Abstract
This study investigated the postprandial plasma metabolome following consumption of four dairy matrices different in texture and structure: cheddar cheese (Cheese), homogenized cheddar cheese (Hom. Cheese), and micellar casein isolate (MCI) with cream (MCI Drink) or a MCI Gel. An acute, randomized, crossover trial in male participants (n = 25) with four test days was conducted. Blood samples were collected during an 8-h postprandial period after consumption of a meal similar in micro- and macronutrients containing one of the four dairy matrices, and the metabolome was analyzed using nuclear magnetic resonance (NMR) spectroscopy. A liquid dairy matrix (MCI Drink) resulted in a faster absorption of amino acids compared to products, representing either a semi-solid (MCI Gel and Hom. Cheese) or solid (Cheese) dairy matrix. For the MCI Gel, plasma concentration of acetic acid and formic acid increased approximately 2 h following consumption, while 3-hydroxybyturate and acetoacetic acid increased approximately 6 h after consumption. The structure and texture of the dairy matrix affected the postprandial absorption of amino acids, as revealed by the plasma metabolome. Our study furthermore pointed at endogenous effects associated with consumption of dairy products containing glucono-δ-lactone.
Keywords: amino acid absorption, food structure, glucono-δ-lactone, NMR metabolomics, cheese
1. Introduction
Dairy products can contain high amounts of saturated fats, which, traditionally, have been associated with increased risk of cardiovascular disease (CVD) [1]; however, studies have indicated that a potentially harmful impact of saturated fatty acids on plasma lipids depends on the type of dairy product consumed, and the association between dairy product consumption may even be neutral or protective [2,3,4].
The complexity between type of dairy product consumed and impact on CVD indicates an effect of the food matrix. Evidence is increasing that the nutritional properties of a food is not only determined by its single nutrients, but also by the complex food structure in which the nutrients are embedded [5,6]. Thus, studies indicate that the food matrix can affect digestion and absorption of the nutrients in a given food [2,7]. In particular, the dairy matrix, comprising both solid matrices (cheese), semi-solid matrices (yoghurt, crème fraiche), and liquids (milk, cream), receives attention [8]. In vitro and in vivo studies have indicated that the physical structure and processing of dairy products may affect bioavailability of nutrients [8]. In vitro studies have shown that the texture and composition of cheeses affect their disintegration upon digestion [9,10]. A low initial hardness, cohesiveness, and chewiness of the cheeses was associated with a higher disintegration at the end of gastric digestion [9]. Lamothe et al. (2017) investigated the release of nutrients in a simulated gastrointestinal environment from three structurally different dairy matrices: milk, yoghurt, and cheese [11]. The results showed that the cheeses were more resistant to protein and lipid digestion than milk and yoghurt. Barbé et al. (2013) investigated protein digestion and amino acid (AA) absorption in six mini pigs consuming four different dairy matrices: skimmed milk and rennet gels, either heated or unheated [12]. They found that heat treatment increased the retention time in the stomach and that gelation delayed gastric emptying. Further, analysis of postprandial plasma samples showed that gelation resulted in delayed and lower AA absorption in the postprandial phase [12]. A recent human study investigated the postprandial blood AA concentration after consumption of different dairy matrices [13]. Here, a higher postprandial blood concentration of essential AA (EAA) was observed after consumption of yoghurt compared to milk and cheese. In addition, higher fat content resulted in a slower protein digestion [13]. Thus, several studies have indicated that the nature of the dairy matrix affects digestion and nutrient absorption; however, human studies investigating the impact of the dairy matrix on AA absorption and postprandial responses are sparse. Therefore, during an acute, randomized, crossover human trial, the present study investigated the effect of consuming four different dairy matrices on blood plasma metabolites during an 8-h postprandial period using 1H nuclear magnetic resonance (1H NMR) spectroscopy. The four experimental dairy matrices, a cheddar cheese (Cheese), a homogenized cheddar cheese (Hom. Cheese), micellar casein isolate with cream (MCI Drink), or a gel made from the MCI Drink (MCI Gel), differed in texture and structure and were served as meals in which the nutrient compositions were similar. This study is part of the interdisciplinary DAIRYMAT project. Detailed description of the study design and primary outcomes [14], a thorough characterization of the experimental dairy products [15], and the effect of the experimental diets on postprandial phospholipids [16] have previously been published.
2. Materials and Methods
2.1. Study Design and Participants
A total of 25 men aged 19–40 years with a body mass index (BMI) between 20.0 and 24.5 kg/m2 were enrolled for this acute, randomized, crossover trial, previously described in detail in Kjølbæk et al., 2021 [14]. The study was conducted from September 2018 to March 2019 at the Department of Nutrition, Exercise, and Sports, University of Copenhagen, Denmark, and consisted of four test days. Use, or use within the previous 3 months, of prescription medication, except non-prescription primary analgesics (non-steroidal anti-inflammatory drugs and paracetamol) and glucocorticoids for topical use, was not allowed. A total of 21 participants completed a minimum of two test days and were included in the analyses, and 18 participants completed all four test days. The participants consumed a standardized meal before 22:00 in the evening prior to the test days. In addition, alcohol, medication, and moderate-to-vigorous physical activities were to be avoided 48 h prior to the test days. Upon arrival at each test day, the participants were weighed and fasting blood samples were collected. Subsequently, one of four iso-energetic test meals and 1.5 g paracetamol was consumed over 15 min. The test meal consisted of one of the dairy matrices, bread and water. During the following 8 h, blood samples were collected at 30, 60, 90, 120, 180, 240, 300, 360, 420, and 480 min. In order to obtain plasma, blood samples were transferred to heparin tubes and centrifuged at 2500 g for 10 min at 4 °C. Plasma samples were stored at −80 °C until analysis. The study was registered at clinicaltrial.gov (NCT03656367) (accessed on 4 September, 2018).
