Abstract
Metabolic syndrome, a complex group of metabolic disorders of energy use and storage, is considered as an important determinant risk factor for many cardiovascular diseases. This study aimed to examine the association between metabolic syndrome (MetS) and dietary pattern among adults in Jiangsu Province of China. Data were from three rounds of cross–sectional nutrition and diet investigation projects in Jiangsu Province of China, which were conducted in 2002, 2007, and 2014 by Jiangsu Provincial Center for Disease Control and Prevention. A total of 13,944 participants with complete food frequency questionnaire (FFQ) were eventually included in this study after further data screening. The 2009 Joint Interim Statement for China was used to define metabolic syndrome. Three distinct dietary patterns were identified by factor analysis: the modern dietary pattern (rich in pork, poultry, vegetables, seafood, pastry food, other animal meats, fruits, milk and its products, soft drink, whole grains, nuts, and seeds, but low in wheat), vegetable oils/condiments/soy products dietary pattern (rich in vegetable oils, other condiments, salt, soy products, and fruits and low in dry legumes), and modern high–wheat dietary pattern (rich in wheat, tubers, fruits, and other animal meats, but low in rice). Higher intake of the modern dietary pattern and modern high–wheat dietary pattern were positively associated with metabolic syndrome in both unadjusted and adjusted models by genders, whereas higher intake of the vegetable oils/condiments/soy products dietary pattern had a negative relationship with metabolic syndrome in both unadjusted and adjusted models by genders (p < 0.05). Our study recommends reducing the consumption of animal meat products, especially processed meat products, and replacing animal oils with vegetable oils as the main supply of daily oils. Furthermore, more prospective and experimental studies are needed to confirm the relationship between dietary patterns and metabolic syndrome.
Keywords: dietary pattern, metabolic syndrome, factor analysis, Jiangsu Province
1. Introduction
Metabolic syndrome (MetS), also known as “Syndrome X” and “Insulin Resistance Syndrome”, is a complex group of metabolic disorders of energy use and storage [1]. This syndrome, characterized by centripetal obesity, dyslipidemia, increased blood pressure, and elevated blood glucose levels, is one of the most serious non–communicable chronic diseases and increases the risk of type 2 diabetes and cardiovascular disease (CVD) [2,3,4]. Unfortunately, regardless of the diagnostic criteria used, the prevalence of metabolic syndrome increases year on year worldwide. From 2011 to 2016, the overall prevalence of metabolic syndrome in the United States was 34.7% (95% CI, 33.1–34.3%), and the prevalence was not significantly different among men and women (35.1% vs. 34.3%; p = 0.47) [5]. A systematic review and meta–analysis of 226,653 Chinese people showed that 24.5% (95% confidence interval (CI): 22.0–26.9%) had metabolic syndrome, with a prevalence of 19.2% in men and 27.0% in women [6]. In addition, the high prevalence of metabolic syndrome in Chinese children is not negligible, rising from 2.3% in 2004 to 3.2% in 2014 [7]. This is an extremely grim figure and bodes well for the future of metabolic syndrome in our country as an increasingly tough health problem. Epidemiological studies showed that environmental factors such as socio–economic status, sedentary lifestyle, and diet are significantly related to the occurrence of metabolic syndrome [4,7]. Among them, diet as a controllable environmental factor plays an important role in the increased prevalence of metabolic syndrome.
The importance of single diet or dietary composition on metabolic syndrome has been assessed [8,9,10]. In current nutritional epidemiological investigations, dietary patterns are more frequently used to assess the complex interactions of nutrients and foods with various diseases and their synergistic effects on the same diseases. Dietary patterns are more representative of the overall dietary habits of the study population. Therefore, examining dietary patterns may be a better predictor of disease risk than individual nutrients or foods [11,12,13]. A systematic review and meta–analysis including 40 observational studies showed that adherence to a “healthy” dietary pattern—characterized as rich in vegetables and fruit, poultry, fish, and whole grains—was associated with a reduced risk of metabolic syndrome, and was more pronounced in men and Asian countries. However, adherence to a “meat/Western” pattern (which is characterized by a high loading of red meat, processed meat, animal fat, eggs, and sweets) increased the risk of metabolic syndrome in stratified analyses based on either geographical region (Asia, Europe, America) or study design [14]. A previous study found that a Mediterranean diet, which is characterized as rich in vegetables, fruit, whole grains, cereal, fish, and seafood products, was associated with a lower frequency of metabolic syndrome and reduced all–cause mortality [15,16].
Recent decades have witnessed the rapid economic development of Jiangsu Province. This rapid economic development has then accelerated the change in the region’s dietary habits and disease profile. The prevalence of metabolic syndrome in Jiangsu Province of China has been reported to be increasing year by year, but few articles have been found to assess the relationship between dietary pattern and metabolic syndrome in this region [17,18,19]. Therefore, the purpose of this study was to examine the association between dietary pattern and metabolic syndrome among adults in Jiangsu Province of China.
2. Methods
2.1. Study Population
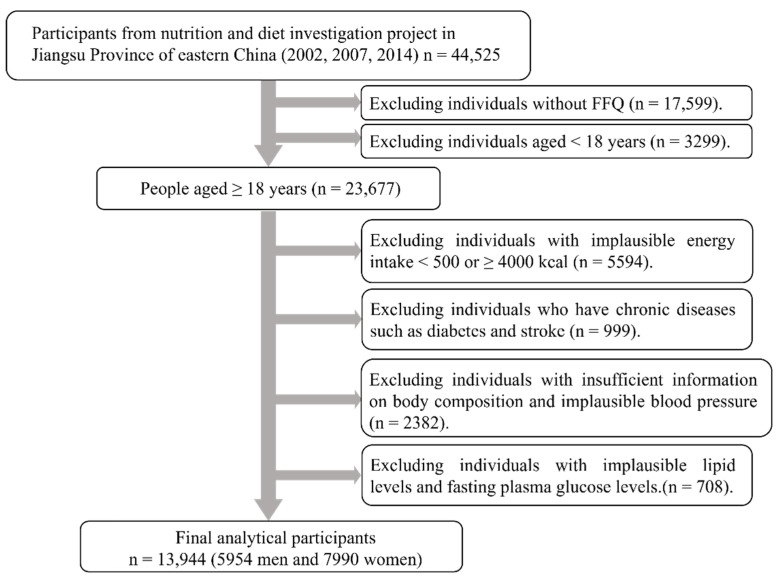
Data were from three rounds of cross–sectional nutrition and diet investigation projects in Jiangsu Province of China, which were conducted in 2002, 2007, and 2014 by Jiangsu Provincial Center for Disease Control and Prevention. The three–round surveys used the same multi–stage stratified cluster random sampling method. The screening process for survey respondents is shown in Figure 1. Of the total 44,525 respondents, those aged 18 years and over with a completed food frequency questionnaire (FFQ) were included in the study (n = 23,677). Then, we excluded data from those with abnormal energy intake (<500 or ≥4000 kcal per day, n = 5594). Among the remaining respondents, we further excluded people with chronic diseases such as diabetes and stroke, as their diet may be altered owing to their chronic diseases (n = 999). Individuals with insufficient information on body composition, blood pressure, lipid levels, and fasting plasma glucose (FPG) were further excluded from this study (n = 3090). A total of 13,944 participants (5954 men and 7990 women) were finally included in this study. This study was approved by the Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention, reference number JSCDC2014236. In addition, all participants signed an informed consent form for this study.
Figure 1.
Flowchart of the selection of research participants from three rounds of cross–sectional nutrition and diet investigation projects. 2002, n = 2295; 2007, n = 6700; 2014, n = 4949.
2.2. Demographic and Lifestyle Survey
Demographic data including age, gender, education level (primary and below, secondary, and senior secondary and above), physical work (low physical work, middle physical work, high physical work, and other work), region (city and rural), and geographical region (divided into southern and northern Jiangsu Province according to geographical location) were surveyed by professionally trained investigators. Smoking/drinking status was defined as who had the habits of smoking and/or drinking during the investigation [18].
2.3. Anthropometric Measurements
All anthropometric measurements were carried out in a comfortable environment with light clothing. Weight was measured in kilogram (kg). Height and waist circumference was measured in centimeter (cm) [20]. All measurements were performed twice using a standard protocol and techniques. Body mass index (BMI) was calculated as weight (kg) divided by square of height (meter (m)) [21].
