], who propose geogenic sources as the most important factor for As uptake.
The established maximum level for Cd and Pb in edible mushrooms is regulated by EU regulations [62,63] and is 0.3 mg kg−1 for Pb and 1 mg kg−1 for Cd, wet weight (3 and 10 mg kg−1 dry weight at 90% moisture content). The maximum amount of As in fresh mushrooms is regulated by Croatian legislation to 0.3 mg kg−1 (3 mg kg−1 dry weight) [64]. According to these regulations, P. multipedata (1/7263) and C. cornucopioides (1/7292) collected in the forest area far from direct anthropogenic influences are not considered safe for consumption due to elevated As and Pb content, respectively. Cd levels were well below the maximum allowable limit in dry products. These results support the conclusions of Fu et al. [32] who advise caution in the consumption of wild mushrooms due to possible accumulation of these potentially toxic elements.
4.2. Macronutrients (Ca, Mg, K, Na)
In general, the highest concentrations of macroelements were determined in mushrooms belonging to the Psathyrellaceae family, while C. comatus (1/7274) was rich in Ca and Na.
The determined concentrations of Ca, Mg, and K were in the usual range for wild mushrooms (100–500 mg kg−1, 800–1800 mg kg−1, and 20–40 g kg−1, respectively; [50]), although the values for individual mushroom species (L. perlatum, M. procera, C. cornucopioides, L. nuda, A. cylindracea, C. comatus) did not always agree with those determined by other researchers [23,52,53,56].
Concentrations of Ca ranged from 128 to 545 mg kg−1 (Table 2), except in P. dryinus (1/7256), where an exceptionally low concentration (26 mg kg−1) was determined. The concentrations of Mg and K varied from 0.939 to 1.88 g kg−1 and 17.1 to 49.5 g kg−1, respectively, except for mushrooms from the family Psathyrellaceae (P. piluliformis (1/7268, 1/7273) and P. multipedata (1/7263)), where slightly higher values of Mg (around 2 g kg−1) and significantly higher values of K (65.3–80.8 g kg−1) were determined. The determined Na concentrations were outside the range suggested by Kalač [52] (100–400 mg kg−1); in the majority of samples they were below 100 mg kg−1, while in mushrooms from the family Psathyrellaceae (P. piluliformis (1/7268, 1/7273) and P. multipedata (1/7263)) and L. leucothites (1/7293) and C. comatus (1/7274) from the family Agaricaeae were in the range of 0.653 to 1.01 g kg−1 (Table 2).
While the uptake of K was correlated with that of Mg and Na (r = 0.6 and 0.7, respectively, p < 0.05; Table S1), taking into account all studied mushrooms, the uptake of Na was significantly increased in species of the family Psathyrellaceae from different sites, but was also site-specific, as samples 1/7273, 1/7274, and 1/7275 from Dotrščina Park had significantly increased values compared to other mushrooms. However, the fact that mushrooms of the same species collected from different sites (L. perlatum (1/7260 and 1/7267), I. gibba (1/7258 and 1/7272) and P. piluliformis (1/7268 and 1/7273)) had similar concentrations of macroelements supports the finding that macronutrient uptake is mostly species-dependent. L. deterrimus (1/7284 and1/7288), a mycorrhizal mushroom, showed different concentrations of K and Na, which could indicate a possible importance of the individual host tree in the uptake of major elements.
4.3. Micronutrients (Cu, Fe, Mn, Mo, Se, Zn)
The obtained values for micronutrients were within the accepted range for wild mushrooms (20–100 mg kg−1 for Cu, 30–150 mg kg−1 for Fe, 10–60 mg kg−1 for Mn, <2 mg kg−1 for Se, 25–200 mg kg−1 for Zn; [52]). Only for Mo, several samples exceeded the values found in Kalač [52] (<0.6 mg kg−1).
The concentrations of Cu and Fe ranged from 2.19 to 84.3 mg kg−1, and 11.0 to 105 mg kg−1, respectively (Table 2). The lowest values were found in P. dryinus (1/7256), and the highest in L. perlatum (1/7260), and no site-, species-, or lifestyle-related trends were observed. A comparison with data from related studies for specific mushrooms (L. perlatum, C. comatus, M. procera, L. nuda, C. cornucopioides, L. leucothites, L. deterrimus, A. cylindracea) revealed similar, but also divergent values [23,40,53,54,55,56,57,65].
The values for Mn ranged from 4.9 mg kg−1 to 38.2 mg kg−1, with the lowest concentrations obtained in P. dryinus (1/7256), L. deterrimus (1/7288), and A. cylindracea (1/7294), and the highest in L. perlatum (1/7267), I. gibba (1/7272), and L. nuda (1/7291). In general, the values obtained for certain species (L. perlatum, M. procera, C. comatus, L. nuda, C. cornucopioides) were lower than those reported in the literature [23,28,33,57,65], although higher values were found for L. nuda [29] and similar values for A. cylindracea and C. comatus [53], L. leucothites [53], and M. procera [23,30].
A positive correlation between Mn and Fe (r = 0.62, p < 0.05) and Ni and Fe (r = 0.53, p < 0.05; Table S1) in the investigated mushrooms suggests their interdependent uptake, as previously reported by Kokkoris et al. [18]. The co-occurrence of Mn and Fe results from the Fe-Mn oxides and oxyhydroxides associated with clay minerals in the soil. Moreover, a strong correlation was observed between V and Fe (r = 0.66, p < 0.05; Table S1) due to its association with Fe oxides [3]. This agrees with previously reported findings that Fe, Mn, and partially Ni in topsoils from the Zagreb area are mostly of geological/pedological origin [66].
The determined concentrations of Mo ranged from 0.007 mg kg−1 to 1.15 mg kg−1. The lowest Mo content was determined in L. deterrimus (1/7284, 1/7288), P. dryinus (1/7256), and P. piluliformis (1/7268, 1/7273), while P. flaccida (1/7262) and L. nuda (1/7291) exceeded the usual content for mushrooms from uncontaminated areas [52]. In general, it was observed that mushrooms growing on wood had lower Mo concentrations.
