Abstract
Fungal contamination of food, especially by mycotoxigenic fungi, not only reduces the quality of the food, but can also cause serious diseases, thus posing a major food safety challenge to humans. Apart from sound food control systems, there is also a continual need to explore antifungal agents that can inhibit fungal growth and mycotoxin production in food. Many types of fatty acids (FAs) and their oxidized derivatives, oxylipins, have been found to exhibit such effects. In this review, we provide an update on the most recent literature on the occurrence and formation of FAs and oxylipins in food, their effects on fungal growth and mycotoxin synthesis, as well as the genetic and molecular mechanisms of actions. Research gaps in the field and needs for further studies in order to realizing the potential of FAs and oxylipins as natural antifungal preservatives in food are also discussed.
Keywords: fatty acids, oxylipins, antifungal, anti-mycotoxin
1. Introduction
Fungal contamination of agricultural and food products is a major cause of food spoilage, which leads to food wastage and substantial economic losses [1]. Fungi are opportunistic organisms that can contaminate foods easily through their spores in the air if the hygiene of the food processing and storage environments is substandard or the air quality not properly controlled [2]. Fungal contamination of foods can also pose a serious health issue due to the production of mycotoxins by certain fungal species. Mycotoxins are secondary metabolites produced by several fungal genera, which can cause a range of adverse health effects on humans, including carcinogenesis, mutagenicity, nephrotoxicity, teratogenicity, neurotoxicity, and immunosuppression [3]. Preventing fungal growth and mycotoxin biosynthesis are important topics in food safety research.
There are a number of strategies, including physical, chemical, and biological methods, that can be used alone or in combination to prevent fungal growth and mycotoxin production [3]. However, most of these methods involve high equipment and high energy costs, or may reduce food quality to some extent, which limits their application in the food industry. Therefore, there is a continual need to explore new and more economical methods, such as natural antifungal agents, for the control of fungal growth and toxin production in foods. Oils (especially nano-essential oils) are new strategies to solve the above problems, not only for being of natural origin, but also because of having proven preservative efficacy against mycotoxin production. However, the high cost of essential oils and the controversy in biosafety have restricted its practical application [4,5,6]. In recent years, fatty acids (FAs) and their derivatives, oxylipins, have emerged as potential natural antifungal agents. FAs are important cellular molecules that are associated with cell membrane structure, energy storage, regulation of inflammatory processes, and various signaling pathways [7,8,9,10]. As components of fats and oils, they are widely present in many foods. A large number of FAs are found to have fungicidal or fungistatic properties and are used as antifungal agents in agriculture and medicines [11,12]. Oxylipins are a family of secondary metabolites derived from the oxidation or further transformation of polyunsaturated fatty acids (PUFAs). They are widely present in organisms from all the kingdoms of nature [13,14]. Oxylipins are found to have a strong impact on fungal development, reproduction, synthesis of secondary metabolites (including mycotoxins), and adaptive responses [14].
Here, we review the structure and formation of FAs and oxylipins in food, their inhibitory effects on fungal growth, and mycotoxin synthesis, as well as the mechanisms of their antimicrobial actions. We also discuss the challenges associated with the exploration of PUFAs and oxylipins as antifungal and anti-mycotoxin agents in the food industry and the research needed to address these challenges.
2. Effects of FAs on the Regulation of Fungal Growth and Mycotoxin Synthesis
Lipids are important physiological molecules related to cellular energy storage (e.g., adipose tissue), membrane structure (phospholipid bilayer), and various signaling pathways [15]. They are also found to be linked with mycotoxin production in foods. It is reported that when A. parasiticus contaminated crop seeds with different lipid contents such as peanut, paddy, sorghum, cowpea, and green gram, it produced the greatest amount of AFB1 in peanut, which has the highest level of lipids. Furthermore, A. parasiticus colonies were larger on powdered seed material than on the defatted samples of the same material [16]. Research has also shown that A. flavus and A. parasiticus produced greater amounts of aflatoxins on oil seeds or the lipid-rich tissues of seeds than on starch rich materials [17,18,19]. Similarly, Rajasekaran et al. [20] found that fungal spread and aflatoxin production in infected cottonseed were closely related to lipid accumulation in the seed, and the seed lipids played an important role in regulating the growth of A. flavus and A. parasiticus and the production of aflatoxins. In addition, A. ochraceus has been shown to infect peanuts and soybeans, and produce more ochratoxin A (OTA) on these high fat content seeds than on corn and wheat [21].
FAs can also regulate the growth and secondary metabolism of fungi; however, the effects are dependent on both the type and concentration of FAs. For example, Gupta et al. [22]. found that when lauric acid was added to a liquid medium for A. parasitica, aflatoxin synthesis by the fungus was significantly promoted, while myristic, palmitic, stearic, and oleic acids were less effective. Table 1 lists various FAs that have been shown to regulate fungal growth and mycotoxin production. In general, the antifungal efficiency of an FA is related to the structure and concentration of the FA. For example, the addition of 10 mM oleic acid promoted aflatoxin synthesis, while at 150 mM, it inhibited its synthesis in vitro. Furthermore, 50 mM oleic acid was found to promote A. parasiticus growth but inhibit aflatoxin synthesis; however, linoleic acid had the opposite effect at the same concentration [23].
Table 1.
Effect of fatty acids in fungal development and mycotoxin production.
| Fatty Acid | Concentration | Fungal Species | Fungal Growth | Mycotoxin Production | Ref. |
|---|---|---|---|---|---|
| lauric acid | 50.00 mM | Aspergillus parasiticus | + | − AF | [23] |
| myristic acid | + | + AF | |||
| palmitic acid | − | − AF | |||
| oleic acid | + | − AF | |||
| linoleic acid | − | + AF | |||
| lauric acid | 2.50 mM | Aspergillus niger | − | u | [24] |
| capric acid | 0.60 mM | Aspergillus fumigatus | − | u | [25] |
| Aspergillus nidulans | − | u | |||
| 0.30 mM | Penicillium commune | − | u | ||
| 0.15 mM | Penicillium roqueforti | − | u | ||
| lauric acid | 0.20 mM | Aspergillus niger | − | u | [26] |
| myristic acid | 0.09 mM | Penicillium glabrum | − | u | |
| 0.13 mM | Aspergillus niger | + | u | ||
| 0.13 mM | Penicillium italicum | − | u | ||
| myristoleic acid | 0.09 mM | Aspergillus niger | − | u | |
| 0.13 mM | Penicillium italicum | − | u | ||
| palmitic acid | 12.00 mM | Botrytis cinerea | − | u | [27] |
| stearic acid | 0.10 mM | Aspergillus flavus | u | + AF | [28] |
| linolenic acid | 1.25 mM | u | − AF |
u: unknown; +: stimulate; −: inhibit; AF; aflatoxin.
