Abstract
Background
There is growing interest towards the use of helmet noninvasive ventilation (NIV) for the management of acute hypoxemic respiratory failure. Gas conditioning through heat and moisture exchangers (HME) or heated humidifiers (HHs) is needed during facemask NIV to provide a minimum level of humidity in the inspired gas (15 mg H2O/L). The optimal gas conditioning strategy during helmet NIV remains to be established.
Methods
Twenty patients with acute hypoxemic respiratory failure (PaO2/FiO2 < 300 mmHg) underwent consecutive 1-h periods of helmet NIV (PEEP 12 cmH2O, pressure support 12 cmH2O) with four humidification settings, applied in a random order: double-tube circuit with HHs and temperature set at 34 °C (HH34) and 37 °C (HH37); Y-piece circuit with HME; double-tube circuit with no humidification (NoH). Temperature and humidity of inhaled gas were measured through a capacitive hygrometer. Arterial blood gases, discomfort and dyspnea through visual analog scales (VAS), esophageal pressure swings (ΔPES) and simplified pressure–time product (PTPES), dynamic transpulmonary driving pressure (ΔPL) and asynchrony index were measured in each step.
Results
Median [IqR] absolute humidity, temperature and VAS discomfort were significantly lower during NoH vs. HME, HH34 and HH37: absolute humidity (mgH2O/L) 16 [12–19] vs. 28 [23–31] vs. 28 [24–31] vs. 33 [29–38], p < 0.001; temperature (°C) 29 [28–30] vs. 30 [29–31] vs. 31 [29–32] vs 32. [31–33], p < 0.001; VAS discomfort 4 [2–6] vs. 6 [2–7] vs. 7 [4–8] vs. 8 [4–10], p = 0.03. VAS discomfort increased with higher absolute humidity (p < 0.01) and temperature (p = 0.007). Higher VAS discomfort was associated with increased VAS dyspnea (p = 0.001). Arterial blood gases, respiratory rate, ΔPES, PTPES and ΔPL were similar in all conditions. Overall asynchrony index was similar in all steps, but autotriggering rate was lower during NoH and HME (p = 0.03).
Conclusions
During 1-h sessions of helmet NIV in patients with hypoxemic respiratory failure, a double-tube circuit with no humidification allowed adequate conditioning of inspired gas, optimized comfort and improved patient–ventilator interaction. Use of HHs or HME in this setting resulted in increased discomfort due to excessive heat and humidity in the interface, which was associated with more intense dyspnea.
Trail Registration Registered on clinicaltrials.gov (NCT02875379) on August 23rd, 2016.
Keywords: Noninvasive ventilation, Respiratory Insufficiency, Humidity, Temperature
Introduction
The ongoing COVID-19 pandemic dramatically highlighted the role of noninvasive management of acute hypoxemic respiratory failure [1–3]. In hypoxemic respiratory failure, there is raising interest on the possible benefit of helmet noninvasive ventilation (NIV), since a growing body of evidence suggests physiological and clinical advantages over both facemask and high-flow nasal oxygen [4–8]. Putative benefits of this technique include the possibility of delivering long-term treatments with high levels of positive end-expiratory pressure (PEEP) [9–11], which improve hypoxemia, mitigate self-inflicted lung injury and may improve clinical outcome [12–14].
For helmet NIV, gas-compressed mechanical ventilators are necessary to provide the high inspiratory flows needed to wash out exhaled CO2 from the high device-related dead space [15]. When a compressed-gas ventilator is used, dry and cold air is delivered to the patient, while a minimum level of absolute humidity of 15 mgH2O/L has been shown to be necessary to avoid patients’ discomfort and airway dryness during NIV [16]. Comfort and tolerability are key determinants of NIV success [17, 18]. Thus, gas conditioning is recommended during facemask NIV with compressed-gas ventilators [19–28], either by heated humidifiers (HH) or heat and moisture exchangers (HME), whose effects appear similar [16, 26–29]. These results may not be applicable to helmet NIV, as the interface is a 18-L mixing chamber that allows some degree of gas heating and humidification by the patient himself; the few available data during helmet continuous positive airway pressure (CPAP) indicate that artificial conditioning of the inspired gas may be necessary only as the flow washing the system exceeds 30–35 L/min [30, 31]. To our knowledge, no data ever assessed the effects during helmet pressure-support ventilation of the different strategies available for gas conditioning.
We conducted a randomized cross-over study to assess the effects of four different gas conditioning strategies during helmet pressure-support ventilation in hypoxemic patients, in terms of comfort, work of breathing, respiratory mechanics, patient–ventilator interaction and gas exchange.
Methods
This prospective, physiological, randomized, cross-over trial was conducted in the 20-bed general intensive care unit (ICU) of a university hospital in Italy between February 2017 and January 2019. Data analysis and manuscript drafting were delayed due to the COVID-19 pandemic. The study protocol was approved by local ethics committee and prospectively registered on clinicaltrials.gov (NCT02875379). All enrolled patients provided written informed consent to participating in the trial and data analysis.
Patients
Non-hypercapnic adult patients deemed by the attending physician to require respiratory support due to acute hypoxemic respiratory failure (PaO2/FiO2 ratio < 300) were screened for the enrollment. Exclusion criteria were: exacerbation of asthma or chronic obstructive pulmonary disease; clinical evidence of acute cardiogenic pulmonary edema; hemodynamic instability (systolic blood pressure < 90 mmHg or mean arterial pressure < 65 mmHg) and/or shock; metabolic acidosis (pH < 7.30 with normo- or hypocapnia); hypercapnia (PaCO2 > 45 mmHg); Glasgow coma scale < 13; recent gastric or abdominal surgery; non-collaborative patient.
