Abstract
Xanthomonas oryzae delivers transcription activator-like effectors (TALEs) into plant cells to facilitate infection. Following economic principles, the redundant TALEs are rarely identified in Xanthomonas. Previously, we identified the Tal2b, which activates the expression of the rice 2-oxoglutarate-dependent dioxygenase gene OsF3H03g to promote infection in the highly virulent strain of X. oryzae pv. oryzicola HGA4. Here, we reveal that another clustered TALE, Tal2c, also functioned as a virulence factor to target rice OsF3H04g, a homologue of OsF3H03g. Transferring Tal2c into RS105 induced expression of OsF3H04g to coincide with increased susceptibility in rice. Overexpressing OsF3H04g caused higher susceptibility and less salicylic acid (SA) production compared to wild-type plants. Moreover, CRISPR–Cas9 system-mediated editing of the effector-binding element in the promoters of OsF3H03g or OsF3H04g was found to specifically enhance resistance to Tal2b- or Tal2c-transferring strains, but had no effect on resistance to either RS105 or HGA4. Furthermore, transcriptome analysis revealed that several reported SA-related and defense-related genes commonly altered expression in OsF3H04g overexpression line compared with those identified in OsF3H03g overexpression line. Overall, our results reveal a functional redundancy mechanism of pathogenic virulence in Xoc in which tandem Tal2b and Tal2c specifically target homologues of host genes to interfere with rice immunity by reducing SA.
Keywords: rice, bacterial leaf streak, disease resistance, TALEs, 2OGD, Xanthomonas oryzae
1. Introduction
During rice production, the Xanthomonas phytopathogen Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas oryzae pv. oryzicola (Xoc) cause bacterial blight (BB) and bacterial leaf streak (BLS) diseases and yield losses of up to 50% and 32% under favorable conditions, respectively [1,2]. Currently, BLS has attracted more attention because of its increasing risk of frequent outbreaks and tremendous damage in Asia and Africa [3,4]. In China, BLS has also attracted attention because it is a plant quarantine disease for restricted seed production and commercial sales [5]. In addition to other Xanthomonas species, Xoc mainly uses the bacterial type III secretion system to deliver dozens of type III effectors, including transcription activator-like effectors (TALEs) and non-TALE effectors (non-TALEs), into rice to serve as virulence or avirulence factors [1]. TALEs belong to a unique family of transcription activators that conservatively consist of N-terminal, nuclear localization signal (NLS) motifs and acidic transcriptional activation domains (ADs) in the C-terminus and tandem repeats composed of 33 to 35 amino acids in the central region [6]. Each central repeat contains two hypervariable residues termed repeat-variable diresidue (RVD) at positions 12 and 13 that determine the recognition of one base in the DNA [7]. In a sequential fashion, each TALE contains up to 33.5 tandem repeats, which determine the specific recognition DNA sequence named the effector-binding elements (EBEs) on target genes [7,8]. After translocation into host nuclei, TALEs usually mimic the plant transcription factors to activate the expression of target genes.
Several non-TALEs have biological virulence functions in Xoc. For example, XopAJ/AvrRxo1 functions as an NAD kinase to suppress ROS burst [9,10] and XopC functions as an atypical kinase that phosphorylates OSK1 to suppress stomatal immunity [11]. Most candidate targeted genes for each TALE in sequenced Xoc strains have been predicted [12,13]. The development of functional analysis of TALEs is proceeding rapidly in Xoc. For instance, Tal2g was the first identified virulent TALE in Xoc BLS256, and it targets EBE in the promoter and activates the expression of the rice sulfate transporter gene.
OsSULTR3;6 promotes infection [13]. Tal2h is a truncated TALE in BLS256 that interacts with and inhibits Xo-1-mediated resistance in Carolina Gold Select rice independent of DNA-binding activity [14,15]. Tal7 targets rice cyclin-D4-1 and GATA zinc finger family protein genes to repress avrXa7/Xa7-mediated defense in rice [16]. However, overexpression of Tal2a in BLS256 decreased virulence by targeting UCH, a rice ubiquitin carboxy-terminal hydrolase gene [17]. Tal2b (also known as TalBR1 and TalAQ3/Tal9b in Xoc and Xoo, respectively) and Tal2c (TalBL1) are clustered in some Xoc strains, such as BLS256 and HGA4 [4]. They were predicted to target genes encoding rice 2-oxoglutarate-dependent dioxygenase (2OGD), LOC_Os03g03034 (OsF3H03g) and LOC_Os04g49194 (OsF3H04g) [18]. Interestingly, a single mutant of Tal2b or Tal2c did not affect virulence in BLS256 [13], suggesting that they may present redundant functions with each other. Recently, we identified that Tal2b could enhance the pathogenicity of RS105 by targeting OsF3H03g to inactivate salicylic acid (SA) [4]. However, the redundancy function of Tal2c and its putative target OsF3H04g remain unclear.
The 2OGD family is involved in oxidative and hydroxylated reactions of different kinds of plant metabolites, and it is classified into four categories: DOXA, DOXB, DOXC and JMJ [19,20]. The DOXA and DXOB families are usually involved in DNA demethylation and proline hydroxylation, respectively. The JMJ family usually functions in histone lysine residue demethylation. The DOXC family may participate in the metabolism of various hormones and secondary metabolites [21], such as hydroxylation of SA, one of the key plant regulators involved in various plant defense responses [22]. In Arabidopsis, DMR6/S5H produces the inactive form of 2,5-dihydroxybenzoic acid (2,5-DHBA) from the substrate of SA. A mutant of DMR6/S5H could result in high accumulation of SA and resistance to Hyaloperonospora parasitica [23,24,25]. The DMR6/S5H homologue, S3H/DLO1, functions in the process of 2,3-dihydroxybenzoic acid (2,3-DHBA) by hydroxylating SA in vivo [26]. Recently, the loss of function of DMR-like genes was found to confer resistance to different phytopathogens in several plant species. For instance, gene editing of StDMR6-1 in potato, ObDMR6 in sweet basil and MusaDMR6 in banana was found to specifically enhance resistance to late blight [27], downy mildew [28] and banana Xanthomonas wilt [29]. Moreover, gene editing of SlDMR6-1 increases broad-spectrum disease resistance to bacteria, oomycetes and fungi in tomato [30]. In rice, the flavanone 3-hydroxylase (F3H) gene OsF3H03g is the target of Tal2b and Tal9b from Xoc and Xoo, respectively [13,18], which is involved in positively regulating resistance to brown planthoppers by accumulating flavonoid content [31], as well as negatively regulating resistance to BB, BLS and sheath blight along with a reduction of SA [4].
The CRISPR–Cas9 system is a powerful tool for studying the function of genes in many organisms [32]. Currently, it has been successfully applied for editing the promoter EBE of several susceptibility genes to generate broad-spectrum resistance to BB and BLS in rice. The sucrose transporter genes OsSWEET11, OsSWEET13 and OsSWEET14 are specific targets of the Xoo TALEs PthXo1, PthXo2 and PthXo3/AvrXa7, and they confer broad-spectrum resistance to BB after EBE edition in rice [33,34,35]. The EBE edited lines in the promoter of OsSULTR3;6 increase resistance to Xoc strains BLS256 and RS105 [36]. Furthermore, gene editing of all three EBEs in the promoters of OsSWEET11, OsSWEET14 and OsSULTR3;6 confers resistance to both BLS and BB without any effect on agronomic traits in rice [37]. Thus, discovery of the recognition of TALEs and susceptible genes provides important guidance to generate resistant rice by editing EBEs.
Recently, we identified a highly virulent strain of Xoc HGA4 that showed four expanded TALEs not found in RS105. We found that Tal2b increases the virulence of RS105 by activating the expression of OsF3H03g [4]. The main objective of this study was to investigate the virulence contributions for other TALEs. Aimed at this goal, we focused on Tal2c, which is one of the four expanded TALEs that also act as a virulence factor to increase pathogenicity after introduction into RS105. Furthermore, we overexpressed (OE) the targeted gene of OsF3H04g, edited EBE editing in the OsF3H04g or OsF3H03g promoter and performed transcriptome analysis of the OsF3H04g OE line. All results suggest that the pair of TALEs, Tal2b and Tal2c, target two 2OGD family genes to redundantly regulate susceptibility to BLS in rice.
