Abstract
Aims
While glycemic control is key in effective type 2 diabetes mellitus management, many patients fail to reach their individualized glycemic goal. This analysis aimed to describe a real-world picture of diabetes management: individualized hemoglobin A1c (HbA1c) goals, rate of goal attainment, HbA1c at each line of therapy, and patient awareness of their glycemic goal. Secondly, we aimed to understand physician satisfaction with HbA1c amongst patients aware vs. those unaware of HbA1c goal.
Methods
Analysis of physicians and the next ten consulting patients with type 2 diabetes mellitus conducted in Europe and the USA including medical record data abstraction/assessment by physicians, a patient-reported survey and a physician survey. Patients were diagnosed for 3 months or more with a known current and target HbA1c. For the sub-analysis assessment of patient awareness of HbA1c goal, in addition to the above, these patients had to have completed a patient-reported questionnaire and answer the question on awareness of HbA1c goal.
Results
A total of 730 physicians provided data on 8794 patients with type 2 diabetes mellitus; 5331 patients were eligible for this analysis. Overall, mean (standard deviation, SD) individualized HbA1c goal was 6.8% (0.68%). Of eligible patients, 39.1% met their HbA1c goal; of 60.9% of patients not reaching their HbA1c goal, the mean distance from individualized HbA1c goal was 0.9% (SD 1.0%). Physicians progressed patients’ antihyperglycemic therapy when HbA1c was 8% or higher. Among 2560 patients who were included in the sub-analysis assessing the effect of patient awareness of their HbA1c goal on multiple parameters, 70.5% were aware of their HbA1c goal; mean HbA1c goal was 6.8% (0.7%) and current mean HbA1c value 7.1% (1.2%). A total of 949 patients in the sub-analysis (39.2%) achieved their goal; achieving HbA1c goal was not related to knowledge of goal. Patients aware of their HbA1c goal were slightly more adherent to their antihyperglycemic medication. They also were prescribed more antihyperglycemic agents, more often on a later therapy line receiving a GLP-1 receptor agonist, SGLT2i, or insulin, and more often tested their blood glucose levels than patients who were unaware. Physicians were not satisfied with the current blood glucose level of one third of their patients, believing that more of those who were aware of their HbA1c goal could achieve better glucose control (32.4% of aware vs. 28.2% of unaware patients; p = 0.003).
Conclusions
Our results showed that the proportion of patients with type 2 diabetes mellitus achieving their goals for glycemic control was suboptimal when compared to current guideline criteria, with only about 40% of patients achieving their individualized HbA1c goal. Treatment intensification was often delayed until HbA1c was 8% and higher. Patients aware of their HbA1c goal were slightly more adherent to their antihyperglycemic medication; however, awareness of HbA1c goal did not enhance goal attainment. This highlights the need for a holistic approach to diabetes management, involving patient education, and patient–physician communication and partnership.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01985-3.
Keywords: Glycemic control, Glycemic goal attainment, Individualized HbA1c goal, Individualized HbA1c, Individualized HbA1c target, Patient goal awareness, Patient target awareness, Real world, Type 2 diabetes mellitus
Key Summary Points
| What is already known about this subject? |
| Whilst guidelines recommend HbA1c targets of < 7% for a majority of patients to reduce rates of development and progression of micro/macrovascular complications, individualized HbA1c goals can vary in real-world clinical practice, with many patients failing to achieve their goal. |
| Evidence suggests that the proportion of patients maintaining target glycemic levels decreases over time, regardless of antihyperglycemic therapy. |
| Patient knowledge of glycemic goal is associated with better glycemic control. |
| What is the key question? |
| What is the real-world HbA1c goal for patients with type 2 diabetes mellitus and how does patient awareness impact attainment? |
| What are the new findings? |
| Around 60% of patients were not at the HbA1c goal set by their physicians, which was 6.8% on average; mean distance from HbA1c goal was 0.9%. |
| With each successive therapy line, physicians tended to wait until HbA1c was progressively higher before changing/adding pharmacologic agents. |
| Patients aware of their HbA1c goal were slightly more adherent to their antihyperglycemic medication; however, awareness of HbA1c goal did not enhance goal attainment. |
| How might this impact on clinical practice in the foreseeable future? |
| Results of this analysis highlighted the need for a holistic approach to diabetes management, involving patient education, and patient–physician communication and partnership. |
Introduction
Glycemic control is the primary goal of diabetes treatment to prevent target organ damage and other disease-related complications. Guidelines recommend a target glycated hemoglobin (HbA1c) value of less than 7.0% (< 53 mmol/mol) for most non-pregnant adults, although these targets are individualized per patient [1–3]. Target values need to be determined individually per patient by the treating physician, with factors influencing this decision including age, comorbidities and complications, or disease duration [3]. Lower HbA1c levels have been observed to reduce rates of development and progression of microvascular complications [4, 5], to maintain reduction in microvascular risk [6], and reduce macrovascular complications [7, 8]. Guidelines also recommend that HbA1c goals are individualized on the basis of patient characteristics, patient preferences and goals, and risk of treatment-related adverse effects such as hypoglycemia and weight gain [1, 2]. Previous real-world studies have focused on the prevalence of adults with type 2 diabetes mellitus achieving an HbA1c goal of < 7.0% despite the recommended individualization of glycemic goals [9–14].
Although knowledge of glycemic goals was associated with better glycemic control [15–17], patient knowledge of their individualized glycemic goals is not well understood on a systematic level [18, 19]. It was estimated that only about one quarter of patients with diabetes understand the meaning of HbA1c or can recall their most recent value [16, 20], while some thought that their HbA1c values above 8% indicated good glycemic control [21]. Understanding patient experiences is essential [22].