2.2. Test Meals
At each test day, the participants consumed one of four dairy matrices served with bread and water [14]. The four dairy matrices were (I) a commercial full-fat cheddar cheese (Cheese) (Arla Foods Ltd., Leeds, UK), (II) a homogenized cheddar cheese (Hom. Cheese), (III) a micellar casein isolate (MCI) with cream and salt (MCI Drink), and (IV) a gel made from the MCI Drink by glucono-δ-lactone addition (GDL) (MCI Gel), as described by Schmidt et al. (2020) [15]. The meals were similar in micro and macronutrient composition, but the dairy matrices differed in texture and structure. Since the dairy matrices differed in carbohydrate and salt content, lactose, potato starch, and salt were added to the bread to adjust for these differences to obtain meals that contained 54 g protein, 72 g carbohydrate, 68 g of fat, and 1.53–1.57 g sodium chloride.
2.3. NMR Spectroscopy
Plasma samples were thawed, and 350 µL plasma was mixed with 350 µL phosphate buffer in D2O (pH 7.4). A volume of 600 µL was subsequently transferred to a 5-mm NMR tube. NMR spectroscopy was conducted using a Bruker Avance IVDr 600 MHz spectrometer, operating at a frequency of 600.13 MHz (Bruker BioSpin, Rheinstetten, Germany). The NMR spectrometer was equipped with a 5 mm 1H TXI probe and a Bruker SampleJet set to 4 °C. An in vitro diagnostics research (IVDr) tool (Avance IVDr, IVDr methods, User manual, version 3, Bruker Corporation, Schwitzerland) was used to analyze the samples. Four experiments were applied in automation for each sample: 1D Nuclear Overhauser Effect Spectroscopy (NOESY), 2D J-resolved experiment (JRES), Carr−Purcell− Meiboom−Gill (CPMG), and difference spectroscopy (DIFF). The CPMG spectra were used for metabolite quantification using the following parameters: dummy scans: 4; number of scans: 32; relaxation delay: 4 s; acquisition time: 3.10 s; spectral width: 19.84 ppm. Plasma metabolites were automatically quantified by B.I.Quant-PS (version 2.0, Bruker BioSpin, Rheinstetten, Germany). In total, 41 plasma metabolites were detected and quantified. The quality of the quantification for the individual metabolites was evaluated, and as a result, 22 metabolites were included in the data analysis. Detection of 22 metabolites in human plasma using the Bruker IVDR platform is consistent with a former ring trial, where quantification of 24 metabolites were reported [17]. Data on analytical variance and reproducibility for the applied NMR IVDr SOP obtained can be found elsewhere [17].
2.4. Statistics
Data were analyzed using mixed effect models to analyze the effects of meal, time, and meal-time interactions. Meal and time were treated as fixed factors and the participant as the random factor. False discovery rate (FDR) adjustment using the Benjamini and Hochberg approach [18] was conducted in order to control for multiple testing. FDR-adjusted p-values are denominated q-values. If significant meal-time interactions were found (q-value < 0.05), Tukey’s multiple comparison test was used to identify time points with significant differences between meals. p-values < 0.05 were considered significant. Data is shown as mean ± SEM. GraphPad Prism (Version 9) was used for statistical analyses and graphs.
3. Results
A total of 21 participants completed a minimum of two test days and were included in the analyses, and 18 participants completed all four test days. Due to the lack of sufficient sample material, a total of 851 plasma samples were analyzed using NMR spectroscopy, and data analysis was performed on a total of 22 plasma metabolites. For all of the analyzed metabolites, except formic acid, a significant effect of time was found (q < 0.05).
