Abstract
Cancer metastasis is responsible for the vast majority of cancer‐related deaths worldwide. In contrast to numerous discoveries that reveal the detailed mechanisms leading to the formation of the primary tumor, the biological underpinnings of the metastatic disease remain poorly understood. Cancer metastasis is a complex process in which cancer cells escape from the primary tumor, settle, and grow at other parts of the body. Epithelial‐mesenchymal transition and anoikis resistance of tumor cells are the main forces to promote metastasis, and multiple components in the tumor microenvironment and their complicated crosstalk with cancer cells are closely involved in distant metastasis. In addition to the three cornerstones of tumor treatment, surgery, chemotherapy, and radiotherapy, novel treatment approaches including targeted therapy and immunotherapy have been established in patients with metastatic cancer. Although the cancer survival rate has been greatly improved over the years, it is still far from satisfactory. In this review, we provided an overview of the metastasis process, summarized the cellular and molecular mechanisms involved in the dissemination and distant metastasis of cancer cells, and reviewed the important advances in interventions for cancer metastasis.
Keywords: cancer, epithelial‐mesenchymal transition, immunotherapy, metastasis, targeted therapy, tumor microenvironment
Cancer metastasis is a complex process in which cancer cells escape from the primary tumor, settle and grow at other parts of the body. A variety of stromal cells, immune cells and other molecular components surrounding the tumor provide signals that enhance the metastatic potential of cancer cells. With the deepening understanding of mechanisms of tumorigenesis and metastasis in recent years, a plethora of treatment approaches have been established in patients with metastatic cancer.
1. INTRODUCTION
Cancer is one of the main diseases threatening human health. According to the Global Cancer Statistics, 2020, there were 18.1 million new cancer cases and 9.6 million cancer‐related deaths worldwide. 1 Despite the continuous development of medical technology, distant metastasis has already appeared at the time of diagnosis for many patients. Furthermore, a large number of cancer patients, both early‐ and late‐stage, may eventually develop metastatic diseases. 2 Metastatic lesions in distant organs are difficult to be cured by current therapeutic approaches, and metastasis accounts for about 90% of death in cancer patients. 3 In other words, the overwhelming problem highlighted by the cancer‐associated deaths is, for the most part, metastatic cancer. In order to develop the methods for preventing or treating metastasis, it is inevitable to understand the cellular and molecular mechanisms of metastasis.
In contrast to numerous discoveries that reveal the detailed mechanisms leading to the formation of the primary tumor, research on metastatic cancer is still lagging behind. Stephen Paget and James Ewing proposed the “seed and soils” and the “mechanical metastasis” hypothesis, respectively, which laid the foundation for the study of tumor metastasis. 4 , 5 , 6 Cancer metastasis is a complicated process, which is regulated by various signaling pathways and modulated by the surrounding extracellular matrix (ECM). Tumor cells spread from the primary tumor mass to distant organs through blood vessels, lymphatic vessels, and transcoelomic routes. 7 , 8 In order to successfully metastasize from the prior site, tumor cells need to go through the following stages: local invasion, intravasation, survival in circulation, extravasation, and colonization. 9 Furthermore, multiple components in the tumor microenvironment (TME), including immune cells, stromal cells, chemokines, and cytokines are involved in a complex crosstalk with tumor cells that affects tumor growth and metastasis. 10 , 11 , 12 Surgery, chemotherapy, and radiotherapy are the three cornerstones of cancer treatment. With the deepening understanding of mechanisms of tumorigenesis and metastasis in recent years, a plethora of treatment approaches, including targeted therapy and immunotherapy have been established. 13 , 14 , 15 However, the underlying mechanisms of cancer metastasis are not yet fully understood, and the strategies for preventing and inhibiting cancer metastasis are also limited.
Here, we provided an overview of the metastasis process, summarized the mechanisms underlying the dissemination and distant metastasis of cancer cells, and reviewed the important advances in interventions targeting cancer metastasis.
2. COMPONENTS AND MECHANISMS INVOLVED IN METASTASIS
Tumor metastasis is one of the hallmarks of tumor malignancy and one of the causes of tumor‐related death. 16 The malignant behavior of tumor metastasis is mainly related to the malignant degree of the primary tumor, but one of the common features is that all metastases need to go through a cascade called “invasion‐metastasis cascade.” 17 The metastasis process begins when the tumor cells gain invasiveness and lose their adhesion to the surrounding matrix including basement membrane (BM) and ECM; tumor cells migrate out of the primary tumor and invade surrounding tissues. 18
Subsequently, disseminated tumor cells penetrate blood vessels or lymph vessels into the circulation and respond to various resistance conditions such as shear force, anoikis, and immune surveillance in the circulation. 19 Only a small percentage of tumor cells can survive in the harsh conditions of circulation. After successfully entering the secondary site, tumor cells adhere to the endothelium of the target organ, and exudate and migrate into the organ parenchyma, which is the “pre‐metastatic niche.” 20 , 21 , 22 They either enter a long‐term dormant state in the form of a single cell, or enter micrometastasis in the form of multiple cells, and finally begin to grow continuously to form clinical metastases (Figure 1). 23 , 24
FIGURE 1.
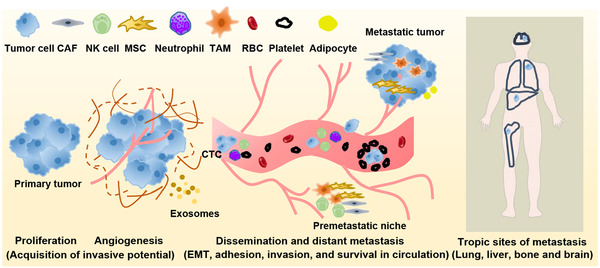
Overview of the metastatic cascade. Carcinoma cells escaping from the primary tumor migrate and invade through the basement membrane and extracellular matrix, enter the blood or lymphatic vessels, intravasate into the circulation, penetrate the blood or lymphatic vessels (extravasation), and adhere and grow in secondary sites. A variety of stromal cells, immune cells, and other molecular components surrounding the tumor provide signals that enhance the metastatic potential of cancer cells. Platelets and neutrophils can protect tumor cells by providing physical protection against shear stress, secreting mediators (such as transforming growth factor‐beta (TGF‐β)), neutralizing the cytotoxicity of NK cells and favoring immune escape. Abbreviations: BM, basement membrane; CAF, cancer‐associated fibroblast; CTC, circulating tumor cell; ECM, extracellular matrix; EMT, epithelial‐to‐mesenchymal transition; MSC, mesenchymal stem cell; NK cell, natural killer cell; RBC, red blood cell; TAM, tumor‐associated macrophage
At the beginning of the whole metastasis process, the most important thing is that there are a group of invasive and plastic tumor cells in the cancer nest, which are collectively referred to as metastasis initiating cells. 25 Cancer stem cells (CSCs) have been proved to have the above characteristics and play a crucial role in tumor metastasis. 26 , 27 In addition to the role of the tumor itself, metastasis is induced by various factors, including genetic alation, abnormal epigenetic modifications, immune escape, and changes in the growth environment. 9 Here, we will describe in detail the major factors and mechanisms involved in the metastasis cascade.
2.1. Key driver of metastasis: Epithelial‐mesenchymal transition (EMT)
The EMT is the transition of epithelial cells to mesenchymal cells under certain physiological and pathological conditions, which was proposed by Greenberg and Hay in 1982. 28 , 29 , 30 Studies in the last decades have shown that EMT is an important molecular event for epithelial tumor cells to gain invasiveness and plays an important role in the development and metastatic dissemination of malignant tumor cells. 31 Tumor cells can obtain a higher mesenchymal phenotype through EMT, which manifests as reduced contact with surrounding cells and matrix, and enhanced cell migration and motility at the beginning of tumor cell invasion and metastasis. 30 Therefore, interrupting or reversing EMT can inhibit the invasion of malignant tumor cells and reduce the rate of tumor metastasis. 32 The process of EMT is shown in Figure 2.
FIGURE 2.
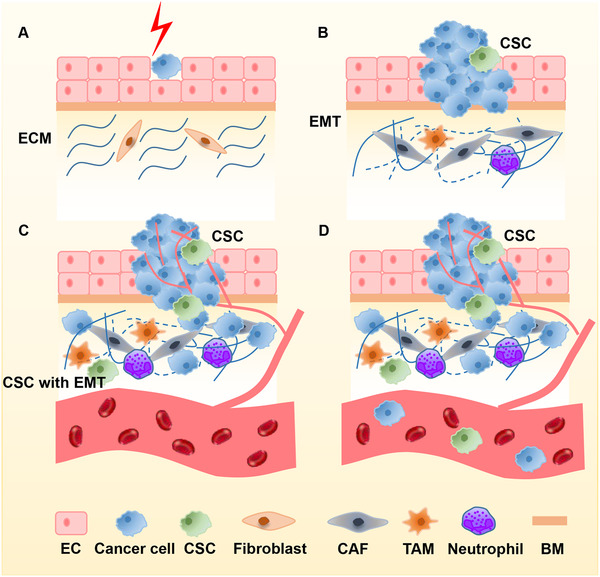
Cancer cells undergo EMT and invade into circulation. (A) A single transformed epithelial cell remains quiescent for a period of time. (B, C). The transformed cells proliferate and generate a small intraepithelial colony, accompanied by the formation of cancer stem cells. Cancer cells destroy the basement membrane, undergo EMT, and migrate and invade through the basement membrane and extracellular matrix. Normal extracellular matrix undergoes cancer‐associated remodel. Meanwhile, cells and molecular components in tumor microenvironment (TME; CAFs, TAMs, neutrophils, MSCs…) surrounding the primary tumor enhance cancer cell survival, proliferation and metastasis. (D) Cancer cells escaping from primary tumors can invade into the circulation as single CTCs or multicellular CTC clusters. Abbreviations: BM, basement membrane; CAF, cancer‐associated fibroblast; CTC, circulating tumor cell; EC, endothelial cell; ECM, extracellular matrix; EMT, epithelial‐to‐mesenchymal transition; MSC, mesenchymal stem cell; TAM, tumor‐associated macrophage
Cancer cells undergoing and molecular EMT exhibit morphological changes, such as decreased expression of epithelial markers (e.g., E‐cadherin, zonula occludens‐1, and occluding) and increased expression of mesenchymal markers (e.g., N‐cadherin protein, fibroblast‐specific protein 1, and fibronectin). 33 , 34 Cell adhesion molecules, such as E‐cadherin, N‐ cadherin, and β‐catenin, are closely related to EMT and are regulated by EMT‐related transcription factors (EMT‐TFs), such as zinc finger E‐box‐binding homeobox 1/2 (ZEB1/2), snai1/2 and twist. 35 , 36 , 37 The down‐regulation of E‐cadherin is accompanied by the up‐regulation of N‐cadherin, which reduces the adhesion of cancer cells to epithelial cells and increases their adhesion to stromal cells, leading to subsequent invasion of tumor cells into matrix. 38 β‐catenin has also been found to promote tumor metastasis, mainly driven by the ectopic expression of β‐catenin. 39 Vimentin has been found to promote cell invasion by regulating the E‐cadherin/β‐catenin complex. 40 EMT‐TFs can be regulated by signaling pathways such as Wnt/β‐catenin, transforming growth factor‐beta (TGF‐β), and Notch; these factors work together to give tumor cells the characteristics required for metastasis under different conditions, thereby promoting the occurrence of metastasis. 41 , 42 , 43 , 44 , 45
EMT is also involved in the process of tumor invasion and colonization in metastasis, which mainly depends on circulating tumor cells (CTCs) and CSCs. 41 , 46 , 47 CTCs are divided into three groups, including epithelial CTCs, hybrid epithelial/mesenchymal phenotype CTCs, and mesenchymal CTCs. 48 With the development of modern medical technology, tumor metastasis can be monitored throughout the process. Compared with epithelial CTCs, mesenchymal CTCs may be easier to metastasize because they are resistant to anoikis and chemotherapy and have a stronger ability to migrate to distant organs. 49 , 50 Previous studies reported that up‐regulating the expression of EMT‐TFs in breast cancer cells increased the expression of CSC‐specific cell markers, improved the ability to form spheroids, and accelerated tumor formation of breast cancer cells in mice. 51 In non‐CSCs, the activation of EMT promotes their conversion to CSCs. 51 , 52 , 53 , 54 Tumor formation and metastasis rely on the tumor‐forming ability of tumor cells, which suggests that CSCs with tumor‐initiating ability are the crucial prerequisite for disseminated cancer cells to establish metastatic colonies. 55
Another mechanism of EMT promoting metastasis is to protect tumor cells from immune cell‐mediated killing. 56 , 57 , 58 Recent researches on breast cancer found that, compared with epithelial cancer cell lines, mesenchymal breast cancer cell lines can recruit more immunosuppressive regulatory T cells (Tregs) and M2 macrophages. 57 Compared with epithelial cancer cells, mesenchymal cancer cells are less responsive to anti‐cytotoxic lymphocyte‐associated protein 4 (CTLA4) immunotherapy. 59 In addition, the expressions of programmed death ligand‐1 (PD‐L1), PD‐L2, and B7 homolog 3 (B7‐H3) are up‐regulated in tumor cells that have undergone EMT, which can facilitate the immune escape of cancer cells. 58 , 60
During dormancy and colonization, the process of mesenchymal cells to transform to epithelial cells (MET) is required to help tumor cells survive in the foreign microenvironment. 31 Indeed, the role of MET in promoting tumor cell colonization at a secondary site during metastatic growth has been confirmed in numerous studies. 31 , 61 It is worth noting that the tightly interconnected transfer network of EMT and MET has been proved to be subject to epigenetic regulation mediated by DNA methylation and histone modification. 62 , 63 , 64 For example, in breast cancer, histone methyltransferase nuclear receptor‐binding SET domain 3 (NSD3) cooperates with enhancer of zeste homolog 2 (EZH2) and RNA polymerase II to stimulate the Notch pathway‐mediated E‐cadherin transcriptional inhibition to promote EMT and trigger tumor invasion and metastasis. 65 During the initiation of EMT, histone demethylase lysine (K) ‐specific demethylase 6A (KDM6A) is inhibited, leading to the transcriptional inhibition of epithelial genes, whereas during the MET process, the expression of KDM6A is restored, thereby reactivating the epithelial gene by the trimethylation of histone 3 lysine 27 (H3K27me3) and reversing the phenotype. 66 , 67 Therefore, in general, a series of epigenetic regulation of tumor cells makes EMT dynamically change, giving tumor cells the ability to survive in different microenvironments.
2.2. The most important soil factor: TME
In addition to the characteristics of tumor cells, numerous studies have shown that TME is the soil for tumor cells and provides the necessary energy for tumor growth (Figure 3). 68 In the past two decades, many achievements have been made regarding the impact of TME on cancer metastasis. 10 , 11 , 69 , 70 TME can promote the occurrence and development of tumor by affecting metabolism, secretion, immunity, structure, and function of tumor cells, thereby playing an essential role in the overall cascade of tumor metastasis. 12 , 71 , 72 The members in TME are generally classified into two categories: cellular components and extracellular components. Cellular components mainly include immune cells, cancer‐associated fibroblasts (CAFs), mesenchymal stem cells (MSCs), and endothelial cells. 73 , 74 , 75 Extracellular components, such as tumor‐secreted extracellular vesicles (EVs), growth factors, chemokines, and cytokines are also closely involved in tumor metastasis. 76 Cellular components and extracellular components restrict and influence each other, which form a mutual feedback network.
FIGURE 3.
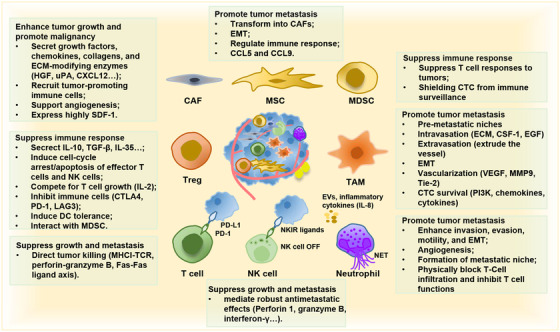
TME involved in the processes of invasion–metastasis cascade. The cellular components in TME can be classified into cancer cells, stromal cells, and immune cells. These cells interact with each other through ligand‐receptor interactions, and the secretion of cytokines, chemokines, exosomes, and extracellular vesicles, forming an evolving microenvironment. Cancer cells that are good at recruiting and establishing a supportive metastatic niche may be able to survive and initiate the process of proliferation and metastasis. The formation of the metastatic niche may occur before the arrival of cancer cells, also known as pre‐metastatic niches. Here, we summarized the role of important cellular components in TME in tumor metastasis
2.2.1. Immune cells
Under normal circumstances, the immune system will recognize foreign antigens to initiate autoimmunity and passive immunity to eliminate harmful pathogens. For this reason, tumor immunity has become a hot spot in the field of tumor research. Studies have pointed out that some types of immune cells are closely related to tumor occurrence and development and play an essential role in tumor metastasis. 72 , 77 Among the immune cells in TME, tumor‐associated macrophages (TAMs), Tregs, and neutrophils are noteworthy for their roles in tumor development and metastasis.
