Abstract
(1) Background: Based on its antiviral activity, anti-inflammatory properties, and functional inhibition effects on the acid sphingomyelinase/ceramide system (FIASMA), we sought to examine the potential usefulness of the H1 antihistamine hydroxyzine in patients hospitalized for COVID-19. (2) Methods: In a multicenter observational study, we included 15,103 adults hospitalized for COVID-19, of which 164 (1.1%) received hydroxyzine within the first 48 h of hospitalization, administered orally at a median daily dose of 25.0 mg (SD = 29.5). We compared mortality rates between patients who received hydroxyzine at hospital admission and those who did not, using a multivariable logistic regression model adjusting for patients’ characteristics, medical conditions, and use of other medications. (3) Results: This analysis showed a significant association between hydroxyzine use and reduced mortality (AOR, 0.51; 95%CI, 0.29–0.88, p = 0.016). This association was similar in multiple sensitivity analyses. (4) Conclusions: In this retrospective observational multicenter study, the use of the FIASMA hydroxyzine was associated with reduced mortality in patients hospitalized for COVID-19. Double-blind placebo-controlled randomized clinical trials of hydroxyzine for COVID-19 are needed to confirm these results, as are studies to examine the potential usefulness of this medication for outpatients and as post-exposure prophylaxis for individuals at high risk for severe COVID-19.
Keywords: COVID-19, SARS-CoV-2, hydroxyzine, FIASMA, treatment, inpatients, mortality, death
1. Introduction
Global spread of the novel coronavirus SARS-CoV-2, the causative agent of coronavirus disease 2019 (COVID-19), has created an unprecedented infectious disease crisis worldwide [1,2,3,4]. The search for an effective treatment for patients with COVID-19 among all available medications is still urgently needed [5,6,7,8].
Antihistamines are widely used in the treatment of urticaria, allergic rhinitis, hay fever, conjunctivitis, and pruritus. They work by competitive binding to H1 receptors and inhibiting the action of histamine, a primary mediator of an early-phase allergic inflammatory response that also modulates the late-phase response characterized by cellular influx of eosinophils, neutrophils, basophils, mononuclear cells, and T lymphocytes [9]. In vivo and in vitro studies have also suggested additional anti-inflammatory properties of H1 antihistamines, including both receptor-dependent and receptor-independent mechanisms [10]. The receptor-dependent mechanisms may involve inhibition of NF-kB dependent cytokines (such as IL-1, IL-2, IL-6, IL-8, IL-12, TNF-α) [11] and adhesion proteins (such as ICAM-1, VCAM-1 and ECAM-1) [10]. The receptor-independent mechanisms, which require higher drug concentrations, may include inhibition of the release of pre-formed mediators, such as histamine and eosinophil proteins, by inflammatory cells as well as eicosanoid generation and oxygen free radicals’ production [10].
Prior research also supports in vitro antiviral effects of the H1 antihistamine hydroxyzine against MERS and hepatitis C virus [12], and recent studies suggest that antihistamine medications could interact with SARS-CoV-2 cellular entry and might be beneficial in reducing disease progression [13,14,15,16].
Among first generation antihistamines, hydroxyzine is one of the most prescribed antihistamines. Beyond its antihistaminic activity, hydroxyzine is also prescribed as a psychotropic medication for its tranquilizer and sedative properties, as it is a weak antagonist of the serotonin 5-HT2A, dopamine D2, and α1-adrenergic receptors. Prior work indicates several biological mechanisms that hydroxyzine may induce which could be beneficial against COVID-19.
First, hydroxyzine belongs to the group of functional inhibitors of acid sphingomyelinase (FIASMA) [17,18], and prior research supports that these molecules could be beneficial in COVID-19 patients, through potential subsequent antiviral and anti-inflammatory effects [7,8,17,19,20,21,22,23,24,25,26,27,28]. In addition to hydroxyzine, this group also comprises other medications commonly used in clinical practice, such as antidepressants (e.g., fluoxetine, fluvoxamine, amitriptyline), calcium channel blockers (e.g., amlodipine [18], bepridil), anti-Parkinson’s drugs (e.g., biperiden) and mucolytics (e.g., ambroxol [8,12,17]). In vitro and in vivo, these pharmacological compounds inhibit ASM, an enzyme that catalyzes the hydrolysis of sphingomyelin into ceramide and phosphorylcholine [20]. Preclinical data indicate that SARS-CoV-2 activates the ASM-ceramide system, resulting in the formation of ceramide-enriched membrane domains that facilitate viral entry and infection by clustering ACE2, the cellular receptor of SARS-CoV-2, and the release of pro-inflammatory cytokines [17,20,21]. The inhibition of the ASM/ceramide system by FIASMA antidepressants prevents infection of Vero E6 cells with SARS-CoV-2 [20]. Importantly, the reconstitution of ceramides in cells treated with these antidepressants restores the infection [20]. In healthy volunteers, oral use of the FIASMA antidepressant amitriptyline prevents infection of freshly isolated nasal epithelial cells, which is also restored after the reconstitution of ceramides in these cells [20]. In an observational multicenter retrospective study, use of a FIASMA medication upon hospital admission was associated with a substantially reduced risk of intubation or death [23]. Finally, plasma levels of certain sphingolipids, particularly sphingomyelins and ceramides, were found to correlate with disease clinical severity [29,30,31], inflammation markers [29,30] and viral load [30,31] in patients with COVID-19. Altogether, these data support the central role of ASM/ceramide system in SARS-CoV-2 infection that could be targeted by hydroxyzine, even if the magnitude of the in vitro FIASMA effect of this treatment (i.e., in vitro residual ASM activity of 43%) is slightly lower than that of fluoxetine (13%) and fluvoxamine (37.4%) [18].
Second, hydroxyzine binds with significant affinity to Sigma-1 receptors (S1Rs) [32], which have been shown to restrict the endonuclease activity of an endoplasmic reticulum stress sensor inositol-requiring enzyme 1 (IRE1) and to reduce cytokine expression without inhibiting classical inflammatory pathways [26,33,34].
Third, hydroxyzine is a medication with highly likely lysosomotropic behavior [35]. Lysosomotropic medications have shown antiviral effects on coronaviruses, likely due to interference with endosomal pathway [36], and are assumed to suppress the cytokine release syndrome and to attenuate the transition from mild to severe SARS-CoV-2 infection/COVID-19 [37,38].
Because prior research supports that severe COVID-19 is characterized by an excessive inflammatory response [39,40] and that viral load could be associated with worsening of symptoms [41], we hypothesized in this report that hydroxyzine could be beneficial in reducing mortality in inpatients with COVID-19. Short-term oral use of hydroxyzine is generally relatively well tolerated, although common side effects include sleepiness, headache, and dry mouth, and less common serious ones include delirium, QT prolongation, and torsade de pointes, particularly among older adults [1]. These risks can be significantly increased if this medication is administered parentally.