The achievement of and distance to the individualized HbA1c goal, and the HbA1c level at which providers intensify patients’ treatment regimens by adding or changing to a second or third antihyperglycemic agent (AHA), have not been well characterized. The objectives of this analysis were to (1) describe individualized HbA1c goal and rate of goal attainment; (2) note HbA1c levels as patients progressed through lines of therapy; (3) understand patient awareness of goal, and association between awareness and goal attainment; and (4) understand differences in number of AHAs, lines of therapy, glucose testing, and physician satisfaction with HbA1c amongst patients aware vs. those unaware of their HbA1c goal.
Methods
Survey Design
Data were drawn from the Adelphi Diabetes Disease Specific Programme™ (DSP), a large, real-world survey of physicians and their patients conducted in Europe and the USA. The DSP comprised physician surveys and medical record data abstraction by physicians, matched with patient-reported surveys. Data were collected in Germany, Italy, Spain, the UK, and the USA between October 2018 and March 2019. Full DSP methodology has been published and validated [23–25].
Upon providing consent to participate, primary care physicians (PCPs) or diabetologists/endocrinologists involved with the management and treatment of patients with type 2 diabetes mellitus (monthly workload ≥ 25 and ≥ 50 patients with type 2 diabetes mellitus, respectively) enrolled the next ten consecutive patients who presented in their offices and met the patient eligibility criteria, at least 18 years old, not in a clinical trial at time of data capture, and currently receiving at least one AHA. Physicians were also asked to include two additional patients treated with either a sodium glucose cotransporter 2 inhibitor (SGLT2i) or a glucagon-like peptide 1 (GLP-1) receptor agonist, or both of these agents (either alone or in combination with other AHAs), to ensure newer AHAs were represented. The research methodology is designed to maximize the number of physicians sampled while minimizing the burden on each physician by limiting the number of patients on whom they report. This also increases the power of the sample size overall. For each patient who met the eligibility criteria, physicians completed a form containing detailed questions, capturing patient demographics, tests performed (including current HbA1c value, i.e., “at time of data collection”), HbA1c goal, comorbid conditions, and current and previous treatment including HbA1c at time of initiation.
Physicians then invited the same patients to complete, on a voluntary basis, a patient-reported form, containing questions about demographics and current condition. Patients also answered the question “Do you have an agreed blood sugar target with your doctor?”. In addition, information on medication adherence was reported using the Adherence to Refills and Medicines Scale for Diabetes (ARMS-D) [26, 27]. The ARMS-D is an 11-item self-report measure of adherence that assesses patients’ ability to take and refill diabetes medications, generating total, refill, and medication-taking subscale scores. Each of the items is structured for a response on a 4-point Likert scale and scored as 1 = “none,” 2 = “some,” 3 = “most,” or 4 = “all” of the time, with higher values indicating poorer adherence (total score ranges from 11 to 44) ) [26, 27].
Physians also completed a workload form to record a 5-day period of overall patient caseload, including consultation of patients with T2DM, irrespective of patients recruited into the survey.
To be included in this retrospective analysis of the Diabetes DSP™, patients had to have a physician-reported current and target HbA1c, and have been diagnosed with type 2 diabetes mellitus for at least 3 months. For assessment of patient awareness of HbA1c goal (the sub-analysis), in addition to the above, patients had to have completed a patient-reported questionnaire and answer the question on awareness of HbA1c goal.
The survey obtained ethics approval from the Western Institutional Review Board, study protocol number 1247198, and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Physicians did not see patient responses, thereby ensuring that future interactions between physicians and their patients were not compromised by patient responses. Patients provided written informed consent for use of their anonymized and aggregated data.
Statistical Analysis
Patient characteristics were summarized using descriptive analyses. Means and standard deviations (SDs) were calculated for continuous variables, and frequency and percentages were calculated for categorical variables. Glycemic control rates were calculated as the proportion of patients with a current HbA1c level lower than the individualized HbA1c goal set by physicians.
Inferential analyses were used to explore differences in patients’ characteristics between those achieving and those not achieving individualized HbA1c goals. Fisher’s exact test was used for binary categorical variables, chi-squared test was used for unordered categorical variables (more than two groups), and a t test was used for continuous variables.
Multivariate regression was performed to determine the relationship between patient knowledge of their HbA1c goal and goal achievement, controlling for age, gender, time since diagnosis, body mass index (BMI), previous HbA1c value, Charlson Comorbidity Index (performed excluding diabetes as a comorbidity) [28], and number of AHAs currently used. A p value of less than 0.05 was taken as statistically significant.
Results
Participant Characteristics
Physician Demographics
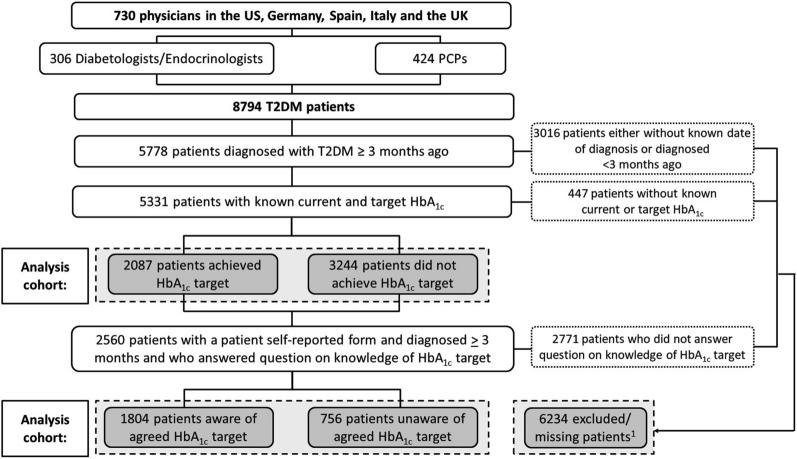
A total of 730 physicians, 306 (58%) PCPs and 424 (42%) diabetologists/endocrinologists, provided data on 8794 patients with type 2 diabetes mellitus (Fig. 1). Country-level differences are reported in Supplementary Table 1.
Fig. 1.