2.2.1.1. TAMs
According to the expression of specific markers, differentiation status, and functional role in the immune system, macrophages are conventionally classified into two major phenotypes, M1 and M2, which can be converted to each other. 78 M1 macrophages promote inflammation responses against tumor cells, whereas M2 macrophages tend to exert immune suppressive effects. M2 macrophages (M2‐TAMs) express abundant arginase‐1, mannose receptor, and scavenger receptors, and secrete a large number of anti‐inflammatory cytokines such as interleukin‐4 (IL‐4), interleukin‐10 (IL‐10), and interleukin‐13 (IL‐13). 79 , 80 In general, researchers tend to consider TAMs as M2‐like phenotype‐acquired macrophages. 81 The cytokines and chemokines secreted by tumor cells or fibroblasts could recruit more M2‐TAMs in TME. 82 , 83 , 84 TAMs are elevated in TME and are associated with poor clinical prognoses. 85
The role of TAMs in promoting metastasis involves multiple mechanisms. First, TAMs participate in the regulation of the EMT process by enhancing the expression of N‐cadherin and snail, secreting various soluble factors such as IL‐1β, IL‐8, tumor necrosis factor‐α (TNF‐α), and TGF‐β, and secreting a number of proteolytic enzymes. 86 , 87 , 88 Second, TAMs promote vascularization of tumor cells through stimulating the formation of new tumor vessels and the remodeling of the established vascular system into a more tortuous and leaky form. 89 , 90 Third, TAMs promote intravasation of tumor cell, favor tumor cell survival in the circulation, and promote extravasation of tumor cells. 91 Last, TAMs are important determinants for the formation of pre‐metastatic niches. Notably, TAMs contribute to the immune escape of tumor cells throughout the metastasis process. 81
2.2.1.2. Tregs
There are mounting studies that convincingly demonstrated that Tregs play a prominent role in promoting metastasis. Tregs have a strong immunosuppressive function in TME by inhibiting adaptive and innate immune responses. 92 , 93 , 94 , 95 , 96 Here, we summarized the main mechanisms. 97 First, Tregs secrete inhibitory cytokines, including IL‐10, TGF‐β, and IL‐35. 98 , 99 , 100 Tregs can inhibit the function of CD8+ T cells and dendritic cells (DCs) through membrane‐bound TGF‐β. For example, Tregs impede CD8+T cell‐mediated anti‐tumor immune responses by inhibiting interleukin‐2 (IL‐2) production and activating the TGF‐β signaling pathway. 92 It has been reported that TGF‐β1 secreted by Tregs in breast cancer can induce the up‐regulation of IL‐17Rb and promote tumor lymph node metastasis. 101 Second, Tregs can kill effector cells by granzymes and perforin and diminish the function of T cells and natural killer (NK) cells through inhibitory receptors, such as CTLA‐4, lymphocyte activation gene 3 (LAG‐3), and programmed cell death 1 (PD‐1). 102 , 103 , 104 Tregs can also suppress CD8+ T cells and NK cells secretion of interferon‐γ (IFN‐γ). 105 Olkhanud et al. found that chemokine receptor (CCR) 4+Tregs are mainly recruited at the lung metastasis site in breast cancer, and the Tregs secret β‐galactoside‐binding proteins to induce the apoptosis of NK cells, thereby promoting tumor metastasis. 95 Third, Tregs affect effector cell functions by interfering with cell metabolism mainly through the following three ways: depriving IL‐2, promoting the production of adenosine, and transferring a large number of cyclic adenosine monophosphate to effector T cells. 106 , 107 , 108 , 109 Fourth, Treg induces DCs tolerance through the expression of inhibitory receptors CTLA‐4 and LAG‐3, with the latter further inhibiting T‐cell capacity through indoleamine 2,3‐dioxygenase. 110 , 111 Finally, factors produced by myeloid‐derived suppressor cells (MDSCs) and Tregs form positive feedback loops to reinforce the suppressive microenvironment. 112 , 113 , 114
In addition, it has been found that Tregs could secrete vascular endothelial growth factor A (VEGF‐A) in metastatic ovarian cancer, thereby promoting angiogenesis and tumor cell dissemination. 115 TGF‐β1 secreted by Tregs can promote tumor metastasis by enhancing EMT. 116 Some preclinical studies have shown that Tregs were closely related to lymph node metastasis and peritoneal metastasis and suggested that Treg may be involved in pre‐metastasis niche reprogramming to promote metastasis. 101 , 117 Therefore, eliminating or alleviating the immunosuppressive state of Tregs may suppress the progression of tumor metastasis.
2.2.1.3. Neutrophils
Neutrophils, as innate immune cells, protect the body from infection by foreign microorganisms and promote inflammatory responses under normal conditions. 118 However, it is worth noting that neutrophils have been found to promote tumor metastasis in a variety of ways, including inhibiting anti‐tumor T cells and influencing tumor cell invasion. 119 In a mouse model of sarcoma, pleomorphic neutrophils were found to reduce the expression of intercellular adhesion molecule 1 in tumor cells, thereby increasing tumor motility and facilitating the initiation of metastasis. 120 In addition, pro‐metastatic neutrophils activate various signaling pathways to promote EMT by secreting pro‐EMT‐related cytokines, including IL‐8 and IL‐17A. 121 , 122 In the process of metastasis, neutrophils bind with CTCs in circulation to protect CTCs from immune killing, increase CTCs invasiveness, and promote metastasis. 123 A large number of neutrophils were found in the secondary sites. 124 , 125 In terms of mechanism, recruited neutrophils promote the formation of pre‐metastatic niche and support angiogenesis mainly by forming an immunosuppressive microenvironment and secreting neutrophil elastase. 126
2.2.2. Cancer‐associated fibroblasts (CAFs)
CAFs are one of the most common fibroblasts in tumor tissues. CAFs are derived from a variety of different fibroblasts, including normal fibroblasts stimulated by exosomes, bone marrow MSCs induced by TGF‐β, and epithelial cells transformed by EMT. 127 , 128 , 129 Since CAFs originate from many kinds of cells and have apparent heterogeneity, there is no clear marker that can distinguish CAFs from normal fibroblasts. CAFs are the primary source of various factors and enzymes of TME and are one of the critical members involved in tumor metastasis.
In previous studies, it was believed that the role of CAFs in tumor metastasis is mainly manifested in the reconstruction of ECM structure. 130 However, current studies have found that CAFs promote EMT‐mediated tumor metastasis in ovarian cancer, bladder cancer, and breast cancer cells in various ways, including the secretion of TGF‐β and exosomes. 131 The increased autocrine and paracrine TGF‐β signaling in the mesenchymal transcription factor forkhead box F2 (FOXF2) deficient base‐like breast cancer cells induces EMT to mediate tumor metastasis. 132 In turn, TGF‐β silences FOXF2 expression by up‐regulating the post‐transcriptional regulator (miR‐182‐5p) of FOXF2 and promotes breast cancer metastasis. 133 In addition, chemokine (C‐X‐C motif) ligand 11 (CXCL11) secreted by CAFs promotes the metastasis of liver cancer by up‐regulating the expression of circUBAP2. 134 In order to gain a deeper understanding of the role of CAFs in the tumor, Wang et al. analyzed CAFs by single‐cell sequencing combined with RNA‐sequence and divided CAFs into four types. 136 One of the subtypes has high glycolytic activity and is termed MeCAFs. It was found that pancreatic ductal adenocarcinoma patients with abundant MeCAFs had a higher risk of metastasis but had a significantly better response to immunotherapy. 136 These results indicate that abnormal glycolysis directly or indirectly affects tumor metastasis. CAFs up‐regulate carnitine palmitoyltransferase 1A (CPT1A) to cause reduction of fatty acid oxidation, leading to peritoneal metastasis of colorectal cancer. 137
Fibroblasts play an important role in both the primary tumor site and the secondary tumor site. Fibroblasts distributed in the metastatic site are called metastasis‐associated fibroblasts (MAFs), which promote angiogenesis and the formation of immunosuppressive microenvironment in pre‐metastatic niche. 138 The main difference between MAFs and primary tumor‐related fibroblasts is that MAFs have a stronger ability to inhibit the anti‐tumor effect of immune cells, which is reflected explicitly in the higher levels of CC motif chemokines ligand 2 (CCL2), CXCL12, and interferon‐related genes secreted by MAFs. 139 Additionally, in metastatic liver cancer and gastric cancer, it was found that the decrease of CD3+ infiltrating lymphocytes and the increase of TAM cells were related to the high levels of MAFs, partly due to the chemokine CXC receptor 4 (CXCR4) signal transduction in α‐smooth muscle actin (α‐SMA)+ MAFs. 140 In another study conducted by Costa et al., fibroblasts that inhibit tumor immunity were discovered, named cancer‐associated fibroblasts subset 1 (CAF‐S1). 141 Furthermore, it was found that the presence of CAF‐S1 in breast cancer promoted bone metastasis of cancer cells. 142
2.2.3. MSCs
More and more studies have recognized the importance of MSCs in regulating tumor metastasis at the initial tumor site and distant metastasis sites. At the beginning of tumor metastasis, MSCs support the generation of the cancer‐promoting microenvironment by transforming into CAFs and increase the motility of cancer cells through secreting growth factors and chemokines in autocrine and paracrine manners. 128 Pendergast et al. co‐cultured MSCs and lung cancer cells and found that MSCs can mediate EMT to promote lung cancer metastasis by activating the abelson‐ matrix metalloprotein 9 (ABL‐MMP9) signaling pathway. 143 In the late stage of tumor metastasis, MSCs participate in the colonization and metastatic growth of tumor cell. For example, in ovarian cancer, MET of cancer‐associated MSCs mediated by Wilms’ tumor 1 (WT1) and EZH2 promote metastatic tumors growth in distant organs. 135
MSCs can also promote tumor metastasis by regulating anti‐tumor immunity. As an immune checkpoint, PD‐L1 is one of the crucial members involved in tumor immune escape and treatment resistance. 144 Studies have found that PD‐L1 is highly expressed on tumor‐associated MSCs, and MSCs can up‐regulate the expression of PD‐L1 in a variety of tumor cells. Hamidreza et al. reported that MSCs promoted the up‐regulation of PD‐L1 expression in breast cancer cells by secreting CCL5. 145 Sun et al. found that IL‐8 secreted by MSCs can increase the expression of PD‐L1 in gastric cancer cells. 146 Further experiments suggested that MSCs can increase the binding of PD‐L1 to the transcription factor CCCTC‐binding factor, thereby affecting the stemness of gastric cancer cells and leading to tumorigenesis. 147
2.2.4. Tumor‐associated endothelial cells (TECs)
The role of TECs in metastasis is complex. In the process of tumor metastasis, TECs play an important role in obtaining anti‐anoikis properties of CTCs, which is one of the mechanisms by which TECs promote tumor cell metastasis to secondary organs. 148 In addition, studies have found that biglycan in TECs is significantly higher than that in normal endothelial cells. 149 TECs‐biglycan activates nuclear factor‐κB (NF‐kB) and extracellular signal‐regulated kinase (ERK) signal transduction to stimulate the metastasis of tumor cells expressing toll‐like receptors (TLR) and promote tumor angiogenesis of tumors by activating the TLR signaling pathway. 150 IL‐6 secreted by tumor cells can activate the signal transducers and activators of transcription 3 (STAT3)‐VEGF pathway of lymphatic endothelial cells and encourage lymphatic metastasis of the tumor. 151
2.2.5. Extracellular matrix (ECM)
ECM is a network of various proteins represented by collagen and macromolecules, which maintain tissue structure outside tumor cells. 18 A hallmark of metastasis is ECM degradation. 152 In the process of metastasis, it is necessary to enhance the invasion ability of tumor cells and reconstruct the ECM structure. 153 ECM reconstruction is a relatively complex process, which requires the participation of multiple chemokines. 154 , 155 Matrix metalloproteinases (MMPs) are a group of enzymes that can directly degrade collagen and multiple connexins in the process of ECM degradation. Among matrix MMPs family, MMP‐2 and MMP‐9 are the two important enzymes involved in the EMT process, which specifically degrade gelatin, collagen, elastin, and fibronectin, thereby inducing invasion and metastasis of tumor cells. 156 Furthermore, Musashi‐1 can promote the degradation of ECM by upregulating Timp3 to encourage the expression of MMP‐9 in breast cancer, and thus regulating cell‐ECM adhesion. 157
2.2.6. Cytokines and chemokines
The involvement of various cytokines/chemokines, along with their receptors and signaling axis in the promotion of tumor metastasis has been well‐studied. The convoluted cross‐talk between various cell types and these secreted factors helps drive the sequence of events that lead to tumor metastasis. 158 , 159 These cytokines/chemokines are involved in ECM remodeling, tumor invasion, EMT, angiogenesis, pre‐metastatic niche reprogramming, extravasation, and modulating stromal cells and immune cells. 115 , 154 , 160 , 161 , 162 The cytokines/chemokines including TNF‐α, IL‐8, CCL20, CXCL5, CXCL12. 163 , 164 , 165 , 166 CXCR2, CXCR3, and CXCR4, up‐regulate EMT‐TFs (snail and ZEB1), promote MMP‐2 expression, and accelerate EMT of cancer cells, thereby promoting tumor metastasis. 160 , 165 , 166 Cytokine IL‐8 mainly down‐regulates E‐cadherin through activating the Wnt/β‐catenin pathway in ovarian cancer, thereby promoting tumor invasion. 163 Some cytokines can promote the expression of chemokines and mesenchymal transformation. For example, TNF‐α has been reported to promote the up‐regulation of CXCL10 and activate the phosphoinositide3‐kinase/protein kinase B (PI3K/AKT) pathway to inhibit the phosphorylation of GSK‐3β, leading to the up‐regulation of snail and promoting tumor metastasis. 160
Cytokines/chemokines, such as VEGF, TGF‐β, IL‐1, CXCL8, and CCL21 are involved in promoting angiogenesis and lymphatic formation. 167 , 168 , 169 , 170 , 171 Among the known pro‐angiogenic factors, VEGF is the most effective cytokine in promoting angiogenesis. By directly binding to receptors on endothelial cells, VEGF induces endothelial cell proliferation and promotes tumor metastasis. 167 VEGF‐C and VEGF‐D in the VEGF family can activate the generation of lymphatic vessels, recruit chemokines, such as CCL21, CCL27, and CCL28, to lymphatic vessels and promote the occurrence of lymph node metastasis. 172 , 173 Cytokines/chemokines also play an indispensable role in promoting the recruitment of immune cells in the tumor microenvironment. For example, the recruitment of Treg requires the participation of CCL20 and CCL22, while the recruitment of TAMs requires the participation of CCL2. 174 , 175 , 176 , 177 Single‐cell sequencing analysis revealed that chemokine CCL5 is an important mediator of CTC immune escape. 162 CCL5 can promote immune escape and metastasis of CTCs by recruiting Tregs, which further suggests that chemokines can promote immune escape. 162 In addition, MDSCs secrete TGF‐β in esophageal cancer, which can increase the expression of PD‐1 on tumor‐infiltrating CD8+ T cells, leading to immunotherapy resistance. 178 , 179
Cytokines and chemokines are involved in the organotropism of tumor metastasis. Lung metastasis is associated with the high levels of CXCL1, CXCL9, and CXCL10 secreted by human pulmonary artery endothelial cells or lung fibroblasts. 179 , 180 Ricardo et. Al. reported that blocking CXCL5/CXCR2 signaling can inhibit the bone colonization of breast cancer cells. 181 Blocking CXCL12/CXCR4 signaling significantly impair the metastasis of breast cancer cells to regional lymph nodes and lung, which indicate that chemokines and their receptors play a key role in determining the destination of metastasis. 182 , 183 Overall, the potential of cytokines and chemokines to induce metastasis is mainly achieved by promoting EMT, angiogenesis, immunosuppression, and pre‐metastatic niche reprogramming.
