Abstract
Chk1 is an evolutionarily conserved protein kinase that regulates cell cycle progression in response to checkpoint activation. In this study, we demonstrated that agents that block DNA replication or cause certain forms of DNA damage induce the phosphorylation of human Chk1. The phosphorylated form of Chk1 possessed higher intrinsic protein kinase activity and eluted more quickly on gel filtration columns. Serines 317 and 345 were identified as sites of phosphorylation in vivo, and ATR (the ATM- and Rad3-related protein kinase) phosphorylated both of these sites in vitro. Furthermore, phosphorylation of Chk1 on serines 317 and 345 in vivo was ATR dependent. Mutants of Chk1 containing alanine in place of serines 317 and 345 were poorly activated in response to replication blocks or genotoxic stress in vivo, were poorly phosphorylated by ATR in vitro, and were not found in faster-eluting fractions by gel filtration. These findings demonstrate that the activation of Chk1 in response to replication blocks and certain forms of genotoxic stress involves phosphorylation of serines 317 and 345. In addition, this study implicates ATR as a direct upstream activator of Chk1 in human cells.
Checkpoints are signaling pathways that monitor the integrity and replication status of the genetic material before cells commit to either replicate (in S phase) or segregate (in mitosis) their DNA (27). Upon activation, checkpoints interface with cyclin-Cdk complexes to block cell cycle progression or alternatively to induce cell death. The DNA replication checkpoint monitors S-phase completion and prevents mitosis in its absence. The DNA damage checkpoint monitors the integrity of the genome and arrests the cell cycle either in G1 before DNA replication (termed the G1 DNA damage checkpoint), in S phase (the S-phase DNA damage checkpoint), or in G2 before mitosis (the G2 DNA damage checkpoint). Eukaryotic cells activate an evolutionarily conserved set of checkpoint proteins that rapidly induce cell cycle arrest to prevent replication or segregation of damaged DNA before repair is completed.
A key component of the DNA damage checkpoint is ATM (ataxia telangiectasia-mutated), a 370-kDa protein kinase (58). The ATM gene is mutated in the human genetic disorder ataxia telangiectasia (58). Cell lines derived from patients lacking ATM are radiosensitive and exhibit defects in checkpoint responses to ionizing radiation (IR), including p53-dependent G1 cell cycle arrest and p53-independent S and G2 cell cycle arrests (31). The kinase activity of ATM is activated in response to double-stranded DNA breaks, and ATM targets several effectors of checkpoint control, including Cds1 (also known as Chk2), Brca1, p95 (nbs1), p53, and Mdm2 (2, 5, 8–10, 15, 23, 33, 34, 41, 42, 47, 69, 74). Checkpoint responses to UV light and base-damaging agents are normal in cells lacking ATM. Taken together, these findings point to a seminal role for ATM in the IR-induced DNA damage checkpoint.
ATR (the ATM- and Rad3-related protein kinase) also contributes to checkpoint control in eukaryotes (13, 32). Unlike ATM, deletion of ATR in mice results in an embryonic lethal phenotype indicating that ATR is an essential gene (6, 17). Another feature that distinguishes these two kinases is their sensitivity to different types of checkpoint signals. As mentioned above, cells lacking ATM are hypersensitive to IR, but not to UV or hydroxyurea (HU), whereas cells overexpressing a kinase-inactive form of ATR are sensitive to UV and HU, as well as to IR. This suggests that ATR plays a more prominent role than ATM during the cellular response to unreplicated DNA (induced by agents such as HU) and to certain DNA-damaging agents, including UV light (14, 68). However, overexpression of ATR complements the radioresistant DNA synthesis defect of cells lacking ATM, demonstrating that these two kinases have overlapping functions in vivo. In support of this, ATM and ATR have been shown to have similar kinase specificities (35, 52). Both prefer phosphorylating serine or threonine residues that are followed by glutamine (SQ/TQ motifs), and as such, ATM and ATR have overlapping substrate specificity in vivo. Examples of substrates shared by ATM and ATR include p53 and Brca1 (40, 62, 63).
Another potential subsrate of ATR is the human Chk1 protein kinase. Chk1 was first identified in fission yeast as an essential component of the DNA damage checkpoint (1, 66). An additional role for Chk1 in the DNA replication checkpoint was revealed when fission yeast cells lacking both Chk1 and a second checkpoint kinase, Cds1, were found to advance into mitosis with unreplicated DNA (4, 43, 72). Homologs of Chk1 have also been found in humans, Drosophila melanogaster, Caenorhabditis elegans, budding yeast, and Xenopus (20, 21, 38, 50, 55, 60). In humans, fission yeast, and Xenopus, Chk1 has been proposed to regulate the G2 checkpoint by phosphorylating the Cdc25 protein phosphatase on a residue or residues that facilitate the binding of 14-3-3 proteins (38, 54, 56, 72, 73).
Like ATR, Chk1 has been shown to be an essential gene in mice (6, 17, 44, 61). These findings unveil essential functions for both ATR and Chk1 in the absence of environmentally imposed genotoxic stress. Embryos and conditional ES cell lines lacking Chk1 also exhibit defective checkpoint responses to replication blocks and DNA-damaging agents, establishing a checkpoint function for mammalian Chk1 in mice (44, 61). Evidence that Chk1 contributes to G2 checkpoint control in human cells comes from studies showing that agents such as UCN-01 and SB-218078, which are potent inhibitors of Chk1, abrogate G2 checkpoint function in human cells (7, 25, 29).
Chk1 is a component of signaling pathways that respond to structures characteristic of DNA damage and/or incomplete DNA replication. In fission yeast, Chk1 responds to DNA damage induced by either IR or UV, and Chk1 also functions in the DNA replication checkpoint (4, 22, 43, 66, 67, 72). Xenopus Chk1 responds to DNA replication blocks and UV damage, but not to DNA with double-strand breaks, which is characteristic of gamma-irradiated DNA (26, 37). The regulation of Chk1 is perhaps best characterized in Xenopus, where it has been demonstrated that Chk1 is phosphorylated and activated by ATR and that the ATR-Chk1 pathway responds to unreplicated DNA and UV-damaged DNA (26). In the case of human Chk1, there is controversy concerning not only what types of DNA structures it responds to, but also whether its kinase activity is elevated in response to genotoxic stress. Some studies have reported that the electrophoretic mobility of human Chk1 is retarded after exposure to IR (7, 44, 56), HU (7, 44), and UV (44, 46). Other studies failed to observe changes in the electrophoretic mobility of Chk1 when cells were treated with these agents (30). In addition, two studies have reported slight increases in Chk1 kinase activity in response to UV and BNP1350, a topoisomerase I inhibitor (46, 71), while other studies have reported that the kinase activity of human Chk1 is not altered in response to genotoxic stress (30, 76). Finally, human Chk1 has been shown to be phosphorylated on serine 345 when cells are treated with UV, IR, or HU in an ATR-dependent manner (44). However, it is not known if additional sites become phosphorylated during genotoxic stress, if phosphorylation contributes to Chk1 activity, or if human Chk1 is a direct target of ATR.
In this study, we investigated the regulation of human Chk1 in response to different types of genotoxic stress. We report that human Chk1 is phosphorylated on serines 317 and 345 in response to checkpoint activation. The kinase activity of human Chk1 is activated upon phosphorylation of serines 317 and 345, and the phosphorylated, activated form of Chk1 elutes more quickly on gel filtration columns. Finally, we report that ATR directly phosphorylates human Chk1 on serines 317 and 345 in vitro and that the phosphorylation of these residues is ATR dependent in vivo.
MATERIALS AND METHODS
Cell lines
293 and HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS), 100 U (each) of penicillin and streptomycin per ml, and 1 mM glutamine (culture medium). AT22IJE T cells expressing ATM (AT+) or not expressing ATM (AT−) (77) were cultured in a mixture of DMEM, 10% FBS, 100 U of penicillin and streptomycin per ml, 1 mM glutamine, and 100 μg of hygromycin per ml. U2OS cells were grown in modified McCoy's 5A medium (Gibco) supplemented with 10% FBS, 100 U of penicillin and streptomycin per ml, and 1 mM glutamine.
Antibodies.
Chk1 was detected with a rabbit polyclonal antibody raised against bacterially-produced glutathione S-transferase (GST)-Chk1 or with commercial monoclonal (SC8408) or polyclonal (SC7898) antibodies purchased from Santa Cruz Biotechnology. Chk1 fusion proteins were precipitated with either anti-c-Myc (9E10)–agarose conjugate (Santa Cruz Biotechnology) or anti-Flag M2 antibody–agarose affinity gel (Sigma Chemical Co.) and detected by Western blotting with c-myc polyclonal antibody (A-14; Santa Cruz Biotechnology). Bound primary antibodies were detected with either horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (ICN/CAPPEL) or HRP–rat anti-rabbit antibody (Zymed), and proteins were visualized by chemiluminescence with the ECL enhanced chemiluminescence reagent (Amersham). Antibodies specific for Chk1 phosphorylated on serine 317 were generated by immunization of rabbits with the phosphopeptide C-VKYSSpSQPEPRT coupled to keyhole limpet hemocyanin.
