Abstract
We aimed to evaluate the prevalence of asthma and its associating environmental factors within a 6–12-year-old population. A cross-sectional, questionnaire-based study was conducted in primary schools located in the capital of Hungary; 3836 eligible parent-reported questionnaires were evaluated. Besides the International Study of Asthma and Allergies in Childhood (ISAAC) phase three core questions for asthma, the survey also assessed various potential risk factors. We introduced the umbrella term cumulative asthma as the union of physician-diagnosed asthma and current wheezing to estimate the lifetime prevalence of asthma. Current wheezing and physician-diagnosed asthma showed a frequency of 9.5% and 6.3%, respectively. They contributed to a cumulative asthma prevalence of 12.6% among the sampled population, with a girl-boy percentage of 37.4% to 62.6%. Air-pollution and weedy areas were associated with greater risk for asthma, while a suburban residence showed lesser odds. Indoor smoking, visible mold, and keeping a dog were defined as risk factors for asthma, while the presence of plants in the bedroom and pet rodents were associated with lower odds ratios. The consumption of fast food, beverages containing additives and margarine were significantly higher in asthmatics, while we found frequent sport activity and cereal intake associated with lower odds ratios for asthma. In this urban environment, we identified an increased asthma prevalence compared to some previously published studies, but the cross-sectional design and the different methodology did not permit us to draw timeframe-dependent conclusions.
Keywords: asthma, schoolchildren, prevalence, nutrition, environment, lifestyle, atopy, allergy, east-central Europe, ISAAC
1. Introduction
Asthma is a heterogeneous disease with time-varying respiratory symptoms (including wheeze) and is often associated with airway hyperresponsiveness and inflammation [1]. Taking the history of the characteristic symptoms is the key to the diagnostic work-up, reinforced with evidence of fluctuating airflow limitation. Questionnaire-based studies are amenable tools to estimate asthma prevalence, especially for large-scale surveys in pediatric focus groups, with whom a physician’s office visit is impractical [2]. The International Study of Asthma and Allergies in Childhood (ISAAC) included three research phases between 1991 and 2012 and developed various questions to investigate asthma and allergy [3]. The rationale behind the ISAAC initiative was to conduct epidemiologic research concerning the more recent symptoms that covered the past 12 months, to minimize recall errors [1]. The core questions of the ISAAC surveys are still favorable tools for such epidemiologic research in respiratory medicine.
Our questionnaire-based study was conducted in Budapest (Hungary). We embedded the ISAAC core questions into a form to evaluate the current asthma prevalence in the 6–12-year-old population. Inner cities are the areas where asthma is more frequent. Urban air pollution has been implicated as one of the factors responsible for the dramatic increase in asthma incidence. Our goal was to assess the prevalence of asthma in our metropolitan area and present the data in a representative survey with a large number of participants almost two decades after the initiation of phase three of the ISAAC project [3].
A secondary aim was to reveal different environmental factors and habits associated with asthma in the region of interest. We examined the association of various indoor and outdoor pollutants (e.g., exposure to industrial air pollution and smoking, exposure to pets), the consumption frequency of nutrients and physical activity,
2. Materials and Methods
2.1. Ethical Considerations
The study was endorsed by the Ethics Committee of the Heim Pál National Pediatric Institute, Budapest (KUT-19/2019). Designing the study and collecting, handling and processing the scientific data was carried out according to the principles of the Helsinki Declaration. Informed consent was obtained from all responders.
2.2. Study Design
This cross-sectional, questionnaire-based study was carried out in September 2019. A total number of twenty-one primary schools in eight districts of Budapest were randomly selected from the listings provided by the Central Data Processing and Registration Office of the Hungarian Ministry of Interior. Parents of 6–12-year-old children, at the first teacher-parent meetings of the school year, were asked to complete the survey. The teachers provided detailed instructions before completion. The questionnaires were collected at the end of teacher-parent meetings or within a week timeframe.
2.3. The Questionnaire
The enrolled parents received the multi-aspect questionnaire. In the present study, we summarized the results related to asthma and its putative risk factors. To address the prevalence of asthma and its risk factors, we included the core questions for asthma according to the phase three manual of the ISAAC supplemented with self-designed queries on physician-diagnosed asthma and risk factors [4].
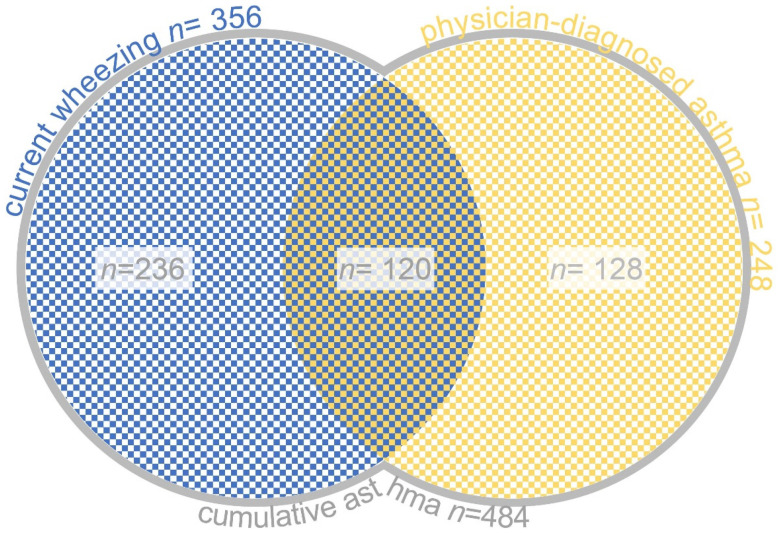
Subjects who responded “Yes” to the ISAAC core question “Has your child had wheezing or whistling in the chest in the past 12 months?” constituted the “current wheezing” group (CW). The prevalence of “physician-diagnosed asthma” (PDA) was determined based on the answers to the question “Has your child had asthma diagnosed by a physician?”. The union of CW and PDA sets defined the “cumulative asthma” group (CA) (Figure 1), best approximating the concept of lifetime asthma among the focused age group of pupils.
Figure 1.
Definition of cumulative asthma: the union of current wheezing and physician-diagnosed asthma sets.
We also included questions dealing with associated atopic conditions (A), including food allergy, asthma and allergic rhinitis.
Environmental (E), lifestyle (L) and nutritional (N) factors were evaluated in association with the CA group. The presence of a given environmental factor was addressed in the form of a yes-no question (E01-E34, Table S1). ZIP codes were registered to distinguish whether a student had a residence in the capital or the suburbs. The frequency of lifestyle factors was determined by questions expecting answers to three predefined choices (L01–L02), except for evaluating smoking behavior (L03), when a polar question was used. The frequency of weekly consumption of different nutrients was initially planned to be evaluated upon three predefined choices: “≥3 times”, “1–2 times”, “Rarely [<1 times]” (N01–16). Despite the given options, parents often responded with other intervals (e.g., 2–3 times a week) covering two choices. To overcome this, answers were rearranged into two intervals during evaluation: “Frequently [≥1 times per week]” or Rarely [<1 times per week]. These derivative answers were marked with the letter “d” in the report (N01d-N16d, Table S2). We asked for anthropometric data of the subjects, including heights and weights, and age and gender related body mass index (BMI) percentiles (BMI-for-age percentiles) were calculated according to the guideline and reference tables of the Association of Hungarian Health Visitors [5].
