Abstract
In recent decades, more than 130 potentially toxic metabolites originating from dinoflagellate species belonging to the genus Karenia or metabolized by marine organisms have been described. These metabolites include the well-known and large group of brevetoxins (BTXs), responsible for foodborne neurotoxic shellfish poisoning (NSP) and airborne respiratory symptoms in humans. Karenia spp. also produce brevenal, brevisamide and metabolites belonging to the hemi-brevetoxin, brevisin, tamulamide, gymnocin, gymnodimine, brevisulcenal and brevisulcatic acid groups. In this review, we summarize the available knowledge in the literature since 1977 on these various identified metabolites, whether they are produced directly by the producer organisms or biotransformed in marine organisms. Their structures and physicochemical properties are presented and discussed. Among future avenues of research, we highlight the need for more toxin occurrence data with analytical techniques, which can specifically determine the analogs present in samples. New metabolites have yet to be fully described, especially the groups of metabolites discovered in the last two decades (e.g tamulamides). Lastly, this work clarifies the different nomenclatures used in the literature and should help to harmonize practices in the future.
Keywords: Karenia spp., marine biotoxins, brevetoxins, metabolic products, shellfish, marine organisms
1. Introduction
The genus Karenia belongs to the clade of the alveolates (with the ciliates and the apicomplexa). It includes 12 known marine species from oceanic and coastal areas. These eukaryotes were formerly classified as microalgae, and belong to the group of photosynthetic protists (autotrophs) [1]. Among the genus Karenia, some species of this dinoflagellate can proliferate and form dense blooms that cause multiple dysfunctions of the hydrosystem, but also have a strong toxic potential for organisms in the food web [2,3]. These massive blooms, referred as “red tides”, can be visible and color the water. Brevetoxins (BTXs), which are produced by Karenia brevis (C.C. Davis) Gert Hansen and Moestrup, 2000, belong to the main group of marine biotoxins associated with these events. These neurotoxins can cause significant mortality in fish, sea birds, and marine mammals [4,5,6]. Humans can also be exposed to BTX through food, leading to human poisoning, called neurotoxic shellfish poisoning (NSP), inhalation or contact (skin or mucous membranes) [7,8]. Shellfish consumption is the main cause of NSP. Symptoms of NSP include nausea, vomiting, diarrhea, paresthesia, cramps, bronchoconstriction, paralysis, seizures and coma. Inhalation or contact exposure can result in irritant effects. To date, NSP events have been limited to the Gulf of Mexico, the east coast of the United States of America (U.S.A.), and New Zealand [9]. No cases of NSP have been reported in Europe to date; however, a recent study has shown the presence of these toxins in shellfish from the French Mediterranean Sea, raising the question of the potential emergence of this group of toxins in areas that have been preserved to date [10,11]. Karenia spp. can also produce the following groups of potentially toxic metabolites in addition to the BTXs: hemi-brevetoxins, brevenals, brevisamides, brevisins, tamulamides, gymnocins, gymnodimines, brevisucenals, and brevisulcatic acids [12].
The use of systematic nomenclature to refer to these metabolites is cumbersome, considering their molecular weight and the large number of analogs. In recent decades, different nomenclatures have been used to name the toxins produced by Karenia spp., leading to a large number of names, sometimes for the same analog, especially for brevetoxins. One reason for this proliferation of names is that isolation and toxicology studies were sometimes carried out from the causative organisms, before pure materials were available and the structures of the metabolites were elucidated [13]. Moreover, one of the earliest nomenclatures was based on the name of the producer identified at that time: Gymnodinium breve C.C. Davis, 1948. For example, “GB-1 toxin” was the name attributed to BTX-1. In 1979, Gymnodinium breve was taxonomically reclassified to Ptychodiscus brevis (C.C. Davis) K.A.Steidinger, 1979. This change led to a nomenclature revision of brevetoxins, which became Ptychodiscus brevis toxins, with the abbreviation PbTx [14]. Eventually, in 2000, the brevetoxin producer was named Karenia brevis (K. brevis) by Hansen and Moestrup [15]. The term brevetoxin is the only name that has withstood the test of time. BTX is now widespread and taken up not only for recently isolated metabolites, but also for the first discovered metabolites, which were initially named following another nomenclature [10,11,16,17,18,19]. Despite the harmonization of the term BTX, several analogs with different names are still encountered, for several other reasons. Moreover, we should recall that this abbreviation is also shared with the batrachotoxins, a small group of steroidal alkaloids.
Considering all the different groups of metabolites produced by Karenia spp., accumulation and biotransformation by marine organisms has been investigated in depth for BTXs and gymnodimines (GYMs), contrary to the other groups [4,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. In 1992–1993, a major NSP outbreak occurred in New Zealand after a K. brevis bloom. A total of 180 people were intoxicated after shellfish consumption, with a toxin profile dominated by the analogue BTX-2. An analysis of the shellfish samples collected during this NSP event, combined with the implementation of specific analytical methods (particularly physicochemical techniques), led to the first evidence suggesting BTX metabolism in shellfish [20,21,31,33,36,37,38,40]. BTX-2, BTX-3 and BTX-B5, known to be produced by K. brevis, were detected in cockles, mussels and oysters collected during this event (Austrovenus stutchburyi, Perna canaliculus and Crassostrea gigas, respectively) [20,21,31,33]. Metabolites that were not identified from K. brevis natural blooms or cultures at that time were also isolated from these shellfish, particularly amino acid conjugates (taurine-BTX-B and cysteine-BTX-B sulfoxide, also called BTX-B1 and BTX-B2, respectively) and fatty acid conjugates (oxidized open D-ring tetradecanoyl-BTX-2 and oxidized open D-ring hexadecanoyl-BTX-2), and sometimes a combination of these transformations (N-tetradecanoyl-cysteine-BTX-B sulfoxide, also called BTX-B4). These preliminary results suggested the metabolism processes of BTXs in shellfish, and further investigations followed, with different shellfish species and other marine organisms either naturally exposed to K. brevis, in cultures, or to pure toxins [17,22,23,30,34,35,39,48,49,50]. BTX profiles varied depending on the species due to different rates of accumulation and elimination. Consequently, their toxicity fluctuates over time, based on the toxin profile. These studies also allowed for the identification of numerous new metabolites. For many of them, chemical structure elucidation relied on a stringent study of their mass spectrometry fragmentation profiles. Confirmation of these structures with more thorough elucidation tools, such as nuclear magnetic resonance (NMR), would require large quantities of samples for isolation and purification purposes, making this goal difficult to achieve.
The aim of this review is to summarize the knowledge collected in recent decades about the potentially toxic metabolites produced by Karenia spp. or reported in other marine organisms. Therefore, metabolites obtained by chemical synthesis (partially or fully from existing compounds) or from in vitro metabolism studies will not be considered [51,52,53,54,55]. We will present and discuss the structures and physicochemical properties of the metabolites reported in the literature. The accumulation and biotransformation of these potentially toxic metabolites by marine organisms will be discussed in depth and will help to identify and highlight future avenues of research. As previously mentioned, the use of different nomenclatures can be disruptive and it is not always easy to switch between the different names used, especially for BTXs. We will tackle this aspect by reporting all the names used in the literature and by preferentially using those which seem to achieve a better overall consistency.
2. Metabolites Produced by Karenia spp.
Among the different Karenia species reported, K. brevis is known to produce BTXs, brevisin, brevisamide, brevenals and tamulamides, whereas brevisulcenals and brevisulcatic acids can be produced by Karenia brevisulcata (F.H. Chang, 1999) G. Hansen & Moestrup, 2000. Gymnocins and gymnodimine-A were discovered from cultures of Karenia mikimotoi (Miyake & Kominami ex Oda) G. Hansen & Moestrup, 2000 [24,56,57,58], whereas gymnodimine-B and -C were first isolated from Karenia selliformis A.J. Haywood, K.A. Steidinger & L. MacKenzie, 2004 [59]. All these groups of metabolites possess different structure skeletons that give them their physicochemical properties (Table 1). To allow for a better follow-up of the numerous compounds reported in this review, an identification number (ID) was attributed to each of them in the following parts of this article (text, tables and figures).
Table 1.
Physicochemical properties of brevetoxins (BTXs) and other potentially toxic metabolites produced by Karenia species.
| Group | Compound Identification Number (ID) | Metabolite | Other Existing Names | Molecular Formula |
Monoisotopic Mass (Da) | LogP 1 | Species | First Identification/Structural Elucidation References |
|---|---|---|---|---|---|---|---|---|
| A-type brevetoxins (BTX-A) |
#1 | BTX-1 | PbTx-1; GB-1; T46, BTX-A | C49H70O13 | 866.5 | 5.8 | K. brevis | [60] |
| #2 | BTX-7 | PbTx-7; GB-7; Aldehyde-reduced PbTx-1 | C49H72O13 | 868.5 | 5.7 | K. brevis | [60] | |
| #3 | BTX-10 | / | C49H74O13 | 870.5 | 5.5 | K. brevis | [13,61] 2 | |
| #4 | Oxidized BTX-1 | Oxidized PbTx-1 PbTx-1-carboxylic acid |
C49H70O14 | 882.5 | / | K. brevis | [35] 3 | |
| #5 | Open A-ring BTX-1 | Open-ring PbTx-1 | C49H72O14 | 884.5 | / | K. brevis | [35] 3 | |
| #6 | Open A-ring, oxidized BTX-1 | Open-ring, oxidized PbTx-1; PbTx-1-open ring-carboxylic acid |
C49H72O15 | 900.5 | / | K. brevis | [35] 3 | |
| #7 | Open A-ring BTX-7 | Open-ring PbTx-7 | C49H74O14 | 886.5 | / | K. brevis | [35] 3 | |
| B-type brevetoxins (BTX-B) |
#8 | BTX-2 | PbTx-2; T47; GB-2, T34, BTX-B | C50H70O14 | 894.5 | 6.4 | K. brevis | [62,63,64] |
| #9 | BTX-3 | PbTx-3; GB-3; T17; Dihydro-BTX-B; Aldehyde-reduced PbTx-2 | C50H72O14 | 896.5 | 6.4 | K. brevis | [65,66] | |
| #10 | BTX-5 | PbTx-5; GB-5; acetylated PbTx-2 | C52H72O15 | 936.5 | 7.1 | K. brevis | [67] | |
| #11 | BTX-6 | PbTx-6; GB-6; 27,28 epoxyde of PbTx-2 | C50H70O15 | 910.5 | 7.3 | K. brevis | [67] | |
| #12 | BTX-9 | PbTx-9; α-methylene reduced PbTx-3 |
C50H74O14 | 898.5 | 6.3 | K. brevis | [13,14,61] | |
| #13 | BTX-B5 | Oxidized BTX-2; Oxidized PbTx-2 |
C50H70O15 | 910.5 | / | K. brevis | [32,33,35] | |
| #14 | Open A-ring BTX-2 | Open A-ring PbTx-2 | C50H72O15 | 912.5 | / | K. brevis | [35] 3 | |
| #15 | Open A-ring, oxidized BTX-2 | Open A-ring, Oxidized PbTx-2; Open A-ring BTX-B5; PbTx-2-open ring-carboxylic acid |
C50H72O16 | 928.5 | / | K. brevis | [35] 3 | |
| #16 | Open A-ring BTX-3 | Open A-ring PbTx-3 | C50H74O15 | 914.5 | / | K. brevis | [35] 3 | |
| Hemi- Brevetoxins (Hemi-BTXs) |
#17 | Hemi-BTX-A | GB-M | / | / | / | K. brevis | [68,69] |
| #18 | Hemi-BTX-B | GB-N | C28H42O7 | 490.3 | 3.5 | K. brevis | [69] | |
| #19 | Hemi-BTX-C | GB-4 | / | / | / | K. brevis | [69] | |
| Brevenals | #20 | Brevenal | / | C39H60O8 | 656.4 | 6.9 | K. brevis | [70] |
| #21 | Dimethyl acetal brevenal | / | C41H66O9 | 702.5 | / | K. brevis | [70] | |
| Brevisamide | #22 | Brevisamide | / | C18H29NO4 | 323.2 | / | K. brevis | [71] |
| Brevisin | #23 | Brevisin | / | C39H62O11 | 706.4 | 3.6 | K. brevis | [72] |
| Tamulamides (Tams) |
#24 | Tam-A | / | C35H45NO10 | 639.3 | 1.0 | K. brevis | [73] |
| #25 | Tam-B | / | C34H43NO10 | 625.3 | 0.7 | K. brevis | [73] | |
| Gymnocins | #26 | Gymnocin-A | / | C55H80O18 | 1028.5 | 3.3 | K. mikimotoi | [56] |
| #27 | Gymnocin-A carboxylic acid | / | C55H80O19 | 1044.5 | 3.6 | K. mikimotoi | [58] | |
| #28 | Gymnocin-A2 | / | C55H80O18 | 1028.5 | / | K. mikimotoi | [58] | |
| #29 | Gymnocin-B | / | C62H92O20 | 1156.6 | 5.0 | K. mikimotoi | [57] | |
| Gymnodimines (GYMs) |
#30 | GYM-A | / | C32H45NO4 | 509.4 | 6.4 | K. mikimotoi K. selliformis | [24] |
| #31 | GYM-B | / | C32H45NO5 | 523.3 | 5.1 | K. selliformis | [74] | |
| #32 | GYM-C | GYM-B isomer | C32H45NO5 | 523.3 | / | K. selliformis | [59] | |
| Brevisucenals (KBTs) |
#33 | KBT-A1 (sodium salt) | KBT-F sulfate ester | C107H159O41SNa | 2155.0 | / | K. brevisulcata | [75] |
| #34 | KBT-A2 (sodium salt) | KBT-G sulfate ester | C108H161O42SNa | 2185.0 | / | K. brevisulcata | [75] | |
| #35 | KBT-F | / | C107H160O38 | 2053.1 | 7.9 | K. brevisulcata | [76,77] | |
| #36 | KBT-G | / | C108H162O39 | 2083.1 | / | K. brevisulcata | [77,78] | |
| #37 | KBT-H | / | C107H160O39 | 2069.1 | / | K. brevisulcata | [78] | |
| #38 | KBT-I | / | C108H162O40 | 2099.1 | / | K. brevisulcata | [78] | |
| Brevisulcatic acids (BSXs) | #39 | BSX-1 | / | C49H72O16 | 916.5 | 3.6 | K. brevisulcata | [77,79] |
| #40 | BSX-2 | / | C47H68O15 | 872,5 | / | K. brevisulcata | [77,80] | |
| #41 | BSX-3 | / | / | 856.5 | / | K. brevisulcata | [77] 4 | |
| #42 | BSX-4 | / | C49H70O15 | 898.5 | 3.8 | K. brevisulcata | [68,79] | |
| #43 | BSX-5 | / | C47H66O14 | 854.4 | / | K. brevisulcata | [77,80] | |
| #44 | BSX-6 | Lactone derivative of BSX-3 | / | 838.5 | / | K. brevisulcata | [77] 4 | |
| #45 | BSX-7 | / | C47H70O14 | 858.5 | / | K. brevisulcata | [80] |
1 LogP predicted from the chemical structure of molecules using the ACD/Labs platform. Octanol/water partition coefficients were predicted using algorithms. 2 Synthesized toxin, unidentified in cultures to date, but postulated to occur naturally, based on structural correlation with BTX-9 and its presence in stationary cultures. 3 Analogs postulated after thorough study of LC-MS/MS fragmentations. 4 Structure not elucidated.