3. Roles of Oxylipins in Regulating Fungal Growth and Mycotoxin Production
Oxylipins are a family of secondary metabolites derived from the oxidation or further transformation of polyunsaturated fatty acids (PUFAs). They are widely present in all organisms and play a significant role in their physiology [13,14]. For fungi, oxylipins are involved in the regulation of cell development, reproduction, synthesis of secondary metabolites (including mycotoxins), and adaptive responses [14]. Oxylipin can also be used as a natural preservative in food to control fungal growth and subsequent mycotoxin production. Lipidomics studies have shown that FA oxidation products, especially FA hydroperoxides, have a significant impact on the accumulation of fumonisin [29]. There is evidence that monohydroxy octadecenoic acid exerts antifungal activity in bread [30]. In particular, it has been shown that 13S-hydroperoxy-octadecadienoic acid (13S-HPODE) and 13S-hydroperoxy-octadecatrienoic acid (13S-HPOTE) inhibit aflatoxin production, while 9S-hydroperoxy-octadecadienoic acid (9S-HPODE) promotes it [31]. Similar to FAs, the effect of oxylipins on fungal growth and mycotoxin synthesis also depends on their structure and concentration (Table 2). Adding 9S-hydroxy-octadecadienoic acid (9S-HODE) to potato agar medium was found to inhibit the formation of conidia of A. ochraceus and promote the synthesis of OTA, while 13S-hydroxy-octadecadienoic acid (13S-HODE) at the same concentration had the opposite effect [21,32]. Another study found that mono- and di-unsaturated FAs with hydroxylation at position 9, 10, 12, or 13 exhibited similar fungal static activity, while unsaturated FAs with hydroxylation at position 2 or 18 appeared to have lower antifungal activity against P. roqueforti and A. niger [33]. Overall, the results of those study indicated that fungi and mycotoxins in foods can be controlled by adding oxylipin to foods or by modifying processing technology to regulate the formation of certain oxylipins in foods. The relationship between the structure and concentration of oxylipins and their antifungal activities provides an avenue for their application in foods for controlling fungal growth and mycotoxin production. However, the situation is complicated by the fact that a specific oxylipin may have different effects on different fungal species. For example, 13S-HPODE increases aflatoxin synthesis by A. parasiticus [34], whereas the same oxylipin decreases aflatoxin production by F. verticillioides [35]. This may prevent the selection of oxylipin with a wide spectrum antifungal application.
Table 2.
Proposed oxylipins with anti-fungal development and anti-mycotoxin production activities.
| Qxylipin | Host Source | Fungal Species | Fungal Growth | Mycotoxin Production | Ref. |
|---|---|---|---|---|---|
| 13S-HPODE | c.a | Aspergillus flavus; Aspergillus parasiticus | u | − AF | [34] |
| peanut | Aspergillus flavus; Fusarium verticillioides | + | + AF | [35] | |
| 13S-HODE | c.a | Aspergillus ochraceus | + | − OTA | [32] |
| 9S-HPODE | soybean | Aspergillus flavus; Aspergillus nidulans | +/− | − AF/ST | [31] |
| corn | Aspergillus flavus; Fusarium verticillioides | + | + AF | [36] | |
| 9S-HOD(T)E | corn | Fusarium verticillioides | n | + AFB1 | [37] |
| 9-HODE | wheat | Fusarium graminearum | u | + DON | [38] |
| 9-HPODE | |||||
| 9S-HODE | c.a | Aspergillus ochraceus | − | + OTA | [21] |
| 9-HODE | maize | Fusarium verticillioides | u | + FB1 | [39] |
| 10-HODE | |||||
| 1-octen-3-ol | c.a | Penicillium paneum | − | u | [40] |
| c.a | Aspergillus nidulans | − | u | [41] | |
| Aspergillus oryzae | Aspergillus flavus | − | + AFB1 | [42] | |
| MeJA | c.a | Aspergillus parasiticus | − | − AF | [43] |
c.a: commercially available; u: unknown; +: stimulate; −: inhibit; n: no effect; AF: aflatoxin; AFB1: aflatoxin B1; FB1: fumonisin B1; ST: sterigmatocystin; OTA: ochratoxin A; DON: deoxynivalenol. 13S-HPODE: 13S-hydroperoxy-octadecadienoic acid; 9S-HPODE: 9S-hydroperoxy-octadecadienoic acid; 13S-HODE: 13S-hydroxy-octadecadienoic acid; 9S-HOD(T)E: 9S-hydroxy-octadecadi(tri)enoic acid; 9-HODE: 9-Hydroxy-octadecadienoic acids; 10-HODE: 10-Hydroxy-octadecadienoic acids; 9-HPODE: 9-hydroperoxy-octadecadienoic acids; MeJA: methyl jasmonate.
