Abstract
Drug delivery systems (DDS) often comprise biopharmaceuticals in aqueous form, making them susceptible to physical and chemical degradation, and therefore requiring low temperature storage in cold supply and distribution chains. Freeze-drying, spray-drying, and spray-freeze-drying are some of the techniques used to convert biopharmaceuticals-loaded DDS from aqueous to solid dosage forms. However, the risk exists that shear and heat stress during processing may provoke DDS damage and efficacy loss. Supercritical fluids (SCF), specifically, supercritical carbon dioxide (scCO2), is a sustainable alternative to common techniques. Due to its moderately critical and tunable properties and thermodynamic behavior, scCO2 has aroused scientific and industrial interest. Therefore, this article reviews scCO2-based techniques used over the year in the production of solid biopharmaceutical dosage forms. Looking particularly at the use of scCO2 in each of its potential roles—as a solvent, co-solvent, anti-solvent, or co-solute. It ends with a comparison between the compound’s stability using supercritical CO2-assisted atomization/spray-drying and conventional drying.
Keywords: biopharmaceuticals, solid dosage forms, drying technologies, sustainable engineering, supercritical carbon dioxide, supercritical carbon dioxide-assisted spray-drying
1. Introduction
Biopharmaceuticals include enzymes, nucleic acids, monoclonal antibodies, and recombinant proteins that are either manufactured or isolated from biological sources for medical application [1]. Due to their greater specificity, affinity, and potency than those of small and chemically synthesized molecules, biopharmaceuticals have aroused tremendous scientific and market interest in recent years. The biopharmaceutical market represented USD 1.6 billion in 2016, increased 102% by 2008 [2] and continues to grow apace. One biopharmaceutical to recently attract attention is recombinant human deoxyribonuclease I (commercially called Dornase alfa) which is widely used in a solution as a cystic fibrosis treatment, as it reduces the viscosity and quantity of airway mucus. Recently, Earhart et al. [3] proposed the use of Dornase alfa for COVID-19 treatment. However, biopharmaceuticals can be challenging to formulate and deliver, owing to their molecular weights that can be up to 500 times that of small molecules. For example, the enzyme golimumab has a molecular weight of 147 kDa whereas quercetin weighs approximately 302 Da, which hinders permeation across biological membranes. It also presents poor biophysical stability, being susceptible to degradation or aggregation depending on the ambient moisture, temperature, or even pH, light, and shear stress [4,5]. Such challenges can be overcome by associating biopharmaceuticals to drug delivery systems [6], such as polymers [7,8,9], lipid-based nanoparticles [10,11,12], metal-organic frameworks [5,13], and dendrimers [14,15]. However, biopharmaceutical-based drug delivery systems are usually formulated in aqueous forms [16] which require storage at temperatures between 2 and 8 °C or even below −70 °C to prevent chemical and physical destabilization [17]. Nevertheless, converting these biopharmaceuticals-loaded drug delivery systems into solid dosage forms (or dry powder formulations) removes the water and minimizes oxidation and hydrolysis degradation, thereby improving stability and reducing cold distribution and supply chain costs [18]. Moreover, solid dosage conversion widens the range of potential administration routes to include intravenous, intraperitoneal, oral, nasal, and pulmonary administration. The latter is particularly attractive as the lung’s physiognomy increases bioavailability due to its large absorptive surface area. Advantages of pulmonary administration include: the ability to use smaller dosage sizes due to the local biodistribution enhancing effect, the avoidance of first-pass hepatic elimination, gut wall metabolism, and/or gastrointestinal tract destruction [19,20].
Freeze-drying is the best-known drying process for biopharmaceuticals [21,22]. Fifty percent of biopharmaceuticals approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2016 were estimated to be freeze-dried [23]. Spray-drying and spray-freeze-drying offer greater control of particle morphology and scalability, while requiring less time. In addition, plentiful data are available for the use of these techniques in biopharmaceutical drying [16,18,24]. Exubera® [25], by Pfizer, was a commercial example of insulin-based solid dosage form for diabetes treatment. This ready-to-inhale powder was mostly made up of mannitol, glycine, and sodium citrate insulin excipients that are produced by spray-drying. In 2007, Exubera® was withdrawn from the market due to low sales [26]. Ibrahim et al. [27] reported using spray-drying for basic fibroblast growth factor (bFGF) as alternative asthma and chronic obstructive pulmonary disease (COPD) therapies. Such techniques, however, are costly and require high levels of energy. What is more, they might also induce heat, cold, or shear stress, resulting in biopharmaceutical degradation.
Recent decades have seen an upsurge of interest in solid dosage forms using supercritical fluids (SCF) since first reported in 1879 by Hannay and Hogarth [28]. The advantages include the ability to operate at mild conditions, their cost-effectiveness, the ability to produce microparticulate protein powders, and their scaling up feasibility [29]. Supercritical CO2 (scCO2) is the most common SCF, with a low critical temperature and moderate critical pressure at 31.1 °C and 7.38 MPa, respectively, making them suitable for processing heat-sensitive compounds [20,30]. Moreover, CO2 is non-flammable, non-toxic, recyclable, inert, chemically stable, and, as it is a gas at normal conditions, it can be removed by simply lowering the pressure [19]. At supercritical conditions, CO2 has liquid-like density and gas-like transport properties. Thus, its polarity and solvation power are superior, and it offers the mass transfer properties of a low viscosity gas in association with high associated diffusivity [31].Moreover, these properties are easily tunable near the critical point via minor temperature or pressure changes [32]. For this reason, scCO2 can be used with different technologies, depending on its solvating behavior. In fact, these processes can be classified into different groups depending on the scCO2 processing role: acting as a solvent, co-solvent, anti-solvent, or as a co-solute [33]. scCO2 use also respects some of the Twelve principles of green chemistry, outlined by Anastas in 1991 [34], rating in fifth place Safer solvents and auxiliaries. Next, we will review the latest works on the bottom-up assembly of solid dosage forms of biopharmaceuticals in drug delivery systems using scCO2 as a sustainable strategy.
2. Supercritical CO2-Based Drying Techniques to Produce Solid Dosage Forms of Biopharmaceuticals in Drug Delivery Systems
2.1. Rapid Expansion of Supercritical Solvent (RESS)
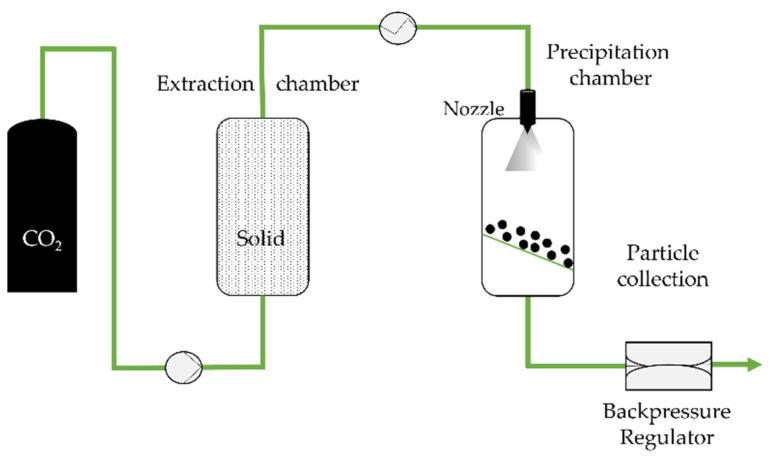
The rapid expansion of supercritical solvent (RESS) is a 1987 technique by Peterson, Matson, and Smith [35,36,37] which consists of saturating supercritical fluids with a solid substrate (and the coating agent), without any organic solvents. Upon depressurization to subcritical pressure using a heated nozzle, the substrate precipitates into a lower pressure precipitation chamber as the solution density drops (Figure 1) (usually near atmospheric pressure) [35,38].
Figure 1.
The rapid expansion of supercritical solvent (RESS) process, adapted from Aguiar-Ricardo et al. [39].
The morphology and physicochemical properties of the resulting powders are related to the processing parameters (temperature, pressure, nozzle geometry) and the chemical structure of the material [40]. RESS has been applied mainly to the production of micronized poor water-soluble compounds. In 1993, Reverchon et al. [41] reported the micronization of salicylic acid. The first particles were in needle form, but the work was optimized, and spherical particles resulted. Years later, Yildiz and co-authors [42] reported the micronization of salicylic acid and paclitaxel, using ethanol as a co-solvent to improve salicylic acid’s solubility in CO2. This highlights, in fact, one of the technique’s limitations. On the one hand, scCO2 presents low solubility for most polymers and pharmaceuticals. On the other, the addition of a second component influences phase behavior. Unfortunately, there is little data available for the multicomponent systems involved in real processes [43].
Years later, a variation in a non-solvent RESS method (RESS-N), introduced by Mishima and co-authors, aroused considerable interest [44]. An organic solvent which does not, in its pure form, solubilize the polymer was added as a co-solvent. Nevertheless, in the presence of those organic solvents, the polymer solubility in CO2 increased significantly. In sum, a suspension of a drug in the ternary system composed by CO2, the non-toxic organic solvent (such as ethanol, isopropanol), and the dissolved polymer was sprayed through the nozzle. The polymer excipient then precipitated around the drug, producing core–shell structured microparticles [45]. Another process modification involved the rapid expansion of a supercritical solution into a liquid solvent (RESOLV) in order to obtain nanoscale particles [46,47]. In this case, however, the supercritical solution was expanded into a liquid solvent instead of a lower pressure precipitation chamber, and particles with a diameter less than 50 nm were obtained [46].
RESS is more often used with poor water-soluble compound processing, yet it (and its derivatives) has been also applied to encapsulating biopharmaceuticals in drug delivery microsystems. In fact, the processing of water-soluble compounds using RESS can be quite challenging due to CO2′s poor solubility in water. Table 1 displays research reporting on the encapsulation of biopharmaceuticals and the final dry powder properties.
Table 1.
Solid dosage form biopharmaceuticals in RESS drug delivery systems.
| Year | Active Compound | Co-Solvent | Solid Dosage Form | Observations | Ref. |
|---|---|---|---|---|---|
| 2000 | Lysozyme * Lipase |
Ethanol | PEG 1 PMMA 2 P(DLLA) 3 P(St) 4 PLGA 5 PEG-PPG 6 |
Lower diameters for ethanol as a co-solvent The size is more influenced by the polymer feed composition |
[44] |
| 2002 | BSA 7 | N/A | Dynasan®114 Gelucire®50-02 |
Similar RESS method Dynasan®114-based microparticle diameter < 50 µm 13% < BSA content < 62% Gelucire®50-02-based microparticles with a mean size of 543 µm 36% < BSA content < 67% |
[48] |
| 2012 | Co-enzyme Q10 | Ethanol Acetone Dichloromethane |
PEG 1 P(DLLA) 3 |
Microparticle diameter between 2–8 µm PEG coating results in higher microparticles Higher coQ10 release for higher microparticles |
[49] |
| 2013 | Insulin | Ethanol | Tripalmitin | RESS is combined with the supercritical assisted drying (SAD) 3.5 µm < PSD 8 < 11 µm Insulin content of 33.1% |
[50] |
1 Poly (ethylene glycol); 2 Poly (methyl methacrylate); 3 Poly (DL-lactic acid); 4 Poly (styrene); 5 Poly (DL-lactide-co-glycolide); 6 Poly (propylene glycol); 7 Bovine serum albumin; 8 Particle size distribution; * RESS-N.
