Abstract
Latent tuberculosis infection (LTBI) represents a major challenge to curing TB disease. Current guidelines for LTBI management include only three older drugs and their combinations—isoniazid and rifamycins (rifampicin and rifapentine). These available control strategies have little impact on latent TB elimination, and new specific therapeutics are urgently needed. In the present mini-review, we highlight some of the alternatives that may potentially be included in LTBI treatment recommendations and a list of early-stage prospective small molecules that act on drug targets specific for Mycobacterium tuberculosis latency.
Keywords: latent tuberculosis infection, dormant Mycobacterium tuberculosis, LTBI treatment, anti-TB drugs
1. Introduction
Tuberculosis (TB), an infection caused by the bacillus Mycobacterium tuberculosis, still remains one of the top 10 causes of death worldwide, especially in low- and lower-middle-income countries [1]. Although TB is preventable and curable, the World Health Organization (WHO) estimates that 7.1 million people fell ill and 1.4 million died from the infection in 2019 [2]. This situation is a result of many factors, such as late diagnosis, co-infection with HIV, and the emergence of multidrug- and extensively drug-resistant (MDR- and XDR-TB) bacilli due to incomplete or inappropriate care. Approximately 0.22 million (3%) of the total new TB cases in 2019 were associated with resistant forms of the infection [2]. However, despite a small share of total TB cases, the number of MDR-TB and XDR-TB cases is increasing every year (0.22 million in 2019 up from 0.20 million in 2018, and 0.17 million in 2017) [2,3,4].
Another global challenge in TB management is the ability of M. tuberculosis to cause latent infection, which can suddenly turn into active disease during the lifetime of infected individuals. LTBI may be defined as infection in which tubercle bacilli are persistent in the host but do not currently cause active disease. Latent TB infection is diagnosed by the absence of clinical symptoms and signs and by positive tuberculin skin (TST) or interferon-gamma release assay (IGRA) blood tests. However, these tests/diagnostic approaches have some limitations [5]. Since there is no gold-standard test for LTBI, the exact number of LTBI cases is difficult to establish [6]. It is thought that one fourth of the world’s population is latently infected with M. tuberculosis, indicating a large reservoir of individuals with a potential risk of developing active TB [7]. The WHO recommends LTBI treatment for people living with HIV, for people who have household contacts with patients with bacteriologically confirmed pulmonary tuberculosis, and for specific groups of patients receiving immunosuppression therapy (anti-TNF treatment, etc.) [8]. Notably, the likelihood of LTBI activation for these categories may increase from 5% to 10% per year throughout their lifetime. Therefore, addressing LTBI is one of the End TB Strategy crucial milestones for TB elimination [3].
The development of small molecules that suppress latent tuberculosis infection is a non-trivial task, since target-based approaches have had little success. Thus, testing candidate molecules on bacterial cells rather than on isolated enzymes may be the preferred method for finding new drugs for TB as it allows the testing of antibacterial molecules under the appropriate physiological state of the pathogen [9,10]. Therefore, reliable and adequate modeling of latent TB both in vitro and in vivo to search for anti-latent TB drugs is currently a crucial task. Although animal models of LTBI (guinea pigs, mice, rabbits, and non-human primates) may greatly simplify the study of the pathogenic mechanisms of M. tuberculosis [11,12,13,14,15,16], due to their relatively high cost and ethical considerations they are unlikely to be applied for primary small molecule screening. Obviously, in vitro models cannot comprehensively reproduce all complex host/pathogen interactions involved in the latent stage of TB, but they are cheaper and save time [17]. Several in vitro models that attempt to mimic latency have been reported [18,19,20,21,22,23,24,25].
2. Differences between the Definitions of Latency, Persistence, and Dormancy of Mycobacterium tuberculosis
The clinical term latency refers to the asymptomatic state of the infection. LTBI is currently diagnosed by using a tuberculin skin test (TST), which measures delayed-type immune response to a purified protein derivative derived from tuberculin and injected under the skin, or by using an interferon-gamma release assay (IGRA), which measures immune response to the tubercle bacteria in whole blood [26]. The term latency was introduced by Clemens von Pirquet, who in 1907 developed the tuberculin test applied to the skin by scarification [27]. It is believed that culture of tubercle cells from sputum or other samples of a latently infected person is impossible, indicating a low bacterial load in such a patient [26].
The term persistence is mostly used to describe the ability of M. tuberculosis to survive and persist in host tissues under stress. Concerning bacterial infections, the term was first introduced in 1944 by Joseph Bigger to characterize a small subgroup in a growing population of staphylococci that was genetically susceptible to drugs but was able to persist after long-term treatment with penicillin [28]. McDermott defined Mycobacterium tuberculosis persistence as the “capacity of drug-susceptible microorganisms to survive drug attacks when subsisting in an animal body” [29]. Thus, persistence arises under the effect of antibiotics, while latency is the result of the host’s immune system activity.
The term dormancy comes from the Latin word “dormire” (“to sleep”) and describes a bacterial phenotype with reduced metabolism and slower cell division [17]. This term is often used in the context of the in vitro model of progressive hypoxia (the Wayne model), where M. tuberculosis cells decrease metabolism and replication rate, and become phenotypically resistant to isoniazid [18].
3. Pathogenesis of Latent Tuberculosis Infection in Humans
Tubercle bacilli that cause LTBI were traditionally assumed to represent a population of dormant non-replicating bacilli with a temporary inability to grow and divide, and with reduced metabolic activity as a result of an adaptive response to immune-mediated mechanisms [30]. Lillebaek et al. showed that the restriction fragment length polymorphism (RFLP) associated with the insertion sequence profiles of latent isolates has not changed for decades, supporting the idea of reduced bacterial replication in the latent state [31]. Colangeli et al. also demonstrated no increase in the number of mutations using whole genome sequencing of M. tuberculosis isolates when comparing cases from over 20 years ago and the present day [32]. So, both latent and active tuberculosis are considered to be caused by a heterogeneous population of mycobacteria, including both actively growing bacilli and bacilli with reduced metabolic activity, only in different proportions [33].
Generally, the pathogenesis of LTBI in humans is characterized by a range of features [34]. Primary granulomas, a pathological hallmark of tuberculosis, are formed mostly in the basis pulmonis under the influence of very small amounts of tubercle bacilli (from 1 to 5). In primarily infected persons, the lesions most often disappear spontaneously and asymptomatically, but a local or systemic disease develops in 5–10% of infected persons (mostly in children) in the next 1–2 years [35]. In more than 90% of cases, tuberculosis infection is latent and asymptomatic; after 3–8 weeks of primary infection, the tuberculin skin test becomes positive—this status persists throughout life. Mycobacterium tuberculosis cells may also migrate with lymph and blood from primary lesions to secondary locations in the apical regions of the lungs where post-primary granulomas form (post-primary TB) [36]. For reasons that remain unclear, in about 10% of cases of the post-primary disease, the host’s immune system is unable to control the infection. Dormant bacteria begin to reactivate and actively divide, which leads to an increase in their concentration in granulomas in the apical regions of the lungs. Taken together, the infection–disease–infection cycle, mediated by the reactivation of dormant M. tuberculosis cells in TST-positive people with latent TB disease, is precisely the mechanism by which M. tuberculosis maintains its survival and spreads to new hosts.
4. In Vitro and In Vivo Models to Imitate Mycobacterium tuberculosis Latency
The Wayne model, developed in 1996, is probably the most studied model of M. tuberculosis dormancy [18]. The idea is based on the gradual oxygen depletion of tubercle bacilli, in which dormant cells are obtained by adaptation to hypoxia. Limitations of the model include the lack of explanation for other aspects of dormancy, such as bacterial adaptation to other stresses; the possibility of growing immediately after recovery; and lack of standardization of the method [37]. Another model that utilizes hypoxia is the low-oxygen recovery assay (LORA) [38]. The model uses a recombinant M. tuberculosis luciferase reporter to provide results of compound activity against non-replicating tubercle bacilli surviving under hypoxic conditions.
Almost 90 years ago, Loebel et al. noticed that nutritional deficiencies lead to decreased growth and metabolic activity of mycobacteria [39]. Much later, Betts et al. modified the Loebel nutrient-deficient model to design a simple method for testing molecules active against persistent bacteria [20]. The principle is based on the complete deprivation of nutrients from a culture medium. To achieve this, the nutrient-rich culture medium is washed with phosphate-buffered saline, which leads to a gradual shutdown of respiration and a switch of viable bacilli to a dormant state. Like the Wayne model, the Loebel–Betts model also cannot comprehensively mimic the environment of granuloma.
