Abstract
Plants from the Ilex genus are known for properties such as antimicrobial and anti-inflammatory activity, can act as antiobesity agents and thus can be helpful in medicine. Some holly species, such as Ilex paraguariensis (widely known in the form of popular beverage: yerba mate), have been investigated, while others have been partially researched or remain unknown. Therefore, we performed qualitative and quantitative phytochemical analyses and screened antimicrobial properties of lesser-studied species (I. aquifolium L., I. aquifolium ‘Argentea Marginata’ and I. × meserveae ‘Blue Angel’). I. paraguariensis was used as a standard species for comparison purposes. Investigations were performed on water extracts due to their expected activity and composition. Antimicrobial research included evaluating minimal inhibitory, bactericidal (Staphylococcus aureus and Escherichia coli) and fungicidal concentration (Candida albicans, Alternaria alternata, Fusarium oxysporum, and Aspergillus niger) of extracts. The influence of the extracts on the production, eradication, and viability of bacterial biofilms was also analysed. It was established that Ilex paraguariensis possesses the richest profile of hydroxycinnamic acids derivatives in terms of component concentration and diversity. Ilex spp., especially I. × meserveae, contain a slightly higher amount of flavonoids and more different flavonoid derivatives than I. paraguariensis. However, the strongest antibacterial activity was shown by I. aquifolium L. and its cultivar ‘Argentea Marginata’ in terms of minimal inhibitory, bactericidal and fungicidal concentration, and biofilm assays. Extracts from both species significantly reduced the biofilm viability of S. aureus as well, which may be of use in the production of multicomponent lavaseptics, antiseptics, diuretics (supporting urinary tract infection therapy) and, due to their action on fungi, additives to growth media for specific fungi. The significant content of saponins enables Ilex extracts to be used as natural emulsifiers, for example, in cosmetics. Moreover, relatively high chlorogenic acid and rutin content may suggest use of Ilex spp. to treat obesity, digestive problems, in chemoprevention, and as preservatives in the food industry.
Keywords: Ilex, antimicrobial activity, biofilm, HPLC, DAD, MS/MS, polyphenols, yerba mate
1. Introduction
Over the past several years, there has been increased interest in searching for a natural approach to food production. This is partly due to the common belief that natural substances are less toxic than artificially synthesised preservatives. Therefore products perceived as natural are bought and consumed more readily, e.g., consuming fruits, herbs and vegetables could improve public health [1].
Yerba mate, an infusion prepared from the leaves of Ilex paraguariensis A. St. Hilaire (Aquifoliaceae), is widely consumed worldwide. In producer countries (e.g., Argentina, Brazil), mate production is an important branch of agriculture and has a special status in the economy. The leaves of I. paraguariensis used for preparing the beverage has become a valuable source of bioactive substances known to have therapeutic efficacy in treating or preventing the development of arthritis, inflammatory diseases, cardiovascular diseases, haemorrhoids, headache, hepatic disorders, and obesity [2,3]. It is known that different yerba mate beverages are used in South American folk medicine, for example, by the Guarani people [4].
Biologically active compounds found in I. paraguariensis may differ depending on extraction methods, genetic and environmental variability as well as harvest time [5,6]. Generally, these extracts contain polyphenols, purines (caffeine, theobromine), vitamins A, B, C, and E, tannins, hydroxycinnamic acids esters of quinic acids (mainly caffeoylquinic acids [5,7,8,9,10]) as well as some triterpene saponins derived from ursolic acid [3,10,11].
Recent inquiries have revealed the antimicrobial potential of some Ilex species extracts, whose spectrum of activity encompasses Gram-positive and Gram-negative bacteria and fungi [12,13,14]. Overall, I. paraguariensis extracts were usually more active against Gram-positive than Gram-negative bacteria species [12,13,14]. It is noteworthy though that activity was retained against Gram-negative pathogens responsible for bacterial diarrhoeas such as Escherichia coli O157:H7 [13], Salmonella enteritidis and Listeria monocytogenes [12]. Therefore, as a popular drink, Yerba mate may be used to treat diarrhoea as a supporting agent with other drugs. It is especially noteworthy that Ilex species extracts were also active against drug-resistant strains [13]. Apart from bacteria, Ilex exhibits antifungal activity. For example, Haraguchi et al. [15] has shown that I. integra is a potent antifungal agent against Candida albicans. Usually, the antimicrobial activity of Ilex species was associated with polyphenols (mainly chlorogenic acids and their derivates [12]) and triterpenoids [15].
Among all of the Ilex genus species, research is generally focused to a large extent on I. paraguariensis due to its importance in the food industry as a source of drinks containing caffeine. In contrast, other Ilex species composition and biological properties usually remain partially investigated or unexplored at all. To our best knowledge, among selected species, only I. paraguariensis has been the subject of intensive phytochemical and pharmacological studies; therefore, we focused on other holly species easily cultivable in European temperate climate: I. aquifolium L., Ilex aquifolium ‘Argentea Marginata’, and Ilex × meserveae ‘Blue Angel’. We decided to use these species and cultivars because of their easy growth in a temperate climate, availability and low environmental requirements. Moreover, these plants have been cultivated in Poland for many years as ornamental plants. Overall, these characteristics may make them promising crop species in the future.
A significant amount of hydroxycinnamic acids esters of quinic acid in these species (previous research [9]), as well as reported antimicrobial activity of I. paraguariensis water extract [14,16] and antibiofilm properties of I. guayusa [17], make holly species candidates for antibacterial research. Moreover, the presence of saponins in the investigated species [18] justifies using water as an extractant. Usually, water is not considered a proper solvent due to the high sugar content in water extracts; sugars can stimulate microbial growth. However, we supposed that at the same time, emulsifying properties of saponins should increase the solubility of expected antimicrobial agents (hydroxycinnamic acids esters of quinic acid) in water. Moreover, this solution may be classified as ‘green chemistry’ and support saving the environment from ‘hard chemicals’.
In summary, our aims were screening research of antimicrobial properties as well as qualitative and quantitative analysis of I. aquifolium L., Ilex aquifolium ‘Argentea Marginata’, and Ilex × meserveae ‘Blue Angel’ leaves water extracts. I. paraguariensis was used as standard species for comparison purposes due to the most exhaustive research of yerba mate. Antimicrobial tests included the evaluation of microorganism growth curves, MIC (minimal inhibitory concentration) and MBC (minimal bactericidal concentration) or MFC (minimal fungicidal concentration). Moreover, antibiofilm properties of extracts against Staphylococcus aureus and Escherichia coli were also investigated. The antibiofilm panel is composed of assays of inhibition of bacterial adhesion, biofilm production, viability and eradication. S. aureus and E. coli were chosen for biofilm tests according to their model status as Gram-positive and Gram-negative bacteria, respectively.
The highlights of our research include:
UHPLC-DAD-MS/MS qualitative and quantitative analysis of I. aquifolium L., I. aquifolium ‘Argentea Marginata’ and I. × meserveae ‘Blue Angel’ water extracts focused on polyphenols components.
Antibacterial and antifungal activity (evaluation of MIC, MBC/MFC and bacteria and fungi growth curves) of Ilex spp water extracts (I. aquifolium L., I. aquifolium ‘Argentea Marginata’ and I. × meserveae ‘Blue Angel’).
Influence of Ilex spp. water extracts (I. aquifolium L., I. aquifolium ‘Argentea Marginata’ and I. × meserveae ‘Blue Angel’) on bacterial adhesion, production, viability and eradication of Staphylococcus aureus and Escherichia coli biofilm.
In the future, purified extracts of investigated Ilex species (or their preparations) may be sources of antibacterial agents, as well part of different multicomponent antimicrobial preparations. However, this requires screening evaluation of basic antimicrobial properties and phytochemical composition.
2. Results
2.1. UHPLC-DAD-MS/MS Phytochemical Profiles and Quantification of Ilex Species Water Extracts
2.1.1. Qualitative Analysis of Ilex Species Water Extracts
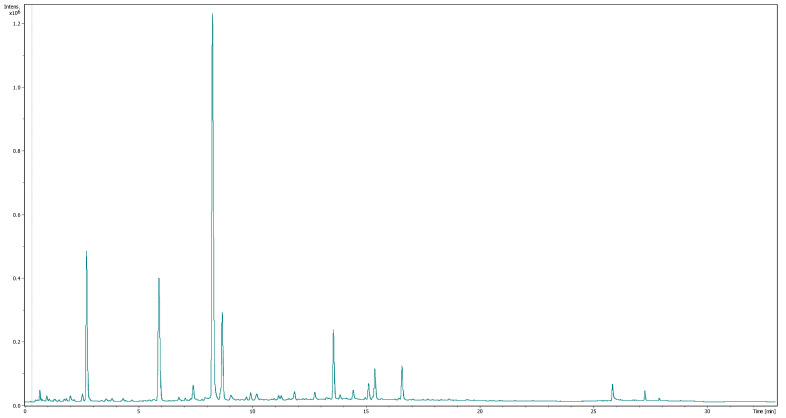
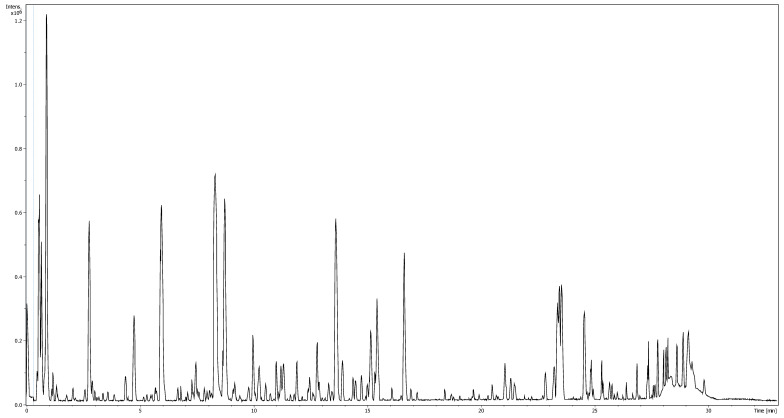
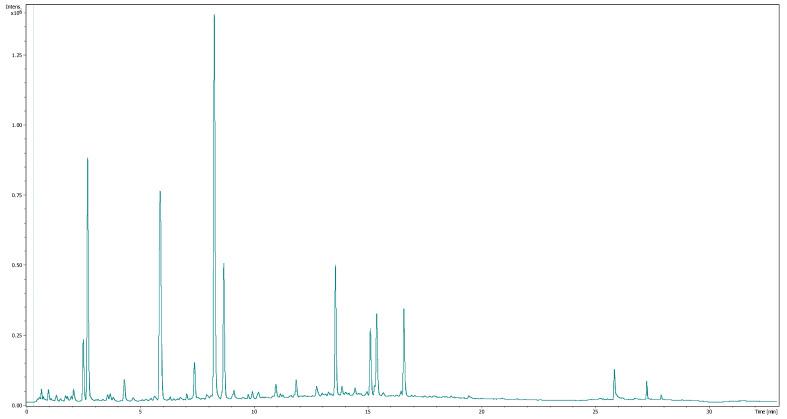
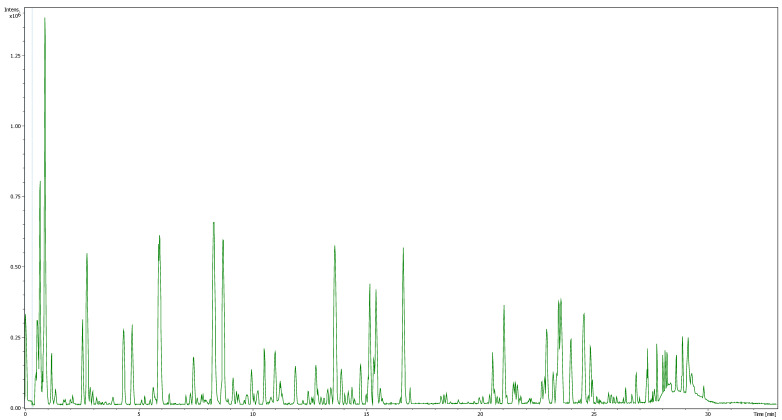
Results are presented in Table 1 (identification of components), Table 2 (presence of components) and Table 3 (quantification of phenolic acid derivates and flavonoids). In our research, MS and MS/MS analyses were supported by an analysis of UV absorption spectra. This approach allowed us to successfully identify most phenolic components by comparing mass and UV spectra with known data on standards and literature. In addition, some isomers were determined according to elution order in analyses performed in similar conditions recorded in the literature. The error between the calculated and experimental m/z for deprotonated ions was lower than 5.0 ppm. Detailed data on UHPLC-DAD-MS/MS qualitative analysis are available in Appendix A (Table A1 and Figure A1, Figure A2, Figure A3, Figure A4, Figure A5, Figure A6, Figure A7 and Figure A8).
Table 1.
UHPLC-DAD-MS/MS identification of components in Ilex leaves water extracts.
