Abstract
Garcinia indica (commonly known as kokum), belonging to the Clusiaceae family (mangosteen family), is a tropical evergreen tree distributed in certain regions of India. It has been used in culinary and industrial applications for a variety of purposes, including acidulant in curries, pickles, health drinks, wine, and butter. In particular, G. indica has been used in traditional medicine to treat inflammation, dermatitis, and diarrhea, and to promote digestion. According to several studies, various phytochemicals such as garcinol, hydroxycitric acid (HCA), cyanidin-3-sambubioside, and cyanidin-3-glucoside were isolated from G. indica, and their pharmacological activities were published. This review highlights recent updates on the various pharmacological activities of G. indica. These studies reported that G. indica has antioxidant, anti-obesity, anti-arthritic, anti-inflammatory, antibacterial, hepatoprotective, cardioprotective, antidepressant and anxiolytic effects both in vitro and in vivo. These findings, together with previously published reports of pharmacological activity of various components isolated from G. indica, suggest its potential as a promising therapeutic agent to prevent various diseases.
Keywords: Garcinia indica, kokum, wild mangosteen, pharmacological activity
1. Introduction
The use of medicinal herbs as medicine is the oldest form of medical treatment known to humanity and has been used in all cultures throughout history [1]. Since time immemorial, humans have recognized their dependence on nature for healthy living, and have relied on a variety of plant resources for medicine to cure numerous diseases [2]. This indigenous knowledge, passed down from generation to generation in different parts of the world, has contributed significantly to the development of traditional medical systems [3], as well as provided a scientific basis for their traditional uses by exploring various biologically active natural products [4]. For instance, between 1981 and 2014, about 26% of new chemical entities were natural products or derived from natural products [5]. They are widely used in the prevention and treatment of clinical diseases as they have the unique advantages of low toxicity and side effects compared with chemical drugs [6]. Among medicinal plants, Clusiaceae contains approximately 50 genera and 600 species [7], and has been extensively used in ethnomedicine to treat a number of disease conditions, including wounds, ulcers, dysentery, cancer, inflammation, and infection [8,9].
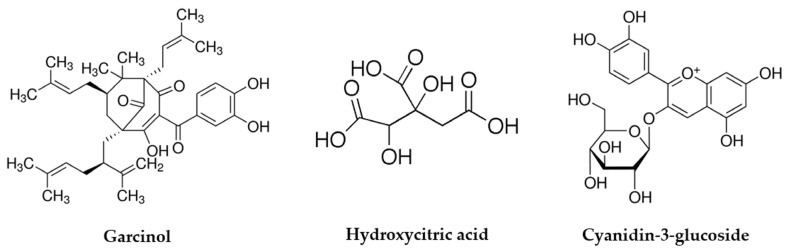
Garcinia belongs to the Clusiaceae family (Mangosteen family) and has multiple application in the culinary, pharmaceutical, and industrial fields [10]. The plants are distributed around the world including tropical Asia, Africa, and Western Polynesia [11]. In the last few decades, they have received considerable attention and extracts of different plant parts of the Garcinia species, e.g., Garcinia brasiliensis, G. cambogia, G. gardneriana, G. pedunculata, and G. mangstana have demonstrated potential effectiveness in the prevention and treatment of non-transmissible chronic diseases [12]. Furthermore, it was reported that they contain a wide range of biologically active metabolites and that the chemical compositions of their extracts are rich in bioactive molecules including hydroxycitric acid (HCA), bioflavonoids, procyanidines and polyisoprenylated benzophenone derivatives including garcinol, xanthochymol and guttiferone isoforms [9,13]. These compounds have been implicated in biological activities such as antioxidant [14], anticancer [15,16], and antiviral effects [17]. In particular, the major bioactive ingredients such as garcinol, HCA, and cyanidin-3-glucoside have been isolated (Figure 1), characterized and evaluated for their therapeutic properties. For example, garcinol showed anti-inflammatory effects by regulating various signaling pathways, molecular binding, and gene expression contributing to inflammation, such as inhibition of the cyclooxygenase-2, 5-lipoxygenase and inducible nitric oxide synthase synthesis [18,19,20]. Also, garcinol was reported to have antioxidant effects through high free-radical, superoxide anion (O2-) scavenging activity [21,22], and to have neuroprotective properties by functioning as a histone acetyltransferase inhibitor (HAT) [23]. In addition, recent studies demonstrated its anticancer effects by inducing apoptosis and cell cycle arrest, inhibiting of angiogenesis, and regulating of gene expression in oncogenic cells [24].
Figure 1.
Chemical structure of bioactive ingredients isolated from G. indica.
Garcinia indica is a plant native to certain regions of India [25]. It is an underexploited slender evergreen tree and is known as wild mangosteen, kokum, and goa butter tree [26]. All parts of G. indica, i.e., fruits, rind, seeds, etc., have been used in various culinary, industrial and pharmaceutical applications, as well as fruit drinks and food [27]. Its pharmacological properties including antioxidant [28], anti-inflammatory activity [29], antimicrobial [30], anticancer [31], and anti-obesity [32] effects have been reported. The present review aims to discuss these recent studies and update the pharmacological properties of G. indica.
2. Methodology
We collected scientific literature on the origin, medicinal uses, and pharmacological activity of G. indica published in the English language up till 2021 from PubMed, Google Scholar, and Web of Science. The search terms were “Garcinia indica”, “kokum”, “pharmacological activity”, “antioxidant”, “anti-obesity”, “anti-inflammatory”, and “anti-diabetic”.
As a result of the literature research, various pharmacological activities of G. indica were reported and reviewed [33,34,35], and a total of 7 papers were additionally reported recently. We comprehensively reviewed and updated the study design, results, and interpretation of each paper.
3. Pharmacological Properties of G. indica
A summary of the pharmacological properties of G. indica is described in Table 1.
Table 1.
Summary of pharmacological studies for Garcinia indica.
