Abstract
In this review, the results of recent and ongoing clinical trials in patients with SLE are discussed. After many unsuccessful trials in the past decade, belimumab was the first biologic specifically designed for SLE that met its primary end point. At the same time, studies on the pathophysiology of SLE have further elucidated the pathways involved in the disease, which has led to the identification of new possible therapeutics and has encouraged the initiation of new trials. These new drugs include biologics that target B cells, T cells and type 1 interferons, and small molecules that inhibit kinases. Other therapeutics aim to restore immunological balance by restoring tolerance. Results from phase II and even phase III trials are promising and it is likely that some of the therapeutics discussed will receive approval in the following years. Hopefully, this will allow for more tailor-made medicine for SLE patients in the future.
Keywords: biologic therapies, SLE, therapy, targeted therapies
Rheumatology key messages
Several new therapeutic approaches targeting different molecular pathways show promising results in trials in SLE.
Anifrolumab is the second biologic that reached its primary end point in a phase III trial.
The possibility of targeting different pathways will hopefully allow for more individualized medicine in the future.
Introduction
SLE is a chronic systemic auto-immune disease that is associated with considerable morbidity and mortality. The disease typically affects women of childbearing age and follows a relapsing–remitting course. The prevalence varies from 12 to 150 cases per 100 000 people.
SLE is characterized by the production of autoantibodies against a range of autoantigens including nuclear components, formation of immune complexes and deposition of immune complexes in a variety of organs, leading to inflammation and organ damage. Environmental and hormonal factors are supposed to influence the development of SLE in genetically susceptible individuals by inducing defects in the innate and adaptive immune system [1]. Immune dysregulation in patients with SLE can occur at the level of cytokines, T cells, B cells and macrophages.
The disease often affects the skin, joints, kidneys and blood, but can virtually affect any organ system resulting in a striking clinical heterogeneity. The 5-year survival of patients with SLE has improved from 50% in the 1950s to >90% since the 1990s [2, 3], but minimal additional improvement has occurred over the past decades and numerous clinical trials for SLE using biologics targeting a specific pathway failed to reach their primary end points [4]. Only one new drug, belimumab, has been approved for the treatment of SLE in >60 years [5]. Hydroxychloroquine, glucocorticoids, and the immunomodulatory agents azathioprine, methotrexate and mycophenolate mofetil are currently the most frequently used drugs. In persistently active or flaring extra-renal disease, additional treatment with belimumab is recommended, while rituximab or cyclophosphamide may be considered in severe, organ-threatening or refractory disease [6, 7].
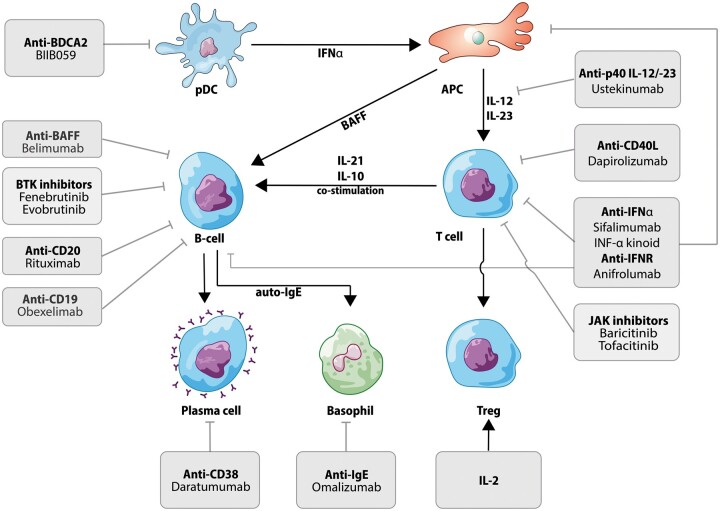
The array of clinical phenotypes of SLE reflects the complex cellular and molecular mechanisms involved in its pathogenesis. Several pathways are involved in each patient but the relative contribution of each pathway varies between individuals [8]. The improved insight into the complex pathogenesis of SLE has inspired the development of multiple clinical trials using new agents directed against recognized targets (Fig. 1). The aim of this review is to discuss the results of recent articles on clinical trials and to summarize ongoing novel clinical trials in patients with SLE (Table 1).
Fig. 1.