2. Results
2.1. Tal2c Acts as a Virulence Factor
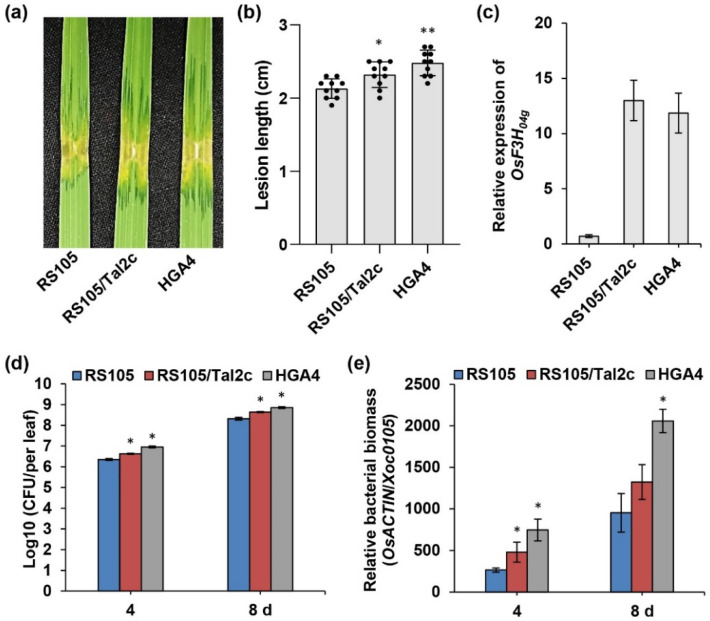
There are four expanded TALEs in Xoc HGA4 but not in RS105, namely Tal2b, Tal2c, Tal2d and Tal2e [4]. Single mutants of Tal2b or Tal2c had no significant effect on the virulence of BLS256 [13]. Tal2b has been identified as a virulence factor for enhanced susceptibility after introduction into RS10 [4]. Thus, we further introduced Tal2c into RS105 for pathogenicity investigation. As shown in Figure 1a,b, RS105/Tal2c (2.32 ± 0.17 cm) caused an intermediate lesion length longer than that of RS105 (2.13 ± 0.13 cm) but less than that of HGA4 (2.48 ± 0.17 cm). Compared with RS105, both RS105/Tal2c and HGA4 induced an increase in OsF3H04g expression by 10-fold at 4 days post inoculation (Figure 1c). Consistent with the phenotype, we also observed that the bacterial population and relative biomass of RS105/Tal2b were larger than those of RS105 but lower than those of HGA4 in rice leaves during infection (Figure 1d,e). These results suggest that Tal2c acts as a virulence factor in Xoc HGA4.
Figure 1.
Tal2c from the Xoc strain HGA4 enhances the pathogenicity of Xoc strain RS105 by increasing the expression of OsF3H04g. (a) Image of lesion expansion and (b) lesion length in ZH11 rice leaves after inoculation with RS105, RS105/Tal2c and HGA4 at 14 days. Data represent the means ± SD, n = 10. Asterisks represent significant differences between RS105 and RS105/Tal2c, RS105 and HGA4 (* p ≤ 0.05, ** p ≤ 0.01, Student’s t-test). (c) Relative expression of OsF3H04g at 4 days post inoculation (dpi) with RS105, RS105/Tal2c and HGA4. The internal control was used, OsACTIN. Data represent the means ± SD, n = 3. (d) Bacterial populations and (e) relative bacterial biomass of RS105, RS105/Tal2c and HGA4 in ZH11 rice at 4 and 8 days after inoculation. Data represent the means ± SD, n = 3. Total genomic DNA of ZH11 rice inoculated with Xoc strains at 0 dpi was used as a control. Asterisks represent significant differences between RS105 and RS105/Tal2c, RS105 and HGA4 (* p ≤ 0.05, Student’s t-test).
2.2. Tal2c Targets OsF3H04g and Tandem Pairs of Tal2b in Xoc
Tal2c was predicted to bind the EBE sequence at the OsF3H04g promoter region and activate the expression of uidA driven under the promoter of OsF3H04g in N. benthamiana using a transient expression system [13,18]. We also found that both Tal2c-containing strains of RS105/Tal2c and HGA4 could activate the expression of OsF3H04g (Figure 1c). To further validate the activation ability of Tal2c, we coexpressed it with green fluorescence protein (GFP) driven under the promoter of OsF3H04g (04gPRO-GFP) or EBE deletion (04gPRO∆EBE-GFP). Compared to each control, coexpressed Tal2c activated strong fluorescence with 04gPRO-GFP but not with 04gPRO∆EBE-GFP (Figure S1). There are different names for homologues to Tal2c in sequenced Xoc strains (Figure S2). Based on the characteristic pair of RVDs at positions 12 and 13 of each central repeat, Tal2c orthologues from different Xoc strains were annotated according to a previous report [38]. Interestingly, we found that Tal2c appeared in tandem with Tal2b in most sequenced Xoc strains (Table 1).
Table 1.
The Tal2b and Tal2c orthologs appeared to be tandem pairs in most Xoc strains.
| Xoc Strains | Tal2b Ortholog | Tal2c Ortholog | Area of Isolation |
|---|---|---|---|
| HGA4 | + | + | China |
| RS105 | − | − | China |
| L8 | + | + | China |
| B8-12 | + | + | China |
| GX01 | + | + | China |
| 0–9 | + | + | China |
| BLS256 | + | + | Philippines |
| BLS279 | + | + | Philippines |
| CFBP2286 | + | + | Malaysia |
| CFBP7331/MAI10 | + | + | Mali |
| CFBP7341/BAI5 | + | + | Burkina |
| CFBP2286/BAI11 | + | + | Burkina |
| BXOR1 | + | + | India |
2.3. Overexpression of OsF3H04g Increases Susceptibility to Xoc in Rice
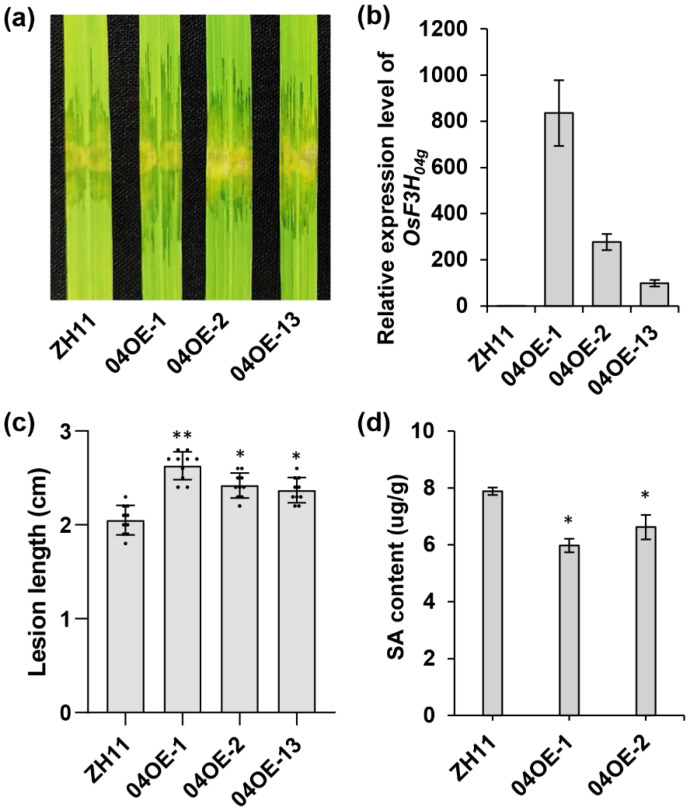
Tal2c enhances the pathogenicity of RS105 and activates the expression of OsF3H04g in rice (Figure 1a,c). To investigate the function of OsF3H04g, we generated 15 OsF3H04g overexpression (OE) lines driven under the ubiquitin promoter in ZH11. Three lines named 04OE-1, 04OE-2 and 04OE-13 were selected for the detection of OsF3H04g expression in the T2 generation (Figure 2a). Compared with wild-type ZH11, the expression of OsF3H04g was specifically increased 834-, 276- and 98-fold in the 04OE-1, 04OE-2 and 04OE-13 lines (Figure 2b). Additionally, disease resistance assays were performed on three OE lines, and they caused more severe symptoms in all OsF3H04g OE lines than in ZH11 after inoculation with RS105 (Figure 2a). The lesion lengths of 04OE-1 (2.63 ± 0.15 cm), 04OE-2 (2.42 ± 0.13 cm) and 04OE-13 (2.37 ± 0.13 cm) were longer than that of ZH11 (2.05 ± 0.16 cm) (Figure 2c). Taken together, we concluded that overexpression of OsF3H04g results in increased susceptibility to RS105. OsF3H03g negatively regulates the resistance to RS105 by inactivating SA in rice [4]. To test the effect of OsF3H04g on SA, we then performed SA quantification on two OsF3H04g OE lines. The results showed that the SA content also decreased in the OsF3H04g OE lines (Figure 2d).
Figure 2.
Overexpression of OsF3H04g increases rice susceptibility to Xoc strain RS105. (a) Photograph of lesion expansion at 14 dpi with RS105 in ZH11 and OsF3H04g OE lines. (b) Quantification of OsF3H04g expression in the ZH11 and OsF3H04g overexpression (OE) lines (04OE-1, 04OE-2 and 04OE-13). OsACTIN was used as an internal control. Data represent the means ± SD, n = 3. (c) Diagram of lesion lengths in ZH11 and OsF3H04g OE lines at 14 dpi with RS105. Data represent the means ± SD, n = 10. Asterisks represent significant differences between ZH11 and OsF3H04g OE lines (* p ≤ 0.05, ** p ≤ 0.01, Student’s t-test). (d) Salicylic acid (SA) content in ZH11 and OsF3H04g OE lines (04OE-1 and 04OE-2). Data represent the means ± SD, n = 3. Asterisks represent significant differences between ZH11 and OsF3H04g OE lines (* p ≤ 0.05, Student’s t-test).