Generation of recombinant adenoviruses.
Wild-type and mutant forms of Chk1 were cloned as Flag-tagged fusions into the adenovirus shuttle vector pAdTrack-CMV (cytomegalovirus) (28). Chk1 (wild type and mutants) cDNAs were amplified with forward primer 3 containing the Flag tag and a KpnI site (5′-ATAGGTACCATGGACTATAAGGACGACGATGATAAGGCAGTGC CCTTTGAGGAAG-3′) and reverse primer 4 containing a stop codon and an XbaI site (5′-ATATCTAGATCATGTGGCAGGAAGCC). PCR products were digested with KpnI and XbaI and cloned into pAdTrack-CMV. The pAdTrack-CMV-based plasmids encoding wild-type and mutant Chk1 proteins were cotransformed with pAdEasy-1 into Escherichia coli BJ5183 to achieve homologous recombination. Recombinant adenoviruses were generated and propagated with the pAdEasy system as described previously (28).
Generation of recombinant baculovirus encoding His-Chk1 and affinity purification of Chk1 antibody.
Chk1 was amplified by PCR with pAdTrack-CMV-Flag-Chk1(wild type) as template with forward primer 5′-GGGAATTCGGTGGAGTCATGGCAGTGC CC and reverse primer 5′-GGGGTACCCTGTGGCAGGAAGCC. The PCR product was digested with EcoRI and KpnI and ligated into pFASTBACHTa (Gibco BRL). Recombinant baculovirus encoding His6-Chk1 was generated with the BAC-TO-BAC Baculovirus Expression System (Gibco BRL) and protocols suggested by the manufacturer. His6-Chk1 protein was purified from infected Sf9 insect cells on Ni-nitrilotriacetic acid agarose (Qiagen), and purified His6-Chk1 was eluted in a mixture of 50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 200 mM imidazole, and 1 mM dithiothreitol (DTT). Four milligrams of His6-Chk1 was coupled to 2 g of CNBr-activated Sepharose 4B (Sigma Chemical Co.) according to the manufacturer's recommendations. Antibodies generated against GST-Chk1 were applied to the column, and Chk1-specific antibodies were eluted with 0.1 M glycine (pH 2.8) and immediately neutralized with 1 M Tris (pH 8.0).
Coupling of antibody to immobilized protein A for immunoprecipitations.
ImmunoPure immobilized protein A agarose (Pierce) and affinity-purified Chk1 antibodies were incubated for 1 h at 4°C in 1 ml of mammalian cell lysis buffer 1 (MCLB1: 50 mM Tris-HCl [pH 8.0], 2 mM DTT, 5 mM EDTA, 0.5% Nonidet P-40, 100 mM NaCl, 1 μM microcystin, 1 mM sodium orthovanadate, 2 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg of aprotinin per ml, 20 μM leupeptin) at a ratio of 14 μg of affinity-purified antibody to 30 μl of packed beads. The antibody-bead mixture was washed once with 1 ml of MCLB1, twice with 1 ml of LiCl buffer (0.5 M LiCl, 50 mM Tris [pH 8.0]), and twice with 1 ml of MCLB1.
Treatment of cells with DNA-damaging agents and caffeine
HeLa cell cultures were incubated in culture medium containing 50 μM cisplatin, 3 μM etoposide, or 3 μM 1-β-d-arabinofuranosylcytosine (ara-C) for 1 h. The medium was removed, and cells were washed once and then incubated for 6 h in culture medium. For UV irradiation, the culture medium was removed, cells were washed twice with phosphate-buffered saline (PBS), and cells were irradiated in uncovered tissue culture dishes with 254-nm UV light at a dose of 10 J/m2 with a Stratalinker (Stratagene). Fresh culture medium was added back, and cells were incubated for an additional 6 h. In some experiments, HeLa cells were incubated in 10 mM caffeine for 2.5 h prior to HU treatment.
Plasmids
Chk1 was amplified by PCR with pAdTrack-CMV-Flag-Chk1(+) as the template with forward primer 1 (5′-AGGGAATCCCATGGCAGTGCCCTTTGTGG-3′) and reverse primer 2 (5′-GCTCTAGAGCTCATGTGGCAGGAAGCC-3′). The PCR product was digested with EcoRI and XbaI and ligated into pcDNA3-myc to generate pcDNA3-myc-Chk1. Alanine was substituted for aspartic acid at position 130 to generate kinase-inactive Chk1 (Chk1D130A). Phosphorylation-site mutants were made with the Quick Change mutagenesis kit (Strategene) by using pcDNA3-myc-Chk1 as a template. In each case, serine was changed to alanine. pcDNA3-myc-Chk1 was used as a template with forward primer 5′-GTGAAGTACTCCAGTGCTCAGCCAGAACCCCGC-3′ and reverse primer 5′-GCGGGGTTCTGGCTGAGCACTGGAGTACTTCAC-3′ to generate pcDNA3-myc-Chk1(S317A). pcDNA3-myc-Chk1 was used as a template with forward primer 5′-CAAGGGATCAGCTTTGCTCAGCCCACATGTCC-3′ and reverse primer 5′-GGACATGTGGGCTGAGCAAAGCTGATCCCTTG-3′ to generate pcDNA3-myc-Chk1(S345A). pcDNA3-myc-Chk1 was used as a template with forward primer 5′-CATATGCTTTTGAATGCTCAGTTACTTGGCACCCC-3′ and reverse primer 5′-GGGGTGCCAAGTAACTGAGCATTCAAAAGCATATG-3′ to generate pcDNA3-myc-Chk1(S357A). pcDNA3-myc-Chk1 was used as a template with forward primer 5′-GGCACCCCAGGATCCGCACAGAACCCCTGGCAG-3′ and reverse primer 5′-CTGCCAGGGGTTCTGTGCGGATCCTGGGGTGCC-3′ to generate pcDNA3-myc-Chk1(S366A). pcDNA3-myc-Chk1 was used as a template with forward primer 5′-GCTGATTGATATTGTGAGCGCACAGAAGGTTTGGCTTCCTGCC-3′ and reverse primer 5′-GGCAGGAAGCCAAACCTTCTGTGCGCTCACAATATCAATCAGC-3′ to generate pcDNA3-myc-Chk1(S468A). pcDNA3-myc-Chk1(S317A) was used as a template with primers described above to generate pcDNA3-myc-Chk1(S317A, S345A). pcDNA3-myc-Chk1(S357A) was used as a template with the primers described above to generate pcDNA3-myc-Chk1(S357A, S366A). Chk1 (wild type and D130A) proteins were also amplified by PCR with forward primer 5 (ATACCCGGGAATGGCAG TGCCCTTTGTGG) and reverse primer 6 (ATAGAATTCTCATGTGGCAGGAAGCC). The PCR products were digested with SmaI and EcoRI and ligated into pGEX2TN. pGEX2TN-Chk1 was used as a template to generate pGEX2TN-Chk1(S317A), pGEX2TN-Chk1(S345A), pGEX2TN-Chk1(S357A), pGEX2TN-Chk1(S366A), and pGEX2TN-Chk1(S468A) by using the methodologies and primers described above.
Purification of soluble GST-Chk1 (wild type and mutants) for kinase assays.
JM109 cells were transformed with plasmids encoding GST-Chk1 (wild type and mutants). Cultures were grown at 37°C to an optical density at 600 nm (OD600) of 0.6, and isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to a final concentration of 0.5 mM. After growing for an additional 3 h at 30°C, cells were pelleted by centrifugation. Cell pellets were washed with PBS buffer and resuspended in STE (100 mM NaCl, 10 mM Tris HCl [pH 8.0], 1 mM EDTA) supplemented with 2 mM PMSF, 10 μg of aprotinin per ml, 20 μM leupeptin, and 1.0 mg of lysozyme per ml. After rocking at 4°C for 20 min, Sarkosyl was added to a final concentration of 1.5%, and lysis was accomplished by sonication. Lysates were clarified by centrifugation, and Triton X-100 was added to a final concentration of 2%. Proteins were precipitated with glutathione-agarose (Sigma Chemical Co.) and washed twice in STE, twice in LiCl buffer (0.5 M LiCl, 50 mM Tris HCl [pH 8.0]), and twice in 50 mM Tris HCl (pH 7.4). GST-fusion proteins were eluted with 20 mM glutathione in 50 mM Tris HCl (pH 7.4).
Purification of GST-Cdc25C200–256
GST-Cdc25C200–256 was produced in bacteria and purified as described previously (53). The protein was eluted from GSH agarose in buffer consisting of 50 mM Tris (pH 7.4), 20 mM glutathione, 2 mM PMSF, 10 μg of aprotinin per ml, and 20 μM leupeptin followed by dialysis in 1 liter of dialyis buffer (25 mM Tris [pH 8.0], 5 mM DTT) overnight at 4°C.