Interventions
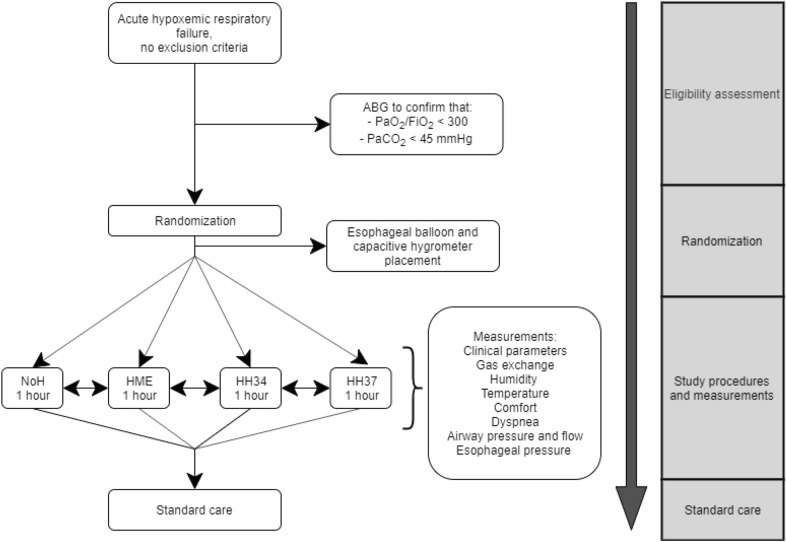
Study flow diagram is shown in Fig. 1.
Fig. 1.
Study flowchart
Patients underwent helmet NIV through a dedicated interface (Dimar, Mirandola, Italy), whose size was chosen according to neck circumference [9]. Patients were connected to a pneumatic mechanical ventilator (Drager Evita XL or Evita Infinity, Lubeck, Germany) [32]. The ventilator was set in pressure-support mode with the following settings: initial pressure support 10–12 cmH2O and then adjusted to attain a peak inspiratory flow of 100 L/min, up to a maximum of 20 cmH2O; PEEP 10–12 cmH2O; flow trigger 2 L/min and eventually increased in the presence of significant autotriggering; fastest pressurization time; expiratory trigger 30% of maximum inspiratory flow and eventually diminished in the case of double-triggering; maximum inspiratory time 1.0–1.2 s. FiO2 was titrated to obtain as SpO2 ≥ 92% and ≤ 98%. These settings were kept unchanged during the study steps.
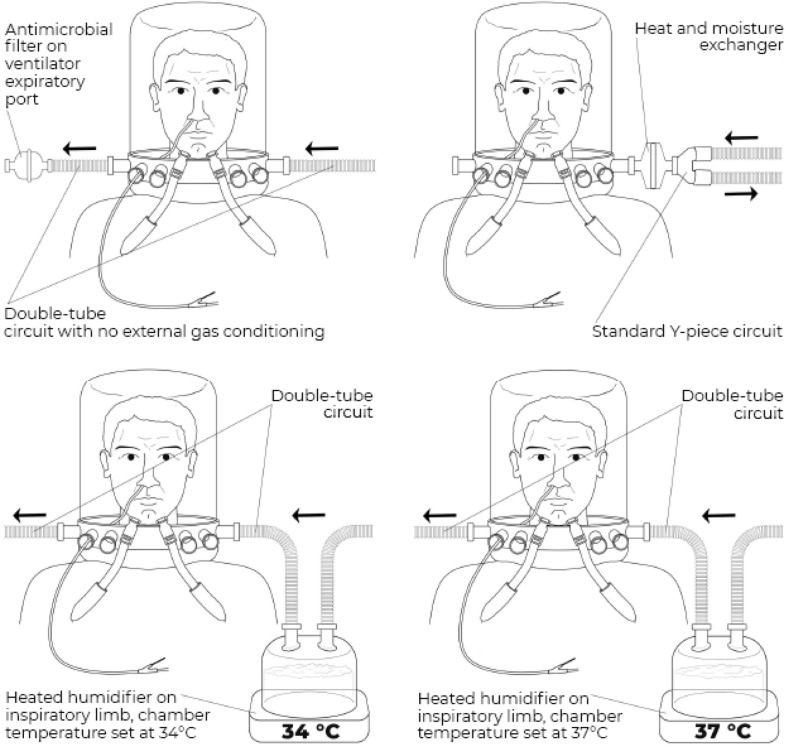
Each patient received four gas conditioning strategies (study steps) in a random order, for a duration of 1 h each (randomization was performed with SAS random allocation software and enrolled patients were assigned to the allocated sequence through sealed opaque envelopes) (Fig. 2):
Heated humidification (MR860, Fisher and Paykel healthcare, New Zealand) with humidification chamber set at 34 °C—double-tube circuit (HH34 step);
Heated humidification (MR860, Fisher and Paykel healthcare, New Zealand) with humidification chamber set at 37 °C—double-tube circuit (HH37 step);
Passive humidification with a heat and moisture exchanger (DAR™, Covidien, Medtronic, USA)—standard circuit with Y-piece (HME step);
No external humidification provided—double-tube circuit (NoH step).
Fig. 2.
Study set-up. The four study steps are illustrated. Black arrows indicate the direction of flow (i.e., arrows pointing towards the patient indicate the inspiratory limb, whereas arrows pointing away from the patient indicate the expiratory limb). Polyfunctional nasogastric tube for the measurement of esophageal pressure is also illustrated. Upper left panel: NoH step (double-tube circuit with no external humidification). Upper right panel: HME step (standard Y-piece circuit with a heat and moisture exchanger). Lower left panel: HH34 step (double-tube circuit with a heated humidifier, with humidification chamber set at 34 °C on the inspiratory limb). Lower right panel: HH37 step (double-tube circuit with a heated humidifier, with humidification chamber set at 37 °C on the inspiratory limb)
To avoid any carry-on effect, between each step, patients received a 15-min washout period on oxygen therapy.
After enrollment, a polyfunctional nasogastric tube provided with an esophageal balloon (Nutrivent, Sidam, Italy) was placed to measure esophageal pressure (PES). Because of the impossibility to perform Baydur maneuver in nonintubated patient [33–35], the esophageal balloon was filled with 4 ml of air, which has been shown to be a non-stress volume providing reliability in a wide pressure range for this specific balloon [36]. To ensure intra-individual reproducibility, esophageal balloon was deflated and, after checking adequate zeroing, re-inflated before all measurements.
A capacitive hygrometer (Dimar, Mirandola, Italy) for measurement of absolute and relative humidity (AH and RH) and temperature was positioned inside the helmet through a dedicated port. The device was calibrated before all measurements.
Measurements
Patient’s demographic and clinical characteristics and baseline room temperature and humidity were collected at study entry. During the study, patients received standard ICU monitoring (5-lead electrocardiogram, invasive blood pressure and SpO2).