The implementation of specific methods for the analysis of metabolites produced by Karenia spp. allowed for the identification of individual analogs belonging to several of these groups of toxins in Australia, Japan, New Zealand, the Gulf of Mexico and the South China Sea (Figure 1) [16,35,45,46,47,56,64,75,76,77,81,82].
Figure 1.
Geographical distribution of potentially toxic metabolites individually and formally identified (blue color for country), displayed by type of samples collected (forms) and by group to which they belong (colors of forms). Map generated using R software and the ggplot2 package [83,84].
2.1. Brevetoxins
BTXs are lipid-soluble and thermostable polycyclic polyethers produced by the dinoflagellate Karenia brevis [13]. In laboratory conditions, the production of BTX-2 (ID #8) has also been shown for Karenia papilionacea A.J. Haywood and K.A. Steidinger, 2004 [85]. These toxins are polyethers with ladder-like structures similar to ciguatoxins (CTXs) (produced by the dinoflagellate Gamberdiscus). All polyether compounds produced by K. brevis are thought to derive from polyepoxide precursors [86,87]. A wide range of BTXs have been identified from K. brevis cultures (Table 1). NMR and X-ray crystallography were the analytical techniques implemented to establish their structures. A-type and B-type BTXs (BTX-A and BTX-B) are the two base skeletons identified (Figure 2). BTX-1 (ID #1) and BTX-2 are the parent algal toxins of the BTX-A and BTX-B types, respectively [88]. The molecular formula of BTX-1 is C49H70O13 and its structure is composed of 10 rings [60,66]. The molecular formula of BTX-2 is C50H70O14 and its structure is composed of 11 rings [14,64]. Both structures carry a lactone function that is thought to be responsible for their biological activity [88]. BTX-2 is the major toxin isolated from K. brevis and the one produced by the largest number of species of the genus Karenia [88].
Figure 2.
Chemical structures of brevetoxins (BTXs) identified from environmental samples and cultures of Karenia brevis.
The structural elucidation of BTX-1 (ID #1) and BTX-2 (ID #8) facilitated the resolution of the structure of all subsequently discovered all related analogs (Figure 2). Each family consists of several compounds, differentiated by the “J-ring” in BTX-A (BTX-1, BTX-7, BTX-10 with respective ID of #1, #2, #3) and at the “K-ring” in BTX-B (BTX-2, BTX-3, BTX-5, BTX-6, BTX-9 with respective ID of #8, #9, #10, #11, #12) [13,14,35,60,61,65,66,67,89]. These different metabolites are reduced, acetylated or epoxidized forms of BTX-1 (ID #1) or BTX-2 (ID #2). Other analogs have been identified from K. brevis cultures and natural blooms, and correspond to the oxidized forms of BTX-1 (ID #4) and BTX-2 (BTX-B5, ID #13), and to the open forms of the first ring (A-ring) of BTX-1 (ID #5), BTX-2 (ID #14), BTX-3 (ID #16) and BTX-7 (ID #7) [35]. Open forms of oxidized BTX-1 (ID #6) and BTX-2 (ID #15) have also been reported (Figure 2). The structures of these open A-ring derivatives were postulated after a thorough study of their tandem mass spectrometry (MS/MS) fragmentations, but could not be elucidated with powerful techniques such as NMR. It is worth noting that some of these open A-ring derivatives were identified with greater abundance than their non-hydrolyzed counterparts.
In 2005, BTX-11 (ID #46), BTX-12 (ID #47) and BTX-tbm (BTX-2 decomposition product without the side chain tail; ID #48) were described as new toxins after their identification in cultures and in the field (Figure 2) [88,90]. Since then, these toxins were never detected. Moreover, several compounds initially identified as BTXs are now considered artifacts (not included in Table 1). BTX-8 (C49H69ClO14; also called BTX-C; ID #49) is generated during chloroform extraction of BTX-2 (ID #8) from K. brevis [35,88,91]. BTX-13 (ID #50) and BTX-14 (ID #51) are likely artifacts of acid-catalyzed methanol addition during purification protocols [88].
BTXs are neurotoxins that primarily target the voltage-gated Na+ channels (NaV) [11]. The neurological nature of the primarily symptoms observed in humans and animals can be explained by the specific affinity of BTXs for NaV channel subtypes and the tissue distribution of NaV channels. The symptoms also involve gastrointestinal and cardiovascular systems. Data on acute toxicity in animals are very limited. The only toxicity study by oral administration allows for an estimation of the median lethal doses (LD50) for BTX-2 and BTX-3 in female mice [14]. BTX-3 was about 10 times more toxic than BTX-2 by oral administration, with LD50 of 520 and 6600 µg/kg bw, respectively. However, by intraperitoneal injection, the two toxins were almost equipotent.
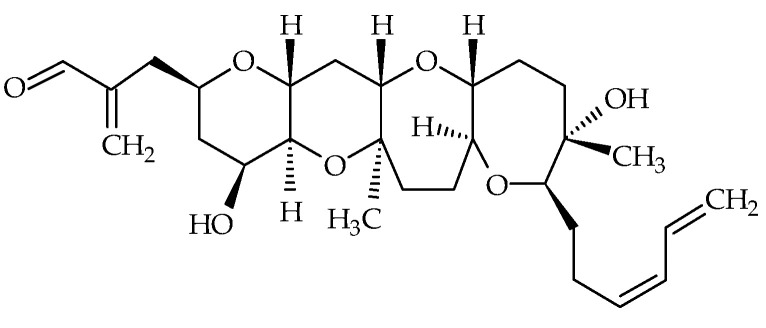
2.2. Hemibrevetoxins
Hemibrevetoxins (Hemi-BTX-A, -B, -C with respective ID of #17, #18, #19), cyclic ethers biosynthesized by K. brevis, were first isolated by Shimizu [68] in 1982 and were reported to have weak ichthyotoxicity. Later, Prasad and Shimizu elucidated the chemical structure of Hemi-BTX-B [69] (Figure 3). Its molecular formula is C28H42O7, and is about half that of BTXs, hence its name (Table 1). The authors suggested that hemibrevetoxins could play an important role in the biosynthesis of BTXs. They also noted cytotoxicity at a concentration of 5 µmol on cultured mouse neuroblastoma cells, and a characteristic rounding of cells, as for BTX-A and BTX-B. Hemi-BTX-A and –C were isolated; however, their chemical structures seem to have never been fully elucidated [68,69].
Figure 3.
Chemical structure of hemibrevetoxin-B (ID #18).
2.3. Brevenals
In 2004, brevenal (ID #20) and its dimethyl acetal (ID #21) were first isolated and characterized from K. brevis cells collected in Florida, USA (Figure 4). These fat-soluble compounds, with molecular formulae C39H60O8 and C41H66O9, respectively, are composed of five ether rings and possess an aldehyde function [16,70,81]. Brevenals are not toxic for fish and antagonize the effects of BTXs. Both competitively displace BTX from its binding site in rat brain synaptosomes. Moreover, brevenal inhibits the bronchoconstriction induced by BTX-2 (ID #8) and BTX-3 (ID #9) in a sheep assay [90]. In 2006, the first synthesis of brevenal was accomplished [92]. This work led to a revision of the structure of brevenal, which corresponds to the C26 epimer of the initially proposed natural product structure (Figure 4).
Figure 4.
Chemical structures of brevenal (ID #20) and dimethyl acetal brevenal (ID #21).
2.4. Brevisamide
In 2008, brevisamide (ID #22) was isolated from K. brevis [71]. Its molecular formula is C18H29NO4 and its structure is composed of a monocyclic ether possessing an amide function. It is also composed of a tetrahydropyran ring bearing a 3,4-dimethylhepta-2,4 dienal side chain (Figure 5). To date, no other analog has been discovered. Brevisamide was synthetized by several authors using different strategies [93,94,95]. Brevisamide may be the biosynthetic precursor of brevisin, based on their respective structures [95]. The toxicity of this metabolite remains unknown, due to the absence of any studies.
Figure 5.
Chemical structure of brevisamide (ID #22).
2.5. Brevisin
In 2009, brevisin (ID #23) was identified from K. brevis cells [72,96]. Its structure consists of two separate fused polyether ring assemblies linked by a methylene group. One of the polycyclic moieties was conjugated with an aldehyde side chain (Figure 6). Brevisin inhibits the binding of [3H]-BTX-3 on sodium channels in rat brain synaptosomes. In 2011, the total synthesis of brevisin was accomplished [97]. To date, studies regarding this secondary metabolite are limited and further investigation is required.
Figure 6.
Chemical structure of brevisin (ID #23).
2.6. Tamulamides
Tamulamides, cyclic polyethers with a ladder-like chemical structure, were isolated from K. brevis cultures [73]. Both are composed of seven cyclic ethers with an amide and aldehyde side chain (Figure 7). The molecular formulae of Tamulamide-A (Tam-A; ID #24) and Tamulamide-B (Tam-B; ID #25) are C35H45NO10 and C34H43NO10, respectively (Table 1). Tam-A and Tam-B compete with [3H]-BTX-3 for its binding site on rat brain synaptosomes. Neither showed toxicity to fish at doses up to 200 nM and cause only slight bronchoconstriction in sheep pulmonary assays [73].
Figure 7.
Chemical structures of tamulamide-A (ID #24) and tamulamide-B (ID #25).
2.7. Gymnocins
Gymnocins are polyethers biosynthesized by K. mikimotoi. Gymnocin-A (ID #26) was the first analog discovered from Japanese cultures of this dinoflagellate, isolated at Kushimoto Bay [56] (Figure 1). Structure elucidation revealed 14 saturated ether rings, bearing a 2-methyl-2-butenal side chain (Figure 8). In 2003, this analog was fully synthetized by Tsukano and Sasaki [98]. Gymnocin-A carboxylic acid (ID #27), Gymnocin-A2 (ID #28), Gymnocin-B (ID #29; composed of 15 adjacent rings ending with the same 2-methyl-2-butenal side chain as gymnocin-A) were isolated and elucidated from K. mikimotoi [57,58] (Table 1). Conventional fish assays showed that Gymnocin-A and Gymnocin-B are weakly toxic compared to BTX-B [56,57]. However, the authors highlighted that fish were directly exposed to dissolved gymnocins, whereas during red tide events, K. mikimotoi cells stuffed the fish gills, allowing direct contact of the gymnocins with the gills. The extremely low solubility of gymnocins to water could prevent them from reaching the fish gills during assays. This mechanism could be involved in the fish mortalities observed in the field, whereas conventional fish assays do not reveal significant toxicity. Gymnocin-A carboxylic acid and Gymnocin-A2 showed moderate cytotoxicity against P388 cells [58].
Figure 8.
Chemical structures of gymnocins.
2.8. Gymnodimines (GYMs)
Initially, gymnodimine-A (GYM-A; ID #30) was isolated from extracts of New Zealand oysters (Tiostrea chilensis) [24] (Figure 1). Isolation of the active substance made it possible to determine its molecular formula as C32H45NO4 (Table 1), whereas its absolute stereochemistry was later determined by X-ray crystallography (Figure 9) [99]. The origin of this toxin was first attributed to the dinoflagellate K. selliformis for two reasons: efflorescence of the latter was observed at the same time; gymnodimine was isolated in cultures of this species [100]. Two other analogs are known to be produced by K. selliformis: GYM-B (ID #31) and GYM-C (ID #32); whereas several others (12-methyl-GYM-A, 12-methyl-GYM-B, GYM-D, 16-desmethyl-GYM-D, GYM-E and more than 30 related gymnodimine-like compounds) have only been identified from Alexandrium ostenfeldii or Alexandrium peruvianum [59,87,101,102,103,104]. The structure of GYM-B is similar to GYM-A, but contains an exocyclic methylene at position C17 and an allylic hydroxyl group at position C18 (Figure 9). GYM-C is an oxidized isomer of GYM-B at position C-18. In 2015, it was demonstrated that GYM-A, GYM-B, and GYM-C could also be produced by A. ostenfeldii [105] (Table 1). The use of phytoplankton net sampling allowed for the identification of GYM-A in Australia (Figure 1), together with K. selliformis cells (Moreton Bay, Queensland), and in China (Daya Bay) [45,46]. In terms of toxicity, GYM-A demonstrated high intraperitoneal toxicity in mice (LD50 of 80–96 μg kg−1), while very low toxicity was reported after oral exposure [106,107]. Moreover, human toxicity is debatable, since no human poisoning could be associated with this toxin.
Figure 9.
Chemical structures of gymnodimines (GYMs) identified from cultures of Karenia selliformis.