Chk1 kinase assays
To monitor the activity of endogenous Chk1, 3 × 106 HeLa cells were untreated or treated with 3 mM HU for 17 h. Cells were lysed in MCLB2 (50 mM Tris-HCl [pH 8.0], 2 mM DTT, 5 mM EDTA, 0.5% Nonidet P-40, 100 mM NaCl, 1 μM microcystin, 1 mM sodium orthovanadate, 2 mM PMSF,10 μg of aprotinin per ml, 20 μM [5 μg/ml] leupeptin, 10 mM β-glycerophosphate, 1 mM sodium fluoride). Clarified lysates, representing 2 mg of total cellular protein, were incubated with 14 μg of affinity-purified Chk1 antibody coupled to protein A agarose overnight at 4°C. Precipitates were washed twice with MCLB2 and twice with incomplete kinase buffer (50 mM Tris Cl [pH 7.4], 1 mM DTT, 10 mM MgCl2). Fifty-microliter kinase reactions were carried out in the presence of incomplete kinase buffer containing 10 μM ATP, 10 μCi of [γ-32P]ATP, and 5 μg of soluble GST-Cdc25C200–256. Reaction mixtures were incubated at 30°C for 25 min and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred to nitrocellulose membrane. Radiolabeled proteins were visualized by autoradiography. The same membranes were then subjected to Western blot analysis with anti-Chk1 monoclonal antibody. For ectopically produced Chk1, clarified lysates, representing 2 mg of total cellular protein, or column fractions were incubated with 30 μl of anti-Flag–agarose (Sigma Chemical Co.) for 2 h at 4°C. Precipitates were washed twice with MCLB2, twice with LiCl buffer, and once with incomplete kinase buffer. Kinase assays were performed as described above.
Phosphatase treatment.
HeLa cells were lysed in MCLB2 (50 mM Tris-Cl [pH 8.0], 100 mM NaCl, 10 mM MgCl2, 0.5% Nonidet P-40, 2 mM DTT) containing 2 mM PMSF,10 μg of aprotinin per ml, and 5 μg of leupeptin per ml. Clarified lysates containing 150 μg of total cellular protein were incubated with 5 U of calf intestinal phosphatase (NEB) in the absence or presence of 10 mM β-glycerophosphate and 10 mM NaF at 37°C for 1 h.
ATR kinase assays.
293 cells (2.8 × 106) were transfected with 30 μg of plasmid encoding kinase-active or -inactive Flag-ATR by using the calcium phosphate transfection system according to the manufacturer's instructions (Life Technologies). Forty-eight hours after transfection, cells were lysed in MCLB3 (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.5% Nonidet P-40, 2 mM DTT, 5 mM EDTA, 10% glycerol) containing 1 μM microcystin, 1 mM sodium orthovanadate, 2 mM PMSF, 10 μg of aprotinin per ml, 5 μg of leupeptin per ml, 10 mM β-glycerophosphate, and 1 mM sodium fluoride. Lysates representing 4 mg of total cell protein were incubated with anti-Flag agarose for 2 h at 4°C. Precipitates were washed twice with MCLB3, once with LiCl buffer, and once with incomplete kinase buffer (20 mM HEPES [pH 7.4], 10 mM MgCl2, 10 mM MnCl2, 50 mM NaCl, 1 mM DTT). Kinase assays (50 μl) were performed in incomplete kinase buffer supplemented with 5 μM ATP, 20 μM β-glycerophosphate, 10 μCi of [γ-32P]ATP, and 1 μg of GST-Chk1 (wild type and mutants) that had been purified from bacteria.
Adenovirus infections and gel filtration.
HeLa cells were either mock infected or infected for 60 min with recombinant adenoviruses encoding Flag-tagged versions of Chk1 (wild type and mutants) at a multiplicity of infection (MOI) of 10 in 1 ml of serum-free DMEM. After 1 h, 5 ml of culture medium was added. After 15 h of infection, cells were either untreated or were incubated with 10 mM HU for 4 h. Cells were lysed in MCLB1. Lysates were passed through a 27-gauge needle 10 times and then clarified by centrifugation at 20,000 × g for 10 min followed by 50,000 rpm for 30 min. Clarified lysates containing 2 to 5 mg of total cellular protein were loaded onto a Superdex 200 10/30 column (Pharmacia) and eluted with 50 mM HEPES (pH 7.4), 150 mM NaCl, and 1 mM EDTA. Columns were run at 0.35 ml/min, and 0.5 ml was collected per fraction. For mock-infected cells, 150 μl from fractions 23 to 30 was resolved by SDS-PAGE. Chk1 was visualized by Western blotting with anti-Chk1 polyclonal antibody (SC7898). For adenovirus-infected cells, fractions were first incubated with 30 μl of packed anti-Flag–agarose. Precipitates were resolved by SDS-PAGE, and Flag-Chk1 (wild type and mutants) proteins were visualized by Western blotting with Chk1 polyclonal antibody (SC7898). When kinase assays were performed, precipitates were first incubated in complete kinase buffer containing GST-Cdc25C200–256. Kinase reactions were resolved by SDS-PAGE, and proteins were transferred to nitrocellulose. Radiolabeled proteins were visualized by autoradiography, and then the membrane was subjected to Western blotting with Chk1 polyclonal antibody (SC7898).
In vivo 32P labeling and two-dimensional peptide mapping analysis.
293 cells (2 × 106) were either mock transfected or transfected with plasmids encoding myc-tagged wild-type and mutant forms of Chk1 by using the Superfect transfection reagent (Qiagen). After 16 h of incubation, cells were incubated in phosphate-free DMEM containing 2 mCi of 32P-labeled inorganic phosphate per ml and 10 mM HU for 4 h. When labeling exogenous Chk1, 1 μM UCN-01 was also included during the labeling period. Cells were lysed in MCLB1. Endogenous Chk1 was immunoprecipitated from mock-transfected cell lysates with affinity-purified polyclonal antibody that had been precoupled to protein A beads. Ectopically expressed Chk1 was immunoprecipitated with anti-myc monoclonal antibody–agarose (Santa Cruz). Immunoprecipitates were subjected to SDS-PAGE on an 8% polyacrylamide gel, and proteins were transferred to nitrocellulose. Radiolabeled Chk1 was digested in a solution containing 0.2 mg of trypsin (Worthington) per ml in 50 mM ammonium bicarbonate for 17 h. Tryptic phosphopeptides were separated in the first dimension by thin-layer chromatography at pH 1.9 and in the second dimension by ascending chromatography in a buffer consisting of n-butanol–pyridine–acetic acid–water in a ratio of 75/50/15/60 (65). Alternatively, tryptic phosphopeptides were incubated with 1.2 U of Asp-N endopeptidase (Roche) per ml in 50 mM ammonium bicarbonate for 17 h and then incubated with Ziptip C18 resin (Millipore) to purify the phosphopeptides prior to two-dimensional phosphopeptide mapping. In some cases, an unlabeled phosphopeptide of the sequence LVQGISFpSQPTCP was resolved by two-dimensional mapping.
RESULTS
Checkpoints induce the phosphorylation and activation of Chk1.
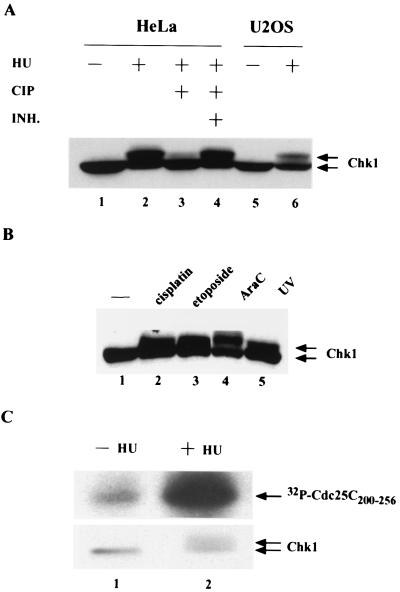
To examine how the Chk1 protein kinase is regulated during checkpoint responses, HeLa and U2OS cells were treated with HU to activate the DNA replication checkpoint (Fig. 1A). U2OS cells contain a functional p53 protein, whereas HeLa cells lack functional p53 due to the expression of the papillomavirus E6 protein, which targets p53 for destruction (59). The electrophoretic mobility of Chk1 was observed to be retarded on SDS gels after HU treatment of both cell types, suggesting that p53 status does not impinge upon this pathway (Fig. 1A, lanes 2 and 6). Phosphatase treatment confirmed that the slower electrophoretic form of Chk1 was due to HU-induced phosphorylation of Chk1 (lanes 3 and 4). Interestingly, the reduced electrophoretic mobility of Chk1 was also observed when HeLa cells were treated with UV light and a variety of chemotherapeutic agents that induce DNA damage or interfere with DNA replication, including cisplatin, ara-C, and etoposide (Fig. 1B).
FIG. 1.