The multiple-stress model was designed to overcome the limitations associated with single-stress conditions. The model proposed by Deb et al. uses a low-nutrient medium that has an acidic pH of 5.0 with a gas mixture of low oxygen and high carbon dioxide (5% O2 + 10% CO2 + 85% N2) [22]. The streptomycin-dependent model represents another in vitro model of M. tuberculosis dormancy [40]. The M. tuberculosis strain underlying the model was isolated from the sputum of a TB patient who was resistant to streptomycin therapy in Japan in 1955 [41]. The M. tuberculosis strain ss18b is characterized by an inability to grow in vitro in the absence of streptomycin [40]. Although the bacteria could not divide, they maintained their viability and ability to reproduce after adding streptomycin to the culture medium.
There are several in vivo models of latent infection that are currently used to explain the disease development. Among them, zebrafish is the cheapest and simplest model that mimics Mycobacterium tuberculosis latency, and it may be used in the early preclinical stages of drug discovery [42]. However, most models of TB latency have been developed in mice: the chronic tuberculosis model [43]; the Cornell model based on the treatment of M. tuberculosis-infected mice with antibiotics (isoniazid and pyrazinamide), as a result of which bacilli are not detected by organ culture [11]; the artificial granuloma model [44]; and the Kramnik model, in which specially grown C3HeB/FeJ mice demonstrated the key features of latent tuberculosis in humans [13]. In guinea pig/rabbit models, granulomas are very similar to those in humans, but these animals are highly susceptible to rapid disease progression [45]. The most useful animal model of LTBI is the non-human primate model—this model most accurately reproduces the clinical, histological, and microbiological characteristics of latent infection in humans [46]. Limitations of the model include high cost and ethical considerations. Although there is not an ideal model, these animal models are used to study the efficacy of molecules active against dormant mycobacteria.
5. Alternatives for Latent Tuberculosis Infection Treatment
Since specific and effective anti-latent TB drugs are still in short supply, traditional antituberculosis drugs—isoniazid, rifampicin, and rifapentine—are used in modern LTBI chemotherapy, and therapeutic regimens are generally based on long-term treatment of latently infected patients with these drugs [6,47,48]. Isoniazid obviously has low efficiency against latent TB infection because it targets bacterial cell wall biosynthesis, which is inactive during dormancy [49]. The role of isoniazid in LTBI therapy is probably to kill emerging bacilli that grow actively as a result of reactivation of dormant cells or transformation of slowly growing persisters into actively growing bacilli susceptible to INH treatment [49]. Rifampicin is used as an alternative agent to reduce the side effects of isoniazid therapy (e.g., hepatotoxicity) [47]. Rifapentine, in turn, has a longer half-life and greater potency against M. tuberculosis than rifampicin [50]. Rifamycins block transcription by binding to the b-subunit of RNA polymerase—an important stage in the life of actively growing mycobacteria—so the effectiveness of the above drugs in the treatment of LTBI is still an open question.
Three anti-TB drugs with original targets have the potential to be selected in LTBI treatment guidelines. Bedaquiline (formerly TMC207 or R207910) was approved to treat active tuberculosis in 2012, being the first anti-TB drug discovered in the past 40 years. The molecule targets mycobacterial F0F1 ATP synthase and thus stops the production of ATP required for cellular energy production [51]. In further study, Andries’ research group observed that TMC207/R207910 treatment leads to a 1.8-log reduction in CFU in the Wayne model and 2.1-log in CFU in the hypoxia-induced model [52]. A killing kinetic study revealed that the compound completely sterilizes dormant bacilli in the Wayne hypoxia model in vitro after 14 days. Andries et al. proposed that ATP synthase contributes to mycobacterial survival despite its downregulation during the latent state and thus may potentially serve as a target for dormant bacteria. Rao et al. also confirmed TMC207/R207910 is highly active against non-replicating mycobacteria under hypoxic conditions [53]. The in vivo efficacy of bedaquiline was determined in several murine models of LTBI [54,55].
Pretomanid (formerly PA-824), belonging to the chemical class of nitroimidazoles, was initially identified as an attractive small molecule for TB treatment in 2000 and was approved for clinical use in 2019 by the U.S. Food and Drug Administration (FDA) and in 2020 by the European Medicines Agency (EMA) [56]. The compound exhibits bactericidal activity not only against actively replicating M. tuberculosis, but also against non-replicating bacteria in the Wayne hypoxia model [56,57]. PA-824 is a prodrug, which is converted to an active metabolite by deazaflavin-dependent nitroreductase (Ddn). Singh et al. suggested that the des-nitro metabolite and reactive nitrogen species, primarily nitric oxide (NO) generated from it, significantly contribute to PA-824’s anaerobic activity against non-replicating tubercle bacilli [58]. To verify the in vivo efficacy of pretomanid against dormant M. tuberculosis, Dutta and Karakousis used a necrotic granuloma murine model of LTBI, which is considered a highly clinically relevant model [59,60]. PA-824 demonstrates relatively limited efficacy against latent M. tuberculosis infection in C3HeB/FeJ mice, reducing bacterial load from Month 1 to Month 4 by only ~1.0-log CFU [60]. Another anti-TB nitroimidazooxazole derivative, FDA-approved delamanid (formerly OPC-67683), has also been investigated against LTBI. Chen et al. reported that the molecule kills more than 50% of the population of dormant bacilli in a modified Wayne model at concentrations of 0.4 mg/L and above [61]. In M. tuberculosis-infected guinea pigs, delamanid completely eradicates bacterial CFU in lungs over time (2.04-log CFU at 4 weeks, 0-log CFU at 8 weeks) compared with the control (5.91-log CFU at 4 weeks, 5.64-log CFU at 8 weeks) [61].
A few drugs from other therapeutic areas are being investigated as repurposable alternatives for LTBI treatment. For example, tafenoquine, an antimalarial agent from the 8-aminoquinoline class, was found to be active in a nutrient-starved dormancy model with a ~2.0-log decrease in CFU [62]. Other works evaluate derivatives of sacubitrile, an antihypertensive drug used in combination with valsartan, and pleuromutilin, an antibiotic, against dormant tubercle cells [63,64].
6. Early-Stage Compounds Active against Dormant and Non-Replicating Bacilli
The search for small-molecule inhibitors of dormant M. tuberculosis is a challenging process. First of all, dormant bacilli are characterized by reduced metabolism, and therefore most bacterial targets are non-druggable and only a few M. tuberculosis enzymes may be inhibited. In addition, all currently available in vivo and in vitro models of TB dormancy have certain limitations, such as high metabolic activity of dormant mycobacteria, lack of difference between the clinical manifestations of active and dormant TB, or an ambiguous assessment of the reduction in bacterial load. However, despite the existing obstacles, a number of recently discovered small molecules with anti-latent TB activity may be exploited as a platform for further in-depth study of the prospective M. tuberculosis molecular targets for LTBI elimination.