2.9. Brevisulcenals and Brevisulcatic Acids
The toxin complex produced by K. brevisulcata was initially designated as Wellington Harbour Toxin (WHT), after the appearance of a bloom in 1998, which devastated all marine life in this New Zealand harbour [108]. This complex is composed of two groups of toxins: brevisulcenals (KBTs for K. brevisulcata toxins) and brevisulcatic acids (BSXs) (Figure 10). KBTs are lipophilic polycyclic ethers with a ladder-like structure [109]. Ten analogs are reported (Table 1); however, only six of them have been well characterized to date: KBT-A1 (ID #33), KBT-A2 (ID #34), KBT-F (ID #35), KBT-G (ID #36), KBT-H (ID #37), and KBT-I (ID #38) [75,76,78,82,110] (Figure 10). The mouse i.p. LD50 for KBT-F and -G are 0.032 and 0.040 mg/kg bw, respectively. KBT-F and KBT-G are strongly haemolytic, cytotoxic to P388 and neuro-2a cells and highly toxic i.p. to mice [82]. Brevisulcatic acids are composed of seven analogs with a common nine cyclic ether backbone. BSX-1 (ID #39), BSX-2 (ID #40), BSX-4 (ID #42) and BSX-5 (ID #43) have known structures and are close to those of BTX-A, especially the ladder arrangement [77,79]. BSX-2, -5, and -7 (ID #45) carry a lactone function. Different side chain substituents make up the various BSX analogs [80]. BSX-3 (ID #41) and BSX-6 (ID #44) were isolated from K. brevisulcata cultures by means of 13C labelled extracts, combined with the use of liquid chromatography with mass spectrometry detection (LC-MS) acquisitions [77]. Their [M + H]+ m/z are 857.5 and 839.5, respectively. However, their molecular formula and chemical structure remain unknown. Harwood et al. [110] suggest that BSX-6 could be an important intermediate in BSX biosynthesis. The mouse i.p. LD50 for BSX-1 is 3.9 mg/kg, while no deaths were seen in mice injected with BSX-2 at 6.6 mg/kg. The LD50 for the lactones BSX-4 and BSX-5 are 1.4 and 1.6 mg/kg, respectively. BSX-4 and -5 are agonists of voltage-gated sodium channels but only weakly haemolytic. Activities in the Neuro-2a cytotoxicity assay were ca 10% of dihydro-brevetoxin-2 and were fully antagonised by saxitoxin [82].
Figure 10.
Chemical structures of brevisulcenals (KBTs) and brevisulcatic acids (BSXs).
3. Biotransformation of Metabolites Produced by Karenia spp. in Shellfish
This section will only focus on the metabolism of BTXs and GYMs in shellfish. For both, individual analogs have been reported worldwide (Figure 1), whereas data are lacking for the other groups of metabolites produced by Karenia spp. [10,20,21,22,23,24,25,26,27,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45,46,47].
3.1. Brevetoxins
3.1.1. Brevetoxins Already Reported from Karenia spp.
Several BTXs known to be produced by Karenia spp. have also been identified in shellfish (Table 2). Whereas BTX-1 (ID #1) has not been found in shellfish, BTX-2 (ID #8) has occasionally been identified in different species [28,29,30,31]. In fact, both toxins are extensively metabolized, and several metabolic pathways were proposed [31,32]. A study in which eastern oysters (Crassostrea virginica) were exposed under controlled conditions to pure BTX-2 revealed that BTX-2 rapidly accumulated and was metabolized into several compounds, including BTX-3 (ID #9), as a reduced form of BTX-2 [28]. Oysters exposed to BTX-3 toxin showed rapid accumulation and significant elimination (about 90%), without any apparent biotransformation, in two weeks. Among the metabolites reported from K. brevis, several oxidized, reduced and A-ring lactone hydrolyzed metabolites of BTX-1 and BTX-2 have also been identified in different shellfish species [20,21,30,31,32,33,34]. It is worth noting that the profile of BTX metabolites and their concentrations varied considerably between bivalve mollusk species. For A-type BTXs, oxidized-BTX-1 (ID #4), open A-ring oxidized BTX-1 (ID #6) and open A-ring BTX-7 (ID #7) were reported in hard clams (mercenaria sp.) and eastern oysters (Crassostrea virginica). For B-type BTXs, BTX-3, BTX-9 (ID #12), and BTX-B5 (ID #13) have been identified in clams (Mercenaria sp., Macrocallista nimbosa), cockles (Austrovenus stutchburyi), gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata), mussels (Perna canaliculus) and oysters (Crassostrea gigas, Crassostrea virginica). BTX-3 was reported at higher level than BTX-2 in shellfish, i.e., in oysters (Crassostea gigas) and cockles (Austrovenus Stutchburyi) in New Zealand [20,31,33], horse conch (Triplofusus giganteus), lightning whelk (Sinistrofulgur sinistrum), banded tulip (Cinctura hunteria), fighting conch (Strombus alatus), pear whelk (Fulguropsis spirata), clam (Mercenaria spp.) and oyster (Crassostrea virginica) in Florida [17,22,111]. BTX-3 and BTX-9 are largely eliminated in oysters after two weeks of depuration [34]. Importantly, open A-ring BTX-1 (ID #5), open A-ring BTX-2 (ID #14), and open A-ring BTX-3 (ID #16), reported from K. brevis environmental samples, were not detected. Their extensive metabolism, due to the reactive α,β-unsaturated aldehyde group in their tail region, could explain their absence [35].
Table 2.
Physicochemical characteristics of brevetoxin (BTX) metabolites reported in marine organisms.
| Group | Compound Identification Number (ID) |
Metabolite | Other Existing Names | Molecular Formula |
Monoisotopic Mass (Da) | LogP 1 | Sample | References |
|---|---|---|---|---|---|---|---|---|
| A-type BTXs | #4 | Oxidized BTX-1 | Oxidized PbTx-1; PbTx-1-carboxylic acid |
C49H70O14 | 882.5 | / | Clams (mercenaria sp.) | [22] |
| #6 | Open A-ring, oxidized BTX-1 | Open A-ring of oxidized PbTx-1; A-type opened A-ring derivative |
C49H72O15 | 900.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,35] | |
| #7 | Open A-ring BTX-7 | Open A-ring PbTx-7 | C49H74O14 | 886.5 | / | Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) |
[35,39,48] | |
| #52 | Taurine-BTX-A | N-taurine conjugate of oxidized BTX-1 | C51H75NO16S | 989.5 | / | Clams (mercenaria sp.) | [22] | |
| #53 | Cysteine–BTX-A | Cysteine–PbTx-A; Cysteine-PbTx-1; Cysteine-BTX-1 |
C52H79NO15S | 989.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) |
[23,35,39,48] | |
| #54 | Cysteine–BTX-A sulfoxide | Cysteine–PbTx-A sulfoxide; Sulfoxide cysteine conjugate of PbTx-1 |
C52H79NO16S | 1005.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) |
[23,35,39,48] | |
| #55 | Open A-ring cysteine-BTX-A | Open A-ring cysteine–PbTx-A | C52H81NO16S | 1007.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,34,35,39] | |
| #56 | Glutathione-BTX-A | Glutathione-PbTx-A | C59H89N3O19S | 1175.6 | / | Oysters (Crassostrea virginica) | [23,34,39] | |
| #57 | Glycine-cysteine-BTX-A | Glycine-cysteine-PbTx-A | C54H82N2O16S | 1046.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[23,39] | |
| #58 | N-hexadecanoyl-cysteine–BTX-A | N-hexadecanoyl-cysteine–PbTx-A | C68H109NO16S | 1227.7 | / | Oysters (Crassostrea virginica) | [39] | |
| #59 | Metabolite not named | / | C51H76O16S | 976.5 | / | Oysters (Crassostrea virginica) | [39] | |
| B-type BTXs | #8 | BTX-2 | PbTx-2; T47; GB-2; T34; BTX-B | C50H70O14 | 894.5 | 6.2 | Cockles (Austrovenus stutchburyi); Mussels (Perna canaliculus); Oysters (Crassostrea virginica, Crassostrea gigas); Sharks (Rhizoprionodon terraenovae, Sphyrna tiburo); Shrimps (Litopenaeus vannamei) |
[10,21,30,31,32,49,64,112] |
| #9 | BTX-3 | PbTx-3; GB-3; T17; dihydro-BTX-B; aldehyde-reduced PbTx-2 | C50H72O14 | 896.5 | 6.4 | Bottlenose dolphins (Tursiops Truncatus); Clams (Mercenaria sp.); Cockles (Austrovenus stutchburyi); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea gigas, Crassostrea virginica); Pinfish (Lagodon rhomboides); Rays (Dasyatis sabina); Sharks (Carcharhinus limbatus, Rhizoprionodon terraenovae, Sphyrna tiburo); Spot (Leiostomus xanthurus) |
[10,17,20,21,22,31,32,33,48,49,50,66] | |
| #16 | Open A-ring BTX-3 | Open-ring PbTx-3 | C50H74O15 | 914.5 | / | Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) |
[17,35,48] | |
| #12 | BTX-9 | Reduced-BTX-2 | C50H74O14 | 898.5 | 6.3 | Oysters (Crassostrea virginica) | [34,39] | |
| #13 | BTX-B5 | Oxidized BTX-2; Oxidized PbTx-2; PbTx-2-carboxylic acid |
C50H70O15 | 910.5 | 6.7 | Clams (Mercenaria sp., Macrocallista nimbosa);
Cockles (Austrovenus stutchburyi); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea gigas, Crassostrea virginica) |
[17,22,23,31,32,33,39] | |
| #15 | Open A-ring, oxidized BTX-2 | Open ring, oxidized PbTx-2; Open A-ring BTX-B5; PbTx-2-open ring-carboxylic acid |
C50H72O16 | 928.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,23,35] | |
| #60 | Oxidized, open D-ring tetradecanoyl-BTX-2 | BTX-B3; Oxidized, open D-ring myristoyl-BTX-2 |
C64H96O17 | 1136.7 | / | Mussels (Perna canaliculus) | [36] | |
| #61 | Oxidized, open D-ring hexadecanoyl-BTX-2 | BTX-B3; Oxidized, open D-ring palmitoyl-BTX-2 |
C66H100O17 | 1164.7 | / | Mussels (Perna canaliculus) | [36] | |
| #62 | Taurine-BTX-B | BTX-B1; N-taurine conjugate of oxidized PbTx-2; N-taurine conjugate of BTX-B5 |
C52H75NO17S 2 | 1017.5 2 | / | Clams (Mercenaria sp.; Macrocallista nimbosa); Cockles (Austrovenus stutchburyi); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea gigas) |
[17,20,22,23,31,32,33,37] | |
| #63 | Cysteine-BTX-B | S-desoxy-BTX-B2; S-deoxy-BTX-B2; Cysteine-BTX-B; Cysteine-PbTx; Cysteine-PbTx-B; Cysteine conjugate of PbTx-2 |
C53H79NO16S | 1017.5 | / | Clams (Mercenaria sp.; Macrocallista nimbosa); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Sharks (Carcharhinus limbatus, Rhizoprionodon terraenovae, Sphyrna tiburo); Spot (Leiostomus xanthurus); Rays (Dasyatis sabina) |
[17,22,23,28,34,35,48,49] | |
| #64 | Cysteine-BTX-B sulfoxide | BTX-B2; Cysteine-PbTx-B sulfoxide; Cysteine-PbTx sulfoxide; Oxidised-S-desoxy-BTX-B2 |
C53H79NO17S | 1033.5 | 4.3 | Clams (Mercenaria sp., Macrocallista nimbosa); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Sharks (Carcharhinus limbatus, Rhizoprionodon terraenovae, Sphyrna tiburo); Spot (Leiostomus xanthurus); Rays (Dasyatis sabina) |
[17,22,23,28,34,35,38,48,49] | |
| #65 | Oxidized cysteine-BTX-2 | Cysteine conjugate of oxidized PbTx-2 | C53H77NO17S | 1031.5 | / | Oysters (Crassostrea virginica) | [39] | |
| #66 | Open A-ring cysteine-BTX-B | Open A-ring S-desoxy-BTX-B2; Open A-ring cysteine–PbTx-B; Open A-ring cysteine–PbTx-2 |
C53H81NO17S | 1035.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,34,35,39] | |
| #67 | Glycine-cysteine-BTX-B | Glycine-cysteine-PbTx-B | C55H82N2O17S | 1074.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,34,39] | |
| #68 | γ-glutamyl-cysteine-BTX-B | γ-glutamyl-cysteine-PbTx-B | C58H86N2O19S | 1146.6 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,34,39] | |
| #69 | Glutathione-BTX-B | Glutathione-PbTx-B | C60H89N3O20S | 1203.6 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,34,39] | |
| #70 | N-tetradecanoyl-cysteine-BTX-B sulfoxide | BTX-B4; N-tetradecanoyl-BTX-B2; N-myristoyl-BTX-B2; N-myristoyl-cysteine- PbTx-B sulfoxide |
C67H105NO18S | 1243.7 | 11.3 | Mussels (Perna canaliculus); Oysters (Crassostrea virginica) | [39,40] | |
| #71 | N-hexadecanoyl-cysteine-BTX-B sulfoxide | BTX-B4; N-hexadecanoyl-BTX-B2; N-palmitoyl-BTX-B2; N-hexadecanoyl-cysteine–PbTx-B sulfoxide |
C69H109NO18S | 1271.7 | 12.3 | Clams (mercenaria sp.); Mussels (Perna canaliculus); Oysters (Crassostrea virginica) |
[22,39,40] | |
| #72 | N- (hydroxy-hexadecenoyl)-cysteine-BTX-B sulfoxide |
N- (hydroxy-hexadecenoyl)-BTX-B2 |
C69H107NO19S | 1285.7 | / | Clams (mercenaria sp.) | [22] | |
| #73 | N-octadecanoyl-cysteine-BTX-B sulfoxide | N-octadecanoyl-BTX-B2 | C71H113NO18S | 1299.8 | / | Clams (mercenaria sp.) | [22] | |
| #74 | N-octadecenoyl-cysteine-BTX-B sulfoxide | N-octadecenoyl-BTX-B2 | C71H111NO18S | 1297.8 | / | Clams (mercenaria sp.) | [22] | |
| #75 | N- (hydroxy-eicosanoyl)-cysteine-BTX-B sulfoxide |
N- (hydroxy-eicosanoyl)-BTX-B2 |
C73H117NO19S | 1343.8 | / | Clams (mercenaria sp.) | [22] | |
| #76 | N-arachidonyl-cysteine-BTX-B sulfoxide | N-arachidonyl-BTX-B2 | C73H109NO18S | 1319.7 | / | Clams (mercenaria sp.) | [22] | |
| #77 | N-didecenoyl-cysteine-BTX-B sulfoxide | N-didecenoyl-BTX-B2 | C73H115NO18S | 1325.8 | / | Clams (mercenaria sp.) | [22] | |
| #78 | N-tetradecanoyl-cysteine-BTX-B | N-tetradecanoyl-cysteine–PbTx-B | C67H105NO18S | 1243.7 | / | Oysters (Crassostrea virginica) | [39] | |
| #79 | N-hexadecanoyl-cysteine-BTX-B | N-hexadecanoyl-cysteine–PbTx-B; N-hexadecanoyl-S-deoxy-BTX-B2 |
C69H109NO17S | 1255.7 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,39] | |
| #80 | N-(hydroxy-hexadecenoyl)-cysteine-BTX-B | N-(hydroxy-hexadecenoyl)-S-deoxy-BTX-B2 | C69H107NO18S | 1269.7 | / | Clams (mercenaria sp.) | [22] | |
| #81 | N-octadecanoyl-cysteine-BTX-B | N-octadecanoyl-cysteine–PbTx-B; N-octadecanoyl-S-deoxy-BTX-B2 |
C71H113NO17S | 1283.8 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,39] | |
| #82 | N-octadecenoyl-cysteine-BTX-B | N-octadecenoyl-cysteine–PbTx-B | C71H111NO17S | 1281.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #83 | N-eicosenoyl-cysteine-BTX-B | N-eicosenoyl-cysteine–PbTx-B | C73H115NO17S | 1309.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #84 | N- (hydroxy-eicosanoyl)-cysteine-BTX-B |
N-(hydroxy-eicosanoyl)-cysteine–PbTx-B; N-(hydroxy-eicosanoyl)-S-deoxy-BTX-B2 |
C73H117NO18S | 1327.8 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) |
[22,39] | |
| #85 | Glycine-(N-tetradecanoyl)-cysteine-BTX-B | Glycine-(N-tetradecanoyl-cysteine)–PbTx-B | C69H108N2O18S | 1284.7 | / | Oysters (Crassostrea virginica) | [39] | |
| #86 | Glycine-(N-hexadecanoyl)-cysteine-BTX-B | Glycine-(N-hexadecanoyl-cysteine)– PbTx-B |
C71H112N2O18S | 1312.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #87 | Glycine–(N-hydroxy-eicosanoyl)-cysteine-BTX-B | Glycine–(N-hydroxy-eicosanoyl)-cysteine–PbTx-B | C75H120N2O19S | 1384.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #88 | Metabolite not named | / | C52H76O17S | 1004.5 | / | Oysters (Crassostrea virginica) | [30,39] |
3.1.2. Fatty Acid Conjugates of Brevetoxins
BTX-B3 is not a single compound, but a mixture of two fatty acid conjugates (myristic and palmitic acids) of the open D-ring of BTX-2 with oxidation of the terminal aldehyde (ID #60 and #61) (Figure 11) [36]. Therefore, the use of the term BTX-B3 is discouraged from a chemical perspective (Table 2). These analogs were isolated from greenshell mussels (Perna canaliculus) involved in the NSP events of 1992–1993. Interestingly, the mixture of these fatty acid conjugates did not kill mice by intraperitoneal injection at a dose of 300 µg/kg [36]. No further fatty acid conjugates of BTXs (without amino-acid conjugation) were reported.