Observational studies of patients with COVID-19 taking medications for other indications can help decide which treatment should be prioritized for randomized clinical trials and minimize the risk of patient exposure to potentially harmful and ineffective treatments. To our knowledge, no prior clinical study has examined the potential usefulness of hydroxyzine in patients with COVID-19.
To this end, we used data from the Assistance Publique-Hôpitaux de Paris (AP-HP) Health Data Warehouse [23,42,43,44,45], which includes data on all patients who have been admitted for COVID-19 to any of 36 Greater Paris University hospitals.
In this report, we examined the association between hydroxyzine use at hospital admission and mortality among adult patients who have been admitted to these medical centers for COVID-19, while adjusting for potential confounders, including patients’ characteristics (such as sex, age, hospital, obesity, current smoking status), medical conditions, and use of other medications. We hypothesized that among patients hospitalized for COVID-19, hydroxyzine use would be associated with reduced mortality.
2. Materials and Methods
2.1. Setting and Cohort Assembly
A multicenter cohort study was conducted at 36 AP-HP hospitals from the beginning of the epidemic in France, i.e., 24 January until 1 May 2020 [23,42,43,44,45]. We included all adults aged 18 years or over who had been hospitalized in these medical centers for COVID-19. COVID-19 was ascertained by a positive reverse-transcriptase-polymerase-chain-reaction (RT-PCR) test on nasopharyngeal or oropharyngeal swab specimens.
This observational study using routinely collected data received approval from the Institutional Review Board of the AP-HP clinical Data Warehouse (decision CSE-20- 20_COVID19, IRB00011591). AP-HP clinical Data Warehouse initiatives ensure patient information and informed consent regarding the different approved studies through a transparency portal in accordance with European Regulation on data protection and authorization n°1980120 from National Commission for Information Technology and Civil Liberties (CNIL).
2.2. Data Sources
We used data from the AP-HP Health Data Warehouse (‘Entrepôt de Données de Santé (EDS)). This warehouse contains all the clinical data available on all inpatient visits for COVID-19 to any of 36 Greater Paris University hospitals. The data obtained included patients’ demographic characteristics, vital signs, laboratory test and RT-PCR test results, medication administration data, current medication lists, current diagnoses, and death certificates.
2.3. Variables Assessed
We obtained data for each patient at the time of the hospitalization regarding patients’ characteristics, other medications, medical indications of hydroxyzine prescription, and medical comorbidities. Patients’ characteristics included sex; age, which was categorized into four classes based on the OpenSAFELY study results [46] (i.e., 18–50, 51–70, 71–80, 81+); hospital, which was categorized into four classes following the administrative clustering of AP-HP hospitals in Paris and its suburbs based on their geographical location (i.e., AP-HP Centre—Paris University, Henri Mondor University Hospitals and at home hospitalization; AP-HP Nord and Hôpitaux Universitaires Paris Seine-Saint-Denis; AP-HP Paris Saclay University; and AP-HP Sorbonne University); obesity, which was defined as having a body mass index higher than 30 kg/m2 or an International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnosis code for obesity (E66.0, E66.1, E66.2, E66.8, E66.9); and self-reported current smoking status. Other medications included any medication prescribed according to compassionate use or as part of a clinical trial (i.e., hydroxychloroquine, azithromycin, remdesivir, tocilizumab, sarilumab, or dexamethasone) and any other antihistamine medication. To take into account medical indications of hydroxyzine prescription, we recorded whether patients had any current diagnosis, based on ICD-10 diagnosis codes recorded during the visit, of any anxiety, insomnia, nausea, urticaria or pruritus (F4, G47, R11, L50 or L29). Finally, medical comorbidities included: any other infectious diseases (A00-B99); neoplasms and diseases of the blood (C00-D89); mental disorders (F01-F99); diseases of the nervous system (G00-G99); cardiovascular disorders (I00-I99); respiratory disorders (J00-J99); digestive disorders (K00-K95); dermatological disorders (L00-L99); diseases of the musculoskeletal system (M00-M99); diseases of the genitourinary system (N00-N99); endocrine disorders (E00-E89); and eye-ear-nose-throat disorders (H00-H95).
2.4. Hydroxyzine Use
Hydroxyzine use was defined as receiving this medication per os within the first 48 h from hospital admission. We used this delay because we considered that, in a context of overwhelming of all hospital units during the COVID-19 peak incidence, patients may not have received or been prescribed the treatment the first day of their admission. To minimize potential confounding effects of late prescription of hydroxyzine, patients who initiated this treatment more than 48 h after hospital admission were excluded from the analyses.
2.5. Outcome
Study baseline was defined as the date of hospital admission for COVID-19. The outcome was in-hospital all-cause mortality from study baseline until 1 May 2020.
2.6. Statistical Analysis
We calculated frequencies of all baseline characteristics described above in patients who received hydroxyzine at hospital admission and in those who did not, and compared them using standardized mean differences (SMD) [47].
To examine the associations between hydroxyzine use and mortality, we performed multivariable logistic regression models. Patients with a baseline prescription of hydroxyzine were compared with a reference group without any hydroxyzine prescription during the hospitalization. To help account for the nonrandomized prescription of hydroxyzine and reduce the effects of confounding, the primary analysis was a multivariable logistic regression that included sex, age, hospital, obesity, self-reported current smoking status, medical conditions, any medication prescribed according to compassionate use or as part of a clinical trial, and any other antihistamine medication.
As a sensitivity analysis, we performed a univariate logistic regression model in a matched analytic sample using a 1:1 ratio, based on the same variables used for the multivariable logistic regression analysis. In this analysis, to reduce the effects of confounding, optimal matching was used to obtain the smallest average absolute distance across all clinical characteristics between exposed patient and non-exposed matched controls. In case of non-balanced covariates, a multivariable logistic regression model adjusting for the non-balanced covariates was also performed.
We also performed five additional analyses. First, to increase our confidence that the results might not be due to unmeasured confounding or indication bias, we examined (i) this association among patients who received hydroxyzine only within the 3 months before hospital admission (and not during the visit) as compared to those who received it during the visit only, and (ii) the change of the magnitude of the effect of potential residual confounding on our results by varying the relationship of each potential confounder with mortality [48]. Second, we reproduced the main analyses among all patients hospitalized for COVID-19 while additionally adjusting by severity criteria at baseline. Severity of COVID-19 was defined as having at least one of the following criteria at hospital admission: respiratory rate >24 breaths/min or <12 breaths/min, resting peripheral capillary oxygen saturation in ambient air <90%, temperature >40 °C, systolic blood pressure <100 mm Hg, or high lactate levels >2 mmol/L [49]. Third, we reproduced the main analyses separately in patients admitted in ICUs and those admitted in normal wards. Fourth, we compared the mortality rate of patients who were prescribed hydroxyzine more than 48 h after admission with (i) those who received this medication within 48 h from hospital admission and (ii) those who never received this treatment during the visit. In the third and fourth analyses, we used a 1:2 ratio in the matched analytic sample to obtain adequate statistical power. Finally, we examined a potential dose-effect relationship by testing the association between the daily dose received (dichotomized at the median dose) with mortality within patients who received hydroxyzine at baseline.