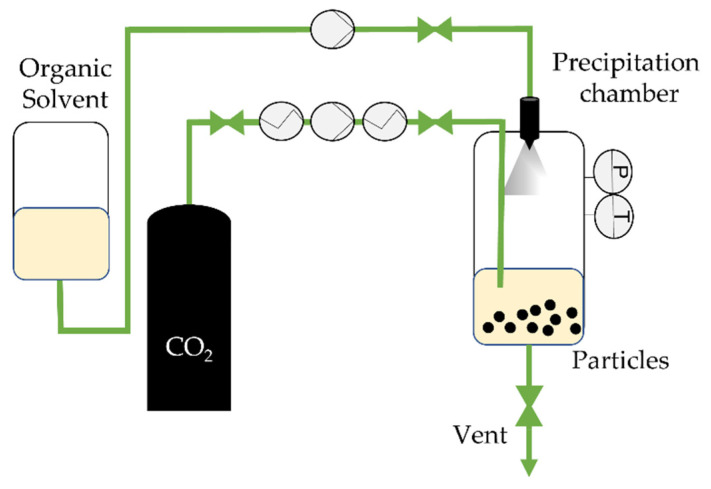
2.2. Particles from Gas-Saturated Solutions (PGSS)
The PGSS technique [51] was introduced in 1994 by Weidner, Knez, and Novak and uses CO2 as a solute. ScCO2 is dissolved into a melted material, consisting of drugs, polymers, biopolymers, oils, or fatty acids in a stirred high-pressure vessel until saturation is achieved [33]. The gas saturated liquid is then expanded through the nozzle to an expansion chamber at ambient pressure and lower temperature where the particles precipitate (Figure 2).
Figure 2.
The particles from gas-saturated solutions (PGSS) technique, adapted from Kerč et al. [52].
P and T–Pressure and temperature indicator and controller in the autoclave, respectively. PGSS can be more effective than RESS insofar as the compound (drug or polymer) does not need to be dissolved in scCO2 [52] and no organic solvents are necessary. Moreover, PGSS enables polar and water-soluble compounds to be processed. For this reason, this technique has been widely used in the pharmaceutical field with different compounds. Initially, compounds were processed without carriers. In 1999, Kerč and co-workers [52] used the technique to micronize nifedipine and felodipine (commonly used in hypertension treatment) in order to increase the dissolution rate and their bioavailability. Using temperatures between 150 °C and 185 °C, nifedipine and felodipine microparticles with an irregular shape showed a mean size between 15 and 30 µm and 42 µm, respectively. Smaller microparticles showed an increased dissolution rate. However, the effective surface area was reduced by the drug’s hydrophobicity and agglomeration. Thus, to reduce agglomeration, PEG4000 was added. Microparticles were produced between 50 and 70 °C since the polymer decreased the drug’s melting point. It was found that the size reduction and the drug/ PEG4000 interaction contributed for an enhanced dissolution rate against the pure micronized drugs. Interestingly, this study allowed the further micronization of drug delivery systems with different carriers. Hao et al. [53] produced poly(DL-lactic acid) (P(DLLA)) microparticles, affirming that solid drug particles can be mixed into the plasticized polymer. Figure 3 shows that scCO2 dissolves into and plasticizes the polymer at temperatures significantly below its glass transition temperature [54].
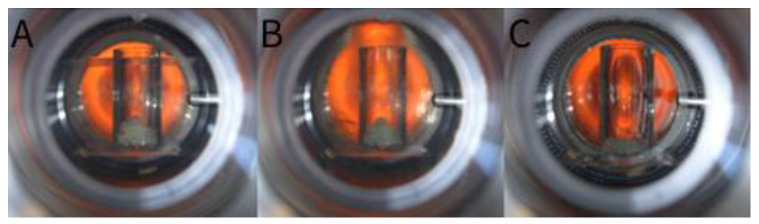
Figure 3.
Plasticization of the P(LLA)-PEG1500-P(LLA) copolymers by scCO2, with rise in working temperature and pressure, from Perenelli et al. [55]. (A) Stage conditions at 29 °C and 71 bar; (B) conditions at 35 °C and 85 bar; and (C) plasticization point at 47 °C and 140 bar. Reproduced with permission. Copyright 2014, Elsevier.
A year later, Hao et al. [56] applied PGSS to produce PEG microparticles and observed that the temperature increase and the pressure decrease favored the production of spherical particles. These works contributed to the development of the CriticalMix™—a solvent-free encapsulation process based on PGSS [57]. Rodrigues et al. [58] reported the encapsulation of theophylline—a bronchodilator—in hydrogenated palm oil, yielding microparticles sized between 2 and 3 µm. Higher working pressures (P) were found to lead to a more spherical size and a more limited distribution. Lower P of 120 or 140 bar led to agglomerated structures. Theophylline encapsulation was lower since the drug was mostly located on the carrier’s surface. More recently, Tokunaga et al. [59] reported the encapsulation of an amino acid, phenylalanine, in Eudragit L100. The work was carried out at 50 °C, between 80 and 120 bar. Microparticles sized from 200 µm to 140 µm were obtained for the respective pressures. At 100 bar, an encapsulation efficiency (EE) of 68.7% was achieved. In fact, the PGSS technique produced microparticles with a wide range of diameters, depending on the working parameters.
PGSS can encapsulate biopharmaceuticals at relatively low temperatures, which makes it attractive for thermolabile compound processing. Moreover, the lack of organic solvents contributes to the integrity of compounds’ activity. In 2005, Whitaker et al. [60] reported the encapsulation of ribonuclease A (RNase A), lysozyme, recombinant human insulin, and salmon calcitonin in P(DLLA) microparticles sized between 10 and 300 µm. Neither enzyme demonstrated an alteration. In turn, insulin activity decreased (although less so in the case of in P(DLLA) insulin encapsulation than for the control insulin powder). As for salmon calcitonin activity, the protein concentration was too low to measure. Salmaso et al. [61] describe an improved PGSS technique (GAMA–gas-assisted melting atomization) applied to the micronization of protein-loaded lipid particles. In this case, the protein and the melted lipid were loaded into a melting chamber that was then pressurized with scCO2 at 150 bar at a working temperature (T) of 40 °C, while undergoing stirring. The loaded lipid particles were dried with a stream of co-axially injected hot air. Vezzù et al. [62] encapsulated RNase A in lipids and polymers and observed that smaller particles were produced at lower temperatures. Table 2 reports the PGSS encapsulation of a range of biopharmaceuticals.
Table 2.
Solid dosage forms of biopharmaceuticals in PGSS drug delivery systems.
| Year | Active Compound | Co-Solvent | Solid Dosage Form | Observations | Ref. |
|---|---|---|---|---|---|
| 2005 | RNase A Lysozyme Insulin Salmon calcitonin |
N/A | P(DLLA) | 10 µm < PS 1 < 300 µm Insulin and salmon calcitonin microparticles with rough morphologies Poor particle size control RNase A and lysozyme retained their enzymatic activity Stored insulin microparticles decrease in activity at 25 °C for 1 week and 1 month Due to the low dosage of calcitonin, the salmon calcitonin was mixed with polymer powder and freeze-dried |
[60] |
| 2009 | Insulin Recombinant human growth hormone (rh-GH) |
1 mL of DMSO 2 containing the protein | Tristearin/Phosphatidylcholine/PEG5000 | Spherical particles with a mean diameter of 197 nm Insulin recovery of 57 ± 8% rh-GH recovery 48 ± 5% Glucose reduction of 50% with the lower dose and 70% at the higher dose, in 1–2 h. |
[61] |
| 2009 | Insulin | 1 mL of DMSO containing the protein | Tristearin/Phosphatidylcholine/PEG/Tween80 Tristearin/Phosphatidylcholine/dioctyl sulfosuccinate |
The authors defined the process as GAMA Binodal size distribution two main particle size populations. The main fraction had a diameter range of 200–400 nm, and a minor fraction had a diameter range of 80–120 nm. |
[63] |
| 2010 | RNase A | 1 mL of DMSO containing the enzyme | Tristearin/Phosphatidylcholine/PEG5000 | The higher the T, the higher the product yield The higher the T, the higher the particle size EE up to 80% The enzyme retained its a residual activity of about 83% |
[62] |
| 2010 | Human growth hormone (hGH) | N/A | P(DLLA) PLGA Excipients 3 |
Rounded particles with few pores Apparent size around 93 μm 56.1 μm < D50 4 < 104.5 μm 97.1% < EE < 100% |
[57] |
| 2011 | Co-enzyme Q10 | N/A | PEG6000 | Particle size of 190 nm 220 nm < Dv50 5 < 2.36 µm Enzyme recovery yield of 89.8% 6 |
[64] |
| 2011 | Human growth hormone (hGH) | N/A | PLGA P(DLLA) Poloxamer 407 |
EE of 98.3 ± 4.6% The structural integrity of hGH is unaffected by scCO |
[65] |
| 2013 | Progesterone (PGN) | N/A | PEG400/PEG4000 (50:50) D-α-tocopheryl PEG1000 succinate (TPGS) Gelucire 44/14 |
At T of approximately 56 °C, process yields of 95.7% for PNG-loaded TPGS86.3% for PNG-loaded PEG93.3% for PNG-loaded Gelucire 44/14 PGN showed high dissolution rates for all the formulations |
[66] |
| 2014 | Bovine serum albumin (BSA) | N/A | P(LLA) -PEG1500- P(LLA) | 19. 07 µm < PS50 < 78.63 µm 29.19% < Process yield < 41.74% 96.85% < EE < 101.75% |
[55] |
1 Particle size; 2 Dimethyl sulfoxide; 3 Poloxamer 188, poloxamer 407, or Solutol HS15; 4 Diameters at 50% cumulative volume; 5 Volume median diameter; 6 After PEG removal.