6.1. Dormancy Survival Two-Component Regulatory System (DosRST)
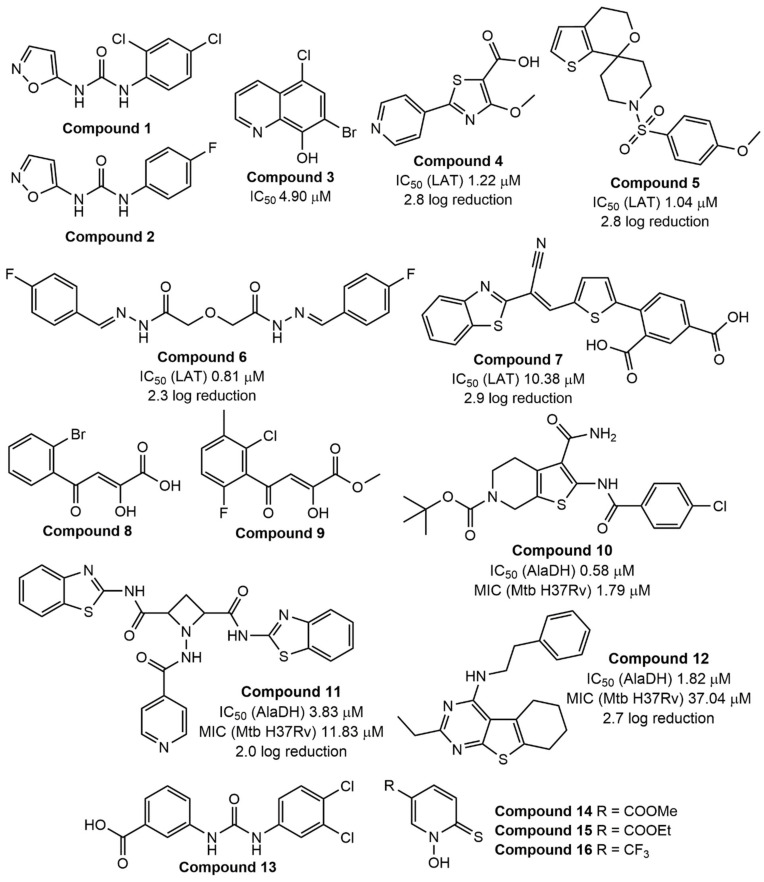
Low oxygen level (hypoxia), nutrient starvation, acidic pH, and other environmental and host immune pressures force active mycobacterial cells to switch into a dormant state with reduced metabolism [65]. M. tuberculosis is known to exploit DosRST to regulate its dormancy and virulence, and, especially, to promote survival during this state [66,67,68,69]. The DosRST regulatory system consists of two heme-based histidine sensor kinases, DosS and DosT, and the response regulator DosR, which are activated under stress conditions [70]. Given the importance of this system for maintaining dormancy in mycobacteria, it may be an attractive target for the control of LTBI. The feasibility of this approach for suppressing tubercle bacilli was first explored by Gupta et al. in 2009 [71]. Later, Zheng et al. screened a large chemical library using the DosRST regulon fluorescent M. tuberculosis reporter strain CDC1551 [72]. They suggested that inhibition of such fluorescence may be associated with inactivation of the DosRST. As a result, HC106A (compound 1, Figure 1) was found to inhibit dosR-dependent GFP fluorescence with an EC50 of 6.9 μM or 2.5 μM, and further to modulate the DosRST signaling by directly targeting the heme sensor [72]. To understand the structure–activity relationships (SAR) of the hit compound, a range of urea derivatives with different substitution patterns were further synthesized [72]. The isoxazole ring was important for the function as its replacement with another hetero/aryl group led to a decrease in fluorescence inhibition. The urea moiety was also needed for better activity. Particular attention has been paid to the role of the 2,4-dichlorophenyl ring. So, 1-(4-fluorophenyl)-3-(isoxazol-5-yl)urea (compound 2, Figure 1) was selected as the most active inhibitor of DosRST with an EC50 of 0.54 μM.
Figure 1.
Early-stage small molecules with established drug targets for dormant M. tuberculosis. Compound 1: 1-(2,4-dichlorophenyl)-3-(isoxazol-5-yl)urea; compound 2: 1-(4-fluorophenyl)-3-(isoxazol-5-yl)urea; compound 3: 7-bromo-5-chloroquinolin-8-ol; compound 4: 4-methoxy-2-(pyridin-4-yl)thiazole-5-carboxylic acid; compound 5: 1-((4-methoxyphenyl)sulfonyl)-4′,5′-dihydrospiro[piperidine-4,7′-thieno[2 ,3-c]pyran]; compound 6: 2,2′-oxybis(N′-(4-fluorobenzylidene)acetohydrazide); compound 7: 4-(5-(2-(benzo[d]thiazol-2-yl)-2-cyanovinyl)thiophen-2-yl)isophthalic acid; compound 8: 4-(2-bromophenyl)-2-hydroxy-4-oxobut-2-enoic acid; compound 9: methyl ester of -4-(2-chloro-6-fluoro-3-methylphenyl)-2-hydroxy-4-oxobut-2-enoic acid; compound 10: tert-butyl ester of 3-carbamoyl-2-(4-chlorobenzamido)-4,7-dihydrothieno[2,3-c]pyridine-6(5H)-carboxylic acid; compound 11: N2,N4-bis(benzo[d]thiazol-2-yl)-1-(isonicotinamido)azetidine-2,4-dicarboxamide; compound 12: 2-ethyl-N-phenethyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-amine; compound 13: 3-(3-(3,4-dichlorophenyl)ureido)benzoic acid; compound 14: methyl ester of 1-hydroxy-6-thioxo-1,6-dihydropyridine-3-carboxylic acid; compound 15: ethyl ester of 1-hydroxy-6-thioxo-1,6-dihydropyridine-3-carboxylic acid; compound 16: 1-hydroxy-5-(trifluoromethyl)pyridine-2(1H)-thione.
6.2. Methionine Aminopeptidase (MetAP)
Bacterial methionine aminopeptidases are metalloenzymes that catalyze the cleavage of amino acid residues at the N-terminal position of peptides and proteins [73]. To validate mycobacterial metalloproteinase (MtMetAP) as a druggable target, Olaleye et al. screened a chemical library and found that inhibitors of these mycobacterial enzymes were active against both replicating and aged non-growing (non-replicating) M. tuberculosis in the Byrne model of persistence [74,75]. Continuing this research, they characterized 7-bromo-5-chloroquinolin-8-ol CLBQ14 (compound 3, Figure 1), a bromine analog of clioquinol, as a suitable inhibitor of non-replicating bacilli [76]. Clioquinol is an antiprotozoal drug from the 8-hydroxyquinoline class that is probably neurotoxic at high doses. Olaleye et al. hypothesized that CLBQ14 does not show the same adverse effect, but they did not confirm or reject this point of view. Moreover, in recent preclinical studies of this compound in rats, the researchers also did not provide a toxicity profile of CLBQ14 [77].
6.3. Lysine ε-Aminotransferase (LAT)
Lysine ε-aminotransferase (LAT), a pyridoxal 5′-phosphate (PLP)-dependent enzyme, was found to be involved in the formation of persisters in mycobacteria [78,79]. A little earlier, Betts et al. determined that this enzyme is activated ~42-fold in their own developed nutrient-starved model of dormancy [20]. To understand the potential of LAT as a therapeutic target, Sriram et al. selected a number of primary hits using an e-pharmacophore approach and synthesized a library of hit-based analogs to assess in vitro activity [80,81,82,83]. Some interesting second-generation hits are presented in Figure 1. Three of the four compounds exhibit acceptable activity towards the target in an LAT enzyme inhibition assay (except for compound 7) together with good bacterial log reduction in a nutrient-starved model of M. tuberculosis dormancy.
6.4. Isocitrate Lyase (ICL)
Isocitrate lyases (ICLs) represent Mg2+-dependent enzymes that catalyze the reversible cleavage of D-isocitrate to glyoxylate and succinate in the glyoxylate shunt [84]. Actually, M. tuberculosis contains two isocitrate lyases with 27% sequence identity, ICL1 (428aa) and ICL2 (766aa), encoded by the icl1 and aceA genes, respectively [85]. Evidence indicates that ICLs are essential proteins not only for virulence [86,87], but also for the survival of non-replicating M. tuberculosis in a nutrient-deprived, oxygen-rich model of latency (the Loebel–Betts model) [88]. Several compounds have been investigated to validate the druggability of the target. 3-Nitropropionate and itaconate (structures not shown) strongly inhibit M. tuberculosis ICLs and, however, were found to be very toxic [89,90]. Bhusal et al. defined the main problems in the development of ICL inhibitors: the highly polar nature of the ICL binding pocket may lead to a drug selectivity issue, and the small size of the pocket will not allow a complete SAR study of the small molecules [91]. Some other studies have identified 4-(4-methoxy-phenyl)-4-oxo-crotonic acid methyl ester (IMBI-3), phenolic N-mono-substituted carbamates, salicylanilide derivatives, and cis-2,3-epoxy-succinic acid (structures not shown) as ICL inhibitors; however, limited data do not permit a conclusion about the usefulness of their further development [92,93,94,95].
6.5. Malate Synthase (GlcB)
While isocitrate lyase catalyzes the first step of the glyoxylate shunt, malate synthase acts in the second step of this pathway and converts glyoxylate into malate using one molecule of acetyl-CoA [96,97]. Krieger et al. suggested that GlcB may be a more “druggable” target than ICL [96]. Using a focused library of glyoxylate-like small molecules, researchers identified (Z)-2-hydroxy-4-oxo-4-phenylbut-2-enoic acid (PDKA) as a suitable GlcB inhibitor with an IC50 value of 2.0 µM [96]. However, PDKA was unstable in cell growth media (half-life t1/2 = 3 days). The PDKA derivative containing an ortho-bromine atom in the 4-phenyl ring (compound 8, Figure 1) exhibited a more pronounced enzyme inhibition effect (IC50 = 0.6 µM), but it is also not sufficiently stable. At the same time, the derivative with three substituents in the phenyl ring was weaker inhibitor of GlcB (IC50 = 5.5 µM) but with a longer half-life. To improve stability, Krieger et al. synthesized and evaluated the pharmacological properties of several alkyl- and benzyl-ester prodrugs of selected PDKA analogs [96]. The methyl ester (compound 9, Figure 1) showed a more favorable in vitro pharmacokinetic profile than other prodrugs and was selected for further detailed studies.