3.1. Amino Acid Concentrations in Plasma
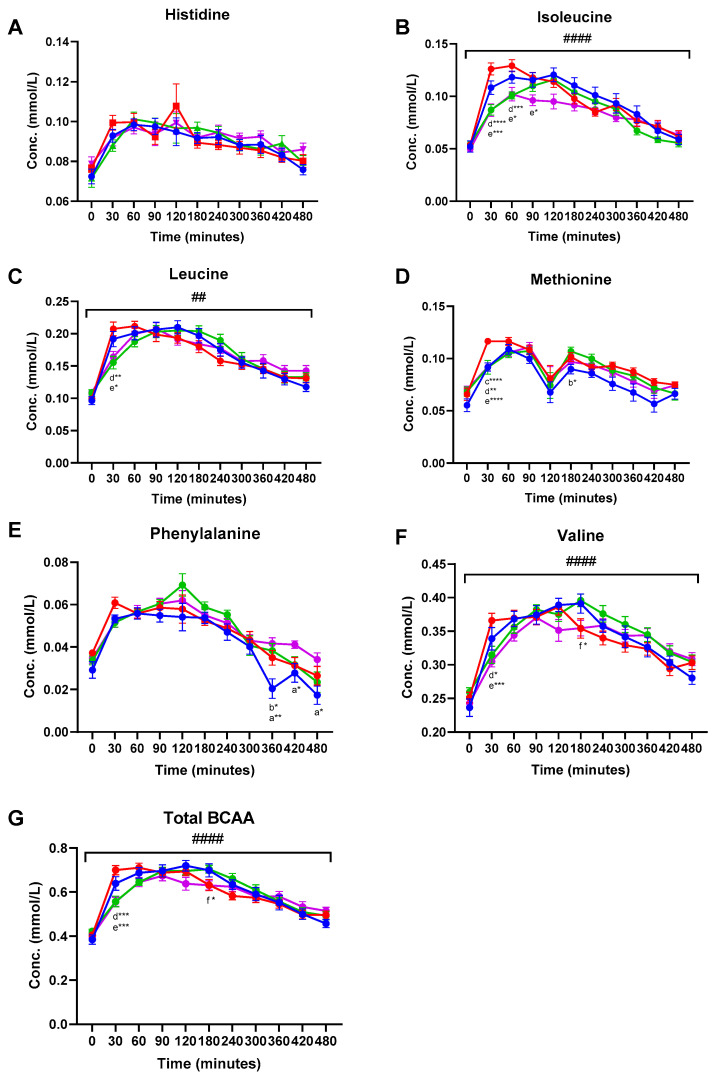
Amino acids (AA) detected and included in the data analysis were alanine, glutamine, glycine, histidine, leucine, isoleucine, methionine, phenylalanine, tyrosine, and valine (Supplementary Material, Table S1). Plasma concentrations of EAA and total branched chain AA (BCAA) are shown in Figure 1. Significant meal-time interactions were found for alanine (q < 0.001), leucine (q = 0.001), isoleucine (q < 0.001), tyrosine (q < 0.001), valine (q < 0.001), and total BCAA (q < 0.001). A near-significant meal-time interaction was found for phenylalanine (q = 0.05). Significant meal effects were observed for isoleucine (q = 0.041), methionine (q = 0.041), and phenylalanine (q = 0.041) (Figure 1 and Table S1). Tukey’s multiple comparisons test was used to identify significant differences between treatment groups at specific time points during the postprandial period. In general, consumption of the MCI Drink resulted in the highest total plasma AA concentration 30 min post-consumption. Hence, significantly higher plasma concentration of leucine, isoleucine, methionine, tyrosine, valine, and total BCAA was found 30 min following consumption of the MCI Drink compared to Cheese and Hom. Cheese (Figure 1 and Table S1).
Figure 1.
Postprandial plasma concentrations (mean ± SEM) of essential amino acids: (A) Histidine, (B) isoleucine, (C) leucine, (D) methionine, (E) phenylalanine, (F) valine, and (G) total branched chain amino acids. ● MCI Drink, ● MCI Gel, ● Hom. Cheese, ● Cheese. # indicate significant meal-time interactions: # (q < 0.05), ## (q < 0.005), ### (q < 0.0005) #### (q < 0.0001). Letters indicate significant differences in post-hoc tests: a MCI gel vs. Cheese, b MCI gel vs. Hom. Cheese, c MCI gel vs. MCI drink, d MCI drink vs. Hom. Cheese, e MCI drink vs. Cheese, f Hom. Cheese vs. Cheese. Asterisks indicate level of significance: * (p < 0.05), ** (p < 0.005), *** (p < 0.0005) **** (p < 0.0001).
Among the remaining analyzed metabolites, significant meal effects were found for acetic acid (q < 0.001) and formic acid (q = 0.041). Significant meal-time interactions were found for acetic acid (q < 0.001), acetoacetic acid (q < 0.001), 3-hydroxybutyric acid (q = 0.037), and pyruvic acid (q < 0.001) (Table 1). From 120 min following consumption of the MCI Gel, an increasing plasma concentration of acetic acid was observed. From 180 to 420 min, significantly higher acetic acid concentrations in plasma were observed after consumption of MCI Gel compared to all of the other three products (Table 1). For formic acid, increased plasma concentrations were also observed from 180 min after MCI Gel consumption compared to the other products, although only reaching significance at 180 min compared to the MCI Drink, at 240 min compared to Hom. Cheese and Cheese, at 360 min compared to MCI Drink, at 420 min compared to MCI Drink and Hom. Cheese, and at 480 min compared to Hom. Cheese and Cheese (Table 1).
Table 1.
Mean concentrations of plasma metabolites. q-values indicate false discovery rate (FDR)-adjusted p-values from mixed effect models for meal-time interactions (qmeal-time), meal effect (qmeal), and time effect (qtime). Letters indicate significant differences in post-hoc tests at a given time point within each metabolite section.
