Abstract
Since nuclear factor of κ‐light chain of enhancer‐activated B cells (NF‐κB) was discovered in 1986, extraordinary efforts have been made to understand the function and regulating mechanism of NF‐κB for 35 years, which lead to significant progress. Meanwhile, the molecular mechanisms regulating NF‐κB activation have also been illuminated, the cascades of signaling events leading to NF‐κB activity and key components of the NF‐κB pathway are also identified. It has been suggested NF‐κB plays an important role in human diseases, especially inflammation‐related diseases. These studies make the NF‐κB an attractive target for disease treatment. This review aims to summarize the knowledge of the family members of NF‐κB, as well as the basic mechanisms of NF‐κB signaling pathway activation. We will also review the effects of dysregulated NF‐κB on inflammation, tumorigenesis, and tumor microenvironment. The progression of the translational study and drug development targeting NF‐κB for inflammatory diseases and cancer treatment and the potential obstacles will be discussed. Further investigations on the precise functions of NF‐κB in the physiological and pathological settings and underlying mechanisms are in the urgent need to develop drugs targeting NF‐κB for inflammatory diseases and cancer treatment, with minimal side effects.
Keywords: cancer, immunity, inflammation, NF‐κB, signal transduction
This year (2021) marks the 35th anniversary of the discovery of NF‐κB. With so many years of in‐depth research on NF‐κB, people have realized that the NF‐κB signaling pathway plays an important role in inflammation, immunity, cell survival and proliferation. This review summarizes the relevant knowledge of the NF‐κB signaling pathway, inflammation, and cancer. The progression of the translational study and drug development targeting NF‐κB for inflammatory diseases and cancer treatment and the potential obstacles will also be discussed.
1. INTRODUCTION
A nuclear transcription factor is a type of protein that binds to a sequence of conserved nucleotides in the gene's promoter region to initiate the transcription of this gene. The nuclear factor of κ‐light chain of enhancer‐activated B cells (NF‐κB) is one of the nuclear transcription factors with an extensive range of biological functions, which exists in almost all types of mammal cells. In 1986, Sen and Baltimore discovered NF‐κB for the first time through its interaction with a defined site in the enhancer of the κ chain of immunoglobulin gene in B cells. 1 Since then, intensive studies have established the critical roles of NF‐κB on diverse biological processes, including cell proliferation, metastasis, response to DNA damage, apoptosis, and immune response through its vast target genes. 2 , 3 Therefore, NF‐κB is tightly correlated with human diseases such as inflammation, cancer, and autoimmune diseases, 4 , 5 and understanding the regulating mechanism and function of the NF‐κB signaling pathway is not only important for revealing the fundamental principles of cellular biology but also crucial to study the pathogenesis and treatment of many human diseases. In this review, we will summarize the progress of NF‐κB study focusing on the crucial roles of this transcriptional factor family in many aspects of inflammation and cancers in the last 35 years, highlighting NF‐κB as a potential therapeutic target for human inflammatory disease and cancer treatment.
2. THE NF‐ΚB PATHWAY
2.1. NF‐κB/Rel protein family
In humans, the NF‐κB superfamily comprises five transcription factors: NF‐κB1 (p50), NF‐κB2 (p52), RelA (p65), RelB, and REL (c‐Rel). 6 These proteins all contain a Rel homology domain (RHD), a conserved N‐terminal domain, which is very important for DNA binding, dimerization, and nuclear localization. According to the similarity of amino acid sequence within the RHD in the C‐terminal, the NF‐κB superfamily is generally divided into two subfamilies: NF‐κB subfamily (p50 and p52) and Rel subfamily (Rel, RelA, and RelB). NF‐κB1 (p50) and NF‐κB2 (p52) are produced by the processing of protein precursor, p105 and p100, respectively, with ankyrin (ANK) repeat domains in the C‐terminal, which form dimers with other NF‐κB family members. The inhibitor of the NF‐κB (IκB) protein family controls the activation process of NF‐κB by interacting with transcriptional factors. The proteins bind and persist in the cytoplasm, while the Rel protein (Rel, RelA, RelB) has no protein precursor but a conserved transactivation domain in the C‐terminal. 7 These members of the NF‐κB family share a highly conserved 3000‐amino‐acid RHD, which plays a crucial role in their function. 8 , 9 , 10 In most cases, all five NF‐κB proteins could form homodimers and heterodimers with each other to start gene transcription. 11 However, RelB is a special exception, which only forms heterodimers. The most common NF‐κB heterodimer is mainly composed of NF‐κB1 (p50) and RelA (p65) (Figure 1). Whether NF‐κB dimer activates transcription or inhibits transcription depends on the DNA regions it binds to and the interaction with other transcription factors. 12 , 13
FIGURE 1.
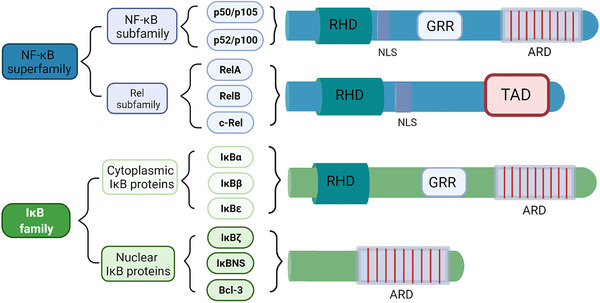
Schematic structures of nuclear factor of κ‐light chain of enhancer‐activated B cells (NF‐κB) superfamily and the inhibitor of NF‐κB (IκB) family. The schematic structures of NF‐κB subfamily proteins, Rel subfamily proteins, and IκB family proteins (cytoplasmic IκB proteins and nuclear IκB proteins) are shown. ARD, ankyrin repeat domain; GRR, glycine‐rich region; NLS, nuclear localization sequence; RHD, Rel homology domain; TAD, transactivation domain
2.2. The IκB protein family
The activity of NF‐κB dimer is regulated by the IκB protein. The IκB family is characterized by ANK repeats that interact with the RHD domain of NF‐κB protein. 9 The IκB protein family has eight members including IκBα, IκBβ, IκBɛ, IκBζ, IκBNS, Bcl‐3, p100, and p105, and every member contains a series of ANK repeat domain (ARD) 14 (Figure 1). The ARD of IκBs forms a slightly curved cylindrical structure, which contains amino acid residues that specifically recognize and bind to NF‐κB dimers. These repetitive sequences can interact with RHD, allowing IκB to bind to NF‐κB, preventing NF‐κB from entering the nucleus. 11 , 15 , 16 The activation of NF‐κB relies on the degradation of IκB protein. Upon activation, IκBα is phosphorylated by IκB kinase (IKK) complex and ubiquitinated and degraded through the proteasome‐dependent pathway, releasing NF‐κB into the nucleus for transcription to activate target genes. NF‐κB interacts with newly synthesized IκB in the nucleus and exits the nucleus into the cytoplasm, forming the cycle of a non‐phosphorylated nuclear‐cytoplasmic shuttling and inactivation. 10 , 16 , 17 It should be noted that the C‐terminal sequence of the precursor protein p100/p105 of p50/p52 also contains the ANK repeat sequence like IκB, which allows p100/p105 to inhibit the activity of NF‐κB like IκB. 18 , 19
2.3. Activation of NF‐κB
The NF‐κB activation signaling pathways have been well studied. There are two types of NFκB activation signaling pathways: the canonical and the noncanonical pathways, 20 relying on distinct molecular mechanisms to activate NF‐κB, as well as the different sets of target genes involved in cell proliferation, differentiation, and immune response. 16 , 21
The IKKs complex is a large multi‐component protein kinases complex, including two homologous catalytically active subunits IKKα (IKK1) and IKKβ (IKK2) and an auxiliary subunit IKKγ (NEMO, NF‐kappaB essential modulator). 22 IKK can be activated by various stimuli such as growth factors, cytokines, stress factors, and microbial components. 17 The two catalytically active IKK subunits participate in different pathways. The noncanonical NF‐κB activation pathway is mainly regulated by IKKα, while the regulation of the canonical NF‐κB activation pathway induced by pro‐inflammatory cytokines and various microbial products requires the participation of IKKβ. The oligomerization of IKKα/β/γ can affect the activity of IKKβ. 17 , 23 , 24 Although IKKγ has no catalytic activity, it is necessary for the canonical pathway. IKKγ can form an IKK complex with other IKKs through its N‐terminal, and its C‐terminal can mediate the interaction with the upstream signal adaptor. 16 , 22 , 25
In the canonical NFκB pathway, the key event is the phosphorylation of IκB protein mediated by IKKs (Figure 2). The adaptor molecule, ubiquitin ligase, and protein kinase activate the IKK complex at different levels. 22 , 26 The canonical activation pathway of NF‐κB is stimulated by various factors, such as tumor necrosis factor receptor (TNFR) superfamily members, ligands of various cytokine receptors, pattern recognition receptors (PRRs, like Toll‐like receptor (TLR) ligands), as well as B‐cell receptor (BCR) and T‐cell receptor (TCR). 16 , 27 , 28 After stimulation and upstream signal transduction, IKKs phosphorylate IκBs, which in turn are ubiquitinated by ubiquitin ligase and degraded by 26S proteasome, losing the ability to bind to NF‐κB. When IκB is degraded, NF‐κB is dissociated from the NF‐κB/IκBs complex and transferred into the nucleus, binds to the target gene promoter region on DNA, and initiate the transcription of the target gene. 5 , 11 , 29 In this activation process, p50/RelA and p50/c‐Rel have mainly involved dimers. In addition, in the canonical NF‐κB pathway, one of the target genes activated is the gene encoding IκBα (Nfkbia), so IκBα is quickly re‐synthesized after degradation, and the newly synthesized IκBα can directly bind to NF‐κB in the nucleus. In this way, NF‐κB is dissociated from the κB‐binding site of DNA. The NF‐κB/IκBs complex is exported back to the cytoplasm to keep the original latent state, fulfilling the cycle of NF‐κB activation and inactivation. Therefore, the activation of the canonical NF‐κB pathway is usually robust and transient. 9 The canonical NF‐κB pathway plays an important role in cell survival and proliferation, tumor cell epithelial to mesenchymal transformation (EMT), angiogenesis, cancer metastasis, and inflammation.
FIGURE 2.
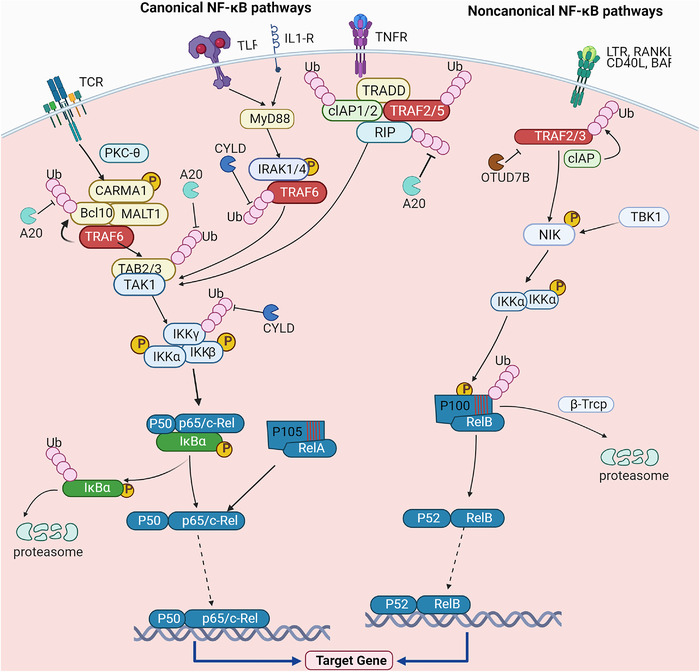
Activation of canonical and noncanonical NF‐κB pathways. In the canonical NF‐κB pathway, protein kinase C‐θ recruits Bcl10 and membrane‐associated lymphoid tissue 1 (MALT1) to form a CARD‐containing MAGUK protein 1 (CARMA1)/Bcl10/membrane‐associated lymphoid tissue (MALT) 1 complex after T‐cell receptor stimulation. TRAF6 is recruited by MALT1, which mediates the ubiquitination of itself and Bcl10 and further activates TAK1. TAK1 phosphorylates and activates IKKβ to induce phosphorylation and degradation of IκBα. After IκBα is degraded, free NF‐κB enter the nucleus to drive target gene transcription. TRADD, the interacting E3 ubiquitin ligases cellular inhibitor of apoptosis (cIAP) 1/2, TRAF2/5, and protein kinase RIP are recruited by tumor necrosis factor receptor (TNFR) stimulation. RIP is then ubiquitinated and recruited to form the TAK1‐IKK complex, which induces NF‐κB activation. Under the stimulation of Toll‐like receptor and interleukin‐1 (IL‐1), myeloid differentiation primary response 88 (MyD88)‐dependent signal transduction induces the recruitment of IRAK1/4 and TRAF6 to activate TAK1‐IKK complex, which then activates NF‐κB. In the noncanonical NF‐κB pathway, specific subsets of the TNFR superfamily are engaged with their ligands to induce the recruitment of TRAF2/3‐cIAP. After TRAF3 is ubiquitinated and degraded, NF‐κB‐induced kinase accumulates in the cytoplasm and then binds to IKKα, mediating p100 phosphorylation and ubiquitination‐dependent processing to produce p52/RelB heterodimers. In these processes, A20, cylindromatosis, and Otud7b play important roles in inhibiting ubiquitination of key molecules of NF‐κB pathways
In the noncanonical NF‐κB pathway, the key event is the processing of the precursor protein p100 of NF‐κB2 rather than the degradation of IκBα (Figure 2). Compared with the canonical pathway with a wide variety of activators, the noncanonical pathway has relatively fewer activators. Noncanonical pathway selectively responds to specific stimuli, including ligands for part of TNF receptor family members, like lymphotoxin (LT) β receptor (LTβR), CD40, B‐cell activation factor family (BAFF), and receptor activator of NF‐κB (RANK). 30 , 31 , 32 , 33 , 34 Under the action of stimuli, the corresponding receptor is activated, leading to degradation of TNF receptor‐associated factor 3 (TRAF3). The protein level of NF‐κB‐induced kinase (NIK) is mediated by TRAF3‐cellular inhibitor of apoptosis (cIAP) under the steady‐state condition. It is currently believed that NIK and IKKα mediate the conversion of p100 to p52, which are the key components of the noncanonical NF‐κB pathway. 35 NF‐κB2 exists in the form of precursor p100 under the non‐stimulated condition. p100 functions as IκB protein, binding to RelB and retaining RelB in the cytoplasm. 36 Together with IKKα, NIK mediates the phosphorylation of p100, which is further conjugated with ubiquitin chains mediated by β‐transducin repeats‐containing proteins (β‐TrCP) E3 ligase. Ubiquitinated p100 is processed to generate p52, forming p52/RelB heterodimer. 30 , 32 , 37
Two NF‐κB pathways have shared characteristics: forming functional NF‐κB dimer and translocating the dimer into nuclear. The difference between these two pathways is that the activation of the canonical pathway is to rely on the degradation of IκBα; meanwhile, in the noncanonical NF‐κB pathway, processing of p100 into p52 does not only generate p52 but also liberate RelB from p100, forming p52/RelB dimer. The resynthesis of NIK is the key to start the downstream pathway, so the activating non‐standard NF‐κB pathway is much slower than the standard pathway. 21 Optimizing target gene expression also requires the interaction of NF‐κB with other transcription factors, such as activator protein 1 (AP1), signal transducer and activator of transcription (STAT) family members, and interferon regulatory factors (IRFs). 38 , 39 , 40
2.4. Regulation of NF‐κB pathway
As mentioned in the previous chapter 2.3, NF‐κB is often blocked in the cytoplasm by IκBs, keeping the entire pathway silent. However, once activated by stimuli (regardless of the various stimuli in the canonical pathway or a subset of specific stimuli in the noncanonical pathway), NF‐κB is activated to induce the production of pro‐inflammatory mediators and molecules leading to inflammation and activation and differentiation of the immune cell. 29 If the NF‐κB activation is dysregulated, diseases such as chronic inflammation, tumors, and autoimmune diseases may occur. Therefore, NF‐κB is strictly regulated to maintain homeostasis.
First of all, as an inhibitory protein of NF‐κB, IκB plays a very important role in controlling the intensity and duration of NF‐κB activity. The target genes of NF‐κB include genes encoding IκB, such as Nfkbia (encoding IκBα) and Nfkbie (encoding IκBε). When the activated NF‐κB dimer enters the nucleus to bind to the κB‐binding site, target genes including Nfkbia are transcribed, and the newly generated IκBα and IκBε proteins bind and drive the NF‐κB dimer from the nucleus to the cytoplasm. This dynamic negative feedback mechanism is critical for keeping the activation of NF‐κB in check. 41 , 42 , 43 Although both IκBα and IκBε have regulatory functions, they function in different ways. Compared with the rapid production and inhibition of IκBα, IκBε provides slower negative feedback regulation with delayed expression. 44 In contrast, another member of the IκB family, IκBβ, directly binds to RelA and c‐Rel in the nucleus to counteract the inhibitory function of IκBα. When the hypo‐phosphorylated IκBβ binds to RelA and c‐Rel, the inhibitory effect mediated by IκBα is impaired, and the continuous transcription of the target gene is maintained. 24 , 45 In addition to driving NF‐κB back to the cytoplasm through the IκB protein, another way to downregulate NF‐κB activation is to degrade NF‐κB dimers in the nucleus directly. For example, elongin‐B‐elongin‐C‐cullin‐SOCS1 (ECS) is a ubiquitin ligase complex that promotes ubiquitination and subsequent degradation of RelA in the nucleus. 46 , 47 , 48 Studies have also reported that the E3 ubiquitin ligase post‐synaptic density‐95, disks‐large, and zonula occludens‐1 and Lin‐11, Isl1 and Mec‐3 (LIM) domain protein 2 directly remove RelA from the DNA binding site and mediate its degradation to suppress NF‐κB transcription activity. 49 , 50 Protein inhibitor of activated STAT 1 (PIAS1) also moves to the promoter region of NF‐κB target genes after phosphorylation of IKKα, inhibiting the binding of RelA‐containing dimers to DNA. 51 , 52 , 53
Secondly, the studies on NF‐κB signaling pathways reveal that ubiquitination is essential for NF‐κB activation. In the canonical NF‐κB pathway, NF‐κB activation mediated by multiple stimuli requires ubiquitination. For example, when interleukin‐1 receptor (IL‐1R) or TLR is activated, the ubiquitination of tumour necrosis factor receptor‐associated factor 6 (TRAF6) requires TRAF6‐regulated IKK activator 1 (TRIKA1, a dimeric ubiquitin‐conjugating enzyme complex, composed of ubiquitin‐conjugating enzyme 13 (Ubc13) and ubiquitin E2 variant 1Uev1A). 54 , 55 The function of TRIKA1 is to synthesize K63‐linked polyubiquitin chains on IKKγ and TRAF6. 54 , 55 , 56 TRAF6 requires TRIKA2 (a complex composed of TAK1, TAB1, and TAB2) to activate IKK, and the activation of TRIKA2 requires ubiquitination of the adaptor protein TAB2/3. 55 , 57 , 58 Similarly, when stimulation goes through TNFR, the activation of IKK depends on K63‐ubiquitination of receptor‐interacting protein 1 (RIP1), and this process is mediated by cIAP1/2. 59 In the noncanonical NF‐κB pathway, p100 ubiquitination is the key event to regulate NF‐κB activation. β‐TrCP acts as a ubiquitin E3 ligase to mediate inducible p100 ubiquitination, another E3 ligase Fbxw7α requires glycogen synthase kinase 3 (GSK3)‐mediated phosphorylation to mediate ubiquitination and degradation of p100. 37 , 60 Another molecule of interest is deubiquitinase (DUB) A20. A20 contains the DUB domain and the C2‐C2 zinc finger E3 ubiquitin ligase domain, which inhibits K63 ubiquitination during the activation of NF‐κB, leading to the decomposition of the IKK complex. A20 also mediates the K48 ubiquitination of RIP1 after inhibiting K63 ubiquitination, causing the degradation of RIP1 and suppressing the signaling pathway induced by TNFα. 61 , 62 , 63 , 64 As a key enzyme that inhibits ubiquitination in the canonical NF‐κB pathway, A20 regulates NF‐κB activity by coordinating with another E3 lineage such as Itch. 65 Meanwhile, the A20 encoding gene, Tnfaip3, is one of the NF‐κB target genes to mediate the inactivation of NF‐κB, suggestive of negative feedback regulation. 66 Similar to A20, the tumor suppressor protein cylindromatosis (CYLD) is also a DUB involved in the regulation of NF‐κB activity. Its function is to remove K63 ubiquitin chain of key molecules upstream of IKK, including TRAF2/6 and IKKγ. 67 , 68 , 69 In noncanonical NF‐κB pathway, ovarian tumor domain‐containing protein 7B (OTUD7B) functions as the DUB of TRAF3 to stabilize TRAF3 protein upon stimulation. 70 OTUD7B deficiency leads to enhanced noncanonical NF‐κB activity. Additionally, OTUD 1 is also a DUB, which inhibits the ubiquitination of p100, leading to the stabilization of p100 under stimulation condition and steady state. 71
Up to date, all the noncanonical NF‐κB‐activating stimuli activate NIK, which means that NIK is essential for noncanonical NF‐κB activation. 72 Considering the importance of NIK, the regulatory factors of NIK are also considered to play an important role in the regulation of NF‐κB. As a negative regulator of NIK, TRAF3 binds to NIK through the N‐terminal region causing NIK degradation in a ubiquitination‐dependent way and keeping NIK protein at a very low level. 73 This process also requires the participation of TRAF2 and ubiquitin E3 ligase cIAP1/2. 74 This constitutive proteasome‐mediated degradation regulated by TRAF2, TRAF3, and cIAP1/2 inhibits NIK. 33 , 74 NIK is also regulated by TBK1. TBK1 mediates the phosphorylation of NIK at Ser862, which is located in the degradation domain of NIK, making the phosphorylated NIK unstable. 75 , 76 Moreover, TBK1 forms a ternary complex with TRAF family member‐associated NF‐κB activator (TANK), and TRAF2 and plays a role upstream of the NIK and IKK complex, a substitute for the receptor signaling complex for TRAF‐mediated NF‐κB activation. 77 Kinase inactive TBK1 inhibits TANK‐mediated NF‐κB activation but does not block TNFα, IL‐1, or CD40‐mediated NF‐κB activation. 77 IKKα also mediates the negative feedback regulation of NIK. IKKα phosphorylates the C‐terminus of NIK and causes NIK degradation to prevent excessive accumulation of NIK. 78
In summary, most of the regulating mechanisms of the NF‐κB pathway are based on the regulation of key molecules or processes of this pathway, such as IKKs, NIK, ubiquitination. These mechanisms also indicate that the activation of NF‐κB is strictly regulated at multiple layers. Due to various stimuli and target genes involved in the NF‐κB pathway, the precise and comprehensive understanding of regulating mechanisms of the NF‐κB pathway requires additional intensive studies in vivo and in vitro.