6.6. L-Alanine Dehydrogenase (L-AlaDH)
L-Alanine dehydrogenase is another enzyme expressed by mycobacteria during the latent state [98]. This enzyme catalyzes a reversible conversion of L-alanine to pyruvate. Griffin et al. also proposed that L-AlaDH maintains an optimal NADH/NAD ratio during mycobacterial cells’ recovery from hypoxic state (anaerobiosis), and the enzyme may be utilized as a potential target for TB dormancy inhibitors [99]. So, Shiram’s research group attempted to develop L-AlaDH inhibitors [100,101,102]. In the articles discussed, they identify a prospective parent structure from each virtual screening and synthesize a range of molecules based on this backbone. Then, they evaluate the activity of the compounds against nutrient-starved dormant bacilli to investigate the SAR. High enzyme-specificity compounds were cytotoxic and, in contrast, low enzyme-specificity molecules showed less cytotoxic effect. In the nutrient-starved model of dormant M. tuberculosis, the bacterial reduction value was approximately 2-log. A few interesting hits (compounds 10–12) are presented in Figure 1. However, the rationale for further optimization of these chemical classes needs to be clarified in detail.
6.7. Cysteine Synthase (CysM)
Cysteine synthase (CysM), one of three pyridoxal phosphate-dependent cysteine synthases in M. tuberculosis, is responsible for the synthesis of cysteine using O-phosphoserine and a sulfur carrier protein CysO [103]. Moreover, cysteine biosynthesis plays an important role during M. tuberculosis dormancy, since cysteine is involved in the biosynthesis of mycothiol, which is used to maintain redox homeostasis by mycobacteria [104]. A diverse library screening campaign by Brunner et al. led to the selection of urea-based hits for follow-up experiments. 3-(3-(3,4-Dichlorophenyl)ureido)benzoic acid (compound 13, Figure 1) shows suitable CysM-specific affinity with a Kd value of 0.32 μM and a 3-log decrease in bacterial count in a nutrient-starved model of dormancy (or the Loebel–Betts model) [105]. A preliminary SAR study revealed that the introduction of an additional substituent in the left phenyl ring or the replacement of two chlorine atoms with various groups in the right phenyl ring had little effect on target-specific affinity. At the same time, the urea moiety is important for binding to the CysM active site. Furthermore, Brunner et al. additionally evaluated these urea-based derivatives for binding with two another cysteine synthases, CysK1 and CysK2, and found some interesting inhibitors [106]. However, in-depth SAR investigation is needed to understand the potential of this chemical class as CysM inhibitors.
6.8. Copper-Mediated Innate Immunity
Copper-mediated innate immunity and its antibacterial properties [107,108,109,110] coupled with the ability of the metal ion to accumulate in bacteria-infected macrophage phagosomes [111] suggest copper as a viable antibacterial weapon. 1-Hydroxy-5-R-pyridine-2(1H)-thiones (compounds 14–16, Figure 1), active against streptomycin-deficient cells of M. tuberculosis 18b strain in vitro [25], form stable, charged lipophilic complexes with Cu2+ ions for transport into M. tuberculosis cells [112]. Subsequent metabolic transformation of the compounds led to the release and accumulation of free ions in the cytoplasm of bacilli. The actual molecular target of copper toxicity is still debated, but it may be scattered across a range of metabolic targets containing or constructing iron–sulfur clusters [113,114,115]. Hence, copper may actually affect a wide variety of cellular processes; this “target plurality” appears to be effective in inhibiting tubercle bacilli at different metabolic stages, including the dormant state.
6.9. Unknown Targets
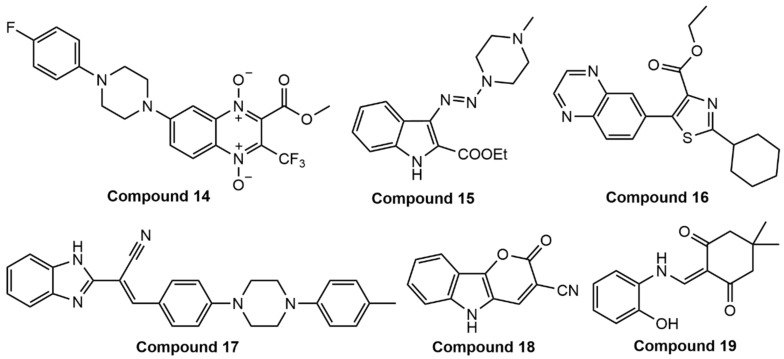
Santivañez-Veliz et al. reported that the quinoxaline-based molecule (compound 14, Figure 2) inhibits M. tuberculosis cells with an MIC value of 0.375 µg/mL in a low-oxygen recovery assay (LORA) [116]. Nikonenko et al. synthesized the 3-triazenoindole-based molecule TU112 (compound 15, Figure 2), which inhibits dormant M. tuberculosis by ~2.0-log in the Salina model, but the toxicity profile of the molecule and the class as a whole strongly needs to be optimized [117]. In another article, a thiazole derivative (compound 16, Figure 2) was found to show more than 90% inhibition of dormant M. tuberculosis H37Ra at 10 µM [118]. Bonnet et al. demonstrated the bactericidal activity of hydrazones (structures not shown) against non-replicating M. tuberculosis in two (hypoxia and starvation) dormancy models [119]. A benzimidazole–acrylonitrile hybrid (compound 17, Figure 2) shows a 2.8-log reduction in mycobacterial count in a nutrient-starved dormancy model [120]. Monakhova et al. investigated a class of pyrano[3,2-b]indolones and found that a particular compound (compound 18, Figure 2) exhibits good activity against M. tuberculosis H37Rv and the streptomycin-starved M. tuberculosis 18b model (ss18b) with MIC99 values of 0.3 and 0.4 μg/mL, respectively [121]. Rather et al. evaluated the potency of a recently discovered compound, PAMCHD (compound 19, Figure 2), against M. tuberculosis in several in vitro models mimicking the latent state [122]. PAMCHD was found to sterilize persister M. tuberculosis cells in a hypoxia-induced model with a 6.5-log CFU decrease, as well as in a nutrient-starved model with a 7.5-log decrease. In some other works, molecular docking has been used to predict drug targets for the compounds discovered [123,124,125,126]. We have included these articles in the present chapter of the review because in all these cases, mechanism-of-action studies are needed to clarify the site of action of the molecules.
Figure 2.
Small molecules active against dormant M. tuberculosis by an unknown mechanism of action. Compound 14: 6-(4-(4-fluorophenyl)piperazin-1-yl)-3-(methoxycarbonyl)-2-(trifluoromethyl)quinoxaline 1,4-dioxide; compound 15: ethyl ester of 3-((4-methylpiperazin-1-yl)diazenyl)-1H-indole-2-carboxylic acid; compound 16: ethyl ester of 2-cyclohexyl-5-(quinoxalin-6-yl)thiazole-4-carboxylic acid; compound 17: 2-(1H-benzo[d]imidazol-2-yl)-3-(4-(4-(p-tolyl)piperazin-1-yl)phenyl)acrylonitrile; compound 18: 2-oxo-2,5-dihydropyrano[3,2-b]indole-3-carbonitrile; compound 19: 2-(((2-hydroxyphenyl)amino)methylene)-5,5-dimethylcyclohexane-1,3-dione.
7. Concluding Remarks and Future Outlook
Latent tuberculosis infection is a serious obstacle to the complete elimination of tuberculosis. Therapeutic and preventive options for LTBI treatment are limited. For example, the only vaccine licensed against TB thus far is Bacille Calmette-Guérin (BCG), which was approved a century ago [127]. BCG vaccination primarily provides consistent protection against the most severe forms of childhood tuberculosis and practically does not provide protection against adult-type tuberculosis [128]. In 2019, GSK reported that the M72/AS01E vaccine candidate reduces the incidence of pulmonary tuberculosis in HIV-negative adults with latent infection. It demonstrated an overall efficacy of 50% for at least three years after vaccination in a Phase IIb study, but there is still no information on additional clinical trials or market launch of the vaccine [129].
Phage therapy may play an important role in TB management. Recently, Guerrero-Bustamante et al. found that a five-phage cocktail minimized the emergence of phage resistance and cross-resistance to multiple phages, and efficiently killed M. tuberculosis strains tested [130]. We hypothesize that the evaluation of bacteriophages against dormant tubercle cells represents an interesting research direction, but more data are needed to clarify this point.
The discovery of small molecules acting on both active and dormant M. tuberculosis cells seems to be the most promising direction. In the present mini-review, we highlighted early-stage compounds targeting the dormancy survival two-component regulatory system and a number of enzymes, such as methionine aminopeptidase, lysine-aminotransferase, or malate synthase, that are specific for the dormant state of tubercle bacilli. In addition, the discovery of multiple-target compounds that can induce apoptosis only in mycobacterial cells, for example, through copper-mediated innate immunity, may also represent an attractive way to target dormant bacteria.