Figure 11.
Chemical structures of fatty acid conjugates of brevetoxins (BTXs).
3.1.3. Amino Acid/Peptide Conjugates of Brevetoxins
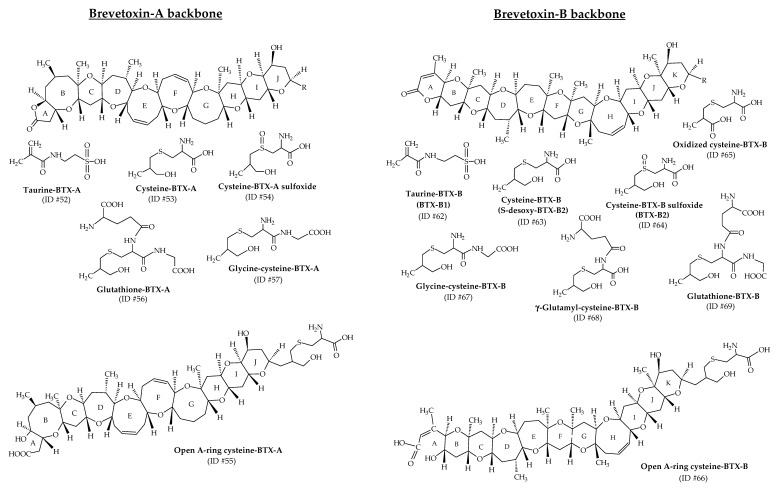
As previously described, taurine-BTX-B (ID #62; also called BTX-B1) and cysteine-BTX-B sulfoxide (ID #64; also called BTX-B2) were the first amino acid conjugates of BTXs identified [37,38], quickly followed by a series of amino acid/peptide conjugates (cysteine, taurine, glycine-cysteine, glutathione, γ-glutamyl-cysteine), among which figure the open A-ring conjugates [22,23,28,34,35,39,48]. Their structures are presented in Figure 12 (ID #52, #53, #54, #55, #56, #57, #62, #63, #64, #65, #66, #67, #68 and #69). Studies from oysters (Crassostrea virginica) exposed to K. brevis cultures demonstrated the accumulation of several metabolic products like cysteine-BTX-A (ID #53), cysteine-BTX-B (ID #63) and their respective sulfoxides (ID #54 and #64), which were then slowly eliminated [28,34,39]. These metabolites were detectable for up to 6 months in oysters. Additionally, cysteine-BTX-B sulfoxide could also be formed during shellfish extraction or storage of the extracts after sulfoxidation of cysteine-BTX-B [4]. It is important to consider this point to better estimate the actual metabolite content in shellfish in the field. A few years later, after repeated K. brevis blooms over a 3-year period in Sarasota Bay (Florida, USA), cysteine-BTX-B and its sulfoxide conjugate were detected in oysters (Crassostrea virginica) and were the most abundant and persistent metabolites among those monitored (BTX-3, cysteine and cysteine sulfoxide conjugates of BTX-A and BTX-B) [113]. Six months after experimental exposure to K. brevis, both conjugates were still detectable in tissues. The following peptide conjugates were later discovered with BTX-A and BTX-B backbone structures: glycine-cysteine–BTX (m/z 1047 and 1075, with respective ID numbers of #57 and #67), γ-glutamyl-cysteine–BTX (m/z 1147; ID #86), and glutathione–BTX (m/z 1176 and 1204, with respective ID numbers of #56 and #69) [39]. As for BTXs directly produced from K. brevis, the lactone group in the A-ring of the conjugate metabolites may open under certain conditions through hydrolysis. Cysteine–BTX-A and cysteine–BTX-B have been identified with their lactone ring opened (ID #55 and #66, respectively) in oysters (Crassostrea virginica) [34,35,39]. Binding these amino acids or peptides on the algal toxins decreases lipophilicity and can require method adjustments. The presence of more polar metabolites than algal BTXs has been demonstrated in oysters (Crassostrea virginica) that were naturally and experimentally exposed to K. brevis [35].
Figure 12.
Chemical structures of the amino acid/peptide metabolites of brevetoxins (BTXs) identified from shellfish.
3.1.4. Fatty Acid Derivatives of Brevetoxin Amino acid Conjugates
In most cases, fatty acids react with amino acid–BTX conjugates (or peptide-BTX conjugates) through amide linkage to form a series of 19 fatty acid–amino acid–BTX conjugates (ID #58 and #70 to #87 presented in Figure 13). A greater number of amino acid–fatty acid conjugates of BTX-B have been identified compared to BTX-A. BTX-B4, the first isolated, is a mixture of N-tetradecanoyl (N-myristoyl) and N-hexadecanoyl (N-palmitoyl) conjugates with the cysteine sulfoxide moiety of BTX-B2 (ID #70 and #71, respectively) [36,40]. Therefore, as for BTX-B3 (ID #60 and #61), the use of the term BTX-B4 is discouraged. Based on MS/MS fragmentations, Wang et al. [39] reported numerous new analogs, all mentioned in Table 2. Several of these metabolites were confirmed in hard clams (Mercenaria sp.) naturally exposed to K. brevis blooms [22].
Figure 13.
Chemical structures of amino acid-fatty acid conjugates of brevetoxins (BTXs) metabolized in shellfish.
3.1.5. Other Brevetoxin Metabolites
Two metabolites, with m/z of 976.5 and 1004.5 (ID #59 and #88, respectively), have been identified from Eastern oysters (Crassostrea virginica) collected in the Gulf of Mexico [30,39]. These compounds have not been named. LC-MS/MS analysis revealed the presence of characteristic fragments of A-type and B-type backbone structure of BTXs, allowing Wang et al. [39] to propose structures and fragmentation pathways (Figure 14). In both cases, the side chain possesses sulfoxide and carboxylic acid functions. These metabolites were much more abundant in field-exposed oysters compared with laboratory-exposed oysters. Since 2004, these BTX metabolites have never been reported in the literature.
Figure 14.
Chemical structures of other brevetoxin (BTX) metabolites identified from shellfish.
3.1.6. Biomarkers of Brevetoxin Exposure in Shellfish
The great diversity of physicochemical properties of BTXs complicates the development of efficient multi-toxin analytical methods. Importantly, the products of shellfish metabolism have different lipophilic properties compared to their precursor. The binding of an amino acid or a peptide to the algal toxin contributes to decreased lipophilicity, while binding of a fatty acid increases it. Therefore, the extraction of metabolites with highly variable polarities constitutes a major difficulty. For this reason, different authors emphasized the relevance of use several metabolites as biomarkers of BTX exposure to monitor the toxicity of shellfish following a K. brevis bloom [4,18,23]. BTX-3 (ID #9), BTX-B5 (ID #13), taurine-BTX-B (BTX-B1; ID #62), cysteine-BTX-B (S-deoxy-BTX-B2, ID #63), and cysteine-BTX-B sulfoxide (BTX-B2, ID #64) constitute relevant candidates due to their relative persistence. Nevertheless, considering the toxin profile differences between shellfish species, the appropriate biomarkers should be chosen carefully depending on the monitored shellfish species. This high specificity may also complicate the broad application of biomarkers of exposure when different species are targeted. Recently, a strategy was successfully applied to monitor BTX-B in gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata) and in one species of clams (Macrocallista nimbosa) [17]. All gastropod samples were contaminated by BTX-3 (ID #9), BTX-B5 (ID #13), open A-ring BTX-3 (ID #16), taurine-BTX-B (ID #62), cysteine-BTX-B (ID #63) and cysteine-BTX-B sulfoxide (ID #64). Clam samples contained the same metabolites, besides BTX-3 and open A-ring BTX-3.
3.1.7. Monitoring Programs for Brevetoxins in Shellfish
In the United States, brevetoxins widely impact the coasts, especially in the Gulf of Mexico. The closure of production areas is based on Karenia spp. cell count or toxin analysis in shellfish tissues. A maximum limit of 20 mouse units per 100 g of tissue is applied using the mouse bioassay (MBA) and, with certain limitations, the enzyme linked immunosorbent assay (ELISA), as described in the National Shellfish Sanitation Program [114]. The re-opening of production areas is based on toxin analysis. With these analytical tools, the identity of the analogs remains unknown. In Australia, production area closures have different strategies depending on the federal states. MBA is implemented in Victoria and New South Wales; however, an LC-MS/MS analysis is also carried out in New South Wales [115,116]. BTX-2 (ID #8), BTX-3 (ID #9), BTX-B5 (ID #13), taurine-BTX-B (BTX-B1; ID #62), cysteine-BTX-B (S-deoxy-BTX-B2; ID #63), and cysteine-BTX-B sulfoxide (BTX-B2; ID #64) are the BTX analogs sought [117]. In New Zealand, BTXs are regulated (maximum permissible level of 0.8 mg BTX-2 equivalent per kg of shellfish meat) and the reference method is based on LC-MS [118]. In France, BTX-2 and BTX-3 have been monitored since 2018 [10] as part of a program on emerging toxins in marine shellfish (EMERGTOX). Recently, a working group was set up by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) to prevent health risks associated with the consumption of shellfish contaminated with BTXs [11,119]. A guidance level of 180 µg BTX-3 eq./kg shellfish meat was established, considering a protective default portion size of 400 g shellfish meat. A monitoring strategy has also been proposed and is divided into three parts [119]. First, the implementation of an ELISA test for the screening of B-type brevetoxin metabolites in shellfish flesh. This approach considers brevetoxins not quantifiable by LC-MS/MS due to the lack of commercial standards. Second, the targeted physicochemical analysis of brevetoxins for which standards are available with an LC-MS/MS method developed for a wide range of polarity. Based on available reference materials and toxicity data, a list of analogs to seek first was established and includes: BTX-1 (ID #1), BTX-2 (ID #8), BTX-3 (ID #9), oxidized open D-ring tetradecanoyl-BTX-2 (BTX-B3; ID #60), oxidized open D-ring hexadecanoyl-BTX-2 (BTX-B3; ID #61), taurine-BTX-B (BTX B1; ID #62), cysteine-BTX-B (S-desoxy-BTX-B2; ID #63), cysteine-BTX-B sulfoxide (BTX-B2; ID #64), N-tetradecanoyl-cysteine-BTX-B sulfoxide (N-myristoyl-BTX-B2; ID #70), and N-hexadecanoyl-cysteine-BTX-B sulfoxide (N-palmitoyl-BTX-B2; ID #71). Lastly, the implementation of a non-targeted analysis using liquid chromatography-high resolution mass spectrometry (LC-HRMS) has been suggested to screen a large number of compounds and identify possible new BTX analogs or degradation products.