Selenium is a micronutrient normally present in mushrooms at 2 mg kg−1 [52]. This is in agreement with the results obtained; the concentrations obtained were mostly <1 mg kg−1, except for the two samples with higher values; 1.59 mg kg−1 in P. multipedata (1/7263) and 1.49 mg kg−1 in L. leucothites (1/7293). The important role of Se in biological functions is supported by its positive correlation with macronutrients K and Mg (r = 0.62 and 0.5, respectively; p < 0.05; Table S1).
Concentrations of Zn ranged from 17.3 mg kg−1 in P. dryinus (1/7256) to 143 mg kg−1 in L. excipuliforme (1/7259). L. perlatum (1/7260 and 1/7267), as an accumulator of Zn [52], showed higher values; however, they were not the highest recorded. Species from the Psathyrellaceae family (P. multipedata (1/7263) and P. piluliformis (1/7268 and 1/7273)) and Russulaceae (L. deterrimus, 1/7284 and 1/7288), which were from different sites, showed similar Zn values, suggesting that species-specific affinities rather than soil geochemistry are important in Zn uptake. Considering specific mushroom species, similar values were previously reported for C. comatus [27,30], M. procera [23,30,40], L. leucothites [67], L. deterrimus [40], and A. cylindracea [53], but different concentrations can also be found [33,53,55,57,65].
4.4. Lithogenic Group of Elements
The group of lithogenic elements (Al, Ba, Li, Ti, Cs, and Rb) was positively correlated with each other (r = 0.50–0.87, p < 0.05; Table S1), indicating a common mechanism of their uptake. Their concentrations were in a narrow range of values, considering the differences between localities. Positive correlations of REY, Sr, and V with Al (Table S1) indicate their common origin from the soil.
Concentrations of Al varied from 7.83 mg kg−1 in P. dryinus (1/7256) to 99.3 mg kg−1 in P. piluliformis (1/7268). Similar values were reported for C. comatus [53] and L. nuda [28], lower for A. cylindracea [53] and C. cornucopioides [28], and higher for L. perlatum [28]. According to the data for a number of wild species [68], the Ba content in mushrooms is mostly below 1.6 mg kg−1. This is in agreement with the present results; only P. piluliformis (1/7268) slightly exceeded this range with a value of 1.73 mg kg−1. Similar concentrations of Ba were previously reported for C. comatus, and higher for A. cylindracea [53]. Lithium is not a commonly occurring element in mushrooms, and the results obtained are consistent with the usual reported range, such as below 0.19 mg kg−1 [69]. The content of Ti was up to 9.7 mg kg−1, in agreement with the usual content in wild mushrooms (<10 mg kg−1, [52]), although Niedzielski et al. [53] found lower content in A. cylindracea and C. comatus. The Cs content was mostly below 0.6 mg kg−1, except in C. cornucopioides (1/7292), where the determined value was 1.96 mg kg−1, confirming an increased input of lithogenic elements, as previously suggested. A strong positive correlation between Cs and Rb (r = 0.85, p < 0.05; Table S1) results from their similar geochemical behaviour [70]. According to data collected by Kalač [52], Rb is present in mushrooms in tens to hundreds of mg kg−1; in this study, the content of Rb varied from 2.59 mg kg−1 in L. perlatum (1/7260) to 299 mg kg−1 in C. cornucopioides (1/7292).
The highest concentrations of Cr and Ni were determined to be 0.761 mg kg−1 and 1.595 mg kg−1, respectively, in C. cornucopioides (1/7292) (Table 2). The concentrations of Ni and Cr were generally lower compared to other studies [33,41,42,55], while similar levels of Ni were determined in A. cylindracea [53]. The results showed equal Ni content in the two samples of L. deterrimus sampled at widely separated sites (1/7284 and 1/7288), while similar values were also observed in mushrooms belonging to the Tricholomataceae family (1/7258, 1/7262, 1/7272, 1/7291) (Table 2); this may indicate that the accumulation of Ni is species-specific. However, the positive correlation of Ni with Fe and V (r = 0.53, p < 0.05; Table S1) suggests its association with Fe oxides and oxyhydroxides from soil. In contrast, the lack of a significant correlation between Cr and other elements suggests that there are multiple factors controlling its bioaccumulation.
4.5. Rare Earth Elements
The REY concentrations obtained (Table 3) are in accordance with the values reported for saprobic macrofungi [71] and aboveground species [72], but are significantly higher compared to the values reported for Suillus luteus [70] and lower compared to the values reported by the ref. [73] for caps of M. procera (Table 5). Moreover, the mushrooms from northwestern Croatia investigated in this study have much lower REY values compared to the mushrooms of the genus Agaricus from the Prašnik area (eastern part of Croatia; [44]), although all of them were grown on a pedological substrate enriched with REEs [43]. The reason for the observed discrepancy is probably the fact that mushrooms of the genus Agaricus are known to easily accumulate metals [11].
Table 5.
The comparison of the determined REY concentration with the literature values for mushrooms from different regions (all expressed in mg/kg). If not otherwise specified, the results refer to the fruiting body.