2.2.7. Tumor‐secreted extracellular vesicles
EVs secreted by tumors are the key mediators of cell‐to‐cell contact in the local and remote microenvironments. 184 EVs are exceptional cargo for various nucleic acid, proteins, and lipids in TME, and play a prominent role in the metastasis of the tumor to distant organs. 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 Tumor‐associated EVs in breast cancer can promote breast cancer colonization by changing the composition and structure of lung tissue fibroblasts. 185 It was found that EVs secreted by bladder cancer cells mediate their intercellular communication with human lymphatic endothelial cells through long non‐coding RNA (lncRNA) ELNAT1 and promote lymph node metastasis in a SUMOylation‐dependent manner. 186
In nasopharyngeal carcinoma (NPC), EVs migrate from highly metastatic to poorly metastatic NPC cells, mediate intercellular communication, and enhance the metastatic potential of poorly metastatic NPC cells by inducing the up‐regulation of epidermal growth factor receptor (EGFR) and down‐regulation of reactive oxygen species (ROS). 191 Mechanistically, overexpression of EGFR mediated by EGFR‐rich EVs down‐regulates intracellular ROS levels through regulating PI3K/AKT pathway, thereby promoting the metastatic potential of NPC with poorly metastatic ability. 191
The role of EVs in tumor metastasis depends on their components; the function of EVs may be different for different tumor metastasis sites. For example, in brain metastasis, EVs mainly mediate pre‐metastasis regulation by destroying the blood‐brain barrier or by distinct increasing the expression of cell migration‐inducing and hyaluronan‐binding protein in brain‐tropic EVs. 187 , 188 In hepatocellular carcinoma, nidogen 1 (NID1) in EVs promote tumor cell colonization and extrahepatic metastasis by enhancing angiogenesis and lung endothelial permeability and promoting the formation of pre‐metastasis niches in the lung. 189 EVs‐NID1 also activates fibroblasts that secrete TNF receptor 1 (TNFR1), promote lung colonization of tumor cells, and augment the growth and motility of cancer cells. 189 EVs in bone metastasis mainly mediate cancer‐induced osteolysis to produce a suitable microenvironment for tumor cells. 190 In addition, EVs can also promote the formation of pre‐metastasis niches by stimulating CAFs, inducing MSC differentiation, and regulating tumor immunity. 183
2.2.8. Neutrophil extracellular traps (NETs)
NETs are net‐like structures composed of DNA histone complexes, and proteins released by neutrophils in response to infection or inflammatory cytokines were discovered by Brinkmann et al. in 2004. 192 NETs are initially discovered to be one of the host defense mechanisms of neutrophils against pathogens, and they are also involved in the progression of sterile inflammation‐related diseases, such as autoimmune diseases, diabetes, and carcinoma. 193 In the absence of infection, NETs can also be stimulated by cancer cells and CAFs, and hijack anti‐tumor immune system to promote tumor cell proliferation and metastasis. 193
The levels of plasma NETs in patients with lung cancer, cervical cancer, and pancreatic cancer are significantly higher than those in healthy controls and are related to tumor recurrence and metastasis. 194 In tumor metastasis, NETs are mainly involved in the spread of cancer cells and awakening dormant tumor cells. NETs can induce EMT of primary tumor cells in the early stage of metastasis and lead to the activation of inflammatory signaling pathways such as NF‐kB and STAT3, thereby enhancing the mobility and invasiveness of cancer cells and promoting metastatic growth. 195 , 196
In the circulation, NETs wrap CTCs to avoid contact between CTCs and immune cells and can also form a physical barrier for immune escape. 197 , 198 The molecular mechanisms by which NETs can trap CTCs have been investigated, and it has been found that expression of β1‐integrin on both cancer cells and NETs is important for the adhesion of CTCs to NETs. 197 , 198 After spreading to other sites, cancer cells from primary tumors often do not start growing immediately but enter a dormant state. Disseminated cancer cells can remain dormant for years or even decades before they relapse or “wake up” as metastatic cancer. 199 Studies have found that the formation of NETs induced by sustained inflammation is required for awakening dormant cancer. 199 Two NET‐associated proteases (neutrophil elastase and MMP9) sequentially cleave laminin, and then NET‐remodeled laminin facilitates the proliferation of dormant cancer cells by activating integrin α3β1 signaling and focaladhesion kinase (FAK)/ERK/myosin light chain kinase (MLCK)/Yes associated protein (YAP) signaling. 199
2.3. A double‐edged knife in tumor metastasis: Autophagy
Autophagy is generally defined as a lysosome‐dependent mechanism of intracellular degradation and recycling process, which is highly conserved in all eukaryotes. 200 , 201 , 202 Several forms of autophagy have been discovered. In all types of autophagy, autophagosomes are the hallmark morphological characteristics, and the formation of autophagosomes is the core process of this dynamic process. 200 , 203 Autophagy is a dynamic physiological process that maintains cell homeostasis when the body encounters stress, including nutrient deprivation, hypoxia, and infection. 204 , 205 It has been reported that autophagy plays a key role in the prevention of cancer and other diseases by promoting cellular senescence and antigen presentation and preventing genomic instability and necrosis. 206 However, autophagy also plays a vital role in the occurrence and progression of tumor. 207 , 208 In many types of tumors, including melanoma, breast cancer, and liver cancer, the expressions of autophagy‐related proteins light chain 3 and beclin‐1 in tumor tissues of patients with distant metastasis are higher than in tumor tissues of patients with non‐metastatic tumor, suggesting that autophagy is closely related to tumor metastasis. 209 , 210 , 211 Partly due to the heterogeneity of tumor cells, autophagy is a double‐edged sword in the process of tumor metastasis. 212
In the early stage of metastasis, autophagy mainly suppresses the occurrence of metastasis by regulating the anti‐tumor immune response. 213 , 214 , 215 Autophagy‐related protein 5(ATG5) is considered to be closely related to autophagy. In pancreatic ductal adenocarcinoma, mice with ATG5 knockout developed more tumors and metastases than control mice. Moreover, compared with the control mice, the expression of cytokines that regulate macrophage chemoattraction and differentiation into M2 macrophages was up‐regulated in the tumors of mice with ATG5 knockout. Meanwhile, the number and activity of M2‐TAMs were substantially higher in ATG5 knockout mice, indicating that autophagy can inhibit TAMs infiltration and limit tumor metastasis. 214 In addition, studies have found that autophagy can stimulate tumor‐related spontaneous inflammation by releasing high mobility group box 1 and enhance the antitumor immune response of DCs to limit metastasis. 215 In the later stage of tumor metastasis, autophagy reduces the adhesion between tumor cells and ECM by regulating the activity of the Rho family so as to promote tumor migration and invasion. 216 In addition, autophagy can enhance tumor invasion and promote the progress of tumor metastasis by inhibiting anoikis of CTCs, maintaining the stemness of CSCs, and reawakening dormant tumor cells. 210 , 217 , 218
Another significant aspect of autophagy in metastasis is involved in the regulation of EMT. P53, an oncogene, also plays an important role in regulating the relationship between EMT and autophagy. 219 , 220 Under normal circumstances, P53 exists in the cytoplasm and inhibits autophagy. However, under cellular stress, P53 translocates from the cytoplasm to the nucleus and induces autophagy. 221 , 222 In addition to autophagy, recent studies have found that P53 inhibits EMT and metastasis by affecting the TFs involved in the EMT process. 223
It is well known that AKT/ mammalian target of rapamycin (mTOR) signaling pathway participates in modulating the EMT process. 224 A large number of studies have found that oncogenes activate mTOR‐related pathways to promote tumor metastasis by inhibiting autophagy. 225 Suppressor of cytokine signaling‐5 (SOCS5), a member of the SOCS protein family, has also been found to promote tumor cell invasion and metastasis in hepatocellular tumors by up‐regulating PI3K/Akt/mTOR‐mediated autophagy pathway. 226 Interestingly, opposite findings have been reported in NPC. Annexin A1 (ANXA1) inhibits autophagy and promotes EMT by activating PI3K/Akt/mTOR pathway. 227 ANXA1 inhibits autophagy by activating PI3K/Akt/mTOR pathway, leading to the up‐regulation of tumor cell migration and invasion capabilities, EMT‐like changes, and metastasis in vivo. 227 During this process, autophagy inhibited by ANXA1 induces EMT‐like alterations, possibly by inhibiting autophagy‐mediated Snail degradation. 227
Autophagy can also be induced by activating the adenosine monophosphate activated protein kinase (AMPK) pathway that promotes tumor metastasis. 228 For example, tight junction protein 1 (CLDN1) promotes EMT and metastasis of esophageal cancer cells by triggering autophagy in vivo and in vitro. 229 Mechanically, CLDN1 induces autophagy by up‐regulating the expression of Unc‐51‐like autophagy activating kinase 1 (ULK1) through AMPK/signal transducer and activator of transcription 1 (STAT1)/ULK1 signaling pathway. 229 , 230 In general, autophagy is a double‐edged sword in the process of tumor metastasis, and the mechanisms involved are very intricate and require further research.
2.4. Lipid metabolism
Under hypoxic conditions, tumor cells mainly rely on glycolysis to obtain sufficient energy. 231 This metabolic mode different from normal cells is also called tumor‐related metabolic rearrangement. 232 Glycolysis is essential to maintain the malignant proliferation behavior of the tumor, but there are relatively few studies on the effect of lipid metabolism on tumor progression. Obesity has been recognized as a risk factor for cancer, so investigating lipid metabolism in the tumor is necessary. 233 , 234 The results of researches in the past 10 years have shown that abnormal lipid metabolism in the TME is one of the key steps involved in tumor metastasis. 235 Therefore, correcting abnormal lipid metabolism may prevent the occurrence of tumor metastasis.
It has been found that a variety of enzymes involved in lipid anabolism and catabolism are related to tumor metastasis, including adenosine triphosphate (ATP) citrate lyase (ACLY), fatty‐acid synthase (FASN), stearoyl‐CoA desaturases (SCD), and monoacylglycerol lipases. 236 ACLY and SCD‐1 are associated with facilitated colon cancer metastasis, and FASN can promote retroperitoneal metastasis of ovarian cancer. 237 , 238 Identifying tumor metastasis initiating cells is considered to be one of the most challenging problems in the field of tumor metastasis research. In recent years, fatty acid receptor CD36 has been shown to play an essential role in the metastasis of oral cancer because CD36 is positively correlated with lymph node metastasis and can be used as a marker of metastasis initiating cells. 239 Palmitic acid or a high‐fat diet can enhance the metastatic ability of CD36+ tumor cells. Subsequent studies have found that CD36+ cells are associated with a poor prognosis of ovarian cancer, and blocking CD36 can attenuate tumor metastasis. 240 These findings suggest that the initiating cells of tumor metastasis may depend on lipids, and blocking CD36 can reverse tumor metastasis.
With the development of proteomics and metabolomics, new discoveries have been made about cancer metabolic disorder and its correlation with metastasis. In breast cancer, it was found that the levels of phospholipid are different between mammary epithelial cells and breast cancer cells, as well as between breast cancer cells with different levels of aggressiveness. 241 In short, lipid metabolic reprogramming is associated with breast cancer carcinogenesis and metastasis. 241 In pancreatic cancer, fatty acid synthesis was found to maintain the stemness of pancreatic cancer cells, indicating that abnormal lipid metabolism can make cancer cells more invasive and promote the spread of tumor cells. 242 Promoting cholesterol biosynthesis may promote tumor metastasis.
More and more molecules regulating lipid metabolism have been proved to promote tumor metastasis by regulating EMT, including apolipoprotein C, sterol regulatory element‐binding transcription protein 1, stromal‐interaction molecule 1, human hydroxysteroid dehydrogenase‐like 2, and cytosolic phospholipase A2α. 243 , 244 , 245 , 246 In triple‐negative breast cancer, nicotinamide adenine dinucleotide phosphate (NADP) steroid dehydrogenase‐like (NSDHL), a cholesterol metabolic enzyme, has been reported to be a potential metastatic driver. 247 The functions of NSDHL rely on its enzyme activity in the biosynthesis of cholesterol and is mediated by the NSDHL‐TGFβR2 signaling pathway. 247
TME has a unique lipid structure, which is characterized by being rich in sphingolipids and cholesterol and is called lipid rafts. 248 The role of lipid rafts in tumor metastasis is different from other lipid metabolism‐related regulatory molecules. Rina et al. reported that palmitoylated CD44 is wrapped in lipid rafts, and its binding to pro‐migration binding partners (such as Ezlin) is restricted, thereby inhibiting cancer metastasis and spread. 249 Moreover, another study reported that squalene synthase could promote lung cancer metastasis by activating TNFR1, NF‐Κb, and matrix metallopeptidase 1, and destroying the lipid raft structure. 250 Therefore, lipid rafts mainly play an inhibitory role in tumorigenesis and metastasis. For cells in TME, abnormal lipid metabolism may be a potential mechanism to promote tumor metastasis. For example, lipid accumulation in TME can lead to CD8+T cell dysfunction and promote TAMs differentiation. 251 , 252 In addition, the abnormal elevation of fatty acid synthase in CAFs may enhance the aggressiveness of tumor cells. 253 Therefore, lipid metabolism reprogramming plays an important role in regulating the formation of the pro‐metastatic TME.
2.5. Long non‐coding RNA
LncRNA is a standard non‐coding RNA with a length of more than 200. 254 Researches in recent years have shown that lncRNA plays an important role in tumorigenesis. 254 In fact, lncRNA plays a double‐edged role in tumor metastasis. LncRNA may be involved in all the processes of metastasis, including EMT, tumor invasion and migration, and tumor cell colonization in secondary sites. We briefly summarized the representative lncRNA that have been shown to be relevant with metastasis in vivo and in vitro (Table 1). 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280
TABLE 1.
Important metastasis‐related long non‐coding RNAs
| Metastasis‐related long non‐coding RNA | Cancer type | Function | Effect | Reference |
|---|---|---|---|---|
| H19 | Bladder cancer | Bind to enhancer of zeste homolog 2 (EZH2) to downregulate E‐cadherin | Inhibit | 255 |
| Prostate cancer | Encode miR‐675 to mediate the down‐regulation of TGF‐β1 | Promote | 258 | |
| Colorectal cancer | Upregulate zinc finger E‐box‐binding homeobox 1/2 protein | Promote | 257 | |
| PNUTS | Breast cancer | Competitive sponge for miR‐205 and miR‐200 and enhancing epithelial‐mesenchymal transition (EMT) | Promote | 259 |
| LINC00460 | Colon cancer | Enhance the expression of high‐mobility group AT‐hook 1 | Promote | 260 |
| Lnc01232 | Pancreatic cancer | Upregulate HNRNPA2B1, and activate the MAPK/ERK signaling | Promote | 261 |
| MALAT1 | Colorectal cancer | Regulate the miR‐106b‐5p via SLAIN2 | Promote | 262 |
| TPA | Breast Cancer | Activate TGF‐β signaling pathway | Promote | 263 |
| PVT1 | Colon Cancer | Downregulate tumor suppressor miR‐152‐3p | Promote | 264 |
| LIMT | Breast cancer | Suppress tumor cells motility | Inhibit | 265 |
| SPRY4‐IT1 | Bladder cancer | Bind to miR‐101‐3p to upregulate the expression of EZH2 | Promote | 266 |
| TRERNA1 | Gastric cancer | Regulating CDH1 to upregulate SNAI1 | Promote | 267 |
| NEF | Hepatocellular carcinoma | Suppress Wnt/β‐catenin signaling to activate expression of FOXA2 | Inhibit | 268 |
| HOXD‐AS1 | Hepatocellular carcinoma | Competitive bind to miR‐130a‐3p to upregulate the expression of EZH2 and MMP2 | Promote | 269 |
| CYTOR | Colon cancer | Activate Wnt/β‐catenin signaling to enhance EMT | Promote | 270 |
| JPX | Lung cancer | Activate Wnt/β‐catenin signaling pathway to upregulate Twist1 expression | Promote | 271 |
| LINC00662 | Colon cancer | Activate extracellular signal‐regulated kinase (ERK) signaling pathway | Promote | 272 |
| ID2‐AS1 | Hepatocellular carcinoma | Activate HDAC8/ID2 signaling pathway to decrease Twist expression | Inhibit | 273 |
| RPPH1 | Colorectal cancer | Interact with TUBB3 to reduce E‐cadherin levels and induce macrophages M2 polarization | Promote | 274 |
| SATB2‐AS1 | Colorectal cancer | Regulate SATB2 to decrease MMP9 and vimentin | Inhibit | 275 |
| NORAD | Lung cancer Breast cancer | Bind and sequester S100P to suppress S100P pro‐metastatic signaling pathway | Inhibit | 276 |
| URRCC | Renal cancer | Enhance EGFL7 expression to suppress P‐AKT/FOXO3 signaling pathway | Promote | 277 |
| FEZF1‐AS1 | Colorectal cancer | Activate PKM2/signal transducers and activators of transcription 3 signaling pathway | Promote | 278 |
| ADAMTS9‐AS2 | Salivary adenoid cystic carcinoma | Activate PI3K/Akt and MEK/ERK signaling pathway | Promote | 279 |
| GAS5 | Pancreatic cancer. | Regulate miR‐221/SOCS3 to suppress EMT and cancer stem cells self‐renewal | Inhibit | 280 |
As mentioned above, EMT is an essential step in the initiation of tumor metastasis. It has been reported that LncRNA H19 can mediate the transformation of tumor cells into mesenchyme and is significantly elevated in bladder cancer, breast cancer, and colorectal cancer. 255 , 256 , 257 H19 directly binds to EZH2 in bladder cancer, resulting in a reduction in EMT epithelial marker E‐cadherin. 255 In colorectal cancer, H19 acts as a competing endogenous RNA, leading to the up‐regulation of ZEB1/2 protein expression. 257 However, in prostate cancer, the opposite phenomenon has been observed, that is, H19 inhibits tumor metastasis, mainly by encoding miR‐675 to mediate the down‐regulation of TGFBI. 258 Similar to H19, lncRNA‐PNUTS serves as a competitive sponge for miR‐205 and miR‐200, leading to the up‐regulation of EMT in breast cancer. 259 LncRNA LINC00460 is increased in colon cancer, and it induces EMT and promoteS tumor growth and metastasis by enhancing the expression of high‐mobility group AT‐hook 1 and decreasing the expression of E‐cadherin. 260
The influence of lncRNA on tumor invasion and migration is also one of the mechanisms by which it affects tumor metastasis. For example, colon cancer‐associated transcript 2 (CCAT2) and RNA associated with metastasis‐11 (RAMS11) have been reported to participate in the process of tumor invasion and migration. CCAT2 is related to the stability of microsatellites; knockdown of CCAT2 inhibits tumor invasion and migration in mouse models. CCAT2 may interact with TCF7L2 to activate Myc transcription and Wnt signaling pathways and thus promote metastasis. 281 , 282 RAMS11 is overexpressed in colon cancer with liver metastasis. Knockout of RAMS11 gene using CRISPR‐Cas9 technology can reduce the invasion and migration of colon cancer cells in vitro and reduce liver metastasis in mouse model. 283 Lnc01232 was found to promote metastasis by inhibiting ubiquitination, upregulating HNRNPA2B1, and activating the mitogen‐activated protein kinase/extracellular signal‐regulated kinase (MAPK/ERK) signaling pathway in pancreatic cancer. 261
As the first lncRNA found to be associated with metastasis, metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) has been shown to be associated with poor prognosis of lung cancer and breast cancer, and its expression is significantly increased in patients with metastatic cancer. 284 , 285 In the breast cancer mice model lacking the promoter of MALAT1 or MALAT1, tumor differentiation and E‐cadherin are increased, whereas lung metastasis is significantly reduced, indicating that MALAT1 may serve as essential factors for tumor cell colonization at distant metastasis sites. 285
With the progress in understanding the functions of lncRNAs, increasing evidence has indicated that lncRNAs play a role in the physiological and pathological processes of malignancies. Although many findings need further verification, it is certain that lncRNAs can carry out diverse functions in carcinogenesis and metastasis. However, the specific role and mechanisms of different lncRNAs in cancer metastasis need to be further elucidated.
3. INTERVENTIONS FOR TUMOR METASTASIS
Metastatic disease is the major contributor to cancer‐related mortality. Therefore, there is an urgent need for effective cancer treatments that can eliminate large solid tumors and disseminated and metastatic nodules, while simultaneously preventing tumor recurrence. With the deepening understanding of the molecular mechanism of tumorigenesis and metastasis in recent years, a plethora of treatment approaches, including targeted therapy and immunotherapy have been established for antitumor treatments. 13 , 286 , 287 Although the emergence of new therapies, such as those based on immune checkpoint inhibitors (ICIs), is rapidly changing the treatment modality of metastatic patients in some cases, the current standard of cancer treatment for localized disease is still usually based on surgery, chemotherapy, and radiotherapy. 288 , 289 In addition, the combination therapies are receiving more attention and are being actively evaluated. 290 We summarize the interventions for metastatic diseases based on preclinical and clinical evidence.