3.2. Gymnodimines (GYMs)
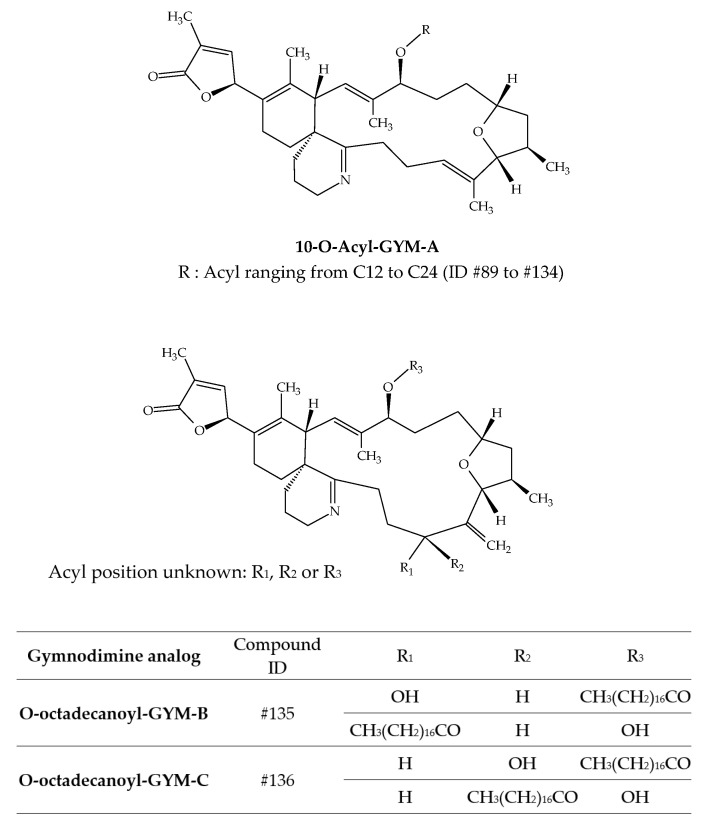
Several studies have demonstrated the accumulation of gymnodimines (GYMs) in different shellfish species (Table 3). The discovery of GYM-A (ID #30) from New Zealand oysters (Tiostrea chilensis) in 1995 immediately highlighted this capacity [24]. A few years later, several batches of clams harvested in Tunisia were neurotoxic to mice by i.p. [27]. Analytical investigations allowed for the unequivocal identification of GYM-A. Several studies on Tunisian samples followed [25,26]. In 2012, GYM-A, GYM-B (ID #31) and GYM-C (ID #32) were detected in the same shellfish species collected in the Gulf of Gabes, along with the presence of K. selliformis [25]. Other studies were carried out in South Africa, Australia, and China (Figure 1) and allowed for the detection of GYM-A in oysters, gastropods, mussels, donax and pen shells [43,44,45,46,47]. In 2013, numerous gymnodimine fatty acid conjugates were identified from grooved carpet shells (Ruditapes decussatus) collected in Tunisia (Gulf of Gabes) [42]. In total, 46 fatty acid conjugates of GYM-A (ID #89 to ID #134) have been reported, with acyl carbon chain lengths comprised between C12:0 and C24:6 (Table 3). The presence of a series of GYM-B and GYM-C fatty acid conjugates was also established, with esters of 16, 18, 20 and 22 carbon chain lengths being the most abundant. However, only the 18:0 esters of GYM-B and GYM-C were specifically identified, with respective ID number of #135 and # ID136 (Figure 15). Some of the GYM-A fatty acids conjugates were also identified in shellfish collected in the South China Sea [41]. Clam (Antigona lamellaris) and pen shell (Atrina pectinata) profiles were different. In clams, the 18:0 ester was the most abundant, followed by the 20:1 ester, whereas the 20:1, 18:0 and 22:2 esters dominated the acyl ester profile in pen shell samples. These authors also experimentally exposed mussels (Mytilus galloprovincialis) to K. selliformis for 96 h. In all, 28 fatty acids esters were detected with acyl carbon chain lengths ranging between C14:0 and C24:6. Interestingly, GYM-A was detected in oysters (Crassostrea sp.) and gastropods (Batillaria zonalis), but no GYM-A esters were observed in these species. Further investigations are required to obtain better knowledge of GYM toxicity. However, considering the significant presence of GYM acyl esters, more occurrence data are required to conduct a risk assessment at a later stage.
Table 3.
Physicochemical characteristics of gymnodimine (GYM) metabolites reported in shellfish (only gymnodimine analogs produced from K. selliformis).
| Gymnodimine (GYM) Metabolite |
Compound Identification Number (ID) | Carboxyl Group of the Ester (Carbon:Unsaturation) |
Molecular Formula |
Monoisotopic Mass (Da) | LogP 1 | Sample | References |
|---|---|---|---|---|---|---|---|
| GYM-A | #30 | / | C32H45NO4 | 507.3 | 6.4 | Clams (Antigona lamella, Ruditapes decussatus); Gastropods (Batillaria zonalis); Oysters (Crassostrea angulata, Crassostrea ariakensis, Crassostrea giga, Dendostrea crenulifrea, Saccostrea glomerata, Tiostrea chilensis); Mussels (Choromytilus meridionalis, Mytilus galloprovincialis, Modiolus proclivis); Pipis (Donax deltoides); Pen shells (Atrina pectinata); |
[24,25,26,27,41,44,45,46,47] |
| GYM-B | #31 | / | C32H45NO5 | 523.3 | 5.1 | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [25,41,42] |
| GYM-C | #32 | / | C32H45NO5 | 523.3 | / | Clams (Ruditapes decussatus) | [25,42] |
| 10-O-dodecanoyl-GYM-A | #89 | 12:0 | C44H67NO5 | 689.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-dodecenoyl-GYM-A | #90 | 12:1 | C44H65NO5 | 687.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetradecanoyl GYM-A | #91 | 14:0 | C46H71NO5 | 717.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetradecenoyl GYM-A | #92 | 14:1 | C46H69NO5 | 715.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetradecatrienoyl-GYM-A | #93 | 14:3 | C46H65NO5 | 711.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-pentadecanoyl-GYM-A | #94 | 15:0 | C47H73NO5 | 731.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-pentadecenoyl-GYM-A | #95 | 15:1 | C47H71NO5 | 729.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-hexadecanoyl-GYM-A | #96 | 16:0 | C48H75NO5 | 745.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) |
[41,42] |
| 10-O-hexadecenoyl-GYM-A | #97 | 16:1 | C48H73NO5 | 743.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-hexadecadienoyl-GYM-A | #98 | 16:2 | C48H71NO5 | 741.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-hexadecatrienoyl-GYM-A | #99 | 16:3 | C48H69NO5 | 739.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-hexadecatetraenoyl-GYM-A | #100 | 16:4 | C48H67NO5 | 737.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heptadecanoyl-GYM-A | #101 | 17:0 | C49H77NO5 | 759.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-heptadecenoyl-GYM-A | #102 | 17:1 | C49H75NO5 | 757.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-heptadecadienoyl-GYM-A | #103 | 17:2 | C49H73NO5 | 755.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecanoyl-GYM-A | #104 | 18:0 | C50H79NO5 | 773.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) |
[41,42] |
| 10-O-octadecenoyl-GYM-A | #105 | 18:1 | C50H77NO5 | 771.6 | / | Clams (Ruditapes decussatus; Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) |
[41,42] |
| 10-O-octadecadienoyl-GYM-A | #106 | 18:2 | C50H75NO5 | 769.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecatrienoyl-GYM-A | #107 | 18:3 | C50H73NO5 | 767.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecatetraenoyl-GYM-A | #108 | 18:4 | C50H71NO5 | 765.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-nonadecanoyl-GYM-A | #109 | 19:0 | C51H81NO5 | 787.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-nonadecenoyl-GYM-A | #110 | 19:1 | C51H79NO5 | 785.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-nonadecadienoyl-GYM-A | #111 | 19:2 | C51H77NO5 | 783.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosanoyl-GYM-A | #112 | 20:0 | C52H83NO5 | 801.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-eicosenoyl-GYM-A | #113 | 20:1 | C52H81NO5 | 799.6 | / | Clams (Ruditapes decussatus; Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) |
[41,42] |
| 10-O-eicosadienoyl-GYM-A | #114 | 20:2 | C52H79NO5 | 797.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) |
[41,42] |
| 10-O-eicosatrienoyl-GYM-A | #115 | 20:3 | C52H77NO5 | 795.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosatetraenoyl-GYM-A | #116 | 20:4 | C52H75NO5 | 793.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosapentaenoyl-GYM-A | #117 | 20:5 | C52H72NO5 | 790.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-heneicosanoyl-GYM-A | #118 | 21:0 | C53H85NO5 | 815.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosenoyl-GYM-A | #119 | 21:1 | C53H83NO5 | 813.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosadienoyl-GYM-A | #120 | 21:2 | C53H81NO5 | 811.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosatrienoyl-GYM-A | #121 | 21:3 | C53H79NO5 | 809.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosatetraenoyl-GYM-A | #122 | 21:4 | C53H77NO5 | 807.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosapentaenoyl-GYM-A | #123 | 21:5 | C53H75NO5 | 805.6 | / | Mussels (M. galloprovincialis) | [41] |
| 10-O-docosanoyl-GYM-A | #124 | 22:0 | C54H87NO5 | 829.7 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-docosenoyl-GYM-A | #125 | 22:1 | C54H85NO5 | 827.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-docosadienoyl-GYM-A | #126 | 22:2 | C54H83NO5 | 825.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) |
[41,42] |
| 10-O-docosatrienoyl-GYM-A | #127 | 22:3 | C54H81NO5 | 823.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosatetraenoyl-GYM-A | #128 | 22:4 | C54H79NO5 | 821.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosapentaenoyl-GYM-A | #129 | 22:5 | C54H77NO5 | 819.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosahexaenoyl-GYM-A | #130 | 22:6 | C54H75NO5 | 817.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-tetracosanoyl-GYM-A | #131 | 24:0 | C56H91NO5 | 857.7 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetracosenoyl-GYM-A | #132 | 24:1 | C56H89NO5 | 855.7 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetracosapentaenoyl-GYM-A | #133 | 24:5 | C56H81NO5 | 847.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-tetracosahexaenoyl-GYM-A | #134 | 24:6 | C56H79NO5 | 845.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| O-octadecanoyl-GYM-B | #135 | 18:0 | C50H79NO6 | 789.6 | / | Clams (Ruditapes decussatus) | [42] |
| O-octadecanoyl-GYM-C | #136 | 18:0 | C50H79NO6 | 789.6 | / | Clams (Ruditapes decussatus) | [42] |
1 LogP predicted from the chemical structure of molecules using the ACD/Labs platform. Octanol/water partition coefficient are predicted using algorithms.
Figure 15.
Chemical structures of fatty acid conjugates of gymnodimines (GYMs).
4. Accumulation and Biotransformation of Metabolites Produced by Karenia spp. in Other Marine Organisms
Blooms of K. brevis are commonly associated with massive mortality for marine organisms. Massive death episodes of fish (prey fishes, sharks, rays), mammals (dolphins, manatees) or shorebirds, all occurring in Florida between 1999 and 2006, were associated with K. brevis blooms and the presence of BTXs [5,49,50,120]. Data regarding the accumulation and biotransformation of the other groups of toxins produced by Karenia spp. are lacking. In several studies, the implementation of biochemical methods of analysis, such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), or receptor-binding assay (RBA), made it possible to measure high levels of BTX-like toxins in animal tissues, but did not enable identification of the specific nature of the BTX analogs. In a few studies conducted on samples collected in the Gulf of Mexico (Figure 1), LC-MS and LC-MS/MS analyses were implemented to obtain this information [2,48,49,50].
In fish, BTX-3 (ID #9) was identified in Lagodon rhomboides and Leiostomus xanthurus, whereas open A-ring BTX-3 (ID #16) and open A-ring BTX-7 (ID #7), not reported in shellfish to date, were also reported [48]. Among the amino-acid conjugates, cysteine-BTX-A (ID #53), cysteine BTX-A sulfoxide (ID #54), cysteine-BTX-B (ID #61) and cysteine-BTX-B sulfoxide (ID #62) were also identified in these fish species. From 301 live or dead sharks and rays from Florida, Flewelling at al. [49] studied the accumulation of BTXs. Some shark embryos were also included. For dead animals, the cause of their death was unknown but sometimes occurred during red-tide episodes of K. brevis blooms. Among these samples, a subset of liver and gill tissues were selected, and several metabolites were sought through LC-MS analysis (BTX-1, BTX-2, BTX-3, BTX-6, BTX-7, BTX-9, BTX-10, cysteine-BTX-B, cysteine-BTX-B sulfoxide and brevenal with respective ID numbers #1, #8, #9, #11, #2, #12, #3, #61, #62, #20). BTXs were detected in 26 of the 27 selected extracts. BTX-3 was quantified in most of the liver and gill tissues (n = 21), whereas BTX-2 was only quantified in gills of two sharks. Cysteine-BTX-B and its sulfoxide were detected in 18 and 9 extracts of gills or livers, respectively.
After major dolphin (Tursiops truncatus) mortality events between 1999 and 2006 in Florida, BTXs and domoic acid (amnesic toxin produced by the Pseudo-nitzschia diatom) were sought in dead animals [50]. Of the 105 animals collected in 2004, 100% of the tested animals were positive for BTXs and 89% for domoic acid. Moreover, dolphin stomach contents frequently consisted of BTX-contaminated menhaden (Brevoortia sp.). For the period 2005–2006, 93% of the 90 dolphins were positive for BTXs, whereas domoic acid was not detected in these animals. Among the samples analyzed by LC-MS, BTX-3 (ID #9) was the predominant toxin in the four stomach contents tested (fishes partially digested) and was also detected in 10 of the 12 livers tested. Two peaks co-eluting with BTX-2 were not attributed to any known toxin.