3. NF‐ΚB AND INFLAMMATION
Inflammation is a protective response to infection and injury, 4 which is usually beneficial and transient, but sometimes inflammation is also harmful. Excessive inflammation causes tissue damage and inflammation‐related diseases. As the central activator of a variety of pro‐inflammatory genes, NF‐κB plays an important role in innate and adaptive immune cells and inflammation. 5 , 79 Due to the diversity of inflammatory factors, including infectious and non‐infectious stimuli, the function of NF‐κB in inflammation is complicated.
3.1. NF‐κB regulates innate immune response and inflammation
The innate immune cells including macrophages, dendritic cells (DCs), and neutrophils, are required to produce inflammatory mediators and regulators to eliminate pathogens, while at the same time avoiding a sustained inflammatory response through negative feedback mechanisms. 80 The PRRs expressed by these cells can detect various microbial components, so‐called pathogen‐associated molecular patterns (PAMPs). PRRs can also sense molecules released by necrotic cells and damaged tissues, called damage‐associate molecular patterns (DAMPs). 81 , 82 The PRRs on innate immune cells induce the expression of pro‐inflammatory cytokines such as TNF, IL‐1, IL‐6, IFN‐I, chemokines, and antimicrobial proteins and mediate an inflammatory response to eliminate pathogens and repair damaged tissue. 83 , 84 Different PRRs families have different structural characteristics and respond to different PAMPs and DAMPs. Five PRR families have been found in mammals, including TLRs, NOD‐like receptors (NLRs), RIG‐I‐like receptors (RLRs), C‐type lectin receptors (CLRs), and cytosolic DNA sensors. 83 , 85 , 86 , 87 The activation of the canonical NF‐κB pathway through these PRRs induces pro‐inflammatory cytokines, chemokines, and other inflammatory mediators. 82 , 88 Moreover, NF‐κB plays an important role in the signal transduction mediated by granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and myeloid progenitor cell differentiation. 89 The development of innate immune cells from myeloid progenitor cells is specifically affected by different members of the NF‐κB transcription factor family. 90
3.1.1. NF‐κB functions in macrophage
Macrophages are the phagocytic innate immune cells against infection, and NF‐κB is crucial to regulate macrophage function. Macrophages can be activated by many PAMPs and DAMPs and secrete cytokines and chemokines. 4 Activated macrophages can differentiate into different phenotypes, including M1 and M2 macrophages. 91 M1 macrophages are characterized by producing pro‐inflammatory cytokines to promote inflammation, while M2 macrophages produce anti‐inflammatory cytokines to inhibit inflammation. 92 , 93 NF‐κB is a key transcription factor of M1 macrophages and is required for the expression of a large number of inflammatory genes. 91 TLR signaling plays an important role in regulating the polarization of macrophages. A clear example is the TLR4 ligand lipopolysaccharide (LPS), which promotes the differentiation of macrophages to the M1 phenotype. 94 Under LPS stimulation, TLR4 recruits toll‐interleukin 1 receptor domain‐containing adaptor protein (TIRAP) and Toll/IL‐IR domain‐containing adapter‐inducing IFN‐beta (TRIF)‐related adaptor molecule (TRAM) and further recruits MyD88 and TRIF for downstream signaling. 94 , 95 The signal from MyD88 activates the IRAK family (IRAK1 and IRAK4), which in turn stimulates TRAF6 and activate TAK1. 94 , 96 , 97 , 98 Subsequently, TAK1 mediates the phosphorylation and activation of IKKs, which in turn phosphorylates IκBα, leading to ubiquitin‐dependent IκBα degradation and NF‐κB activation, and the expression of pro‐inflammatory cytokines. 56 , 99 , 100 TRIF‐dependent signaling pathways can mediate inflammatory cytokines by recruiting TRAF6 and RIP1 to activate the canonical NF‐κB pathway. 28 , 54 , 96 , 101 TRAF3 can also recruit TRIF‐dependent signaling pathways, activate TBK1 and IKKɛ and phosphorylate IRF3, and then induce the dimerization of IRF3, leading to the transcription of pro‐inflammatory cytokines and Type I IFNs. 28 , 91 , 102 NF‐κB can also regulate other pathways to regulate the inflammatory function of macrophages. IKKβ can inhibit the activation of STAT1 in macrophages and suppress its inflammatory function. 103 In general, NF‐κB is a key mediator of the inflammatory response of macrophages and mediates the pro‐inflammatory signaling functions. It was found in mice that if both c‐Rel and p50 proteins are defective, the innate immune response to bacterial sepsis will be impaired, and macrophages were able to exert normal immune functions. 104
3.1.2. NF‐κB regulates DC function
As primary antigen‐presenting cells (APCs), DCs present antigens to T cells and activate the adaptive immune response as a bridge to link innate immunity with adaptive immunity. 105 Under the stimulation of PRRs, DCs sense infection and tissue damage through the canonical NF‐κB pathway and mature into APCs. 106 DCs also express members of the TNFR superfamily such as CD40, LTβR, and RANK, which can stimulate the noncanonical NF‐κB pathway. 107 It has been found that both canonical and noncanonical NF‐κB pathways regulated DC development and maturation. 108 For example, the increase of RelB expression level is related to DC maturation, and RelB‐deficient DCs cannot induce antigen‐specific T‐cell responses in vitro and in vivo. 109 , 110 A recent study found that RelB‐deficient mice have spontaneous allergic airway inflammation, but the adoptive transfer of RelB‐sufficient DCs can reverse this phenotype. 111 RelB is considered a component of the noncanonical NF‐κB transcription factor, but the canonical pathway NF‐κB is also critical to the RelB activity in DCs 112 , 113 since RelB activity in DCs is negatively regulated by IκBα and IκBε. 106 In addition to RelB, targeted deletion of IKKβ in DCs can prevent the accumulation of non‐lymphoid tissue DCs in lymph nodes and impair the transformation of regulatory T cells. 114 In short, it is clear that RelB plays an important role in the development and maturation of DCs, but it is necessary to explore the regulatory mechanism of other NF‐κB pathway components in DCs.
3.2. Functions of NF‐κB in adaptive immunity
Inflammation also involves adaptive immunity. After activation, T cells and B cells proliferate and differentiate into effector cells. These effector cells mediate different immune responses, including the secretion of cytokines, cytotoxic T lymphocyte response, and the production of antibodies by B cells. 29 It is worthy of special attention that the activated CD4+ T cells can differentiate into effector T‐cell subsets with different functions, including Th1, Th2, Th9, Th17, Tfh, and regulatory T (Treg) cells. 115 , 116 , 117 The differentiation of CD4+ T cells is regulated not only by the cytokines secreted by APCs and other innate immune cells but also by T‐cell intrinsic factors. 4 Th1 cells produce pro‐inflammatory cytokines IL‐12 and Interferon‐γ (IFN‐γ) to activate macrophages and mediate the immune response to intracellular pathogens and participate in inflammation. 115 Th2 cells release IL‐4, IL‐5, and IL‐13 and stimulate the response of mast cells, eosinophils and basophils to pathogens. 118 , 119 Th17 cells can produce IL‐17 and IL‐22 to recruit neutrophils and monocytes to the site of inflammation and mediate immune responses against pathogens or autoantigens. 117 Treg cells are produced during the intrathymic development and can produce cytokines, such as IL‐10 and transforming growth factor‐β (TGF‐β), to suppress the immune response. 115 , 117 , 120 Similar to CD4+ T cells, activated naive CD8+ T cells can also proliferate and differentiate into various effector and memory cell types, including T effector cells, T memory stem cells, and T central memory cells, which are responsible for the elimination of tumor cells and viral infections cells. 121 , 122 , 123 While B lymphocytes develop in the bone marrow, they differentiate into plasma cells that can produce pathogen‐specific antibodies after activation. 124 Memory B cell is formed in the germinal center after the initial infection. In the case of re‐infection, memory B cells quickly produce antibodies and play an important role in the secondary immune response. 125
3.2.1. NF‐κB in T cell
Many studies have proved that NF‐κB is necessary for the differentiation of effector T cells and the recall response of memory T cells. 126 , 127 , 128 , 129 The initial activation of primitive T cells through TCR and costimulatory signals depends on the canonical NF‐κB pathway. 130 TCR activates the canonical NF‐κB pathway through the CARD‐containing MAGUK protein 1 (CARMA1)/Bcl10/membrane‐associated lymphoid tissue (MALT) 1 complex, which requires protein kinase C‐θ‐mediated phosphorylation of CARD‐containing MAGUK protein 1 (CARMA1). 131 , 132 , 133 After T cell activation, some TNFRs are induced to mediate the activation of the noncanonical NF‐κB pathway. 134 The noncanonical NF‐κB pathway plays a role in regulating the development of natural killer T cells and γδT cells, and this function is mediated by medullary thymic epithelial cells. 135 , 136 , 137
CD4+ T cells differentiate into different effector cells after stimulation, thereby participating in various immune responses. In mice, inhibiting the activation of NF‐κB by expressing an anti‐degradation IκBα in T cells can reduce the differentiation of Th1 cells. 138 The generation of Th1 cells also requires c‐Rel. The main function of c‐Rel is to mediate Th1 polarization cytokines in APC. Mice with c‐Rel deficiency have impaired Th1‐mediated immune response and impaired IFN‐γ production. 139 The production of IFN‐γ relies on RelA; and another family member, RelB, also plays an important role in Th1 differentiation through regulating T‐bet. 140 NF‐κB synergized with the IL‐4 to promote the development of Th2 cells by triggering the activation of the transcription factor STAT‐6. 141 , 142
An initial study found that T‐cell‐specific IKKβ‐deficient mice have impaired T‐cell activation and are completely resistant to experimental autoimmune encephalomyelitis, a Th17‐dependent autoimmune disease. 143 More studies later proved that NF‐κB regulated Th17 cells. For example, the defect of RelA in DCs results in reduced production of IL‐1α, IL‐1β, and IL‐6 under LPS stimulation, which affects the Th17 differentiation. 144 , 145 In CD4+ T cells, c‐Rel mediates the expression of IL‐21, an important cytokine for Th17 and Tfh cell differentiation, upon TCR stimulation. Experiments have confirmed that c‐Rel‐deficient mice have defects in Th17 and Tfh differentiation. 146 Another NF‐κB family member, p52, can promote the pathological function of Th17 cells in neuroinflammation by regulating GM‐CSF. 147 In addition, as a key adaptor in TCR signaling, the lack of CARMA1 impairers Th17 development. 145 The function of NF‐κB on Th9 cell differentiation is less known. A study found that OX40, a member of the TNFR family, activated TRAF6 and NIK in CD4+ T cells, which further activated the noncanonical NF‐κB pathway and promoted the production of Th9 cells. 148
Tregs are crucial to prevent autoimmunity and chronic inflammation. 149 Although NF‐κB is proved to promote T‐cell activation and effector T‐cell differentiation, NF‐κB is also involved in Treg generation. The Treg development in the thymus and the expression of the forkhead box P3 (Foxp3) depend on c‐Rel. 150 , 151 , 152 In addition, RelA and c‐Rel, which are activated by canonical NF‐κB signals, participate in mTEC differentiation by regulating the transcription of RelB and also regulate the negative selection of autoreactive T cells and development of regulatory T cells. 153 p105 deficiency can also make CD4+ T cells more resistant to Treg‐mediated suppression of inflammation. 154 In the mice lacking canonical NF‐kB pathway components (including CARMA1, IKK, BCL10, and TAK1), it was found that fewer Tregs were produced. 155 A significant decrease in Tregs' development was also observed in mice with a deletion in CYLD, the negative regulator of IKKs. 156 It is worth noting that Ubc13 maintains the immunosuppressive function of Tregs through the canonical NF‐κB signaling pathway and prevents Tregs from acquiring an inflammatory phenotype. 157 The development of Treg may also be affected by the ablation of non‐canonical NF‐kB signaling. 158 For example, a study found a decrease of Treg in mice with NIK knockout. 159
3.2.2. NF‐κB and B cell
NF‐κB is essential for the development, survival, and function of B cells. 160 , 161 The activation of NF‐κB family members p52 and RelB and the upstream kinases NIK and IKKα can participate in the formation of germinal centers. 162 , 163 , 164 , 165 , 166 The initiation and maintenance of germinal center (GC) require the cooperation of different cells, including B cells, Tfh cells, follicular regulatory T cells, macrophages and follicular DCs. 167 , 168 Under the stimulation of LTβR, the noncanonical NF‐κB pathway in stromal cells mediate the production of chemokines, including CXCL13 and CCL21, to provide migration signals to B cells and promote GC formation. 169 This process also promotes the B cells proliferation with high‐affinity BCR and clearance of the low‐affinity B cells. 170 , 171 These high‐affinity B cells differentiate into plasma cells and memory B cells and initiate humoral immune responses. 172 In RelB‐deficient mice, the induction of CXCL13 and CCL21 is reduced, leading to defects in the microstructure of the spleen and defects in the development and structure of lymphatic organs. 169 In addition, recent studies have shown that in NIK‐knockout (KO) mice, the deficiency of NIK reduces the number of GC B cells and class‐switching. 173 , 174 It was found that deleting nfkb2 and Relb genes in B cells of germinal centers would lead to the collapse of established germinal centers. 163 , 169 , 175 In short, NF‐κB plays an important role in regulating B‐cell survival, differentiation, and maintenance of germinal centers.
3.3. NF‐κB is involved in inflammatory diseases
3.3.1. Rheumatoid arthritis (RA)
RA is an autoimmune and inflammatory disease characterized by chronic inflammation caused by immune cells infiltrating into the synovium, which is associated with the destruction of cartilage and bone, and progressive joint destruction occurs over time. 176 , 177 Studies on animal models and human patients have confirmed that NF‐κB is an important inflammatory mediator in RA. 4 Studies have found that NF‐κB is activated in the synovial tissue of a mouse arthritis model, and the activation of NF‐κB increases as the disease progresses. 178 Similarly, in the early studies, NF‐κB activation was detected in the synovial tissue of RA patients, and it was determined that NF‐κB plays an important role in joint inflammation. 179 , 180 , 181 The pro‐inflammatory effect of NF‐κB in RA has long been recognized; both the canonical and the noncanonical pathways are involved in different aspects of the pathogenesis of RA. 182 , 183 For example, inflammatory bone loss in RA patients involves the abnormal generation and activation of osteoclasts, and the occurrence of osteoclasts requires RANK, which can stimulate the canonical and noncanonical NF‐κB pathways in osteoclast precursor cells. 184 , 185 , 186 In addition to the participation of osteoclasts, the pathogenesis of RA also involves many cell types, including innate immune cells, inflammatory T cells, B cells, and synovial fibroblasts. 187 NF‐κB mediates the production of pro‐inflammatory cytokines (such as TNFα, IL‐1, and IL‐6) of innate immune cells, promotes the recruitment of inflammatory cells, and causes joint inflammation. 188 , 189 In addition, the excessive production of inflammatory cytokines and the induction of osteoclasts promote the destruction of bone and cartilage and aggravate disease progression. 190 , 191 , 192 In particular, as a key component of noncanonical NF‐κB pathways, NIK is highly expressed in synovial endothelial cells of RA patients, and NIK enhances CXCL12 expression in endothelial cells to promote pathogenic angiogenesis and synovial inflammation. 193 , 194 Moreover, the study also found that NIK‐deficient mice have resistance to antigen‐induced arthritis caused by T cell. 195 , 196 As mentioned earlier, T cells are also involved in the pathogenesis of RA, especially Th17 cells, which can recruit synovial tissues, release pro‐inflammatory cytokines in synovial tissues, and induce synovitis. 130 , 176 The inflammatory factors required for Th17‐cell differentiation (such as IL‐1, IL‐6, and IL‐23) are induced by NF‐κB. 4 , 176 , 197 B cells are also involved in the pathogenesis of RA. In addition to producing autoantibodies, B cells regulate other immune cells and produce cytokines. Studies have found that the level of the B‐cell‐activating factor cloning to the tumor necrosis factor family (BAFF) in serum and synovial fluid of RA patients is usually increased, which is associated with the severity of the disease, while BAFF mainly activates noncanonical NF‐κB pathway to facilitates survival and maturation of B cells. 198 , 199
Because NF‐κB functions many aspects of RA, inhibiting NF‐κB is a potential therapeutic target for RA. 199 , 200 It is well accepted that the biological agents such as anti‐TNFα‐neutralizing antibodies (etanercept, infliximab, and adalimumab, etc.) and anti‐IL‐6‐neutralizing antibody (tocilizumab) target NF‐κB pathway to prevent joint destruction of RA patients. 201 Recent studies have found that iguratimod (IGU) is a potential treatment of RA. IGU is a new type of disease‐modifying antirheumatic drug, which inhibits the production of immunoglobulin by inhibiting NF‐κB without affecting the proliferation of B cells, the production of various inflammatory cytokines and osteoclasts. 202 , 203 In addition, salvianolic acid B and Ephedra gerardiana aqueous ethanolic extract have also been found to inhibit joint inflammation by suppressing NF‐κB in animal models. 204 , 205 Drugs targeting NF‐κB have been found to not only suppress inflammation but also effectively maintain bone mass and control the bone destruction of RA. 176 Furthermore, the latest study tried to treat collagen‐induced arthritis mice with an injection of β‐arrestin‐2 (βArr2) adenovirus and found that βArr2 effectively reduced ankle joint inflammation by inhibiting the NF‐κB pathway and NLRP3 inflammasome. 206 The latest clinical trials have also found that the activation of NF‐κB in RA patients treated with β‐d‐mannuronic acid is significantly reduced, as well as the levels of IL‐6 and TNFα in serum, reflecting the prospect of this treatment. 207 The possibility of serious side effects caused by inhibiting NF‐κB should be considered since serious adverse reactions such as embryonic death have been reported. 208 , 209 , 210 , 211 We need to carry out further more basic and clinical research on the molecular mechanisms of RA and NF‐κB to find the best drugs for RA treatment.
