“Strapline”
The human endometrium exhibits complex signaling cascades mediated by spatial-temporal cellular interactions. Understanding the normal endometrial microenvironment is the first step towards understanding diseases of endometrial dysfunction.
A healthy endometrium is essential for female reproduction. Multiple conditions exist counterproductive to successful reproduction through endometrial dysfunction (Figure 1). Unfortunately, studying endometrial dysfunction is limited by the paucity of model systems that recapitulate the human endometrium well. Non-human primates offer significant parallels to humans, but the expense, expertise, and ethical concerns make studies with non-human primates limited to specialized centers1. Mouse models are low-cost and have a long-standing history of providing robust transcriptomic data. Importantly, they allow extensive manipulation of genes, signaling pathways, and cellular crosstalk. However, embryonic implantation in rodents is significantly different from that in humans. Additionally, rodents do not spontaneously menstruate1,2. As such, studies on human endometrium are crucial to understanding the role of this tissue in female health and disease. In this issue of Nature Genetics, Garcia-Alonso et al.3 provide a comprehensive, cell-level transcriptomic view of the human endometrium.
Figure 1: The endometrium plays a crucial role in female health.
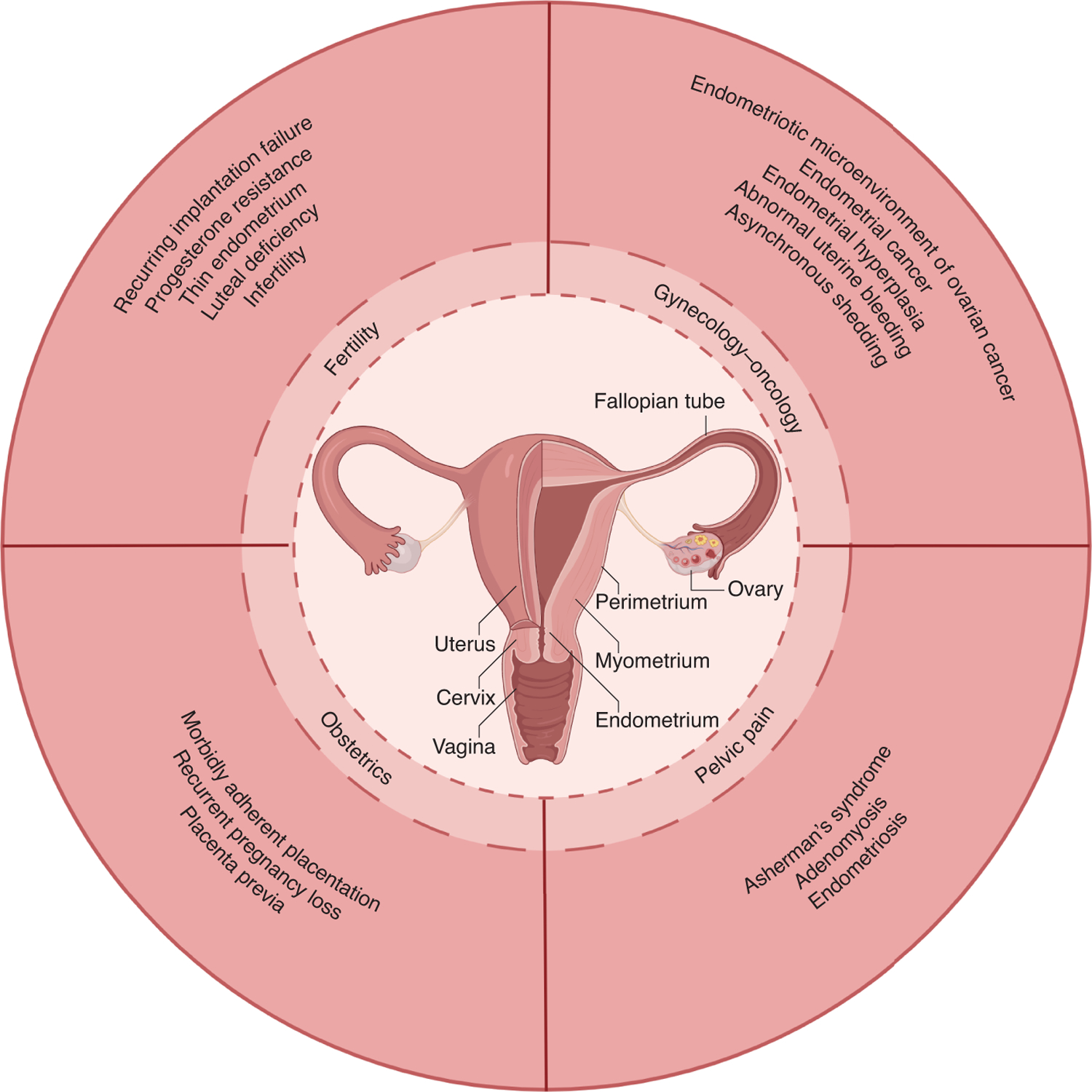
Endometrial dysfunction can be broken into several broad categories, including fertility, obstetrics, pelvic pain, and gynecology/oncology. The datasets from the spatial-temporal transcriptomic profiling and organoid models can be used to study these diseases of endometrial dysfunction.