| Croatia | Poland | Czech Republic | Italy | |||
|---|---|---|---|---|---|---|
| Element |
Agaricus sp. (Caps and Stipes) [44] |
Agaricus sp., Trichaptum biforme [12] |
M. procera (Caps and Stipes) [34,35] |
Edible [48] | Boletaceae (Edible) [49] | Different Fungal Species [38] |
| La | 1.373 | 0.083 ± 0.049 | 0.06 | <0.01–0.08 | 0.023 | 0.015–0.488 |
| Ce | 4.137 | 0.18 ± 0.091 | 0.12 | 0.042 | 0.025–0.843 | |
| Pr | 0.526 | 0.017 ± 0.009 | 0.04 | 0.02–1.8 | 0.0056 | <DL–0.109 |
| Nd | 2.137 | 0.058 ± 0.003 | 0.17 | 0.11–0.45 | 0.020 | 0.011–0.446 |
| Sm | 0.467 | 0.012 ± 0.006 | 0.03 | 0.0041 | 0.0021–0.0822 | |
| Eu | 0.099 | 0.0027 ± 0.018 | <0.01 | 0.00068 | <DL–0.019 | |
| Gd | 0.305 | 0.011 ± 0.006 | 0.05 | <0.01–0.05 | 0.0023 | 0.002–0.081 |
| Tb | 0.057 | 0.0018 ± 0.0009 | 0.03 | 0.00059 | <DL–0.012 | |
| Dy | 0.285 | 0.010 ± 0.005 | <0.01 | 0.0022 | 0.001–0.0724 | |
| Ho | 0.062 | 0.0023 ± 0.0012 | 0.04 | <0.01–0.30 | 0.00042 | 0.0003–0.015 |
| Er | 0.163 | 0.0070 ± 0.0035 | 0.72 | 0.0013 | 0.0005–0.0429 | |
| Tm | 0.027 | 0.0011 ± 0.0006 | 0.03 | 0.00017 | 0.0001–0.006 | |
| Yb | 0.150 | 0.0073 ± 0.0037 | 0.02 | 0.0013 | <DL–0.041 | |
| Lu | 0.027 | 0.0011 ± 0.0005 | 0.02 | 0.00013 | <DL–0.0058 | |
| Y | 0.693 | 0.074 ± 0.039 | 0.04 | 0.009–0.549 | ||
| ∑REY | 9.797 | 0.481 | 0.23 | 0.104 | ||
In general, the results indicate a large variability between the different species studied in terms of their ability for REY uptake and suggest that both the soil substrate and the mushroom species influence the accumulation of this group of elements in the mushroom tissue.
4.6. Factors Influencing the Element Uptake
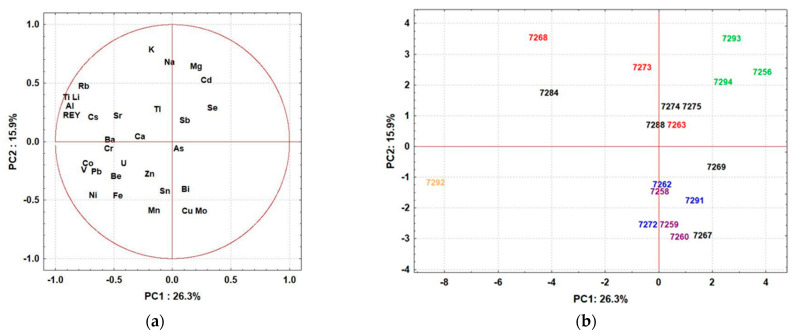
In order to identify the role of different factors affecting the intake of elements, such as species, lifestyle, dietary habits, and geographical location, the relationship between the obtained element concentrations was investigated using principal component analysis (PCA). The eigenvalues of the first two principal components (PCs) were greater than 1, indicating their significance. The first two PCs explained 42.2% of the total variability among 32 variables; the first component (PC1) contributed 26.3%, while the second corresponded to 15.9% of the total variance of the data set. PCA results are presented on the PCA loading plot (Figure 2a) and PCA score plot (Figure 2b), which illustrate the alignment of variables (elements) and samples (mushrooms) with respect to principal components.
Figure 2.
Principal component analysis: PC1–PC2 loading (a) and score plot (b).
Based on these parameters, the following can be observed:
-
(i)
Mushrooms belonging to the Psathyrellaceae family (1/7263, 1/7268, and 1/7273; colored red in Figure 2b) showed high variability in element distribution, suggesting that soil geochemistry/properties are the main factor determining the uptake of elements. However, P. piluliformis (1/7268 and 1/7273) collected from widely separated sites showed similarities in terms of high uptake of macronutrients (K, Na, Mg) and low uptake of Mo and Cu, indicating that the uptake of these elements is species-specific.
-
(ii)
L. leucothites (1/7293) and A. cylindracea (1/7294) sampled from the same site in the city of Zagreb, and P. dryinus (1/7256) (all colored green in Figure 2b) from another site within the urban area were characterized by high Cd concentrations; these mushrooms were collected near a road and, therefore, the elevated Cd levels could be explained by traffic pollution. However, elevated levels of other elements derived from traffic pollution, such as Pb, were not observed.
-
(iii)
Mushrooms from the Tricholomataceae family (1/7262, 1/7272, and 1/7291; colored blue) were grouped independently of sampling sites, suggesting that species-specific affinities in element uptake predominate. These mushrooms were described by slightly higher levels of Cu, Mn, and Mo and lower levels of Cd and macronutrients.
-
(iv)
Significant similarity in the content of elements was observed in different mushrooms sampled within the green area at the Jelenovac site: L. excipuliforme (1/7259), I. gibba (1/7258), L. perlatum (1/7260) (all colored purple in Figure 2b) and P. flaccida (1/7262) (colored blue in Figure 2b). This strongly suggests that geochemistry and soil properties are the main factors controlling elemental uptake, as these samples are characterized by higher levels of Cu, Mn, and Mo and lower levels of K, Mg, and Na.
-
(v)
PCA analysis confirmed the unique elemental composition of C. cornucopioides (1/7292; colored orange in Figure 2b), which is distinguished from all other samples by its significantly elevated values of Co, Ni, Pb, and REY.