Checkpoint-induced phosphorylation and activation of Chk1. (A) HeLa and U20S cells were untreated (−) or were treated (+) with 3 mM HU for 20 h. Cellular lysates containing 150 μg of protein were either resolved directly by SDS-PAGE (lanes 1, 2, 5, and 6) or were treated with 5 U of calf intestinal phosphatase (CIP) in the absence (lane 3) or in the presence (+ INH) of phosphatase inhibitors, including 10 mM β-glycerophosphate and 10 mM NaF (lane 4). Chk1 was detected by Western blotting with Chk1-specific polyclonal antibody (SC7898). (B) HeLa cells were cultured in DMEM alone (lane 1) or DMEM containing 50 μM cisplatin (lane 2), 3 μM etoposide (lane 3), or 3 μM ara-C (lane 4) for 1 h, after which the medium was replaced with fresh DMEM, and then the cells were cultured for an additional 6 h. In addition, HeLa cells were irradiated with 254-nm UV light at a dose of 10 J/m2 (lane 5). Cellular lysates containing 150 μg of protein were resolved directly by SDS-PAGE on an 8% polyacrylamide gel. Chk1 was detected by Western blotting with Chk1-specific polyclonal antibody (SC7898). (C) HeLa cells were untreated (−) or were treated (+) with 3 mM HU for 17 h. Clarified cellular lysates were incubated with affinity-purified Chk1 antibodies, and kinase assays were performed in vitro in the presence of 5 μg of GST-Cdc25C200–256. Reactions were resolved by SDS-PAGE, and proteins were transferred to nitrocellulose. Radiolabeled GST-Cdc25C200–256was visualized by autoradiography. Levels of Chk1 in each immunoprecipitate were determined by Western blotting with monoclonal antibody specific for Chk1 (SC8408).
To determine whether the protein kinase activity of Chk1 was regulated by phosphorylation, immune complex kinase assays were performed in vitro (Fig. 1C). Endogenous Chk1 was immunoprecipitated from HeLa cells that had been cultured in the absence (lane 1) or in the presence (lane 2) of HU. Chk1 immunoprecipitates were monitored for their ability to phosphorylate GST-Cdc25C200–256, a protein consisting of amino acids 200 to 256 of Cdc25C fused to GST. This substrate has previously been used to monitor the activity of Chk1 (25, 56). Interestingly, the protein kinase activity of Chk1 isolated from HU-treated cells was measured to be approximately fivefold higher than that isolated from untreated cells.
Gel filtration analysis of human Chk1.
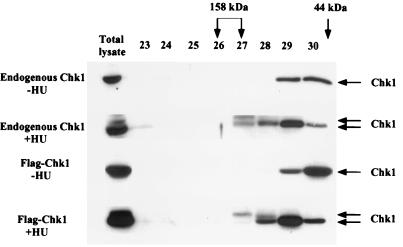
Gel filtration analysis was performed to monitor the elution profile of Chk1 before and after HU treatment (Fig. 2). In the absence of HU treatment, Chk1 eluted in fractions 29 and 30, consistent with it being a monomer in cells. However, after HU treatment, Chk1 was also detected in fractions that eluted earlier (Fig. 2 [and see Fig. 7]). Interestingly, the phosphorylated form of Chk1 that migrates with a slower electrophoretic mobility on SDS gels was preferentially found in the fractions that elute earlier. These findings suggest that the phosphorylated form of Chk1 either multimerizes, binds to cellular proteins, or undergoes conformational changes causing it to elute with protein standards of 100 to 158 kDa. To determine whether ectopically expressed Chk1 behaved like endogenous Chk1, we produced a recombinant adenovirus expressing Flag-tagged Chk1. Lysates prepared from infected HeLa cells were resolved by gel filtration, and selected fractions were incubated with Flag-agarose to specifically precipitate Flag-tagged Chk1. Precipitates were analyzed for the presence of Chk1 by Western blotting. As seen in Fig. 2, in the absence of HU treatment, Flag-Chk1 eluted in the same fractions as endogenous Chk1 (fractions 29 and 30). After HU treatment, Flag-Chk1 was observed to also elute in fractions 27 and 28, as did endogenous Chk1.
FIG. 2.
Analysis of Chk11 by gel filtration. HeLa cells were either mock infected or infected with recombinant adenovirus encoding Flag-tagged Chk1. After 15 h of infection, cells were either untreated (−HU) or were incubated with 10 mM HU (+HU) for 4 h. Clarified lysates were analyzed directly by SDS-PAGE and Western blotting (Total lysate) or were loaded onto a Superdex 200 10/30 column. For mock-infected cells, 150 μl from fractions 23 to30 was resolved by SDS-PAGE. For adenovirus-infected cells, fractions 23 to 30 were first incubated with anti-Flag–agarose. Chk1 and Flag-Chk1 were visualized by Western blotting with Chk1 polyclonal antibody (SC7898). Fractions containing protein standards of 158 and 44 kDa are indicated. The 44-kDa protein standard eluted primarily in fractions 30 and 31.
FIG. 7.
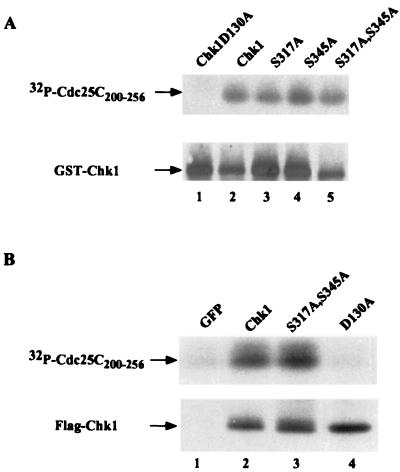
Phosphorylation site mutants retain intrinsic protein kinase activity. (A) Chk1, Chk1(D130A), Chk1(S317A), Chk1(S345A), and Chk1(S317A, S345A) were purified as GST-fusion proteins in bacteria and tested for their ability to phosphorylate GST-Cdc25C200–256 in vitro. Reaction products were resolved by SDS-PAGE, and proteins were transferred to nitrocellulose. Phosphorylated Cdc25C200–256 was detected by autoradiography, and levels of GST-Chk1 (wild type and mutants) were visualized by Western blotting with Chk1-specific polyclonal antibody (SC7898). (B) HeLa cells were infected with recombinant adenoviruses encoding GFP (lane 1), Flag-Chk1 (lane 2), Flag-Chk1(S317A, S345A) (lane 3), and Flag-Chk1(D130A). Lysates were prepared and incubated with Flag-agarose. Precipitates were tested for their ability to phosphorylate GST-Cdc25C200–256 in vitro. Reaction products were resolved by SDS-PAGE, and proteins were transferred to nitrocellulose. Phosphorylated Cdc25C200–256 was detected by autoradiography, and levels of GST-Chk1 (wild type and mutants) were visualized by Western blotting with Chk1-specific polyclonal antibody (SC7898).
Checkpoint-induced phosphorylation of Chk1 is ATM independent but caffeine sensitive.
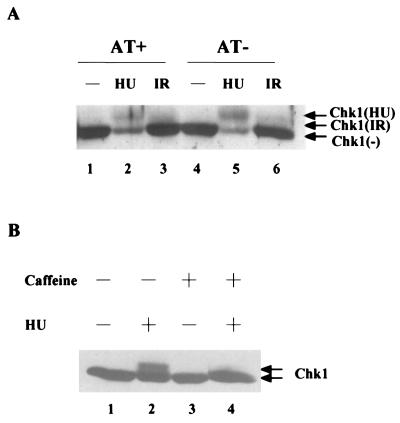
To determine whether the HU-induced phosphorylation of Chk1 required functional ATM, immortalized fibroblasts derived from a patient lacking ATM (AT cells), stably transfected with either the ATM expression vector (AT+) or an empty vector (AT−), were treated with HU. The electrophoretic mobility of Chk1 was then monitored by immunoblotting (Fig. 3A). The slower electrophoretic form of Chk1 was detected in both AT+ (lane 2) and AT− (lane 5) cells, although reduced levels of Chk1 protein were observed in both cell lines after HU treatment. We also monitored the electrophoretic mobility of Chk1 after treatment of AT+ and AT− cells with ionizing radiation. As seen in Fig. 3A (lanes 3 and 6), the electrophoretic mobility of a fraction of Chk1 was found be retarded in both AT+ and AT− cells, although to a lesser extent than that observed with HU.
FIG. 3.
Checkpoint-induced phosphorylation of Chk1 is ATM independent but caffeine sensitive. (A) AT+ and AT− cells were untreated (lanes 1 and 4) or were treated with 3 mM HU (lanes 2 and 5) or irradiated with 10 Gy of IR (lanes 3 and 6). Cellular lysates containing 150 μg of protein were resolved directly by SDS-PAGE on an 8% polyacrylamide gel. Chk1 was detected by Western blotting with Chk1-specific polyclonal antibody (SC7898). (B) HeLa cells were cultured in DMEM lacking caffeine (lanes 1 and 2) or containing 10 mM caffeine (lanes 3 and 4) for 2.5 h. Cells were cultured for an additional 20 h in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 3 mM HU. Cellular lysates containing 150 μg of protein were resolved directly by SDS-PAGE on an 8% polyacrylamide gel. Chk1 was detected by Western blotting with Chk1-specific polyclonal antibody (SC7898).