3.3.2. Inflammatory bowel disease (IBD)
IBD is a chronic, recurrent and non‐infectious gastrointestinal (GI) disease, including ulcerative colitis (UC) and Crohn's disease, accompanied by long‐term abdominal pain, diarrhea, and bloody stools. 212 , 213 , 214 It is believed that the interaction between immunological, environmental, and genetic factors is regarded as the pathogenesis of IBD. 215 , 216 , 217 , 218 , 219 In genetically susceptible individuals, when the balance of immune response to commensal bacteria is disturbed, the GI homeostasis is failed and the inflammation occurs. 214 Both innate immunity and adaptive immunity play important roles in the pathogenesis of IBD; NF‐κB is involved in both aspects of immunity as an important inflammatory signaling pathway in the pathogenesis of IBD. 4 , 215 Early studies have reported that constitutive NF‐κB activation was found in the inflamed intestinal tissues of IBD patients. 220 , 221 In addition, NFKB1 genes encoding p105 and p50 were found to be associated with IBD. 222 , 223 , 224 Mice carrying knock‐in mutations of NFKB1 to block the production of p105 can develop IBD‐like intestinal inflammation. 154 Similarly, Nfkb2 also promotes the expression of RelA‐driven pro‐inflammatory genes in the intestinal epithelium and aggravates inflammatory cell infiltration and colon tissue damage. 225 Consistent with these findings, decoy oligodeoxynucleotides targeting the DNA‐binding activity of NF‐κB showed potential therapeutic prospects in mice model. 226 Genetic defects of negative regulators of NF‐κB pathway can promote colon inflammation and even cancer. For example, in a colitis‐related cancer model, compared with the control group, CYLD‐deficient mice are more susceptible to colonic inflammation, and the incidence of tumors is significantly increased. 227 Deletion of A20 in intestinal epithelial cells and myeloid cells induces ileitis and severe colitis. 228 Although NF‐κB participates in the pathogenesis of IBD, its functions in innate immune cells and epithelial cells are different. Loss of IKKβ in myeloid cells inhibits pro‐inflammatory cytokines expression, attenuating colitis and colitis‐related cancers. 229 Contrary to the pro‐inflammatory effect in myeloid cells, NF‐κB has a protective effect in intestinal epithelial cells and is a key regulator of epithelial integrity and intestinal immune homeostasis. 230 Using the conditional KO of IKKβ and IKKγ in intestinal epithelial cells, it shows the excessive and abnormal immune response in the intestine. 229 , 230 It has been confirmed that the IKKβ‐dependent gene expression in the intestinal epithelium is essential for intestinal immune homeostasis by promoting mucosal immunity and limiting chronic inflammation. 231 In the intestinal epithelium, NF‐κB not only plays a protective role but also participates in the regulation of the intestinal tight junction barrier. The latest research shows that the destruction of matrix metalloproteinase‐9 (MMP‐9)‐induced Caco‐2 intestinal epithelial tight junction barrier is regulated by the NF‐κB pathway. 232 This finding suggests that the function of NF‐κB in the intestinal epithelium is complex and to be further studied. As a mediator between the immune response and the intestinal commensal microbiota, TLRs also play a key role in maintaining intestinal homeostasis. 233 The enhanced expression of TLRs leads to a hyper‐activated downstream signal cascade including NF‐κB and increased inflammatory cytokines and IBD. 214 , 233 Under normal physiological conditions, the expression level of TLR4 in the intestinal epithelium is low to maintain the integrity of the mucosa and protect against invading bacteria; however, its expression is upregulated in IBD, activating the downstream cascade and leading to inflammation. 233 , 234
The current treatment strategy for IBD is to control mucosal inflammation and inhibit overwhelming immune responses. 235 , 236 Commonly used drugs include 5‐aminosalicylic acid, glucocorticoid, immune‐suppressor, and biological agents. 237 A biological agent that has attracted much attention is the anti‐TNF antibody infliximab, which has shown good therapeutic effects in IBD. 238 , 239 The success of infliximab indicates that inhibiting the inflammatory target genes of NF‐κB is one of the effective treatment strategies for IBD. In addition, for glucocorticosteroid treatment, the activity of NF‐κB is decreased in UC patients who respond to the treatment, which may be due to the increase in the level of glucocorticoid receptors that regulate the activity of NF‐κB. 240 There are also some medicines in the development stages that show certain therapeutic prospects. A recent study found that butyrolactone‐I reduces the production of IL‐1, IL‐6, and TNFα by inhibiting the TLR4/NF‐κB and mitogen‐activated protein kinases (MAPK) signaling pathways and reduces the inflammatory response of dextran sulfate sodium‐induced colitis in mice. 241 Another study found that a type of 2,3‐dihydro‐flavonoid has a beneficial effect on 2,4,6‐trinitrobenzene sulfonic acid‐induced colitis, possibly by inhibiting NF‐κB p65 phosphorylation to reduce inflammatory mediators produce. 242
3.3.3. Atherosclerosis
Atherosclerosis is a multi‐step inflammatory process in the arterial wall, characterized by the accumulation of low‐density lipoprotein (LDL) particles and immune cells in the subendothelial space. Both innate immunity and adaptive immunity are involved in this disease. 243 , 244 NF‐κB regulates the expression of genes involved in the pathogenesis of atherosclerosis. 245 , 246 The first step in atherosclerosis is the injury and activation of endothelial cells. In endothelial cells, NF‐κB mediates the expression of inflammatory cytokines, chemokines, and cell adhesion molecules and promotes the recruitment of monocytes to the arterial intima. 4 , 247 , 248 Then, the recruited monocytes differentiate into macrophages, and after ingesting LDL particles, they will eventually become lipid‐rich foam cells, which is a hallmark of the arterial lesion. 245 In foam cells, NF‐κB is activated, pro‐inflammatory cytokines are secreted to enhance inflammation; the vascular smooth muscle cells are recruited to the inflamed sites, and finally atherosclerosis is formed. 19 , 245 Considering the pro‐inflammatory function of NF‐κB and inflammation play crucial roles in the process of atherosclerosis, inhibiting NF‐κB may be a target for the prevention and treatment of atherosclerosis. Inhibition of NF‐κB reduces the expression of inflammatory cytokines and chemokines and suppresses the induction of adhesion molecules in endothelial cells, to reduce the recruitment of macrophages to plaques and vascular inflammation. In an apolipoprotein E (ApoE)‐deficient mouse model fed by a high‐cholesterol diet, NF‐κB was inhibited by ablation of IKKγ or increased expression of IκBα, and atherosclerotic plaque formation was significantly reduced. 249 Studies have also found that in macrophages, the overexpression of trans‐dominant and non‐degradable forms of IκBα specifically inhibits NF‐κB activation, which can reduce lipid load and foam cell formation. 250 Similarly, one study has shown that the lack of IκBα in myeloid cells may promote atherosclerosis by reducing leukocyte recruitment to plaque. 251 However, the role of macrophage‐specific NF‐κB in the development of atherosclerosis is still incompletely known. 245
Targeting NF‐κB‐dependent inflammation is a very promising strategy for atherosclerosis treatment. A large clinical trial used anti‐IL‐1β antibody (canakinumab) for anti‐inflammatory treatment in patients with atherosclerosis and previous myocardial infarction. The results showed that, compared with placebo, the recurrence rate of cardiovascular events was significantly reduced in the group with treatment. 252 The results of this clinical trial demonstrate the effectiveness of anti‐inflammatory treatments in the management of atherosclerotic diseases. Recent studies have also confirmed the therapeutic value of NF‐κB as a target for atherosclerosis using animal models. The traditional Chinese medicine Qing‐Xue‐Xiao‐Zhi formula inhibits TLR4‐mediated NF‐κB pathway, affects cholesterol metabolism and promotes lipid efflux, and inhibits macrophage‐mediated inflammation, which has a therapeutic effect on atherosclerosis. 253 Another clinical trial has also found that crocin may reduce the expression of lectin‐like oxidized LDL receptor 1 and NF‐κB by increasing expression of sirtuin 1 and 5′‐adenosine monophosphate‐activated protein kinase (AMPK), demonstrating the potential therapeutic value of coronary heart disease. 254 The latest research has found that Paeonia lactiflora extract also exhibits a certain therapeutic potential for atherosclerosis. In vitro P. lactiflora extract can inhibit TNFα‐induced nuclear translocation of NF‐κB p65 from the cytoplasm and NF‐κB activity; in vivo oral administration of P. lactiflora extract can improve TNFα‐induced macrophages activation. The infiltration of immune cells to the vascular endothelium and the expression of Il6 and Tnfa in the mouse aorta contribute to the early stage of atherosclerosis. 255
3.3.4. Corona virus disease 2019 (COVID‐19)
In the past 2 years, COVID‐19 has had a catastrophic impact on the health and lives worldwide. According to the current vaccination situation and drug development, this impact will continue for a certain time. COVID‐19 patients show a wide range of clinical features, including cough and fever, and even some patients develop septic shock, acute respiratory distress syndromes, and multiple organ failure. 256 , 257 , 258 Compared with asymptomatic patients or mildly symptomatic patients, the clinical manifestations of severe cases suggest excessive activation and imbalance of systemic inflammation. 256 , 259 In particular, cytokine storm syndrome (CSS) occurs in critically ill patients, with significantly increased cytokine and chemokine. 260 , 261 , 262 The overwhelming production of these cytokines is related to the severity of COVID‐19, and the NF‐κB signaling pathway plays an important role in it. 263 , 264 Activated NF‐κB upregulates the production of inflammatory cytokines (such as TNFα, IL‐1β, IL‐6, and IL‐8), which is essential for a comprehensive cytokine storm. 265 , 266 Multiplication of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) promotes the production and accumulation of dsRNA. 267 Protein kinase R (PKR) can trigger the innate immune response and terminate the translation process to prevent replication of the virus in infected cells. PKR combined with dsRNA can activate IKK, trigger the degradation of IκBα and IKKβ, and activate canonical NF‐κB pathway. 268 , 269 SARS‐CoV‐2 spikes and nucleocapsid (N) protein induced NF‐κB activation, which in turn significantly increased the expression of pro‐inflammatory cytokines. The spike protein subunit 1 of SARS‐CoV‐2 has been identified as an effective cytokine storm inducer in COVID‐19. 270 This subunit has a high binding affinity to the angiotensin‐converting enzyme 2 receptor and can activate NF‐κB to produce cytokines. 271 In addition, SARS‐CoV‐2 N protein can also promote NF‐κB activation and increase the expression of cytokines by recruiting TAK1 and IKK complexes after binding to viral RNA. 262 SARS‐CoV‐2 mediates inflammation by activating NF‐κB, so the development of drugs that inhibit NF‐κB is currently considered to be one of the potential treatment strategies for COVID‐19. 272 A clinical trial used the BTK inhibitor acalabrutinib to treat severe COVID‐19 patients, and the result showed that C‐reactive protein and IL‐6 levels were reduced, and patients' oxygen saturation improved. 273 Another study used diosmectite in the SARS‐CoV‐2 model, as diosmectite can bind to SARS‐CoV‐2 components and inhibit NF‐κB activation and CXCL10 secretion and inhibit downstream inflammation, indicating the potential value of diosmectite for COVID‐19‐related diarrhea. 274 It is worth noting that traditional Chinese medicine has shown considerable prospects in the treatment of COVID‐19. A clinical study found that Yindan Jiedu granules may inhibit the production of inflammatory cytokines by targeting the NF‐κB pathway, thereby shortening the course of COVID‐19 and delaying its progression. 275 In in vitro experiments, it was also found that another traditional Chinese medicine, Liu Shen capsule, can inhibit SARS‐CoV‐2 infection by downregulating cytokine‐induced virus expression and regulating the activity of the NF‐κB/MAPK signaling pathway. 276 Despite the intensive efforts and large‐scale drug screening, an effective and safe antiviral treatment plan has not been developed. Nucleotide analogs inhibit RNA‐dependent RNA polymerase (RdRp) to suppress dsRNA‐induced NF‐κB activation and prevent virus replication. One example is remdesivir, which shows a certain clinical effect, compared to a placebo. 277 In addition, the latest drug development is the oral antiviral drugs molnupiravir and paxlovid, which have shown good treatment efficiency in clinical tries. 278 , 279 As an important inflammation‐related pathway, NF‐κB is still a potential therapeutic target for COVID‐19 treatment, especially for critically ill patients and CSS.
4. NF‐ΚB AND CANCER
4.1. Activation of NF‐κB in cancer
v‐Rel is an oncogene of avian Rev‐T retrovirus, isolated from a turkey liver lymphoma by Theilen and Robinson in 1958, 280 which is the earliest evidence that NF‐κB is related to cancer. However, the carcinogenicity of the rel gene is only found in birds, not in humans. Although the gene rel is not a bona fide oncogene, the constitutive‐activated NF‐κB has been examined in most tumors, which participates in a variety of cancer‐related biological procedures. 16
In most cancers, the activation of NF‐κB is enhanced, and this enhancement in NF‐κB activation is often due to increased stimuli of the NF‐κB pathway, such as increased TNFα and IL‐1 in the tumor microenvironment. 281 , 282 On the other hand, NF‐κB has a certain tumor‐suppressing function confirmed using tumor cell line and mouse models but not fully demonstrated in human cancers yet. 283 , 284 Mutations of NF‐κB are found in many cancers, although the mutation frequency of RelA and RelB is much lower than that of REL, p50, and p52. 7 It has been reported that REL gene amplification in lymphoma leads to an increased expression of REL protein, as well as a C‐terminal truncated p100 protein that lacks the ANK repeat inhibitory sequence. 285 , 286 , 287 In addition to NF‐κB family proteins, mutations have been found in the core components and regulators of the NF‐κB pathway, which affect both canonical and noncanonical NF‐κB activation. For example, studies have identified a loss‐of‐function mutation of IκB family proteins in lymphoma, glioblastoma, and nasopharyngeal carcinoma. 288 , 289 , 290 , 291 Meanwhile, studies have also reported that mutations of IKKα and IKKβ have been found in several cancers, 292 , 293 , 294 although the mutation of IKK is relatively rare. The reason may be that IKK also participates in many other signaling pathways except for NF‐κB. 295 The functional versatility of IKK may reduce the possibility of mutations. Mutations in critical regulating molecules that affect the activation and function of NF‐κB are identified in tumor cells. Considering NF‐κB as a family of the conserved transcription factor, the most common mutations that enhance NF‐κB signaling may occur in the upstream molecules and downstream target genes. 7 In diffuse large B‐cell lymphoma (DLBCL) and multiple myeloma (MM), proteins such as CD79 and MyD88 have been found to activate NF‐κB through gain‐of‐function mutations. It was also found that loss‐of‐function mutations of CYLD, A20, TRAF3, and other negative regulators also cause abnormal activation of NF‐κB. 296 , 297 , 298 , 299 , 300 , 301 Similarly, TNFα coded by an NF‐κB target gene TNFA acts as a strong activator of NF‐κB, functions in many tumor cells with constitutive activation of NF‐κB in an autocrine manner (usually stromal). 301 , 302 Taking specific tumor types as an example, DLBCL is the most studied malignant tumor with NF‐κB mutation. 297 Studies have shown that mutations in the three proteins (REL, IκB, p300) are involved in the NF‐κB activation in human B‐cell lymphoma cell line RC‐K8. 287 , 288 Most B‐cell malignancies not only have an oncogenic driving force activated by mutated NF‐κB but also have mutations in many other pathways. The subtype of DLBCL lymphoma with the activation of the canonical NF‐κB pathway is named by the activated B‐cell (ABC) subtype. Meanwhile, the abnormal activation of the noncanonical NF‐κB pathway is also detected more in other DLBCL subtypes. 299 , 300 The mutations in the NF‐κB signaling pathway involved in cancers are summarized in Table 1.
TABLE 1.
Mutations in the nuclear factor of κ‐light chain of enhancer‐activated B cells (NF‐κB) pathway identified in cancers
| Cancer type | Protein | Gene | Mutation type | Effect | Ref. |
|---|---|---|---|---|---|
| Solid | |||||
| Bladder cancer | TRAF2/3 | TRAF2/3 | Mutations, deletions, amplifications | Decrease | 7 , 426 |
| TRAF4 | TRAF4 | Mutations, amplifications | Increase | 7 , 426 | |
| Breast cancer | TRAF4/5/6 | TRAF4/5/6 | Mutations, amplifications | Increase | 7 , 426 |
| Cervical cancer | p50/p105 | NFKB1 | Point mutations | Increase | 427 |
| TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| Colon cancer | TRAF1 | TRAF1 | Point mutations | Increase | 428 |
| TRAF6 | TRAF6 | Mutations, amplifications | Increase | 7 , 426 | |
| Cylindromatosis | CYLD | CYLD | Mutations, deletions | Decrease | 69 |
| Esophageal cancer | TRAF4/5/6 | TRAF4/5/6 | Mutations, amplifications | Increase | 7 , 426 |
| Gastric cancer | p50/p105 | NFKB1 | Point mutations | Increase | 3 |
| MYD88 | MYD88 | Mutations, deletions | Increase | 299 , 429 | |
| TRAF1 | TRAF1 | Point mutations | Increase | 7 , 426 | |
| TRAF2/3 | TRAF2/3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| TRAF6 | TRAF6 | Mutations, amplifications | Increase | 7 , 426 , 430 | |
| Glioblastoma | IκBα | NFKBIA | Mutations, deletions | Decrease | 290 , 431 |
| Head and neck cancer | TRAF2/3 | TRAF2/3 | Mutations, deletions, amplifications | Decrease | 7 , 426 |
| TRAF6 | TRAF6 | Mutations, amplifications | Increase | 7 , 426 | |
| Liver cancer | p50/p105 | NFKB1 | Point mutations | Increase | 3 |
| TRAF5/6 | TRAF5/6 | Mutations, amplifications | Increase | 7 , 426 | |
| Lung cancer | TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 |
| TRAF4/5/6 | TRAF4/5/6 | Mutations, amplifications | Increase | 7 , 426 | |
| Melanoma | TRAF1 | TRAF1 | Point mutations | Increase | 426 , 432 |
| TRAF2/3 | TRAF2/3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| TRAF4/5 | TRAF4/5 | Mutations, amplifications | Increase | 7 , 426 | |
| Nasopharyngeal carcinoma | IκBα | NFKBIA | Mutations, deletions | Decrease | 291 |
| A20 | TNFAIP3 | Point mutations | Decrease | 291 | |
| CYLD | CYLD | Mutations, deletions | Decrease | 69 | |
| TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| Ovarian cancer | p50/p105 | NFKB1 | Point mutations | Increase | 3 |
| TRAF2/3 | TRAF2/3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| TRAF4/5/6 | TRAF4/5/6 | Mutations, amplifications | Increase | 7 , 426 | |
| Pancreatic cancer | TRAF4 | TRAF4 | Mutations, amplifications | Increase | 7 , 426 |
| Prostate cancer | IKKβ | IKBKB | Point mutations | Increase | 293 |
| TRAF2 | TRAF2 | Mutations | Decrease | 7 , 426 | |
| TRAF5/6 | TRAF5/6 | Mutations, amplifications | Increase | 7 , 426 | |
| Uterine cancer | TRAF2/3 | TRAF2/3 | Mutations, deletions, amplifications | Decrease | 7 , 426 |
| TRAF4/5/6 | TRAF4/5/6 | Mutations, amplifications | Increase | 7 , 426 | |
| Hematologic malignancy | |||||
| Chronic lymphocytic leukemia | IκBε | NFKBIE | Deletions, point mutations | Decrease | 433 |
| BCL3 | BCL3 | Translocations | Increase | 434 | |
| Chronic myelogenous leukemia | TRAF1 | TRAF1 | Point mutations | Increase | 435 |
| Diffuse large B‐cell lymphoma | p52/p100 | NFKB2 | C‐terminal truncations | Increase | 285 |
| REL | REL | Point mutations, amplifications, | Increase | 287 | |
| REL | REL | Truncations | Decrease | 287 | |
| IκBα | NFKBIA | Mutations, deletions | Decrease | 285 | |
| IKKβ | IKBKB | Point mutations | Increase | 293 | |
| CD79A/B | CD79A/B | Point mutations | Increase | 436 | |
| BCL10 | BCL10 | Point mutations, chromosomal translocations | Increase | 437 | |
| LUBAC | HOIP, HOIL, SHARPIN | Point mutations | Increase | 438 | |
| MALT1 | MALT1 | Chromosomal translocations, point mutations, amplifications | Increase | 439 | |
| A20 | TNFAIP3 | Point mutations | Decrease | 440 | |
| CARD11 | CARMA1 | Chromosomal translocation; point mutation | Increase | 441 | |
| p300 | EP300 | Deletions | Decrease | 442 , 443 | |
| TRAF2 | TRAF2 | Mutations | Decrease | 444 | |
| TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| CBP | CREBBP | Deletions | Decrease | 442 , 443 | |
| MYD88 | MYD88 | Mutations, deletions | Increase | 299 | |
| Hodgkin lymphoma | NIK | MAP3K14 | Gene fusion, point mutations | Increase | 31 |
| Leukemia | CARD11 | CARMA1 | Chromosomal translocation; point mutation | Increase | 441 |
| p300 | EP300 | Deletions | Decrease | 442 , 443 | |
| CBP | CREBBP | Deletions | Decrease | 442 , 443 | |
| Mantle cell lymphoma | TRAF2 | TRAF2 | Mutations | Decrease | 444 |
| Marginal zone lymphoma | TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 |
| Multiple myeloma | RELA (p65) | RELA | Point mutations | Increase | 445 |
| IκBβ | NFIKBB | Point mutations | Decrease | 446 | |
| IKKβ | IKBKB | Point mutations | Increase | 293 | |
| NIK | MAP3K14 | Gene fusion, point mutations | Increase | 446 | |
| CYLD | CYLD | Mutations, deletions | Decrease | 69 | |
| TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
| B‐cell lymphoma | CD79A/B | CD79A/B | Point mutations | Increase | 436 |
| p52/p100 | NFKB2 | C‐terminal truncations | Increase | 285 | |
| BCL10 | BCL10 | Point mutations, chromosomal translocations | Increase | 437 | |
| MALT1 | MALT1 | Chromosomal translocations, point mutations, amplifications | Increase | 439 | |
| A20 | TNFAIP3 | Point mutations | Decrease | 440 | |
| CARD11 | CARMA1 | Chromosomal translocation; point mutation | Increase | 441 | |
| T‐cell lymphoma | p52/p100 | NFKB2 | C‐terminal truncations | Increase | 285 |
| Waldenström's macroglobulinemia | MYD88 | MYD88 | Mutations, deletions | Increase | 447 |
| TRAF3 | TRAF3 | Mutations, deletions, amplifications | Decrease | 7 , 426 | |
Abbreviations: Bcl3, B‐cell leukemia/lymphoma 3; CARD11, caspase recruitment domain‐containing protein 11; CARMA1, CARD‐containing MAGUK protein 1.; CBP, CREB binding protein; CYLD, cylindromatosis; HOIL, heme‐oxidized IRP2 ubiquitin ligase; HOIP, HOIL‐1L‐interacting protein; IKKβ, IκB kinase β; IκB, inhibitor of NF‐κB; LUBAC, linea ubiquitin assembly complex; MALT1, mucosa‐associated lymphoid tissue lymphoma translocation protein 1; MyD88, myeloid differentiation primary response gene 88; NIK, NF‐κB‐induced kinase; SHARPIN, shank‐associated RH domain interactor; TRAF, TNF‐R‐associated factor.
4.2. NF‐κB and cancer cell survival and proliferation
One important function of the NF‐κB is to regulate cell survival. In tumor cells, the activation of NF‐κB usually leads to impaired cell apoptosis. 303 The deletion or inactivation of genes encoding key molecules in the NF‐κB pathway promotes cell apoptosis. For example, inactivation of the v‐Rel oncogene leads to apoptosis of transformed lymphocytes, and KO of the Rela gene leads to apoptosis of mouse fibroblasts and embryonic hepatocytes under the TNFα stimulation. 210 , 304 NF‐κB target genes encode anti‐apoptotic molecules such as Bcl2, Bcl‐xL, and inhibitor of apoptosis (IAP). Upregulation of NF‐κB activity has been detected in various tumor cells, and the expression of target genes of anti‐apoptotic NF‐κB is also increased. 7 It is noteworthy that NF‐κB is not only anti‐apoptotic, the activation of NF‐κB is also required for cell apoptosis. Studies have found that inhibition of NF‐κB reduced the apoptosis induced by drugs in many tumor cells. 305 , 306 , 307 Therefore, NF‐κB may also be a cell type‐specific repressor of anti‐apoptotic genes or an activator of pro‐apoptotic genes. 308 , 309 , 310 In addition, the recent researches suggest that many molecules (such as various anti‐cancer drugs, non‐coding RNA, microRNA, etc.) regulate the apoptosis of cancer cell through the NF‐κB pathway and other coordinated pathways (such as AMPK, AKT, glycogen synthase kinase 3, metabolism pathways). 311 , 312 , 313 , 314 , 315 , 316 , 317
In addition to apoptosis, proliferation is also the fundamental cellular procedure. NF‐κB activates genes that regulate cell proliferation, such as cyclin D1/D2/D3. 318 , 319 , 320 In addition to cyclins, NF‐κB also regulates cell proliferation by induction of critical enzymes. The expression of E3 ubiquitin ligase mouse double minute 2 protein is NF‐κB‐dependent, which affects the stability of p53 and cell proliferation. 321 In inflammatory immune cells such as macrophages and neutrophils, the activation of NF‐κB activates the expression of inflammatory cytokines such as TNFα, IL‐1β, and IL‐6, thereby promoting the proliferation of malignant cells and tumor stromal cells. 16 , 322 , 323 Taken together, it has been well‐accepted that NF‐κB plays an important role in the regulation of tumor cell proliferation and apoptosis, and its functions and mechanisms are multi‐faceted.