For all associations, we performed residual analyses to assess the fit of the data, checked assumptions, and examined the potential influence of outliers [50]. Statistical significance was fixed a priori at two-sided p-value < 0.05. All analyses were conducted in R software version 3.6.3 (R Project for Statistical Computing).
3. Results
3.1. Characteristics of the Cohort
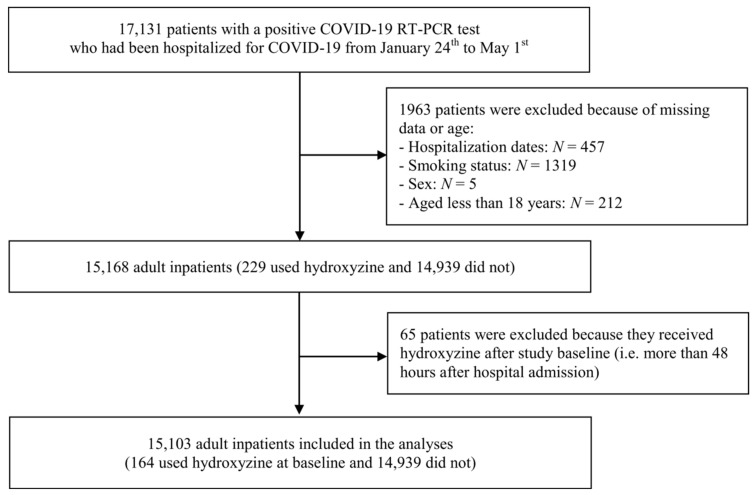
Of the 17,131 patients hospitalized for laboratory-confirmed COVID-19, 1963 patients (11.5%) were excluded because of missing data or their young age (i.e., less than 18 years old of age). Of the remaining 15,168 adult patients, 65 patients (0.4%) were excluded because they received hydroxyzine after more than 48 h after hospital admission. Of the remaining 15,103 adult inpatients, 164 (1.1%) used hydroxyzine within the first 48 h of hospitalization, with a mean delay between hospital admission and first prescription of 0.9 days (SD = 0.6; interquartile range (IQR) = 0–1.5)), at a median daily dose at baseline of 25.0 mg (SD = 29.5, IQR = 25.0–50.0 mg) (Figure 1).
Figure 1.
Study cohort.
RT-PCR test results were obtained after a median delay of 1.2 days (SD = 12.7; IQR = 0.6–7.2 days) from hospital admission date. This median delay was of 1.0 days in the exposed (SD = 12.9; IQR = 0.7–7.0 days) group, and of 1.2 days in the non-exposed (SD = 12.7; IQR = 0.6–7.2) group (Two-Sample Brown–Mood Median Test, Z = 1.83; p = 0.067). Over a mean follow-up of 14.5 days (SD = 17.9; median = 8 days; IQR = 1–24 days), 1589 patients (10.5%) died at the time of data cutoff on 1 May 2020. Among patients who received hydroxyzine medication at baseline, the mean follow-up was 14.4 days (SD = 15.4, median = 10 days; IQR = 5–18 days), while it was of 14.5 days (SD = 24.0, median = 8 days; IQR = 1–24 days) in those who did not (Welch Two Sample t-test, t = 0.9; p = 0.926).
All patients’ characteristics, except for any other antihistamine medication, were significantly associated with death. A multivariable logistic regression model showed that age, sex, hospital, obesity, and most ICD-10 disorder categories, neoplasms and diseases of the blood, mental disorders, cardiovascular disorders, respiratory disorders, dermatological disorders, diseases of the genitourinary system, and eye-ear-nose-throat disorders were significantly and independently associated with mortality (Table S1).
The distribution of patients’ characteristics according to hydroxyzine use is shown in Table 1. In the full sample, hydroxyzine use substantially differed according to all baseline characteristics (Table 1). The direction of these associations indicated older age and overall greater medical severity of patients receiving hydroxyzine than those who did not. In the matched analytic sample, these differences were substantially reduced and only sex, hospital, obesity and medication according to compassionate use or as part of a medical trial remained substantially different between groups (Table 1).
Table 1.
Characteristics of inpatients with COVID-19 receiving or not receiving hydroxyzine.
| Exposed to Hydroxyzine (N = 164) | Not Exposed to Hydroxyzine (N = 14,939) | Non-Exposed Matched Group (N = 164) |
Exposed to Hydroxyzine vs. Not Exposed to Hydroxyzine |
Exposed to Hydroxyzine vs. Non-Exposed Matched Group |
|
|---|---|---|---|---|---|
| Standardized mean differences | Standardized mean differences in the matched analytic sample | ||||
| N (%) | N (%) | N (%) | SMD | SMD | |
| Patients’ characteristics | |||||
| Age | 0.300 | 0.074 | |||
| 18 to 50 years | 44 (26.8%) | 5774 (38.7%) | 39 (23.8%) | ||
| 51 to 70 years | 65 (39.6%) | 4716 (31.6%) | 66 (40.2%) | ||
| 71 to 80 years | 30 (18.3%) | 1833 (12.3%) | 32 (19.5%) | ||
| More than 80 years | 25 (15.2%) | 2616 (17.5%) | 27 (16.5%) | ||
| Sex | 0.126 | 0.161 | |||
| Women | 76 (46.3%) | 7865 (52.6%) | 63 (38.4%) | ||
| Men | 88 (53.7%) | 7074 (47.4%) | 101 (61.6%) | ||
| Hospital | 0.557 | 0.147 | |||
| AP-HP Centre—Paris University, Henri Mondor University Hospitals and at home hospitalization | 36 (22.0%) | 6987 (46.8%) | 45 (27.4%) | ||
| AP-HP Nord and Hôpitaux Universitaires Paris Seine-Saint-Denis | 56 (34.1%) | 4059 (27.2%) | 51 (31.1%) | ||
| AP-HP Paris Saclay University | 31 (18.9%) | 1830 (12.2%) | 33 (20.1%) | ||
| AP-HP Sorbonne University | 41 (25.0%) | 2063 (13.8%) | 35 (21.3%) | ||
| Obesity a | 0.253 | 0.105 | |||
| Yes | 38 (23.2%) | 2009 (13.4%) | 31 (18.9%) | ||
| No | 126 (76.8%) | 12,930 (86.6%) | 133 (81.1%) | ||
| Smoking b | 0.194 | 0.017 | |||
| Yes | 24 (14.6%) | 1263 (8.5%) | 23 (14.0%) | ||
| No | 140 (85.4%) | 13,676 (91.5%) | 141 (86.0%) | ||
| Other medications | |||||
| Medication according to compassionate use or as part of a clinical trial c | 0.482 | 0.107 | |||
| Yes | 52 (31.7%) | 1838 (12.3%) | 44 (26.8%) | ||
| No | 112 (68.3%) | 13,101 (87.7%) | 120 (73.2%) | ||
| Any other antihistamine medication | 0.276 | <0.001 | |||
| Yes | 10 (6.1%) | 153 (1.0%) | 10 (6.1%) | ||
