Abstract
SS is a chronic, autoimmune condition characterized by lymphocytic infiltration of the exocrine glands and B-cell dysfunction. Current treatment strategies are largely empirical and offer only symptomatic relief for patients. There are no proven treatments that alter disease progression or treat the systemic manifestations of disease. B-cell depletion is used in patients with systemic disease but its overall clinical efficacy has not been demonstrated in two large randomized controlled trials. Studies are now focussing on alternative strategies to target B-cells, including co-stimulation targets, with promising data. It is increasingly clear that clinical trials in SS will require patient stratification and relevant and sensitive outcome measures to identify successful treatment modalities.
Keywords: SS, biologics, B-cells, BAFF, CD40, stratification, outcome measures
Rheumatology key messages
The efficacy of rituximab remains unclear, but it may be useful for certain systemic manifestations of Sjogren’s syndrome.
Other B-cell-targeting therapies show promise but data from larger trials are needed.
Future clinical trials in Sjogren’s syndrome require patient stratification, and relevant and sensitive outcome measures.
Introduction
Primary SS is a chronic, autoimmune condition characterized by lymphocytic infiltration of the exocrine glands, which leads to glandular dysfunction and eventual irreversible tissue damage. The primary clinical manifestations are ocular and oral dryness, but 30–40% of patients develop systemic features and there is an increased lifetime risk of lymphoma, estimated to be 5–10% [1, 2]. Patients carry a large symptom burden with a subsequent reduction in health-related quality of life and work productivity [3]. Current treatment strategies are empirical and rely on symptomatic management, with no biological or synthetic DMARDs proven to alter the progression of disease [4].
Evidence of B-cell pathology in SS
B-cell dysfunction and hyperactivity is a hallmark of SS. Serum B-cell activating factor (BAFF) is elevated in patients and upregulated within salivary glands. It is not clear if this is a factor in the initiation of disease or a consequence of it, but it does suggest that BAFF is critical to the B-cell microenvironment at the site of disease, where it may promote the survival of autoreactive cells. Indeed, BAFF transgenic mice have been shown to develop a syndrome similar to SS [5].
The autoantibodies, RF, anti-Ro and anti-La, emerge a median of 4–6 years prior to the development of symptoms [6]. Other evidence of B-cell hyperactivity include hypocomplementemia, hypergammaglobulinaemia and raised levels of kappa free light chains and beta 2 microglobulin [7]. Indeed, serum levels of BAFF, kappa free light chains and beta 2 microglobulin are associated with clinical disease activity, as measured by the EULAR SS Disease Activity Index (ESSDAI) [8]. Immunophenotyping studies confirm changes in the peripheral blood B-cell compartment; with abnormalities in memory B-cells and the retention of this subset in salivary gland tissue [9, 10].
The observed glandular lymphocytic infiltrate becomes more organized with time; with an increase in B-cell infiltrate and germinal centre formation as disease progresses [11]. The functional germinal centre formation, identified in the salivary glands of a proportion of patients, may play a role in the perpetuation of disease by enabling further inflammatory cell recruitment and the local production of autoantibodies [12]. Clonal B-cell expansion is seen within the glandular tissue and malignant transformation, in clonally expanded cells, may lead to the development of lymphoma in 5–10% of patients [13, 14]. The selective pressures involved in this process are not fully defined but chronic stimulation by an autoantigen is one postulated mechanism.
Treatment strategies
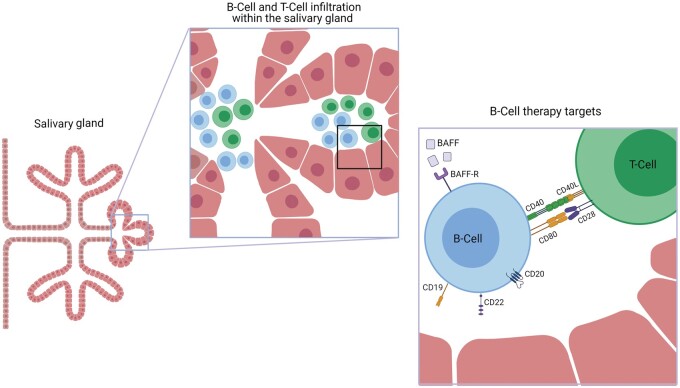
Strategies to identify potential treatments in SS have focussed on targeting B-cells, with the aim of providing symptomatic benefit and preventing disease progression. The glandular tissue, and site of disease, is infiltrated with T-cells at an early stage in disease. This is thought to lead to an epithelitis and, as disease progresses, T-cell help is required to form ectopic lymphoid structures (ELS). This interaction between T and B-cells provides a further potential drug target. The potential mechanisms to target B-cells may be divided into: those directly targeting B-cells, or B-cell homeostasis, and those focussing on co-stimulation and antigen presentation (Fig. 1). The large unmet need in this condition, and the identification of potential molecular targets, has led to an increasing number of randomized controlled trials (RCTs) in SS. In this review, we will focus on biologic therapies that target B-cells directly or indirectly (Table 1).
