Abstract
The global increase in multidrug-resistant infections caused by various pathogens has raised concerns in human and veterinary medicine. This has renewed interest in the development of alternative methods to antibiotics, including the use of bacteriophages for controlling bacterial infections. The aim of this review is to present potential uses of bacteriophages as an alternative to antibiotics in the control of bacterial infections caused by multidrug-resistant bacteria posing a risk to humans, with particular emphasis on foodborne and zoonotic pathogens. A varied therapeutic and immunomodulatory (activation or suppression) effect of bacteriophages on humoral and cellular immune response mechanisms has been demonstrated. The antibiotic resistance crisis caused by global antimicrobial resistance among bacteria creates a compelling need for alternative safe and selectively effective antibacterial agents. Bacteriophages have many properties indicating their potential suitability as therapeutic and/or prophylactic agents. In many cases, bacteriophages can also be used in food quality control against microorganisms such as Salmonella, Escherichia coli, Listeria, Campylobacter and others. Future research will provide potential alternative solutions using bacteriophages to treat infections caused by multidrug-resistant bacteria.
Keywords: bacteriophages, foodborne pathogens, antimicrobial resistance, zoonotic bacteria
1. Introduction
Zoonotic pathogens cause problems all over the world, including diseases such as anthrax, brucellosis, bovine tuberculosis, hydatid disease, echinococcosis, trichinellosis, rabies, highly pathogenic avian influenza, Nipah/Hendra disease and bovine spongiform encephalopathy. In 2015, the WHO reported that more than 600 million people (1 in 10) worldwide fell ill as a result of foodborne infections [1,2]. According to the European Food Safety Authority (EFSA), the most frequently reported human foodborne diseases were campylobacteriosis and salmonellosis. However, the most dangerous pathogens for humans were identified as foodborne pathogenic bacteria found in livestock products, including enterohaemorrhagic Escherichia coli (EHEC; O157:H7), Shigella sp., Enterococcus spp. or Listeria spp. Multidrug-resistant pathogens isolated from human outbreaks, cattle, swine, and poultry were most often S. aureus, Streptococcus spp., Vibrio sp. and Yersinia spp. [3,4]. According to Niu et al. [5], these bacteria can also be transmitted to food products by direct contact with animals or indirectly by vectors such as insects, rodents, wild birds, or irrigation water.
The global increase in multidrug-resistant infections and antibiotic failures in control of pathogens has raised concerns in human and veterinary medicine. An official report of the European Food Safety Authority (EFSA) regarding zoonotic and indicator bacteria isolated from humans, animals, and food showed that a high proportion (28.6%) of human Salmonella strains were resistant to three or more antimicrobials, and 34.9% of E. coli strains isolated from pigs were resistant to more than six antibiotics [6].
There has been a marked increase in the antibiotic resistance of Gram-negative bacteria via a variety of mechanisms, such as antibiotic target modification, antibiotic degradation, and modulation of permeability through the bacterial membrane. These mechanisms have limited the development of novel antibiotics. The most resistant strains of bacteria are carbapenem-resistant Enterobacteriaceae, extensively drug-resistant (XDR) Pseudomonas aeruginosa, and XDR Acinetobacter baumannii. Understanding the mechanisms of resistance of multidrug-resistant bacteria is the main goal in the development of modern antibacterial agents [7].
Global livestock production is faced with an alarming increase in bacterial resistance, including among zoonotic pathogens. For example, Donkor et al., [8] showed higher antimicrobial resistance in livestock than in humans, with animal E. coli isolates exhibiting high levels of resistance to tetracycline and penicillin. This has led to renewed interest among scientists to develop alternative methods to antibiotics, including the use of bacteriophages, since the beginning of the 21st century [9].
Widespread multidrug resistance among bacteria necessitates the search for alternative methods of controlling infections, including pre- and probiotics, vaccines, bacteriophages, nanoparticles, antimicrobial peptides (AMPs) and others. An example is the use of bacteriophages to reduce or eliminate pathogenic bacteria in livestock production, as biocontrol agents to control foodborne pathogens and to reduce contamination on food-contact surfaces [9]. An important contribution to research on the use of bacteriophages to control bacteria, including zoonotic pathogens, is the development and implementation of new legal regulations in the EU regarding restrictions or complete bans on the use of selected groups of chemotherapeutics in individual sectors of animal production. An example of such legislative action is the EU Council Directive 2019/6 [10] coming into force in January 2022.
1.1. General Characteristics of Bacteriophages
Due to the widespread nature of bacteriophages (phages) associated with crops, live animals, and human intestinal environments, humans have direct and indirect contact with them. Many studies have demonstrated the common presence of bacteriophages in various fermented foods, such as yogurt and cheese. The application of specific bacteriophages to foods helps to reduce foodborne pathogenic bacteria [5].
Bacteriophages are bacterial viruses, causing complete lysis of a susceptible bacterial culture [11]. Interactions between phages and bacteria can be regarded as parasitism, as most virulent phage replication necessarily results in bacterial death. Certain interactions can be termed mutualistic, while some temperate phages encode benefits for the phenotypic properties of the host bacteria [12] According to Batinovic et al. [13], the prevalence of bacteriophages in the environment has been a natural phenomenon for billions of years, resulting in a balance of commensal and pathogenic bacteria. Phages and bacteria are the oldest and most ubiquitous microorganisms on Earth, likely having originated approximately 3 billion years ago [14,15].
Phages are prevalent in a variety of environments, including water, forest groundcover, food products, wastewater, and animal and human waste [16]. Bacteriophages have also been detected in commercial products, such as sera and human vaccines, as well as inside the human mouth (dental plaque and saliva) and in the gastrointestinal tracts of animals and humans [17].
Although bacteriophages may be present autonomously outside the host, all phages require the bacterial cell as a host for multiplication. Most phages are highly specific for host cell surface receptors such as receptor binding proteins (RBPs) or LPS [18,19].
1.2. History of Bacteriophages
Bacteriophages were first discovered more than 100 years ago by two microbiologists, Frederick Twort from England and the French Canadian Felix d’Herelle [20,21]. The first experimental and successful phage therapy was carried out by D’Herelle in the control of fowl typhoid in chickens (95–100% survival) [22]. He also coined the term ‘bacteriophage’, meaning ‘bacteria eater’. Finally, in 1940, electron microscopes were used to identify the viral nature and morphology of phages [23]. Bacteriophages have been used in various types of therapies in humans, e.g., in dermatological, ophthalmological, urological, paediatric, otolaryngology and surgical infections. The significant therapeutic success of these treatments had a major impact on the development of phage therapy in the pre-antibiotic era. This was crucial, as the only treatment available in the first two decades of the 20th century was serum therapy (e.g., for pneumococci or the diphtheria bacterium), so bacteriophage therapy began to dominate in human medicine [24].
The discovery of the antimicrobial properties of Penicillium notatum in 1928 by Alexander Fleming culminated in the successful development of the first major antibiotic, penicillin, in 1941 [25], which marked the beginning of the antibiotic era and naturally inhibited the development of bacteriophage therapy.
At present, as bacterial resistance to antibiotics is increasing significantly worldwide, phages are one of the factors with potential to replace them [26]. The best known bacteriophage centres in the world are the Eliava Institute of Bacteriophages, Microbiology, and Virology (EIBMV) of the Georgian Academy of Sciences, in Tbilisi, Georgia, and the Hirszfeld Institute of Immunology and Experimental Therapy (HIIET) of the Polish Academy of Sciences, in Wroclaw, Poland. Both institutes offer phage therapy against many bacterial and fungal pathogens, e.g., Staphylococcus spp., Klebsiella sp., Proteus sp., E. coli, and Pseudomonas sp., as well as other enteric pathogens [27,28,29].
1.3. Classification of Bacteriophages
Bacteriophages are the most widespread life forms on Earth. By 2018 year more than 650 strains of bacteriophages had been deposited in the American Type Culture Collection (ATCC) and >27,000 bacteriophage nucleotide sequences had been deposited in the International Nucleotide Sequence Database Collaboration (INSDC) [30]. The total number of these bacterial viruses has been estimated at 1032, which is 10 times the number of characterized bacteria. In water, the total count of bacteriophages has been estimated at 104 to 108 virions/mL−1 [31].
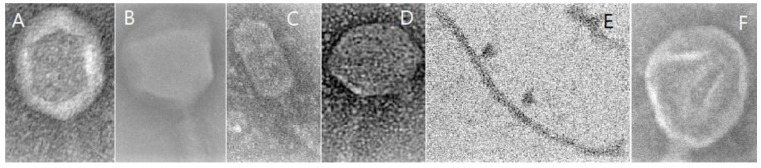
The classification of bacteriophages is based on the type of nucleic acid (ssRNA, dsRNA, ssDNA, dsDNA), the structure of the capsid (e.g., helical, pleomorphic, icosahedral, filamentous/thread-like, complex/polyhedral), which is built of structural proteins, and their life cycle, bacterial target, and site (Figure 1). The phage taxonomy criteria applied by the International Committee on Taxonomy of Viruses (ICTV) were nucleic acid composition and virion morphology [9]. In 2015 the Bacterial and Archaeal Viruses Subcommittee (BAVS) classified phages into 873 species, 204 genera and 14 subfamilies [32]. The classification of bacteriophages is still ongoing, and in 2018 the ICTV presented a new classification of these bacterial viruses into 142 families, 81 subfamilies and 4978 species [9]. Most bacteriophages (96%) belong to the order Caudovirales, which is grouped into three main families: Myoviridae, Podoviridae and Siphoviridae [32]. Most bacteriophages contain double-stranded DNA, and the nucleic acid is coated with a protein capsid. Some phages have an additional layer (envelope) [17]. As new bacteriophages are continually being detected, their classification is constantly modified. The latest classification of viruses, based on the virus taxonomy proposed by the ICTV, was presented in our previous paper [33].
Figure 1.
Examples of capsid structures in bacteriophages in TEM microscopy. (A) Helical; (B) Polyhedral; (C) Prolate; (D) Icosahedral; (E) Filamentous; (F) Pleomorphic-like.
1.4. Life Cycles of Phages
The life cycle of phages is an important element of infections of bacteria. Phages can be categorized into types based on their virulence: lytic (virulent, productive) and lysogenic (temperate, dormant). Virulent phages follow a lytic cycle in the bacterial cell and lyse it to release a newly created population of phages [34].