3.1. Surgery, chemotherapy, and radiotherapy
Surgery, chemotherapy, and radiotherapy are the three cornerstones of tumor treatment. According to the purpose of treatment, surgical treatment can be divided into radical surgery, local surgery, and palliative surgery. 291 For patients with early‐stage tumor, radical surgery can prevent and reduce tumor recurrence and metastasis. However, for most patients, due to the insidious tumor‐related symptoms, distant metastasis has already occurred at the time of diagnosis. In the past, it was considered that metastatic tumors cannot be treated surgically, but a number of recent studies and clinical practices have confirmed that local surgery can be performed on some patients with localized metastasis, which can reduce the tumor burden and may achieve the goal of radical cure. 292 , 293 , 294 , 295 For example, palliative surgery for patients with intraperitoneal metastases such as liver cancer and ovarian cancer can alleviate their symptoms and improve their quality of life. 296 , 297
For intervention and treatment of metastatic tumors, surgery alone is not enough. Dormant CTCs is the main cause of early postoperative metastasis. Therefore, many clinical studies have been carried out to reduce the probability of tumor metastasis. Neoadjuvant chemotherapy and adjuvant chemotherapy are currently widely used in the treatment of cancer because these interventions can kill CTCs and reduce the possibility of metastasis while reducing the size of the preoperative tumor and preserving some functional organs. 298 , 299 , 300 , 301 Except for a small number of patients, chemotherapy, such as 5‐FU, Adriamycin, and platinum, is the first‐line choice for most patients with advanced cancer. 301 , 302 , 303 , 304 It has been observed that diverse malignancy patients with metastatic lesions benefit from chemotherapy, especially patients with lymphoma and leukemia, and some patients can even obtain a durable response. 305 , 306
However, conventional maximum‐dose chemotherapy cannot be tolerated by many patients with advanced cancer due to severe side effects and ultimately leads to treatment failure. In addition, it has been found that chemotherapy may induce the production of pro‐metastatic cytokines and chemokines, thereby inducing tumor metastasis. 307 Therefore, many studies have been carried out to optimize chemotherapy regimens, and some progress has been made. Recent studies have shown that metronomic chemotherapy can inhibit angiogenesis and reduce the expression of pro‐metastatic cytokines and chemotaxis, thereby reducing tumor recurrence and the distant spread of tumor cells. 308 , 309 These results indicate that low‐dose metronomic chemotherapy may be effective in the prevention and treatment of tumor metastasis.
Radiotherapy has been recognized as a radical treatment for some early‐stage cancer, such as NPC, and progress has also been made in the treatment of patients with metastatic cancer. 295 , 310 Compared with radiotherapy alone, the combination of radiotherapy and other therapies has better therapeutic effects on metastatic cancer, especially when combined with chemotherapy. For locally advanced NPC, concurrent chemoradiotherapy has been approved as a standard regimen by the National Comprehensive Cancer Network Guidelines. 310 Recently, two Phase III clinical trials from China proposed that induction chemotherapy before concurrent chemoradiotherapy can significantly improve the prognosis of patients with NPC. 311 , 312 It can be reflected that multidisciplinary combination therapy will be the main means to improve the prognosis of patients with metastatic cancer.
3.2. Targeted therapy for metastasis
For most patients with advanced‐stage cancer, surgery and radiotherapy are difficult to achieve satisfactory therapeutic effects, which is also the reason for the high tumor‐related mortality. In the process of tumor metastasis from initiation to colonization, the occurrence of each step is the result of the joint action of some specific genes and signaling pathways. Blocking one of these steps may block the formation of metastases. Therefore, the development of drugs targeting these targets may provide an alternative for patients with advanced tumors.
3.2.1. Targeting EMT and cell motility
Blocking EMT is one of the key strategies to prevent tumor cells from spreading from the primary tumor site. As a marker of mesenchymal cells, N‐cadherin is elevated in metastatic tumors and is associated with a poor prognosis. 33 , 38 ADH‐1 is the first humanized antibody selectively targeting N‐cadherin, which has been proved to improve the prognosis of patients with tumor‐expressing N‐cadherin. 313 Recently, a study showed that blocking N‐cadherin by ADH‐1 can also inhibit the expression of PD‐L1 and the recruitment of Tregs in TME and augment the cytotoxicity of tumor‐infiltrating lymphocytes (TILs) against tumor cells. 314 The steroid receptor coactivator (SRC) tyrosine kinase family is one of the central members that mediate EMT. 45 Drugs targeting SRC, including dasatinib, bosutinib, and saracatinib, have been proved to inhibit tumor growth and prolong patient survival. 315 When combined with other treatment modalities, the anti‐tumor effect is stronger. In a Phase III prospective clinical trial (NCT01584648), the 3‐year progression‐free survival rate of patients receiving dabrafenib plus trametinib was 22% and 12% for patients receiving dabrafenib monotherapy, and the 3‐year overall survival rate was 44% and 32%, respectively. Furthermore, in the subgroup with normal lactate dehydrogenase and less than three metastatic sites, the 3‐year overall survival rate of patients receiving combination therapy reached 62%. 316
The motility of tumor cells is one of the critical factors that determine whether the metastasis can proceed smoothly, and the integrin family can affect cell motility by regulating cell adhesion. 37 Therefore, targeting the integrin family can weaken the motility of tumor cells. Currently, drugs targeting the integrin family mainly include cilengitide, intetumumab, 264RAD, and MK‐0429, which have been proven to prolong the survival of cancer patients in preclinical studies and prospective clinical studies. 317 , 318 , 319 , 320 The allosterisms of actin and myosin in ECM promote structural remodeling and tumor cell polarization during tumor cell migration; Ras homolog gene family, member A (RhoA), MMP, and non‐muscle Myosin‐II are involved in the allosterisms of these two proteins. 130 Small molecule inhibitors of molecules regulating actin and myosin have been shown to attenuate tumor cell motility and inhibit tumor metastasis in vivo and in vitro, but their effectiveness in humans needs to be confirmed by further clinical studies. 156 , 321
After separation from ECM, only a small percentage of cells can survive and achieve subsequent metastasis. The main reason is that this small part of tumor cells has acquired the properties of anti‐anoikis. 19 , 322 Therefore, tumor metastasis can be further inhibited by promoting tumor cell anoikis and eliminating anoikis resistance. T0070907 inhibits PPAR‐γ and leads to cell death by reducing adhesion and inducing anoikis. 323 In addition, galectin‐3 inhibitors have been shown to be effective in the treatment of thyroid cancer. The mechanism is mainly attributed to the inhibition of anoikis resistance, which is expected to provide a new strategy for inhibiting tumor metastasis. 324
3.2.2. Targeting CAFs
CAFs have also been proven to be important members involved in promoting tumor metastasis, and targeting CAFs have gradually attracted more attention in the treatment of metastatic tumor. 130 The activity of CAFs promoting tumor metastasis is mediated by a variety of signaling pathways, especially TGF‐β and EGFR signaling pathways, which may provide targets for anti‐metastasis. 325 , 326 , 327 In a variety of malignancies, including bladder cancer, breast cancer, colorectal cancer, and pancreatic cancer, TGF‐β1 can induce the transformation of resident normal fibroblasts into CAFs and lead to the differential expression of α‐SMA and fibroblast activation protein (FAP) genes (specific markers of CAFs) through the typical TGF‐β signaling pathway. 161 , 328 , 329 , 330 These data suggest that TGF‐β1 plays a role in promoting CAFs production.
As the first oral small‐molecule selective inhibitor of TGF‐β1, galunisertib has been proved to have anti‐tumor effects in clinical trials of liver cancer, pancreatic cancer, and glioma. 331 , 332 , 333 In addition, the therapeutic effect of combination gemcitabine with galunisertib is significantly better than gemcitabine monotherapy in advanced pancreatic cancer (combination group 8.9 months vs. monotherapy group 7.1 months). 332 In a Phase II trial, the median overall survival of galunisertib combined with the multi‐target tyrosine kinase inhibitor (TKI) sorafenib in the treatment of metastatic liver cancer was 18.8 months. 331 Researchers have also developed a fusion protein, M7824, which blocks both PD‐L1 and TGF‐β signaling pathways; M7824 has shown potent anti‐tumor activity in preclinical studies. 334 Subsequently, a Phase I clinical study was conducted in advanced lung cancer patients, and M7824 showed tolerable toxicities and preliminary anti‐tumor effects. 335
In addition, a variety of cytokines secreted by CAFs act as ligands on the janus kinase‐signal transducer and activator of transcription (JAK/STAT) pathway, participate in the activation of this pathway, and promote tumor metastasis. 336 , 337 , 338 For example, CAFs‐derived IL‐6 can activate the STAT3 pathway to promote EMT of tumor cells, thereby facilitating metastasis. 62 , 339 Therefore, antagonizing those cytokines or blocking JAK/STAT signaling can inhibit the occurrence of tumor metastasis. The JAK inhibitor ruxolitinib has been shown to inhibit the invasion and migration of breast cancer, lung cancer, and colorectal cancer cells in vivo. 336 , 340 , 341 Two Phase II clinical trials have shown that ruxolitinib combined with gemcitabine improved overall survival in patients with advanced HER2‐negative cancer or advanced pancreatic cancer. 342 The IL‐6 receptor inhibitor tocilizumab and IL‐6 antagonist siltuximab have been shown to augment the anti‐tumor activity of chemotherapeutic agents in preclinical trials, and further clinical studies are expected. 343 , 344
Blocking the activity of CAFs directly can also play an anti‐tumor metastasis role. FAP is one of the markers of activated CAFs, and targeting FAP could transfer activated CAFs into a quiescent state. The monoclonal antibody targeting FAP (sibrutuzumab) and the small molecule inhibitors of FAP (F19 and PT100) were well‐tolerated, but no obvious clinical anti‐tumor effect was observed in Phase I clinical trials, and subsequent structural adjustments may be needed. 345 , 346 , 347
3.2.3. Targeting angiogenesis
Angiogenesis is considered to be one of the components involved in tumor metastasis. A variety of small‐molecule tyrosine kinase inhibitors and monoclonal antibody against angiogenesis have been approved as first‐line or second‐line therapy in a variety of advanced malignancies, including pazopanib targeting vascular endothelial growth factor receptor (VEGFR), imatinib targeting platelet‐derived growth factor receptor (PDGFR), bevacizumab targeting VEGF, and multi‐targeted receptor TKI anlotinib. 348 , 349 , 350 , 351 In addition, the combined application of existing drugs and the development of new drugs inhibiting angiogenesis are being explored in pre‐clinical and clinical trials. 352 Several clinical trials of advanced lung cancer have shown that the combination of targeted drugs such as gefitinib, erlotinib, and anlotinib with radiotherapy can enhance the efficacy of anti‐brain metastasis and are expected to be applied in clinical practice. 354 , 355 , 356 There has been a large amount of literature reviewing the therapies targeting angiogenesis, so we will not describe them in detail.
3.3. Immunotherapy
3.3.1. Targeting TAMs and Tregs
Regulating tumor immune microenvironment is another crucial therapeutic strategy to interfere with tumor metastasis. There is increasing evidence that TAMs and Tregs in the microenvironment promote tumor development and metastasis. 93 , 353 Targeting TAM and Tregs is a promising strategy to modify the immunosuppressive TME and prevent metastasis.
A large number of studies have shown that CC chemokine receptors are essential mediators involved in tumor metastasis. 357 Among them, CCR1, CCR2, CCR3, and CCR5 promote the recruitment of TAMs in the TME, and CCR4 mainly mediates the recruitment of Tregs, indicating that CC chemokines can be used as potential anti‐tumor pharmacological targets. 82 , 175 In recent years, CCR inhibitors have shown strong anti‐tumor effects in preclinical and clinical studies. 358 For example, in a Phase I clinical study, CCR5 antagonists have demonstrated their ability to inhibit liver metastasis in colon cancer. 359 A preclinical experiment has shown that mogamulizumab, which antagonizes CCR4, can reduce tumor lung metastasis, and a Phase III clinical trial has demonstrated that antibodies targeting CCR4 are effective in treating patients with cutaneous T lymphoma. 360 Furthermore, mogamulizuma combined with the immune checkpoint inhibitor nivolumab may provide a new strategy for the treatment of advanced or metastatic solid tumors. 361 Another monoclonal antibody targeting CCR, AMG 820, also showed preliminary anti‐tumor ability. 362
Colony‐stimulating factor 1 (CSF1) can enhance macrophage recruitment and activation into a pro‐tumoral TAM phenotype and plays a role in promoting metastasis. 363 , 364 The pleiotropic signaling of CSF1 receptor (CSF1R) supports multiple functions of macrophage, including proliferation, differentiation, and migration. 365 The CSF1/CSF1R axis has received much attention, and approaches targeting the ligands or receptors are currently being clinically developed. Pexidartinib, which target KIT, CSF1R, and Fms‐like tyrosine kinase 3 (FLT3), has been approved by the US Food and Drug Administration (FDA) for the treatment of symptomatic tenosynovial giant cell tumor in adult patients. 366 Emactuzumab, a humanized monoclonal antibody targeting CSF1/CSF1R, inhibit tumor cell proliferation and metastasis by blocking the activity of CSF1R‐dependent TAMs, suppressing the recruitment of TAMs to the microenvironment, and enhancing the T‐cell infiltration. 367 However, the anti‐tumor ability of emactuzumab is not evident in a Phase I clinical trial, and further studies are needed. 367
The CD4+ Treg cell population in the TME is the main cell group leading to the inactivation of effector T cells and is also one of the factors that promote tumor progression and metastasis. Therefore, targeting CD4+ Treg cells directly is a promising strategy to effectively prevent tumor metastasis and progression. IT1208, a defucosylated humanized anti‐CD4 monoclonal antibody, demonstrated tolerable toxicities and encouraging preliminary efficacy in a Phase I clinical trial involving 11 patients with advanced solid tumors. 368 In addition, a microsatellite‐stable colon cancer patient receiving IT1208 treatment showed increased infiltration of both CD4+ and CD8+ T cells in the tumor and achieved a durable partial response. 368 These results suggest that Tregs‐targeted drugs, such as IT1208, are expected to provide a new idea for immunotherapy, but further researches are needed.
3.3.2. ICIs
A major hallmark of T‐cell exhaustion is the increased expression of multiple immune checkpoints, such as PD‐1, CTLA‐4, LAG‐3, T‐cell immunoglobulin‐3 (TIM‐3), and T‐cell immunoglobulin and ITIM domain (TIGIT). 369 , 370 , 371 Immune checkpoints have distinct ligands and inhibit anti‐tumor activity of T cell through multiple mechanisms; T cell function decreases with the increase of immune checkpoint expression. 373 Recent advances in cancer immunotherapy have shown that ICIs, including inhibitors of PD‐1, PD‐L1, CTLA‐4, LAG‐3, TIM‐3, and TIGIT, can improve the clinical response and survival of patients with a broad spectrum of metastatic cancers, such as melanoma, non‐small lung cancer, and renal cell cancer (Table 2). 13 , 369 , 371 , 374 , 375 , 376 ICIs limit the inhibitory pathway of T cells and stimulate the activation of effector T cells to enhance the anti‐tumor immune response. So far, at least eight different ICIs have been approved by the US FDA for the treatment of more than a dozen different cancers, including melanoma, kidney cancer, lymphoma, colorectal cancer, lung cancer, head and neck carcinoma, liver cancer, and sarcoma. 377
TABLE 2.
Important immune checkpoint inhibitors under clinical trial and on the market
| Target | Phase I | Phase II | Phase III | On the market |
|---|---|---|---|---|
| PD‐1 | CS1003 | MGA012 | Cemiplimab | Nivolumab |
| ZKAB001 | GLS‐010 | Camrelizumab | Pembrolizumab | |
| MK‐3475 | Balstilimab | HLX10 | Sintilimab | |
| PF‐06801591 | SG001 | Penpulimab | Tislelizumab | |
| AGEN1777 | BGB A317 | REGN2810 | Dostarlimab | |
| 609A | Retifanlimab | Spartalizumab | Toripalimab | |
| AMP‐224 | Zimberelimab | JS001 | ||
| PF‐06801591 | ||||
| INCMGA00012 | ||||
| BCD‐100 | ||||
| IBI308 | ||||
| JNJ‐63723283 | ||||
| PD‐L1 | LY3300054 | STI‐3031 | ZKAB001 | Atezolizumab |
| KN035 | CS1001 | SHR‐1316 | Avelumab | |
| BMS‐936559 | BGB‐A333 | Durvalumab | ||
| HLX20 | LP002 | Camrelizumab | ||
| MSB2311 | Bintrafusp alfa | |||
| BCD‐135 | ||||
| CTLA4 | CS1002 | Quavonlimab | Tremelimumab | Ipilimumab |
| BCD‐145 | AGEN1884 | MDX‐010 | ||
| ADU‐1604 | BCD‐217 | |||
| ONC‐392 | BMS‐986218 | |||
| ADG126 | CP 675,206 | |||
| ADG116 | IBI310 | |||
| TIGIT | JS006 | EOS‐448 | BGB‐A1217 | |
| ASP8374 | Ociperlimab | Tiragolumab | ||
| COM902 | BMS‐986207 | |||
| AZD2936 | Etigilimab | |||
| EOS‐448 | ||||
| IBI939 | ||||
| LAG‐3 | REGN3767 | IMP321 | ||
| TSR‐033 | Relatlimab | |||
| Sym022 | LAG525 | |||
| INCAGN02385 | ||||
| TIM‐3 | Sym023 | TSR‐022 | ||
| INCAGN2390 | MBG453 | |||
| LY3321367 | BMS‐986258 | |||
| SHR‐1702 | INCAGN02390 | |||
| Cobolimab | ||||
| VISTA | JNJ‐61610588 | |||
| B7‐H3 | MGD009 | Enoblituzumab | ||
| Dual PD‐1/PD‐L1 | IBI318 | |||
| Dual PD‐1/TIGIT | AZD2936 | |||
| Dual PD‐1/TIM‐3 | RO7121661 | AZD7789 | ||
| Dual PD‐1/LAG‐3 | RO7247669 | MGD013 | ||
| MGD013 | ||||
| Dual PD‐1/VEGF | AK112 | |||
| Dual PD‐1/CTLA4 | AK104 | |||
| BCD‐217 | ||||
| Dual PD‐L1/LAG‐3 | FS118 | RO7247669 | ||
| IBI323 | ||||
| Dual PD‐L1/TIM‐3 | LY3415244 | RO7121661 | ||
| Dual PD‐L1/4‐1BB | ABL503 | |||
| Dual PD‐L1/VISTA | CA‐170 | |||
| Dual PD‐L1/TGF‐β | Y101D | SHR1701 | M7824 |
Note: All the data source information is from ClinicalTrials.gov.
Abbreviations: CTLA4, cytotoxic lymphocyte‐associated protein 4; LAG‐3, lymphocyte activation gene 3; PD‐1, programmed cell death 1; PD‐L1, programmed death ligand‐1; TIGIT, T cell immunoglobulin and ITIM domain; TIM‐3, T‐cell immunoglobulin‐3.