PGSS has also been modified to encapsulate bioactive molecules in solid lipid nanoparticles (SLN). Couto et al. [67] reported the encapsulation of Vitamin B2 into fully hydrogenated canola oil using a modified PGSS technique, where a stream of water and a stabilizer (PEG) were added to suspend SLN in water, yielding loaded SLN in aqueous bulk.
2.3. Carbon Dioxide-Assisted Nebulization with a Bubble Dryer (CAN-BD)
In 1997, Sievers et al. [68] proposed a PGSS improvement: the carbon dioxide-assisted nebulization with a bubble dryer (CAN-BD). This technique enables the use of any water-soluble compound, as well as the nebulization of inorganic salts or proteins [69]. Here, CO2 serves as a co-solute. An aqueous stream containing the drug and any excipients or stabilizers (typically 1 to 10% of the total solids dissolved [33]) is mixed with supercritical CO2 in a low-dead-volume mixing tee, forming a gas–liquid emulsion. This is then rapidly decompressed through a flow restrictor, forming an aerosol of droplets containing dissolved CO2. Upon expanding the dissolved CO2, the droplets rupture, and a fine aerosol, usually of diameters below 5 µm, is produced (Figure 4). It is important to point out that the expansion is enhanced since CO2 is one of the most soluble gases in water (1.6 mole% at 63 °C and 1500 psi). Dry particles are obtained in a furnace [70], and micronized particles are then collected on a filter.
Figure 4.
The carbon dioxide-assisted nebulization with a bubble dryer (CAN-BD) technique, adapted from Sievers et al. [69]. P, T: Pressure and temperature indicator and controller in the low-dead-volume mixing tee, respectively.
Due to the use of the aqueous stream, CAN-BD has been widely applied to processing water-soluble compounds. In 2001, Sellers et al. [71] reported the encapsulation of proteins (lysozyme and lactate dehydrogenase) using sucrose and mannitol as excipients for pulmonary delivery. A buffer was used to neutralize any potential acidification of the solution upon the addition CO2 to avoid damaging the protein. The proteins were also found to maintain their activity after rehydration due to the excipients and surfactant. Years later, Sievers et al. [72] reported the encapsulation of hepatitis B surface antigen (HBsAg) and a live-attenuated measles virus by trehalose and sucrose. In addition to achieving powder stability after 43 days of storage, they were also able to provide pulmonary administration. In 2008, Cape et al. [73] reported an impressive review of the CAN-BD applied to biopharmaceutical processing. Curiously, since then, CAN-BD has been mostly used to produce vaccines, such as the Edmonston-Zagreb live-attenuated measles virus vaccine [74,75,76], and inhalable antibiotics [77]. To our knowledge, no data on biopharmaceuticals in solid dosage forms have been reported.
2.4. Supercritical Assisted Atomization (SAA)/Supercritical CO2-Assisted Spray-Drying (SASD)
Supercritical assisted atomization (SAA) is based on the PGSS and CAN-BD techniques. First patented by Reverchon in 2001, it consists of the production of nano and microparticles from liquid formulations [78]. This one-step process is very similar to spray-drying but is aided by scCO2 and it can also be called supercritical CO2-assisted spray drying (SASD) [31,43]. scCO2 acts as a co-solute and is solubilized into a liquid solution containing the drug and/or a carrier system. The resulting near-equilibrium mixture flowing out of the saturator is atomized using a nozzle in a precipitation chamber at near atmospheric pressure, where it is dried with a hot air/nitrogen flow [43,79] which serves to avoid the Joule–Thomson effect upon decompression. Two atomization processes take place: (i) droplets at the nozzle are produced by pneumatic atomization; and (ii) CO2 is quickly released from inside the droplets [80]. Figure 5 illustrates a SAA/SASD process.
Figure 5.
The supercritical CO2-assisted spray drying (SASD) apparatus at NOVA’s laboratory. P, T: Pressure and temperature indicator and controller in the static-mixer, respectively.
A series of SAA/SASD studies have been published over the years reporting microparticle production, even for poorly water-soluble compounds. In 2002, Reverchon and co-authors reported the micronization of various compounds using SAA, from salts to corticosteroids [81]. Next, several demonstrated the micronization of antibiotics for inhalation [82,83,84]. The research continued, and later polymeric microparticles (chitosan [85], poly(methyl methacrylate), and poly-L-lactide [86]) were produced using the technique. Reverchon, in 2007, proposed to produce a composite, in which ampicillin trihydrate and chitosan were selected as a model drug and carrier, respectively, upscaling the technique for the production of a drug delivery system [87]. Several projects have developed drug delivery systems for inhalation. Cabral et al. [88] reported the production of inhalable ibuprofen-loaded chitosan microparticles. Nano-in-microparticles were also developed for effective pulmonary drug delivery using dendrimers [89] and gold nanoparticles [90] as well as gold-coated magnetite nanocomposites [91]. As in the case of RESS, SAA/SASD has also undergone modifications, such as the introduction of a hydrodynamic cavitation mixer (HCM) [92], to improve mass transfer. Smaller diameter microparticles were reported with a narrower particle size distribution than those produced without HCM.
SAA/SASD for encapsulating and delivering biopharmaceuticals (Table 3) has been studied for a large range of compounds from proteins [30] to nucleic acids [93]. Additionally, the encapsulation of a hydrophilic dye as a molecule model (5(6)-carboxyfluorescein) has recently been encapsulated in liposomes [94] upon encapsulating trehalose using SASD. The liposomes kept their structure upon resuspension and the encapsulation efficiency was above 95%. The powders showed a mass median aerodynamic diameter of 1.75 ± 0.04 µm and a fine particle fraction of 65 ± 3%, making them fit for pulmonary administration. Storage stability assays at relative humidities of 4%, 50%, and 78% at 20 °C for 7 and 30 days then revealed that the dry powder formulations kept their liposome structural stability at relative humidities of 4% and 50% at 20 °C for 30 days.
Table 3.
Solid dosage forms of biopharmaceuticals in drug delivery systems produced by SAA/SASD methods.
| Year | Active Compound | Nanocarrier | Co-Solvent | Solid Dosage Form | Observations | Ref. |
|---|---|---|---|---|---|---|
| 2009 | Lysozyme | N/A | Ethanol | N/A | Spherical microparticles 1.0 µm < PSD < 4.0 µm Lysozyme remained stable with biological activity from 95% to 100%. |
[80] |
| 2009 | Lysozyme Trypsin |
N/A | N/A | N/A | 80% of trypsin and 65% of lysozyme particles have a diameter smaller than 5 µm | [95] |
| 2010 | Gentamicin sulfate * | N/A | N/A | BSA | Mean diameter of 2 µm 1.70 µm < D50 > 2.24 µm EE > 95.6% |
[96] |
| 2011 | BSA | N/A | N/A | N/A | Well-defined, hollow, and spherical BSA microparticles 0.3 µm < PSD < 5.0 µm |
[97] |
| 2011 | BSA | N/A | N/A | N/A | The solubility of BSA is dependent on processing temperature | [98] |
| 2011 | Lysozyme | N/A | Ethanol | N/A | SAA-HCM 1 0.2 µm < PS < 5.0 µm Lysozyme kept 85% of its activity |
[99] |
| 2013 | Insulin | N/A | N/A | N/A | SAA-HCM 0.5 µm < PS < 5.0 µm |
[100] |
| 2015 | Trypsin | N/A | N/A | Chitosan | SAA-HCM 0.2 µm < PS < 4.0 µm LE 2 up to 91.8% Trypsin retained > 70% of its enzymatic activity |
[101] |
| 2017 | BSA | N/A | Acetonitrile | PLGA | 1.7 µm < MMAD 3 < 3.5 µm FPF 4 of 43% BSA showed both chemical and structural stability |
[30] |
| 2018 | Parathyroid hormone | N/A | N/A | Chitosan oligosaccharide | SAA-HCM 1.0 µm < MMAD < 5.0 µm FPF of 63.51% LE up to 92.8% |
[102] |
| 2020 | SiRNA 5 | Mesoporous silica nanoparticles Poly-L-arginine Hyaluronic acid | Ethanol | Chitosan (CHT) |
3.0 µm < Dv,50 < 4.0 µm FPF of 44.4% EEsiRNA of 11.4% onto LBL nanosystems Entrapment efficiency of the LbL nanoparticles of 28.7% in CHT powder 90% of gene silencing from CHT-LbL siRNA |
[93] |
1 Supercritical fluid assisted atomization introduced by a hydrodynamic cavitation mixer; 2 Loading efficiency; 3 Fine particle fraction; 4 Mass median aerodynamic diameter; 5 Small interfering RNA. * This work was selected since BSA was used as a microcarrier.
An adaptation of SAA to produce liposomes was recently reported [103]. Supercritical assisted Liposome formation (SuperLip) is a continuous operative process, in which biopharmaceuticals can be encapsulated in liposomes, with high reproducibility [104,105]. It is important to point out that these vesicles are obtained in aqueous bulk instead of solid dosage forms.
2.5. Depressurization of an Expanded Liquid Organic Solution (DELOS)
Ventosa and co-workers [106] reported the depressurization of an expanded liquid organic solution (DELOS) crystallization technique twenty years ago, and it has been commonly used for producing nano- and micro-sized crystalline particles. In this process, at certain temperature and pressure conditions, a compressed gas, e.g., CO2, is completely miscible with the organic solvent, acting as a co-solvent, unlike in other techniques [107]. This is a three-step process. First, the solute is dissolved in the organic solvent, below the saturation limit, at working temperature and atmospheric pressure. Then, an expanded liquid mixture is obtained by adding the compressed fluid, at high working pressure, which should not exceed the critical point of the CO2/solvent mixture. Finally, depressurization takes place by way of using a non-returning valve upon the rapid reduction of the pressure of the expanded liquid mixture, leading to the precipitation of nano- or microparticles [33,107] (Figure 6).
Figure 6.
The DELOS process, adapted from Ventosa et al. [106]. P, T: Pressure and temperature indicator and controller in the high-pressure vessel and before and after the non-return valve.
Since 2001, DELOS has been applied to the micronization of polymers, surfactants, [108] and poorly water-soluble compounds such as naproxen and ibuprofen [109,110,111]. This process uses less CO2; the filtration takes place at atmospheric pressure, and the lack of nozzles improves scalability [112]. A technique for developing unilamellar nanovesicles based on DELOS was developed that was similar to the above-mentioned SuperLip [113]. Depressurization of an expanded liquid organic solution into aqueous solution (DELOS-susp) is mainly applied to produce nanovesicles to carry nucleic acids and enzymes [50,114,115]. However, loaded liposomes are obtained in aqueous bulk rather than in the dried form.