Participant flow diagram. HbA1c glycated hemoglobin, PCPs primary care physicians, T2DM type 2 diabetes mellitus. 1These patients did not have a known physician-reported current or target HbA1c, were diagnosed with type 2 diabetes mellitus less than 3 months ago, chose not to complete a self-reported record form, or chose not to answer the question on awareness of their HbA1c goal. Patients may or may not have been aware of their HbA1c goal
Patient Demographics
Of 5331 patients eligible for the analysis, 1126 (54.0%) patients were male with no differences across countries or previous waves of the survey. The median (IQR) duration of diabetes was 4.7 (2.0–9.6) years (Table 1). Physicians reported that after diabetes, the most common cardiovascular-metabolic comorbid condition across the patient cohort was hypertension (67.1%), followed by CKD (6.8%) and coronary artery disease (6.6%).
Table 1.
Patient demographic and clinical characteristics by achievement of HbA1c goal
| Overall (N = 5331) | Patients achieving HbA1c goal (N = 2087) | Patients not achieving HbA1c goal (N = 3244) | p value | |
|---|---|---|---|---|
| Characteristic | ||||
| Age, years, mean (SD) | 59.9 (12.1) | 60.7 (12.1) | 58.0 (12.0) | < 0.0001 (TT) |
| Male, n (%) | 2934 (55.0) | 1126 (54.0) | 1808 (55.7) | 0.2045 (FE) |
| Body mass index, kg/m2, mean (SD) | 30.1 (5.5) | 29.7 (5.3) | 30.4 (5.6) | < 0.0001 (TT) |
| Duration of diabetes, years, median (IQR) | 4.7 (2.0, 9.6) | 4.3 (2.0, 8.9) | 4.9 (2.0, 10.0) | 0.005 (MW) |
| HbA1c assessment, mean (SD) | ||||
| Individualized HbA1c goal | 6.8 (0.7) | 6.8 (0.6) | 6.8 (0.8) | 0.1039 (TT) |
| Most recent HbA1c | 7.3 (1.2) | 6.5 (0.6) | 7.7 (1.3) | < 0.0001 (TT) |
| Distance from individualized HbA1c goala | 0.5 (1.0) | − 0.3 (0.3) | 0.9 (1.0) | < 0.0001 (TT) |
| Comorbidities (physician-reported), n (%) | ||||
| Missing, n | 1 | 0 | 1 | – |
| Atrial fibrillation | 275 (5.2) | 104 (5.0) | 171 (5.3) | 0.6575 (FE) |
| Chronic kidney disease | 362 (6.8) | 147 (7.0) | 215 (6.6) | 0.0811 (FE) |
| Coronary heart/arterial disease | 351 (6.6) | 129 (6.2) | 222 (6.8) | 0.3654 (FE) |
| Heart failure | 134 (2.5) | 53 (2.5) | 81 (2.5) | 0.9288 (FE) |
| Hypertension | 3286 (61.7) | 1348 (64.6) | 1938 (59.8) | 0.0004 (FE) |
| Post-myocardial infarction | 177 (3.3) | 61 (2.9) | 116 (3.6) | 0.2104 (FE) |
| Peripheral vascular disease | 154 (2.9) | 55 (2.6) | 99 (3.1) | 0.4028 (FE) |
| Post-stroke | 46 (0.9) | 25 (1.2) | 21 (0.6) | 0.0473 (FE) |
Total sample included where there are no missing data reported
FE Fisher’s exact test, HbA1c glycated hemoglobin, IQR interquartile range, MW Mann–Whitney, SD standard deviation, TT t test
aFor those patients who did not attain their HbA1c goal
Non-eligible patients are described in the Supplementary File.
Individualized HbA1c Goals, Patient Attainment of Their HbA1c Goal and HbA1c at Pharmacologic Change/Addition
Individualized HbA1c Assessment
Overall, patients had a mean (SD) individualized HbA1c goal of 6.8% (0.68%). Of those patients with a known current and target HbA1c (n = 5331), 39.1% met their individualized HbA1c goal (n = 2087). Among those patients not reaching their individualized HbA1c goal (n = 3244; 60.9%), the mean (SD, median) distance from individualized HbA1c goal was 0.9% (1.0%; 0.6%). Individual country data also showed that patients in the UK had the longest disease duration and highest current HbA1c, HbA1c goal, and HbA1c at pharmacologic change/addition compared with all other countries; Germany had the shortest disease duration and lowest HbA1c at change/addition second lowest current HbA1c, HbA1c goal, indicating good correlation between disease duration and the HbA1c difference. Overall, characteristics of patients not achieving their HbA1c goal were similar to those achieving their goal (Table 1).
HbA1c at Change/Addition of Pharmacologic Agents by Line of Therapy
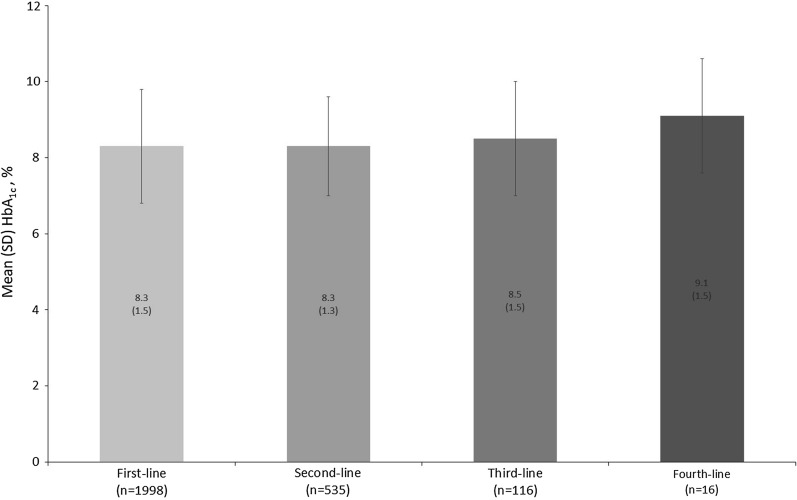
Physicians’ decision to change/add patients’ pharmacologic agents at lines of therapy typically occurred above an HbA1c of 8%. Pharmacologic change/addition from first-line of therapy to second-, third-, and fourth-line of therapy was made when HbA1c levels were 8.3% (n = 1998), 8.3% (n = 535), 8.5% (n = 116), and 9.1% (n = 16), respectively (Fig. 2).