The lytic cycle includes the adsorption, penetration, biosynthesis, assembly and release of bacteriophages from the infected bacterium. During this process many phages use specific proteins located on the surface of the bacterial cell as receptors. During the adsorption phase, the bacteriophage adheres to the bacterial cell, and phage proteins bind to specific receptors, such as teichoic and lipoteichoic acid for Gram-positive bacteria or LPS for Gram-negative bacteria [35]. The next phase, penetration, consists of destruction of the bacterial wall by bacteriophage enzymes and insertion of the genetic material into the bacterial cell. This is followed by the formation of capsid structures for nucleic acid and protein replication, accompanied by inhibition of replication of bacterial DNA. The phage genetic material is transcribed in the bacterial cell by RNA polymerases to produce mRNA, which supresses host intracellular synthesis as a consequence of bacteriophage multiplication [36]. Tens, hundreds or thousands of replicated phages are released by means of lysis of the bacterial cytoplasmic membrane by a phage protein (holin) and the formation of pores by endolysin encoded by double-stranded phage DNA and peptidoglycan hydrolases. The duration of the entire lytic cycle may be 20–40 min or up to 1–2 h [9,37].
Lysogenic infection via phages involves integration of their genetic material into the chromosome of the infected bacteria (prophage), which does not destroy the bacterial cell or produce a new population of bacteriophages. It leads to the integration of the phage genetic material with the bacterial DNA and its transmission into a new population of bacteria. This kind of bacteriophage is called a temperate phage, and in cells carrying a prophage it is referred to as lysogenic. Nevertheless, the viral prophage, also called an endogenous phage (a latent form of phage), can become activated by abnormal environmental conditions and other external stress factors that can damage the bacterial genetic material, including sunlight, UV radiation, some alkylating cytostatics (chlorambucil, cyclophosphamide, ifosfamide, estramustine or chlormethine) or mutagenic antibiotics such as mitomycin C. In some cases, the prophage is excised incorrectly from the chromosome, taking with it neighbouring bacterial genes. This is one of the main means of horizontal gene transfer (HGT) among bacteria, which is also one of the main methods in molecular biology [9]. Phages which have been defined as temporary include E. coli Lambda [38], with activity against E. coli and other Enterobacteriaceae; phage Mu, specific for Salmonella, Citrobacter and Erwinia; MM1 Streptococcus pneumoniae; and φ11 S. aureus [39].
In another type of life cycle involving chronic infection, bacteriophages infect the bacterial cell, in which new phage populations arise without destroying the bacteria. The chronic infection lifestyle is found in rod-shaped (filamentous) single-stranded DNA phages and in plasmaviruses that infect mycoplasmas. In the chronic infection lifestyle, phages are gradually eliminated from the bacteria over a long period without destruction of the cell [40].
2. The Spectrum of Use of Bacteriophages
The specificity of phage activity means that they infect only the bacteria specific for them (called the host) via external receptors, which determines the phage host range. Therefore, the use of phage therapy relies on a detailed and accurate characterization of the bacteria, including pathotypes and serotypes. Bacteriophages can be used in a variety of forms and methods to control and eliminate bacteria, including therapy, food protection and sanitation procedures [1,9]. Examples of the scope of the use of bacteriophages are presented in Table 1.
Table 1.
Examples of the use of bacteriophages in controlling bacteria.
| Scope of Use | Example | Host Pathogens | References |
|---|---|---|---|
| Treatment of human and animals | Gastroenteric, respiratory, urinary tract and skin infections, otitis, keratitis | E. coli, Salmonella spp., S. aureus, Pseudomonas spp., Enterococcus spp., Acinetobacter baumanni | [1] |
| Prophylaxis and treatment | Neonatal diarrhoeal infections in calves; | E. coli, | [41] |
| Campylobacter infections in broiler chickens; | Campylobacter jejuni | [42] | |
| Salmonella infections in chickens | Salmonella spp. | [43] | |
| Decontaminants | Biocontrol agents against food- and beverage-borne pathogens | Control of LAB growth during ethanol fermentation | [44,45] |
| Biosanitization | On equipment surfaces to eradicate biofilms in food production; on plastic, glass, and ceramic surfaces in hospitals | S. aureus, E. coli, P. aeruginosa, L. monocytogenes Acinetobacter baumannii | [46,47] |
| Bio-preservation | Highly processed products with a short shelf life | Listeria monocytogenes; Campylobacter spp. | [48] |
| Agriculture | Biocontrol of plant pathogens, i.e., potato and tomato diseases; onion scab; lettuce and leek diseases; fruit tree diseases; cultivated mushrooms |
Pseudomonas spp., Xanthomonas spp., Erwinia spp., Ralstonia spp., Agrobacterium spp., Xylella spp., Pectobacterium spp., Dickeya spp. Pleurotus ostreatus |
[49] |
| Aquaculture | Biocontrol of fish pathogens in commercial fish farming | Mainly to Vibrio spp., less to Edwardsiella spp., Lactococcus spp., Pseudomonas spp., Aeromonas spp., Flavobacterium spp. | [49] |
Bacteriophages can potentially be used as biological control agents, especially in the reduction and elimination of bacterial contamination in foods, e.g., by Salmonella, Listeria monocytogenes, Campylobacter spp. or E. coli O157:H7 [15,50]. The high efficacy and safety of bacteriophage therapy is due in part to their specificity for selected bacteria: a single species, serotype, or strain. This is beneficial because the commensal gut microbiota is not destroyed. Another advantage is that, due to the self-replication of bacteriophages at the site of application, repetition of the application is often unnecessary. In many cases, no side effects of clinical treatment are observed, indicating a high level of safety that has been confirmed in many studies [51]. However, the application of bacteriophages in live animals or humans induces a cellular immune response, which could lead to the inactivation of phages, rendering them ineffective in eliminating bacteria [52,53,54].
In many experimental phage therapies a beneficial effect was observed as a significant reduction in bacterial content or elimination of the pathogens. Phages have been used to control Shiga-toxin-producing E. coli (ETEC) infections in newborn ruminants, including calves and lambs, or other livestock species, such as piglets [41,55]. They have been exploited to control bacterial infections in humans in many countries, including Poland, Georgia, Russia, France, Belgium, Switzerland and the USA [56,57,58]. Phage therapies have been applied against infections caused by numerous pathogens, especially multidrug-resistant bacteria, e.g., Acinetobacter, Burkholderia, Citrobacter, Enterobacter, Enterococcus, Escherichia coli, Klebsiella, Morganella, Proteus, Pseudomonas, Shigella spp., Shigella flexneri, Staphylococcus, Salmonella, Serratia and Stenotrophomonas. According to the Eliava Phage Therapy Centre, bacteriophage therapy against Enterococcus faecalis, E. coli (O11, O18, O20, O25, O26, O44, O55, O113, O125 and O128), Proteus vulgaris, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella spp., and Shigella spp. showed positive results in 35–50% of human patients [59,60].
Bacteriophage Interactions during Phage Therapy
Bacteriophages are regarded as the most applicable ecological and alternative means of elimination of pathogens (control and prevention of infections) due to their natural origin and numerous advantages, including the following:
lysis of bacteria usually highly resistant to antibiotic therapy, living in a biofilm;
high degree of safety for commensal and symbiotic flora;
possibility of use with other bacteriophages as a cocktail or with other antibacterials;
complete biodegradability of bacteriophages, making them safe for the organism and the environment [17].
However, phage therapy may carry a risk of immunological reactions, which is linked to the protein structures of bacteriophages. The immune response to bacteriophages depends on the location of the bacterial infection and the route of administration of the phages. The activity of bacteriophages also relies on their ability to penetrate epithelial cells and potentially spread to the bloodstream, lymph and internal organs such as the lung, liver, kidney and brain [61]. Bacteriophages can activate dendritic cells to synthesize pro-inflammatory factors (including IL-6, IL-1α, IL-1β and TNF-α) and to induce changes in the expression profile of these cell surface proteins and activation of the NF-κB signalling pathway [62].
The results of many studies confirm that bacteriophages can be phagocytosed by mammalian cells [63]. For example, Geier et al. [64] demonstrated rapid removal of wild-type phage λ from the circulatory system in humans. According to the authors, phagocytosis via immune cells is the main process of elimination of bacteriophages in mammals, and this mechanism takes place during lysis of bacteria by bacteriophages, which increases the activity of phagocytic cells, including PMN cells. The higher number of neutrophils at the site of infection is necessary to remove phage-resistant bacteria; this neutrophil-phage cooperation process has been confirmed in the resolution of P. aeruginosa infections [53,65]. However, some studies [66] have confirmed that bacteriophages can also express anti-inflammatory properties by decreasing the expression of TNFα and monocyte chemoattractant protein-1, which reduces ROS production by neutrophils and protects the epithelia against damage.
Some bacteriophages can also be a natural component of the intestinal microbiota and consumed food [67]. The oral administration of phages against Staphylococcus, Klebsiella, Escherichia, Proteus and Pseudomonas also induces the production of antibodies [68]. There has been no evidence of immunological disorders following phage ingestion per os at any concentration [69]. Topical application of phages to animals and humans also caused no side effects [70].
Minor problems have been observed in the case of other internal organs and blood vessels, which are not natural environments for phages. Here the immunogenic and immunomodulatory effects of phages can be observed. Bacteriophages can have non-specific effects on the immunological functions of various immune cells, including PMNCs, as well as on cytokine production and the induction of specific antibodies against non-phage antigens [71]. For example, resident liver macrophages (also called Kupffer cells) are able to eliminate bacteriophages by phagocytosis four times faster than spleen macrophages. The natural innate immune response is usually sufficient to eliminate pathogens before the activation of adaptive immune mechanisms. Bacteriophages can activate immune mechanisms and thus affect the metabolic activity of immune cells. However, bacteriophages can inhibit the production and release of reactive oxygen species in response to pathogens, which could decrease innate antibacterial immunity. [72]. Phages can induce antibodies that neutralize them, which can inhibit the antibacterial effect of phages in the form of lysis of targeted bacteria [71,73]. It is not currently clear how long this type of antibody will remain in the body, as knowledge of the kinetic aspect of bacteriophage activity is insufficient. Moreover, the titre of these antibodies depends on many factors, including the route of application (local application causes a minor increase in antibodies) and its frequency [74]. Some information about the influence of bacteriophages on immune responses in animal’s model has been presented in Table 2.
Table 2.