2.4. Blood Pressure Measurement and Biochemical Indicator
Blood pressure was likewise measured in a quiet and comfortable environment. The blood pressure of all respondents was measured three times by a professional investigator and the final result was averaged from the three measurements [22]. All blood samples were collected in the morning after fasting during the night. All samples were subjected to rigorous quality control analysis by the Jiangsu Centre for Disease Control and Prevention, which measured the concentrations of triglyceride (TG), high density lipoprotein cholesterol (HDL), and FPG.
2.5. Definition of Metabolic Syndrome
The metabolic syndrome as a collective term for a group of risk factors has not been defined uniformly for more than a decade, and in this survey, we mainly used the definition of metabolic syndrome from the 2009 Joint Interim Statement for China [23]: (1) elevated waist circumference: waist circumference ≥85 cm in males, ≥80 cm in females; (2) elevated TG: TG >1.70 mmol/L (150 mg/dL) or those who have been treated for high TG; (3) reduced HDL: HDL <1.0 mmol/L (40 mg/dL) in males, <1.30 mmol/L (50 mg/dL) in females or those who have been treated for low HDL; (4) elevated blood pressure: systolic blood pressure (SBP) ≥130 and/or diastolic blood pressure (DBP) ≥85 mmHg or those who were treated for hypertension; (5) elevated FPG: FPG ≥5.6 mmol/L (100 mg/dL) or those who received anti–hyperglycemia treatment. Among the five points above, metabolic syndrome can be confirmed if three or more of them were met.
2.6. Dietary Assessment
All dietary information was collected by a standardized semi–quantitative food frequency questionnaire (FFQ) [24]. The FFQ contained hundreds of kinds of food, which basically covered dietary intake of residents in Jiangsu Province for one year. The food frequency questionnaire included all the foods consumed daily by residents of Jiangsu Province. The food model was used to allow the investigators to recall the amount of foods eaten. At the same time, participants were asked to recount the frequency of personal food consumption (number of times per day, week, month, and year) as well as the number of servings. Ultimately, food intake was transformed into g/day for data analysis. Then, all foods were divided into 23 food groups according to the Chinese Dietary Guidelines and the available explanations, which represent the dietary habits of Jiangsu residents in the last decade or so, as shown in Table 1 [25].
Table 1.
Food groupings used in factor analysis.
| Food Group | Example of the Food Group |
|---|---|
| Rice | Rice, rice flour |
| Wheat | noodles, pasta, plain bread |
| Whole grains | Barley, buckwheat, millet, corn |
| Tubers | Sweet potato, potato, Chinese yam, taro |
| Vegetables | Spinach, canola, carrot, spinach, preserved vegetables |
| Soy products | Soybeans, soymilk, tofu |
| Dry legumes | Black beans, red lentils, kidney beans, green beans |
| Fruits | Fresh and canned (no added sugar) fruits |
| Eggs | Whole eggs, yolk, white, preserved eggs |
| Milk and its products | Whole milk, skim milk, flavored milk, cheese, yogurt |
| Poultry | Chicken, duck meat |
| Pork | pork and its products |
| Other animal meat | beef, lamb, and those products |
| Animal viscera | Viscera products of animals |
| Seafood | Fresh fish, dried fish, shellfish, shrimp |
| Nuts and seeds | Sesame, sunflower, peanuts, walnuts, almonds, hazelnuts, pine–nuts |
| Vegetable oils | Soybean oil, peanut oil, sesame oil |
| Animal oils | Butter, lard, sheep oil |
| Salt | Salt |
| Other condiments | Sauce, soy sauce, monosodium glutamate |
| Wine | Beer, rice wine, white wine |
| Soft drink | Fruit or flavored drinks, fruit juice, soft drinks |
| Pastry snacks | Cakes, pancake, mooncake |
2.7. Statistical Analysis
Factor analysis (FA) was performed to infer dietary patterns in different genders. Orthogonal rotations using Kaiser’s criterion (eigenvalues > 1.3) were utilized to establish the number of factors. Only the absolute values of factor loading >0.26 were included in the analysis, as these items represented the foods with the strongest relationships to the identified factors. The dietary patterns were named according to the magnitude of the factor scores and the interpretability of the overall diet. Factor scores were further divided into three quartiles. Participants were divided into three groups based on the dietary pattern obtained. All categorical variables were expressed using numbers (percentages), and continuous variables were expressed using means ± standard deviations (SD). Chi–square tests and generalized linear models were used to explore differences in the general characteristics of participants. Multivariable logistic regression analysis was used to compare the odds ratios (ORs) and 95%CI to analyze the relationship between dietary patterns and metabolic syndrome by gender. Data management and statistical analysis were performed using IBM SPSS Statistics software Version 26.0 and OriginLab (2021) Data analysis and graphing software. All analyses were defined as statistically different at p–values (two sides) <0.05.
3. Results
3.1. Determination of Dietary Patterns
Dietary patterns from different genders were identified by factor analysis. Both the Kaiser–Meyer–Olkin index (0.704 in males and 0.705 in females) and Bartlett’s test (p < 0.001) showed that the sample was suitable for FA.
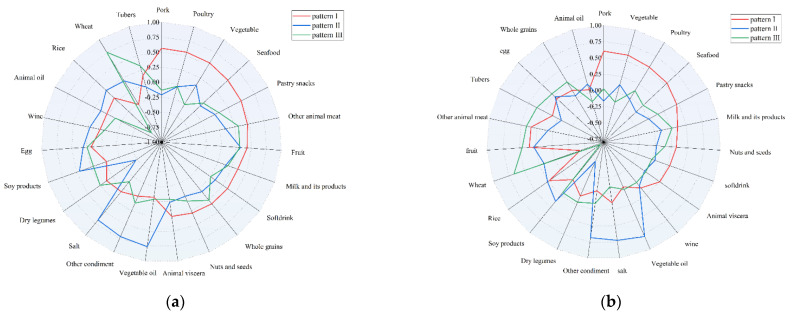
Among males, finally, three dietary patterns were established and named as the modern dietary pattern (pattern I), vegetable oils/condiments/soy products dietary pattern (pattern II), and modern high–wheat pattern dietary pattern (pattern III) according to the highest factor loading of food items and interpretability, as shown in Figure 2a and Table 2. The modern dietary pattern (pattern I), vegetable oil/condiment/soy products dietary pattern (pattern II), and modern high–wheat pattern dietary pattern (pattern III) can explain 10.732%, 10.310%, and 8.149% of the variance, respectively.
Figure 2.
Radar chart of different dietary patterns from factor analysis. (a) radar chart in males; (b) radar chart in females.
Table 2.
Factor loadings and dietary patterns for the 23 food groups derived from factor analysis.
| Food Groups | Men | Women | ||||
|---|---|---|---|---|---|---|
| Pattern I | Pattern II | Pattern III | Pattern I | Pattern II | Pattern III | |
| Pork | 0.564 | 0.603 | ||||
| Poultry | 0.556 | 0.553 | ||||
| Vegetables | 0.544 | −0.266 | 0.592 | |||
| Seafood | 0.512 | 0.535 | ||||
| Pastry snacks | 0.494 | 0.466 | ||||
| Other animal meats | 0.462 | 0.310 | 0.341 | 0.414 | ||
| Fruits | 0.429 | 0.319 | 0.306 | 0.347 | 0.279 | 0.458 |
| Milk and its products | 0.348 | 0.362 | 0.275 | |||
| Soft drink | 0.345 | 0.279 | ||||
| Whole grains | 0.325 | 0.286 | ||||
| Nuts and seeds | 0.290 | 0.322 | ||||
| Animal viscera | ||||||
| Vegetable oils | 0.758 | 0.784 | ||||
| Other condiments | 0.717 | 0.684 | ||||
| Salt | 0.673 | 0.727 | ||||
| Dry legumes | −0.477 | −0.479 | ||||
| Soy products | 0.450 | 0.374 | ||||
| Eggs | 0.309 | 0.291 | ||||
| Wine | ||||||
| Animal oils | ||||||
| Rice | 0.262 | −0.773 | −0.739 | |||
| Wheat | −0.266 | 0.753 | –0.418 | 0.666 | ||
| Tubers | 0.322 | 0.359 | ||||
Factor loadings of <0.26 in absolute terms were excluded for simplicity; pattern I: modern dietary pattern; pattern II: vegetable oils/condiments/soy products dietary pattern; pattern III: modern high–wheat pattern dietary pattern.