2.6. Supercritical CO2 as Anti-Solvent
Since scCO2 is a poor solvent for most pharmaceutical compounds and polymers, some approaches use it as an anti-solvent. In a supercritical anti-solvent (SAS), a solute is dissolved into an organic solvent and precipitates when scCO2 is added. In fact, the precipitation occurs by the diffusion of CO2 in the organic solvent, expanding the liquid mixture, decreasing its density, and consequently, its solvation power, until nucleation and precipitation occur [116]. The mass transfer between the CO2 and the organic solution decreases the surface tension, which is strong enough to control droplet shape [117]. Figure 7 illustrates this process.
Figure 7.
The SAS process, adapted from Aguiar-Ricardo et al. [39]. P, T: Pressure and temperature indicator and controller in the precipitation chamber, respectively.
The CO2 is pumped until a desired pressure in the heated precipitator is achieved. The organic solvent with the solute(s) is then injected by nozzle. In the case of a drug/polymer system, both precipitate under supersaturation and the drug are trapped into the polymer matrix [118]. The loaded polymer precipitates on a filter and the organic solvent/CO2 is recovered for further separation [119]. Impressive reviews related to pharmaceutical encapsulation using the SAS and SAS-based methods have been published [117,119]. However, the use of organic solvents to dissolve the solute may represent an obstacle to biopharmaceutical applications [118]. Even so, some research has reported successful biopharmaceutical drying with the SAS technique (Table 4). In 1993, Yeo et al. [120] reported the first production of microparticulate insulin powders using SAS, obtaining powders with diameters mostly less than 4 µm. The blood glucose level decreased with the administration of the insulin microparticles. Further results showed that SAS can compromise proteins’ secondary structure, although precipitated insulin recovered its native structure upon redissolution in aqueous media [121]. Some modifications of the SAS process have been proposed to overcome this drawback, where CO2 also acts as an anti-solvent. However, this review reported little focused attention on this aerosol solvent extraction system (ASES) [122] using solution-enhanced dispersion by supercritical fluids (SEDS) [123], precipitation with a compressed fluid anti-solvent (PCA) [124] and SCF-assisted extraction of emulsions (SFEE) [119,125].
Table 4.
Solid dosage forms of biopharmaceuticals in SAS drug delivery systems.
| Year | Active Compound | Co-Solvent | Solid Dosage Form | Observations | Ref. |
|---|---|---|---|---|---|
| 1993 | Insulin | DMSO DMFA 1 |
N/A | 90% of the particles with a diameter smaller than 4 µm 10% of the particles with a diameter smaller than 1 µm Blood glucose level decreases over the time |
[120] |
| 1999 | Lysozyme | DCM 2 | (a) P(LLA) 3 (b) PLGA |
PCA (a) 250 µm < Diameter < 500 µm (b) 5 µm < Diameter < 60 µm CO2 at high velocity through an annular region in a coaxial nozzle results in spherical and uniform particles |
[124] |
| 2001 | Insulin | (a) DCM (b) DCM- DMSO (50:50,%v/v) |
P(LLA) 3 | (a) 1 µm < Diameter < 3 µm (b) 0.5 µm < Diameter < 2 µm |
[118] |
| 2009 | Lysozyme | Water/EtOH | N/A | SEDS Fiber formation at higher pressures 0.1 µm < PSD < 0.4 µm Lysozyme activity was recovered from all spherical particles |
[126] |
| 2009 | BSA | DCM | P(LLA) 3 | PS < 2.5 µm The secondary BSA structure is not affected BSA content of 17.11% |
[127] |
| 2009 | Lysozyme | DMSO | N/A | PCA: PS < 100 nm GAS: 233 nm < PS < 302 nm Formation of the lysozyme particles involves spinodal decomposition |
[128] |
| 2012 | Insulin | DMSO/Acetone | HPMCP 4 | 138 nm < PS < 342 nm EE up to 100% 10. 76% < Insulin loading < 16.04% |
[129] |
1 N-N-Dimethylformamide; 2 Dichloromethane; 3 Poly-(L-lactide); 4 Hydroxypropyl methyl cellulose phthalate.
3. Biopharmaceutical Stability during Freeze-Drying, Spray-Drying, and Supercritical CO2-Assisted Atomization/Spray-Drying
Biopharmaceutical stability after processing is an interesting drying issue. Despite the advantages of freeze-drying, including the freedom from the need for high temperatures and moisture control, biopharmaceuticals are submitted to different stresses, such as crystallization, dehydration changes, ionic strength changes, interfacial stress, and ice crystal formation [24]. In fact, the drying of enzymes and proteins without excipients can lead to dimers and aggregates [130]. Zhai et al. [131] reported a herpes simplex virus 2 activity recovery of 22% for a minimum number of excipients, whereas a higher recovery (80%) was found when higher percentages of excipients were used. However, recent studies have shown that the effectiveness of the excipients on biopharmaceutical stabilization depends mainly on the protein/enzyme structure [132]. For this reason, the solute concentration and solute–excipient–buffer interaction should be considered for a stable biopharmaceutical solid dosage form, using freeze-drying. Spray-drying is an attractive technique for biopharmaceuticals insofar as it is simple and easy to scale-up, plus it offers particle size control and good aerosolization. However, the biopharmaceutical is subject to thermal, atomization, interfacial, and mechanical stresses [24]. Ajmera et al. [133] reported that the combination of two amino acids—arginine and glycine—was mandatory for catalase stability during spray-drying. Without excipients, the catalase lost around 50% of its activity due to the destruction of hydrophobic interactions during drying and the breakup of intermolecular hydrogen bonds upon removing the hydration shell. With the presence of the amino acids, catalase activity increases to 60–80%. A recent study from Fernandes et al. [134] showed a SOD (Cu, Zn-superoxide dismutase) activity retention between 50–80%, with no loss of SOD conformational stability. They observed, as in the case of freeze-drying, a stabilizing effect induced by the presence of excipients (trehalose and leucine). Ji et al. [135] showed a total lysozyme bioactivity of approximately 71.5% without excipients. A notable increase in bioactivity was observed upon the addition of trehalose, Tween 20, or phosphate-buffered saline (PBS), and at a lower inlet temperature (80 °C).
Biopharmaceuticals processed with SAA/SASD are also submitted to atomization stress. However, several studies have shown that protein stability suffers only a slight change during the process. The tunable properties of scCO2 mean that particle engineering can speed up the process. Du et al. [99] found that an increase of PCO2 led to a loss of relative activity, due to the acidity of CO2, with a consequent pH change that provoked protein denaturation. The authors also observed a relative activity of 95% when the mixer was at 100 °C. It is important to call attention to the fact that no excipients were used during drying. Another study from Shen et al. [101] showed the processing of trypsin in SAA-HCM. Free trypsin, without excipients, presented a relative activity of 74.3%, whereas the relative activity of trypsin from spray-drying was 66.1%. Silva et al. [93] also reported a stable nucleic acid after SASD. It would be interesting to study SOD activity from the solid dosage SASD forms to compare with those from freeze-drying and spray-drying. All of these studies show that SAA/SASD is not merely an effective process for particle morphology control and scalability. Processing in mild conditions might also reinforce biopharmaceutical stability without compromising its conformational structure.
4. Conclusions and Future Perspectives
This work reviews scCO2-based techniques for producing solid dosage forms of biopharmaceuticals. scCO2 provides green and sustainable alternatives to conventional techniques, such as spray-drying for producing dry powders, as it reduces the heat and shear stress and, even when using organic solvents, their amount is lower. The encapsulation of biopharmaceuticals in solid dosage forms using RESS, PGSS, CAN-BD, SAA/SASD, DELOS, and SAS has been intensively reviewed. RESS showed good size control. However, the poor solubility of drugs and polymers in scCO2 hindered particle recovery leading to scale-up limitations and other disadvantages. In contrast, PGSS presents easy scalability, requires no organic solvents, and can take place at low temperatures. However, since it requires the solubilization of scCO2 in the melted compound, the technique is limited to pharmaceutical compounds in which CO2 is highly soluble. Moreover, the fact that the solute must be melted represents a potential issue for heat-sensitive materials. CAN-BD is an interesting method as a wide range of compounds can be processed, although high temperatures are required. SAA/SASD presents good particle size control, high drying and atomization efficiency, and easy tunability to produce microparticles. Yet, small amounts of organic solvents might be required to improve the drying process. Using the DELOS technique, low pressures can be applied for optimum precipitation. However, the process is not applied to biopharmaceuticals (probably since biopharmaceutical crystallization might occur). Finally, SAS was found to be attractive for processing insoluble compounds in CO2 (as is the case for most drugs). However, the use of organic solvents to dissolve the solute may represent an obstacle to biopharmaceutical applications. Unfortunately, throughout the literature, there is less data about producing solid dosage forms of biopharmaceuticals than that looking into the same system for poorly water-soluble and low molecular weight molecules, excepting SAA/SASD. As a matter of fact, even scCO2 techniques present impressive and potential results; biopharmaceuticals are very sensitive to their environment and their processing may prove more challenging. For this reason, future research into the development of nano-in-microparticles solid dosage forms is predicted to increase. Biopharmaceuticals are first encapsulated in a nanosystem that is then encapsulated in microparticles. We believe that this scCO2-based technique will potentially produce loaded solid dosage forms, without compromising the biopharmaceutical structure or stability.
Author Contributions
Conceptualization, C.C., M.L.C., and A.A.-R.; writing—original draft preparation, C.C.; writing—review and editing, T.C., M.L.C., and A.A.-R.; supervision, A.A.-R. All authors have read and agreed to the published version of the manuscript.