An experimental study on shrimps (Litopenaeus vannamei) demonstrated that these marine organisms could represent a vector of BTXs [112]. The authors found by LC-MS levels of 80 and 90 µg eq BTX-2/kg in the digestive glands and muscles, respectively. It should be noted that this contamination started to appear in the digestive gland and the muscle after 30 and 45 days of exposure, respectively, and with relatively low cellular concentrations of K. brevis (103 to 106 cells/L). Macrobenthic invertebrates (including polychaetes and crustaceans belonging to amphipods and isopods) collected during a K. brevis bloom in Florida also demonstrated their capacity to accumulate BTXs [2]. However, the identity of the analogs was not determined due to the analytical tool implemented for the analysis (ELISA test).
5. Conclusions
In this review, we summarized and gathered the knowledge reported in the literature over recent decades of over 130 potentially toxic metabolites reported from Karenia spp. or metabolized by marine organisms. The structures and certain properties of these metabolites are presented. This information could constitute an interesting basis in view of library-building, particularly for physicochemical analysis using liquid chromatography combined with high-resolution mass spectrometry (LC-HRMS). Such an approach could be an interesting tool to simultaneously screen the different analogs mentioned here, and could also allow us to discover new analogs. The accumulation and biotransformation by marine organisms of the metabolites produced by Karenia spp. was also assessed in depth for BTXs and GYMs. We point out the need for more information on BTXs in marine organisms other than shellfish. Occurrence data would also be valuable for several groups of potentially toxic metabolites discovered in the last two decades. These data, combined with additional toxicology assays, could be used to carry out a risk assessment. Lastly, we compiled all the names reported for metabolites in the existing literature. Among the different nomenclatures, we tried to select the terms that allowed us to obtain a better overall consistency, hoping that this work will be useful to harmonize practices in the future.
Acknowledgments
The authors sincerely thank the other experts who participated in the working group set up by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) to prevent health risks associated with the consumption of shellfish contaminated with BTXs in France, that is to say: Katia Comte, Estelle Chaix, Nicolas Delcourt, César Mattei, Jordi Molgó and Raphaele Le Garrec.
Author Contributions
Z.A. and V.H. contributed equally to this manuscript. Conceptualization, V.H. and Z.A.; investigation, V.H. and Z.A.; writing—original draft preparation, V.H. and Z.A.; writing—review and editing, E.A., M.-Y.D.B., N.A., V.H. and Z.A.; visualization, V.H.; supervision, V.H. and Z.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest. Public DOIs are available on the following website: https://dpi.sante.gouv.fr/, accessed on 22 November 2021.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adl S.M., Bass D., Lane C.E., Lukeš J., Schoch C.L., Smirnov A., Agatha S., Berney C., Brown M.W., Burki F., et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019;66:4–119. doi: 10.1111/jeu.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bricelj V.M., Haubois A.-G., Sengco M.R., Pierce R.H., Culter J.K., Anderson D.M. Trophic Transfer of Brevetoxins to the Benthic Macrofaunal Community during a Bloom of the Harmful Dinoflagellate Karenia Brevis in Sarasota Bay, Florida. Harmful Algae. 2012;16:27–34. doi: 10.1016/j.hal.2012.01.001. [DOI] [Google Scholar]
- 3.Brand L.E., Campbell L., Bresnan E. Karenia: The Biology and Ecology of a Toxic Genus. Harmful Algae. 2012;14:156–178. doi: 10.1016/j.hal.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plakas S.M., Dickey R.W. Advances in Monitoring and Toxicity Assessment of Brevetoxins in Molluscan Shellfish. Toxicon. 2010;56:137–149. doi: 10.1016/j.toxicon.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Flewelling L.J., Naar J.P., Abbott J.P., Baden D.G., Barros N.B., Bossart G.D., Dechraoui Bottein M.-Y., Hammond D.G., Haubold E.M., Heil C.A., et al. Red Tides and Marine Mammal Mortalities. Nature. 2005;435:755–756. doi: 10.1038/nature435755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen M., Xu J., Tsang T.Y., Au D.W.T. Toxicity Comparison between Chattonella Marina and Karenia Brevis Using Marine Medaka (Oryzias Melastigma): Evidence against the Suspected Ichthyotoxins of Chattonella Marina. Chemosphere. 2010;80:585–591. doi: 10.1016/j.chemosphere.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Berdalet E., Fleming L.E., Gowen R., Davidson K., Hess P., Backer L.C., Moore S.K., Hoagland P., Enevoldsen H. Marine Harmful Algal Blooms, Human Health and Wellbeing: Challenges and Opportunities in the 21st Century. J. Mar. Biol. Assoc. UK. 2016;96:61–91. doi: 10.1017/S0025315415001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young N., Sharpe R.A., Barciela R., Nichols G., Davidson K., Berdalet E., Fleming L.E. Marine Harmful Algal Blooms and Human Health: A Systematic Scoping Review. Harmful Algae. 2020;98:101901. doi: 10.1016/j.hal.2020.101901. [DOI] [PubMed] [Google Scholar]
- 9.Watkins S.M., Reich A., Fleming L.E., Hammond R. Neurotoxic Shellfish Poisoning. Mar. Drugs. 2008;6:431–455. doi: 10.3390/md6030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amzil Z., Derrien A., Terre Terrillon A., Duval A., Connes C., Marco-Miralles F., Nézan E., Mertens K.N. Monitoring the Emergence of Algal Toxins in Shellfish: First Report on Detection of Brevetoxins in French Mediterranean Mussels. Mar. Drugs. 2021;19:393. doi: 10.3390/md19070393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnich N., Abadie E., Amzil Z., Dechraoui Bottein M.-Y., Comte K., Chaix E., Delcourt N., Hort V., Mattei C., Molgó J., et al. Guidance Level for Brevetoxins in French Shellfish. Mar. Drugs. 2021;19:520. doi: 10.3390/md19090520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruana A.M.N., Amzil Z. Chapter 13—Microalgae and Toxins. In: Levine I.A., Fleurence J., editors. Microalgae in Health and Disease Prevention. Academic Press; Cambridge, MA, USA: 2018. pp. 263–305. [Google Scholar]
- 13.Baden D.G. Brevetoxins: Unique Polyether Dinoflagellate Toxins. FASEB J. 1989;3:1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- 14.Poli M.A., Mende T.J., Baden D.G. Brevetoxins, Unique Activators of Voltage-Sensitive Sodium Channels, Bind to Specific Sites in Rat Brain Synaptosomes. Mol. Pharm. 1986;30:129–135. [PubMed] [Google Scholar]
- 15.Daugbjerg N., Hansen G., Larsen J., Moestrup Ø. Phylogeny of Some of the Major Genera of Dinoflagellates Based on Ultrastructure and Partial LSU RDNA Sequence Data, Including the Erection of Three New Genera of Unarmoured Dinoflagellates. Phycologia. 2000;39:302–317. doi: 10.2216/i0031-8884-39-4-302.1. [DOI] [Google Scholar]
- 16.Shen H., Song X., Zhang Y., Zhang P., Li J., Song W., Yu Z. Profiling of Brevetoxin Metabolites Produced by Karenia Brevis 165 Based on Liquid Chromatography-Mass Spectrometry. Toxins. 2021;13:354. doi: 10.3390/toxins13050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham A., Flewelling L.J., El Said K.R., Odom W., Geiger S.P., Granholm A.A., Jackson J.T., Bodager D. An Occurrence of Neurotoxic Shellfish Poisoning by Consumption of Gastropods Contaminated with Brevetoxins. Toxicon. 2021;191:9–17. doi: 10.1016/j.toxicon.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Abraham A., El Said K.R., Flewelling L.J. Role of Biomarkers in Monitoring Brevetoxins in Karenia Brevis Exposed Shellfish. Food Saf. 2018;6:33–43. doi: 10.14252/foodsafetyfscj.2017021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on Marine Biotoxins in Shellfish—Emerging Toxins: Brevetoxin Group. EFSA J. 2010;8:1677. doi: 10.2903/j.efsa.2010.1677. [DOI] [Google Scholar]
- 20.Nozawa A., Tsuji K., Ishida H. Implication of Brevetoxin B1 and PbTx-3 in Neurotoxic Shellfish Poisoning in New Zealand by Isolation and Quantitative Determination with Liquid Chromatography-Tandem Mass Spectrometry. Toxicon. 2003;42:91–103. doi: 10.1016/S0041-0101(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 21.Ishida H., Muramatsu N., Nukaya H., Kosuge T., Tsuji K. Study on Neurotoxic Shellfish Poisoning Involving the Oyster, Crassostrea Gigas, in New Zealand. Toxicon. 1996;34:1050–1053. doi: 10.1016/0041-0101(96)00076-1. [DOI] [PubMed] [Google Scholar]
- 22.Abraham A., Wang Y., El Said K.R., Plakas S.M. Characterization of Brevetoxin Metabolism in Karenia Brevis Bloom-Exposed Clams (Mercenaria sp.) by LC-MS/MS. Toxicon. 2012;60:1030–1040. doi: 10.1016/j.toxicon.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Abraham A., El Said K.R., Wang Y., Jester E.L.E., Plakas S.M., Flewelling L.J., Henry M.S., Pierce R.H. Biomarkers of Brevetoxin Exposure and Composite Toxin Levels in Hard Clam (Mercenaria sp.) Exposed to Karenia Brevis Blooms. Toxicon. 2015;96:82–88. doi: 10.1016/j.toxicon.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Seki T., Satake M., Mackenzie L., Kaspar H.F., Yasumoto T. Gymnodimine, a New Marine Toxin of Unprecedented Structure Isolated from New Zealand Oysters and the Dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995;36:7093–7096. doi: 10.1016/0040-4039(95)01434-J. [DOI] [Google Scholar]
- 25.Ben Naila I., Hamza A., Gdoura R., Diogène J., de la Iglesia P. Prevalence and Persistence of Gymnodimines in Clams from the Gulf of Gabes (Tunisia) Studied by Mouse Bioassay and LC–MS/MS. Harmful Algae. 2012;18:56–64. doi: 10.1016/j.hal.2012.04.004. [DOI] [Google Scholar]
- 26.Marrouchi R., Dziri F., Belayouni N., Hamza A., Benoit E., Molgó J., Kharrat R. Quantitative Determination of Gymnodimine-A by High Performance Liquid Chromatography in Contaminated Clams from Tunisia Coastline. Mar. Biotechnol. 2010;12:579–585. doi: 10.1007/s10126-009-9245-7. [DOI] [PubMed] [Google Scholar]
- 27.Biré R., Krys S., Frémy J.-M., Dragacci S., Stirling D., Kharrat R. First Evidence on Occurrence of Gymnodimine in Clams from Tunisia. J. Nat. Toxins. 2002;11:269–275. [PubMed] [Google Scholar]
- 28.Plakas S.M., El Said K.R., Jester E.L.E., Ray Granade H., Musser S.M., Dickey R.W. Confirmation of Brevetoxin Metabolism in the Eastern Oyster (Crassostrea Virginica) by Controlled Exposures to Pure Toxins and to Karenia Brevis Cultures. Toxicon. 2002;40:721–729. doi: 10.1016/S0041-0101(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 29.Poli M.A., Musser S.M., Dickey R.W., Eilers P.P., Hall S. Neurotoxic Shellfish Poisoning and Brevetoxin Metabolites: A Case Study from Florida. Toxicon. 2000;38:981–993. doi: 10.1016/S0041-0101(99)00191-9. [DOI] [PubMed] [Google Scholar]
- 30.Dickey R., Jester E., Granade R., Mowdy D., Moncreiff C., Rebarchik D., Robl M., Musser S., Poli M. Monitoring Brevetoxins during a Gymnodinium Breve Red Tide: Comparison of Sodium Channel Specific Cytotoxicity Assay and Mouse Bioassay for Determination of Neurotoxic Shellfish Toxins in Shellfish Extracts. Nat. Toxins. 1999;7:157–165. doi: 10.1002/(SICI)1522-7189(199907/08)7:4<157::AID-NT52>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Ishida H., Nozawa A., Nukaya H., Tsuji K. Comparative Concentrations of Brevetoxins PbTx-2, PbTx-3, BTX-B1 and BTX-B5 in Cockle, Austrovenus Stutchburyi, Greenshell Mussel, Perna Canaliculus, and Pacific Oyster, Crassostrea Gigas, Involved Neurotoxic Shellfish Poisoning in New Zealand. Toxicon. 2004;43:779–789. doi: 10.1016/j.toxicon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Ishida H., Nozawa A., Nukaya H., Rhodes L., McNabb P., Holland P.T., Tsuji K. Confirmation of Brevetoxin Metabolism in Cockle, Austrovenus Stutchburyi, and Greenshell Mussel, Perna Canaliculus, Associated with New Zealand Neurotoxic Shellfish Poisoning, by Controlled Exposure to Karenia Brevis Culture. Toxicon. 2004;43:701–712. doi: 10.1016/j.toxicon.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Ishida H., Nozawa A., Hamano H., Naoki H., Fujita T., Kaspar H.F., Tsuji K. Brevetoxin B5, a New Brevetoxin Analog Isolated from Cockle Austrovenus Stutchburyi in New Zealand, the Marker for Monitoring Shellfish Neurotoxicity. Tetrahedron Lett. 2004;45:29–33. doi: 10.1016/j.tetlet.2003.10.124. [DOI] [Google Scholar]