4. Pathways of Oxylipin Formation
Oxylipins, which are ubiquitous in all organisms, are the metabolites of PUFAs such as arachidonic acid (ARA), linoleic acid (LA), α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), formed by enzyme and non-enzyme oxidation of these FAs. Non-enzymatic pathways for the formation of oxylipins can occur through free radical-mediated oxidation of reactive oxygen species (ROS) [15,44,45]. ROS—mediated reaction can produce hydrogen peroxides from PUFAs, which are then rapidly reduced to a mixture of monohydroxy PUFAs [46]. The three main enzymes involved in their formation are lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome P450 (CYP). LOX is reported to catalyze the formation of hydroperoxide from PUFAs with stereospecificity, and then convert them into monohydroxy PUFA (e.g., hydroxy-octadecadienoic, hydroxy-pentaenoic, and hydroxyl-docosahexaenoic acids), which can be further oxidized to corresponding ketones (e.g., keto-octadecadienoic acid, keto-eicosatetraenoic acid) [15,47,48]. CYP has ω-hydroxylation and cyclooxygenase activities. CYP cyclooxygenase catalyzes the formation of epoxide (e.g., epoxyeicosatrienoic, epoxy eicosatetraenoic, and epoxy docosapentaenoic acids), while CYP hydroxylase catalyzes the formation of medium-chain hydroxide and ω-hydroxylation (e.g., 20-hydroxypentaenoic and 18-hydroxyeicosapentaenoic acids) [15,47,49]. COX enzyme converts AA and EPA into peroxides hydroperoxide-endoperoxide prostaglandin G2 and peroxides hydroperoxide-endoperoxide prostaglandin G3 [15].
Plant oxylipins act as signals to modulate fungal developmental processes, including sporogenesis and biosynthesis of mycotoxins. These oxylipins are primarily derived from linolenic (C18:3 n-3), linoleic (C18:2 n-3), and hexadecatrienoic (C16:3) acids [50]. Over the last decade, understanding of the biosynthesis, metabolism, and regulation of plant oxylipins, especially jasmonate, has improved [13,50,51,52]. To some extent, plant oxylipins are recognized as part of the plant defense mechanisms against pathogenic attack. Compared with higher plants and mammals, the characteristics of fungal oxylipin synthesis are not yet comprehensively understood. Fungal oxylipin synthases, primarily including dioxygenases, lipoxygenase, and monooxygenase, are believed to mediate ω-oxidation, while multifunctional β-oxidase and hydroperoxide lyase are involved in the formation of volatile compounds. These oxylipin synthases catalyze C18 PUFA to form various compounds via multiple pathways in fungi (for a review see reference [14]).
5. Mechanisms Underlying the Antifungal Actions of FAs and Oxylipins
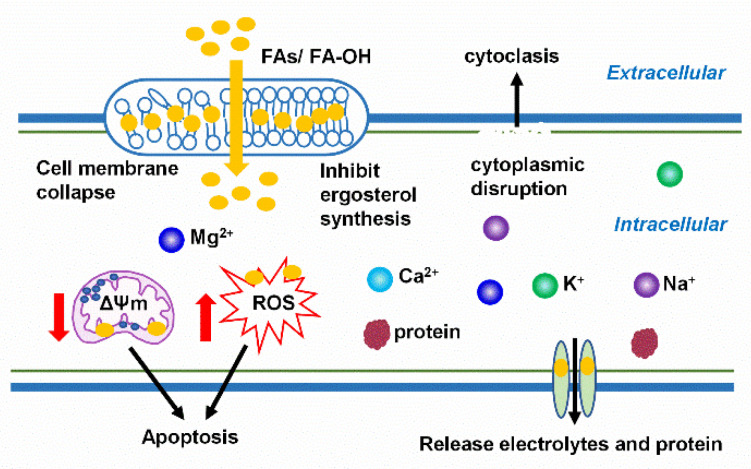
With regard to the antifungal mechanisms of FAs and oxylipins, it appears that the most important target of antifungal actions of FAs is the cell membrane (Figure 1). Avis and Bélange [53] reported that the general antifungal mechanism of FAs involves direct interactions with fungal cell membrane. Antifungal FAs insert themselves into the lipid bilayer of the fungal membrane and physically interfere with the membrane, resulting in an increase in the fluidity of the membrane. The increase in membrane fluidity causes general disruption of the cell membrane, which leads to conformational changes in membrane proteins, release of intracellular components, cytoplasmic disruption, and ultimately, cell disintegration. Vuyisile et al. [54] proposed that certain PUFAs increase the unsaturation index of the cellular membrane and accumulation of intracellular ROS in C. albicans and C. dubliniensis. These authors also found that C18:4 n-3, in particular, could cause apoptosis, probably by causing an increase in ROS production and loss of mitochondrial transmembrane potential (ΔΨm). Hydroxy FAs penetrate through fungal membrane bilayers to increase membrane permeability and release intracellular electrolytes and proteins [25,53,55]. However, monohydroxy unsaturated FAs with -OH in C9–C13 position inhibit the growth of the food spoilage fungi P. roqueforti and A. niger, indicating that fungal resistance to monohydroxy unsaturated fatty acids (HUFA) is related to the sterol content of the fungi biomass [33]. This suggests that food components that affect fungal sterol synthesis may enhance the antifungal activity of FAs and oxylipins.
Figure 1.
Antifungal mechanisms of free fatty acids and hydroxy fatty acids. Free fatty acids or hydroxy fatty acids (FAs/FA-OH) insert themselves into the lipid bilayer of the fungal membrane and result in general disruption of the cell membrane, low sterol content, release of intracellular components, cytoplasmic disruption, and ultimately, cell disintegration; they also cause an increase in ROS production and loss of mitochondrial transmembrane potential (ΔΨm) and ultimately, apoptosis.
