Abstract
Fucosylation is an oligosaccharide modification that plays an important role in immune response and malignancy, and specific fucosyltransferases (FUTs) catalyze the three types of fucosylations: core-type, Lewis type, and H type. FUTs regulate cancer proliferation, invasiveness, and resistance to chemotherapy by modifying the glycosylation of signaling receptors. Oligosaccharides on PD-1/PD-L1 proteins are specifically fucosylated, leading to functional modifications. Expression of FUTs is upregulated in renal cell carcinoma, bladder cancer, and prostate cancer. Aberrant fucosylation in prostate-specific antigen (PSA) could be used as a novel biomarker for prostate cancer. Furthermore, elucidation of the biological function of fucosylation could result in the development of novel therapeutic targets. Further studies are needed in the field of fucosylation glycobiology in urological malignancies.
Keywords: fucosylation, fucosyltransferase, prostate cancer, bladder cancer, kidney cancer
1. Introduction
Fucosylation is an oligosaccharide modification that plays an important role in immune response and malignancies. Changes in glycosylation status affect cellular function via glycosylated proteins, such as growth factor receptors and adhesion molecules.
To date, advances in glycomics have identified several types of biomarkers, including fucosylation-related factors, that are associated with certain types of cancers.
One of the representative fucosylation-related cancer biomarkers is fucosylated alpha-fetoprotein (AFP), which is named the AFP-L3 fraction. It has been widely used as a specific cancer biomarker for hepatocellular carcinoma (HCC) since 1996 in Japan and 2005 in the United States [1,2] because it is more specific than alpha-fetoprotein. Another famous one is sialic Lewis a (sLea), also known as CA19-9, which has been clinically used as a cancer biomarker for pancreatic cancer [3].
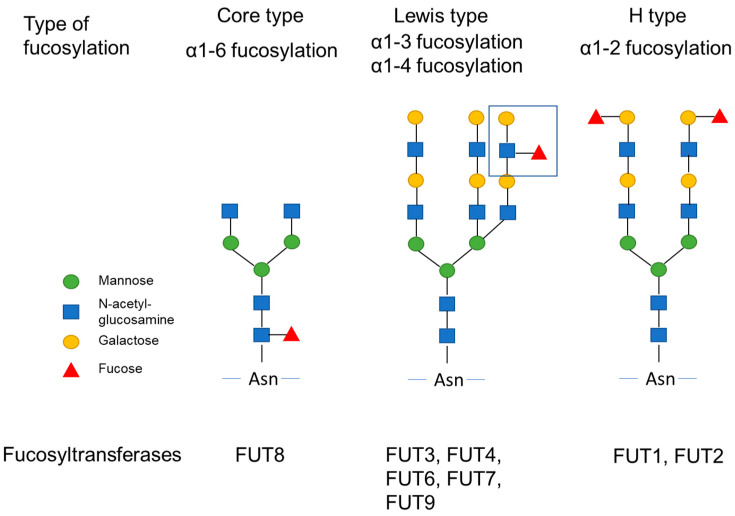
Glycosylation, including fucosylation of the protein, occurs within the Golgi endoplasmic reticulum. Fucosylation is dependent on fucosyltransferase (FUT) activity and the level of its donor substrate, guanosine diphosphate (GDP)-fucose synthetic enzyme. In addition, GDP-mannose-4,6-dehydratase (GMDS) is a key enzyme involved in the synthesis of GDP-fucose [4]. Three types of fucosylation exist: α1-2 fucose (H type), α1-3/α1-4 fucose (Lewis type), and α1-6 fucose (core type) (Figure 1). Each type of fucosylation is catalyzed by specific FUTs [5,6,7,8]. Eleven different FUTs are classified into four groups. FUT1 and FUT2 catalyze the synthesis of α1-2 fucose, FUT3, 4, 5, 6, 7, and 9 catalyze the synthesis of α1-3/α1-4 fucose, and only FUT8 catalyzes the synthesis of α1-6 fucose [5]. FUT activity has not been reported for either FUT10 or FUT11.
Figure 1.
Fucosylation and fucosyltransferases. Three types of fucosylation, α1-2 fucose (H type), α1-3/α1-4 fucose (Lewis type), and α1-6 fucose (core type) are shown. Each fucosylation are catalyzed by specific fucosyltransferases.
This review will discuss the importance of fucosylation as a biomarker in malignancies, especially urological cancers, and the possible biological and clinical role of fucosylation based on recent findings.
2. Biological Function of Fucosyltransferases and GDP-Fucose Synthetic Enzyme
Fucosylation is the enzymatic attachment of L-Fucose to oligosaccharides on glycoproteins and glycolipids or proteins. It is one of the most important post-translational modifications, which can confer unique functions to oligosaccharides or proteins. L-fucose is equivalent to 6-deoxy-L-galactose and has two structural differences from other hexoses in mammals. One is the lack of a hydroxyl group on the carbon at the 6-position and the other is the L-configuration. Fucosylation is closely associated with various physiological events and is reported to be upregulated in several types of cancers. Fucosylation is regulated by FUTs, and FUTs regulate cancer proliferation, invasiveness, and resistance to chemotherapy by modifying the glycosylation of signaling receptors. Each FUT has been reported to play a significant role in various types of cancers (Table 1).
Table 1.
Biological function of fucosyltransferases.
| Fucosyltransferase | Type of Fucosylation | Function in Cancer | References |
|---|---|---|---|
| FUT1 | α1-2 fucosylation | Migration, invasion, epithelial-mesenchymal transition, and drug resistance | [6,7,8] |
| FUT2 | α1-2 fucosylation | Migration, invasion, and epithelial-mesenchymal transition (EMT) | [6] |
| FUT3 | α1-3/α1-4 fucosylation | Activation of TGF-β signaling pathway | [9,10] |
| FUT4 | α1-3/α1-4 fucosylation | Drug resistance, invasion, migration, EMT, and cell adhesion | [11,12,13] |
| FUT5 | α1-3/α1-4 fucosylation | Activation of PI3K/Akt signaling pathway | [14] |
| FUT6 | α1-3/α1-4 fucosylation | Activation of PI3K/Akt signaling pathway | [14] |
| FUT7 | α1-3/α1-4 fucosylation | Proliferation, migration, invasion, and EMT; activation of MAPK and PI3K/Akt signaling pathway via EGFR | [15,16] |
| FUT8 | α1-6 fucosylation | Invasion, migration, and EMT; activation of TGF-β and EGFR signaling pathway | [17,18,19,20,21,22] |
| FUT9 | α1-3/α1-4 fucosylation | Cancer stemness | [23] |
| FUT10 | No fucoysltransferase activity | ||
| FUT11 | No fucoysltransferase activity |
FUT1 and FUT2 encode α1-2 FUTs. Glycan products of FUT1 and FUT2, such as Globo H and Lewis Y, are highly expressed in malignant tissues. Overexpression of FUT1 or FUT2 promotes the migration and invasion of tumor cells in breast cancer cell lines and metastasis in vivo, whereas FUT1 or FUT2 knockdown reduces Globo H and decreases mesenchymal-like markers, such as fibronectin, vimentin, and twist [9]. FUT1 expression is associated with chemotherapy resistance in ovarian cancer through interaction with apoptotic pathways [10,11].