| No | 154 (93.9%) | 14,786 (99.0%) | 154 (93.9%) | ||
| Medical indications of hydroxyzine prescription | |||||
| Anxiety, insomnia, nausea, urticaria or pruritus | 0.276 | <0.001 | |||
| Yes | 14 (8.5%) | 349 (2.3%) | 14 (8.5%) | ||
| No | 150 (91.5%) | 14,590 (97.7%) | 150 (91.5%) | ||
| Medical comorbidities | |||||
| Other infectious diseases d | 0.347 | 0.052 | |||
| Yes | 25 (15.2%) | 740 (5.0%) | 22 (13.4%) | ||
| No | 139 (84.8%) | 14,199 (95.0%) | 142 (86.6%) | ||
| Neoplasms and diseases of the blood e | 0.386 | 0.048 | |||
| Yes | 30 (18.3%) | 873 (5.8%) | 27 (16.5%) | ||
| No | 134 (81.7%) | 14,066 (94.2%) | 137 (83.5%) | ||
| Mental disorders f | 0.371 | 0.033 | |||
| Yes | 28 (17.1%) | 824 (5.5%) | 26 (15.9%) | ||
| No | 136 (82.9%) | 14,115 (94.5%) | 138 (84.1%) | ||
| Diseases of the nervous system g | 0.349 | 0.017 | |||
| Yes | 23 (14.0%) | 618 (4.1%) | 24 (14.6%) | ||
| No | 141 (86.0%) | 14,321 (95.9%) | 140 (85.4%) | ||
| Cardiovascular disorders h | 0.372 | 0.066 | |||
| Yes | 48 (29.3%) | 2118 (14.2%) | 53 (32.3%) | ||
| No | 116 (70.7%) | 12,821 (85.8%) | 111 (67.7%) | ||
| Respiratory disorders i | 0.787 | 0.012 | |||
| Yes | 97 (59.1%) | 3452 (23.1%) | 98 (59.8%) | ||
| No | 67 (40.9%) | 11,487 (76.9%) | 66 (40.2%) | ||
| Digestive disorders j | 0.238 | 0.041 | |||
| Yes | 15 (9.2%) | 509 (3.4%) | 17 (10.4%) | ||
| No | 149 (90.9%) | 14,430 (96.6%) | 147 (89.6%) | ||
| Dermatological disorders k | 0.206 | <0.001 | |||
| Yes | 7 (4.3%) | 148 (1.0%) | 7 (4.3%) | ||
| No | 157 (95.7%) | 14,791 (99.0%) | 157 (95.7%) | ||
| Diseases of the musculoskeletal system l | 0.292 | <0.001 | |||
| Yes | 15 (9.2%) | 360 (2.4%) | 15 (9.2%) | ||
| No | 149 (90.9%) | 14,579 (97.6%) | 149 (90.9%) | ||
| Diseases of the genitourinary system m | 0.291 | 0.017 | |||
| Yes | 24 (14.6%) | 879 (5.9%) | 25 (15.2%) | ||
| No | 140 (85.4%) | 14,060 (94.1%) | 139 (84.8%) | ||
| Endocrine disorders n | 0.434 | 0.064 | |||
| Yes | 53 (32.3%) | 2148 (14.4%) | 58 (35.4%) | ||
| No | 111 (67.7%) | 12,791 (85.6%) | 106 (64.6%) | ||
| Eye-Ear-Nose-Throat disorders o | 0.199 | <0.001 | |||
| Yes | 7 (4.3%) | 160 (1.1%) | 7 (4.3%) | ||
| No | 157 (95.7%) | 14,779 (98.9%) | 157 (95.7%) |
a Defined as having a body-mass index higher than 30 kg/m2 or an International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnosis code for obesity (E66.0, E66.1, E66.2, E66.8, E66.9). b Current smoking status was self-reported. c Any medication prescribed as part of a clinical trial or according to compassionate use (e.g., hydroxychloroquine, azithromycin, remdesivir, tocilizumab, sarilumab or dexamethasone). d Assessed using ICD-10 diagnosis codes for certain infectious and parasitic diseases (A00-B99). e Assessed using ICD-10 diagnosis codes for neoplasms (C00-D49) and diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism (D50-D89). f Assessed using ICD-10 diagnosis codes for mental, behavioral and neurodevelopmental disorders (F01-F99). g Assessed using ICD-10 diagnosis codes for diseases of the nervous system (G00-G99). h Assessed using ICD-10 diagnosis codes for diseases of the circulatory system (I00-I99). i Assessed using ICD-10 diagnosis codes for diseases of the respiratory system (J00-J99). j Assessed using ICD-10 diagnosis codes for diseases of the digestive system (K00-K95). k Assessed using ICD-10 diagnosis codes for diseases of the skin and subcutaneous tissue (L00-L99). l Assessed using ICD-10 diagnosis codes for diseases of the musculoskeletal system and connective tissue (M00-M99). m Assessed using ICD-10 diagnosis codes for diseases of the genitourinary system (N00-N99). n Assessed using ICD-10 diagnosis codes for endocrine, nutritional and metabolic diseases (E00-E89). o Assessed using ICD-10 diagnosis codes for diseases of the eye and adnexa (H00-H59) and diseases of the ear and mastoid process (H60-H95). SMD > 0.1 in bold indicate substantial differences. Abbreviation: SMD, standardized mean difference.
3.2. Study Endpoint
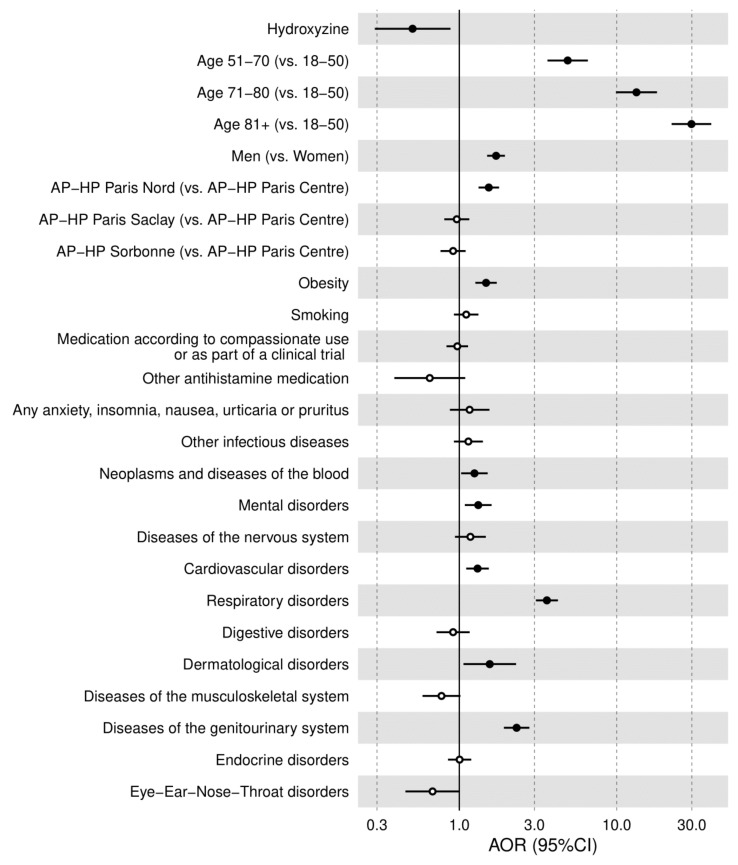
Among patients receiving hydroxyzine, death occurred in 18 patients (11.0%), while 1571 non-exposed patients (10.5%) had this outcome (Table 2). Despite the older age and the overall greater medical severity of patients receiving hydroxyzine at baseline than those who did not, the univariate unadjusted association between hydroxyzine use and mortality was not statistically significant (OR, 1.05, 95%CI, 0.64–1.72, p = 0.849) (Table 2). When taking into account differences in baseline characteristics between exposed and non-exposed patients, the multivariable logistic regression adjusting for age, sex, hospital, obesity, current smoking status, any medication prescribed according to compassionate use or as part of a clinical trial, any other antihistamine medication, and all medical conditions showed that hydroxyzine use was significantly associated with reduced mortality (AOR, 0.51; 95%CI, 0.29–0.88, p = 0.016) (Table 2; Figure 2).