2.4. Statistical Analysis and Data Visualization
The data was characterized by standard descriptive statistics: frequencies (percentages) and means for categorical and quantitative data, respectively. Binomial logistic regression and chi-square were used to compare frequencies, and the t-test was used to compare the means of groups. Individual regression models were applied for the different explanatory variables. The response variable was cumulative asthma in all models. There was no reference group in the case of BMI percentages, so we used the chi-squared test only. Results were considered statistically significant at p < 0.05. In the case of categorical variables, odds ratios (OR) and 95% confidence intervals (95% CI) were calculated. Prevalence estimates were calculated by dividing positive responses to the given question by the total number of completed questionnaires. Percentages were calculated by dividing the frequency by the total number of observations, excluding missing answers, and then multiplying by 100. All analyzes were performed with the R-3.6.2 for Windows statistical program software [6].
3. Results
3.1. Data Acquisition, Refinement and Demographic Parameters of the Study Group
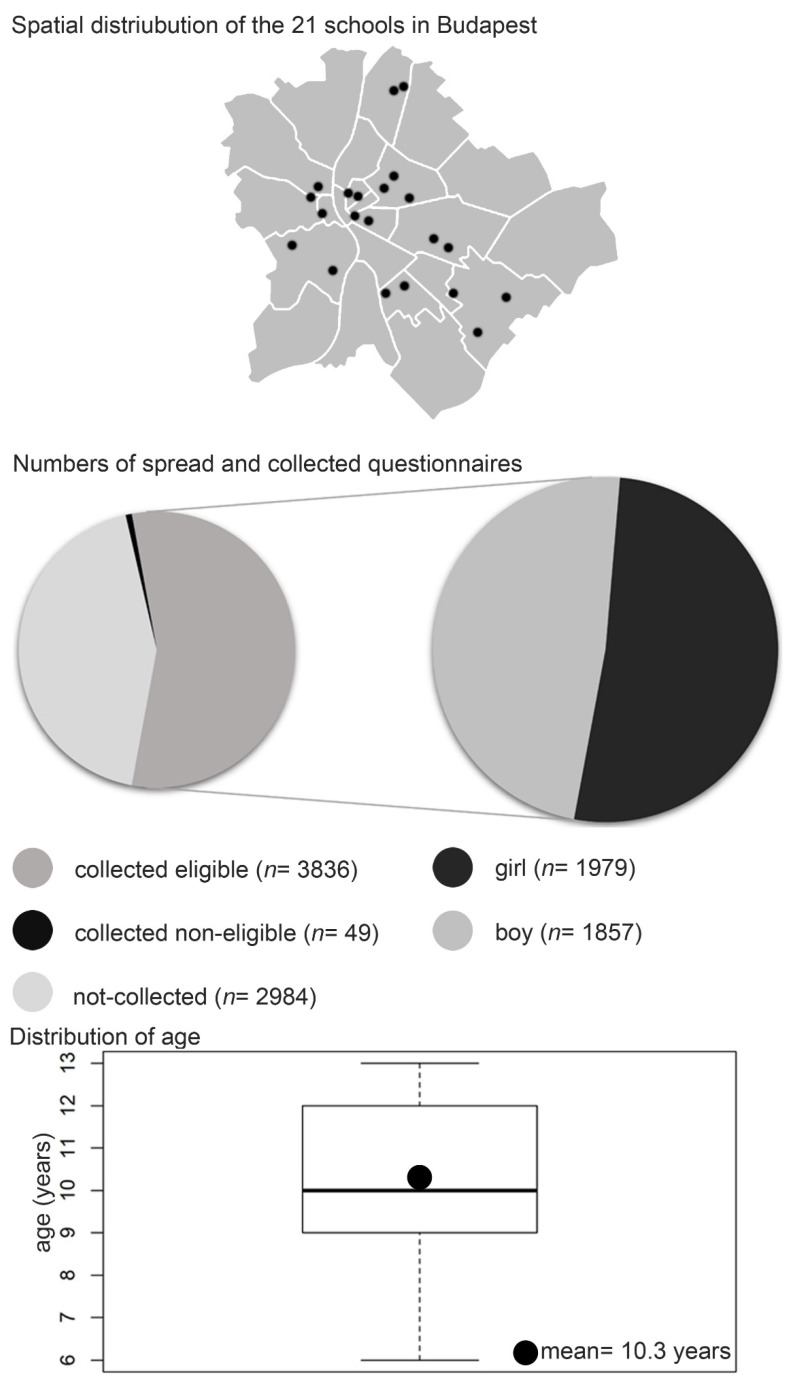
A total of 6869 questionnaires were distributed in the 21 primary schools. Altogether 3885 forms were returned, of which 49 had to be ruled out due to technical reasons or the subject’s inappropriate age. Of the 3836 eligible students 51.6% (n = 1979) were girls and 48.4% (n = 1857) were boys. The mean age of the children was 10.33 years +/− 1.68 (Figure 2).
Figure 2.
The schematic map of Budapest demonstrates the distribution of the selected primary schools. The Pie chart demonstrates the ratio of the collected forms and the gender-specific rate of the eligible.
3.2. Prevalence of Asthma
In the last 12 months according to the ISAAC core questions, 9.3% of responders (n = 356) experienced wheezing. This set of subjects represented the current wheezing (CW) group. The symptoms’ frequency and characteristics are also included in Table 1. Physician-diagnosed asthma (PDA) was 6.5% (n = 248) of the pupils regardless of being symptomatic or not in the previous year (Table 1 and Table 2). Thus, cumulative asthma (CA) had a prevalence of 12.6% (n = 484) among the 6–12-year-old schoolchildren in the sampled environment (Table 2). Among girls, asthmatics had a significantly lower proportion (OR = 0.52, CI: 0.42–0.63, p < 0.0001), and girl-boy percentage was 37.4% (n = 181) to 62.6% (n = 303) in the CA group.
Table 1.
The prevalence of asthma-related respiratory symptoms.
| Has Your Child Ever Had Wheezing or Whistling in the Chest at Any Time in the Past? | ||
|---|---|---|
| Yes | 834 | 21.7% |
| No | 3002 | 78.3% |
| Has Your Child Had Wheezing or Whistling in the Chest in the Past 12 Months? | ||
| Yes | 356 | 9.3% |
| No | 3480 | 90.7% |
| How Many Attacks of Wheezing Has Your Child Had in the Past 12 Months? | ||
| none | 3598 | 93.8% |
| 1–3 | 183 | 4.8% |
| 4–12 | 42 | 1.1% |
| ≥ 12 | 13 | 0.3% |
| In the Past 12 Months, How Often, on Average, Has Your Child’s Sleep Been Disturbed Due to Wheezing? | ||
| Never woken with wheezing | 3588 | 93.5% |
| Less than one night per week | 162 | 4.2% |
| One or more nights per week | 86 | 2.3% |
| In the Past 12 Months, Has Wheezing Ever Been Severe Enough to Limit Your Child’s Speech to Only One or Two Words at a Time between Breaths? | ||
| Yes | 41 | 1.1% |
| No | 3795 | 98.9% |
| Has Your Child Ever Had Asthma? | ||
| Yes | 290 | 7.6% |
| No | 3546 | 92.4% |
| In the Past 12 Months, Has Your Child’s Chest Sounded Wheezy during or after Exercise? (Physical Education, Running, Walking on Stairs) | ||
| Yes | 227 | 5.9% |
| No | 3609 | 94.1% |
| In the Past 12 Months, Has Your Child Had a Dry Cough at Night Apart from a Cough Associated with a Cold or Chest Infection? | ||
| Yes | 422 | 11% |
| No | 3414 | 89% |
| Has Your Child Had Asthma Diagnosed by a Physician? | ||
| Yes | 248 | 6.5% |
| No | 3588 | 93.5% |
Table 2.