| NB | Component | RT | UV λnm Max |
MS 1 m/z |
MS 2 Base Peak m/z | MS2 Secondary Peaks—m/z (Abundance) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Quinic acid a,b,c | 0.92 | 258 | 191.0207 | 111.0087 | - | standard |
| 2 | Isomer of lithospermoside I b,c | 2.58 | 258 | 328.1101 | 130.0336 | 121.034 (20.16), 148.043 (19.10) | [19] |
| 3 | Isomer of lithospermoside II b,c | 2.75 | 259 | 328.1043 | 130.0305 | 121.0295 (40.06), 102.0358 (36.89), 125.0218 (3.17) | [19] |
| 4 | Unidentified | 3.39 | 258 | 371.0984 | 191.0563 | 135.0455 (5.18), 161.0237 (3.29), 173.0456 (2.60) | - |
| 5 | * 3-Hydroxy-4-O-glucosylbenzoic acid b,c | 3.90 | 259 | 315.0720 | 108.0227 | 152.0121 (80.47) | [20,21,22] |
| 6 | Caffeoylglucose I b,c | 4.09 | 326 | 341.0889 | 161.0264 | 135.0467 (22.08), 179.0353 (13.14) | [8,22,23] |
| 7 | * Canthoside B b,c | 4.75 | 257 | 463.1454 | 233.0671 | 293.0870 (58.98), 125.0252 (50.23), 123.0448 (34.74), 149.0461 (32.35), 191.0550 (17.67), 131.0351 (10.15), 105.0348 | [24,25] |
| 8 | Caffeoylglucose II b,c | 4.81 | 327 | 341.0885 | 161.0266 | 135.0475 (55.38), 179.0356 (14.39), 221.051 (7.01) | [8,22,23] |
| 9 | Theobromine a | 5.07 | 273 | - | - | - | standard |
| 10 | Caffeoylglucose III b,c | 5.14 | 327 | 341.0878 | 161.0267 | 135.0473 (49.92), 179.0386 (18.17), 221.051 (3.04) | [8,22,23] |
| 11 | * p-Hydroxybenzoic acid a,b,c | 5.50 | 280 | 137.0246 | 108.0238 | 136.0166 (75.91) | [26] |
| 12 | cis-3-Caffeoylquinic acid b,c | 5.51 | 321 | 353.0886 | 191.0594 | 135.0474 (63.62), 179.0375 (38.53) | [8] |
| 13 | Neochlorogenic acid (5-CQA) b,c | 5.94 | 324 | 353.0888 | 191.0586 | 135.0477 (69.82), 179.0377 (45.58), 161.0266 (5.09) | [8] |
| 14 | Caffeoylglucose IV b,c | 6.47 | 326 | 341.0890 | 161.0266 | 135.0472 (24.92), 179.0381 (17.14), 203.0384 (3.96), 221.0474 (2.19) | [8,22,23] |
| 15 | 6-Caffeoylglucose b,c | 7.21 | 324 | 341.0890 | 161.0263 | 135.0481 (63.45), 179.0377 (63.45), 221.0475 (42.31), 281.0661 (5.44) | [8,22,23] |
| 16 | 3-p-Coumaroylquinic acid b,c | 7.46 | 313 | 337.0938 | 119.0519 | 163.0417 (82.87), 191.0586 (14.73) | [8] |
| 17 | Caffeoylquinic acid b,c | 7.61 | 324 | 353.0882 | 135.0464 | 191.0570 (96.13), 173.0450 (80.28), 179.0354 (67.36), 93.0365 (10.89), 155.0352 (8.34), 111.0467 (8.22), 137.0241 (7.54) | [8] |
| 18 | Caffeoylglucose VI b,c | 8.05 | 324 | 341.0890 | 161.0268 | 135.0475 (80.13), 179.0367 (76.09) 221.0472 (44.08), 281.0662 (8.54), 203.0385 (2.33) | [8,22,23] |
| 19 | Caffeic acid a, b, c | 8.17 | 321 | 179.0371 | 135.0473 | 117.0362 (6.09) | standard |
| 20 | Chlorogenic acid (3-CQA) a,b,c | 8.30 | 324 | 353.0883 | 191.0584 | 161.0265 (2.08) | standard |
| 21 | Caffeine a | 8.52 | 278 | - | - | - | standard |
| 22 | 3-Feruloylquinic acid b,c | 8.63 | 325 | 367.1036 | 134.0399 | 193.0528 (72.78), 149.0625 (10.02), 117.0361 (9.64) | [8] |
| 23 | Cryptochlorogenic acid (4-CQA) b,c | 8.71 | 324 | 353.0892 | 135.0473 | 191.0583 (97.91), 179.0374 (81.84), 161.0269 (8.46), | [8,9] |
| 24 | Quercetin-di-glucoside b,c | 9.62 | 352, 253 | 625.1411 | 625.1416 | 463.0865 (80.18), 301.0368 (15.78), 299.0190 (9.67) | [27,28] |
| 25 | Quercetin-deoxyhexoside-hexoside b,c | 9.81 | 353, 255 | 609.1471 | - | - | [29] |
| 26 | cis-5-Caffeoylquinic acid b,c | 9.97 | 325 | 353.0878 | 191.0585 | - | [8] |
| 27 | 4-p-Coumaroylquinic acid b,c | 10.23 | 312 | 337.0940 | 173.0478 | 191.058 (55.34), 119.0522 (37.71), 163.0418 (34.04), 137.0269 (13.06) | [8] |
| 28 | * Flavonoid-derivate b,c | 10.51 | 353, 255 | 667.1523 | - | - | [29] |
| 29 | Kaempferol-di-hexoside b,c | 10.66 | - | 609.1463 | 447.0917 | 285.0413 (73.38), 609.1441 (41.45), 283.0255 (24.85), 327.0522 (8.38), 489.0952 (4.21) | [28] |
| 30 | Kaempferol-rutinoside-hexoside b,c | 10.86 | 346, 265 | 755.2039 | 593.1502 | 755.2028 (25.24), 447.0918 (4.80), 285.0397 (4.05) | [30,31] |
| 31 | Unidentified | 10.92 | 282 | 537.1980 | 375.1456 | 357.1344 (33.02), 537.1990 (32.01), 489.2040 (25.25), 119.0356 (21.60), 327.1211 (19.19), 321.1131 (17.17), 339.1247 | - |
| 32 | Unidentified | 11.06 | - | 593.1519 | 593.1487 | 473.1092 (30.56), 353.0676 (16.77), 383.0765 (7.87), 503.1181 | - |
| 33 | * Isorhamnetin-3-O-gentiobioside b,c | 11.06 | 345, 254 | 639.1576 | 477.1040 | 315.0504 (61.04), 639.1571 (28.77), 313.0387 (12.37) | [32,33] |
| 34 | 4-Feruloylquinic acid b,c | 11.20 | 324 | 367.1045 | 173.0476 | 134.0394 (41.39), 193.0527 (22.24) | [8,9] |
| 35 | * Isorhamnetin-rutinoside-glucoside b,c | 11.21 | 352, 254 | 785.2152 | 623.1603 | 785.2078 (15.49), 315.0503 (6.79), 477.0992 (6.61) | [32,33] |
| 36 | 5-Feruloylquinic acid b,c | 11.31 | 325 | 367.1046 | 191.0583 | 134.0403 (24.8), 193.0538 (23.18),173.0476 (16.98), 149.0632 (4.52) | [8,9] |
| 37 | Caffeoylquinic lactone b,c | 11.41 | - | 335.0787 | - | - | [8] |
| 38 | Isorhamnetin derivate b,c | 12.00 | 349, 253 | 725.1572 | 519.1137 | 681.1659 (63.20) 477.1007 (27.15) 315.0503 (16.33), 561.1252 (13.86), 357.0587 (5.06) | - |
| 39 | Myricetin-hexoside b,c | 12.38 | 355, 254 | 479.1206 | 317.0683 | 299.0591 (15.88), 479.1203 (13.93), 289.0719 (11.26) 0.001640, 165.0210 | [34] |
| 40 | Quercetin-pentoside-hexoside b,c | 12.84 | 352, 256 | 595.1310 | 300.0282 | 595.1303 (55.26), 179.0017 (2.11) | [35,36] |
| 41 | Quercetin-7-O-rutinoside b,c | 13.34 | 353, 256 | 609.1457 | 609.1456 | 300.0291 (47.5) | [29] |
| 42 | Rutin (Quercetin-3-O-rutinoside) a,b,c | 13.67 | 353, 256 | 609.1445 | 609.1441 | 300.0312 (43.16) | standard |
| 43 | Quercetin-glucoside b,c | 13.95 | 352, 256 | 463.0878 | 300.0301 | - | [9,29] |
| 44 | Unidentified | 14.51 | 392, 263 | 701.1363 | 701.1355 | 335.0193 (67.64), 509.0718 (9.63), 191.0564 (4.69), 669.0322 (2.02) | - |
| 45 | Unidentified | 14.51 | 385, 251 | 493.1338 | 331.0837 | - | - |
| 46 | Flavonoid c | 14.80 | 354, 257 | 547.2386 | 149.0481 | 191.0585 (30.48), 131.0365 (15.69), 101.0264 (11.61) | - |
| 47 | Kaempferol-3-O-rutinoside b,c | 15.05 | 348, 265 | 593.1495 | 285.0415 | - | [8,9,37] |
| 48 | 3,4-Dicaffeoylquinic acid b,c | 15.19 | 325 | 515.1185 | 173.0477 | 179.0372 (99.12), 353.0887 (54.25), 191.0583 (46.01), 161.0262 (19.59), 135.0474 (16.21) | [8,9] |
| 49 | Kaempferol-3-O-glucoside b,c | 15.38 | 345, 254 | 447.0928 | 284.0331 | 447.0938 (14.96), 255.0305 (12.35), 227.0358 (6.66) | [8,38] |
| 50 | Isorhamnetin-3-O-rutinoside b,c | 15.38 | 353 | 623.1621 | 315.0518 | 623.1618 (90.32) | [39] |
| 51 | 3,5-Dicaffeoylquinic acid b,c | 15.46 | 326 | 515.1184 | 191.058 | 179.0371 (66.61), 353.0882 (45.97), 135.0472 (11.41), 161.0263 (4.97) | [8,9] |
| 52 | Isorhamnetin-3-O-glucoside b,c | 15.76 | 353, 255 | 477.1032 | 314.0442 | 477.1005 (36.22), 286.0504 (8.18), 299.0195 (4.73) | [33,40] |
| 53 | Dicaffeoylquinic acid b | 15.76 | 325 | 515.1204 | 191.0566 | 179.0358 (73.65), 353.0878 (30.52), 173.0459 (16.28), 135.0447 (11.64), 161.0257 (11.13), 335.0779 (5.08), 359.0566 (2.56), 155.0362 (2.03) | [8] |
| 54 | Isorhamnetin glucoside b,c | 16.10 | 253 | 477.1051 | 314.0431 | 299.0193 (93.25), 477.1052 (51.68), 271.0265 (10.28) | [40,41] |
| 55 | Quercetin-rutinoside b,c | 16.25 | 284 | 609.1829 | 301.0717 | 609.1807 (5.26) | [29] |
| 56 | Dicaffeoylquinic acid b,c | 16.31 | 325 | 515.1197 | 173.0458 | 179.0358 (84.27), 707.1842 (54.47), 191.0560 (38.01) 161.0247 (13.78), 135.0454 (10.60), 354.0899 (8.68), 155.0340 (4.89), 335.0758 (2.88), 163.0756 (2.37), 243.0645 (2.37) |
[8] |
| 57 | 4,5-dicaffeoylquinic acid b,c | 16.66 | 326 | 515.1188 | 173.0479 | 179.0373 (77.99), 353.0885 (65.03), 191.0586 (30.17), 135.0477 (10.58), 161.0271 (2.79) | [8,9] |
| 58 | * Hexoside derivate c | 16.95 | - | 431.1917 | 207.1381 | 251.1300 (94.96), 113.0225 (16.89), 335.0227 (15.87), 119.0341, 331 (11.20), 99.0457 (10.80), 101.0234 (9.81), 163.1470 (2.64) | - |
| 59 | Isorhamnetin 3-O-acetylglucoside | 17.05 | 351 | 519.1147 | 314.0451 | 299.0217 (15.41), 519.1172 (6.32) | [41,42] |
| 60 | * Hexoside derivate c | 17.13 | 310 | 429.1771 | 205.1236 | 249.1115 (74.12), 161.1358 (44.20), 99.0439 (9.41), 153.0933 (3.39) | - |
| 61 | * Hexoside derivate c | 17.49 | - | 431.1919 | 207.1392 | 251.1292 (81.26), 99.0452 (15.10), 113.0234 (10.61), 189.1290 (9.72), 119.0352 (9.70), 101.0266 | - |
| 62 | Caffeoylferuoyl-quinic acid b | 17.79 | 326 | 529.1342 | 193.0529 | 367.1029 (17.65), 173.0467 (6.66), 134.0391 (3.39), 191.0622 (2.19) | [8] |
| 63 | Caffeoylferuoyl-quinic acid b | 17.98 | 326 | 529.1322 | 191.0583 | 179.0366 (44.92), 353.0898 (14.56), 173.0496 (12.08), 193.0512 (11.45), 135.0465 (10.73), 367.1057 (10.36) 161.0259 (8.01) |
[8] |
| 64 | Caffeoylquinic acid b,c | 18.51 | 326 | 515.1176 | 173.0479 | 179.0374 (90.57), 353.0892 (58.97), 191.0573 (45.39), 135.0479 (13.9), 161.0254 (13.9 5.49) | [8] |
| 65 | Caffeoylferuoyl-quinic acid b | 18.71 | 325 | 529.1338 | 173.0478 | 193.0519 (21.1), 367.1043 (19.77), 137.0254 (2.43) | [8] |
| 66 | Caffeoylferuoyl-quinic acid b | 19.01 | - | 529.1322 | 173.0475 | 179.0368 (79.05), 191.057 (73.88), 353.0882 (52.19), 135.0483 (10.6), 367.103 (9.32), 161.0303 (6.47) 137.0279 (4.76) |
[8] |
Table legend; NB—number; RT—retention time; * tentatively identified component (lack of accurate spectral literature database; suspected isomer of a known component or unclear UV spectrum); a component identified by comparison with a standard; b component identified by comparison of UV and/or MS/MS spectra with literature; c component identified by prediction with MS/MS spectra;—lack of experimental UV spectra/ions or not applicable.
Table 2.
Presence of components in Ilex leaves water extracts.
| Nb | Component | RT | I.AQ | I.’AM’ | I.×M | I.PA |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 0.92 | + | + | + | + |
| 2 | Isomer of lithospermoside I | 2.58 | + | + | tr | - |
| 3 | Isomer of lithospermoside II | 2.75 | + | + | + | - |
| 4 | Unidentified | 3.39 | + | + | + | tr |
| 5 | * 3-Hydroxy-4-O-glucosylbenzoic acid | 3.90 | + | + | + | tr |
| 6 | Caffeoylglucose I | 4.09 | tr | tr | tr | + |
| 7 | * Canthoside B | 4.75 | + | + | + | tr |
| 8 | Caffeoylglucose II | 4.81 | - | - | - | + |
| 9 | Theobromine | 5.07 | - | - | - | + |
| 10 | Caffeoylglucose III | 5.14 | - | - | - | + |
| 11 | p-Hydroxybenzoic acid | 5.50 | + | tr | + | + |
| 12 | cis-3-Caffeoylquinic acid | 5.51 | tr | tr | tr | + |
| 13 | Neochlorogenic acid (5-CQA) | 5.94 | + | + | + | + |
| 14 | Caffeoylglucose IV | 6.47 | tr | - | - | + |
| 15 | 6-Caffeoylglucose | 7.21 | tr | tr | - | + |
| 16 | 3-p-Coumaroylquinic acid | 7.46 | + | + | + | + |
| 17 | Caffeoylquinic acid isomer I | 7.61 | tr | tr | tr | + |
| 18 | Caffeoylglucose VI | 8.05 | tr | tr | tr | + |
| 19 | Caffeic acid | 8.17 | tr | tr | tr | + |
| 20 | Chlorogenic acid (3-CQA) | 8.30 | + | + | + | + |
| 21 | Caffeine | 8.52 | - | - | - | + |
| 22 | 3-Feruloylquinic acid | 8.63 | + | + | tr | + |
| 23 | Cryptochlorogenic acid (4-CQA) | 8.71 | + | + | + | + |
| 24 | Quercetin-di-glucoside | 9.62 | tr | - | + | - |
| 25 | Quercetin-deoxyhexoside-hexoside | 9.81 | tr | tr | + | tr |
| 26 | cis-5-Caffeoylquinic acid | 9.97 | + | + | + | + |
| 27 | 4-p-Coumaroylquinic acid | 10.23 | + | + | + | + |
| 28 | Flavonoid-derivate | 10.51 | - | - | + | - |
| 29 | Kaempferol-di-hexoside | 10.66 | - | - | + | - |
| 30 | Kaempferol-rutinoside-hexoside | 10.86 | - | - | + | tr |
| 31 | Unidentified | 10.92 | + | + | + | - |
| 32 | Unidentified | 11.06 | - | - | + | - |
| 33 | * Isorhamnetin-3-O-gentiobioside | 11.06 | - | - | + | - |
| 34 | 4-Feruloylquinic acid | 11.20 | + | + | tr | + |
| 35 | * Isorhamnetin-rutinoside-glucoside | 11.21 | - | - | + | - |
| 36 | 5-Feruloylquinic acid | 11.31 | + | + | + | + |
| 37 | Caffeoylquinic lactone | 11.41 | - | - | - | tr |
| 38 | Isorhamnetin derivate | 12.00 | - | - | + | - |
| 39 | Myricetin-hexoside | 12.38 | - | - | + | - |
| 40 | Quercetin-pentoside-hexoside | 12.84 | + | + | + | tr |
| 41 | Quercetin-7-O-rutinoside | 13.34 | + | + | + | tr |
| 42 | Rutin (Quercetin-3-O-rutinoside) | 13.67 | + | + | + | + |
| 43 | Quercetin-glucoside | 13.95 | + | + | + | + |
| 44 | Unidentified | 14.51 | + | + | + | - |
| 45 | Unidentified | 14.51 | - | - | + | - |
| 46 | Unidentified | 14.80 | + | + | - | + |
| 47 | Kaempferol-3-O-rutinoside | 15.05 | + | + | + | + |
| 48 | 3,4-Dicaffeoylquinic acid | 15.19 | + | + | tr | + |
| 49 | Kaempferol-3-O-glucoside | 15.38 | tr | - | tr | tr |
| 50 | Isorhamnetin-3-O-rutinoside | 15.38 | + | + | + | tr |
| 51 | 3,5-Dicaffeoylquinic acid | 15.46 | + | + | tr | + |
| 52 | Isorhamnetin-3-O-glucoside | 15.76 | tr | + | + | tr |
| 53 | Dicaffeoylquinic acid | 15.76 | + | + | - | + |
| 54 | Isorhamnetin glucoside | 16.10 | - | - | + | - |
| 55 | Quercetin-rutinoside | 16.25 | - | - | + | - |
| 56 | Dicaffeoylquinic acid | 16.31 | tr | tr | - | + |
| 57 | 4,5-dicaffeoylquinic acid | 16.66 | + | + | + | + |
| 58 | Hexoside derivate | 16.95 | + | + | + | - |
| 59 | Isorhamnetin 3-O-acetylglucoside | 17.05 | - | - | + | - |
| 60 | Hexoside derivate | 17.13 | tr | - | + | - |
| 61 | Hexoside derivate | 17.49 | tr | - | + | - |
| 62 | Caffeoylferuoyl-quinic acid | 17.79 | tr | tr | - | + |
| 63 | Caffeoylferuoyl-quinic acid | 17.98 | - | - | - | + |
| 64 | Caffeoylquinic acid | 18.51 | + | + | - | + |
| 65 | Caffeoylferuoyl-quinic acid | 18.71 | + | + | - | + |
| 66 | Caffeoylferuoyl-quinic acid | 19.01 | tr | - | - | + |
Table legend: + component present;—component absent; * tentatively identyfied component (lack of accurate spectral literature database); tr—trace ions (deprotonated molecules) were observed; I.PA—yerba mate, I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
Table 3.