| Pharmacological Activity |
Tested Substance | In Vitro/ In Vivo |
Model | Dose/Concentration | Ref. |
|---|---|---|---|---|---|
| Antioxidant | Aqueous extract | In vivo | Wister albino rats | 400 and 800 mg/kg | [36] |
| Fruit extract | In vitro | - | 1.5 mM | [37] | |
| Garcinol-enriched fraction | In vivo | C57BL/6 male mice | 25, 50, and 100 mg/kg | [38] | |
| Aqueous extract | In vitro | - | - | [39] | |
| In vivo | Wister albino rats | 250 and 500 mg/kg | |||
| G. indica fruit rind powder | In vivo | Swiss albino mice, Wister rats | 0.5, 1, and 2% w/w | [40] | |
| Anti-obesity | Garcinol-enriched fraction | In vivo | C57BL/6 male mice | 25, 50, and 100 mg/kg | [38] |
| Fruit extract | In vitro | 3T3-L1 preadipocytes | 1 and 2 µg/mL | [41] | |
| In vivo | C57BL/6 male mice | 0.01% w/w | |||
| Anti-arthritic | Garcinol-enriched fraction | In vivo | Male Wistar rats | 10 mg/kg | [42] |
| Anti-inflammatory | Garcinol-enriched fraction | In vivo | C57BL/6 male mice | 25, 50, and 100 mg/kg | [38] |
| Antidepressant and anxiolytic effect |
G. indica fruit rind powder | In vivo | Swiss albino mice, Wister rats | 0.5, 1, and 2% | [40] |
| Antibacterial | Fruit extract | In vitro | - | 1.5 mM | [37] |
| Hepatoprotective | Aqueous extract | In vivo | Wister albino rats | 400 and 800 mg/kg | [36] |
| Cardioprotective | Garcinol-enriched fraction | In vivo | C57BL/6 male mice | 25, 50, and 100 mg/kg | [38] |
| Aqueous extract | In vivo | Wister albino rats | 250 and 500 mg/kg | [39] |
3.1. Antioxidant Effects
The overproduction of oxidants can cause oxidative damage to biomolecules such as lipids, DNA, and proteins, increasing the risk of cancer, and cardiovascular and other diseases [43,44]. Antioxidants may reduce oxidative stress through various mechanisms [45]. In the past few years, there have been many reports on the antioxidant activity of G. indica [46,47,48,49,50,51,52,53]. Panda V et al. [36] reported the antioxidant effect of aqueous extracts of G. indica in an animal model of oxidative stress induced with ethanol (EtOH). Malondialdehyde (MDA), a lipid peroxidation marker, was elevated in the EtOH-treated group compared with the normal group. Treatment with the aqueous extracts of G. indica reversed these results. The reduced glutathione (GSH) level in the EtOH-treated group was significantly reduced compared with the normal group (p < 0.001). Treatment of the EtOH-treated group with aqueous extracts of G. indica (400 and 800 mg/kg) significantly restored the decreased levels of GSH (p < 0.05 and p < 0.01, respectively). In addition, superoxide dismutase (SOD) and catalase (CAT) activities were also markedly lower in the EtOH-treated group compared with the normal group (p < 0.01). The reduction in SOD and CAT activity levels were improved in a dose-dependent manner when treated with the aqueous extracts of G. indica. In particular, the high-dose group treated with 800 mg/kg of aqueous extracts of G. indica recovered to near normal levels. Furthermore, treatment with aqueous extracts of G. indica significantly ameliorated glutathione peroxidase and glutathione reductase activity, which were decreased by EtOH treatment (p < 0.01).
Nanobiotechnology is a new field of research related to the convergence of biology and nanotechnology [54]. A recent study successfully demonstrated the use of plant extracts for the biogenic synthesis of silver nanoparticles (AgNPs) with antioxidant activity [55]. However, the synthesis of AgNPs was mainly achieved by physical and chemical methods of metal nanoparticle synthesis, which includes the use of hazardous chemicals that limit their potential use in biomedical applications [56]. To overcome these disadvantages, biosynthesis using various biological agents such as bacteria, fungi, plants, and their extracts is a green chemistry approach that is inexpensive, does not use hazardous chemicals, and has excellent biocompatibility and low toxicity [57]. Sangaonkar, G.M. et al. [37] optimized parameters for a simple, stable and benign biosynthesis method of AgNPs using G. indica fruit extract. Then, they performed 1,1-diphenyl-2-picrylhydrazyl (DPPH) free-radical scavenging, reducing power activity, and hydrogen peroxide and nitric oxide (NO) radical scavenging activity to evaluate the potential antioxidant activity of AgNPs biologically synthesized with G. indica fruit extract. They found that the tested range of AgNPs (20, 40, 60, 80, and 100 µg/mL) inhibited DPPH activity in a dose-dependent manner and exhibited a 57% inhibitory effect at the maximum concentration. Similar to the DPPH activity, the NO radical scavenging activity of AgNPs was confirmed to be dose-dependent, but lower compared with the DPPH activity. Furthermore, A 100 μg/mL AgNPs, which was less than half of the dose of butylated hydroxytoluene used as a positive control, resulted in 27% NO radical scavenging. Similar to DPPH and NO radical scavenging activity, the inhibitory response of biological AgNPs to reducing power was increased in a dose-dependent manner. Whereas, as a result of H2O2 radical scavenging activity, the inhibitory activity of AgNPs at the highest concentration was 65%, which was similar to the inhibitory activity of 72% using the same test concentration of standard ascorbic acid. In conclusion, G. indica fruit extract containing significant amounts of natural antioxidants such as HCA can be used in environmentally friendly AgNPs, preventing the use of toxic chemicals. Therefore, G. indica has important applications in biomedical fields as a good source of reducing and capping agents.
Barve, K. [38] performed lipid peroxide, GSH, CAT, and SOD assays using C57BL/6 male mice to measure the antioxidant activity of the garcinol-enriched fraction (GEF). This fraction was prepared from the hexane extract of G. indica. The extracts were loaded on a silica column, and hexane and ethyl acetate were eluted in ascending order of polarity to obtain various fractions by flash chromatography at a flow rate of 15 mL/min. The collected fractions (12 mL) were confirmed for the presence of garcinol by thin-layer chromatography, and this flash chromatography procedure was repeated 3 times to obtain sufficient GEF. Lipid peroxidation and changes in the oxido-redox state were measured for enzymatic and non-enzymatic markers. Lipid peroxide, an indicator of oxidative stress, was twice as high in disease-induced animals fed a modified Western diet compared with normal animals (p < 0.001). Simvastatin treatment was used as a positive control and animals treated with GEF (50 and 100 mg/kg) showed significantly reduced effects (p < 0.05 and p < 0.01, respectively), although they were higher than those in normal animals. GSH levels in disease-induced animals were significantly depleted compared with those in normal animals (p < 0.001). Treatment with GEF (25, 50, and 100 mg/kg) strikingly ameliorated depleted GSH levels in a dose-dependent manner, although this did not reach normal levels. CAT and SOD activity levels were significantly reduced in disease-induced animals (p < 0.001 and p < 0.0001, respectively). GEF treatment at 25 mg/kg reversed CAT activity levels, but this did not reach statistical significance. However, GEF treatment at 50 and 100 mg/kg significantly restored the CAT activity levels to levels similar to those of normal animals. Of note, only 100 mg/kg GEF treatment significantly increased the SOD activity level.
In another study conducted by Patel et al. [39], the IC50 value for the DPPH scavenging activity of an aqueous extract of G. indica was estimated to be 231.85 ± 21.56 μg/mL. They also used animal models to measure biomarkers related to oxidative stress, including SOD, CAT, and thiobarbituric acid reactive substance (TBARS). The group treated with 85 mg/kg of isoprenaline (ISO) showed significantly reduced SOD and CAT values (5.38 ± 0.31 unit/g of tissue and 0.54 ± 0.02 µmol of H2O2 consumed/min/g of tissue, respectively) compared to the normal group (13.55 ± 0.62 unit/g of tissue and 2.66 ± 0.24 µmol of H2O2 consumed/min/g of tissue, respectively). Treatment of the ISO-induced toxicity group with an aqueous extract of G. indica at 250 and 500 mg/kg slightly restored the SOD levels (6.12 ± 0.93 and 7.56 ± 0.22 unit/g of tissue, respectively) and slightly improved the CAT levels (1.1 ± 0.17 and 1.08 ± 0.19 µmol of H2O2 consumed/min/g of tissue, respectively). Furthermore, treatment with ISO increased the TBARS level (3.82 ± 0.21 nmol MDA/g of tissue) compared with the normal group (1.65 ± 0.12 nmol MDA/g of tissue). The tested concentrations of aqueous extracts of G. indica did not affect these values (2.55 ± 0.25 and 2.17 ± 0.13 nmol MDA/g of tissue, respectively) and treatment of the normal control group with 500 mg/kg G. indica aqueous extract did not change the levels of any of the three biomarkers related to oxidative stress. They used the lower dose range because the antioxidant enzyme levels were not restored in the 250 and 500 mg/kg G. indica-treated groups.