| Time (min) | 0 | 30 | 60 | 90 | 120 | 180 | 240 | 300 | 360 | 420 | 480 | qmeal-time | qmeal | qtime |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | ||||||||||||||
| Cheese | 0.070 | 0.063 | 0.036 | 0.029 | 0.030 ab | 0.025 a | 0.018 a | 0.030 a | 0.038 a | 0.036 a | 0.027 a | <0.001 | <0.001 | <0.001 |
| Hom. Cheese | 0.057 | 0.046 | 0.029 | 0.017 | 0.023 a | 0.026 a | 0.026 a | 0.034 a | 0.032 a | 0.033 a | 0.034 ab | |||
| MCI Drink | 0.057 | 0.044 | 0.024 | 0.027 | 0.033 ab | 0.023 a | 0.020 a | 0.028 a | 0.029 a | 0.031 a | 0.027 a | |||
| MCI Gel | 0.066 | 0.050 | 0.037 | 0.032 | 0.050 b | 0.056 b | 0.073 b | 0.084 b | 0.095 b | 0.066 b | 0.057 b | |||
| Acetoacetic acid | ||||||||||||||
| Cheese | 0.070 | 0.063 | 0.036 | 0.029 | 0.030 | 0.025 | 0.018 | 0.030 | 0.038 ab | 0.036 | 0.027 | <0.001 | 0.831 | 0.003 |
| Hom. Cheese | 0.057 | 0.046 | 0.029 | 0.017 | 0.023 | 0.026 | 0.026 | 0.034 | 0.032 ac | 0.033 | 0.034 | |||
| MCI Drink | 0.057 | 0.044 | 0.024 | 0.027 | 0.033 | 0.023 | 0.020 | 0.028 | 0.029 c | 0.031 | 0.027 | |||
| MCI Gel | 0.066 | 0.050 | 0.037 | 0.032 | 0.050 | 0.056 | 0.073 | 0.084 | 0.095 b | 0.066 | 0.057 | |||
| Acetone | ||||||||||||||
| Cheese | 0.035 | 0.035 | 0.033 | 0.037 | 0.036 | 0.039 | 0.036 | 0.031 | 0.032 | 0.035 | 0.036 | 0.112 | 0.992 | 0.001 |
| Hom. Cheese | 0.035 | 0.033 | 0.038 | 0.036 | 0.034 | 0.038 | 0.031 | 0.032 | 0.031 | 0.035 | 0.032 | |||
| MCI Drink | 0.032 | 0.044 | 0.039 | 0.042 | 0.036 | 0.042 | 0.035 | 0.037 | 0.035 | 0.035 | 0.032 | |||
| MCI Gel | 0.024 | 0.032 | 0.037 | 0.037 | 0.030 | 0.039 | 0.035 | 0.030 | 0.036 | 0.034 | 0.038 | |||
| Citric acid | ||||||||||||||
| Cheese | 0.148 | 0.157 | 0.156 | 0.151 | 0.142 | 0.139 | 0.153 | 0.148 | 0.145 | 0.147 | 0.162 | 0.061 | 0.992 | <0.001 |
| Hom. Cheese | 0.143 | 0.157 | 0.167 | 0.161 | 0.126 | 0.150 | 0.141 | 0.139 | 0.139 | 0.162 | 0.150 | |||
| MCI Drink | 0.142 | 0.161 | 0.149 | 0.141 | 0.149 | 0.137 | 0.156 | 0.141 | 0.141 | 0.153 | 0.158 | |||
| MCI Gel | 0.130 | 0.166 | 0.157 | 0.148 | 0.127 | 0.150 | 0.148 | 0.158 | 0.148 | 0.165 | 0.168 | |||
| Creatine | ||||||||||||||
| Cheese | 0.008 | 0.013 | 0.011 | 0.010 | 0.007 | 0.007 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.963 | 0.992 | <0.001 |
| Hom. Cheese | 0.007 | 0.014 | 0.010 | 0.012 | 0.005 | 0.009 | 0.007 | 0.008 | 0.008 | 0.006 | 0.006 | |||
| MCI Drink | 0.010 | 0.014 | 0.012 | 0.011 | 0.006 | 0.006 | 0.007 | 0.005 | 0.005 | 0.006 | 0.003 | |||
| MCI Gel | 0.007 | 0.013 | 0.012 | 0.011 | 0.009 | 0.008 | 0.008 | 0.005 | 0.007 | 0.006 | 0.006 | |||
| Creatinine | ||||||||||||||
| Cheese | 0.098 | 0.107 | 0.103 | 0.105 | 0.104 | 0.099 | 0.099 | 0.094 | 0.095 | 0.094 | 0.094 | 0.691 | 0.992 | <0.001 |
| Hom. Cheese | 0.098 | 0.106 | 0.111 | 0.101 | 0.104 | 0.102 | 0.096 | 0.095 | 0.094 | 0.095 | 0.093 | |||
| MCI Drink | 0.099 | 0.109 | 0.105 | 0.102 | 0.103 | 0.097 | 0.096 | 0.094 | 0.095 | 0.093 | 0.093 | |||
| MCI Gel | 0.092 | 0.109 | 0.110 | 0.102 | 0.107 | 0.098 | 0.096 | 0.094 | 0.089 | 0.094 | 0.090 | |||
| Dimethylsulfone | ||||||||||||||
| Cheese | 0.008 | 0.007 | 0.009 | 0.010 | 0.007 | 0.008 | 0.008 | 0.007 | 0.007 | 0.007 | 0.007 | 0.649 | 0.992 | 0.043 |
| Hom. Cheese | 0.008 | 0.010 | 0.008 | 0.009 | 0.007 | 0.009 | 0.008 | 0.008 | 0.010 | 0.008 | 0.008 | |||
| MCI Drink | 0.010 | 0.011 | 0.009 | 0.006 | 0.004 | 0.007 | 0.008 | 0.009 | 0.007 | 0.009 | 0.009 | |||
| MCI Gel | 0.006 | 0.007 | 0.007 | 0.005 | 0.004 | 0.006 | 0.007 | 0.006 | 0.008 | 0.007 | 0.008 | |||