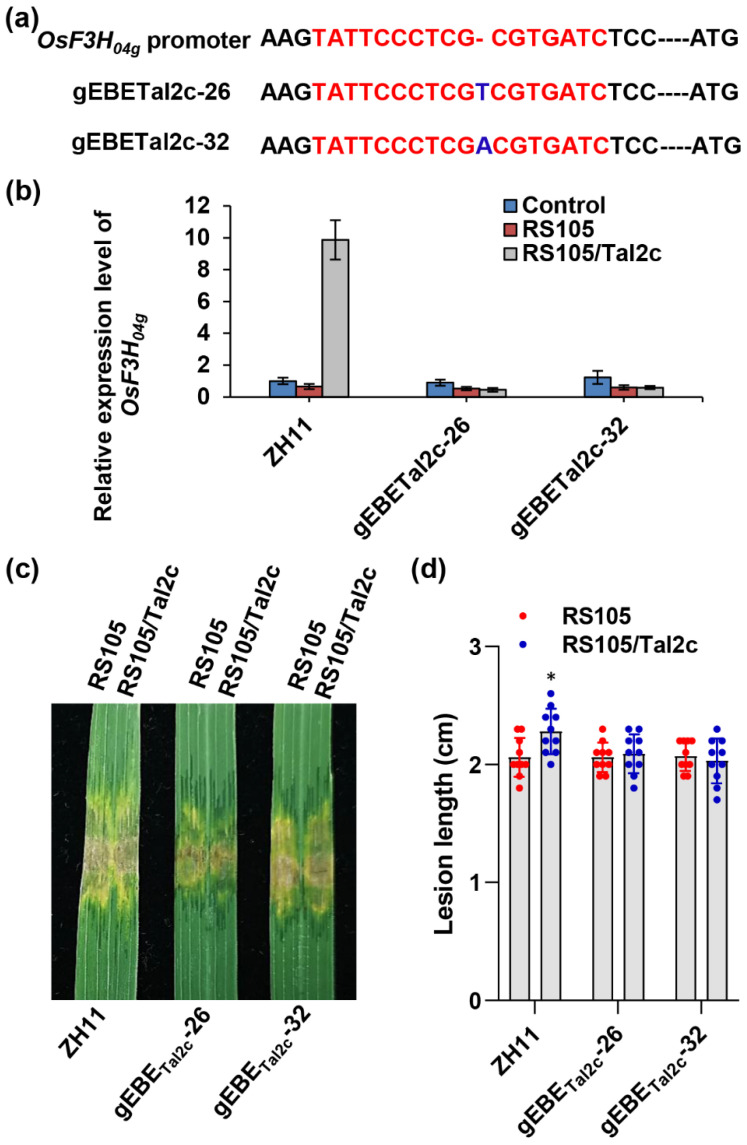
2.4. Editing EBE of the OsF3H04g Promoter Compromised Tal2c-Mediated Susceptibility in Rice
Tal2c was predicted to bind EBE in the promoter of OsF3H04g to activate gene expression and promote infection. Thus, we designed guide RNA and performed gene editing of the EBE in the OsF3H04g promoter. Two individual lines were identified to homozygously insert a “T” and “A” base in lines gEBETal2c-26 and gEBETal2c-32, respectively (Figure 3a and Figure S3). We first measured the induction of OsF3H04g by inoculation with RS105 and RS105/Tal2c on the gEBETal2c-26 and gEBETal2c-32 lines. We observed that the induced expression of OsF3H04g by RS105/Tal2c was abolished in the two lines, gEBETal2c-26 and gEBETal2c-32, compared to the wild-type ZH11 (Figure 3b). In accordance with the deficient induction of OsF3H04g, the disease symptoms caused by RS105/Tal2c in gEBETal2c-26 and gEBETal2c-32 were milder than those in ZH11, while the disease symptoms caused by RS105 were similar to those in ZH11 and the two EBE gene-edited lines (Figure 3c). As shown in Figure 3d, similar lesion lengths caused by RS105 were observed in ZH11 (2.06 ± 0.16 cm), gEBETal2c-26 (2.06 ± 0.13 cm) and gEBETal2c-32 (2.07 ± 0.12 cm). However, the lesion lengths caused by RS105/Tal2c in gEBETal2c-26 (2.09 ± 0.17 cm) and gEBETal2c-32 (2.03 ± 0.19 cm) were shorter than that in ZH11 (2.28 ± 0.19 cm) and similar to those after inoculation with RS105. The above results indicated that the induction expression of OsF3H04g determined by EBE is required for the virulence of Tal2c.
Figure 3.
Gene editing of EBE in the OsF3H04g promoter decreases rice susceptibility to Xoc strain RS105/Tal2c. (a) Genotypes of the EBE gene editing lines (gEBETal2c-26 and gEBETal2c-32) in the OsF3H04g promoter. The sequences in red represent the Tal2c binding sites, and the bases in blue represent the insertion in the OsF3H04g promoter. (b) Relative expression level of OsF3H04g in ZH11, gEBETal2c-26 and gEBETal2c-32 lines at 4 dpi with RS105 and RS105/Tal2c. OsACTIN was used as an internal control. Data represent the means ± SD, n = 3. (c) Photograph of lesion expansion and (d) diagram of the lesion lengths in the ZH11, gEBETal2c-26 and gEBETal2c-32 lines at 14 dpi with RS105 and RS105/Tal2c. Data represent the means ± SD, n = 10. Asterisks represent significant differences between RS105 and RS105/Tal2c in ZH11 rice (* p ≤ 0.05, Student’s t-test).
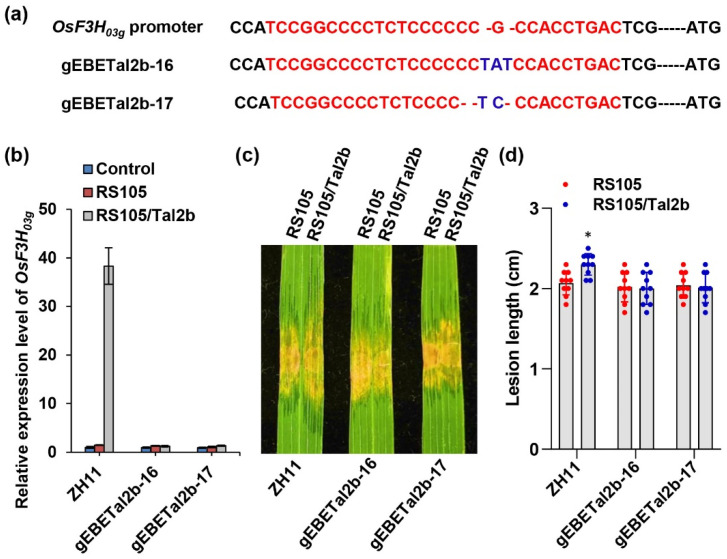
2.5. Gene Editing of EBE in the OsF3H03g Promoter Specifically Attenuates Rice Susceptibility to RS105/Tal2b
We previously demonstrated that Tal2b could activate the expression of OsF3H03g and thus contribute to the virulence of Xoc HGA4 [4]. However, OsF3H03g was involved in negatively regulating rice immunity, and gene editing of the CDS of the gene resulted in broad resistance to Xoc and Xoo strains, regardless of whether they carried Tal2b [4]. To mine additional evidence to support the hypothesis that Tal2b redundantly functions with Tal2c, we also performed gene editing of EBE in the promoter of the Xoc susceptible gene OsF3H03g in ZH11. The two lines gEBETal2b-16 and gEBETal2b-17 were identified as homozygous substitutions of “TAT” to “G” and “TC” to “CCG” in the EBE region, respectively (Figure S4 and Figure 4a). RS105/Tal2b was abolished to induce the expression of OsF3H03g in both gEBETal2b-16 and gEBETal2b-17 (Figure 4b). After inoculation with RS105 or RS105/Tal2b on ZH11, gEBETal2b-16 and gEBETal2b-17, five out of six inoculations caused similar disease symptoms and lesion lengths, except RS105/Tal2b, which caused milder symptoms and longer lesion lengths on ZH11 compared with the five inoculations (Figure 4c,d). Similar to the above specific interaction between Tal2c and OsF3H04g, these results suggest that OsF3H03g is specifically required for Tal2b-mediated virulence.
Figure 4.