Among them, immune checkpoint therapy‐targeting PD‐1/PD‐L1 pathway has achieved remarkable success in various types of tumors. For example, the most dramatic effects have been observed in metastatic melanoma, a malignancy that only slightly responds to conventional chemotherapy, and the historical average survival time of metastatic melanoma patients is less than 1 year. 378 Surprisingly, when combined with anti‐CTLA4 and anti‐PD‐1 therapy, nearly 60% of the patients can achieve radiographic responses, with a median survival time of more than 3 years. 379 , 380 In a Phase II trial, 42 patients with brain metastases from non–small‐cell lung cancer (NSCLC) were recruited to receive PD‐1 targeting inhibitor pembrolizumab, 11 of whom responded, and the median follow‐up time was 8.3 months. 381 In addition, clinical studies have shown that the PD‐L1 inhibitor atezolizumab can significantly improve the prognosis of patients with advanced or metastatic solid tumors. 382 Pembrolizumab and atezolizumab have been approved as first‐line or second‐line therapies for some patients with advanced or metastatic solid tumors, such as NSCLC, kidney cancer, breast cancer, and melanoma. 381 , 382 , 383
However, only a limited number of patients can benefit from immunotherapy, and some patients who initially respond to immunotherapy may eventually relapse and progress. 384 Therefore, a large number of pre‐clinical and clinical studies have investigated the combination of immunotherapy with other therapies, including chemotherapy, radiotherapy, or targeted therapy, as well as the combination of ICIs targeting different immune checkpoints to overcome the dilemma. To date, many therapies combined with immunotherapy and chemotherapy or anti‐angiogenic drugs have been recommended as first‐line treatments for multiple solid tumors. 385 , 386 For example, in patients with squamous NSCLC, the addition of pembrolizumab to chemotherapy (carboplatin plus paclitaxel or nab‐paclitaxel) result in significantly better overall survival and progression‐free survival than chemotherapy alone. 387 As a result, the combination of pembrolizumab with chemotherapy is recommended as the first‐line treatment for patients with advanced squamous NSCLC. 388 In advanced renal cell carcinoma, the FDA approved pembrolizumab combined with lenvatinib or axitinib as the first‐line treatment. 389 , 391 Among them, pembrolizumab combined with axitinib is the first anti‐PD‐1 antibody plus targeted drug combination therapy approved by the FDA. 390 , 391 Moreover, it has been reported that radiotherapy can improve the response of patients with metastatic NSCLC to immunotherapy. 392 Overall, a broad range of immunotherapy‐based combination therapies have been approved for the treatment of cancer, and a large number of preclinical studies and clinical trials are ongoing to explore more possibilities for combination therapies in the treatment of metastatic cancer.
3.4. Others
In recent years, due to the abundance of natural herbal compounds and the diversity of their chemical compositions, they have received more and more attention as anti‐tumor drugs. Triptolide, anthocyanidins, and gigantol have been confirmed to inhibit the proliferation and invasion of NSCLC cells, glioblastoma cells, and bladder cancer cells in vivo and in vitro. 393 , 394 , 395 Studies have found that the anti‐tumor effects of certain compounds are mainly achieved by inhibiting EMT. 394 , 395 In addition, the chemical compound DZ‐50 and the natural product curcumol can inhibit tumor metastasis by weakening the resistance of tumor cells to anoikis. 396 , 397 However, their anti‐tumor effects need to be confirmed by further clinical studies.
The failure of metastatic cancer treatment may be partly due to the inability of drugs to persist in the blood or lymph and the inability of drugs to cross certain natural barriers. For example, for patients with metastatic brain cancer, the difficulty in treatment is that most anti‐tumor drugs cannot cross the blood‐brain barrier. The emergence of nanomedicine has brought revolutionary updates to tumor treatment. Due to the small molecular weight of nanometers and the ability to carry specific pharmacophores to distant metastatic regions, nano‐related chemotherapy and nano‐related immunotherapy have demonstrated outstanding potential in tackling metastatic cancer. 398 , 399
The combination of different nano‐polymer materials (such as hydrogels, micelles, and nanoparticles) and chemotherapeutic drugs, small molecule inhibitors, or immunotherapy drugs, has successfully improved the control rate of metastatic tumor. 400 , 401 , 402 , 403 For example, losartan‐loaded injectable peptide hydrogel can inhibit the formation of CAFs collagen, thereby increasing the concentration of adriamycin at the metastatic sites and killing tumor cells. 400 Succinobucol is a small molecule inhibitor targeting vascular cell adhesion molecule‐1, which is believed to be able to reverse lung metastasis, but the results in in vivo experiments are not satisfactory. Then, He et al. successfully designed succinobucol on ph‐responsive wormlike micelles as a novel nanomedicine. Both in vivo and in vitro experiments demonstrated that succinobucol could be successfully delivered to the site of metastasis through ph‐responsive wormlike micelles, thus improving treatment efficiency. 401 In addition, nanotechnology can enhance the anti‐tumor effect of immunotherapy by rebuilding the tumor immune microenvironment, enhancing the anti‐tumor effect of immune cells, enhancing the effectiveness of anti‐tumor cytokines, and awakening tumor cells to release antigens. 398 , 399 Moreover, nanotechnology may reduce the side effects of immunotherapy.
4. CONCLUSIONS AND FUTURE PERSPECTIVES
Metastasis accounts for the great majority of cancer‐related deaths, which is the result of the interaction between tumor cells and TME components. In contrast to numerous discoveries that have revealed the detailed mechanisms leading to the formation of primary tumor, the biological underpinnings of metastatic disease are still poorly understood. However, some progress has been made in elucidating the cellular and molecular mechanisms that drive cancer metastasis over the past decades.
Despite the diversity of tumor types, the mechanisms of metastasis have some commonalities. The invasion–metastasis cascade involves angiogenesis, detachment of metastatic cells from the primary tumor, invasion through the BM and ECM rounding tumor, intravasation of the metastatic cells into the blood and lymphatic vessels and survival during hematogenous transit, adhesion of these cells to the endothelium of capillaries, extravasation through vascular walls into the parenchyma of distant tissues, and colonization at the target organ site. 17 , 23 , 404 The EMT and anoikis resistance of tumor cells are the main forces to promote the metastasis cascade, and multiple components in TME are inducers to ensure the success of metastasis by reprogramming the pre‐metastasis niche and promoting metastatic cell growth. 21 , 35 , 325 EMT modulates cell‐to‐cell or cell‐to‐matrix adhesion and allows cells to increase their migration and invasion capabilities by forming invasive protrusions. 405 Metastatic cells develop an ability to resist anoikis, enabling them to adapt and survive when detached from the ECM or in the absence of the ECM. 19 The interactions between tumor and ECM components are essential for EMT and acquisition of tumor invasive abilities, and TME is also closely involved with all the processes of metastasis. 69 As presented earlier, immune cells (e.g., TAMs, Treg, and neutrophil), stromal cells (e.g., CAFs, MSCs, and TECs), chemokines, cytokines, and growth factors in TME, are involved in a complex crosstalk with tumor cells that affects tumor growth and metastasis. 69 , 79 , 138 , 406 Furthermore, recent studies have emphasized the role of autophagy, lipid metabolism, and lncRNA in tumor metastasis. 212 , 240 , 255
Those discoveries have brought new insight into our understanding of cancer metastasis. For most patients, due to the insidious tumor‐related symptoms, distant metastasis has already appeared at the time of diagnosis. 1 In addition to the three cornerstones of tumor treatment, surgery, chemotherapy, and radiotherapy, novel treatment approaches, including targeted therapy and immunotherapy, have been established in patients with metastatic cancer. According to the chronological order and biological characteristics of metastasis, intervention strategies can be roughly classified into four types: early prevention, blocking metastasis‐specific pathways, reducing angiogenesis, and enhancing anti‐tumor immunity. For low‐grade early malignancies, preoperative neoadjuvant chemotherapy and postoperative adjuvant chemotherapy can prevent and reduce the probability of recurrence and metastasis to a certain extent. 298 , 301 For advanced or metastatic tumors, targeted therapies, especially drugs targeting metastasis‐specific pathways and angiogenesis have been approved for the treatment of most types of tumors. 15 What is more exciting is that immunotherapy, especially ICIs, is revolutionizing the treatment of malignancies and has demonstrated their potential as “cancer terminators.” The advent of immunotherapy and combination therapies based on immunotherapy has provided more options for patients with metastatic cancer. Despite many challenges, we hold an optimistic view of the future prospect of immunotherapy.
In summary, this review provided our current understanding of the mechanisms that underlie the dissemination and metastatic outgrowth of cancer cells. As presented above, although many critical obstacles are still lying ahead, tumor metastasis‐related pathways and TME represent novel and attractive directions, which may change the landscape of cancer therapy in the future. However, further researches and more endeavors in this area are needed.
CONFLICT OF INTEREST
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
ETHICS APPROVAL
Not applicable.
AUTHOR CONTRIBUTIONS
ML and YJ: data curation, formal analysis, and writin—goriginal draft. BX: data curation and formal analysis. XZ: conceptualization, supervision, validation, and writing—review editing. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81772863 and 82072958) and the China Postdoctoral Science Foundation‐funded project (No. 2020M683119). This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Liu M, Yang J, Xu B, Zhang X. Tumor metastasis: Mechanistic Insights and therapeutic interventions. MedComm. 2021;2:587–617. 10.1002/mco2.100
Mengmeng Liu and Jing Yang contributed equally to this work.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Jiang WG, Sanders AJ, Katoh M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35:S244‐S275. [DOI] [PubMed] [Google Scholar]
- 3. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679‐695. [DOI] [PubMed] [Google Scholar]
- 4. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98‐101. [PubMed] [Google Scholar]
- 5. Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9(8):808. [DOI] [PubMed] [Google Scholar]
- 6. Scanlon EF. James Ewing lecture. The process of metastasis. Cancer. 1985;55(6):1163‐1166. [DOI] [PubMed] [Google Scholar]
- 7. Brown M, Assen FP, Leithner A, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359(6382):1408‐1411. [DOI] [PubMed] [Google Scholar]
- 8. Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352(6282):169‐175. [DOI] [PubMed] [Google Scholar]
- 9. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26(1):19‐34. [DOI] [PubMed] [Google Scholar]
- 11. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou Z, Lu ZR. Molecular imaging of the tumor microenvironment. Adv Drug Deliv Rev. 2017;113:24‐48. [DOI] [PubMed] [Google Scholar]
- 13. Edwards SC, Hoevenaar WHM, Coffelt SB. Emerging immunotherapies for metastasis. Br J Cancer. 2021;124(1):37‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chyuan IT, Chu CL, Hsu PN. Targeting the tumor microenvironment for improving therapeutic effectiveness in cancer immunotherapy: focusing on immune checkpoint inhibitors and combination therapies. Cancers (Basel). 2021;13(6):1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang H, Kuo YH, Smith ZI, Spangler J. Targeting cancer metastasis with antibody therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(4):e1698. [DOI] [PubMed] [Google Scholar]
- 16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ‐specific colonization. Nat Rev Cancer. 2009;9(4):274‐284. [DOI] [PubMed] [Google Scholar]
- 18. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4‐27. [DOI] [PubMed] [Google Scholar]
- 19. Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272(2):177‐185. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Cao X. Characteristics and significance of the pre‐metastatic niche. Cancer Cell. 2016;30(5):668‐681. [DOI] [PubMed] [Google Scholar]
- 21. Peinado H, Zhang H, Matei IR, et al. Pre‐metastatic niches: organ‐specific homes for metastases. Nat Rev Cancer. 2017;17(5):302‐317. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Pan J, Barsky L, et al. Characteristics of pre‐metastatic niche: the landscape of molecular and cellular pathways. Mol Biomed. 2021;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563‐572. [DOI] [PubMed] [Google Scholar]
- 24. Klein CA. Framework models of tumor dormancy from patient‐derived observations. Curr Opin Genet Dev. 2011;21(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 25. Celia‐Terrassa T, Kang Y. Distinctive properties of metastasis‐initiating cells. Genes Dev. 2016;30(8):892‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755‐768. [DOI] [PubMed] [Google Scholar]
- 27. Malanchi I, Santamaria‐Martinez A, Susanto E, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481(7379):85‐89. [DOI] [PubMed] [Google Scholar]
- 28. Yang J, Antin P, Berx G, et al. Guidelines and definitions for research on epithelial‐mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21(6):341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial‐mesenchymal transitions in development and disease. Cell. 2009;139(5):871‐890. [DOI] [PubMed] [Google Scholar]
- 30. Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30(10):764‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35(9):479‐488. [DOI] [PubMed] [Google Scholar]
- 33. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial‐mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548‐558. [DOI] [PubMed] [Google Scholar]
- 34. Cano A, Perez‐Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial‐mesenchymal transitions by repressing E‐cadherin expression. Nat Cell Biol. 2000;2(2):76‐83. [DOI] [PubMed] [Google Scholar]
- 35. Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13(16):4769‐4776. [DOI] [PubMed] [Google Scholar]
- 36. van Roy F. Beyond E‐cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14(2):121‐134. [DOI] [PubMed] [Google Scholar]
- 37. Li ZH, Zhou Y, Ding YX, Guo QL, Zhao L. Roles of integrin in tumor development and the target inhibitors. Chin J Nat Med. 2019;17(4):241‐251. [DOI] [PubMed] [Google Scholar]
- 38. Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N‐cadherin promotes motility in human breast cancer cells regardless of their E‐cadherin expression. J Cell Biol. 1999;147(3):631‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morgan RG, Pearn L, Liddiard K, et al. Gamma‐catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of beta‐catenin. Leukemia. 2013;27(2):336‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB. Upregulation of vimentin and aberrant expression of E‐cadherin/beta‐catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010;23(2):213‐224. [DOI] [PubMed] [Google Scholar]
- 41. Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial‐mesenchymal transition. Cells. 2020;9(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan X, Wu H, Han N, et al. Notch signaling and EMT in non‐small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang N, Ji J, Zhou D, et al. The interaction of the senescent and adjacent breast cancer cells promotes the metastasis of heterogeneous breast cancer cells through Notch signaling. Int J Mol Sci. 2021;22(2):849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cai J, Fang L, Huang Y, et al. Simultaneous overactivation of Wnt/beta‐catenin and TGFbeta signalling by miR‐128‐3p confers chemoresistance‐associated metastasis in NSCLC. Nat Commun. 2017;8:15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patel A, Sabbineni H, Clarke A, Somanath PR. Novel roles of Src in cancer cell epithelial‐to‐mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016;157:52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qi LN, Xiang BD, Wu FX, et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018;78(16):4731‐4744. [DOI] [PubMed] [Google Scholar]
- 47. Fan F, Samuel S, Evans KW, et al. Overexpression of snail induces epithelial‐mesenchymal transition and a cancer stem cell‐like phenotype in human colorectal cancer cells. Cancer Med. 2012;1(1):5‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan X, Ma F, Li C, et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial‐mesenchymal transition markers in the first‐line chemotherapy of HER2‐negative metastatic breast cancer. Cancer Commun (Lond). 2019;39(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Q, Rong Y, Yi K, Huang L, Chen M, Wang F. Circulating tumor cells in hepatocellular carcinoma: single‐cell based analysis, preclinical models, and clinical applications. Theranostics. 2020;10(26):12060‐12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mani SA, Guo W, Liao MJ, et al. The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chaffer CL, Brueckmann I, Scheel C, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem‐like state. Proc Natl Acad Sci U S A. 2011;108(19):7950‐7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo W, Keckesova Z, Donaher JL, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okuda H, Kobayashi A, Xia B, et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem‐like cells with macrophages and stromal cells. Cancer Res. 2012;72(2):537‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ye X, Tam WL, Shibue T, et al. Distinct EMT programs control normal mammary stem cells and tumour‐initiating cells. Nature. 2015;525(7568):256‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiang Y, Zhan H. Communication between EMT and PD‐L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2020;468:72‐81. [DOI] [PubMed] [Google Scholar]
- 57. Dongre A, Rashidian M, Reinhardt F, et al. Epithelial‐to‐mesenchymal transition contributes to Immunosuppression in breast carcinomas. Cancer Res. 2017;77(15):3982‐3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Noman MZ, Janji B, Abdou A, et al. The immune checkpoint ligand PD‐L1 is upregulated in EMT‐activated human breast cancer cells by a mechanism involving ZEB‐1 and miR‐200. Oncoimmunology. 2017;6(1):e1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kursunel MA, Taskiran EZ, Tavukcuoglu E, et al. Small cell lung cancer stem cells display mesenchymal properties and exploit immune checkpoint pathways in activated cytotoxic T lymphocytes. [published online ahead of print, 2021 Jul 6]. Cancer Immunol Immunother. 2021. 10.1007/s00262-021-02998-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lou Y, Diao L, Cuentas ER, et al. Epithelial‐mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016;22(14):3630‐3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017;11(7):755‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin WH, Chang YW, Hong MX, et al. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT‐MET switch and cancer metastasis. Oncogene. 2021;40(4):791‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee J, You JH, Kim MS, Roh JL. Epigenetic reprogramming of epithelial‐mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020;37:101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Skrypek N, Goossens S, De Smedt E, Vandamme N, Berx G. Epithelial‐to‐mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends Genet. 2017;33(12):943‐959. [DOI] [PubMed] [Google Scholar]