Quantification of polyphenols in Ilex leaves water extracts (average from three experiments).
| No. | Component | RT | I.AQ | I.’AM’ | I.×M | I.PA |
|---|---|---|---|---|---|---|
| Caffeoylglucoses [mg ChE g−1] * | ||||||
| 6 | Caffeoylglucose I | 4.09 | - | - | - | 0.70 ± 0.00 |
| 14 | Caffeoylglucose IV | 6.47 | - | - | - | 1.15 ± 0.01 |
| 15 | 6-Caffeoylglucose | 7.21 | - | - | - | 1.14 ± 0.01 |
| p-Coumaroylquinic acid derivate [mg pCE g−1] ** | ||||||
| 16 | 3-p-Coumaroylquinic acid | 7.49 | 0.23 ± 0.01 | 0.10 ± 0.00 | - | - |
| 27 | 4-p-Coumaroylquinic acid | 10.23 | 0.12 ± 0.00 | 0.05 ± 0.00 | - | 0.06 ± 0.00 |
| Caffeoylquinic derivates [mg ChE g−1] * | ||||||
| 13 | Neochlorogenic acid (5-CQA) | 5.94 | 5.13 ± 0.03 | 4.53 ± 0.03 | 1.70 ± 0.01 | 19.71 ± 0.02 |
| 20 | Chlorogenic acid (3-CQA) | 8.30 | 23.29 ± 0.17 | 11.64 ± 0.11 | 16.68 ± 0.05 | 17.14 ± 0.06 |
| 23 | Cryptochlorogenic acid (4-CQA) | 8.71 | 5.33 ± 0.01 | 4.29 ± 0.01 | 2.23 ± 0.02 | 11.70 ± 0.57 |
| 48 | 3,4-Dicaffeoylquinic acid | 15.19 | 1.41 ± 0.00 | 2.49 ± 0.04 | - | 4.80 ± 0.01 |
| 51 | 3,5-Dicaffeoylquinic acid | 15.46 | 2.43 ± 0.00 | 3.48 ± 0.01 | - | 13.27 ± 0.07 |
| 57 | 4,5-Dicaffeoylquinic acid | 16.66 | 2.39 ± 0.00 | 3.53 ± 0.05 | 0.90 ± 0.00 | 8.85 ± 0.04 |
| Feruloylquinic derivates [mg ChE g−1] * | ||||||
| 22 | 3-Ferruoylquinic acid | 8.63 | 0.72 ± 0.00 | 0.81 ± 0.00 | - | 0.63 ± 0.00 |
| 34 | 4- Ferruoylquinic acid | 11.2 | 0.67 ± 0.01 | 0.63 ± 0.00 | 0.68 ± 0.01 | 0.80 ± 0.01 |
| 36 | 5- Ferruoylquinic acid | 11.31 | 0.99 ± 0.02 | 0.77 ± 0.01 | 0.77 ± 0.01 | 0.89 ± 0.02 |
| Flavonoids [mg RuE g−1] *** | ||||||
| 24 | Quercetin-di-glucoside | 9.62 | - | - | 0.26 ± 0.01 | - |
| 25 | Quercetin-deoxyhexoside-hexoside | 9.81 | - | - | 1.13 ± 0.01 | - |
| 28 | Flavonoid-derivate | 10.51 | - | - | 0.23 ± 0.00 | - |
| 30 | Kaempferol-rutinoside-hexoside | 10.86 | - | - | 0.27 ± 0.00 | - |
| 33 | * Isorhamnetin-3-O-gentiobioside | 11.06 | - | - | 0.31 ± 0.01 | - |
| 35 | * Isorhamnetin-rutinoside-glucoside | 11.21 | - | - | 0.46 ± 0.01 | - |
| 38 | Isorhamnetin derivate | 12.00 | - | - | 0.25 ± 0.00 | - |
| 39 | Myricetin-hexoside | 12.38 | - | - | 0.18 ± 0.00 | - |
| 40 | Quercetin-pentoside-hexoside | 12.84 | 0.59 ± 0.01 | 0.47 ± 0.00 | 0.47 ± 0.00 | - |
| 41 | Quercetin-7-O-rutinoside | 13.34 | 0.26 ± 0.00 | 0.26 ± 0.00 | 0.20 ± 0.00 | - |
| 42 | Rutin | 13.67 | 4.25 ± 0.02 | 4.37 ± 0.03 | 2.21 ± 0.01 | 2.88 ± 0.02 |
| 43 | Quercetin-glucoside | 13.95 | 0.47 ± 0.00 | 0.50 ± 0.00 | 0.46 ± 0.01 | 0.51 ± 0.02 |
| 47 | Kaempferol-3-O-rutinoside | 15.05 | 0.20 ± 0.00 | - | 0.38 ± 0.01 | 0.23 ± 0.01 |
| 49 | Isorhamnetin-3-O-rutinoside | 15.38 | - | - | 0.87 ± 0.12 | - |
| 52 | Isorhamnetin-3-O-glucoside | 15.76 | - | 0.23 ± 0.00 | 0.30 ± 0.00 | - |
| 59 | Isorhamnetin 3-O-acetylglucoside | 17.05 | - | 0.17 ± 0.00 | 0.28 ± 0.01 | - |
| Sum of hydroxycinnamic acids derivatives **** | 81.31 ± 1.81 | 42.71 ± 0.19 | 32.31 ± 0.05 | 22.96 ± 0.03 | ||
| Sum of flavonoids [mg RuE g−1] *** | 3.91 ± 0.09 | 5.78 ± 0.00 | 6.00 ± 0.02 | 8.26 ± 0.16 | ||
Table legend: I.PA—yerba mate, I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’; * [mg ChE g−1]—concertation as chlorogenic acid equivalent (mg) per g of herb; ** [mg pCE g−1]—concertation as p-coumaric acid equivalent (mg) per g of herb; *** [mg RuE g−1]—concertation in rutin equivalent (mg) per g of herb; - = -components absent or too low concertation to quantification; **** Sum of hydroxycinnamic acids derivatives was calculated as sum of mg [ChE g−1] and mg [pCE g−1].
UHPLC-DAD-MS/MS revealed the presence of 66 non-saponin components. These substances were divided into several groups: hydroxycinnamic acids and their derivates (28 compounds), flavonoid glycosides (24 compounds), methylxanthines (two compounds) and others (19 compounds). Extracts were also rich in saponins, but these were analysed previously and, therefore, not in our focus [18]. Components were detectable in MS negative mode (NEG), except for methylxanthines which were ionised only in the positive mode (POS). Therefore, ESI-NEG was used as a basic ionisation method.
The hydroxycinnamic acids derivatives group were dominated by caffeic acid esters (mainly mono- and dicaffeoyl quinic acids). Among monoesters the main components were chlorogenic (3-caffeoylquinic acid; 3-CQA), neochlorogenic (5-CQA) and cryptochlorogenic acid (4-CQA) while dicaffeoylesters included 3,5-dicaffeoylquinic acid (3,5-CQA), 4,5-dicaffeoylquinic acid (4,5-CQA) and 3,4-dicaffeoylquinic acid (3,4-CQA). Apart from mono and dicaffeoyl esters, samples contained monoferuloyl, mono-p-coumaroyl and caffeoylferuloylquinic acids and some other phenols such as caffeoylglucose. Generally, the greatest variety of phenolic acid derivatives was observed for I. paraguariensis and the poorest for I. × meserveae.
The second considerable group of compounds, flavonoids, were mainly glycosides of quercetin, kaempferol and isorhamnetin. It was found that analysed Ilex taxons possess very similar profiles of flavonoids. In all of the samples, rutin is by far the most abundant of flavonoids. Among all investigated samples, I. × meserveae exhibited the most diverse profile of flavonoids.
The third group, methylxanthines, included caffeine, theobromine and trace of theophylline, present only in I. paraguariensis leaves.
The last group, named ‘other components’, included mainly unidentified substances, phenols and components which were not derivatives of hydroxycinnamic acids (e.g., cyanogenic glucosides). Components in this group were usually tentatively identified due to the lack of accurate UV and (or) MS/MS data. Moreover, in some cases, MS/MS generally agreed with the literature, but UV did not. These components may have similar skeletons to those proposed, but the substituents are probably different.
2.1.2. Quantitative UHPLC-DAD Analysis of Ilex Species Water Extracts
Results of the analysis are presented in Table 3. Phenolic acid derivatives were calculated as mg of chlorogenic or p-coumaric acid equivalents per gram of dry mass (mg ChE g−1 and mg pCE g−1, respectively) and flavonoids as rutin equivalents per gram of dry mass (mg RuE g−1). The calculated standard deviation for each measurement was lower than 5%. The highest concentration of polyphenols was observed for I. paraguariensis, I. aquifolium, I. aquifolium ‘Argentea Marginata’ and I. × meserveae, respectively.
In our work, I. paraguariensis had a large amount of hydroxycinnamic acids derivates (81.31 ± 1.81 mg ChAE g−1). The rest of the investigated Ilex leaves possessed significantly lower phenolic acid derivates per gram (from 42.71 in I. aquifolium to 22.96 mg ChE g−1 in I. × meserveae). Generally, I. paraguariensis contained more hydroxycinnamic derivates, mainly mono and dicaffeoyl quinic acids, than European Ilex species. Moreover, various caffeoylquinic acids dominated in different samples. I. paraguariensis contained a similar amount of neochlorogenic and chlorogenic acids, while the other species contained mostly chlorogenic acid; the concentration of neochlorogenic acid was about four times lower. However, in the case of dicaffeoylquinic acids, 3,5-dicaffeoylquinic acid was the most abundant in this group for all samples.
For flavonoids, I. paraguariensis contained the lowest (3.62 mg RuE g−1) amount and I. × meserveae ‘Blue Angel’ the highest (8.26 mg RuE g−1). The highest amount of single flavonoid was observed for rutin in all samples: from 2.21 in I. × meserveae to 4.37 mg RuE g−1 in I. aquifolium ‘Argentea Marginata’. The remaining flavonoids were found below 1.00 mg RuE g−1 except for quercetin-rutinoside (1.13 mg in I. × meserveae). Generally, differences in amounts of flavonoids between samples were lower than in the case of hydroxycinnamic acids derivatives. Usually, those were caused by the absence of some flavonoid derivatives in most cases.
2.2. Evaluation of the Antimicrobial Activity of Ilex spp. Water Extracts
Tested extracts of Ilex spp. were subjected to multistage microbiological tests to determine their activity against selected strains of bacteria and fungi. Tests were performed to determine their antimicrobial efficacy against both free cells forms and biofilms.
2.2.1. Evaluation of Bacterial and Fungal Growth Curves
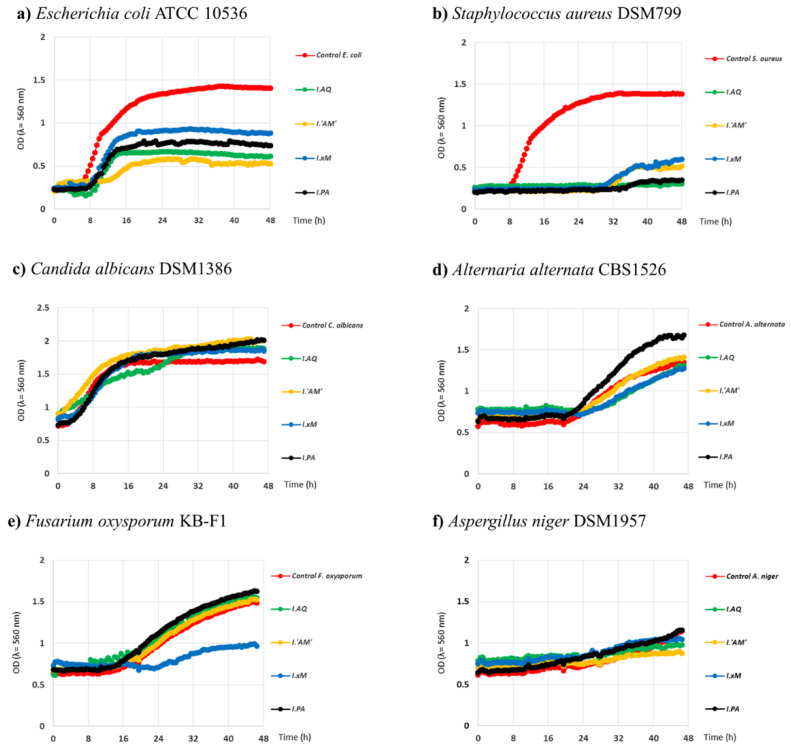
The evaluation results of bacterial and fungal growth curves are presented in Figure 1.
Figure 1.
Growth curves of selected strains of bacteria and fungi under the influence of water extracts from Ilex spp in time 48 h. Legend: Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
As shown in Figure 1, all Ilex extracts decreased the growth rate of S. aureus and E. coli. A more pronounced effect was observed against S. aureus than E. coli. The growth curves for the mentioned strains of bacteria under the influence of the tested water extracts showed a weakened growth expressed as optical density below 1 OD and for S. aureus a growth delay of between 8-16 h (I. aquifolium ‘Argentea Marginata’ and I. paraguariensis) in relation to control. This strongly suggests that the extracts may be bacteriostatic. The growth curves under the influence of Ilex spp. extracts for the fungal strains were very similar to the controls. Only the activity of I. × meserveae ‘Blue Angel’ extract was different, where a strong inhibition of the growth of the F. oxysporum strain was observed. There were differences between the activity of the extracts as well. The most significant effect was observed for I. aquifolium; I. paraguariensis, and I. aquifolium ‘Argentea Marginata’.
In the case of fungi, the results were variable. For most cases, extracts exhibited very low growth stimulation or had no effect. The greatest growth stimulation was observed for I. aquifolium ‘Argentea Marginata’ used against C. albicans, especially at the beginning. After 48h, the remaining extracts showed similar effects. Clear stimulation of growth was also exhibited by I. paraguariensis against A. alternata. Among all tested extracts, clear growth inhibition of fungi was found only for I. × meserveae ‘Blue Angel’ against F. oxysporum. Its growth was inhibited for 24 h and then only slightly increased.
2.2.2. Analysis of Minimum Inhibitory Concentration (MIC) and Fungicidal and Bactericidal Concentrations (MFC/MBC)
Results of the analysis of antimicrobial screening are presented in Table 4. Measurements of MIC and MBC demonstrated that the most potent antibacterial effect was observed against S. aureus and E. coli for I. aquifolium and I. aquifolium ‘Argentea Marginata’ extracts and were in the range of 0.26 mg mL−1–1.02 mg mL−1. Bactericidal concentration was obtained for concentrations ranging from 0.26 mg mL−1 to 2.06 mg mL−1. For selected fungal strains, the strongest activity was observed for C. albicans and A. alternata reaching 1.02 mg mL−1, for the abovementioned extracts. Extracts tested on other fungal strains were less potent, often reaching values above 2.06 mg mL−1.
Table 4.
Minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC *) of Ilex water extracts (average from three experiments).
| Extract | MIC & MBC */MFC * (mg mL−1) | |||||
|---|---|---|---|---|---|---|
|
Escherichia coli ATCC 10536 |
Staphylococcus aureus DSM799 |
Candida albicans DSM1386 |
Alternaria alternata CBS1526 |
Fusariumoxysporum KB-F1 |
Aspergillus niger DSM1957 |
|
| I.AQ | 0.51 0.51 * |
0.26 0.26 * |
1.02 >2.06 * |
1.02 2.06 * |
2.06 >2.06* |
2.06 >2.06 * |
| I.’AM’ | 1.02 2.06 * |
0.51 1.02 * |
2.06 >2.06 * |
1.02 2.06 * |
2.06 >2.06 * |
2.06 >2.06 * |
| I.×M | 1.02 2.06 * |
1.02 2.06 * |
2.06 >2.06 * |
2.06 >2.06 * |
1.02 2.06 * |
2.06 >2.06 * |
| I.PA | 1.02 2.06 * |
1.02 2.06 * |
2.06 >2.06 * |
2.06 2.06 * |
2.06 2.06 * |
2.06 >2.06 * |
Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’; * values of minimal bactericidal (MBC) or fungicidal concentration (MFC).
2.2.3. Activity of Tested Extracts against Biofilm
The next stage of the research was to determine the activity of the tested Ilex spp. extracts against the biofilm of selected bacterial strains. In the first stage of the study, the extracts were most active against E. coli and S. aureus for which bacteriostatic activity was previously relatively low. This part of our study determined both the influence of the extracts on the adhesion of bacteria and the ability to form biofilm under flow conditions with medium on a glass surface and under conditions without medium flow in 96-well titration plates. Due to the presence of chlorogenic acids and their derivatives, as well as saponins, Ilex species are promising candidates for agents used against bacterial biofilm formation.
Influence of Ilex spp. Extracts on Adhesion Process and Biofilm Formation of Bacterial Strains in the Microfluidic Flow System
This study determined the effect of the tested extracts on the adhesion process and the ability to create a biofilm of E. coli and S. aureus in the microfluidic flow system of medium during 17 h long incubation. Therefore, growth curves from the experiment, which established 1/2 MIC values of Ilex spp. were used. The experiment duration was adjusted to the optimal flow conditions in the test channels (the biofilm may clog the channels preventing the flow of medium if left to grow for a sufficiently long time). The results are shown in Figure 2 and Figure 3.
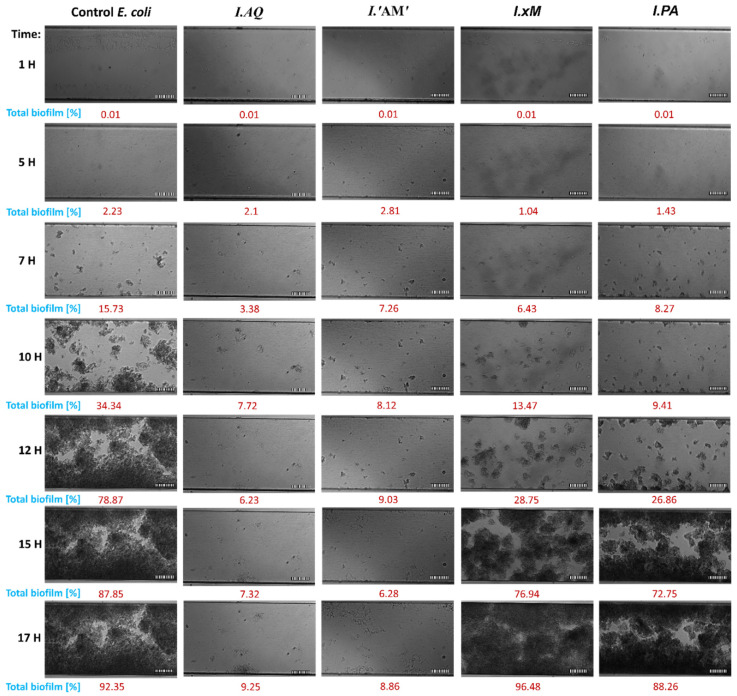
Figure 2.
Imaging of the channel cross section with E. coli biofilms during 17 h of incubation at flow conditions (0.5 dyn cm2). Biofilms were formed by bacteria treated with Ilex spp. water extracts (1/2 MIC). Biofilms were visualised using differential interference contrast (DIC); Control—untreated bacteria; scale bar = 100 µm. Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
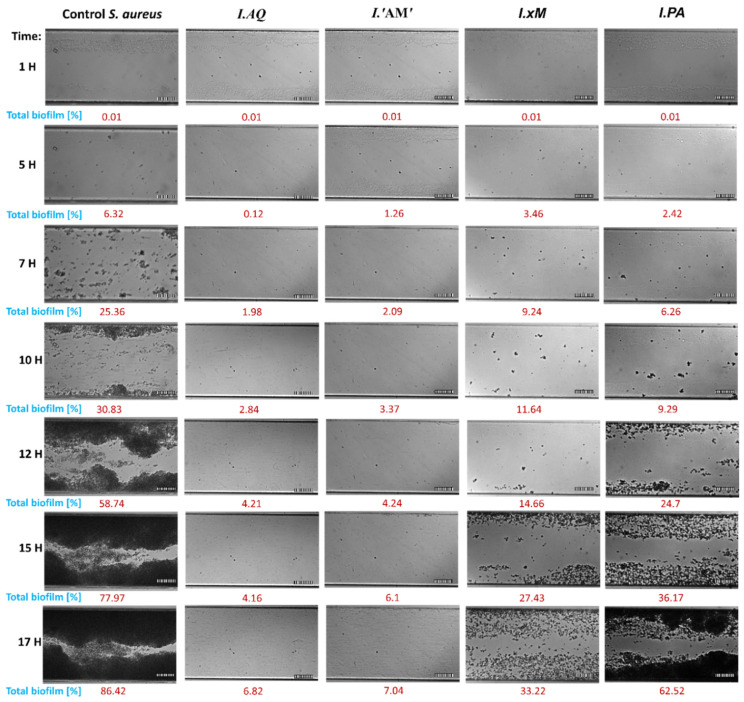
Figure 3.
Imaging of the channel cross section with of S. aureus biofilms after 17 h of incubation at flow conditions (0.5 dyn cm2). Biofilms were formed by bacteria treated with water Ilex spp. extracts (1/2 MIC). Biofilms visualized using differential interference contrast (DIC); Control—untreated bacteria; scale bar = 100 µm. Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
A detailed analysis of the results of the influence of the tested extracts on the adhesion and biofilm formation of E. coli showed a substantial delay in the bacterial adhesion process. Generally, the critical point of the experiments was reached at 12 h when the control exhibited 78.87% of total biofilm formation. At this critical point, I. aquifolium ‘Argentea Marginata’, I. aquifolium, I. paraguariensis and I. × meserveae showed 6.23, 9.03, 26.86 and 28.75% of total biofilm formation, respectively. However, the strongest effect was observed at the 15 h mark of the experiment. At this point, I. aquifolium ‘Argentea Marginata’ exhibited 6.28% and I. aquifolium 7.32% of total biofilm formation in comparison to 87.85% in control and above 72% in the remaining samples. Strong inhibition of biofilm formation caused by I. aquifolium ‘Argentea Marginata’ and I. aquifolium slightly decreased by the end of the experiment, when they exhibited 8.86% and 9.25% of total biofilm formation, respectively. At this time, the remaining samples showed no effect on biofilm formation or, at best, a very weak inhibition.
The results of extract activity against S. aureus were very similar. A strong influence on the inhibition of the adhesion process up to the 12 h mark was observed. The amount of biofilm formed under the influence of the extracts was also significantly reduced compared to the control (86.42%). The strongest activity was, again, demonstrated by I. aquifolium and I. aquifolium ‘Argentea Marginata’ extracts, inhibiting biofilm formation up to 10% during the experiment.
Influence of Extracts on Biofilm Formation
In this experiment, the influence of Ilex spp. on E. coli and S. aureus biofilm production on polystyrene surfaces over a 24 h period was observed. Results are shown in Figure 4.
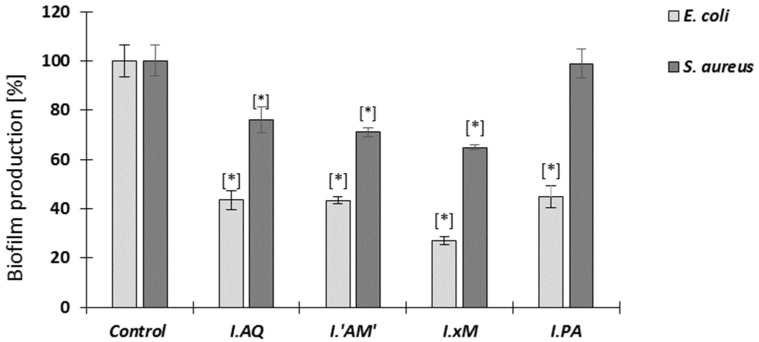
Figure 4.
Influence of Ilex spp. extracts (at 1/2 MIC concentration) on biofilm production E. coli and S. aureus after 24 h. The control is bacteria without extracts; mean ± SD, n = 3; * statistically different from the control p < 0.05. Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
An analysis of the results of the influence on the biofilm production of the tested extracts showed significant inhibition of the tested bacterial strains. It was significantly potent in the case of E. coli, where a steep reduction of the amount of biofilm was noticed (from 55% to less than 40% compared to the control). For S. aureus, the inhibition of biofilm production by most extracts was also noticeable, ranging from 70% to 80%; however, it was not as effective as in the case of E. coli. It is also worth mentioning that the extract of I. paraguariensis showed no effect on the production of S. aureus biofilm.
Influence of Extracts on Biofilm Eradication
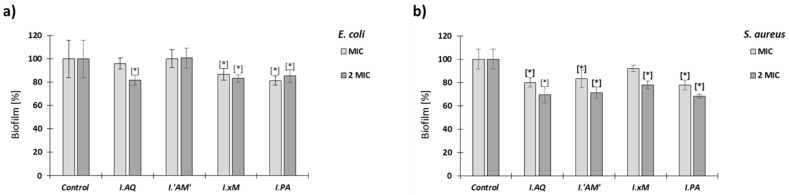
In this experiment, the tested extracts’ ability to eradicate 24 h biofilm of E. coli and S. aureus was determined at two concentrations corresponding to the MIC and 2 MIC. The results are shown in Figure 5.
Figure 5.
Influence of water Ilex extracts (MIC and 2 MIC) on biofilm eradication E. coli (a) and S. aureus (b) after 24 h. The control is biofilm not treated with extracts; mean ± SD, n = 3; * statistically different from the control p < 0.05. Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
Eradication of the tested bacterial biofilms for all tested extracts was quite low and similar to each other both in the concentration of MIC and 2 MIC. The results showed that eradication of S. aureus biofilm by Ilex spp. extracts was stronger than E. coli biofilm (with 70—80% biofilm preservation compared to the control).
Biofilm Viability Measured by Fluorescence Microscopy after the Action of Ilex ssp. Extracts
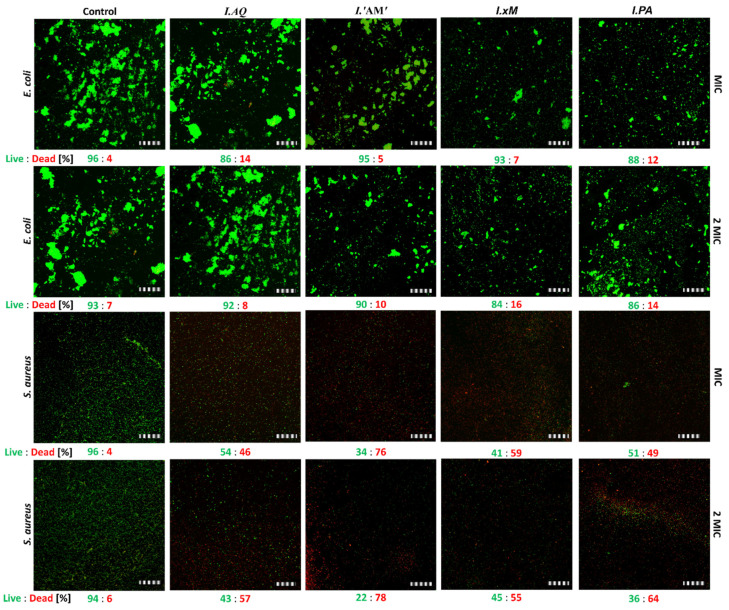
In this experiment, the E. coli and S. aureus biofilms were treated by extracts of Ilex spp. at two concentrations (MIC and 2 MIC). The samples were stained by Film TracerTM LIVE/DEADTM Biofilm Viability Kit. Bacteria killing percentage in biofilm condition was analysed by counting the live/dead bacteria and results are shown in Figure 6.
Figure 6.
E. coli and S. aureus killing percentage in the biofilm condition analysed by counting the live/dead bacteria. Legend: I.PA—I. paraguariensis; I.AQ—I. aquifolium; I.’AM’—I. aquifolium ‘Argentea Marginata’; I.×M—I. × meserveae ‘Blue Angel’.
Comparison of bactericidal effects of Ilex ssp. water extracts (MIC and 2 MIC). Examples of fluorescence images showing the top view of 24 h developed biofilm formed by E. coli and S. aureus. The control is a living biofilm. The samples were stained by a FilmTracerTM LIVE/DEADTM Biofilm Viability Kit. Bacteria killing percentage in biofilm conditions was analysed by counting the live/dead bacteria. Scale bar = 100 µm. Results determining the viability of the biofilm under the influence of the tested extracts showed a strong differentiation between the tested bacterial strains. The viability of the E. coli biofilm was similar (circa 90%) for all extracts compared to the untreated control.
3. Discussion
3.1. Comparison of Phytochemical Composition of Ilex spp.
The presence of hydroxycinnamic acids derivatives, methylxanthines, flavonoids and saponins in the Ilex family is widely known [5,8,17,33]. Saponins were not in the scope of this investigation because they were analysed in previous research [18], as described earlier.
The most considerable group of compounds was hydroxycinnamic acids derivatives in qualitative and quantitative terms. This was composed mainly of monoesters and diesters of hydroxycinnamic acids (usually caffeic acid) and, to a lesser degree, of glucose monohydroxycinnamic acids esters. The tested yerba mate exhibited a similar amount of hydroxycinnamic acids derivatives (81.23 mg g−1) to literature data (from 66 to 97 mg g−1 [5,8]), while European Ilex species contained from about a three to four times lower amount of these components. The profile of these components was also the richest for yerba mate. Among these substances, to our best knowledge, some components such as caffeoylglucoses (components 6, 15 and 18) and p-coumaroylquinic acids (components 16 and 27) were reported the first time for I. aquifolium ‘Argentea Marginata’ and I. × meserveae, as well as feruloylquinic acids (components 22, 34 and 36) for I. × meserveae,. They were previously found in another Ilex species, however [5,8,9,10].
In the case of flavonoids, in all tested samples, lower amounts than those of hydroxycinnamic acid derivatives were found. Most of the identified flavonoids were reported previously for I. paraguariensis [5,8,9]. Among all investigated samples, I. × meserveae exhibited the richest profile of flavonoids. Some derivatives of rhamnetin and isorhamnetin (components 33, 35, 38, 50, 52, 54 and 59), quercetin (components 24, 25, 40 and 55) kaempferol (components 29, 47 and 49) and myricetin (component 39) are reported for the first time in this paper. According to literature data [5,8,9,10,33,43] main flavonoids of Ilex species are glucosides of quercetin, kaempferol and isorhamnetin and, therefore, most new components were predicted to be derivatives of these aglycones.
Apart from polyphenol components, the main difference between yerba mate and other samples was the absence of methylxanthines in European Ilex species. Some older literature [10] described the presence of theobromine in I. aquifolium, but our research did not confirm this observation.