Among females, three similar dietary patterns were finally identified and named as the modern dietary pattern (pattern I), vegetable oils/condiments/soy products dietary pattern (pattern II), and modern high–wheat pattern dietary pattern (pattern III), which can be seen in Figure 2b and Table 2. The modern dietary pattern (pattern I), vegetable oil/condiment/soy products dietary pattern (pattern II), and modern high–wheat pattern dietary pattern (pattern III) can explain 10.766%, 10.049%, and 8.537% of the variance, respectively.
3.2. Basic Information of Participants
Of the 13,944 respondents, 28.5% (95%CI: 27.8–29.2%) had metabolic syndrome, with a significantly higher prevalence among women (36.1% (95%CI: 30.6–32.6%)) than men (24.4% (95%CI: 23.3–25.5%)) (p < 0.001). The average of waist circumstance in men was 83.0 ± 9.7 cm, which was higher than that in women (79.8 ± 10.0 cm) (p < 0.001). In addition, smoking and alcohol consumption rates were higher in men than those in women, with statistically significant differences (p < 0.001) (table not shown).
3.3. Characteristics of the Participants in Dietary Patterns
General characteristics of participants across the three dietary patterns across tertiles are shown in Table 3.
Table 3.
General characteristics of the participants under the three dietary patterns by genders.
| Tertile of Each Pattern Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dietary Pattern | Men | Women | ||||||
| T1 | T2 | T3 | p Value | T1 | T2 | T3 | p Value | |
| Pattern I | ||||||||
| Age (years) | 54.4 ± 15.2 a | 52.3 ± 14.9 | 49.0 ± 15.4 | <0.001 | 52.7 ± 16.0 a | 50.6 ± 14.8 | 48.4 ± 14.5 | <0.001 |
| BMI (kg/m2) | 23.4 ± 3.3 a | 23.6 ± 3.2 | 24.0 ± 3.3 | <0.001 | 24.0 ± 3.6 a | 23.7 ± 3.5 | 23.8 ± 3.6 | 0.007 |
| Energy intake (kcal/d) | 2189.5 ± 618.9 c | 2228.4 ± 653.2 | 2454.5 ± 683.0 | <0.001 | 1939.0 ± 556.4 c | 1968.1 ± 563.8 | 2222.3 ± 598.8 | <0.001 |
| Waist circumference (cm) | 81.6 ± 9.6 a | 82.8 ± 9.5 | 84.6 ± 9.8 | <0.001 | 80.4 ± 10.2 a | 79.4 ± 9.9 | 79.7 ± 10.0 | <0.001 |
| SBP (mm Hg) | 129.3 ± 20.1 | 129.7 ± 19.0 | 128.3 ± 17.6 | 0.061 | 127.3 ± 22.3 a | 126.2 ± 20.6 | 124.6 ± 19.7 | <0.001 |
| DBP (mm Hg) | 82.1 ± 11.4 b | 82.8 ± 10.5 | 82.6 ± 10.4 | 0.088 | 79.9 ± 11.9 c | 79.4 ± 10.2 | 78.6 ± 10.2 | <0.001 |
| TG (mmol/L) | 1.4 ± 1.5 a | 1.6 ± 1.7 | 1.9 ± 2.0 | <0.001 | 1.4 ± 1.1 a | 1.5 ± 1.2 | 1.6 ± 1.3 | <0.001 |
| HDL–C (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.879 | 1.3 ± 0.3 a | 1.3 ± 0.3 | 1.3 ± 0.4 | <0.001 |
| FPG (mmol/L) | 5.0 ± 1.1 a | 5.1 ± 1.3 | 5.1 ± 1.2 | <0.001 | 5.0 ± 1.2 | 5.0 ± 1.1 | 5.1 ± 1.1 | 0.019 |
| Education level | <0.001 | <0.001 | ||||||
| Primary school or less | 1130 (47.7) c | 947 (40.0) | 770 (32.5) | 1834 (68.8) c | 1478 (55.5) | 1168 (43.9) | ||
| Junior high school | 891 (37.6) | 981 (41.4) | 1027 (43.4) | 696 (26.1) c | 850 (31.9) | 972 (36.5) | ||
| High school and higher | 349 (14.7) c | 441 (18.6) | 572 (24.1) | 134 (5.0) c | 335 (12.6) | 523 (19.6) | ||
| Physical work | <0.001 | <0.001 | ||||||
| Low physical work | 806 (40.6) c | 968 (48.8) | 1111 (55.9) | 1536 (57.7) | 1508 (56.6) c | 1669 (62.7) | ||
| Middle physical work | 119 (6.0) a | 228 (11.5) | 237 (11.9) | 57 (2.1) c | 154 (5.8) | 186 (7.0) | ||
| High physical work | 780 (39.3) a | 462 (23.3) | 344 (17.3) | 876 (32.9) c | 679 (25.5) | 507 (19.0) | ||
| Other physical work | 279 (14.1) | 326 (16.4) | 294 (14.8) | 195 (7.3) c | 322 (12.1) | 301 (11.3) | ||
| Region | 0.278 | <0.001 | ||||||
| City | 610 (30.7) | 564 (28.4) | 589 (29.7) | 812 (30.5) | 916 (34.4) | 755 (28.4) | ||
| Rural | 1374 (69.3) | 1420 (71.6) | 1397 (70.3) | 1852 (69.5) b | 1747 (65.6) | 1908 (71.6) | ||
| Geographical region | <0.001 | <0.001 | ||||||
| Southern Jiangsu | 672 (33.9) a | 1347 (67.9) | 1447 (72.9) | 682 (25.6) a | 1676 (62.9) | 1926 (72.3) | ||
| Northern Jiangsu | 1312 (66.1) | 637 (32.1) | 539 (27.1) | 1982 (74.4) | 987 (37.1) | 737 (27.7) | ||
| Marital status | <0.001 | <0.001 | ||||||
| Unmarried | 71 (3.6) c | 79 (4.0) | 114 (5.7) | 60 (2.3) c | 58 (2.2) | 90 (3.4) | ||
| Married | 1791 (90.3) | 1813 (91.4) | 1812 (91.2) | 2253 (84.6) c | 2356 (88.5) | 2394 (89.9) | ||
| Divorced | 16 (0.8) | 13 (0.7) | 14 (0.7) | 23 (0.9) | 20 (0.8) | 25 (0.9) | ||
| Widowed | 106 (5.3) c | 79 (4.0) | 46 (2.3) | 328 (12.3) c | 229 (8.6) | 154 (5.8) | ||
| Smoking behavior | 0.007 | 0.126 | ||||||
| No | 974 (49.1) | 887 (44.7) | 972 (48.9) | 2596 (97.4) | 2609 (98.0) | 2616 (98.2) | ||
| Yes | 1010 (50.9) b | 1097 (55.3) | 1014 (51.1) | 70 (2.6) c | 167 (2.0) | 287 (1.8) | ||
| Alcohol consumption | 0.100 | <0.001 | ||||||
| Non–drinker | 1142 (57.6) | 1075 (54.2) | 1113 (56.0) | 2606 (97.8) | 2558 (96.1) | 2544 (95.5) | ||
| Current drinker | 842 (42.4) | 909 (45.8) | 873 (44.0) | 58 (2.2) a | 105 (3.9) | 119 (4.5) | ||
| Pattern II | ||||||||
| Age (years) | 54.1 ± 15.4 a | 52.5 ± 15.2 | 49.2 ± 15.0 | <0.001 | 52.5 ± 15.8 a | 50.0 ± 15.4 | 49.2 ± 14.2 | <0.001 |
| BMI (kg/m2) | 23.8 ± 3.2 c | 23.6 ± 3.3 | 23.5 ± 3.2 | 0.112 | 23.7 ± 3.5 c | 23.8 ± 3.6 | 24.0 ± 3.6 | 0.002 |
| Energy intake (kcal/d) | 1969.0 ± 716.0 a | 2277.1 ± 549.6 | 2626.2 ± 534.8 | <0.001 | 1832.6 ± 669.5 a | 2033.9 ± 508.2 | 2262.7 ± 485.2 | <0.001 |
| Waist circumference (cm) | 84.0 ± 9.9 a | 82.8 ± 9.6 | 82.4 ± 9.5 | <0.001 | 79.9 ± 9.9 | 79.6 ± 10.2 | 79.9 ± 9.9 | 0.473 |
| SBP (mm Hg) | 129.8 ± 17.8 c | 130.0 ± 19.8 | 127.5 ± 19.1 | <0.001 | 126.9 ± 20.2 a | 125.7 ± 21.7 | 125.5 ± 20.9 | 0.034 |
| DBP (mm Hg) | 82.1 ± 10.4 c | 82.7 ± 10.8 | 82.9 ± 11.2 | 0.045 | 79.3 ± 10.5 | 78.7 ± 10.9 c | 79.8 ± 11.1 | 0.002 |
| TG (mmol/L) | 1.9 ± 2.0 a | 1.6 ± 1.6 | 1.4 ± 1.5 | <0.001 | 1.7 ± 1.4 a | 1.5 ± 1.2 | 1.3 ± 1.1 | <0.001 |
| HDL–C (mmol/L) | 1.3 ± 0.4 a | 1.3 ± 0.4 | 1.2 ± 0.4 | <0.001 | 1.3 ± 0.4 a | 1.3 ± 0.3 | 1.2 ± 0.3 | <0.001 |
| FPG (mmol/L) | 5.3 ± 1.4 a | 5.1 ± 1.1 | 4.9 ± 1.1 | <0.001 | 5.2 ± 1.0 a | 5.0 ± 1.0 | 4.9 ± 1.3 | <0.001 |
| Education level | 0.001 | <0.001 | ||||||