4.3. NF‐κB and tumorigenesis
The journal from tumorigenesis to tumor progression is a long‐term procedure controlled by multiple factors. Most tumors usually require multiple gene mutations. 324 Inflammation and NF‐κB affect the tumor occurrence by promoting the production of reactive oxygen species and reactive nitrogen species, leading to DNA damage and carcinogenic mutations. 282 Chronic inflammation and NF‐κB also cause chromosomal instability, aneuploidy, and epigenetic changes, resulting in the occurrence and progression of tumors. 282 In addition, one of the mechanisms by which NF‐κB promotes tumorigenesis is that when cells with damaged DNA strands enter the cell cycle, NF‐κB activation integrates mutations into the two DNA strands and transmits them to daughter cells. 325 , 326 Another mechanism by which NF‐κB stimulates tumorigenesis is to induce mutant‐related enzymes such as activation‐induced cytidine deaminase that deaminates cytosine residues and causes the conversion of cytosine to thymine, increasing mutations probability. 327 Furthermore, by preventing p53‐dependent apoptosis, NF‐κB activation increases the number of DNA‐damaged cells that accumulate oncogenic mutations. 328 In addition to gene mutation, viral infection is the risk factor leading to tumorigenesis, such as hepatitis B virus (HBV), human papillomavirus, and Epstein–Barr virus (EBV), and so forth. Some viruses promote tumorigenesis through chronic NF‐κB‐dependent inflammation or the continuous activated transcriptional activity induced by viral genes. Viruses also directly encode NF‐κB‐stimulating factors. EBV that causes B‐cell lymphoma and nasopharyngeal carcinoma encode LMP1 protein that functions as CD40 homolog to prevent apoptosis of EBV‐infected B‐cell by upregulating NF‐κB activation. 329 Kaposi's sarcoma‐associated herpesvirus that causes sarcoma and lymphoma encodes viral FLICE inhibitory protein (vFLIP) that hijacks both canonical and noncanonical NF‐κB pathways promoting cell survival and proliferation. 329 Besides direct transcribing cell proliferation‐related genes, NF‐κB also induces the expression of proinflammatory cytokines such as IL‐1β, IL‐6, and TNFα, which play a key role in the NF‐κB‐dependent tumor cell proliferation. 330 Meanwhile, NF‐κB also regulates apoptosis of tumor cells. NF‐κB blocks the death of tumor cells induced by the activation of oncogenes Ras. 331 NF‐κB‐dependent Rac guanosine triphosphatase effectively inhibits the p53‐independent apoptosis response induced by high levels of Ras activity. Rac mutants that could not activate NF‐kB are defective in inhibiting Ras‐induced apoptosis. In particular, under hypoxic conditions, NF‐κB enhances the expression of hypoxia‐inducible factor 1α, thereby enhancing the early survival of tumor cells. 332 , 333
NF‐κB is important for tumor angiogenesis. 334 Among the singling pathways contributing to tumor angiogenesis, NF‐κB is critical to activate angiogenesis‐related genes. Study has confirmed that B7‐H3 activates NF‐κB pathway to upregulate the expression of vascular endothelial growth factor‐A (VEGFA) in colorectal cancer and promotes angiogenesis. 335 In contrast, NF‐κB interacting long noncoding RNA (NKILA) was found to inhibit the IL‐6 production and angiogenesis through NF‐κB. 336 NF‐κB in cancer cells promotes EMT. 337 The expression of major EMT molecules (including Twist, Zinc finger transcription factor Snail2 (Slug) and Smad‐interacting protein1) is NF‐κB‐dependent, which initiate EMT and identify malignant phenotypes by enhancing the stemness and migration of cancer cells. 338 , 339 Additionally, NF‐κB activation also promotes EMT through other mechanisms, such as the matrix‐degrading enzyme. 340 MMPs induced by NF‐κB promote the release of TGF‐β, EMT process, and hypoxia that contribute to metastatic dissemination. 341 , 342 , 343 In addition, NF‐κB and cytokines (such as VEGF and TGF‐β) can directly stimulate the expression of metastasis‐related genes and promote tumor metastasis, such as exercise‐inducing factors and chemokines. 344 , 345 Inflammation and NF‐κB can also directly stimulate metastatic dissemination through EMT, increase the extravasation of cancer cells into the blood and lymphatic vessels, and prevent the death of connective tissue tumor cells. 346 Taking liver cancer as an example, the functions of NF‐κB in tumorigenesis are schematically shown in Figure 3.
FIGURE 3.
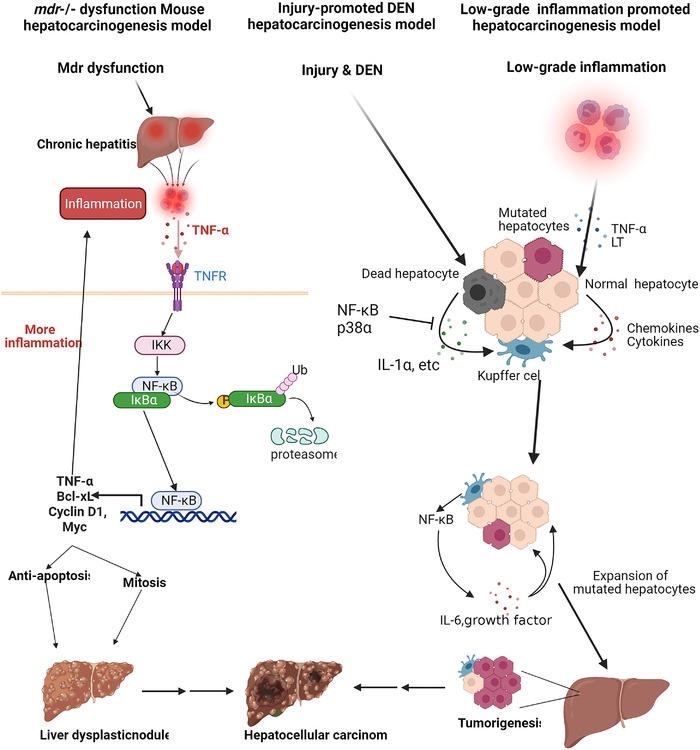
NF‐κB signaling pathway in tumorigenesis. Schematic diagram of the mechanism of gene mutation, exogenous carcinogenic stimulation, and inflammation leading to liver cancer. In the mice model, the deletion of Mdr gene leads to the accumulation of bile acids and induces chronic inflammation of the liver. After inflammatory cells migrate to the liver matrix, they release TNFα and invade the liver parenchymal cells between the liver epithelium. TNFα activates the NF‐κB signaling pathway, resulting in the expression of apoptosis‐inhibiting proteins, pro‐proliferation proteins and TNFα and promotes the development of dysplastic nodules into hepatocellular carcinoma. In the damage‐promoted diethylnitrosamine liver cancer model, Kupffer cells are activated by IL‐1α released by dead liver cells. In the low‐grade inflammation‐promoting hepatocellular carcinoma model, the hepatocytes with activated NF‐κB produce cytokines and chemokines. These cytokines and chemokines activate Kupffer cells. In these two models, activated Kupffer cells produce cytokines and growth factors, which promote the expansion of mutated liver cells and the development of liver cancer
4.4. NF‐κB and cancer stem cells (CSCs)
CSCs are malignant cells that have the ability to self‐renew and differentiate into highly differentiated malignant cells. CSCs are the major reason for cancer recurrence, metastasis, and treatment resistance. 347 , 348 CSCs were first identified in leukemia and then isolated as CD34+CD38– cells in the 1990s. 349 , 350 The following studies indicate that in other hematologic malignancies and solid tumors, CSCs express other different surface markers (such as CD133, nestin, and CD44). 351 , 352 The activities of cancer stem cells are controlled by many intracellular and extracellular factors and various signaling pathways. The functions of NF‐κB pathway in CSCs are complicated. NF‐κB is constitutively activated in various CSCs (including leukemia, glioblastoma, prostate, ovary, breast, pancreatic, and colon cancer), which mediates inflammation, cell proliferation, survival, maintenance, and expansion. 353 NF‐κB could work alone or synergize with other signaling pathways to induce and promote the self‐renewal, proliferation, and metastasis of CSCs by mediating the expression of stem‐cell‐related transcriptional factors and genes (such as Nanog, Sox2, Olig2, CD 44, NKX3.1 and Krüppel‐like factor 4 (KLF4)). 354 In breast cancer, NF‐κB activation causes CSC proliferation by activating Notch signaling in a cell‐involuntary way. 355 In addition, high levels of NIK also induce the activation of the noncanonical NF‐κB pathway to regulate the self‐renewal and metastasis of breast CSCs. 356 Therefore, drugs targeting NF‐κB pathway could be used to inhibit the proliferation and metastasis of cancer stem cells. The study has shown that in breast cancer stem cells, the anti‐alcoholism drug disulfiram inhibits TGF‐β through the extracellular regulated protein kinases(ERK)/NF‐κB/Snail pathway to induce tumor metastasis. 357 Sulforaphane also inhibits the self‐renewal of triple‐negative breast cancer stem cells by inhibiting NF‐κB p65 subunit translocation and down‐regulating p52 and its transcriptional activity. 358 The canonical NF‐κB pathway enhances the expression of the transcription factor Sry‐related HMG box 9 (Sox9), which is associated with enhanced pancreatic CSCs and invasiveness. 359 In ovarian cancer, CD44+ CSCs enhance self‐renewal and metastasis by upregulating the expression of RelA, RelB, and IKKα and enhancing the activation of p50/RelA dimer. 360 In colorectal cancer, the inflammatory mediator prostaglandin E2 activates NF‐κB through prostaglandin E Receptor 4‐phosphoinositide 3‐kinase to promote the formation, maintenance, and metastasis of CSCs. 361 The transcription factor Foxp3 also interacts with NF‐κB, to inhibit the expression of the NF‐κB target gene COX2, and affect the self‐renewal and metastasis of colorectal CSCs. 362
4.5. NF‐κB and tumor microenvironment
Tumorigenesis’ progress and metastasis rely on the environment where the tumor cells are located. The tumor microenvironment is composed of tumor cells, a variety of stromal cells, and immune cells, together with cytokines, chemokine, and other mediators, especially endothelial cells, cancer‐associated fibroblasts (CAFs), myeloid‐derived suppressor cells (MDSCs), and other numerous immune cells are critical for the tumor microenvironment. 363 , 364 , 365 NF‐κB functions as the key regulator in many types of cells to shape the tumor microenvironment.
Cytokines produced by stromal cells, cancer cells, and immune cells are the key soluble factors for tumorigenesis, metastasis, and inflammation. 330 Tumorigenesis is associated with tumor‐promoting cytokines, so it is important to understand the correlation between NF‐κB and pro‐tumorigenic cytokines. Tumor necrosis factor α (TNFα) is also one of the well‐studied tumor‐promoting cytokines, and its expression is enhanced in many cancers, which often indicates a poor prognosis. 302 , 366 , 367 TNFα is mainly produced by activated neutrophils and macrophages, which induces other proinflammatory cytokines, including IL‐6 and IL‐1β, and accelerates tumorigenesis. 302 Similar to TNFα, IL‐6 is another important and abundant proinflammatory cytokine in the tumor microenvironment. The functions of this NF‐κB‐dependent cytokine on inflammation and tumorigenesis have been well studied. 229 , 367 , 368 IL‐6, mainly produced by malignant tumor cells and activated immune cells, initiates cancer‐related inflammation and stem cell expansion in an autocrine manner. 367 , 369 In addition, IL‐1α/β are also key proinflammatory cytokines produced by cancer cells and immune cells, which are NF‐κB‐dependent and promote the activation of NF‐κB and MAPK pathways and tumorigenesis. 370 , 371 IL‐17A activates NF‐κB and MAPK signaling pathways, thereby promoting tumorigenesis. 372 , 373 , 374 The other IL‐1 family cytokine, IL‐33 is mainly expressed by epithelial cells, fibroblasts, and tumor cells, with a crucial function in allergy, autoimmunity, inflammation, and cancer. 375 , 376 Another important cytokine is TGF‐β, which is produced by a variety of cells, including fibroblasts, myeloid cells, T cells, and cancer cells. TGF‐β is essential for the differentiation of Treg and TH17 cells, making it a powerful inducer of tumor invasion and metastasis. 330 , 343
NF‐κB drives chemokine expression in tumor cells, stromal cells, and immune cells in the tumor microenvironment, especially CAFs. 377 , 378 , 379 The activation of NF‐κB and other transcription factors (such as AP1 and STAT3) induces the expression of chemokines, which recruit more immune cells and further aggravate the inflammatory response. 380 , 381 Meanwhile, chemokines are important to stimulate the growth and metastasis of primary tumors, synthesizing growth factors, and angiogenesis. Although the functions of chemokines are complicated and uncertain in tumorigenesis and metastasis, chemokines still are important targets for cancer immunotherapy. 364 , 382 , 383 For example, chemokine (C‐C motif) ligand 5 (CCL5) induces the formation of eukaryotic initiation factor 4F translation initiation complex in an mechanistic target of rapamycin (mTOR)‐dependent way, which mediates the quick upregulation of cyclin D1, c‐Myc, and defender against cell death‐1 (Dad‐1) protein expression to promote tumor cell proliferation in breast cancer. 384 In addition, in human pancreatic cancer and mouse pancreatic tumor models (Pan02), the CCL5 level on tumor cells is elevated, while CD4+Foxp3– effector T cells preferentially express C‐C chemokine receptor 5 (CCR5). When the CCR5/CCL5 signal is disturbed, either by inhibiting CCL5 expression on tumor cells or systemically administrating CCR5 inhibitors, the migration of Treg cells to the tumor microenvironment is reduced, which indicates that the chemokine CCL5 is required for Treg cells migration. 385 Tumor‐related cytokines are mainly expressed by inflammatory cells, such as tumor‐associated macrophages (TAMs) and neutrophils. NF‐κB is mainly activated in these cells to induce the expression of cytokines (TNFα, IL‐1β, IL‐6, etc.), thereby promoting tumor survival and proliferation. 16 , 323 As the most abundant immune cells in the microenvironment, macrophages produce inflammatory cytokines and chemokines and produce proteases such as cysteine cathepsins, involved in activating cytokines to promote tumor development. 330 , 365 M1 macrophages activated by IFN‐γ and LPS can produce proinflammatory cytokines, chemokines, and enzymes to promote inflammation. In contrast, M2 macrophages induced by IL‐4, IL‐10, and IL‐13 can inhibit inflammation by releasing anti‐inflammatory mediators. 92 , 386 , 387 M1 macrophages promote inflammation, while M2 macrophages create an immunosuppressive tumor microenvironment. The increase in p50 activity determines the M1 to M2‐ polarization of macrophages. 387 , 388 , 389 Inhibiting NF‐κB transforms M2 macrophages to M1 macrophages because the polarization of M1 macrophages through IL‐1R and MyD88 into M2 macrophages requires IKKβ‐mediated activation of NF‐κB. Moreover, the impaired NF‐κB activation in TAMs also promotes the tumor‐killing activity of macrophages. When NF‐κB signaling is inhibited in TAMs, they become M1 phenotype with anti‐tumor cytotoxicity. 390 NF‐κB could be a target for manipulating the phenotype of macrophages in the tumor microenvironment.
DCs are also important immune cells modulating anti‐tumor immunity in the tumor microenvironment. The canonical NF‐κB pathway in DCs can be inhibited by signals from the immune checkpoint molecule programmed cell death protein 1 (PD‐1), thereby inhibiting the production of cytokines. 391 In contrast, the ligand of checkpoint like programmed cell death 1 ligand 1 (PD‐L1) is an NF‐κB target gene. The upregulation of PD‐L1 expression in cancer cells relies on NF‐κB activation triggered by many stimuli and activators, including oncogenes, stress, inflammatory cytokines, and chemotherapeutic drugs. 392 In addition to macrophages and DCs, it has also been reported that IL‐1β induces NF‐κB activation in MDSCs to suppress the function of tumor microenvironment (TME), leading to tumor proliferation. 393 In natural killer (NK) cells that directly kill tumor cells to exert anti‐tumor activity, the expression of cytotoxic effector molecules (such as perforin and granzyme B) ia also affected by NF‐κB. 394 , 395 , 396 The activation of NF‐κB in NK cells can be stimulated by exogenous anti‐cancer drugs, like paclitaxel. 397
As the important components of the tumor microenvironment, T cells and B cells in the tumor microenvironment could have tumor‐promoting or anti‐tumor functions. 398 As mentioned above, the important stimuli of the NF‐κB pathway include TCR and BCR. The activation of conventional T cells requires the participation of the canonical NF‐κB pathway, which is required for CD8+ T cell proliferation and anti‐tumor immune response. 399 , 400 The development of Treg cells requires the participation of p65 and Rel. Rel is not only essential for the optimal steady‐state expansion of Treg cells in peripheral blood 150 but also for the development of thymic Treg cells. p65 is crucial for Tregs maturation, and the maintenance of immune tolerance 401 IKKβ‐dependent NF‐κB is activated in B cells that induce a cytokine LT, a heterotrimeric member of the TNF family that activates IKKα. 402 The LT produced by B cells can activate IKKα in tumor cells. IKKα phosphorylates a site of the transcription factor E2F transcription factor 1 (E2F1) to promote E2F1 translocation and recruit to the target of the Bmi1 gene. The IKKα–E2F1–Bmi1 cascade activated by B cells controls prostate regeneration and tumor recurrence and regulates the renewal function of tumor stem cells. 403
The component that cannot be ignored in the tumor microenvironment is fibroblasts. Normal fibroblasts undergo changes in a self‐intrinsic way, and under the stimulation of tumor‐induced alterations tissue structure, TGF‐β, and hypoxia, they become tumor‐associated fibroblasts (CAFs) and express a large number of inflammatory factors and chemokines. 378 , 379 For example, IL‐1β is induced by the NF‐κB signaling pathway to promote inflammation, cancer cell proliferation, angiogenesis, and tumor metastasis. 404 For example, in pancreatic ductal adenocarcinoma, CAFs mediate the exosomal metastasis and paracrine of tumor cells through producing cytokines (such as GM‐CSF and IL‐6), and this process requires the participation of NF‐κB. In breast cancer, locked nucleic acid (LNA)‐i‐miR‐221 inhibits miR‐221‐mediated NF‐κB activation to reduce the secretion of tumor‐promoting cytokines of CAFs. 405 In brief, NF‐κB has complex functions on the different types of cells in the tumor microenvironment and might become a potentially effective therapeutic target. The function of the NF‐κB in the tumor microenvironment is shown in Figure 4.
FIGURE 4.
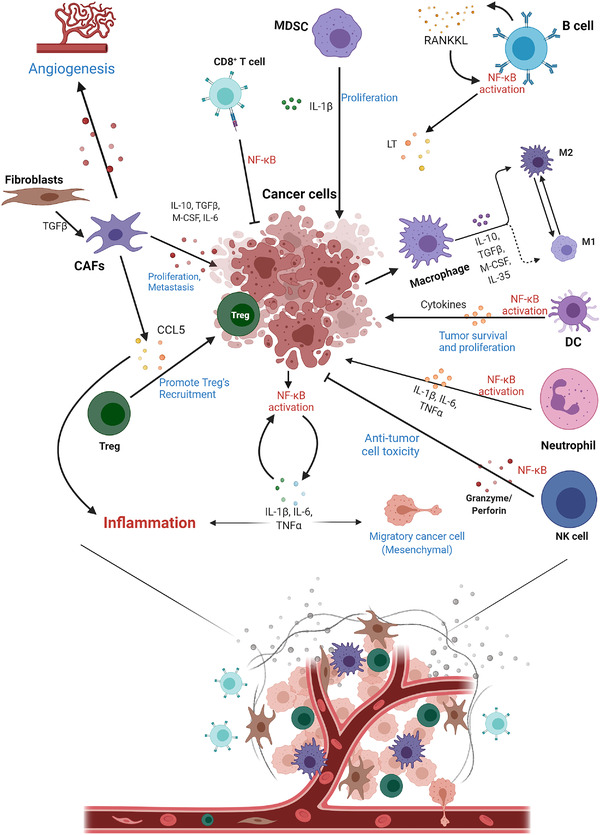
NF‐κB pathway modulates tumor microenvironment. The NF‐κB signaling pathway is tightly correlated to the various components of the tumor microenvironment. NF‐κB can not only drive the expression of chemokines in stromal cells, tumor cells, and immune cells in the tumor microenvironment but can also be activated in each cell to transcriptionally enhance the expression of cytokines and promote tumor proliferation and metastasis
4.6. NF‐κB and inflammation in cancer
The relationship between inflammation and tumor has become an important field of cancer research. It is well‐studied that inflammation is associated with autoimmune diseases, infection, and cancers. 5 The well‐established examples of inflammation leading to cancer are HBV infection and hepatocellular carcinoma (HCC), and chronic Helicobacter pylori infection and mucous MALT lymphoma and gastric cancer. 25 , 330 NF‐κB is essential for the activation, differentiation, and effector functions of inflammatory T cells and innate immune cells and participate in the regulation of inflammasomes. 406
In addition to NF‐κB, the activation of other transcription factors such as AP1 and STAT3 can also induce the expression of chemokines, which recruit more immune cells (including T cells, B cells, macrophages, neutrophils, etc.) to aggravate the inflammatory response further. 380 , 407 As early as 2004, two studies showed the key roles of NF‐κB in inflammation‐driven colitis‐associated cancer and HCC. 229 , 408 These results indicate that tumorigenic pathogens cause chronic infection and inflammation, and chronic inflammation sequentially leads to malignant tumors by increasing cellular stress response and recruiting inflammatory immune cells. 5 , 409 However, chronic inflammation does not necessarily lead to cancer. As a chronic inflammatory disease, IBD is associated with colorectal cancer, but RA and psoriasis are chronic inflammatory diseases without obvious tumor outcomes. 410 Another relevant evidence is that the loss of IKKβ in myeloid cells suppresses experimental colitis and colitis‐related cancers. 229 Another area worthy of attention is tertiary lymphoid tissues. Clinical studies have shown that there are tertiary lymphoid tissues in the tumor microenvironment, and the presence of tertiary lymphoid tissues suggests a better prognosis. 411 , 412 The formation of tertiary lymphoid tissues is achieved by LTβR‐mediated noncanonical NF‐κB activation pathway to induce the expression of adhesion molecules and chemokines (like CCL19, CCL21, CXCL12, and CXCL13). 413 , 414 In general, the relationship between the NF‐κB signaling pathway and inflammation is profound and complex, and the precise mechanisms of NF‐κB, inflammation, and cancer in the current context need to be further studied. The functions of NF‐κB in the cross‐talking between immune cells and tumor cells are shown in Figure 5.
FIGURE 5.