Targeted therapies used for the treatment or currently used in clinical trials in SLE
APC: antigen presenting cell; BAFF: B-cell activating factor; BDCA2: blood dendritic cell antigen 2; BTK: Bruton’s tyrosine kinase; CD40L: CD40 ligand; IFNR: IFN receptor; JAK: Janus kinase
Table 1.
Ongoing registered clinical trials in SLE
| Therapy | Target | Trial phase | Participants (target) | Trial registration |
|---|---|---|---|---|
| B cells | ||||
| Belimumab/rituximab | BAFF/CD20 | III | 292 | NCT03312907 |
| Belimumab/rituximab | BAFF/CD20 | II (LN) | 70 | NCT03312907 |
| Ianalumab/iscalimab | BAFF/CD40 | II | 120 | NCT03656562 |
| Dapirolizumab | CD40L | III | 450 | NCT04294667 |
| Rozibafusp alpha | ICOSL/BAFF | II | 320 | NCT04058028 |
| RC-18 | TACI-Fc fusion protein | II (completed) | 249 | NCT02885610 |
| Cytokines/chemokines | ||||
| Secukinumab | Anti-IL17 mAb | III (LN) | 460 | NCT04181762 |
| BOS161721 | Anti-IL21 mAb | I/II (completed) | 143 | NCT03371251 |
| Guselkumab | Anti-IL23 mAb | II (LN) | 60 | NCT04376827 |
| BT063 | Anti-IL10 mAb | II (completed) | 36 | NCT02554019 |
| Aldesleukin | IL2 | II (completed) | 16 | NCT03312335 |
| IL2 | II | 500 | NCT04077684 | |
| ILT-101 | IL2 | II (completed) | 100 | NCT02955617 |
| LY3471851 (NKTR-358) | IL2 | II | 280 | NCT04433585 |
| AMG592 | IL2 | I | 29 | NCT03451422 |
| AMG592 | IL2 | II | 320 | NCT04058028 |
| PF-06835375 | Anti-CXCR5 antagonist | I | 112 | NCT03334851 |
| Kinases | ||||
| AC0058TA | BTK | I | 32 | NCT03878303 |
| Orelabrutinib (ICP-022) | BTK | I/II (completed) | 60 | NCT04305197 |
| Branebrutinib | BTK | II | 185 | NCT04186871 |
| Elsubrutinib/upadacitinib | BTK/JAK1 | II | 325 | NCT03978520 |
| Elsubrutinib/upadacitinib | BTK/JAK1 | II | 260 | NCT04451772 |
| Baricitinib | JAK1/2 | III | 1100 | NCT03843125 |
| Baricitinib | JAK1/2 | III | 750 | NCT03616964 |
| Baricitinib | JAK1/2 | III | 809 | NCT03616912 |
| PF-06700841 | JAK1/TYK2 | II | 448 | NCT03845517 |
| BMS986165 | TYK2 | II | 360 | NCT03920267 |
| BMS986165 | TYK2 | II | 360 | NCT03252587 |
| BMS986165 | TYK2 | II (LN) | 78 | NCT03943147 |
| Cenerimod | S1P | II | 500 | NCT03742037 |
| Other | ||||
| Itolizumab | Anti-CD6 mAb | I | 60 | NCT04128579 |
| Iberdomide (CC-220) | Cereblon E3 ligase modulator | II (completed) | 42 | NCT02185040 |
| Curcumin | II | 68 | NCT03953261 | |
| SM934 | Artesimin analogue | II | 48 | NCT03951259 |
| Mesenchymal stem cells | II | 81 | NCT02633163 | |
| Lenabasum (JBT-101) | CB2 agonist | II | 100 | NCT03093402 |
| KZR-616 | Immunoproteasome | II | 68 | NCT03393013 |
BAFF: B cell activating factor; BTK: Bruton’s tyrosine kinase; CD40L: CD40 ligand; JAK: Janus kinase; mAb: monoclonal antibody; TYK: tyrosine kinase.
Methods
To identify articles relevant to this review, a PubMed search was conducted using the MeSH terms: (SLE) with filters: Clinical trial or Randomized Controlled Trial. Papers published between 1 January 2019 and 1 of February 2021 were selected. Our search revealed 73 original articles. Additionally, a search was conducted on clinicaltrials.gov to identify ongoing trials.