The results presented showed that mushrooms belonging to the same species or family collected in different areas had considerably different elemental contents, suggesting different soil geochemical composition and/or anthropogenic influences as the main factors determining elemental content. Since the main distribution of trace elements in the studied areas did not indicate an anthropogenic influence, it can be assumed that soil geochemical properties are the most important factor determining these variations in chemical composition. The influence of different soil properties and geochemistry on the uptake of elements by mushrooms may be more evident in studies comparing soils with markedly different geological composition, as in the study by Nikkarinen and Mertanen [74] and Alaimo et al. [37], than when comparing mushrooms growing in areas where differences in soil geochemistry are less pronounced. However, several elements, such as As, Ni, Zn, and macronutrients, showed similar behavior in certain mushroom species or families, indicating an important influence of species-specific affinity for certain elements. Finally, both factors—soil geochemistry and species characteristics—have an important influence on the uptake of elements, as also shown by other studies [36,38,39,52], although the prevalence of each factor varies from species to species and depends on the element in question.
4.7. Health Risk Assessment
The data (THQ and HI values) obtained in the assessment of non-carcinogenic health risk due to metal ingestion from the consumption of the wild mushrooms studied are presented in Table 6. Toxicity was evaluated based on the calculated HI values. If the HI value for a mixture is less than 1, it is considered unlikely that assessed exposure from mushroom consumption will result in significant toxicity. On the other hand, there is great concern about potential toxicity when the HI value is greater than 1 [48].
Table 6.
Non-carcinogenic risk data (expressed by health risk indexes THQ and HI) obtained in the health risk assessment of metals through the consumption of the edible wild mushrooms from Croatia. The RfD data are expressed as µg kg−1 day−1.
| Target Hazard Quotient (THQ) | Hazard Index (HI) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Ba | Be | Cd | Cu | Fe | Mn | Ni | Pb | Sb | Sr | U | Zn | ||
| 1/7256 | 0.026 | 0.000 | 0.000 | 0.280 | 0.023 | 0.006 | 0.016 | 0.007 | 0.008 | 0.002 | 0.000 | 0.000 | 0.024 | 0.392 |
| 1/7258 | 0.436 | 0.003 | 0.000 | 0.156 | 0.479 | 0.056 | 0.063 | 0.011 | 0.016 | 0.015 | 0.000 | 0.000 | 0.102 | 1.337 |
| 1/7259 | 0.540 | 0.001 | 0.000 | 0.146 | 0.397 | 0.031 | 0.040 | 0.013 | 0.130 | 0.006 | 0.000 | 0.001 | 0.196 | 1.501 |
| 1/7260 | 0.742 | 0.001 | 0.000 | 0.129 | 0.866 | 0.060 | 0.081 | 0.015 | 0.060 | 0.006 | 0.000 | 0.001 | 0.149 | 2.111 |
| 1/7262 | 0.660 | 0.003 | 0.000 | 0.059 | 0.296 | 0.029 | 0.092 | 0.007 | 0.027 | 0.013 | 0.001 | 0.001 | 0.137 | 1.325 |
| 1/7263 | 5.047 | 0.002 | 0.000 | 0.150 | 0.801 | 0.029 | 0.034 | 0.011 | 0.041 | 0.070 | 0.001 | 0.001 | 0.134 | 6.321 |
| 1/7267 | 0.752 | 0.001 | 0.000 | 0.111 | 0.690 | 0.047 | 0.112 | 0.010 | 0.102 | 0.004 | 0.000 | 0.000 | 0.174 | 2.004 |
| 1/7268 | 0.119 | 0.004 | 0.000 | 0.163 | 0.116 | 0.052 | 0.067 | 0.007 | 0.031 | 0.012 | 0.001 | 0.000 | 0.122 | 0.694 |
| 1/7269 | 0.300 | 0.001 | 0.000 | 0.158 | 0.582 | 0.036 | 0.034 | 0.007 | 0.051 | 0.005 | 0.000 | 0.000 | 0.100 | 1.273 |
| 1/7272 | 0.905 | 0.002 | 0.000 | 0.089 | 0.538 | 0.045 | 0.107 | 0.014 | 0.015 | 0.007 | 0.000 | 0.000 | 0.092 | 1.815 |
| 1/7273 | 0.207 | 0.001 | 0.000 | 0.201 | 0.205 | 0.040 | 0.075 | 0.005 | 0.048 | 0.003 | 0.000 | 0.000 | 0.096 | 0.882 |
| 1/7274 | 0.670 | 0.001 | 0.000 | 0.200 | 0.435 | 0.033 | 0.043 | 0.004 | 0.019 | 0.011 | 0.001 | 0.000 | 0.080 | 1.496 |
| 1/7275 | 0.011 | 0.002 | 0.000 | 0.048 | 0.097 | 0.019 | 0.063 | 0.004 | 0.014 | 0.004 | 0.000 | 0.000 | 0.063 | 0.324 |
| 1/7284 | 0.875 | 0.003 | 0.000 | 0.244 | 0.170 | 0.037 | 0.024 | 0.010 | 0.021 | 0.002 | 0.001 | 0.001 | 0.182 | 1.570 |
| 1/7288 | 0.356 | 0.001 | 0.000 | 0.226 | 0.091 | 0.017 | 0.014 | 0.010 | 0.022 | 0.003 | 0.000 | 0.000 | 0.141 | 0.883 |
| 1/7291 | 0.812 | 0.001 | 0.000 | 0.073 | 0.421 | 0.028 | 0.110 | 0.009 | 0.063 | 0.004 | 0.000 | 0.000 | 0.080 | 1.602 |
| 1/7292 | 0.016 | 0.002 | 0.000 | 0.226 | 0.338 | 0.045 | 0.074 | 0.033 | 0.472 | 0.004 | 0.000 | 0.001 | 0.088 | 1.299 |
| 1/7293 | 0.425 | 0.001 | 0.000 | 1.869 | 0.269 | 0.014 | 0.021 | 0.003 | 0.035 | 0.036 | 0.000 | 0.000 | 0.103 | 2.776 |
| 1/7294 | 0.000 | 0.000 | 0.000 | 1.626 | 0.460 | 0.013 | 0.017 | 0.005 | 0.027 | 0.003 | 0.000 | 0.000 | 0.125 | 2.278 |
| RfD | 0.3 a | 200 a | 2 a | 1 a | 40 b | 700 b | 140 a | 20 a | 3.5 b | 0.4 a | 600 a | 3 a | 300 a | |
The risk assessment revealed that 14 of the 19 mushrooms studied, from both urban and forest areas, had HI levels above 1; therefore, consumers of these mushrooms are exposed to a significant non-carcinogenic health risk due to the high metal content. As, Cd, and Cu, which have the highest THQ values, account for the largest proportion of total toxicity.