| Primary school or less | 770 (38.8) | 833 (42.0) c | 715 (36.0) | 1468 (55.1) c | 1456 (54.7) | 1556 (58.4) | ||
| Junior high school | 816 (41.1) | 795 (40.1) | 837 (42.1) | 813 (30.5) | 864 (32.4) | 841 (31.6) | ||
| High school and higher | 399 (20.1) | 355 (17.9) | 434 (21.9) | 382 (14.3) c | 343 (12.9) | 267 (10.0) | ||
| Physical work | <0.001 | <0.001 | ||||||
| Low physical work | 1011 (50.9) | 935 (47.2) | 939 (47.3) | 1600 (60.1) b | 1502 (56.4) | 1611 (60.5) | ||
| Middle physical work | 212 (10.7) | 197 (9.9) | 175 (8.8) | 144 (5.4) c | 149 (5.6) | 104 (3.9) | ||
| High physical work | 521 (26.2) | 509 (25.7) | 556 (28.0) | 646 (24.3) | 707 (26.5) | 709 (26.6) | ||
| Other physical work | 241 (12.1) b | 342 (17.2) | 316 (15.9) | 273 (10.3) | 305 (11.5) | 240 (9.0) | ||
| Region | <0.001 | <0.001 | ||||||
| City | 498 (25.1) a | 593 (29.9) | 672 (33.8) | 744 (27.9) a | 856 (32.1) | 883 (33.1) | ||
| Rural | 1487 (74.9) | 1390 (70.1) | 1314 (66.2) | 1919 (72.1) | 1807 (67.9) | 1781 (66.9) | ||
| Geographical region | <0.001 | <0.001 | ||||||
| Southern Jiangsu | 1286 (64.8) | 1202 (60.6) | 978 (49.2) | 1703 (64.0) | 1411 (53.0) | 1170 (43.9) | ||
| Northern Jiangsu | 699 (35.2) a | 781 (39.4) | 1008 (50.8) | 960 (36.0) a | 1252 (47.0) | 1494 (56.1) | ||
| Marital status | 0.160 | 0.597 | ||||||
| Unmarried | 81 (4.1) | 73 (3.7) | 110 (5.5) | 57 (2.1) | 80 (3.0) | 71 (2.7) | ||
| Married | 1813 (91.3) | 1816 (91.6) | 1787 (90.0) | 2341 (87.9) | 2322 (87.2) | 2340 (87.8) | ||
| Divorced | 13 (0.7) | 15 (0.8) | 15 (0.8) | 21 (0.8) | 24 (0.9) | 23 (0.9) | ||
| Widowed | 78 (3.9) | 79 (4.0) | 74 (3.7) | 244 (9.2) | 237 (8.9) | 230 (8.6) | ||
| Smoking behavior | <0.001 | 0.032 | ||||||
| No | 991 (49.9) | 992 (50.0) | 850 (42.8) | 2617 (98.3) | 2612 (98.1) | 2592 (97.3) | ||
| Yes | 994 (50.1) c | 991 (50.0) | 1136 (57.2) | 46 (1.7) c | 51 (1.9) | 72 (2.7) | ||
| Alcohol consumption | <0.001 | 0.001 | ||||||
| Non–drinker | 1183 (59.6) | 1153 (58.1) | 994 (50.1) | 2586 (97.1) | 2582 (97.0) | 2540 (95.3) | ||
| Current drinker | 802 (40.4) c | 830 (41.9) | 992 (49.9) | 77 (2.9) c | 81 (3.0) | 124 (4.7) | ||
| Pattern III | ||||||||
| Age (years) | 52 ± 14.2 c | 52.7 ± 15.7 | 51.0 ± 16.0 | 0.002 | 52.2 ± 14.5 c | 51.4 ± 15.5 | 48.1 ± 15.3 | <0.001 |
| BMI (kg/m2) | 23.4 ± 3.2 c | 23.5 ± 3.2 | 24.0 ± 3.3 | <0.001 | 23.7 ± 3.6 c | 23.7 ± 3.5 | 24.2 ± 3.6 | <0.001 |
| Energy intake (kcal/d) | 2492.1 ± 546.2 a | 2047.1 ± 699.1 | 2333.4 ± 654.4 | <0.001 | 2115.7 ± 542.9 b | 1881.0 ± 631.7 | 2132.6 ± 548.9 | <0.001 |
| Waist circumference (cm) | 81.9 ± 9.2 a | 83.0 ± 9.7 | 84.2 ± 10.1 | <0.001 | 79.6 ± 9.7 c | 79.4 ± 9.9 | 80.5 ± 10.4 | <0.001 |
| SBP (mm Hg) | 128.4 ± 18.5 | 129.3 ± 18.2 | 129.6 ± 20.1 | 0.123 | 126.6 ± 20.8 c | 126.6 ± 20.6 | 124.8 ± 21.3 | 0.001 |
| DBP (mm Hg) | 82.0 ± 10.5 c | 82.3 ± 10.2 | 83.3 ± 11.6 | <0.001 | 78.7 ± 10.7 a | 79.5 ± 10.6 | 79.6 ± 11.3 | 0.008 |
| TG (mmol/L) | 1.5 ± 1.7 b | 1.9 ± 1.8 | 1.5 ± 1.7 | <0.001 | 1.4 ± 1.0 b | 1.7 ± 1.4 | 1.4 ± 1.2 | <0.001 |
| HDL–C (mmol/L) | 1.3 ± 0.4 b | 1.3 ± 0.4 | 1.2 ± 0.3 | <0.001 | 1.2 ± 0.3 b | 1.3 ± 0.3 | 1.3 ± 0.3 | <0.001 |
| FPG (mmol/L) | 5.0 ± 1.1 a | 5.2 ± 1.3 | 5.1 ± 1.2 | <0.001 | 5.0 ± 1.1 a | 5.1 ± 1.1 | 5.0 ± 1.2 | <0.001 |
| Education level | <0.001 | <0.001 | ||||||
| Primary school or less | 889 (44.8) a | 713 (35.9) | 716 (36.1) | 1659 (62.3) b | 1369 (51.4) | 1452 (54.5) | ||
| Junior high school | 810 (40.8) | 829 (41.8) | 809 (40.8) | 794 (29.8) | 875 (32.9) | 849 (31.9) | ||
| High school and higher | 285 (14.4) a | 443 (22.3) | 460 (23.2) | 210 (7.9) b | 419 (15.7) | 363 (13.6) | ||
| Physical work | <0.001 | <0.001 | ||||||
| Low physical work | 791 (39.9) b | 1112 (56.0) | 982 (49.5) | 1232 (46.3) a | 1693 (63.6) | 1788 (67.1) | ||
| Middle physical work | 198 (10.0) a | 236 (11.9) | 150 (7.6) | 134 (5.0) | 178 (6.7) c | 85 (3.2) | ||
| High physical work | 618 (31.1) a | 363 (18.3) | 605 (30.5) | 985 (37.0) a | 483 (18.1) | 594 (22.3) | ||
| Other physical work | 377 (19.0) c | 274 (13.8) | 248 (12.5) | 312 (11.7) c | 309 (11.6) | 197 (7.4) | ||
| Region | 0.291 | <0.001 | ||||||
| City | 567 (28.6) | 612 (30.8) | 584 (29.4) | 792 (29.7) c | 725 (27.2) | 966 (36.3) | ||
| Rural | 1417 (71.4) | 1373 (69.2) | 1401 (70.6) | 1871 (70.3) | 1938 (72.8) | 1698 (63.7) | ||
| Geographical region | <0.001 | <0.001 | ||||||
| Southern Jiangsu | 1438 (72.5) | 1538 (77.5) | 490 (24.7) | 1693 (63.6) | 1927 (72.4) | 664 (24.9) | ||
| Northern Jiangsu | 546 (27.5) a | 447 (22.5) | 1495 (75.3) | 970 (36.4) a | 736 (27.6) | 2000 (75.1) | ||
| Marital status | 0.409 | <0.001 | ||||||
| Unmarried | 73 (3.7) | 91 (4.6) | 100 (5.0) | 43 (1.6) | 77 (2.9) | 88 (3.3) | ||
| Married | 1817 (91.6) | 1811 (91.2) | 1788 (90.1) | 2326 (87.3) | 2316 (87.0) | 2361 (88.6) | ||
| Divorced | 13 (0.7) | 14 (0.7) | 16 (0.8) | 17 (0.6) | 23 (0.9) | 28 (1.1) | ||
| Widowed | 81 (4.1) | 69 (3.5) | 81 (4.1) | 277 (10.4) a | 247 (9.3) | 187 (7.0) | ||
| Smoking behavior | <0.001 | <0.001 | ||||||
| No | 888 (44.8) | 926 (46.6) | 1019 (51.3) | 2590 (97.3) | 2612 (98.1) | 2619 (98.3) | ||
| Yes | 1096 (55.2) c | 1059 (53.4) | 966 (48.7) | 73 (2.7) a | 51 (1.9) | 45 (1.7) | ||
| Alcohol consumption | 0.024 | <0.001 | ||||||
| Non–drinker | 1061 (53.5) | 1127 (56.8) | 1142 (57.5) | 2543 (95.5) | 2560 (96.1) | 2605 (97.8) | ||
| Current drinker | 923 (46.5) c | 858 (43.2) | 843 (42.5) | 120 (4.5) c | 103 (3.9) | 59 (2.2) | ||
T: tertile; pattern I: modern dietary pattern; pattern II: vegetable oils/condiments/soy products dietary pattern; pattern III: modern high–wheat pattern dietary pattern; a vs. other tertiles p < 0.05; b vs. tertile 2 p < 0.05; c vs. tertile 3 p < 0.05; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; HDL, high–density lipoprotein cholesterol; FPG, fasting plasma glucose.