At the end of each study step, clinical parameters (heart rate, arterial blood pressure, SpO2,) and arterial blood gases were collected, and patients were asked to rate the degree of dyspnea and device-related discomfort according to visual analog scales (VAS) for critically ill patients [5]. The examiner subjectively visually evaluated the presence and degree of condensation inside the helmet (“fog effect”) according to a 1-to-3 observation scale.
At the end of each step, after a stable breathing pattern was established and after ensuring stability of humidity and temperature measurements, a pressure transducer measured (sampling rate = 200 Hz) PES and a Fleisch-type pneumotachograph (n.2, Metabo, Lausanne, Switzerland) measured inspiratory flow and airway pressure (Paw). All signals were amplified, low-pass filtered, digitalized and transmitted to a personal computer with dedicated softwares for waveforms analysis (ICU Lab, Kleistek, Bari, Italy) and temperature and humidity data analysis (Humidity Dimar, E + E Elektronik Ges.m.b.H, Austria). All data from this recording were analyzed and results were averaged for each study step.
PES, Paw and flow tracings where then reviewed offline and the following parameters were computed for each step:
Respiratory mechanics and work of breathing indices: patient’s respiratory rate (number of negative deflections of PES per minute); ventilator respiratory rate (number of ventilator-delivered breaths per minute); inspiratory effort (ΔPES, the negative inspiratory swing of PES); simplified PES pressure–time product per breath (PTPES: area under the curve of esophageal pressure during inspiration, chest wall recoil pressure was neglected due to the impossibility of performing occlusions, PES) and per minute; dynamic transpulmonary driving pressure (ΔPL, the positive tidal swing in transpulmonary pressure, calculated as the difference between airway pressure and PES) [5, 34, 35, 37]. ΔPL, albeit primarily influenced by tidal volume, also includes a resistive component due to airflow; we assumed that resistive pressure did not change significantly between the four study steps, thus allowing to take ΔPL as a surrogate for transpulmonary driving pressure.
-
Patient–ventilator synchrony: the following asynchronies were diagnosed by in-phase reading of esophageal and airway tracings [35, 38, 39], according to existing definitions and diagnostic criteria [39–41]:
- Ineffective efforts: negative flexion of esophageal pressure (i.e., active inspiratory effort) without a corresponding delivery of a mechanical breath by the ventilator.
- Autotriggering: delivery of a mechanical breath by the ventilator without a corresponding negative flexion of esophageal pressure.
- Double cycling: delivery of two mechanical breaths by the ventilator corresponding to a single continuing negative flexion of esophageal pressure.
- Premature expiratory cycling: termination of a mechanical breath delivered by the ventilator (as assessed by the flow tracing) preceding the end of the corresponding patient’s active inspiratory effort as assessed by the esophageal pressure tracing.
The overall burden of asynchronies was assessed by the asynchrony index, defined as the total number of asynchronies divided by the total respiratory rate (computed as the sum of the number of ventilator-delivered breaths, triggered or not, and ineffective efforts). Inspiratory and expiratory trigger delay asynchronies were not considered, since helmet NIV is known to be characterized by significant physiological trigger delays due to the compliance of the interface and its associated slow internal pressure buildup and decay [5, 11, 42].
For all calculations, start of inspiration was determined at the instant of PES initial decay, and end of inspiration was determined at the point of the respiratory cycle where 25% of time elapsed from PES maximal negative deflection towards its return to baseline [43].
Endpoints
Primary endpoints were VAS discomfort, work of breathing indices and patient–ventilator synchrony. Main secondary endpoints were: humidity and temperature inside the interface, degree of fog effect, VAS dyspnea, respiratory rate, expired helmet minute volume, dynamic transpulmonary driving pressure.
Sample size calculation
Given the physiological design of the study, we did not perform a formal sample size calculation. Consistent with previous investigations on the topic with similar endpoints [16, 30, 31], we planned to enroll 20 patients.
Statistical analysis
Qualitative data are expressed as number of events (%) and continuous data as median [interquartile range]. Ordinal qualitative variables and non-normal quantitative variables distributions in the four study step were compared with the Wilcoxon/Kruskal–Wallis sum of rank test. Paired comparisons between steps were performed with the Wilcoxon test. All analysis was performed applying a bilateral hypothesis and results with p ≤ 0.05 were considered significant. Statistical analysis was performed with JMP® Pro, Version 13 (SAS Institute Inc., Cary, NC, USA). Manuscript figures were prepared with GraphPad Prism (GraphPad Software, La Jolla, CA USA).
Results
Twenty patients were included. Patients’ demographic and baseline most relevant clinical features are shown in Table 1; main results of the study are displayed in Table 2.
Table 1.
Demographics and baseline characteristics of enrolled patients
| Age, years | 72 [64–77] |
| Female sex , no. (%) | 7 (35%) |
| Height, cm | 168 [161–170] |
| Body mass index, kg/m2 | 28 [24–29] |
| Ideal body weight, kg | 64 [57–66] |
| SAPS II | 43 [34–51] |
| SOFA at study inclusion | 4 [3–8] |
| Reason for ICU admission, no. (%) | |
| Medical | 20 (100%) |
| Surgical | 0 (0%) |
| Cause of respiratory failure, no. (%) | |
| Pulmonary, infectious | 13 (65%) |
| Pulmonary, non-infectious | 5 (25%) |
| Extrapulmonary | 2 (10%) |
| Length of noninvasive respiratory support before enrollment, hours | 0 [0–14] |
| Baseline respiratory support device at enrollment, no. (%) | |
| Venturi mask | 1 (5%) |
| High-flow nasal oxygen | 16 (80%) |
| Facemask noninvasive ventilation | 1 (5%) |
| Helmet noninvasive ventilation | 2 (10%) |
| Characteristics at enrollment | |
| PaO2/FiO2 ratio, mmHg | 169 [112–279] |
| PaCO2, mmHg | 33 [29–35] |
| pH | 7.45 [7.41–7.50] |
| Heart rate, bpm | 91 [81–109] |
| Mean arterial pressure, mmHg | 93 [84–103] |
| Room temperature, °C | 25 [24, 25] |
| Room absolute humidity, mgH2O/L | 11 [9–12] |
| Room relative humidity, % | 50 [41–52] |
| Sedation/analgesia during the study*, no. (%) | 3 (15%) |
| Glasgow coma scale at study inclusion | 15 [15–15] |
| Need for endotracheal intubation, no. (%) | 9 (45%) |
| In-intensive care unit mortality, no. (%) | 7 (35%) |
Data are expressed as median [interquartile range], if not otherwise specified
*Two patients received remifentanil continuous infusion during the study, without dosage changes over the course of the study
Table 2.