The prevalence of current wheezing (CW), physician-diagnosed asthma (PDA) and cumulative asthma (CA) in the sample population.
| Type of Asthma | Frequency | Percentage | |
|---|---|---|---|
| Current wheezing (CW) | No | 3480 | 90.7% |
| Yes | 356 | 9.3% | |
| Total | 3836 | 100% | |
| Physician-diagnosed asthma (PDA) | No | 3588 | 93.5% |
| Yes | 248 | 6.5% | |
| Total | 3836 | 100% | |
| Cumulative asthma (CA) | No | 3552 | 87.4% |
| Yes | 484 | 12.6% | |
| Total | 3836 | 100% |
3.3. The Severity of Asthma and Associating Atopic Diseases
The ISAAC core questions also describe the severity of a subject’s disease. Table 3 demonstrates the frequency of such symptoms (including sleep disturbances, speech limiting wheezing and wheezing related to physical exercises) in the CA group.
Table 3.
The frequency of asthma symptoms indicates the severity in the CA group.
| Severity Indicator | n in CA | % in CA | |
|---|---|---|---|
| In the past 12 months, how often, on average, has your child’s sleep been disturbed due to wheezing? | Never woken with wheezing | 292 | 60.33% |
| Less than one night per week | 119 | 24.59% | |
| One or more nights per week | 73 | 15.01% | |
| In the past 12 months, has wheezing ever been severe enough to limit your child’s speech to only one or two words at a time between breaths? | Yes | 37 | 7.64% |
| No | 447 | 92.36% | |
| In the past 12 months, has your child’s chest sounded wheezy during or after exercise? (physical education, running, walking on stairs) | Yes | 149 | 30.79% |
| No | 335 | 69.21% |
Eczema, food allergy and allergic rhinitis had significant associations with CA (Table 4). Physician-diagnosed allergic rhinitis had the greatest odds for asthma (OR = 6.21, CI: 4.90–7.86, p < 0.0001) out of this sequence.
Table 4.
The association between CA asthma and other manifestations of atopy.
| QID | Atopic Condition: Has Your Child Ever Had…? |
n (%) in CA | p-Values | OR | CI | |
|---|---|---|---|---|---|---|
| A1 | any allergic diseases | Yes (n = 1206) | 331 (27.45) | <0.0001 | 6.12 | 4.99–7.55 |
| No (n = 2630) | 153 (5.82) | |||||
| A2 | Eczema | Yes (n = 491) | 86 (17.52) | 0.0005 | 1.57 | 1.21–2.02 |
| No (n = 3345) | 398 (11.90) | |||||
| A3 | food allergy | Yes (n = 242) | 61 (25.21) | <0.0001 | 2.53 | 1.85–3.42 |
| No (n = 3595) | 423 (11.77) | |||||
| A4 | allergic rhinitis | Yes (n = 357) | 124 (34.73) | <0.0001 | 4.61 | 3.61–5.88 |
| No (n = 3479) | 360 (10.35) | |||||
| A5 | Has your child been diagnosed with allergic rhinitis by a physician? | Yes (n = 373) | 149 (39.95) | <0.0001 | 6.21 | 4.90–7.86 |
| No (n = 3463) | 335 (9.67) |
QID: question ID, OR: odds ratio, CI: confidence interval.
3.4. Environmental Risk Factors
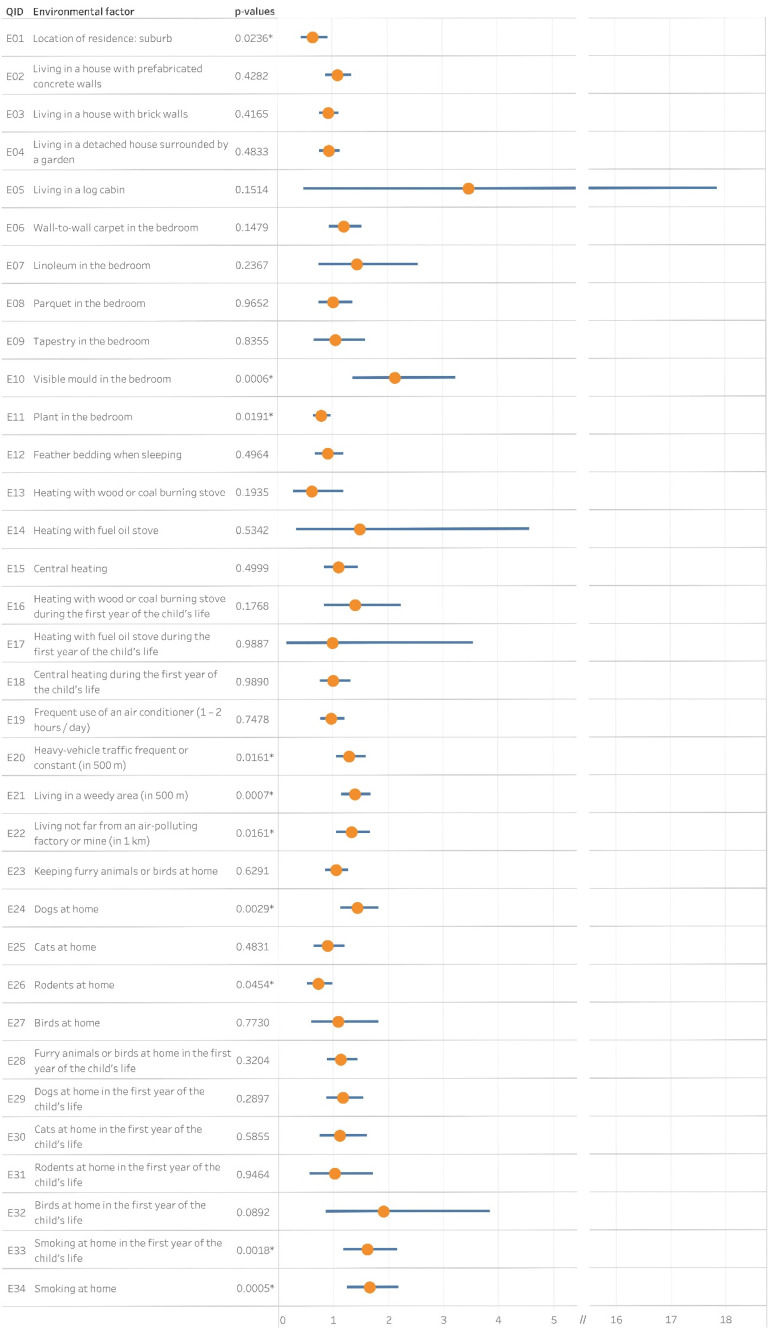
Thirty-four questions aimed to analyze any possible relationships between the prevalence of cumulative asthma and local environmental risk factors (Table S1). Figure 3 visualizes the statistical association between CA and the risk factors.