Finally, tackling the spread of tuberculosis from two ends—small-molecule drug discovery and vaccination—holds great promise for fighting this dangerous and persistent pathogen.
Funding
A.E. and V.M. were financially supported by the Russian Science Foundation (Project No. 21-15-00042, rscf.ru/project/21-15-00042).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization The top 10 causes of death. [(accessed on 15 September 2021)]. Available online: Who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.WHO . Global Tuberculosis Report 2020. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
- 3.WHO . Global Tuberculosis Report 2019. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 4.WHO . Global Tuberculosis Report 2018. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 5.Muñoz L., Stagg H.R., Abubakar I. Diagnosis and Management of Latent Tuberculosis Infection. Cold Spring Harb. Perspect. Med. 2015;5:a017830. doi: 10.1101/cshperspect.a017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . Latent Tuberculosis Infection—Executive Summary. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 7.Cohen A., Mathiasen V.D., Schön T., Wejse C. The global prevalence of latent tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2019;54:1900655. doi: 10.1183/13993003.00655-2019. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. World Health Organization; Geneva, Switzerland: 2018. [PubMed] [Google Scholar]
- 9.Koul A., Arnoult E., Lounis N., Guillemont J.E.G., Andries K. The challenge of new drug discovery for tuberculosis. Nat. Cell Biol. 2011;469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 10.Payne D.J., Gwynn M.N., Holmes D.J., Pompliano D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 11.McCune R.M., Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J. Exp. Med. 1956;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCune R.M., Feldmann F.M., Lambert H.P., McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J. Exp. Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramnik I., Beamer G. Mouse models of human TB pathology: Roles in the analysis of necrosis and the development of host-directed therapies. Semin. Immunopathol. 2016;38:221–237. doi: 10.1007/s00281-015-0538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesavan A.K., Brooks M., Tufariello J., Chan J., Manabe Y.C. Tuberculosis genes expressed during persistence and reactivation in the resistant rabbit model. Tuberculosis. 2009;89:17–21. doi: 10.1016/j.tube.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenaerts A.J., Hoff D., Aly S., Ehlers S., Andries K., Cantarero L., Orme I.M., Basaraba R.J. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by r207910. Antimicrob. Agents Chemother. 2007;51:3338–3345. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin P.L., Rodgers M., Smith L., Bigbee M., Myers A., Bigbee C., Chiosea I., Capuano S.V., Fuhrman C., Klein E., et al. Quantitative Comparison of Active and Latent Tuberculosis in the Cynomolgus Macaque Model. Infect. Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veatch A.V., Kaushal D. Opening Pandora’s Box: Mechanisms of Mycobacterium tuberculosis Resuscitation. Trends Microbiol. 2018;26:145–157. doi: 10.1016/j.tim.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wayne L.G., Hayes L.G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y., Mangan J.A., Dhillon J., Sole K.M., Mitchison D.A., Butcher P.D., Coates A.R.M. Detection of mRNA Transcripts and Active Transcription in Persistent Mycobacterium tuberculosis Induced by Exposure to Rifampin or Pyrazinamide. J. Bacteriol. 2000;182:6358–6365. doi: 10.1128/JB.182.22.6358-6365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts J.C., Lukey P.T., Robb L.C., McAdam R.A., Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 21.Shleeva M.O., Bagramyan K., Telkov M.V., Mukamolova G.V., Young M., Kell D., Kaprelyants A.S. Formation and resuscitation of ‘non-culturable’ cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology. 2002;148:1581–1591. doi: 10.1099/00221287-148-5-1581. [DOI] [PubMed] [Google Scholar]
- 22.Deb C., Lee C.-M., Dubey V.S., Daniel J., Abomoelak B., Sirakova T.D., Pawar S., Rogers L., Kolattukudy P.E. A Novel In Vitro Multiple-Stress Dormancy Model for Mycobacterium tuberculosis Generates a Lipid-Loaded, Drug-Tolerant, Dormant Pathogen. PLoS ONE. 2009;4:e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sala C., Dhar N., Hartkoorn R., Zhang M., Ha Y.H., Schneider P., Cole S.T. Simple Model for Testing Drugs against Nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2010;54:4150–4158. doi: 10.1128/AAC.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shleeva M.O., Kudykina Y.K., Vostroknutova G.N., Suzina N.E., Mulyukin A.L., Kaprelyants A.S. Dormant ovoid cells of Mycobacterium tuberculosis are formed in response to gradual external acidification. Tuberculosis. 2011;91:146–154. doi: 10.1016/j.tube.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Salina E., Ryabova O., Kaprelyants A., Makarov V. New 2-Thiopyridines as Potential Candidates for Killing both Actively Growing and Dormant Mycobacterium tuberculosis Cells. Antimicrob. Agents Chemother. 2013;58:55–60. doi: 10.1128/AAC.01308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carranza C., Pedraza-Sanchez S., de Oyarzabal-Mendez E., Torres M. Diagnosis for latent tuberculosis infection: New alternatives. Front. Immunol. 2020;11:2006. doi: 10.3389/fimmu.2020.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman S.T. Clemens von Pirquet: A Remarkable Life and Career. J. Pediatr. Infect. Dis. Soc. 2016;6:376–379. doi: 10.1093/jpids/piw063. [DOI] [PubMed] [Google Scholar]
- 28.Bigger J.W. Treatment of staphylococcal infections with penicillin by intermittent sterilization. Lancet. 1944;244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 29.McDedmott W. Microbial persistence. Yale J. Biol. Med. 1958;30:257–291. [PMC free article] [PubMed] [Google Scholar]
- 30.Gold B., Nathan C. Targeting Phenotypically Tolerant Mycobacterium tuberculosis. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.TBTB2-0031-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillebaek T., Dirksen A., Vynnycky E., Baess I., Thomsen V.Ø., Andersen Å.B. Stability of DNA Patterns and Evidence of Mycobacterium tuberculosis Reactivation Occurring Decades after the Initial Infection. J. Infect. Dis. 2003;188:1032–1039. doi: 10.1086/378240. [DOI] [PubMed] [Google Scholar]
- 32.Colangeli R., Arcus V.L., Cursons R.T., Ruthe A., Karalus N., Coley K., Manning S.D., Kim S., Marchiano E., Alland D. Whole genome sequencing of Mycobacterium tuberculosis reveals slow growth and low mutation rates during latent infections in humans. PLoS ONE. 2014;9:e91024. doi: 10.1371/journal.pone.0091024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhar N., McKinney J., Manina G. Phenotypic Heterogeneity in Mycobacterium tuberculosis. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.TBTB2-0021-2016. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad S. Pathogenesis, Immunology, and Diagnosis of Latent Mycobacterium tuberculosis Infection. Clin. Dev. Immunol. 2011;2011:1–17. doi: 10.1155/2011/814943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardona P.J., Ruiz-Manzano J. On the nature of Mycobacterium tuberculosis-latent bacilli. Eur. Respir. J. 2004;24:1044–1051. doi: 10.1183/09031936.04.00072604. [DOI] [PubMed] [Google Scholar]
- 36.Hunter R.L. The Pathogenesis of Tuberculosis: The Early Infiltrate of Post-primary (Adult Pulmonary) Tuberculosis: A Distinct Disease Entity. Front. Immunol. 2018;9:2108. doi: 10.3389/fimmu.2018.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson S., Harrison J., Cox J. Modelling a Silent Epidemic: A Review of the In Vitro Models of Latent Tuberculosis. Pathogens. 2018;7:88. doi: 10.3390/pathogens7040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho S.H., Warit S., Wan B., Hwang C.H., Pauli G.F., Franzblau S.G. Low-Oxygen-Recovery Assay for High-Throughput Screening of Compounds against Nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007;51:1380–1385. doi: 10.1128/AAC.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loebel R.O., Shorr E., Richardson H.B. The Influence of Foodstuffs upon the Respiratory Metabolism and Growth of Human Tubercle Bacilli. J. Bacteriol. 1933;26:139–166. doi: 10.1128/jb.26.2.139-166.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M., Sala C., Hartkoorn R.C., Dhar N., Mendoza-Losana A., Cole S.T. Streptomycin-starved Mycobacterium tuberculosis 18b, a drug discovery tool for latent tuberculosis. Antimicrob. Agents Chemother. 2012;56:5782–5789. doi: 10.1128/AAC.01125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto T. Experimental studies on the mechanism of infection and immunity in tuberculosis from the analytical standpoint of streptomycin-dependent tubercle bacilli. 1. Isolation and biological characteristics of a streptomycin-dependent mutant, and effect of streptomycin administration on its pathogenicity in guinea pigs. Kekkaku. 1955;30:4–8. [PubMed] [Google Scholar]