Examples of the influence of bacteriophages on immune responses in animals.
| Kind of Phage | Form of Application | Animal Model | Influence on Immune Parameters | References |
|---|---|---|---|---|
| Pseudomonas spp. bacteriophage (PA1Ø) | 100 µL of PA1Ø (5 × 104 PFU; 5 × 107 PFU (10 MOI) or 5 × 108 PFU (100 MOI) in a single i.p. dose | 4–5-week-old male ICR mice weighing 24–26 g | Increase in phagocytosis (killing effect of PA1Ø + PMN up to 6 h after application) | [65] |
| Pseudomonas aeruginosa phage PAK_P1 | Intranasally at a curative dose of 1.0 × 108 PFU or 1.0 × 109 PFU | Wild-type BALB/c (C), wild-type C57Bl6/J (B6) | Increase in neutrophil activity, NK cells; reduced production of IFNγ and TNFα | [75] |
| Cronobacter sakazakii ES2 phage | Phage suspension 106 PFU·mL−1 in vitro | 6–8-week-old C57BL/6 mice | Increase in expression of maturation markers CD86, CD40, and MHC II; stimulation of induction of NF-κBp65-mediated-IL-12p40; stimulation of IL-12 expression; suppression of IL-6, TNF-α, IL-1β, and IFN-γ |
[62] |
| E. coli T4 phage | Intraperitoneal injection at 20 µg/mouse | Female C57Bl6/J (6–8-week-old) mice | No effect on production of cytokines IL-1α, IL-6, IL-12, and TNF-α; minor changes in expression of MHC II, CD40, CD86, and CD80 |
[76] |
| E.coli T4 bacteriophages | T4 phages 5 × 108 PFU/mL | 8–12-week-old female C57BL/6 mice | Inhibition of specific antibody response; reduction in bacteria-induced ROS production by phagocytic cells; antitumour response; activation of T cells for IFN-γ production | [77] |
| E. coli P1 and P2 phages | 106 PFU/mL in vitro | Mice | Stimulation of TNFα; stimulation of macrophage activity in vitro | [78] |
| Wild-type E. coli T7 phage | 109 PFU/mL injected in vitro into tail vein of mice | Adult female C57BL/6J, SCID (C57BL/6J-Prkdcscid), B-cell-deficient (C57BL/10-Igh-6tm1Cgn) and T-cell deficient (C57BL/6J-Hfh11nu) mice | Spontaneous antibodies, mainly IgM, observed in sera; slight effect on NK activation; anti-inflammatory effect—ROS suppression | [79] |
| Wild-type E.coli φ26, φ27, φ29 | 107–108 PFU/mL for 5 days per rectum as suppositories | 25 newborn HF calves aged 1 d to 2 weeks | Significant increase in IgG and IgA production stimulation of nonspecific immune response—IFNγ, lysozyme; activation of acute phase response SAA and HP |
[41] |
| Wild-type E.coli phage and bacteriophage genomes NC-A: MK310182; NC-B: MK310183; NC-G: MK310184 | 3 × 107 PFU/mL of phage mixture with drinking water | 8-week-old germ-free Swiss Webster mice | Whole bacteriophages and phage DNA stimulated IFN-γ via nucleotide-sensing receptor TLR9 | [80] |
| S. aureus vB_SauM_JS25 phage | MAC-T cells pre-treated with vB_SauM_JS25 phage 108 PFU/well for 3 h | In vitro MAC-T bovine mammary epithelial cells | Reduction in TNF-α, IL-1β, IL-6, IL-8, and IL-10 | [54] |
| Staphylococcus spp. bacteriophage A3R or 676Z | 3 doses of 1010 PFU/mouse in drinking water and peritoneally | C57BL/6J normal male mice | Induction of specific antibodies in blood (IgM, IgG, IgA) | [81] |
| Klebsiella pneumoniae MTCC109 bacteriophage PA43 | Intranasal application of 109 PFU BPA43 phage after 2 h of bacterial infection | BALB/c mice, 6–8 weeks old, weight 20–25 g | Suppression of local inflammatory reaction in lungs; suppression of migration of lymphocytes and macrophages | [82] |
Antiphage antibodies are probably one of the most important factors influencing the efficacy of phage therapy. However, the activation of the production of neutralizing antibodies by phages need not be a problem during the initial phase of treatment of bacterial infections, because bacteriophage activity is much faster than the production of phage-neutralizing antibodies [27]. However, these antibodies can affect the efficacy of treatment during the second phase of therapy. This necessitates the implementation of additional solutions, such as the following:
repeating phage administration two or more times, because bacteriophages can multiply at the site of application during infection of the host bacteria;
increasing the phage concentration in the solution, because a high level of phages protects against complete destruction by neutralizing antibodies;
using different phages, especially during the second and subsequent cycles of application during treatment, because resistance differs from one phage to another [27]. In addition to the increase in neutralizing antibodies during phage therapies, the concentration of class M and G immunoglobulins increases as well and continues to increase with subsequent applications of phage preparations [83,84].
Besides activating humoral response mechanisms, phages also play a significant role in the modulation of cellular immunity against them. For example, s.c. application of MS-2 phages induced a hypersensitivity reaction in guinea pigs [85]. It has been suggested that the cellular response plays only a minor role in phage inactivation, as observed in the case of phage T7 in T cell-deficient mice [79]. In another study [86], phages had an immunosuppressive effect by suppressing the activation of T lymphocytes during the development of transplantation tolerance.
While bacteriophage therapies have been an effective tool in control of bacterial infections in various animal species, phages are also currently used for typing and diagnosis of specific bacterial species and for control of foodborne pathogens in food.
3. Commercial Products with Bacteriophages for Elimination of Foodborne Zoonotic Pathogens
Foodborne infections are the most important global health problem, contributing significantly to hospitalizations and deaths worldwide despite many advances in pathogen surveillance. Traditional food sanitation techniques using antimicrobial methods (including pasteurization, high pressure, irradiation or chemical treatment) can reduce pathogens in foods in varying degrees. However, these methods may damage equipment and adversely affect the organoleptic qualities (and possibly the nutritional value) of foods. The most important problem with using chemicals is that they eliminate ‘good’ microbes, which are beneficial in natural preservation of foods [87]. Therefore, it seems preferable to use an effective natural and ecological alternative such as bacteriophages for biocontrol of foodborne pathogens. Bacteriophages are mainly used in three sectors of the food industry to ensure food safety: primary production, biopreservation and biosanitization. As components of commercial products, they are currently finding application in the elimination of pathogens from food products of animal origin (meat products, milk and dairy products) or plant origin (fresh fruits and vegetables).
The number of commercial bacteriophage products approved for use in food safety in various countries is continually increasing. Many commercial companies around the world have shown interest in information about the use of bacteriophages as antibacterial tools to control foodborne pathogens, e.g., in the United States (AmpliPhi Biosciences (VI, USA), Enbiotix (Boston, MA, USA); Intralytix), the United Kingdom (Novolytics, Sarum Biosciences and Fixed Phage, Bolton, UK), EU countries such as France (Pherecydes Pharma, Romainville, Ile-de-France, France) and Portugal (Technophage and InnoPhage, Lisbon, Portugal), and other countries [88]. Detailed information about commercial bacteriophage products used for biocontrol of foodborne pathogens in food is presented in Table 3.
Table 3.
Examples of commercial bacteriophage products used in biocontrol of foodborne pathogens in various foods.
| Commercial Phage Product | Target Bacteria | Company | Target Food Products | Country Approving Product | References |
|---|---|---|---|---|---|
| SalmoLyse® | Salmonella spp. | Intralytix, Inc., USA | Raw pet food ingredients; meat products: chicken, tuna, turkey; plant products: cantaloupe, lettuce | USA | [89] |
| SalmoFreshTM | Salmonella spp. | Intralytix Inc., USA | Poultry, fish and shellfish, fresh and processed fruits and vegetables | USA, Canada, Israel | [90] |
| PhageGuard S SalmonelexTM | Salmonella spp. | Micreos Food Safety/Nederlands | Fresh poultry meat | USA, Canada, Australia, Israel | [91] |
| Bafasal® |
Salmonella spp., Aeromonas spp. Pseudomonas spp., Yersinia spp. |
Proteon Pharmaceuticals (Łódź, Poland) | Regulatory-approved poultry feed | Poland | [9] |
| EnkoPhagum |
Salmonella spp., Shigella spp.; enteropathogenic E. coli; Staphylococcus spp. |
Brimrose Technology Corporation (Sparks Glencoe, MD, USA) | Meat products | Georgia | [92] |
| BacWash TM | Salmonella spp. | OmniLytics Inc. (Sandy, UT, USA) | For disinfection of skin of live animals prior to slaughter | USA | [4] |
| Biotector® S |
Salmonella Gallinarum S. Pullorum |
CJ CheilJedang Research Institute of Biotechnology (Seoul, Korea) | In animal feed to control Salmonella in poultry | South Korea | [93] |
| PhageGuard STM | Salmonella | Micreos Food Safety BV (Wageningen, The Netherlands) | Fresh poultry meat | Netherlands, Australia, Canada, USA | [87,94] |
| EcoShield TM | Escherichia coli O157:H7 | Intralytix Inc. (Columbia, MD, USA) | Kosher meat (ground beef); vegetables (tomatoes, broccoli, spinach); lettuce and cantaloupe; leafy greens | USA | [9,91,95] |
| Secure Shield E1 | Escherichia coli O157:H7 | FINK TEC GmbH (Hamm, Germany) | Beef carcasses | USA | [96] |
| EcoShield PX™ | Stx Escherichia coli O157:H7 | Intralytix, Inc., Baltimore, MD, USA | Fresh-cut leafy greens; foods of plant origin, beef, chicken | USA, Canada, Israel | [90,95] |
| ShigaShield™ (ShigActive™) |
Shigella spp. | Intralytix, Inc., Baltimore, MD, USA | Beef, poultry, dairy products, including cheese; fruit and vegetable surfaces | USA | [90,97] |
| ListShield™ | Listeria monocytogenes | Intralytix, Inc., Baltimore, MD, USA | Food biopreservative in meat and poultry products | USA, Canada, Israel | [9,87,90] |
| Listex P100 PhageGuard Listex™ |
Listeria monocytogenes | Micreos Food Safety, Wageningen, Netherlands | Beef and turkey meat; fish and shellfish; dairy products; red smear soft cheese, smoked salmon and fresh salmon; frozen vegetables | USA, Australia, New Zealand, Israel, Switzerland, the Netherlands | [87,98] |
| ListPhage™ | Listeria monocytogenes | Intralytix, Inc., Baltimore, MD, USA | Pet food | USA, EU | [91] |
| Agriphage™ | Xanthomonas campestris pv. vesicatoria, Pseudomonas syringae pv. tomato | OmniLytics Inc., USA | Foods of plant origin, especially tomatoes and peppers | USA | [91] |
| Agriphage-Fire Blight | Erwinia amylovora | OmniLytics Inc., USA | Surfaces of apples and pears | USA | [91] |
| Biolyse™ | Erwinia, Pectobacterium, Pseudomonas | APS Biocontrol Ltd./Dundee, UK | Vegetables, including potatoes | UK, Europe | [91] |
4. Advantages and Disadvantages of Bacteriophage Therapy
Phages have several advantages over antibiotics as therapeutic agents, such as activity against all types of bacteria, including MDR-pathogens. Their narrow antibacterial spectrum (which protects the natural microbiome), the low level of side effects, and their extensive distribution when administered systemically are also worth noting. They also may exert an effect on the inflammatory response, and their low production cost and high efficacy are significant benefits [31,99]. Many studies have confirmed the beneficial effects of the use of bacteriophages, shown as follows:
Bacteriophages show high specificity for their target pathogens and kill only pathogens without destroying the physiological saprophytic flora; the narrow host range of phages is also a useful feature in prophylaxis of infections caused by enteric bacteria [100].