Gene editing of EBE in the OsF3H03g promoter reduces rice susceptibility to Xoc strain RS105/Tal2b. (a) Genotypes of the EBE gene editing lines (gEBETal2b-16 and gEBETal2b-17) in the OsF3H03g promoter. The sequences in red represent the Tal2b binding sites, and the bases in blue represent the substitution in the OsF3H03g promoter. (b) Relative expression level of OsF3H03g in the ZH11, gEBETal2b-16 and gEBETal2b-17 lines at 4 dpi with RS105 and RS105/Tal2b. OsACTIN was used as an internal control. Data represent the means ± SD, n = 3. (c) Photograph of lesion expansion and (d) diagram of lesion length in the ZH11, gEBETal2b-16 and gEBETal2b-17 lines at 14 dpi with RS105 and RS105/Tal2b. Data represent the means ± SD, n = 10. Asterisks represent significant differences between RS105 and RS105/Tal2b in ZH11 rice (* p ≤ 0.05, Student’s t-test).
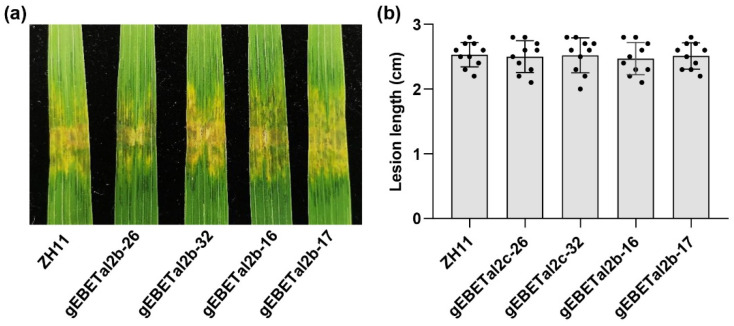
2.6. Single Gene Editing of EBE in OsF3H04g or OsF3H03g Does Not Affect Resistance to Xoc HGA4
We found that gene editing of EBE in OsF3H04g or OsF3H03g reduced rice susceptibility to Xoc strains RS105/Tal2c or RS105/Tal2b, respectively. However, neither of them had any significant effect on rice susceptibility to Xoc strain RS105, which contains Tal2b and Tal2c [4]. The hypothesis is that Tal2b and Tal2c are redundant pairs of TAL effectors; thus, gene editing of EBE in OsF3H04g or OsF3H03g would not affect resistance to Xoc strains containing Tal2b and Tal2c. We then performed a disease assay for inoculation of HGA4 after gene editing of EBE in OsF3H04g or OsF3H03g plants. The results showed that no significant difference in lesion expansion caused by HGA4 was observed in gEBETal2c-26 and gEBETal2c-32 compared with ZH11 or in gEBETal2b-16 and gEBETal2b-17 (Figure 5a). The lesion lengths in gEBETal2c-26 (2.5 ± 0.24 cm) and gEBETal2c-32 (2.52 ± 0.27 cm), gEBETal2b-16 (2.47 ± 0.25 cm) and gEBETal2b-17 (2.51 ± 0.20 cm) were similar to that in ZH11 (2.53 ± 0.19 cm) (Figure 5b). Thus, the results also supported that Tal2b and Tal2c contained in HGA4 may have redundant functions.
Figure 5.
Single mutants of EBE in either the OsF3H04g or OsF3H03g promoter had no effect on rice susceptibility to Xoc strain HGA4. (a) Image of lesion expansion in ZH11, gEBETal2b-16, gEBETal2b-17, gEBETal2c-26 and gEBETal2c-32 lines at 14 dpi with HGA4. (b) Diagram of lesion lengths in ZH11, gEBETal2b-16, gEBETal2b-17, gEBETal2c-26 and gEBETal2c-32 lines at 14 dpi with HGA4. Data represent the means ± SD, n = 10.
2.7. Comparative Analysis of the Transcriptome Profiles of the OsF3H04g and OsF3H03g Overexpression Lines
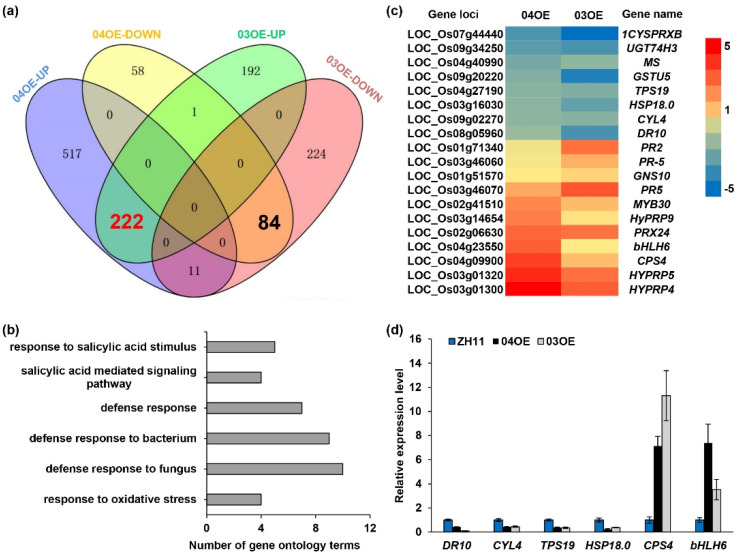
Previously, we reported that overexpression of OsF3H03g could cause transcriptional reprogramming of numerous defense response genes using RNA-seq [4]. Simultaneously, RNA-seq of OsF3H04g OE plants was also performed for a parallel analysis of the differentially expressed genes (DEGs). As shown in Figure 6a, compared to ZH11, 750 upregulated and 143 downregulated DEGs were identified in the OsF3H04g OE line 04OE-1 (Table S1). We then performed a comparison of the DEGs between the line 04OE-1 and the OsF3H03g OE line 03OE-2. A total of 222 upregulated and 84 downregulated DEGs were identified as common DEGs, with 29.6% and 53.5% of the upregulated DEGs and 58.7% and 26.3% of downregulated DEGs sharing in the OsF3H04g and OsF3H03g OE lines, respectively (Figure 6a; Table S2). We further performed a gene ontology (GO) analysis for these 306 common DEGs. Six functional categories related to the defense response were enriched, with 5, 4, 7, 9 and 10 DEGs belonging to the response to salicylic acid stimulus, salicylic-acid-mediated signaling pathway, defense response and defense response to bacterium and fungus, respectively (Figure 6b). Otherwise, four DEGs belonged to the category of response to oxidative stress (Figure 6b). Overexpression of both OsF3H03g and OsF3H04g resulted in enhanced susceptibility and a reduction in SA levels in rice (Figure 2) [4]. We then analyzed the expression of SA-related and defense-related genes among the common DEGs (Figure 6c). SA-related genes, including the pathogen-induced defense-responsive gene DR10 [39] and the cyclase-like gene CYL4 [40], were downregulated in both rice OE lines. Additionally, the SA and JA signaling regulator gene bHLH6 (LOC_Os04g23550), which negatively regulates rice immunity [41], was found to have enhanced expression in both OsF3H04g and OsF3H03g OE lines (Figure 6c,d). Among the GO functional categories of defense response, a small heat shock protein gene HSP18.0-CI [42,43] and a terpene synthase gene OsTPS19 [44] have been identified as positively regulating resistance to phytopathogens in rice and were classified into the commonly downregulated DEGs in both OsF3H04g and OsF3H03g OE lines (Figure 6c,d). In addition, the syn-copalyl diphosphate synthase gene OsCPS4 [45], which negatively regulates rice resistance to Xoo and Magnaporthe grisea, showed increased expression in both OsF3H04g and OsF3H03g OE lines (Figure 6c,d). Overall, the OsF3H04g- and OsF3H03g-overexpressing rice lines share a large number of common DEGs, suggesting that they may have similar biological functions in regulating rice immunity.
Figure 6.
OsF3H04g negatively regulates the rice defense response to Xoc. (a) Venn diagram of the upregulated and downregulated genes in OsF3H04g (04OE-1) and OsF3H03g (03OE-2) OE lines. (b) Gene ontology terms of defense response-related pathways for the common differentially expressed genes (DEGs). The 222 upregulated and 84 downregulated genes in the OsF3H04g and OsF3H03g OE lines were analyzed and enriched. (c) Heatmap of common DEGs related to defense response, SA response and signaling pathway. (d) qRT–PCR analysis of the expression of SA-related genes and defense response genes in OsF3H04g and OsF3H03g OE lines. OsACTIN was used as an internal control. Data represent the means ± SD, n = 3.