| Formic acid | ||||||||||||||
| Cheese | 0.020 | 0.021 | 0.024 | 0.024 | 0.015 | 0.019 ab | 0.023 a | 0.021 | 0.021 ab | 0.022 ab | 0.014 a | 0.212 | 0.041 | 0.061 |
| Hom. Cheese | 0.019 | 0.026 | 0.024 | 0.021 | 0.015 | 0.020 ab | 0.021 a | 0.022 | 0.019 ab | 0.021 a | 0.019 a | |||
| MCI Drink | 0.022 | 0.022 | 0.019 | 0.026 | 0.018 | 0.016 a | 0.022 ab | 0.018 | 0.015 a | 0.020 a | 0.021 ab | |||
| MCI Gel | 0.024 | 0.021 | 0.024 | 0.021 | 0.021 | 0.027 b | 0.030 b | 0.028 | 0.028 b | 0.030 b | 0.029 b | |||
| Glucose | ||||||||||||||
| Cheese | 5.533 | 6.514 a | 5.191 | 5.322 | 5.322 ab | 5.408 | 5.140 | 5.398 | 5.480 | 5.432 | 5.337 ab | <0.001 | 0.992 | <0.001 |
| Hom. Cheese | 5.554 | 6.338 ac | 5.270 | 5.103 | 5.123 a | 5.296 | 5.279 | 5.270 | 5.376 | 5.349 | 5.265 a | |||
| MCI Drink | 5.808 | 5.500 b | 4.629 | 5.355 | 5.830 b | 5.564 | 5.411 | 5.518 | 5.428 | 5.462 | 5.465 b | |||
| MCI Gel | 5.427 | 5.476 bc | 4.898 | 5.137 | 5.591 ab | 5.541 | 5.507 | 5.463 | 5.344 | 5.368 | 5.304 ab | |||
| 3-hydroxybutyric acid | ||||||||||||||
| Cheese | 0.088 | 0.073 | 0.046 | 0.040 | 0.042 | 0.058 | 0.053 | 0.078 | 0.098 ab | 0.113 | 0.139 | 0.037 | 0.992 | <0.001 |
| Hom. Cheese | 0.136 | 0.075 | 0.047 | 0.043 | 0.042 | 0.060 | 0.073 | 0.072 | 0.082 ab | 0.129 | 0.120 | |||
| MCI Drink | 0.074 | 0.096 | 0.061 | 0.056 | 0.062 | 0.052 | 0.049 | 0.050 | 0.059 a | 0.085 | 0.084 | |||
| MCI Gel | 0.074 | 0.094 | 0.059 | 0.042 | 0.049 | 0.063 | 0.080 | 0.079 | 0.121 b | 0.109 | 0.139 | |||
| Lactic acid | ||||||||||||||
| Cheese | 0.771 | 0.796 | 1.048 a | 1.067 a | 0.955 | 0.832 | 0.841 | 0.701 | 0.691 | 0.695 | 0.670 | <0.001 | 0.438 | <0.001 |
| Hom. Cheese | 0.807 | 0.828 | 1.100 a | 1.110 a | 1.023 | 0.850 | 0.797 | 0.685 | 0.678 | 0.717 | 0.724 | |||
| MCI Drink | 0.835 | 0.737 | 0.807 b | 0.800 b | 0.884 | 0.826 | 0.854 | 0.720 | 0.690 | 0.634 | 0.643 | |||
| MCI Gel | 0.778 | 0.740 | 0.831 b | 0.900 ab | 0.846 | 0.773 | 0.678 | 0.697 | 0.626 | 0.680 | 0.773 | |||
| Pyruvic acid | ||||||||||||||
| Cheese | 0.061 | 0.064 | 0.100 a | 0.103 a | 0.078 | 0.073 | 0.062 | 0.057 | 0.053 | 0.049 | 0.044 | <0.001 | 0.992 | <0.001 |
| Hom. Cheese | 0.062 | 0.068 | 0.091 ab | 0.103 a | 0.076 | 0.075 | 0.064 | 0.057 | 0.046 | 0.048 | 0.043 | |||
| MCI Drink | 0.068 | 0.066 | 0.075 ab | 0.074 b | 0.073 | 0.076 | 0.073 | 0.065 | 0.054 | 0.045 | 0.045 | |||
| MCI Gel | 0.064 | 0.056 | 0.071 b | 0.083 ab | 0.065 | 0.070 | 0.058 | 0.052 | 0.047 | 0.046 | 0.049 | |||
3.2. Plasma Metabolites
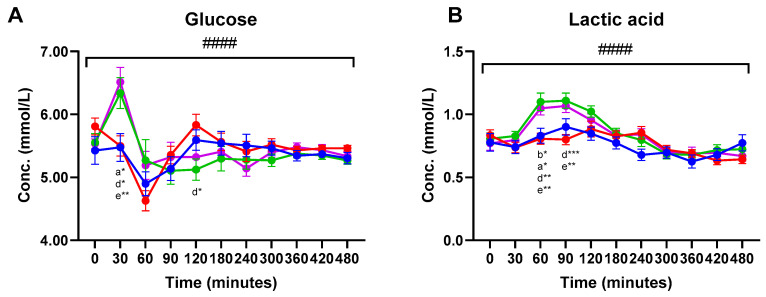
Significant meal-time interactions were found for postprandial plasma concentrations of glucose and lactic acid (q < 0.0001) (Figure 2). For both glucose and lactic acid, increased plasma concentrations were observed within 30 min after consumption of Cheese and Hom. Cheese compared to the two MCI products. Thus, Cheese consumption significantly increased glucose plasma concentrations at 30 min compared to the MCI Drink and MCI Gel. The Hom. Cheese significantly increased glucose concentrations at 30 min compared to the MCI Drink. A near-significant higher glucose concentration was observed 30 min after consumption of Hom. Cheese compared to the MCI Gel (p = 0.060). For lactic acid, Cheese and Hom. Cheese significantly increased plasma concentrations 60 min following consumption compared to the two MCI products. At 90 min, lactic acid concentrations were significantly increased after consumption of Cheese and Hom. Cheese compared to the MCI Drink (Figure 2).