Among males, participants in higher tertile of modern dietary pattern (pattern I) were more likely to be younger and more concentrated in the southern Jiangsu Province (p < 0.001); had a greater portion of participants who were unmarried; had higher education level and lower physical work; and had higher energy intake, BMI level, waist circumference, TG, and FPG compared with participants in the lowest quartile of pattern I (p < 0.05), while participants from higher quartile of the vegetable oils/condiments/soy products dietary pattern (pattern II) were more likely to be younger and more concentrated in urban areas and the southern Jiangsu Province (p < 0.001); had smoking and drinking behavior; and had higher energy intake and DBP level, but lower waist circumference, SBP, TG, HDL, and FPG levels compared with the participants in the lowest quartile of pattern II (p < 0.05). Meanwhile, participants in higher quartile of the modern high–wheat dietary pattern (pattern III) were likely to be younger and more concentrated in northern Jiangsu Province (p < 0.05); had a greater portion of individuals who had lower education levels and lower physical work; had lower rates of smoking and drinking; and had higher BMI level, waist circumference, DBP, and FPG levels, but lower energy intake compared with the participants in the lowest quartile of pattern III (p < 0.001).
Among females, individuals in higher tertile of the modern dietary pattern (pattern I) were more likely to be younger and more concentrated in rural areas and the southern Jiangsu Province (p < 0.001); had a greater portion of participants who were married; had a higher education level and lower physical work; and had higher energy intake, BMI, waist circumference, TG, and FPG levels, but lower SBP, DBP, and HDL levels compared with participants in the lowest quartile of pattern I (p < 0.05), while participants from higher quartile of the vegetable oils/condiments/soy products dietary pattern (pattern II) were more likely to be younger and more concentrated in urban areas and northern Jiangsu Province (p < 0.001); had smoking and drinking behavior; and had higher energy intake, BMI, and DBP levels, but lower SBP, TG, HDL, and FPG levels compared with the participants in the lowest quartile of pattern II (p < 0.05). Meanwhile, participants in higher quartile of the modern high–wheat dietary pattern (pattern III) were likely to be younger and more concentrated in urban areas and the northern Jiangsu Province (p < 0.001); had a greater portion of individuals who had higher education levels and lower physical work; had lower rates of smoking and drinking; and had higher waist circumference, BMI, DBP, and FPG levels, but lower SBP level compared with the participants in the lowest quartile of pattern III (p < 0.05).
3.4. Association between Dietary Patterns and Metabolic Syndrome by Gender
The results of the relationship between dietary patterns and metabolic syndrome in different genders using multivariate logistic regression are displayed in Table 4. Among males, participants in the higher tertiles of the modern dietary pattern (pattern I) and modern high–wheat pattern dietary pattern (pattern III) have a positive influence on metabolic syndrome in both unadjusted model and adjusted model (composed to T1, OR = 1.530, 95% CI: 1.271–1.842 in pattern I; OR = 1.360, 95% CI, 1.172–1.578 in pattern III, respectively, p < 0.05). On the other hand, the higher intake of vegetable oils/condiments/soy products dietary pattern score was negatively associated with metabolic syndrome in both unadjusted model and adjusted model (compared with T1, OR = 0.692, 95% CI: 0.598–0.800, p < 0.05). Similar to males, both the modern dietary pattern (pattern I) and modern high–wheat pattern dietary pattern (pattern III) with higher scores among females were positively associated with metabolic syndrome, but the difference was that such a positive association was only seen in the adjusted model (compared with T1, OR = 1.289, 95% CI: 1.104–1.505 in pattern I; OR = 1.203, 95% CI, 1.030–1.406 in pattern III, respectively, p < 0.05). Conversely, participants with a higher score of vegetable oils/condiments/soy products dietary pattern were negatively correlated with metabolic syndrome in both unadjusted model and adjusted model (compared with T1, OR = 0.863, 95% CI: 0.746–0.977, p < 0.05).
Table 4.
Odds ratios and 95% confidence intervals for metabolic syndrome across tertiles of dietary patterns by genders.
| Group | Dietary Pattern | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Men | pattern I | OR (95%CI) | OR (95%CI) | OR (95%CI) |
| T1 | 1.000 | 1.000 | 1.000 | |
| T2 | 1.285 (1.105–1.494) | 1.161 (0.969–1.389) | 1.159 (0.968–1.388) | |
| T3 | 1.678 (1.449–1.943) | 1.533 (1.273–1.845) | 1.530 (1.271–1.842) | |
| pattern II | ||||
| T1 | 1.000 | 1.000 | 1.000 | |
| T2 | 0.769 (0.667–0.887) | 0.829 (0.700–0.983) | 0.829 (0.700–0.983) | |
| T3 | 0.692 (0.598–0.800) | 0.867 (0.720–1.043) | 0.871 (0.723–1.048) | |
| pattern III | ||||
| T1 | 1.000 | 1.000 | 1.000 | |
| T2 | 1.462 (1.262–1.695) | 1.213 (1.014–1.450) | 1.210 (1.012–1.446) | |
| T3 | 1.360 (1.172–1.578) | 1.162 (0.954–1.416) | 1.164 (0.956–1.419) | |
| Women | pattern I | |||
| T1 | 1.000 | 1.000 | 1.000 | |
| T2 | 0.922 (0.821–1.035) | 1.104 (0.956–1.275) | 1.109 (0.960–1.281) | |
| T3 | 0.977 (0.871–1.096) | 1.280 (1.096–1.493) | 1.289 (1.104–1.505) | |
| pattern II | ||||
| T1 | 1.000 | 1.000 | 1.000 | |
| T2 | 0.841 (0.750–0.943) | 0.872 (0.760–1.001) | 0.872 (0.759–1.001) | |
| T3 | 0.818 (0.729–0.918) | 0.860 (0.744–0.994) | 0.863 (0.746–0.997) | |
| pattern III | ||||
| T1 | 1.000 | 1.000 | 1.000 | |
| T2 | 1.086 (0.967–1.220) | 1.005 (0.873–1.158) | 1.006 (0.873–1.158) | |
| T3 | 1.121 (0.999–1.259) | 1.208 (1.034–1.412) | 1.203 (1.030–1.406) |
Model 1: unadjusted model; Model 2: adjusted for age, education level, physical work, marital status, region, geographical regions, body mass index, and energy intake; Model 3: additionally adjusted for smoking behavior and alcohol consumption as cluster groups to analyze the association; pattern I: modern dietary pattern; pattern II: vegetable oils/condiments/soy products dietary pattern; pattern III: modern high–wheat pattern dietary pattern.