3. Discussion
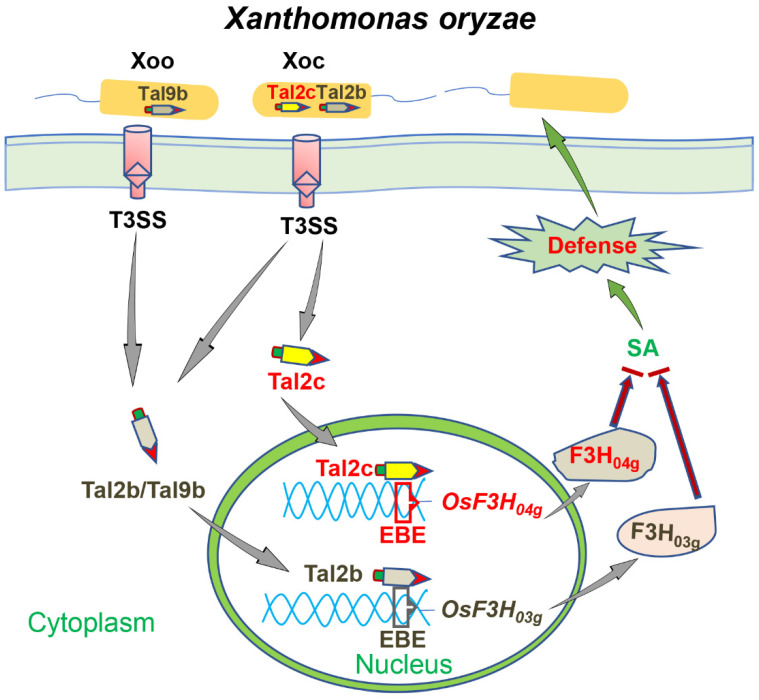
In this work, we revealed that the TALE Tal2c acts as a virulence factor that shows a redundant function with previously reported Tal2b in most Xoc strains, including the highly virulent strain HGA4. The detailed interaction between the two TALEs and targets is illustrated in Figure 7, and these interactions are further described in the following main results. We identified that Tal2c binds to the EBE of the OsF3H04g promoter to promote infection in rice. Overexpression of OsF3H04g resulted in increased susceptibility, decreased SA accumulation and shared common DEGs with the OsF3H03g-OE line. The EBE editing lines of OsF3H03g and OsF3H04g specifically confer resistance to Tal2b- or Tal2c-containing strains. Overall, we significantly broadened the knowledge of TALE-mediated susceptibility to Xoc.
Figure 7.
Working model of redundant Tal2b and Tal2c from Xanthomonas oryzae pv. oryzicola. Xoc delivers two TALEs, Tal2b and Tal2c, into rice cells using a type III secretion system (T3SS). Tal2b and Tal2c then enter the nucleus to directly bind to EBEs of the 2-oxoglutarate-dependent dioxygenase (2OGD) genes OsF3H03g and OsF3H04g promoters and activate gene expression, respectively. However, Xanthomonas oryzae pv. oryzae delivers only a Tal2b homologue, Tal9b, to directly bind to EBE of OsF3H03g promoter to promote infection. Finally, OsF3H03g and OsF3H04g work as similar functions in the negative regulation of defense and SA content in rice against Xoc.
Most Xoc and Xoo strains carry dozens of TALEs that have been delivered into rice cells to support infection [46]. To date, several TALE-induced target genes have been identified that support the virulence of Xoo pathogens. For example, PthXo1 and OsSWEET11 [47], PthXo2 and OsSWEET13 [48], and OsSWEET14 are targeted by four TALEs, including PthXo3, TalC, AvrXa7, Tal5 [48,49,50,51], PthXo6 and OsTFX1 [52], PthXo7 and OsTFIIAγ1 [52]. However, only a few Xoc TALEs and targets have been identified as promoting infection, such as Tal2g and OsSULTR3;6 [13], Tal7 and Cyclin-D4-1 [16], and Tal2b and OsF3H03g [4]. A recent study also demonstrated that a TalI-deficient mutant compromised bacterial virulence and growth and acted as a virulence factor of Xoc without any predicted target in rice [53]. Previously, we identified that the highly virulent strain of Xoc HGA4 contains four expanded TALEs, namely, Tal2b, Tal2c, Tal2d and Tal2e [4]. Among them, Tal2b is a virulence factor targeting OsF3H03g after introduction into RS105, which does not contain the four TALEs [4]. Tal2c from Xoc (also known as TalBL) could activate the expression of putative target genes OsF3H04g (OsDOX-2) in Nicotiana benthamiana transient experiments [18]. In this study, we identified that Tal2c from Xoc HGA4 also acts as a virulence factor to promote infection in rice by introducing Tal2c into RS105 (Figure 1). Its target gene OsF3H04g was indeed a susceptibility gene (Figure 2). Thus, we provided a novel interaction that provides further insights into the virulence of TALE.
In previous studies, classical interactions between TALEs and targeted susceptibility genes have demonstrated that several TALEs in different Xoo strains target EBEs in the promoters of sugar transporter family genes OsSWEET11, OsSWEET13 and OsSWEET14 to promote infection in rice [47,49,51,54]. Due to the similar biological functions of their targets, these Xoo TALEs could be substituted with each other to defeat xa13-mediated resistance [49]. Recently, we reported that Tal2b from Xoc strain HGA4 targets the EBE of rice OsF3H03g as a virulence factor [4]. In this study, we found that another TALE, Tal2c, from HGA4 targets the EBE of rice OsF3H04g as a virulence factor. Both OsF3H03g- and OsF3H04g-overexpressing rice lines showed increased susceptibility to Xoc, decreased SA contents and altered the expression of defense response genes (Figure 2 and Figure 6). Thus, similar to Xoo, Tal2b and Tal2c from HGA4 may target similar functional enzymes to reduce the rice defense response to Xoc. Compared with the diverse TALEs in different Xoo strains, Tal2b and Tal2c clustered in most of the sequenced genomes of Xoc strains (Table 1), suggesting a redundant relationship. In addition, gene editing of the EBE in the OsF3H03g or OsF3H04g promoter correspondingly enhanced rice resistance to Xoc strains RS105/Tal2b or RS105/Tal2c but had no effect on rice resistance to Xoc strain HGA4, which carries both Tal2b and Tal2c (Figure 5). Thus, the Tal2b and Tal2c orthologues target functionally similar enzymes of the OsF3H family to redundantly regulate the rice defense response to Xoc. This could explain why a single mutant of Tal2b or Tal2c orthologue did not cause a significant loss of pathogenicity, as previously reported [13].
SA is an important hormone in the regulation of the plant defense response to a variety of phytopathogens [55]. To successfully infect plants, bacterial pathogens have evolved different tactics to block SA-mediated defense [22,46]. One of the tactics is disturbing the biosynthesis of SA. For example, HopI1 from Pseudomonas syringae pv. maculicola ES436 is translocated to the chloroplast and targets Hsp70 to suppress SA accumulation in the host [56,57]. The second tactic interferes with the SA signaling pathway. For example, P. syringae can generate coronatine to simulate JA signaling to antagonize SA signaling [58]. Otherwise, P. syringae secretes AvrPtoB to facilitate the ubiquitination and degradation of NPR1, the master regulator of SA signaling, to subvert plant defense [59]. The last tactic leads to the direct degradation of SA. There are many examples, such as Ralstonia solanacearum, which utilizes the Nag pathway to degrade SA and causes wilt disease in tomato [60], and Candidatus Liberibacter asiaticus, which secretes the hydroxylase SahA to degrade SA and causes huanglongbing (HLB) in citrus plants [61]. Recently, we reported that the Xoc strain HGA4 delivers a TALE Tal2b into rice to activate OsF3H03g to decrease the SA content, which may occur through hydroxylation into 2,5-DHBA to promote infection in rice. Xoo also carries TALE Tal9b to target EBE in the OsF3H03g promoter to promote infection in rice [4,18]. In this study, we also found that Tal2c from HGA4 activates the similar-functioning enzyme OsF3H04g to decrease SA content and increase a larger bacterial population (Figure 1 and Figure 2). However, both Tal2b and Tal2c are not necessarily required for maintaining complete pathogenicity because the strain RS105 could successfully infect rice in the absence of the two TALEs. Thus, our findings suggest a role to understand the mechanism by which Xoc and Xoo deliver TALEs to activate host SA metabolic enzymes to decrease the SA content. It would be better to maintain better living conditions for growth rather than interfering with key immunity in the host.
Previously, we found that OsF3H03g plays a negative role in SA-mediated defense against Xoc [4]. Here, we also found that overexpression of OsF3H04g resulted in increased susceptibility to Xoc (Figure 2). These two rice genes were closely homologous to AtDMR6, which participates in SA metabolism in Arabidopsis [18,25]. These results were consistent with our findings that overexpression of OsF3H03g and OsF3H04g reduced the SA level and altered the expression of several SA-related genes in rice (Figure 2 and Figure 6). Thus, we concluded that OsF3H04g also plays a negative role in regulating SA-mediated defense against Xoc. We also observed the differences caused by overexpression of OsF3H03g and OsF3H04g. For instance, the SA response genes OsbHLH187, OsWRKY45 and OsNPR3 have been previously identified as exhibiting decreased and upregulated expression, respectively, in OsF3H03g OE and gene editing lines [4]. However, OsbHLH187 and OsWRKY45 were found to activate expression in the OsF3H04g OE line (Table S2). Moreover, a severe reduction in SA was identified in OsF3H03g OE lines [4], while a mild decrease in SA was observed in OsF3H04g OE lines (Figure 2d). These results implied that OsF3H04g may have other functions in the regulation of the rice defense response in addition to participating in SA-related defense against Xoc. Heterogeneous expression of OsF3H03g (LOC_Os03g03034) or OsF3H04g (LOC_Os04g49194) in the Arabidopsis AtF3H gene-deficient mutant tt6 did not recover anthocyanin accumulation [62]. Overexpression of OsF3H03g in rice could improve flavonoid and anthocyanin contents and result in increased resistance to rice brown planthoppers [31]. Thus, whether OsF3H04g plays a role in the regulation of flavonoid and anthocyanin accumulation in rice needs further study.