Main results of the study
| NoH | HME | HH34 | HH37 | p | |
|---|---|---|---|---|---|
| Gas exchange | |||||
| PaO2/FiO2 ratio, mmHg | 260 [216–320] | 252 [207–317] | 232 [188–303] | 234 [201–292] | 0.73 |
| PaCO2, mmHg | 30 [28–36] | 34 [30–37] | 31 [29–35] | 34 [29–38] | 0.46 |
| pH | 7.47 [7.42–7.49] | 7.44 [7.41–7.48] | 7.46 [7.42–7.49] | 7.47 [7.41–7.49] | 0.73 |
| Gas conditioning, self-assessed symptoms and fog effect | |||||
| Absolute humidity, mgH2O/L | 16 [12–19]αβγ | 28 [23–31]αε | 28 [24–31]βζ | 33 [29–38]γζ | < 0.001 |
| Relative humidity, % | 52 [40–63]αβγ | 92 [81–97]αε | 96 [83–100]β | 100 [93–100]γε | < 0.001 |
| Helmet temperature, °C | 29 [28–30]α | 30 [29–31]ε | 31 [29–32]ζ | 32 [31–33]αεζ | < 0.001 |
| Dyspnea, VAS | 2 [1–5] | 3 [1–5] | 4 [1–5] | 4 [1–7] | 0.46 |
| Discomfort, VAS | 4 [2–6]γ | 6 [2–7] | 7 [4–8] | 8 [4–10]γ | 0.03 |
| Fog effect, 1–3 scale | 0 [0–0]αβγ | 1 [1–3]αε | 2 [1–3]βζ | 3 [3–3]γζ | < 0.001 |
| Respiratory mechanics and work of breathing indices | |||||
| Patient respiratory rate, bpm | 26 [22–31] | 26 [22–32] | 27 [21–33] | 26 [22–31] | 0.99 |
| Ventilator respiratory rate, bpm | 31 [27–36] | 31 [25–34] | 32 [24–34] | 30 [23–33] | 0.43 |
| Helmet minute ventilation, L*min−1 | 21 [20–27] | 19 [16–24]δε | 27 [23–30]δ | 24 [20–32]ε | 0.009 |
| ΔPES, cmH2O | 7 [4–9] | 9 [5–11] | 6 [4–10] | 7 [5–11] | 0.54 |
| ΔPL, cmH2O | 21 [17–23] | 17 [15–21] | 20 [18–23] | 21 [16–24] | 0.21 |
| PTPES per minute, cmH2O*sec*min−1 | 82 [40–127] | 109 [32–172] | 74 [34–137] | 61 [39–112] | 0.74 |
| Patient–ventilator asynchronies | |||||
| Asynchrony index (AI), % | 19 [4–51] | 7 [2–33] | 18 [5–35] | 16 [4–40] | 0.78 |
| Ineffective efforts, % | 1 [0–2] | 1 [0–2] | 1 [0–5] | 2 [0–6] | 0.55 |
| Proportion of AI due to ineffective efforts, % | 14 [0–71] | 15 [0–63] | 19 [0–64] | 22 [0–61] | 0.99 |
| Double cycling, % | 1 [0–5]α | 0 [0–1]αε | 0 [0–3] | 1 [0–3]ε | 0.05 |
| Proportion of AI due to double cycling, % | 7 [0–30]α | 0 [0–7]αε | 0.3 [0–12] | 11 [0–26]ε | 0.07 |
| Premature expiratory cycling, % | 7 [1–40] | 2 [0–19] | 6 [0–16] | 4 [0–14] | 0.72 |
| Proportion of AI due to premature expiratory cycling, % | 42 [9–80] | 24 [0–74] | 22 [6–56] | 21 [13–56] | 0.66 |
| Autotriggering, % | 0 [0–1]βγ | 0 [0–2]δ | 1 [0–8]βδ | 1 [0–6]γ | 0.03 |
| Proportion of AI due to autotriggering, % | 0 [0–2]βγ | 2 [0–30] | 14 [2–28]β | 16 [3–39]γ | 0.01 |
| Hemodynamics | |||||
| Heart rate, bpm | 83 [80–109] | 90 [75–108] | 90 [75–108] | 90 [76–106] | 0.98 |
| Mean arterial pressure, mmHg | 87 [73–104] | 88 [78–106] | 88 [72–101] | 83 [74–103] | 0.61 |
Data are expressed as median [interquartile range]
α indicates a p < 0.05 for paired comparisons between NoH and HME
β indicates a p < 0.05 for paired comparisons between NoH and HH34
γ indicates a p < 0.05 for paired comparisons between NoH and HH37
δ indicates a p < 0.05 for paired comparisons between HME and HH34
ε indicates a p < 0.05 for paired comparisons between HME and HH37
ζ indicates a p < 0.05 for paired comparisons between HH34 and HH37
Gas exchange
Median [interquartile range] PaO2/FiO2 ratio at enrollment was 169 mmHg [112–279]. No differences in PaO2/FiO2, PaCO2, pH, heart rate and mean arterial pressure were detected between the study steps (Table 2).