B cells
As producers of auto-antibodies, B cells have a key role in auto-immune diseases. Rituximab (anti-CD20) was the first biologic used in SLE that specifically targets the B cell. While successful in many autoimmune diseases including rheumatoid arthritis and ANCA associated vasculitis [9, 10], two major trials in SLE and lupus nephritis failed to achieve their primary end point [11, 12]. The reasons for the failure of these two trials are extensively discussed elsewhere [13, 14], but include selection of patients, trial design and primary outcome parameter. However, data from national registries demonstrated reduction in disease activity score and concomitant glucocorticoid use in rituximab treated patients [15–17].
Belimumab
Belimumab is a monoclonal antibody (mAb) targeting B cell activating factor (BAFF), which is a regulator of B cell survival. In two landmark phase III trials (BLISS-52 and BLISS-72) [5, 18], patients who received belimumab on top of standard of care had a higher SLE Responder Index 4 (SRI4) response rate compared with placebo. Patients with active lupus nephritis were excluded from these trials. Extension studies of phase II and III trials demonstrated long-term safety and tolerability of belimumab, as well as the suggestion of long-term efficacy [19, 20].
A recent phase III randomized, double-blind, placebo-controlled trial (RCT) investigated belimumab in 448 lupus nephritis patients [21]. Significantly more patients who received belimumab achieved the primary efficacy renal response compared with placebo at week 104. Complete renal response, a major secondary outcome, was more frequent in patients who received belimumab and their risk of a renal-related event or death was lower compared with placebo. The safety profile of belimumab was equal to that of previous trials. The rates of serious adverse events and other adverse events were similar between standard of care and standard of care plus belimumab. The results of this trial led to the approval of belimumab for treatment of lupus nephritis in March 2021.
In an ongoing phase II RCT in 93 childhood SLE patients, the addition of belimumab to standard of care did not result in more adverse events at week 52 [22]. Although the study was not powered to determine superiority, numerically more patients on belimumab achieved the primary end point SRI4 compared with placebo, but statistical significance was not reached. However, this study did result in the approval of belimumab for SLE treatment in children of 5 years or older.
Currently, two ongoing trials are investigating the combination of rituximab and belimumab [23, 24]. In the BLISS-BELIEVE trial, belimumab is administered subcutaneously. Rituximab is then added 4–8 weeks after the initiation of belimumab, while in the BEAT Lupus trial, belimumab is administered intravenously 4–8 weeks after two infusions of rituximab. Results of these trials are expected this year.
XmAb5871
XmAb5871 (obexelimab) is a humanized anti-CD19 antibody Fc-engineered for increased affinity to FcgRIIb. XmAb5871 therefore targets B cells. One hundred and four patients with moderate to severe, non-organ threatening disease were enrolled in a RCT [25]. Only hydroxychloroquine and 10 mg prednisolone per day were allowed as immunosuppressive co-medication. Patients were given 2 × 80 mg Depo-Medrol at the start followed by XmAb5871 or placebo every 14 days. The primary end point (no loss of improvement) did not reach statistical difference, but a trend favouring XmAb5871 was observed. Because of the unique primary end point used in this trial, the results are difficult to interpret.
Dapirolizumab
CD40L, which is primarily expressed on T cells but also on other cells such as B cells and pDCs, plays an important role in the regulation of the immune response through T cell activation, B cell activitation and differentiation, and the release of pro-inflammory cytokines by pDCs. Dapirolizumab is a PEGylated mAb with specificity for CD40L. This drug is particularly interesting, because PEGylated Abs barely pass the placental membrane and it is potentially safe to use during pregnancy, which is a major issue. In a phase IIb, dose-ranging RCT in 182 SLE patients, dapirolizumab or placebo was given on top of standard of care [26]. While numerically higher response rates were observed in all three dosages of dapirolizumab compared with placebo, a statistically significant difference in BILAG based Composite Lupus Assessment (BICLA) response as the primary end point at week 24 was not achieved. The results from this trial seemed promising enough, as a phase III trial in 450 patients is currently recruiting (NCT04294667).
IFNs
One of the hallmark features of SLE is the so called IFN gene signature [27]. IFNs play a key role in host defence against viruses and belong to an evolutionarily old part of the innate immune system. Increased levels of type 1 IFNs in serum is a typical feature of SLE. Targeting type 1 IFNs, therefore, has garnered much interest as a possible new therapeutic pathway.