The mushrooms that do not pose a risk to human health are those growing on wood—1/7256, 1/7268, 1/7273, and 1/7275. The only mushroom growing in soil that is considered safe for consumption is L. deterrimus (1/7288), which was growing in grassland within the forest area.
Therefore, a diet that includes many wild mushrooms can be very risky. Dowlati et al. [48] conducted a comprehensive review, meta-analysis, and evaluation of non-carcinogenic health risk to humans based on the collected data on the concentrations of toxic metals in edible mushrooms from different countries of the world. The health risk assessment revealed that consumers from 8 out of 19 countries studied were exposed to a significant non-carcinogenic health risk.
5. Conclusions
In the studied mushrooms, the most abundant elements were essential nutrients, whose uptake seems to be more under a genetic influence and less influenced by site-specific characteristics.
The content of toxic elements was low, and no influence of traffic pollution could be detected, as similar concentrations of Pb were found in mushrooms from forest and urban sites. While the concentrations of metals did not show variations according to substrate (wood and soil), it was observed that mushrooms growing on wood generally had lower levels of As and Mo.
Nonetheless, the health risk assessment revealed a high non-carcinogenic risk of metals in 14 out of 19 mushrooms studied from both urban and forest areas, suggesting that these mushrooms should be consumed with caution.
Despite a limited data set, the results suggest that both the geochemical composition of substrate and the species-specific affinities influence the uptake of elements, with the predominance of one of these factors depending on both the mushroom species and the element of interest. The diversity of factors affecting the chemical composition of edible mushrooms should certainly be considered not only in future studies, but also in the evaluation of edible mushrooms as a source of certain metals in the diet.
Acknowledgments
A.M. and Z.T. are grateful to Dunja Šamec and Vedran Bahun for freeze-drying of samples for this study and to Margita Jadan for generating DNA barcode sequences (ITS rDNA) used for molecular identification of mushrooms.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof7121068/s1, Table S1: title Spearman correlation coefficients of the investigated elements in mushrooms. Correlation coefficients marked in red are significant at p < 0.05.
Author Contributions
Conceptualization, M.I. and Ž.F.; sampling: A.M. and Z.T.; methodology, M.F.T.; data curation: M.I., M.F.T. and Ž.F.; writing—original draft preparation, M.I. and Ž.F.; writing—review and editing, M.I., M.F.T. and Ž.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been partially supported by Croatian Science Foundation under the project ForFungiDNA (IP-2018-01-1736).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katz A. Alternative Medicine for Prostate Cancer: Diet Vitamins Minerals and Supplements. In: Su L.-M., editor. Early Diagnosis and Treatment of Cancer Series: Prostate Cancer. Elsevier; Amsterdam, The Netherlands: 2010. [Google Scholar]
- 2.Marchand L.R.A., Stewart J. Breast Cancer. In: Rakel D., editor. Integrative Medicine. Lancet; London, UK: 2018. [Google Scholar]
- 3.Kabata-Pendias A. Trace Elements in Soils and Plants. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 4.Falandysz J., Kawano M., Swieczkowski A., Brzostowski A., Dadej M. Total mercury in wild-grown higher mushrooms and underlying soil from Wdzydze Landscape Park Northern Poland. Food Chem. 2003;81:21–26. doi: 10.1016/S0308-8146(02)00344-8. [DOI] [Google Scholar]
- 5.Kalač P. A review of edible mushroom radioactivity. Food Chem. 2001;75:29–35. doi: 10.1016/S0308-8146(01)00171-6. [DOI] [Google Scholar]
- 6.Svoboda L., Zimmermannova K., Kalač P. Concentrations of mercury cadmium lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Sci. Total Environ. 2000;246:61–67. doi: 10.1016/S0048-9697(99)00411-8. [DOI] [PubMed] [Google Scholar]
- 7.Falandysz J., Borovička J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2012;97:477–501. doi: 10.1007/s00253-012-4552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das N. Heavy metal biosorption by mushrooms. Nat. Prod. Radiance. 2005;4:454–459. [Google Scholar]
- 9.García M.A., Alonso J., Fernandez M.I., Melgar M.J. Lead content in edible wild mushrooms in Northwest Spain as indicator of environmental contamination. Arch. Environ. Contam. Toxicol. 1998;34:330–335. doi: 10.1007/s002449900326. [DOI] [PubMed] [Google Scholar]
- 10.García M.A., Alonso J., Melgar M.J. Lead in edible mushrooms; Levels and bioaccumulation factors. J. Hazard. Mater. 2009;167:777–783. doi: 10.1016/j.jhazmat.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Kalač P., Svoboda L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000;69:273–281. doi: 10.1016/S0308-8146(99)00264-2. [DOI] [Google Scholar]
- 12.Ivanić M., Fiket Ž., Medunić G., Furdek Turk M., Marović G., Senčar J., Kniewald G. Multi-element composition of soil mosses and mushrooms and assessment of natural and artificial radioactivity of a pristine temperate rainforest system (Slavonia, Croatia) Chemosphere. 2019;215:668–677. doi: 10.1016/j.chemosphere.2018.10.108. [DOI] [PubMed] [Google Scholar]