Gas conditioning
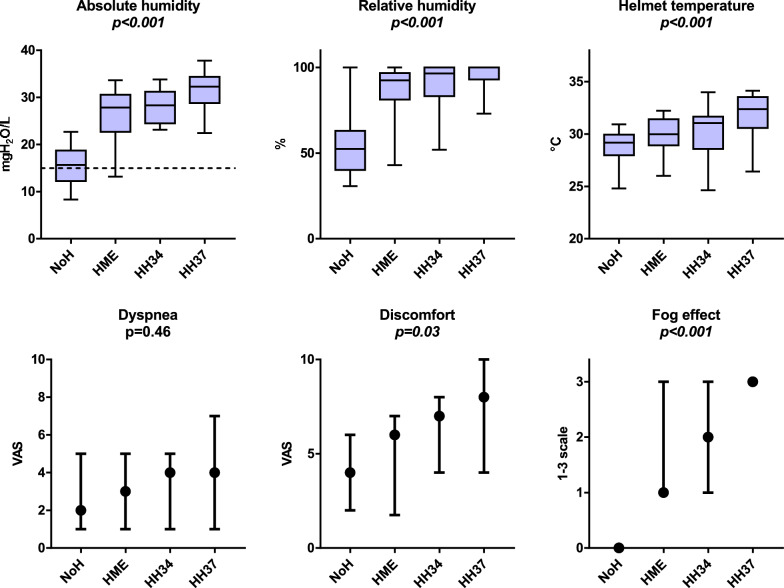
These results are shown in Table 2 and Fig. 3. Absolute humidity, relative humidity and temperature inside the helmet interface were significantly lower when the NoH strategy was applied (all p < 0.001), displaying an increasing trend from the steps NoH to HME to HH34 to HH37. Median absolute humidity was above 15 mgH2O/L in all steps, notably being 16 [12–19] mgH2O/L in the NoH step.
Fig. 3.
Upper panels show absolute humidity, relative humidity and temperature in the four study steps. Boxes indicate median [interquartile range] values, whiskers indicate minimum and maximum values. Dotted line in the absolute humidity panel marks the threshold of 15 mgH2O/L that has been indicated as the lowest humidity value to avoid discomfort during facemask NIV and CPAP [16]. When no external gas conditioning was applied (NoH step) both temperature and humidity were lower, albeit median absolute humidity was above 15 mgH2O/L, the 25th percentile being 12 mgH2O/L (i.e., most patients received an adequate level of humidification). Lower panels show VAS dyspnea, VAS discomfort and subjective observator-assessed fog effect (on a scale from 1 to 3) in the four steps (black circles indicate median value, range indicates interquartile range). Discomfort and fog effect were lowest in the NoH step
Self-assessed symptoms and fog effect
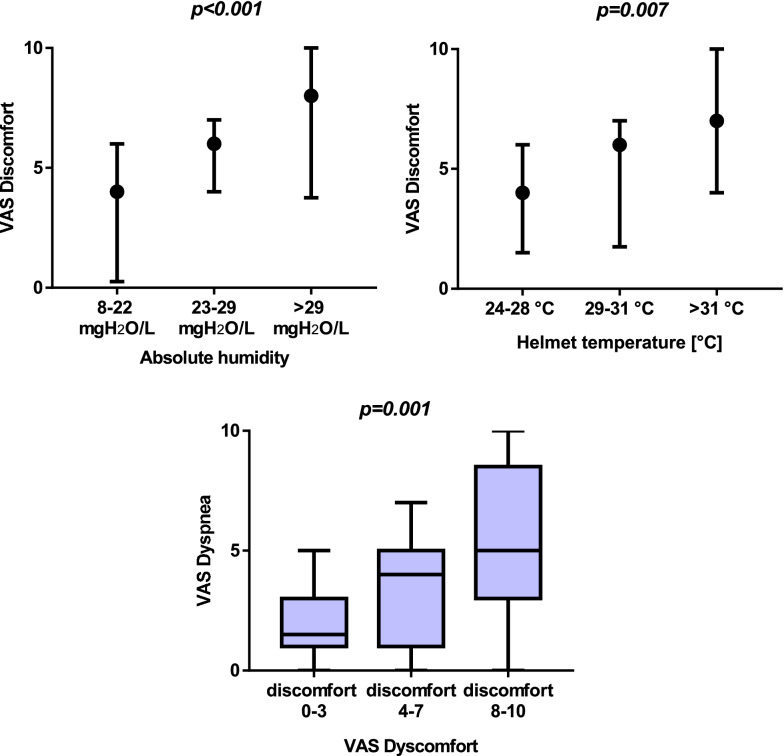
These results are shown in Table 2 and Fig. 3. VAS discomfort and observator-assessed fog effect were lower in the NoH step, increasing towards the higher-humidification steps (p = 0.03 and p < 0.001, respectively). VAS dyspnea was not significantly different between the study steps (p = 0.46). Across all study steps (all measurements taken into account), VAS discomfort increased at raising levels of absolute humidity (p < 0.001) and temperature (p = 0.007), as displayed in Fig. 4. Higher discomfort was associated to higher dyspnea (p = 0.001—Fig. 4).
Fig. 4.
Upper panels show the relationship between absolute humidity (left) and temperature (right), respectively, and VAS discomfort. Values of humidity and temperature on the x axis are divided in lower, middle and upper tertiles in the whole cohort of measurements (i.e., all measurements from the four study steps). Medians (black circles) and interquartile ranges are displayed. Discomfort increased at raising humidity and temperature across all steps. Lower panel illustrates the relationship between VAS discomfort (presented on the x axis as low, intermediate and high discomfort levels) and VAS dyspnea in the whole cohort of measurements, showing how raising discomfort is associated to increasing subjective dyspnea (independently of the gas conditioning strategy used). Data in this panel are presented as a boxes-and-whiskers plot, the boxes indicating median [interquartile range] and the whiskers indicating minimum and maximum value
Respiratory mechanics and work of breathing
These results are displayed in Table 2. Neither patient nor ventilator respiratory rate were different between the study steps (p = 0.99 and p = 0.43, respectively). Total minute ventilation (i.e., the total system minute fresh gas flow) was slightly higher in HH34 and HH37 when compared to the non-actively humidified steps (p = 0.009). The analyzed work of breathing indices (ΔPES, dynamic transpulmonary driving pressure and simplified esophageal PTPES) along with ΔPL did not differ between steps.
Patient–ventilator interaction
As shown in Table 2, the asynchrony index was not significantly different between the four steps (p = 0.78), with a median asynchrony index of 16% [4–40%]. Overall, the most represented asynchrony was premature expiratory cycling, with an index of 4% when all measurements were taken into account [0–16%]. The rate of autotriggering, albeit low in all steps, was significantly higher in HH34 and HH37 steps (p = 0.03).
Discussion
The main results of this randomized cross-over physiological study on gas conditioning during helmet pressure-support NIV can be summarized as follows:
Levels of humidity and temperature are significantly lower when no external gas conditioning is applied, when compared with the use of HME or HHs. Even with a NoH strategy, most patients are above the absolute humidity threshold of 15 mgH2O/L, which has been described as the minimum required humidification during NIV [16].