We also monitored the effects of caffeine on the HU-induced phosphorylation of Chk1, because both ATM and ATR are inhibited by caffeine (3, 9, 49, 57, 75). As seen in Fig. 3B, the ability of HU to induce the phosphorylation of Chk1 was completely abrogated when cells were pretreated with 10 mM caffeine (lane 4). Taken together, these observations suggest that the HU-induced phosphorylation of Chk1 is independent of ATM, but may be dependent on ATR or another caffeine-sensitive kinase.
Chk1 is phosphorylated on serines 317 and 345 in vivo.
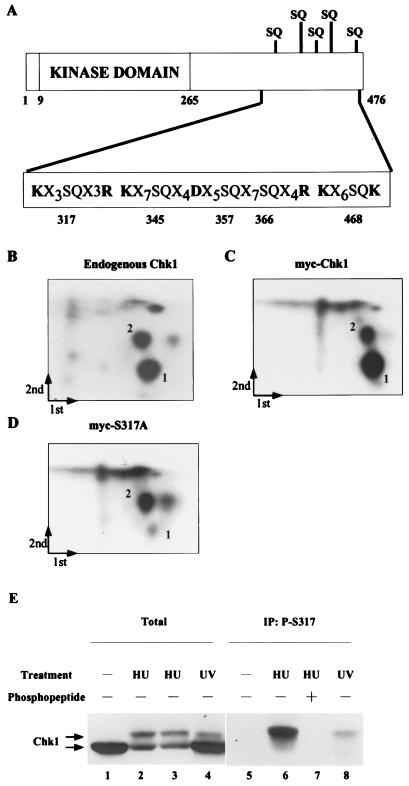
Two-dimensional phosphopeptide mapping of Chk1 was performed to identify residues that were phosphorylated in response to HU treatment. HU-treated HeLa cells were incubated with 32P-labeled inorganic phosphate, and endogenous Chk1 was isolated by immunoprecipitation. In addition, HeLa cells were transfected with plasmids encoding myc-tagged Chk1. Transfected cells were incubated with 32P-labeled inorganic phosphate in the presence of both HU and 1 μM UCN-01. UCN-01 inhibits the activity of Chk1 kinase, but not the activity of the kinases that lie upstream of Chk1 (7, 25, 29). We observed enhanced phosphorylation of ectopically expressed Chk1, but not that of endogenous Chk1 when UCN-01 was included during the labeling period. Although the mechanistic basis for this is not known, UCN-01 is expected to inhibit the kinase activity of ectopically produced Chk1, thus allowing cells to overproduce Chk1 in a kinase-inactive form. 32P-labeled endogenous Chk1 and myc-tagged Chk1 were digested with trypsin, and peptides were separated in two dimensions. As seen in Fig. 4, two predominant phosphospeptides (denoted 1 and 2) were evident in the case of endogenous (Fig. 4B) and ectopically expressed (Fig. 4C) Chk1. Given the caffeine sensitivity of Chk1 phosphorylation, we examined the Chk1 protein sequence for the presence of SQ and TQ residues. Serine and threonine residues followed by glutamine are preferred sites of phosphorylation for the ATM and ATR kinases (35, 52). As seen in Fig. 4A, there are five SQ residues located within the C terminus of Chk1 (serines 317, 345, 357, 366, and 468). A mutant form of Chk1 containing alanine in place of serine at position 317 was expressed as a myc-tagged fusion protein in HeLa cells. Two-dimensional tryptic phosphopeptide mapping demonstrated that mutation of serine 317 resulted in the disappearance of phosphopeptide 1 (Fig. 4D). This indicates that serine 317 is phosphorylated in HU-treated cells.
FIG. 4.
Chk1 is phosphorylated on serine 317 in vivo. (A). The structure of Chk1 is schematically represented to show the location of the kinase domain (amino acids 9 to 265), the SQ-rich region at the C terminus, and trypsin and Asp-N cleavage sites relative to potential phosphorylation sites. (B to D) 293 cells were either mock transfected or transfected with plasmids expressing myc-Chk1 (wild type) or myc-Chk1(S317A). Cells were incubated with 32P-labeled inorganic phosphate in the presence of HU. Radiolabeled Chk1 was digested with trypsin, and the peptides were subjected to two-dimensional phosphopeptide mapping. Arrows indicate the direction of the first and second dimensions, and the origin is located where the two arrows meet. (E) HeLa cells were untreated (lanes 1 and 5) or were treated with either 3 mM HU (lanes 2, 3, 6, and 7) or 20 J of UV m2 (lanes 4 and 8). Lysates were prepared and analyzed as directed by SDS-PAGE (lanes 1 to 4) or were first incubated with the S317 phosphospecific antibody in the absence (lanes 5, 6, and 8) or presence (lane 7) of competing phosphopeptide immunogen. Chk1 was detected by immunoblotting with a monoclonal antibody specific for Chk1 (SC8408).
To directly monitor the phosphorylation of serine 317 in vivo, we generated an antibody that recognizes Chk1 when it is phosphorylated on serine 317. HeLa cells were either untreated or were treated with HU or UV (Fig. 4E). Lysates were prepared and analyzed directed by SDS-PAGE (lanes 1 to 4) or were incubated with the S317 phosphospecific antibody prior to SDS-PAGE (lanes 5 to 8). As expected, HU and UV treatments caused the appearance of the slower electrophoretic (phosphorylated) form of Chk1. Interestingly, the phosphospecific antibody immunoprecipated only the slower electrophoretic (phosphorylated) form of Chk1 found in HU- and UV-treated cells (lanes 6 and 8). Preincubation of the antibody with the phosphopeptide immunogen blocked the immunoprecipitation of S317-phosphorylated Chk1 (lane 7). These results verify that Chk1 is phosphorylated on serine 317 in vivo in response to HU and UV treatments.
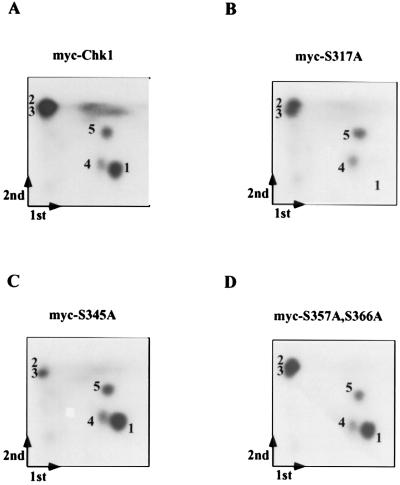
Because serines 345, 357, and 366 are contained within a single, large tryptic peptide (Fig. 4A), we digested myc-tagged Chk1 (wild type and mutants) with trypsin followed by Asp-N endopeptidase. As seen in Fig. 5A, five phosphopeptides were visualized under these conditions. Mutation of serine 317 resulted in the loss of phosphopeptide 1 (Fig. 5B), whereas mutation of serine 345 resulted in the loss of phosphopeptide 2 (Fig. 5C [and see Fig. 8]). S345 has previously been shown to be phosphorylated in response to checkpoint activation (44). Replacement of serines 357 and 366 with alanine did not cause the loss of any of the five phosphopeptides identified for wild-type Chk1 (Fig. 5D). In addition, replacement of serine 468 with alanine did not alter the two-dimensional phosphopeptide mapping results, suggesting that this residue is not phosphorylated in vivo (data not shown). These findings also indicate that serines 317 and 345 are sites of Chk1 phosphorylation in vivo.
FIG. 5.
Chk1 is also phosphorylated on serine 345 in vivo. 293 cells were transfected with plasmids expressing myc-Chk1 (wild type), myc-Chk1(S317A), myc-Chk1(S345A), and myc-Chk1(S357A, S366A). Cells were incubated with 32P-labeled inorganic phosphate in the presence of HU. Radiolabeled Chk1 (wild type and mutants) was digested with trypsin followed by Asp-N endopeptidase, and peptides were subjected to two-dimensional phosphopeptide mapping. Arrows indicate the direction of the first and second dimensions, and the origin is located where the two arrows meet.
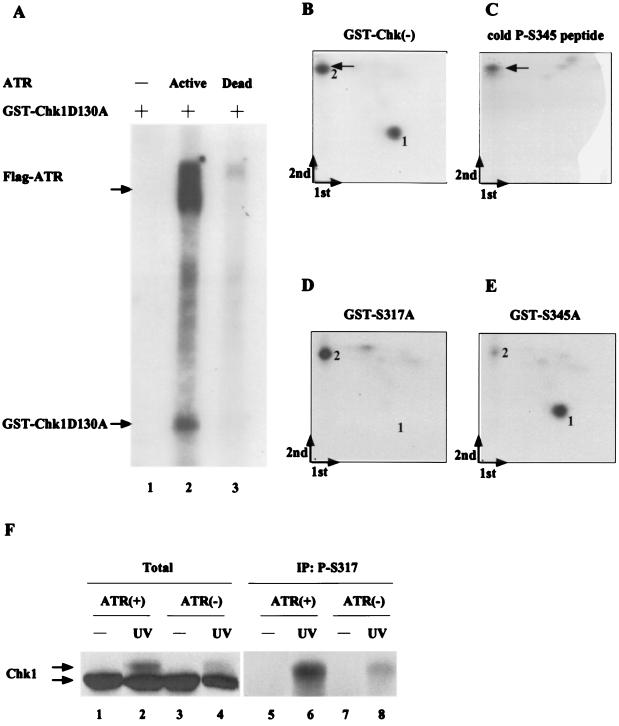
FIG. 8.