Anifrolumab
Anifrolumab, a human mAb targeting the type I IFN receptor subunit 1, surprisingly did not achieve its primary end point SRI4 in the first phase III trial in SLE (TULIP-1) [28], after promising results in a phase II trial. However, BICLA, another composite index for response was achieved significantly more frequently in patients allotted to anifrolumab than to placebo. In the second phase III trial in 362 patients, monthly infusions of 300 mg anifrolumab resulted in a higher percentage of patients with a BICLA response at week 52 than did placebo (48% vs 32%, respectively) [29]. The success of the second phase III trial of anifrolumab might in part be attributable to the selection of a different primary outcome parameter.
Interestingly, the presence of an IFN gene signature at baseline was not a predictor for response to anifrolumab. A possible explanation might be that the majority of patients (83%) had a high IFN gene signature at baseline. No significant differences in the number of adverse and serious adverse events between the anifrolumab and placebo groups were observed, except for herpes zoster (7.2% for anifrolumab and 1.1% for placebo). US Food and Drug Administration and European Medicines Agency approval for anifrolumab are pending.
Sifalimumab
In a small phase II open label trial in 66 Japanese patients, sifalimumab, an anti-IFN-α mAb, was shown to be safe and well tolerated [30]. Serious adverse events occurred in 30% of patients in the first 52 weeks and were mainly instances of SLE flares. A concern with the use of Abs directed against IFN in the current COVID-19 pandemic is that ‘endogenic’ auto-antibodies against IFN-α, induced by SARS-CoV-2 infection, are associated with severe COVID-19 [31].
BIIB059
BIIB059 indirectly targets type I IFN production by targeting a receptor of the plasmacytoid dendritic cell (blood dendritic cell antigen 2, BDCA2) [32]. In a double-blind RCT, BIIB059 was first given to 54 healthy volunteers. In the second part of the trial, BIIB059 administration in 12 patients with SLE decreased expression of IFN response genes in blood, reduced immune infiltrates in skin lesion biopsies, and clinically decreased skin disease activity. BIIB059 was generally well tolerated although one SLE patient experienced nine serious adverse events, which were interpreted as unrelated to the BIIB059. A phase II study in cutaneous lupus with or without SLE has recently completed and results of this trial are awaited (NCT02847598).
IFN-α kinoid
The IFN-α kinoid (IFN-K) is an immunotherapeutic vaccine aimed to induce antibodies against IFN-α. A phase II RCT in 185 SLE patients was recently conducted [33]. Neutralizing anti-IFN-α2b serum antibodies were detected in 91% of treated patients at week 36. While the first co-primary end point of neutralization of the IFN signature was achieved, the clinical end point of a modified BICLA was not. A secondary clinical outcome parameter, Lupus Low Disease Activity State, reached its end point (53% of patients assigned to IFN-K compared with 30% of patients assigned to placebo). While adverse events and serious adverse events were comparable in both groups, significantly more patients in the IFN-K group had upper respiratory tract infections compared with placebo (17.6% vs 5.4%). The increased rate of upper respiratory tract infections underlines the safety concern for severe COVID-19, as previously mentioned.
Omalizumab
Omalizumab, a mAb targeting IgE, is currently used in IgE mediated asthma, among others. Its presumed method of action in SLE is by hampering plasmacytoid dendritic cells and basophil activation and thereby reducing type I IFN production [34]. A small RCT in 16 patients demonstrated an improvement in SLEDAI 2000 (SLEDAI-2K) scores in the omalizumab group compared with the placebo group at week 16. Subjects receiving omalizumab showed a trend toward improvement in IFN gene signature.
Cytokines
IL-2
Deficiency of regulatory T cells (Tregs) has been implicated in many autoimmune diseases, including SLE. Tregs have an important role in tolerance and control of inflammation. A previous open clinical trial, in which IL-2 was administered to patients with varying autoimmune diseases, demonstrated specific Treg expansion and activation without effector T cell activation [35]. Six out of 46 patients in this trial had SLE. A subsequent RCT treated 60 SLE patients in a 1:1 ratio with IL-2 or placebo [36]. At the primary end point at week 12, the SRI4 response rates were 55% for IL-2 and 30% for placebo, which did not reach statistical significance. However, at week 24, SRI4 response rates did significantly differ (66% and 37%, respectively). Furthermore, complete remission of lupus nephritis was more frequent in patients treated with IL-2 than placebo (7/13, 53.85% vs 2/12, 16.67%, respectively). A second phase II trial in 500 SLE patients is currently recruiting. This study will investigate three different dosages of IL-2 compared with placebo (NCT04077684).