Dhamija I et al. [40] measured antioxidant activity to elucidate the mechanism of antidepressant effect in various animal models. G. indica at 1% w/w significantly decreased monoamine oxidase levels (MAO-A and MAO-B) in brain homogenates, similar to a group treated with fluoxetine. By measuring the MDA concentration in the brain homogenate, a direct correlation was observed between the dose of G. indica and a decrease in the MDA level, suggesting a decrease in free-radical production.
3.2. Anti-Obesity Effects
Recently, as the prevalence of overweight and obesity has reached epidemic levels, the incidence of various comorbidities such as type 2 diabetes, cardiovascular disease, and cancer has rapidly increased, causing enormous health and economic burdens [58,59]. Medicines and bariatric surgery are the main strategies to prevent and treat obesity, but there are side effects [60]. For this reason, various natural products and their components are being tested to improve obesity with minimal side effects [61]. An anti-obesity effect of garcinol isolated from G. indica was reported [62,63,64].
As mentioned above, Barve, K. [38] confirmed the reduction of the oxidative stress response of GEF. In addition, he conducted research into the anti-obesity effects of GEF. C57BL/6 male mice were randomly divided into 6 groups of 6 mice each: Group 1 (normal control); Group 2 (disease-induced control by a modified Western diet); Group 3 (positive control receiving 8 mg/kg of simvastatin); and Groups 4, 5, and 6 (treated with 25, 50, and 100 mg/kg of GEF). Compared with Group 1, the weights of animals in all groups were significantly increased at 12 weeks. Group 2 weights continued to increase significantly until 16 weeks (p < 0.05), but those in Groups 3, 4, 5, and 6 treated with simvastatin and GEF decreased significantly from 12 to 16 weeks (p < 0.001 and p < 0.0001, respectively). Group 2 showed significant negative changes in the lipid profile indicative of disease induction. Groups 3, 4, 5, and 6 showed a decrease in the increased total cholesterol and triglyceride levels induced by a Western diet in a concentration-dependent manner, In particular, the values of Group 6 were similar to those of Group 1 (p < 0.01 and p < 0.0001, respectively). Furthermore, treatment with different concentrations of GEF elevated high-density cholesterol levels in a concentration-dependent manner and significantly decreased low density cholesterol levels to those of positive control levels (p < 0.0001).
Tung YC et al. [41] investigated the potential anti-obesity effects of G. indica fruit extract (GIE) in a 3T3-L1 cell model and high-fat diet (HFD)-induced obese animal model. First, 3T3-L1 preadipocytes were used for the following experiments after differentiation induced by differentiation medium (DMI) containing 5 μg/mL insulin, 0.5 mM 3-isobutylmethylxanthine, 1 μM dexamethasone, and 2 μM rosiglitazone. After confirming the lipid content by Oil Red O staining, the groups treated with 1 and 2 μg/mL GIE showed significantly reduced triglyceride levels to 63.5% and 65.6% without cytotoxic effects, compared with the group treated with only DMI. However, treatment with GIE in the tested range did not affect the amount of glycerol in the medium. The weights of C57BL/6 male mice fed a normal diet (ND) or HFD for 12 weeks were 27.5 and 37.4 g, respectively. The body weights of the group supplemented with 0.01% GIE in HFD were significantly reduced to 34.4 g (p < 0.05), and no effects on liver, kidney, or spleen weight were observed. A significantly reduced weight of mesenteric, perigonadal and retroperitoneal fat was noted in the group supplemented with GIE. Treatment with GIE also reduced the ratio of body fat compared with the HFD group. In addition, the histopathological examination of liver and the perigonadal adipose tissue of mice demonstrated that GIE treatment reduces the adipocyte size and liver fat accumulation. They further investigated the expressions of proteins related to adipogenesis, lipolysis and β-oxidation to elucidate the mechanisms involved in these effects. There was no significant difference in PPARγ and C/EBPα, proteins related to adipogenesis, between the ND and HFD groups, whereas PPARγ and C/EBPα protein levels were significantly decreased after supplementation with GIE compared with the HFD group. Although there was no significant difference in the protein levels of CPT-1A between the ND and HFD groups, supplementation with GIE was significantly elevated in the protein levels of CPT-1A compared to the HFD group. Moreover, a significant increase in PPARα protein levels was observed in the GIE supplemented group compared with the HFD group. These results suggest that GIE exerts anti-obesity effects in 3T3-L1 adipocytes and animal models by inhibiting adipogenesis and increasing β-oxidation.
3.3. Anti-Arthritic Activity
Arthritis is highly prevalent worldwide and can be divided into types, the most common of which are rheumatoid arthritis, osteoarthritis, psoriatic arthritis and inflammatory arthritis [65]. The exact cause of arthritis is not yet clear, and although it is commonly used to treat various types of anti-inflammatory arthritis, it is associated with serious side effects such as gastric bleeding and an increased risk of other cardiovascular problems [66]. Consequently, it is very important to explore effective and safe anti-arthritis drug candidates isolated from natural products.
Warriar, P. et al. [42] evaluated the anti-arthritic effects of GEF in rheumatoid arthritis. An extract (9 gm) of G. indica was adsorbed onto silica and loaded. Thereafter, fractions were obtained through stepwise gradient elution with n-hexane-ethyl acetate, including a fraction (800 mg) showing the maximum concentration of garcinol. A rat model of adjuvant arthritis (AA) was developed with a single injection of 0.1 mL of Complete Freund’s Adjuvant (CFA). Rats were divided into 4 groups of 6 each as follows: Group 1 (normal control); Group 2 (disease control of CFA alone); Group 3 (positive control receiving 10 mg/kg of diclofenac sodium); Group 4 (treated with 10 mg/kg of GEF). To examine the major lesions and determine the effects of the therapeutic agents, the volume of the left hind paw of rats was measured on days 0, 1, 5, 12, 16, and 21 using an electronic transfusion meter. To determine the severity of the secondary lesions, the volume of the right hind paw was also measured. In addition, to evaluate the severity of AA, the ears, nose, tail, forepaws, and hind paws of the rats were visually examined for inflammatory lesions, and arthritis severity was scored according to the redness, swelling, and presence of nodules [67]. The CFA-treated group developed primary arthritis in the left hind paw, and compared with the normal group, a significant level of swelling was observed and maintained for 21 days (p < 0.0001). Treatment with GEF significantly suppressed paw swelling from days 5 to 21, similar to the positive control group, when compared with the disease control group. On the other hand, the GEF-treated group also had a decrease in the swelling of the right hind paw, but this was not statistically significant. CFA-treated rats showed a significant increase in the arthritis index compared with the control group, reaching a maximum at 5 days, and then gradually decreasing. The groups treated with 10 mg/mL of GEF and diclofenac had a significantly reduced arthritis index compared with the disease control animal group (p < 0.01). Treatment of CFA significantly reduced the stair climbing activity of all animals from days 5 to 21 compared with the normal group demonstrating the induction of hyperalgesia (p < 0.0001). However, the group treated with GEF and diclofenac had improved scores from days 12 and 16, which were significantly increased until day 21. In addition, the motility score, which was reduced by CFA treatment, steadily and significantly recovered from days 16 to 21 in the GEF-treated group. This study indicated that GEF has anti-arthritic activity in an animal model of arthritis induced with CFA.