To summarise, European Ilex species contained a mix of hydroxycinnamic acids derivates and some flavonoids, similar to I. paraguariensis. Observed qualitative and quantitative differences were probably caused by differences in metabolism in hot and colder climates. A potentially lower amount of polyphenols and lack of methylxanthine alkaloids in temperate climates may be caused by lower pressure of pests (evolutionary alkaloids work as antipest components) [44].
3.2. Potential Utilisation of Ilex spp. in Obesity, Digestive Problems and Food Industry
According to some papers, yerba mate may be used to reduce body weight due to its high content of caffeoylquinic acids [45,46]. Despite a lower amount of hydroxycinnamic derivates, European species may potentially be used for this purpose due to the relatively high amount of chlorogenic acids [47], absence of methylxanthines and ease of cultivation. The main disadvantage of methylxanthines, especially caffeine, lies in their potential hypertension inducing effect, which is undesirable when reducing body mass. Moreover, a relatively high content of rutin may also be helpful in the reduction of body mass due to the potential normalisation of lipid metabolism. Rutin is known to lower liver weight and decrease enzymes activity, as well as total plasma cholesterol and LDL [48]. Chlorogenic acid is also an insulin sensitiser [46], which may also help normalise metabolism. It is also worth adding that chlorogenic acid and similar components increase the secretion and production of bile [49] and exhibit an antiulcer effect [50]. Therefore, Ilex spp. are potential candidates for different formulations to treat digestive system conditions (herbs or extracts mix).
The last potential application is associated with the strong antioxidant properties of mono and dicaffeoylesters of quinic acid. Due to the relatively high amount of these components in Ilex water extracts, they may be used in chemoprevention or as food preservatives. For example, our previous research demonstrated the possibility of using I. × meserveae as a marinade component to prevent the peroxidation of meat [51].
However, the possibility of internal use of European Ilex species requires considerable further and more profound research, the most critical factor being their unclear toxicological status.
3.3. Toxicological Status of Ilex spp.
It was reported that the main toxins of Ilex species are saponins [52,53] and cyanogenic glycosides [54]. Saponins from I. opaca are said to be hemolytic [53], although their bioavailability is very low. This is fortunate, as it substantially decreases their risk of becoming systemic toxins. In the case of I. aquifolium, some authors claimed that only berries are toxic due to the presence of high quantities of saponins [55] and cyanogenic glucosides [54], while the leaves are nontoxic [55]. However, other authors claimed that leaves are also toxic [52]. It is known that I. aquifolium leaves upset the gastrointestinal system (vomiting, diarrhoea, depression, and anorexia) in animals after treatment [52]. Authors connected this effect with irritation of the gastrointestinal system by the rigid texture of leaves and the presence of saponins [52]. However, the potentially toxic effects of I. aquifolium leaves extracts remain unknown. In our opinion, it is possible that the harmful effect may be exhibited only by some chemotypes of I. aquifolium, which contain a significant amount of saponins and/or cyanogenic glycosides, or specific profile of these components. It is widely known that holly leaves were successfully used as fodder in some parts of the UK, which supports our point [56]. Moreover, saponins are also present in yerba mate, which is not known for toxic effects even in the case of prolonged periods of administration [3,57]. In traditional Chinese medicine, applications of Ilex species include gastrointestinal disorders [57]. The toxicological status of I. × meserveae and I. paraguariensis is clearer than that of I. aquifolium. It is known that saponins present in the hydroalcoholic dry extract as well as in water infusions from leaves of these species do not demonstrate nephrotoxicity but, conversely, have a protective role on kidney status in animals fed a regular diet and in a high-cholesterol diet [18].
In summary, I. × meserveae and I. paraguariensis are promising candidates for internal application, while I. aquifolium and I. aquifolium ‘Argentea Marginata’ additional tests are required.
3.4. Antimicrobial Potential of Ilex spp.
It is quite significant that the strongest antibacterial agents (in terms of MIC and biofilm tests) are usually I. aquifolium and I. aquifolium ‘Argentea Marginata’. Comparison with the literature has shown that our water extracts of yerba mate had a higher MIC value against S. aureus than water extracts investigated by others [13,14]. In the case of E. coli, the results were inconclusive; MIC was higher [13] or lower [14]. Different extraction techniques probably caused the observed effect. Moreover, all extracts exhibited lower activity against E. coli and fungi (in most cases) than S. aureus, as well as a shorter (8–16 h) inhibited growth period of E. coli and fungi. This proved the higher resistance of E. coli and fungi, which may be caused by a non-specific efflux pump in E. coli and differences in the structure of cell barriers between Gram-positive and Gram-negative bacteria and fungi.
Correlation between the composition of Ilex spp. water extracts and antimicrobial activity was ambiguous. It is known that hydroxycinnamic acid derivatives such as chlorogenic acid possess antibacterial and antibiofilm activity [58,59]; the antimicrobial potential of flavonoids is also well documented [60]. However, in our tests, I. paraguariensis did not show the highest activity in MIC and biofilm assays despite the fact it contained the greatest quantity of polyphenols. This seems to indicate that antimicrobial activity is probably correlated with a different group of components. Apart from polyphenols, all tested Ilex species contain microbiologically active saponins as well, which may be responsible for their properties. Saponins possess a documented ability to decrease water surface tension and disrupt the biological functions of cell membranes. Therefore, it is expected that saponins should extirpate bacterial biofilms, which was proven for different saponins such as those from Quillaja species [61]. Moreover, the antibiofilm effect is also known for extracts from I. guayusa with saponins suspected to be active components [17] as well as antifungal effects of saponins aglycones from I. integra [15]. Based on the fact that I. aquifolium and I. aquifolium ‘Argentea Marginata’ contained the greatest quantities of saponins among the investigated subspecies as published prior to this work [18], we suspect that these components may be responsible for the observed strong antibacterial properties of both species. It may also be the case that hydroxycinnamic acid derivatives and flavonoids may work as auxiliary antibacterial agents. The literature [62] discusses synergistic antimicrobial effects of saponins and polyphenols, and the conclusions support our reasoning. The mechanism of this synergistic effect is not fully known, but we suspect it has a multifactorial nature. From one point of view, saponins may increase the concentration of hydroxycinnamic acid derivatives and flavonoids in bacterial cells by disrupting cell membranes and biofilms. On the other hand, saponins may also increase polyphenol activity against cell barriers via an unknown mechanism or mechanisms.
The ability of biofilm formation is one of the most important virulence factors of human pathogenic bacteria. Moreover, it is also responsible for the easy spread of bacteria across hospital and non-hospital environments. For these reasons, antibiofilm tests should be separately discussed.
Investigation of the effect of extracts on adhesion and biofilm formation under flow conditions showed significant inhibition of the adhesion process caused by I. aquifolium and I. aquifolium ‘Argentea Marginata’ extracts for both strains. Reduction of bacterial surface adhesion caused by Ilex spp. extracts may be a result of different factors. In the beginning, polyphenols and saponins may directly inactivate adhesins or inhibit their production in the case of S. aureus, as well as disrupt the function of fimbriae of E. coli [63]. Additionally, they may also disrupt the quorum quenching mechanism [64].
Biofilm formation assays under flow conditions did not allow us to investigate all aspects of biofilm formation, and therefore we decided to use additional tests. We chose to use polystyrene surface tests to evaluate the production of biofilms in static conditions during 24h.
Polystyrene tests are a standard in E. coli biofilm research, and this material is widely used in the medicinal industry for the production of covers, diagnostic instruments, and disposable tableware, among others. This aspect is also important for S. aureus, which is known for the ease of spreading in hospital environments and often cause serious infections of wounds. Assays performed on polystyrene surfaces showed that all extracts except that from I. paraguariensis caused a decline in biofilm production. It is also worth noting that, in all cases, the effect was stronger against E. coli than S. aureus.
Eradication and viability were the last investigated aspects of antibiofilm assays. It is known that, usually, eradication of a biofilm is more difficult than prevention of its formation due to the protection of bacterial cells by extracellular polymeric substances (EPS) and other rather complex phenomena [65]. All the extracts that we researched eradicated the biofilm of E. coli to a lesser degree than that of S. aureus. Moreover, E. coli biofilms were found to exhibit lower viability on Ilex extracts than those of S. aureus. This proves that mature biofilms of S. aureus are more sensitive to Ilex extracts than those of E. coli. This is probably caused by compounds from the extracts such as saponins and polyphenols penetrating through water channels inside the biofilm structure, reducing its viability [64]. Emulsifying properties of saponins may also facilitate this process. Higher resistance of E. coli biofilms is probably a result of different compositions of the biofilm matrix.
In summary, due to the unclear toxicity status of I. aquifolium and I. aquifolium ‘Argentea Marginata’, their antimicrobial potential should be used for purposes of external application only, at least until there are enough data to assure safety of the more systemic application. One f such function may be the production of multicomponent lavaseptics or surface antiseptics due to the high ability of the extracts of reducing S. aureus biofilm viability. Another external application safe for humans is using the Ilex species water extracts in antiseptics and skin cosmetics. Only when the toxicological status of I. aquifolium and I. aquifolium ‘Argentea Marginata’ water extracts is fully explained and classified as safe for humans should internal application be considered. Due to activity against E. coli and diuretic effects [11,66], European Ilex species may be a candidate for medicines in diarrhoea and/or urinary tract infections. In Chinese medicine, some Ilex species (I. latifolia and I. kudingcha) are traditionally used in diarrhoea [57] and yerba mate beverages are used in urinary infections [4] and exhibit a diuretic effect [11]. However, European Ilex species possess a distinct advantage, as their lack of caffeine prevents them from increasing symptoms of diarrhoea, which caffeine is known to cause [67]. I. × meserveae due to relatively high amount of chlorogenic acid and rutin, as well as more clear toxicological status internal application, especially in the reduction of body mass and as an appetiser, should be considered.
4. Materials and Methods
4.1. Plant Material and Reagents
Leaves of Ilex aquifolium L., I. aquifolium ‘Argentea Marginata’ and I. × meserveae ‘Blue Angel’ (I. aquifolium × I. rugosa) were obtained from the plant collection of the Department of Horticulture, Wrocław University of Environmental and Life Sciences. Species and cultivar identification was performed by prof. Przemysław Bąbelewski from the Department of Horticulture, Wrocław University of Environmental and Life Sciences. Plants in the Ilex collection were marked according to the latest tree and shrub catalogues compiled by Polish scientists working for the Polish Nurserymen’s Association. Fresh plant material was air-dried at 35–40 °C for 24 h. I. paraguariensis ‘la oracion’ (dry plant material) was purchased from Carbales Company (Buenos Aires, Argentina).
Acetonitrile HPLC grade was obtained from Sigma-Aldrich (Saint Louis, MO, USA) and MS grade chemicals (Acetonitrile, 99% formic acid, methanol, water) were purchased from Merck (Darmstadt, Germany). UHPLC deionised water was prepared on-site.
Analytical standards included caffeine and theobromine (Sigma-Aldrich, St. Louis, MO, USA), caffeic acid (Fluka, St. Gallen, Switzerland), chlorogenic acid (Roth, Karlsruhe, Germany), rutin (Merck, Darmstadt, Germany), p-coumaric acid (Extrasynthese, Genay, France).
Mueller-Hinton agar and Sabouraud agar were obtained from Oxoid (Hampshire, UK).
4.2. Preparation of Water Extracts
Boiling water was poured onto the plant material (20 mL per 1 g), then kept at 80 °C for 3 h with mixing. After this time, extracts were stored at room temperature for 21 h and then filtered by Whatmann filtrate paper. One mL was used for UHPLC-DAD-MS/MS analysis; this was frozen at −20 °C and lyophilised for 24 h. After lyophilisation, extracts were mechanically standardised by grinding in a mortar. Lyophilised plant extracts were then used for antimicrobial assays.
4.3. Qualitative UHPLC-DAD-MS/MS Analysis of Water Extracts
Analysis was performed on Thermo Scientific™ UltiMate™ 3000 system (Thermo Fischer Scientific™ Dionex™, Waltham, MA, USA), equipped with an autosampler and DAD detector set at 280, 320 and 360 nm. Spectral data were recorded in the 200–600 nm range. Chromatographic separation was performed on Accucore RP-MS 2.6 μm, 100 × 2.1 mm, column (Thermo Fisher Scientific, Waltham, MA, USA) thermostated at 35 ± 2 °C. Injection volume was set to 1 μL. The mobile phase consisted of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B). The flow rate was set at 0.4 mL.min−1 and the separation was obtained using gradient: 100% of solvent A, decreasing to 70% within 22 min, decreasing to 0% at minute 25.0, isocratic for 2 min and increasing to 100% at minute 33.0.
UHPLC-DAD-MS/MS was performed at a similar setting and chromatographic conditions, additionally using a Compact QqTOF MS/MS detector (Bruker, Darmstadt, Germany). The MS detector was used in electrospray negative mode. Parameters of analysis for both modes were the same: the ion source temperature was set at 210 °C, nebuliser gas pressure was set at 2.0 bar, dry gas (nitrogen) flow 8.0 L/min and temperature at 210 °C. The capillary voltage was set at 4.5 kV. The collision energy was set at 8.0 eV. Internal calibration was obtained using 10 mM solution of sodium formate. For ESI-MS/MS experiments, collision energy was set at 35.0 eV and nitrogen was used as the collision gas. The scan range was set from 30 to 1300 m/z. Before the analysis, all the extracts were dissolved in ethanol and filtered through CHROMAFIL® 0.22 µm, Ø13 mm, H-PTFE membrane syringe filter (Macherey-Nagel, Düren, Germany).
4.4. Quantification of Polyphenols in Ilex Species
Quantification was performed according to the methods described in Section 4.3. Standard compounds were dissolved in pure methanol and subsequently diluted to obtain calibration curves in the range of concentrations from 1.000 to 0.016 µg mL−1. Amounts of different compounds in the samples were calculated based on the calibration curve of the appropriate standard or corresponding parent compound. Caffeoyl and feruloyl quinic acids and caffeoylglucoses were expressed as chlorogenic acid equivalents, monomers of p-coumaroyl quinic acid as p-coumaric acid equivalent and flavonoids as rutin equivalents.
4.5. Strains and Growth Conditions
In this study, we used the following bacterial (Escherichia coli ATCC 10536, Staphylococcus aureus DSM799) and fungal strains (Candida albicans DSM1386, Alternaria alternata CBS1526, Fusarium oxysporum KB-F1, Aspergillus niger DSM1957). Bacterial strains were cultured in Mueller Hinton II Broth BD (MHB) and fungal strains in MHB enriched with 2% glucose. The strains were incubated aerobically for 24 h at 37 °C (E. coli, S. aureus and C. albicans) or for 48 h at 28 °C for the remaining ones. Overnight, microorganism cultures were centrifuged, washed with PBS (pH 7.4) and suspended in fresh MHB to obtain suitable optical density.
4.6. Minimal Inhibitory and Fungicidal Concentrations
Values of the minimal inhibitory concentrations (MIC) were determined according to the modified protocol described previously [68,69].
Overnight, bacterial strains cultures were suspended in PBS (pH 7.4) (0.5 McF) and for fungal turbidity adjusted to an OD (Optical Density) of 0.8, all finally diluted to obtain 105 CFU mL−1. Microorganism suspensions (10 µL) were added into the wells of the microplate containing serial dilutions (2556–2564 µg mL −1) of the tested water extracts and filled with 100 µL of MHB medium. Microplates were incubated aerobically for 24 h at 37 °C (E. coli, S. aureus, and C. albicans) or, for other strains, 48 h at 28 °C. As a growth control, a fungal suspension in MHB without extracts was used; for the sterile control, MHB without microorganisms, and for the blank control, a fungal suspension in PBS. Each control and extract dilution were performed in duplicate and the experiment was repeated three times. The MIC values were determined spectrophotometrically (590 nm) using Asys Hitachi 340, Driver version: 4.02 (Biogenet, Poland). To determine the minimal bactericidal and fungicidal concentration (MBC and MFC), 100 µL of fungal suspension from each dilution of the tested compound was transferred to a Mueller Hinton II Agar BD (MHA) plate. MBC and MFC were expressed as the concentration of the Ilex spp. extracts that reduced the number of colony-forming units by 99.9% after 48 h of incubation at 37 °C or at 28 °C.