Figure 2.
Postprandial plasma concentrations (mean ± SEM) of (A) glucose and (B) lactic acid. ● MCI Drink, ● MCI Gel, ● Hom. Cheese, ● Cheese. # indicate significant meal-time interactions: #### (q <0.0001). Letters indicate significant differences in post-hoc tests: a MCI gel vs. Cheese, b MCI gel vs. Hom. Cheese, c MCI gel vs. MCI drink, d MCI drink vs. Hom. Cheese, e MCI drink vs. Cheese, f Hom. Cheese vs. Cheese. Asterisks indicate level of significance: * (p <0.05), ** (p <0.005), *** (p <0.0005)). For 3-hydroxybutyric acid, a significantly higher plasma concentration was found at 360 min following consumption of the MCI Gel compared to the MCI Drink (Table 1). For acetoacetic acid, significant differences were found at 360 min. Thus, significantly higher plasma concentrations of acetoacetic acid were found after consumption of MCI Gel compared to the MCI Drink and Hom. Cheese, and a significantly higher concentration was found after Cheese consumption compared to the MCI Drink. For pyruvic acid, significant differences were found after 60 and 90 min. At 60 min, Cheese consumption resulted in significantly higher plasma pyruvic acid concentrations compared to MCI Gel and near-significant differences were higher compared to the MCI Drink (p = 0.069). After 90 min, significantly higher concentrations were found after consumption of Cheese and Hom. Cheese compared to the MCI Drink.
4. Discussion
The present study investigated the effect of four different dairy matrices, different in texture and structure, and consumed within a meal similar in micro- and macronutrients, on the plasma metabolome. In general, the MCI Drink resulted in the highest plasma concentration of AAs 30 min after consumption, indicating a faster absorption of AAs after consumption of the liquid food matrix. Thus, significantly higher plasma concentrations of isoleucine, leucine, valine, methionine, tyrosine, and total BCAA were found 30 min following consumption of MCI Drink compared to Cheese and Hom. Cheese. When comparing the MCI Drink and MCI Gel, a significantly higher concentration after consumption of the MCI Drink was only reached for methionine after 30 min. Horstman et al. (2021) previously found that consumption of yoghurt resulted in a higher postprandial blood concentration of EAA compared to milk and cheese [13]. The influence of the dairy matrix on postprandial AA absorption might be associated with the accessibility of proteins to digestion enzymes, which has previously been correlated to protein digestion kinetics in cheese [11]. Previous in vitro studies found that disintegration upon digestion was affected by texture and composition of the dairy matrix [9,10,11]. For cheeses, disintegration of the food matrix is necessary in order for pepsin to be able to access the proteins, and Lamothe et al. (2017) found that, in contrast to milk and yoghurt, which were disintegrated from the beginning of the digestion, cheese was gradually disintegrated during digestion [11]. The faster AA absorption after the MCI Drink and MCI Gel consumption might also be related to the gastric emptying rate, since previous studies have indicated that dairy matrices with increased viscosity decrease the rate of gastric emptying [12,19,20]. The clinical impact of the observed differences in AA absorption rate among the dairy products remains to be further elucidated. Although AA availability, in particular BCAA, is expected to be of importance for postprandial muscle protein synthesis, the exact threshold in plasma concentration remains to be established. A recent study found no significant differences in milk and insect (lesser mealworm) protein sources’ potential to stimulate muscle protein synthesis, even though postprandial plasma concentration of leucine was markedly and significantly higher after ingestion of milk protein compared with insect protein [21]. Of other clinical effects, it could be speculated that higher postprandial blood concentration of BCAA could stimulate secretion of insulin; however, insulin analyses did not reveal a higher insulin level at 30–60 min postprandial for the MCT Drink [14].
Increased plasma concentrations of glucose and lactic acid were observed 30 min and 60–90 min following consumption of the Cheese and Hom. Cheese, respectively, compared to the MCI products. The increased lactic acid concentrations in plasma following consumption of the cheese products compared to the MCI products likely reflects a higher lactic acid content in the cheese products. Similarly, Cheese and Hom. Cheese increased pyruvic acid concentration 60–90 min following consumption. This increase might derive from pyruvic acid formed during fermentation and thus may be contained within the cheese products.
Since the cheese products did not contain lactose, lactose was added to the bread served with the products in order to adjust for these differences [14]. The lactose consumed through the bread might have been more accessible and more easily degraded compared to the lactose within the MCI products. Hence, this might account for the differences seen in the glucose response between the cheese products and the MCI products.
Intriguingly, from 120 min following consumption of the MCI Gel, a pronounced increase in plasma acetic acid was found. Similarly, but less pronounced, the concentration of formic acid increased from 180 min following consumption of the MCI Gel. The observed increase in these two carboxylic acids likely originates from endogenous formation following consumption of the MCI Gel. In contrast to the three other dairy matrices, GDL was added (3 g/100 g) in order to make the gelled product. GDL hydrolyses to form gluconic acid and lowers the pH [22]. Tsukahara et al. (2002) previously found that gluconic acid stimulated the bacterial formation of short-chain fatty acids, including acetate, in pig cecal digesta in vitro [23]. In addition, a previous study found that ingestion of gluconic acid increased the presence of fecal Bifidobacteria in humans [24]. Thus, the increased acetic acid concentrations in plasma might be caused by fermentation in the intestine following consumption of the MCI Gel. Formate can be formed as a byproduct from the production of acetate [25], which might explain the increase in formic acid concurrently with increased acetic acid concentrations. Later in the postprandial course (360 min), intake of the MCI Gel was associated with a significantly higher level of plasma 3-hydroxybutyrate and acetoacetic acid. The ketone bodies, 3-hydroxybutyrate and acetoacetic acid, are synthesized in the liver from short-chain fatty acids [26], and the observed increase in ketone bodies is thus most likely linked with increased serum acetate. This observation furthermore corresponds well with the in vitro digestion of the dairy matrices, where the MCI Gel resulted in the overall highest release of free fatty acids [15].