4. Discussion
Metabolic syndrome is a cluster of risk factors that increases the risk of type 2 diabetes by five times and cardiovascular disease by three times, and has unfortunately become a global public health problem [2]. Diet, as an important part of lifestyle, has been demonstrated to be significantly associated with metabolic syndrome [26,27]. In this cross–sectional study, three dietary patterns including the modern dietary pattern, vegetable oils/condiments/soy products dietary pattern, and modern high–wheat dietary pattern were established using factor analysis to analyze the relationship between diet and metabolic syndrome.
The modern dietary pattern, which was high in pork, poultry, vegetables, seafood, pastry food, other animal meats, fruits, milk and its products, soft drink, whole grains, nuts, and seeds, but low in wheat, was found to have a positive association with metabolic syndrome in both men and women. This dietary pattern was similar to the modern dietary pattern obtained previously from China Health and Nutrition Survey (CHNS), which was found to be positively associated with general and central obesity [28,29]. In addition, the modern dietary pattern in our study contained a high consumption of animal meats, including processed meat and red meat, which were consistent with the Western dietary pattern. The Western dietary pattern, which is rich in red meat, processed meat, pasta, and rice, has been found to promote inflammatory responses and CVDs [30]. Data from the cohort study indicated that the Western dietary pattern was negatively associated with HDL cholesterol and positively associated with all other response variables (LDL–cholesterol, systolic and diastolic blood pressure, fasting glucose, and insulin) [27]. Soft drinks including sugar–sweetened beverages (SSBs) and artificially sweetened beverages (ASBs) were also the main components of modern dietary pattern in our study. A systematic review and meta–analysis demonstrated that the high consumption of SSBs and ASBs was positively associated with metabolic syndrome [31]. Besides, the increased intake of ASBs and SSBs also contributed to the increased risk of CVD death and all–cause mortality [32]. Energy provided by sugar is highly likely to cause weight gain if it is not eventually metabolized and balanced by physical activity. Numerous epidemiological surveys have found that obesity was a major risk factor for insulin resistance, type 2 diabetes, dyslipidemia, and hypertension, which are components of metabolic syndrome [2,13]. Although the dangers of soft drinks have reached a point where we have to be aware of them, the market for soft drinks in China is still booming. A CHNS survey found that the beverage consumption prevalence rate rose from 22.4% in 2004 to 33.1% in 2011 [33]. Moreover, the consumption of pastry snacks rich in high energy density was another important components of modern dietary patterns. Despite the general interest in the idea of consuming snacks, ready–to–eat, highly processed snacks are becoming more readily available and consumed. Such highly processed snacks tend to be higher in energy, which leads to an excessive accumulation of energy in the body and, ultimately, to obesity or other metabolic diseases [34]. Therefore, it is important to raise concerns about reducing the public’s obsession with soft drinks and pastry snacks. Notably, the modern dietary pattern also contains some beneficial food components, such as fish, vegetables, whole grains, and fruits. A systematic review and meta–analysis demonstrated that fish consumption might have a preventive role in the development of metabolic syndrome only in men [35]. Some studies hold the opposite opinion that people with metabolic syndrome might have a higher intake of protein, meat, and fish [36]. In addition, a study from Hangzhou of China showed that adherence to a seafood diet had no association with the risk of metabolic syndrome [37]. The relationship between various seafood products, such as fish, and metabolic syndrome is still confusing. Hence, it is necessary to conduct further studies to determine the ability of fish and long–chain n–3 fatty acid depletion to improve metabolic syndrome and its composition. Numerous clinical studies have demonstrated the beneficial effects of vegetables and fruits on health [38,39]. The importance of fruits and vegetables was reflected in various dietary guidelines [25,40]. However, in our study, vegetables and fruits did not provide sufficient protection against metabolic syndrome. This might be due to the high intake of meat in modern dietary patterns, which offset the beneficial effects of fruits and vegetables.
The modern high–wheat dietary pattern, which was high in wheat, tubers, fruits, and other animal meats, but low in rice (listed here are only the parts that are the same for both genders), was more common in northern Jiangsu Province. This dietary pattern was more inclined to the integration of the traditional Northern dietary pattern and Western dietary pattern [41,42,43]. Zuo Hui, et al. noted that the adherence of people in Jiangsu region to a high–wheat dietary pattern led to a positive association with insulin resistance, which is thought to be the underlying mechanism for metabolic syndrome after adjustment for age and sex. Further, the positive association remained after adjustment for income, total energy intake, and physical activity, although it disappeared after an additional increase in BMI [44]. Notably, an interesting observation was that the modern dietary pattern, where men and women consumed less wheat, was positively associated with metabolic syndrome, while the modern high–wheat dietary pattern characterized by a high intake of wheat and a low intake of rice was still positively associated with metabolic syndrome. The main reason for this phenomenon might be that it was not just rice or wheat alone that was responsible for the development of metabolic syndrome. This result might be best explained by the focus on the overall diet rather than the impact of a single food or nutrient on health. The high consumption of milk and its products was contained in this dietary pattern. In a systematic review and meta–analysis, the results have shown that higher intake of dairy products significantly reduces the risk of metabolic syndrome by 17% in cross–sectional studies and by 14% in cohort studies [45]. However, the other study held the opposite opinion that the more cheese the elderly eat, the higher the risk of metabolic syndrome [46]. However, in our study, all kinds of milk and dairy products were grouped together into one food subgroup, so it remained controversial whether they were beneficial. Therefore, subsequent studies should consider separating the different types of dairy products to explore the relationship with metabolic syndrome. Fewer articles have been written on the relationship between eggs and the metabolic syndrome. A systematic review and meta–analysis of a prospective study suggested that egg intake was associated with an increased incidence of type 2 diabetes in the general population and an increased incidence of CVD co–morbidity in patients with diabetes [47]. On the contrary, data from Korean health examinees study showed that a higher weekly intake of eggs was associated with a reduced risk of metabolic syndrome and its components in women [48]. Therefore, the protective effect of eggs remains to be proven by more studies.
To our surprise, the vegetable oils/condiments/soy products dietary pattern, dominated by a high score of vegetable oils, other condiments, salt, soy products, and fruits and low in dry legumes, was found to be negatively correlated with metabolic syndrome. Salty foods act as ‘addictive substances’, stimulating the brain’s reward and pleasure mechanisms to crave salty foods, which may also be responsible for the increased incidence of obesity–related diseases [49]. In addition, a high salt intake has been reported as an independent risk factor for hypertension and might likewise increase the incidence of metabolic syndrome in healthy people [50,51,52]. Therefore, we used salt as a separate food grouping to examine whether salt also increased the prevalence of metabolic syndrome in the whole diet at the beginning of the study. The result was surprising as the factor scores for salt and other condiments were higher in the vegetable oil/condiment/soy products dietary pattern, whereas this pattern showed a protective effect against metabolic syndrome in both the unadjusted model and the model adjusted for genders. The reason for this result may be that other components of this dietary pattern have the opposite effect, e.g., vegetable oil. Different from animal oils containing large amounts of saturated fatty acids, vegetable oils contain high levels of unsaturated fatty acids and have been reported in numerous studies to improve lipocalin concentrations, insulin resistance, and body composition in people by increasing their levels in foods, even without changing other components of the diet or fat intake [53,54,55]. Most observational studies have found a positive association between monounsaturated fatty acids’ (MUFAs) and polyunsaturated fatty acids’ (PUFAs) (both n–3 and n–6 subtypes) intake and metabolic syndrome components. The benefits of diets rich in MUFA or PUFA, including low–fat diets, in reducing metabolic syndrome were also supported by clinical trials [56,57,58]. Another major reason was the higher intake of soy products in this dietary pattern. Research has found that the regular intake of cultivated soy foods, which are unique to Asia, appeared to be associated with a lower prevalence of metabolic syndrome [59,60]. In addition, soy intake tends to have a gender–dependent effect on the risk of metabolic syndrome and specific cancers [61,62]. The consumption of fruits and eggs (only in men) has been described in the above two dietary pattern discussions as beneficial for metabolic syndrome. Therefore, it is reasonable to assume that this dietary pattern was negatively associated with metabolic syndrome. However, more research is probably needed to prove it.