Subjective discomfort is lower when a double-tube circuit with no humidification is used, as it is the formation of fog effect inside the interface.
Overall, discomfort increased at raising levels of humidity and temperature, provided that the great majority of measurements above or around the abovementioned safety margin of 15 mgH2O/L. Increasing levels of discomfort appear to be related to worsening dyspnea in these patients.
The gas conditioning strategy does not appear to influence overall patient–ventilator synchrony, with the exception of an increased rate of autotriggering when HHs are used.
Respiratory rate, inspiratory effort, dynamic transpulmonary driving pressure and work of breathing are not affected by the gas conditioning strategy used. A slightly increased total helmet minute ventilation is seen when heated humidification is applied.
Two studies addressed the issue of gas conditioning during helmet continuous positive airway pressure (CPAP), and showed that humidification and comfort were optimal when no gas conditioning strategy was used during ventilator-delivered CPAP, while the application of a HH was needed at high continuous fresh gas flows (> 40 L min−1) with a PEEP valve [30, 31]. Notably, ventilator-delivered CPAP implies a lower fresh gas flow than continuous-flow CPAP, usually below the threshold of 30 L min−1, which is the minimum value of fresh gas flow suggested to avoid CO2 retention in the interface and significant rebreathing [15].
No previous data exist regarding the optimal gas conditioning strategy during helmet pressure-support ventilation, where system washout flow is equal or slightly superior to the system minute ventilation, typically lower than during high-flow CPAP but higher than during ventilator-delivered CPAP. This implies that existing data on helmet CPAP cannot be extrapolated to the setting of helmet pressure-support ventilation.
Our study demonstrates that, during helmet pressure-support ventilation, a double-tube circuit with no humidification allows adequate conditioning of the inspired gas, improves patient’s comfort and, when compared to active conditioning through heated humidifiers, may reduce ventilator autotriggering. Patient’s comfort appeared essential to prevent dyspnea. Both dyspnea and discomfort are important factors for successful noninvasive ventilation and avoiding the need for endotracheal intubation [17, 18, 44–47].
Helmet NIV is receiving growing attention and is being proposed as a first-line strategy in the noninvasive management of acute hypoxemic respiratory failure, as it may have physiological advantages and improve outcomes over facemask NIV and high-flow nasal oxygen [4–8]. Our study shines light on the rather unexplored but relevant issue of gas conditioning in the specific setting of helmet NIV and its effects on comfort and other patient-centered outcomes.
Our data indicate that an adequate minimal level of humidification is provided with all strategies during helmet NIV, including the absence of external gas conditioning. This is likely due to the relatively low total system washout flow (in all steps median helmet minute ventilation was below 30 L/min) when compared to high-flow CPAP, where usual flows range around 50 L/min: this allows the helmet interface to efficiently work as a mixing chamber where patient’s expired air directly contributes to the heating and humidification of ventilator-delivered dry inspired gas, without being massively washed-out as in high-flow CPAP [30]. Provided that minimal acceptable gas conditioning is guaranteed, our data indicate that excessive humidity and temperature may generate discomfort [31]: in our cohort over-humidification, rather than under-humidification, appeared to be related to patients’ discomfort. This explains the worsening discomfort when heated humidifiers are applied in this setting. Importantly, reduction of discomfort mediates concurrent reductions in the dyspnea score.
The gas conditioning strategy does not appear to influence gas exchange, work of breathing and overall patient–ventilator interaction (as measured by the asynchrony index). When individual asynchronies were analyzed, a small increase in the autotriggering rate with heated humidifiers was apparent, likely due to increased water condensation in the inspiratory limb of the circuit [48].
Despite the physiological nature of the study, our results may have relevant clinical implications. During helmet pressure-support noninvasive ventilation in hypoxemic patients, a double-tube circuit with no external humidification applied may be an acceptable gas conditioning strategy, since it does not affect work of breathing and oxygenation and appears to improve patient’s comfort and patient–ventilator interaction.
Our study has limitations:
The duration of each study step was limited to 1 h, which may preclude generalizability of the results to patients subjected to longer NIV treatments. Specifically, such a limited time could be insufficient to accurately assess respiratory discomfort directly related to higher airway dryness in case of under-humidification. However, study measurements were taken at the end of the 60-min periods after ensuring stability of physiological data, and the randomized cross-over design with 60-min consecutive periods of the present investigation is consistent with previous studies comparing different gas conditioning systems during CPAP or NIV with similar physiological endpoints [16, 28–30, 49]. We believe that this makes the results of our manuscript reproducible for longer-term treatments.
The small sample size may have precluded the possibility to draw specific conclusions on secondary outcome measures and particularly subgroup analyses. Nevertheless, similar sample sizes have been selected for physiological studies focusing on similar outcomes, and results on the primary outcomes show a strong statistical significance.
PaO2/FiO2 ratio, albeit below 300 mmHg, varies significantly between patients, with a relevant proportion of patients exhibiting mild hypoxemia (PaO2/FiO2 between 200 and 300). This may have diluted the sample of moderate-to-severe patients, which are expected to display higher inspiratory effort and total minute ventilation: it is possible that a sample of more severe patients with higher helmet minute ventilation could reach higher median washout flows, possibly exceeding 30–40 L/min and thus physiologically resembling the abovementioned condition of high-flow CPAP [30]. In such a setting, our conclusions may not be applicable, as the risk of under-humidification could dictate the use of external gas conditioning strategies.
Conclusion
During helmet pressure support NIV, a double-tube circuit with no external gas conditioning ensures adequate humidity and heat of the inspired gas, improves comfort and patient–ventilator interaction. Use of HHs or HME in this setting may generate excessive humidity and heat in the interface, finally increasing discomfort, which is associated to worse dyspnea.
Acknowledgements
The authors are grateful to Gabriele Esposito, PhD, for drafting Figure 2.