Fig. 1.
Lymphocytic infiltration of the salivary gland in SS and potential therapeutic targets
T-cell help is required to form ectopic lymphoid structures (ELS) in the salivary gland. Strategies to treat SS include: a direct effect on B-cells, B-cell activating factor (BAFF), its receptor (BAFF-R) or co-stimulation molecules. BAFF: B-cell activating factor, BAFF-R: B-cell activating factor receptor. Created with BioRender.com.
Table 1.
Clinical trials targeting B-cells in SS
| Drug | Phase | Type of study | Molecular target | Primary endpoint | Results | Trial identifier |
|---|---|---|---|---|---|---|
| Abatacept | 3 | Randomized, double-blind, placebo-controlled | CD80/CD86 | Change in ESSDAI at day 169 | No significant difference in ESSDAI at day 169 between abatacept and placebo. Significant improvement in disease-relevant biomarkers. | NCT02915159/ |
| 2016-001948-19 [15] | ||||||
| Abatacept | 2 | Randomized, placebo controlled, crossover assignment | CD80/CD86 | Salivary gland changes on ultrasound at week 32 | Recruiting | NCT03411850 |
| Abatacept | 3 | Randomized, double-blind, placebo-controlled, parallel assignment | CD80/CD86 | Change in ESSDAI at week 24 | No significant difference in ESSDAI at week 24 | NCT02067910/2014-000417-31 [16] |
| Abatacept | 2 | Open-label | CD80/CD86 | Stimulated whole salivary gland function at week 24 | Salivary gland function did not change at week 24. | 2009-015558-40 [17] |
| ESSDAI, ESSPRI, RF and IgG levels decreased significantly during abatacept treatment and increased post-treatment. Fatigue and HR-QoL improved during treatment. | ||||||
| Belimumab | 2 | Open label, non-randomized, single group assignment | BAFF | Improvement in 2 of 5 areas: ≥30% reduction in dryness, fatigue, musculoskeletal pain, physician’s systemic activity VAS, ≥25% reduction B-cell activation markers (or ≥25% increase in C4) at week 28 | Significant reduction in mean score for dryness, fatigue and pain VAS. Primary end point reached. | NCT01160666 (NCT01008982) [18] |
| Belimumab and Rituximab | 2 | Randomized, double-blind, placebo-controlled, parallel assignment | BAFF | Number of SAEs and AESIs at week 68 | Study completed | NCT02631538/2015-000400-26 |
| CD20 | ||||||
| Ianalumab (VAY736) | 2 | Randomized parallel assignment | BAFF receptor | Dose response measured by change in multi-dimensional disease activity assessed by physician at week 24 | Active, not recruiting | NCT02962895/2016-003292-22 |
| Iscalimab (CFZ533) | 2 | Randomized, double-blind, placebo-controlled, parallel assignment | CD40 | Change in ESSDAI (cohort 1 moderate to severe disease) and ESSPRI (cohort 2 low systemic involvement with high symptom burden) at week 24 | Recruiting | NCT03905525 |
| Iscalimab (CFZ533) | 2 | Double-blind, non-randomized, parallel assignment | CD40 | Incidence of treatment-emergent adverse events, change in haematology, serum chemistry laboratory results and vital signs from baseline to each study visit | Recruiting—an extension of NCT03905525 | NCT04541589/ 2020-001942-20 |
| Iscalimab (CFZ533) | 2 | Randomized, double-blind, placebo-controlled, parallel group | CD40 | Change in ESSDAI at week 12 | Significant mean reduction in ESSDAI with i.v. iscalimab vs placebo. | NCT02291029/ 2013-004808-19 [19] |
| No significant difference in ESSDAI change between SC iscalimab and placebo. | ||||||
| Ravagalimab | 2 | Double-blind, placebo-controlled, parallel assignment | CD40 | Change in ESSDAI at week 24 | Study ongoing | 2019-003131-31 |
| Rituximab | 2 & 3 | Randomized double blind placebo-controlled study | CD20 | 30% improvement in VAS for 2 of 4 from global disease activity, pain, fatigue, and dryness at week 24 | Primary end point not reached. | NCT00740948 [20] |
| Rituximab | 3 | Randomized, double-blind, placebo-controlled, parallel study | CD20 | ≥30% reduction in oral dryness or fatigue on VAS score at week 48 |
Primary end point not reached. Significant improvement in unstimulated salivary flow. |
2010-021430-64 [21] |
| SAR441344 | 2 | Randomized, double-blind, placebo-controlled, parallel assignment | CD40L | Change in ESSDAI at week 12 | Recruiting | NCT04572841/2 |
| 020-000511-77 | ||||||
| Telitacicept (RC18) | 2 | Randomized, placebo-controlled, parallel assignment | BAFF/APRIL | Change in ESSDAI at week 24 | Recruiting | NCT04078386 |
| Tibulizumab (LY3090106) | 2 & 3 | Open label single group assignment | BAFF and IL17 | Change in unstimulated salivary flow rate or salivary gland ultrasound score at week 12 | Not recruiting | NCT04563195 |
| VIB4920 | 2 | Randomized, double-blind, placebo-controlled | CD40L | Change in ESSDAI or ESSPRI at day 169 | Recruiting | NCT04129164/2019-002713-19 |
Studies identified from search of phase 2–4 trials in SS from clinicaltrials.gov and clinicaltrialsregister.eu (identification number supplied).