- 42.Yang H.J., Wang D., Wen X., Weiner D.M., Via L.E. One size fits all? Not in in vivo modeling of tuberculosis chemotherapeutics. Front. Cell. Infect. Microbiol. 2021;11:613149. doi: 10.3389/fcimb.2021.613149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manabe Y.C., Bishai W.R. Latent Mycobacterium tuberculosis–persistence, patience and winning by waiting. Nat. Med. 2000;6:1327–1329. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 44.Karakousis P.C., Yoshimatsu T., Lamichhane G., Woolwine S.C., Nuermberger E.L., Grosset J., Bishai W.R. Dormancy Phenotype Displayed by Extracellular Mycobacterium tuberculosis within Artificial Granulomas in Mice. J. Exp. Med. 2004;200:647–657. doi: 10.1084/jem.20040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuermberger E.L. Preclinical Efficacy Testing of New Drug Candidates. Microbiol. Spectr. 2017;5:3. doi: 10.1128/microbiolspec.TBTB2-0034-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flynn J.L., Capuano S.V., Croix D., Pawar S., Myers A., Zinovik A., Klein E. Non-human primates: A model for tuberculosis research. Tuberculosis. 2003;83:116–118. doi: 10.1016/S1472-9792(02)00059-8. [DOI] [PubMed] [Google Scholar]
- 47.Kim H.W., Kim J.S. Treatment of Latent Tuberculosis Infection and Its Clinical Efficacy. Tuberc. Respir. Dis. 2018;81:6–12. doi: 10.4046/trd.2017.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turetz M.L., Ma K.C. Diagnosis and management of latent tuberculosis. Curr. Opin. Infect. Dis. 2016;29:205–211. doi: 10.1097/QCO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 49.Vilchèze C., Jacobs W.R., Jr. The Isoniazid Paradigm of Killing, Resistance, and Persistence in Mycobacterium tuberculosis. J. Mol. Biol. 2019;431:3450–3461. doi: 10.1016/j.jmb.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sterling T.R., Villarino M.E., Borisov A.S., Shang N., Gordin F., Bliven-Sizemore E., Hackman J., Hamilton C.D., Menzies D., Kerrigan A., et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N. Engl. J. Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 51.Andries K., Verhasselt P., Guillemont J., Göhlmann H.W., Neefs J.M., Winkler H., Van Gestel J., Timmerman P., Zhu M., Lee E., et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 52.Koul A., Vranckx L., Dendouga N., Balemans W., Wyngaert I.V.D., Vergauwen K., Göhlmann H.W., Willebrords R., Poncelet A., Guillemont J., et al. Diarylquinolines Are Bactericidal for Dormant Mycobacteria as a Result of Disturbed ATP Homeostasis. J. Biol. Chem. 2008;283:25273–25280. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- 53.Rao S.P.S., Alonso S., Rand L., Dick T., Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tasneen R., Li S.Y., Peloquin C.A., Taylor D., Williams K.N., Andries K., Mdluli K.E., Nuermberger E.L. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2011;55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T., Li S.-Y., Williams K.N., Andries K., Nuermberger E.L. Short-Course Chemotherapy with TMC207 and Rifapentine in a Murine Model of Latent Tuberculosis Infection. Am. J. Respir. Crit. Care Med. 2011;184:732–737. doi: 10.1164/rccm.201103-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stover C.K., Warrener P., VanDevanter D., Sherman D.R., Arain T.M., Langhorne M.H., Anderson S.W., Towell J.A., Yuan Y., McMurray D.N., et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nat. Cell Biol. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 57.Lenaerts A.J., Gruppo V., Marietta K.S., Johnson C.M., Driscoll D.K., Tompkins N.M., Rose J.D., Reynolds R.C., Orme I.M. Preclinical Testing of the Nitroimidazopyran PA-824 for Activity against Mycobacterium tuberculosis in a Series of In Vitro and In Vivo Models. Antimicrob. Agents Chemother. 2005;49:2294–2301. doi: 10.1128/AAC.49.6.2294-2301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh R., Manjunatha U., Boshoff H.I.M., Ha Y.H., Niyomrattanakit P., Ledwidge R., Dowd C.S., Lee I.Y., Kim P., Zhang L., et al. PA-824 Kills Nonreplicating Mycobacterium tuberculosis by Intracellular NO Release. Science. 2008;322:1392–1395. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutta N.K., Illei P.B., Jain S.K., Karakousis P.C. Characterization of a novel necrotic granuloma model of latent tuberculosis infection and reactivation in mice. Am. J. Pathol. 2014;184:2045–2055. doi: 10.1016/j.ajpath.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dutta N., Karakousis P.C. PA-824 is as effective as isoniazid against latent tuberculosis infection in C3HeB/FeJ mice. Int. J. Antimicrob. Agents. 2014;44:564–566. doi: 10.1016/j.ijantimicag.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Hashizume H., Tomishige T., Nakamura I., Matsuba M., Fujiwara M., Kitamoto R., Hanaki E., Ohba Y., Matsumoto M. Delamanid Kills Dormant Mycobacteria In Vitro and in a Guinea Pig Model of Tuberculosis. Antimicrob. Agents Chemother. 2017;61:e02402-16. doi: 10.1128/AAC.02402-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sidrônio M.G.S., Branco A.P.O.C., Abbadi B.L., Macchi F., Silveira M.D., Lock G.D.A., Costa T.D., de Araújo D.M., Cibulski S., Bizarro C.V., et al. Effects of tafenoquine against active, dormant and resistant Mycobacterium tuberculosis. Tuberculosis. 2021;128:102089. doi: 10.1016/j.tube.2021.102089. [DOI] [PubMed] [Google Scholar]
- 63.Konduri S., Pogaku V., Prashanth J., Krishna V.S., Sriram D., Basavoju S., Behera J.N., Rao K.P. Sacubitril-Based Urea and Thiourea Derivatives as Novel Inhibitors for Anti-Tubercular against Dormant Tuberculosis. ChemistrySelect. 2021;6:3869–3874. doi: 10.1002/slct.202004724. [DOI] [Google Scholar]
- 64.Lemieux M.R., Siricilla S., Mitachi K., Eslamimehr S., Wang Y., Yang D., Pressly J.D., Kong Y., Park F., Franzblau S., et al. An antimycobacterial pleuromutilin analogue effective against dormant bacilli. Bioorganic Med. Chem. 2018;26:4787–4796. doi: 10.1016/j.bmc.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dutta N.K., Karakousis C. Latent Tuberculosis Infection: Myths, Models, and Molecular Mechanisms. Microbiol. Mol. Biol. Rev. 2014;78:343–371. doi: 10.1128/MMBR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zahrt T.C., Deretic V. Mycobacterium tuberculosis signal transduction system required for persistent infections. Proc. Natl. Acad. Sci. USA. 2001;98:12706–12711. doi: 10.1073/pnas.221272198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyagi J.S., Sharma D. Signal transduction systems of mycobacteria with special reference to M. tuberculosis. Curr. Sci. 2004;86:93–102. [Google Scholar]
- 68.Honaker R.W., Leistikow R.L., Bartek I.L., Voskuil M.I. Unique Roles of DosT and DosS in DosR Regulon Induction and Mycobacterium tuberculosis Dormancy. Infect. Immun. 2009;77:3258–3263. doi: 10.1128/IAI.01449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sivaramakrishnan S., Ortiz de Montellano P.R. The DosS-DosT/DosR mycobacterial sensor system. Biosensors. 2013;3:259–282. doi: 10.3390/bios3030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim M.-J., Park K.-J., Ko I.-J., Kim Y.M., Oh J.-I. Different Roles of DosS and DosT in the Hypoxic Adaptation of Mycobacteria. J. Bacteriol. 2010;192:4868–4875. doi: 10.1128/JB.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta R.K., Thakur T., Desiraju G.R., Tyagi J.S. Structure-Based Design of DevR Inhibitor Active against Nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 2009;52:6324–6334. doi: 10.1021/jm900358q. [DOI] [PubMed] [Google Scholar]