To the best of our knowledge, this was the first time that such a large sample size from three rounds of cross–sectional nutrition surveys had been used to study the relationship between dietary patterns and metabolic syndrome using multivariate logistic regression in Jiangsu Province of China. Second, all three rounds of the nutrition survey had a rigorous experimental design, with participant data including demographic information, lifestyle, dietary intake, anthropometric data, and biochemical measurements, providing a variety of data information and homogenization of data analysis. However, the present investigation also had some weaknesses. Firstly, because of the cross–sectional nature, we are unable to understand the causal relationship between dietary patterns by gender and metabolic syndrome. Secondly, selection bias may be unavoidable as we have removed no FFQs or other data that lead to incomplete data. Thirdly, factor analysis is an a posteriori methodological approach that relies on learned knowledge and experience in grouping foods, but the final presentation of the data does not rely on any prior knowledge, and thus the reproducibility and validity of the data are poor. Finally, although we included the physical work, the physical activities of residents were not included, which may lead to bias.
5. Conclusions
Three dietary patterns were obtained through factor analysis in both males and females in Jiangsu Province of China: the modern dietary pattern, the vegetable oils/condiments/soy products dietary pattern, and the modern high–wheat dietary pattern. The modern dietary pattern and modern high–wheat dietary pattern had a positive association with metabolic syndrome in both males and females. The vegetable oils/condiments/soy products dietary pattern was negatively associated with metabolic syndrome in both males and females. Our study recommends that the consumption of animal meat products, especially processed meat products, should be reduced and that vegetable oils should replace animal oils as the main supply of daily oil in Jiangsu Province of China. Furthermore, more prospective and experimental studies are needed to confirm the relationship between dietary patterns and metabolic syndrome.
Acknowledgments
The authors thank all the participants, researchers, and collaborators for their contribution in anthropometric measurements, biochemical and nutritional evaluation, and database management.
Author Contributions
Y.W. made interpretation of data and drafted the work; T.T. collected all the samples; D.P., D.X. and Y.L. conducted the experiments and analyzed the data; J.Z., W.X., H.X. and S.W. helped with the experiments; G.S. and Y.D. designed and supervised the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81872618).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention, reference number JSCDC2014236.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCracken E., Monaghan M., Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill S., O’Driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 3.Saklayen M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H., Li X., Adams H., Kubena K., Guo S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018;20:128. doi: 10.3390/ijms20010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirode G., Wong R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA. 2020;323:2526–2528. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R., Li W., Lun Z., Zhang H., Sun Z., Kanu J.S., Qiu S., Cheng Y., Liu Y. Prevalence of metabolic syndrome in mainland China: A meta-analysis of published studies. BMC Public Health. 2016;16:296. doi: 10.1186/s12889-016-2870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song P., Yu J., Chang X., Wang M., An L. Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey. Nutrients. 2017;9:79. doi: 10.3390/nu9010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson J.J., Eisenmann J.C., Norman G.J., Ortiz K.A., Young P.C. Dietary Fiber and Nutrient Density Are Inversely Associated with the Metabolic Syndrome in US Adolescents. J. Am. Diet. Assoc. 2011;111:1688–1695. doi: 10.1016/j.jada.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Covas M.I., Rafael D., Fitó M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015;113((Suppl. 2)):S19–S28. doi: 10.1017/S0007114515000136. [DOI] [PubMed] [Google Scholar]
- 10.Chiva-Blanch G., Urpi-Sarda M., Ros E., Valderas-Martinez P., Casas R., Arranz S., Guillén M., Lamuela–Raventós R.M., Llorach R., Andres–Lacueva C., et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013;32:200–206. doi: 10.1016/j.clnu.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Hu F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Chan R., Leung J., Woo J. Dietary Patterns and Risk of Frailty in Chinese Community–Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients. 2015;7:7070–7084. doi: 10.3390/nu7085326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calton E.K., James A.P., Pannu P.K., Soares M.J. Certain dietary patterns are beneficial for the metabolic syndrome: Reviewing the evidence. Nutr. Res. 2014;34:559–568. doi: 10.1016/j.nutres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Fabiani R., Naldini G., Chiavarini M. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta–Analysis. Nutrients. 2019;11:2056. doi: 10.3390/nu11092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotos–Prieto M., Ortolá R., Ruiz–Canela M., Garcia-Esquinas E., Martínez–Gómez D., Lopez–Garcia E., Martínez–González M.Á, Rodriguez-–Artalejo F. Association between the Mediterranean lifestyle, metabolic syndrome and mortality: A whole–country cohort in Spain. Cardiovasc. Diabetol. 2021;20:5. doi: 10.1186/s12933-020-01195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Daniele N., Noce A., Vidiri M.F., Moriconi E., Marrone G., Annicchiarico–Petruzzelli M., D’Urso G., Tesauro M., Rovella V., De Lorenzo A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget. 2017;8:8947–8979. doi: 10.18632/oncotarget.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X.S., Guo Z.R., Zhou H., Shi Z.M., Wu M., Zhang J., Sun G.X., Zhou Z.Y., Pan X.Q., Yao C.L. Study on the prevalence of metabolic syndrome among 35–74 year–olds in Jiangsu Province. Chin. J. Epidemiol. 2006;27:751–756. [PubMed] [Google Scholar]
- 18.Tian T., Zhang J., Zhu Q., Xie W., Wang Y., Dai Y. Predicting value of five anthropometric measures in metabolic syndrome among Jiangsu Province, China. BMC Public Health. 2020;20:1317. doi: 10.1186/s12889-020-09423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo H., Shi Z., Hu X., Wu M., Guo Z., Hussain A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism. 2009;58:1102–1108. doi: 10.1016/j.metabol.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 20.WHO Expert Committee Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 21.Zhou B.F., Cooperative Meta-Analysis Group of the Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut–off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 22.World Health Organization, Guidelines Sub–Committee International Society of Hypertension Guidelines for the Management of Hypertension. Blood Press. Suppl. 1999;1:9–43. [PubMed] [Google Scholar]
- 23.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W., Hasegawa K., Chen J. The use of food–frequency questionnaires for various purposes in China. Public Health Nutr. 2002;5:829–833. doi: 10.1079/PHN2002374. [DOI] [PubMed] [Google Scholar]
- 25.Wang S.S., Lay S., Yu H.N., Shen S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B. 2016;17:649–656. doi: 10.1631/jzus.B1600341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asghari G., Yuzbashian E., Mirmiran P., Hooshmand F., Najafi R., Azizi F. Dietary Approaches to Stop Hypertension (DASH) Dietary Pattern Is Associated with Reduced Incidence of Metabolic Syndrome in Childrenand Adolescents. J. Pediatr. 2016;174:178–184. doi: 10.1016/j.jpeds.2016.03.077. [DOI] [PubMed] [Google Scholar]
- 27.Drake I., Sonestedt E., Ericson U., Wallström P., Orho-Melander M. A Western dietary pattern is prospectively associated with cardio–metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 2018;119:1168–1176. doi: 10.1017/S000711451800079X. [DOI] [PubMed] [Google Scholar]