Fig. 2.
Mean HbA1c at change/addition of line of therapy. HbA1c glycated hemoglobin, SD standard deviation. HbA1c value is physician-reported
Patient Awareness of Their Individual Glycemic Goal and Effect on Goal Attainment and Physician-Reported Assessment of Patient HbA1c Testing
Patient Demographics, HbA1c Value and Goal
Of 5331 patients with type 2 diabetes mellitus diagnosed for at least 3 months with a known current and target HbA1c, 2560 (48.0%) patients qualified for the sub-analysis of whether they were aware of their HbA1c goal agreed with their physician.
Of these 2560 patients, 1804 (70.5%) patients were aware of their HbA1c goal. Overall, patients in this sub-analysis had a mean (SD) age of 58.4 (11.7) years and 1396 (54.5%) were male (Table 2). There were no statistically significant differences in the demographics of patients who were aware and unaware of their HbA1c goal. Overall, patients in the sub-analysis were currently using a mean of two AHAs, including metformin (62.5%) and very long-acting insulin (13.7%).
Table 2.
Physician and patient characteristics by awareness of agreed HbA1c goal
| Overall (N = 2560) | Patients unaware of agreed HbA1c goal (N = 756) | Patients aware of HbA1c goal (N = 1804) | p value | |
|---|---|---|---|---|
| Physician-level information | ||||
| Physician characteristics | ||||
| Physician specification | < 0.0001 (FE) | |||
| Primary care physician | 1556 (60.8) | 507 (67.1) | 1049 (58.1) | |
| Specialist (diabetologist/endocrinologist) | 1004 (39.2) | 249 (32.9) | 755 (41.9) | |
| Physician qualifying year | < 0.0001 (CH) | |||
| Before 1982 | 147 (5.7) | 49 (6.5) | 98 (5.4) | |
| 1982–1994 | 912 (35.6) | 287 (38.0) | 625 (34.6) | |
| 1995–2004 | 991 (38.7) | 237 (31.3) | 754 (41.8) | |
| 2005–2015 | 464 (18.1) | 166 (22.0) | 298 (16.5) | |
| After 2015 | 46 (1.8) | 17 (2.2) | 29 (1.6) | |
| Physician 5-day workload, mean patients with T2DM, n (SD) | 44.8 (35.1) | 43.2 (35.4) | 45.4 (35.0) | 0.1341 (TT) |
| Patient-level information | ||||
| Patient demographics | ||||
| Age, years, mean (SD) | 58.4 (11.7) | 59.0 (12.5) | 58.2 (11.3) | 0.0819 (TT) |
| Male, n (%) | 1396 (54.5) | 413 (54.6) | 983 (54.5) | 0.9653 (FE) |
| Body mass index, kg/m2, mean (SD) | 29.8 (5.3) | 29.9 (5.3) | 29.8 (5.3) | 0.8081 (TT) |
| Physician-reported clinical characteristics | ||||
| Median time since diagnosis, years (IQR) | 4.3 (1.9, 8.7) | 4.4 (2.0, 8.9) | 4.3 (1.9, 8.6) | 0.4012 (MW) |
| Mean most recent HbA1c | ||||
| Missing, n | 107 | 34 | 73 | |
| Mean % (SD) | 7.1 (1.2) | 7.1 (1.2) | 7.1 (1.1) | 0.5659 (TT) |
| Mean target HbA1c | ||||
| Missing, n | 31 | 18 | 13 | |
| Mean % (SD) | 6.8 (0.7) | 6.8 (0.9) | 6.8 (0.7) | 0.1705 (TT) |
| At (or below) current HbA1c % goal | ||||
| Missing, n | 136 | 51 | 85 | |
| n (%) | 949 (39.2) | 292 (41.4) | 657 (38.2) | 0.1430 (FE) |
| Current therapy class, n (%) | ||||
| Missing, n | 1 | 1 | 0 | |
| Metformin | 1600 (62.5) | 499 (66.1) | 1101 (61.0) | 0.0176 (FE) |
| Metformin monotherapy | 669 (26.1) | 267 (35.4) | 402 (22.3) | < 0.0001 (FE) |
| SU | 263 (10.3) | 76 (10.1) | 187 (10.4) | 0.8865 (FE) |
| DPP4i | 259 (10.1) | 80 (10.6) | 179 (9.9) | 0.6152 (FE) |
| SGLT2i | 407 (15.9) | 101 (13.4) | 306 (17.0) | 0.0243 (FE) |
| GLP-1 | 510 (19.9) | 98 (13.0) | 412 (22.8) | < 0.0001 (FE) |
| Very rapid-acting insulin | 134 (5.2) | 32 (4.2) | 102 (5.7) | 0.1727 (FE) |
| Very long-acting insulin | 350 (13.7) | 76 (10.1) | 274 (15.2) | 0.0005 (FE) |
| Any insulin | 532 (20.8) | 114 (15.1) | 418 (23.2) | < 0.0001 (FE) |
| Mean number of drugs in current regimen | ||||
| Missing, n | 1 | 1 | 0 | |
| n (SD) | 1.93 (0.89) | 1.77 (0.88) | 1.99 (0.88) | < 0.0001 (TT) |
| Mean current line of therapy | ||||
| Missing, n | 467 | 115 | 352 | |
| n (SD) | 1.68 (0.80) | 1.57 (0.72) | 1.73 (0.83) | < 0.0001 (TT) |
| Charlson Comorbidity Index score (SD)a | 0.3 (0.9) | 0.4 (0.9) | 0.3 (0.8) | 0.1758 (TT) |
| Patient-reported adherence and therapy satisfaction | ||||
| Mean total ARMS-D score (range 11–44)b | ||||
| Missing, n | 73 | 19 | 54 | |