Funding
C. Costa, T. Casimiro and A. Aguiar-Ricardo are grateful for the financial support of the Associate Laboratory for Green Chemistry-LAQV, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, Portugal, which is financed by national funds from FCT/MCTES (UIDB/50006/2020 and UIDP/50006/2020). C. Costa thanks FCT (Fundação para a Ciência e Tecnologia) and ESF (European Social Fund) through POCH (Programa Operacional Capital Humano) for her PhD grant ref. PD/BD/142880/2018 and Project PD/00184/2012-PDQS. M.L. Corvo is grateful for the financial support of the Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa, Lisbon, Portugal, which is supported in part by UID/DTP/04138/2020 and UIDP/04138/2020 from FCT/MCTES, Portugal.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He W., Xing X., Wang X., Wu D., Wu W., Guo J., Mitragotri S. Nanocarrier-Mediated Cytosolic Delivery of Biopharmaceuticals. Adv. Funct. Mater. 2020;30:1910566. doi: 10.1002/adfm.201910566. [DOI] [Google Scholar]
- 2.Kesik-Brodacka M. Progress in Biopharmaceutical Development. Biotechnol. Appl. Biochem. 2018;65:306–322. doi: 10.1002/bab.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earhart A.P., Holliday Z.M., Hofmann H.V., Schrum A.G. Consideration of Dornase Alfa for the Treatment of Severe COVID-19 Acute Respiratory Distress Syndrome. New Microbes New Infect. 2020;35:100689. doi: 10.1016/j.nmni.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitragotri S., Burke P.A., Langer R. Overcoming the Challenges in Administering Biopharmaceuticals: Formulation and Delivery Strategies. Nat. Rev. Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y., Wang H., Zhang S., Zhao Y., Gao J., Zheng Y., Zhao P., Zhang Z., Zaworotko M.J., Cheng P., et al. Antibodies@MOFs: An In Vitro Protective Coating for Preparation and Storage of Biopharmaceuticals. Adv. Mater. 2019;31:1805148. doi: 10.1002/adma.201805148. [DOI] [PubMed] [Google Scholar]
- 6.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X.J. Therapeutic SiRNA: State of the Art. Signal Transduct. Target. Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing Y., Xu Z., Liu T., Shi L., Kohane D., Guo S. Synthesis of Poly(Acyclic Orthoester)s: Acid-Sensitive Biomaterials for Enhancing Immune Responses of Protein Vaccine. Angew. Chem. Int. Ed. 2020;59:7235–7239. doi: 10.1002/anie.202001169. [DOI] [PubMed] [Google Scholar]
- 8.Edmans J.G., Murdoch C., Santocildes-Romero M.E., Hatton P.V., Colley H.E., Spain S.G. Incorporation of Lysozyme into a Mucoadhesive Electrospun Patch for Rapid Protein Delivery to the Oral Mucosa. Mater. Sci. Eng. C. 2020;112:110917. doi: 10.1016/j.msec.2020.110917. [DOI] [PubMed] [Google Scholar]
- 9.Shigemitsu H., Kubota R., Nakamura K., Matsuzaki T., Minami S., Aoyama T., Urayama K., Hamachi I. Protein-Responsive Protein Release of Supramolecular/Polymer Hydrogel Composite Integrating Enzyme Activation Systems. Nat. Commun. 2020;11:3859. doi: 10.1038/s41467-020-17698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa C., Liu Z., Simões S.I., Correia A., Rahikkala A., Seitsonen J., Ruokolainen J., Aguiar-Ricardo A., Santos H.A., Corvo M.L. One-Step Microfluidics Production of Enzyme-Loaded Liposomes for the Treatment of Inflammatory Diseases. Colloids Surf. B Biointerfaces. 2021;199:111556. doi: 10.1016/j.colsurfb.2020.111556. [DOI] [PubMed] [Google Scholar]
- 11.Costa C., Liu Z., Martins J.P., Correia A., Figueiredo P., Rahikkala A., Li W., Seitsonen J., Ruokolainen J., Hirvonen S.-P., et al. All-in-One Microfluidic Assembly of Insulin-Loaded pH-Responsive Nano-in-Microparticles for Oral Insulin Delivery. Biomater. Sci. 2020:3270–3277. doi: 10.1039/D0BM00743A. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., Shen H., Xu J., Liu L., Tan J., Li M., Xu N., Luo S., Wang J., Yang F., et al. Liver-Targeted SiRNA Lipid Nanoparticles Treat Hepatic Cirrhosis by Dual Antifibrotic and Anti-Inflammatory Activities. ACS Nano. 2020;14:6305–6322. doi: 10.1021/acsnano.0c02633. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Guo Z., Kordanovski M., Kaltbeitzel J., Zhang H., Cao Z., Gu Z., Wich P.R., Lord M., Liang K. Metal-Organic Frameworks as Protective Matrices for Peptide Therapeutics. J. Colloid Interface Sci. 2020;576:356–363. doi: 10.1016/j.jcis.2020.05.057. [DOI] [PubMed] [Google Scholar]
- 14.Khaitov M., Nikonova A., Shilovskiy I., Kozhikhova K., Kofiadi I., Vishnyakova L., Nikolskii A., Gattinger P., Kovchina V., Barvinskaia E., et al. Silencing of SARS-CoV-2 with Modified SiRNA-Peptide Dendrimer Formulation. Allergy. 2021;76:2840–2854. doi: 10.1111/all.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohr A., Tsapis N., Foged C., Andreana I., Yang M., Fattal E. Treatment of acute lung inflammation by pulmonary delivery of anti-TNF-α siRNA with PAMAM dendrimers in a murine model. Eur. J. Pharm. Biopharm. 2020;156:114–120. doi: 10.1016/j.ejpb.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto J.T., Faulhammer E., Dieplinger J., Dekner M., Makert C., Nieder M., Paudel A. Progress in Spray-Drying of Protein Pharmaceuticals: Literature Analysis of Trends in Formulation and Process Attributes. Dry Technol. 2021;39:1415–1446. doi: 10.1080/07373937.2021.1903032. [DOI] [Google Scholar]
- 17.Krammer F. SARS-CoV-2 Vaccines in Development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 18.Langford A., Bhatnagar B., Walters R., Tchessalov S., Ohtake S. Drying Technologies for Biopharmaceutical Applications: Recent Developments and Future Direction. Dry Technol. 2018;36:677–684. doi: 10.1080/07373937.2017.1355318. [DOI] [Google Scholar]
- 19.Aguiar-Ricardo A., Bonifácio V.D.B., Casimiro T., Correia V.G. Supercritical Carbon Dioxide Design Strategies: From Drug Carriers to Soft Killers. Phil. Trans. R. Soc. A. 2015;373:20150009. doi: 10.1098/rsta.2015.0009. [DOI] [PubMed] [Google Scholar]
- 20.Silva A.S., Tavares M.T., Aguiar-Ricardo A. Sustainable Strategies for Nano-in-Micro Particle Engineering for Pulmonary Delivery. J. Nanopart. Res. 2014;16 doi: 10.1007/s11051-014-2602-0. [DOI] [Google Scholar]
- 21.Wang J.L., Hanafy M.S., Xu H., Leal J., Zhai Y., Ghosh D., Williams R.O., Smyth H.D.C., Cui Z. Aerosolizable SiRNA-Encapsulated Solid Lipid Nanoparticles Prepared by Thin-Film Freeze-Drying for Potential Pulmonary Delivery. Int. J. Pharm. 2021;596:120215. doi: 10.1016/j.ijpharm.2021.120215. [DOI] [PubMed] [Google Scholar]
- 22.Corvo M.L., Boerman O.C., Oyen W.J.G., Van Bloois L., Cruz M.E.M., Crommelin D.J.A., Storm G. Intravenous Administration of Superoxide Dismutase Entrapped in Long Circulating Liposomes II. In Vivo Fate in a Rat Model of Adjuvant Arthritis. Biochim. Biophys. Acta-Biomembr. 1999;1419:325–334. doi: 10.1016/S0005-2736(99)00081-4. [DOI] [PubMed] [Google Scholar]
- 23.Fissore D., McCoy T. Editorial: Freeze-Drying and Process Analytical Technology for Pharmaceuticals. Front. Chem. 2018;6:622. doi: 10.3389/fchem.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emami F., Vatanara A., Park E.J., Na D.H. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics. 2018;10:131. doi: 10.3390/pharmaceutics10030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellary S., Barnett A.H. Inhaled Insulin (Exubera®): Combining Efficacy and Convenience. Diabetes Vasc. Dis. Res. 2006;3:179–185. doi: 10.3132/dvdr.2006.027. [DOI] [PubMed] [Google Scholar]
- 26.Bailey C.J., Barnett A.H. Why Is Exubera Being Withdrawn? BMJ. 2007;335:1156. doi: 10.1136/bmj.39409.507662.94. [DOI] [Google Scholar]
- 27.Ibrahim B.M., Jun S.W., Lee M.Y., Kang S.H., Yeo Y. Development of Inhalable Dry Powder Formulation of Basic Fibroblast Growth Factor. Int. J. Pharm. 2010;385:66–72. doi: 10.1016/j.ijpharm.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Hannay J.B., Hogarth J. On the Solubility of Solids in Gases. Philos. Trans. R. Soc. 1879;29:324–326. doi: 10.1038/scientificamerican04241880-3585bsupp. [DOI] [Google Scholar]
- 29.Jovanovic N., Bouchard A., Hofland G.W., Witkamp G.-J., Crommelin D.J.A., Jiskoot W. Ph.D. Thesis. Utrecht University; Utrecht, The Netherlands: 2007. Stabilization of Proteins in Dry Powder Formulations Using Supercritical Fluid Technology. [DOI] [PubMed] [Google Scholar]
- 30.Tavares M., Cabral R.P., Costa C., Martins P., Fernandes A.R., Casimiro T., Aguiar-Ricardo A. Development of PLGA Dry Powder Microparticles by Supercritical CO2-Assisted Spray-Drying for Potential Vaccine Delivery to the Lungs. J. Supercrit. Fluids. 2017;128:235–243. doi: 10.1016/j.supflu.2017.06.004. [DOI] [Google Scholar]
- 31.Aguiar-Ricardo A. Building Dry Powder Formulations Using Supercritical CO2 Spray Drying. Curr. Opin. Green Sustain. Chem. 2017;5:12–16. doi: 10.1016/j.cogsc.2017.03.005. [DOI] [Google Scholar]