FUT3 overexpression promotes the fucosylation of transforming growth factor (TGF)βR-I, resulting in the activation of the TGF-β signaling pathway in colorectal cancer [10]. The FUT3 gene is also known as the Lewis gene, and the high expression of FUT3 and its product, tetrasaccharide Sialic Lewis x (sLex), has been reported in several types of malignant tumors, such as breast cancer [12], pancreatic cancer [13], ovarian carcinoma, and colorectal cancer [14]. sLex is the well-known ligand of E-selectin [15], which mediates cell–cell adhesion. This adhesion is necessary for leukocyte rolling on the endothelium when inflammation takes place, but tumor cells utilize its mechanism to extravasate to distant places during metastasis, resulting in the metastasis of cancer [16]. A recent study reported that the abilities of proliferation, invasion, and migration in gastric carcinoma can be inhibited by FUT3 gene silencing [17]. In addition, FUT3 also specifically catalyzes another well-known fucosylated glycan, sialic Lewis a (sLea, CA19-9). sLea is another ligand for E-selectin and a serum biomarker of pancreatic cancer and colorectal cancer in the prediction of prognosis and surveillance of recurrence [18].
FUT4 is frequently upregulated in lung cancer, and FUT4 knockdown increases chemosensitivity to cisplatin by suppressing FOXO1-induced apoptosis [19]. FUT4 overexpression promotes lung cancer invasion, migration, epithelial-mesenchymal transition (EMT), and cell adhesion. Genome-wide RNA-seq and immunoprecipitation–mass spectrometry showed that FUT4 is associated with membrane trafficking, cell cycle, and major oncogenic signaling pathways [20]. Metastasis-associated lung adenocarcinoma transcript 1(MALAT1) in exosomes increased FUT4-mediated fucosylation and phosphorylation of the PI3K/Akt/mTOR pathway and promoted invasion and metastasis in colon cancer cells [21].
FUT5 or FUT6 expression modulates the activity of the PI3K/Akt signaling pathway in colorectal cancer cells [22]. FUT7 is overexpressed in follicular thyroid carcinoma and is associated with a poor prognosis. The extent of glycan α1-3 fucosylation on epidermal growth factor receptor (EGFR) was positively correlated with EGFR activation. FUT7 enhances EGF-induced progression of follicular thyroid carcinoma cells through mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathways [23]. In bladder cancer, FUT7 expression is correlated with tumor-infiltrating lymphocytes and promotes tumor proliferation, migration, invasion, and EMT [24].
FUT8 is the only FUT that catalyzes core fucosylation (α1-6 fucosylation) [25]. Core fucosylation of TGF-β receptor by FUT8 promotes TGF-β signaling and epithelial-mesenchymal transition in breast cancer cells [26]. In mice, FUT8 knockout induces severe growth retardation, early death during postnatal development, and emphysema-like changes in the lungs. Core fucosylation plays an important role in growth factor receptors, such as TGF-β1 and EGFR-mediated biological functions [27]. Blocking core fucosylation of TGF-βRII and ALK5 in renal tubular cells inhibits TGF-β1 signaling by suppressing the phosphorylation and translocation of Smad2/3 to the nucleus, resulting in the inhibition of EMT [28]. FUT8 overexpression is also observed in cancer-associated fibroblasts (CAFs) in non-small-cell lung carcinoma, and FUT8 in CAFs promotes the formation of an invasive and malignant tumor microenvironment via core fucosylation of EGFR [29]. Overexpression of FUT8 in prostate cancer cells reduces the number of extracellular vesicles secreted by prostate cancer cells and increases the abundance of proteins associated with cell motility and prostate cancer metastasis [30].
In colon cancer cells, FUT9 expression is associated with the expression of genes related to cancer stemness. Lewis X, the markers of stemness (CD44 expression, ALDH, Sox2), and tumor-sphere formation, chemoresistance to 5-FU treatment, and proliferation of tumor in vivo were increased in FUT9-expressing murine colon adenocarcinoma cells compared with control cells [31].
FUT activity has not been confirmed for either FUT10 or FUT11 [2], but a meta-analysis of microarray data showed that FUT11 expression was associated with tumor progression in clear cell renal cell carcinoma [32].
GDP-Fucose Synthetic Enzyme
GDP-fucose synthetic enzyme, also known as TSTA3, is the key enzyme involved in fucosylation. TSTA3 produces GDP-L-fucose, which is the only donor of fucosylation. TSTA3 is amplified in esophageal squamous cell carcinoma, and TSTA3 overexpression results in increased cell invasion and tumor metastasis. TSTA3 may play a role in promoting invasion by increasing the core fucosylation of LAMP2 and the terminal fucosylation of ERBB2 [33]. In glioblastoma cell lines, Golgi phospholipoprotein 3 (GOLPH3) regulates EGFR via terminal sialylation and fucosylation. Changes in the glycosylation pattern of EGFR lead to changes in its localization and tracking [34]. In a breast cancer cell line, the fucosylation inhibitor 2-fluorofucoseS decreases NF-κB activity by increasing IκBα [35].
3. Fucosylation in Cancer Immunology
The development of immune checkpoint inhibitors has changed the treatment strategy for advanced renal cell carcinoma and urothelial carcinoma [36]. Recently, the evaluation of glycosylation profiles has gained increasing interest in several types of cancers, especially in immunotherapeutic targets, such as PD-1 and PD-L1 [37,38,39]. The N-glycans of PD-1 and PD-L1 are highly core fucosylated, and fucosylation also plays an important role in the regulation of PD-1 and PD-L1 functions. Inhibition of FUT8 by genetic ablation or inhibitors reduces cell-surface expression of PD-1 and enhances T cell activation, resulting in the improvement of anti-tumor response in mice [40].
The T cell receptor is also a highly core fucosylated glycoprotein. The core fucosylation of CD4+ T cells is significantly increased in systemic lupus erythematosus patients, and loss of FUT8 reduced CD4+ T cell activation [41]. Levels of core fucosylation of T cell receptors are increased in patients with inflammatory bowel disease, and core fucosylation of the T cell receptor is required for T cell signaling, the production of inflammatory cytokines, and induction of colitis in mice [42]. Core fucosylation is required for signal transduction via T cell receptors (TCRs), and the ablation of core fucosylation results in abnormal T cell development in the thymus due to attenuated signaling via TCRs [43]. The formation of lung adenocarcinoma is markedly reduced in FUT8−/−OT-I mice. Removal of core fucosylation from PD-1 impaired its expression on CD8+ cytotoxic T lymphocytes (CTLs), resulting in enhanced CTL activation and cytotoxicity, leading to tumor eradication. Loss of core fucosylation significantly enhanced PD-1 ubiquitination, leading to the degradation of PD-1 in the proteasome [44]. Inflammatory cytokine production by macrophages was suppressed in FUT8-deficient mice. Core fucose is essential for CD14-dependent toll-like receptor (TLR) 4 and TLR2 signaling in mouse macrophages [45]. Levels of α1-3 fucosylated AGP (fAGP) increased along with disease progression and decreased in response to chemotherapy treatments in patients with lung cancer. Thus, Serum fAGP level is a biomarker for predicting the clinical efficacy of immunotherapy with nivolumab [46].