Garcia-Alonso and colleagues’ study begins with robust cataloging of spatial and molecular features of normal human endometrium through the menstrual cycle. The incorporation of superficial endometrial biopsies of living donors, full-thickness samples from cadaveric donors, and published data4 using single-cell RNA sequencing, single-nuclei RNA sequencing, and high-resolution spatial transcriptomics is a strength. This spatiotemporal profiling covered human endometrial cells in both proliferative and secretory phases of the menstrual cycle. The analysis discerned 14 unique cell clusters with localization onto corresponding endometrial compartments or niche areas, while previous work described only six unique cell clusters4. Identification, characterization, and localization of novel cell types across the menstrual cycle will open new research areas. Further, the creation of a publicly available, open-source web server (www.reproductivecellatlas.org) with an easy user interface speaks to the impact of this work as a research resource into the future.
The authors3 focused on epithelial cells as glandular and luminal epithelium are the vital functional cells for endometrial function and pregnancy5. Embryo implantation occurs during the window of implantation during the progesterone-dominant secretory phase (also known as the luteal phase). Improper timing of the window of implantation due to slow progression through the secretory phase may be a cause of implantation failure. Understanding the critical molecular events in luminal and glandular epithelial cell differentiation through the menstrual cycle is essential for understanding implantation failure and other causes of infertility. Spatial transcriptomics showed that in the secretory phase, there is increased luminal expression of cytokeratin 5 (KRT5) and prostaglandin-endoperoxide synthase 1 (PTGS1 or Cox-1) and increased glandular expression of secretoglobin family 2A member 2 (SCGB2A2). As a potentially useful biomarker for the dynamics of the endometrium, investigators can discern luminal epithelium from glandular epithelium and time the menstrual phase more accurately.
With every menstrual cycle, the endometrium must regenerate the functional layer of the endometrium. Previous studies showed that cells within a proposed stem cell niche within the basal endometrial layer expressed SOX9 plus other markers, including SSEA1, LGR5, β-catenin, or N-cadherin6. However, these studies were limited because they could not examine the cell-level transcriptome of the cells within the niche. The study by Garcia-Alonso et al.3, focusing on SOX9+, stratified the epithelial cells into SOX9+LGR5+ and SOX9+LGR5- populations. Spatial characterization showed that proliferative SOX9+LGR5+ cells map to the regenerating cells within the proposed stem cell niche. As endometrial pathology is thought to arise from the regenerative capacity of this endometrial stem cell niche6, the authors highlight the translational relevance of this detailed data on endometrial epithelial cells and examined two disease datasets – endometrial cancer and endometriosis. Both datasets show enrichment in markers (SOX9+LGR5+) associated with the proliferative menstrual cycle phase. This data fits with the estrogen dependence and proliferative nature of endometrial cancer and the progesterone resistance phenotype of endometriosis7. Future studies are needed to confirm if these findings will be recapitulated in other non-TCGA endometrial cancer datasets. Further confirmation is necessary for non-peritoneal forms of endometriosis, as the mutational and molecular landscape of endometriosis lesions (i.e., deep infiltrating endometriosis, superficial peritoneal, iatrogenic, endometriomas, rectovaginal nodules)8, and the presence or absence of progesterone receptor expression9 may provide different results.