- 32.Della Porta G., De Vittori C., Reverchon E. Supercritical Assisted Atomization: A Novel Technology for Microparticles Preparation of an Asthma-Controlling Drug. AAPS PharmSciTech. 2005;6:E421–E428. doi: 10.1208/pt060352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes A.V.M., Duarte C.M.M. Dense CO2 as a Solute, Co-Solute or Co-Solvent in Particle Formation Processes: A Review. Materials. 2011;4:2017–2041. doi: 10.3390/ma4112017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastas P., Eghbali N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010;39:301–312. doi: 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]
- 35.Matson D.W., Petersen R.C., Smith R.D. Production of Powders and Films by the Rapid Expansion of Supercritical Solutions. J. Mater. Sci. 1987;22:1919–1928. doi: 10.1007/BF01132917. [DOI] [Google Scholar]
- 36.Matson D.W., Fulton J.L., Petersen R.C., Smith R.D. Rapid Expansion of Supercritical Fluid Solutions: Solute Formation of Powders, Thin Films, and Fibers. Ind. Eng. Chem. Res. 1987;26:2298–2306. doi: 10.1021/ie00071a021. [DOI] [Google Scholar]
- 37.Petersen R.C., Matson D.W., Smith R.D. Rapid Precipitation of Low Vapor Pressure Solids from Supercritical Fluid Solutions: The Formation of Thin Films and Powders. J. Am. Chem. Soc. 1986;108:2100–2102. doi: 10.1021/ja00268a066. [DOI] [Google Scholar]
- 38.Reverchon E., Adami R. Nanomaterials and Supercritical Fluids. J. Supercrit. Fluids. 2006;37:1–22. doi: 10.1016/j.supflu.2005.08.003. [DOI] [Google Scholar]
- 39.Aguiar-Ricardo A., Costa E. Supercritical Fluid Manufacture. In: Hickey A.J., da Rocha S.R.P., editors. Pharmaceutical Inhalation Aerosol Technology. 3rd ed. CRC Press; Boca Raton, FL, USA: 2019. pp. 1–21. [Google Scholar]
- 40.Gupta R.B., Shim J.-J. Solubility in Supercritical Carbon Dioxide. 1st ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 1–18. [Google Scholar]
- 41.Reverchon E., Donsi G., Gorgoglione D. Salicylic Acid Solubilization in Supercritical CO2 and Its Micronization by RESS. J. Supercrit. Fluids. 1993;6:241–248. doi: 10.1016/0896-8446(93)90034-U. [DOI] [Google Scholar]
- 42.Yildiz N., Tuna Ş., Döker O., Çalimli A. Micronization of Salicylic Acid and Taxol (Paclitaxel) by Rapid Expansion of Supercritical Fluids (RESS) J. Supercrit. Fluids. 2007;41:440–451. doi: 10.1016/j.supflu.2006.12.012. [DOI] [Google Scholar]
- 43.Costa C., Casimiro T., Aguiar-Ricardo A. Optimization of Supercritical CO2-Assisted Atomization: Phase Behavior and Design of Experiments. J. Chem. Eng. Data. 2018;63:885–896. doi: 10.1021/acs.jced.7b00820. [DOI] [Google Scholar]
- 44.Mishima K., Matsuyama K., Tanabe D., Yamauchi S., Young T.J., Johnston K.P. Microencapsulation of Proteins by Rapid Expansion of Supercritical Solution with a Nonsolvent. AIChE J. 2000;46:857–865. doi: 10.1002/aic.690460418. [DOI] [Google Scholar]
- 45.Matsuyama K., Mishima K., Hayasččhi K.I., Ishikawa H., Matsuyama H., Harada T. Formation of Microcapsules of Medicines by the Rapid Expansion of a Supercritical Solution with a Nonsolvent. J. Appl. Polym. Sci. 2003;89:742–752. doi: 10.1002/app.12201. [DOI] [Google Scholar]
- 46.Pathak P., Meziani M.J., Desai T., Sun Y.P. Nanosizing Drug Particles in Supercritical Fluid Processing. J. Am. Chem. Soc. 2004;126:10842–10843. doi: 10.1021/ja046914t. [DOI] [PubMed] [Google Scholar]
- 47.Xiang S.T., Chen B.Q., Kankala R.K., Wang S.B., Chen A.Z. Solubility Measurement and RESOLV-Assisted Nanonization of Gambogic Acid in Supercritical Carbon Dioxide for Cancer Therapy. J. Supercrit. Fluids. 2019;150:147–155. doi: 10.1016/j.supflu.2019.04.008. [DOI] [Google Scholar]
- 48.Ribeiro Dos Santos I., Richard J., Pech B., Thies C., Benoit J.P. Microencapsulation of Protein Particles within Lipids Using a Novel Supercritical Fluid Process. Int. J. Pharm. 2002;242:69–78. doi: 10.1016/S0378-5173(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 49.Vergara-Mendoza M.D.S., Ortiz-Estrada C.H., González-Martínez J., Quezada-Gallo J.A. Microencapsulation of Coenzyme Q 10 in Poly(Ethylene Glycol) and Poly(Lactic Acid) with Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2012;51:5840–5846. doi: 10.1021/ie2014839. [DOI] [Google Scholar]
- 50.Segura M.F., Gallego S., Sánchez de Toledo J., Soriano A., Ventosa N., Veciana J., Boloix A., Segovia Ramos N.V. Nanovesicles and Its Use for Nucleic Acid Delivery. 2020/229469. WO Patent. 2020 November 19;
- 51.Weidner E., Knez Ž., Novak Z. Process for Preparing Particles or Powders. 1995/021688. WO Patent. 1995 August 17;
- 52.Kerč J., Srčič S., Knez Ž., Senčar-Božič P. Micronization of Drugs Using Supercritical Carbon Dioxide. Int. J. Pharm. 1999;182:33–39. doi: 10.1016/S0378-5173(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 53.Hao J., Whitaker M.J., Wong B., Serhatkulu G., Shakesheff K.M., Howdle S.M. Plasticization and Spraying of Poly (DL-lactic Acid) Using Supercritical Carbon Dioxide: Control of Particle Size. J. Pharm. Sci. 2004;93:1083–1090. doi: 10.1002/jps.20002. [DOI] [PubMed] [Google Scholar]
- 54.Shieh Y.-T., Su J.-H., Manivannan G., Lee P.H.C., Sawan S.P., Spall W.D. Interaction of Supercritical Carbon Dioxide with Polymers. I. Crystalline Polymers. J. Appl. Polym. Sci. 1996;59:695–705. doi: 10.1002/(SICI)1097-4628(19960124)59:4<695::AID-APP15>3.0.CO;2-P. [DOI] [Google Scholar]
- 55.Perinelli D.R., Bonacucina G., Cespi M., Naylor A., Whitaker M., Palmieri G.F., Giorgioni G., Casettari L. Evaluation of P(L)LA-PEG-P(L)LA as Processing Aid for Biodegradable Particles from Gas Saturated Solutions (PGSS) Process. Int. J. Pharm. 2014;468:250–257. doi: 10.1016/j.ijpharm.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 56.Hao J., Whitaker M.J., Serhatkulu G., Shakesheff K.M., Howdle S.M. Supercritical Fluid Assisted Melting of Poly(Ethylene Glycol): A New Solvent-Free Route to Microparticles. J. Mater. Chem. A. 2005;15:1148–1153. doi: 10.1039/b411187g. [DOI] [Google Scholar]
- 57.Jordan F., Naylor A., Kelly C.A., Howdle S.M., Lewis A., Illum L. Sustained Release HGH Microsphere Formulation Produced by a Novel Supercritical Fluid Technology: In Vivo Studies. J. Control Release. 2010;141:153–160. doi: 10.1016/j.jconrel.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues M., Peiriço N., Matos H., Gomes de Azevedo E., Lobato M.R., Almeida A.J. Microcomposites Theophylline/Hydrogenated Palm Oil from a PGSS Process for Controlled Drug Delivery Systems. J. Supercrit. Fluids. 2004;29:175–184. doi: 10.1016/S0896-8446(03)00034-2. [DOI] [Google Scholar]
- 59.Tokunaga S., Ono K., Ito S., Sharmin T., Kato T., Irie K., Mishima K., Satho T., Harada T., Aida T.M., et al. Microencapsulation of Drug with Enteric Polymer Eudragit L100 for Controlled Release Using the Particles from Gas Saturated Solutions (PGSS) Process. J. Supercrit. Fluids. 2021;167:105044. doi: 10.1016/j.supflu.2020.105044. [DOI] [Google Scholar]
- 60.Whitaker M.J., Hao J., Davies O.R., Serhatkulu G., Stolnik-Trenkic S., Howdle S.M., Shakesheff K.M. The Production of Protein-Loaded Microparticles by Supercritical Fluid Enhanced Mixing and Spraying. J. Control Release. 2005;101:85–92. doi: 10.1016/j.jconrel.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 61.Salmaso S., Bersani S., Elvassore N., Bertucco A., Caliceti P. Biopharmaceutical Characterisation of Insulin and Recombinant Human Growth Hormone Loaded Lipid Submicron Particles Produced by Supercritical Gas Micro-Atomisation. Int. J. Pharm. 2009;379:51–58. doi: 10.1016/j.ijpharm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 62.Vezzù K., Borin D., Bertucco A., Bersani S., Salmaso S., Caliceti P. Production of Lipid Microparticles Containing Bioactive Molecules Functionalized with PEG. J. Supercrit. Fluids. 2010;54:328–334. doi: 10.1016/j.supflu.2010.05.013. [DOI] [Google Scholar]
- 63.Salmaso S., Elvassore N., Bertucco A., Caliceti P. Production of Solid Lipid Submicron Particles for Protein Delivery Using a Novel Supercritical Gas-Assisted Melting Atomization Process. J. Pharm. Sci. 2009;98:640–650. doi: 10.1002/jps.21434. [DOI] [PubMed] [Google Scholar]
- 64.Hu X., Guo Y., Wang L., Hua D., Hong Y., Li J. Coenzyme Q10 Nanoparticles Prepared by a Supercritical Fluid-Based Method. J. Supercrit. Fluids. 2011;57:66–72. doi: 10.1016/j.supflu.2011.01.007. [DOI] [Google Scholar]
- 65.Kelly C.A., Howdle S.M., Naylor A., Coxhill G., Tye L.C., Illum L., Lewis A.L. Stability of Human Growth Hormone in Supercritical Carbon Dioxide. J. Pharm. Sci. 2012;101:56–67. doi: 10.1002/jps.22747. [DOI] [PubMed] [Google Scholar]
- 66.Falconer J.R., Wen J., Zargar-Shoshtari S., Chen J.J., Farid M., Tallon S.J., Alany R.G. Preparation and Characterization of Progesterone Dispersions Using Supercritical Carbon Dioxide. Drug Dev. Ind. Pharm. 2014;40:458–469. doi: 10.3109/03639045.2013.768630. [DOI] [PubMed] [Google Scholar]
- 67.Couto R., Alvarez V., Temelli F. Encapsulation of Vitamin B2 in Solid Lipid Nanoparticles Using Supercritical CO2. J. Supercrit. Fluids. 2017;120:432–442. doi: 10.1016/j.supflu.2016.05.036. [DOI] [Google Scholar]