In addition, it is reported that Immunoglobulin G (IgG) is also highly core fucosylated. Loss of fucosylation also has significant biological consequences toward IgG antibodies. IgG antibodies are crucial for protection against invading pathogens. A highly conserved N-linked glycan within the IgG-Fc tail is essential for IgG function and the lack of IgG core fucosylation results in 50–100 times higher activity of antibody-dependent cellular cytotoxicity [4]. Therefore, defucosylated IgG antibodies are already used in anticancer therapies for their increased activity through Fc receptors (FcγRIIIa) [47,48]. Another study also reported that fucosylation affects tumor immune surveillance and a deficiency of fucosylation leads to escape from NK cell-mediated tumor immune surveillance through regulating death-ligand-induced apoptosis via tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) signaling [4].
4. Fucosylation in Renal Cell Carcinoma
Renal cell carcinoma (RCC) accounts for 3% of all malignancies in the United States. If detected in the early stage, RCC can be cured by surgery, with a 5-year survival rate of 70–90%. However, locally advanced disease has a great risk of recurrence, and metastatic disease is often fatal with a 5-year survival rate of less than 10%. RCC is usually detected incidentally by ultrasonography or computed tomography (CT), but there are no specific biomarkers for early detection and/or prediction of disease recurrence.
Metabolically, many transcriptomic and proteomic studies have reported the increased glucose and glutamine utilization associated with the Warburg effect in clear cell RCC (ccRCC), the most prevalent subtype of RCC [49,50]. The alternation of glucose and glutamine utilization may have effects on cell surface glycosylation including fucosylation in RCC tissue since the biosynthesis of the sugar components of glycoproteins, proteoglycans, and glycolipids are reportedly linked with glucose and glutamine metabolism.
As mentioned above, fucosylation is known to affect various cellular functions such as cell–cell adhesion, signal transduction, and immune recognition in malignant tumors. Vascular-growth-factor-targeted therapy and immunotherapy are the major therapeutic targets in RCC; changes in fucosylations are expected to have an impact on potential therapeutic effects.
Richard R. Drake et al. assessed the N-glycosylation patterns and compositional differences between tumor and non-tumor regions of ccRCC specimens using an imaging mass spectrometry approach and transcriptomic gene array data. Their results showed that multiantennary N-glycans with bisecting N-acetylglucosamine and multiple fucose residues, which are abundant in the proximal tubule, are not detected in ccRCC tissues, whereas multiple tumor-specific N-glycans with tri- and tetra-antennary structures and various levels of fucosylation and sialylation were present in ccRCC tissues. In addition, the analysis of transcriptomic gene array data revealed that the expression of the FUT3 and FUT6 genes coding fucosyltransferases responsible for α1-3/α1-4 fucosylation were significantly decreased in all ccRCC tissues compared with matched non-malignant tissues [51].
Tousi et al. focused on N-glycosylation of clusterin (apolipoprotein J), which is known to be upregulated in different types of RCC cell lines and tissues, and N-glycosylation of plasma clusterin in patients with RCC was examined using multi-dimensional high-performance liquid chromatography. The plasma levels of bi-antennary digalactosyl disialylated (A2G2S(3)2) glycans were significantly decreased, whereas the levels of a core fucosylated bi-antennary digalactosyl disialylated glycan (FA2G2S(6)2) and tri-antennary trigalactosyl disialylated glycan (A3G3S(6)2) were increased in the plasma samples after radical nephrectomy. Core fucosylation occurred in glycans of clusterin in the event of RCC [52]. The same group also assessed the individual N-glycosylation sites of plasma clusterin from patients with ccRCC using liquid chromatography followed by Tandem mass spectrometry. The core fucosylated biantennary digalactosylated disialylated (FA2G2S2) glycan in clusterin was reduced in the plasma of patients with clear cell RCC compared with plasma after nephrectomy [53]. Meng, L et al. performed immunohistochemical analysis of FUT3 expression in ccRCC and showed that high expression of FUT3 was associated with poor prognosis in ccRCC patients who underwent radical nephrectomy. FUT3 is an enzyme with α1-3 FUT and α1-4 FUT activities and catalyzes the Lewis blood group antigen [54].
The biological function of fucosylation in RCC remains to be elucidated, but these changes in fucosylation associated with RCC can be used to distinguish between cancer and non-cancer samples and suggest the potential for new therapeutic interventions and diagnostic markers
5. Fucosylation in Urothelial Carcinoma
Bladder cancer is the most frequently diagnosed urinary tract carcinoma, and urothelial carcinoma accounts for 90% of all bladder cancers. Despite significant advancements in medical treatment, bladder cancer detected at later stages has a poor prognosis [55]. There are also problems with non-muscle-invasive bladder cancer. The high recurrence rate after transurethral resection and progression to muscle-invasive disease is a challenging problem for patients [56]. Cystoscopy remains the gold standard tool for diagnosis and follow-up monitoring of the disease, but it is an invasive and costly procedure. As well as cystoscopy, urinary cytology has an established role in bladder cancer diagnosis and follow-up monitoring. However, it has poor sensitivity, particularly for low-grade tumors, and does not serve as a prognostic tool [57]. Although new biomarkers, such as Bladder EpiCheck, have been developed [58], there is an urgent need for the development and implementation in clinical practice of a potential therapeutic target and a non-invasive diagnostic biomarker for bladder cancer.
Core fucosylated N-glycans have been reported to be highly expressed, whereas terminal fucosylation of N-glycans is underexpressed in bladder cancer cell lines [59]. The abundant core fucosylated N-glycans in bladder cancer might be associated with prognosis or become a promising target molecule for future therapy. Terminal fucosylation occurs in N- and O-glycans through α1-2, α1-3, and α1-4 linkages, which are induced by FUTs ranging from FUT1 to FUT11, except for FUT8. Fucosylation by FUT1 through α1-2 linkages promotes bladder cancer progression [60]. However, the involvement of other FUTs in bladder cancer has not been reported, and further investigation is required. Lewis antigens found on cancer cell surfaces are another type of fucosylation product of oligosaccharides. Lewis X antigen (3-fucosyl-N-acetyl-lactosamine, CD15) is not detected in normal urothelial cells but is overexpressed in the malignant urothelium. It was reported that the diagnostic accuracy of bladder cancer detection can be enhanced by the Lewis X antigen [61]. Furthermore, the expression of Lewis X antigen in bladder cancer increases in proportion to stage, grade, and metastatic potential [62]. In contrast, lower expression of Lewis X antigen on human neutrophil glycoproteins is associated with better prognosis in patients with bladder cancer after total cystectomy [63].
Moreover, it is known that growth factor receptor signaling drives cancer progression. Other cancer types modulate growth factor receptor signaling, such as TGFβ, EGFR, vascular endothelial growth factor receptor, and c-Met, through fucosylation. Although bladder cancer progression is correlated with these signaling pathways [64,65], the relationship between fucosylation and bladder cancer progression has not been fully investigated. Jia Guo et al. compared N-glycan profiles of TGFβ-treated vs. control bladder cancer cells by MALDI-TOF/TOF-MS and revealed that fucosylation was increased in TGFβ-treated cells. This finding was consistent with the results of lectin microarray analysis, which showed that the Fucα1-2Galβ1-4GlcNAc structure recognized by Lectin ulex europaeus agglutinin-I (UEA-I) was approximately two-fold higher in TGFβ-treated cells than in control cells. These results may suggest that fucosylation plays an important role in TGFβ-induced EMT of bladder cancer [66].