- 13.Mleczek M., Niedzielski P., Kalač P., Budka A., Siwulski M., Gąsecka M., Rzymski P., Magdziak Z., Sobieralski K. Multielemental analysis of 20 mushroom species growing near a heavily trafficked road in Poland. Environ. Sci. Pollut. Res. 2016;23:16280–16295. doi: 10.1007/s11356-016-6760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sager M. Urban Soils and Road Dust—Civilization Effects and Metal Pollution—A Review. Environments. 2020;7:98. doi: 10.3390/environments7110098. [DOI] [Google Scholar]
- 15.Rodríguez-Seijo A., Arenas-Lago D., Andrade M.L., Vega F.A. Identifying sources of Pb pollution in urban soils by means of MC-ICP-MS and TOF-SIMS. Environ. Sci. Pollut. Res. 2015;22:7859–7872. doi: 10.1007/s11356-014-4027-9. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho M.L., Pimentel A.C., Fernandes B. Study of heavy metals in wild edible mushrooms under different pollution conditions by X-ray fluorescence spectrometry. Anal. Sci. 2005;21:747–750. doi: 10.2116/analsci.21.747. [DOI] [PubMed] [Google Scholar]
- 17.Petkovšek S.A.S., Pokorny B. Lead and cadmium in mushrooms from the vicinity of two large emission sources in Slovenia. Sci. Total Environ. 2013;443:944–954. doi: 10.1016/j.scitotenv.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Kokkoris V., Massas I., Polemis E., Koutrotsios G., Zervakis G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece) Sci. Total Environ. 2019;685:280–296. doi: 10.1016/j.scitotenv.2019.05.447. [DOI] [PubMed] [Google Scholar]
- 19.Schlecht M.T., Säumel I. Wild growing mushrooms for the Edible City? Cadmium and lead content in edible mushrooms harvested within the urban agglomeration of Berlin. Ger. Environ. Pollut. 2015;204:298–305. doi: 10.1016/j.envpol.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Świsłowski P., Dołhańczuk-Śródka A., Rajfur M. Bibliometric analysis of European publications between 2001 and 2016 on concentrations of selected elements in mushrooms. Environ. Sci. Pollut. Res. 2020;27:22235–22250. doi: 10.1007/s11356-020-08693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelot D., Siobud E., Dore J.C., Viel C., Poirier F. Update of metal content profiles in mushrooms – toxicological implications and tentative approach to the mechanisms of bioaccumulation. Toxicon. 1998;36:1997–2012. doi: 10.1016/S0041-0101(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 22.Svoboda L., Kalač P., Špička J., Janoušková D. Leaching of cadmium lead and mercury from fresh and differently preserved edible mushroom Xerocomus badius during soaking and boiling. Food Chem. 2002;79:41–45. doi: 10.1016/S0308-8146(02)00175-9. [DOI] [Google Scholar]
- 23.Falandysz J., Szymczyk K., Ichihashi H., Bielawski L., Gucia M., Frankowska A., Yamasaki S.I. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001;18:503–513. doi: 10.1080/02652030119625. [DOI] [PubMed] [Google Scholar]
- 24.Malinowska E., Szefer P., Falandaysz J. Metals bioaccumulation by bay bolete Xerocomus badius from selected sites in Poland. Food Chem. 2004;84:405–416. doi: 10.1016/S0308-8146(03)00250-4. [DOI] [Google Scholar]
- 25.Nowakowski P., Markiewicz-Żukowska R., Soroczyńska J., Puścion-Jakubik A., Mielcarek K., Borawska M.H., Socha K. Evaluation of toxic element content and health risk assessment of edible wild mushrooms. J. Food Compos. Anal. 2021;96:103698. doi: 10.1016/j.jfca.2020.103698. [DOI] [Google Scholar]
- 26.Alonso J., García M., Pérez-López M., Melgar M.J. The concentrations and bioconcentration factors of copper and zinc in edible mushrooms. Arch. Environ. Contam. Toxicol. 2003;44:180–188. doi: 10.1007/s00244-002-2051-0. [DOI] [PubMed] [Google Scholar]
- 27.Mendil D., Uluözlü Ö.D., Tüzen M., Hasdemir E., Sarı H. Trace metal levels in mushroom samples from Ordu Turkey. Food Chem. 2005;91:463–467. doi: 10.1016/j.foodchem.2004.06.028. [DOI] [Google Scholar]
- 28.Sesli E., Tuzen M., Soylak M. Evaluation of trace metal contents of some wild edible mushrooms from Black sea region Turkey. J. Hazard. Mater. 2008;160:462–467. doi: 10.1016/j.jhazmat.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Turkekul I., Elmastas M., Tüzen M. Determination of iron copper manganese zinc lead and cadmium in mushroom samples from Tokat Turkey. Food Chem. 2004;84:389–392. doi: 10.1016/S0308-8146(03)00245-0. [DOI] [Google Scholar]
- 30.Yamaç M., Yıldız D., Sarıkürkcü C., Çelikkollu M., Solak M.H. Heavy metals in some edible mushrooms from the Central Anatolia Turkey. Food Chem. 2007;103:263–267. doi: 10.1016/j.foodchem.2006.07.041. [DOI] [Google Scholar]
- 31.Aruguete D.M., Aldstadt J.H., Mueller G.M. Accumulation of several heavy metals and lanthanides in mushrooms (Agaricales) from the Chicago region. Sci. Total Environ. 1998;224:43–56. doi: 10.1016/S0048-9697(98)00319-2. [DOI] [Google Scholar]
- 32.Fu Z., Liu G., Wang L. Assessment of potential human health risk of trace element in wild edible mushroom species collected from Yunnan Province China. Environ. Sci. Pollut. Res. Int. 2020;27:29218–29227. doi: 10.1007/s11356-020-09242-w. [DOI] [PubMed] [Google Scholar]