Ustekinumab
Ustekinumab, an effective biologic for psoriasis, psoriatic arthritis and inflammatory bowel disease, targets the p40 subunit of IL‐12 and IL‐23. A phase II trial in 102 patients reported significantly greater SRI4 response rates in the ustekinumab group (62%) vs the placebo group (33%) at the primary end point analysis at week 24 [37]. This response rate was maintained at week 48 in the ustekinumab group [38]. However, the subsequent phase III trial was terminated prematurely after a pre-planned interim efficacy analysis failed to show efficacy. No new safety issues were reported. Current analyses focus on elucidating the differences in outcome between the phase II and III trials. It seems unlikely that ustekinumab will be further developed for SLE treatment.
Kinase inhibitors
Baricitinib
In a 24-week double-blind phase II RCT of 314 SLE patients with cutaneous lupus and/or arthritis, 2 or 4 mg daily baricitinib, an oral Janus kinase (JAK)1/2 inhibitor, demonstrated significantly better reduction in skin disease and/or arthritis compared with placebo in the 4 mg baricitinib subgroup. Serious infections and SAEs were more frequent in the 4 mg subgroup while AEs were similar between the groups [39]. The results of two phase III RCTs on the efficacy and safety of 2 or 4 mg daily baricitinib compared with placebo are currently expected.
Tofacitinib
In a single centre open-label case series of 10 SLE patients, treatment with the oral JAK1/3 inhibitor tofacitinib, 5 mg twice daily, resulted in complete resolution of arthritis in four out of four patients, complete resolution of mucocutaneous manifestations in six out of nine patients and partial or no response in the remaining three patients with rash. SLEDAI-2K and physician global assessment scores improved significantly [40].
Voclosporin
Voclosporin, a novel high potency calcineurin inhibitor, in combination with mycophenolate mofetil and rapidly tapered oral glucocorticoids (vs placebo) demonstrated superior efficacy compared with standard therapy in a phase III RCT in 357 patients with active lupus nephritis (AURORA) after 52 weeks [41]. The incidence of SAEs was similar between the groups. Based on this successful trial, approval of voclosporin for the treatment of lupus nephritis is expected in the near future.
Sirolimus
Sirolimus, a mammalian target of rapamycin inhibitor of antigen-induced T cell proliferation, was investigated in a single-arm, open-label phase I/II trial in patients with active, therapy-resistant SLE [42]. In the 29 patients who completed treatment during 12 months, significant reductions in disease activity (assessed using SLEDAI and BILAG) and glucocorticoid use were reported. A single-arm, open-label phase II study in 20 patients with connective tissue disease-related refractory thrombocytopenia, of whom 14 were diagnosed with SLE, reported an overall response rate of 71.4% and complete remission rate of 64.3% in this subgroup [43]. No severe side effects were reported.
Leflunomide
Leflunomide, an inhibitor of dihydroorotate dehydrogenase leading to a decrease in B and T cells, given orally with a loading dose of 40 mg/day for 3 days and followed by 20 mg/day, was demonstrated as effective as intravenously cyclophosphamide (0.8–1.0 g monthly) in combination with prednisone in the induction treatment of proliferative lupus nephritis in an RCT of 24 weeks in 100 Chinese patients [44]. Rates of complete and partial clinical remission and rates of adverse events were similar between the groups.
Bruton’s tyrosine kinase inhibitors
Bruton’s tyrosine kinase (BTK) inhibitors have multiple effects on B cells and macrophages. A phase II RCT of fenebrutinib 150 or 200 mg/day vs placebo in 260 patients with non-renal SLE reported an SRI4 response of 51% in the fenebrutinib 150 mg/day group, 52% in the 200 mg/day group, and 44% in the placebo group, which differences were not significant [45]. A phase II RCT with another oral BTK inhibitor, evobrutinib (in three different dosages), vs placebo in 469 patients with active SLE reported no treatment effect of evobrutinib vs placebo at any dose [46]. A phase II trial studying elsubrutinib (BTK inhibitor) alone or in combination with upadacitinib (JAK inhibitor) compared with placebo is ongoing (NCT03978520).