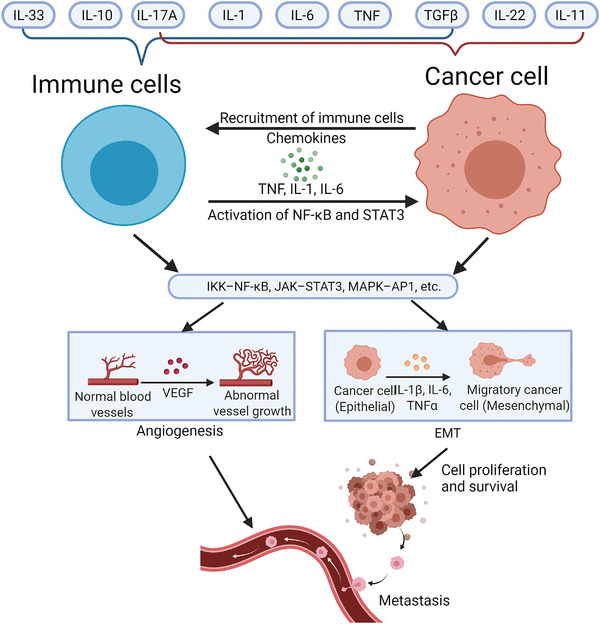
NF‐κB and inflammation in cancer. NF‐κB is involved in the interaction between immune cells and tumor cells. Cytokines such as TNFα, IL‐1, IL‐6, IL‐17A, and transforming growth factor‐β target both cancer cells and immune cells. IL‐1 and IL‐22 mostly target cancer cells, whereas IL‐10 and IL‐33 mainly act on immune cells. These cytokines activate the IκB kinase (IKK)‐NF‐κB, JAK‐signal transducer and activator of transcription 3 (STAT3), and MAPK‐AP1 signaling pathways of immune cells and cancer cells. The activation of NF‐κB in immune cells produces pro‐inflammatory cytokines, chemokines, and growth factors, such as TNFα, IL‐1, IL‐6, and vascular endothelial growth factor, thereby maintaining chronic inflammation and promoting angiogenesis. In cancer cells, pro‐inflammatory cytokines activate NF‐κB and STAT3 pathways, thereby stimulating cancer cell proliferation and survival, epithelial to mesenchymal transformation, invasion, angiogenesis, and metastasis
4.7. The therapeutic application of NF‐κB in cancer
With emerging research on NF‐κB and tumors, it is generally believed that NF‐κB is a valuable target for human cancer treatment. Considering the tumor‐promoting effects of NF‐κB, the current therapeutic strategy is inhibiting the activation of NF‐κB. These inhibitors include microbial and viral proteins, microRNA, non‐coding RNA, antioxidants, engineered active peptides, and various natural products to downregulate the canonical and noncanonical NF‐κB pathways. 415 Due to the complexity of upstream stimulators and downstream target genes of NF‐κB, more than 1000 inhibitors could block the NF‐κB signaling pathway. 416 Most of these inhibitors are found to have certain anti‐tumor effects using tumor cell models or animal models; their effects on human cancer treatment are unknown. Efforts have been made to develop specific and effective inhibitors with few side effects. A good example is bortezomib, a proteasome inhibitor, which has shown the inhibitory effect in MM. 417 As mentioned earlier, NF‐κB is continuously activated in myeloma. Therefore, as a proteasome inhibitor, bortezomib was conceived to inhibit the degradation of ubiquitinated IκB, allowing IκB to bind to NF‐κB dimers and stay in the cytoplasm to inhibit transcription factor activity. In addition to bortezomib, carfilzomib and ixazomib are also approved as proteasome inhibitors for MM treatment by inhibiting NF‐κB. 296 Two glucocorticoids prednisone and dexamethasone are also approved for the clinical treatment of MM. The treatment mechanism is to interfere with the phosphorylation of RNA polymerase II required for NF‐κB‐dependent transcription initiation. 418 Except for inhibiting critical components of the NF‐κB pathway, blocking its downstream target genes or upstream stimuli is also a practicable therapeutic strategy. For example, denosumab, a monoclonal antibody that inhibits RANK ligand, and immunomodulatory drugs thalidomide, lenalidomide, and pomalidomide, which inhibit TNFα and IL‐1β, are also approved for the treatment of MM. 419 , 420 Similarly, in some DLBCL subtypes that exhibit continuous NF‐κB activation, BTK inhibitor has also been shown to have therapeutic value as a key molecule for BCR to activate the NF‐κB pathway. 421 Other anti‐TNFα antibodies, including infliximab, adalimumab, and golimumab, have also been found to improve the therapeutic effect in breast cancer patients. 422
Considering that these drugs do not directly target the core molecules of the NF‐κB pathway, but their upstream and downstream molecules, it is difficult to clarify how much the inhibitor functions on NF‐κB per se. Except for MM and some DLBCL subtypes, the strategy of directly inhibiting NF‐κB seems unsuccessful in other cancers. The success of the drugs mentioned above in MM and DLBCL cannot be replicated in other tumor treatments, suggesting NF‐κB has universal and complicated functions that could not be a fixed therapeutic target like PD‐1/PD‐L. Inhibition of NF‐κB may enhance the cancer treatment response to most traditional treatments and treatments developed in recent years such as immune checkpoint inhibitors; however, long‐term inhibition of the NF‐κB pathway perhaps causes serious side effects, especially impaired immune response and compensated anti‐tumor immune function. 423 Given that NF‐κB regulates many physiological functions, the balance between the anti‐cancer effect and the side effects that impair normal physiological functions should be considered. Moreover, under drug‐mediated pressure, NF‐κB might cooperate with other signaling pathways to regulate pro‐survival genes (such as cyclin D1 and Bcl‐2) and upregulate the transcription of the genes, which helps tumor cells to develop drug resistance. Therefore, inhibiting NF‐κB alone without affecting other signaling pathways seems to be an unachievable presence.
One alternative strategy is to combine NF‐κB inhibitors with other treatments (such as chemotherapy and radiotherapy). For example, pentoxifylline (an REL inhibitor) combined with PD‐1 immunotherapy enhances the therapeutic effect. 424 The combined usage of multiple NF‐κB inhibitors is also one of the feasible strategies. The study has reported using multiple NF‐κB inhibitors in combination and found that it improves the clinical treatment effect in MM. 425 At present, there are many studies on NF‐κB inhibitors, which will not be summarized and discussed in this review, but most of the studies are conducted on in vitro cell models and animal models, and their value of human cancer treatment warrants further investigation. Recent clinical trials of cancer treatment targeting the NF‐κB signaling pathway are summarized in Table 2.
TABLE 2.
Summary of part of recent NF‐κB pathway‐related inhibitor clinical trials
| Cancer type | Target | Main drug | Phase | NCT number | Enrollment | Ref |
|---|---|---|---|---|---|---|
| MM | 20S PI | Ixazomib | III | NCT01564537 | 722 | 425 |
| KRAS G12D‐mutant/p53‐deficient NSCLC | 26S PI | Bortezomib | II | NCT01833143 | 16 | 448 |
| Classic Hodgkin lymphoma | 26S PI | Bortezomib | II | NCT00967369 | 20 | / |
| Head and neck cancer | 26S PI | Bortezomib | I | NCT00011778 | 25 | / |
| MM | SINE+26S PI | Selinexor + bortezomib + dexamethasone | II | NCT02343042 | 42 | 449 |
| Head and neck adenoid cystic carcinoma | 26S PI | Bortezomib | II | NCT00077428 | 25 | 450 |
| Advanced oesophagogastric adenocarcinoma | 26S PI | Bortezomib | I | / | 18 | 451 |
| Advanced gastric adenocarcinoma | 26S PI | Bortezomib | II | / | 16 | 452 |
| Endocrine‐resistant metastatic breast cancer | 26S PI | Bortezomib | II | / | 9 | 453 |
| Locally recurrent or metastatic squamous cell carcinoma of the head and neck | 26S PI | Bortezomib | II | / | 61 | 454 |
| Malignant gliomas | 26S PI + DNA alkylating agents | Bortezomib + Temozolomide | II | / | 10 | 455 |
| Plasma cell myeloma | 20S PI | Ixazomib | II | NCT02765854 | 90 | / |
| Waldenström's macroglobulinemia | 20S PI | Ixazomib | II | NCT02400437 | 26 | 456 |
| Waldenström's macroglobulinemia | 20S PI+BTK | Carfilzomib + ibrutinib | III | NCT04263480 | 184 | / |
| Advanced solid tumors | Na‐K ATPase | PBI‐05204 | I | / | 46 | 457 |
| CLL | BTK | Ibrutinib | II | NCT01500733 | 86 | 458 |
| Early‐stage chronic lymphocytic leukemia | NF‐κB inhibitor | Omega‐3 fatty acids | II | NCT00899353 | 16 | 459 |
| Advanced solid tumors and lymphomas | IKK inhibitor | Bardoxolone methyl | I | / | 44 | 460 |
| Primary CNS lymphoma | BTK | Ibrutinib | I | NCT02315326 | 13 | 461 |
| Waldenström's macroglobulinemia | BTK | BGB‐3111 | III | NCT03053440 | 229 | / |
| NSCLC | RANKL | Denosumab | III | NCT02129699 | 509 | 462 |
| Giant cell tumor of bone | RANKL | Denosumab | III | NCT03259152 | 30 | / |
| Urothelial carcinoma | RANKL | Denosumab | II | NCT03520231 | 50 | / |
| Melanoma | RANKL | Denosumab | I | NCT03161756 | 72 | / |
| MM | BCL‐2 | Venetoclax | I/II | NCT01794520 | 51 | 463 |
| MM | IL‐1R | Anakinra | I | NCT02492750 | 14 | / |
| ALL | Phosphodiesterase | Pentoxifylline | III | NCT02451774 | 44 | / |
| Triple negative breast cancer | CD40 | CDX‐1140 | I | NCT05029999 | 45 | / |
| Metastatic melanoma | CD40 | APX005M | I | NCT03597282 | 22 | / |
| NSCLC | CD40 | APX005M | I | NCT03123783 | 400 | / |
| Soft tissue sarcoma | CD40 | APX005M | II | NCT03719430 | 27 | |
| Metastatic pancreatic adenocarcinoma | CD40 | APX005M | I | NCT03214250 | 129 | 464 |
| Melanoma | CD40 | SEA‐CD40 | II | NCT04993677 | 200 | / |
| NSCLC | CD40 | SEA‐CD40 | I | NCT02376699 | 159 | / |
| Melanoma | CD40 | CP‐870,893 | I | NCT01103635 | 25 | / |
| DLBCL | CD40 | SGN‐40 | II | NCT00529503 | 151 | / |
| Non‐Hodgkin lymphoma, DLBCL | BAFF‐R | VAY736 | I | NCT04903197 | 86 | / |
Abbreviations: ALL, acute lymphoblastic leukemia; BTK, Bruton's tyrosine kinase; CLL, chronic lymphocytic leukemia; diffuse large B‐cell lymphoma. All NCT numbers were obtained from https://clinicaltrials.gov/; IKK, IkappaB kinases; MM, multiple myeloma; NSCLC, non‐small cell lung cancer; PI, proteasome inhibitor; RANKL, receptor activator of NF‐κB ligand; SINE, selective inhibitor of nuclear export.
5. CONCLUSION
Since NF‐κB was discovered in 1984, extraordinary efforts have been being made to understand the functions and regulating mechanisms of NF‐κB for 35 years, leading to significant progress. There has been convincing evidence to support the connection between NF‐κB, cancer, and inflammation, and it has been accepted that the NF‐κB pathway plays a key role in immune homeostasis, chronic inflammation, tumorigenesis, and development. Based on the numerous upstream stimuli and downstream target genes of NF‐κB currently known, we conclude that NF‐κB has a wide range of functions, like a hub with a huge communication network. Nevertheless, there are still many unknowns about the comprehensive mechanisms and functions of NF‐κB that we need to further explore. Recently, more and more drugs targeting NF‐κB have been developed and examined. It should be noted that considering the diversity of upstream stimulus and downstream target genes of NF‐κB, to complete inhibition of NF‐κB as a therapeutic method might not be a practicable strategy. Future investigation on NF‐κB as an anti‐cancer target should focus on inhibiting its tumor‐promoting effects caused by pathological NF‐κB activation while avoiding affecting their normal physiological functions; meanwhile, the combination of multiple drugs targeting different key regulators of the NF‐κB pathway, even multiple pathways, is also attractive.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors declare that ethics approval was not needed for this study.
AUTHOR CONTRIBUTIONS
T.Z., H.Z., and H.H. conceived, wrote, and edited the manuscript; C.M. and Z.Z. provided significant assistance.
ACKNOWLEDGMENTS
We wish to thank the timely help given by Dr. Yang Wang and Ms. Yueyi Li for composing the figures. All figures are created with BioRender.com.
Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF‐κB signaling in inflammation and cancer. MedComm. 2021;2:618–653. 10.1002/mco2.104
Contributor Information
Huiyuan Zhang, Email: hyzhang@scu.edu.cn.
Hongbo Hu, Email: hongbohu@scu.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer‐binding protein Nf‐kappa B by a posttranslational mechanism. Cell. 1986;47(6):921‐928. [DOI] [PubMed] [Google Scholar]
- 2. Bottex‐Gauthier C, Pollet S, Favier A, Vidal DR. The Rel/NF‐kappa‐B transcription factors: complex role in cell regulation. Pathol Biol (Paris). 2002;50(3):204‐211. [DOI] [PubMed] [Google Scholar]
- 3. Perkins ND. The Rel/NF‐kappa B family: friend and foe. Trends Biochem Sci. 2000;25(9):434‐440. [DOI] [PubMed] [Google Scholar]
- 4. Liu T, Zhang L, Joo D, Sun SC. NF‐κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taniguchi K, Karin M. NF‐κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309‐324. [DOI] [PubMed] [Google Scholar]
- 6. Sun SC, Chang JH, Jin J. Regulation of nuclear factor‐κB in autoimmunity. Trends Immunol. 2013;34(6):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilmore TD. NF‐κB and human cancer: what have we learned over the past 35 years? Biomedicines. 2021;9(8):889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wertz IE, Dixit VM. Signaling to NF‐kappaB: regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2(3):a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayden MS, Ghosh S. Shared principles in NF‐kappaB signaling. Cell. 2008;132(3):344‐362. [DOI] [PubMed] [Google Scholar]
- 10. Chen LF, Greene WC. Shaping the nuclear action of NF‐kappaB. Nat Rev Mol Cell Biol. 2004;5(5):392‐401. [DOI] [PubMed] [Google Scholar]
- 11. Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF‐kappaB activity. Faseb J. 2007;21(11):2642‐2654. [DOI] [PubMed] [Google Scholar]
- 12. Mulero MC, Wang VY, Huxford T, Ghosh G. Genome reading by the NF‐κB transcription factors. Nucleic Acids Res. 2019;47(19):9967‐9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji Z, He L, Regev A, Struhl K. Inflammatory regulatory network mediated by the joint action of NF‐kB, STAT3, and AP‐1 factors is involved in many human cancers. Proc Natl Acad Sci U S A. 2019;116(19):9453‐9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuster M, Annemann M, Plaza‐Sirvent C, Schmitz I. Atypical IκB proteins–nuclear modulators of NF‐κB signaling. Cell Commun Signal. 2013;11(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun SC. Non‐canonical NF‐κB signaling pathway. Cell Res. 2011;21(1):71‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karin M, Greten FR. NF‐kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749‐759. [DOI] [PubMed] [Google Scholar]
- 17. Israël A. The IKK complex, a central regulator of NF‐kappaB activation. Cold Spring Harb Perspect Biol. 2010;2(3):a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beinke S, Ley SC. Functions of NF‐kappaB1 and NF‐kappaB2 in immune cell biology. Biochem J. 2004;382(Pt 2):393‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mussbacher M, Salzmann M, Brostjan C, et al. Cell type‐specific roles of NF‐κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napetschnig J, Wu H. Molecular basis of NF‐κB signaling. Annu Rev Biophys. 2013;42:443‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vallabhapurapu S, Karin M. Regulation and function of NF‐kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693‐733. [DOI] [PubMed] [Google Scholar]
- 22. Sun SC, Ley SC. New insights into NF‐kappaB regulation and function. Trends Immunol. 2008;29(10):469‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruland J. Return to homeostasis: downregulation of NF‐κB responses. Nat Immunol. 2011;12(8):709‐714. [DOI] [PubMed] [Google Scholar]
- 24. Rao P, Hayden MS, Long M, et al. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature. 2010;466(7310):1115‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karin M. Nuclear factor‐kappaB in cancer development and progression. Nature. 2006;441(7092):431‐436. [DOI] [PubMed] [Google Scholar]
- 26. Häcker H, Karin M. Regulation and function of IKK and IKK‐related kinases. Sci STKE. 2006;2006(357):re13. [DOI] [PubMed] [Google Scholar]
- 27. Zhang H, Sun SC. NF‐κB in inflammation and renal diseases. Cell Biosci. 2015;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawai T, Akira S. The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat Immunol. 2010;11(5):373‐384. [DOI] [PubMed] [Google Scholar]
- 29. Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF‐κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cildir G, Low KC, Tergaonkar V. Noncanonical NF‐κB signaling in health and disease. Trends Mol Med. 2016;22(5):414‐429. [DOI] [PubMed] [Google Scholar]
- 31. Maubach G, Feige MH, Lim MCC, Naumann M. NF‐kappaB‐inducing kinase in cancer. Biochim Biophys Acta Rev Cancer. 2019;1871(1):40‐49. [DOI] [PubMed] [Google Scholar]
- 32. Sun SC. The noncanonical NF‐κB pathway. Immunol Rev. 2012;246(1):125‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vallabhapurapu S, Matsuzawa A, Zhang W, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK‐dependent alternative NF‐kappaB signaling. Nat Immunol. 2008;9(12):1364‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonizzi G, Karin M. The two NF‐kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280‐288. [DOI] [PubMed] [Google Scholar]
- 35. Xiao G, Harhaj EW, Sun SC. NF‐kappaB‐inducing kinase regulates the processing of NF‐kappaB2 p100. Mol Cell. 2001;7(2):401‐409. [DOI] [PubMed] [Google Scholar]
- 36. Betts JC, Nabel GJ. Differential regulation of NF‐kappaB2(p100) processing and control by amino‐terminal sequences. Mol Cell Biol. 1996;16(11):6363‐6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang C, Zhang M, Sun SC. Beta‐TrCP binding and processing of NF‐kappaB2/p100 involve its phosphorylation at serines 866 and 870. Cell Signal. 2006;18(8):1309‐1317. [DOI] [PubMed] [Google Scholar]
- 38. Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF‐kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF‐κB signaling pathways. Nat Immunol. 2011;12(8):695‐708. [DOI] [PubMed] [Google Scholar]
- 40. Zhong B, Tien P, Shu HB. Innate immune responses: crosstalk of signaling and regulation of gene transcription. Virology. 2006;352(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 41. Sun SC, Ganchi PA, Ballard DW, Greene WC. NF‐kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259(5103):1912‐1915. [DOI] [PubMed] [Google Scholar]
- 42. Tam WF, Sen R. IkappaB family members function by different mechanisms. J Biol Chem. 2001;276(11):7701‐7704. [DOI] [PubMed] [Google Scholar]
- 43. Whiteside ST, Epinat JC, Rice NR, Israël A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF‐kappa B activity. Embo J. 1997;16(6):1413‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kearns JD, Basak S, Werner SL, Huang CS, Hoffmann A. IkappaBepsilon provides negative feedback to control NF‐kappaB oscillations, signaling dynamics, and inflammatory gene expression. J Cell Biol. 2006;173(5):659‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scheibel M, Klein B, Merkle H, et al. IkappaBbeta is an essential co‐activator for LPS‐induced IL‐1beta transcription in vivo. J Exp Med. 2010;207(12):2621‐2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamura T, Sato S, Haque D, et al. The Elongin BC complex interacts with the conserved SOCS‐box motif present in members of the SOCS, ras, WD‐40 repeat, and ankyrin repeat families. Genes Dev. 1998;12(24):3872‐3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakagawa R, Naka T, Tsutsui H, et al. SOCS‐1 participates in negative regulation of LPS responses. Immunity. 2002;17(5):677‐687. [DOI] [PubMed] [Google Scholar]
- 48. Kinjyo I, Hanada T, Inagaki‐Ohara K, et al. SOCS1/JAB is a negative regulator of LPS‐induced macrophage activation. Immunity. 2002;17(5):583‐591. [DOI] [PubMed] [Google Scholar]
- 49. Torrado M, Senatorov VV, Trivedi R, Fariss RN, Tomarev SI. Pdlim2, a novel PDZ‐LIM domain protein, interacts with alpha‐actinins and filamin A. Invest Ophthalmol Vis Sci. 2004;45(11):3955‐3963. [DOI] [PubMed] [Google Scholar]
- 50. Tanaka T, Grusby MJ, Kaisho T. PDLIM2‐mediated termination of transcription factor NF‐kappaB activation by intranuclear sequestration and degradation of the p65 subunit. Nat Immunol. 2007;8(6):584‐591. [DOI] [PubMed] [Google Scholar]
- 51. Liu B, Mink S, Wong KA, et al. PIAS1 selectively inhibits interferon‐inducible genes and is important in innate immunity. Nat Immunol. 2004;5(9):891‐898. [DOI] [PubMed] [Google Scholar]
- 52. Liu B, Yang Y, Chernishof V, et al. Proinflammatory stimuli induce IKKalpha‐mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129(5):903‐914. [DOI] [PubMed] [Google Scholar]
- 53. Liu B, Yang R, Wong KA, et al. Negative regulation of NF‐kappaB signaling by PIAS1. Mol Cell Biol. 2005;25(3):1113‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deng L, Wang C, Spencer E, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin‐conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103(2):351‐361. [DOI] [PubMed] [Google Scholar]
- 55. Wang C, Deng L, Hong M, et al. TAK1 is a ubiquitin‐dependent kinase of MKK and IKK. Nature. 2001;412(6844):346‐351. [DOI] [PubMed] [Google Scholar]
- 56. Xia ZP, Sun L, Chen X, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461(7260):114‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen ZJ. Ubiquitin signalling in the NF‐kappaB pathway. Nat Cell Biol. 2005;7(8):758‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kanayama A, Seth RB, Sun L, et al. TAB2 and TAB3 activate the NF‐kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15(4):535‐548. [DOI] [PubMed] [Google Scholar]
- 59. Mahoney DJ, Cheung HH, Mrad RL, et al. Both cIAP1 and cIAP2 regulate TNFalpha‐mediated NF‐kappaB activation. Proc Natl Acad Sci U S A. 2008;105(33):11778‐11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Busino L, Millman SE, Scotto L, et al. Fbxw7α‐ and GSK3‐mediated degradation of p100 is a pro‐survival mechanism in multiple myeloma. Nat Cell Biol. 2012;14(4):375‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wertz IE, O'Rourke KM, Zhou H, et al. De‐ubiquitination and ubiquitin ligase domains of A20 downregulate NF‐kappaB signalling. Nature. 2004;430(7000):694‐699. [DOI] [PubMed] [Google Scholar]
- 62. Boone DL, Turer EE, Lee EG, et al. The ubiquitin‐modifying enzyme A20 is required for termination of Toll‐like receptor responses. Nat Immunol. 2004;5(10):1052‐1060. [DOI] [PubMed] [Google Scholar]
- 63. Hitotsumatsu O, Ahmad RC, Tavares R, et al. The ubiquitin‐editing enzyme A20 restricts nucleotide‐binding oligomerization domain containing 2‐triggered signals. Immunity. 2008;28(3):381‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF‐induced NF‐kappaB and cell death responses in A20‐deficient mice. Science. 2000;289(5488):2350‐2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shembade N, Harhaj NS, Parvatiyar K, et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin‐editing enzyme A20. Nat Immunol. 2008;9(3):254‐262. [DOI] [PubMed] [Google Scholar]
- 66. Hymowitz SG, Wertz IE. A20: from ubiquitin editing to tumour suppression. Nat Rev Cancer. 2010;10(5):332‐341. [DOI] [PubMed] [Google Scholar]
- 67. Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF‐kappaB. Nature. 2003;424(6950):797‐801. [DOI] [PubMed] [Google Scholar]
- 68. Trompouki E, Hatzivassiliou E, Tsichritzis T, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF‐kappaB activation by TNFR family members. Nature. 2003;424(6950):793‐796. [DOI] [PubMed] [Google Scholar]
- 69. Massoumi R. CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011;7(2):285‐297. [DOI] [PubMed] [Google Scholar]