3.4. Anti-Inflammatory Effects
Inflammation is the immune system’s first biological response to infection, injury, or irritation [68]. There is evidence that anti-inflammatory effects are mediated through the modulation of various inflammatory cytokines [69]. Interleukin 6 (IL-6) is produced rapidly and transiently in response to infection and tissue damage and contributes to host defense through the stimulation of acute phase responses, as well as hematopoietic and immune responses [70]. A study conducted by Barve, K. [38] showed that, after 16 weeks, IL-6 levels in the plasma and thoracic aorta were significantly increased in induced hyperlipidemic animals compared with normal animals (p < 0.0001 and p < 0.001, respectively). In animals treated with 25 mg/kg GEF, IL-6 levels in the thoracic aorta were decreased but not significant, and IL-6 levels were significantly decreased in animals treated with 50 or 100 mg/kg GEF (p < 0.01). In addition, plasma IL-6 levels were significantly reduced in a dose-dependent manner by treatment with all concentrations of GEF (p < 0.0001).
3.5. Antidepressant and Anxiolytic Effects
Depression and anxiety are the most common mental disorders and are the leading cause of psychosocial dysfunction [71]. Pharmacotherapy is the first line treatment of these disorders, but it can impose some problems, including sedation and amnesia, causing tolerance, psychomotor effects, and dependence [72]. These considerations emphasized the importance of finding new psychopharmacological drugs with an immediate onset of action and fewer side effects [73]. In this regard, natural products represent promising candidates for the pharmacological treatment of these pathologies [74].
Dhamija, I. et al. [40] investigated the antidepressant and anti-anxiety effects of G. indica using mouse and rat models in which the rind of G. indica was triturated with a mortar and pestle and mixed with feed. Elevated plus maze (EPM), light–dark model (LDM), and hole-board tests (HBT) were used to evaluate anxiolytic effects, and the forced swim test (FST), tail suspension test (TST), reserpine-induced hypothermia model, measurement of locomotor activity, and estimations of biochemical were used as methods to measure antidepressant activity. Positive controls received 1 mg/kg diazepam (standard anxiolytic), 15 mg/kg imipramine and 20 mg/kg fluoxetine (standard antidepressant agents), and 0.5, 1, and 2% w/w of G. indica rind mixed into their food. In the LDM, a significant increase in the length of time animals stayed in the lighted compartments indicated anxiolytic activity and the reverse of this represented anxiogenic activity. In the HBT models, head-dipping behavior reflected the animal’s emotional state. Administration of G. indica resulted in a significant increase in the length of time mice stayed in the light box in the LDM (p < 0.05). In addition, treatment with 0.5, 1, and 2% w/w of G. indica increased the number of head dips. In the EPM, anxiolytic effects were found in the state of open arms by increasing the length of stay and the number of entries. On the other hand, spending less time with arms outstretched reflected anxiety effect. G. indica at concentrations of 0.5, 1, and 2% w/w dose-dependently improved the time spent and number of entries in the open arms of the EPM (p < 0.05). Immobility refers to a condition in animals known as helplessness that can be converted by antidepressants such as fluoxetine and imipramine. A decrease in immobility indicates the absence of depression. G. indica at the concentrations tested in the FST and TST models significantly decreased animal immobility (p < 0.05). The rectal temperature in mouse using a reserpine-induced hypothermia model reflected the depletion of noradrenaline and serotonin in the brain, and 1% w/w G. indica significantly reversed the reserpine-induced hypothermia. (p < 0.05). These results indicate that G. indica fruit rind as a functional food exhibits significant antidepressant and anti-anxiety effects, without impairing motor activity and not involving CNS stimulation.
3.6. Antibacterial Activity
Bacterial resistance to antibiotics has rapidly increased and is one of the major threats to public health in the 21st century [75]. Natural products have historically been successful as a source of new antibiotics, and most existing classes of antibiotics are derived from natural compounds [76]. The potential for antibacterial effects of G. indica has been reported [77,78,79].
Sangaonkar, G.M. et al. [37] noted the antioxidant effect and the antibacterial effects of the biogenic synthesis of AgNPs using G. indica fruit extract. It was reported that AgNPs exhibit potential antibacterial activity against methicillin resistant Staphylococcus aureus (MRSA) by a mechanism different from that of antibiotics [80]. Based on this potential effect of AgNPs, they tested the antimicrobial activity 20 μg/μL AgNPs against 7 bacterial pathogens (Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enterica typhi, Proteus vulgaris, Serratia marcescens) and found that it inhibited the growth of E. coli, B. subtilis, S. aureus, and P. aeruginosa, with a clear region of inhibition in the range of 12–15 mm. However, S. enterica typhi, P. vulgaris, and S. marcescens were not inhibited by AgNP treatment. As a result of measuring the minimum inhibitory concentration using the micro-broth dilution method reported by Siddiq et al. [81], the inhibitory power of AgNPs was estimated to be 20 mg/mL for E. coli, B. subtilis, and S. aureus, and 40 mg/mL for P. aeruginosa. In addition, the minimum bactericidal concentration for E. coli, B. subtilis, and S. aureus was the same as the minimum inhibitory concentration, and 160 mg/mL for P. aeruginosa. These results suggest that AgNPs biosynthesized with G. indica fruit extract have important applications in biomedical fields as an antibacterial agent.
3.7. Hepatoprotective Activity
As mentioned above, Panda, V. et al. [36] determined the antioxidant effects of G. indica through various mechanisms. Significantly increased levels of serum enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase were observed in an EtOH-treated animal group compared with the normal animal group (p < 0.001). These activities were due to the release of enzymes into the blood following extensive liver damage caused by the EtOH. Treatment with 400 or 800 mg/kg aqueous extracts of G. indica reduced AST activity (p < 0.001). However, ALT and AST activities were significantly improved only in the 800 mg/kg of the G. indica-treated group, similar to treatment with silymarin, which protects hepatocytes from toxins (p < 0.05). Moreover, increased serum triglyceride (sTG) levels and decreased total protein (TP) and albumin (Alb) levels were observed in EtOH-fed rats compared with the normal group. These effects suggested the decreased activity of lipase enzyme responsible for lipoprotein uptake by extrahepatic tissues. Treatment with aqueous extracts of G. indica restored these increased sTG levels and decreased TP levels in a dose-dependent manner. However, treatment with aqueous extracts of G. indica ameliorated Alb levels, although this did not reach statistical significance. Finally, light microscopy histopathological studies of liver sections fixed in 10% buffered formalin, embedded in paraffin, cut to 5 µm, and stained with hematoxylin and eosin (H/E), showed a moderate-to-marked degree of histoarchitectural changes in animals treated with EtOH. There were moderate-to-severe abscesses and bleeding, and the central vein revealed congestion of sinusoids. Moderate mononuclear inflammatory infiltration was observed in all zones, and many hepatocytes showed degenerative changes. However, the group treated with 400 mg/kg aqueous extracts of G. indica had mild-to-moderate mononuclear inflammatory infiltration in all areas with moderate fat changes and few regenerated cells. In the group treated with 800 mg/kg aqueous extracts of G. indica, slight or minimal fat changes were present around the dilated central vein, and many regenerative cells were observed. This histology was similar to that of the silymarin-treated group, a proven hepatoprotective, with a tendency to be more normal compared with the group treated with 400 mg/kg aqueous extracts of G. indica. These findings suggest that the hepatoprotective effect of aqueous extracts of G. indica in ethanol-induced hepatotoxicity may be due to increased endogenous antioxidant levels and the inhibition of lipid peroxidation in the liver.