Immunomodulators
Iguratimod
Iguratimod is a synthetic small molecule that can inhibit nuclear factor-κB (NF-κB) activation and consequently reduces immunoglobulin production [47]. Its modulatory effect is through B cell differentiation. In an open-label study [48], 14 refractory lupus nephritis patients, 10 of whom had recent treatment failure and four had repeated relapses with inadequate initial responses, were enrolled. Out of 12 patients, five had a complete and seven a partial renal response, of whom three had a relapse within 144 weeks of follow-up. There was one serious adverse event (anaemia), which fully recovered after cessation of iguratimod. A phase II trial in 120 lupus nephritis patients is currently recruiting (NCT02936375).
Umbilical cord derived mesenchymal stem cells
A study in Chinese patients aimed to investigate the role of peripheral tolerogenic CD1c+ dendritic cells and the levels of serum FLT3L, a marker for common dendritic cells in SLE [49]. A decrease in CD1c+ dendritic cells and serum levels of FLT3L was observed in SLE patients compared with healthy controls. Next, 21 SLE patients with refractory disease received infusions with umbilical cord derived mesenchymal stem cells. After transplantation, the frequency and absolute number of CD1c+ DCs were significantly increased after 24 and 72 h. Serum FLT3L levels also increased. Clinically, the SLEDAI score significantly declined in the first 11 patients with 6 months of follow-up, during which two patients achieved complete remission and two patients flared. No adverse events were reported. Mechanistically, it was shown that FLT3L promotes the proliferation and inhibits the apoptosis of tolerogenic CD1c+ dendritic cells, which might be the mode of action to suppress inflammation in SLE. A new phase II trial further explores the potential of umbilical cord derived mesenchymal stem cells in lupus nephritis (NCT03673748).
Ongoing registered clinical trials in SLE
Currently, several clinical trials investigating novel molecular pathways in patients with SLE are ongoing. An overview of selected studies and their molecular target is summarized in Table 1.
Conclusion
The need for new therapies in SLE is high as persistent disease activity despite currently approved therapies is common and toxicity of drugs such as glucocorticoids and cyclophosphamide majorly impacts quality of life. Treatment of SLE is still challenging due to the wide clinical and pathophysiological heterogeneity of the disease, which also poses a challenge to clinical trial design. Targeting an identified pathogenic pathway in SLE will only benefit the subgroup of patients in whom that pathway is relevant, which underlines the need for tailored therapy.
In the past two years, several new therapies targeting different molecular pathways have demonstrated encouraging results in clinical trials in SLE. After the approval of belimumab, trials using fully humanized monoclonals targeting B cells or combinations of biologics have been initiated. A second group of drugs target interferon, be it directly, such as anifrolumab and sarilumab, or more indirectly, such as BIIB059 or omalizumab. We are eagerly awaiting the results of phase III trials using small molecules such as the JAK inhibitor baricitinib, which has shown potent effects in other rheumatic diseases and the calcineurin inhibitor voclosporin. The oral route of administration is preferred by most patients and the short half-life provides a favourable safety aspect.
Because most SLE patients are females of childbearing potential, dapirolizumab pegol deserves special attention. PEGylated mAbs do not or only minimally transfer across the blood–placental barrier, thus potentially allowing safe continuation during pregnancy [50].
A fourth group of potential new treatments covered in our review contains immunomodulators. These drugs are interesting because their mode of action is to restore tolerance. Future studies should elucidate whether their safety profile is also more favourable compared with immunosuppressants.
The road is long, but we are confident that several new targeted therapies will receive approval in the next decade, which will hopefully allow for more tailor-made medicine for SLE patients in the future.
Funding: This paper is published as part of a supplement supported by a grant from UCB Pharma.
Disclosure statement: I.E.M.B reports personal fees from Eli Lilly, MSD, Amgen, UCB Pharma, GSK, Roche and Sanofi Genzyme outside the submitted work. M.W.P.T has declared no conflicts of interest.
Data availability statement
The authors declare that all data supporting the findings of this study are available within the article.