| Mean (SD) | 15.0 (3.7) | 15.5 (3.9) | 14.8 (3.6) | < 0.0001 (TT) |
| Mean ARMS-D refill subscale score (range 4–16) | ||||
| Missing, n | 47 | 13 | 34 | |
| Mean (SD) | 6.2 (1.7) | 6.5 (1.7) | 6.1 (1.7) | < 0.0001 (TT) |
| Mean ARMS-D medication-taking subscale score (range 7–28) | ||||
| Missing, n | 58 | 15 | 43 | |
| Mean (SD) | 8.8 (2.6) | 9.0 (2.7) | 8.7 (2.5) | 0.0108 (TT) |
| Physician-reported glucose testing | ||||
| Proportion of patients self-testing glucose levels | ||||
| Missing, n | 7 | 2 | 5 | |
| n (%) | 1745 (68.4) | 412 (54.6) | 1333 (74.1) | < 0.0001 (FE) |
| Are physicians satisfied with the patient’s current blood glucose level? n (%) | 0.0012 (CH) | |||
| Yes | 1494 (58.4) | 455 (60.2) | 1039 (57.6) | |
| No, but this is the best that can be realistically achieved | 236 (9.2) | 88 (11.6) | 148 (8.2) | |
| No, and I believe that better control can be achieved | 830 (32.4) | 213 (28.2) | 617 (34.2) | |
| Consulting physician specialty | Overall (N = 2560) | Primary care physician (N = 1556) | Specialist (N = 1004) | |
| Patient-reported awareness of agreed HbA1c goal, n (%) | ||||
| Aware | 1804 (70.5) | 1049 (67.4) | 755 (75.2) | < 0.0001 (FE) |
ARMS-D Adherence to Refills and Medications Scale for Diabetes, CS chi-squared test, DPP4i dipeptidyl peptidase 4 inhibitor, FE Fisher’s exact test, GLP-1 glucagon-like peptide 1 receptor agonist, HbA1c glycated hemoglobin, IQR interquartile range, MW Mann–Whitney, SD standard deviation, SGLT2i sodium glucose cotransporter 2 inhibitors, SU sulfonylurea, TT t test
Total sample included where there are no missing data reported
aScale of 0, 1–2, 3–4, and ≥ 5; each increasing level represents an increase in the cumulative mortality attributable to comorbid disease
bScale of 1, none of the time to 4, and all of the time; higher ARMS total and subscale scores indicate poorer adherence
cScale of 1, very dissatisfied; 2, dissatisfied; 3, neither dissatisfied nor satisfied; 4, satisfied; and 5, very satisfied
As 70.9% of the patient sample (6234/8794) did not qualify for this sub-analysis, demographics and outcomes were also compared between those qualifying and not qualifying for the sub-analysis (i.e., patients with an unknown HbA1c goal, diagnosed with type 2 diabetes mellitus for less than 3 months, patients who did not chose to complete a self-reported record form, or those who chose not to answer the question on awareness of their HbA1c goal) to investigate potential for bias. Minimal differences were seen between patients qualifying and not qualifying for this sub-analysis (Supplementary Table 2).
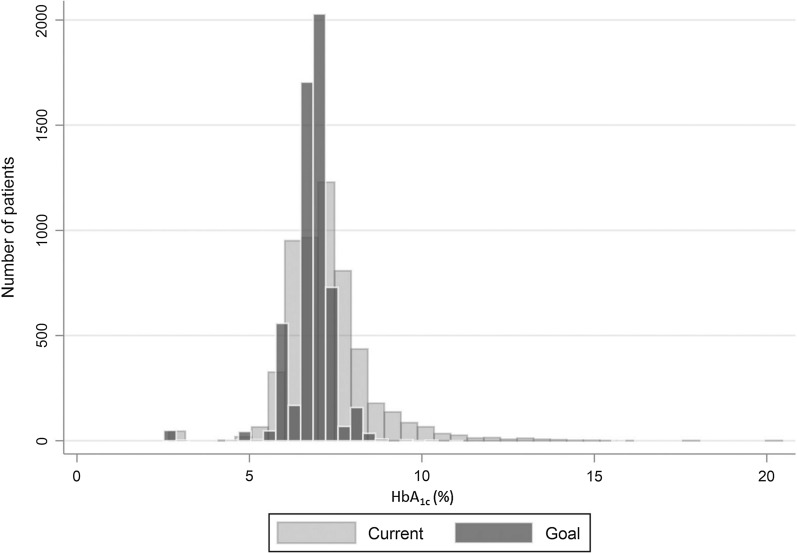
The individualized mean HbA1c goal was 6.8% (0.7%) for the combined 2560 patients who were aware and unaware of their HbA1c goal (Table 2). A higher proportion of patients aware of their HbA1c goal were in the 6% to 7.5% patient groupings when compared to other HbA1c goal groupings i.e. < 6% and 7.5%+ (Supplementary Table 3). Their current mean HbA1c value was 7.1% (1.2%). Distribution of current and target HbA1c among patients is shown in Fig. 3.
Fig. 3.
Distribution of current and target HbA1c among patients. HbA1c glycated hemoglobin
Awareness of Glycemic Goal and Effect on Goal Attainment
Although the majority of patients included in the sub-analysis were aware of their HbA1c goal, physicians reported that only 949 patients (39.2%) of these patients were successful in achieving their goal.
Achieving HbA1c goal was not related to knowledge of goal; 38.2% of patients aware of their HbA1c goal achieved it vs. 41.4% of those unaware of their goal (p = 0.143) (Table 2).
Age, gender, BMI, time since diagnosis, most recent previous HbA1c measure, or Charlson Comorbidity Index [28] did not differ among patients aware and unaware of their HbA1c goal (Table 2). Multivariate logistic regression controlling for these factors showed that patients who were aware of their HbA1c goal were not more likely to meet it than those who were unaware (odds ratio 0.925; 95% confidence interval 0.721–1.19; p = 0.541).