Figure 3.
The association between cumulative asthma and indoor and outdoor environmental factors. Yellow dots and blue bars stand for the corresponding odds ratios and confidence intervals, respectively. QID: Question ID, *: p <0.05.
The odds of the cumulative asthma group were lower among schoolchildren who lived in the suburbs than those who had their residence in the capital (OR = 0.64, CI: 0.43–0.93, p = 0.0236).
Among indoor causative agents, smoking at home had a crucial role in the prevalence of asthma. Whether the child had been exposed during the first year of life (OR = 1.61, CI: 1.18–2.16, p = 0.0018) or at the time of the survey (OR = 1.65, CI: 1.24–2.18, p = 0.0005), smoking associated with asthma. Visible mold (OR = 2.13, CI: 1.36–3.24, p = 0.0006) in the children’s bedroom contributed to the occurrence of asthma, though, the presence of a plant (OR = 0.79, CI: 0.65–0.96, p = 0.0191) yielded lower odds ratio. Dog (OR = 1.44, CI: 1.13–1.82, p = 0.0029) keeping associated with CA, while rodent ownership (OR = 0.72, CI: 0.52–0.98, p = 0.0454) showed lower odds ratio. On the contrary, cat, bird, or furry animal keeping did not.
The proximity of any air-polluting factories (OR = 1.33, CI: 1.05–1.68, p = 0.0161), heavy-vehicle traffic (OR = 1.29, CI: 1.05–1.59, p = 0.0161) or a weedy area (OR = 1.39, CI: 1.15–1.69, p = 0.0007) had a significant association with the prevalence of CA.
3.5. Lifestyle Factors
Sports activity in a frequent manner (≥3 times a week) was significantly associated with the absence of asthma (OR = 0.69, CI: 0.52–0.92, p = 0.0098) (Table 5). Visual display time did not associate with the prevalence of CA. Although environmental tobacco smoke increased asthma risk (OR = 1.65, CI: 1.24–2.18, p = 0.0005), pupils who smoked showed no significant association with CA. The ratio of smokers among students was just 1.04% (n = 4) though.
Table 5.
Statistical analysis of lifestyle factors; results revealed an inverse association between sports activity and cumulative asthma.
| QID | Lifestyle Factors: Presence or Frequency of Behavioral Risk Factor during the Last 12 Months |
n (%) in CA | p-Values | OR | CI | |
|---|---|---|---|---|---|---|
| L1 | Weekly frequency of sport activities? |
≥3 times (n = 1879) |
214 (11.39) | 0.0098 | 0.69 | 0.52–0.92 |
| 1–2 times (n = 1474) |
194 (13.16) | 0.1554 | 0.81 | 0.61–1.09 | ||
| Never or occasionally (n = 483) |
76 (15.73) | |||||
| L2 | Average time per week spent on watching TV and / or computer | ≥3 h (n = 1948) | 259 (13.30) | 0.0965 | 1.39 | 0.96–2.08 |
| 1–3 h (n = 1501) | 185 (12.33) | 0.2312 | 1.27 | 0.87–1.92 | ||
| <1 h (n = 322) | 32 (9.94) | |||||
| None responders (n = 65) | 8 (12.31) | |||||
| L3 | The child smokes occasionally or frequently | Yes (n = 4) | 1 (25.00) | 0.4687 | 2.31 | 0.11–18.10 |
| No (n = 3832) | 483 (12.60) |
QID: question ID, OR: odds ratio, CI: confidence interval.
3.6. Nutritional Factors
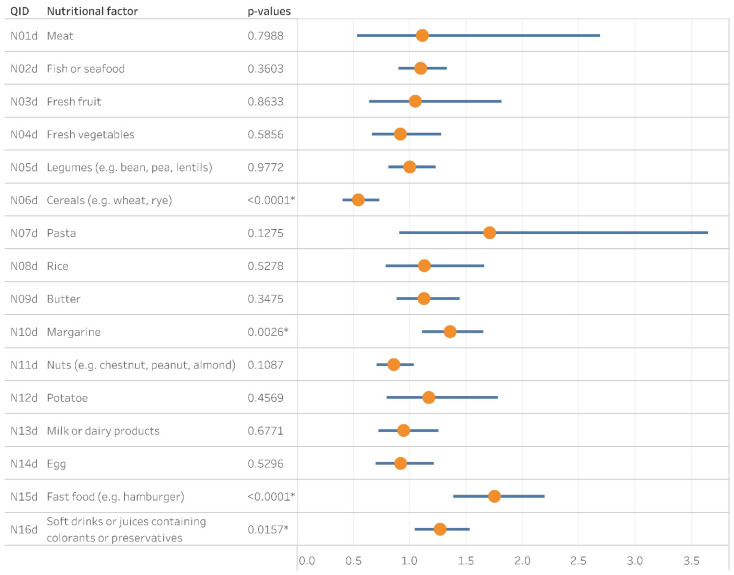
The weekly consumption of a particular ingredient was derived from the original questions as per the method section (Table S2). Frequently eating fast food (e.g., hamburgers) or drinking soft drinks including artificial additives was associated with the prevalence of CA (OR = 1.75, CI: 1.39–2.19, p < 0.0001 and OR = 1.27, CI: 1.05–1.53, p = 0.0157 respectively). Weekly consumption of margarine had a significant association with the development of asthma (OR = 1.35, CI: 1.11–1.65, p = 0.0026). The intake of cereals at least once a week seemed preventive for asthma (OR = 0.54, CI: 0.41–0.73, p < 0.0001) (Figure 4).
Figure 4.
The Forest plot depicts the contribution of nutritional factors to the prevalence of cumulative asthma. QID: Question ID; Yellow dots and blue bars stand for the corresponding odds ratios and confidence intervals, respectively; *: p <0.05.
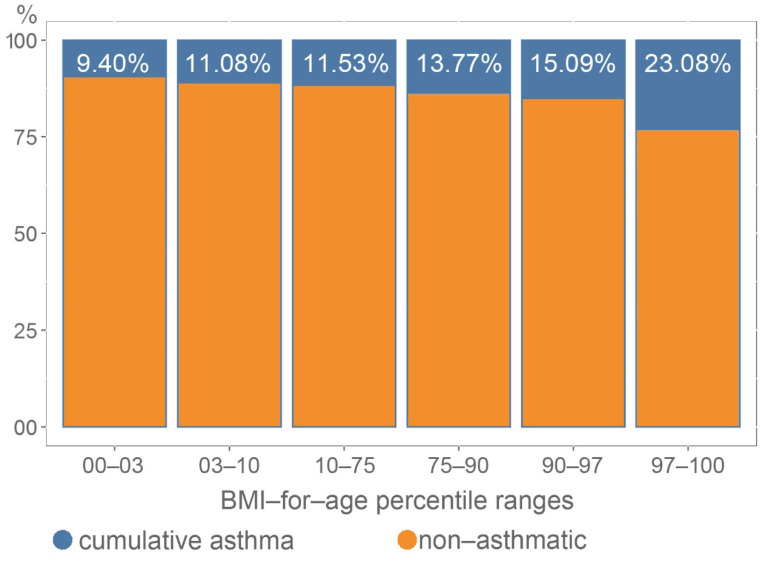
Based on the available data, we were able to calculate the BMI-for-age percentile of a child in the case of only 3295 participants. Among them, we identified an association (Chi-square = 16.26 df = 5 p = 0.0062) between the percentile values and cumulative asthma (Figure 5, Table S3). Children with higher BMI were more likely to have asthma.