It is important to note that there are concerns about the risk of hepatotoxicity related to garcinia food supplements from a recent report by the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) [82]. As a result of several previous studies [83,84,85], G. gummi-gutta (G. cambogia) and HCA, its main active ingredient, can cause herb-induced hepatotoxicity (HILI) including elevated liver enzyme levels, hepatitis, cholestasis, and jaundice. Furthermore, these acute liver damages are known to persist even after discontinuation of their intake. Although no research results have been reported on the direct association between G. indica and acute liver injury, more scientific and clinical investigations on the effect of G. indica on liver health since G. indica also contain HCA.
3.8. Cardioprotective Activity
Atherosclerosis is one of the leading causes of cardiovascular disease, with significant mortality and morbidity worldwide. Barve, K. [38] calculated various indicators to predict the risk of atherosclerosis and subsequent cardiovascular disease using lipid values as follows: atherogenic index of plasma (AIP) = (log (triglycerides/high-density cholesterol)), cardiac risk ratio (CRR) = (total cholesterol/high-density cholesterol), atherogenic coefficient (AC) = (total cholesterol–high-density cholesterol/high-density cholesterol). The values of AIP, CPR, and AC were significantly higher at the end of 12 weeks in the group of animals fed a modified Western diet compared with the group of normal animals (p < 0.01, p < 0.001 and p < 0.0001, respectively). However, treatment with all tested concentrations of GEF significantly reduced these values (p < 0.0001), almost normalizing the levels, and attenuating the risk of atherosclerosis. Histopathological studies using light microscopy were performed by staining tissue sections of the thoracic aorta of mice with H/E. Then, lesions were scored for global pathological changes (lesion scores) as follows: score 0 = no change, score 1 = minimal changes, score 2 = minor changes, score 3 = moderate changes, and score 4 = significant changes. In the disease-induced animals, histopathological analysis of the aorta showed thickening of the vessel wall, and the lesion score of 3.33. Treatment with 25 or 50 mg/kg GEF improved some of these changes, resulting in a lesion score of 2.33, but this was not statistically significant. On the other hand, treatment with simvastatin or 100 mg/kg GEF showed completely normal vasculature with lesion scores of 0.66 and 1 (p < 0.0001 and p < 0.001, respectively).
Patel et al. [39] evaluated the cardioprotective effects of aqueous extracts of G. indica by ISO-induced myocardial injury in Wistar albino rats. The aqueous extract of G. indica did not affect the levels of cardiac injury markers including serum troponin I, lactate dehydrogenase, creatinine kinase-MB, ALT, AST, and uric acid, which were significantly increased by ISO treatment. In addition, histopathological analysis demonstrated that the animals treated with aqueous G. indica extract had improved edema, infiltration, and necrosis levels, but no statistical significance was observed compared with the ISO control group. The results of these experiments suggest that aqueous extracts of G. indica do not have a cardioprotective effect against myocardial damage, and further studies with larger sample sizes and higher dose ranges may be needed to evaluate its cardioprotective effects.
4. Pharmacological Effects of Garcinol, a Major Active Constituent from G. indica
As mentioned above, various pharmacological activities of extracts and fractions of G. indica have been reported [33]. G. indica contains a major active compound called garcinol. This component is a yellow-colored, fat soluble pigment found in the rinds of G. indica at a level of 2–3%, which can be separated from the fruit rinds by EtOH and hexane extraction [35]. Garcinol shows powerful antioxidant activity, since it contains both phenolic hydroxyl groups and a β-diketone moiety, and in this respect, it resembles the structure of curcumin [48]. In addition, like the various pharmacological activities of G. indica mentioned above, there are many reported mechanisms by which garcinol also acts as an antioxidant, anti-inflammatory, or anticancer effects. It inhibited free-radical DPPH and was shown to have antioxidant activity on arachidonic acid metabolism and NO radical synthesis. These activities are involved in inflammation and carcinogenesis. Garcinol effectively inhibited inducible nitric oxide synthase (iNOS) synthesis by suppressing the activation of nuclear factor NFκB, and diminished the production of extracellular signal-regulated kinase 1/2, cyclooxygenase-2, and prostaglandins. In addition, it inhibited the activation of 5-lipoxygenase, which is responsible for the production of inflammatory molecules such as leukotrienes. These actions have inspired studies on inflammation-related cancers. In addition to reducing inflammation, the mechanisms underlying anticancer effects such as induction of apoptosis, inhibition of cell growth and proliferation, stimulation of cell cycle arrest and prevention of cancer cell metastasis have been identified. Consequently, numerous preclinical studies have reviewed the antitumor potential of garcinol in a variety of oncological variants, including colon, breast, prostate, head, and neck cancer, and hepatocellular carcinoma [86,87]. Furthermore, the antioxidant and anti-inflammatory properties of garcinol could be extended to a neuroprotective role, and garcinol acts as a potent natural HAT inhibitor, and has presented promising results in molecular interactions studies against MAO-B and catechol-O-methyltransferase, as well as in l-DOPA-induced dyskinesia. It has recently been reported the ability of garcinol to modulate memory and cognition by affecting nerve growth and survival and altering the neurochemical state of the brain [88].
5. Conclusions
G. indica has various pharmacological activities including antioxidant, anti-obesity, anti-arthritis, anti-inflammatory, antibacterial, hepatoprotective, cardioprotective, anti-depressant and anti-anxiety effects. These characteristics are consistent with the previously reported activity of abundant phytochemical components such as garcinol, HCA, cyanidin-3-sambubioside and cyanidin-3-glucoside isolated from G. indica. These studies suggest the potential of G. indica as a promising therapeutic agent for controlling and preventing various diseases. However, given that most of the trials have been conducted either in vitro or in vivo, further study at the clinical level is needed to establish the efficacy and safety of G. indica in humans.