Patients who were aware of their individual HbA1c goal were using more AHAs in their current treatment regimen than those who were unaware of their HbA1c goal; 68.4% vs. 55% of patients, respectively, received more than one AHA (p < 0.0001). Those receiving vs. not receiving a GLP-1 receptor agonist, SGLT2i, or insulin were more aware of their HbA1c goal (p < 0.05). Patients aware of their HbA1c goal were also on a later line of therapy than patients who were unaware; 53.3% of patients aware of HbA1c goal were on second-line of therapy or later vs. 44.8% of patients unaware of goal.
While both patient groups who were aware and unaware of their agreed HbA1c goal reported good adherence to their AHAs, those who were aware of their goal reported slightly higher adherence (ARMS-D total score 14.8 vs. 15.5, respectively; p < 0.0001).
Physician-Reported Glucose Testing and Patient Awareness
Physicians reported that those patients who were aware of their HbA1c goal were more likely to test their blood glucose levels than patients who were unaware (74.1% vs. 54.6%; p < 0.0001) (Table 2). Physicians were not satisfied with the current blood glucose level of approximately one third of these patients. They believed that a higher proportion of patients who were aware of their HbA1c goal could achieve better glucose control as opposed to those who were unaware (32.4% vs. 28.2%, respectively; p = 0.003). Physicians were more satisfied with the current blood glucose level of patients aware of their HbA1c goal in the sub-analysis than those excluded patients not aware of their HbA1c goal (58.4% vs. 49.0%; p < 0.0001).
Physician Profiles
The physicians’ year of medical qualification and 5-day workload of patients with T2DM did not affect whether their patients were aware of an agreed HbA1c goal or not. However, patients of diabetologists/endocrinologists were more aware of their agreed HbA1c goal than patients of PCPs (75.2% vs. 67.4%; p < 0.0001) (Table 2).
Discussion
Major international guidelines recommend the determination of glycemic goals on an individual level based on the respective patient’s clinical profile. Data on individual glycemic goals and the proportion of patients achieving them are generally not available from large datasets, such as claims data. Therefore, we aimed to assess this important question in a survey of 8794 patients with type 2 diabetes mellitus in the USA and Europe. We found that two thirds of patients were not at the HbA1c goal set by their physician, which was on average 6.8%, and that physicians were not changing/adding AHAs until HbA1c was above 8%. The HbA1c goal of 6.8% in our analysis is comparable to patient-reported HbA1c goals set by physicians in other countries (6.1–6.9%), where 26–70% of patients reported that they had a specific HbA1c goal [29].
Waiting to change/add therapies could suggest therapeutic inertia, whereby physicians delaying intensification of treatment regimen of patients with type 2 diabetes mellitus when appropriate to achieve good glycemic control [30]. Moreover, it may also indicate that guideline recommendations of a target HbA1c of < 7.0% [1–3] are not being fully implemented by physicians in clinical practice. Studies have previously found that a considerable proportion of patients with type 2 diabetes mellitus with suboptimal glycemic control experience a delay in receiving treatment intensification with AHAs [31, 32]. The average time to treatment intensification from one to two AHAs agents in patients with HbA1c ≥ 7.0% was 2.9 years, 1.9 years in patients with HbA1c ≥ 7.5%, and 1.6 years in patients with HbA1c ≥ 8.0% [31]. Evidence suggests that patients with type 2 diabetes mellitus do not receive intensified treatment for over a year after monotherapy failure, with half of patients waiting over 5 years [33].
Similarly to our analysis, other studies confirm that patients do not receive treatment intensification until their HbA1c is > 8 [33, 34]. Of concern, therapy for around half of patients with an HbA1c of 8 to ≥ 9% is not intensified [35]. Patients with type 2 diabetes mellitus who intensified treatment earlier have been found to have higher mean HbA1c levels, suggesting that physicians react to disease severity [33, 36]. Early treatment intensification also resulted in patients achieving a greater mean decline in HbA1c level [33, 36]. Moreover, patients receiving rapid treatment intensification appear to achieve a maintained HbA1c reduction faster than patients with delayed treatment intensification or no second-line therapy, despite a higher HbA1c at baseline [37].
Challenges for healthcare systems such as poor communication between healthcare providers, lack of a coordinated care plan, and time limitations may also play a role in inertia with later therapy lines [38, 39].
Over two thirds of patients in our analysis (68.1%) were utilizing more than one AHA; most were receiving metformin with fewer on insulin, a GLP-1 receptor agonist, and/or a SGLT2i. A study with a cohort of around 21,000 newly treated patients with type 2 diabetes mellitus (76% initiated on metformin) in the Republic of Ireland found that about 20% of those who remained on their initial therapy were non-persistent to their treatment (i.e., treatment gap of more than 12 weeks within 365 days of treatment initiation) [40]. Of those changing treatment regimens, treatment additions were more frequent than changes. After metformin, treatment additions were sulfonylurea followed by a dipeptidyl peptidase 4 inhibitor, and changes were most frequently to a sulfonylurea followed by a metformin combination product [40].
Although the majority of patients (n = 1804, 70.5%) included in the sub-analysis were aware of their HbA1c goal, interestingly, awareness of HbA1c goal did not enhance goal attainment. Other studies have reported that two thirds or more of patients with type 2 diabetes mellitus did not know their last HbA1c [20, 41]. In one study, the few patients who knew their last HbA1c value reported a biomedically accurate level of diabetes control and better understanding of diabetes care compared with those who did not know their HbA1c value [20]. However, such knowledge of HbA1c did not translate into improved diabetes self-management [20].