- 72.Zheng H., Colvin C.J., Johnson B.K., Kirchhoff P.D., Wilson M., Jorgensen-Muga K., Larsen S.D., Abramovitch R.B. Inhibitors of Mycobacterium tuberculosis DosRST signaling and persistence. Nat. Chem. Biol. 2017;13:218–225. doi: 10.1038/nchembio.2259. [DOI] [PubMed] [Google Scholar]
- 73.Gonzales T., Robert-Baudouy J. Bacterial aminopeptidases: Properties and functions. FEMS Microbiol. Rev. 1996;18:319–344. doi: 10.1111/j.1574-6976.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 74.Olaleye O., Raghunand T.R., Bhat S., He J., Tyagi S., Lamichhane G., Gu P., Zhou J., Zhang Y., Grosset J., et al. Methionine Aminopeptidases from Mycobacterium tuberculosis as Novel Antimycobacterial Targets. Chem. Biol. 2010;17:86–97. doi: 10.1016/j.chembiol.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.John S.F., Aniemeke E., Ha N.P., Chong C.R., Gu P., Zhou J., Zhang Y., Graviss E.A., Liu J.O., Olaleye O.A. Characterization of 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazone as a novel inhibitor of methionine aminopeptidases from Mycobacterium tuberculosis. Tuberculosis. 2016;101:S73–S77. doi: 10.1016/j.tube.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 76.Olaleye O., Raghunand T.R., Bhat S., Chong C., Gu P., Zhou J., Zhang Y., Bishai W.R., Liu J.O. Characterization of clioquinol and analogues as novel inhibitors of methionine aminopeptidases from Mycobacterium tuberculosis. Tuberculosis. 2011;91:S61–S65. doi: 10.1016/j.tube.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekpenyong O., Gao X., Ma J., Cooper C., Nguyen L., Olaleye O.A., Liang D., Xie H. Pre-clinical pharmacokinetics, tissue distribution and physicochemical studies of CLBQ14, a novel methionine aminopeptidase inhibitor for the treatment of infectious diseases. Drug Des. Dev. Ther. 2020;14:1263–1277. doi: 10.2147/DDDT.S238148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan X., Li Y., Du Q., Huang Q., Guo S., Xu M., Lin Y., Liu Z., Xie J. Mycobacterium lysine ε-aminotransferase is a novel alarmone metabolism related persister gene via dysregulating the intracellular amino acid level. Sci. Rep. 2016;6:19695. doi: 10.1038/srep19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rittershaus E.S., Baek S.-H., Krieger I.V., Nelson S.J., Cheng Y.-S., Nambi S., Baker R.E., Leszyk J.D., Shaffer S.A., Sacchettini J.C., et al. A Lysine Acetyltransferase Contributes to the Metabolic Adaptation to Hypoxia in Mycobacterium tuberculosis. Cell Chem. Biol. 2018;25:1495–1505.e3. doi: 10.1016/j.chembiol.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devi P.B., Sridevi J.P., Kakan S.S., Saxena S., Jeankumar V.U., Soni V., Anantaraju H.S., Yogeeswari P., Sriram D. Discovery of novel lysine ε-aminotransferase inhibitors: An intriguing potential target for latent tuberculosis. Tuberculosis. 2015;95:786–794. doi: 10.1016/j.tube.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Parthiban B.D., Saxena S., Chandran M., Jonnalagadda P.S., Yadav R., Srilakshmi R.R., Perumal Y., Dharmarajan S. Design and development of Mycobacterium tuberculosis lysine ε-aminotransferase inhibitors for latent tuberculosis infection. Chem. Biol. Drug Des. 2016;87:265–274. doi: 10.1111/cbdd.12655. [DOI] [PubMed] [Google Scholar]
- 82.Reshma R.S., Jeankumar V.U., Kapoor N., Saxena S., Bobesh K.A., Vachaspathy A.R., Kolattukudy P.E., Sriram D. Mycobacterium tuberculosis lysine-ε-aminotransferase a potential target in dormancy: Benzothiazole based inhibitors. Bioorganic Med. Chem. 2017;25:2761–2771. doi: 10.1016/j.bmc.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 83.Alluri K.K., Reshma R.S., Suraparaju R., Gottapu S., Sriram D. Synthesis and evaluation of 4′,5′-dihydrospiro[piperidine-4,7′-thieno[2,3-c]pyran] analogues against both active and dormant Mycobacterium tuberculosis. Bioorganic Med. Chem. 2018;26:1462–1469. doi: 10.1016/j.bmc.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 84.Höner Zu Bentrup K., Miczak A., Swenson D.L., Russell D.G. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 1999;181:7161–7167. doi: 10.1128/JB.181.23.7161-7167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pham T.V., Murkin A., Moynihan M.M., Harris L., Tyler P.C., Shetty N., Sacchettini J.C., Huang H.-L., Meek T.D. Mechanism-based inactivator of isocitrate lyases 1 and 2 from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2017;114:7617–7622. doi: 10.1073/pnas.1706134114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKinney J.D., Höner zu Bentrup K., Muñoz-Elías E.J., Miczak A., Chen B., Chan W.T., Swenson D., Sacchettini J.C., Jacobs W.R., Jr., Russell D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 87.Muñoz-Elías E.J., McKinney J.D. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005;11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gengenbacher M., Rao S.P.S., Pethe K., Dick T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology. 2009;156:81–87. doi: 10.1099/mic.0.033084-0. [DOI] [PubMed] [Google Scholar]
- 89.Sharma V., Sharma S., Hoener zu Bentrup K., McKinney J.D., Russell D.G., Jacobs W.R., Jr., Sacchettini J.C. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol. 2000;7:663–668. doi: 10.1038/77964. [DOI] [PubMed] [Google Scholar]
- 90.Kwai B.X.C., Collins A.J., Middleditch M.J., Sperry J., Bashiri G., Leung I.K.H. Itaconate is a covalent inhibitor of the Mycobacterium tuberculosis isocitrate lyase. RSC Med. Chem. 2020;12:57–61. doi: 10.1039/D0MD00301H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhusal R.P., Bashiri G., Kwai B.X.C., Sperry J., Leung I.K.H. Targeting isocitrate lyase for the treatment of latent tuberculosis. Drug Discov. Today. 2017;22:1008–1016. doi: 10.1016/j.drudis.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 92.Krátký M., Vinšová J., Novotná E., Mandíková J., Wsól V., Trejtnar F., Ulmann V., Stolaříková J., Fernandes S., Bhat S., et al. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis. 2012;92:434–439. doi: 10.1016/j.tube.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y., Zhou S., Deng Q., Li X., Meng J., Guan Y., Li C., Xiao C. Identification of a novel inhibitor of isocitrate lyase as a potent antitubercular agent against both active and non-replicating Mycobacterium tuberculosis. Tuberculosis. 2016;97:38–46. doi: 10.1016/j.tube.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Krátký M., Janďourek O., Baranyai Z., Novotná E., Stolaříková J., Bősze S., Vinšová J. Phenolic N-monosubstituted carbamates: Antitubercular and toxicity evaluation of multi-targeting compounds. Eur. J. Med. Chem. 2019;181:111578. doi: 10.1016/j.ejmech.2019.111578. [DOI] [PubMed] [Google Scholar]
- 95.Pham T.V., Mellott D.M., Moghadamchargari Z., Chen K., Krieger I., Laganowsky A., Sacchettini J.C., Meek T.D. Covalent inactivation of Mycobacterium tuberculosis isocitrate lyase by cis-2,3-epoxy-succinic acid. ACS Chem. Biol. 2021;16:463–470. doi: 10.1021/acschembio.0c00740. [DOI] [PubMed] [Google Scholar]
- 96.Krieger I.V., Freundlich J.S., Gawandi V.B., Roberts J.P., Gawandi V.B., Sun Q., Owen J.L., Fraile M.T., Huss S.I., Lavandera J.-L., et al. Structure-Guided Discovery of Phenyl-diketo Acids as Potent Inhibitors of M. tuberculosis Malate Synthase. Chem. Biol. 2012;19:1556–1567. doi: 10.1016/j.chembiol.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puckett S., Trujillo C., Wang Z., Eoh H., Ioerger T.R., Krieger I., Sacchettini J., Schnappinger D., Rhee K.Y., Ehrt S. Glyoxylate detoxification is an essential function of malate synthase required for carbon assimilation in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2017;114:E2225–E2232. doi: 10.1073/pnas.1617655114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeong J.-A., Oh J.-I. Alanine dehydrogenases in mycobacteria. J. Microbiol. 2019;57:81–92. doi: 10.1007/s12275-019-8543-7. [DOI] [PubMed] [Google Scholar]