- 70. Hu H, Brittain GC, Chang JH, et al. OTUD7B controls non‐canonical NF‐κB activation through deubiquitination of TRAF3. Nature. 2013;494(7437):371‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li Y, Yang JY, Xie X, et al. Preventing abnormal NF‐κB activation and autoimmunity by Otub1‐mediated p100 stabilization. Cell Res. 2019;29(6):474‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun SC. Controlling the fate of NIK: a central stage in noncanonical NF‐kappaB signaling. Sci Signal. 2010;3(123):pe18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF‐kappaB‐inducing kinase by tumor necrosis factor receptor‐associated factor 3‐induced degradation. J Biol Chem. 2004;279(25):26243‐26250. [DOI] [PubMed] [Google Scholar]
- 74. Zarnegar BJ, Wang Y, Mahoney DJ, et al. Noncanonical NF‐kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9(12):1371‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bram RJ. TBK1 suppression of IgA in the NIK of time. Nat Immunol. 2012;13(11):1027‐1029. [DOI] [PubMed] [Google Scholar]
- 76. Jin J, Xiao Y, Chang JH, et al. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF‐κB signaling. Nat Immunol. 2012;13(11):1101‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pomerantz JL, Baltimore D. NF‐kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK‐related kinase. Embo J. 1999;18(23):6694‐6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Razani B, Zarnegar B, Ytterberg AJ, et al. Negative feedback in noncanonical NF‐kappaB signaling modulates NIK stability through IKKalpha‐mediated phosphorylation. Sci Signal. 2010;3(123):ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Barnabei L, Laplantine E, Mbongo W, Rieux‐Laucat F, Weil R. NF‐κB: at the borders of autoimmunity and inflammation. Front Immunol. 2021;12:716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Saleh HA, Yousef MH, Abdelnaser A. The anti‐inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF‐κB‐mediated inflammation. Front Immunol. 2021;12:606069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3):a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805‐820. [DOI] [PubMed] [Google Scholar]
- 84. Gong T, Liu L, Jiang W, Zhou R. DAMP‐sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95‐112. [DOI] [PubMed] [Google Scholar]
- 85. Cao X. Self‐regulation and cross‐regulation of pattern‐recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16(1):35‐50. [DOI] [PubMed] [Google Scholar]
- 86. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16‐34. [DOI] [PubMed] [Google Scholar]
- 87. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783‐801. [DOI] [PubMed] [Google Scholar]
- 88. Hayden MS, Ghosh S. NF‐κB in immunobiology. Cell Res. 2011;21(2):223‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ebner K, Bandion A, Binder BR, de Martin R, Schmid JA. GMCSF activates NF‐kappaB via direct interaction of the GMCSF receptor with IkappaB kinase beta. Blood. 2003;102(1):192‐199. [DOI] [PubMed] [Google Scholar]
- 90. Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R. Genetic approaches in mice to understand Rel/NF‐kappaB and IkappaB function: transgenics and knockouts. Oncogene. 1999;18(49):6888‐6895. [DOI] [PubMed] [Google Scholar]
- 91. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1‐M2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209‐212. [DOI] [PubMed] [Google Scholar]
- 94. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 95. Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS‐TLR4 signaling to IRF‐3/7 and NF‐kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198(7):1043‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akira S, Takeda K. Toll‐like receptor signalling. Nat Rev Immunol. 2004;4(7):499‐511. [DOI] [PubMed] [Google Scholar]
- 97. Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR‐associated factor (TRAF) 6 is essential for MyD88‐dependent pathway but not toll/IL‐1 receptor domain‐containing adaptor‐inducing IFN‐beta (TRIF)‐dependent pathway in TLR signaling. J Immunol. 2004;173(5):2913‐2917. [DOI] [PubMed] [Google Scholar]
- 98. Kawagoe T, Sato S, Matsushita K, et al. Sequential control of Toll‐like receptor‐dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9(6):684‐691. [DOI] [PubMed] [Google Scholar]
- 99. Barton GM, Kagan JC. A cell biological view of Toll‐like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9(8):535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6(11):1087‐1095. [DOI] [PubMed] [Google Scholar]
- 101. Kumar H, Kawai T, Akira S. Toll‐like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621‐625. [DOI] [PubMed] [Google Scholar]
- 102. Kawai T, Akira S. Signaling to NF‐kappaB by Toll‐like receptors. Trends Mol Med. 2007;13(11):460‐469. [DOI] [PubMed] [Google Scholar]
- 103. Hallam S, Escorcio‐Correia M, Soper R, Schultheiss A, Hagemann T. Activated macrophages in the tumour microenvironment‐dancing to the tune of TLR and NF‐kappaB. J Pathol. 2009;219(2):143‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Courtine E, Cagnard N, Mazzolini J, et al. Combined loss of cRel/p50 subunits of NF‐κB leads to impaired innate host response in sepsis. Innate Immun. 2012;18(5):753‐763. [DOI] [PubMed] [Google Scholar]
- 105. Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17‐58. [DOI] [PubMed] [Google Scholar]
- 106. Shih VF, Davis‐Turak J, Macal M, et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF‐κB pathways. Nat Immunol. 2012;13(12):1162‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Summers deLuca L, Gommerman JL. Fine‐tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol. 2012;12(5):339‐351. [DOI] [PubMed] [Google Scholar]
- 108. Gerondakis S, Grumont R, Gugasyan R, et al. Unravelling the complexities of the NF‐kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25(51):6781‐6799. [DOI] [PubMed] [Google Scholar]
- 109. Seki T, Yamamoto M, Taguchi Y, et al. Visualization of RelB expression and activation at the single‐cell level during dendritic cell maturation in Relb‐Venus knock‐in mice. J Biochem. 2015;158(6):485‐495. [DOI] [PubMed] [Google Scholar]
- 110. Li M, Zhang X, Zheng X, et al. Immune modulation and tolerance induction by RelB‐silenced dendritic cells through RNA interference. J Immunol. 2007;178(9):5480‐5487. [DOI] [PubMed] [Google Scholar]
- 111. Nair PM, Starkey MR, Haw TJ, et al. RelB‐deficient dendritic cells promote the development of spontaneous allergic airway inflammation. Am J Respir Cell Mol Biol. 2018;58(3):352‐365. [DOI] [PubMed] [Google Scholar]
- 112. Ammon C, Mondal K, Andreesen R, Krause SW. Differential expression of the transcription factor NF‐kappaB during human mononuclear phagocyte differentiation to macrophages and dendritic cells. Biochem Biophys Res Commun. 2000;268(1):99‐105. [DOI] [PubMed] [Google Scholar]
- 113. Kobayashi T, Walsh PT, Walsh MC, et al. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19(3):353‐363. [DOI] [PubMed] [Google Scholar]
- 114. Baratin M, Foray C, Demaria O, et al. Homeostatic NF‐κB signaling in steady‐state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity. 2015;42(4):627‐639. [DOI] [PubMed] [Google Scholar]
- 115. Krebs CF, Steinmetz OM. CD4(+) T cell fate in glomerulonephritis: a tale of Th1, Th17, and novel treg subtypes. Mediators Inflamm. 2016;2016:5393894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Golubovskaya V, Wu L. Different subsets of T Cells, memory, effector functions, and CAR‐T immunotherapy. Cancers (Basel). 2016;8(3):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Walker JA, McKenzie ANJ. T(H)2 cell development and function. Nat Rev Immunol. 2018;18(2):121‐133. [DOI] [PubMed] [Google Scholar]
- 119. Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011;132(4):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19(3):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8(+) T cell differentiation. Nat Rev Immunol. 2018;18(5):340‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Opferman JT, Ober BT, PG Ashton‐Rickardt. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283(5408):1745‐1748. [DOI] [PubMed] [Google Scholar]
- 123. Youngblood B, Hale JS, Kissick HT, et al. Effector CD8 T cells dedifferentiate into long‐lived memory cells. Nature. 2017;552(7685):404‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Seifert M, Küppers R. Human memory B cells. Leukemia. 2016;30(12):2283‐2292. [DOI] [PubMed] [Google Scholar]
- 126. Murray SE, Polesso F, Rowe AM, et al. NF‐κB–inducing kinase plays an essential T cell–intrinsic role in graft‐versus‐host disease and lethal autoimmunity in mice. J Clin Invest. 2011;121(12):4775‐4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Y, Wang H, Zhou X, et al. Cell intrinsic role of NF‐κB‐inducing kinase in regulating T cell‐mediated immune and autoimmune responses. Sci Rep. 2016;6:22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yu J, Wang Y, Yan F, et al. Noncanonical NF‐κB activation mediates STAT3‐stimulated IDO upregulation in myeloid‐derived suppressor cells in breast cancer. J Immunol. 2014;193(5):2574‐2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rowe AM, Murray SE, Raué HP, et al. A cell‐intrinsic requirement for NF‐κB‐inducing kinase in CD4 and CD8 T cell memory. J Immunol. 2013;191(7):3663‐3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sun SC. The non‐canonical NF‐κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17(9):545‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hu H, Sun SC. Ubiquitin signaling in immune responses. Cell Res. 2016;26(4):457‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Thome M. CARMA1, BCL‐10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4(5):348‐359. [DOI] [PubMed] [Google Scholar]
- 133. Roncagalli R, Mingueneau M, Grégoire C, Malissen M, Malissen B. LAT signaling pathology: an “autoimmune” condition without T cell self‐reactivity. Trends Immunol. 2010;31(7):253‐259. [DOI] [PubMed] [Google Scholar]
- 134. Croft M. The role of TNF superfamily members in T‐cell function and diseases. Nat Rev Immunol. 2009;9(4):271‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Elewaut D, Shaikh RB, Hammond KJ, et al. NIK‐dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med. 2003;197(12):1623‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197(12):1613‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mair F, Joller S, Hoeppli R, et al. The NFκB‐inducing kinase is essential for the developmental programming of skin‐resident and IL‐17‐producing γδ T cells. Elife. 2015;4:e10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aronica MA, Mora AL, Mitchell DB, et al. Preferential role for NF‐kappa B/Rel signaling in the type 1 but not type 2 T cell‐dependent immune response in vivo. J Immunol. 1999;163(9):5116‐5124. [PubMed] [Google Scholar]
- 139. Hilliard BA, Mason N, Xu L, et al. Critical roles of c‐Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110(6):843‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Balasubramani A, Mukasa R, Hatton RD, Weaver CT. Regulation of the Ifng locus in the context of T‐lineage specification and plasticity. Immunol Rev. 2010;238(1):216‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li‐Weber M, Giaisi M, Baumann S, Pálfi K, Krammer PH. NF‐kappa B synergizes with NF‐AT and NF‐IL6 in activation of the IL‐4 gene in T cells. Eur J Immunol. 2004;34(4):1111‐1118. [DOI] [PubMed] [Google Scholar]
- 142. Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50(1):87‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Greve B, Weissert R, Hamdi N, et al. I kappa B kinase 2/beta deficiency controls expansion of autoreactive T cells and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2007;179(1):179‐185. [DOI] [PubMed] [Google Scholar]
- 144. Brüstle A, Brenner D, Knobbe CB, et al. The NF‐κB regulator MALT1 determines the encephalitogenic potential of Th17 cells. J Clin Invest. 2012;122(12):4698‐4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Molinero LL, Cubre A, Mora‐Solano C, Wang Y, Alegre ML. T cell receptor/CARMA1/NF‐κB signaling controls T‐helper (Th) 17 differentiation. Proc Natl Acad Sci U S A. 2012;109(45):18529‐18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chen G, Hardy K, Bunting K, et al. Regulation of the IL‐21 gene by the NF‐κB transcription factor c‐Rel. J Immunol. 2010;185(4):2350‐2359. [DOI] [PubMed] [Google Scholar]
- 147. Yu J, Zhou X, Nakaya M, et al. T cell‐intrinsic function of the noncanonical NF‐κB pathway in the regulation of GM‐CSF expression and experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2014;193(1):422‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xiao X, Balasubramanian S, Liu W, et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat Immunol. 2012;13(10):981‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T Cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302‐316. [DOI] [PubMed] [Google Scholar]
- 150. Isomura I, Palmer S, Grumont RJ, et al. c‐Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206(13):3001‐3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ruan Q, Kameswaran V, Tone Y, et al. Development of Foxp3(+) regulatory t cells is driven by the c‐Rel enhanceosome. Immunity. 2009;31(6):932‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor‐kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31(6):921‐931. [DOI] [PubMed] [Google Scholar]
- 153. Riemann M, Andreas N, Fedoseeva M, et al. Central immune tolerance depends on crosstalk between the classical and alternative NF‐κB pathways in medullary thymic epithelial cells. J Autoimmun. 2017;81:56‐67. [DOI] [PubMed] [Google Scholar]
- 154. Chang M, Lee AJ, Fitzpatrick L, Zhang M, Sun SC. NF‐kappa B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J Immunol. 2009;182(5):3131‐3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shi JH, Sun SC. TCR signaling to NF‐κB and mTORC1: expanding roles of the CARMA1 complex. Mol Immunol. 2015;68(2 Pt C):546‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Miraghazadeh B, Cook MC. Nuclear factor‐kappaB in autoimmunity: man and mouse. Front Immunol. 2018;9:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chang JH, Xiao Y, Hu H, et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector‐like T cells. Nat Immunol. 2012;13(5):481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhu M, Fu Y. The complicated role of NF‐kappaB in T‐cell selection. Cell Mol Immunol. 2010;7(2):89‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Murray SE. Cell‐intrinsic role for NF‐kappa B‐inducing kinase in peripheral maintenance but not thymic development of Foxp3+ regulatory T cells in mice. PLoS One. 2013;8(9):e76216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Feng B, Cheng S, Pear WS, Liou HC. NF‐kB inhibitor blocks B cell development at two checkpoints. Med Immunol. 2004;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gerondakis S, Siebenlist U. Roles of the NF‐kappaB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2(5):a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mesin L, Ersching J, Victora GD. Germinal center B Cell dynamics. Immunity. 2016;45(3):471‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Caamaño JH, Rizzo CA, Durham SK, et al. Nuclear factor (NF)‐kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell‐mediated immune responses. J Exp Med. 1998;187(2):185‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Franzoso G, Carlson L, Poljak L, et al. Mice deficient in nuclear factor (NF)‐kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187(2):147‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yamada T, Mitani T, Yorita K, et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF‐kappa B‐inducing kinase. J Immunol. 2000;165(2):804‐812. [DOI] [PubMed] [Google Scholar]
- 166. Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKalpha‐dependent signals. Proc Natl Acad Sci U S A. 2007;104(15):6359‐6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126(5):898‐907; quiz 908‐899. [DOI] [PubMed] [Google Scholar]
- 168. Denton AE, Linterman MA. Stromal networking: cellular connections in the germinal centre. Curr Opin Immunol. 2017;45:103‐111. [DOI] [PubMed] [Google Scholar]
- 169. Weih DS, Yilmaz ZB, Weih F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol. 2001;167(4):1909‐1919. [DOI] [PubMed] [Google Scholar]
- 170. Bannard O, Cyster JG. Germinal centers: programmed for affinity maturation and antibody diversification. Curr Opin Immunol. 2017;45:21‐30. [DOI] [PubMed] [Google Scholar]
- 171. Ramezani‐Rad P, Rickert RC. Murine models of germinal center derived‐lymphomas. Curr Opin Immunol. 2017;45:31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Suan D, Sundling C, Brink R. Plasma cell and memory B cell differentiation from the germinal center. Curr Opin Immunol. 2017;45:97‐102. [DOI] [PubMed] [Google Scholar]
- 173. Brightbill HD, Jackman JK, Suto E, et al. Conditional deletion of NF‐κB‐inducing Kinase (NIK) in adult mice disrupts mature B cell survival and activation. J Immunol. 2015;195(3):953‐964. [DOI] [PubMed] [Google Scholar]
- 174. Hahn M, Macht A, Waisman A, Hövelmeyer N. NF‐κB‐inducing kinase is essential for B‐cell maintenance in mice. Eur J Immunol. 2016;46(3):732‐741. [DOI] [PubMed] [Google Scholar]
- 175. De Silva NS, Anderson MM, Carette A, et al. Transcription factors of the alternative NF‐κB pathway are required for germinal center B‐cell development. Proc Natl Acad Sci U S A. 2016;113(32):9063‐9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jimi E, Takakura N, Hiura F, Nakamura I, Hirata‐Tsuchiya S. The role of NF‐κB in physiological bone development and inflammatory bone diseases: is NF‐κB inhibition “killing two birds with one stone”? Cells. 2019;8(12):1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205‐2219. [DOI] [PubMed] [Google Scholar]
- 178. Han Z, Boyle DL, Manning AM, Firestein GS. AP‐1 and NF‐kappaB regulation in rheumatoid arthritis and murine collagen‐induced arthritis. Autoimmunity. 1998;28(4):197‐208. [DOI] [PubMed] [Google Scholar]
- 179. Marok R, Winyard PG, Coumbe A, et al. Activation of the transcription factor nuclear factor‐kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996;39(4):583‐591. [DOI] [PubMed] [Google Scholar]
- 180. Gilston V, Jones HW, Soo CC, et al. NF‐kappa B activation in human knee‐joint synovial tissue during the early stage of joint inflammation. Biochem Soc Trans. 1997;25(3):518s. [DOI] [PubMed] [Google Scholar]
- 181. Asahara H, Asanuma M, Ogawa N, Nishibayashi S, Inoue H. High DNA‐binding activity of transcription factor NF‐kappa B in synovial membranes of patients with rheumatoid arthritis. Biochem Mol Biol Int. 1995;37(5):827‐832. [PubMed] [Google Scholar]
- 182. Vinay DS, Kwon BS. Targeting TNF superfamily members for therapeutic intervention in rheumatoid arthritis. Cytokine. 2012;57(3):305‐312. [DOI] [PubMed] [Google Scholar]
- 183. Noort AR, Tak PP, Tas SW. Non‐canonical NF‐κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Novack DV. Role of NF‐κB in the skeleton. Cell Res. 2011;21(1):169‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yao Z, Xing L, Boyce BF. NF‐kappaB p100 limits TNF‐induced bone resorption in mice by a TRAF3‐dependent mechanism. J Clin Invest. 2009;119(10):3024‐3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Baum R, Gravallese EM. Bone as a target organ in rheumatic disease: impact on osteoclasts and osteoblasts. Clin Rev Allergy Immunol. 2016;51(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Feldmann M, Maini RN. Anti‐TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163‐196. [DOI] [PubMed] [Google Scholar]
- 188. Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF‐kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford). 2008;47(5):584‐590. [DOI] [PubMed] [Google Scholar]
- 189. Davignon JL, Hayder M, Baron M, et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2013;52(4):590‐598. [DOI] [PubMed] [Google Scholar]
- 190. Selmi C. Autoimmunity in 2018. Clin Rev Allergy Immunol. 2019;56(3):375‐384. [DOI] [PubMed] [Google Scholar]
- 191. Favero M, Giusti A, Geusens P, et al. OsteoRheumatology: a new discipline? RMD Open. 2015;1(1):e000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ceccarelli F, Saccucci M, Di Carlo G, et al. Periodontitis and rheumatoid arthritis: the same inflammatory mediators? Mediators Inflamm. 2019;2019:6034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Maijer KI, Noort AR, de Hair MJ, et al. Nuclear factor‐κB‐inducing kinase is expressed in synovial endothelial cells in patients with early arthritis and correlates with markers of inflammation: a prospective cohort study. J Rheumatol. 2015;42(9):1573‐1581. [DOI] [PubMed] [Google Scholar]
- 194. Noort AR, van Zoest KP, Weijers EM, et al. NF‐κB‐inducing kinase is a key regulator of inflammation‐induced and tumour‐associated angiogenesis. J Pathol. 2014;234(3):375‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Aya K, Alhawagri M, Hagen‐Stapleton A, et al. NF‐(kappa)B‐inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115(7):1848‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Yang C, McCoy K, Davis JL, et al. NIK stabilization in osteoclasts results in osteoporosis and enhanced inflammatory osteolysis. PLoS One. 2010;5(11):e15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8(5):337‐348. [DOI] [PubMed] [Google Scholar]
- 198. Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine. 2015;76(2):537‐544. [DOI] [PubMed] [Google Scholar]
- 199. Hayden MS, Ghosh S. Regulation of NF‐κB by TNF family cytokines. Semin Immunol. 2014;26(3):253‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Jimi E, Ghosh S. Role of nuclear factor‐kappaB in the immune system and bone. Immunol Rev. 2005;208:80‐87. [DOI] [PubMed] [Google Scholar]
- 201. Abbasi M, Mousavi MJ, Jamalzehi S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019;234(7):10018‐10031. [DOI] [PubMed] [Google Scholar]
- 202. Li J, Bao J, Zeng J, et al. Iguratimod: a valuable remedy from the Asia Pacific region for ameliorating autoimmune diseases and protecting bone physiology. Bone Res. 2019;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Mimori T, Harigai M, Atsumi T, et al. Safety and effectiveness of iguratimod in patients with rheumatoid arthritis: final report of a 52‐week, multicenter postmarketing surveillance study. Mod Rheumatol. 2019;29(2):314‐323. [DOI] [PubMed] [Google Scholar]
- 204. Xia ZB, Yuan YJ, Zhang QH, et al. Salvianolic acid B suppresses inflammatory mediator levels by downregulating NF‐κB in a rat model of rheumatoid arthritis. Med Sci Monit. 2018;24:2524‐2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Uttra AM, Alamgeer, Shahzad M , Shabbir A, Jahan S. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund Complete Adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL‐1β, IL‐6, TNF‐α, NF‐kB and upregulating IL‐4 and IL‐10. J Ethnopharmacol. 2018;224:482‐496. [DOI] [PubMed] [Google Scholar]