Terminal fucosylation of oligosaccharides mediated by FUT1 is correlated with bladder cancer progression. It should be investigated whether abundant expression of core fucosylation mediated by FUT8 in bladder cancer patients could be a predictor of prognosis. Although growth factor receptor signaling activated by fucosylation has been reported in other cancer types, it has not been investigated in bladder cancer. Further research in this field is required.
As a biomarker for bladder cancer, fucosylated-glycoisoform of integrin alpha-3 (ITGA3) from the urine of bladder cancer patients was utilized for bladder cancer diagnosis. ITGA3 was recognized with its specific lectin, UEA-I, and Islam MK. et al. reported the detection of urinary ITGA3 glycoisoform with the use of UEA conjugated on nanoparticles (the ITGA3-UEA assay). The assay is capable of discriminating bladder cancer from clinically challenging age-matched benign conditions (p = 0.007) [67].
Thus, targeting fucosylation may be useful in both the treatment and diagnosis of bladder cancer.
6. Fucosylation in Prostate Cancer
Prostate cancer is the second most frequently diagnosed cancer among men and was the cause of an estimated 385,000 deaths worldwide in 2018 [68]. Glycosylation is a common post-translational modification of secreted proteins that play a key role in many important biological processes in prostate cancer [69,70].
Core-type fucosylation is the major type of glycosylation in prostate cancer [71], and the levels of core-fucosylated glycans are significantly increased in serum samples derived from prostate cancer patients compared with patients with benign prostate hyperplasia [72,73]. The fucosylation of glycan structures is higher in the serum samples derived from patients with metastatic prostate cancer than in healthy males, showing area under the curve (AUC) values greater than 0.9 in several specific N-glycans [74]. Fucosylation of glycans plays an important role in prostate cancer development and progression. TRAMP mice deficient in α1-3 FUT activity exhibited a lower incidence of prostate cancer formation and a lower rate of tumor progression, as evidenced by significantly smaller prostate weights [75]. Fucosylated glycopeptides are overexpressed in aggressive prostate cancer cell lines compared with nonaggressive prostate cancer cells [76,77].
FUT8 is considered to be the only FUT involved in core-fucosylation based on the fact that FUT8 knockout mice showed no oligosaccharide structures with core fucose [78]. Analysis of fucosylation of serum haptoglobin showed that core-type fucosylation is more characteristic in prostate cancer than in gastrointestinal cancer [71]. High expression of FUT8 and haptoglobin was observed in prostate cancer cell lines, and serum levels of fucosylated haptoglobin were associated with high-grade prostate cancer [79]. FUT8 overexpression in LNCaP cells increased cell migration, whereas FUT8 knockdown in PC3 cells decreased cell motility [80]. FUT8 was overexpressed in androgen-resistant LAPC4 cells compared with androgen-dependent LAPC4 cells, suggesting the functional role of fucosylated enzymes associated with aggressive prostate cancer [81].
As described above, fucosylation is upregulated in malignancies as compared with that in benign disease. Thus, the detection of fucosylated proteins could be a biomarker of urological malignancies, especially in prostate cancer. Prostate-specific antigen (PSA) is a glycoprotein that is commonly used as a biomarker for prostate cancer diagnosis. However, a single PSA test is not sufficient to distinguish between prostate cancer and benign prostate hyperplasia (BPH). To develop novel biomarkers, there is an unmet clinical need to refine the PSA test. PSA has one N-glycosylation site (Asn-69) to which various types of oligosaccharides can be attached [69,82]. In 2004, Ohyama et al. demonstrated that free serum PSA from patients with prostate cancer exhibits increased binding to Maackia amurensis agglutinin (MAA) lectin, which recognizes α2,3-linked sialic acid, compared with that in patients with BPH [83]. Since then, a number of reports have been published on PSA glycosylation. Fucosylation in PSA has been studied by several groups. Fukushima et al. demonstrated that there is an elevated expression of α1,2-Fucosylation of PSA during prostate carcinogenesis, using Trichosanthes japonica agglutinin-II (TJA-II) column chromatography [84]. Dwek et al. demonstrated that serum α1,2-Fucosylated PSA was significantly higher in men with prostate cancer than in men with BPH using an enzyme-linked immunosorbent lectin assay with Ulex europaeus (UEA-1) [85]. Li et al. demonstrated that the fucosylated total PSA was significantly increased in high-risk prostate cancer and correlated with the tumor Gleason score, using a magnetic bead-based immunoassay in which fucosylated total PSA was detected by Aleuria aurantia lectin (AAL) [86]. AAL exhibits broader specificity towards fucosylation, whereas PhoSL and Lens culinaris agglutinin (LCA) show specificity toward core-type fucosylated N-glycans [87]. Since a lectin generally has a weak binding affinity to fucose, the development of lectin-antibody ELISA for serum fucosylated markers is difficult due to the existence of abundant proteins in the sera. Ishikawa et al. developed microfluidic electrophoresis immunoassay systems to measure the α2,3-linked sialyl N-glycan-carrying PSA (S2,3PSA) using MAL lectin [88]. They found that the ratio of serum S2,3PSA to non-sialylated PSA could predict prostate cancer better than serum PSA. Fujita et al. showed that the ratio of serum fucosylated PSA could differentiate high-risk prostate cancer from biopsy-negative or high-risk prostate cancer using PhoSL to detect core-type fucosylated free PSA using a microcapillary electrophoresis-based immunoassay system [89]. When S2,3PSA and core-type fucosylated PSA (FucPSA) were simultaneously measured by automated micro-total immunoassay systems, S2,3PSA was not correlated with FucPSA in patients with prostate cancer. Thus, the simultaneous measurement of S2,3PSA and FucPSA (SF index) increased the accuracy of the detection of high-risk prostate cancer. The SF index showed good discrimination for high Gleason score cancer (AUC 0.842; 95%CI, 0.782–0.903), compared with the single PSA test (AUC 0.632, 95%CI 0.543–0.721), S2,3PSA (AUC 0.711, 95%CI 0.629–0.793), and FucPSA (AUC 0.738, 95%CI 0.657–0.819) [90]. Thus, fucosylated PSA, especially core-type fucosylated PSA, can be a useful biomarker for prostate cancer diagnosis.
7. Therapeutic Target of Fucosylation
Since fucosylation is associated with tumor growth and invasion, inhibition of fucosylation has been attempted to treat tumors. Fluorinated fucose analogs inhibit the activity of FUTs, including FUT8, and inhibit the proliferation of several types of cancer cells [91]. The L-fucose analog 2-fluoro-L-fucose (2FF) inhibits core fucosylation by interfering with the normal synthesis of GDP-fucose, resulting in the inhibition of cell proliferation and migration in liver cancer cell lines [92]. The development of specific FUT inhibitors or neutral antibodies for core fucose could lead to the development of a new class of drugs for cancer. Miyoshi et al. revealed that fucosylation controls the sensitivity to NK cells through regulating tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) signaling-induced apoptosis. Although the increase in fucosylation plays important roles at an early stage of carcinogenesis, tumor cells could escape from NK-cell-mediated tumor surveillance by defucosylation induced by genetic mutation in several types of advanced cancers [93].