- 99.Giffin M.M., Shi L., Gennaro M.L., Sohaskey C.D. Role of alanine dehydrogenase of Mycobacterium tuberculosis during recovery from hypoxic nonreplicating persistence. PLoS ONE. 2016;11:e0155522. doi: 10.1371/journal.pone.0155522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saxena S., Samala G., Sridevi J.P., Devi P.B., Yogeeswari P., Sriram D. Design and development of novel Mycobacterium tuberculosis l-alanine dehydrogenase inhibitors. Eur. J. Med. Chem. 2015;92:401–414. doi: 10.1016/j.ejmech.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 101.Reshma R.S., Saxena S., Bobesh K.A., Jeankumar V.U., Gunda S., Yogeeswari P., Sriram D. Design and development of new class of Mycobacterium tuberculosis l-alanine dehydrogenase inhibitors. Bioorganic Med. Chem. 2016;24:4499–4508. doi: 10.1016/j.bmc.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 102.Samala G., Devi P.B., Saxena S., Gunda S., Yogeeswari P., Sriram D. Anti-tubercular activities of 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d ]pyrimidin-4-amine analogues endowed with high activity toward non-replicative Mycobacterium tuberculosis. Bioorganic Med. Chem. 2016;24:5556–5564. doi: 10.1016/j.bmc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 103.Schnell R., Sriram D., Schneider G. Pyridoxal-phosphate dependent mycobacterial cysteine synthases: Structure, mechanism and potential as drug targets. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2015;1854:1175–1183. doi: 10.1016/j.bbapap.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Sareen D., Newton G.L., Fahey R.C., Buchmeier N.A. Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman. J. Bacteriol. 2003;185:6736–6740. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brunner K., Maric S., Reshma R.S., Almqvist H., Seashore-Ludlow B., Gustavsson A.-L., Poyraz O., Yogeeswari P., Lundbäck T., Vallin M., et al. Inhibitors of the Cysteine Synthase CysM with Antibacterial Potency against Dormant Mycobacterium tuberculosis. J. Med. Chem. 2016;59:6848–6859. doi: 10.1021/acs.jmedchem.6b00674. [DOI] [PubMed] [Google Scholar]
- 106.Brunner K., Steiner E.M., Reshma R.S., Sriram D., Schnell R., Schneider G. Profiling of in vitro activities of urea-based inhibitors against cysteine synthases from Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2017;27:4582–4587. doi: 10.1016/j.bmcl.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 107.Becker K., Skaar E.P. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol. Rev. 2014;38:1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Djoko K.Y., Goytia M.M., Donnelly P.S., Schembri M.A., Shafer W.M., McEwan A.G. Copper(II)-Bis(Thiosemicarbazonato) Complexes as Antibacterial Agents: Insights into Their Mode of Action and Potential as Therapeutics. Antimicrob. Agents Chemother. 2015;59:6444–6453. doi: 10.1128/AAC.01289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ladomersky E., Petris M.J. Copper tolerance and virulence in bacteria. Metallomics. 2015;7:957–964. doi: 10.1039/C4MT00327F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neyrolles O., Wolschendorf F., Mitra A., Niederweis M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 2015;264:249–263. doi: 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rowland J., Niederweis M. Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload. Tuberculosis. 2012;92:202–210. doi: 10.1016/j.tube.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salina E.G., Huszár S., Zemanová J., Keruchenko J., Riabova O., Kazakova E., Grigorov A., Azhikina T., Kaprelyants A., Mikušová K., et al. Copper-related toxicity in replicating and dormant Mycobacterium tuberculosis caused by 1-hydroxy-5-R-pyridine-2(1H)-thiones. Metallomics. 2018;10:992–1002. doi: 10.1039/C8MT00067K. [DOI] [PubMed] [Google Scholar]
- 113.Macomber L., Imlay J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chillappagari S., Seubert A., Trip H., Kuipers O.P., Marahiel M.A., Miethke M. Copper Stress Affects Iron Homeostasis by Destabilizing Iron-Sulfur Cluster Formation in Bacillus subtilis. J. Bacteriol. 2010;192:2512–2524. doi: 10.1128/JB.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson M.D., Kehl-Fie T.E., Rosch J.W. Copper intoxication inhibits aerobic nucleotide synthesis in Streptococcus pneumonia. Metallomics. 2015;7:786–794. doi: 10.1039/C5MT00011D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santivañez-Veliz M., Perez-Silanes S., Torres E., Moreno-Viguri E. Design and synthesis of novel quinoxaline derivatives as potential candidates for treatment of multidrug-resistant and latent tuberculosis. Bioorganic Med. Chem. Lett. 2016;26:2188–2193. doi: 10.1016/j.bmcl.2016.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nikonenko B.V., Kornienko A., Majorov K., Ivanov P., Kondratieva T., Korotetskaya M., Apt A.S., Salina E., Velezheva V. In Vitro Activity of 3-Triazeneindoles against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 2016;60:6422–6424. doi: 10.1128/AAC.00998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karale U.B., Krishna V.S., Krishna E.V., Choudhari A.S., Shukla M., Gaikwad V.R., Mahizhaveni B., Chopra S., Misra S., Sarkar D., et al. Synthesis and biological evaluation of 2,4,5-trisubstituted thiazoles as antituberculosis agents effective against drug-resistant tuberculosis. Eur. J. Med. Chem. 2019;178:315–328. doi: 10.1016/j.ejmech.2019.05.082. [DOI] [PubMed] [Google Scholar]
- 119.Bonnett S.A., Dennison D., Files M., Bajpai A., Parish T. A class of hydrazones are active against non-replicating Mycobacterium tuberculosis. PLoS ONE. 2018;13:e0198059. doi: 10.1371/journal.pone.0198059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sirim M.M., Krishna V.S., Sriram D., Unsal Tan O. Novel benzimidazole-acrylonitrile hybrids and their derivatives: Design, synthesis and antimycobacterial activity. Eur. J. Med. Chem. 2020;188:112010. doi: 10.1016/j.ejmech.2019.112010. [DOI] [PubMed] [Google Scholar]
- 121.Monakhova N., Korduláková J., Vocat A., Egorova A., Lepioshkin A., Salina E.G., Nosek J., Repková E., Zemanová J., Jurdáková H., et al. Design and synthesis of pyrano[3,2-b]indolones showing antimycobacterial activity. ACS Infect. Dis. 2021;7:88–100. doi: 10.1021/acsinfecdis.0c00622. [DOI] [PubMed] [Google Scholar]
- 122.Rather M.A., Bhat Z.S., Lone A.M., Maqbool M., Bhat B.A., Ahmad Z. In vitro potency of 2-(((2-hydroxyphenyl)amino)methylene)-5,5-dimethylcyclohexane-1,3-dione against drug-resistant and non-replicating persisters of Mycobacterium tuberculosis. J. Glob. Antimicrob. Resist. 2021;25:202–208. doi: 10.1016/j.jgar.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 123.Krishna V.S., Zheng S., Rekha E.M., Nallangi R., Sai Prasad D.V., George S.E., Guddat L.W., Sriram D. Design and development of ((4-methoxyphenyl)carbamoyl) (5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-yl)amide analogues as Mycobacterium tuberculosis ketol-acid reductoisomerase inhibitors. Eur. J. Med. Chem. 2020;193:112178. doi: 10.1016/j.ejmech.2020.112178. [DOI] [PubMed] [Google Scholar]
- 124.Konduri S., Bhargavi D., Prashanth J., Krishna V.S., Sriram D., Rao K.P. Design and Synthesis of “Chloropicolinate Amides and Urea Derivatives” as Novel Inhibitors for Mycobacterium tuberculosis. ACS Omega. 2021;6:1657–1667. doi: 10.1021/acsomega.0c05690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Konduri S., Prashanth J., Krishna V.S., Sriram D., Behera J.N., Siegel D., Rao K.P. Design and synthesis of purine connected piperazine derivatives as novel inhibitors of Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2020;30:127512. doi: 10.1016/j.bmcl.2020.127512. [DOI] [PubMed] [Google Scholar]
- 126.Verma J., Subbarao N. Designing novel inhibitors against cyclopropane mycolic acid synthase 3 (PcaA): Targeting dormant state of Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2021;39:6339–6354. doi: 10.1080/07391102.2020.1797534. [DOI] [PubMed] [Google Scholar]
- 127.Hatherill M., White R.G., Hawn T.R. Clinical Development of New TB Vaccines: Recent Advances and Next Steps. Front. Microbiol. 2020;10:3154. doi: 10.3389/fmicb.2019.03154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E.M., Rodrigues L.C., Smith P., Lipman M., Whiting P., et al. Protection by BCG Vaccine against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin. Infect. Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 129.Tait D.R., Hatherill M., Van Der Meeren O., Ginsberg A.M., Van Brakel E., Salaun B., Scriba T.J., Akite E.J., Ayles H.M., Bollaerts A., et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2019;381:2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 130.Guerrero-Bustamante C.A., Dedrick R.M., Garlena R.A., Russell D.A., Hatfull G.F. Toward a phage cocktail for tuberculosis: Susceptibility and tuberculocidal action of mycobacteriophages against diverse Mycobacterium tuberculosis strains. mBio. 2021;12:e00973-21. doi: 10.1128/mBio.00973-21. [DOI] [PMC free article] [PubMed] [Google Scholar]