- 34.Plakas S.M., Wang Z., El Said K.R., Jester E.L.E., Granade H.R., Flewelling L., Scott P., Dickey R.W. Brevetoxin Metabolism and Elimination in the Eastern Oyster (Crassostrea Virginica) after Controlled Exposures to Karenia Brevis. Toxicon. 2004;44:677–685. doi: 10.1016/j.toxicon.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Abraham A., Plakas S.M., Wang Z., Jester E.L.E., El Said K.R., Granade H.R., Henry M.S., Blum P.C., Pierce R.H., Dickey R.W. Characterization of Polar Brevetoxin Derivatives Isolated from Karenia Brevis Cultures and Natural Blooms. Toxicon. 2006;48:104–115. doi: 10.1016/j.toxicon.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Morohashi A., Satake M., Murata K., Naoki H., Kaspar H.F., Yasumoto T. Brevetoxin B3, a New Brevetoxin Analog Isolated from the Greenshell Mussel Perna Canaliculus Involved in Neurotoxic Shellfish Poisoning in New Zealand. Tetrahedron Lett. 1995;36:8995–8998. doi: 10.1016/0040-4039(95)01969-O. [DOI] [Google Scholar]
- 37.Ishida H., Nozawa A., Totoribe K., Muramatsu N., Nukaya H., Tsuji K., Yamaguchi K., Yasumoto T., Kaspar H., Berkett N., et al. Brevetoxin B1, a New Polyether Marine Toxin from the New Zealand Shellfish, Austrovenus Stutchburyi. Tetrahedron Lett. 1995;36:725–728. doi: 10.1016/0040-4039(94)02326-7. [DOI] [Google Scholar]
- 38.Murata K., Satake M., Naoki H., Kaspar H.F., Yasumoto T. Isolation and Structure of a New Brevetoxin Analog, Brevetoxin B2, from Greenshell Mussels from New Zealand. Tetrahedron. 1998;54:735–742. doi: 10.1016/S0040-4020(97)10336-2. [DOI] [Google Scholar]
- 39.Wang Z., Plakas S.M., El Said K.R., Jester E.L.E., Granade H.R., Dickey R.W. LC/MS Analysis of Brevetoxin Metabolites in the Eastern Oyster (Crassostrea Virginica) Toxicon. 2004;43:455–465. doi: 10.1016/j.toxicon.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Morohashi A., Satake M., Naoki H., Kaspar H.F., Oshima Y., Yasumoto T. Brevetoxin B4 Isolated from Greenshell Mussels Perna Canaliculus, the Major Toxin Involved in Neurotoxic Shellfish Poisoning in New Zealand. Nat. Toxins. 1999;7:45–48. doi: 10.1002/(SICI)1522-7189(199903/04)7:2<45::AID-NT34>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Ji Y., Che Y., Wright E.J., McCarron P., Hess P., Li A. Fatty Acid Ester Metabolites of Gymnodimine in Shellfish Collected from China and in Mussels (Mytilus Galloprovincialis) Exposed to Karenia Selliformis. Harmful Algae. 2020;92:101774. doi: 10.1016/j.hal.2020.101774. [DOI] [PubMed] [Google Scholar]
- 42.De la Iglesia P., McCarron P., Diogène J., Quilliam M.A. Discovery of Gymnodimine Fatty Acid Ester Metabolites in Shellfish Using Liquid Chromatography/Mass Spectrometry. Rapid Commun. Mass Spectrom. 2013;27:643–653. doi: 10.1002/rcm.6491. [DOI] [PubMed] [Google Scholar]
- 43.Li A., Sun G., Qiu J., Fan L. Lipophilic Shellfish Toxins in Dinophysis Caudata Picked Cells and in Shellfish from the East China Sea. Environ. Sci. Pollut. Res. 2015;22:3116–3126. doi: 10.1007/s11356-014-3595-z. [DOI] [PubMed] [Google Scholar]
- 44.Liu R., Liang Y., Wu X., Xu D., Liu Y., Liu L. First Report on the Detection of Pectenotoxin Groups in Chinese Shellfish by LC–MS/MS. Toxicon. 2011;57:1000–1007. doi: 10.1016/j.toxicon.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Jiang T., Liu L., Li Y., Zhang J., Tan Z., Wu H., Jiang T., Lu S. Occurrence of Marine Algal Toxins in Oyster and Phytoplankton Samples in Daya Bay, South China Sea. Chemosphere. 2017;183:80–88. doi: 10.1016/j.chemosphere.2017.05.067. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi E., Yu Q., Eaglesham G., Connell D.W., McBroom J., Costanzo S., Shaw G.R. Occurrence and Seasonal Variations of Algal Toxins in Water, Phytoplankton and Shellfish from North Stradbroke Island, Queensland, Australia. Mar. Environ. Res. 2007;64:429–442. doi: 10.1016/j.marenvres.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Krock B., Pitcher G.C., Ntuli J., Cembella A.D. Confirmed Identification of Gymnodimine in Oysters from the West Coast of South Africa by Liquid Chromatography–Tandem Mass Spectrometry. Afr. J. Mar. Sci. 2009;31:113–118. doi: 10.2989/AJMS.2009.31.1.12.783. [DOI] [Google Scholar]
- 48.Fire S.E., Flewelling L.J., Naar J., Twiner M.J., Henry M.S., Pierce R.H., Gannon D.P., Wang Z., Davidson L., Wells R.S. Prevalence of Brevetoxins in Prey Fish of Bottlenose Dolphins in Sarasota Bay, Florida. Mar. Ecol. Prog. Ser. 2008;368:283–294. doi: 10.3354/meps07643. [DOI] [Google Scholar]
- 49.Flewelling L., Adams D., Naar J., Atwood K., Granholm A., O’Dea S., Landsberg J. Brevetoxins in Sharks and Rays (Chondrichthyes, Elasmobranchii) from Florida Coastal Waters. Mar. Biol. 2010;157:1937–1953. doi: 10.1007/s00227-010-1463-z. [DOI] [Google Scholar]
- 50.Twiner M.J., Flewelling L.J., Fire S.E., Bowen-Stevens S.R., Gaydos J.K., Johnson C.K., Landsberg J.H., Leighfield T.A., Mase-Guthrie B., Schwacke L., et al. Comparative Analysis of Three Brevetoxin-Associated Bottlenose Dolphin (Tursiops Truncatus) Mortality Events in the Florida Panhandle Region (USA) PLoS ONE. 2012;7:e42974. doi: 10.1371/journal.pone.0042974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radwan F.F.Y., Ramsdell J.S. Characterization of In Vitro Oxidative and Conjugative Metabolic Pathways for Brevetoxin (PbTx-2) Toxicol. Sci. 2006;89:57–65. doi: 10.1093/toxsci/kfj013. [DOI] [PubMed] [Google Scholar]
- 52.Guo F., An T., Rein K.S. Human Metabolites of Brevetoxin PbTx-2: Identification and Confirmation of Structure. Toxicon. 2010;56:648–651. doi: 10.1016/j.toxicon.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gawley R.E., Rein K.S., Jeglitsch G., Adams D.J., Theodorakis E.A., Tiebes J., Nicolaou K.C., Baden D.G. The Relationship of Brevetoxin ‘Length’ and A-Ring Functionality to Binding and Activity in Neuronal Sodium Channels. Chem. Biol. 1995;2:533–541. doi: 10.1016/1074-5521(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 54.Jeglitsch G., Rein K., Baden D.G., Adams D.J. Brevetoxin-3 (PbTx-3) and Its Derivatives Modulate Single Tetrodotoxin-Sensitive Sodium Channels in Rat Sensory Neurons. J. Pharm. Exp. 1998;284:516–525. [PubMed] [Google Scholar]
- 55.Purkerson-Parker S.L., Fieber L.A., Rein K.S., Podona T., Baden D.G. Brevetoxin Derivatives That Inhibit Toxin Activity. Chem. Biol. 2000;7:385–393. doi: 10.1016/S1074-5521(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 56.Satake M., Shoji M., Oshima Y., Naoki H., Fujita T., Yasumoto T. Gymnocin-A, a Cytotoxic Polyether from the Notorious Red Tide Dinoflagellate, Gymnodinium Mikimotoi. Tetrahedron Lett. 2002;43:5829–5832. doi: 10.1016/S0040-4039(02)01171-1. [DOI] [Google Scholar]
- 57.Satake M., Tanaka Y., Ishikura Y., Oshima Y., Naoki H., Yasumoto T. Gymnocin-B with the Largest Contiguous Polyether Rings from the Red Tide Dinoflagellate, Karenia (Formerly Gymnodinium) Mikimotoi. Tetrahedron Lett. 2005;46:3537–3540. doi: 10.1016/j.tetlet.2005.03.115. [DOI] [Google Scholar]
- 58.Tanaka Y., Satake M., Yotsu-Yamashita M., Oshima Y. Gymnocin-A Carboxylic Acid and Gymnocin-A2, Cytotoxic Polyethers from the Red Tide Dinoflagellate Karenia Mikimotoi. Heterocycles. 2013;87:2037–2046. doi: 10.3987/COM-12-12814. [DOI] [Google Scholar]
- 59.Miles C.O., Wilkins A.L., Stirling D.J., MacKenzie A.L. Gymnodimine C, an Isomer of Gymnodimine B, from Karenia Selliformis. J. Agric. Food Chem. 2003;51:4838–4840. doi: 10.1021/jf030101r. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu Y., Chou H.N., Bando H., Duyne G.V., Clardy J.C. Structure of Brevetoxin A (GB-1 Toxin), the Most Potent Toxin in the Florida Red Tide Organism Gymnodinium Breve (Ptychodiscus Brevis) J. Am. Chem. Soc. 1986;108:514–515. doi: 10.1021/ja00263a031. [DOI] [PubMed] [Google Scholar]
- 61.Baden D., Mende T.J., Trainer V.L. Derivatized Brevetoxins and Their Use as Quantitative Tools in Detection. Mycotoxins. 1988;1988:1–2. doi: 10.2520/myco1975.1988.1Supplement_1. [DOI] [Google Scholar]
- 62.Baden D.G. Metabolism and Toxinology of the Marine Dinoflagellate, Gymnodinium Breve. University of Miami; Coral Gables, FL, USA: 1977. [Google Scholar]
- 63.Catterall W.A., Risk M. Toxin T46 from Ptychodiscus Brevis (Formerly Gymnodinium Breve) Enhances Activation of Voltage-Sensitive Sodium Channels by Veratridine. Mol. Pharm. 1981;19:345–348. [PubMed] [Google Scholar]
- 64.Lin Y.Y., Risk M., Ray S.M., Van Engen D., Clardy J., Golik J., James J.C., Nakanishi K. Isolation and Structure of Brevetoxin B from the “Red Tide” Dinoflagellate Ptychodiscus Brevis (Gymnodinium Breve) J. Am. Chem. Soc. 1981;103:6773–6775. doi: 10.1021/ja00412a053. [DOI] [Google Scholar]
- 65.Baden D.G., Mende T.J. Toxicity of Two Toxins from the Florida Red Tide Marine Dinoflagellate, Ptychodiscus Brevis. Toxicon. 1982;20:457–461. doi: 10.1016/0041-0101(82)90009-5. [DOI] [PubMed] [Google Scholar]
- 66.Chou H.-N., Shimizu Y. A New Polyether Toxin from Gymnodinium Breve Davis. Tetrahedron Lett. 1982;23:5521–5524. doi: 10.1016/S0040-4039(00)85883-9. [DOI] [Google Scholar]
- 67.Chou H.-N., Shimizu Y., Van Duyne G., Clardy J. Isolation and Structures of Two New Polycyclic Ethers from Gymnodinium Breve Davis (=Ptychodiscus Brevis) Tetrahedron Lett. 1985;26:2865–2868. doi: 10.1016/S0040-4039(00)98857-9. [DOI] [Google Scholar]
- 68.Shimizu Y. Recent progress in marine toxin research. Pure Appl. Chem. 1982;54:1973–1980. doi: 10.1351/pac198254101973. [DOI] [Google Scholar]
- 69.Prasad A.V.K., Shimizu Y. The Structure of Hemibrevetoxin-B: A New Type of Toxin in the Gulf of Mexico Red Tide Organism. J. Am. Chem. Soc. 1989;111:6476–6477. doi: 10.1021/ja00198a098. [DOI] [Google Scholar]
- 70.Bourdelais A.J., Jacocks H.M., Wright J.L.C., Bigwarfe P.M., Jr., Baden D.G. A New Polyether Ladder Compound Produced by the Dinoflagellate Karenia Brevis. J. Nat. Prod. 2005;68:2–6. doi: 10.1021/np049797o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satake M., Bourdelais A.J., Van Wagoner R.M., Baden D.G., Wright J.L.C. Brevisamide: An Unprecedented Monocyclic Ether Alkaloid from the Dinoflagellate Karenia Brevis That Provides a Potential Model for Ladder-Frame Initiation. Org. Lett. 2008;10:3465–3468. doi: 10.1021/ol801243n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satake M., Campbell A., Van Wagoner R.M., Bourdelais A.J., Jacocks H., Baden D.G., Wright J.L.C. Brevisin: An Aberrant Polycyclic Ether Structure from the Dinoflagellate Karenia Brevis and Its Implications for Polyether Assembly. J. Org. Chem. 2009;74:989–994. doi: 10.1021/jo802183n. [DOI] [PubMed] [Google Scholar]
- 73.Truxal L.T., Bourdelais A.J., Jacocks H., Abraham W.M., Baden D.G. Characterization of Tamulamides A and B, Polyethers Isolated from the Marine Dinoflagellate Karenia Brevis. J. Nat. Prod. 2010;73:536–540. doi: 10.1021/np900541w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miles C.O., Wilkins A.L., Stirling D.J., MacKenzie A.L. New Analogue of Gymnodimine from a Gymnodinium Species. J. Agric. Food Chem. 2000;48:1373–1376. doi: 10.1021/jf991031k. [DOI] [PubMed] [Google Scholar]