Soluble recombinant human TRAIL or agonistic antibodies targeting TRAIL receptors may be promising proapoptotic anti-tumor therapeutic agents in patients with several types of tumors, but many types of tumor cells have been shown to be resistant to TRAIL [94,95,96]. However, it is expected that combination therapy of TRAIL and demethylation agents could be promising immunotherapy. Further studies should clarify how fucosylation affects cancer immunology, and then TRAIL therapy could be a more effective means of treatment. Fucosylation is a promising target for cancer therapeutics.
8. Conclusions
In conclusion, fucosylation plays an important role in urological cancers. Fucosylated proteins could be a promising biomarker for urological malignancies, especially prostate cancer. Furthermore, elucidation of the biological function of fucosylation could result in the development of novel therapeutic targets. Further studies are needed in the near future in the field of fucosylation in urological malignancies.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science KAKENHI [grant numbers 18K09132].
Author Contributions
Conceptualization, K.F. and H.U.; writing—original draft preparation, K.F., M.H., K.H. and E.T.; writing—review and editing, E.M. and N.N.; visualization, K.F.; supervision, E.M. and N.N.; project administration, H.U.; funding acquisition, K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Japan Society for the Promotion of Science KAKENHI [grant numbers 18K09132].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Osaka University submitted a patent for the detection of prostate cancer. Kazutoshi Fujita, Eiji Miyoshi, and Norio Nonomura are listed as co-inventors. Other authors have no conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aoyagi Y., Isemura M., Suzuki Y., Sekine C., Soga K., Ozaki T., Ichida F. Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet. 1985;2:1353–1354. doi: 10.1016/S0140-6736(85)92643-1. [DOI] [PubMed] [Google Scholar]
- 2.Connor J.A. Making sense of the Food and Drug Administration. Semin. Pediatr. Surg. 2006;15:293–301. doi: 10.1053/j.sempedsurg.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Szymendera J.J. Clinical usefulness of three monoclonal antibody-defined tumor markers: CA 19-9, CA 50, and CA 125. Tumour Biol. 1986;7:333–342. [PubMed] [Google Scholar]
- 4.Miyoshi E., Moriwaki K., Terao N., Tan C.C., Terao M., Nakagawa T., Matsumoto H., Shinzaki S., Kamada Y. Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules. 2012;2:34–45. doi: 10.3390/biom2010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyoshi E., Moriwaki K., Nakagawa T. Biological function of fucosylation in cancer biology. J. Biochem. 2008;143:725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- 6.Weston B.W., Nair R.P., Larsen R.D., Lowe J.B. Isolation of a novel human α(1,3)fucosyltransferase gene and molecular comparison to the human lewis blood group α(1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J. Biol. Chem. 1992;267:4152–4160. doi: 10.1016/S0021-9258(19)50641-X. [DOI] [PubMed] [Google Scholar]
- 7.Larsen R.D., Ernst L.K., Nair R.P., Lowe J.B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:β-D-galactoside 2-α-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc. Natl. Acad. Sci. USA. 1990;87:6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagidani S., Uozumi N., Ihara Y., Miyoshi E., Yamaguchi N., Taniguchi N. Purification and cDNA cloning of GDP-L-Fuc: N-acetyl-β-D-glucosaminide: α1-6 fucosyltransferase (α1-6 FucT) from human gastric cancer MKN45 cells. J. Biochem. 1997;121:626–632. doi: 10.1093/oxfordjournals.jbchem.a021631. [DOI] [PubMed] [Google Scholar]
- 9.Lai T.Y., Chen I.J., Lin R.J., Liao G.S., Yeo H.L., Ho C.L., Wu J.C., Chang N.C., Lee A.C.L., Yu A.L. Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells. Cell Death Discov. 2019;5:74. doi: 10.1038/s41420-019-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Zheng M., Qi Y., Wang H., Liu M., Liu Q., Lin B. Lewis(y) antigen-mediated positive feedback loop induces and promotes chemotherapeutic resistance in ovarian cancer. Int. J. Oncol. 2018;53:1774–1786. doi: 10.3892/ijo.2018.4496. [DOI] [PubMed] [Google Scholar]
- 11.Li F.F., Sha D., Qin X.Y., Li C.Z., Lin B. Alpha1,2-fucosyl transferase gene, the key enzyme of Lewis y synthesis, promotes taxol resistance of ovarian carcinoma through apoptosis-related proteins. Neoplasma. 2018;65:515–522. doi: 10.4149/neo_2018_170823N552. [DOI] [PubMed] [Google Scholar]
- 12.do Nascimento J.C.F., Beltrão E.I.C., Rocha C.R.C. High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconj. J. 2020;37:263–275. doi: 10.1007/s10719-020-09914-2. [DOI] [PubMed] [Google Scholar]
- 13.Bassagañas S., Allende H., Cobler L., Ortiz M.R., Llop E., de Bolós C., Peracaula R. Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines. Cytokine. 2015;75:197–206. doi: 10.1016/j.cyto.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Mare L., Caretti A., Albertini R., Trinchera M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013;45:792–797. doi: 10.1016/j.biocel.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Lasky L.A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem. 1995;64:113–140. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 16.Burdick M.M., Henson K.A., Delgadillo L.F., Choi Y.E., Goetz D.J., Tees D.F.J., Benencia F. Expression of E-selectin ligands on circulating tumor cells: Cross-regulation with cancer stem cell regulatory pathways? Front. Oncol. 2012;2:103. doi: 10.3389/fonc.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Y.J., Zheng X.F., Lu C.H., Jiang Q., Liu Q., Xin Y.H. Effect of FUT3 gene silencing with miRNA on proliferation, invasion and migration abilities of human KATO-III gastric cancer cell line. Cell. Mol. Biol. 2016;62:15–20. [PubMed] [Google Scholar]
- 18.Pinho S.S., Reis C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 19.Gao W., Liang J., Ye Y., Lu J., Lin T., Wang N., Dong J., Pan J. FUT4siRNA augments the chemosensitivity of non-small cell lung cancer to cisplatin through activation of FOXO1-induced apoptosis. BMC Cancer. 2020;20:895. doi: 10.1186/s12885-020-07324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H.H., Lin S.Y., Weng R.R., Juan Y.H., Chen Y.W., Hou H.H., Hung Z.C., Oswita G.A., Huang Y.J., Guu S.Y., et al. Fucosyltransferase 4 shapes oncogenic glycoproteome to drive metastasis of lung adenocarcinoma. EBioMedicine. 2020;57:102846. doi: 10.1016/j.ebiom.2020.102846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J., Xiao Y., Liu B., Pan S., Liu Q., Shan Y., Li S., Qi Y., Huang Y., Jia L. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2020;39:54. doi: 10.1186/s13046-020-01562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang L., Gao C., Li Y., Sun M., Xu J., Li H., Jia L., Zhao Y. MiR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017;8:e2968. doi: 10.1038/cddis.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin H., Liu J., Yu M., Wang H., Thomas A.M., Li S., Yan Q., Wang L. FUT7 promotes the malignant transformation of follicular thyroid carcinoma through α1,3-fucosylation of EGF receptor. Exp. Cell Res. 2020;393:112095. doi: 10.1016/j.yexcr.2020.112095. [DOI] [PubMed] [Google Scholar]