Figure 5.
Association between cumulative asthma and BMI-for-age percentiles. (Chi-square = 16.26 df = 5 p = 0.0062).
4. Discussion
In the present study the prevalence of current wheezing based on the corresponding ISAAC core question “Has your child had wheezing or whistling in the chest in the past 12 months?” was 9.3% among the 6–12-year-old children in the metropolitan area of Budapest, the capital of Hungary. Physician-diagnosed asthma accounted for 6.5% of prevalence. Union of these two sets of students defined a cumulative asthma prevalence at 12.6%. Hungary participated with two centers in the phase three study of ISAAC in 2003, but none represented the capital. A survey (ISAAC ID 047002) covering schools of two cities from the southeastern lowland of Hungary involved solely the 13–14 age group. Out of the 2899 students who had completed the written questionnaire 204 (7.1%) reported wheezing in the previous year [7]. The other study (ISAAC ID 047001) indicated a 6.6% and 5% prevalence of current wheeze in the 6–7-year-old and 13–14-year-old groups respectively [7]. The overall higher prevalence of the contemporary sampling frame’s current wheezing can be either due to e.g., the urban environment or a gradually increasing prevalence in the fifteen-year timeframe.
We found cumulative asthma significantly lower among girls than in boys. There are controversial results regarding the sex-specific prevalence of childhood asthma, especially in a lifetime analysis. However, current symptom prevalence has been reported with an increasing boy-girl ratio [8].
From a Central European perspective, the prevalence of asthma shows heterogeneity. Aberle et al. reported 7.9% of current wheezing and 4.1% of diagnosed asthma among 10–11-year-old students in neighboring Croatia [9]. Their survey provided evidence that more boys (63.2%) had asthma. The gender-specific relations are consistent with our results. However, the geographical features of the investigated area resembled Hungary, the level of urbanization did not approximate Budapest, which can explain the higher prevalence in our settings. Results of the Croatian study are more comparable with and more congruent to the former ISAAC surveys conducted in Hungary (ISAAC ID 047001 & 047002) [7].
An almost population-wide screening of primary school students was carried out in Tyrol, Austria [10]. The mean age of the participants was 8.4 years (SD ± 1.2). Besides the current wheezing, defined by the ISAAC manual, they used an extended definition for asthma by massing subjects with current wheeze or who had used an asthma spray ever or recurrent wheezy bronchitis or a doctor diagnosis. 10.3% of the total study population had current wheezing, which is 1% higher than in our subjects. Doctor-diagnosed asthma was at 3.4%. Based on their residence, Tyrolean students were sub-classified into farm children, rural children and Innsbruck-town children, and their exposure to particular agents (e.g., hay-loft, animal shed, or farm milk). They found living on a farm as protective but only for those with regular exposure to farming agents. In our study, children commuting from the suburbs had a lower risk of asthma. With no history of exposure to particular agents, 21.3% of Innsbruck-town children fulfilled the extended criteria of asthma. This is 1.70 times higher than our cumulative asthma results, which can be due to either the regional differences or the different sampling protocols.
In a study from Romania, asthma-like symptoms (dominantly dry cough) were reported among 20% of the participating students [11]; 48% of the subjects were exposed to environmental tobacco smoke, one of the expected triggers responsible for the high rate of symptoms.
Out of our participants, 9.65% (n = 370) shared their home with a smoker relative, which is a remarkable difference compared to the Romanian data. Sixty-eight (18.15%) out of them fit into the CA group and verified tobacco exposure as a major risk factor for asthma. Indoor tobacco fumes also impact other respiratory outcomes in children, including pneumonia, night cough and croup [12].
Increasing evidence has confirmed mold as another predisposing factor in children [13,14,15,16,17]. Visible mold can be a source of fungal spores and other volatile organic compounds. These viable and non-viable particles are sufficient enough to increase the risk of asthma [15]. In our sample, moldy surfaces doubled the chance of asthma development. Fagbule and Ekanem found that mold was only harmful in the bedroom, and on the contrary, it had a somehow protective effect elsewhere at home [18]. The authors noticed that children could also benefit from indoor plants [18]. Our study also revealed this favorable correlation between the presence of plants in the bedroom and asthma prevalence. It is challenging to explain this phenomenon because potted plants’ soil could serve as an origin for fungal and other biological agents.
The literature is controversial on the role of pet allergens in asthma prevalence [19,20,21]. We found that overall pet ownership did not associate with asthma in accordance with current meta-analyses [21]; however, pet-specific risks differed. A UK birth cohort showed that early childhood ownership could have a prophylactic effect but surrounding rabbits or rodents could contribute to non-atopic asthma with a higher odds [22]. Exposure to mouse antigens could associate with wheezing [23]. Among the subjects of the current study, a rodent’s presence resulted in lower odds of asthma. Inconsistency might be a result of a non-species-specific investigation. In addition, rodents were subjects of leisure pet ownership in our study, but the exposure to their antigens can also be from pests invading households. Such circumstances may also associate with other pollutive agents and a lower socioeconomic environment. Our observations concluded dog-keeping as a potential risk factor of asthma, but cat-ownership did not. An explanation could be the so-called cat paradox [22,24,25]. The increased odds associated with dog ownership argue with the contemporary view [21]. The latter statistical relationship seems plausible but might be casual; a more detailed assessment of pet ownership should be conducted in the future.
Outdoor air pollution also plays a role in asthma development. In the current setting, the proximity of any air-polluting factories or heavy vehicle traffic is associated with the risk of asthma. Although numerous reports support these findings, from an environmental health point-of-view the particular composition and concentration of airborne pollutants instead reflect the association [26,27,28,29].
The protective effect of suburban living can also be a surrogate reference of this association between air pollution and asthma because exposure to ubiquitous atmospheric agents is suspected to be lower at those sites.
Living in a highly weeded area is considered a risk factor of asthma development with an OR of 1.33. It is also associated with allergic rhinitis prevalence in the same environment, where common ragweed Ambrosia artemisiifolia is the most widespread cause of allergy-associated symptoms [30]. However, a higher level of pollen load showed a non-significant association with allergy risk in Budapest [31]. Despite the geographical distance, it is worth noting that 14% of asthmatic children of the 3–11-year-old population sensitized against ragweed in a US-based study [32].