In conclusion, the present study demonstrated that intake of a liquid dairy matrix (MCI Drink) resulted in a faster absorption of AAs compared to products representing either a semi-solid (MCI Gel or Hom. Cheese) or solid (Cheese) dairy matrix. In addition, approximately 2 h following consumption of the MCI Gel, plasma concentration of acetic acid and formic acid increased. This might result from fermentation of gluconic acid originating from the GDL used to form the gelled product. Hence, the dairy matrix affected the concentration of plasma metabolites during an 8-h postprandial period. While postprandial differences in circulating metabolites can likely affect, e.g., satiety regulation, further studies are warranted to elucidate associations between the postprandial metabolome and endogenous responses.
Acknowledgments
The authors thank Kristian Wejse Sanggaard, Arla Food Ingredients, Emilien Rouy, Mette Bakman, and Arla Foods Amba, for their participation in fruitful discussions of the project. We also thank the participants, as well as the staff (kitchen staff, laboratory technicians, and master thesis students), involved in the trial at the Department of Nutrition, Exercise, and Sports, University of Copenhagen. Data was generated through accessing the research infrastructure at Aarhus University, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13124280/s1, Table S1: Mean concentrations of plasma amino acids.
Author Contributions
Conceptualization, L.K., K.J.J., A.A., M.H., A.R. and H.C.B.; methodology, R.T., K.L.E. and H.C.B.; software, R.T. and K.L.E.; formal analysis, R.T. and K.L.E.; investigation, L.K. and A.R.; resources, M.H. and H.C.B.; data curation, R.T. and K.L.E.; writing—original draft preparation, R.T. and H.C.B.; writing—review and editing, R.T., H.C.B., K.L.E., L.K., K.J.J., A.A., M.H. and A.R.; project administration, H.C.B., M.H. and A.R.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was part of the DAIRYMAT project funded by Arla Food for Health. The intervention products were produced by Arla Innovation Center, Denmark.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the regional committee on biomedical research in Denmark (H-18024447). The study was registered at clinicaltrial.gov (NCT03656367).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study. All personal data were handled confidentially and stored in accordance with applicable law, with respect to the General Data Protection Regulation and Danish Data Protection Act. No. 502 of 23 May 2018.
Data Availability Statement
Pseudo-anonymized data can be made available upon request before 2029 via a data sharing contract. From 2029, fully anonymized data can be transferred.
Conflicts of Interest
R.T., K.L.E., L.K., M.H., A.R. and H.C.B. declare no conflict of interest. K.J.J. is employed by Arla Foods Amba, Denmark. AA is currently employed by the Novo Nordisk Foundation to establish a National Centre for Healthy Weight in Denmark. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lovegrove J.A. Dietary dilemmas over fats and cardiometabolic risk. Proc. Nutr. Soc. 2020;79:11–21. doi: 10.1017/S0029665119000983. [DOI] [PubMed] [Google Scholar]
- 2.Hjerpsted J., Leedo E., Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am. J. Clin. Nutr. 2011;94:1479–1484. doi: 10.3945/ajcn.111.022426. [DOI] [PubMed] [Google Scholar]
- 3.Feeney E.L., Barron R., Dible V., Hamilton Z., Power Y., Tanner L., Flynn C., Bouchier P., Beresford T., Noronha N., et al. Dairy matrix effects: Response to consumption of dairy fat differs when eaten within the cheese matrix-a randomized controlled trial. Am. J. Clin. Nutr. 2018;108:667–674. doi: 10.1093/ajcn/nqy146. [DOI] [PubMed] [Google Scholar]
- 4.Drouin-Chartier J.-P., Tremblay A., Maltais-Giguère J., Charest A., Guinot L., Rioux L.-E., Labrie S., Britten M., Lamarche B., Turgeon S., et al. Differential impact of the cheese matrix on the postprandial lipid response: A randomized, crossover, controlled trial. Am. J. Clin. Nutr. 2017;106:1358–1365. doi: 10.3945/ajcn.117.165027. [DOI] [PubMed] [Google Scholar]
- 5.Thorning T.K., Bertram H.C., Bonjour J.-P., de Groot L., Dupont D., Feeney E., Ipsen R., Lecerf J.M., Mackie A., McKinley M.C., et al. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017;105:1033–1045. doi: 10.3945/ajcn.116.151548. [DOI] [PubMed] [Google Scholar]
- 6.Parada J.J.M. Aguilera, Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007;72:R21–R32. doi: 10.1111/j.1750-3841.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 7.Grundy M.M., Grassby T., Mandalari G., Waldron K.W., Butterworth P.J., Berry S.E., Ellis P.R. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015;101:25–33. doi: 10.3945/ajcn.114.088328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fardet A., Dupont D., Rioux L.E., Turgeon S.L. Influence of food structure on dairy protein, lipid and calcium bioavailability: A narrative review of evidence. Crit. Rev. Food Sci. Nutr. 2019;59:1987–2010. doi: 10.1080/10408398.2018.1435503. [DOI] [PubMed] [Google Scholar]