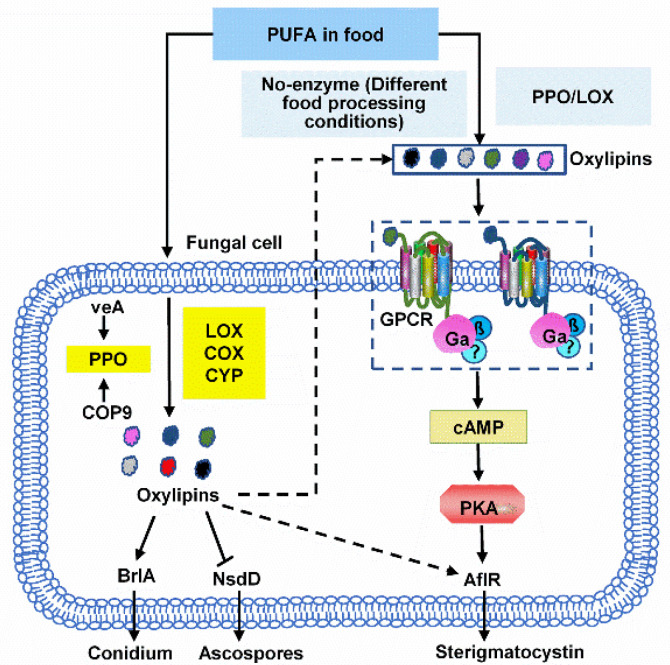
PUFAs have also been shown to affect fungal reproduction. Fungal oxygenase catalyzed intracellular and extracellular PUFA to form oxylipins, which has been shown to play a significant role in regulating the growth of sexual and asexual fungal spores (conidia). In A. nidulans, the precocious sexual inducer (psi) factor is the first extracellular C18 PUFA derived oxylipin signal found to regulate spore development [56]. Psi-factor biosynthetic genes, named PPO (psi-producing oxygenases), are also involved in regulating fungal spore development and mycotoxin synthesis. Overexpression of ppoA results in a six-fold reduction in the ratio of asexual to sexual spore development [57]. Asexual development is significantly delayed and reduced in double ∆ppoA and ∆ppoC and triple ∆ppoA, ∆ppoB, and ∆ppoC mutants, accompanied by a premature increase in ascospore production [58]. In these processes, the light sensor protein VeA and the protein degradation machinery COP9 are involved in the regulation of the PPO genes [59,60]. The cleistothecial (NsdD)-specific and conidiophore (BrlA)-specific transcription factors regulate the sporulation program of the fungus [61,62] (Figure 2). In the IRT4 strain, in which the dioxygenase genes (ppoA, ppoB, ppoC and ppoD) and one lipoxygenase gene (loxA) are downregulated, A. flavus was reported to change from asexual development to sclerotium production, but aflatoxin synthesis increased [63,64], indicating that the bioactive lipoproteins produced by PPO and LOX enzymes play a key role in regulating spore production and mycotoxin biosynthesis. Another study reported that the lipoxygenase-deficient A. ochraceous produced lower levels of linoleic acid-derived 13S-HPODE and displayed remarkably lower ochratoxin production, delayed conidia formation, and increased sclerotia production [65]. Moreover, mutation in a F. sporotrichioides PPO orthologue was found to reduce T-2 toxin production [66]. These findings demonstrate that it is possible to control the growth of fungi and the synthesis of mycotoxins by regulating the activity of PPO and LOX or the concentrations of oxylipins.
Figure 2.
Hypothetical model of the regulation of fungal growth and toxin synthesis signal transmission in Aspergillus by oxylipins. Highlighted in yellow and blue are the fungal and food oxylipin biosynthetic enzymes. Polyunsaturated fatty acid substrates in food are processed by fungal-secreted lipoxygenase for oxylipin production. Fungi can sense and exploit oxylipins from food substrates to regulate GPCR-, cAMP/PKA- and Ppo-mediated growth, sporulation, and mycotoxin production. In these processes, the light sensor protein VeA and the protein degradation machinery COP9 are involved in the regulation of the PPO genes. Oxylipins regulate sporulation through cleistothecial (NsdD)- and conidiophore (BrlA)-specific transcription factors, as well as secondary metabolism (e.g., sterigmatocystin) through AflR.
Oxylipins may also influence fungal growth and mycotoxin synthesis through their effect on G protein (GTP hydrolyzing proteins). G protein signaling has been shown to regulate the synthesis of aflatoxin and its precursor, sterigmatocystin (ST). The Gα subunit FadA and its RGS FlbA are part of an adenylate cyclase/cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) (PkaA) pathway, which controls ST production in A. nidulans via transcriptional and posttranscriptional regulation of aflR [67,68,69]. In recent years, studies have shown that oxylipins, as ligands, are sensed by G-protein coupled receptors (GPCRs) to regulate physiological processes in Aspergillus [70,71,72]. Affeldt and colleagues [70] presented evidence indicating that oxylipins stimulate a burst in cAMP, a downstream event of GPCR activation in A. nidulans, and that the GPCR mutant ΔgprD prevents the cAMP accumulation. Therefore, G protein responds to oxylipin signaling molecules and activates the cAMP/PKA pathway to regulate the growth and secondary metabolism of Aspergillus (Figure 2). In addition to Aspergillus, GPCRs encoded by various fungi, including Magnaporthe grisea, Cryptococcus neoformans, Neurospora crassa, Verticillium spp., and Trichoderma spp., have also been identified [73], but it is not clear whether FAs and their oxygenates activate intracellular signaling pathways through GPCRs to regulate toxin synthesis. The G protein coupled signaling pathway could serve as an important target for controlling fungal growth and its secondary metabolites. For instance, oxylipin receptors could represent potentially rich targets for antifungal agent development.
6. Oxylipins in Foods
Measurement of lipid oxidation is a common quality control practice in the food industry for foods with a high level of fats or oil. Commonly used parameters include peroxide value (POV) and thiobarbituric acid reactive substances (TBARS), which represent the hydroperoxide value and the secondary oxidation products of lipids and fatty acids, respectively. However, neither of these measures provides precise information about specific oxidation products, including oxylipins. At present, there are only a few reports on the concentration of oxylipins, and the effects of different processing conditions on their formation in food. Mechanically deboned meat produces a large amount of 9,10,13-trihydroxy-11-octadecenoic acid (9,10,13-THODE) during storage, and 9,10,13-THODE can be used as a marker for the presence of mechanically deboned meat in various minced meat products [74]. During the processing of Chinese sausages, LOX-catalyzed lipid oxidation occurs mainly during the curing stage and the early stages of drying. As the oxidation process progresses, 3-HODE, 9-HODE, 9,10-dihydroxyoctadecenoic acid (9,10-DHODE), and 9,10,13-THODE levels continue to increase. The oxidation catalyzed by LOX is replaced by non-enzymatic oxidation in the later drying stage [75]. Heat treatment reduces the content of oxylipins in milk, such as the concentrations of linoleic acid derivatives 9,10-dihydroxyoctadecenoic acid (9,10-DiHOME), 13-HODE, and 9-HODE. Furthermore, the concentrations of 13-hydroxyoctadecatrienoic acid (13-HOTrE) and 9-HOTrE of α-linolenic acid after high-temperature short-time and ultra-high temperature treatments are lower than those in raw and pasteurized milk. Compared with POV and TBARS, the concentration of 12,13-DiHOME is reduced by 50% during the heat treatment of milk, which can be used as a sensitive marker of early oxidation of lipids [76]. Therefore, changes in the concentrations of certain oxylipins in food are more representative of the effect of processing conditions on lipid oxidation than POV and TBARS values.