- 24.Liu M., Zheng Q., Chen S., Liu J., Li S. Fut7 promotes the epithelial–mesenchymal transition and immune infiltration in bladder urothelial carcinoma. J. Inflamm. Res. 2021;14:1069–1084. doi: 10.2147/JIR.S296597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastian K., Scott E., Elliott D.J., Munkley J. Fut8 alpha-(1,6)-fucosyltransferase in cancer. Int. J. Mol. Sci. 2021;22:455. doi: 10.3390/ijms22010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu C.F., Wu M.Y., Lin Y.C., Kannagi R., Yang R.B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017;19:111. doi: 10.1186/s13058-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Gu J., Miyoshi E., Honke K., Taniguchi N. Phenotype Changes of Fut8 Knockout Mouse: Core Fucosylation Is Crucial for the Function of Growth Factor Receptor(s) Methods Enzymol. 2006;417:11–22. doi: 10.1016/S0076-6879(06)17002-0. [DOI] [PubMed] [Google Scholar]
- 28.Lin H., Wang D., Wu T., Dong C., Shen N., Sun Y., Sun Y., Xie H., Wang N., Shan L. Blocking core fucosylation of TGF-β1 receptors downregulates their functions and attenuates the epithelial-mesenchymal transition of renal tubular cells. Am. J. Physiol.-Ren. Physiol. 2011;300:1017–1025. doi: 10.1152/ajprenal.00426.2010. [DOI] [PubMed] [Google Scholar]
- 29.Li F., Zhao S., Cui Y., Guo T., Qiang J., Xie Q., Yu W., Guo W., Deng W., Gu C., et al. α1,6-Fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC) Am. J. Cancer Res. 2020;10:816–837. [PMC free article] [PubMed] [Google Scholar]
- 30.Clark D.J., Schnaubelt M., Hoti N., Hu Y., Zhou Y., Gooya M., Zhang H. Impact of Increased FUT8 Expression on the Extracellular Vesicle Proteome in Prostate Cancer Cells. J. Proteome Res. 2020;19:2195–2205. doi: 10.1021/acs.jproteome.9b00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanas A., Zaal A., van der Haar Àvila I., Kempers M., Kruijssen L., de Kok M., Popovic M.A., van der Horst J.C., van Vliet S.J. Fut9-driven programming of colon cancer cells towards a stem cell-like state. Cancers. 2020;12:2580. doi: 10.3390/cancers12092580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zodro E., Jaroszewski M., Ida A., Wrzesiński T., Kwias Z., Bluyssen H., Wesoly J. FUT11 as a potential biomarker of clear cell renal cell carcinoma progression based on meta-analysis of gene expression data. Tumor Biol. 2014;35:2607–2617. doi: 10.1007/s13277-013-1344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Gao Y., Zhang X., Guo M., Yang J., Cui H., Kong P., Niu X., Bi Y., Xu J., et al. TSTA3 facilitates esophageal squamous cell carcinoma progression through regulating fucosylation of LAMP2 and ERBB2. Theranostics. 2020;10:11339–11358. doi: 10.7150/thno.48225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arriagada C., Cavieres V.A., Luchsinger C., González A.E., Muñoz V.C., Cancino J., Burgos P.V., Mardones G.A. GOLPH3 regulates EGFR in T98G glioblastoma cells by modulating its glycosylation and ubiquitylation. Int. J. Mol. Sci. 2020;21:8880. doi: 10.3390/ijms21228880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doud E.H., Shetty T., Abt M., Mosley A.L., Corson T.W., Mehta A., Yeh E.S. Nf-κb signaling is regulated by fucosylation in metastatic breast cancer cells. Biomedicines. 2020;8:600. doi: 10.3390/biomedicines8120600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancini M., Righetto M., Noessner E. Checkpoint Inhibition in Bladder Cancer: Clinical Expectations, Current Evidence, and Proposal of Future Strategies Based on a Tumor-Specific Immunobiological Approach. Cancers. 2021;13:6016. doi: 10.3390/cancers13236016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirwan A., Utratna M., O’Dwyer M.E., Joshi L., Kilcoyne M. Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. Biomed Res. Int. 2015;2015:490531. doi: 10.1155/2015/490531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C.W., Lim S.O., Xia W., Lee H.H., Chan L.C., Kuo C.W., Khoo K.H., Chang S.S., Cha J.H., Kim T., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veillon L., Fakih C., Abou-El-Hassan H., Kobeissy F., Mechref Y. Glycosylation Changes in Brain Cancer. ACS Chem. Neurosci. 2018;9:51–72. doi: 10.1021/acschemneuro.7b00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okada M., Chikuma S., Kondo T., Hibino S., Machiyama H., Yokosuka T., Nakano M., Yoshimura A. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 2017;20:1017–1028. doi: 10.1016/j.celrep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Liang W., Mao S., Sun S., Li M., Li Z., Yu R., Ma T., Gu J., Zhang J., Taniguchi N., et al. Core fucosylation of the T cell receptor is required for T cell activation. Front. Immunol. 2018;9:78. doi: 10.3389/fimmu.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii H., Shinzaki S., Iijima H., Wakamatsu K., Iwamoto C., Sobajima T., Kuwahara R., Hiyama S., Hayashi Y., Takamatsu S., et al. Core Fucosylation on T Cells, Required for Activation of T-Cell Receptor Signaling and Induction of Colitis in Mice, Is Increased in Patients with Inflammatory Bowel Disease. Gastroenterology. 2016;150:1620–1632. doi: 10.1053/j.gastro.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Liang W., Mao S., Li M., Zhang N., Sun S., Fang H., Zhang J., Gu J., Wang J., Li W. Ablation of core fucosylation attenuates the signal transduction via T cell receptor to suppress the T cell development. Mol. Immunol. 2019;112:312–321. doi: 10.1016/j.molimm.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Zhang N., Li M., Xu X., Zhang Y., Liu Y., Zhao M., Li P., Chen J., Fukuda T., Gu J., et al. Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. Eur. J. Immunol. 2020;50:1820–1833. doi: 10.1002/eji.202048543. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama K., Wakamatsu K., Fujii H., Shinzaki S., Takamatsu S., Kitazume S., Kamada Y., Takehara T., Taniguchi N., Miyoshi E. Core fucose is essential glycosylation for CD14-dependent Toll-like receptor 4 and Toll-like receptor 2 signalling in macrophages. J. Biochem. 2019;165:227–237. doi: 10.1093/jb/mvy098. [DOI] [PubMed] [Google Scholar]