References
- 1. Kaul A, Gordon C, Crow MK. et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- 2. Merrell M, Shulman LE.. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chronic Dis 1955;1:12–32. [DOI] [PubMed] [Google Scholar]
- 3. Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC.. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum 2012;41:830–9. [DOI] [PubMed] [Google Scholar]
- 4. Dall'Era M, Bruce IN, Gordon C. et al. Current challenges in the development of new treatments for lupus. Ann Rheum Dis 2019;78:729–35. [DOI] [PubMed] [Google Scholar]
- 5. Furie R, Petri M, Zamani O. et al. ; BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fanouriakis A, Kostopoulou M, Alunno A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 7. Ryden-Aulin M, Boumpas D, Bultink I. et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med 2016;3:e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol 2020;21:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen SB, Emery P, Greenwald MW. et al. ; REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793–806. [DOI] [PubMed] [Google Scholar]
- 10. Stone JH, Merkel PA, Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merrill JT, Neuwelt CM, Wallace DJ. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62: 222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rovin BH, Furie R, Latinis K. et al. ; LUNAR Investigator Group. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215–26. [DOI] [PubMed] [Google Scholar]
- 13. Ramos L, Isenberg D.. Rituximab: the lupus journey. Curr Treatment Options Rheumatol 2015;1:30–41. [Google Scholar]
- 14. Dorner T, Lipsky PE.. Beyond pan-B-cell-directed therapy—new avenues and insights into the pathogenesis of SLE. Nat Rev Rheumatol 2016;12:645–57. [DOI] [PubMed] [Google Scholar]
- 15. Terrier B, Amoura Z, Ravaud P. et al. ; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010;62:2458–66. [DOI] [PubMed] [Google Scholar]
- 16. Dooley MA, Houssiau F, Aranow C. et al. ; BLISS-52 and -76 Study Groups. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013;22:63–72. [DOI] [PubMed] [Google Scholar]
- 17. McCarthy EM, Sutton E, Nesbit S. et al. ; British Isles Lupus Assessment Group Biologics Register. Short-term efficacy and safety of rituximab therapy in refractory systemic lupus erythematosus: results from the. Rheumatology (Oxford) 2018;57:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Navarra SV, Guzman RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 19. van Vollenhoven RF, Navarra SV, Levy RA. et al. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a phase III study extension. Rheumatology (Oxford) 2020;59:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallace DJ, Ginzler EM, Merrill JT. et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furie R, Rovin BH, Houssiau F. et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020;383:1117–28. [DOI] [PubMed] [Google Scholar]
- 22. Brunner HI, Abud-Mendoza C, Viola DO. et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis 2020;79:1340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones A, Muller P, Dore CJ. et al. Belimumab after B cell depletion therapy in patients with systemic lupus erythematosus (BEAT Lupus) protocol: a prospective multicentre, double-blind, randomised, placebo-controlled, 52-week phase II clinical trial. BMJ Open 2019;9:e032569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teng YKO, Bruce IN, Diamond B. et al. Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol. BMJ Open 2019;9:e025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merrill JT, June J, Koumpouras F. et al. Results of a phase 2, double-blind, randomized, placebo-controlled study of a reversible B cell inhibitor, XmAb®5871, in systemic lupus erythematosus (SLE) [abstract #L14]. Arthritis Rheumatol 2018;70 (Suppl 10). [Google Scholar]
- 26. Furie R, Bruce I, Dörner T. et al. Efficacy and safety of dapirolizumab pegol in patients with moderately to severely active systemic lupus erythematosus: a randomized, placebo-controlled study. Arthritis Rheumatol 2019;71 (Suppl 10), https://acrabstracts.org/abstract/efficacy-and-safety-of-dapirolizumab-pegol-in-patients-with-moderately-to-severely-active-systemic-lupus-erythematosus-a-randomized-placebo-controlled-study/ [Google Scholar]
- 27. Ronnblom L, Leonard D.. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med 2019;6:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Furie RA, Morand EF, Bruce IN. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol 2019;1:E208–19. [DOI] [PubMed] [Google Scholar]