Numerous studies support that oxylipins produced by plants or fungi are important molecules regulating fungal growth and secondary metabolism. Therefore, oxylipins formed in food can also be used as antifungal and anti-mycotoxin agents in the food system. For instance, Gao et al. [21] observed that the accumulation of 13S-HODE inhibited the production of OTA in soybean culture medium, but its mechanism is unclear. Understanding of the formation characteristics of oxylipins not only can provide a better understanding of the lipid oxidation process in food, but can also enable exploration of their application for the control of fungal growth and mycotoxin production in food. Furthermore, the actual application of oxylipins in foods requires a detailed understanding of its different antifungal and anti-mycotoxin mechanisms, as each oxylipin content may have different action sites.
7. Conclusions and Remarks
In this review, we summarized current literature on the effect of FAs and oxylipins on fungal growth and mycotoxin synthesis. Studies on their mechanisms of action indicate that cell membrane, specific enzymes, and metabolic pathways are the targets of these compounds. The antifungal and anti-mycotoxin activities of FAs and oxylipins may provide new strategies for the prevention and control of fungal growth and mycotoxin production in food. However, to date, there are only a limited number of studies that have explored their potential in food applications. Several research questions need to be addressed before such potential can be realized. First, many antifungal and anti-mycotoxin compounds have shown high activities in model systems, but their efficacy in complex food matrices are poor. Understanding of the formation characteristics of oxylipins is helpful to improve their efficacy in real food systems. Second, the antifungal and anti-mycotoxin properties of FAs and oxylipins are highly dependent on their concentrations. Research is needed to screen FA and oxylipins with wide spectrum antifungal and anti-mycotoxin activity. Third, the stability of highly reactive FAs and oxylipins in different foods and under various processing and storage conditions require further investigation. Some antifungal hydroxyl FAs have been found to be heat stable in bread making [30]. For this, nanoencapsulation improves efficacy and stability of FAs and oxylipins. Fourth, FAs and their derivatives are odorous and can affect the sensory properties of food; these needs to be taken into account when considering using these compounds in foods. Finally, there is a lack of knowledge regarding the toxicity characteristics of various oxylipins. The safety of these compounds must be ascertained by acute oral toxicity in mice before they can be used as food additives.
Author Contributions
Conceptualization, L.S.; resources, L.S.; writing—original draft preparation, M.Q., Y.W. and J.Z.; data curation, M.Q. and Q.D.; writing—review and editing, M.Q., L.S., Y.W. and J.Z.; supervision, L.S. and Y.W.; project administration, L.S. and Y.W.; funding acquisition, L.S., Y.W. and Q.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (NSFC) [31871898], Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams [2021KJ149, 2021KJ151], Characteristic innovation project of colleges and universities in Guangdong Province [2020KQNCX025], Zhanjiang Competitive Allocation Project of Science and Technology Development Special Fund [200915134541577, 2020A03009] and Guangdong Provincial key research and development program [2021B0202060001].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
In this work, we summarized fatty acids and oxylipins are used as natural preservatives in food to control fungal growth and mycotoxin production. The better understanding on the mode of action of antifungal fatty acids and oxylipin provides important insights on their applications in foods.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garnier L., Penland M., Thierry A., Maillard M.B., Jardin J., Coton M., Leyva-Salas M., Coton E., Valence F., Mounier J. Antifungal activity of fermented dairy ingredients: Identification of antifungal compounds. Int. J. Food Microbiol. 2020;322:108574. doi: 10.1016/j.ijfoodmicro.2020.108574. [DOI] [PubMed] [Google Scholar]
- 2.Faizan A.S., Bowen Y., Fengwei T., Jianxin Z., Hao Z., Wei C. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019;18:1403–1436. doi: 10.1111/1541-4337.12481. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y., Yamdeu J.G., Gong Y., Caroline O. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020;19:1521–1560. doi: 10.1111/1541-4337.12562. [DOI] [PubMed] [Google Scholar]
- 4.Liao W., Badri W., Dumas E., Ghnimi S., Elaissari A., Saurel R., Gharsallaoui A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Appl. Sci. 2021;11:5778. doi: 10.3390/app11135778. [DOI] [Google Scholar]
- 5.Maurya A., Prasad J., Das S., Dwivedy A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021;5:133. doi: 10.3389/fsufs.2021.653420. [DOI] [Google Scholar]
- 6.Chaudhari A.K., Singh V.K., Das S., Dubey N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food. Chem. Toxicol. 2021;149:112019. doi: 10.1016/j.fct.2021.112019. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Ruan W., Li J., Xu H., Wang J., Gao Y., Wang J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia. 2008;166:93–102. doi: 10.1007/s11046-008-9124-1. [DOI] [PubMed] [Google Scholar]
- 8.Christinat N., Morin-Rivron D., Masoodi M. High-throughput quantitative lipidomics analysis of nonesterified fatty acids in human plasma. Proteome Res. 2016;15:2228–2235. doi: 10.1021/acs.jproteome.6b00198. [DOI] [PubMed] [Google Scholar]