- 46.Yokobori T., Yazawa S., Asao T., Nakazawa N., Mogi A., Sano R., Kuwano H., Kaira K., Shirabe K. Fucosylated α1-acid glycoprotein as a biomarker to predict prognosis following tumor immunotherapy of patients with lung cancer. Sci. Rep. 2019;9:14503. doi: 10.1038/s41598-019-51021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shields R.L., Lai J., Keck R., O’Connell L.Y., Hong K., Gloria Meng Y., Weikert S.H.A., Presta L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara C., Grau S., Jäger C., Sondermann P., Brünker P., Waldhauer I., Hennig M., Ruf A., Rufer A.C., Stihle M., et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantuck A.J., Zisman A., Belldegrun A.S. The changing natural history of renal cell carcinoma. J. Urol. 2001;166:1611–1623. doi: 10.1016/S0022-5347(05)65640-6. [DOI] [PubMed] [Google Scholar]
- 50.von Roemeling C.A., Marlow L.A., Radisky D.C., Rohl A., Larsen H.E., Wei J., Sasinowska H., Zhu H., Drake R., Sasinowski M., et al. Functional genomics identifies novel genes essential for clear cell renal cell carcinoma tumor cell proliferation and migration. Oncotarget. 2014;5:5320–5334. doi: 10.18632/oncotarget.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drake R.R., McDowell C., West C., David F., Powers T.W., Nowling T., Bruner E., Mehta A.S., Angel P.M., Marlow L.A., et al. Defining the human kidney N-glycome in normal and cancer tissues using MALDI imaging mass spectrometry. J. Mass Spectrom. 2020;55:e4490. doi: 10.1002/jms.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tousi F., Bones J., Iliopoulos O., Hancock W.S., Hincapie M. Multidimensional liquid chromatography platform for profiling alterations of clusterin N-glycosylation in the plasma of patients with renal cell carcinoma. J. Chromatogr. A. 2012;1256:121–128. doi: 10.1016/j.chroma.2012.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gbormittah F.O., Bones J., Hincapie M., Tousi F., Hancock W.S., Iliopoulos O. Clusterin glycopeptide variant characterization reveals significant site-specific glycan changes in the plasma of clear cell renal cell carcinoma. J. Proteome Res. 2015;14:2425–2436. doi: 10.1021/pr501104j. [DOI] [PubMed] [Google Scholar]
- 54.Meng L., Xu L., Yang Y., Zhou L., Chang Y., Shi T., Tan C., An H., Zhu Y., Xu J. High expression of FUT3 is linked to poor prognosis in clear cell renal cell carcinoma. Oncotarget. 2017;8:61036–61047. doi: 10.18632/oncotarget.17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyazaki J., Nishiyama H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017;24:730–734. doi: 10.1111/iju.13376. [DOI] [PubMed] [Google Scholar]
- 56.van Rhijn B.W.G., Burger M., Lotan Y., Solsona E., Stief C.G., Sylvester R.J., Witjes J.A., Zlotta A.R. Recurrence and Progression of Disease in Non-Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. Eur. Urol. 2009;56:430–442. doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 57.Lotan Y., Roehrborn C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109–118. doi: 10.1016/S0090-4295(02)02136-2. [DOI] [PubMed] [Google Scholar]
- 58.Mancini M., Righetto M., Zumerle S., Montopoli M., Zattoni F. The Bladder EpiCheck Test as a Non-Invasive Tool Based on the Identification of DNA Methylation in Bladder Cancer Cells in the Urine: A Review of Published Evidence. Int. J. Mol. Sci. 2020;21:6542. doi: 10.3390/ijms21186542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang G., Tan Z., Lu W., Guo J., Yu H., Yu J., Sun C., Qi X., Li Z., Guan F. Quantitative glycome analysis of N-glycan patterns in bladder cancer vs. normal bladder cells using an integrated strategy. J. Proteome Res. 2015;14:639–653. doi: 10.1021/pr5006026. [DOI] [PubMed] [Google Scholar]
- 60.Lu Y.C., Chen C.N., Chu C.Y., Lu J.H., Wang B.J., Chen C.H., Huang M.C., Lin T.H., Pan C.C., Chen S.S.A., et al. Calreticulin activates β1 integrin via fucosylation by fucosyltransferase 1 in J82 human bladder cancer cells. Biochem. J. 2014;460:69–78. doi: 10.1042/BJ20131424. [DOI] [PubMed] [Google Scholar]
- 61.Sheinfeld J., Reuter V.E., Melamed M.R., Fair W.R., Morse M., Sogani P.C., Herr H.W., Whitmore W.F., Cordon-Cardo C. Enhanced bladder cancer detection with the Lewis X antigen as a marker of neoplastic transformation. J. Urol. 1990;143:285–288. doi: 10.1016/S0022-5347(17)39935-4. [DOI] [PubMed] [Google Scholar]
- 62.Blanas A., Sahasrabudhe N.M., Rodríguez E., van Kooyk Y., van Vliet S.J. Fucosylated antigens in cancer: An alliance toward tumor progression, metastasis, and resistance to chemotherapy. Front. Oncol. 2018;8:39. doi: 10.3389/fonc.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pal S.K., Pham A., Vuong W., Liu X., Lin Y., Ruel N., Yuh B.E., Chan K., Wilson T., Lerner S.P., et al. Prognostic Significance of Neutrophilic Infiltration in Benign Lymph Nodes in Patients with Muscle-invasive Bladder Cancer. Eur. Urol. Focus. 2017;3:130–135. doi: 10.1016/j.euf.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Sim W.J., Iyengar P.V., Lama D., Lui S.K.L., Ng H.C., Haviv-Shapira L., Domany E., Kappei D., Tan T.Z., Saie A., et al. c-Met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat. Commun. 2019;10:4349. doi: 10.1038/s41467-019-12241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Z., Zhang M., Chen G., Wang W., Zhang P., Yue Y., Guan Z., Wang X., Fan J. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int. J. Oncol. 2019;54:1555–1566. doi: 10.3892/ijo.2019.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo J., Li X., Tan Z., Lu W., Yang G., Guan F. Alteration of N-glycans and expression of their related glycogenes in the epithelial-mesenchymal transition of HCV29 bladder epithelial cells. Molecules. 2014;19:20073–20090. doi: 10.3390/molecules191220073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Islam M.K., Syed P., Dhondt B., Gidwani K., Pettersson K., Lamminmäki U., Leivo J. Detection of bladder cancer with aberrantly fucosylated ITGA3. Anal. Biochem. 2021;628:114283. doi: 10.1016/j.ab.2021.114283. [DOI] [PubMed] [Google Scholar]
- 68.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 69.Munkley J., Mills I.G., Elliott D.J. The role of glycans in the development and progression of prostate cancer. Nat. Rev. Urol. 2016;13:324–333. doi: 10.1038/nrurol.2016.65. [DOI] [PubMed] [Google Scholar]
- 70.Scott E., Munkley J. Glycans as biomarkers in prostate cancer. Int. J. Mol. Sci. 2019;20:1389. doi: 10.3390/ijms20061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi S., Sugiyama T., Shimomura M., Kamada Y., Fujita K., Nonomura N., Miyoshi E., Nakano M. Site-specific and linkage analyses of fucosylated N-glycans on haptoglobin in sera of patients with various types of cancer: Possible implication for the differential diagnosis of cancer. Glycoconj. J. 2016;33:471–482. doi: 10.1007/s10719-016-9653-7. [DOI] [PubMed] [Google Scholar]
- 72.Saldova R., Fan Y., Fitzpatrick J.M., Watson R.W.G., Rudd P.M. Core fucosylation and α2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 2011;21:195–205. doi: 10.1093/glycob/cwq147. [DOI] [PubMed] [Google Scholar]