4.7. Evaluation of Bacterial and Fungal Growth Curves
The biological activity of each tested extract of Ilex sp. was assessed on selected bacterial and fungal strains. The cultures were grown in an MHB medium. The tests were performed in an automated Bioscreen C system (Automated Growth Curve Analysis System, Lab systems, Finland). The working volume in the wells of the Bioscreen plate was 300 µL, comprised of 280 µL of culture medium and 10 µL of cell solution (to obtain final concentration 1 × 105 CFU mL−1). Extracts were dissolved in water and used at a final concentration of 0.1% (w/v). The temperature was controlled at 37 °C for bacteria and 28 °C for fungi. The optical density of the cell suspensions was measured automatically at λ = 560 nm in regular intervals of 30 min for the dependence on optical density of cultivation time. The cell cultures were continuously shaken. Each culture was grown in five replications. The data were analysed using spreadsheet software (Excel 2013 MS Office) and the averages of the triplicates for each type of culture medium were calculated. The averages were used to generate the growth curves for each strain studied, constructed as a function of the incubation time and the absorbance of the culture medium. The resulting microbial growth curves were compared to control cultures in a medium supplemented with water (without extract) [70].
4.8. Influence of Extracts on Adhesion Process and Biofilm Formation of Bacterial Strains in the Microfluidic Flow System
A microfluidic flow system BioFlux 1000, with 48-well plates (Fluxion Biosciences, Alamenda, AZ, USA), was used to study adhesion and biofilm formation in continuous flow. The bacterial strains (E. coli and S. aureus) were diluted to obtain 105 CFU mL −1 in 1 mL of Tryptic Soy Broth BD™ medium (TSB) and flushed into the channels. The extracts were applied at half of their MICs to avoid growth inhibition. Initially, the inoculum (900 µL) was incubated without flow for adhesion of bacteria, the wells were filled, and flow initiated at 0.5 dyn per 1 cm2. The plate was incubated at 37 °C for 17 h period in the incubator chamber (Carl Zeiss Pecon Incubator XL S1, Erbach, Germany). Parameters such as flow shear, adhesion time, and inoculum concentration were tested for biofilm production in the microfluidic system. Imaging was performed at 1 h intervals on an Axio Inverted Observer fluorescence microscope 7 (Carl Zeiss, Erbach, Germany) equipped with an Orca Flash 40 camera (Hamamatsu, Hamamatsu-city, Japan) and 20× objective (Differential Interference Contrast (DIC) mode). Scale bar = 100 µm. Three positions per channel were imaged per extract. Quantification of total biofilm was performed with the BioFlux software (Fluxion, Alameda, AZ USA) [71].
4.9. Influence of Extracts on Biofilm Formation on Polystyrene
To evaluate the influence of Ilex spp. water extracts on E. coli and S. aureus ability to produce biofilm, the strains (105 CFU mL−1) were incubated for 24 h in the presence of extracts in the TSB medium at 37 °C. The extracts were applied at half of their MICs to avoid growth inhibition. Samples (200 uL) were transferred to a 96-well microplate and incubated with shaking (400 RPM) for 24 h at 37 °C. Microplates were washed three times with distilled water and each well was stained with crystal violet for 10 min. Wells were washed three times and the bound crystal violet was dissolved with a 100% 2-propanol, 1% SDS, 50 mM HCl mixture. Absorbance was measured at λ = 590 nm as described by Piecuch et al. Untreated bacterial cells were used as the biofilm growth control. The experiment was conducted with three independent repetitions [72].
4.10. Influence of Extracts on Biofilm Eradication
Bacterial biofilm eradication on polystyrene surfaces (96-hole microplate) was assessed with 200 µL of E. coli and S. aureus cultures, diluted to obtain 105 CFU mL −1 in TSB medium, and pipetted into wells of microplates. Cultures were incubated at 37 °C for 24 h with shaking (400 rpm). After this time, microplates were washed three times with sterile physiological salt solution, then the Ilex spp. extracts were added to final concentrations MIC and 2 MIC (respectively for strains) and incubated for 2h at 37 °C with shaking (400 rpm). The microplates’ holes were washed three times with sterile physiologic salt solution and then the biofilm was stained with a 10 min. incubation with 100 μL 2.45 μM of crystal violet. The microplates were then gently washed three times with sterile physiological salt solution. Crystal violet bound with biofilm on the surface of the plate was dissolved by washing with 200 µL of washing solution (isopropanol, HCl 50 mM, SDS 1%). Absorbance was then read at λ = 590 nm (ASYS UVM 340 Biogenet). Untreated cells were used as control. The experiment was carried out in triplicate [73].
4.11. Biofilm Viability on Fluorescence Microscopy after the Action of Ilex Extracts
Aliquots of 3 mL of each cultured E. coli and S. aureus in TSB medium 105 CFU/mL were added to the wells of sterile six-well plates. Sterile microscopic slides (Ø 15 mm) were put in the wells and the resultant cultures were incubated at 37 °C for 24 h with shaking (400 RPM). The slides were washed twice with sterile physiological salt solution and transferred to fresh six-well plates. The biofilm on glass for each culture was treated with Ilex spp. extracts in two concentrations (MIC and 2 MIC), then were stained with 3 μL of propidium iodide (Ex λ = 543 nm) and 3 μL SYTO 9 (Ex λ = 488) for 3 min using LIVE/DEAD BacLight Bacterial Viability Kit (Thermo Fisher Scientific). Imaging was performed on an Axio Inverted Observer fluorescence microscope 7 (Carl Zeiss, Erbach, Germany) equipped with an Orca Flash 40 camera (Hamamatsu, Hamamatsu-city, Japan) and 10× objective Scale bar = 100 µm. Acquired images were processed and analysed using Fiji/ImageJ software ver. 1.53c (NIH). First, maximum intensity projections (MIP) were obtained from stacks of images. The areas from binarised images were then transferred onto original live and dead channel MIP images and mean fluorescence intensities of all detected objects per field of view were calculated using the ImageJ’s Analyze Particles function [73].
5. Statistical Analysis
Variance analysis was performed using software Statistica 13 ver. 13.3.721.0 (ANOVA one-way analysis). A probability value of p < 0.05 was considered significant. One-way ANOVA analysis was performed for influence of extracts on biofilm formation and the influence of extracts on biofilm eradication. Standard deviations were calculated for qualitative and quantitative UHPLC-DAD-MS/MS analyses.
6. Conclusions
Our research established that water extracts obtained from leaves of Ilex aquifolium L., I. aquifolium ‘Argentea Marginata’, I. × meserveae ‘Blue Angel’ are a reliably rich source of polyphenols, especially chlorogenic acids and their derivates. Their potential applications should, therefore, be associated with known activities of these groups of components, including externally and internally applied preparations. Due to the presence of saponins, external applications at this moment are to be considered first, for those are safer. Taking obtained data under consideration, we can assume that extracts from Ilex genus can play an important role as a potential source of antimicrobial agents. Potentially, a purified fraction of Ilex may be used in multicomponent lavaseptics, antiseptics and cosmetics. Internal application of Ilex aquifolium L., I. aquifolium ‘Argentea Marginata’, I. × meserveae ‘Blue Angel’ requires further research due to their unclear toxicological status for humans. Potentially, those could still be used as components of digestive composition (both herbs and extracts) as well as for auxiliary treatments in urinary tract infections caused by E. coli.
In the future, we wish to develop studies of the antimicrobial effects of Ilex spp. The planned research includes multiple approaches. The basic path is the evaluation of antimicrobial effects against other microorganisms, as well as comparison of different types of extracts and screening of other Ilex species, their cultivars and components such as fruits. Another approach is the purification of water extracts from ballast material such as sugars and potential division into fractions and subfractions. Next, the obtained fractions and subtractions may be used to research interactions between saponins and polyphenols in their antimicrobial activity mechanism of action. Moreover, research of potential synergism between Ilex extracts and other antimicrobial agents (such as antibiotics) should also be performed. The rising resistance of microorganisms to antibiotics has required searching for new therapies and new antibacterial agents. Multicomponent mixtures of antibacterial agents may comprise these therapies due to the expected weaker production of multi-drug resistant strains and potential lower doses of single antibacterial agents.
Acknowledgments
We thank Marta Piksa for helping to run the experiment.
Appendix A
Appendix A contains additional table and figures about UHPLC-DAD-MS/MS analyses of Ilex samples. They are listed below:
Table A1.
Expanded results of UHPLC-DAD-MS/MS analysis of analysed Ilex leaves extracts.
| NB | Component | RT | UV λnm Max |
MS 1 m/z |
MS 2 Base Peak m/z | MS2 Secondary Peaks—m/z (Abundance) | [M−H]− Formula | Error [Da] | Error [ppm] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid a,b,c | 0.92 | 258 | 191.0207 | 111.0087 | - | C6H7O7 | −0.9 | −4.8 | standard |
| 2 | Isomer of lithospermoside I b,c | 2.58 | 258 | 328.1101 | 130.0336 | 121.034 (20.16), 148.043 (19.10) | C14H18NO8 | −0.6 | −1.8 | [19] |
| 3 | Isomer of lithospermoside II b,c | 2.75 | 259 | 328.1043 | 130.0305 | 121.0295 (40.06), 102.0358 (36.89), 125.0218 (3.17) | C14H18NO8 | −0.6 | −1.8 | [19] |
| 4 | Unidentified | 3.39 | 258 | 371.0984 | 191.0563 | 135.0455 (5.18), 161.0237 (3.29), 173.0456 (2.60) | C16H19O10 | 0.0 | −0.1 | - |
| 5 | * 3-Hydroxy-4-O-glucosylbenzoic acid b,c | 3.90 | 259 | 315.0720 | 108.0227 | 152.0121 (80.47) | C13H15O9 | 0.2 | 0.6 | [20,21,22] |
| 6 | Caffeoylglucose I b,c | 4.09 | 326 | 341.0889 | 161.0264 | 135.0467 (22.08), 179.0353 (13.14) | C15H17O9 | −1.1 | −3.2 | [8,22,23] |
| 7 | * Canthoside B b,c | 4.75 | 257 | 463.1454 | 233.0671 | 293.0870 (58.98), 125.0252 (50.23), 123.0448 (34.74), 149.0461 (32.35), 191.0550 (17.67), 131.0351 (10.15), 105.0348 | C19H27O13 | 0.4 | 0.8 | [24,25] |
| 8 | Caffeoylglucose II b,c | 4.81 | 327 | 341.0885 | 161.0266 | 135.0475 (55.38), 179.0356 (14.39), 221.051 (7.01) | C15H17O9 | −0.9 | −2.7 | [8,22,23] |
| 9 | Theobromine a | 5.07 | 273 | - | - | - | - | − | − | standard |
| 10 | Caffeoylglucose III b,c | 5.14 | 327 | 341.0878 | 161.0267 | 135.0473 (49.92), 179.0386 (18.17), 221.051 (3.04) | C15H17O9 | −0.9 | −2.7 | [8,22,23] |
| 11 | * p-Hydroxybenzoic acid a,b,c | 5.50 | 280 | 137.0246 | 108.0238 | 136.0166 (75.91) | C7H5O3 | [26] | ||
| 12 | cis-3-Caffeoylquinic acid b,c | 5.51 | 321 | 353.0886 | 191.0594 | 135.0474 (63.62), 179.0375 (38.53) | C16H17O9 | −0.8 | −2.2 | [8] |
| 13 | Neochlorogenic acid (5-CQA) b,c | 5.94 | 324 | 353.0888 | 191.0586 | 135.0477 (69.82), 179.0377 (45.58), 161.0266 (5.09) | C16H17O9 | −1.2 | −3.3 | [8] |
| 14 | Caffeoylglucose IV b,c | 6.47 | 326 | 341.0890 | 161.0266 | 135.0472 (24.92), 179.0381 (17.14), 203.0384 (3.96), 221.0474 (2.19) | C15H17O9 | −1.2 | −3.5 | [8,22,23] |
| 15 | 6-Caffeoylglucose b, c | 7.21 | 324 | 341.0890 | 161.0263 | 135.0481 (63.45), 179.0377 (63.45), 221.0475 (42.31), 281.0661 (5.44) | C15H17O9 | −1.2 | −3.5 | [8,22,23] |
| 16 | 3-p-Coumaroylquinic acid b,c | 7.46 | 313 | 337.0938 | 119.0519 | 163.0417 (82.87), 191.0586 (14.73) | C16H17O8 | −0.9 | −2.8 | [8] |
| 17 | Caffeoylquinic acid b,c | 7.61 | 324 | 353.0882 | 135.0464 | 191.0570 (96.13), 173.0450 (80.28), 179.0354 (67.36), 93.0365 (10.89), 155.0352 (8.34), 111.0467 (8.22), 137.0241 (7.54) | [8] | |||
| 18 | Caffeoylglucose VI b,c | 8.05 | 324 | 341.0890 | 161.0268 | 135.0475 (80.13), 179.0367 (76.09) 221.0472 (44.08), 281.0662 (8.54), 203.0385 (2.33) | C15H17O9 | −1.1 | −3.4 | [8,22,23] |
| 19 | Caffeic acid a, b, c | 8.17 | 321 | 179.0371 | 135.0473 | 117.0362 (6.09) | C9H7O4 | −1.2 | −3.5 | standard |
| 20 | Chlorogenic acid (3-CQA) a,b,c | 8.30 | 324 | 353.0883 | 191.0584 | 161.0265 (2.08) | C16H17O9 | 0.7 | 1.9 | standard |
| 21 | Caffeine a | 8.52 | 278 | - | - | - | - | standard | ||
| 22 | 3-Feruloylquinic acid b,c | 8.63 | 325 | 367.1036 | 134.0399 | 193.0528 (72.78), 149.0625 (10.02), 117.0361 (9.64) | C17H19O9 | −0.1 | −0.3 | [8] |
| 23 | Cryptochlorogenic acid (4-CQA) b,c | 8.71 | 324 | 353.0892 | 135.0473 | 191.0583 (97.91), 179.0374 (81.84), 161.0269 (8.46), | C16H17O9 | −1.4 | −3.9 | [8,9] |
| 24 | Quercetin-di-glucoside b,c | 9.62 | 352, 253 | 625.1411 | 625.1416 | 463.0865 (80.18), 301.0368 (15.78), 299.0190 (9.67) | C27H29O17 | 0.1 | 0.1 | [27,28] |
| 25 | Quercetin-deoxyhexoside-hexoside b,c | 9.81 | 353, 255 | 609.1471 | - | - | C27H29O16 | −1.0 | −1.6 | [29] |
| 26 | cis-5-Caffeoylquinic acid b,c | 9.97 | 325 | 353.0878 | 191.0585 | - | C16H17O9 | −1.2 | −3.5 | [8] |
| 27 | 4-p-Coumaroylquinic acid b,c | 10.23 | 312 | 337.0940 | 173.0478 | 191.058 (55.34), 119.0522 (37.71), 163.0418 (34.04), 137.0269 (13.06) | C16H17O8 | −1.4 | −3.9 | [8] |