Author Contributions
Conceptualization, C.H.L. and C.-I.C.; methodology, S.H.L. and C.-I.C.; writing—original draft preparation, S.H.L. and H.S.L.; writing—review and editing, C.H.L. and C.-I.C.; supervision, C.H.L. and C.-I.C.; project administration, C.-I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the BK21 FOUR program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education (MOE, Korea).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnes J., Anderson L.A., Phillipson J.D. Herbal Medicines. 3rd ed. Pharmaceutical Press; London, UK: 2007. pp. 1–23. [Google Scholar]
- 2.Kunle O.F., Egharevba H.O., Ahmadu P.O. Standardization of Herbal Medicines—A Review. Int. J. Biodivers. Conserv. 2012;4:101–112. doi: 10.5897/IJBC11.163. [DOI] [Google Scholar]
- 3.Jachak S.M., Saklani A. Challenges and Opportunities in Drug Discovery from Plants. Curr. Sci. 2007;92:1251–1257. [Google Scholar]
- 4.Imam M.Z., Akter S. Musa Paradisiaca L. and Musa Sapientum L.: A Phytochemical and Pharmacological Review. J. Appl. Pharm. Sci. 2011;1:14–20. [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 6.An P., Zhang L.-J., Peng W., Chen Y.-Y., Liu Q.-P., Luan X., Zhang H. Natural Products Are an Important Source for Proteasome Regulating Agents. Phytomedicine. 2021;93:153799. doi: 10.1016/j.phymed.2021.153799. [DOI] [PubMed] [Google Scholar]
- 7.Di Stasi L.C., Hiruma-Lima C.A. Plantas Medicinais Na Amazônia e Na Mata Atlântica. 2nd ed. Editora Unesp; Sâo Paulo, Brazil: 2002. pp. 87–112. [Google Scholar]
- 8.de Melo M.S., Quintans J.d.S.S., Araújo A.A. de S.; Duarte, M.C.; Bonjardim, L.R.; Nogueira, P.C. de L.; Moraes, V.R. de S.; Araújo-Júnior, J.X. de; Ribeiro, Ê.A.N.; Quintans-Júnior, L.J. A Systematic Review for Anti-Inflammatory Property of Clusiaceae Family: A Preclinical Approach. Evid.-Based Complement. Altern. Med. 2014;2014:e960258. doi: 10.1155/2014/960258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acuña U.M., Dastmalchi K., Basile M.J., Kennelly E.J. Quantitative High-Performance Liquid Chromatography Photo-Diode Array (HPLC-PDA) Analysis of Benzophenones and Biflavonoids in Eight Garcinia Species. J. Food Compos. Anal. 2012;25:215–220. doi: 10.1016/j.jfca.2011.10.006. [DOI] [Google Scholar]
- 10.Hemshekhar M., Sunitha K., Santhosh M.S., Devaraja S., Kemparaju K., Vishwanath B.S., Niranjana S.R., Girish K.S. An Overview on Genus Garcinia: Phytochemical and Therapeutical Aspects. Phytochem. Rev. 2011;10:325–351. doi: 10.1007/s11101-011-9207-3. [DOI] [Google Scholar]
- 11.Chen T.-H., Tsai M.-J., Fu Y.-S., Weng C.-F. The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia Linii with Comprehensive Review of the Garcinia Family. Biomolecules. 2019;9:641. doi: 10.3390/biom9110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do Espirito Santo B.L.S., Santana L.F., Kato Junior W.H., de Araújo F.d.O., Bogo D., Freitas K. de C.; Guimarães, R. de C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F. de O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules. 2020;25:4513. doi: 10.3390/molecules25194513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao A.V.R., Sarma M.R., Venkataraman K., Yemul S.S. A Benzophenone and Xanthone with Unusual Hydroxylation Patterns from the Heartwood of Garcinia Pedunculata. Phytochemistry. 1974;13:1241–1244. doi: 10.1016/0031-9422(74)80109-3. [DOI] [Google Scholar]
- 14.Yamaguchi F., Saito M., Ariga T., Yoshimura Y., Nakazawa H. Free Radical Scavenging Activity and Antiulcer Activity of Garcinol from Garcinia Indica Fruit Rind. J. Agric. Food Chem. 2000;48:2320–2325. doi: 10.1021/jf990908c. [DOI] [PubMed] [Google Scholar]
- 15.Ito C., Itoigawa M., Miyamoto Y., Onoda S., Rao K.S., Mukainaka T., Tokuda H., Nishino H., Furukawa H. Polyprenylated Benzophenones from Garcinia Assigu and Their Potential Cancer Chemopreventive Activities. J. Nat. Prod. 2003;66:206–209. doi: 10.1021/np020372g. [DOI] [PubMed] [Google Scholar]
- 16.Pan M.-H., Chang W.-L., Lin-Shiau S.-Y., Ho C.-T., Lin J.-K. Induction of Apoptosis by Garcinol and Curcumin through Cytochrome c Release and Activation of Caspases in Human Leukemia HL-60 Cells. J. Agric. Food Chem. 2001;49:1464–1474. doi: 10.1021/jf001129v. [DOI] [PubMed] [Google Scholar]
- 17.De Meyts P., Whittaker J. Structural Biology of Insulin and IGF1 Receptors: Implications for Drug Design. Nat. Rev. Drug Discov. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 18.Liao C.-H., Sang S., Liang Y.-C., Ho C.-T., Lin J.-K. Suppression of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in Downregulating Nuclear Factor-Kappa B Pathway by Garcinol. Mol. Carcinog. 2004;41:140–149. doi: 10.1002/mc.20050. [DOI] [PubMed] [Google Scholar]
- 19.Koeberle A., Northoff H., Werz O. Identification of 5-Lipoxygenase and Microsomal Prostaglandin E2 Synthase-1 as Functional Targets of the Anti-Inflammatory and Anti-Carcinogenic Garcinol. Biochem. Pharmacol. 2009;77:1513–1521. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Hong J., Sang S., Park H.-J., Kwon S.J., Suh N., Huang M.-T., Ho C.-T., Yang C.S. Modulation of Arachidonic Acid Metabolism and Nitric Oxide Synthesis by Garcinol and Its Derivatives. Carcinogenesis. 2006;27:278–286. doi: 10.1093/carcin/bgi208. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi F., Ariga T., Yoshimura Y., Nakazawa H. Antioxidative and Anti-Glycation Activity of Garcinol from Garcinia Indica Fruit Rind. J. Agric. Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- 22.Kolodziejczyk J., Masullo M., Olas B., Piacente S., Wachowicz B. Effects of Garcinol and Guttiferone K Isolated from Garcinia Cambogia on Oxidative/Nitrative Modifications in Blood Platelets and Plasma. Platelets. 2009;20:487–492. doi: 10.3109/09537100903165182. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanyam K., Altaf M., Varier R.A., Swaminathan V., Ravindran A., Sadhale P.P., Kundu T.K. Polyisoprenylated Benzophenone, Garcinol, a Natural Histone Acetyltransferase Inhibitor, Represses Chromatin Transcription and Alters Global Gene Expression. J. Biol. Chem. 2004;279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 24.Kopytko P., Piotrowska K., Janisiak J., Tarnowski M. Garcinol-A Natural Histone Acetyltransferase Inhibitor and New Anti-Cancer Epigenetic Drug. Int. J. Mol. Sci. 2021;22:2828. doi: 10.3390/ijms22062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padhye S., Ahmad A., Oswal N., Sarkar F.H. Emerging Role of Garcinol, the Antioxidant Chalcone from Garcinia Indica Choisy and Its Synthetic Analogs. J. Hematol. Oncol. 2009;2:38. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khare C.P. Indian Medicinal Plants: An Illustrated Dictionary. 2nd ed. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2008. pp. 278–279. [Google Scholar]