- 33.Zhu F., Qu L., Fan W., Qiao M., Hao H., Wang X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province China. Environ. Monit. Assess. 2011;179:191–199. doi: 10.1007/s10661-010-1728-5. [DOI] [PubMed] [Google Scholar]
- 34.Stefanović V., Trifković J., Djurdjić S., Vukojević V., Tešić Ž., Mutić J. Study of silver selenium and arsenic concentration in wild edible mushroom Macrolepiota procera health benefit and risk. Environ. Sci. Pollut. Res. 2016;23:22084–22098. doi: 10.1007/s11356-016-7450-2. [DOI] [PubMed] [Google Scholar]
- 35.Stefanović V., Trifković J., Mutić J., Tešić Ž. Metal accumulation capacity of parasol mushroom (Macrolepiota procera) from Rasina region (Serbia) Environ. Sci. Pollut. Res. 2016;23:13178–13190. doi: 10.1007/s11356-016-6486-7. [DOI] [PubMed] [Google Scholar]
- 36.Alaimo M.G., Dongarrà G., La Rosa A., Tamburo E., Vasquez G., Varrica D. Major and trace elements in Boletus aereus and Clitopilus prunulus growing on volcanic and sedimentary soils of Sicily (Italy) Ecotoxicol. Environ. Saf. 2018;157:182–190. doi: 10.1016/j.ecoenv.2018.03.080. [DOI] [PubMed] [Google Scholar]
- 37.Alaimo M.G., Saitta A., Ambrosio E. Bedrock and soil geochemistry influence the content of chemical elements in wild edible mushrooms (Morchella group) from South Italy (Sicily) Acta Mycol. 2019;54:1122. doi: 10.5586/am.1122. [DOI] [Google Scholar]
- 38.Cocchi L., Vescovi L., Petrini L., Petrin E. Heavy metals in edible mushrooms in Italy. Food Chem. 2006;98:277–284. doi: 10.1016/j.foodchem.2005.05.068. [DOI] [Google Scholar]
- 39.Giannaccini G., Betti L., Palego L., Mascia G., Schmid L., Lanza M., Mela A., Fabbrini L., Biondi L., Lucacchini A. The trace element content of top-soil and wild edible mushroom samples collected in Tuscany Italy. Environ. Monit. Assess. 2012;184:7579–7595. doi: 10.1007/s10661-012-2520-5. [DOI] [PubMed] [Google Scholar]
- 40.Širić I., Kos I., Kasap A., Petković F.Z., Držaić V. Heavy metals bioaccumulation by edible saprophytic mushrooms. J. Cent. Eur. Agric. 2016;17:884–900. [Google Scholar]
- 41.Širić I., Humar M., Kasap A., Kos I., Mioč B., Pohleven F. Heavy metal bioaccumulation by wild edible saprophytic and ectomycorrhizal mushrooms. Environ. Sci. Pollut. Res. 2016;23:18239–18252. doi: 10.1007/s11356-016-7027-0. [DOI] [PubMed] [Google Scholar]
- 42.Širić I., Kasap A., Bedeković D., Falandysz J. Lead, cadmium and mercury contents and bioaccumulation potential of wild edible saprophytic and ectomycorrhizal mushrooms Croatia. J. Environ. Sci. Health B. 2017;52:156–165. doi: 10.1080/03601234.2017.1261538. [DOI] [PubMed] [Google Scholar]
- 43.Salminen R., Batista M.J., Bidovec M., Demetriades A., De Vivo B., De Vos W., Duris M., Gilucis A., Gregorauskiene V., Halamić J., et al. FOREGS Geochemical Atlas of Europe Part 1: Background Information Methodology and Maps. Geological Survey of Finland Espoo. 2005. [(accessed on 17 March 2021)]. p. 526. Available online: http://weppi.gtk.fi/publ/foregsatlas/
- 44.Fiket Ž., Medunić G., Furdek Turk M., Ivanić M., Kniewald G. Influence of soil characteristics on rare earth fingerprints in mosses and mushrooms: Example of a pristine temperate rainforest (Slavonia Croatia) Chemosphere. 2017;179:92–100. doi: 10.1016/j.chemosphere.2017.03.089. [DOI] [PubMed] [Google Scholar]
- 45.Mešić A., Šamec D., Jadan M., Bahun V., Tkalčec Z. Integrated morphological with molecular identification and bioactive compounds of 23 Croatian wild mushrooms samples. Food Biosci. 2020;37:100720. doi: 10.1016/j.fbio.2020.100720. [DOI] [Google Scholar]
- 46.Filipović Marijić V., Raspor B. Site-specific gastrointestinal metal variability in relation to the gut content and fish age of indigenous european chub from the Sava River. Water Air Soil Pollut. 2012;223:4769–4783. doi: 10.1007/s11270-012-1233-2. [DOI] [Google Scholar]
- 47.Fiket Ž., Mikac N., Kniewald G. Mass fractions of forty-six major and trace elements including rare earth elements in sediment and soil reference materials used in environmental studies. Geostand. Geoanal. Res. 2017;41:123–135. doi: 10.1111/ggr.12129. [DOI] [Google Scholar]
- 48.Dowlati M., Sobhi H.R., Esrafili A., FarzadKia M., Yeganeh M. Heavy metals content in edible mushrooms: A systematic review, meta-analysis and health risk assessment. Trends Food. Sci. Technol. 2021;109:527–535. doi: 10.1016/j.tifs.2021.01.064. [DOI] [Google Scholar]
- 49.USEPA . Reference Dose (RfD): Description and Use in Health Risk Assessment. USEPA; Washington, DC, USA: 1993. [Google Scholar]
- 50.Cvetković J.S., Mitić V.D., Stankov-Jovanović V.P., Dimitrijević M.V., Nikolić-Mandić S.D. Elemental composition of wild edible mushrooms from Serbia. Anal. Lett. 2015;48:2107–2121. doi: 10.1080/00032719.2015.1010118. [DOI] [Google Scholar]
- 51.Dimitrijević M.V., Mitić V.D., Cvetković J.S., Jovanović V.P.S., Mutić J.J., Mandić S.D.N. Update on element content profiles in eleven wild edible mushrooms from family Boletaceae. Eur. Food Res. Technol. 2016;242:1–10. doi: 10.1007/s00217-015-2512-0. [DOI] [Google Scholar]
- 52.Kalač P. Trace element contents in European species of wild growing edible mushrooms: A review for the period 2000–2009. Food Chem. 2010;122:2–15. doi: 10.1016/j.foodchem.2010.02.045. [DOI] [Google Scholar]