- 75.Satake M., Irie R., Holland P.T., Harwood D.T., Shi F., Itoh Y., Hayashi F., Zhang H. Brevisulcenals-A1 and A2, Sulfate Esters of Brevisulcenals, Isolated from the Red Tide Dinoflagellate Karenia Brevisulcata. Toxins. 2021;13:82. doi: 10.3390/toxins13020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamamoto Y., Tachibana K., Holland P.T., Shi F., Beuzenberg V., Itoh Y., Satake M. Brevisulcenal-F: A Polycyclic Ether Toxin Associated with Massive Fish-Kills in New Zealand. J. Am. Chem. Soc. 2012;134:4963–4968. doi: 10.1021/ja212116q. [DOI] [PubMed] [Google Scholar]
- 77.Shi F. Chemical and Toxicological Investigation of the Toxic Dinoflagellate, Karenia Brevisulcata. Lincoln University; Christchurch, New Zealand: 2012. [Google Scholar]
- 78.Satake M., Irie R., Hamamoto Y., Tachibana K., Holland P.T., Harwood D.T., Shi F., Beuzenberg V., Itoh Y., Hayashi F., et al. Brevisulcenal-G, -H, and -I, Polycyclic Ether Marine Toxins from the Dinoflagellate Karenia Brevisulcata. Heterocycles. 2018;96:2096–2105. doi: 10.3987/COM-18-14014. [DOI] [Google Scholar]
- 79.Suzuki R., Irie R., Harntaweesup Y., Tachibana K., Holland P.T., Harwood D.T., Shi F., Beuzenberg V., Itoh Y., Pascal S., et al. Brevisulcatic Acids, Marine Ladder-Frame Polyethers from the Red Tide Dinoflagellate Karenia Brevisulcata in New Zealand. Org. Lett. 2014;16:5850–5853. doi: 10.1021/ol502700h. [DOI] [PubMed] [Google Scholar]
- 80.Irie R., Suzuki R., Kazuo T., Holland P., Harwood T.D., Shi F., McNabb P., Beuzenberg V., Hayashi F., Zhang H., et al. Brevisulcatic Acids from a Marine Microalgal Species Implicated in a Toxic Event in New Zealand. Heterocycles. 2016;92:45. doi: 10.3987/COM-15-13332. [DOI] [Google Scholar]
- 81.Bourdelais A.J., Campbell S., Jacocks H., Naar J., Wright J.L.C., Carsi J., Baden D.G. Brevenal Is a Natural Inhibitor of Brevetoxin Action in Sodium Channel Receptor Binding Assays. Cell. Mol. Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holland P.T., Shi F., Satake M., Hamamoto Y., Ito E., Beuzenberg V., McNabb P., Munday R., Briggs L., Truman P., et al. Novel Toxins Produced by the Dinoflagellate Karenia Brevisulcata. Harmful Algae. 2012;13:47–57. doi: 10.1016/j.hal.2011.10.002. [DOI] [Google Scholar]
- 83.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 84.Hadley W. Ggplot2: Elegant Graphics for Data Analysis. Springer; New York, NY, USA: 2016. [Google Scholar]
- 85.Fowler N., Tomas C., Baden D., Campbell L., Bourdelais A. Chemical Analysis of Karenia Papilionacea. Toxicon. 2015;101:85–91. doi: 10.1016/j.toxicon.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Nakanishi K. The Chemistry of Brevetoxins: A Review. Toxicon. 1985;23:473–479. doi: 10.1016/0041-0101(85)90031-5. [DOI] [PubMed] [Google Scholar]
- 87.Van Wagoner R.M., Misner I., Tomas C.R., Wright J.L.C. Occurrence of 12-Methylgymnodimine in a Spirolide-Producing Dinoflagellate Alexandrium Peruvianum and the Biogenetic Implications. Tetrahedron Lett. 2011;52:4243–4246. doi: 10.1016/j.tetlet.2011.05.137. [DOI] [Google Scholar]
- 88.Baden D.G., Bourdelais A.J., Jacocks H., Michelliza S., Naar J. Natural and Derivative Brevetoxins: Historical Background, Multiplicity, and Effects. Environ. Health Perspect. 2005;113:621–625. doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baden D.G., Mende T.J., Szmant A.M., Trainer V.L., Edwards R.A., Roszell L.E. Brevetoxin Binding: Molecular Pharmacology versus Immunoassay. Toxicon. 1988;26:97–103. doi: 10.1016/0041-0101(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 90.Abraham W.M., Bourdelais A.J., Sabater J.R., Ahmed A., Lee T.A., Serebriakov I., Baden D.G. Airway Responses to Aerosolized Brevetoxins in an Animal Model of Asthma. Am. J. Respir. Crit. Care Med. 2005;171:26–34. doi: 10.1164/rccm.200406-735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golik J., James J.C., Nakanishi K., Lin Y.-Y. The Structure of Brevetoxin C. Tetrahedron Lett. 1982;23:2535–2538. doi: 10.1016/S0040-4039(00)87389-X. [DOI] [Google Scholar]
- 92.Fuwa H., Ebine M., Bourdelais A.J., Baden D.G., Sasaki M. Total Synthesis, Structure Revision, and Absolute Configuration of (−)-Brevenal. J. Am. Chem. Soc. 2006;128:16989–16999. doi: 10.1021/ja066772y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herrmann A.T., Martinez S.R., Zakarian A. A Concise Asymmetric Total Synthesis of (+)-Brevisamide. Org. Lett. 2011;13:3636–3639. doi: 10.1021/ol201283n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakai T., Fukuta A., Nakamura K., Nakano M., Mori Y. Total Synthesis of Brevisamide Using an Oxiranyl Anion Strategy. J. Org. Chem. 2016;81:3799–3808. doi: 10.1021/acs.joc.6b00484. [DOI] [PubMed] [Google Scholar]
- 95.Lee J., Oh H.-S., Kang H.-Y. A Formal Total Synthesis of (−)-Brevisamide, a Marine Monocyclic Ether Amide. Tetrahedron Lett. 2015;56:1099–1102. doi: 10.1016/j.tetlet.2015.01.065. [DOI] [Google Scholar]
- 96.Van Wagoner R.M., Satake M., Bourdelais A.J., Baden D.G., Wright J.L.C. Absolute Configuration of Brevisamide and Brevisin: Confirmation of a Universal Biosynthetic Process for Karenia Brevis Polyethers. J. Nat. Prod. 2010;73:1177–1179. doi: 10.1021/np100159j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuranaga T., Ohtani N., Tsutsumi R., Baden D.G., Wright J.L.C., Satake M., Tachibana K. Total Synthesis of (−)-Brevisin: A Concise Synthesis of a New Marine Polycyclic Ether. Org. Lett. 2011;13:696–699. doi: 10.1021/ol102925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsukano C., Sasaki M. Total Synthesis of Gymnocin-A. J. Am. Chem. Soc. 2003;125:14294–14295. doi: 10.1021/ja038547b. [DOI] [PubMed] [Google Scholar]
- 99.Stewart M., Blunt J.W., Munro M.H., Robinson W.T., Hannah D.J. The Absolute Stereochemistry of the New Zealand Shellfish Toxin Gymnodimine. Tetrahedron Lett. 1997;38:4889–4890. doi: 10.1016/S0040-4039(97)01050-2. [DOI] [Google Scholar]
- 100.Medhioub W., Guéguen M., Lassus P., Bardouil M., Truquet P., Sibat M., Medhioub N., Soudant P., Kraiem M., Amzil Z. Detoxification Enhancement in the Gymnodimine-Contaminated Grooved Carpet Shell, Ruditapes Decussatus (Linné) Harmful Algae. 2010;9:200–207. doi: 10.1016/j.hal.2009.10.002. [DOI] [Google Scholar]
- 101.Otero A., Chapela M.-J., Atanassova M., Vieites J.M., Cabado A.G. Cyclic Imines: Chemistry and Mechanism of Action: A Review. Chem. Res. Toxicol. 2011;24:1817–1829. doi: 10.1021/tx200182m. [DOI] [PubMed] [Google Scholar]
- 102.Harju K., Koskela H., Kremp A., Suikkanen S., de la Iglesia P., Miles C.O., Krock B., Vanninen P. Identification of Gymnodimine D and Presence of Gymnodimine Variants in the Dinoflagellate Alexandrium Ostenfeldii from the Baltic Sea. Toxicon. 2016;112:68–76. doi: 10.1016/j.toxicon.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 103.Strangman W., Anttila M., Tomas C., Wright J.L.C. (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-Tetramethyl-18-Methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-Hexadecahydro-2H-14,17-Epoxybenzo[2,3]Cyclohexadeca[1,2-b]Pyridine-7-Yl]-3-Methylfuran-2(5H)-One (12-Methylgymnodimine B) Molbank. 2016;2016:M896. doi: 10.3390/M896. [DOI] [Google Scholar]
- 104.Zurhelle C., Nieva J., Tillmann U., Harder T., Krock B., Tebben J. Identification of Novel Gymnodimines and Spirolides from the Marine Dinoflagellate Alexandrium Ostenfeldii. Mar. Drugs. 2018;16:446. doi: 10.3390/md16110446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salgado P., Riobó P., Rodríguez F., Franco J.M., Bravo I. Differences in the Toxin Profiles of Alexandrium Ostenfeldii (Dinophyceae) Strains Isolated from Different Geographic Origins: Evidence of Paralytic Toxin, Spirolide, and Gymnodimine. Toxicon. 2015;103:85–98. doi: 10.1016/j.toxicon.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 106.Munday R., Towers N.R., Mackenzie L., Beuzenberg V., Holland P.T., Miles C.O. Acute Toxicity of Gymnodimine to Mice. Toxicon. 2004;44:173–178. doi: 10.1016/j.toxicon.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 107.Kharrat R., Servent D., Girard E., Ouanounou G., Amar M., Marrouchi R., Benoit E., Molgó J. The Marine Phycotoxin Gymnodimine Targets Muscular and Neuronal Nicotinic Acetylcholine Receptor Subtypes with High Affinity. J. Neurochem. 2008;107:952–963. doi: 10.1111/j.1471-4159.2008.05677.x. [DOI] [PubMed] [Google Scholar]
- 108.Chang F.H. Gymnodinium Brevisulcatum sp. Nov. (Gymnodiniales, Dinophyceae), a New Species Isolated from the 1998 Summer Toxic Bloom in Wellington Harbour, New Zealand. Phycologia. 1999;38:377–384. doi: 10.2216/i0031-8884-38-5-377.1. [DOI] [Google Scholar]
- 109.Keyzers R.A. The Isolation of Biologically Active Secondary Metabolites from New Zealand Marine Organisms. Victoria University; Wellington, New Zealand: 2003. [Google Scholar]
- 110.Harwood D.T., Shi F., Satake M., Holland P.T. A Sensitive LC-MS/MS Assay for Brevisulcenal and Brevisulcatic Acid Toxins Produced by the Dinoflagellate Karenia Brevisulcata. Toxicon. 2014;84:19–27. doi: 10.1016/j.toxicon.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 111.Naar J., Kubanek J., Weidner A., Flewelling L., Bourdelais A., Steidinger K., Baden D.G. Brevetoxin Depuration in Shellfish via Production of Non-Toxic Metabolites: Consequences for Seafood Safety and the Environmental Fate of Biotoxins. Harmful Algae. 2004;10:488–490. [PMC free article] [PubMed] [Google Scholar]
- 112.Pérez Linares J., Ochoa J.L., Gago Martínez A. Retention and Tissue Damage of PSP and NSP Toxins in Shrimp: Is Cultured Shrimp a Potential Vector of Toxins to Human Population? Toxicon. 2009;53:185–195. doi: 10.1016/j.toxicon.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 113.Plakas S.M., Jester E.L.E., El Said K.R., Granade H.R., Abraham A., Dickey R.W., Scott P.S., Flewelling L.J., Henry M., Blum P., et al. Monitoring of Brevetoxins in the Karenia Brevis Bloom-Exposed Eastern Oyster (Crassostrea Virginica) Toxicon. 2008;52:32–38. doi: 10.1016/j.toxicon.2008.04.174. [DOI] [PubMed] [Google Scholar]
- 114.U.S. Food and Drug Administration (FDA) National Shellfish Sanitation Program (NSSP). Guide for the Control of Molluscan Shellfish. [(accessed on 22 November 2021)];2019 :1–491. Available online: https://www.fda.gov/media/143238/download.
- 115.NSW Government . Marine Biotoxin Managment Plan—NSW Shellfish Program. NSW Government; Newington, NSW, Australia: 2015. [(accessed on 11 November 2021)]. pp. 1–44. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/industry/marine_biotoxin_management_plan.pdf. [Google Scholar]
- 116.Victorian Fisheries Authority Marine Biotoxin Management Plan. [(accessed on 2 September 2021)]; Available online: https://vfa.vic.gov.au/aquaculture/publications/shellfish-quality-asurance/marine-biotoxin-management-plan.
- 117.McNabb P.S., Selwood A.I., Van Ginkel R., Boundy M., Holland P.T. Determination of Brevetoxins in Shellfish by LC/MS/MS: Single-Laboratory Validation. J. AOAC Int. 2012;95:1097–1105. doi: 10.5740/jaoacint.11-272. [DOI] [PubMed] [Google Scholar]
- 118.New Zealand Government . Regulated Control Scheme—Bivalve Molluscan Shellfish for Human Consumption. New Zealand Government; Wellington, New Zealand: 2021. [(accessed on 22 November 2021)]. pp. 1–68. Available online: https://www.mpi.govt.nz/dmsdocument/30282-Animal-Products-Notice-Regulated-Control-Scheme-Bivalve-Molluscan-Shellfish-for-Human-Consumption-2018. [Google Scholar]
- 119.ANSES . Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the State of Knowledge on Brevetoxins in Shellfish, Data on Toxicity, Occurrence and Brevetoxin-Producing Microalgae. ANSES; Maisons-Alfort, France: 2021. [(accessed on 22 November 2021)]. pp. 1–18. Available online: https://www.anses.fr/en/system/files/ERCA2020SA0020EN.pdf. [Google Scholar]
- 120.Van Deventer M., Atwood K., Vargo G.A., Flewelling L.J., Landsberg J.H., Naar J.P., Stanek D. Karenia Brevis Red Tides and Brevetoxin-Contaminated Fish: A High Risk Factor for Florida’s Scavenging Shorebirds? Botanica Marina. 2012;55:31–37. doi: 10.1515/bot.2011.122. [DOI] [Google Scholar]