- 9.Corrêa-Oliveira R., Fachi J.L., Vieira A., Sato F.T., Vinolo M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016;5:73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura I., Ichimura A., Ohue-Kitano R., Igarashi M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020;100:171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 11.Walters D., Raynor L., Mitchell A., Walker R., Walker K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia. 2004;157:87–90. doi: 10.1023/B:MYCO.0000012222.68156.2c. [DOI] [PubMed] [Google Scholar]
- 12.Clément M., Tremblay J., Lange M., Thibodeau J., Belhumeur P. Purification and Identification of Bovine Cheese Whey Fatty Acids Exhibiting In Vitro Antifungal Activity. J. Dairy Sci. 2008;91:2535–2544. doi: 10.3168/jds.2007-0806. [DOI] [PubMed] [Google Scholar]
- 13.Dimitrios I.T., Nancy P.K. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007;15:109–118. doi: 10.1016/j.tim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Gessler N.N., Filippovich Y.S., Bachurina G.P., Kharchenko E.A., Groza N.V., Belozerskaya T.A. Oxylipins and Oxylipin Synthesis Pathways in Fungi. Appl. Biochem. Microbiol. 2017;53:628–639. doi: 10.1134/S0003683817060060. [DOI] [Google Scholar]
- 15.Gabbs M., Leng S., Devassy J.G., Monirujjaman M., Aukema H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. Am. Int. Rev. J. 2015;6:513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy M.J., Shetty H.S., Fanelli C., Lacey J. Role of seed lipids in Aspergillus parasiticus growth and aflatoxin production. J. Sci. Food Agr. 1992;59:177–181. doi: 10.1002/jsfa.2740590207. [DOI] [Google Scholar]
- 17.Brown R.L., Cotty P.J., Cleveland T.E., Widstrom N.W. Living maize embryo influences accumulation of aflatoxin in maize kernels. J. Food Protect. 1993;56:967–971. doi: 10.4315/0362-028X-56.11.967. [DOI] [PubMed] [Google Scholar]
- 18.Keller N.P., Butchko R.A.E., Sarr B., Phillips T.D. A visual pattern of mycotoxin production in maize kernels by Aspergillus spp. Phytopathology. 1994;84:483–488. doi: 10.1094/Phyto-84-483. [DOI] [Google Scholar]
- 19.Mellon J.E., Dowd M.K., Cotty P.J. Substrate Utilization by Aspergillus flavus in Inoculated Whole Corn Kernels and Isolated Tissues. J. Agric. Food Chem. 2005;53:2351–2357. doi: 10.1021/jf040276g. [DOI] [PubMed] [Google Scholar]
- 20.Rajasekaran K., Ford G., Sethumadhavan K., Carter-Wientjes C., Bland J., Cao H., Bhatnagar D. Aspergillus flavus growth and aflatoxin production as influenced by total lipid content during growth and development of cottonseed. J. Crop. Improv. 2017;31:91–99. doi: 10.1080/15427528.2016.1263811. [DOI] [Google Scholar]
- 21.Gao J., Li C., Li K., Peng M., Liang Z. Effects of Oxylipins on Spore Production and Ochratoxin A Synthesis of Aspergillus ochraceus and Grain Infection. Food Sci. 2019;40:116–121. doi: 10.7506/spkx1002-6630-20180319-239. (In Chinese with English abstract) [DOI] [Google Scholar]
- 22.Gupta S.R., Prasanna H.R., Viswanathan L., Venkitasubramanian T.A. Carboxylic acids as carbon sources for aflatoxin production. Experientia. 1974;30:1244–1246. doi: 10.1007/BF01945162. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari R.P., Mittal V., Singh G., Bhalla T.C., Saini S.S., Vadehra D.V. Effect of fatty acids on aflatoxin production by Aspergillus parasiticus. Folia Microbiol. 1986;31:120–123. doi: 10.1007/BF02926829. [DOI] [PubMed] [Google Scholar]
- 24.Řiháková Z., Plocková M., Filip V. Antifungal activity of lauric acid derivatives against Aspergillus Niger. Eur. Food Res. Technol. 2001;213:488–490. doi: 10.1007/s002170100416. [DOI] [Google Scholar]
- 25.Sjögren J., Magnusson J., Broberg A., Schnürer J., Kenne L. Antifungal 3-Hydroxy Fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbio. 2003;69:7554–7557. doi: 10.1128/AEM.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altieri C., Cardillo D., Bevilacqua A., Singaglia M. Inhibition of Aspergillus spp. and Penicillium spp. by fatty acids and their monoglycerides. J. Food Protect. 2007;70:1206–1212. doi: 10.4315/0362-028X-70.5.1206. [DOI] [PubMed] [Google Scholar]
- 27.Leyva M.O., Vicedo B., Finiti I., Del Amo G., Real M.D., García-Agustin P., González-Bosch C. Preventative and post-infection control of Botrytis cinerea in tomato plants by hexanoic acid. Plant Physiol. 2008;57:1038–1046. doi: 10.1111/j.1365-3059.2008.01891.x. [DOI] [Google Scholar]
- 28.Yan S., Liang Y., Zhang J., Chen Z., Liu C. Autoxidated linolenic acid inhibits aflatoxin biosynthesis in Aspergillus flavus via oxylipin species. Fungal Genet. Biol. 2015;81:229–237. doi: 10.1016/j.fgb.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Righetti L., Lucini L., Giorni P., Locatelli S., Dall’Asta C., Battilani P. Lipids as key markers in maize response to fumonisin accumulation. J. Agric. Food Chem. 2019;67:4064–4070. doi: 10.1021/acs.jafc.8b06316. [DOI] [PubMed] [Google Scholar]
- 30.Black B.A., Zannini E., Curtis J.M., Gänzle M.G. Antifungal hydroxy fatty acids produced during sourdough fermentation: Microbial and enzymatic pathways, and antifungal activity in bread. Appl. Environ. Microbiol. 2013;79:1866–1873. doi: 10.1128/AEM.03784-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burrow G.B., NesbilttI T.C., Dunlap J., Keller N.P. Seed lipoxygenase products modulate Aspergillus mycotoxin biosynthesis. Mol. Plant Microbe Interact. 1997;10:380–387. doi: 10.1094/MPMI.1997.10.3.380. [DOI] [Google Scholar]