- 206. Cao F, Huang C, Cheng J, He Z. β‐arrestin‐2 alleviates rheumatoid arthritis injury by suppressing NLRP3 inflammasome activation and NF‐ κB pathway in Macrophages. Bioengineered. Published online November 17, 2021. 10.1080/21655979.2021.2003678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Mortazavi‐Jahromi SS, Ahmadzadeh A, Rezaieyazdi Z, et al. The role of β‐d‐mannuronic acid, as a new non‐steroidal anti‐inflammatory drug on expression of miR‐146a, IRAK1, TRAF6, NF‐κB and pro‐inflammatory cytokines following a clinical trial in rheumatoid arthritis patients. Immunopharmacol Immunotoxicol. 2020;42(3):228‐236. [DOI] [PubMed] [Google Scholar]
- 208. Schmidt‐Supprian M, Bloch W, Courtois G, et al. NEMO/IKK gamma‐deficient mice model incontinentia pigmenti. Mol Cell. 2000;5(6):981‐992. [DOI] [PubMed] [Google Scholar]
- 209. Tanaka M, Fuentes ME, Yamaguchi K, et al. Embryonic lethality, liver degeneration, and impaired NF‐kappa B activation in IKK‐beta‐deficient mice. Immunity. 1999;10(4):421‐429. [DOI] [PubMed] [Google Scholar]
- 210. Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF‐kappa B. Nature. 1995;376(6536):167‐170. [DOI] [PubMed] [Google Scholar]
- 211. Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284(5412):321‐325. [DOI] [PubMed] [Google Scholar]
- 212. Dejban P, Rahimi N, Takzare N, Dehpour AR. Biochemical and histopathological evidence for the beneficial effects of modafinil on the rat model of inflammatory bowel disease: involvement of nitric oxide pathway. Pharmacol Rep. 2020;72(1):135‐146. [DOI] [PubMed] [Google Scholar]
- 213. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Dejban P, Nikravangolsefid N, Chamanara M, Dehpour A, Rashidian A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF‐kappaB pathway. Phytother Res. 2021;35(2):835‐845. [DOI] [PubMed] [Google Scholar]
- 215. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 216. Roggenbuck D, Reinhold D, Baumgart DC, et al. Autoimmunity in Crohn's disease‐a putative stratification factor of the clinical phenotype. Adv Clin Chem. 2016;77:77‐101. [DOI] [PubMed] [Google Scholar]
- 217. O'Toole A, Korzenik J. Environmental triggers for IBD. Curr Gastroenterol Rep. 2014;16(7):396. [DOI] [PubMed] [Google Scholar]
- 218. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504‐1517. [DOI] [PubMed] [Google Scholar]
- 219. Connelly TM, Berg AS, Harris L, 3rd , et al. Genetic determinants associated with early age of diagnosis of IBD. Dis Colon Rectum. 2015;58(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 220. Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115(2):357‐369. [DOI] [PubMed] [Google Scholar]
- 221. Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42(4):477‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Karban AS, Okazaki T, Panhuysen CI, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13(1):35‐45. [DOI] [PubMed] [Google Scholar]
- 223. Glas J, Török HP, Tonenchi L, et al. Role of the NFKB1 ‐94ins/delATTG promoter polymorphism in IBD and potential interactions with polymorphisms in the CARD15/NOD2, IKBL, and IL‐1RN genes. Inflamm Bowel Dis. 2006;12(7):606‐611. [DOI] [PubMed] [Google Scholar]
- 224. Kaustio M, Haapaniemi E, Göös H, et al. Damaging heterozygous mutations in NFKB1 lead to diverse immunologic phenotypes. J Allergy Clin Immunol. 2017;140(3):782‐796. [DOI] [PubMed] [Google Scholar]
- 225. Chawla M, Mukherjee T, Deka A, et al. An epithelial Nfkb2 pathway exacerbates intestinal inflammation by supplementing latent RelA dimers to the canonical NF‐κB module. Proc Natl Acad Sci U S A. 2021;118(25):e2024828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Fichtner‐Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1‐ and Th2‐mediated inflammatory bowel disease with NF‐kappa B decoy oligonucleotides. J Clin Invest. 2005;115(11):3057‐3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF‐kappaB and increased susceptibility to colitis‐associated tumorigenesis in CYLD‐deficient mice. J Clin Invest. 2006;116(11):3042‐3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Vereecke L, Vieira‐Silva S, Billiet T, et al. A20 controls intestinal homeostasis through cell‐specific activities. Nat Commun. 2014;5:5103. [DOI] [PubMed] [Google Scholar]
- 229. Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis‐associated cancer. Cell. 2004;118(3):285‐296. [DOI] [PubMed] [Google Scholar]
- 230. Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446(7135):557‐561. [DOI] [PubMed] [Google Scholar]
- 231. Zaph C, Troy AE, Taylor BC, et al. Epithelial‐cell‐intrinsic IKK‐beta expression regulates intestinal immune homeostasis. Nature. 2007;446(7135):552‐556. [DOI] [PubMed] [Google Scholar]
- 232. Al‐Sadi R, Engers J, Haque M, et al. Matrix Metalloproteinase‐9 (MMP‐9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF‐κB activation. PLoS One. 2021;16(4):e0249544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Lu Y, Li X, Liu S, Zhang Y, Zhang D. Toll‐like receptors and inflammatory bowel disease. Front Immunol. 2018;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Toiyama Y, Araki T, Yoshiyama S, et al. The expression patterns of Toll‐like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg Today. 2006;36(3):287‐290. [DOI] [PubMed] [Google Scholar]
- 235. Barros LL, Farias AQ, Rezaie A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: prevalence, diagnosis and treatment. World J Gastroenterol. 2019;25(31):4414‐4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Lega S, Dubinsky MC. What are the targets of inflammatory bowel disease management. Inflamm Bowel Dis. 2018;24(8):1670‐1675. [DOI] [PubMed] [Google Scholar]
- 237. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(3):s1‐s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Papamichael K, Lin S, Moore M, et al. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:2040622319838443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of Infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57(8):929‐942. [DOI] [PubMed] [Google Scholar]
- 240. Flood L, Innala E, Löfberg R, Wikström AC. Patients with ulcerative colitis responding to steroid treatment up‐regulate glucocorticoid receptor levels in colorectal mucosa. J Crohns Colitis. 2008;2(2):123‐130. [DOI] [PubMed] [Google Scholar]
- 241. Chen S, Zhang Y, Niu X, et al. Coral‐derived endophytic fungal product, Butyrolactone‐I, alleviates LPS induced intestinal epithelial cell inflammatory response through TLR4/NF‐κB and MAPK signaling pathways: an in vitro and in vivo studies. Front Nutr. 2021;8:748118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Ran X, Li Y, Chen G, et al. Farrerol ameliorates TNBS‐induced colonic inflammation by inhibiting ERK1/2, JNK1/2, and NF‐κB signaling pathway. Int J Mol Sci. 2018;19(7):2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317‐325. [DOI] [PubMed] [Google Scholar]
- 244. Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31(1):5‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Fiordelisi A, Iaccarino G, Morisco C, Coscioni E, Sorriento D. NFkappaB is a key player in the crosstalk between inflammation and cardiovascular diseases. Int J Mol Sci. 2019;20(7):1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Yu XH, Zheng XL, Tang CK. Nuclear factor‐κB activation as a pathological mechanism of lipid metabolism and atherosclerosis. Adv Clin Chem. 2015;70:1. [DOI] [PubMed] [Google Scholar]
- 247. Kempe S, Kestler H, Lasar A, Wirth T. NF‐kappaB controls the global pro‐inflammatory response in endothelial cells: evidence for the regulation of a pro‐atherogenic program. Nucleic Acids Res. 2005;33(16):5308‐5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Monaco C, Andreakos E, Kiriakidis S, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101(15):5634‐5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell‐specific NF‐kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8(5):372‐383. [DOI] [PubMed] [Google Scholar]
- 250. Ferreira V, van Dijk KW, Groen AK, et al. Macrophage‐specific inhibition of NF‐kappaB activation reduces foam‐cell formation. Atherosclerosis. 2007;192(2):283‐290. [DOI] [PubMed] [Google Scholar]
- 251. Goossens P, Vergouwe MN, Gijbels MJ, et al. Myeloid IκBα deficiency promotes atherogenesis by enhancing leukocyte recruitment to the plaques. PLoS One. 2011;6(7):e22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119‐1131. [DOI] [PubMed] [Google Scholar]
- 253. Li Y, Zhang L, Ren P, et al. Qing‐Xue‐Xiao‐Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF‐κB pathway regulation. Phytomedicine. 2021;93:153812. [DOI] [PubMed] [Google Scholar]
- 254. Abedimanesh N, Motlagh B, Abedimanesh S, et al. Effects of crocin and saffron aqueous extract on gene expression of SIRT1, AMPK, LOX1, NF‐κB, and MCP‐1 in patients with coronary artery disease: a randomized placebo‐controlled clinical trial. Phytother Res. 2020;34(5):1114‐1122. [DOI] [PubMed] [Google Scholar]
- 255. Kim MJ, Kang HH, Seo YJ, et al. Paeonia lactiflora root extract and its components reduce biomarkers of early atherosclerosis via anti‐inflammatory and antioxidant effects in vitro and in vivo. Antioxidants (Basel). 2021;10(10):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Attiq A, Yao LJ, Afzal S, Khan MA. The triumvirate of NF‐κB, inflammation and cytokine storm in COVID‐19. Int Immunopharmacol. 2021;101(Pt B):108255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS‐CoV‐2. Clin Chim Acta. 2020;509:280‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Wu Y, Ma L, Cai S, et al. RNA‐induced liquid phase separation of SARS‐CoV‐2 nucleocapsid protein facilitates NF‐κB hyper‐activation and inflammation. Signal Transduct Target Ther. 2021;6(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Su CM, Wang L, Yoo D. Activation of NF‐κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS‐CoV‐2. Sci Rep. 2021;11(1):13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kandasamy M. NF‐κB signalling as a pharmacological target in COVID‐19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(3):561‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Liao QJ, Ye LB, Timani KA, et al. Activation of NF‐kappaB by the full‐length nucleocapsid protein of the SARS coronavirus. Acta Biochim Biophys Sin (Shanghai). 2005;37(9):607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Wang W, Ye L, Ye L, et al. Up‐regulation of IL‐6 and TNF‐alpha induced by SARS‐coronavirus spike protein in murine macrophages via NF‐kappaB pathway. Virus Res. 2007;128(1‐2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Hillen HS, Kokic G, Farnung L, et al. Structure of replicating SARS‐CoV‐2 polymerase. Nature. 2020;584(7819):154‐156. [DOI] [PubMed] [Google Scholar]
- 268. Garcia MA, Gallego P, Campagna M, et al. Activation of NF‐kB pathway by virus infection requires Rb expression. PLoS One. 2009;4(7):e6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Meusel TR, Kehoe KE, Imani F. Protein kinase R regulates double‐stranded RNA induction of TNF‐alpha but not IL‐1 beta mRNA in human epithelial cells. J Immunol. 2002;168(12):6429‐6435. [DOI] [PubMed] [Google Scholar]
- 270. Yang H, Lyu Y, Hou F. SARS‐CoV‐2 infection and the antiviral innate immune response. J Mol Cell Biol. 2020;12(12):963‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Kircheis R, Haasbach E, Lueftenegger D, et al. NF‐κB pathway as a potential target for treatment of critical stage COVID‐19 patients. Front Immunol. 2020;11:598444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Kanan T, Kanan D, Al Shardoub EJ, Durdagi S. Transcription factor NF‐κB as target for SARS‐CoV‐2 drug discovery efforts using inflammation‐based QSAR screening model. J Mol Graph Model. 2021;108:107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID‐19. Sci Immunol. 2020;5(48):eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Poeta M, Cioffi V, Buccigrossi V, et al. Diosmectite inhibits the interaction between SARS‐CoV‐2 and human enterocytes by trapping viral particles, thereby preventing NF‐kappaB activation and CXCL10 secretion. Sci Rep. 2021;11(1):21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Feng Y, Zhu B, Liu Y, et al. Yindan Jiedu granules exhibit anti‐inflammatory effect in patients with novel Coronavirus disease (COVID‐19) by suppressing the NF‐κB signaling pathway. Phytomedicine. 2021:153784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Ma Q, Pan W, Li R, et al. Liu Shen capsule shows antiviral and anti‐inflammatory abilities against novel coronavirus SARS‐CoV‐2 via suppression of NF‐κB signaling pathway. Pharmacol Res. 2020;158:104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID‐19–final report. N Engl J Med. 2020;383(19):1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Mahase E. Covid‐19: UK becomes first country to authorise antiviral molnupiravir. Bmj. 2021;375:n2697. [DOI] [PubMed] [Google Scholar]
- 279. Mahase E. Covid‐19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. [DOI] [PubMed] [Google Scholar]
- 280. Robinson FR, Twiehaus MJ. Isolation of tha avian reticuloendothelial virus (strain T). Avian Dis. 1974;18(2):278‐288. [PubMed] [Google Scholar]
- 281. Ben‐Neriah Y, Karin M. Inflammation meets cancer, with NF‐κB as the matchmaker. Nat Immunol. 2011;12(8):715‐723. [DOI] [PubMed] [Google Scholar]
- 282. DiDonato JA, Mercurio F, Karin M. NF‐κB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379‐400. [DOI] [PubMed] [Google Scholar]
- 283. Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF‐kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF‐kappaB. Proc Natl Acad Sci U S A. 1998;95(5):2307‐2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Gapuzan ME, Yufit PV, Gilmore TD. Immortalized embryonic mouse fibroblasts lacking the RelA subunit of transcription factor NF‐kappaB have a malignantly transformed phenotype. Oncogene. 2002;21(16):2484‐2492. [DOI] [PubMed] [Google Scholar]
- 285. Courtois G, Gilmore TD. Mutations in the NF‐kappaB signaling pathway: implications for human disease. Oncogene. 2006;25(51):6831‐6843. [DOI] [PubMed] [Google Scholar]
- 286. Weniger MA, Küppers R. Molecular biology of Hodgkin lymphoma. Leukemia. 2021;35(4):968‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Gilmore TD, Gerondakis S. The c‐Rel transcription factor in development and disease. Genes Cancer. 2011;2(7):695‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Kalaitzidis D, Davis RE, Rosenwald A, Staudt LM, Gilmore TD. The human B‐cell lymphoma cell line RC‐K8 has multiple genetic alterations that dysregulate the Rel/NF‐kappaB signal transduction pathway. Oncogene. 2002;21(57):8759‐8768. [DOI] [PubMed] [Google Scholar]
- 289. Lake A, Shield LA, Cordano P, et al. Mutations of NFKBIA, encoding IkappaB alpha, are a recurrent finding in classical Hodgkin lymphoma but are not a unifying feature of non‐EBV‐associated cases. Int J Cancer. 2009;125(6):1334‐1342. [DOI] [PubMed] [Google Scholar]
- 290. Bredel M, Scholtens DM, Yadav AK, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364(7):627‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Zheng H, Dai W, Cheung AK, et al. Whole‐exome sequencing identifies multiple loss‐of‐function mutations of NF‐κB pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 2016;113(40):11283‐11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Greenman C, Stephens P, Smith R, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Pflueger D, Terry S, Sboner A, et al. Discovery of non‐ETS gene fusions in human prostate cancer using next‐generation RNA sequencing. Genome Res. 2011;21(1):56‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Meyer AN, Gallo LH, Ko J, et al. Oncogenic mutations in IKKβ function through global changes induced by K63‐linked ubiquitination and result in autocrine stimulation. PLoS One. 2018;13(10):e0206014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Antonia RJ, Hagan RS, Baldwin AS. Expanding the view of IKK: new substrates and new biology. Trends Cell Biol. 2021;31(3):166‐178. [DOI] [PubMed] [Google Scholar]
- 296. Vrábel D, Pour L, Ševčíková S. The impact of NF‐κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 2019;34:56‐66. [DOI] [PubMed] [Google Scholar]
- 297. Staudt LM. Oncogenic activation of NF‐kappaB. Cold Spring Harb Perspect Biol. 2010;2(6):a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Matthews GM, de Matos Simoes R, Dhimolea E, et al. NF‐κB dysregulation in multiple myeloma. Semin Cancer Biol. 2016;39:68‐76. [DOI] [PubMed] [Google Scholar]
- 299. Young RM, Phelan JD, Wilson WH, Staudt LM. Pathogenic B‐cell receptor signaling in lymphoid malignancies: new insights to improve treatment. Immunol Rev. 2019;291(1):190‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Eluard B, Nuan‐Aliman S, Faumont N, et al. The alternative RelB NF‐κB subunit is a novel critical player in diffuse large B‐cell lymphoma. Blood. Publsihed online July 7, 2021. 10.1182/blood.2020010039 [DOI] [PubMed] [Google Scholar]
- 301. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B‐cell lymphoma. N Engl J Med. 2018;378(15):1396‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361‐371. [DOI] [PubMed] [Google Scholar]
- 303. Barkett M, Gilmore TD. Control of apoptosis by Rel/NF‐kappaB transcription factors. Oncogene. 1999;18(49):6910‐6924. [DOI] [PubMed] [Google Scholar]
- 304. White DW, Roy A, Gilmore TD. The v‐Rel oncoprotein blocks apoptosis and proteolysis of I kappa B‐alpha in transformed chicken spleen cells. Oncogene. 1995;10(5):857‐868. [PubMed] [Google Scholar]
- 305. Bian X, McAllister‐Lucas LM, Shao F, et al. NF‐kappa B activation mediates doxorubicin‐induced cell death in N‐type neuroblastoma cells. J Biol Chem. 2001;276(52):48921‐48929. [DOI] [PubMed] [Google Scholar]
- 306. Bessho R, Matsubara K, Kubota M, et al. Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor kappa B (NF‐kappa B) activation, prevents apoptosis in human promyelocytic leukemia HL‐60 cells and thymocytes. Biochem Pharmacol. 1994;48(10):1883‐1889. [DOI] [PubMed] [Google Scholar]
- 307. Ivanov VN, Ronai Z. p38 protects human melanoma cells from UV‐induced apoptosis through down‐regulation of NF‐kappaB activity and Fas expression. Oncogene. 2000;19(26):3003‐3012. [DOI] [PubMed] [Google Scholar]
- 308. Grimm T, Schneider S, Naschberger E, et al. EBV latent membrane protein‐1 protects B cells from apoptosis by inhibition of BAX. Blood. 2005;105(8):3263‐3269. [DOI] [PubMed] [Google Scholar]
- 309. Wang Z, Zhang B, Yang L, Ding J, Ding HF. Constitutive production of NF‐kappaB2 p52 is not tumorigenic but predisposes mice to inflammatory autoimmune disease by repressing Bim expression. J Biol Chem. 2008;283(16):10698‐10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor‐kappaB motifs in 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin‐mediated regulation of Fas and Fas ligand expression. Mol Pharmacol. 2007;71(1):145‐157. [DOI] [PubMed] [Google Scholar]
- 311. Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF‐κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME‐mediated cancer cell pyroptosis. Cell Cycle. 2020;19(10):1089‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Yi S, Liu G, Wu Y, Liang Q, Li L. Baicalein suppresses the growth of the human thyroid cancer cells by inducing mitotic catastrophe, apoptosis and autophagy via NF‐kB signalling pathway. J Buon. 2020;25(1):389‐394. [PubMed] [Google Scholar]
- 313. Yang S, Zhang X, Qu H, et al. Cabozantinib induces PUMA‐dependent apoptosis in colon cancer cells via AKT/GSK‐3β/NF‐κB signaling pathway. Cancer Gene Ther. 2020;27(5):368‐377. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 314. Wang B, Mao JH, Wang BY, et al. Exosomal miR‐1910‐3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF‐κB signaling pathway. Cancer Lett. 2020;489:87‐99. [DOI] [PubMed] [Google Scholar]
- 315. Ren X, Chen C, Luo Y, et al. lncRNA‐PLACT1 sustains activation of NF‐κB pathway through a positive feedback loop with IκBα/E2F1 axis in pancreatic cancer. Mol Cancer. 2020;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Song L, Chen X, Mi L, et al. Icariin‐induced inhibition of SIRT6/NF‐κB triggers redox mediated apoptosis and enhances anti‐tumor immunity in triple‐negative breast cancer. Cancer Sci. 2020;111(11):4242‐4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Anaya‐Eugenio GD, Eggers NA, Ren Y, et al. Apoptosis induced by (+)‐Betulin through NF‐κB inhibition in MDA‐MB‐231 breast cancer cells. Anticancer Res. 2020;40(12):6637‐6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS, Jr . NF‐kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19(8):5785‐5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Iwanaga R, Ozono E, Fujisawa J, et al. Activation of the cyclin D2 and cdk6 genes through NF‐kappaB is critical for cell‐cycle progression induced by HTLV‐I Tax. Oncogene. 2008;27(42):5635‐5642. [DOI] [PubMed] [Google Scholar]
- 320. Wang Z, Sicinski P, Weinberg RA, Zhang Y, Ravid K. Characterization of the mouse cyclin D3 gene: exon/intron organization and promoter activity. Genomics. 1996;35(1):156‐163. [DOI] [PubMed] [Google Scholar]
- 321. Nam SY, Ko YS, Jung J, et al. A hypoxia‐dependent upregulation of hypoxia‐inducible factor‐1 by nuclear factor‐κB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104(1):166‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Cerhan JR, Anderson KE, Janney CA, et al. Association of aspirin and other non‐steroidal anti‐inflammatory drug use with incidence of non‐Hodgkin lymphoma. Int J Cancer. 2003;106(5):784‐788. [DOI] [PubMed] [Google Scholar]
- 323. Becker C, Fantini MC, Schramm C, et al. TGF‐beta suppresses tumor progression in colon cancer by inhibition of IL‐6 trans‐signaling. Immunity. 2004;21(4):491‐501. [DOI] [PubMed] [Google Scholar]
- 324. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759‐767. [DOI] [PubMed] [Google Scholar]
- 325. Joyce D, Albanese C, Steer J, et al. NF‐kappaB and cell‐cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12(1):73‐90. [DOI] [PubMed] [Google Scholar]