Table 2.
Association between hydroxyzine use and mortality in the full sample and in the matched analytic sample.
| Number of Events/Number of Patients |
Crude Logistic Regression Analysis |
Multivariable Logistic Regression Analysis a |
Multivariable Logistic Regression Analysis b |
Number of Events/ Number of Patients |
Univariate Logistic Regression in the Matched Analytic Sample (1:1) | Multivariable Logistic Regression Analysis in the Matched Analytic Sample (1:1) c |
|
|---|---|---|---|---|---|---|---|
| N (%) | OR (95%CI; p-value) |
AOR (95%CI; p-value) |
AOR (95%CI; p-value) |
N (%) | OR (95%CI; p-value) |
AOR (95%CI; p-value) |
|
| Hydroxyzine | 18/164 (11.0%) | 1.05 (0.64–1.72; 0.849) | 0.53 (0.31–0.91; 0.023 *) | 0.51 (0.29–0.88; 0.016 *) | 18/164 (11.0%) | 0.44 (0.24–0.81; 0.008 *) | 0.42 (0.22–0.80; 0.008 *) |
| No hydroxyzine | 1571/14,939 (10.5%) | Ref. | Ref. | Ref. | 36/164 (22.0%) | Ref. | Ref. |
a Adjusted by age; sex; anxiety, insomnia, nausea, urticaria or pruritus; other infectious diseases; neoplasms and diseases of the blood; mental disorders; diseases of the nervous system; cardiovascular disorders; respiratory disorders; digestive disorders; dermatological disorders; diseases of the musculoskeletal system; diseases of the genitourinary system; endocrine disorders; and eye-ear-nose-throat disorders. b Adjusted by age; sex; hospital; obesity; smoking status; medication according to compassionate use or as part of a clinical trial; any other antihistamine medication; anxiety, insomnia, nausea, urticaria or pruritus; other infectious diseases; neoplasms and diseases of the blood; mental disorders; diseases of the nervous system; cardiovascular disorders; respiratory disorders; digestive disorders; dermatological disorders; diseases of the musculoskeletal system; diseases of the genitourinary system; endocrine disorders; and eye-ear-nose-throat disorders. c Adjusted by sex; hospital; obesity; and medication according to compassionate use or as part of a clinical trial. * p-value is significant (p < 0.05). Abbreviations: OR, odds ratio; AOR, adjusted odds ratio; CI, confidence interval.
Figure 2.
Multivariable logistic regression analysis for the association between hydroxyzine use and mortality in the full sample (N = 15,103). Dots in black indicate p < 0.05.
In sensitivity analyses, the univariate logistic regression model in the matched analytic sample showed similar results as the multivariable logistic regression model (OR, 0.42; 95%CI, 0.22–0.80 p = 0.008) (Table 2).
Additional analyses indicated that patients who were prescribed hydroxyzine in the three months before but not during the visit were significantly at higher risk of death than those who received this medication only during the visit (Table S2). This result suggests that the association between hydroxyzine use and reduced mortality is rather due to hydroxyzine use than to unmeasured characteristics of patients who were prescribed this medication, even if we cannot rule out that long-term side effects of prior use of this treatment might explain this finding.
The quantitative bias analysis with observed imbalances showed fairly robust odds’ ratios under a wide range of assumed associations between potential confounders and the outcome. Associations for an apparent odds ratio of 0.51 are presented in Figure S1.
The association between hydroxyzine use and decreased mortality remained significant when additionally adjusting for clinical severity of COVID-19 at baseline (AOR, 0.49; 95%CI, 0.28–0.86, p = 0.013) (Table S3).
The association between hydroxyzine use and decreased mortality remained significant in both patients admitted to ICUs and those hospitalized in normal wards (Table S4). However, the magnitude of the effect among patients admitted in ICUs should be considered with caution given the very limited numbers of patients taking hydroxyzine (N = 24) and events (N = 2) in this group.
Taking hydroxyzine more than 48 h from hospital admission was significantly associated with increased mortality compared to taking hydroxyzine at baseline (i.e., within 48 h from hospital admission), and was not significantly different than not receiving this medication during the hospitalization (Table S5).
Finally, exposure to higher (median daily dose = 25 mg, IQR = 25.0–50.0 mg) than lower doses (median daily dose = 12.5 mg, IQR = 10.6–12.5 mg) of hydroxyzine at baseline was not significantly associated with mortality (Table S6).
4. Discussion
In this multicenter retrospective observational study involving a relatively large sample of patients admitted to the hospital for COVID-19, our results suggest that hydroxyzine use at baseline, administered orally at a median daily dose of 25.0 mg (SD = 29.5) for a median duration of 14.4 days (SD = 15.4), was significantly and substantially associated with reduced mortality, independently of patients’ characteristics, other medications, medical indications of hydroxyzine prescription and medical comorbidities. Although these findings should be interpreted with caution due to the observational design of the study, they nonetheless provide support for conducting randomized double-blind placebo-controlled clinical trials with hydroxyzine against COVID-19.