- 28.Xu X., Hall J., Byles J., Shi Z. Dietary Pattern Is Associated with Obesity in Older People in China: Data from China Health and Nutrition Survey (CHNS) Nutrients. 2015;7:8170–8188. doi: 10.3390/nu7095386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhen S., Ma Y., Zhao Z., Yang X., Wen D. Dietary pattern is associated with obesity in Chinese children and adolescents: Data from China Health and Nutrition Survey (CHNS) Nutr. J. 2018;17:68. doi: 10.1186/s12937-018-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christ A., Lauterbach M., Latz E. Western Diet and the Immune System: An Inflammatory Connection. Immunity. 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Narain A., Kwok C.S., Mamas M.A. Soft drink intake and the risk of metabolic syndrome: A systematic review and meta–analysis. Int. J. Clin. Pract. 2017;71:e12927. doi: 10.1111/ijcp.12927. [DOI] [PubMed] [Google Scholar]
- 32.Pan B., Ge L., Lai H., Wang Q., Wang Q., Zhang Q., Yin M., Li S., Tian J., Yang K., et al. Association of soft drink and 100% fruit juice consumption with all–cause mortality, cardiovascular diseases mortality, and cancer mortality: A systematic review and dose–response meta–analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2021;61:1–12. doi: 10.1080/10408398.2021.1937040. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Liu R., Zhao Y., Shi Z. High Consumption of Soft Drinks Is Associated with an Increased Risk of Fracture: A 7–Year Follow–Up Study. Nutrients. 2020;12:530. doi: 10.3390/nu12020530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njike V.Y., Smith T.M., Shuval O., Shuval K., Edshteyn I., Kalantari V., Yaroch A.L. Snack Food, Satiety, and Weight. Adv. Nutr. 2016;7:866–878. doi: 10.3945/an.115.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tørris C., Molin M., Cvancarova Småstuen M. Fish consumption and its possible preventive role on the development and prevalence of metabolic syndrome—A systematic review. Diabetol. Metab. Syndr. 2014;6:112. doi: 10.1186/1758-5996-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng M., Wang H., Wang Z., Du W., Ouyang Y., Zhang B. Relationship between dietary factors and the number of altered metabolic syndrome components in Chinese adults: A cross–sectional study using data from the China Health and Nutrition Survey. BMJ Open. 2017;7:e014911. doi: 10.1136/bmjopen-2016-014911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J.B., Lin L.P., Li –Y.D., Zhen P.F. Association of three dietary patterns with metabolic syndrome in the middle–aged population in Hangzhou. Prev. Med. 2018;30:1222–1225. [Google Scholar]
- 38.Turati F., Rossi M., Pelucchi C., Levi F., La Vecchia C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015;113((Suppl. 2)):S102–S110. doi: 10.1017/S0007114515000148. [DOI] [PubMed] [Google Scholar]
- 39.Alissa E.M., Ferns G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017;57:1950–1962. doi: 10.1080/10408398.2015.1040487. [DOI] [PubMed] [Google Scholar]
- 40.Kromhout D., Spaaij C.J., de Goede J., Weggemans R.M. The 2015 Dutch food–based dietary guidelines. Eur. J. Clin. Nutr. 2016;70:869–878. doi: 10.1038/ejcn.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batis C., Sotres–Alvarez D., Gordon–Larsen P., Mendez M.A., Adair L., Popkin B. Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. Br. J. Nutr. 2014;111:1441–1451. doi: 10.1017/S0007114513003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batis C., Mendez M.A., Gordon-Larsen P., Sotres-Alvarez D., Adair L., Popkin B. Using both principal component analysis and reduced rank regression to study dietary patterns and diabetes in Chinese adults. Public Health Nutr. 2016;19:195–203. doi: 10.1017/S1368980014003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C., Shi Z., Lv J., Guo Y., Bian Z., Du H., Chen Y., Tao R., Huang Y., Chen J., et al. Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank. Nutrients. 2017;9:232. doi: 10.3390/nu9030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo H., Shi Z., Yuan B., Dai Y., Pan X., Wu G., Hussain A. Dietary patterns are associated with insulin resistance in Chinese adults without known diabetes. Br. J. Nutr. 2013;109:1662–1669. doi: 10.1017/S0007114512003674. [DOI] [PubMed] [Google Scholar]
- 45.Chen G.C., Szeto I.M., Chen L.H., Han S.F., Li Y.J., van Hekezen R., Qin L.Q. Dairy products consumption and metabolic syndrome in adults: Systematic review and meta–analysis of observational studies. Sci. Rep. 2015;5:14606. doi: 10.1038/srep14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babio N., Becerra-Tomás N., Martínez–González M.Á., Corella D., Estruch R., Ros E., Sayón-Orea C., Fitó M., Serra–Majem L., Arós F. Consumption of Yogurt, Low–Fat Milk, and Other Low–Fat Dairy Products Is Associated with Lower Risk of Metabolic Syndrome Incidence in an Elderly Mediterranean Population. J. Nutr. 2015;145:2308–2316. doi: 10.3945/jn.115.214593. [DOI] [PubMed] [Google Scholar]
- 47.Shin J.Y., Xun P., Nakamura Y., He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: A systematic review and meta–analysis. Am. J. Clin. Nutr. 2013;98:146–159. doi: 10.3945/ajcn.112.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin S., Lee H.W., Kim C.E., Lim J., Lee J.K., Lee S.A., Kang D. Egg Consumption and Risk of Metabolic Syndrome in Korean Adults: Results from the Health Examinees Study. Nutrients. 2017;9:687. doi: 10.3390/nu9070687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cocores J.A., Gold M.S. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med. Hypotheses. 2009;73:892–899. doi: 10.1016/j.mehy.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 50.Takase H., Machii M., Nonaka D., Ohno K., Dohi Y. Excessive salt intake is a significant predictor for future development of metabolic syndrome in the general population. Eur. Heart. J. 2020;41((Suppl. 2)) doi: 10.1093/ehjci/ehaa946.3058. [DOI] [Google Scholar]
- 51.Pilic L., Pedlar C.R., Mavrommatis Y. Salt–sensitive hypertension: Mechanisms and effects of dietary and other lifestyle factors. Nutr. Rev. 2016;74:645–658. doi: 10.1093/nutrit/nuw028. [DOI] [PubMed] [Google Scholar]
- 52.Chen J. Sodium sensitivity of blood pressure in Chinese populations. Curr. Hypertens. Rep. 2010;12:127–134. doi: 10.1007/s11906-009-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norris L.E., Collene A.L., Asp M.L., Hsu J.C., Liu L.F., Richardson J.R., Li D., Bell D., Osei K., Jackson R.D., et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009;90:468–476. doi: 10.3945/ajcn.2008.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asp M.L., Collene A.L., Norris L.E., Cole R.M., Stout M.B., Tang S.Y., Hsu J.C., Belury M.A. Time–dependent effects of safflower oil to improve glycemia, inflammation and blood lipids in obese, post–menopausal women with type 2 diabetes: A randomized, double–masked, crossover study. Clin. Nutr. 2011;30:443–449. doi: 10.1016/j.clnu.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H.E., Larsson A., Johansson L., Ahlström H., Arner P., Dahlman I., et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 56.Julibert A., Bibiloni M.D.M., Tur J.A. Dietary fat intake and metabolic syndrome in adults: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2019;29:887–905. doi: 10.1016/j.numecd.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 57.Marangoni F., Agostoni C., Borghi C., Catapano A.L., Cena H., Ghiselli A., La Vecchia C., Lercker G., Manzato E., Pirillo A., et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 2020;292:88–90. doi: 10.1016/j.atherosclerosis.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Molendi–Coste O., Legry V., Leclercq I.A. Why and How Meet n–3 PUFA Dietary Recommendations? Gastroenterol Res. Pract. 2011;2011:364040. doi: 10.1155/2011/364040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nanri A., Mizoue T., Takahashi Y., Kirii K., Inoue M., Noda M., Tsugane S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J. Nutr. 2010;140:580–586. doi: 10.3945/jn.109.116020. [DOI] [PubMed] [Google Scholar]
- 60.Jun S.H., Shin W.K., Kim Y. Association of Soybean Food Intake and Cardiometabolic Syndrome in Korean Women: Korea National Health and Nutrition Examination Survey (2007 to 2011) Diabetes Metab. J. 2020;44:143–157. doi: 10.4093/dmj.2019.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan A., Franco O.H., Ye J., Demark–Wahnefried W., Ye X., Yu Z., Li H., Lin X. Soy protein intake has sex–specific effects on the risk of metabolic syndrome in middle–aged and elderly Chinese. J. Nutr. 2008;138:2413–2421. doi: 10.3945/jn.108.097519. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins D.J., Kendall C.W., Connelly P.W., Jackson C.J., Parker T., Faulkner D., Vidgen E. Effects of high– and low–isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle–aged men and women. Metabolism. 2002;51:919–924. doi: 10.1053/meta.2002.33352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.