- 32.Gao J., Liang Z. Effect of oxylipins on ochratoxin A production by Aspergillus ochraceus in soybean culture medium. Microbiol. China. 2020;47:1721–1729. doi: 10.13344/j.microbiol.china.190812. [DOI] [Google Scholar]
- 33.Liang N., Dacko A., Tan A.K., Xiang S., Curtis J.M., Gänzle M.G. Structure-function relationships of antifungal monohydroxy unsaturated fatty acids (HUFA) of plant and bacterial origin. Food Res. Internat. 2020;134:109237. doi: 10.1016/j.foodres.2020.109237. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri A.A., Fanelli C., Panfili G., Passi S., Fasella P. Lipoperoxidation and aflatoxin biosynthesis by Aspergillus parasiticus and Aspergillus flavus. J. Gen. Appl. Microbiol. 1983;129:3447–3452. doi: 10.1099/00221287-129-11-3447. [DOI] [Google Scholar]
- 35.Tsitsigiannis D.I., Kunze S., Willis D.K., Feussner I., Keller N.P. Aspergillus infection inhibits the expression of peanut 13S-HPODE-forming seed lipoxygenases. Mol. Plant Microbe Interact. 2005;18:1081–1089. doi: 10.1094/MPMI-18-1081. [DOI] [PubMed] [Google Scholar]
- 36.Wilson R.A., Gardner H.W., Keller N.P. Cultivar dependent expression of a maize lipoxygenase responsive to seed infesting fungi. Mol. Plant Microbe Interact. 2001;14:980–987. doi: 10.1094/MPMI.2001.14.8.980. [DOI] [PubMed] [Google Scholar]
- 37.Gao X.Q., Shim W.B., Gobel C., Kunze S., Feussner I., Meeley R., Balint-Kurti P., Kolomiets M. Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol. Plant Microbe Interact. 2007;20:922–933. doi: 10.1094/MPMI-20-8-0922. [DOI] [PubMed] [Google Scholar]
- 38.Nobili C., D’Angeli S., Altamura M.M., Scala V., Fabbri A.A., Reverberi M., Fanelli C. ROS and 9-oxylipins are correlated with deoxynivalenol accumulation in the germinating caryopses of Triticum aestivum after Fusarium graminearum infection. Eur. J. Plant Pathol. 2014;139:429–444. doi: 10.1007/s10658-014-0401-1. [DOI] [Google Scholar]
- 39.Scala V., Camera E., Ludovici M., Dall’Asta C., Cirlini M., Giorni P., Battilani P., Bello C., Fabbri A.A., Fanelli C., et al. Fusarium verticillioides and maize interaction in vitro: Relationship between oxylipin cross-talk and fumonisin synthesis. World Mycotoxin J. 2013;6:343–351. doi: 10.3920/WMJ2012.1527. [DOI] [Google Scholar]
- 40.Chitarra G.S., Abee T., Rombouts F.M., Posthumus M.A., Dijksterhuis J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Appl. Environ. Microbiol. 2004;70:2823–2829. doi: 10.1128/AEM.70.5.2823-2829.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrero-Garcia E., Garzia A., Cordobés S., Espeso E.A., Ugalde U. Carbon oxylipins inhibit germination and growth, and stimulate aerial conidiation in Aspergillus Nidulans. Fungal Biol. 2011;115:393–400. doi: 10.1016/j.funbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Singh D., Son S.Y., Lee C.H. Interactions modulating the growth and secondary metabolism. Sci. Rep. 2020;10:11116. doi: 10.1038/s41598-020-68096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meimaroglou D.M., Galanopoulou D., Markaki P. Study of the effect of methyl jasmonate concentration on aflatoxin B1 biosynthesis by Aspergillus parasiticus in yeast extract sucrose medium. Int. J. Microbiol. 2009;2009:842626. doi: 10.1155/2009/842626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vigor C., Bertrand-Michel J., Pinot E., Oger C., Vercauteren J., Le Faouder P., Galano J.M., Lee J.C.Y., Durand T. Non-enzymatic lipid oxidation products in biological systems: ASSESSMENT of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 45.Shearer G.C., Walker R.E. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot. Essent. Fat. Acids. 2018;137:26–38. doi: 10.1016/j.plefa.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp. Gerontol. 2001;36:1425–1457. doi: 10.1016/S0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Z.-X., Majchrzak-Hong S., Keyes G.S., Iadarola M.J., Mannes A.J., Ramsden C.E. Lipidomic profiling of targeted oxylipins with ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018;410:6009–6029. doi: 10.1007/s00216-018-1222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan C.N., Dalli J., Colas R.A., Winkler J.W., Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capdevila J.H., Falck J.R. Biochemical and molecular characteristics of the cytochrome P450 arachidonic acid monooxygenase. Prostag. Prostaglandins Other Lipid Mediat. 2000;62:271–292. doi: 10.1016/S0090-6980(00)00085-X. [DOI] [PubMed] [Google Scholar]
- 50.Gao X., Kolomiets M.V. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 2009;28:79–88. doi: 10.1080/15569540802420584. [DOI] [Google Scholar]
- 51.Prost I., Dhondt S., Rothe G., Vicente J., Rodriguez M.J., Kift N., Carbonne F., Griffiths G., Esquerré-Tugayé M.-T., Rosahl S., et al. Evaluation of the Antimicrobial Activities of Plant Oxylipins Supports Their Involvement in Defense against Pathogens. Plant Physiol. 2005;139:1902–1913. doi: 10.1104/pp.105.066274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wasternack C., Feussner I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant Biol. 2018;69:363–386. doi: 10.1146/annurev-arplant-042817-040440. [DOI] [PubMed] [Google Scholar]
- 53.Avis T.J., Belanger R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001;67:956–960. doi: 10.1128/AEM.67.2.956-960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thibane V.S., Ells R., Hugo A., Albertyn J., van Rensburg W.J.J., Van Wyk P.W.J., Kock J.L.F., Pohl C.H. Polyunsaturated fatty acids cause apoptosis in C. albicans and C. dubliniensis biofilms. Biochim. Biophys. Acta. 2012;1820:1463–1468. doi: 10.1016/j.bbagen.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Pohl C.H., Kock J.L.F., Thibane V.S. Antifungal free fatty acids: A review. In: Méndez-Vilas A., editor. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. Formatex Research Center; Badajoz, Spain: 2011. [(accessed on 10 October 2021)]. pp. 61–71. Available online: https://www.researchgate.net/publication/266463207. [Google Scholar]