Our study has several limitations. First, there are two possible major potential inherent biases in observational studies: unmeasured confounding and confounding by indication. Specifically, information on the precise indication for the prescription of hydroxyzine and whether the prescription was ongoing or first started at this time were not available. We tried to minimize the effects of confounding by hydroxyzine indication in different ways. First, we used a multivariable regression model to minimize the effects of confounding [35,36], taking into account main medical indications for hydroxyzine, including anxiety, insomnia, nausea, urticaria or pruritus, as well as a wide range of medical comorbidities, including any other infectious diseases, neoplasms and diseases of the blood, mental disorders, diseases of the nervous system, cardiovascular disorders, respiratory disorders, digestive disorders (K00-K95), dermatological disorders, diseases of the musculoskeletal system, diseases of the genitourinary system, endocrine disorders, and eye-ear-nose-throat disorders. Second, we performed a univariate Cox regression model as a sensitivity analysis in a matched analytic sample, that showed similar results. Third, although some amount of unmeasured confounding may remain, our analyses adjusted for numerous potential confounders and a quantitative bias analysis with observed imbalances suggested that residual confounding was unlikely to affect our results. Finally, the association was only observed in patients who received hydroxyzine within the first 48 h from hospital admission and not in those who received it only within the 3 months before hospital admission. Second, there were missing data for some baseline characteristic variables, including baseline clinical and biological markers of severity of COVID-19, which may be explained by the overwhelming of all hospital units during the COVID-19 peak incidence, and potential for inaccuracies in the electronic health records in this context. However, the associations observed between baseline characteristics and mortality are in line with prior epidemiological data [46]. Third, inflation of type I error might have occurred in secondary exploratory analyses due to multiple testing. Fourth, our results rely on a relatively limited number of patients who were prescribed hydroxyzine at baseline (N = 164), and limited statistical power may be responsible for the overestimation of the magnitude of the association [51]. Finally, despite the multicenter inpatient design, our results may not be generalizable to other settings or regions.
Multiple and possibly interrelated biological mechanisms may underlie this potential positive effect of hydroxyzine against COVID-19. First, hydroxyzine is a functional inhibitor of acid sphingomyelinase [17,18], and prior research supports that these molecules could be beneficial in COVID-19 patients, through potential subsequent antiviral and anti-inflammatory effects [7,8,17,19,20,21,22,23,24,25,26,27,28]. Second, hydroxyzine binds with significant affinity to Sigma-1 receptors (S1Rs) [32], which have been shown to restrict the endonuclease activity of an endoplasmic reticulum stress sensor inositol-requiring enzyme 1 (IRE1) and to reduce cytokine expression without inhibiting classical inflammatory pathways [26,34,35]. Finally, hydroxyzine is a medication with highly likely lysosomotropic behavior [36], and lysosomotropic medications have shown antiviral effects on coronaviruses, likely due to interference with endosomal pathway [36], and are assumed to suppress the cytokine release syndrome and to attenuate the transition from mild to severe SARS-CoV-2 infection/COVID-19 [37,38].
In this multicenter observational retrospective study involving patients hospitalized for COVID-19, hydroxyzine use was significantly and substantially associated with reduced mortality, independently of patients’ characteristics, other medications, medical indications of hydroxyzine prescription and medical comorbidities. Double-blind placebo-controlled randomized clinical trials of hydroxyzine for COVID-19 are needed to confirm these results, as are observational and clinical studies to examine the potential usefulness of this medication for outpatients with mild-to-moderate COVID-19 infection at high risk of progressing to severe COVID-19 and as post-exposure prophylaxis for individuals at high risk for severe COVID-19.
Acknowledgments
The authors thank the EDS APHP COVID consortium integrating the APHP Health Data Warehouse team as well as all the APHP staff and volunteers who contributed to the implementation of the EDS-COVID database and operating solutions for this database. Collaborators of the EDS APHP COVID consortium are: Pierre-Yves Ancel, Alain Bauchet, Nathanaël Beeker, Vincent Benoit, Mélodie Bernaux, Ali Bellamine, Romain Bey, Aurélie Bourmaud, Stéphane Breant, Anita Burgun, Fabrice Carrat, Charlotte Caucheteux, Julien Champ, Sylvie Cormont, Christel Daniel, Julien Dubiel, Catherine Ducloas, Loic Esteve, Marie Frank, Nicolas Garcelon, Alexandre Gramfort, Nicolas Griffon, Olivier Grisel, Martin Guilbaud, Claire Hassen-Khodja, François Hemery, Martin Hilka, Anne-Sophie Jannot, Jerome Lambert, Richard Layese, Judith Leblanc, Léo Lebouter, Guillaume Lemaitre, Damien Leprovost, Ivan Lerner, Kankoe Levi Sallah, Aurélien Maire, Marie-France Mamzer, Patricia Martel, Arthur Mensch, Thomas Moreau, Antoine Neuraz, Nina Orlova, Nicolas Paris, Bastien Rance, Hélène Ravera, Antoine Rozes, Elisa Salamanca, Arnaud Sandrin, Patricia Serre, Xavier Tannier, Jean-Marc Treluyer, Damien Van Gysel, Gaël Varoquaux, Jill Jen Vie, Maxime Wack, Perceval Wajsburt, Demian Wassermann, Eric Zapletal.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10245891/s1, Figure S1: Quantitative bias analysis based on the observed residual differences in age (A) and other patients characteristics (B) with an apparent OR of 0.51, Table S1: Associations of baseline clinical characteristics with mortality in the cohort of patients who had been admitted to the hospital for COVID-19 (N = 15,103), Table S2: Association between hydroxyzine use within the 3 months prior to hospital admission and not during the visit for COVID-19 versus patients with hydroxyzine use in the first 48 h of hospitalization for COVID-19 and mortality (N = 15,078), Table S3: Association between hydroxyzine use and mortality in the full sample and in the matched analytic sample, while adjusting for clinical severity of COVID-19 at baseline, Table S4: Association between hydroxyzine use and mortality in the full sample and in the matched analytic sample separately among patients admitted in ICUs and those admitted in normal wards, Table S5. Comparison of the mortality rate of patients who were prescribed hydroxyzine more than 48 h after admission with those who received this medication within 48 h from hospital admission and those who never received this treatment during the visit, Table S6: Associations between hydroxyzine daily dose at baseline and mortality among patients receiving hydroxyzine (N = 149).
Author Contributions
Conceptualization, M.S.-R. and N.H.; Methodology M.S.-R. and N.H.; Software, M.S.-R. and R.V.; Validation, F.L. and N.B.; Formal Analysis, M.S.-R. and R.V.; Investigation, M.S.-R., N.H. and P.D.L.M.; Resources, F.L.; Data Curation, N.B., A.N., N.P., C.D., A.G., G.L., M.B. and A.B.(Ali Bellamine); Writing—Original Draft Preparation, M.S.-R. and N.H.; Writing—Review and Editing, F.L., R.V., N.B., C.B., M.O., C.L., P.M., G.L., P.D.L.M., E.S., A.B.(Ali Bellamine) and A.B.(Anita Burgun); Visualization, M.S.-R. and P.D.L.M.; Supervision, N.H. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This observational study using routinely collected data received approval from the Institutional Review Board of the AP-HP clinical Data Warehouse (decision CSE-20-20_COVID19, IRB00011591, 8 April 2020). All procedures related to this work adhered to the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Informed Consent Statement
AP-HP clinical Data Warehouse initiative ensures patients’ information and informed consent regarding the different approved studies through a transparency portal in accordance with European Regulation on data protection and authorization n°1980120 from National Commission for Information Technology and Civil Liberties (CNIL).