Next, Garcia-Alonso et al.3 explored the effects of the cells within the microenvironment on epithelial cell identity. CellPhoneDB v3, a mathematical modeling pipeline that correlates spatial features of receptors and their ligands to infer cell-cell communication, was used to determine key signaling pathways. Importantly, spatial transcriptomics with CellPhoneDB v3 identified a simple mechanism whereby the balance of WNT and NOTCH regulates luminal and glandular identity. Importantly, cells surrounding the glandular epithelium express high levels of the WNT inhibitor, DKK1, affecting the cell identity of the glandular epithelium. DKK1 is a known progesterone-regulated gene7 involved in the secretory phase of the menstrual cycle. Highlighting the translational nature of this work, studies have shown members of the WNT and NOTCH signaling pathways are critical for pregnancy, including endometrial embryo receptivity, appropriate uterine development, and endometrial diseases2,5,10. The robust datasets from spatial transcriptomics analysis, single-cell RNA sequencing, and single-nuclei RNA sequencing validated that human endometrial epithelial organoids11 respond in vitro similar to the endometrium in vivo. Furthermore, the use of WNT or NOTCH inhibitors showed that luminal and glandular identity and differentiation were dependent on both estrogen and progesterone, respectively. These results suggest that organoids recapitulate human endometrium in vivo and can be manipulated to test signaling pathways in vitro. Future studies will be needed to determine the contributions of other cell types such as immune or stromal cells to the microenvironment.
Garcia-Alonso et al.3 provide robust datasets on normal endometrium, including identification of unique cell types. Notably, the characterization and localization of immune cell types within the endometrium will allow future studies on the role of the endometrial immune niche in diseases such as endometriosis12. The identification and molecular characterization of SOX9+LGR+ stem-like cells may advance research beyond endometriosis and endometrial cancer and into endometriosis-associated ovarian cancers or other diseases of the female reproductive tract.
Garcia-Alonso et al.3 provide further confirmation of organoids as a valid model system. Recently organoids, composed of glandular epithelial cells, or assembloids, composed of epithelium and stroma, have been explored across multiple endometrial diseases, normal human endometrium, and interactions with embryos11,13–15. The authors show that in vitro organoids recapitulate the molecular and steroid-hormone responsiveness of the in vivo endometrium. Critically, such well-validated model systems are essential for discovering new treatments for diseases of endometrial dysfunction (Figure 1). Endometrial dysfunction represents multiple diseases across the spectrum of fertility to infertility. These diseases clinically range from annoying (i.e., asynchronous shedding, abnormal uterine bleeding) to deadly (i.e., endometrial cancer, ovarian cancer, morbidly adherent placentation). Treatments for most of these diseases remain highly surgical, while medical management focuses on steroid hormone therapy. Translational applications of the robust datasets will allow for the discovery of novel treatments, and the use of well-validated models will allow for efficient pre-clinical testing.
Footnotes
The authors declare no competing interests.
References
- 1.Greaves E, Critchley HOD, Horne AW & Saunders PTK Acta Obstet Gynecol Scand 96, 644–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurya VK, DeMayo FJ & Lydon JP Front Cell Dev Biol 9, 640907 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Alonso L Nature Genetics (2021). [Google Scholar]
- 4.Wang W et al. Nat Med 26, 1644–1653 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Kelleher AM, DeMayo FJ & Spencer TE Endocr Rev 40, 1424–1445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tempest N, Maclean A & Hapangama DK Int J Mol Sci 19(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burney RO et al. Endocrinology 148, 3814–26 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Bulun SE et al. Endocr Rev 40, 1048–1079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores VA, Vanhie A, Dang T & Taylor HS J Clin Endocrinol Metab 103, 4561–4568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Menkhorst E & Dimitriadis E FASEB J 35, e21784 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Boretto M et al. Development 144, 1775–1786 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Vallve-Juanico J, Houshdaran S & Giudice LC Hum Reprod Update 25, 564–591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boretto M et al. Nat Cell Biol 21, 1041–1051 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Turco MY et al. Nat Cell Biol 19, 568–577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawlings TM et al. Elife 10(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