With a decline in beta-cell function and mass, more treatments fail to control glycemic levels and there is an increasing risk of the development of complications [42]. Patients may become more aware of their goal if they have more recalcitrant disease because it makes goal achievement more difficult. Our analysis indicated that patients who were aware of their HbA1c goal were on more agents and insulin, and to consult with a specialist rather than a PCP. They had also used more lines of therapy and tested their glucose level more frequently. Patients on metformin monotherapy, typically those who are easier to treat, met their goal even though they were less likely to know what that goal was.
Our findings should be considered in light of the survey limitations. The non-random sample of physicians led to over-representation of specialists based on national PCP-to-specialist ratios [43]. Potential differences between PCPs and diabetologists/endocrinologists in knowledge level and diabetes management may have affected patient treatment and clinical outcomes. Furthermore, the patient population was not truly random because of the inclusion of the next ten consecutive consulting patients and two additional patients receiving SGLT2i or GLP-1 therapy. Additionally, analysis of the overall patient population excluded 39.4% of patients for whom current HbA1c or individualized HbA1c goals were unavailable, or who had been diagnosed with type 2 diabetes mellitus for less than 3 months. Although unsurprising, the reduction of patient numbers evaluated at each change/addition in line of therapy was also recognized. Lastly, this analysis included patients from different European countries and the USA; therefore, findings may have been affected by country variations in clinical practice and may differ should data be drawn from one or more other countries. Further research is required at a country level. Of note, data was collected prior to the emergence of coronavirus disease (COVID-19) and does not reflect shifting practice patterns caused by the pandemic.
In conclusion, we demonstrated that the proportion of patients with type 2 diabetes mellitus achieving their goals for glycemic control remained suboptimal when compared to current guideline criteria. Despite the availability of many new antihyperglycemic medications, about 60% of patients with type 2 diabetes mellitus did not achieve their individualized HbA1c goal. Intensification of treatment was often delayed until HbA1c was 8% or higher. Results of this analysis highlighted the need for a holistic approach to diabetes management, involving patient education, and patient–physician communication and partnership.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Diabetes DSP. Merck & Co., Inc., Kenilworth, NJ, USA, and Pfizer Ltd., Tadworth, UK, did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the Adelphi Diabetes DSP. The DSP is a wholly owned Adelphi product. Merck & Co., Inc., Kenilworth, NJ, USA, and Pfizer Ltd., Tadworth, UK, are two of multiple subscribers to the DSP. The journal’s Rapid Service Fee was sponsored by Merck & Co.
Medical Writing
Medical writing was provided by Sue Libretto, PhD, of Sue Libretto Publications Consultant Ltd (Hertfordshire, UK).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
DL responsible for clinical oversight and guidance as lead author. Study set-up and data collection were led by VH. Analysis design was conducted by all authors with statistical analyses prepared by GM. DL, RB and VH wrote the manuscript supported by a medical writer. All authors had access to the aggregated data, participated in manuscript development, provided critical feedback and approved the final manuscript, assume responsibility for the accuracy and completeness of the data, and vouch for the study’s fidelity to the protocol.
Prior Presentation
This is an original work and is not under consideration by any other journal.
The data were presented in part at the Virtual 56th European Association for the Study of Diabetes (EASD) Annual Meeting, September 21–25, 2020: Boggs R et al. Impact of T2DM patients’ knowledge of their individualized HbA1c goal on glycemic control; and the International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE) All Access, September 16–19, 2020: Lautsch D et al. Individualized glycemic control in type 2 diabetes in Europe and the United States.
Disclosures
Dominik Lautsch, Robert Boggs, Tongton Wang, Claudio Gonzalez, Swapnil Rajpathak and Seema Malkani are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Euan McLeod is an employee of Pfizer Ltd., Tadworth, UK, and may own stock and/or hold stock options in Pfizer Ltd., Tadworth, UK. Gary Milligan, James Carroll and Victoria Higgins are employees of Adelphi Real World, Bollington, UK.
Compliance with Ethics Guidelines
The survey obtained ethics approval from the Western Institutional Review Board, study protocol number 1247198, and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Physicians did not see patient responses, thereby ensuring that future interactions between physicians and their patients were not compromised by patient responses. Patients provided written informed consent for use of their anonymized and aggregated data.
Data Availability
All data relevant to the analysis are included in the article. All data that support the findings of this survey are the intellectual property of Adelphi Real World. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHA
Antihyperglycemic agent
- ARMS-D
Adherence to Refills and Medicines Scale for Diabetes
- BMI
Body mass index
- CKD
Chronic kidney disease
- COVID-19
Coronavirus disease
- CS
Chi-squared test
- DSP
Disease Specific Programme™
- FE
Fisher’s exact test
- GLP-1
Glucagon-like peptide 1
- HbA1c
Hemoglobin A1c
- PCP
Primary care physician
- SD
Standard deviation
- SGLT2i
Sodium glucose cotransporter 2 inhibitor
- SU
Sulfonylurea
- TT
t test
- UK
United Kingdom
- USA
United States of America
References
- 1.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. Recommendations for managing type 2 diabetes in primary care, 2017. www.idf.org/managing-type2-diabetes. Accessed 01 Mar 2021.