Data Availability Statement
Data from the AP-HP Health Data Warehouse can be obtained upon request at https://eds.aphp.fr//, (accessed on 20 May 2020).
Conflicts of Interest
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no support from any organization for the submitted work; N. H. has received personal fees and non-financial support from Lundbeck, outside the submitted work. F. L. has received speaker and consulting fees from Janssen-Cilag, Euthérapie-Servier, and Lundbeck, outside the submitted work. C. L. reports personal fees and non-financial support from Janssen-Cilag, Lundbeck, Otsuka Pharmaceutical, and Boehringer Ingelheim, outside the submitted work. Other authors declare no financial relationships with any organisation that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoertel N., Blachier M., Blanco C., Olfson M., Massetti M., Rico M.S., Limosin F., Leleu H. A Stochastic Agent-Based Model of the SARS-CoV-2 Epidemic in France. Nat. Med. 2020;26:1417–1421. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- 2.Hoertel N., Blachier M., Sánchez-Rico M., Limosin F., Leleu H. Impact of the Timing and Adherence to Face Mask Use on the Course of the COVID-19 Epidemic in France. J. Travel Med. 2021;28:taab016. doi: 10.1093/jtm/taab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoertel N., Blachier M., Blanco C., Olfson M., Massetti M., Limosin F., Leleu H. Facing the COVID-19 Epidemic in NYC: A Stochastic Agent-Based Model of Various Intervention Strategies. medRxiv. 2020 doi: 10.1101/2020.04.23.20076885. [DOI] [Google Scholar]
- 4.Matta J., Wiernik E., Robineau O., Carrat F., Touvier M., Severi G., de Lamballerie X., Blanché H., Deleuze J.-F., Gouraud C., et al. Association of Self-Reported COVID-19 Infection and SARS-CoV-2 Serology Test Results with Persistent Physical Symptoms among French Adults during the COVID-19 Pandemic. JAMA Intern. Med. 2021;8:e216454. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevance A., Gourion D., Hoertel N., Llorca P.-M., Thomas P., Bocher R., Moro M.-R., Laprévote V., Benyamina A., Fossati P., et al. Ensuring mental health care during the SARS-CoV-2 epidemic in France: A narrative review. L’Encephale. 2020;46:S3–S13. doi: 10.1016/j.encep.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoertel N. Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality among Patients with COVID-19? JAMA Netw. Open. 2021;4:e2136510. doi: 10.1001/jamanetworkopen.2021.36510. [DOI] [PubMed] [Google Scholar]
- 8.Hoertel N., Sánchez-Rico M., Cougoule C., Gulbins E., Kornhuber J., Carpinteiro A., Becker K.A., Reiersen A.M., Lenze E.J., Seftel D., et al. Repurposing Antidepressants Inhibiting the Sphingomyelinase Acid/Ceramide System against COVID-19: Current Evidence and Potential Mechanisms. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenwasser L. New Insights into the Pathophysiology of Allergic Rhinitis. Allergy Asthma Proc. 2007;28:10–15. doi: 10.2500/aap.2007.28.2977. [DOI] [PubMed] [Google Scholar]
- 10.Mandhane S.N., Shah J.H., Bahekar P.C., Mehetre S.V., Pawar C.A., Bagad A.S., Chidrewar G.U., Rao C.T., Rajamannar T. Characterization of Anti-Inflammatory Properties and Evidence for No Sedation Liability for the Novel Antihistamine SUN-1334H. Int. Arch. Allergy Immunol. 2010;151:56–69. doi: 10.1159/000232571. [DOI] [PubMed] [Google Scholar]
- 11.Liu T., Zhang L., Joo D., Sun S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villoutreix B.O., Beaune P.H., Tamouza R., Krishnamoorthy R., Leboyer M. Prevention of COVID-19 by Drug Repurposing: Rationale from Drugs Prescribed for Mental Disorders. Drug Discov. Today. 2020;25:1287. doi: 10.1016/j.drudis.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villoutreix B.O., Krishnamoorthy R., Tamouza R., Leboyer M., Beaune P. Chemoinformatic Analysis of Psychotropic and Antihistaminic Drugs in the Light of Experimental Anti-SARS-CoV-2 Activities. Adv. Appl. Bioinforma. Chem. 2021;14:71–85. doi: 10.2147/AABC.S304649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas M.D., Saponi-Cortes J.M.R., Zamorano J. Hydroxyzine Inhibits SARS-CoV-2 Spike Protein Binding to ACE2 in a Qualitative in Vitro Assay. bioRxiv. 2021 doi: 10.1101/2021.01.04.424792. [DOI] [Google Scholar]
- 15.Reznikov L.R., Norris M.H., Vashisht R., Bluhm A.P., Li D., Liao Y.-S.J., Brown A., Butte A.J., Ostrov D.A. Identification of Antiviral Antihistamines for COVID-19 Repurposing. Biochem. Biophys. Res. Commun. 2021;538:173–179. doi: 10.1016/j.bbrc.2020.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge S., Wang X., Hou Y., Lv Y., Wang C., He H. Repositioning of Histamine H1 Receptor Antagonist: Doxepin Inhibits Viropexis of SARS-CoV-2 Spike Pseudovirus by Blocking ACE2. Eur. J. Pharmacol. 2021;896:173897. doi: 10.1016/j.ejphar.2021.173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornhuber J., Hoertel N., Gulbins E. The Acid Sphingomyelinase/Ceramide System in COVID-19. Mol. Psychiatry. 2021:1–8. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornhuber J., Muehlbacher M., Trapp S., Pechmann S., Friedl A., Reichel M., Mühle C., Terfloth L., Groemer T.W., Spitzer G.M., et al. Identification of Novel Functional Inhibitors of Acid Sphingomyelinase. PLoS ONE. 2011;6:e23852. doi: 10.1371/journal.pone.0023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornhuber J., Tripal P., Reichel M., Mühle C., Rhein C., Muehlbacher M., Groemer T.W., Gulbins E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A Novel Pharmacological Group of Drugs with Broad Clinical Applications. Cell. Physiol. Biochem. 2010;26:9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]
- 20.Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S., et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020;1:100142. doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpinteiro A., Gripp B., Hoffmann M., Pöhlmann S., Hoertel N., Edwards M.J., Kamler M., Kornhuber J., Becker K.A., Gulbins E. Inhibition of Acid Sphingomyelinase by Ambroxol Prevents SARS-CoV-2 Entry into Epithelial Cells. J. Biol. Chem. 2021;296:100701. doi: 10.1016/j.jbc.2021.100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloer S., Brunotte L., Goretzko J., Mecate-Zambrano A., Korthals N., Gerke V., Ludwig S., Rescher U. Targeting the Endolysosomal Host-SARS-CoV-2 Interface by Clinically Licensed Functional Inhibitors of Acid Sphingomyelinase (FIASMA) including the Antidepressant Fluoxetine. Emerg. Microbes Infect. 2020;9:2245–2255. doi: 10.1080/22221751.2020.1829082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoertel N., Sánchez-Rico M., Gulbins E., Kornhuber J., Carpinteiro A., Lenze E.J., Reiersen A.M., Abellán M., de la Muela P., Vernet R., et al. Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study. Clin. Pharmacol. Ther. 2021;110:1498–1511. doi: 10.1002/cpt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darquennes G., Le Corre P., Le Moine O., Loas G. Association between Functional Inhibitors of Acid Sphingomyelinase (Fiasmas) and Reduced Risk of Death in COVID-19 Patients: A Retrospective Cohort Study. Pharmaceuticals. 2021;14:226. doi: 10.3390/ph14030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seftel D., Boulware D.R. Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19. Open Forum Infect. Dis. 2021;8:ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., Miller J.P., Yang L., Yingling M., Avidan M.S., et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients with Symptomatic COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reis G., dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., dos Santos C.V.Q., de Souza Campos V.H., Nogueira A.M.R., de Almeida A.P.F.G., et al. Effect of Early Treatment with Fluvoxamine on Risk of Emergency Care and Hospitalisation among Patients with COVID-19: The TOGETHER Randomised, Platform Clinical Trial. Lancet Glob. Health. 2021;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoertel N., Sánchez-Rico M., Gulbins E., Kornhuber J., Carpinteiro A., Abellán M., de la Muela P., Vernet R., Beeker N., Neuraz A., et al. Association between Psychotropic Medications Functionally Inhibiting Acid Sphingomyelinase and Reduced Risk of Intubation or Death among Individuals with Mental Disorder and Severe COVID-19: An Observational Study. medRxiv. 2021 doi: 10.1101/2021.02.18.21251997. [DOI] [Google Scholar]
- 29.Marín-Corral J., Rodríguez-Morató J., Gomez-Gomez A., Pascual-Guardia S., Muñoz-Bermúdez R., Salazar-Degracia A., Pérez-Terán P., Restrepo M.I., Khymenets O., Haro N., et al. Metabolic Signatures Associated with Severity in Hospitalized COVID-19 Patients. Int. J. Mol. Sci. 2021;22:4794. doi: 10.3390/ijms22094794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dei Cas M., Ottolenghi S., Morano C., Rinaldo R., Roda G., Chiumello D., Centanni S., Samaja M., Paroni R. Link between Serum Lipid Signature and Prognostic Factors in COVID-19 Patients. Sci. Rep. 2021;11:21633. doi: 10.1038/s41598-021-00755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torretta E., Garziano M., Poliseno M., Capitanio D., Biasin M., Santantonio T.A., Clerici M., Lo Caputo S., Trabattoni D., Gelfi C. Severity of COVID-19 Patients Predicted by Serum Sphingolipids Signature. Int. J. Mol. Sci. 2021;22:10198. doi: 10.3390/ijms221910198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vela J.M. Repurposing Sigma-1 Receptor Ligands for COVID-19 Therapy? Front. Pharmacol. 2020;11:582310. doi: 10.3389/fphar.2020.582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., Gaultier A. Modulation of the Sigma-1 Receptor–IRE1 Pathway Is Beneficial in Preclinical Models of Inflammation and Sepsis. Sci. Transl. Med. 2019;11:eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Front. Pharmacol. 2021;12:652688. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum P.R., Rubin D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 36.Norinder U., Tuck A., Norgren K., Munic Kos V. Existing Highly Accumulating Lysosomotropic Drugs with Potential for Repurposing to Target COVID-19. Biomed. Pharmacother. Biomed. Pharmacother. 2020;130:110582. doi: 10.1016/j.biopha.2020.110582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaess M., Kaiser L., Sommerfeld O., Rentschler S., Csuk R., Deigner H.-P. Rational Drug Repurposing: Focus on Lysosomotropism, Targets in Disease Process, Drug Profile, and Pulmonary Tissue Accumulation in SARS-CoV-2 Infection/COVID-19. Front. Pharmacol. 2020;11:584881. doi: 10.3389/fphar.2020.584881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou D., Dai S.-M., Tong Q. COVID-19: A Recommendation to Examine the Effect of Hydroxychloroquine in Preventing Infection and Progression. J. Antimicrob. Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: Combining Antiviral and Anti-Inflammatory Treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagunas-Rangel F.A. Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-C-Reactive Protein Ratio in Patients with Severe Coronavirus Disease 2019 (COVID-19): A Meta-Analysis. J. Med. Virol. 2020;92:1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M., Peiris M., Poon L.L., Zhang W. Viral Dynamics in Mild and Severe Cases of COVID-19. Lancet Infect. Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.-S., Neuraz A., Salamanca E., Paris N., Daniel C., Gramfort A., et al. Association between Antidepressant Use and Reduced Risk of Intubation or Death in Hospitalized Patients with COVID-19: Results from an Observational Study. Mol. Psychiatry. 2021;26:5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 43.Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Neuraz A., Alvarado J.M., Daniel C., Paris N., Gramfort A., Lemaitre G., et al. Dexamethasone Use and Mortality in Hospitalized Patients with Coronavirus Disease 2019: A Multicentre Retrospective Observational Study. Br. J. Clin. Pharmacol. 2021;87:3766–3775. doi: 10.1111/bcp.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoertel N., Sánchez-Rico M., Vernet R., Jannot A.-S., Neuraz A., Blanco C., Lemogne C., Airagnes G., Paris N., Daniel C., et al. Observational Study of Chlorpromazine in Hospitalized Patients with COVID-19. Clin. Drug Investig. 2021;41:221–233. doi: 10.1007/s40261-021-01001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoertel N., Sánchez-Rico M., Vernet R., Jannot A.-S., Neuraz A., Blanco C., Lemogne C., Airagnes G., Paris N., Daniel C., et al. Observational Study of Haloperidol in Hospitalized Patients with COVID-19. PLoS ONE. 2021;16:e0247122. doi: 10.1371/journal.pone.0247122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin P.C. Using the Standardized Difference to Compare the Prevalence of a Binary Variable between Two Groups in Observational Research. Commun. Stat.-Simul. Comput. 2009;38:1228–1234. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 48.Schneeweiss S. Sensitivity Analysis and External Adjustment for Unmeasured Confounders in Epidemiologic Database Studies of Therapeutics. Pharmacoepidemiol. Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 49.Haut Conseil de la Santé Publique Statement on the Management at Home or in a Care Facility of Suspected or Confirmed COVID-19 Patients. [(accessed on 5 May 2021)]. Available online: https://www.hcsp.fr.
- 50.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 51.Serdar C.C., Cihan M., Yücel D., Serdar M.A. Sample Size, Power and Effect Size Revisited: Simplified and Practical Approaches in Pre-Clinical, Clinical and Laboratory Studies. Biochem. Med. 2021;31:010502. doi: 10.11613/BM.2021.010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the AP-HP Health Data Warehouse can be obtained upon request at https://eds.aphp.fr//, (accessed on 20 May 2020).