| 28 | * Flavonoid-derivate b,c | 10.51 | 353, 255 | 667.1523 | - | - | C29H31O18 | −0.7 | −1 | [29] |
| 29 | Kaempferol-di-hexoside b,c | 10.66 | - | 609.1463 | 447.0917 | 285.0413 (73.38), 609.1441 (41.45), 283.0255 (24.85), 327.0522 (8.38), 489.0952 (4.21) | C27H29O16 | −0.2 | −0.3 | [28] |
| 30 | Kaempferol-rutinoside-hexoside b,c | 10.86 | 346, 265 | 755.2039 | 593.1502 | 755.2028 (25.24), 447.0918 (4.80), 285.0397 (4.05) | C33H39O20 | 0.2 | 0.2 | [30,31] |
| 31 | Unidentified | 10.92 | 282 | 537.1980 | 375.1456 | 357.1344 (33.02), 537.1990 (32.01), 489.2040 (25.25), 119.0356 (21.60), 327.1211 (19.19), 321.1131 (17.17), 339.1247 | C26H33O12 | −0.3 | −0.5 | - |
| 32 | Unidentified | 11.06 | - | 593.1519 | 593.1487 | 473.1092 (30.56), 353.0676 (16.77), 383.0765 (7.87), 503.1181 | C27H29O15 | −0.7 | −0.1 | - |
| 33 | * Isorhamnetin-3-O-gentiobioside b,c | 11.06 | 345, 254 | 639.1576 | 477.1040 | 315.0504 (61.04), 639.1571 (28.77), 313.0387 (12.37) | C28H31O17 | −1.0 | −1.6 | [32,33] |
| 34 | 4-Feruloylquinic acid b,c | 11.20 | 324 | 367.1045 | 173.0476 | 134.0394 (41.39), 193.0527 (22.24) | C17H19O9 | −1.0 | −2.8 | [8,9] |
| 35 | * Isorhamnetin-rutinoside-glucoside b,c | 11.21 | 352, 254 | 785.2152 | 623.1603 | 785.2078 (15.49), 315.0503 (6.79), 477.0992 (6.61) | C34H41O21 | −0.7 | −0.8 | [32,33] |
| 36 | 5-Feruloylquinic acid b,c | 11.31 | 325 | 367.1046 | 191.0583 | 134.0403 (24.8), 193.0538 (23.18),173.0476 (16.98), 149.0632 (4.52) | C17H19O9 | −1.2 | −3.2 | [8,9] |
| 37 | Caffeoylquinic lactone b,c | 11.41 | - | 335.0787 | - | - | −1.5 | −3.5 | [8] | |
| 38 | Isorhamnetin derivate b,c | 12.00 | 349, 253 | 725.1572 | 519.1137 | 681.1659 (63.20) 477.1007 (27.15) 315.0503 (16.33), 561.1252 (13.86), 357.0587 (5.06) | C31H33O20 | −0.1 | −0.2 | - |
| 39 | Myricetin-hexoside b,c | 12.38 | 355, 254 | 479.1206 | 317.0683 | 299.0591 (15.88), 479.1203 (13.93), 289.0719 (11.26) 0.001640, 165.0210 | C22H23O12 | −1.1 | −2.4 | [34] |
| 40 | Quercetin-pentoside-hexoside b,c | 12.84 | 352, 256 | 595.1310 | 300.0282 | 595.1303 (55.26), 179.0017 (2.11) | C26H27O16 | −0.5 | −0.9 | [35,36] |
| 41 | Quercetin-7-O-rutinoside b,c | 13.34 | 353, 256 | 609.1457 | 609.1456 | 300.0291 (47.5) | C27H29O16 | 1.5 | 2.3 | [29] |
| 42 | Rutin (Quercetin-3-O-rutinoside) a,b,c | 13.67 | 353, 256 | 609.1445 | 609.1441 | 300.0312 (43.16) | C27H29O16 | 1.6 | 2.6 | standard |
| 43 | Quercetin-glucoside b,c | 13.95 | 352, 256 | 463.0878 | 300.0301 | - | C21H19O12 | 0.4 | 0.9 | [9,29] |
| 44 | Unidentified | 14.51 | 392, 263 | 701.1363 | 701.1355 | 335.0193 (67.64), 509.0718 (9.63), 191.0564 (4.69), 669.0322 (2.02) | C32H29O18 | −0.3 | −0.05 | - |
| 45 | Unidentified | 14.51 | 385, 251 | 493.1338 | 331.0837 | - | C23H25O12 | −1.8 | −3.7 | - |
| 46 | Flavonoid c | 14.80 | 354, 257 | 547.2386 | 149.0481 | 191.0585 (30.48), 131.0365 (15.69), 101.0264 (11.61) | C25H39O13 | 1.0 | 1.9 | - |
| 47 | Kaempferol-3-O-rutinoside b,c | 15.05 | 348, 265 | 593.1495 | 285.0415 | - | C27H29O15 | 1.7 | 2.9 | [8,9,37] |
| 48 | 3,4-Dicaffeoylquinic acid b,c | 15.19 | 325 | 515.1185 | 173.0477 | 179.0372 (99.12), 353.0887 (54.25), 191.0583 (46.01), 161.0262 (19.59), 135.0474 (16.21) | C25H23O12 | 1.0 | 2.0 | [8,9] |
| 49 | Kaempferol-3-O-glucoside b,c | 15.38 | 345, 254 | 447.0928 | 284.0331 | 447.0938 (14.96), 255.0305 (12.35), 227.0358 (6.66) | C21H19O11 | 0.5 | 1.0 | [8,38] |
| 50 | Isorhamnetin-3-O-rutinoside b,c | 15.38 | 353 | 623.1621 | 315.0518 | 623.1618 (90.32) | C28H31O16 | −0.4 | −0.6 | [39] |
| 51 | 3,5-Dicaffeoylquinic acid b,c | 15.46 | 326 | 515.1184 | 191.058 | 179.0371 (66.61), 353.0882 (45.97), 135.0472 (11.41), 161.0263 (4.97) | C25H23O12 | 1.1 | 2.1 | [8,9] |
| 52 | Isorhamnetin-3-O-glucoside b,c | 15.76 | 353, 255 | 477.1032 | 314.0442 | 477.1005 (36.22), 286.0504 (8.18), 299.0195 (4.73) | C22H21O12 | 0.7 | 1.5 | [33,40] |
| 53 | Dicaffeoylquinic acid b | 15.76 | 325 | 515.1204 | 191.0566 | 179.0358 (73.65), 353.0878 (30.52), 173.0459 (16.28), 135.0447 (11.64), 161.0257 (11.13), 335.0779 (5.08), 359.0566 (2.56), 155.0362 (2.03) | C25H23O12 | −0.7 | −1.4 | [8] |
| 54 | Isorhamnetin glucoside b,c | 16.10 | 253 | 477.1051 | 314.0431 | 299.0193 (93.25), 477.1052 (51.68), 271.0265 (10.28) | C22H21O12 | −1.2 | −2.5 | [40,41] |
| 55 | Quercetin-rutinoside b,c | 16.25 | 284 | 609.1829 | 301.0717 | 609.1807 (5.26) | C28H33O15 | [29] | ||
| 56 | Dicaffeoylquinic acid b,c | 16.31 | 325 | 515.1197 | 173.0458 | 179.0358 (84.27), 707.1842 (54.47), 191.0560 (38.01) 161.0247 (13.78), 135.0454 (10.60), 354.0899 (8.68), 155.0340 (4.89), 335.0758 (2.88), 163.0756 (2.37), 243.0645 (2.37) |
C25H23O12 | −0.2 | −0.5 | [8] |
| 57 | 4,5-dicaffeoylquinic acid b,c | 16.66 | 326 | 515.1188 | 173.0479 | 179.0373 (77.99), 353.0885 (65.03), 191.0586 (30.17), 135.0477 (10.58), 161.0271 (2.79) | C25H23O12 | 0.7 | 1.4 | [8,9] |
| 58 | * Hexoside derivate c | 16.95 | - | 431.1917 | 207.1381 | 251.1300 (94.96), 113.0225 (16.89), 335.0227 (15.87), 119.0341, 331 (11.20), 99.0457 (10.80), 101.0234 (9.81), 163.1470 (2.64) | C20H31O10 | 0.5 | 1.3 | - |
| 59 | Isorhamnetin 3-O-acetylglucoside | 17.05 | 351 | 519.1147 | 314.0451 | 299.0217 (15.41), 519.1172 (6.32) | C24H23O13 | −0.1 | −0.2 | [41,42] |
| 60 | * Hexoside derivate c | 17.13 | 310 | 429.1771 | 205.1236 | 249.1115 (74.12), 161.1358 (44.20), 99.0439 (9.41), 153.0933 (3.39) | C20H29O10 | −0.5 | −1.1 | - |
| 61 | * Hexoside derivate c | 17.49 | - | 431.1919 | 207.1392 | 251.1292 (81.26), 99.0452 (15.10), 113.0234 (10.61), 189.1290 (9.72), 119.0352 (9.70), 101.0266 | C20H31O10 | 0.7 | 1.4 | - |
| 62 | Caffeoylferuoyl-quinic acid b | 17.79 | 326 | 529.1342 | 193.0529 | 367.1029 (17.65), 173.0467 (6.66), 134.0391 (3.39), 191.0622 (2.19) | C26H25O12 | 1.0 | 1.8 | [8] |
| 63 | Caffeoylferuoyl-quinic acid b | 17.98 | 326 | 529.1322 | 191.0583 | 179.0366 (44.92), 353.0898 (14.56), 173.0496 (12.08), 193.0512 (11.45), 135.0465 (10.73), 367.1057 (10.36) 161.0259 (8.01) |
C26H25O12 | 2.0 | 4.0 | [8] |
| 64 | Caffeoylquinic acid b,c | 18.51 | 326 | 515.1176 | 173.0479 | 179.0374 (90.57), 353.0892 (58.97), 191.0573 (45.39), 135.0479 (13.9), 161.0254 (13.9 5.49) | C25H23O12 | 1.9 | 3.7 | [8] |
| 65 | Caffeoylferuoyl-quinic acid b | 18.71 | 325 | 529.1338 | 173.0478 | 193.0519 (21.1), 367.1043 (19.77), 137.0254 (2.43) | C26H25O12 | 1.3 | 2.5 | [8] |
| 66 | Caffeoylferuoyl-quinic acid b | 19.01 | - | 529.1322 | 173.0475 | 179.0368 (79.05), 191.057 (73.88), 353.0882 (52.19), 135.0483 (10.6), 367.103 (9.32), 161.0303 (6.47) 137.0279 (4.76) |
C26H25O12 | 1.9 | 3.7 | [8] |
Table legend; NB—number; RT—retention time; [M–H+]− formula—calculated experimental deprotonated molecular formula; * tentatively identyfied component (lack of accurate spectral literature database; suspected isomer of a known component or unclear UV spectrum); a component identified by comparison with a standard; b component identified by comparison of UV and/or MS/MS spectra with literature; c component identified by prediction with MS/MS spectra;—lack of experimental UV spectra/ions or not applicable.
Figure A1.
Ilex paraguariensis A.St.-Hil. leaves water extract, UV chromatogram, 280 nm.
Figure A2.
Ilex paraguariensis A.St.-Hil. leaves water extract, basic peak chromatogram, negative mode.
Figure A3.
Ilex aquifolium L. leaves water extract, UV chromatogram, 280 nm.
Figure A4.
Ilex aquifolium L. leaves water extract, basic peak chromatogram, negative mode.
Figure A5.
Ilex aquifolium ‘Argentea Marginata’ leaves water extract, UV chromatogram, 280 nm.
Figure A6.
Ilex aquifolium ‘Argentea Marginata’, basic peak chromatogram, negative mode.
Figure A7.
Ilex × meserveae ‘Blue Angel’ leaves water extract, UV chromatogram, 280 nm.
Figure A8.
Ilex × meserveae ‘Blue Angel’, basic peak chromatogram, negative mode.
Author Contributions
Conceptualization A.Z.-W., E.P., P.O. and J.S.; methodology E.P., P.O., J.S., A.Z.-W., M.W., B.Ż. and A.D.-M.; software E.P. and P.O.; validation, E.P. and P.O.; formal analysis E.P. and P.O.; investigation E.P., P.O., J.S., B.Ż., A.Z.-W. and A.D.-M.; resources P.B., A.Z.-W., B.Ż., E.P., P.O. and J.S.; data curation, E.P. and P.O.; writing—original draft preparation E.P., P.O., A.Z.-W. and J.S.; writing—review and editing J.S., A.Z.-W., A.S., B.Ż., P.B., R.K., W.W. and M.W.; visualisation E.P.; supervision, A.S., B.Ż., R.K. and P.B.; project administration, A.S.; funding acquisition E.P., P.O., A.S. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the National Science Centre Poland (NCN) number UMO–2015/19/B/NZ9/02971 and Wroclaw Centre of Biotechnology (KNOW 2014-2018). This work was also funded by the Wroclaw Medical University (statutory research SUB.A.130.21.031 and SUB.D110.21.101.). The BioFlux system (Fluxion, San Francisco, CA, USA) is funded by the National Centre for Research and Development Poland (NCBiR); No. IA/SP/453975/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Research data is available from authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds I. paraguariensis, I. aquifolium L., I. aquifolium ‘Argentea Marginata’ and Ilex × meserveae ‘Blue Angel’ are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoffmann K., Boeing H., Volatier J.-L., Becker W. Evaluating the potential health gain of the World Health Organization’s recommendation concerning vegetable and fruit consumption. Public Health Nutr. 2003;6:765–772. doi: 10.1079/PHN2003500. [DOI] [PubMed] [Google Scholar]
- 2.Bastos D., Oliveira D., Matsumoto R., Carvalho P., Ribeiro M. Yerba mate: Pharmacological properties, research and bio-technology. Med. Aromat. Plant Sci. Biotechnol. 2007;1:37–46. [Google Scholar]
- 3.Bracesco N., Sanchez A., Contreras V., Menini T., Gugliucci A. Recent advances on Ilex paraguariensis research: Minireview. J. Ethnopharmacol. 2011;136:378–384. doi: 10.1016/j.jep.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Kujawska M. Yerba Mate (Ilex paraguariensis) beverage: Nutraceutical ingredient or conveyor for the intake of medicinal plants? Evidence from Paraguayan Folk Medicine. Evid.-Based Complement. Altern. Med. 2018;2018:6849317. doi: 10.1155/2018/6849317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo L., Goya L., Lecumberri E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int. 2007;40:393–405. doi: 10.1016/j.foodres.2006.10.016. [DOI] [Google Scholar]
- 6.Grujić N., Lepojević Z., Srdjenovic B., Vladic J., Sudji J. Effects of different extraction methods and conditions on the phenolic composition of Mate tea extracts. Molecules. 2012;17:2518–2528. doi: 10.3390/molecules17032518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzan S., Hernandes A., Reichert C.L., Donaduzzi C., Pires V.A., Junior A.G., Junior E.L. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia. 2013;86:115–122. doi: 10.1016/j.fitote.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Mateos R., Baeza G., Sarriá B., Bravo L. Improved LC-MSn characterization of hydroxycinnamic acid derivatives and flavonols in different commercial mate (Ilex paraguariensis) brands. Quantification of polyphenols, methylxanthines, and antioxidant activity. Food Chem. 2018;241:232–241. doi: 10.1016/j.foodchem.2017.08.085. [DOI] [PubMed] [Google Scholar]
- 9.Zwyrzykowska A., Kupczyński R., Jarosz B., Szumny A., Kucharska A.Z. Qualitative and quantitative analysis of polyphenolic compounds in Ilex sp. Open Chem. 2015;13 doi: 10.1515/chem-2015-0142. [DOI] [Google Scholar]
- 10.Alikaridis F. Natural constituents of Ilex species. J. Ethnopharmacol. 1987;20:121–144. doi: 10.1016/0378-8741(87)90084-5. [DOI] [PubMed] [Google Scholar]
- 11.Heck C., de Mejia E. Yerba Mate Tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007;72:R138–R151. doi: 10.1111/j.1750-3841.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 12.Martin J.G.P., Porto E., de Alencar S.M., Gloria E., Corrêa C.B., Cabral I.S.R. Antimicrobial activity of yerba mate (Ilex paraguariensis St. Hil.) against food pathogens. Rev. Argent. Microbiol. 2013;45:93–98. doi: 10.1016/s0325-7541(13)70006-3. [DOI] [PubMed] [Google Scholar]
- 13.Burris K.P., Davidson P.M., Stewart C.N., Jr., Harte F.M. Antimicrobial activity of yerba mate (Ilex paraguariensis) aqueous extracts against Escherichia coli O157:H7 and Staphylococcus aureus. J. Food Sci. 2011;76:M456–M462. doi: 10.1111/j.1750-3841.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 14.Noureddine T., El Husseini Z., Nehme A., Massih R.A. Antibacterial activity of Ilex paraguariensis (Yerba Mate) against Gram-positive and Gram-negative bacteria. J. Infect. Dev. Ctries. 2018;12:712–719. doi: 10.3855/jidc.10380. [DOI] [PubMed] [Google Scholar]
- 15.Haraguchi H., Kataoka S., Okamoto S., Hanafi M., Shibata K. Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phyther. Res. 1999;13:151–156. doi: 10.1002/(SICI)1099-1573(199903)13:2<151::AID-PTR391>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Filip R., Davicino R., Anesini C. Antifungal activity of the aqueous extract of Ilex paraguariensis against Malassezia furfur. Phytotherapy Res. 2009;24:715–719. doi: 10.1002/ptr.3004. [DOI] [PubMed] [Google Scholar]
- 17.Córdova V., FernandoLic M., Caiza C., Pamela E. Actividad antibiofilm de los extractos de plantas Urtica dioica L., Ilex guayusa y Uncaria tomentosa en Staphylococcus aureus. 2019. [(accessed on 7 November 2021)]. Universidad Técnica de Ambato—Facultad de Ciencias de la Salud—Carrera de Laboratorio Clínico. Available online: https://repositorio.uta.edu.ec/jspui/handle/123456789/29295.