The distribution of phages in the body following systemic administration is much more extensive than in the case of antibiotics, in part due to the lack of or very low level of resistance of bacteria [31].
Because the mechanism of action of phages against the host bacteria is different to that of antibiotics, they are highly effective against many pathogens, especially against multidrug-resistant bacteria [36].
Phages replicate at the site of infection even after a single application, because they multiply inside the bacterial cell and therefore are released at the site of infection [101].
Bacteriophages are resistant to stress factors during food production [91].
Phage therapy is theoretically cheaper than antibiotic therapy due to the simplicity of production [99]. The unit costs of production as well as the costs of isolation and characterization are comparable or even lower than the costs of classical chemotherapeutic products [102].
There is no withdrawal period in livestock due to the lack of residue in tissues as soon as therapy is completed [103].
There are no side effects or allergic reactions because most bacteriophages consist mainly of proteins and genetic material (DNA or RNA).
However, in addition to the positive effects of phage therapies, widespread use of bacteriophages is limited by obstacles such as the following:
Due to their high specificity for a single type of bacteria, bacteriophages have a narrow host range [104].
Bacteriophages may neutralize antibodies, which may prevent a portion of the administered phage dose from adhering to the target bacteria [104].
Bacteriophages have poor stability in the environment, e.g., sunlight, UV, low pH <3.5, or high temperature >50°C [17,105].
Only lytic phages are admissible in phage therapy because lysogenic (temporary) phages may be a source of horizontal transfer of bacterial toxins or antibiotic resistance [31].
The duration of survival of phages is varied, depending in part on the presence of the host bacteria. Their activity is also influenced by the environment within the organism in which it is administered, and therefore the survival of phages must be monitored at the site of administration in order to assess their antimicrobial activity [99].
Information about the kinetics of phages remains insufficient, especially the degree of adsorption, the number of replications necessary for a therapeutic effect, the latent period, and their elimination from the body by phagocytic cells [106].
5. Bacteriophage Efficacy in Experimental Models
There are many methods of application of phages in therapies for humans and animals, including intraperitoneal, subcutaneous or intramuscular injection or oral, intragastric, rectal, topical or intranasal administration. Forms of administration of phages during therapy include sprays, aerosols, lozenges, compresses, mouthwash, suppositories, throat rinses, bandages, eye or ear drops and tampons [107]. In many studies in humans and animals, the form of application and type of administration has been associated with the type and location of the disease. In earlier studies [108,109,110,111], the best therapeutic effect was observed after direct application of phages to the target bacteria, as in the case of bacterial dysentery caused by Shigella; intestinal dysbacteriosis caused by E. coli and Proteus spp.; lung and pleural infections caused by Staphylococcus; suppurative skin infections caused by Pseudomonas, Staphylococcus, Klebsiella, Proteus, and E. coli; and infections of the skin or nasal mucosa caused by Klebsiella spp.
Some studies have explored the use of phages for control and treatment of neonatal enterotoxigenic E. coli infections in cattle, poultry and pigs [40,112]. Bacteriophages have also been used in controlling systemic infections with foodborne pathogens, including Salmonella spp., E. coli, Campylobacter spp., Vibro spp., Pseudomonas aeruginosa, and other pathogens, such as Staphylococcus spp., Streptococcus spp., Klebsiella spp., Acinetobacter spp., and even Mycobacterium spp. These experiments were carried out in experimental mouse or rat models, as well as in chickens, rabbits, calves, pigs and sheep. Examples of the effects of experimental phage therapies in different animal species and in the control of various pathogens are presented in Table 4.
Table 4.
Examples of major experimental studies on phage therapy in animals.
| Animal Species | Pathogen Species | Phage Treatment | Results | Treatment Procedure | References |
|---|---|---|---|---|---|
| Cattle–newborn Holstein-Friesian heifers |
E. coli O9:K30.99 106 CFU mL−1 |
Oral administration of phage cocktail (B44/1 and B44/2), 1011 PFU mL−1 | 100 % reduction of mortality in calves; Significant reduction (93%) of morbidity of bacterial diarrhoea; high protection against ETEC infections |
Treatment of diarrhoea | [113] |
| Cattle–Holstein-Friesian dairy cows |
Staphylococcus
aureus |
Direct infusions into teats with bacteriophage K cocktail (CS1, DW2) (108 PFU ml−1) |
About 10,000-fold reduction of S. aureus in udder; lower presence of somatic cells in milk |
Treatment of subclinical mastitis | [114] |
| 20 female BALB/cJRj (SPF) mice | Staphylococcus aureus causing mastitis in cows | Inoculation with 108 PFU of ISP phage mixture into mammary glands | Significant reduction of bacterial count; reduction or lack of clinical changes in mammary glands | Antibacterial activity and therapeutic effect | [115] |
| 280 Holstein-Friesian lactating cows with metritis during the first and second lactations |
Escherichia coli strains causing metritis | Intravaginal administration of 20 mL 10-phage cocktail 109 PFU mL−1 at 230, 260 and 275 days of gestation |
Lack of antibacterial effect; no prophylactic effect in prevention of metritis; increased incidence of retained placenta |
Failure of therapeutic and prophylactic effect in metritis | [116] |
| 25 newborn Holstein-Friesian heifers aged 0–14 days old | E. coli causing diarrhoea in newborn calves | Rectal application as suppositories of phage cocktail (26, 27, 29 at 107 to 109 PFU mL−1) mixed with Lactobacillus spp. strains for 5 days | Significant reduction of clinical signs and duration of diarrhoea <24h; significant reduction of ETEC content in faeces 2 log10 CFU/mL; protection against re-infection for 3 weeks after treatment; immunomodulatory effect | Prophylactic and therapeutic effect against diarrhoea | [41] |
| Holstein-Friesian dairy cows with clinical or subclinical mastitis | S. aureus strains obtained from cows with subclinical and clinical mastitis, pig farm and human infections | 0.1 mL phage cocktail (STA1.ST29, EB1.ST11, and 27) 1.2 × 108 PFU/mL or 1.2 × 109 PFU/mL against S. aureus inoculated into about 5.0 mL of milk obtained from cows with mastitis |
Significant reduction of S. aureus in milk–2 log10 CFU/mL in vitro | Antibacterial activity | [117] |
| 3 female Yorkshire pigs weighing~60 kg | S. aureus ulcers | S. aureus F44/10 and F125/10, inoculated topically at 108 to 109 PFU | Slight reduction of S. aureus strains, reduction of ulcerous changes | Therapeutic effect on skin ulcers | [118] |
| 16 small pigs 3 to 4 weeks old |
Salmonella enterica ser. Typhimurium at 5 × 108 CFU mL |
Microencapsulated alginate beads containing 16-phage cocktail (SEP-1, SGP-1, STP-1, SS3eP-1, STP-2, SChP-1, SAP-1, SAP-2), ∼109 to 1010 PFU/mL by gavage | Significant early reduction (99%) in concentration of S. Typhimurium 2 to 3 log10 CFU/g in the ileum, caecum and tonsils; significant influence on health status and AWG of pigs |
Prophylactic and therapeutic effect | [119] |
| 3-week-old weaned pigs | E. coli (ETEC); O149:H10:F4 | Oral administration of phage cocktail GJ1–GJ7 or mono-phage: prophylactic 1010 PFU/pig or therapeutic 108 PFU/pig | Significant reduction of diarrhoea; reduction of duration of diarrhoea <2 days, mean diarrhoea score, and mean composite diarrhoea score significant reduction of ETEC strains; protection against diarrhoea |
Prophylactic and therapeutic effect against diarrhoea | [120] |
| Weaned pigs >4 weeks old | Oral challenge with 5 mL of 109 CFU/mL Salmonella Typhimurium | Microencapsulated phage cocktail in feed (5 × 1011 PFU) for 5 days before challenge with Salmonella Typhimurium | Reduction of S. Typhimurium in ileum and caecum by about 1 log10 CFU/g | Therapeutic and prophylactic effect | [121] |
| 4-week-old weaned pigs | Salmonella enterica serovar Typhimurium | 5 mL of a 8- phage cocktail at 109 PFU/mL (SEP-1, SGP-1, STP-1, SS3eP-1, STP-2, SChP-1, SAP-1, SAP-2) | Significant reduction of Salmonella Typhimurium; 100% lytic activity against 34 Salmonella reference strains and 92.5% lytic activity against 107 wild strains | Therapeutic effect in diarrhoea | [122] |
| Merino cross wethers sheep (1 year of age) | S. aureus strain ATCC 25923 | Phage cocktail CTSA 2 × 108 PFU/mL applied to right and left sinuses | Reduction of tissue damage; reduction of S. aureus colonization | Therapeutic and antibacterial activity | [123] |
| 20 Canadian Arcott rams weighing 50 kg | E. coli O157:H7(109 CFU/mL | Oral administration of E. coli phage cocktail P5, P8 and P11 (1010 PFU) administered orally 5 times using a sterile 60-mL syringe and stomach tube | Significant reduction~2 log10 CFU of intestinal E. coli O157:H7 in sheep; total elimination of bacteria in 30% of animals | Prophylactic and therapeutic effect | [124] |
| Ross broiler chickens at 34 d of age | S. enterica ser. Enteritidis P125109; S. enterica serotype Typhimurium 4/74; S. enterica serotype Hadar 18 | Bacteriophage suspensions as antacid administered by oral gavage 9.0 or 11.0 log10 PFU of φ151 (S. enterica ser. Enteritidis), φ25 (S. enterica ser. Hadar), or φ10 (S. enterica ser. Typhimurium) | Significant reduction of S. enterica ser. Enteritidis and Typhimurium caecal colonization by ≥4.2 log10 CFU within 24 h | Therapeutic and prophylactic effect | [125] |
| Young chicks | Salmonella Typhimurium DT104 | Single oral dose of phage FO1 of 109 (PFU)/chick in encapsulated form | Reduction of Salmonella Typhimurium strains in caecum | Antibacterial effect | [126] |
| Vrolix chicks aged 20 days | Campylobacter jejuni | 3-bacteriophage cocktail 5 × 108 PFU of CP14, CP81 or CP68 | Reduction of C. jejuni strains in caeca by approx. 3 log10 CFU units | Antibacterial and protective effect | [127] |
| Chickens |