- 27.Swami S.B., Thakor N.J., Patil S.C. Kokum (Garcinia Indica) and Its Many Functional Components as Related to the Human Health: A Review. J. Food Res. Technol. 2014;2:130–142. [Google Scholar]
- 28.Tamil Selvi A., Joseph G.S., Jayaprakasha G.K. Inhibition of Growth and Aflatoxin Production in Aspergillus Flavus by Garcinia Indica Extract and Its Antioxidant Activity. Food Microbiol. 2003;20:455–460. doi: 10.1016/S0740-0020(02)00142-9. [DOI] [Google Scholar]
- 29.Khatib N.A., Kiran P., Patil P.A. Evaluation of Anti Inflammatory Activity of Garcinia Indica Fruit Rind Extracts in Wistar Rats. Int. J. Res. Ayurveda Pharm. IJRAP. 2010;1:449–454. [Google Scholar]
- 30.Varalakshmi K.N., Sangeetha C.G., Shabeena A.N., Sunitha S.R., Vapika J. Antimicrobial and Cytotoxic Effects of Garcinia Indica Fruit Rind Extract. Am.-Eurasian J. Agric. Environ. Sci. 2010;7:652–656. [Google Scholar]
- 31.Ding M., Feng R., Wang S.Y., Bowman L., Lu Y., Qian Y., Castranova V., Jiang B.-H., Shi X. Cyanidin-3-Glucoside, a Natural Product Derived from Blackberry, Exhibits Chemopreventive and Chemotherapeutic Activity. J. Biol. Chem. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- 32.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Dietary Cyanidin 3-O-β-D-Glucoside-Rich Purple Corn Color Prevents Obesity and Ameliorates Hyperglycemia in Mice. J. Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 33.Baliga M.S., Bhat H.P., Pai R.J., Boloor R., Palatty P.L. The Chemistry and Medicinal Uses of the Underutilized Indian Fruit Tree Garcinia Indica Choisy (Kokum): A Review. Food Res. Int. 2011;44:1790–1799. doi: 10.1016/j.foodres.2011.01.064. [DOI] [Google Scholar]
- 34.Parasharami V., Kunder G., Desai N. Recent Pharmacological Advances of Endangered Species of South India: Garcinia Indica Choisy. J. Sci. Res. Rep. 2015;8:1–10. doi: 10.9734/JSRR/2015/14550. [DOI] [Google Scholar]
- 35.Jagtap P., Bhise K., Prakya V. A Phytopharmacological Review on Garcinia Indica. Int. J. Herb. Med. 2015;3:2–7. [Google Scholar]
- 36.Panda V., Ashar H., Srinath S. Antioxidant and Hepatoprotective Effect of Garcinia Indica Fruit Rind in Ethanol-Induced Hepatic Damage in Rodents. Interdiscip. Toxicol. 2012;5:207–213. doi: 10.2478/v10102-012-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangaonkar G.M., Pawar K.D. Garcinia Indica Mediated Biogenic Synthesis of Silver Nanoparticles with Antibacterial and Antioxidant Activities. Colloids Surf. B Biointerfaces. 2018;164:210–217. doi: 10.1016/j.colsurfb.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 38.Barve K. Garcinol Enriched Fraction from the Fruit Rind of Garcinia Indica Ameliorates Atherosclerotic Risk Factor in Diet Induced Hyperlipidemic C57BL/6 Mice. J. Tradit. Complement. Med. 2019;11:95–102. doi: 10.1016/j.jtcme.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel K.J., Panchasara A.K., Barvaliya M.J., Purohit B.M., Baxi S.N., Vadgama V.K., Tripathi C.B. Evaluation of Cardioprotective Effect of Aqueous Extract of Garcinia Indica Linn. Fruit Rinds on Isoprenaline-Induced Myocardial Injury in Wistar Albino Rats. Res. Pharm. Sci. 2015;10:388–396. [PMC free article] [PubMed] [Google Scholar]
- 40.Dhamija I., Parle M., Kumar S. Antidepressant and Anxiolytic Effects of Garcinia Indica Fruit Rind via Monoaminergic Pathway. 3 Biotech. 2017;7:131. doi: 10.1007/s13205-017-0766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tung Y.-C., Shih Y.-A., Nagabhushanam K., Ho C.-T., Cheng A.-C., Pan M.-H. Coleus Forskohlii and Garcinia Indica Extracts Attenuated Lipid Accumulation by Regulating Energy Metabolism and Modulating Gut Microbiota in Obese Mice. Food Res. Int. 2021;142:110143. doi: 10.1016/j.foodres.2021.110143. [DOI] [PubMed] [Google Scholar]
- 42.Warriar P., Barve K., Prabhakar B. Anti-Arthritic Effect of Garcinol Enriched Fraction Against Adjuvant Induced Arthritis. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13:49–56. doi: 10.2174/1872213X12666181120091528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ames B.N., Shigenaga M.K., Gold L.S. DNA Lesions, Inducible DNA Repair, and Cell Division: Three Key Factors in Mutagenesis and Carcinogenesis. Environ. Health Perspect. 1993;101:35–44. doi: 10.1289/ehp.93101s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Temple N.J. Antioxidants and Disease: More Questions than Answers. Nutr. Res. 2000;20:449–459. doi: 10.1016/S0271-5317(00)00138-X. [DOI] [Google Scholar]
- 45.Hur S.J., Lee S.Y., Kim Y.-C., Choi I., Kim G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- 46.Deore A.B., Sapakal V.D., Naikwade N.S. Antioxidant and Hepatoprotective Activity of Garcinia Indica Linn Fruit Rind. Int. J Compr. Pharm. 2011;2:1–5. [Google Scholar]
- 47.Singh T., Kasture S.B., Mohanty P.K., Jaliwala Y., Karchuli M. In Vitro Antioxidative Activity of Phenolic and Flavonoid Compounds Extracted from Fruit of Garcinia Indica. Int. J. Pharm. Life Sci. 2011;2:613–616. [Google Scholar]
- 48.Mishra A., Bapat M.M., Tilak J.C., Devasagayam T.P.A. Antioxidant Activity of Garcinia Indica (Kokam) and Its Syrup. Curr. Sci. 2006;91:90–93. [Google Scholar]
- 49.Rawri R.K., Bharathi K., Jayaveera K.N., Asdaq S.M.B. In Vitro Antioxidant Properties of Garcinia Indica Linn Alcoholic Fruits Extracts. J. Pharm. Sci. Innov. 2013;2:9–12. doi: 10.7897/2277-4572.02328. [DOI] [Google Scholar]
- 50.Krishnamurthy N., Sampathu S.R. Antioxydant Principles of Kokum Rind. J. Food Sci. Technol. Mysore. 1988;25:44–45. [Google Scholar]
- 51.Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of Cytotoxicity and Inhibition of Intercellular Communication by Antioxidant Catechins Isolated from Chinese Green Tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 52.Darji K.K., Shetgiri P., D’mello P.M. Evaluation of Antioxidant and Antihyperlipidemic Activity of Extract of Garcinia Indica. Int. J. Pharm. Sci. Res. 2010;1:175–181. [Google Scholar]
- 53.Sang S., Liao C.-H., Pan M.-H., Rosen R.T., Lin-Shiau S.-Y., Lin J.-K., Ho C.-T. Chemical Studies on Antioxidant Mechanism of Garcinol: Analysis of Radical Reaction Products of Garcinol with Peroxyl Radicals and Their Antitumor Activities. Tetrahedron. 2002;58:10095–10102. doi: 10.1016/S0040-4020(02)01411-4. [DOI] [Google Scholar]
- 54.Astier Y., Bayley H., Howorka S. Protein Components for Nanodevices. Curr. Opin. Chem. Biol. 2005;9:576–584. doi: 10.1016/j.cbpa.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic Synthesis of Silver Nanoparticles and Their Antioxidant and Antibacterial Activity. Appl. Nanosci. 2016;6:755–766. doi: 10.1007/s13204-015-0473-z. [DOI] [Google Scholar]
- 56.Vo-Dinh T. Nanotechnology in Biology and Medicine: Methods, Devices, and Applications. 1st ed. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 57.Kulkarni N., Muddapur U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014;2014:e510246. doi: 10.1155/2014/510246. [DOI] [Google Scholar]