Abbreviations
- ICU
Intensive care unit
- NIV
Noninvasive ventilation
- PEEP
Positive end-expiratory pressure
- HH
Heated humidifier
- NoH
No humidification
- HME
Heat and moisture exchanger
- ΔPL
Dynamic transpulmonary driving pressure
- ΔPES
Esophageal pressure inspiratory swing
- PTPES
Esophageal pressure inspiratory pressure–time product
Authors' contributions
DLG, FB and MA designed the study. FB, GMA, LSM, VR, BM, GM, SDA and RM conducted the study on enrolled patients. FB, LSM, TM, MC, CDB and DLG analyzed the data. FB and DLG drafted the first draft of the manuscript. GB, RM, MAP and MA interpreted the results and revised the first draft of the manuscript. MA organized the study as an overall supervisor. All the authors approved the final version of the manuscript and agreed on submitting it to Annals of Intensive Care. All authors read and approved the final manuscript.
Funding statement
This work is supported by a research Grant by SIAARTI (2017 MSD award).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by local Ethics Committee and informed consent was obtained by enrolled patients according to committee recommendation.
Consent for publication
Not applicable.
Competing interests
DLG has received payments for travel expenses by Maquet, Getinge and Air Liquide. MA has received payments for Board participation from Maquet, Air Liquide and Chiesi. DLG and MA disclose a research grant by General Electric Healthcare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Filippo Bongiovanni, Email: filippo.bongiovanni02@gmail.com.
Domenico Luca Grieco, Email: dlgrieco@outlook.it.
Gian Marco Anzellotti, Email: g.anzellotti@yahoo.it.
Luca Salvatore Menga, Email: luca.salvatore.menga@gmail.com.
Teresa Michi, Email: teresa.michi@gmail.com.
Melania Cesarano, Email: mel.cesare@gmail.com.
Valeria Raggi, Email: raggi.valeria@gmail.com.
Cecilia De Bartolomeo, Email: cecilia.debartolomeo8@gmail.com.
Benedetta Mura, Email: benedettamura@virgilio.it.
Giovanna Mercurio, Email: giovimercurio@gmail.com.
Sonia D’Arrigo, Email: sonia.darrigo@virgilio.it.
Giuseppe Bello, Email: gsppbll@gmail.com.
Riccardo Maviglia, Email: riccardo.maviglia@policlinicogemelli.it.
Mariano Alberto Pennisi, Email: marianoalberto.pennisi@unicatt.it.
Massimo Antonelli, Email: Massimo.Antonelli@unicatt.it.
References
- 1.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 2.Gorman E, Connolly B, Couper K, Perkins GD, McAuley DF. Non-invasive respiratory support strategies in COVID-19. Lancet Respir Med. 2021;9:553–556. doi: 10.1016/S2213-2600(21)00168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021;47:851–866. doi: 10.1007/s00134-021-06459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grieco DL, Menga LS, Raggi V, Bongiovanni F, Anzellotti GM, Tanzarella ES, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201:303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 6.Ferreyro BL, Angriman F, Munshi L, Del Sorbo L, Ferguson ND, Rochwerg B, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure. JAMA [Internet]. 2020;324:57. https://jamanetwork.com/journals/jama/fullarticle/2767025 [DOI] [PMC free article] [PubMed]
- 7.Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri D, Jinah R, Burns KEA, Angriman F, Ferreyro B, Munshi L, et al. Helmet non-invasive ventilation compared to facemask non-invasive ventilation and high flow nasal cannula in acute respiratory failure: a systematic review and meta-analysis. Eur Respir J. 2021 doi: 10.1183/13993003.01269-2021. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli M, Conti G, Pelosi P, Gregoretti C, Pennisi MA, Costa R, et al. New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet—a pilot controlled trial. Crit Care Med. 2002;30:602–608. doi: 10.1097/00003246-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Vargas F, Thille A, Lyazidi A, Campo FR, Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37:1921–1928. doi: 10.1097/CCM.0b013e31819fff93. [DOI] [PubMed] [Google Scholar]
- 11.Mojoli F, Iotti GA, Currò I, Pozzi M, Via G, Venti A, et al. An optimized set-up for helmet noninvasive ventilation improves pressure support delivery and patient-ventilator interaction. Intensive Care Med. 2013;39:38–44. doi: 10.1007/s00134-012-2686-x. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Gao Y, Chen R, Cheng Z. Noninvasive ventilation with helmet versus control strategy in patients with acute respiratory failure: a systematic review and meta-analysis of controlled studies. Crit Care. 2016;20:265. doi: 10.1186/s13054-016-1449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T, Fujino Y, Amato MBP, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195:985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 14.Morais CCA, Koyama Y, Yoshida T, Plens GM, Gomes S, Lima CAS, et al. High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med. 2018;197:1285–1296. doi: 10.1164/rccm.201706-1244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taccone P, Hess D, Caironi P, Bigatello LM. Continuous positive airway pressure delivered with a “helmet”: effects on carbon dioxide rebreathing. Crit Care Med. 2004;32:2090–2096. doi: 10.1097/01.ccm.0000142577.63316.c0. [DOI] [PubMed] [Google Scholar]
- 16.Lellouche F, Maggiore SM, Lyazidi A, Deye N, Taillé S, Brochard L. Water content of delivered gases during non-invasive ventilation in healthy subjects. Intensive Care Med. 2009;35:987–995. doi: 10.1007/s00134-009-1455-y. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 18.Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. 2009;374:250–259. doi: 10.1016/S0140-6736(09)60496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54:71–84. [PubMed] [Google Scholar]
- 20.Esquinas Rodriguez AM, Scala R, Soroksky A, BaHammam A, de Klerk A, Valipour A, et al. Clinical review: humidifiers during non-invasive ventilation–key topics and practical implications. Crit Care. 2012;16:203. doi: 10.1186/cc10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomita M, Smaldone GC. Humidification and noninvasive ventilation. Respir Care. 2007;52:24–25. [PubMed] [Google Scholar]
- 22.Shelly MP, Lloyd GM, Park GR. A review of the mechanisms and methods of humidification of inspired gases. Intensive Care Med. 1988;14:1–9. doi: 10.1007/BF00254114. [DOI] [PubMed] [Google Scholar]