- 326. Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP. Inflammation‐induced cell proliferation potentiates DNA damage‐induced mutations in vivo. PLoS Genet. 2015;11(2):e1004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Shimizu T, Marusawa H, Endo Y, Chiba T. Inflammation‐mediated genomic instability: roles of activation‐induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103(7):1201‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Gudkov AV, Gurova KV, Komarova EA. Inflammation and p53: a tale of two stresses. Genes Cancer. 2011;2(4):503‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Charostad J, Nakhaie M, Dehghani A, Faghihloo E. The interplay between EBV and KSHV viral products and NF‐κB pathway in oncogenesis. Infect Agent Cancer. 2020;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Joneson T, Bar‐Sagi D. Suppression of Ras‐induced apoptosis by the Rac GTPase. Mol Cell Biol. 1999;19(9):5892‐5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Gilkes DM, Semenza GL. Role of hypoxia‐inducible factors in breast cancer metastasis. Future Oncol. 2013;9(11):1623‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Görlach A, Bonello S. The cross‐talk between NF‐kappaB and HIF‐1: further evidence for a significant liaison. Biochem J. 2008;412(3):e17‐e19. [DOI] [PubMed] [Google Scholar]
- 334. Zhou W, Yang L, Nie L, Lin H. Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am J Cancer Res. 2021;11(2):301‐317. [PMC free article] [PubMed] [Google Scholar]
- 335. Wang R, Ma Y, Zhan S, et al. B7‐H3 promotes colorectal cancer angiogenesis through activating the NF‐κB pathway to induce VEGFA expression. Cell Death Dis. 2020;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Luo LH, Rao L, Luo LF, et al. Long non‐coding RNA NKILA inhibited angiogenesis of breast cancer through NF‐κB/IL‐6 signaling pathway. Microvasc Res. 2020;129:103968. [DOI] [PubMed] [Google Scholar]
- 337. Ding D, Xi P, Zhou J, Wang M, Cong YS. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF‐κB‐dependent transcription. Faseb J. 2013;27(11):4375‐4383. [DOI] [PubMed] [Google Scholar]
- 338. Pires BR, Mencalha AL, Ferreira GM, et al. NF‐kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS One. 2017;12(1):e0169622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Scheel C, Weinberg RA. Cancer stem cells and epithelial‐mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22(5‐6):396‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF‐kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188‐4197. [DOI] [PubMed] [Google Scholar]
- 341. Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8(20):3267‐3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Drabsch Y, ten Dijke P. TGF‐β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31(3‐4):553‐568. [DOI] [PubMed] [Google Scholar]
- 343. Meulmeester E, Ten Dijke P. The dynamic roles of TGF‐β in cancer. J Pathol. 2011;223(2):205‐218. [DOI] [PubMed] [Google Scholar]
- 344. Malki A, ElRuz RA, Gupta I, et al. Molecular mechanisms of colon cancer progression and metastasis: recent insights and advancements. Int J Mol Sci. 2020;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540‐550. [DOI] [PubMed] [Google Scholar]
- 346. Huber MA, Azoitei N, Baumann B, et al. NF‐kappaB is essential for epithelial‐mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114(4):569‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Chen W, Dong J, Haiech J, Kilhoffer MC, Zeniou M. Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells Int. 2016;2016:1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717‐728. [DOI] [PubMed] [Google Scholar]
- 349. Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645‐648. [DOI] [PubMed] [Google Scholar]
- 350. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730‐737. [DOI] [PubMed] [Google Scholar]
- 351. Shimokawa M, Ohta Y, Nishikori S, et al. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545(7653):187‐192. [DOI] [PubMed] [Google Scholar]
- 352. Shibata M, Hoque MO. Targeting cancer stem cells: a strategy for effective eradication of cancer. Cancers (Basel). 2019;11(5):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Vazquez‐Santillan K, Melendez‐Zajgla J, Jimenez‐Hernandez L, Martínez‐Ruiz G, Maldonado V. NF‐κB signaling in cancer stem cells: a promising therapeutic target? Cell Oncol (Dordr). 2015;38(5):327‐339. [DOI] [PubMed] [Google Scholar]
- 354. Rinkenbaugh AL, Baldwin AS. The NF‐κB pathway and cancer stem cells. Cells. 2016;5(2):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Yamamoto M, Taguchi Y, Ito‐Kureha T, et al. NF‐κB non‐cell‐autonomously regulates cancer stem cell populations in the basal‐like breast cancer subtype. Nat Commun. 2013;4:2299. [DOI] [PubMed] [Google Scholar]
- 356. Vazquez‐Santillan K, Melendez‐Zajgla J, Jimenez‐Hernandez LE, et al. NF‐kappaΒ‐inducing kinase regulates stem cell phenotype in breast cancer. Sci Rep. 2016;6:37340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Han D, Wu G, Chang C, et al. Disulfiram inhibits TGF‐β‐induced epithelial‐mesenchymal transition and stem‐like features in breast cancer via ERK/NF‐κB/Snail pathway. Oncotarget. 2015;6(38):40907‐40919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Burnett JP, Lim G, Li Y, et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017;394:52‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Sun L, Mathews LA, Cabarcas SM, et al. Epigenetic regulation of SOX9 by the NF‐κB signaling pathway in pancreatic cancer stem cells. Stem Cells. 2013;31(8):1454‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Gonzalez‐Torres C, Gaytan‐Cervantes J, Vazquez‐Santillan K, et al. NF‐κB participates in the stem cell phenotype of ovarian cancer cells. Arch Med Res. 2017;48(4):343‐351. [DOI] [PubMed] [Google Scholar]
- 361. Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149(7):1884‐1895.e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Liu S, Zhang C, Zhang K, et al. FOXP3 inhibits cancer stem cell self‐renewal via transcriptional repression of COX2 in colorectal cancer cells. Oncotarget. 2017;8(27):44694‐44704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. DeBerardinis RJ. Tumor microenvironment, metabolism, and immunotherapy. N Engl J Med. 2020;382(9):869‐871. [DOI] [PubMed] [Google Scholar]
- 364. Atretkhany KN, Drutskaya MS, Nedospasov SA, Grivennikov SI, Kuprash DV. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol Ther. 2016;168:98‐112. [DOI] [PubMed] [Google Scholar]
- 365. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(1):i104‐108. [DOI] [PubMed] [Google Scholar]
- 367. Taniguchi K, Karin M. IL‐6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54‐74. [DOI] [PubMed] [Google Scholar]
- 368. Chang Q, Daly L, Bromberg J. The IL‐6 feed‐forward loop: a driver of tumorigenesis. Semin Immunol. 2014;26(1):48‐53. [DOI] [PubMed] [Google Scholar]
- 369. He G, Dhar D, Nakagawa H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL‐6 signaling. Cell. 2013;155(2):384‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Voronov E, Apte RN. IL‐1 in colon inflammation, colon carcinogenesis and invasiveness of colon cancer. Cancer Microenviron. 2015;8(3):187‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Garlanda C, Dinarello CA, Mantovani A. The interleukin‐1 family: back to the future. Immunity. 2013;39(6):1003‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Song X, Qian Y. IL‐17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25(12):2335‐2347. [DOI] [PubMed] [Google Scholar]
- 373. Yang B, Kang H, Fung A, et al. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm. 2014;2014:623759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Wang K, Kim MK, Di Caro G, et al. Interleukin‐17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41(6):1052‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Lu B, Yang M, Wang Q. Interleukin‐33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy. J Mol Med (Berl). 2016;94(5):535‐543. [DOI] [PubMed] [Google Scholar]
- 376. Liew FY, Girard JP, Turnquist HR. Interleukin‐33 in health and disease. Nat Rev Immunol. 2016;16(11):676‐689. [DOI] [PubMed] [Google Scholar]
- 377. Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A. 2014;111(41):14776‐14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582‐598. [DOI] [PubMed] [Google Scholar]
- 379. Koliaraki V, Pallangyo CK, Greten FR, Kollias G. Mesenchymal cells in colon cancer. Gastroenterology. 2017;152(5):964‐979. [DOI] [PubMed] [Google Scholar]
- 380. Bando H, Toi M. Tumor angiogenesis, macrophages, and cytokines. Adv Exp Med Biol. 2000;476:267‐284. [DOI] [PubMed] [Google Scholar]
- 381. Karin M, Lin A. NF‐kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221‐227. [DOI] [PubMed] [Google Scholar]
- 382. Jing H, Lee S. NF‐κB in cellular senescence and cancer treatment. Mol Cells. 2014;37(3):189‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22(11):557‐566. [DOI] [PubMed] [Google Scholar]
- 384. Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF‐7 cells through mTOR‐dependent mRNA translation. Biochem Biophys Res Commun. 2009;387(2):381‐386. [DOI] [PubMed] [Google Scholar]
- 385. Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5‐dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182(3):1746‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541‐566. [DOI] [PubMed] [Google Scholar]
- 388. Porta C, Rimoldi M, Raes G, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106(35):14978‐14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Lawrence T. Macrophages and NF‐κB in cancer. Curr Top Microbiol Immunol. 2011;349:171‐184. [DOI] [PubMed] [Google Scholar]
- 390. Hagemann T, Lawrence T, McNeish I, et al. “Re‐educating” tumor‐associated macrophages by targeting NF‐kappaB. J Exp Med. 2008;205(6):1261‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Karyampudi L, Lamichhane P, Krempski J, et al. PD‐1 blunts the function of ovarian tumor‐infiltrating dendritic cells by inactivating NF‐κB. Cancer Res. 2016;76(2):239‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Antonangeli F, Natalini A, Garassino MC, et al. Regulation of PD‐L1 expression by NF‐κB in cancer. Front Immunol. 2020;11:584626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin‐1beta induces gastric inflammation and cancer and mobilizes myeloid‐derived suppressor cells in mice. Cancer Cell. 2008;14(5):408‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503‐510. [DOI] [PubMed] [Google Scholar]
- 395. Zhou J, Zhang J, Lichtenheld MG, Meadows GG. A role for NF‐kappa B activation in perforin expression of NK cells upon IL‐2 receptor signaling. J Immunol. 2002;169(3):1319‐1325. [DOI] [PubMed] [Google Scholar]
- 396. Huang C, Bi E, Hu Y, et al. A novel NF‐kappaB binding site controls human granzyme B gene transcription. J Immunol. 2006;176(7):4173‐4181. [DOI] [PubMed] [Google Scholar]
- 397. Kubo M, Morisaki T, Matsumoto K, et al. Paclitaxel probably enhances cytotoxicity of natural killer cells against breast carcinoma cells by increasing perforin production. Cancer Immunol Immunother. 2005;54(5):468‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Ward JP, Gubin MM, Schreiber RD. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. Adv Immunol. 2016;130:25‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Evaristo C, Spranger S, Barnes SE, et al. Cutting edge: engineering active IKKβ in T cells drives tumor rejection. J Immunol. 2016;196(7):2933‐2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Gerondakis S, Fulford TS, Messina NL, Grumont RJ. NF‐κB control of T cell development. Nat Immunol. 2014;15(1):15‐25. [DOI] [PubMed] [Google Scholar]
- 401. Oh H, Grinberg‐Bleyer Y, Liao W, et al. An NF‐κB transcription‐factor‐dependent lineage‐specific transcriptional program promotes regulatory T cell identity and function. Immunity. 2017;47(3):450‐465.e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B‐cell‐derived lymphotoxin promotes castration‐resistant prostate cancer. Nature. 2010;464(7286):302‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Ammirante M, Kuraishy AI, Shalapour S, et al. An IKKα‐E2F1‐BMI1 cascade activated by infiltrating B cells controls prostate regeneration and tumor recurrence. Genes Dev. 2013;27(13):1435‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Erez N, Truitt M, Olson P, ST Arron, Hanahan D. Cancer‐associated fibroblasts are activated in incipient neoplasia to orchestrate tumor‐promoting inflammation in an NF‐kappaB‐dependent manner. Cancer Cell. 2010;17(2):135‐147. [DOI] [PubMed] [Google Scholar]
- 405. Santolla MF, Lappano R, Cirillo F, et al. miR‐221 stimulates breast cancer cells and cancer‐associated fibroblasts (CAFs) through selective interference with the A20/c‐Rel/CTGF signaling. J Exp Clin Cancer Res. 2018;37(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Sparmann A, Bar‐Sagi D. Ras‐induced interleukin‐8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447‐458. [DOI] [PubMed] [Google Scholar]
- 408. Pikarsky E, Porat RM, Stein I, et al. NF‐kappaB functions as a tumour promoter in inflammation‐associated cancer. Nature. 2004;431(7007):461‐466. [DOI] [PubMed] [Google Scholar]
- 409. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Waldner MJ, Neurath MF. Colitis‐associated cancer: the role of T cells in tumor development. Semin Immunopathol. 2009;31(2):249‐256. [DOI] [PubMed] [Google Scholar]
- 411. Lin L, Hu X, Zhang H, Hu H. Tertiary lymphoid organs in cancer immunology: mechanisms and the new strategy for immunotherapy. Front Immunol. 2019;10:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Sautès‐Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307‐325. [DOI] [PubMed] [Google Scholar]
- 413. Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424‐433. [DOI] [PubMed] [Google Scholar]
- 414. Furtado GC, Marinkovic T, Martin AP, et al. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A. 2007;104(12):5026‐5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Suhail M, Tarique M, Muhammad N, et al. A critical transcription factor NF‐κB as a cancer therapeutic target and its inhibitors as cancer treatment options. Curr Med Chem. 2021;28(21):4117‐4132. [DOI] [PubMed] [Google Scholar]
- 416. Gilmore TD, Garbati MR. Inhibition of NF‐κB signaling as a strategy in disease therapy. Curr Top Microbiol Immunol. 2011;349:245‐263. [DOI] [PubMed] [Google Scholar]
- 417. Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907‐4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine‐2 phosphorylation of the RNA polymerase II carboxy‐terminal domain. Genes Dev. 2000;14(18):2314‐2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS, Jr . Inhibition of NF‐kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276(25):22382‐22387. [DOI] [PubMed] [Google Scholar]
- 420. Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double‐blind, double‐dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370‐381. [DOI] [PubMed] [Google Scholar]
- 421. Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560(7718):387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Mercogliano MF, Bruni S, Elizalde PV, Schillaci R. Tumor necrosis factor α blockade: an opportunity to tackle breast cancer. Front Oncol. 2020;10:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Lin Y, Bai L, Chen W, Xu S. The NF‐kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14(1):45‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Wang W, Tam WF, Hughes CC, Rath S, Sen R. c‐Rel is a target of pentoxifylline‐mediated inhibition of T lymphocyte activation. Immunity. 1997;6(2):165‐174. [DOI] [PubMed] [Google Scholar]
- 425. Dash AB, Zhang J, Shen L, et al. Clinical benefit of ixazomib plus lenalidomide‐dexamethasone in myeloma patients with non‐canonical NF‐κB pathway activation. Eur J Haematol. 2020;105(3):274‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Zhu S, Jin J, Gokhale S, et al. Genetic alterations of TRAF proteins in human cancers. Front Immunol. 2018;9:2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Yang D, Zhang W, Liang J, et al. Single cell whole genome sequencing reveals that NFKB1 mutation affects radiotherapy sensitivity in cervical cancer. Oncotarget. 2018;9(7):7332‐7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Luo J, Song J, Zhang H, et al. Melatonin mediated Foxp3‐downregulation decreases cytokines production via the TLR2 and TLR4 pathways in H. pylori infected mice. Int Immunopharmacol. 2018;64:116‐122. [DOI] [PubMed] [Google Scholar]
- 430. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Patanè M, Porrati P, Bottega E, et al. Frequency of NFKBIA deletions is low in glioblastomas and skewed in glioblastoma neurospheres. Mol Cancer. 2013;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Mansouri L, Papakonstantinou N, Ntoufa S, Stamatopoulos K, Rosenquist R. NF‐κB activation in chronic lymphocytic leukemia: a point of convergence of external triggers and intrinsic lesions. Semin Cancer Biol. 2016;39:40‐48. [DOI] [PubMed] [Google Scholar]
- 434. Lenardo M, Siebenlist U. Bcl‐3‐mediated nuclear regulation of the NF‐kappa B trans‐activating factor. Immunol Today. 1994;15(4):145‐147. [DOI] [PubMed] [Google Scholar]
- 435. Ljungström V, Cortese D, Young E, et al. Whole‐exome sequencing in relapsing chronic lymphocytic leukemia: clinical impact of recurrent RPS15 mutations. Blood. 2016;127(8):1007‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF‐κB in lymphoid malignancies. Immunol Rev. 2012;246(1):359‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Gehring T, Seeholzer T, Krappmann D. BCL10 ‐ bridging CARDs to immune activation. Front Immunol. 2018;9:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Yang Y, Staudt LM. Protein ubiquitination in lymphoid malignancies. Immunol Rev. 2015;263(1):240‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Ruland J, Hartjes L. CARD‐BCL‐10‐MALT1 signalling in protective and pathological immunity. Nat Rev Immunol. 2019;19(2):118‐134. [DOI] [PubMed] [Google Scholar]
- 440. Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12(11):774‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Juilland M, Thome M. Role of the CARMA1/BCL10/MALT1 complex in lymphoid malignancies. Curr Opin Hematol. 2016;23(4):402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Haery L, Thompson RC, Gilmore TD. Histone acetyltransferases and histone deacetylases in B‐ and T‐cell development, physiology and malignancy. Genes Cancer. 2015;6(5‐6):184‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23(24):4225‐4231. [DOI] [PubMed] [Google Scholar]
- 444. Compagno M, Lim WK, Grunn A, et al. Mutations of multiple genes cause deregulation of NF‐kappaB in diffuse large B‐cell lymphoma. Nature. 2009;459(7247):717‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Trecca D, Guerrini L, Fracchiolla NS, et al. Identification of a tumor‐associated mutant form of the NF‐kappaB RelA gene with reduced DNA‐binding and transactivating activities. Oncogene. 1997;14(7):791‐799. [DOI] [PubMed] [Google Scholar]
- 446. Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367(9):826‐833. [DOI] [PubMed] [Google Scholar]
- 448. Drilon A, Schoenfeld AJ, Arbour KC, et al. Exceptional responders with invasive mucinous adenocarcinomas: a phase 2 trial of bortezomib in patients with KRAS G12D‐mutant lung cancers. Cold Spring Harb Mol Case Stud. 2019;5(2):a003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Bahlis NJ, Sutherland H, White D, et al. Selinexor plus low‐dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132(24):2546‐2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Argiris A, Ghebremichael M, Burtness B, et al. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: a trial of the Eastern Cooperative Oncology Group (E1303). Cancer. 2011;117(15):3374‐3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Turkington RC, Purcell C, James CR, et al. A phase I trial of bortezomib in combination with epirubicin, carboplatin and capecitabine (ECarboX) in advanced oesophagogastric adenocarcinoma. Invest New Drugs. 2014;32(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 452. Shah MA, Power DG, Kindler HL, et al. A multicenter, phase II study of bortezomib (PS‐341) in patients with unresectable or metastatic gastric and gastroesophageal junction adenocarcinoma. Invest New Drugs. 2011;29(6):1475‐1481. [DOI] [PubMed] [Google Scholar]
- 453. Trinh XB, Sas L, Van Laere SJ, et al. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine‐resistant metastatic breast cancer. Oncol Rep. 2012;27(3):657‐663. [DOI] [PubMed] [Google Scholar]
- 454. Gilbert J, Lee JW, Argiris A, et al. Phase II 2‐arm trial of the proteasome inhibitor, PS‐341 (bortezomib) in combination with irinotecan or PS‐341 alone followed by the addition of irinotecan at time of progression in patients with locally recurrent or metastatic squamous cell carcinoma of the head and neck (E1304): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2013;35(7):942‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Raizer JJ, Chandler JP, Ferrarese R, et al. A phase II trial evaluating the effects and intra‐tumoral penetration of bortezomib in patients with recurrent malignant gliomas. J Neurooncol. 2016;129(1):139‐146. [DOI] [PubMed] [Google Scholar]
- 456. Castillo JJ, Meid K, Flynn CA, et al. Ixazomib, dexamethasone, and rituximab in treatment‐naive patients with Waldenström macroglobulinemia: long‐term follow‐up. Blood Adv. 2020;4(16):3952‐3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Hong DS, Henary H, Falchook GS, et al. First‐in‐human study of pbi‐05204, an oleander‐derived inhibitor of akt, fgf‐2, nf‐κΒ and p70s6k, in patients with advanced solid tumors. Invest New Drugs. 2014;32(6):1204‐1212. [DOI] [PubMed] [Google Scholar]
- 458. Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF‐κB signaling and reduces tumor proliferation in tissue‐resident cells of patients with CLL. Blood. 2014;123(21):3286‐3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Fahrmann JF, Ballester OF, Ballester G, et al. Inhibition of nuclear factor kappa B activation in early‐stage chronic lymphocytic leukemia by omega‐3 fatty acids. Cancer Invest. 2013;31(1):24‐38. [DOI] [PubMed] [Google Scholar]
- 460. Hong DS, Kurzrock R, Supko JG, et al. A phase I first‐in‐human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res. 2012;18(12):3396‐3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Peters S, Danson S, Hasan B, et al. A randomized open‐label phase III trial evaluating the addition of denosumab to standard first‐line treatment in advanced NSCLC: the European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR Trial. J Thorac Oncol. 2020;15(10):1647‐1656. [DOI] [PubMed] [Google Scholar]
- 463. Kaufman JL, Gasparetto C, Schjesvold FH, et al. Targeting BCL‐2 with venetoclax and dexamethasone in patients with relapsed/refractory t(11;14) multiple myeloma. Am J Hematol. 2021;96(4):418‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. O'Hara MH, O'Reilly EM, Varadhachary G, et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open‐label, multicentre, phase 1b study. Lancet Oncol. 2021;22(1):118‐131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