Campylobacter jejuni; S. enterica serovar Enteritidis |
Direct inoculation onto chicken skin, C. jejuni typing phage 12673 at 106 PFU/cm2 of skin; S. enterica serovar Enteritidis phage P22, phage 29C, 103 PFU/cm2 of skin |
Significant reduction of Campylobacter up to 2 log10 per unit area of skin within 48 h; reduction of C. jejuni ~2 log10 on experimentally contaminated chicken skin after phage application | Therapeutic and antibacterial effect | [128] |
| Ross strain 308 commercial chicken broilers | Salmonella enterica | 3-phage cocktail, liposome/alginate, encapsulated, 1010 PFU/animal for 9 days | Significant decrease in Salmonella spp. concentration (~50%) in caeca | Antibacterial activity | [129] |
| Broiler chickens (Cobb 500) at 1 d of age | E. coli ser 02 | Sprayed with 200 mL of 8 × 108 PFU/mL phage SPR02 | Significant reduction of mortality by >10% | Antibacterial and protective effect | [130] |
| 8-day-old quail | Oral challenge with 100 μL of 1.2 × 109 CFU ml−1 S. Enteritidis | Oral application of 100 μL of 106 PFU ml−1 bacteriophage for 3 days | Reduction of S. Enteritidis in caecal tonsils of Japanese quails to 33.3 and 20%, 24 h and 7 days after application; prophylactic effect against S. Enteritidis colonization, increase in resistance against Salmonella challenge | Prophylactic effect | [43] |
| 2-day-old New Zealand White rabbits | Oral infection with Vibrio cholerae 8 × 108 CFU | Oral application of 3 phages (Phi_2, 24 and X29) 109 PFU | Reduction of bacteria count up to 4 log10 CFU/g; full protection against clinical signs of disease |
Prophylactic and therapeutic effects | [131] |
| 120 eight-week-old female BALB/c mice |
Mycobacterium ulcerans as ulcerous infections |
Single dose of mycobacteriophage D29 108 PFU/mouse administered 33 days post infection |
Progressive reduction of footpad swelling by day 150 post-infection significant reduction of M. ulcerans~1.5–2 log10 CFU/ml |
Therapeutic effect and antipathogenic activity effect | [132] |
| Mice | Pseudomonas aeruginosa | Bacteriophage PAK_P1 intranasally at curative dose of 1.0 × 108 PFU/mL or prophylactic dose of 1.0 × 109 PFU (MOI 100) | Prophylaxis of acute respiratory infections caused by P. aeruginosa; significant reduction of clinical signs; resistance to infection; stimulation of immune response | Therapeutic and prophylactic effect | [75] |
| BALB-C female mice aged 10 weeks | Pseudomonas aeruginosa | Single dose of phage MMI-Ps1 107 PFU suspension by intranasal application | Prophylaxis against P. aeruginosa infection; significant reduction of bacterial content in lungs about 2 log10 | Protective and antibacterial effect | [133] |
| Female mice C57BL/6 mice, aged 7 to 8 weeks | Acinetobacter baumanni | A. baumanni phage Bϕ-C62 inoculated intranasally (1 × 1010 PFU/ml | 100% survival after challenge with A. baumanni | Therapeutic effect, slight immunostimulatory effect | [134] |
| BALB/c mice aged 6–8 weeks | Klebsiella pneumonia-induced pneumonia | Bacteriophage suspension 2 × 109 PFU/mouse applied in a single dose i.n. | Significant decrease in duration of illness and microscopic lesions; suppression of necrosis, bronchiolitis, and infiltration of inflammatory cells | Therapeutic effect | [82] |
| BALB/c mice | Klebsiella pneumoniae B5055 | 50 μL of 108 PFU/mL single and 5-phage cocktail applied topically at wound site (Kpn1, Kpn2, Kpn3, Kpn4 and Kpn5) |
Significant reduction of K. pneumoniae load to 4.32, 4.64, 4.42, 4.11 and 4.27 log CFU/mL; rapid healing of wounds in all phage-treated groups | Therapeutic and antibacterial activity | [135] |
| Male Wistar rats; 9–10 weeks old | Staphylococcus aureus-associated pneumonia | Intravenous application of cocktail of 4 phages (2–3 × 109 PFU/mL of 2003, 2002, 3A, and K | Increase in survival from 0% to 58% significant reduction of bacterial content in the lung (1.2 × 106 CFU/g of tissue for survivors vs. 1.2 × 109 CFU/g for nonsurviving animals); reduction of lung damage | Therapeutic and immunomodulatory effect; antibacterial activity | [136] |
| New Zealand White infant rabbits (aged 3 days) and CD-1 infant mice (aged 4 and 5 days) |
Vibrio cholerae; oral administration of 5 × 108 CFU/rabbit or mouse |
Oral administration of phage cocktail (3 × 107 or 108 PFU/rabbit or mouse) | Protective effect against cholera via significant reduction of caecal colonization by V. cholerae; protection against cholera-like diarrhoea | Prophylactic and therapeutic effect | [137] |
| New Zealand White rabbits 2-day-old | Vibrio cholera 5 × 108 CFU per animal | Phage Phi_1 at 1 × 109 PFU/animal orally administered either 6 h before or 6 h after bacterial challenge | Protection against clinical signs of cholera; lack of diarrhoea; significant reduction of 2–4 log10 CFU/g V. cholera |
Prophylactic and therapeutic effect | [131] |
| Female C57BL6/SJL mice as cow mastitis infection model | Streptococcus dysgalactiae NRRL B-65273, S. agalactiae NRRL B-65272, and S. uberis NRRL B-65274 | Direct application into mammary gland: Streptococcus spp. phage endolysins 25 μg/gland for λSA2, 250 μg/gland for B30, and 12.5 (λSA2) + 125 (B30) μg/gland | Significant reduction of S. dysgalactiae content by 3.5 log10 CFU; S. agalactiae (2 log); S. uberis (4 log); protection against clinical signs of mastitis | Therapeutic effect and antibacterial activity | [138] |
6. Conclusions
To sum up, bacteriophages have many properties indicating their potential suitability as therapeutic or/and prophylactic agents. Future research on the scope of phages will provide a good picture of their potential to treat infections caused by multidrug-resistant bacteria. However, as bacteriophages are essentially ‘living’ drugs, the study of their use for therapy or biocontrol spans from purely clinical observations to molecular analysis to considerations of immunology and ecology. Due to the antibiotic resistance crisis, there is a compelling need for alternative safe and selectively effective antibacterial agents.
Abbreviations
| AMPs | antimicrobial peptides |
| ATCC | American Type Culture Collection |
| BALB | Bagg Albino Mouse |
| BAVS | Bacterial and Archaeal Viruses Subcommittee |
| CD | cluster of differentiation |
| CFU | colony-forming unit |
| EFSA | European Food Safety Authority |
| EHEC | Enterohaemorrhagic Escherichia coli |
| ETEC | Enterotoxigenic Escherichia coli |
| EFSA | European Food Safety Authority |
| EIBMV | Eliava Institute of Bacteriophages, Microbiology, and Virology |
| HF | Holstein–Friesian |
| HIIET | Hirszfeld Institute of Immunology and Experimental Therapy |
| HGT | horizontal gene transfer |
| Hp | haptoglobin |
| ICR | Institute of Cancer Research |
| ICTV | International Committee on Taxonomy of Viruses |
| IFNγ | Interferon gamma |
| Ig | immunoglobulin |
| i.p | intraperitoneally |
| i.n | intranasal |
| INSDC | International Nucleotide Sequence Database Collaboration |
| IL | interleukin |
| LAB | lactic acid bacteria |
| LPS | lipopolysaccharide |
| MAC-T | mammary alveolar cells |
| MDR | multidrug-resistant |
| MHC | major histocompatibility complex |
| MOI | multiplicity of infection |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer |
| PFU | plaque-forming units |
| PMN | polymorphonuclear |
| RBPs | receptor-binding proteins |
| ROS | reactive oxygen species |
| mRNA | messenger RNA |
| SAA | serum amyloid A |
| ssRNA | single-stranded ribonucleic acid |
| dsRNA | double-stranded ribonucleic acid |
| ssDNA | single-stranded deoxyribonucleic acid |
| dsDNA | double-stranded deoxyribonucleic acid |
| SPF | specific free pathogens |
| stx | Shiga toxin |
| TEM | transmission electron microscopy |
| TNF-α | tumour necrosis factor α |
| UV | ultraviolet |
| WHO | World Health Organization |
Author Contributions
Writing the original draft, literature review and collection, M.M.M.A.; literature review and resources, visualization, M.D.; conception of the manuscript, writing and editing of the manuscript, R.U.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ssekatawa K., Byarugabaa D.K., Katoa C.D., Wampandea E.M., Ejobia F., Tweyongyerea R., Nakavumaa J.L. A review of phage mediated antibacterial applications. Alexandria J. Med. 2021;57:1–20. doi: 10.1080/20905068.2020.1851441. [DOI] [Google Scholar]
- 2.World Health Organization . World Health Organization Estimates of the Global Burden of Foodborne Diseases. WHO; Geneva, Switzerland: 2015. [(accessed on 22 November 2021)]. Available online: http://apps.who.int. [Google Scholar]
- 3.Alali W.Q., Thakur S., Berghaus R.D., Martin M.P., Gebreyes W.A. Prevalence anddistribution of Salmonella in organic andconventional broiler poultry farms. Foodborne Pathog. Dis. 2010;7:1363–1371. doi: 10.1089/fpd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 4.Sillankorva S.M., Oliveira H., Azeredo J. Bacteriophages and their role in food safety. Inter. J. Microbiol. 2012;2012:863945. doi: 10.1155/2012/863945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu Y.D., Cook S.R., Wang J., Klima C.L., Hsu Y., Kropinski A.M., Turner D., McAllister T.A. Comparative analysis of multiple inducible phages from Mannheimia haemolytica. BMC Microbiol. 2015;15:175. doi: 10.1186/s12866-015-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Food Safety Authority (EFSA) The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2018;17:e05598. doi: 10.2903/j.efsa.2019.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichenberger E.M., Thaden J.T. Epidemiology and mechanisms of resistance of extensively drug resistant Gram-negative bacteria. Antibiotics. 2019;8:37. doi: 10.3390/antibiotics8020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donkor E.S., Newman M.J., Yeboah-Manu D. Epidemiological aspects of non-human antibiotic usage and resistance: Implications for the control of antibiotic resistance in Ghana. Trop. Med. Int. Health. 2012;17:462–468. doi: 10.1111/j.1365-3156.2012.02955.x. [DOI] [PubMed] [Google Scholar]
- 9.Żbikowska K., Michalczuk M., Dolka B. The use of bacteriophages in the poultry industry. Animals. 2020;10:872. doi: 10.3390/ani10050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regulation (EU) 2019/6 Of The European Parliament And Of The Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Offical J. EU.Union. 2019. [(accessed on 22 November 2021)]. pp. 43–167. Available online: https://eur-lex.europa.eu/eli/reg/2019/6/oj.