Previously, we found that gene editing in the CDS region caused a deficient function of OsF3H03g and broad resistance to BB and BLS. However, constitutive activation of resistance results in agronomic trait costs [4]. Recent studies have shown that gene editing of the EBE in the promoter of the Tal2g target OsSULTR3;6 enhances rice resistance to different Xoc strains without any observed changes in most agronomic traits [36,37]. In this study, we found that gene editing of EBEs in the OsF3H03g or OsF3H04g promoter conferred rice with resistance to Xoc strains RS105/Tal2b and RS105/Tal2c, respectively, but did not enhance rice resistance to the wild-type Xoc strains RS105 and HGA4 (Figure 3 and Figure 4). Additional Xoc strains carry both Tal2b and Tal2c orthologues (Table 1). We proposed that double gene editing of both EBEs in the OsF3H03g and OsF3H04g promoters and the pyramiding of gene editing of all EBEs in OsSULTR3;6 could greatly improve rice production and provide broad-spectrum resistance to Xoc.
4. Materials and Methods
4.1. Plant Materials and Growth Condition
Rice ZH11 (Oryza sativa L. ssp. japonica) variety which is moderately susceptible to BLS was used for gene expression pattern and transgenic manipulation. The rice seeds were grown in the greenhouse under the temperature of 28 ± 2 °C, humidity of 85% to 100% and photoperiod of 12 h. Nicotiana benthamiana was used for the transient expression, and it was grown under the temperature of 22 ± 2 °C, humidity of 40% to 50% and photoperiod of 16 h in growth chamber.
4.2. Transient Expression
The 3282 bp DNA fragment containing coding sequence (CDS) of Tal2c gene was amplified from HGA4 genomic DNA, and subsequently cloned into the pCXSN-MYC vector to construct pSN-Tal2c [63]. The 1000 bp promoter fragment upstream initiation site of OsF3H04g gene was cloned into pCXGFP-P vector to generate 04gPRO-GFP, and the DNA fragment which deleted the EBE was generated by overlapping PCR and then cloned into pCXGFP-P vector to generate 04gPROΔEBE-GFP. Above plasmids were transformed into Agrobacterium tumefaciens GV3101 separately. As previously reported [64], the A. tumefaciens carried-different vectors were co-infiltrated into N. benthamiana cells, the GFP signal was observed at 3 days after infiltration using fluorescence microscope (Nikon, Tokyo, Japan).
4.3. Construction of the RS105/Tal2c Strain and Bacterial Inoculation
The Tal2c gene, including the 129 bp promoter and 3282 bp CDS, was amplified from HGA4 genomic DNA with special primers (Table S3) and then cloned into the pVSP61 vector to generate pVSP61-Tal2c. The pVSP61-Tal2c vector was transformed into RS105 competent cells according to previous studies [4,65]. The Xoc strains RS105, HGA4 and RS105/Tal2c were incubated on PSA plates for 2 to 3 days. The bacteria were eluted from the plates, and the OD600 was adjusted to 0.5 with sterile water separately. The bacterial suspensions were infiltrated into 8-week-old rice leaves using a 2 mL no-needle syringe. The bacterial growth curve and relative bacterial biomass were determined at 4 and 8 dpi with RS105 or RS105/Tal2c according to a previous study [4].
4.4. RNA Manipulation and RNA-seq
Eight-week-old ZH11 rice leaves were inoculated with Xoc strains and then collected at 4 dpi for RNA extraction using Monzol® Reagent (Monad, Wuhan, China). First strand cDNA was synthesized using MonScript™ RTIII All-in-One Mix with dsDNase (Monad, Wuhan, China). cDNA was used for qRT–PCR with MonAmp™ SYBR® Green qPCR Mix (Monad, Wuhan, China) in a qTOWER3G Real-Time PCR System (Analytikjena, Jena, Germany). The primers used for qRT–PCR are listed in Table S3.
As previously reported [4], the 04gOE-1 was grown for 8 weeks along with ZH11, and leaves were collected for RNA extraction using a plant RNA kit (OMEGA Bio-Tek, Norcross, GA, USA). Three different repeats of RNA were used to construct libraries and perform RNA-seq by BGISEQ-500 (Beijing Genomics Institution, Shenzhen, China) according to previous reports [42,43,65]. The accession number (PRJNA781784) was obtained from the NCBI Sequence Read Archive (SRA) after submitting the raw sequence reads. The significant DEGs were screened by absolute log2-ratio values ≥1 in 04gOE vs. ZH11 (simultaneously sequenced with 04gOE and 03gOE which previously uploaded files as PRJNA730674) and p ≤ 0.05. The DEGs in OsF3H03g OE line were obtained from NCBI SRA with accession number PRJNA730674 [4]. The common DEGs were identified by Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/index.html, accessed on 8 September 2021), and gene ontology (GO) analysis was performed by PlantRegMap (http://plantregmap.gao-lab.org/go.php, accessed on 26 September 2021) and Oryzabase (https://shigen.nig.ac.jp/rice/oryzabase/gene/advanced/search, accessed on 26 September 2021).
4.5. Vector Construction and Rice Transformation for OsF3H04g Overexpression and EBE Gene Editing Plasmids
To construct the overexpression rice, the 1023 bp fragment of OsF3H04g was amplified from ZH11 rice cDNA and cloned into the pCXUN-MYC vector to generate pUN-F3H04g. Gene editing of the rice genome was implemented by the CRISPR–Cas9 system according to previous reports [65,66]. The CRISPR–Cas9-mediated gene editing of gEBETal2c (5′-TATTCCCTCGCGTGATC-3′) in the OsF3H04g promoter and gEBETal2b (5′-TCCGGCCCCTCTCCCCCCGCCACCTGAC-3′) in the OsF3H03g promoter was designed with the U3-gRNA targets 5′-GCCGGCCGGAGATCACGCGA-3′ and 5′-AAGTCGAGTCAGGTGGCGGG-3′, respectively. The above gRNA targets were cloned into the pYLCRISPR/Cas9-MH vector [66]. The above vectors were transformed into A. tumefaciens EHA105 and then subjected to A. tumefaciens-mediated callus transformation in ZH11 rice.
4.6. Hormone Treatment
The leaves of wild-type ZH11 and OsF3H04g overexpression rice lines were collected for hormone quantitative analysis. In total, 100 mg of leaf tissue of ZH11 and OsF3H04g overexpression rice lines was ground in liquid nitrogen and then used for hormone extraction according to the previous report [4,67].
5. Conclusions
In this study, we validated the virulence function of Tal2c by introducing it into RS105. It activated the expression of OsF3H04g to reduce SA content as well as to increase susceptibility in rice. We compared the interaction of Tal2c and OsF3H04g with previously identified Tal2b and OsF3H03g using transcriptome profiling and EBE gene editing. Intriguingly, our data suggest that the pair of Tal2b and Tal2c acts as redundant TALEs by specifically hijacking homologues of rice 2OGD gene. Thus, our research uncovers a virulence mechanism of Xoc and provides guidance for breeding BLS disease-resistant rice.
Acknowledgments
We are grateful to Yaoguang Liu, Gongyou Chen and Wenxian Sun for providing CRISPR-Cas9 gene editing system, RS105 strain and pVSP61 vector, respectively.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222413628/s1.