Interactions between ATR and Chk1 in vitro and in vivo. (A) 293 cells were transfected with plasmids encoding kinase-active (ATR+) and -inactive (ATR−) forms of Flag-tagged ATR. Lysates were prepared and incubated with anti-Flag–agarose. Precipitates were monitored for their ability to phosphorylate bacterially produced kinase-inactive Chk1 fused to GST (GST-Chk1D130A). Kinase reactions were resolved by SDS-PAGE, proteins were transferred to nitrocellulose, and radiolabeled Chk1 was visualized by autoradiography. (B to E) 293 cells were transfected with plasmid encoding Flag-tagged ATR. Lysates were prepared and incubated with anti-Flag–agarose. Kinase reactions were carried out in the presence of bacterially produced GST-Chk1(D130A), GST-Chk1(D130A, S317A), and GST-Chk1(D130A, S345A). Radiolabeled Chk1 proteins were digested with trypsin followed by Asp-N endopeptidase, and peptides were subjected to two-dimensional phosphopeptide mapping. The first and second dimensions of separation are indicated, and the origin is located where the two arrows meet. Phosphopeptides from proteolyzed GST-Chk1(D130A) were mixed with unlabeled phosphopeptide (LVQGISFpSQPTCP) prior to mapping (B), or the phosphopeptide was resolved alone (C) and visualized by staining with ninhydrin. The arrows indicate the position of the unlabeled phosphopeptide. (F) 293 cells were transfected with plasmids encoding kinase-active (ATR+) and -inactive (ATR−) forms of Flag-tagged ATR. Transfected cells were untreated (lanes 1, 3, 5, and 7) or were treated with 20 J of UV m2 (lanes 2, 4, 6, and 8). Lysates were prepared and analyzed directed by SDS-PAGE (lanes 1 to 4) or were first incubated with the S317 phosphospecific antibody prior to SDS-PAGE (lanes 5 to 8). Chk1 was detected by immunoblotting with a monoclonal antibody specific for Chk1 (SC8408).
HU-induced activation of Chk1 requires phosphorylation of serines 317 and 345.
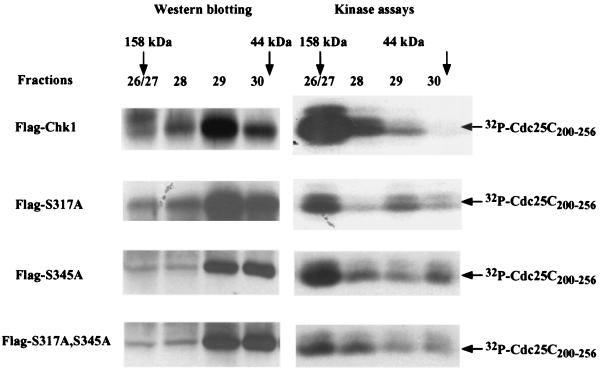
To address the functional significance of phosphorylation of serines 317 and 345, recombinant adenoviruses expressing single- and double-phosphorylation-site mutants of Chk1 were generated. HeLa cells were infected with wild-type and mutant viruses in the presence of HU. Lysates were prepared and fractionated by gel filtration. Selected fractions were analyzed for Chk1 protein and for Chk1 kinase activity. As seen in Fig. 6 and as shown earlier (Fig. 2), the phosphorylated, activated form of Chk1 eluted in fractions consistent with a molecular size of ∼100 to 158 kDa (fractions 26 to 28). The bulk of the hypo- or unphosphorylated, inactive Chk1 migrated in fractions 29 to 30, as expected for monomeric protein. Mutation of either S317 or S345 to alanine reduced the amount of Chk1 in the fractions eluting earlier (fractions 26 to 28). In addition, the kinase activity associated with Chk1(S317A) and Chk1(S345A) in fractions 26 to 28 was markedly reduced compared with that of wild-type Chk1. Finally, the mutant of Chk1 containing alanine in place of serines 317 and 345 eluted primarily in fractions 29 and 30 and was poorly activated by HU treatment. Taken together, these findings suggest that phosphorylation of both S317 and S345 contributes to the activation of Chk1 and its faster elution on gel filtration columns.
FIG. 6.
HU-induced activation of Chk1 requires phosphorylation of serines 317 and 345. (A) HeLa cells were infected with recombinant adenovirus encoding Flag-Chk1 (wild type), Flag-Chk1(S317A), Flag-Chk1(S345A), or Flag-Chk1(S317A, S345A). After 15 h of infection, cells were incubated with 10 mM HU for 4 h. Clarified lysates were loaded onto a Superdex 200 10/30 column. Fractions 26 and 27 were pooled. Fractions 26/27, 28, 29, and 30 were incubated with anti-Flag–agarose. Precipitates were monitored for their ability to phosphorylate GST-Cdc25C200–25. Kinase reactions were resolved by SDS-PAGE, and proteins were transferred to nitrocellulose. Radiolabeled GST-Cdc25C200–256 was visualized by autoradiography, Chk1 was visualized by Western blotting with Chk1-specific polyclonal antibody (SC7898).
To determine whether the intrinsic protein kinase activity of Chk1 was altered as a consequence of amino acid substitutions, we monitored the kinase activity of bacterially produced Chk1 (wild type and mutants). Kinase-dead Chk1, wild-type Chk1, Chk1(S317A), Chk1(S345A), and Chk1(S317A, S345A) were expressed and purified from bacteria as GST-fusion proteins and were tested for their ability to phosphorylate GST-Cdc25C200–256 in vitro. As seen in Fig.7A, each phosphorylation site mutant of Chk1 had kinase activity similar to that of wild-type Chk1 in this assay. Wild-type and mutant forms of Chk1 expressed in HeLa cells were also examined for kinase activity (Fig. 7B). Lysates prepared from cells infected with adenoviruses encoding green fluorescent protein (GFP; lane 1), Flag-Chk1 (lane 2), Flag-Chk1(S317A, S345A), and kinase-inactive Flag-Chk1(D130A) were incubated with anti- Flag–agarose, and precipitates were tested for their ability to phosphorylate GST-Cdc25C200–256 in vitro. The double-phosphorylation mutant of Chk1 (lane 3) had kinase activity similar to that of wild-type Chk1 (lane 2) in this assay. These results indicate that the failure of the phosphorylation site mutants of Chk1 to be fully activated when cells are treated with HU (Fig. 6) reflects an inability of these mutants to be phosphorylated rather than intrinsic defects in kinase activity as a result of amino acid substitution.
Phosphorylation of Chk1 by ATR.
We next asked whether Chk1 is a direct substrate of ATR in vitro. Kinase-active and -inactive forms of ATR were tested for their ability to phosphorylate Chk1 in vitro. A kinase-inactive mutant of Chk1(D130A) fused to GST was used as a substrate in these assays. As seen in Fig. 8A, Chk1 was phosphorylated by kinase-active (lane 2) but not kinase-inactive (lane 3) ATR. Radiolabeled Chk1 was isolated and digested with trypsin followed by Asp-N endopeptidase. Peptides were separated in two dimensions, and phosphopeptides were visualized by autoradiography. As seen in Fig. 8B, two predominant phosphopeptides were detected under these conditions. Mutation of serine 317 caused loss of phosphopeptide 1 (Fig. 8D), whereas mutation of serine 345 caused loss of phosphopeptide 2 (Fig. 8E). To further confirm that phosphopeptide 2 represented the peptide containing phosphorylated serine 345, we synthesized the phosphopeptide expected after digestion of Chk1 with both trypsin and Asp-N endopeptidase (LVQGISFpSQPTCP) and monitored its position after two-dimensional phosphopeptide mapping. As seen in Fig. 8B and C, the synthetic phosphopeptide comigrated with phosphopeptide 2, confirming that phosphopeptide 2 contains S345. These results demonstrate that Chk1 is phosphorylated on serines 317 and 345 by ATR in vitro and indicate that Chk1 may also be a direct target of ATR in vivo.
To examine the contribution made by ATR to S317 phosphorylation in vivo, we expressed either kinase-active or -inactive ATR in 293 cells and examined S317 phosphorylation after UV treatment. As seen in Fig. 8F, UV-induced phosphorylation of Chk1 was attenuated in cells expresssing kinase-inactive ATR (lane 4) relative to kinase-active ATR (lane 2). Immunoprecipitation with the S317 phosphospecific antibody demonstrated that phosphorylation of Chk1 on serine 317 in response to UV was reduced in cells expressing kinase-inactive ATR (lane 8). It has previously been reported that S345 is phosphorylated in response to HU, UV, and IR in an ATR-dependent manner in vivo (44). Taken together, these results demonstrate that Chk1 is inducibly phosphorylated on S317 and S345 in response to various stimuli that cause checkpoint activation and that phosphorylation of Chk1 on both S317 and S345 in vivo is ATR dependent.