- 29. Morand EF, Furie R, Tanaka Y. et al. ; TULIP-2 Trial Investigators. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 30. Takeuchi T, Tanaka Y, Matsumura R. et al. Safety and tolerability of sifalimumab, an anti-interferon-alpha monoclonal antibody, in Japanese patients with systemic lupus erythematosus: a multicenter, phase 2, open-label study. Mod Rheumatol 2020;30:93–100. [DOI] [PubMed] [Google Scholar]
- 31. Bastard P, Rosen LB, Zhang Q. et al. ; HGID Lab. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furie R, Werth VP, Merola JF. et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest 2019;129:1359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houssiau FA, Thanou A, Mazur M. et al. IFN-α kinoid in systemic lupus erythematosus: results from a phase IIb, randomised, placebo-controlled study. Ann Rheum Dis 2020;79:347–55. [DOI] [PubMed] [Google Scholar]
- 34. Hasni S, Gupta S, Davis M. et al. Safety and tolerability of omalizumab: a randomized clinical trial of humanized anti-IgE monoclonal antibody in systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenzwajg M, Lorenzon R, Cacoub P. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann Rheum Dis 2019;78:209–17. [DOI] [PubMed] [Google Scholar]
- 36. He J, Zhang R, Shao M. et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2020;79:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Vollenhoven RF, Hahn BH, Tsokos GC. et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 2018;392:1330–9. [DOI] [PubMed] [Google Scholar]
- 38. van Vollenhoven RF, Hahn BH, Tsokos GC. et al. Maintenance of efficacy and safety of ustekinumab through one year in a phase II multicenter, prospective, randomized, double-blind, placebo-controlled crossover trial of patients with active systemic lupus erythematosus. Arthritis Rheumatol 2020;72:761–8. [DOI] [PubMed] [Google Scholar]
- 39. Wallace DJ, Furie RA, Tanaka Y. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:222–31. [DOI] [PubMed] [Google Scholar]
- 40. You H, Zhang G, Wang Q. et al. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis 2019;78:1441–3. [DOI] [PubMed] [Google Scholar]
- 41. Teng YKO, Parikh SV, Saxena A. et al. O11. AURORA phase 3 study demonstrates voclosporin statistical superiority over standard of care in lupus nephritis (LN). Lupus Sci Med 2020;7:doi: 10.1136/lupus-2020-eurolupus.24 [Google Scholar]
- 42. Lai ZW, Kelly R, Winans T. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 2018;391:1186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu C, Wang Q, Xu D, Li M, Zeng X.. Sirolimus for patients with connective tissue disease-related refractory thrombocytopenia: a single-arm, open-label clinical trial. Rheumatology (Oxford) 2021;60:2629–34. [DOI] [PubMed] [Google Scholar]
- 44. Zhang M, Qi C, Zha Y. et al. Leflunomide versus cyclophosphamide in the induction treatment of proliferative lupus nephritis in Chinese patients: a randomized trial. Clin Rheumatol 2019;38: 859–67. [DOI] [PubMed] [Google Scholar]
- 45. Isenberg DA, Furie R, Jones N. et al. Efficacy, safety, and pharmacodynamic effects of the Bruton’s tyrosine kinase inhibitor, fenebrutinib (GDC-0853), in moderate to severe systemic lupus erythematosus: results of a phase 2 randomized controlled trial. Arthritis Rheumatol 2019;71 (Suppl 10), https://acrabstracts.org/abstract/efficacy-safety-and-pharmacodynamic-effects-of-the-brutons-tyrosine-kinase-inhibitor-fenebrutinib-gdc-0853-in-moderate-to-severe-systemic-lupus-erythematosus-results-of-a-phase-2-rando/ [DOI] [PubMed] [Google Scholar]
- 46. Wallace DJ, Dörner T, Pisetsky D, et al. Efficacy and safety of evobrutinib (M2951) in adult patients with systemic lupus erythematosus who received standard of care therapy: a phase II, randomized, double-blind, placebo-controlled dose ranging study. Arthritis Rheumatol 2020;72 (Suppl 10), https://acrabstracts.org/abstract/efficacy-and-safety-of-evobrutinib-m2951-in-adult-patients-with-systemic-lupus-erythematosus-who-received-standard-of-care-therapy-a-phase-ii-randomized-double-blind-placebo-controlled-dose-rang/ [Google Scholar]
- 47. Tanaka K, Yamamoto T, Aikawa Y. et al. Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatology (Oxford) 2003;42:1365–71. [DOI] [PubMed] [Google Scholar]
- 48. Kang Y, Yan Q, Fu Q. et al. Iguratimod as an alternative induction therapy for refractory lupus nephritis: a preliminary investigational study. Arthritis Res Ther 2020;22:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yuan X, Qin X, Wang D. et al. Mesenchymal stem cell therapy induces FLT3L and CD1c+ dendritic cells in systemic lupus erythematosus patients. Nat Commun 2019;10:2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mariette X, Forger F, Abraham B. et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018;77:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article.