- 58.Apovian C.M. Obesity: Definition, Comorbidities, Causes, and Burden. Am. J. Manag. Care. 2016;22:10. [PubMed] [Google Scholar]
- 59.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015;4:363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 60.Lu M., Cao Y., Xiao J., Song M., Ho C.-T. Molecular Mechanisms of the Anti-Obesity Effect of Bioactive Ingredients in Common Spices: A Review. Food Funct. 2018;9:4569–4581. doi: 10.1039/C8FO01349G. [DOI] [PubMed] [Google Scholar]
- 61.Conforti F., Pan M.-H. Natural Products in Anti-Obesity Therapy. Molecules. 2016;21:1750. doi: 10.3390/molecules21121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee P.-S., Teng C.-Y., Kalyanam N., Ho C.-T., Pan M.-H. Garcinol Reduces Obesity in High-Fat-Diet-Fed Mice by Modulating Gut Microbiota Composition. Mol. Nutr. Food Res. 2019;63:e1800390. doi: 10.1002/mnfr.201800390. [DOI] [PubMed] [Google Scholar]
- 63.Jena B.S., Jayaprakasha G.K., Singh R.P., Sakariah K.K. Chemistry and Biochemistry of (−)-Hydroxycitric Acid from Garcinia. J. Agric. Food Chem. 2002;50:10–22. doi: 10.1021/jf010753k. [DOI] [PubMed] [Google Scholar]
- 64.Tsuda T., Ueno Y., Aoki H., Koda T., Horio F., Takahashi N., Kawada T., Osawa T. Anthocyanin Enhances Adipocytokine Secretion and Adipocyte-Specific Gene Expression in Isolated Rat Adipocytes. Biochem. Biophys. Res. Commun. 2004;316:149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 65.Hong J.-I., Park I.Y., Kim H.A. Understanding the Molecular Mechanisms Underlying the Pathogenesis of Arthritis Pain Using Animal Models. Int. J. Mol. Sci. 2020;21:533. doi: 10.3390/ijms21020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang C.-H. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int. J. Mol. Sci. 2019;20:1646. doi: 10.3390/ijms20071646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel H. Drug Discovery and Evaluation: Pharmacological Assays. 2nd ed. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2007. pp. 902–942. [Google Scholar]
- 68.Zhu F., Du B., Xu B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018;58:1260–1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 69.Dinarello C.A. Anti-Inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka T., Narazaki M., Kishimoto T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edition F. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; Washington, DC, USA: 2013. [Google Scholar]
- 72.Grundmann O., Nakajima J.-I., Kamata K., Seo S., Butterweck V. Kaempferol from the Leaves of Apocynum Venetum Possesses Anxiolytic Activities in the Elevated plus Maze Test in Mice. Phytomedicine. 2009;16:295–302. doi: 10.1016/j.phymed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 73.Abouhosseini Tabari M., Hajizadeh Moghaddam A., Maggi F., Benelli G. Anxiolytic and Antidepressant Activities of Pelargonium Roseum Essential Oil on Swiss Albino Mice: Possible Involvement of Serotonergic Transmission. Phytother. Res. 2018;32:1014–1022. doi: 10.1002/ptr.6038. [DOI] [PubMed] [Google Scholar]
- 74.Benelli G., Maggi F., Nicoletti M. Ethnopharmacology in the Fight against Plasmodium Parasites and Brain Disorders: In Memoriam of Philippe Rasoanaivo. J. Ethnopharmacol. 2016;193:726–728. doi: 10.1016/j.jep.2016.07.077. [DOI] [PubMed] [Google Scholar]
- 75.Woolhouse M., Waugh C., Perry M.R., Nair H. Global Disease Burden Due to Antibiotic Resistance—State of the Evidence. J. Glob. Health. 2016;6:010306. doi: 10.7189/jogh.06.010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cattoir V., Felden B. Future Antibacterial Strategies: From Basic Concepts to Clinical Challenges. J. Infect. Dis. 2019;220:350–360. doi: 10.1093/infdis/jiz134. [DOI] [PubMed] [Google Scholar]
- 77.Negi P.S., Jayaprakasha G.K. Control of Foodborne Pathogenic and Spoilage Bacteria by Garcinol and Garcinia Indica extracts, and Their Antioxidant Activity. J. Food Sci. 2004;69:FMS61–FMS65. doi: 10.1111/j.1365-2621.2004.tb13372.x. [DOI] [Google Scholar]
- 78.Pasha C., Sayeed S., Ali S., Khan Z. Antisalmonella Activity of Selected Medicinal Plants. Turk. J. Biol. 2009;33:59–64. [Google Scholar]
- 79.Kunder G., Parasharami V. Evidence to Prove Why Garcinia Indica Choisy Leaves Does Not Respond to Hairy Root Induction by Agrobacterium Rhizogenes Mediated Transformation along with Positive Antimicrobial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:720–730. [Google Scholar]
- 80.Ahmed S., Ahmad M., Swami B.L., Ikram S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siddiq A.M., Parandhaman T., Begam A.F., Das S.K., Alam M.S. Effect of Gemini Surfactant (16-6-16) on the Synthesis of Silver Nanoparticles: A Facile Approach for Antibacterial Application. Enzym. Microb. Technol. 2016;95:118–127. doi: 10.1016/j.enzmictec.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 82.AESAN Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the Risk Associated with the Consumption of Food Supplements that Contain Garcinia Gummi-Gutta as an Ingredient. [(accessed on 15 December 2021)]; Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_cc_ingles/GARCINIA_FOOD_SUPPLEMENTS.pdf.
- 83.Lunsford K.E., Bodzin A.S., Reino D.C., Wang H.L., Busuttil R.W. Dangerous Dietary Supplements: Garcinia Cambogia-Associated Hepatic Failure Requiring Transplantation. World J. Gastroenterol. 2016;22:10071–10076. doi: 10.3748/wjg.v22.i45.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crescioli G., Lombardi N., Bettiol A., Marconi E., Risaliti F., Bertoni M., Menniti Ippolito F., Maggini V., Gallo E., Firenzuoli F., et al. Acute Liver Injury Following Garcinia Cambogia Weight-Loss Supplementation: Case Series and Literature Review. Intern. Emerg. Med. 2018;13:857–872. doi: 10.1007/s11739-018-1880-4. [DOI] [PubMed] [Google Scholar]
- 85.Sharma A., Akagi E., Njie A., Goyal S., Arsene C., Krishnamoorthy G., Ehrinpreis M. Acute Hepatitis Due to Garcinia Cambogia Extract, an Herbal Weight Loss Supplement. Case Rep. Gastrointest. Med. 2018;2018:9606161. doi: 10.1155/2018/9606171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu C., Ho P.C.-L., Wong F.C., Sethi G., Wang L.Z., Goh B.C. Garcinol: Current Status of Its Anti-Oxidative, Anti-Inflammatory and Anti-Cancer Effects. Cancer Lett. 2015;362:8–14. doi: 10.1016/j.canlet.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Aggarwal V., Tuli H.S., Kaur J., Aggarwal D., Parashar G., Chaturvedi Parashar N., Kulkarni S., Kaur G., Sak K., Kumar M., et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines. 2020;8:103. doi: 10.3390/biomedicines8050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deb S., Phukan B.C., Mazumder M.K., Dutta A., Paul R., Bhattacharya P., Sandhir R., Borah A. Garcinol, a Multifaceted Sword for the Treatment of Parkinson’s Disease. Neurochem. Int. 2019;128:50–57. doi: 10.1016/j.neuint.2019.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.