- 4.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 7.Cosentino F, ESC Scientific Document Group et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association 9. Glycemic targets: standards of medical care in diabetes. Diabetes Care. 2021;44(Suppl 1):S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 9.Dalal MR, Grabner M, Bonine N, Stephenson JJ, DiGenio A, Bieszk N. Are patients on basal insulin attaining glycemic targets? Characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract. 2016;121:17–26. doi: 10.1016/j.diabres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama H, Oishi M, Takamura H, et al. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40) BMJ Open Diabetes Res Care. 2016;4(1):e000294. doi: 10.1136/bmjdrc-2016-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425–1432. doi: 10.2337/dc16-1974. [DOI] [PubMed] [Google Scholar]
- 12.Blonde L, Patel C, Bookhart B, Pfeifer M, Chen YW, Wu B. A real-world analysis of glycemic control among patients with type 2 diabetes treated with canagliflozin versus dapagliflozin. Curr Med Res Opin. 2018;34(6):1143–1152. doi: 10.1080/03007995.2018.1458709. [DOI] [PubMed] [Google Scholar]
- 13.Blonde L, Brunton SA, Chava PSJ, et al. Achievement of target A1C < 70% (< 53 mmol/mol) by US type 2 diabetes patients treated with basal insulin in both randomized controlled trials and clinical practice. Diabetes Spectr. 2019;32(2):93–103. doi: 10.2337/ds17-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson S, Boye KS, Mody R, et al. Real-world effectiveness of dulaglutide in patients with type 2 diabetes mellitus: a literature review. Diabetes Ther. 2020;11(7):1437–1466. doi: 10.1007/s13300-020-00839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berikai P, Meyer PM, Kazlauskaite R, Savoy B, Kozik K, Fogelfeld L. Gain in patients' knowledge of diabetes management targets is associated with better glycemic control. Diabetes Care. 2007;30(6):1587–1589. doi: 10.2337/dc06-2026. [DOI] [PubMed] [Google Scholar]
- 16.Beard E, Clark M, Hurel S, Cooke D. Do people with diabetes understand their clinical marker of long-term glycemic control (HbA1c levels) and does this predict diabetes self-care behaviours and HbA1c? Patient Educ Couns. 2010;80(2):227–232. doi: 10.1016/j.pec.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Trivedi H, Gray LJ, Seidu S, et al. Self-knowledge of HbA1c in people with type 2 diabetes mellitus and its association with glycaemic control. Prim Care Diabetes. 2017;11(5):414–420. doi: 10.1016/j.pcd.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Milo RB, Connelly CD. Predictors of glycemic management among patients with type 2 diabetes. J Clin Nurs. 2019;28(9–10):1737–1744. doi: 10.1111/jocn.14779. [DOI] [PubMed] [Google Scholar]
- 19.Gopalan A, Kellom K, McDonough K, Schapira MM. Exploring how patients understand and assess their diabetes control. BMC Endocr Disord. 2018;18(1):79. doi: 10.1186/s12902-018-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heisler M, Piette JD, Spencer M, Kieffer E, Vijan S. The relationship between knowledge of recent HbA1c values and diabetes care understanding and self-management. Diabetes Care. 2005;28(4):816–822. doi: 10.2337/diacare.28.4.816. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson MO, Long JA, Zhu J. Low health literacy predicts misperceptions of diabetes control in patients with persistently elevated A1C. Diabetes Educ. 2015;41(3):309–319. doi: 10.1177/0145721715572446. [DOI] [PubMed] [Google Scholar]
- 22.Finer S, Robb P, Cowan K, Daly A, Shah K, Farmer A. Setting the top 10 research priorities to improve the health of people with type 2 diabetes: a diabetes UK-James Lind Alliance Priority Setting Partnership. Diabet Med. 2018;35(7):862–870. doi: 10.1111/dme.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 24.Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi: 10.2147/DMSO.S120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. doi: 10.1111/j.1524-4733.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- 27.Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013;102(2):96–104. doi: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Simacek K, Curran C, Fenici P, Garcia-Sanchez R. Patient perceptions of their glycemic control and its influence on type 2 diabetes outcomes: an international survey of online communities. Patient Prefer Adherence. 2019;13:295–307. doi: 10.2147/PPA.S186801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12. doi: 10.1016/j.pcd.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–3417. doi: 10.2337/dc13-0331:3411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535–1540. doi: 10.2337/diacare.27.7.1535. [DOI] [PubMed] [Google Scholar]
- 33.Desai U, Kirson NY, Kim J, et al. Time to treatment intensification after monotherapy failure and its association with subsequent glycemic control among 93,515 patients with type 2 diabetes. Diabetes Care. 2018;41(10):2096–2104. doi: 10.2337/dc17-0662. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes G, Sawhney B, Hannachi H. Distance to glycemic goal at the time of treatment intensification in patients with type 2 diabetes mellitus failing metformin monotherapy in the United States. Curr Med Res Opin. 2020;36(5):741–748. doi: 10.1080/03007995.2020.1722623. [DOI] [PubMed] [Google Scholar]
- 35.Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Clinical inertia in type 2 diabetes management: evidence from a large, real-world data set. Diabetes Care. 2018;41(7):e113–e114. doi: 10.2337/dc18-0116. [DOI] [PubMed] [Google Scholar]
- 36.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. doi: 10.1186/s12933-015-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson L, Das R, Farquhar R, Langerman H, Barnett AH. Consequences of delaying treatment intensification in type 2 diabetes: evidence from a UK database. Curr Med Res Opin. 2016;32(9):1465–1475. doi: 10.1185/03007995.2016.1157462. [DOI] [PubMed] [Google Scholar]
- 38.Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43(6):501–511. doi: 10.1016/j.diabet.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Rushforth B, McCrorie C, Glidewell L, Midgley E, Foy R. Barriers to effective management of type 2 diabetes in primary care: qualitative systematic review. Br J Gen Pract. 2016;66(643):e114–e127. doi: 10.3399/bjgp16X683509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimes RT, Bennett K, Tilson L, Usher C, Smith SM, Henman MC. Initial therapy, persistence and regimen change in a cohort of newly treated type 2 diabetes patients. Br J Clin Pharmacol. 2015;79(6):1000–1009. doi: 10.1111/bcp.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harwell TS, Dettori N, McDowall JM. Do persons with diabetes know their (A1C) number? Diabetes Educ. 2002;28(1):99–105. doi: 10.1177/014572170202800111. [DOI] [PubMed] [Google Scholar]
- 42.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–S156. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hing E, Hsiao C-J. State variability in supply of office-based primary care providers: United States, 2012. National Center for Health Statistics. NCHS Data Brief No. 151, May 2014. https://www.cdc.gov/nchs/data/databriefs/db151.pdf. Accessed 17 Feb 2021. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the analysis are included in the article. All data that support the findings of this survey are the intellectual property of Adelphi Real World. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.