DISCUSSION
In this study, regulation of the human Chk1 protein kinase was examined after exposure of cells to agents that induce various forms of DNA damage or interfere with DNA replication. There has been controversy concerning not only what DNA structures human Chk1 responds to, but also whether the kinase activity of Chk1 is elevated in response to genotoxic stress. It has been reported that human Chk1 is inducibly phosphorylated on serine 345 in response to HU and UV and, to a lesser extent, IR (44). In addition, dominant-negative forms of ATR interfere with the phosphorylation of Chk1 on serine 345 in vivo (44). However, it has not been determined if human Chk1 becomes phosphorylated on additional sites in response to genotoxic stress in vivo, if phosphorylation regulates the kinase activity of human Chk1, or if human Chk1 is a direct target of ATR.
In this study, serines 317 and 345 were identified as sites of phosphorylation in vivo and as sites of ATR phosphorylation in vitro. Furthermore, expression of a kinase-inactive form of ATR interfered with the UV-induced phosphorylation of Chk1 on serine 317 in vivo. It has previously been reported that kinase-inactive ATR also interferes with the UV-induced phosphorylation of Chk1 on serine 345 in vivo. These findings in conjunction with the role of ATR in regulating cellular responses to UV and HU argue that human Chk1 may be directly phosphorylated on serines 317 and 345 by ATR in vivo. Xenopus Chk1 contains four SQ/TQ consensus sites within its C terminus, and all four can be phosphorylated by ATR in vitro (26). Expression of a mutant form of Chk1 lacking all four phosphorylation sites abrogated checkpoint responses of Xenopus extracts to replication blocks and UV-damaged DNA (26). These findings highlight the conserved nature of the ATR-Chk1 checkpoint pathway in eukaryotic organisms.
Human Chk1 has five ATM or ATR consensus phosphorylation sites (SQ/TQ) within its C terminus. These include serines 317, 345, 357, 366, and 468, each of which is followed by glutamine. Serines 317 and 345 are the only two found conserved in Chk1 from other species, including fission yeast, C. elegans, Xenopus, and Drosophila. In addition, residues inclusive of and surrounding serines 317 and 345 in human Chk1 comprise optimal ATM or ATR consensus phosphorylation sites (35). Interestingly deletion of the C terminus of human and Xenopus Chk1 potently activates its intrinsic kinase activity, suggesting that the C terminus functions as a negative regulator (12, 51). Phosphorylation of Chk1 by ATR may serve to relieve this C-terminal inhibitory function to activate Chk1 during the cellular response to genotoxic stress. In addition to activating the protein kinase activity of Chk1, phosphorylation also caused Chk1 to elute faster on gel filtration columns. Additional studies will be required to determine if the faster elution profile of the phosphorylated form of human Chk1 is a result of Chk1 binding to cellular proteins, multimerizing, or undergoing conformational changes. In checkpoint-activated extracts, Xenopus Chk1 has been shown to bind to claspin, a 215-kDa protein, and the interaction between Xenopus Chk1 and claspin is required for checkpoint-induced activation of Chk1 (37).
Although Chk1 is highly conserved throughout evolution, the signals that Chk1 responds to have diverged in eukaryotic organisms. In fission yeast, Chk1 responds to DNA damage induced by either IR or methyl methanesulfonate as well as UV (66, 67). In Xenopus, Chk1 is activated by DNA replication blocks and UV-damaged DNA, but not by double-stranded DNA breaks of the type induced by IR. In this study, we report that human Chk1 is activated by HU, UV, etoposide, cisplatin and ara-C, but poorly by IR. HU blocks DNA replication by inhibiting ribonuceotide reductase, but may subsequently induce DNA damage. ara-C is a nucleoside analog that incorporates into DNA and interferes with DNA polymerases, which in turn interferes with DNA chain elongation during both DNA replication and DNA repair (48, 64). UV and cisplatin perturb the helical structure of the DNA duplex and as such inhibit DNA replication (19). Etoposide is a DNA topoisomerase II inhibitor that causes double-stranded DNA breaks (11). IR also induces double-stranded DNA breaks, but does not signal efficiently to human Chk1. One function of DNA topoisomerase II is to disentangle intertwined daughter chromatids after DNA replication and prior to mitosis. Thus, inhibition of topoisomerase II by etoposide also leads to activation of the chromatid catenation checkpoint (18), and this may explain in part why etoposide but not IR leads to Chk1 activation. Thus, human Chk1 is activated when DNA replication is directly blocked (i.e., by agents such as HU) or disabled (ara-C, UV, cisplatin), but is poorly activated by double-stranded DNA breaks induced by IR. In addition, Chk1 may be activated when cells recognize mismatches that result from attempted replication across modified or damaged bases. An essential role for Chk1 in genome surveillance during DNA replication may explain why disruption of Chk1 results in early embryonic lethality in mice (44, 61).
Data reported in this study and elsewhere indicate that ATR lies directly upstream of Chk1 in pathways that respond to unreplicated DNA and certain forms of DNA damage. There are likely to be several downstream cellular targets of Chk1 in these pathways as well. In humans, fission yeast, and Xenopus, Chk1 has been shown to phosphorylate Cdc25 on a residue or residues that facilitate the binding of 14-3-3 proteins (38, 54, 56, 72, 73). The functional significance of 14-3-3–Cdc25 interactions has been demonstrated in several organisms by expression of mutants of Cdc25 that cannot bind to 14-3-3 proteins. Perturbations in the DNA replication checkpoint and/or the G2-DNA damage checkpoint have been observed in cells expressing the non-14-3-3 binding mutants of Cdc25 (39, 54, 72, 73). Loss of 14-3-3 binding leads to the nuclear accumulation of Cdc25 in fission yeast, Xenopus, and human tissue culture cells (16, 24, 25, 36, 45, 70, 73). Thus, one function of Chk1 may be to keep Cdc25C out of the nucleus as part of the cellular response to checkpoint activation. The Cdc25A protein phosphatase has also been proposed to be a target of human Chk1. A recent study reported that the G1 cell cycle arrest induced by UV-damaged DNA requires ubiquitin-mediated proteolysis of Cdc25A, and this in turn requires Chk1 (46). Chk1 has also been shown to directly phosphorylate Cdc25A in vitro, although the functional significance was not examined (56). Further studies will be required to identify additional substrates of human Chk1.
ACKNOWLEDGMENTS
We are grateful to J. Schwarz and C. Ryan for assistance with production of recombinant adenoviruses and J. Gales for production and purification of antibodies. We thank K. Cimprich and R. Abraham for providing ATR reagents and M. Linder for helpful discussions. We thank J. Hurov, C. Lovly, and M.-S. Chen for comments on the manuscript.