- 23.Burton JD. Effects of dry anaesthetic gases on the respiratory mucous membrane. Lancet (London, England) 1962;1:235–238. doi: 10.1016/s0140-6736(62)91187-x. [DOI] [PubMed] [Google Scholar]
- 24.Branson RD, Gentile MA. Is humidification always necessary during noninvasive ventilation in the hospital? Respir Care. 2010;55:209–216. [PubMed] [Google Scholar]
- 25.Fontanari P, Burnet H, Zattara-Hartmann MC, Jammes Y. Changes in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol. 1996;81:1739–1743. doi: 10.1152/jappl.1996.81.4.1739. [DOI] [PubMed] [Google Scholar]
- 26.American Association for Respiratory Care. Restrepo RD, Walsh BK. Humidification during invasive and noninvasive mechanical ventilation: 2012. Respir Care. 2012;57:782–788. doi: 10.4187/respcare.01766. [DOI] [PubMed] [Google Scholar]
- 27.Lellouche F, L’Her E, Abroug F, Deye N, Rodriguez PO, Rabbat A, et al. Impact of the humidification device on intubation rate during noninvasive ventilation with ICU ventilators: results of a multicenter randomized controlled trial. Intensive Care Med. 2014;40:211–219. doi: 10.1007/s00134-013-3145-z. [DOI] [PubMed] [Google Scholar]
- 28.Lellouche F, Pignataro C, Maggiore SM, Girou E, Deye N, Taillé S, et al. Short-term effects of humidification devices on respiratory pattern and arterial blood gases during noninvasive ventilation. Respir Care. 2012;57:1879–1886. doi: 10.4187/respcare.01278. [DOI] [PubMed] [Google Scholar]
- 29.Lellouche F, Maggiore SM, Deye N, Taillé S, Pigeot J, Harf A, et al. Effect of the humidification device on the work of breathing during noninvasive ventilation. Intensive Care Med. 2002;28:1582–1589. doi: 10.1007/s00134-002-1518-9. [DOI] [PubMed] [Google Scholar]
- 30.Chiumello D, Chierichetti M, Tallarini F, Cozzi P, Cressoni M, Polli F, et al. Effect of a heated humidifier during continuous positive airway pressure delivered by a helmet. Crit Care. 2008;12:R55. doi: 10.1186/cc6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueta K, Tomita T, Uchiyama A, Ohta N, Iguchi N, Goto Y, et al. Influence of humidification on comfort during noninvasive ventilation with a helmet. Respir Care. 2013;58:798–804. doi: 10.4187/respcare.01735. [DOI] [PubMed] [Google Scholar]
- 32.Scala R, Naldi M. Ventilators for noninvasive ventilation to treat acute respiratory failure. Respir Care. 2008;53:1054–1080. [PubMed] [Google Scholar]
- 33.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 34.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 35.Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42:1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 36.Mojoli F, Chiumello D, Pozzi M, Algieri I, Bianzina S, Luoni S, et al. Esophageal pressure measurements under different conditions of intrathoracic pressure. An in vitro study of second generation balloon catheters. Minerva Anestesiol. 2015;81:855–864. [PubMed] [Google Scholar]
- 37.Yoshida T, Grieco DL, Brochard L. Guiding ventilation with transpulmonary pressure. Intensive Care Med. 2019;45:535–538. doi: 10.1007/s00134-018-5483-3. [DOI] [PubMed] [Google Scholar]
- 38.Dres M, Rittayamai N, Brochard L. Monitoring patient-ventilator asynchrony. Curr Opin Crit Care. 2016;22:246–253. doi: 10.1097/MCC.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 39.Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med. 2006;32:34–47. doi: 10.1007/s00134-005-2828-5. [DOI] [PubMed] [Google Scholar]
- 40.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–1522. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 41.Grieco DL, Bitondo MM, Aguirre-Bermeo H, Italiano S, Idone FA, Moccaldo A, et al. Patient-ventilator interaction with conventional and automated management of pressure support during difficult weaning from mechanical ventilation. J Crit Care. 2018;48:203–210. doi: 10.1016/j.jcrc.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 42.Costa R, Navalesi P, Antonelli M, Cavaliere F, Craba A, Proietti R, et al. Physiologic evaluation of different levels of assistance during noninvasive ventilation delivered through a helmet. Chest. 2005;128:2984–2990. doi: 10.1378/chest.128.4.2984. [DOI] [PubMed] [Google Scholar]
- 43.Rittayamai N, Beloncle F, Goligher EC, Chen L, Mancebo J, Richard JCM, et al. Effect of inspiratory synchronization during pressure-controlled ventilation on lung distension and inspiratory effort. Ann Intensive Care. 2017;7:100. doi: 10.1186/s13613-017-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menga LS, Cese LD, Bongiovanni F, Lombardi G, Michi T, Luciani F, et al. High failure rate of noninvasive oxygenation strategies in critically ill subjects with acute hypoxemic respiratory failure due to COVID-19. Respir Care. 2021;66:705–714. doi: 10.4187/respcare.08622. [DOI] [PubMed] [Google Scholar]
- 45.Mercurio G, D’Arrigo S, Moroni R, Grieco DL, Menga LS, Romano A, et al. Diaphragm thickening fraction predicts noninvasive ventilation outcome: a preliminary physiological study. Crit Care. 2021;25:219. doi: 10.1186/s13054-021-03638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dangers L, Montlahuc C, Kouatchet A, Jaber S, Meziani F, Perbet S, et al. Dyspnoea in patients receiving noninvasive ventilation for acute respiratory failure: prevalence, risk factors and prognostic impact: a prospective observational study. Eur Respir J. 2018;52:1702637. doi: 10.1183/13993003.02637-2017. [DOI] [PubMed] [Google Scholar]
- 47.Menga LS, Grieco DL, Rosà T, Cesarano M, Delle Cese L, Berardi C, et al. Dyspnoea and clinical outcome in critically ill patients receiving noninvasive support for COVID-19 respiratory failure: post hoc analysis of a randomised clinical trial. ERJ open Res. 2021 doi: 10.1183/23120541.00418-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrone G, Cipriani F, Spinazzola G, Festa O, Arcangeli A, Proietti R, et al. A bench study of 2 ventilator circuits during helmet noninvasive ventilation. Respir Care. 2013;58:1474–1481. doi: 10.4187/respcare.02060. [DOI] [PubMed] [Google Scholar]
- 49.Natalini D, Grieco DL, Santantonio MT, Mincione L, Toni F, Anzellotti GM, et al. Physiological effects of high-flow oxygen in tracheostomized patients. Ann Intensive Care. 2019;9:114. doi: 10.1186/s13613-019-0591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.