Measurement of physical activity is challenging to carry out: besides the duration and frequency, the intensity is supposed to be a question of interest. Questionnaire-based research is a limiting factor of good quality and comparable data. Our current survey reported a significant association when children realizing vigorous activity more than three times a week were less likely to be in the cumulative asthma group. Visual display time as a reference for sedentary lifestyle correlated with the odds ratio of asthma, but the relationship was not statistically significant.
The ISAAC Phase Three summarized similar tendencies, but the methodology was different [33]. Other studies with different approaches reported an ambiguous correlation between physical activity and asthma prevalence [34,35,36,37,38]. We must emphasize that asthma has also been reported as a barrier to physical activity; thus, in the future, we should analyze this relation through an interdisciplinary lens [34]. Emerging technologies, including wearable biosensors or smartphone applications, may serve as more accurate physical activity data resources in the future.
Dietary patterns influence the risk of asthma development. A Mediterranean-style diet, rich in fruits, vegetables and whole grains while taking less meat and dairy in, and other plant-based foods have associated with reduced odds for asthma [39,40,41,42,43]. A Westernized diet, including a predominant amount of animal products with higher fat intake and lower fiber consumption, is a risk factor for asthma [41]. It would be welcome to keep adherence to a healthier diet where it is already historically and geographically predisposed; trends showed an increased prevalence of asthma symptoms in the Mediterranean and Latin America [41,44]. In the current sampled population, such emblematic meals of urbanized living like fast-food and drinks with artificial additives are associated with cumulative asthma.
Regular consumption of margarine also showed an association with cumulative asthma. A high rate of fat intake is also attributed to the Westernized diet, but there is more emphasis placed on the composition of fat fractions. According to the lipid hypothesis, an increased intake of polyunsaturated fatty acids (PUFAs) over saturated fat can increase asthma prevalence and allergic sensitization, but controversial results are also available [41,42,45,46]. Margarine can serve as a significant source of PUFAs. Therefore, we should be aware of its potential causative role in asthma and atopy development [45,47,48,49].
Frequent intake of cereals showed an association with decreased odds of cumulative asthma. The literature has supported this finding, though the protective mechanism is not completely understood yet [41,42,43,46]. Our survey did not evaluate prior cereal consumption, although the early introduction of cereal grains into the diet is substantial to avoid sensitization [50].
High energy diet is a predisposing factor for obesity. Obesity is associated with worse asthma control and an increased risk of exacerbations in all ages [41]. Besides the fact that obese and overweight children are more likely to have asthma, according to the current view, obesity-related asthma in childhood is a separate entity with a Th1 cell polarization [41,51,52]. Our observation reinforced the conception of the association between asthma and BMI-for-age percentiles, but the phenotyping of obese asthmatics was beyond our scope.
5. Limitations and Strengths
Some limitations should be considered when interpreting our results. First, the prevalence of asthma was measured based on questionnaires, without performing clinical tests. Second, such a survey cannot collect data regarding the demographic and health features of non-responders. Members of the focus group can also differ in health literacy which can either influence the understanding of the questions and reporting current prior and current medical conditions.
Current wheezing symptoms were related to the past 12 months, which may involve further study limitations. On the other hand, the reported current wheeze supports defining a group of children who have not been diagnosed with asthma symptoms due to undisclosed reasons. The cumulative asthma group was set up for a better approximation of the composition of childhood asthma prevalence. Thus, the outcomes may have been either over or underestimated.
The food consumption frequency results refer to the one-year pre-survey period. Keeping in mind the wide age-group of the subjects, this is a short period to define putative etiologic relationships. Longitudinal surveys or twin studies would be more appropriate tools.
The strengths of this study are that we used a standardized method and expanded the questionnaire with numerous environmental and lifestyle-related questions, stressing the main environmental factors associated with asthma. The database is large enough to reflect the population of Budapest. We added comparable considerations of the associating factors. Our cross-sectional survey can serve as a basis for further longitudinal studies to have a clearer view of the prevalence and characteristics of childhood asthma.
6. Conclusions
Our study delivered data on the recent prevalence of asthma among 6–12-year-old children in Budapest, Hungary. We found the prevalence of current wheezing 9.3% among the subjects, in comparison 6.5% had already been diagnosed with asthma by a physician. We introduced the term “cumulative asthma” to estimate the lifetime prevalence of asthma. This group included 12.6% of the individuals.
In this urban environment, we identified an increased asthma prevalence compared to some previously published studies, but the cross-sectional design and the different methodology did not permit us to draw timeframe-dependent conclusions.
Besides the outdoor and indoor environmental factors, we also analyzed the contribution of lifestyle and nutritional determinants. Suburban residence, plant in the bedroom, rodent ownership, frequent consumption of cereals and regular sport activity are associated with lower odds ratios for asthma. In comparison, nearby heavy vehicle traffic or air polluting factories, weedy areas, visible mold, indoor smoking, dog ownership, the consumption of fast food, beverages containing additives and margarine, and high BMI showed significant association with asthma.
Though the statistical associations revealed are not numerous, the majority fit in the existing literature. The controversial impact of rodent and dog ownership requires a better understanding.
Our study serves large-scale descriptive data on the local prevalence of asthma which has not been examined in the capital area. Novel devices and prospective, interventional studies with interdisciplinary approaches may help us reduce the burden of asthma by comprehending its triggers.
Acknowledgments
The authors would like to thank the pupils, their parents and the heads, teachers and secretaries of the schools for their help and co-operation during the data collection phase. They also would like to express their special thanks of gratitude to Zsolt Abonyi-Tóth (Data Processor Ltd.) for contribution to the statistical analysis. They are also grateful to Zsófa Pók Körmendiné, the librarian at Heim Pál National Pediatric Institute Budapest for the reference retrievals.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph182413403/s1, Table S1: Dataset of environmental risk factor analysis Table S2: Dataset of nutritional risk factor analysis, Table S3: Dataset of cumulative asthma prevalence in BMI-for-age percentile ranges
Author Contributions
Conceptualization, D.M., G.G., A.H. (Alpár Horváth), G.T., G.K., A.H. (Andor Hirschberg), G.M. and M.S.; methodology, D.M., G.G., A.H. (Alpár Horváth), G.M. and M.S.; data curation, D.M., G.G., A.H. (Alpár Horváth), G.M. and M.S.; validation, D.M., G.G., A.H. (Alpár Horváth), G.M. and M.S.; investigation, D.M., G.G., A.H. (Alpár Horváth) and M.S.; writing—original draft preparation, D.M., G.G., A.H. (Alpár Horváth), G.M. and M.S.; writing—review and editing, G.T., G.K., A.H. (Andor Hirschberg); visualization, D.M.; project administration, G.G. and M.S.; resources, G.G., A.H. (Alpár Horváth) and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Heim Pál National Pediatric Institute, Budapest (KUT-19/2019).
Informed Consent Statement
Informed consent was obtained from all responders, parents and/or legal guardians as children under 16 years of age were involved in the study.
Data Availability Statement
Raw data are available from the corresponding author upon reasonable request. The datasets derived during this study are included in this published article (and its supplementary information files).