- 65. Jeong GY, Park MK, Choi HJ, et al. NSD3‐induced methylation of H3K36 activates notch signaling to drive breast tumor initiation and metastatic progression. Cancer Res. 2021;81(1):77‐90. [DOI] [PubMed] [Google Scholar]
- 66. Terashima M, Ishimura A, Wanna‐Udom S, Suzuki T. Epigenetic regulation of epithelial‐mesenchymal transition by KDM6A histone demethylase in lung cancer cells. Biochem Biophys Res Commun. 2017;490(4):1407‐1413. [DOI] [PubMed] [Google Scholar]
- 67. Taube JH, Sphyris N, Johnson KS, et al. The H3K27me3‐demethylase KDM6A is suppressed in breast cancer stem‐like cells, and enables the resolution of bivalency during the mesenchymal‐epithelial transition. Oncotarget. 2017;8(39):65548‐65565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giraldo NA, Sanchez‐Salas R, Peske JD, et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Neophytou CM, Panagi M, Stylianopoulos T, Papageorgis P. The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities. Cancers (Basel). 2021;13(9):2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ye LY, Chen W, Bai XL, et al. Hypoxia‐induced epithelial‐to‐mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76(4):818‐830. [DOI] [PubMed] [Google Scholar]
- 72. Hibino S, Kawazoe T, Kasahara H, et al. Inflammation‐induced tumorigenesis and metastasis. Int J Mol Sci. 2021;22(11):5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Maia A, Wiemann S. Cancer‐associated fibroblasts: implications for cancer therapy. Cancers (Basel). 2021;13(14):3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557‐563. [DOI] [PubMed] [Google Scholar]
- 75. Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017;108(10):1921‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maacha S, Bhat AA, Jimenez L, et al. Extracellular vesicles‐mediated intercellular communication: roles in the tumor microenvironment and anti‐cancer drug resistance. Mol Cancer. 2019;18(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang L, Lin PC. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 2017;47:185‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1‐m2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728‐5739. [DOI] [PubMed] [Google Scholar]
- 81. Lin Y, Xu J, Lan H. Tumor‐associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska‐Bosiacka I. CC chemokines in a tumor: a review of pro‐cancer and anti‐cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. 2020;21(20):7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol. 2004;14(3):155‐160. [DOI] [PubMed] [Google Scholar]
- 84. Marcovecchio PM, Thomas G, Salek‐Ardakani S. CXCL9‐expressing tumor‐associated macrophages: new players in the fight against cancer. J Immunother Cancer. 2021;9(2):e002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti‐cancer therapy. Eur J Cancer. 2006;42(6):717‐727. [DOI] [PubMed] [Google Scholar]
- 86. Fu XT, Dai Z, Song K, et al. Macrophage‐secreted IL‐8 induces epithelial‐mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46(2):587‐596. [DOI] [PubMed] [Google Scholar]
- 87. Helm O, Held‐Feindt J, Grage‐Griebenow E, et al. Tumor‐associated macrophages exhibit pro‐ and anti‐inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135(4):843‐861. [DOI] [PubMed] [Google Scholar]
- 88. Vasiljeva O, Papazoglou A, Kruger A, et al. Tumor cell‐derived and macrophage‐derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66(10):5242‐5250. [DOI] [PubMed] [Google Scholar]
- 89. Lin EY, Li JF, Gnatovskiy L, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238‐11246. [DOI] [PubMed] [Google Scholar]
- 90. Lin EY, Pollard JW. Tumor‐associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064‐5066. [DOI] [PubMed] [Google Scholar]
- 91. Lewis CE, De Palma M, Naldini L. Tie2‐expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin‐2. Cancer Res. 2007;67(18):8429‐8432. [DOI] [PubMed] [Google Scholar]
- 92. Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor‐specific CD8 T cell cytotoxicity through TGF‐beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102(2):419‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T‐cell effector differentiation by modulating IL‐2 homeostasis. Proc Natl Acad Sci U S A. 2011;108(18):7529‐7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Du Y, Chen X, Lin XQ, Wu W, Huang ZM. Tumor‐derived CD4+CD25+ Tregs inhibit the maturation and antigen‐presenting function of dendritic cells. Asian Pac J Cancer Prev. 2015;16(7):2665‐2669. [DOI] [PubMed] [Google Scholar]
- 95. Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69(14):5996‐6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang L, Guo Y, Liu S, et al. Targeting regulatory T cells for immunotherapy in melanoma. Mol Biomed. 2021;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Toomer KH, Malek TR. Cytokine signaling in the development and homeostasis of regulatory T cells. Cold Spring Harb Perspect Biol. 2018;10(3):a028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Budhu S, Schaer DA, Li Y, et al. Blockade of surface‐bound TGF‐beta on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci Signal. 2017;10(494):eaak9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wei X, Zhang J, Gu Q, et al. Reciprocal expression of IL‐35 and IL‐10 defines two distinct effector Treg subsets that are required for maintenance of immune tolerance. Cell Rep. 2017;21(7):1853‐1869. [DOI] [PubMed] [Google Scholar]
- 101. Huang SC, Wei PC, Hwang‐Verslues WW, et al. TGF‐beta1 secreted by Tregs in lymph nodes promotes breast cancer malignancy via up‐regulation of IL‐17RB. EMBO Mol Med. 2017;9(12):1660‐1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene‐driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210(11):2435‐2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Camisaschi C, Casati C, Rini F, et al. LAG‐3 expression defines a subset of CD4(+) CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol. 2010;184(11):6545‐6551. [DOI] [PubMed] [Google Scholar]
- 104. Delgoffe GM, Woo SR, Turnis ME, et al. Stability and function of regulatory T cells is maintained by a neuropilin‐1‐semaphorin‐4a axis. Nature. 2013;501(7466):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu C, Chikina M, Deshpande R, et al. Treg cells promote the SREBP1‐dependent metabolic fitness of tumor‐promoting macrophages via repression of CD8(+) T cell‐derived interferon‐gamma. Immunity. 2019;51(2):381‐397.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine‐A2A adenosine receptor pathway. Front Immunol. 2012;3:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Carmenate T, Ortiz Y, Enamorado M, et al. Blocking IL‐2 signal in vivo with an IL‐2 antagonist reduces tumor growth through the control of regulatory T cells. J Immunol. 2018;200(10):3475‐3484. [DOI] [PubMed] [Google Scholar]
- 108. Spolski R, Li P, Leonard WJ. Biology and regulation of IL‐2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18(10):648‐659. [DOI] [PubMed] [Google Scholar]
- 109. Park YJ, Ryu H, Choi G, et al. IL‐27 confers a protumorigenic activity of regulatory T cells via CD39. Proc Natl Acad Sci U S A. 2019;116(8):3106‐3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu J, Zhang H, Jia L, Sun H. Effects of Treg cells and IDO on human epithelial ovarian cancer cells under hypoxic conditions. Mol Med Rep. 2015;11(3):1708‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ihara F, Sakurai D, Takami M, et al. Regulatory T cells induce CD4(‐) NKT cell anergy and suppress NKT cell cytotoxic function. Cancer Immunol Immunother. 2019;68(12):1935‐1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid‐derived suppressor cells in hepatocellular carcinoma patients induces CD4(+) CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234‐243. [DOI] [PubMed] [Google Scholar]
- 113. Morello S, Pinto A, Blandizzi C, Antonioli L. Myeloid cells in the tumor microenvironment: role of adenosine. Oncoimmunology. 2016;5(3):e1108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF‐beta‐secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202(7):919‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226‐230. [DOI] [PubMed] [Google Scholar]
- 116. Shi C, Chen Y, Chen Y, Yang Y, Bing W, Qi J. CD4(+) CD25(+) regulatory T cells promote hepatocellular carcinoma invasion via TGF‐beta1‐induced epithelial‐mesenchymal transition. Onco Targets Ther. 2019;12:279‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nunez NG, Tosello Boari J, Ramos RN, et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat Commun. 2020;11(1):3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223‐1248. [DOI] [PubMed] [Google Scholar]
- 119. Coffelt SB, Kersten K, Doornebal CW, et al. IL‐17‐producing gamma delta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Donadio AC, Remedi MM, Frede S, Bonacci GR, Chiabrando GA, Pistoresi‐Palencia MC. Decreased expression of intercellular adhesion molecule‐1 (ICAM‐1) and urokinase‐type plasminogen activator receptor (uPAR) is associated with tumor cell spreading in vivo. Clin Exp Metastasis. 2002;19(5):437‐444. [DOI] [PubMed] [Google Scholar]
- 121. Li XJ, Peng LX, Shao JY, et al. As an independent unfavorable prognostic factor, IL‐8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial‐mesenchymal transition and activation of AKT signaling. Carcinogenesis. 2012;33(7):1302‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li S, Cong X, Gao H, et al. Tumor‐associated neutrophils induce EMT by IL‐17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Szczerba BM, Castro‐Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553‐557. [DOI] [PubMed] [Google Scholar]
- 124. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis‐initiating breast cancer cells. Nature. 2015;528(7582):413‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tyagi A, Sharma S, Wu K, et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre‐metastatic niche in lung. Nat Commun. 2021;12(1):474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gong L, Cumpian AM, Caetano MS, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013;12(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ringuette Goulet C, Bernard G, Tremblay S, Chabaud S, Bolduc S, Pouliot F. Exosomes induce fibroblast differentiation into cancer‐associated fibroblasts through TGF‐beta signaling. Mol Cancer Res. 2018;16(7):1196‐1204. [DOI] [PubMed] [Google Scholar]
- 128. Yeon JH, Jeong HE, Seo H, et al. Cancer‐derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer‐associated fibroblasts. Acta Biomater. 2018;76:146‐153. [DOI] [PubMed] [Google Scholar]
- 129. Tan HX, Cao ZB, He TT, Huang T, Xiang CL, Liu Y. TGFbeta1 is essential for MSCs‐CAFs differentiation and promotes HCT116 cells migration and invasion via JAK/STAT3 signaling. Onco Targets Ther. 2019;12:5323‐5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Erdogan B, Webb DJ. Cancer‐associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45(1):229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer‐associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang QS, Kong PZ, Li XQ, Yang F, Feng YM. FOXF2 deficiency promotes epithelial‐mesenchymal transition and metastasis of basal‐like breast cancer. Breast Cancer Res. 2015;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lu JT, Tan CC, Wu XR, et al. FOXF2 deficiency accelerates the visceral metastasis of basal‐like breast cancer by unrestrictedly increasing TGF‐beta and miR‐182‐5p. Cell Death Differ. 2020;27(10):2973‐2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu G, Sun J, Yang ZF, et al. Cancer‐associated fibroblast‐derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR‐4756/IFIT1/3 axis. Cell Death Dis. 2021;12(3):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fan H, Atiya HI, Wang Y, et al. Epigenomic reprogramming toward mesenchymal‐epithelial transition in ovarian‐cancer‐associated mesenchymal stem cells drives metastasis. Cell Rep. 2020;33(10):108473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang Y, Liang Y, Xu H, et al. Single‐cell analysis of pancreatic ductal adenocarcinoma identifies a novel fibroblast subtype associated with poor prognosis but better immunotherapy response. Cell Discov. 2021;7(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Peng S, Chen D, Cai J, et al. Enhancing cancer‐associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol Oncol. 2021;15(5):1391‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang Z, Liu J, Huang H, et al. Metastasis‐associated fibroblasts: an emerging target for metastatic cancer. Biomark Res. 2021;9(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gui Y, Aguilar‐Mahecha A, Krzemien U, et al. Metastatic breast carcinoma‐associated fibroblasts have enhanced protumorigenic properties related to increased IGF2 expression. Clin Cancer Res. 2019;25(23):7229‐7242. [DOI] [PubMed] [Google Scholar]
- 140. Chen IX, Chauhan VP, Posada J, et al. Blocking CXCR4 alleviates desmoplasia, increases T‐lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A. 2019;116(10):4558‐4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Costa A, Kieffer Y, Scholer‐Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463‐479.e10. [DOI] [PubMed] [Google Scholar]
- 142. Bonneau C, Elies A, Kieffer Y, et al. A subset of activated fibroblasts is associated with distant relapse in early luminal breast cancer. Breast Cancer Res. 2020;22(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gu JJ, Hoj J, Rouse C, Pendergast AM. Mesenchymal stem cells promote metastasis through activation of an ABL‐MMP9 signaling axis in lung cancer cells. PLoS One. 2020;15(10):e0241423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kalbasi A, Ribas A. Tumour‐intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Aboulkheyr Es H, Bigdeli B, Zhand S, Aref AR, Thiery JP, Warkiani ME. Mesenchymal stem cells induce PD‐L1 expression through the secretion of CCL5 in breast cancer cells. J Cell Physiol. 2021;236(5):3918‐3928. [DOI] [PubMed] [Google Scholar]
- 146. Sun L, Wang Q, Chen B, et al. Gastric cancer mesenchymal stem cells derived IL‐8 induces PD‐L1 expression in gastric cancer cells via STAT3/mTOR‐c‐Myc signal axis. Cell Death Dis. 2018;9(9):928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sun L, Huang C, Zhu M, et al. Gastric cancer mesenchymal stem cells regulate PD‐L1‐CTCF enhancing cancer stem cell‐like properties and tumorigenesis. Theranostics. 2020;10(26):11950‐11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983‐1992. [DOI] [PubMed] [Google Scholar]
- 149. Maishi N, Ohba Y, Akiyama K, et al. Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Sci Rep. 2016;6:28039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hu L, Zang MD, Wang HX, et al. Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Mol Oncol. 2016;10(9):1473‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS. Breast cancer cells condition lymphatic endothelial cells within pre‐metastatic niches to promote metastasis. Nat Commun. 2014;5:4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kai F, Laklai H, Weaver VM. Force matters: biomechanical regulation of cell invasion and migration in disease. Trends Cell Biol. 2016;26(7):486‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yuzhalin AE, Lim SY, Kutikhin AG, Gordon‐Weeks AN. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim Biophys Acta Rev Cancer. 2018;1870(2):207‐228. [DOI] [PubMed] [Google Scholar]
- 154. Savino W, Mendes‐Da‐Cruz DA, Smaniotto S, Silva‐Monteiro E, Villa‐Verde DM. Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix. J Leukoc Biol. 2004;75(6):951‐961. [DOI] [PubMed] [Google Scholar]
- 155. Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156(3):932‐938. [PubMed] [Google Scholar]
- 156. Jacob A, Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol. 2015;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bi X, Lou P, Song Y, et al. Msi1 promotes breast cancer metastasis by regulating invadopodia‐mediated extracellular matrix degradation via the Timp3‐Mmp9 pathway. Oncogene. 2021;40(29):4832‐4845. [DOI] [PubMed] [Google Scholar]
- 158. Yao M, Brummer G, Acevedo D, Cheng N. Cytokine Regulation of metastasis and tumorigenicity. Adv Cancer Res. 2016;132:265‐367. [DOI] [PubMed] [Google Scholar]
- 159. Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26(3‐4):453‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wang Z, Ao X, Shen Z, et al. TNF‐alpha augments CXCL10/CXCR3 axis activity to induce epithelial‐mesenchymal transition in colon cancer cell. Int J Biol Sci. 2021;17(11):2683‐2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tan HX, Xiao ZG, Huang T, Fang ZX, Liu Y, Huang ZC. CXCR4/TGF‐beta1 mediated self‐differentiation of human mesenchymal stem cells to carcinoma‐associated fibroblasts and promoted colorectal carcinoma development. Cancer Biol Ther. 2020;21(3):248‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sun YF, Wu L, Liu SP, et al. Dissecting spatial heterogeneity and the immune‐evasion mechanism of CTCs by single‐cell RNA‐seq in hepatocellular carcinoma. Nat Commun. 2021;12(1):4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wen J, Zhao Z, Huang L, Wang L, Miao Y, Wu J. IL‐8 promotes cell migration through regulating EMT by activating the Wnt/beta‐catenin pathway in ovarian cancer. J Cell Mol Med. 2020;24(2):1588‐1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ren T, Zhu L, Cheng M. CXCL10 accelerates EMT and metastasis by MMP‐2 in hepatocellular carcinoma. Am J Transl Res. 2017;9(6):2824‐2837. [PMC free article] [PubMed] [Google Scholar]
- 165. Cheng Y, Song Y, Qu J, et al. The chemokine receptor CXCR4 and c‐MET cooperatively promote epithelial‐mesenchymal transition in gastric cancer cells. Transl Oncol. 2018;11(2):487‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhao J, Ou B, Han D, et al. Tumor‐derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk‐1/Snail and AKT/GSK3beta/beta‐catenin pathways. Mol Cancer. 2017;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86(10):937‐950. [DOI] [PubMed] [Google Scholar]
- 168. Voronov E, Shouval DS, Krelin Y, et al. IL‐1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100(5):2645‐2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor‐beta signal transduction in angiogenesis and vascular disorders. Chest. 2005;128(6):585S‐590S. [DOI] [PubMed] [Google Scholar]
- 170. Bae WJ, Ahn JM, Byeon HE, Kim S, Lee D. PTPRD‐inactivation‐induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin. J Exp Clin Cancer Res. 2019;38(1):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Xiong Y, Huang F, Li X, et al. CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer. Int J Oncol. 2017;51(1):75‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Karnezis T, Farnsworth RH, Harris NC, et al. CCL27/CCL28‐CCR10 chemokine signaling mediates migration of lymphatic endothelial cells. Cancer Res. 2019;79(7):1558‐1572. [DOI] [PubMed] [Google Scholar]
- 173. Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor‐C and C‐C chemokine receptor 7 in tumor cell‐lymphatic cross‐talk promote invasive phenotype. Cancer Res. 2009;69(1):349‐357. [DOI] [PubMed] [Google Scholar]
- 174. Kitamura T, Qian BZ, Soong D, et al. CCL2‐induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis‐associated macrophages. J Exp Med. 2015;212(7):1043‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Marshall LA, Marubayashi S, Jorapur A, et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J Immunother Cancer. 2020;8(2):e000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gobert M, Treilleux I, Bendriss‐Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000‐2009. [DOI] [PubMed] [Google Scholar]
- 177. Zhang CY, Qi Y, Li XN, et al. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomed Pharmacother. 2015;69:242‐248. [DOI] [PubMed] [Google Scholar]
- 178. Chen X, Wang L, Li P, et al. Dual TGF‐beta and PD‐1 blockade synergistically enhances MAGE‐A3‐specific CD8(+) T cell response in esophageal squamous cell carcinoma. Int J Cancer. 2018;143(10):2561‐2574. [DOI] [PubMed] [Google Scholar]