- 11.Orlova E. Bacteriophages and Their Structural Organisation. 1st ed. IntechOpen; London, UK: 2012. pp. 3–30. [Google Scholar]
- 12.Lenski R.E. Advances in Microbial Ecology. Springer; Chicago, IL, USA: 1988. Dynamics of interactions between bacteria and virulent bacteriophage; pp. 1–44. [Google Scholar]
- 13.Batinovic S., Wassef F., Knowler S.A., Rice D.T.F., Stanton C.R., Rose J., Tucci J., Nittami T., Vinh A., Drummond G.R., et al. Bacteriophages in natural and artificial environments. Pathogens. 2019;8:100. doi: 10.3390/pathogens8030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brüssow H. Bacteria between protists and phages: From antipredation strategies to the evolution of pathogenicity. Mol. Microbiol. 2007;65:583–589. doi: 10.1111/j.1365-2958.2007.05826.x. [DOI] [PubMed] [Google Scholar]
- 15.Vikram A., Tokman J.I., Woolston J., Sulakvelidze A. Phage Biocontrol Improves Food Safety by Significantly Reducing the Level and Prevalence of Escherichia coli O157:H7 in Various Foods. J. Food Prot. 2021;83:668–676. doi: 10.4315/0362-028X.JFP-19-433. [DOI] [PubMed] [Google Scholar]
- 16.Viazis S., Akhtar M., Feirtag J., Brabban A.D., Diez-Gonzalez F. Isolation and characterization of lytic bacteriophages against enterohaemorrhagic Escherichia coli. J. Appl. Microbiol. 2011;110:1323–1331. doi: 10.1111/j.1365-2672.2011.04989.x. [DOI] [PubMed] [Google Scholar]
- 17.Dec M., Wernicki A., Urban-Chmiel R. Efficacy of experimental phage therapies in livestock. Anim. Health Res. Rev. 2020;21:69–83. doi: 10.1017/S1466252319000161. [DOI] [PubMed] [Google Scholar]
- 18.De Jonge P.A., Nobrega F.L., Brouns S.J., Dutilh B.E. Molecular and evolutionary determinants of bacteriophage host range. Trends Microb. 2019;27:51–63. doi: 10.1016/j.tim.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Dunne M., Rupf B., Tala M., Qabrati X., Ernst P., Shen Y., Sumrall E., Heeb L., Plückthun P., Loessner M.J., et al. Reprogramming Bacteriophage Host Range through Structure-Guided Design of Chimeric Receptor Binding Proteins. Cell Reports. 2019;29:1336–1350. doi: 10.1016/j.celrep.2019.09.062. [DOI] [PubMed] [Google Scholar]
- 20.Twort F.W. An investigation on the nature of ultramicroscopic viruses. Lancet. 1915;186:1241–1243. doi: 10.1016/S0140-6736(01)20383-3. [DOI] [Google Scholar]
- 21.d’Herelle F. Sur un microbe invisible antagoniste des bacilles dysentériques. Comptes Rendus de l’Académie Sci. Paris. 1917;165:173–175. [Google Scholar]
- 22.d’Herelle F. Sur le role du microbe bacteriophage dans la typhose aviare. C. R. Acad. Sci. 1919;169:932–934. [Google Scholar]
- 23.Duckworth D.H. Who discovered bacteriophage? Bacteriol. Rev. 1976;40:793. doi: 10.1128/br.40.4.793-802.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abedon S.T., Thomas-Abedon C., Thomas A., Mazure H. Bacteriophage prehistory: Is or is not Hankin, 1896, a phage reference. Bacteriophage. 2011;1:174–178. doi: 10.4161/bact.1.3.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opal S.M. The evolution of the understanding of sepsis, infection, and the host response: A brief history. Crit. Care Clin. 2009;25:637–663. doi: 10.1016/j.ccc.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Rello J., Bunsow E., Perez A. What if there were no new antibiotics? A look at alternatives. Expert Rev. Clin. Pharmacol. 2016;9:1547–1555. doi: 10.1080/17512433.2016.1241141. [DOI] [PubMed] [Google Scholar]
- 27.Sulakvelidze A., Alavidze Z., Morris J.G. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutter E., De Vos D., Gvasalia G., Alavidze Z., Gogokhia L., Kuhl S., Abedon S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 29.Górski A., Miedzybrodzki R., Weber-Da˛browska B., Fortuna W., Letkiewicz S., Rogóż P., Jończyk-Matysiak E., Dabrowska K., Majewska J., Borysowski J. Phage Therapy: Combating Infections with Potential for Evolving from Merely a Treatment for Complications to Targeting Diseases. Front. Microbiol. 2016;7:1515. doi: 10.3389/fmicb.2016.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Nucleotide Sequence Database Collaboration. [(accessed on 28 October 2021)]. Available online: https://www.insdc.org/
- 31.Wittebole X., De Roock S., Opal S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adriaenssens E.M., Krupovic M., Knezevic P., Ackermann H.W., Barylski J., Brister Clokie R.M.C., Duffy S., Dutilh B.E., Edwards R.A., Enault F., et al. Taxonomy of prokaryotic viruses: 2016 update from the ICTV bacterial and archaeal viruses subcommittee. Archiv. Virol. 2017;162:1153–1157. doi: 10.1007/s00705-016-3173-4. [DOI] [PubMed] [Google Scholar]
- 33.Pyzik E., Radzki R.P., Urban-Chmiel R. Experimental Phage Therapies in Companion Animals with A Historical Rewiev. Curr. Clin. Pharmacol. 2021;16:17–29. doi: 10.2174/1574884715666200330105411. [DOI] [PubMed] [Google Scholar]
- 34.García P., Rodríguez L., Rodríguez A., Martínez B. Food biopreservation: Promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci. Technol. 2010;21:373–382. doi: 10.1016/j.tifs.2010.04.010. [DOI] [Google Scholar]
- 35.Rakhuba D.V., Kolomiets E.I., Dey E.S., Novik G.I. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol. J. Microbiol. 2010;59:145–155. doi: 10.33073/pjm-2010-023. [DOI] [PubMed] [Google Scholar]
- 36.Hanlon G.W. Bacteriophages: An appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agen. 2007;30:118–128. doi: 10.1016/j.ijantimicag.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Wernicki A., Nowaczek A., Urban-Chmiel R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017;14:179. doi: 10.1186/s12985-017-0849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark D.P., Pazdernik N.J. Molecular Biology. 2nd ed. Elsevier Inc.; Waltham, MA, USA: 2013. Chapter 7. Cloning Genes for Analysis; pp. 194–226. Academic Cell Update Edition. [Google Scholar]
- 39.Hyman P., Abedon S.T. Bacteriophage Host Range and Bacterial Resistance, in Advances in Applied Microbiology. Elsevier; Amsterdam, The Netherlands: 2010. pp. 217–248. [DOI] [PubMed] [Google Scholar]
- 40.Clokie M.R., Millard A.D., Letarov A.V., Heaphy S. Phages in nature. Bacteriophage. 2011;1:31–45. doi: 10.4161/bact.1.1.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alomari M.M.M., Dec M., Nowaczek A., Puchalski A., Wernicki A., Kowalski C.J., Urban-Chmiel R. Therapeutic and Prophylactic Effect of the Experimental Bacteriophage Treatment to Control Diarrhea Caused by E. coli in Newborn Calves. ACS Infect. Dis. 2021;7:2093–2101. doi: 10.1021/acsinfecdis.1c00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagenaar J.A., Van Bergen M.A., Mueller M.A., Wassenaar T.M., Carlton R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 2005;109:275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Ahmadi M., Karimi Torshizi M.A., Rahimi S., Dennehy J.J. Prophylactic bacteriophage administration more effective than post-infection Administration in Reducing Salmonella enterica serovar Enteritidis shedding in quail. Front. Microbiol. 2016;7:1253. doi: 10.3389/fmicb.2016.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazi M., Annapure U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Tech. 2016;53:1355–1362. doi: 10.1007/s13197-015-1996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva J., Sauvageau D. Bacteriophages as antimicrobial agents against bacterial contaminants in yeast fermentation processes. J. Biotech. Biof. 2014;7:123. doi: 10.1186/s13068-014-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy B., Ackermann H.W., Pandian S., Picard G., Goulet J. Biological inactivation of adhering Listeria monocytogenes by listeriaphages and a quaternary ammonium compound. Appl. Environ. Microbiol. 1993;59:2914–2917. doi: 10.1128/aem.59.9.2914-2917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho Y.H., Tseng C.C., Wang L.S., Chen Y.T., Ho G.J., Lin T.Y., Wang L.Y., Chen L.K. Application of bacteriophage-containing aerosol against nosocomial transmission of carbapenem-resistant acinetobacter baumannii in an intensive care unit. PLoS ONE. 2016;11:e0168380. doi: 10.1371/journal.pone.0168380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le T.S., Southgate P.C., O’Connor W., Poole S., Kurtböke D.I. Bacteriophages as biological control agents of enteric bacteria contaminating edible oysters. Curr. Microbiol. 2018;75:611–619. doi: 10.1007/s00284-017-1424-6. [DOI] [PubMed] [Google Scholar]
- 49.Sieiro C., Areal-Hermida L., Pichardo-Gallardo Á., Almuiña-González R., de Miguel T., Sánchez S., Sánchez-Pérez Á., Villa T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics. 2020;9:493. doi: 10.3390/antibiotics9080493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai J., Kim Y.T., Ryu S., Lee J.H. Biocontrol and rapid detection of food-borne pathogens using bacteriophages and endolysins. Front. Microbiol. 2016;7:1–15. doi: 10.3389/fmicb.2016.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sultan I., Rahman S., Jan A.T., Siddiqui M.T., Mondal A.H., Haq Q.M.R. Antibiotics, resistome and resistance mechanisms: A bacterial perspective. Front. Microbiol. 2018;9:2066. doi: 10.3389/fmicb.2018.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park K., Cha K.E., Myung H. Observation of inflammatory responses in mice orally fed with bacteriophage T 7. J. Appl. Microb. 2014;117:627–633. doi: 10.1111/jam.12565. [DOI] [PubMed] [Google Scholar]
- 53.Van Belleghem J.D., Clement F., Merabishvili M., Lavigne R., Vaneechoutte M. Pro-and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Hou X., Sun L., He T., Wei R., Pang M., Wang R. Staphylococcus aureus bacteriophage suppresses LPS-induced inflammation in MAC-T bovine mammary epithelial cells. Front. Microbiol. 2018;9:1614. doi: 10.3389/fmicb.2018.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith H.W., Huggins M.B., Shaw K.M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 1987;133:1127–1135. doi: 10.1099/00221287-133-5-1127. [DOI] [PubMed] [Google Scholar]