Author Contributions
Methodology, T.W., H.Z. and Y.Y.; validation, T.W., X.D. and Z.C.; investigation, T.W., H.Z. and B.Y.; data analysis, T.W., H.L., Y.B. and H.Y.; project administration, T.W., X.D. and Z.C.; writing—original draft preparation, T.W. and Z.C.; writing—review and editing, T.W. and Z.C.; funding acquisition, X.D. and Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation (31771748, 31872925, 32072500), the Shandong Modern Agricultural Technology & Industry system (SDAIT-17-06) and Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong Province (2019KJF023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data from this study can be found in the Rice Genome Annotation Project website (http://rice.plantbiology.msu.edu/, accessed on 25 November 2021), NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 25 November 2021) and the Xanthomonas Resource (http://www.xanthomonas.org/t3e.html, accessed on 25 November 2021) under the following accession number: OsF3H04g (LOC_Os04g49494), Tal2c of BLS256 (XOC_1570) and HGA4 (CP064794). Raw sequence reads of transcriptome sequencing for ZH11 and OsF3H03g transgenic rice were obtained from the NCBI Sequence Read Archive (SRA) with accession number of PRJNA730674. Raw sequence reads of transcriptome sequencing for OsF3H04g transgenic rice were performed in this study and uploaded to SRA to achieve the accession number PRJNA781784.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niño-Liu D.O., Ronald P.C., Bogdanove A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006;7:303–324. doi: 10.1111/j.1364-3703.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 2.Ke Y., Deng H., Wang S. Advances in understanding broad-spectrum resistance to pathogens in rice. Plant J. 2017;90:738–748. doi: 10.1111/tpj.13438. [DOI] [PubMed] [Google Scholar]
- 3.Jiang N., Yan J., Liang Y., Shi Y., He Z., Wu Y., Zeng Q., Liu X., Peng J. Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa L.)—An updated review. Rice. 2020;13:3. doi: 10.1186/s12284-019-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu T., Zhang H., Yuan B., Liu H., Kong L., Chu Z., Ding X. Tal2b targets and activates the expression of OsF3H03g to hijack OsUGT74H4 and synergistically interfere with rice immunity. New Phytol. 2021:nph17877. doi: 10.1111/nph.17877. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Wang S. Disease resistance. In: Zhang Q., Wing R.A., editors. Genetics and Genomics of Rice. Springer; New York, NY, USA: 2013. pp. 161–175. [DOI] [Google Scholar]
- 6.White F.F., Potnis N., Jones J.B., Koebnik R. The type III effectors of Xanthomonas. Mol. Plant Pathol. 2009;10:749–766. doi: 10.1111/j.1364-3703.2009.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 8.Mak A.N., Bradley P., Cernadas R.A., Bogdanove A.J., Stoddard B.L. The crystal structure of TAL effector PthXo1 bound to its DNA target. Science. 2012;335:716–719. doi: 10.1126/science.1216211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Chang Q., Feng W., Zhang B., Wu T., Li N., Yao F., Ding X., Chu Z. Domain dissection of AvrRxo1 for suppressor, avirulence and cytotoxicity functions. PLoS ONE. 2014;9:e113875. doi: 10.1371/journal.pone.0113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuebel F., Rocker A., Edelmann D., Schessner J., Brieke C., Meinhart A. 3′-NADP and 3′-NAADP, two metabolites formed by the bacterial type III effector AvrRxo1. J. Biol. Chem. 2016;291:22868–22880. doi: 10.1074/jbc.M116.751297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S., Li S., Wang J., Li Q., Xin X.F., Zhou S., Wang Y., Li D., Xu J., Luo Z.Q., et al. A bacterial kinase phosphorylates OSK1 to suppress stomatal immunity in rice. Nat. Commun. 2021;12:5479. doi: 10.1038/s41467-021-25748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Quintero A.L., Rodriguez-R L.M., Dereeper A., López C., Koebnik R., Szurek B., Cunnac S. An improved method for TAL effectors DNA-binding sites prediction reveals functional convergence in TAL repertoires of Xanthomonas oryzae strains. PLoS ONE. 2013;8:e68464. doi: 10.1371/journal.pone.0068464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cernadas R.A., Doyle E.L., Niño-Liu D.O., Wilkins K.E., Bancroft T., Wang L., Schmidt C.L., Caldo R., Yang B., White F.F., et al. Code-assisted discovery of TAL effector targets in bacterial leaf streak of rice reveals contrast with bacterial blight and a novel susceptibility gene. PLoS Pathog. 2014;10:e1003972. doi: 10.1371/journal.ppat.1003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read A.C., Rinaldi F.C., Hutin M., He Y., Triplett L.R., Bogdanove A.J. Suppression of Xo1-mediated disease resistance in rice by a truncated, non-DNA-binding TAL effector of Xanthomonas oryzae. Front. Plant Sci. 2016;7:1516. doi: 10.3389/fpls.2016.01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read A.C., Hutin M., Moscou M.J., Rinaldi F.C., Bogdanove A.J. Cloning of the rice Xo1 resistance gene and interaction of the Xo1 protein with the defense-suppressing Xanthomonas effector Tal2h. Mol. Plant Microbe Interact. 2020;33:1189–1195. doi: 10.1094/MPMI-05-20-0131-SC. [DOI] [PubMed] [Google Scholar]
- 16.Cai L., Cao Y., Xu Z., Ma W., Zakria M., Zou L., Cheng Z., Chen G. A transcription activator-like effector Tal7 of Xanthomonas oryzae pv. oryzicola activates rice gene Os09g29100 to suppress rice immunity. Sci. Rep. 2017;7:5089. doi: 10.1038/s41598-017-04800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hummel A.W., Wilkins K.E., Wang L., Cernadas R.A., Bogdanove A.J. A transcription activator-like effector from Xanthomonas oryzae pv. oryzicola elicits dose-dependent resistance in rice. Mol. Plant Pathol. 2017;18:55–66. doi: 10.1111/mpp.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mücke S., Reschke M., Erkes A., Schwietzer C.A., Becker S., Streubel J., Morgan R.D., Wilson G.G., Grau J., Boch J. Transcriptional reprogramming of rice cells by Xanthomonas oryzae TALEs. Front. Plant Sci. 2019;10:162. doi: 10.3389/fpls.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai Y., Ono E., Mizutani M. Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 2014;78:328–343. doi: 10.1111/tpj.12479. [DOI] [PubMed] [Google Scholar]
- 20.Nadi R., Mateo-Bonmatí E., Juan-Vicente L., Micol J.L. The 2OGD superfamily: Emerging functions in plant epigenetics and hormone metabolism. Mol. Plant. 2018;11:1222–1224. doi: 10.1016/j.molp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Farrow S.C., Facchini P.J. Functional diversity of 2-oxoglutarate/Fe (II)-dependent dioxygenases in plant metabolism. Front. Plant Sci. 2014;5:524. doi: 10.3389/fpls.2014.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi G., Chen J., Chang M., Chen H., Hall K., Korin J., Liu F., Wang D., Fu Z.Q. Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol. Plant. 2018;11:1427–1439. doi: 10.1016/j.molp.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 23.van Damme M., Huibers R.P., Elberse J., Van den Ackerveken G. Arabidopsis DMR6 encodes a putative 2OG-Fe (II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant J. 2008;54:785–793. doi: 10.1111/j.1365-313X.2008.03427.x. [DOI] [PubMed] [Google Scholar]
- 24.Zeilmaker T., Ludwig N.R., Elberse J., Seidl M.F., Berke L., Van Doorn A., Schuurink R.C., Snel B., Van den Ackerveken G. DOWNY MILDEW RESISTANT 6 and DMR6-LIKE OXYGENASE 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J. 2015;81:210–222. doi: 10.1111/tpj.12719. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Zhao L., Zhao J., Li Y., Wang J., Guo R., Gan S., Liu C.J., Zhang K. S5H/DMR6 encodes a salicylic acid 5-hydroxylase that fine-tunes salicylic acid homeostasis. Plant Physiol. 2017;175:1082–1093. doi: 10.1104/pp.17.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K., Halitschke R., Yin C., Liu C., Gan S. Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA. 2013;110:14807–14812. doi: 10.1073/pnas.1302702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieu N.P., Lenman M., Wang E.S., Petersen B.L., Andreasson E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021;11:4487. doi: 10.1038/s41598-021-83972-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasley J.A.R., Navet N., Tian M. CRISPR/Cas9-mediated mutagenesis of sweet basil candidate susceptibility gene ObDMR6 enhances downy mildew resistance. PLoS ONE. 2021;16:e0253245. doi: 10.1371/journal.pone.0253245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathi J.N., Ntui V.O., Shah T., Tripathi L. CRISPR/Cas9-mediated editing of DMR6 orthologue in banana (Musa spp.) confers enhanced resistance to bacterial disease. Plant Biotechnol. J. 2021;19:1291–1293. doi: 10.1111/pbi.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomazella D.P.T., Seong K., Mackelprang R., Dahlbeck D., Geng Y., Gill U.S., Qi T., Pham J., Giuseppe P., Lee C.Y., et al. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA. 2021;118:e2026152118. doi: 10.1073/pnas.2026152118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Z., Tan J., Zhou C., Yang X., Yang F., Zhang S., Sun S., Miao X., Shi Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa) Plant Biotechnol. J. 2019;17:1657–1669. doi: 10.1111/pbi.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manghwar H., Lindsey K., Zhang X., Jin S. CRISPR/Cas system: Recent advances and future prospects for genome editing. Trends Plant Sci. 2019;24:1102–1125. doi: 10.1016/j.tplants.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z., Xu X., Gong Q., Li Z., Li Y., Wang S., Yang Y., Ma W., Liu L., Zhu B., et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol. Plant. 2019;12:1434–1446. doi: 10.1016/j.molp.