- 73.Totten S.M., Adusumilli R., Kullolli M., Tanimoto C., Brooks J.D., Mallick P., Pitteri S.J. Multi-lectin Affinity Chromatography and Quantitative Proteomic Analysis Reveal Differential Glycoform Levels between Prostate Cancer and Benign Prostatic Hyperplasia Sera. Sci. Rep. 2018;8:6509. doi: 10.1038/s41598-018-24270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kyselova Z., Mechref Y., Al Bataineh M.M., Dobrolecki L.E., Hickey R.J., Vinson J., Sweeney C.J., Novotny M.V. Alterations in the serum glycome due to metastatic prostate cancer. J. Proteome Res. 2007;6:1822–1832. doi: 10.1021/pr060664t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barthel S.R., Hays D.L., Yazawa E.M., Opperman M., Walley K.C., Nimrichter L., Burdick M.M., Gillard B.M., Moser M.T., Pantel K., et al. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 2013;73:942–952. doi: 10.1158/0008-5472.CAN-12-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah P., Wang X., Yang W., Eshghi S.T., Sun S., Hoti N., Chen L., Yang S., Pasay J., Rubin A., et al. Integrated proteomic and glycoproteomic analyses of prostate cancer cells reveal glycoprotein alteration in protein abundance and glycosylation. Mol. Cell. Proteom. 2015;14:2753–2763. doi: 10.1074/mcp.M115.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou J., Yang W., Hu Y., Höti N., Liu Y., Shah P., Sun S., Clark D., Thomas S., Zhang H. Site-Specific Fucosylation Analysis Identifying Glycoproteins Associated with Aggressive Prostate Cancer Cell Lines Using Tandem Affinity Enrichments of Intact Glycopeptides Followed by Mass Spectrometry. Anal. Chem. 2017;89:7623–7630. doi: 10.1021/acs.analchem.7b01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., et al. Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujita K., Shimomura M., Uemura M., Nakata W., Sato M., Nagahara A., Nakai Y., Takamatsu S., Miyoshi E., Nonomura N. Serum fucosylated haptoglobin as a novel prognostic biomarker predicting high-Gleason prostate cancer. Prostate. 2014;74:1052–1058. doi: 10.1002/pros.22824. [DOI] [PubMed] [Google Scholar]
- 80.Wang X., Chen J., Li Q.K., Peskoe S.B., Zhang B., Choi C., Platz E.A., Zhang H. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24:935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Höti N., Yang S., Hu Y., Shah P., Haffner M.C., Zhang H. Overexpression of α (1,6) fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 2018;21:137–146. doi: 10.1038/s41391-017-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilgunn S., Conroy P.J., Saldova R., Rudd P.M., O’Kennedy R.J. Aberrant PSA glycosylation—A sweet predictor of prostate cancer. Nat. Rev. Urol. 2013;10:99–107. doi: 10.1038/nrurol.2012.258. [DOI] [PubMed] [Google Scholar]
- 83.Ohyama C., Hosono M., Nitta K., Oh-eda M., Yoshikawa K., Habuchi T., Arai Y., Fukuda M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology. 2004;14:671–679. doi: 10.1093/glycob/cwh071. [DOI] [PubMed] [Google Scholar]
- 84.Fukushima K., Satoh T., Baba S., Yamashita K. α1,2-Fucosylated and β-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology. 2009;20:452–460. doi: 10.1093/glycob/cwp197. [DOI] [PubMed] [Google Scholar]
- 85.Dwek M.V., Jenks A., Leathem A.J.C. A sensitive assay to measure biomarker glycosylation demonstrates increased fucosylation of prostate specific antigen (PSA) in patients with prostate cancer compared with benign prostatic hyperplasia. Clin. Chim. Acta. 2010;411:1935–1939. doi: 10.1016/j.cca.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Li Q.K., Chen L., Ao M.H., Chiu J.H., Zhang Z., Zhang H., Chan D.W. Serum fucosylated prostate-specific antigen (PSA) improves the differentiation of aggressive from non-aggressive prostate cancers. Theranostics. 2015;5:267–276. doi: 10.7150/thno.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubén L.C., Laura M.R., Almudena F.B., Emilio G.M. Glycan array analysis of Pholiota squarrosa lectin and other fucose-oriented lectins. Glycobiology. 2021;31:459–476. doi: 10.1093/glycob/cwaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishikawa T., Yoneyama T., Tobisawa Y., Hatakeyama S., Kurosawa T., Nakamura K., Narita S., Mitsuzuka K., Duivenvoorden W., Pinthus J.H., et al. An Automated Micro-Total Immunoassay System for Measuring Cancer-Associated α2,3-linked Sialyl N-Glycan-Carrying Prostate-Specific Antigen May Improve the Accuracy of Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2017;18:470. doi: 10.3390/ijms18020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujita K., Hatano K., Tomiyama E., Hayashi Y., Matsushita M., Tsuchiya M., Yoshikawa T., Date M., Miyoshi E., Nonomura N. Serum core-type fucosylated prostate-specific antigen index for the detection of high-risk prostate cancer. Int. J. Cancer. 2021;148:3111–3118. doi: 10.1002/ijc.33517. [DOI] [PubMed] [Google Scholar]
- 90.Hatano K., Yoneyama T., Hatakeyama S., Tomiyama E., Tsuchiya M., Nishimoto M., Yoshimura K., Miyoshi E., Uemura H., Ohyama C., et al. Simultaneous analysis of serum α2,3-linked sialylation and core-type fucosylation of prostate-specific antigen for the detection of high-grade prostate cancer. Br. J. Cancer. 2021 doi: 10.1038/s41416-021-01637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dai Y., Hartke R., Li C., Yang Q., Liu J.O., Wang L.X. Synthetic Fluorinated l -Fucose Analogs Inhibit Proliferation of Cancer Cells and Primary Endothelial Cells. ACS Chem. Biol. 2020;15:2662–2672. doi: 10.1021/acschembio.0c00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Y., Fukuda T., Hang Q., Hou S., Isaji T., Kameyama A., Gu J. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci. Rep. 2017;7:11563. doi: 10.1038/s41598-017-11911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moriwaki K., Noda K., Furukawa Y., Ohshima K., Uchiyama A., Nakagawa T., Taniguchi N., Daigo Y., Nakamura Y., Hayashi N., et al. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology. 2009;137:188–198.e2. doi: 10.1053/j.gastro.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Ashkenazi A., Herbst R.S. To kill a tumor cell: The potential of proapoptotic receptor agonists. J. Clin. Investig. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koschny R., Holland H., Sykora J., Haas T.L., Sprick M.R., Ganten T.M., Krupp W., Bauer M., Ahnert P., Meixensberger J., et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin. Cancer Res. 2007;13:3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen T., Zhang X.D., Hersey P. Relative Resistance of Fresh Isolates of Melanoma to Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)-induced Apoptosis. Cancer Res. 2001;7:966s–973s. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.