- 18.Kuropka P., Zwyrzykowska-Wodzińska A., Kupczyński R., Włodarczyk M., Szumny A., Nowaczyk R. The effect of Ilex × meserveae S. Y. Hu extract and its fractions on renal morphology in rats fed with normal and high-cholesterol diet. Foods. 2021;10:818. doi: 10.3390/foods10040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda K., Yasutomi K., Mori I. Structure of a new cyanoglucoside from Ilex warburgii Loesn. Chem. Lett. 1983;12:149–150. doi: 10.1246/cl.1983.149. [DOI] [Google Scholar]
- 20.Tirloni C.A.S., Silva A.O., Palozi R., Vasconcelos P.C.D.P., Souza R.I.C., Dos Santos A.C., de Almeida V.P., Budel J.M., Souza L., Junior A.G. Biological characterization of an edible species from Brazilian biodiversity: From pharmacognostic data to ethnopharmacological investigation. J. Med. Food. 2018;21:1276–1287. doi: 10.1089/jmf.2018.0010. [DOI] [PubMed] [Google Scholar]
- 21.Zheleva-Dimitrova D., Sinan K.I., Etienne O.K., Zengin G., Gevrenova R., Mahomoodally M.F., Lobine D., Mollica A. Chemical composition and biological properties of Synedrella nodiflora (L.) Gaertn: A comparative investigation of different extraction methods. Process. Biochem. 2020;96:202–212. doi: 10.1016/j.procbio.2020.06.002. [DOI] [Google Scholar]
- 22.Jaiswal R., Halabi E.A., Karar M.G.E., Kuhnert N. Identification and characterisation of the phenolics of Ilex glabra L. Gray (Aquifoliaceae) leaves by liquid chromatography tandem mass spectrometry. Phytochemistry. 2014;106:141–155. doi: 10.1016/j.phytochem.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal R., Matei M.F., Glembockyte V., Patras M.A., Kuhnert N. Hierarchical key for the LC–MSn identification of all ten regio- and stereoisomers of caffeoylglucose. J. Agric. Food Chem. 2014;62:9252–9265. doi: 10.1021/jf501210s. [DOI] [PubMed] [Google Scholar]
- 24.Kanchanapoom T., Kasai R., Yamasaki K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry. 2002;61:461–464. doi: 10.1016/S0031-9422(02)00140-1. [DOI] [PubMed] [Google Scholar]
- 25.Nurazah Z., Idris A.S., Kushairi A., RamLi U.S. Metabolite profiling of oil palm towards understanding basal stem rot (BSR) disease. J. Oil Palm Res. 2013;25:58–71. [Google Scholar]
- 26.Okińczyc P., Widelski J., Szperlik J., Żuk M., Mroczek T., Skalicka-Woźniak K., Sakipova Z., Widelska G., Kuś P.M. Impact of plant origin on Eurasian propolis on phenolic profile and classical antioxidant activity. Biomolecules. 2021;11:68. doi: 10.3390/biom11010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Cobo A., Gómez-Caravaca A.M., Pasini F., Caboni M.F., Carretero A.S., Gutierrez A.F. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT. 2016;73:505–513. doi: 10.1016/j.lwt.2016.06.049. [DOI] [Google Scholar]
- 28.Cádiz-Gurrea M.D.L.L., Fernández-Arroyo S., Joven J., Segura-Carretero A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013;50:197–204. doi: 10.1016/j.foodres.2012.09.038. [DOI] [Google Scholar]
- 29.Zhou C., Luo Y., Lei Z., Wei G. UHPLC-ESI-MS Analysis of purified flavonoids fraction from stem of Dendrobium denneaum Paxt. and its preliminary study in inducing apoptosis of HepG2 Cells. Evid.-Based Complement. Altern. Med. 2018;2018:8936307. doi: 10.1155/2018/8936307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legay S., Guerriero G., André C., Guignard C., Cocco E., Charton S., Boutry M., Rowland O., Hausman J. MdMyb93 is a regulator of suberin deposition in russeted apple fruit skins. N. Phytol. 2016;212:977–991. doi: 10.1111/nph.14170. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J., Wu Y., Long P., Ho C.-T., Wang Y., Kan Z., Cao L., Zhang L., Wan X., Zhou J., et al. LC-MS-based metabolomics reveals the chemical changes of polyphenols during high-temperature roasting of large-leaf yellow tea. J. Agric. Food Chem. 2018;67:5405–5412. doi: 10.1021/acs.jafc.8b05062. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M., Sakurai K., Fujii H., Saito K. Identification of indicator components for the discrimination of Cassia plants in health teas and development of analytical method for the components. J. AOAC Int. 2014;97:1195–1201. doi: 10.5740/jaoacint.13-038. [DOI] [PubMed] [Google Scholar]
- 33.Zhou S.-X., Yao Z.-R., Li J., Tu P.-F. Flavonoids from the leaves of Ilex cornuta. Chin. J. Nat. Med. 2012;10:84–87. doi: 10.3724/SP.J.1009.2012.00084. [DOI] [Google Scholar]
- 34.Fang Z., Zhang M., Wang L. HPLC-DAD-ESIMS analysis of phenolic compounds in bayberries (Myrica rubra Sieb. et Zucc.) Food Chem. 2007;100:845–852. doi: 10.1016/j.foodchem.2005.09.024. [DOI] [Google Scholar]
- 35.Marksa M., Zymone K., Ivanauskas L., Radušienė J., Pukalskas A., Raudone L. Antioxidant profiles of leaves and inflorescences of native, invasive and hybrid Solidago species. Ind. Crop. Prod. 2020;145:112123. doi: 10.1016/j.indcrop.2020.112123. [DOI] [Google Scholar]
- 36.Kołodziejczyk-Czepas J., Sieradzka M., Moniuszko-Szajwaj B., Nowak P., Oleszek W., Stochmal A. Phenolic fractions from nine Trifolium species modulate the coagulant properties of blood plasma in vitro without cytotoxicity towards blood cells. J. Pharm. Pharmacol. 2018;70:413–425. doi: 10.1111/jphp.12872. [DOI] [PubMed] [Google Scholar]
- 37.Ouyang M.-A. Glycosides from the leaves of Ilex latifolia. Chin. J. Chem. 2010;19:885–892. doi: 10.1002/cjoc.20010190915. [DOI] [Google Scholar]
- 38.Peres R.G., Tonin F., Tavares M.F.M., Rodriguez-Amaya D.B. HPLC-DAD-ESI/MS identification and quantification of phenolic compounds in Ilex paraguariensis beverages and on-line evaluation of individual antioxidant activity. Molecules. 2013;18:3859–3871. doi: 10.3390/molecules18043859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo L., Dou L.-L., Duan L., Liu K., Bi Z.-M., Li P., Liu E.-H. Comprehensive analysis of chemical constituents in Xingxiong injection by high performance liquid chromatography coupled with mass spectrometry. Chin. J. Nat. Med. 2015;13:711–720. doi: 10.1016/S1875-5364(15)30071-6. [DOI] [PubMed] [Google Scholar]
- 40.El-Hawiet A.M., Toaima S.M., Asaad A.M., Radwan M.M., El-Sebakhy N.A. Chemical constituents from Astragalus annularis Forssk. and A. trimestris L., Fabaceae. Rev. Bras. Farm. 2010;20:860–865. doi: 10.1590/S0102-695X2010005000047. [DOI] [Google Scholar]
- 41.Matić I.Z., Juranić Z., Šavikin K., Zdunić G., Nađvinski N., Gođevac D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytotherapy Res. 2012;27:852–858. doi: 10.1002/ptr.4807. [DOI] [PubMed] [Google Scholar]
- 42.Khoza B.S., Chimuka L., Mukwevho E., Steenkamp P.A., Madala N.E. The effect of temperature on pressurised hot water extraction of pharmacologically important metabolites as analysed by UPLC-qTOF-MS and PCA. Evid.-Based Complement. Altern. Med. 2014;2014:914759. doi: 10.1155/2014/914759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez M.A.D.P., Pelotto J.P., Basualdo N. Distribution of flavonoid aglycones in Ilex species (Aquifoliaceae) Biochem. Syst. Ecol. 1997;25:619–622. doi: 10.1016/S0305-1978(97)00054-9. [DOI] [Google Scholar]
- 44.Levin D.A. Alkaloid-bearing plants: An ecogeographic perspective. Am. Nat. 1976;110:261–284. doi: 10.1086/283063. [DOI] [Google Scholar]
- 45.Luís Â.F.S., Domingues F.D.C., Amaral L.M.J.P. The anti-obesity potential of Ilex paraguariensis: Results from a meta-analysis. Braz. J. Pharm. Sci. 2019;55 doi: 10.1590/s2175-97902019000217615. [DOI] [Google Scholar]
- 46.Meng S., Cao J., Feng Q., Peng J., Hu Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A Review. Evid.-Based Complement. Altern. Med. 2013;2013:801457. doi: 10.1155/2013/801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho A.-S., Jeon S.-M., Kim M.-J., Yeo J., Seo K.-I., Choi M.-S., Lee M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Ziaee A., Zamansoltani F., Nassiri-Asl M., Abbasi E. Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin. Pharmacol. Toxicol. 2009;104:253–258. doi: 10.1111/j.1742-7843.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhu L., Wang L., Cao F., Liu P., Bao H., Yan Y., Dong X., Wang N., Wang Z., Gong P. Modulation of transport and metabolism of bile acids and bilirubin by chlorogenic acid against hepatotoxicity and cholestasis in bile duct ligation rats: Involvement of SIRT1-mediated deacetylation of FXR and PGC-1α. J. Hepato-Biliary-Pancreat. Sci. 2018;25:195–205. doi: 10.1002/jhbp.537. [DOI] [PubMed] [Google Scholar]
- 50.Shimoyama A.T., Santin J.R., Machado I.D., Silva A., de Melo I.L.P., Mancini-Filho J., Farsky S.H.P. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012;386:5–14. doi: 10.1007/s00210-012-0807-2. [DOI] [PubMed] [Google Scholar]
- 51.Salejda A.M., Szmaja A., Bobak Ł., Zwyrzykowska-Wodzińska A., Fudali A., Bąbelewski P., Bienkiewicz M., Krasnowska G. Effect of Ilex × meserveae aqueous extract on the quality of dry-aged beef. J. Food Qual. 2021;2021:8848279. doi: 10.1155/2021/8848279. [DOI] [Google Scholar]
- 52.Peterson M.E., Talcott P.A. Small Animal Toxicology. Elsevier-Saunders; St. Louis, MO, USA: 2006. [Google Scholar]
- 53.West L.G., McLaughlin J.L., Eisenbeiss G.K. Saponins and triterpenes from Ilex opaca. Phytochemistry. 1977;16:1846–1847. doi: 10.1016/0031-9422(71)85115-4. [DOI] [Google Scholar]
- 54.Willems M. A cyanogenic glucoside from Ilex aquifolium. Phytochemistry. 1988;27:1852–1853. doi: 10.1016/0031-9422(88)80458-8. [DOI] [Google Scholar]
- 55.Evens Z.N., Stellpflug S. Holiday plants with toxic misconceptions. West. J. Emerg. Med. 2012;13:538–542. doi: 10.5811/westjem.2012.8.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spray M. Holly as a fodder in England. Agric. Hist. Rev. 1981;29:97–110. [Google Scholar]
- 57.Hao D., Gu X., Xiao P., Liang Z., Xu L., Peng Y. Research progress in the phytochemistry and biology of Ilex pharmaceutical resources. Acta Pharm. Sin. B. 2013;3:8–19. doi: 10.1016/j.apsb.2012.12.008. [DOI] [Google Scholar]
- 58.Karunanidhi A., Thomas R., van Belkum A., Neela V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. BioMed Res. Int. 2012;2013:392058. doi: 10.1155/2013/392058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luís Â., Silva F., Sousa S., Duarte A.P., Domingues F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling. 2014;30:69–79. doi: 10.1080/08927014.2013.845878. [DOI] [PubMed] [Google Scholar]
- 60.Cushnie T.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleck J.D., Betti A.H., da Silva F.P., Troian E.A., Olivaro C., Ferreira F., Verza S.G., Fleck J.D., Betti A.H., da Silva F.P., et al. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules. 2019;24:171. doi: 10.3390/molecules24010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J.M., Jin Z.X., Chen T., Gu Q.P. Correlation of antibacterial activity with secondary metabolites content in Sargentodoxa cuneata tablets. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;35:273–280. doi: 10.3785/j.issn.1008-9292.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Ruhal R., Kataria R. Biofilm patterns in Gram-positive and Gram-negative bacteria. Microbiol. Res. 2021;251:126829. doi: 10.1016/j.micres.2021.126829. [DOI] [PubMed] [Google Scholar]
- 64.Paluch E., Rewak-Soroczyńska J., Jędrusik I., Mazurkiewicz E., Jermakow K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020;104:1871–1881. doi: 10.1007/s00253-020-10349-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verderosa A.D., Totsika M., Fairfull-Smith K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019;7:824. doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazur M., Zwyrzykowska-Wodzinska A., Wojtas E., Zachwieja A., Salejda A.M. Effect of yerba mate (Ilex paraguariensis A. St.-Hil.) supplementation on oxidative stress in ruminants. Chil. J. Agric. Res. 2019;79:316–322. doi: 10.4067/S0718-58392019000200316. [DOI] [Google Scholar]
- 67.Wald A., Back C., Bayless T.M. Effect of caffeine on the human small intestine. Gastroenterology. 1976;71:738–742. doi: 10.1016/S0016-5085(76)80353-8. [DOI] [PubMed] [Google Scholar]
- 68.Paluch E., Piecuch A., Obłąk E., Lamch Ł., Wilk K.A. Antifungal activity of newly synthesized chemodegradable dicephalic-type cationic surfactants. Colloids Surfaces B Biointerfaces. 2018;164:34–41. doi: 10.1016/j.colsurfb.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 69.Clinical and Laboratory Standards Institute (CLSI) Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria that Grow Aerobically. 9th ed. Volume 32. CLSI; Wayne, PA, USA: 2012. CLSI Document M07-A9; Approved Standard. [Google Scholar]
- 70.Wu Y., Griffiths M., McKellar R. A comparison of the bioscreen method and microscopy for the determination of lag times of individual cells of Listeria monocytogenes. Lett. Appl. Microbiol. 2000;30:468–472. doi: 10.1046/j.1472-765x.2000.00748.x. [DOI] [PubMed] [Google Scholar]
- 71.Kolderman E., Bettampadi D., Samarian D., Dowd S., Foxman B., Jakubovics N., Rickard A.H. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS ONE. 2015;10:e0121835. doi: 10.1371/journal.pone.0121835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piecuch A., Lamch Ł., Paluch E., Obłąk E., Wilk K. Biofilm prevention by dicephalic cationic surfactants and their interactions with DNA. J. Appl. Microbiol. 2016;121:682–692. doi: 10.1111/jam.13204. [DOI] [PubMed] [Google Scholar]
- 73.Paluch E., Szperlik J., Lamch Ł., Wilk K.A., Obłąk E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-88244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data is available from authors.