- 53.Niedzielski P., Mleczek M., Budka A., Rzymski P., Siwulski M., Jasińska A., Gąsecka M., Budzyńska S. A screening study of elemental composition in 12 marketable mushroom species accessible in Poland. Eur. Food Res. Technol. 2017;243:1759–1771. doi: 10.1007/s00217-017-2881-7. [DOI] [Google Scholar]
- 54.Sarikurkcu C., Popović-Djordjević J., Solak M.H. Wild edible mushrooms from Mediterranean region: Metal concentrations and health risk assessment. Ecotoxicol. Environ. Saf. 2020;190:110058. doi: 10.1016/j.ecoenv.2019.110058. [DOI] [PubMed] [Google Scholar]
- 55.Sevindik M., Rasul A., Hussain G., Anwar H., Zahoor M.K., Sarfraz I., Kamran K.S., Akgul H., Akata I., Selamoglu Z. Determination of anti-oxidative anti-microbial activity and heavy metal contents of Leucoagaricus leucothites. Pak. J. Pharm. Sci. 2018;31:2163–2168. [PubMed] [Google Scholar]
- 56.Jorhem L., Sundström B. Levels of some trace elements in edible fungi. Z. Lebensm. Unters. Forsch. 1995;201:311–316. doi: 10.1007/BF01192723. [DOI] [PubMed] [Google Scholar]
- 57.Brzezicha-Cirocka J., Mędyk M., Falandysz J., Szefer P. Bio-and toxic elements in edible wild mushrooms from two regions of potentially different environmental conditions in eastern Poland. Environ. Sci. Pollut. Res. 2016;23:21517–21522. doi: 10.1007/s11356-016-7371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melgar M.J., Alonso J., García M.A. Cadmium in edible mushrooms from NW Spain: Bioconcentration factors and consumer health implications. Food Chem. Toxicol. 2016;88:13–20. doi: 10.1016/j.fct.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Sardans J., Peñuelas J. Trace element accumulation in the moss Hypnum cupressiforme Hedw.; and the trees Quercus ilex L.; and Pinus halepensis Mill.; in Catalonia. Chemosphere. 2005;60:1293–1307. doi: 10.1016/j.chemosphere.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 60.Vetter J. Arsenic content of some edible mushroom species. Eur. Food Res. Technol. 2004;219:71–74. doi: 10.1007/s00217-004-0905-6. [DOI] [Google Scholar]
- 61.Falandysz J., Rizal L.M. Arsenic and its compounds in mushrooms: A review. J. Environ. Sci. Health C. 2016;34:217–232. doi: 10.1080/10590501.2016.1235935. [DOI] [PubMed] [Google Scholar]
- 62.Commission Regulation (EC) No 629/2008 of 2 July 2008 Amending Regulation. (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2008. L 173/6–173/9.
- 63.Commission Regulation (EC) No 2015/1005 of 25 June 2015 Amending Regulation. (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. 2015. L 161/9–161/13.
- 64.Official Gazette Regulations on Maximum Levels of Certain Contaminants in Foodstuffs; National Journal Zagreb Croatia 2008, 154/08. [(accessed on 9 December 2021)]. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2008_12_154_4198.html.
- 65.Brzezicha-Cirocka J., Grembecka M., Grochowska I., Falandysz J., Szefer P. Elemental composition of selected species of mushrooms based on a chemometric evaluation. Ecotoxicol. Environ. Saf. 2019;173:353–365. doi: 10.1016/j.ecoenv.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 66.Romić M., Romić D. Heavy metals distribution in agricultural topsoils in urban area. Environ. Geol. 2003;43:795–805. doi: 10.1007/s00254-002-0694-9. [DOI] [Google Scholar]
- 67.Borovička J., Řanda Z. Distribution of iron cobalt zinc and selenium in macrofungi. Mycol. Prog. 2007;6:249. doi: 10.1007/s11557-007-0544-y. [DOI] [Google Scholar]
- 68.Demirbaş A. Concentrations of 21 metals in 18 species of mushrooms growing in the East Black Sea region. Food Chem. 2001;75:453–457. doi: 10.1016/S0308-8146(01)00236-9. [DOI] [Google Scholar]
- 69.Vetter J. Lithium content of some common edible wild-growing mushrooms. Food Chem. 2005;90:31–37. doi: 10.1016/j.foodchem.2004.03.019. [DOI] [Google Scholar]
- 70.Zocher A.L., Kraemer D., Merschel G., Bau M. Distribution of major and trace elements in the bolete mushroom Suillus luteus and the bioavailability of rare earth elements. Chem. Geol. 2018;483:491–500. doi: 10.1016/j.chemgeo.2018.03.019. [DOI] [Google Scholar]
- 71.Borovička J., Kubrová J., Rohovec J., Řanda Z., Dunn C.E. Uranium thorium and rare earth elements in macrofungi: What are the genuine concentrations? Biometals. 2011;24:837–845. doi: 10.1007/s10534-011-9435-4. [DOI] [PubMed] [Google Scholar]
- 72.Mleczek M., Niedzielski P., Kalač P., Siwulski M., Rzymski P., Gąsecka M. Levels of platinum group elements and rare-earth elements in wild mushroom species growing in Poland. Food Addit. Contam. 2015;33:86–94. doi: 10.1080/19440049.2015.1114684. [DOI] [PubMed] [Google Scholar]
- 73.Falandysz J., Chudzińska M., Barałkiewicz D., Drewnowska M., Hanć A. Toxic elements and bio-metals in Cantharellus mushrooms from Poland and China. Environ. Sci. Pollut. Res. Int. 2017;24:11472–11482. doi: 10.1007/s11356-017-8554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nikkarinen M., Mertanen E. Impact of geological origin on trace element composition of edible mushrooms. J. Food Compos. Anal. 2004;17:301–310. doi: 10.1016/j.jfca.2004.03.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.