- 56.Cooper R.A., Bjarnsholt T., Alhede M. Biofilms in wounds: A review of present knowledge. J. Wound. Care. 2014;23:570–580. doi: 10.12968/jowc.2014.23.11.570. [DOI] [PubMed] [Google Scholar]
- 57.Reardon S. Phage therapy gets revitalized: The rise of antibiotic resistance rekindles interest in a century-old virus treatment. Nature. 2014;510:15–17. doi: 10.1038/510015a. [DOI] [PubMed] [Google Scholar]
- 58.Kutter E.M., Kuhl S.J., Abedon S.T. Re-establishing a place for phage therapy in western medicine. Future Microbiol. 2015;10:685–688. doi: 10.2217/fmb.15.28. [DOI] [PubMed] [Google Scholar]
- 59.Alomari M.M.M., Nowaczek A., Dec M., Urban-Chmiel R. Antibacterial activity of bacteriophages isolated from poultry against Shiga-toxic strains of Esherichia coli isolated from calves. Med. Weter. 2016;72:699–703. doi: 10.21521/mw.5585. [DOI] [Google Scholar]
- 60.Cisek A.A., Dąbrowska I., Gregorczyk K.P., Wyżewski Z. Phage therapy in bacterial infections treatment: One hundred years after the discovery of bacteriophages. Curr. Microbiol. 2017;74:1–7. doi: 10.1007/s00284-016-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Podlacha M., Grabowski Ł., Kosznik-Kawśnicka K., Zdrojewska K., Stasiłojć M., Węgrzyn G., Alicja Węgrzyn A. Interactions of Bacteriophages with Animal and Human Organisms—Safety Issues in the Light of Phage Therapy. Int. J. Mol. Sci. 2021;22:8937. doi: 10.3390/ijms22168937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ann T.W., Kim S.J., Lee Y.D., Park J.H., Chang H.I. The Immune-Enhancing Effect of the Cronobacter Sakazakii ES2 Phage Results in the Activation of Nuclear Factor-KB and Dendritic Cell Maturation via the Activation of IL-12p40 in the Mouse Bone Marrow. Immunol. Lett. 2014;157:1–8. doi: 10.1016/j.imlet.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Van Belleghem J.D., Dąbrowska K., Vaneechoutte M., Barr J.J., Bollyky P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses. 2019;11:10. doi: 10.3390/v11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geier M.R., Trigg M.E., Merril C.R. Fate of bacteriophage lambda in non-immune germ-free mice. Nature. 1973;246:221–223. doi: 10.1038/246221a0. [DOI] [PubMed] [Google Scholar]
- 65.Tiwari B.R., Kim S., Rahman M., Kim J. Antibacterial efficacy of lytic Pseudomonas bacteriophage in normal and neutropenic mice models. J. Microbiol. 2011;49:994–999. doi: 10.1007/s12275-011-1512-4. [DOI] [PubMed] [Google Scholar]
- 66.Tóthová L., Celec P., Bábíˇcková J., Gajdošová J., Al-Alami H., Kamodyova N., Drahovská H., Liptáková A., Tur ˇna J., Hodosy J. Phage Therapy of Cronobacter-Induced Urinary Tract Infection in Mice. Med. Sci. Monit. 2011;17:173–178. doi: 10.12659/MSM.881844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reyes A., Semenkovich N.P., Whiteson K., Rohwer F., Gordon J.I. Going viral: Next generation sequencing applied to human gut phage populations. Nat. Rev. Microbiol. 2012;10:607–610. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dabrowska K., Switała-Jelen K., Opolski A., Weber-Dabrowska B., Gorski A. Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 2005;98:7–13. doi: 10.1111/j.1365-2672.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 69.Sarker S.A., McCallin S., Barretto C., Berger B., Pittet A.C., Sultana S., Krause L., Huq S., Bibiloni R., Bruttin A., et al. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology. 2012;434:222–232. doi: 10.1016/j.virol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Wright A., Hawkins C.H., Änggård E.E., Harper D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009;34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 71.Górski A., Miedzybrodzki R., Borysowski J., Dabrowska K., Wierzbicki P., Ohams M., Korczak-Kowalska G., Olszowska-Zaremba N., Łusiak-Szelachowska M., KŁak M., et al. Phage as a Modulator of Immune Responses: Practical Implications for Phage Therapy. Adv. Virus Res. 2012;83:41–71. doi: 10.1016/B978-0-12-394438-2.00002-5. [DOI] [PubMed] [Google Scholar]
- 72.Przerwa A., Zimecki M., Switała-Jelen K., Dabrowska K., Krawczyk E., Łuczak M., Weber-Dabrowska B., Syper D., Miedzybrodzki R., Górski A. Effects of bacteriophages on free radical production and phagocytic functions. Med. Microbiol. Immunol. 2006;195:143–150. doi: 10.1007/s00430-006-0011-4. [DOI] [PubMed] [Google Scholar]
- 73.Ly-Chatain M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014;18:51. doi: 10.3389/fmicb.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sulakvelidze A., Kutter E. Bacteriophage Therapy in Humans. Bacteriophages: Biology and Applications. CRC Press; Boca Raton, FL, USA: 2004. pp. 381–436. [Google Scholar]
- 75.Roach D.R., Leung C.Y., Henry M., Morello E., Singh D., Di Santo J.P. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microb. 2017;22:38.e–47.e. doi: 10.1016/j.chom.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 76.Miernikiewicz P., Dąbrowska K., Piotrowicz A., Owczarek B., Wojas-Turek J., Kicielińska J., Rossowska J., Pajtasz-Piasecka E., Hodyra K., Macegoniuk K., et al. T4 phage and its head surface proteins do not stimulate inflammatory mediator production. PLoS ONE. 2013;8:e71036. doi: 10.1371/journal.pone.0071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pajtasz-Piasecka E., Rossowska J., Duś D., Weber-Dabrowska B., Zabłocka A., Górski A. Bacteriophages support anti-tumor response initiated by DC-based vaccine against murine transplantable colon carcinoma. Immunol. Lett. 2008;116:24–32. doi: 10.1016/j.imlet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Yıldızlı G., Coral G., Ayaz F. Immunostimulatory Activities of Coliphages on In Vitro Activated Mammalian Macrophages. Inflammation. 2020;43:595–604. doi: 10.1007/s10753-019-01140-9. [DOI] [PubMed] [Google Scholar]
- 79.Srivastava A.S., Kaido T., Carrier E. Immunological factors that affect the in vivo fate of T7 phage in the mouse. J. Virol. Meth. 2004;115:99–104. doi: 10.1016/j.jviromet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Gogokhia L., Buhrke K., Bell R., Hoffman B., Brown D.G., Hanke-Gogokhia C., Ajami N.J., Wong M.C., Ghazaryan A., John F., et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microb. 2019;25:285–299. doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Majewska J., Kaźmierczak Z., Lahutta K., Lecion D., Szymczak A., Miernikiewicz P., Drapała J., Harhala M., Marek-Bukowiec K., Jędruchniewicz N., et al. Induction of Phage-Specific Antibodies by Two Therapeutic Staphylococcal Bacteriophages Administered per os. Front. Immunol. 2019;10:2607. doi: 10.3389/fimmu.2019.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anand T., Virmani N., Kumar S., Mohanty A.K., Pavulraj S., Bera B.C., Vaid R.K., Ahlawat U., Tripathi B.N. Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J. Glob. Antimicrob. Resist. 2020;21:34–41. doi: 10.1016/j.jgar.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Biswas B., Adhya S., Washart P., Paul B., Trostel A.N., Powell B., Troste A.N., Powell B., Carlton R., Merril C.R. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 2002;70:204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capparelli R., Nocerino N., Iannaccone M., Ercolini D., Parlato M., Chiara M., Iannelli D. Bacteriophage therapy of Salmonella enterica: A fresh appraisal of bacteriophage therapy. J. Infect. Dis. 2010;201:52–61. doi: 10.1086/648478. [DOI] [PubMed] [Google Scholar]
- 85.Langbeheim H., Teitelbaum D., Arnon R. Cellular immune response toward MS-2 phage and a synthetic fragment of its coat protein. Cell Immunol. 1978;38:193–197. doi: 10.1016/0008-8749(78)90046-1. [DOI] [PubMed] [Google Scholar]
- 86.Górski A., Ważna E., Dąbrowska B.W., Dąbrowska K., Świtała-Jeleń K., Międzybrodzki R. Bacteriophage translocation. FEMS Immunol. Med. Microbiol. 2006;46:313–319. doi: 10.1111/j.1574-695X.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 87.Moye Z.D., Woolston J., Sulakvelidze A. Bacteriophage applications for food production and processing. Viruses. 2018;10:205. doi: 10.3390/v10040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abedon S.T. Ecology of anti-biofilm agents II. Bacteriophage exploitation and biocontrol of biofilm bacteria. Pharmaceuticals. 2015;8:559–589. doi: 10.3390/ph8030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soffer N., Abuladze T., Woolston J., Li M., Hanna L.F., Heyse S., Charbonneau D., Sulakvelidze A. Bacteriophages safely reduce Salmonella contamination in pet food and raw pet food ingredients. Bacteriophage. 2016;6:e1220347. doi: 10.1080/21597081.2016.1220347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Food Safety. [(accessed on 28 October 2021)]. Available online: https://www.intralytix.com/index.php?page=food.
- 91.Połaska M., Sokołowska B. Bacteriophages—a new hope or a huge problem in the food industry. AIMS Microbiol. 2019;5:324–346. doi: 10.3934/microbiol.2019.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agricultural Biocontrol Applications. [(accessed on 28 October 2021)]. Available online: https://www.brimrosetechnology.com/biocontrol.
- 93.Sommer J., Trautner C., Witte A.K., Fister S., Schoder D., Rossmanith P., Mester P.J. Don’t shut the stable door after the phage has bolted—the importance of bacteriophage inactivation in food environments. Viruses. 2019;11:468. doi: 10.3390/v11050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.PhageGuard. [(accessed on 28 October 2021)]. Available online: www.phageguard.com.
- 95.Vikram A., Woolston J., Sulakvelidze A. Phage biocontrol applications in food production and processing. Curr. Iss. Mol. Biol. 2020;40:267–302. doi: 10.21775/cimb.040.267. [DOI] [PubMed] [Google Scholar]
- 96.U. S. Food & Drug [(accessed on 28 October 2021)]. Available online: https://fda.report/media/111308/GRAS-Notice-000724.