Conflicts of Interest
The authors report no conflicts of interest pertaining to the submitted work. A.H. and G.T. are full-time employees of Chiesi Hungary Ltd. G.G. reports personal fees from Astra-Zeneca, Chiesi, BMS, MSD, Berlin Chemie, Boehringer Ingelheim, Roche, Novartis, Pfizer, Ipsen, Mylen, Orion outside the submitted work. D.M. declares personal fees from Sanofi-Aventis outside the submitted work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. The Global Initiative for Asthma (GINA); Fontana, WI, USA: 2021. [Google Scholar]
- 2.The Global Initiative for Asthma (GINA) The Global Asthma Report 2018. The Global Initiative for Asthma (GINA); Fontana, WI, USA: 2018. [Google Scholar]
- 3.Ellwood P., Asher M.I., Beasley R., Clayton T.O., Stewart A.W., ISAAC Steering Committee. ISAAC Phase Three Study Group . ISAAC Phase Three Manual. ISAAC International Data Centre; Auckland, New Zealand: 2000. p. 94. [Google Scholar]
- 4.Mallol J., Crane J., von Mutius E., Odhiambo J., Keil U., Stewart A., Group I.P.T.S. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergol Immunopathol. 2013;41:73–85. doi: 10.1016/j.aller.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Ágfalvi R., Blatniczky L., Darvay S., Joubert K. 3sz Módszertani Levél—Útmutató és Táblázatok a Gyermekkori Tápláltság Megítéléséhez [3rd Metholdological Letter—Guideline and Tables for the Evaluation of Childhood Nutrition] Magyar Védőnők Egyesülete (MAVE); Budapest, Hungary: 2004. [Google Scholar]
- 6.R Core Team . R: A Language and Environment for Statistical Computing, R-3.6.2. for Windows. R Foundation for Statistical Computing; Vienna, Austria: 2019. [Google Scholar]
- 7.ISAAC Phase Three Study Group ISAAC Phase Three Data. [(accessed on 4 January 2020)]. Available online: http://isaac.auckland.ac.nz/phases/phasethree/results/results.php.
- 8.Bjerg A., Sandstrom T., Lundback B., Ronmark E. Time trends in asthma and wheeze in Swedish children 1996–2006: Prevalence and risk factors by sex. Allergy. 2010;65:48–55. doi: 10.1111/j.1398-9995.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 9.Aberle N., Kljaic Bukvic B., Blekic M., Vuckovic M., Bardak D., Gudelj A., Cancarevic G., Franic M. Allergic Diseases and Atopy among Schoolchildren in Eastern Croatia. Acta Clin. Croat. 2018;57:82–90. doi: 10.20471/acc.2018.57.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horak E., Morass B., Ulmer H., Genuneit J., Braun-Fahrlander C., von Mutius E., Group G.S. Prevalence of wheezing and atopic diseases in Austrian schoolchildren in conjunction with urban, rural or farm residence. Wien. Klin. Wochenschr. 2014;126:532–536. doi: 10.1007/s00508-014-0571-z. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y., Lin S., Lawrence W.R., Lin Z., Gurzau E., Csobod E., Neamtiu I.A. Evidence from SINPHONIE project: Impact of home environmental exposures on respiratory health among school-age children in Romania. Sci. Total Environ. 2018;621:75–84. doi: 10.1016/j.scitotenv.2017.11.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuge Y., Qian H., Zheng X., Huang C., Zhang Y., Li B., Zhao Z., Deng Q., Yang X., Sun Y., et al. Effects of parental smoking and indoor tobacco smoke exposure on respiratory outcomes in children. Sci. Rep. 2020;10:4311. doi: 10.1038/s41598-020-60700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennie D.C., Karunanayake C.P., Lawson J.A., Kirychuk S., McMullin K., Abonyi S., Seeseequasis J., MacDonald J., Dosman J.A., Pahwa P. Domestic Risk Factors for Atopic and non-Atopic Asthma in First Nations Children Living in Saskatchewan, Canada. Children. 2020;7:38. doi: 10.3390/children7050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe R.A., Thornton C.R., Tyrrell J., Nikolaou V., Osborne N.J. Variable risk of atopic disease due to indoor fungal exposure in NHANES 2005–2006. Clin. Exp. Allergy. 2015;45:1566–1578. doi: 10.1111/cea.12549. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z., Reponen T., Hershey G.K. Fungal Exposure and Asthma: IgE and Non-IgE-Mediated Mechanisms. Curr. Allergy Asthma Rep. 2016;16:86. doi: 10.1007/s11882-016-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caillaud D., Leynaert B., Keirsbulck M., Nadif R. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018;27:1701037. doi: 10.1183/16000617.0137-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W., Liu Q., Chen Y., Yang B., Huang X., Li Y., Zhang J.J. Effects of indoor environment and lifestyle on respiratory health of children in Chongqing, China. J. Thorac. Dis. 2020;12:6327–6341. doi: 10.21037/jtd.2020.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagbule D., Ekanem E.E. Some environmental risk factors for childhood asthma: A case-control study. Ann. Trop. Paediatr. 1994;14:15–19. doi: 10.1080/02724936.1994.11747686. [DOI] [PubMed] [Google Scholar]
- 19.Fretzayas A., Kotzia D., Moustaki M. Controversial role of pets in the development of atopy in children. World J. Pediatr. 2013;9:112–119. doi: 10.1007/s12519-013-0412-6. [DOI] [PubMed] [Google Scholar]
- 20.Poowuttikul P., Saini S., Seth D. Inner-City Asthma in Children. Clin. Rev. Allergy Immunol. 2019;56:248–268. doi: 10.1007/s12016-019-08728-x. [DOI] [PubMed] [Google Scholar]
- 21.Lodrup Carlsen K.C., Roll S., Carlsen K.H., Mowinckel P., Wijga A.H., Brunekreef B., Torrent M., Roberts G., Arshad S.H., Kull I., et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS ONE. 2012;7:e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collin S.M., Granell R., Westgarth C., Murray J., Paul E., Sterne J.A., John Henderson A. Pet ownership is associated with increased risk of non-atopic asthma and reduced risk of atopy in childhood: Findings from a UK birth cohort. Clin. Exp. Allergy. 2015;45:200–210. doi: 10.1111/cea.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oluwole O., Arinola G.O., Huo D., Olopade C.O. Household biomass fuel use, asthma symptoms severity, and asthma underdiagnosis in rural schoolchildren in Nigeria: A cross-sectional observational study. BMC Pulm. Med. 2017;17:3. doi: 10.1186/s12890-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erwin E.A., Custis N., Ronmark E., Wickens K., Sporik R., Woodfolk J.A., Platts-Mills T.A. Asthma and indoor air: Contrasts in the dose response to cat and dust-mite. Indoor Air. 2005;15((Suppl. 10)):33–39. doi: 10.1111/j.1600-0668.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 25.Takkouche B., Gonzalez-Barcala F.J., Etminan M., Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: A meta-analysis. Allergy. 2008;63:857–864. doi: 10.1111/j.1398-9995.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 26.Bowatte G., Lodge C., Lowe A.J., Erbas B., Perret J., Abramson M.J., Matheson M., Dharmage S.C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: A systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70:245–256. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- 27.Gentile D.A., Morphew T., Elliott J., Presto A.A., Skoner D.P. Asthma prevalence and control among schoolchildren residing near outdoor air pollution sites. J. Asthma. 2020:1–11. doi: 10.1080/02770903.2020.1840584. [DOI] [PubMed] [Google Scholar]