- 68.Sievers R.E., Karst U. Methods for Fine Particle Formation. 5639441. U.S. Patent. 1997 June 17;
- 69.Sievers R.E. Formation of Aqueous Small Droplet Aerosols Assisted by Supercritical Carbon Dioxide. Aerosol Sci. Technol. 1999;30:3–15. doi: 10.1080/713834046. [DOI] [Google Scholar]
- 70.Xu C., Watkins B.A., Sievers R.E., Jing X., Trowga P., Gibbons C.S., Vecht A. Submicron-Sized Spherical Yttrium Oxide Based Phosphors Prepared by Supercritical CO2-Assisted Aerosolization and Pyrolysis. Appl. Phys. Lett. 1997;71:1643–1645. doi: 10.1063/1.120004. [DOI] [Google Scholar]
- 71.Sellers S.P., Clark G.S., Sievers R.E., Carpenter J.F. Dry Powders of Stable Protein Formulations from Aqueous Solutions Prepared Using Supercritical CO2-assisted Aerosolization. J. Pharm. Sci. 2001;90:785–797. doi: 10.1002/jps.1032. [DOI] [PubMed] [Google Scholar]
- 72.Sievers R.E., Quinn B.P., Cape S.P., Searles J.A., Braun C.S., Bhagwat P., Rebits L.G., McAdams D.H., Burger J.L., Best J.A., et al. Near-Critical Fluid Micronization of Stabilized Vaccines, Antibiotics and Anti-Virals. J. Supercrit. Fluids. 2007;42:385–391. doi: 10.1016/j.supflu.2007.03.001. [DOI] [Google Scholar]
- 73.Cape S.P., Villa J.A., Huang E.T.S., Yang T.-H., Carpenter J.F., Sievers R.E. Preparation of Active Proteins, Vaccines and Pharmaceuticals as Fine Powders Using Supercritical or Near-Critical Fluids. Pharm. Res. 2008;25:1967–1990. doi: 10.1007/s11095-008-9575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kisich K.O., Higgins M.P., Park I., Cape S.P., Lindsay L., Bennett D.J., Winston S., Searles J., Sievers R.E. Dry Powder Measles Vaccine: Particle Deposition, Virus Replication, and Immune Response in Cotton Rats Following Inhalation. Vaccine. 2011;29:905–912. doi: 10.1016/j.vaccine.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Burger J.L., Cape S.P., Braun C.S., McAdams D.H., Best J.A., Bhagwat P., Pathak P., Rebits L.G., Sievers R.E. Stabilizing Formulations for Inhalable Powders of Live-Attenuated Measles Virus Vaccine. J. Aerosol Med. Pulm. Drug Deliv. 2008;21:25–34. doi: 10.1089/jamp.2007.0658. [DOI] [PubMed] [Google Scholar]
- 76.Lin W.-H., Griffin D.E., Rota P.A., Papania M., Cape S.P., Bennett D., Quinn B., Sievers R.E., Shermer C., Powell K., et al. Successful Respiratory Immunization with Dry Powder Live-Attenuated Measles Virus Vaccine in Rhesus Macaques. Proc. Natl. Acad. Sci. USA. 2011;108:2987–2992. doi: 10.1073/pnas.1017334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manion J.R., Cape S.P., McAdams D.H., Rebits L.G., Sievers R.E. Inhalable Antibiotics Manufactured Through Use of Near-Critical or Supercritical Fluids. Aerosol Sci. Technol. 2012;46:403–410. doi: 10.1080/02786826.2011.634453. [DOI] [Google Scholar]
- 78.Reverchon E. Process For The Production Of Micro And/Or Nano Particles. 7276190. U.S. Patent. 2001 October 2;
- 79.Adeoye O., Costa C., Casimiro T., Aguiar-Ricardo A., Cabral-Marques H. Preparation of Ibuprofen/Hydroxypropyl-γ-Cyclodextrin Inclusion Complexes Using Supercritical CO2-Assisted Spray Drying. J. Supercrit. Fluids. 2018;133:479–485. doi: 10.1016/j.supflu.2017.11.009. [DOI] [Google Scholar]
- 80.Adami R., Osséo L.S., Reverchon E. Micronization of Lysozyme by Supercritical Assisted Atomization. Biotechnol. Bioeng. 2009;104:1162–1170. doi: 10.1002/bit.22470. [DOI] [PubMed] [Google Scholar]
- 81.Reverchon E. Supercritical-Assisted Atomization To Produce Micro- and/or Nanoparticles of Controlled Size and Distribution. Ind. Eng. Chem. Res. 2002;41:2405–2411. doi: 10.1021/ie010943k. [DOI] [Google Scholar]
- 82.Reverchon E., Porta G. Della Spada, A. Ampicillin Micronization by Supercritical Assisted Atomization. J. Pharm. Pharmacol. 2010;55:1465–1471. doi: 10.1211/0022357022043. [DOI] [PubMed] [Google Scholar]
- 83.Reverchon E., Porta G. Della Micronization of Antibiotics by Supercritical Assisted Atomization. J. Supercrit. Fluids. 2003;26:243–252. doi: 10.1016/S0896-8446(02)00162-6. [DOI] [Google Scholar]
- 84.Reverchon E., Spada A. Erythromycin Micro-Particles Produced by Supercritical Fluid Atomization. Powder Technol. 2004;141:100–108. doi: 10.1016/j.powtec.2004.02.017. [DOI] [Google Scholar]
- 85.Reverchon E., Antonacci A. Chitosan Microparticles Production by Supercritical Fluid Processing. Ind. Eng. Chem. Res. 2006;45:5722–5728. doi: 10.1021/ie060233k. [DOI] [Google Scholar]
- 86.Reverchon E., Antonacci A. Polymer Microparticles Production by Supercritical Assisted Atomization. J. Supercrit. Fluids. 2007;39:444–452. doi: 10.1016/j.supflu.2006.03.005. [DOI] [Google Scholar]
- 87.Reverchon E., Antonacci A. Drug–Polymer Microparticles Produced by Supercritical Assisted Atomization. Biotechnol. Bioeng. 2007;97:1626–1637. doi: 10.1002/bit.21370. [DOI] [PubMed] [Google Scholar]
- 88.Cabral R.P., Sousa A.M.L., Silva A.S., Paninho A.I., Temtem M., Costa E., Casimiro T., Aguiar-Ricardo A. Design of Experiments Approach on the Preparation of Dry Inhaler Chitosan Composite Formulations by Supercritical CO2-Assisted Spray-Drying. J. Supercrit. Fluids. 2016;116:26–35. doi: 10.1016/j.supflu.2016.04.001. [DOI] [Google Scholar]
- 89.Restani R.B., Silva A.S., Pires R.F., Cabral R., Correia I.J., Casimiro T., Bonifácio V.D.B., Aguiar-Ricardo A. Nano-in-Micro POxylated Polyurea Dendrimers and Chitosan Dry Powder Formulations for Pulmonary Delivery. Part. Part. Syst. Charact. 2016;33:851–858. doi: 10.1002/ppsc.201600123. [DOI] [Google Scholar]
- 90.Silva A.S., Sousa A.M., Cabral R.P., Silva M.C., Costa C., Miguel S.P., Bonifácio V.D.B., Casimiro T., Correia I.J., Aguiar-Ricardo A. Aerosolizable Gold Nano-in-Micro Dry Powder Formulations for Theragnosis and Lung Delivery. Int. J. Pharm. 2017;519:240–249. doi: 10.1016/j.ijpharm.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 91.Silva M., Silva A., Fernandez-Lodeiro J., Casimiro T., Lodeiro C., Aguiar-Ricardo A. Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation. Materials. 2017;10:74. doi: 10.3390/ma10010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cai M.-Q., Guan Y.-X., Yao S.-J., Zhu Z.-Q. Supercritical Fluid Assisted Atomization Introduced by Hydrodynamic Cavitation Mixer (SAA-HCM) for Micronization of Levofloxacin Hydrochloride. J. Supercrit. Fluids. 2008;43:524–534. doi: 10.1016/j.supflu.2007.07.008. [DOI] [Google Scholar]
- 93.Silva A.S., Shopsowitz K.E., Correa S., Morton S.W., Dreaden E.C., Casimiro T., Aguiar-Ricardo A., Hammond P.T. Rational Design of Multistage Drug Delivery Vehicles for Pulmonary RNA Interference Therapy. Int. J. Pharm. 2020;591:119989. doi: 10.1016/j.ijpharm.2020.119989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Costa C., Nobre B., Matos A.S., Silva A.S., Casimiro T., Corvo M.L., Aguiar-Ricardo A. Inhalable Hydrophilic Molecule-Loaded Liposomal Dry Powder Formulations Using Supercritical CO2–Assisted Spray-Drying. J. CO2 Util. 2021;53:101709. doi: 10.1016/j.jcou.2021.101709. [DOI] [Google Scholar]
- 95.Zalepugin D.Y., Tilkunova N.A., Fronchek E.V., Gallyamov M.O., Chernyshova I.V., Mishin V.S., Yashin Y.S., Grigoryev T.E., Gamzazade A.I., Khokhlov A.R. Production of New Haemostatic Materials by Deposition of Dispersed Proteins onto Porous Matrices Using Supercritical Carbon Dioxide. Russ. J. Phys. Chem. B. 2010;4:1047–1050. doi: 10.1134/S1990793110070018. [DOI] [Google Scholar]
- 96.Della Porta G., Adami R., Della Porta G., Adami R., Del Gaudio P., Prota L., Aquino R., Reverchon E. Albumin/Gentamicin Microspheres Produced by Supercritical Assisted Atomization: Optimization of Size, Drug Loading and Release. J. Pharm. Sci. 2010;90:4720–4729. doi: 10.1002/jps.22173. [DOI] [PubMed] [Google Scholar]
- 97.Wang Q., Guan Y.X., Yao S.J., Zhu Z.Q. Controllable Preparation and Formation Mechanism of BSA Microparticles Using Supercritical Assisted Atomization with an Enhanced Mixer. J. Supercrit. Fluids. 2011;56:97–104. doi: 10.1016/j.supflu.2010.12.002. [DOI] [Google Scholar]
- 98.Adami R., Liparoti S., Reverchon E. A New Supercritical Assisted Atomization Configuration, for the Micronization of Thermolabile Compounds. Chem. Eng. J. 2011;173:55–61. doi: 10.1016/j.cej.2011.07.036. [DOI] [Google Scholar]
- 99.Du Z., Guan Y.X., Yao S.J., Zhu Z.Q. Supercritical Fluid Assisted Atomization Introduced by an Enhanced Mixer for Micronization of Lysozyme: Particle Morphology, Size and Protein Stability. Int. J. Pharm. 2011;421:258–268. doi: 10.1016/j.ijpharm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Du Z., Tang C., Guan Y.X., Yao S.J., Zhu Z.Q. Bioactive Insulin Microparticles Produced by Supercritical Fluid Assisted Atomization with an Enhanced Mixer. Int. J. Pharm. 2013;454:174–182. doi: 10.1016/j.ijpharm.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Shen Y.B., Guan Y.X., Yao S.J. Supercritical Fluid Assisted Production of Micrometric Powders of the Labile Trypsin and Chitosan/Trypsin Composite Microparticles. Int. J. Pharm. 2015;489:226–236. doi: 10.1016/j.ijpharm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 102.Hong D.X., Yun Y.L., Guan Y.X., Yao S.J. Preparation of Micrometric Powders of Parathyroid Hormone (PTH1–34)-Loaded Chitosan Oligosaccharide by Supercritical Fluid Assisted Atomization. Int. J. Pharm. 2018;545:389–394. doi: 10.1016/j.ijpharm.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 103.Santo I.E., Campardelli R., Albuquerque E.C., de Melo S.V., Della Porta G., Reverchon E. Liposomes Preparation Using a Supercritical Fluid Assisted Continuous Process. Chem. Eng. J. 2014;249:153–159. doi: 10.1016/j.cej.2014.03.099. [DOI] [Google Scholar]