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Oliva R., Ji C., Atienza-Grande G., Huguet-Tapia J.C., Perez-Quintero A., Li T., Eom J.S., Li C., Nguyen H., Liu B., et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019;37:1344–1350. doi: 10.1038/s41587-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C., Li W., Zhou Z., Chen H., Xie C., Lin Y. A new rice breeding method: CRISPR/Cas9 system editing of the Xa13 promoter to cultivate transgene-free bacterial blight-resistant rice. Plant Biotechnol. J. 2020;18:313–315. doi: 10.1111/pbi.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X., Xu Z., Li Z., Zakria M., Zou L., Chen G. Increasing resistance to bacterial leaf streak in rice by editing the promoter of susceptibility gene OsSULRT3;6. Plant Biotechnol. J. 2021;19:1101–1103. doi: 10.1111/pbi.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni Z., Cao Y., Jin X., Fu Z., Li J., Mo X., He Y., Tang J., Huang S. Engineering resistance to bacterial blight and bacterial leaf streak in rice. Rice. 2021;14:38. doi: 10.1186/s12284-021-00482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkins K.E., Booher N.J., Wang L., Bogdanove A.J. TAL effectors and activation of predicted host targets distinguish Asian from African strains of the rice pathogen Xanthomonas oryzae pv. oryzicola while strict conservation suggests universal importance of five TAL effectors. Front. Plant Sci. 2015;6:536. doi: 10.3389/fpls.2015.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao W., Liu H., Li Y., Li X., Xu C., Long M., Wang S. A rice gene of de novo origin negatively regulates pathogen-induced defense response. PLoS ONE. 2009;4:e4603. doi: 10.1371/journal.pone.0004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin Y., Shen X., Wang N., Ding X. Characterization of a novel cyclase-like gene family involved in controlling stress tolerance in rice. J. Plant Physiol. 2015;181:30–41. doi: 10.1016/j.jplph.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Meng F., Yang C., Cao J., Chen H., Pang J., Zhao Q., Wang Z., Qing F.Z., Liu J. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J. Integr. Plant Biol. 2020;62:1552–1573. doi: 10.1111/jipb.12922. [DOI] [PubMed] [Google Scholar]
- 42.Ju Y., Tian H., Zhang R., Zuo L., Jin G., Xu Q., Ding X., Li X., Chu Z. Overexpression of OsHSP18.0-CI enhances resistance to bacterial leaf streak in rice. Rice. 2017;10:12. doi: 10.1186/s12284-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang W., Ju Y., Zuo L., Shang L., Li X., Li X., Feng S., Ding X., Chu Z. OsHsfB4d binds the promoter and regulates the expression of OsHsp18.0-CI to resistant against Xanthomonas oryzae. Rice. 2020;13:28. doi: 10.1186/s12284-020-00388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X., Chen H., Yuan J.S., Köllner T.G., Chen Y., Guo Y., Zhuang X., Chen X., Zhang Y.J., Fu J., et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol. J. 2018;16:1778–1787. doi: 10.1111/pbi.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu X., Zhang J., Brown B., Li R., Rodríguez-Romero J., Berasategui A., Liu B., Xu M., Luo D., Pan Z., et al. Inferring roles in defense from metabolic allocation of rice diterpenoids. Plant Cell. 2018;30:1119–1131. doi: 10.1105/tpc.18.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boch J., Bonas U. Xanthomonas AvrBs3 family-type III effectors: Discovery and function. Annu. Rev. Phytopathol. 2010;48:419–436. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- 47.Yang B., Sugio A., White F.F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA. 2006;103:10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Streubel J., Pesce C., Hutin M., Koebnik R., Boch J., Szurek B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013;200:808–819. doi: 10.1111/nph.12411. [DOI] [PubMed] [Google Scholar]
- 49.Antony G., Zhou J., Huang S., Li T., Liu B., White F.F., Yang B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22:3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y., Streubel J., Balzergue S., Champion A., Boch J., Koebnik R., Feng J., Verdier V., Szurek B. Colonization of rice leaf blades by an African strain of Xanthomonas oryzae pv. oryzae depends on a new TAL effector that induces the rice nodulin-3 Os11N3 gene. Mol. Plant Microbe Interact. 2011;24:1102–1113. doi: 10.1094/MPMI-11-10-0254. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J., Peng Z., Long J., Sosso D., Liu B., Eom J.S., Huang S., Liu S., Vera Cruz C., Frommer W.B., et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015;82:632–643. doi: 10.1111/tpj.12838. [DOI] [PubMed] [Google Scholar]
- 52.Sugio A., Yang B., Zhu T., White F.F. Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAγ1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA. 2007;104:10720–10725. doi: 10.1073/pnas.0701742104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long J., Wang Z., Chen X., Liu Y., Zhang M., Song C., Dong H. Identification of a TAL effector in Xanthomonas oryzae pv. oryzicola enhancing pathogen growth and virulence in plants. Physiol. Mol. Plant Pathol. 2021;114:101620. doi: 10.1016/j.pmpp.2021.101620. [DOI] [Google Scholar]
- 54.Doucouré H., Pérez-Quintero A.L., Reshetnyak G., Tekete C., Auguy F., Thomas E., Koebnik R., Szurek B., Koita O., Verdier V., et al. Functional and genome sequence-driven characterization of tal effector gene repertoires reveals novel variants with altered specificities in closely related Malian Xanthomonas oryzae pv. oryzae strains. Front. Microbiol. 2018;9:1657. doi: 10.3389/fmicb.2018.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding P., Ding Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020;25:549–565. doi: 10.1016/j.tplants.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Jelenska J., van Hal J.A., Greenberg J.T. Pseudomonas syringae hijacks plant stress chaperone machinery for virulence. Proc. Natl. Acad. Sci. USA. 2010;107:13177–13182. doi: 10.1073/pnas.0910943107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jelenska J., Yao N., Vinatzer B.A., Wright C.M., Brodsky J.L., Greenberg J.T. A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr. Biol. 2007;17:499–508. doi: 10.1016/j.cub.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng X.Y., Spivey N.W., Zeng W., Liu P.P., Fu Z.Q., Klessig D.F., He S.Y., Dong X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe. 2012;11:587–596. doi: 10.1016/j.chom.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen H., Chen J., Li M., Chang M., Xu K., Shang Z., Zhao Y., Palmer I., Zhang Y., McGill J., et al. A bacterial type III effector targets the master regulator of salicylic acid signaling, NPR1, to subvert plant immunity. Cell Host Microbe. 2017;22:777–788. doi: 10.1016/j.chom.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Lowe-Power T.M., Jacobs J.M., Ailloud F., Fochs B., Prior P., Allen C. Degradation of the plant defense signal salicylic acid protects Ralstonia solanacearum from toxicity and enhances virulence on tobacco. mBio. 2016;7:e00656-16. doi: 10.1128/mBio.00656-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J., Pang Z., Trivedi P., Zhou X., Ying X., Jia H., Wang N. ‘Candidatus Liberibacter asiaticus’ encodes a functional salicylic acid (SA) hydroxylase that degrades SA to suppress plant defenses. Mol. Plant Microbe Interact. 2017;30:620–630. doi: 10.1094/MPMI-12-16-0257-R. [DOI] [PubMed] [Google Scholar]
- 62.Shih C., Chu H., Tang L., Sakamoto W., Maekawa M., Chu I., Wang M., Lo C. Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta. 2008;228:1043–1054. doi: 10.1007/s00425-008-0806-1. [DOI] [PubMed] [Google Scholar]
- 63.Chen S., Songkumarn P., Liu J., Wang G.L. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009;150:1111–1121. doi: 10.1104/pp.109.137125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue X., Geng T., Liu H., Yang W., Zhong W., Zhang Z., Zhu C., Chu Z. Foliar application of silicon enhances resistance against Phytophthora infestans through the ET/JA- and NPR1- dependent signaling pathways in potato. Front. Plant Sci. 2021;12:609870. doi: 10.3389/fpls.2021.609870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu T., Peng C., Li B., Wu W., Kong L., Li F., Chu Z., Liu F., Ding X. OsPGIP1-mediated resistance to bacterial leaf streak in rice is beyond responsive to the polygalacturonase of Xanthomonas oryzae pv. oryzicola. Rice. 2019;12:90. doi: 10.1186/s12284-019-0352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma X., Zhu Q., Chen Y., Liu Y. CRISPR/Cas9 platforms for genome editing in plants: Developments and applications. Mol. Plant. 2016;9:961–974. doi: 10.1016/j.molp.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 67.Li X., Han H., Chen M., Yang W., Liu L., Li N., Ding X., Chu Z. Overexpression of OsDT11, which encodes a novel cysteine-rich peptide, enhances drought tolerance and increases ABA concentration in rice. Plant Mol. Biol. 2017;93:21–34. doi: 10.1007/s11103-016-0544-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data from this study can be found in the Rice Genome Annotation Project website (http://rice.plantbiology.msu.edu/, accessed on 25 November 2021), NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 25 November 2021) and the Xanthomonas Resource (http://www.xanthomonas.org/t3e.html, accessed on 25 November 2021) under the following accession number: OsF3H04g (LOC_Os04g49494), Tal2c of BLS256 (XOC_1570) and HGA4 (CP064794). Raw sequence reads of transcriptome sequencing for ZH11 and OsF3H03g transgenic rice were obtained from the NCBI Sequence Read Archive (SRA) with accession number of PRJNA730674. Raw sequence reads of transcriptome sequencing for OsF3H04g transgenic rice were performed in this study and uploaded to SRA to achieve the accession number PRJNA781784.