This work was supported by a grant from the National Institutes of Health. H.P.-W. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Al-Khodairy F, Fotou E, Sheldrick K S, Griffiths D J F, Lehmann A R, Carr A M. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 3.Blasina A, Price B D, Turenne G A, McGowan C H. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 1999;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- 4.Boddy M N, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- 5.Brown A, Lee C-H, Schwarz J K, Mitiku N, Griffith D, Piwnica-Worms H, Chung J H. A human Cds1-related kinase that functions downstream of ATM in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown E J, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 7.Busby E C, Leistritz D F, Abraham R T, Karnitz L M, Sarkaria J N. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–2212. [PubMed] [Google Scholar]
- 8.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1689. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi P, Eng W K, Zhu Y, Mattern M R, Mishra R, Hurle M R, Zhang X, Annan R S, Lu Q, Faucette L F, Scott G F, Li X, Carr S A, Johnson R K, Winkler J D, Zhou B-B S. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 10.Chehab N H, Malikzay A, Stavridi E S, Halazonetis T D. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A Y, Liu L F. DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Luo C, Deng Y, Ryan K, Register J, Margosiak S, Tempczyk-Russell A, Nguyen B, Myers P, Lundgren K, Kan C C, O'Connor P M. The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation. Cell. 2000;100:681–692. doi: 10.1016/s0092-8674(00)80704-7. [DOI] [PubMed] [Google Scholar]
- 13.Cimprich K A, Shin T B, Keith C T, Schreiber S L. cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc Natl Acad Sci USA. 1996;93:2850–2855. doi: 10.1073/pnas.93.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cliby W A, Roberts C J, Cimprich K A, Stringer C M, Lamb J R, Schreiber S L, Friend S H. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortez D, Wang Y, Qin J, Elledge S J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 16.Dalal S N, Schweitzer C M, Gan J, DeCaprio J A. Cytoplasmic localization of human cdc25C during interphase requires an intact 14–3-3 binding site. Mol Cell Biol. 1999;19:4465–4479. doi: 10.1128/mcb.19.6.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr A M, Lehmann A R, Hoeijmakers J H. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol. 2000;10:479–482. doi: 10.1016/s0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 18.Downes C S, Clarke D J, Mullinger A M, Gimenez-Abian J F, Creighton A M, Johnson R T. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature. 1994;372:467–470. doi: 10.1038/372467a0. [DOI] [PubMed] [Google Scholar]
- 19.Eastman A. The formation, isolation and characterization of DNA adducts by the anti-cancer platinum complexes. Pharmacol Ther. 1987;34:155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 20.Flaggs G, Plug A W, Dunks K M, Mundt K E, Ford J C, Quiggle M R E, Taylor E M, Westphal C H, Ashley T, Hoekstra M F, Carr A M. ATM-dependent interactions of a mammalian Chk1 homolog with meiotic chromosomes. Curr Biol. 1997;7:977–986. doi: 10.1016/s0960-9822(06)00417-9. [DOI] [PubMed] [Google Scholar]
- 21.Fogarty P, Campbell S D, Abu-Shumays R, Phalle B S, Yu K R, Uy G L, Goldberg M L, Sullivan W. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- 22.Francesconi S, Grenon M, Bouvier D, Baldacci G. p56chk1 protein kinase is required for the DNA replication checkpoint at 37oC in fission yeast. EMBO J. 1997;16:1332–1341. doi: 10.1093/emboj/16.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatei M, Scott S P, Filippovitch I, Soronika N, Lavin M F, Weber B, Khanna K K. Role for ATM in DNA damage-induced phosphorylation of BRCA1. Cancer Res. 2000;60:3299–3304. [PubMed] [Google Scholar]
- 24.Graves P R, Lovly C M, Uy G L, Piwnica-Worms H. Localization of human Cdc25C is regulated both by nuclear export and 14–3-3 binding. Oncogene. 2001;20:1839–1851. doi: 10.1038/sj.onc.1204259. [DOI] [PubMed] [Google Scholar]
- 25.Graves P R, Yu L, Schwarz J K, Gales J, Sausville E A, O'Connor P M, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z, Kumagai A, Wang S X, Dunphy W G. Requirement for atr in phosphorylation of chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14:2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 28.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson J R, Gilmartin A, Imburgia C, Winkler J D, Marshall L A, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- 30.Kaneko Y S, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Terasawa M, Tachibana A, Ikeda K, Nakanishi M, Kaneko Y. Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene. 1999;18:3673–3681. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 31.Kastan M B, Lim D-S. The many substrates and functions of ATM. Nat Rev. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 32.Keegan K S, Holtzman D A, Plug A W, Christenson E R, Brainerd E E, Flaggs G, Bentley N J, Taylor E M, Meyn M S, Moss S B, Carr A M, Ashley T, Hoekstra M F. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- 33.Khanna K K, Keating K E, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller S P. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1999;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 34.Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA. 2000;96:14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S T, Lim D S, Canman C E, Kastan M B. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai A, Dunphy W G. Binding of 14–3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 1999;13:1067–1072. doi: 10.1101/gad.13.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumagai A, Dunphy W G. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 38.Kumagai A, Guo Z, Emami K H, Wang S X, Dunphy W G. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumagai A, Yakowec P S, Dunphy W G. 14–3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakin N D, Hann B C, Jackson S P. The ataxia-telangiectasia related protein ATR mediates DNA-dependent phosphorylation of p53. Oncogene. 1999;18:3989–3995. doi: 10.1038/sj.onc.1202973. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Ting N S, Zheng L, Chen P L, Ziv Y, Shiloh Y, Lee E Y, Lee W H. Functional link of BRCA1 and ataxia telangiectasia gene product in DNA damage response. Nature. 2000;406:210–215. doi: 10.1038/35018134. [DOI] [PubMed] [Google Scholar]
- 42.Lim D S, Kim S T, Xu B, Maser R S, Lin J, Petrini J H, Kastan M B. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay H D, Griffiths D J, Edwards R J, Christensen P U, Murray J M, Osman F, Walworth N, Carr A M. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Guntuku S, Cui X S, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower L A, Elledge S J. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14–3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 46.Mailand N, Falck J, Lukas C, Syljuasen R G, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 47.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge S J. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikita T, Beardsley G P. Functional consequences of the arabinosylcytosine structural lesion in DNA. Biochemistry. 1988;27:4698–4705. doi: 10.1021/bi00413a018. [DOI] [PubMed] [Google Scholar]
- 49.Moser B A, Brondello J-M, Baber-Furnari B, Russell P. Mechanism of caffeine-induced checkpoint override in fission yeast. Mol Cell Biol. 2000;20:4288–4294. doi: 10.1128/mcb.20.12.4288-4294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Dev Biol. 1999;207:432–444. doi: 10.1006/dbio.1998.9178. [DOI] [PubMed] [Google Scholar]
- 51.Oe T, Nakajo N, Katsuragi Y, Okazaki K, Sagata N. Cytoplasmic occurrence of the Chk1/Cdc25 pathway and regulation of Chk1 in Xenopus oocytes. Dev Biol. 2001;229:250–261. doi: 10.1006/dbio.2000.9968. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill T, Dwyer A J, Ziv Y, Chan D W, Lees-Miller S P, Abraham R H, Lai J H, Hill D, Shiloh Y, Cantley L C, Rathbun G A. Utilization of orientated peptide libraries to identify substrate motifs selected by ATM. J Biol Chem. 2000;275:22719–22727. doi: 10.1074/jbc.M001002200. [DOI] [PubMed] [Google Scholar]
- 53.Peng C Y, Graves P R, Ogg S, Thoma R S, Byrnes III M J, Wu Z, Stephenson M T, Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14–3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- 54.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge S J. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 57.Sarkaria J N, Busby E C, Tibbetts R S, Roos P, Taya Y, Karnitz L M, Abraham R T. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 58.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle D A, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali S R, Simmons A, Clines G A, Sartiel A, Gatti R A, Chessa L, Sanal O, Lavin M F, Jaspers N G J, Taylor A M R, Arlett C F, Miki T, Weissman S M, Lovett M, Collins F S, Shiloh Y. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 59.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 60.Sibon O C M, Stevenson V A, Theurkauf W E. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- 61.Takai H, Tominaga K, Motoyama N, Minamishima Y A, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 62.Tibbetts R S, Brumbaugh K M, Williams J M, Sarkaria J N, Cliby W A, Shieh S Y, Taya Y, Prives C, Abraham R T. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tibbetts R S, Cortez D, Brumbaugh K M, Scully R, Livingston D, Elledge S J, Abraham R T. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Townsend A J, Cheng Y-C. Sequence-specific effects of ara-5-aza-CTP and ara-CTP on DNA synthesis by purified human DNA polymerases in vitro: visualization of chain elongation on a defined template. Mol Pharmacol. 1987;32:330–339. [PubMed] [Google Scholar]
- 65.van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by electrophoresis and chromatography on thin-layer cellulose plates. Electrophoresis. 1994;15:544–554. doi: 10.1002/elps.1150150173. [DOI] [PubMed] [Google Scholar]
- 66.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 67.Walworth N C, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 68.Wright J A, Keegan K S, Herendeen D R, Bentley N J, Carr A M, Hoekstra M F, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc Natl Acad Sci USA. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu X, Ranganathan V, Weisman D S, Heine W F, Ciccone D N, O'Neil T B, Crick K E, Pierce K S, Lane W S, Rathbun G, Livingston D M, Weaver D T. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature. 2000;405:477–482. doi: 10.1038/35013089. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Wonkler K, Yoshida M, Kornbluth S. Maintenance of G2 arrest in the Xenopus oocyte: a role for 14–3-3-mediated inhibition of Cdc25 nuclear import. EMBO J. 1999;18:2174–2183. doi: 10.1093/emboj/18.8.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin M B, Guo B, Vanhoefer U, Azrak R G, Minderman H, Frank C, Wrzosek C, Slocum H K, Rustum Y M. Characterization of protein kinase chk1 essential for the cell cycle checkpoint after exposure of human head and neck carcinoma A253 cells to a novel topoisomerase I inhibitor BNP1350. Mol Pharmacol. 2000;57:453–459. doi: 10.1124/mol.57.3.453. [DOI] [PubMed] [Google Scholar]
- 72.Zeng Y, Forbes K C, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- 73.Zeng Y, Piwnica-Worms H. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14–3-3 binding. Mol Cell Biol. 1999;19:7410–7419. doi: 10.1128/mcb.19.11.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao S, Weng Y-C, Yuan S-S F, Lin Y-T, Hsu H C, Lin S-C, Gerbino E, Song M-H, Zdzienicka M Z, Gatti R A, Shay J W, Ziv Y, Shiloh Y, Lee E Y-H P. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature. 2000;405:473–477. doi: 10.1038/35013083. [DOI] [PubMed] [Google Scholar]
- 75.Zhou B B, Chaturvedi P, Spring K, Scott S P, Johanson R A, Mishra R, Mattern M R, Winkler J D, Khanna K K. Caffeine abolishes the mammalian G(2)/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J Biol Chem. 2000;275:10342–10348. doi: 10.1074/jbc.275.14.10342. [DOI] [PubMed] [Google Scholar]
- 76.Zhou B B, Elledge S J. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 77.Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen T J, Tsarfati I, Shiloh Y. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene. 1997;15:159–167. doi: 10.1038/sj.onc.1201319. [DOI] [PubMed] [Google Scholar]