- 179. Pein M, Insua‐Rodriguez J, Hongu T, et al. Metastasis‐initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat Commun. 2020;11(1):1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chao CC, Lee CW, Chang TM, Chen PC, Liu JF. CXCL1/CXCR2 paracrine axis contributes to lung metastasis in osteosarcoma. Cancers (Basel). 2020;12(2):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Romero‐Moreno R, Curtis KJ, Coughlin TR, et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat Commun. 2019;10(1):4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50‐56. [DOI] [PubMed] [Google Scholar]
- 183. Tao SC, Guo SC. Role of extracellular vesicles in tumour microenvironment. Cell Commun Signal. 2020;18(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Urabe F, Patil K, Ramm GA, Ochiya T, Soekmadji C. Extracellular vesicles in the development of organ‐specific metastasis. J Extracell Vesicles. 2021;10(9):e12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Barenholz‐Cohen T, Merkher Y, Haj J, et al. Lung mechanics modifications facilitating metastasis are mediated in part by breast cancer‐derived extracellular vesicles. Int J Cancer. 2020;147(10):2924‐2933. [DOI] [PubMed] [Google Scholar]
- 186. Chen C, Zheng H, Luo Y, et al. SUMOylation promotes extracellular vesicle‐mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Invest. 2021;131(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Rodrigues G, Hoshino A, Kenific CM, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21(11):1403‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Morad G, Carman CV, Hagedorn EJ, et al. Tumor‐derived extracellular vesicles breach the intact blood‐brain barrier via transcytosis. ACS Nano. 2019;13(12):13853‐13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Mao X, Tey SK, Yeung CLS, et al. Nidogen 1‐enriched extracellular vesicles facilitate extrahepatic metastasis of liver cancer by activating pulmonary fibroblasts to secrete tumor necrosis factor receptor 1. Adv Sci (Weinh). 2020;7(21):2002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ma Q, Liang M, Wu Y, et al. Small extracellular vesicles deliver osteolytic effectors and mediate cancer‐induced osteolysis in bone metastatic niche. J Extracell Vesicles. 2021;10(4):e12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Li F, Zhao X, Sun R, et al. EGFR‐rich extracellular vesicles derived from highly metastatic nasopharyngeal carcinoma cells accelerate tumour metastasis through PI3K/AKT pathway‐suppressed ROS. J Extracell Vesicles. 2020;10(1): e12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532‐1535. [DOI] [PubMed] [Google Scholar]
- 193. Yang D, Liu J. Neutrophil extracellular traps: a new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Res. 2021;40(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Qi JL, He JR, Liu CB, et al. Pulmonary staphylococcus aureus infection regulates breast cancer cell metastasis via neutrophil extracellular traps (NETs) formation. MedComm. 2020;1(2):188‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhu T, Zou X, Yang C, et al. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelialmesenchymal transition. Int J Mol Med. 2021;48(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Martins‐Cardoso K, Almeida VH, Bagri KM, et al. Neutrophil extracellular traps (NETs) promote pro‐metastatic phenotype in human breast cancer cells through epithelial‐mesenchymal transition. Cancers (Basel). 2020;12(6):1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Najmeh S, Cools‐Lartigue J, Rayes RF, et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1‐integrin mediated interactions. Int J Cancer. 2017;140(10):2321‐2330. [DOI] [PubMed] [Google Scholar]
- 198. Cools‐Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;. 123(8):3446‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409): eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102‐1109. [DOI] [PubMed] [Google Scholar]
- 201. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728‐741. [DOI] [PubMed] [Google Scholar]
- 202. Kim KH, Lee MS. Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10(6):322‐337. [DOI] [PubMed] [Google Scholar]
- 203. Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21(8):439‐458. [DOI] [PubMed] [Google Scholar]
- 204. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Yadav AK, Yadav PK, Chaudhary GR, et al. Autophagy in hypoxic ovary. Cell Mol Life Sci. 2019;76(17):3311‐3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383(16):1564‐1576. [DOI] [PubMed] [Google Scholar]
- 208. Ishaq M, Ojha R, Sharma AP, Singh SK. Autophagy in cancer: Recent advances and future directions. Semin Cancer Biol. 2020;66:171‐181. [DOI] [PubMed] [Google Scholar]
- 209. Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18(2):370‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Peng YF, Shi YH, Ding ZB, et al. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy. 2013;9(12):2056‐2068. [DOI] [PubMed] [Google Scholar]
- 211. Zhao H, Yang M, Zhao J, Wang J, Zhang Y, Zhang Q. High expression of LC3B is associated with progression and poor outcome in triple‐negative breast cancer. Med Oncol. 2013;30(1):475. [DOI] [PubMed] [Google Scholar]
- 212. Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double‐edged sword. Curr Opin Cell Biol. 2010;22(2):241‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Jiang GM, Tan Y, Wang H, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Gorgulu K, Diakopoulos KN, Ai J, et al. Levels of the autophagy‐related 5 protein affect progression and metastasis of pancreatic tumors in mice. Gastroenterology. 2019;156(1):203‐217.e20. [DOI] [PubMed] [Google Scholar]
- 215. Teo Hansen Selno A, Schlichtner S, Yasinska IM, et al. High mobility group box 1 (HMGB1) induces toll‐like receptor 4‐mediated production of the immunosuppressive protein galectin‐9 in human cancer cells. Front Immunol. 2021;12:675731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kadandale P, Stender JD, Glass CK, Kiger AA. Conserved role for autophagy in Rho1‐mediated cortical remodeling and blood cell recruitment. Proc Natl Acad Sci U S A. 2010;107(23):10502‐10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Vera‐Ramirez L, Vodnala SK, Nini R, Hunter KW, Green JE. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat Commun. 2018;9(1):1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Maycotte P, Jones KL, Goodall ML, Thorburn J, Thorburn A. Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Mol Cancer Res. 2015;13(4):651‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Tang Q, Su Z, Gu W, Rustgi AK. Mutant p53 on the path to metastasis. Trends Cancer. 2020;6(1):62‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hu J, Kong S, Dong T, et al. Autophagy modulates mesenchymal‐to‐endothelial transition via p53. Aging (Albany NY). 2020;12(21):22112‐22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22(2):181‐185. [DOI] [PubMed] [Google Scholar]
- 222. Yang Y, Karsli‐Uzunbas G, Poillet‐Perez L, et al. Autophagy promotes mammalian survival by suppressing oxidative stress and p53. Genes Dev. 2020;34(9‐10):688‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kim T, Veronese A, Pichiorri F, et al. p53 regulates epithelial‐mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208(5):875‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial‐mesenchymal transition (EMT) process. Biochimie. 2019;165:229‐234. [DOI] [PubMed] [Google Scholar]
- 225. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhang M, Liu S, Chua MS, et al. SOCS5 inhibition induces autophagy to impair metastasis in hepatocellular carcinoma cells via the PI3K/Akt/mTOR pathway. Cell Death Dis. 2019;10(8):612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zhu JF, Huang W, Yi HM, et al. Annexin A1‐suppressed autophagy promotes nasopharyngeal carcinoma cell invasion and metastasis by PI3K/AKT signaling activation. Cell Death Dis. 2018;9(12):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mihaylova MM, Shaw RJ. The AMPK signaling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Wu J, Gao F, Xu T, et al. CLDN1 induces autophagy to promote proliferation and metastasis of esophageal squamous carcinoma through AMPK/STAT1/ULK1 signaling. J Cell Physiol. 2020;235(3):2245‐2259. [DOI] [PubMed] [Google Scholar]
- 230. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Allen AE, Locasale JW. Glucose metabolism in cancer: The saga of pyruvate kinase continues. Cancer Cell. 2018;33(3):337‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Biswas SK. Metabolic reprogramming of immune cells in Cancer Progression. Immunity. 2015;43(3):435‐449. [DOI] [PubMed] [Google Scholar]
- 233. Annett S, Moore G, Robson T. Obesity and cancer metastasis: molecular and translational perspectives. Cancers (Basel). 2020;12(12):3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Fu Y, Zou T, Shen X, et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm. 2020;2(1):27‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Luo X, Cheng C, Tan Z, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. He Y, Gao M, Cao Y, Tang H, Liu S, Tao Y. Nuclear localization of metabolic enzymes in immunity and metastasis. Biochim Biophys Acta Rev Cancer. 2017;1868(2):359‐371. [DOI] [PubMed] [Google Scholar]
- 237. Wen J, Min X, Shen M, et al. ACLY facilitates colon cancer cell metastasis by CTNNB1. J Exp Clin Cancer Res. 2019;38(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Jiang L, Wang H, Li J, et al. Up‐regulated FASN expression promotes transcoelomic metastasis of ovarian cancer cell through epithelial‐mesenchymal transition. Int J Mol Sci. 2014;15(7):11539‐11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis‐initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41‐45. [DOI] [PubMed] [Google Scholar]
- 240. Ladanyi A, Mukherjee A, Kenny HA, et al. Adipocyte‐induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37(17):2285‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Doria ML, Cotrim Z, Macedo B, et al. Lipidomic approach to identify patterns in phospholipid profiles and define class differences in mammary epithelial and breast cancer cells. Breast Cancer Res Treat. 2012;133(2):635‐648. [DOI] [PubMed] [Google Scholar]
- 242. Brandi J, Dando I, Pozza ED, et al. Proteomic analysis of pancreatic cancer stem cells: Functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310‐322. [DOI] [PubMed] [Google Scholar]
- 243. Fu H, He Y, Qi L, et al. cPLA2alpha activates PI3K/AKT and inhibits Smad2/3 during epithelial‐mesenchymal transition of hepatocellular carcinoma cells. Cancer Lett. 2017;403:260‐270. [DOI] [PubMed] [Google Scholar]
- 244. Wang C, Yang Z, Xu E, et al. Apolipoprotein C‐II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin Transl Med. 2021;11(8): e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Zhang N, Zhang H, Liu Y, et al. SREBP1, targeted by miR‐18a‐5p, modulates epithelial‐mesenchymal transition in breast cancer via forming a co‐repressor complex with Snail and HDAC1/2. Cell Death Differ. 2019;26(5):843‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Zhao H, Yan G, Zheng L, et al. STIM1 is a metabolic checkpoint regulating the invasion and metastasis of hepatocellular carcinoma. Theranostics. 2020;10(14):6483‐6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Chen M, Zhao Y, Yang X, et al. NSDHL promotes triple‐negative breast cancer metastasis through the TGFbeta signaling pathway and cholesterol biosynthesis. Breast Cancer Res Treat. 2021;187(2):349‐362. [DOI] [PubMed] [Google Scholar]
- 248. Greenlee JD, Subramanian T, Liu K, King MR. Rafting down the metastatic cascade: the role of lipid rafts in cancer metastasis, cell Death, and clinical outcomes. Cancer Res. 2021;81(1):5‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Murai T, Maruyama Y, Mio K, Nishiyama H, Suga M, Sato C. Low cholesterol triggers membrane microdomain‐dependent CD44 shedding and suppresses tumor cell migration. J Biol Chem. 2011;286(3):1999‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Yang YF, Jan YH, Liu YP, et al. Squalene synthase induces tumor necrosis factor receptor 1 enrichment in lipid rafts to promote lung cancer metastasis. Am J Respir Crit Care Med. 2014;190(6):675‐687. [DOI] [PubMed] [Google Scholar]
- 251. Manzo T, Prentice BM, Anderson KG, et al. Accumulation of long‐chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J Exp Med. 2020;217(8):e20191920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Su P, Wang Q, Bi E, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor‐associated macrophages. Cancer Res. 2020;80(7):1438‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Gong J, Lin Y, Zhang H, et al. Reprogramming of lipid metabolism in cancer‐associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020;11(4):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Peng WX, Koirala P, Mo YY. LncRNA‐mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661‐5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non‐coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E‐cadherin expression. Cancer Lett. 2013;333(2):213‐221. [DOI] [PubMed] [Google Scholar]
- 256. Zhou W, Ye XL, Xu J, et al. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR‐200b/c and let‐7b. Sci Signal. 2017;10(483):eaak9557. [DOI] [PubMed] [Google Scholar]
- 257. Liang WC, Fu WM, Wong CW, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513‐22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Zhu M, Chen Q, Liu X, et al. lncRNA H19/miR‐675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281(16):3766‐3775. [DOI] [PubMed] [Google Scholar]
- 259. Grelet S, Link LA, Howley B, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19(9):1105‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Hou P, Meng S, Li M, et al. LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. J Exp Clin Cancer Res. 2021;40(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Meng LD, Shi GD, Ge WL, et al. Linc01232 promotes the metastasis of pancreatic cancer by suppressing the ubiquitin‐mediated degradation of HNRNPA2B1 and activating the A‐Raf‐induced MAPK/ERK signaling pathway. Cancer Lett. 2020;494:107‐120. [DOI] [PubMed] [Google Scholar]
- 262. Zhuang M, Zhao S, Jiang Z, et al. MALAT1 sponges miR‐106b‐5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility. EBioMedicine. 2019;41:286‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Li Q, Mo W, Ding Y, Ding X. Study of lncRNA TPA in promoting invasion and metastasis of breast cancer mediated by TGF‐beta signaling pathway. Front Cell Dev Biol. 2021;9:688751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lai SW, Chen MY, Bamodu OA, et al. Exosomal lncRNA PVT1/VEGFA axis promotes colon cancer metastasis and stemness by downregulation of tumor suppressor miR‐152‐3p. Oxid Med Cell Longev. 2021;2021:9959807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Sas‐Chen A, Aure MR, Leibovich L, et al. LIMT is a novel metastasis inhibiting lncRNA suppressed by EGF and downregulated in aggressive breast cancer. EMBO Mol Med. 2016;8(9):1052‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Liu D, Li Y, Luo G, et al. LncRNA SPRY4‐IT1 sponges miR‐101‐3p to promote proliferation and metastasis of bladder cancer cells through up‐regulating EZH2. Cancer Lett. 2017;388:281‐291. [DOI] [PubMed] [Google Scholar]
- 267. Wu H, Hu Y, Liu X, et al. LncRNA TRERNA1 function as an enhancer of SNAI1 promotes gastric cancer metastasis by regulating epithelial‐mesenchymal transition. Mol Ther Nucleic Acids. 2017;8:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Liang WC, Ren JL, Wong CW, et al. LncRNA‐NEF antagonized epithelial to mesenchymal transition and cancer metastasis via cis‐regulating FOXA2 and inactivating Wnt/beta‐catenin signaling. Oncogene. 2018;37(11):1445‐1456. [DOI] [PubMed] [Google Scholar]
- 269. Wang H, Huo X, Yang XR, et al. STAT3‐mediated upregulation of lncRNA HOXD‐AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Yue B, Liu C, Sun H, et al. A positive feed‐forward loop between LncRNA‐CYTOR and Wnt/beta‐catenin signaling promotes metastasis of colon Cancer. Mol Ther. 2018;26(5):1287‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Pan J, Fang S, Tian H, et al. lncRNA JPX/miR‐33a‐5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/beta‐catenin signaling. Mol Cancer. 2020;19(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Cheng B, Rong A, Zhou Q, Li W. LncRNA LINC00662 promotes colon cancer tumor growth and metastasis by competitively binding with miR‐340‐5p to regulate CLDN8/IL22 co‐expression and activating ERK signaling pathway. J Exp Clin Cancer Res. 2020;39(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Zhou Y, Huan L, Wu Y, et al. LncRNA ID2‐AS1 suppresses tumor metastasis by activating the HDAC8/ID2 pathway in hepatocellular carcinoma. Cancer Lett. 2020;469:399‐409. [DOI] [PubMed] [Google Scholar]
- 274. Liang ZX, Liu HS, Wang FW, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes‐mediated macrophage M2 polarization. Cell Death Dis. 2019;10(11):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Xu M, Xu X, Pan B, et al. LncRNA SATB2‐AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol Cancer. 2019;18(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Tan BS, Yang MC, Singh S, et al. LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene. 2019;38(28):5612‐5626. [DOI] [PubMed] [Google Scholar]
- 277. Zhai W, Zhu R, Ma J, et al. A positive feed‐forward loop between LncRNA‐URRCC and EGFL7/P‐AKT/FOXO3 signaling promotes proliferation and metastasis of clear cell renal cell carcinoma. Mol Cancer. 2019;18(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Bian Z, Zhang J, Li M, et al. LncRNA‐FEZF1‐AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res. 2018;24(19):4808‐4819. [DOI] [PubMed] [Google Scholar]
- 279. Xie S, Yu X, Li Y, et al. Upregulation of lncRNA ADAMTS9‐AS2 promotes salivary adenoid cystic carcinoma metastasis via PI3K/Akt and MEK/Erk signaling. Mol Ther. 2018;26(12):2766‐2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Liu B, Wu S, Ma J, et al. LncRNA GAS5 reverses EMT and tumor stem cell‐mediated gemcitabine resistance and metastasis by targeting miR‐221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:472‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23(9):1446‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Redis RS, Vela LE, Lu W, et al. Allele‐specific reprogramming of cancer metabolism by the long non‐coding RNA CCAT2. Mol Cell. 2016;61(4):640. [DOI] [PubMed] [Google Scholar]
- 283. Silva‐Fisher JM, Dang HX, White NM, et al. Long non‐coding RNA RAMS11 promotes metastatic colorectal cancer progression. Nat Commun. 2020;11(1):2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Ji P, Diederichs S, Wang W, et al. MALAT‐1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early‐stage non‐small cell lung cancer. Oncogene. 2003;22(39):8031‐8041. [DOI] [PubMed] [Google Scholar]
- 285. Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30(1):34‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Wang X, Adjei AA. Lung cancer and metastasis: new opportunities and challenges. Cancer Metastasis Rev. 2015;34(2):169‐171. [DOI] [PubMed] [Google Scholar]
- 287. Ost P, Bossi A, Decaestecker K, et al. Metastasis‐directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67(5):852‐863. [DOI] [PubMed] [Google Scholar]
- 288. Creutzberg CL, Lu KH, Fleming GF. Uterine Cancer: Adjuvant therapy and management of metastatic disease. J Clin Oncol. 2019;37(27):2490‐2500. [DOI] [PubMed] [Google Scholar]
- 289. Meko J, Rusch VW. Neoadjuvant therapy and surgical resection for locally advanced non‐small cell lung cancer. Semin Radiat Oncol. 2000;10(4):324‐332. [DOI] [PubMed] [Google Scholar]
- 290. Pitroda SP, Chmura SJ, Weichselbaum RR. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019;20(8):e434‐e442. [DOI] [PubMed] [Google Scholar]
- 291. Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12(2):115‐124. [DOI] [PubMed] [Google Scholar]
- 292. Guner A, Son T, Cho I, et al. Liver‐directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer. 2016;19(3):951‐960. [DOI] [PubMed] [Google Scholar]
- 293. Evrard S, Torzilli G, Caballero C, Bonhomme B. Parenchymal sparing surgery brings treatment of colorectal liver metastases into the precision medicine era. Eur J Cancer. 2018;104:195‐200. [DOI] [PubMed] [Google Scholar]