- 9.Fang X., Rioux L.-E., Labrie S., Turgeon S.L. Commercial cheeses with different texture have different disintegration and protein/peptide release rates during simulated in vitro digestion. Int. Dairy J. 2016;56:169–178. doi: 10.1016/j.idairyj.2016.01.023. [DOI] [Google Scholar]
- 10.Fang X., Rioux L.-E., Labrie S., Turgeon S.L. Disintegration and nutrients release from cheese with different textural properties during in vitro digestion. Food Res. Int. 2016;88:276–283. doi: 10.1016/j.foodres.2016.04.008. [DOI] [Google Scholar]
- 11.Lamothe S., Rémillard N., Tremblay J., Britten M. Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment. Food Res. Int. 2017;92:138–146. doi: 10.1016/j.foodres.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Barbé F., Ménard O., Le Gouar Y., Buffière C., Famelart M.-H., Laroche B., Le Feunteun S., Dupont D., Rémond D. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem. 2013;136:1203–1212. doi: 10.1016/j.foodchem.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Horstman A.M.H., Ganzevles R.A., Kudla U., Kardinaal A.F.M., van den Borne J.J.G.C., Huppertz T. Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix. Int. Dairy J. 2021;113:104890. doi: 10.1016/j.idairyj.2020.104890. [DOI] [Google Scholar]
- 14.Kjølbæk L., Schmidt J.M., Rouy E., Jensen K.J., Astrup A., Bertram H.C., Hammershøj M., Raben A. Matrix structure of dairy products results in different postprandial lipid responses: A randomized crossover trial. Am. J. Clin. Nutr. 2021;114:1729–1742. doi: 10.1093/ajcn/nqab220. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt J.M., Kjølbæk L., Jensen K.J., Rouy E., Bertram H.C., Larsen T., Raben A., Astrup A., Hammershøj M. Influence of type of dairy matrix micro- and macrostructure on in vitro lipid digestion. Food Funct. 2020;11:4960–4972. doi: 10.1039/D0FO00785D. [DOI] [PubMed] [Google Scholar]
- 16.Thøgersen R., Lindahl I.E.I., Khakimov B., Kjølbæk L., Juhl Jensen K., Astrup A., Hammershøj M., Raben A., Bertram H.C. Progression of Postprandial Blood Plasma Phospholipids Following Acute Intake of Different Dairy Matrices: A Randomized Crossover Trial. Metabolites. 2021;11:454. doi: 10.3390/metabo11070454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiménez B., Holmes E., Heude C., Tolson R.F., Harvey N., Lodge S.L., Chetwynd A.J., Cannet C., Fang F., Pearce J.T.M., et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018;90:11962–11971. doi: 10.1021/acs.analchem.8b02412. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 19.Sanggaard K.M., Holst J.J., Rehfeld J.F., Sandström B., Raben A., Tholstrup T. Different effects of whole milk and a fermented milk with the same fat and lactose content on gastric emptying and postprandial lipaemia, but not on glycaemic response and appetite. Br. J. Nutr. 2004;92:447–459. doi: 10.1079/BJN20041219. [DOI] [PubMed] [Google Scholar]
- 20.Gaudichon C., Mahé S., Roos N., Benamouzig R., Luengo C., Huneau J.F., Sick H., Bouley C., Rautureau J., Tome D. Exogenous and endogenous nitrogen flow rates and level of protein hydrolysis in the human jejunum after [15N]milk and [15N]yoghurt ingestion. Br. J. Nutr. 1995;74:251–260. doi: 10.1079/BJN19950128. [DOI] [PubMed] [Google Scholar]
- 21.Hermans W.J.H., Senden J.M., Churchward-Venne T.A., Paulussen K.J.M., Fuchs C.J., Smeets J.S.J., van Loon J.J.A., Verdijk L.B., van Loon L.J.C. Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double-blind randomized trial. Am. J. Clin. Nutr. 2021;114:934–944. doi: 10.1093/ajcn/nqab115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Kruif C.G. Skim Milk Acidification. J. Colloid Interface Sci. 1997;185:19–25. doi: 10.1006/jcis.1996.4548. [DOI] [PubMed] [Google Scholar]
- 23.Tsukahara T., Koyama H., Okada M., Ushida K. Stimulation of butyrate production by gluconic acid in batch culture of pig cecal digesta and identification of butyrate-producing bacteria. J. Nutr. 2002;132:2229–2234. doi: 10.1093/jn/132.8.2229. [DOI] [PubMed] [Google Scholar]
- 24.Asano T., Yuasa K., Kunugita K., Teraji T., Mitsuoka T. Effects of Gluconic Acid on Human Faecal Bacteria. Microb. Ecol. Health Dis. 1994;7:247–256. [Google Scholar]
- 25.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 26.Trapecar M., Communal C., Velazquez J., Maass C.A., Huang Y.J., Schneider K., Wright C.W., Butty V., Eng G., Yilmaz O., et al. Gut-Liver Physiomimetics Reveal Paradoxical Modulation of IBD-Related Inflammation by Short-Chain Fatty Acids. Cell Syst. 2020;10:223–239. doi: 10.1016/j.cels.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pseudo-anonymized data can be made available upon request before 2029 via a data sharing contract. From 2029, fully anonymized data can be transferred.