- 97.Mai V., Ukhanova M., Reinhard M.K., Li M., Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. 2015;5:e1088124. doi: 10.1080/21597081.2015.1088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards): Scientific opinion on the evaluation of the safety and efficacy of Listex TM P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016;14:1–94. [Google Scholar]
- 99.Chhibber S., Kumari S. Application of Therapeutic Phages in Medicine, Bacteriophages, Ipek Kurtboke, IntechOpen. 2012. [(accessed on 22 November 2021)]. Available online: https://www.intechopen.com/chapters/32283. [DOI]
- 100.Barrow P.A., Soothill J.S. Bacteriophage therapy and prophylaxis: Rediscovery and renewed assessment of potential. Trends Microbiol. 1997;5:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 101.Matsuzaki S., Rashel M., Uchiyama J., Sakurai S., Ujihara T., Kuroda M., Ikeuchi M., Tani T., Fujieda M., Wakiguchi H., et al. Bacteriophage therapy: A revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 2005;11:211–219. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 102.Loc-Carrillo C., Abedon S.T. Pros and cons of phage therapy. Bacteriophage. 2011;1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elbreki M., Ross R.P., Hill C., O’Mahony J., McAuliffe O., Coffey A. Bacteriophages and their derivatives as biotherapeutic agents in disease prevention and treatment. J. Viruses. 2014;2014:382539. doi: 10.1155/2014/382539. [DOI] [Google Scholar]
- 104.Oliveira J., Castilho F., Cunha A., Pereira M.J. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquaculture Inter. 2012;20:879–910. doi: 10.1007/s10499-012-9515-7. [DOI] [Google Scholar]
- 105.Iriarte F.B., Balogh B., Momol M.T., Smith L.M., Wilson M., Jones J.B. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl. Environ. Microbiol. 2007;73:1704–1711. doi: 10.1128/AEM.02118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pirnay J.P., Blasdel B.G., Bretaudeau L., Buckling A., Chanishvili N., Jason R., Corte-Real S., Debarbieux L., Dublanchet A., De VosJérôme D., et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015;32:2173–2179. doi: 10.1007/s11095-014-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ryan E.M., Gorman S.P., Donnelly R.F., Gilmore B.F. Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 2011;63:1253–1264. doi: 10.1111/j.2042-7158.2011.01324.x. [DOI] [PubMed] [Google Scholar]
- 108.Babalova E.G., Katsitadze K.T., Sakvarelidze L.A., Imnaishvili N.S., Sharashidze T.G., Badashvili V.A., Kiknadze G.P., Meipariani A.N., Gendzekhadze N.D., Machavariani E.V., et al. Preventive value of dried dysentery bacteriophage. Zhurnal Mikrobiol. 1968;45:143–145. [PubMed] [Google Scholar]
- 109.Litvinova A.M., Chtetsova V.M., Kavtreva I.G. Evaluation of efficacy of the use of coli-Proteus bacteriophage in intestinal dysbacteriosis in premature infants. Voprosy Okhrany Materinstva i Detstva. 1978;23:42–44. [PubMed] [Google Scholar]
- 110.Meladze G.D., Mebuke M.G., Chkhetia N.S., Kiknadze N.I., Koguashvili G.G., Timoshuk I.I., Larionova N.G., Vasadze G.K. The efficacy of staphylococcal bacteriophage in treatment of purulent diseases of lungs and pleura. Grudn. Khir. 1982;1:53–56. [PubMed] [Google Scholar]
- 111.Bogovazova G.G., Voroshilova N.N., Bondarenko V.M. The efficacy of Klebsiella pneumoniae bacteriophage in the therapy of experimental Klebsiella infection. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 1991;4:5–8. [PubMed] [Google Scholar]
- 112.Johnson R.P., Gyles C.L., Huff W.E., Ojha S., Huff G.R., Rath N.C., Donoghue A.M. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim. Health Res. Rev. 2008;9:201–215. doi: 10.1017/S1466252308001576. [DOI] [PubMed] [Google Scholar]
- 113.Smith H.W., Huggins M.B. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 1983;129:2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- 114.O’Flaherty S., Ross R.P., Meaney W., Fitzgerald G.F., Elbreki M.F., Coffey A. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microb. 2005;71:1836–1842. doi: 10.1128/AEM.71.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ngassam-Tchamba C., Duprez J.N., Fergestad M., De Visscher A., L’Abee-Lund T., De Vliegher S., Wasteson Y., Touzain F., Blanchard Y., Lavigne R., et al. In Vitro and in Vivo Assessment of Phage Therapy against Staphylococcus Aureus Causing Bovine Mastitis. J. Glob. Antimicrob. Resist. 2020;22:762–770. doi: 10.1016/j.jgar.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 116.Meira E.B.S., Rossi R.S., Teixeira A.G., Kaçar C., Oikonomou G., Gregory L. Bicalho, R.C. The effect of prepartum intravaginal bacteriophage administration on the incidence of retained placenta and metritis. J. Dairy Sci. 2013;96:7658–7665. doi: 10.3168/jds.2013-6774. [DOI] [PubMed] [Google Scholar]
- 117.Titze I., Lehnherr T., Lehnherr H., Krömker V. Efficacy of bacteriophages against Staphylococcus aureus isolates from bovine mastitis. Pharmaceuticals. 2020;13:35. doi: 10.3390/ph13030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendes J.J., Leandro C., Corte-Real S., Barbosa R., Cavaco-Silva P., Melo-Cristino J., Górski A., Garcia M. Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair. Regen. 2013;21:595–603. doi: 10.1111/wrr.12056. [DOI] [PubMed] [Google Scholar]
- 119.Wall S.K., Zhang J., Rostagno M.H., Ebner P.D. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 2010;76:48–53. doi: 10.1128/AEM.00785-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jamalludeen N., Johnson R.P., Shewen P.E., Gyles C.L. Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet. Microbiol. 2009;136:135–141. doi: 10.1016/j.vetmic.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 121.Saez A.C., Zhang J., Rostagno M.H., Ebner P.D. Direct Feeding of Microencapsulated Bacteriophages to Reduce Salmonella Colonization in Pigs. Foodborne Pathog. Dis. 2011;8:1269–1274. doi: 10.1089/fpd.2011.0905. [DOI] [PubMed] [Google Scholar]
- 122.Seo B.J., Song E.T., Lee K., Kim J.W., Jeong C.G., Moon S.H., Son J.S., Kang S.H., Cho H.S., Jung B.Y., et al. Evaluation of the broad-spectrum lytic capability of bacteriophage cocktails against various Salmonella serovars and their effects on weaned pigs infected with Salmonella Typhimurium. J. Vet. Med. Sci. 2018;80:851–860. doi: 10.1292/jvms.17-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drilling A., Morales S., Boase S., Jervis-Bardy J., James C., Jardeleza C., Tan N.C., Cleland E., Speck P., Vreugde S., et al. Safety and efficacy of topical bacteriophage and ethylenediaminetetraacetic acid treatment of Staphylococcus aureus infection in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2014;4:176–186. doi: 10.1002/alr.21270. [DOI] [PubMed] [Google Scholar]
- 124.Bach S.J., Johnson R.P., Stanford K., McAllister T.A. Bacteriophages reduce Escherichia coli O157: H7 levels in experimentally inoculated sheep. Can. J. Anim. Sci. 2009;89:285–293. doi: 10.4141/CJAS08083. [DOI] [Google Scholar]
- 125.Atterbury R.J., Van Bergen M.A.P., Ortiz F., Lovell M.A., Harris J.A., De Boer A., Wagenaar J.A., Allen V.M., Barrow P.A. Bacteriophage Therapy To Reduce Salmonella Colonization of Broiler Chickens. Appl. Environ. Microbiol. 2007;73:4543–4549. doi: 10.1128/AEM.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma Y.-H., Islam G.S., Wu Y., Sabour P.M., Chambers J.R., Wang Q., Wu S.X.Y., Griffiths M.W. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poultry Sci. 2016;95:2911–2920. doi: 10.3382/ps/pew260. [DOI] [PubMed] [Google Scholar]
- 127.Hammerl J.A., Jäckel C., Alter T., Janzcyk P., Stingl K., Knüver M.T., Hertwing S. Reduction of Campylobacter jejuni in Broiler Chicken by Successive Application of Group II and Group III Phages. PLoS ONE. 2014;9:e114785. doi: 10.1371/journal.pone.0114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goode D., Allen V.M., Barrow P.A. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 2003;69:5032–5036. doi: 10.1128/AEM.69.8.5032-5036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colom J., Cano-Sarabia M., Otero J., Aríñez-Soriano J., Cortés P., Maspoch D., Llagostera M. Microencapsulation with alginate/CaCO 3: A strategy for improved phage therapy. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep41441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El-Gohary F.A., Huff W.E., Huff G.R., Rath N.C., Zhou Z.Y., Donoghue A.M. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens1. Poult. Sci. 2014;93:2788–2792. doi: 10.3382/ps.2014-04282. [DOI] [PubMed] [Google Scholar]
- 131.Bhandare S., Colom J., Baig A., Ritchie J.M., Bukhari H., Shah M.A., Sarkar B.L., Su J., Wren B., Barrow P., et al. Reviving Phage Therapy for the Treatment of Cholera. J. Infect. Dis. 2019;219:786–794. doi: 10.1093/infdis/jiy563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trigo G., Martins T.G., Fraga A.G., Longatto-Filho A., Castro A.G., Azeredo J., Pedrosa J. Phage therapy is effective against infection by Mycobacterium ulcerans in a murine footpad model. PLoS Negl. Trop Dis. 2013;7:e2183. doi: 10.1371/journal.pntd.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.El-Aziz A.M.A., Elgaml A., Ali Y.M. Bacteriophage Therapy Increases Complement-Mediated Lysis of Bacteria and Enhances Bacterial Clearance After Acute Lung Infection With Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019;219:1439–1447. doi: 10.1093/infdis/jiy678. [DOI] [PubMed] [Google Scholar]
- 134.Jeon J., Ryu C.-M., Lee J.-Y., Park J.-H., Yong D., Lee K. In Vivo Application of Bacteriophage as a Potential Therapeutic Agent To Control OXA-66-Like Carbapenemase-Producing Acinetobacter baumannii Strains Belonging to Sequence Type 357. Appl. Environ. Microbiol. 2016;82:4200–4208. doi: 10.1128/AEM.00526-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chadha P., Katare O.P., Chhibber S. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microb. Pathogen. 2016;99:68–77. doi: 10.1016/j.micpath.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 136.Prazak J., Iten M., Cameron D.R., Save J., Grandgirard D., Resch G., Goepfert C., Leib S.L., Takala J., Jakob S.M., et al. Bacteriophages Improve Outcomes in Experimental Staphylococcus aureus Ventilator-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2019;200:1126–1133. doi: 10.1164/rccm.201812-2372OC. [DOI] [PubMed] [Google Scholar]
- 137.Yen M., Cairns L.S., Camilli A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nature Comm. 2017;8:1–7. doi: 10.1038/ncomms14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmelcher M., Powell A.M., Camp M.J., Pohl C.S., Donovan D.M. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl. Micorb. Biotechnol. 2015;99:8475–8486. doi: 10.1007/s00253-015-6579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]