- 294. Xu J, Fan J, Qin X, et al. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (version 2018). J Cancer Res Clin Oncol. 2019;145(3):725‐736. [DOI] [PubMed] [Google Scholar]
- 295. Bhattacharya IS, Hoskin PJ. Stereotactic body radiotherapy for spinal and bone metastases. Clin Oncol (R Coll Radiol). 2015;27(5):298‐306. [DOI] [PubMed] [Google Scholar]
- 296. Birgisson H, Enblad M, Artursson S, Ghanipour L, Cashin P, Graf W. Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2020;46(12):2283‐2291. [DOI] [PubMed] [Google Scholar]
- 297. Mercier F, Mohamed F, Cazauran JB, et al. An update of peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy. Int J Hyperthermia. 2019;36(1):744‐752. [DOI] [PubMed] [Google Scholar]
- 298. Radovich M, Jiang G, Hancock BA, et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple‐negative breast Cancer: preplanned secondary analysis of the BRE12‐158 randomized clinical trial. JAMA Oncol. 2020;6(9):1410‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Pierga JY, Bidard FC, Autret A, et al. Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY‐1 and ‐2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2017;28(1):103‐109. [DOI] [PubMed] [Google Scholar]
- 300. Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol. 2017;3(4):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Kosuge T, Sakamoto Y, Ueno H. Postoperative adjuvant therapy. J Hepatobiliary Pancreat Sci. 2011;18(6):792‐796. [DOI] [PubMed] [Google Scholar]
- 302. Xiong HQ, Ajani JA. Treatment of colorectal cancer metastasis: the role of chemotherapy. Cancer Metastasis Rev. 2004;23(1‐2):145‐163. [DOI] [PubMed] [Google Scholar]
- 303. Meyer M, Seetharam M. First‐line therapy for metastatic soft tissue sarcoma. Curr Treat Options Oncol. 2019;20(1):6. [DOI] [PubMed] [Google Scholar]
- 304. Decatris MP, Sundar S, O'Byrne KJ. Platinum‐based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev. 2004;30(1):53‐81. [DOI] [PubMed] [Google Scholar]
- 305. Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94(11):1266‐1287. [DOI] [PubMed] [Google Scholar]
- 306. Ansell SM. Hodgkin lymphoma:diagnosis and treatment. Mayo Clin Proc. 2015;90(11):1574‐1583. [DOI] [PubMed] [Google Scholar]
- 307. Karagiannis GS, Condeelis JS, Oktay MH. Chemotherapy‐induced metastasis: molecular mechanisms, clinical manifestations, therapeutic interventions. Cancer Res. 2019;79(18):4567‐4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Shaked Y, Emmenegger U, Man S, et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106(9):3058‐3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Chan TS, Hsu CC, Pai VC, et al. Metronomic chemotherapy prevents therapy‐induced stromal activation and induction of tumor‐initiating cells. J Exp Med. 2016;213(13):2967‐2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Colevas AD, Yom SS, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw. 2018;16(5):479‐490. [DOI] [PubMed] [Google Scholar]
- 311. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509‐1520. [DOI] [PubMed] [Google Scholar]
- 312. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124‐1135. [DOI] [PubMed] [Google Scholar]
- 313. Perotti A, Sessa C, Mancuso A, et al. Clinical and pharmacological phase I evaluation of Exherin (ADH‐1), a selective anti‐N‐cadherin peptide in patients with N‐cadherin‐expressing solid tumours. Ann Oncol. 2009;20(4):741‐745. [DOI] [PubMed] [Google Scholar]
- 314. Sun Y, Jing J, Xu H, et al. N‐cadherin inhibitor creates a microenvironment that protect TILs from immune checkpoints and Treg cells. J Immunother Cancer. 2021;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Rivera‐Torres J, San Jose E. Src tyrosine kinase inhibitors: new perspectives on their immune, antiviral, and senotherapeutic potential. Front Pharmacol. 2019;10:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K‐mutant melanoma: long‐term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin‐targeting arginine‐glycine‐aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17(8):1225‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Heidenreich A, Rawal SK, Szkarlat K, et al. A randomized, double‐blind, multicenter, phase 2 study of a human monoclonal antibody to human alphanu integrins (intetumumab) in combination with docetaxel and prednisone for the first‐line treatment of patients with metastatic castration‐resistant prostate cancer. Ann Oncol. 2013;24(2):329‐336. [DOI] [PubMed] [Google Scholar]
- 319. Eberlein C, Kendrew J, McDaid K, et al. A human monoclonal antibody 264RAD targeting alphavbeta6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32(37):4406‐4416. [DOI] [PubMed] [Google Scholar]
- 320. Pickarski M, Gleason A, Bednar B, Duong LT. Orally active alphavbeta3 integrin inhibitor MK‐0429 reduces melanoma metastasis. Oncol Rep. 2015;33(6):2737‐2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Li X, Wang J. Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int J Biol Sci. 2020;16(12):2014‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Alanko J, Mai A, Jacquemet G, et al. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol. Nov 2015;17(11):1412‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Schaefer KL, Wada K, Takahashi H, et al. Peroxisome proliferator‐activated receptor gamma inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Res. 2005;65(6):2251‐2259. [DOI] [PubMed] [Google Scholar]
- 324. Lee JJ, Hsu YC, Li YS, Cheng SP. Galectin‐3 inhibitors suppress anoikis resistance and invasive capacity in thyroid cancer cells. Int J Endocrinol. 2021;2021:5583491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Calon A, Tauriello DV, Batlle E. TGF‐beta in CAF‐mediated tumor growth and metastasis. Semin Cancer Biol. 2014;25:15‐22. [DOI] [PubMed] [Google Scholar]
- 326. Madeo A, Maggiolini M. Nuclear alternate estrogen receptor GPR30 mediates 17beta‐estradiol‐induced gene expression and migration in breast cancer‐associated fibroblasts. Cancer Res. 2010;70(14):6036‐6046. [DOI] [PubMed] [Google Scholar]
- 327. Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer‐associated fibroblasts induce epithelial‐mesenchymal transition of breast cancer cells through paracrine TGF‐beta signalling. Br J Cancer. 2014;110(3):724‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Lamprecht S, Sigal‐Batikoff I, Shany S, et al. Teaming up for trouble: cancer cells, transforming growth factor‐beta1 signaling and the epigenetic corruption of stromal naive fibroblasts. Cancers (Basel). 2018;10(3):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Tan HX, Gong WZ, Zhou K, et al. CXCR4/TGF‐beta1 mediated hepatic stellate cells differentiation into carcinoma‐associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol Ther. 2020;21(3):258‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Huang M, Fu M, Wang J, et al. TGF‐beta1‐activated cancer‐associated fibroblasts promote breast cancer invasion, metastasis and epithelial‐mesenchymal transition by autophagy or overexpression of FAP‐alpha. Biochem Pharmacol. 2021;188:114527. [DOI] [PubMed] [Google Scholar]
- 331. Kelley RK, Gane E, Assenat E, et al. A phase 2 study of galunisertib (TGF‐beta1 Receptor Type I Inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol. 2019;10(7):e00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Melisi D, Garcia‐Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first‐line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119(10):1208‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Rodon J, Carducci MA, Sepulveda‐Sanchez JM, et al. First‐in‐human dose study of the novel transforming growth factor‐beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21(3):553‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD‐L1 and TGF‐beta. Sci Transl Med. 2018;10(424):eaan5488. [DOI] [PubMed] [Google Scholar]
- 335. Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a Bifunctional fusion protein targeting PD‐L1 and TGFbeta, in advanced solid tumors. Clin Cancer Res. 2018;24(6):1287‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Subramaniam KS, Omar IS, Kwong SC, et al. Cancer‐associated fibroblasts promote endometrial cancer growth via activation of interleukin‐6/STAT‐3/c‐Myc pathway. Am J Cancer Res. 2016;6(2):200‐213. [PMC free article] [PubMed] [Google Scholar]
- 337. Biffi G, Oni TE, Spielman B, et al. IL1‐Induced JAK/STAT Signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9(2):282‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Heichler C, Scheibe K, Schmied A, et al. STAT3 activation through IL‐6/IL‐11 in cancer‐associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69(7):1269‐1282. [DOI] [PubMed] [Google Scholar]
- 339. Karakasheva TA, Lin EW, Tang Q, et al. IL‐6 mediates cross‐talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res. 2018;78(17):4957‐4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Patel MR, Dash A, Jacobson BA, et al. JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non‐small cell lung cancer models. Cancer Gene Ther. 2019;26(11‐12):411‐418. [DOI] [PubMed] [Google Scholar]
- 341. Cataldi M, Shah NR, Felt SA, Grdzelishvili VZ. Breaking resistance of pancreatic cancer cells to an attenuated vesicular stomatitis virus through a novel activity of IKK inhibitor TPCA‐1. Virology. 2015;485:340‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, double‐blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol. 2015;33(34):4039‐4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Alraouji NN, Al‐Mohanna FH, Ghebeh H, et al. Tocilizumab potentiates cisplatin cytotoxicity and targets cancer stem cells in triple‐negative breast cancer. Mol Carcinog. 2020;59(9):1041‐1051. [DOI] [PubMed] [Google Scholar]
- 344. Guo Y, Nemeth J, O'Brien C, et al. Effects of siltuximab on the IL‐6‐induced signaling pathway in ovarian cancer. Clin Cancer Res. 2010;16(23):5759‐5769. [DOI] [PubMed] [Google Scholar]
- 345. Welt S, Divgi CR, Scott AM, et al. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell‐surface protein of reactive tumor stromal fibroblasts. J Clin Oncol. 1994;12(6):1193‐1203. [DOI] [PubMed] [Google Scholar]
- 346. Nemunaitis J, Vukelja SJ, Richards D, et al. Phase I trial of PT‐100 (PT‐100), a cytokine‐inducing small molecule, following chemotherapy for solid tumor malignancy. Cancer Invest. 2006;24(6):553‐561. [DOI] [PubMed] [Google Scholar]
- 347. Scott AM, Wiseman G, Welt S, et al. A phase I dose‐escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein‐positive cancer. Clin Cancer Res. 2003;9(5):1639‐1647. [PubMed] [Google Scholar]
- 348. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2012;379(9829):1879‐1886. [DOI] [PubMed] [Google Scholar]
- 349. Carlisle BG, Zheng T, Kimmelman J. Imatinib and the long tail of targeted drug development. Nat Rev Clin Oncol. 2020;17(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 350. Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin(R)) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [DOI] [PubMed] [Google Scholar]
- 351. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi‐targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti‐angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Cornel AM, Mimpen IL, Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel). 2020;12(7):1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Akamatsu H, Harada H, Tokunaga S, et al. A phase II study of gefitinib with concurrent thoracic radiotherapy in Patients With unresectable, stage III non‐small‐cell lung cancer harboring EGFR mutations (WJOG6911L). Clin Lung Cancer. 2019;20(1):e25‐e27. [DOI] [PubMed] [Google Scholar]
- 355. Yang Z, Zhang Y, Li R, et al. Whole‐brain radiotherapy with and without concurrent erlotinib in NSCLC with brain metastases: a multicenter, open‐label, randomized, controlled phase III trial. Neuro Oncol. 2021;23(6):967‐978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Kong C, Zhu X, Jiang M, et al. Anlotinib in combination with whole brain radiotherapy for advanced non‐small‐cell lung cancer with brain metastases progressive or developed after at least one lines of prior treatment. Int J Radiat Oncol Biol Phys. 2021;111(3S):e569. [Google Scholar]
- 357. Korbecki J, Kojder K, Siminska D, et al. CC chemokines in a tumor: A review of pro‐cancer and anti‐cancer properties of the ligands of receptors CCR1, CCR2, CCR3, and CCR4. Int J Mol Sci. 2020;21(21):8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Miao M, De Clercq E, Li G. Clinical significance of chemokine receptor antagonists. Expert Opin Drug Metab Toxicol. 2020;16(1):11‐30. [DOI] [PubMed] [Google Scholar]
- 359. Halama N, Zoernig I, Berthel A, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by Anti‐CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587‐601. [DOI] [PubMed] [Google Scholar]
- 360. Kim YH, Bagot M, Pinter‐Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T‐cell lymphoma (MAVORIC): an international, open‐label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192‐1204. [DOI] [PubMed] [Google Scholar]
- 361. Doi T, Muro K, Ishii H, et al. A Phase I study of the anti‐CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2019;25(22):6614‐6622. [DOI] [PubMed] [Google Scholar]
- 362. Papadopoulos KP, Gluck L, Martin LP, et al. First‐in‐human study of AMG 820, a monoclonal anti‐colony‐stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(19):5703‐5710. [DOI] [PubMed] [Google Scholar]
- 363. Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor‐infiltrating macrophages and improves response to T‐cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74(18):5057‐5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Hung JY, Horn D, Woodruff K, et al. Colony‐stimulating factor 1 potentiates lung cancer bone metastasis. Lab Invest. 2014;94(4):371‐381. [DOI] [PubMed] [Google Scholar]
- 365. Rojo R, Raper A, Ozdemir DD, et al. Deletion of a csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat Commun. 2019;10(1):3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Lamb YN. Pexidartinib: first approval. Drugs. 2019;79(16):1805‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Gomez‐Roca CA, Italiano A, Le Tourneau C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2‐like macrophages. Ann Oncol. 2019;30(8):1381‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Shitara K, Ueha S, Shichino S, et al. First‐in‐human phase 1 study of IT1208, a defucosylated humanized anti‐CD4 depleting antibody, in patients with advanced solid tumors. J Immunother Cancer. 2019;7(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG‐3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8(2):e001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD‐1 and CTLA‐4. Mol Cancer. 2019;18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD‐1, PD‐L1 and CTLA‐4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425‐1434. [DOI] [PubMed] [Google Scholar]
- 373. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Kraman M, Faroudi M, Allen NL, et al. FS118, a bispecific antibody targeting LAG‐3 and PD‐L1, enhances T‐Cell activation resulting in potent antitumor activity. Clin Cancer Res. 2020;26(13):3333‐3344. [DOI] [PubMed] [Google Scholar]
- 375. Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276(1):112‐120. [DOI] [PubMed] [Google Scholar]
- 376. Saleh R, Toor SM, Elkord E. Targeting TIM‐3 in solid tumors: innovations in the preclinical and translational realm and therapeutic potential. Expert Opin Ther Targets. 2020;24(12):1251‐1262. [DOI] [PubMed] [Google Scholar]
- 377. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA‐approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). 2020;12(3):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320‐330. [DOI] [PubMed] [Google Scholar]
- 379. Larkin J, Chiarion‐Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Wolchok JD, Chiarion‐Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and Ipilimumab in advanced Melanoma. N Engl J Med. 2017;377(14):1345‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long‐term results and biomarker analysis from a non‐randomised, open‐label, phase 2 trial. Lancet Oncol. 2020;21(5):655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288‐2301. [DOI] [PubMed] [Google Scholar]
- 383. Esteva FJ, Hubbard‐Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20(3):e175‐e186. [DOI] [PubMed] [Google Scholar]
- 384. Li SJ, Chen JX, Sun ZJ. Improving antitumor immunity using antiangiogenic agents: Mechanistic insights, current progress, and clinical challenges. Cancer Commun (Lond). 2021;41(9):830‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219‐235. [DOI] [PubMed] [Google Scholar]
- 386. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti‐angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2040‐2051. [DOI] [PubMed] [Google Scholar]
- 388. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. [DOI] [PubMed] [Google Scholar]
- 389. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for qdvanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289‐1300. [DOI] [PubMed] [Google Scholar]
- 390. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380(12):1116‐1127. [DOI] [PubMed] [Google Scholar]
- 391. Escudier B. Combination therapy as first‐line treatment in metastatic renal‐cell carcinoma. N Engl J Med. 2019;380(12):1176‐1178. [DOI] [PubMed] [Google Scholar]
- 392. Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non‐small‐cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467‐475. [DOI] [PubMed] [Google Scholar]
- 393. Reno TA, Kim JY, Raz DJ. Triptolide inhibits lung cancer cell migration, invasion, and metastasis. Ann Thorac Surg. 2015;100(5):1817‐1824, discussion 1824–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Zhao M, Sun Y, Gao Z, et al. Gigantol attenuates the metastasis of human bladder cancer cells, possibly through Wnt/EMT signaling. Onco Targets Ther. 2020;13:11337‐11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Ouanouki A, Lamy S, Annabi B. Anthocyanidins inhibit epithelial‐mesenchymal transition through a TGFbeta/Smad2 signaling pathway in glioblastoma cells. Mol Carcinog. 2017;56(3):1088‐1099. [DOI] [PubMed] [Google Scholar]
- 396. Ning N, Liu S, Liu X, et al. Curcumol inhibits the proliferation and metastasis of melanoma via the miR‐152‐3p/PI3K/AKT and ERK/NF‐kappaB signaling pathways. J Cancer. 2020;11(7):1679‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Ning L, Ma H, Jiang Z, et al. Curcumol suppresses breast cancer cell metastasis by inhibiting MMP‐9 Via JNK1/2 and AKT‐dependent NF‐kappaB signaling pathways. Integr Cancer Ther. 2016;15(2):216‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Zhang P, Zhai Y, Cai Y, Zhao Y, Li Y. Nanomedicine‐based immunotherapy for the treatment of cancer metastasis. Adv Mater. 2019;31(49):e1904156. [DOI] [PubMed] [Google Scholar]
- 399. Liang C, Xu L, Song G, Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis‐targeted drug delivery, phototherapy, and immunotherapy. Chem Soc Rev. 2016;45(22):6250‐6269. [DOI] [PubMed] [Google Scholar]
- 400. Hu C, Liu X, Ran W, et al. Regulating cancer associated fibroblasts with losartan‐loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. 2017;144:60‐72. [DOI] [PubMed] [Google Scholar]
- 401. He X, Yu H, Bao X, et al. PH‐responsive wormlike micelles with sequential metastasis targeting inhibit lung metastasis of breast cancer. Adv Healthc Mater. 2016;5(4):439‐448. [DOI] [PubMed] [Google Scholar]
- 402. Xiao J, Duan X, Yin Q, et al. The inhibition of metastasis and growth of breast cancer by blocking the NF‐κB signaling pathway using bioreducible PEI‐based/p65 shRNA complex nanoparticles. Biomaterials. 2013;34(21):5381‐5390. [DOI] [PubMed] [Google Scholar]
- 403. Xu P, Yu H, Zhang Z, et al. Hydrogen‐bonded and reduction‐responsive micelles loading atorvastatin for therapy of breast cancer metastasis. Biomaterials. 2014;35(26):7574‐7587. [DOI] [PubMed] [Google Scholar]
- 404. Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407‐424. [DOI] [PubMed] [Google Scholar]
- 405. Aiello NM, Kang Y. Context‐dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