- 56.Champe S.P., El-Zayat A.A. Isolation of a sexual sporulation hormone from Aspergillus nidulans. J. Bacteriol. 1989;171:3982–3988. doi: 10.1128/jb.171.7.3982-3988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsitsigiannis D.I., Zarnowski R., Keller N.P. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 2004;279:11344–11353. doi: 10.1074/jbc.M310840200. [DOI] [PubMed] [Google Scholar]
- 58.Tsitsigiannis D.I., Kowieski T.M., Zarnowski R., Keller N.P. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot. Cell. 2004;3:1398–1411. doi: 10.1128/EC.3.6.1398-1411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim H.S., Han K.Y., Kim K.J., Han D.M., Jahng K.Y., Chae K.S. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 2002;37:72–80. doi: 10.1016/S1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 60.Busch S., Eckert S.E., Krappmann S., Braus G.H. The COP9 signalosome is an essential regulator of development in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2003;49:717–730. doi: 10.1046/j.1365-2958.2003.03612.x. [DOI] [PubMed] [Google Scholar]
- 61.Han S., Adams T.H. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genomics. 2001;266:260–270. doi: 10.1007/s004380100552. [DOI] [PubMed] [Google Scholar]
- 62.Han K.H., Han K.Y., Yu J.H., Chae K.S., Jahng K.Y., Han D.M. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 2001;41:299–309. doi: 10.1046/j.1365-2958.2001.02472.x. [DOI] [PubMed] [Google Scholar]
- 63.Horowitz B.S., Zarnowski R., Sharpee W.C., Keller N.P. Morphological transitions governed by density dependence and lipoxygenase activity in Aspergillus flavus. Appl. Environ. Microbiol. 2008;74:5674–5685. doi: 10.1128/AEM.00565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown S.H., Scott J.B., Bhaheetharan J., Sharpee W.C., Milde L., Wilson R.A., Keller N.P. Oxygenase coordination is required for morphological transition and the host-fungus interaction of Aspergillus flavus. Mole. Plant Microbe Interact. 2009;22:882–894. doi: 10.1094/MPMI-22-7-0882. [DOI] [PubMed] [Google Scholar]
- 65.Fabbri A.A. Lipoperoxidation affects ochratoxin A biosynthesis in Aspergillus ochraceus and its interaction with wheat seeds. Appl. Microbiol. Biotechnol. 2010;85:1935–1946. doi: 10.1007/s00253-009-2220-4. [DOI] [PubMed] [Google Scholar]
- 66.McDonald T., Devi T., Shimizu K., Sim S.-C., Keller N.P. Signaling events connecting mycotoxin biosynthesis and sporulation in Aspergillus and Fusarium spp. In: Yoshizawa T., editor. New Horizon of Mycotoxicology for Assuring Food Safety, Proceedings of International Symposium of Mycotoxicology. Bookish Co.; Takamatsu, Japan: 2003. pp. 139–147. [DOI] [Google Scholar]
- 67.Yu J.H., Wieser J., Adams T.H. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 1996;15:5184–5190. doi: 10.1002/j.1460-2075.1996.tb00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimizu K., Keller N.P. Genetic involvement of cAMP dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimizu K., Hicks J.K., Huang T.P., Keller N.P. Pka, Ras and RGS protein interactions regulate activity of AflR, a Zn(II)2Cys6 transcription factor in Aspergillus nidulans. Genetics. 2003;165:1095–1104. doi: 10.1093/genetics/165.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Affeldt K.J., Brodhagen M., Keller N.P. Aspergillus oxylipin signaling and quorum sensing pathways depend on G protein-coupled receptors. Toxins. 2012;4:695–717. doi: 10.3390/toxins4090695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Souza W.R., Morais E.R., Krohn N.G., Savoldi M., Goldman M.H.S., Rodrigues F., Caldana C., Semelka C., Tikunov A.P., Macdonald J.M., et al. Identification of metabolic pathways influenced by the G-Protein coupled receptors GprB and GprD in Aspergillus nidulans. PLoS ONE. 2013;8:62088. doi: 10.1371/journal.pone.0062088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Affeldt K.J., Carrig J., Amare M., Keller N.P. Global survey of canonical Aspergillus flavus G protein-coupled receptors. mBio. 2014;5:01501–01514. doi: 10.1128/mBio.01501-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lafon A., Han K.H., Seo J.A., Yu J.H., d’Enfert C. G-protein and cAMP-mediated signaling in aspergilli: A genomic perspective. Fungal Genet. Biol. 2006;43:490–502. doi: 10.1016/j.fgb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 74.Püssa T., Raudsepp P., Toomik P., Pällin R., Mäeorg U., Kuusik S., Soidla R., Rei M. A study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J. Food Compos. Anal. 2009;22:307–314. doi: 10.1016/j.jfca.2009.01.014. [DOI] [Google Scholar]
- 75.Bian H., Ma J., Geng Z., Liu T., Sun C., Wang D., Zhang M., Xu W. Changes of hydroxyl-linoleic acids during Chinese-style sausage processing and their relationships with lipids oxidation. Food Chem. 2019;296:63–68. doi: 10.1016/j.foodchem.2019.05.183. [DOI] [PubMed] [Google Scholar]
- 76.Dias F.F.G., Augusto-Obara T.R., Hennebelle M., Chantieng S., Ozturk G., Taha A.Y., de Moura J.M.L.N. Effects of industrial heat treatments on bovine milk oxylipins and conventional markers of lipid oxidation. Prostag. Leukotr. Ess. 2020;152:102040. doi: 10.1016/j.plefa.2019.102040. [DOI] [PubMed] [Google Scholar]