- 104.Campardelli R., Espirito Santo I., Albuquerque E.C., de Melo S.V., Della Porta G., Reverchon E. Efficient Encapsulation of Proteins in Submicro Liposomes Using a Supercritical Fluid Assisted Continuous Process. J. Supercrit. Fluids. 2016;107:163–169. doi: 10.1016/j.supflu.2015.09.007. [DOI] [Google Scholar]
- 105.Trucillo P., Campardelli R., Reverchon E. A Versatile Supercritical Assisted Process for the One-Shot Production of Liposomes. J. Supercrit. Fluids. 2019;146:136–143. doi: 10.1016/j.supflu.2019.01.015. [DOI] [Google Scholar]
- 106.Ventosa N., Sala S., Veciana J., Torres J., Llibre J. Depressurization of an Expanded Liquid Organic Solution (DELOS): A New Procedure for Obtaining Submicron- Or Micron-Sized Crystalline Particles. Cryst. Growth Des. 2001;1:299–303. doi: 10.1021/cg0155090. [DOI] [Google Scholar]
- 107.Ventosa N., Sala S., Veciana J. DELOS Process: A Crystallization Technique Using Compressed Fluids-1. Comparison to the GAS Crystallization Method. J. Supercrit. Fluids. 2003;26:33–45. doi: 10.1016/S0896-8446(02)00189-4. [DOI] [Google Scholar]
- 108.Muntó M., Ventosa N., Veciana J. Synergistic Solubility Behaviour of a Polyoxyalkylene Block Co-Polymer and Its Precipitation from Liquid CO2-Expanded Ethanol as Solid Microparticles. J. Supercrit. Fluids. 2008;47:290–295. doi: 10.1016/j.supflu.2008.07.019. [DOI] [Google Scholar]
- 109.Muntó M., Ventosa N., Sala S., Veciana J. Solubility Behaviors of Ibuprofen and Naproxen Drugs in Liquid “CO2-Organic Solvent” Mixtures. J. Supercrit. Fluids. 2008;47:147–153. doi: 10.1016/j.supflu.2008.07.013. [DOI] [Google Scholar]
- 110.Sala S., Córdoba A., Moreno-Calvo E., Elizondo E., Muntó M., Rojas P.E., Larrayoz M.À., Ventosa N., Veciana J. Crystallization of Microparticulate Pure Polymorphs of Active Pharmaceutical Ingredients Using CO 2-Expanded Solvents. Cryst. Growth Des. 2012;12:1717–1726. doi: 10.1021/cg200356x. [DOI] [Google Scholar]
- 111.Elizondo E., Larsen J., Hatzakis N.S., Cabrera I., Bjørnholm T., Veciana J., Stamou D., Ventosa N. Influence of the Preparation Route on the Supramolecular Organization of Lipids in a Vesicular System. J. Am. Chem. Soc. 2012;134:1918–1921. doi: 10.1021/ja2086678. [DOI] [PubMed] [Google Scholar]
- 112.Elizondo E., Veciana J., Ventosa N. Nanostructuring Molecular Materials as Particles and Vesicles for Drug Delivery, Using Compressed and Supercritical Fluids. Nanomedicine. 2012;7:1391–1408. doi: 10.2217/nnm.12.110. [DOI] [PubMed] [Google Scholar]
- 113.Cano-Sarabia M., Ventosa N., Sala S., Patiño C., Arranz R., Veciana J. Preparation of Uniform Rich Cholesterol Unilamellar Nanovesicles Using CO2-Expanded Solvents. Langmuir. 2008;24:2433–2437. doi: 10.1021/la7032109. [DOI] [PubMed] [Google Scholar]
- 114.Merlo-Mas J., Tomsen-Melero J., Corchero J.L., González-Mira E., Font A., Pedersen J.N., García-Aranda N., Cristóbal-Lecina E., Alcaina-Hernando M., Mendoza R., et al. Application of Quality by Design to the Robust Preparation of a Liposomal GLA Formulation by DELOS-Susp Method. J. Supercrit. Fluids. 2021;173:105204. doi: 10.1016/j.supflu.2021.105204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cabrera I., Elizondo E., Esteban O., Corchero J.L., Melgarejo M., Pulido D., Córdoba A., Moreno E., Unzueta U., Vazquez E., et al. Multifunctional Nanovesicle-Bioactive Conjugates Prepared by a One-Step Scalable Method Using CO2 -Expanded Solvents. Nano Lett. 2013;13:3766–3774. doi: 10.1021/nl4017072. [DOI] [PubMed] [Google Scholar]
- 116.Davies O.R., Lewis A.L., Whitaker M.J., Tai H., Shakesheff K.M., Howdle S.M. Applications of Supercritical CO2 in the Fabrication of Polymer Systems for Drug Delivery and Tissue Engineering. Adv. Drug Deliv. Rev. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 117.Kalani M., Yunus R. Application of Supercritical Antisolvent Method in Drug Encapsulation: A Review. Int. J. Nanomed. 2011;6:1429–1442. doi: 10.2147/IJN.S19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elvassore N., Bertucco A., Caliceti P. Production of Protein-Loaded Polymeric Microcapsules by Compressed CO2 in a Mixed Solvent. Ind. Eng. Chem. Res. 2001;40:795–800. doi: 10.1021/ie0004904. [DOI] [Google Scholar]
- 119.Franco P., De Marco I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes. 2020;8:938. doi: 10.3390/pr8080938. [DOI] [Google Scholar]
- 120.Yeo S.-D., Lim G.-B., Debendetti P.G., Bernstein H. Formation of Microparticulate Protein Powder Using a Supercritical Fluid Antisolvent. Biotechnol. Bioeng. 1993;41:341–346. doi: 10.1002/bit.260410308. [DOI] [PubMed] [Google Scholar]
- 121.Yeo S., Debenedetti P.G., Patro S.Y., Przybycien T.M. Secondary Structure Characterization of Microparticulate Insulin Powders. J. Pharm. Sci. 1994;83:1651–1656. doi: 10.1002/jps.2600831203. [DOI] [PubMed] [Google Scholar]
- 122.Bleich J., Kleinebudde P., Müller B.W. Influence of Gas Density and Pressure on Microparticles Produced with the ASES Process. Int. J. Pharm. 1994;106:77–84. doi: 10.1016/0378-5173(94)90278-X. [DOI] [Google Scholar]
- 123.Palakodaty S., York P., Pritchard J. Supercritical Fluid Processing of Materials from Aqueous Solutions: The Application of SEDS to Lactose as a Model Substance. Pharm. Res. 1998;15:1835–1843. doi: 10.1023/A:1011949805156. [DOI] [PubMed] [Google Scholar]
- 124.Young T.J., Johnston K.P., Mishima K., Tanaka H. Encapsulation of Lysozyme in a Biodegradable Polymer by Precipitation with a Vapor-over-Liquid Antisolvent. J. Pharm. Sci. 1999;88:640–650. doi: 10.1021/js980237h. [DOI] [PubMed] [Google Scholar]
- 125.Kankala R.K., Zhang Y.S., Wang S.-B., Lee C.-H., Chen A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017;6:1700433. doi: 10.1002/adhm.201700433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodrigues M.A., Li J., Padrela L., Almeida A., Matos H.A., de Azevedo E.G. Anti-Solvent Effect in the Production of Lysozyme Nanoparticles by Supercritical Fluid-Assisted Atomization Processes. J. Supercrit. Fluids. 2009;48:253–260. doi: 10.1016/j.supflu.2008.06.006. [DOI] [Google Scholar]
- 127.Kang Y., Yang C., Ouyang P., Yin G., Huang Z., Yao Y., Liao X. The Preparation of BSA-PLLA Microparticles in a Batch Supercritical Anti-Solvent Process. Carbohydr. Polym. 2009;77:244–249. doi: 10.1016/j.carbpol.2008.12.029. [DOI] [Google Scholar]
- 128.Fusaro F., Kluge J., Mazzotti M., Muhrer G. Compressed CO2 Antisolvent Precipitation of Lysozyme. J. Supercrit. Fluids. 2009;49:79–92. doi: 10.1016/j.supflu.2008.12.005. [DOI] [Google Scholar]
- 129.Jin H.Y., Xia F., Zhao Y.P. Preparation of Hydroxypropyl Methyl Cellulose Phthalate Nanoparticles with Mixed Solvent Using Supercritical Antisolvent Process and Its Application in Co-Precipitation of Insulin. Adv. Powder Technol. 2012;23:157–163. doi: 10.1016/j.apt.2011.01.007. [DOI] [Google Scholar]
- 130.Roy I., Gupta M.N. Freeze-Drying of Proteins: Some Emerging Concerns. Biotechnol. Appl. Biochem. 2004;39:165–177. doi: 10.1042/BA20030133. [DOI] [PubMed] [Google Scholar]
- 131.Zhai S., Hansen R.K., Taylor R., Skepper J.N., Sanches R., Slater N.K.H. Effect of Freezing Rates and Excipients on the Infectivity of a LiveViral Vaccine during Lyophilization. Biotechnol. Prog. 2004;20:1113–1120. doi: 10.1021/bp034362x. [DOI] [PubMed] [Google Scholar]
- 132.Arsiccio A., Pisano R. The Preservation of Lyophilized Human Growth Hormone Activity: How Do Buffers and Sugars Interact? Pharm. Res. 2018;35:131. doi: 10.1007/s11095-018-2410-9. [DOI] [PubMed] [Google Scholar]
- 133.Ajmera A., Scherließ R. Stabilisation of Proteins via Mixtures of Amino Acids during Spray Drying. Int. J. Pharm. 2014;463:98–107. doi: 10.1016/j.ijpharm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 134.Fernandes D.A., Leandro P., Costa E., Corvo M.L. Dry Powder Inhaler Formulation of Cu,Zn-Superoxide Dismutase by Spray Drying: A Proof-of-Concept. Powder Technol. 2021;389:131–137. doi: 10.1016/j.powtec.2021.05.008. [DOI] [Google Scholar]
- 135.Ji S., Thulstrup P.W., Mu H., Hansen S.H., van de Weert M., Rantanen J., Yang M. Investigation of Factors Affecting the Stability of Lysozyme Spray Dried from Ethanol-Water Solutions. Int. J. Pharm. 2017;534:263–271. doi: 10.1016/j.ijpharm.2017.10.021. [DOI] [PubMed] [Google Scholar]