- 28.Khreis H., Nieuwenhuijsen M.J. Traffic-Related Air Pollution and Childhood Asthma: Recent Advances and Remaining Gaps in the Exposure Assessment Methods. Int. J. Environ. Res. Public Health. 2017;14:312. doi: 10.3390/ijerph14030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khreis H., Kelly C., Tate J., Parslow R., Lucas K., Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017;100:1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Sultesz M., Horvath A., Molnar D., Katona G., Mezei G., Hirschberg A., Galffy G. Prevalence of allergic rhinitis, related comorbidities and risk factors in schoolchildren. Allergy Asthma Clin. Immunol. 2020;16:98. doi: 10.1186/s13223-020-00495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voros K., Bobvos J., Varro J.M., Malnasi T., Koi T., Magyar D., Rudnai P., Paldy A. Impacts of long-term ragweed pollen load and other potential risk factors on ragweed pollen allergy among schoolchildren in Hungary. Ann. Agric. Environ. Med. 2018;25:307–313. doi: 10.26444/aaem/82624. [DOI] [PubMed] [Google Scholar]
- 32.Ogershok P.R., Warner D.J., Hogan M.B., Wilson N.W. Prevalence of pollen sensitization in younger children who have asthma. Allergy Asthma Proc. 2007;28:654–658. doi: 10.2500/aap.2007.28.3055. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell E.A., Beasley R., Bjorksten B., Crane J., Garcia-Marcos L., Keil U., Group I.P.T.S. The association between BMI, vigorous physical activity and television viewing and the risk of symptoms of asthma, rhinoconjunctivitis and eczema in children and adolescents: ISAAC Phase Three. Clin. Exp. Allergy. 2013;43:73–84. doi: 10.1111/cea.12024. [DOI] [PubMed] [Google Scholar]
- 34.Glazebrook C., McPherson A.C., Macdonald I.A., Swift J.A., Ramsay C., Newbould R., Smyth A. Asthma as a barrier to children’s physical activity: Implications for body mass index and mental health. Pediatrics. 2006;118:2443–2449. doi: 10.1542/peds.2006-1846. [DOI] [PubMed] [Google Scholar]
- 35.Lang D.M., Butz A.M., Duggan A.K., Serwint J.R. Physical activity in urban school-aged children with asthma. Pediatrics. 2004;113:e341–e346. doi: 10.1542/peds.113.4.e341. [DOI] [PubMed] [Google Scholar]
- 36.Pike K.C., Griffiths L.J., Dezateux C., Pearce A. Physical activity among children with asthma: Cross-sectional analysis in the UK millennium cohort. Pediatr. Pulmonol. 2019;54:962–969. doi: 10.1002/ppul.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eijkemans M., Mommers M., de Vries S.I., van Buuren S., Stafleu A., Bakker I., Thijs C. Asthmatic symptoms, physical activity, and overweight in young children: A cohort study. Pediatrics. 2008;121:e666–e672. doi: 10.1542/peds.2007-1236. [DOI] [PubMed] [Google Scholar]
- 38.Eijkemans M., Mommers M., Draaisma J.M., Thijs C., Prins M.H. Physical activity and asthma: A systematic review and meta-analysis. PLoS ONE. 2012;7:e50775. doi: 10.1371/journal.pone.0050775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro-Rodriguez J.A., Garcia-Marcos L., Alfonseda Rojas J.D., Valverde-Molina J., Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. J. Pediatr. 2008;152:823–828.e2. doi: 10.1016/j.jpeds.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Arvaniti F., Priftis K.N., Papadimitriou A., Papadopoulos M., Roma E., Kapsokefalou M., Anthracopoulos M.B., Panagiotakos D.B. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10-12 years old children: The PANACEA study. Pediatr. Allergy Immunol. 2011;22:283–289. doi: 10.1111/j.1399-3038.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 41.Alwarith J., Kahleova H., Crosby L., Brooks A., Brandon L., Levin S.M., Barnard N.D. The role of nutrition in asthma prevention and treatment. Nutr. Rev. 2020;78:928–938. doi: 10.1093/nutrit/nuaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.H., Ellwood P.E., Asher M.I. Diet and asthma: Looking back, moving forward. Respir Res. 2009;10:49. doi: 10.1186/1465-9921-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatzi L., Apostolaki G., Bibakis I., Skypala I., Bibaki-Liakou V., Tzanakis N., Kogevinas M., Cullinan P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62:677–683. doi: 10.1136/thx.2006.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asher M.I., Garcia-Marcos L., Pearce N.E., Strachan D.P. Trends in worldwide asthma prevalence. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.02094-2020. [DOI] [PubMed] [Google Scholar]
- 45.Black P.N., Sharpe S. Dietary fat and asthma: Is there a connection? Eur. Respir. J. 1997;10:6–12. doi: 10.1183/09031936.97.10010006. [DOI] [PubMed] [Google Scholar]
- 46.Ellwood P., Asher M.I., Bjorksten B., Burr M., Pearce N., Robertson C.F. Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: An ecological analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) data. ISAAC Phase One Study Group. Eur. Respir. J. 2001;17:436–443. doi: 10.1183/09031936.01.17304360. [DOI] [PubMed] [Google Scholar]
- 47.Nagel G., Linseisen J. Dietary intake of fatty acids, antioxidants and selected food groups and asthma in adults. Eur. J. Clin. Nutr. 2005;59:8–15. doi: 10.1038/sj.ejcn.1602025. [DOI] [PubMed] [Google Scholar]
- 48.Sausenthaler S., Kompauer I., Borte M., Herbarth O., Schaaf B., Berg A., Zutavern A., Heinrich J., Group L.S. Margarine and butter consumption, eczema and allergic sensitization in children. The LISA birth cohort study. Pediatr. Allergy Immunol. 2006;17:85–93. doi: 10.1111/j.1399-3038.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- 49.Bolte G., Frye C., Hoelscher B., Meyer I., Wjst M., Heinrich J. Margarine consumption and allergy in children. Am. J. Respir. Crit. Care Med. 2001;163:277–279. doi: 10.1164/ajrccm.163.1.2006004. [DOI] [PubMed] [Google Scholar]
- 50.Poole J.A., Barriga K., Leung D.Y., Hoffman M., Eisenbarth G.S., Rewers M., Norris J.M. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006;117:2175–2182. doi: 10.1542/peds.2005-1803. [DOI] [PubMed] [Google Scholar]
- 51.Rastogi D., Canfield S.M., Andrade A., Isasi C.R., Hall C.B., Rubinstein A., Arens R. Obesity-associated asthma in children: A distinct entity. Chest. 2012;141:895–905. doi: 10.1378/chest.11-0930. [DOI] [PubMed] [Google Scholar]
- 52.Rastogi D., Holguin F. Metabolic Dysregulation, Systemic Inflammation, and Pediatric Obesity-related Asthma. Ann. Am. Thorac. Soc. 2017;14:S363–S367. doi: 10.1513/AnnalsATS.201703-231AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available from the corresponding author upon reasonable request. The datasets derived during this study are included in this published article (and its supplementary information files).