AESI: adverse event of special interest; APRIL: a proliferating-inducing ligand; BAFF: B-cell activating factor; BLyS: B lymphocyte stimulator; BTK: Bruton’s tyrosine kinase; CD40L: CD40 ligand; ESSDAI: European League Against Rheumatism Disease Activity Index; ESSPRI: European League Against Rheumatism Sjogren’s Syndrome Patient Reported Index; HR-QoL: health-related quality of life; i.v.: intravenous; MFI: multidimensional fatigue inventory; RF: rheumatoid factor; SAE: serious adverse event; SC: subcutaneous; VAS: visual analogue scale.
B-cell depletion
Rituximab is a chimeric monoclonal antibody against CD20 found on the majority of B-cells, with the exception of stem cells, pro B-cells and plasma cells. It has been the most studied biologic therapy in SS over the last two decades. The strong evidence for B-cell involvement in pathobiology, combined with the success and safety of B-cell depletion in rheumatoid arthritis, led to an early focus on B-cell depletion as a potential systemic treatment.
Two open label studies showed promising results with improvements in subjective measures of disease, including pain and fatigue, and an increase in salivary gland function [22, 23]. This work led to two small randomized, double blind trials which appeared to confirm the safety profile and the potential benefit on fatigue and stimulated salivary flow [24, 25]. Rituximab was also shown to demonstrate efficacy on disease activity, over standard DMARDS, in early disease, and biopsy results suggested that rituximab reduced the formation of ELS and germinal centres in glandular tissue [26].
There have been two large RCTs looking at the effectiveness of rituximab on SS. The TEARS and TRACTISS studies examined the effects of a single course of rituximab and two courses of rituximab, respectively.
The French Tolerance and Effectiveness of Rituximab in SS (TEARS) trial followed 120 patients after one course of rituximab. The primary end point of at least a 30 mm decrease in the visual analogue scale (VAS, 0–100mm) for two of four domains: dryness, pain, fatigue and global assessment of disease activity, was not reached at 24 weeks. In addition, early responses seen in fatigue at 6 weeks were not sustained at 24 weeks [20]. The primary end point in this study was stringent and subjective which, although critical to patient well-being, may not be a sensitive outcome measure. The choice of primary end point may have contributed to the disappointing outcome. Improvements in the secondary outcomes of salivary flow and laboratory response were seen and so more objective measures of disease may be more appropriate in such studies. Further work on this study population demonstrated an improvement in the salivary gland echo structure at 24 weeks. Ultrasound grading has been suggested as a potential sensitive outcome measure, as it assesses the target tissue [27].
The large UK multicentre TRACTISS study was an RCT with 133 patients, which compared patients receiving two courses of rituximab at 0, 2, 24 and 26 weeks to placebo. This design had the potential to identify sustained improvements gained from a second course of B-cell depletion. The primary end point, of a 30% improvement in fatigue VAS and oral dryness VAS, at 48 weeks was not achieved. The placebo response rate was 36.8% compared with 39.8% in the rituximab group. Unstimulated salivary flow was shown to remain stable over the duration of the follow-up period in the rituximab group, while it deteriorated in the placebo group. However, there was no similar benefit seen in lacrimal flow or stimulated salivary flow. The study concluded that, although well tolerated, B-cell depletion was neither clinically nor cost effective [21].
In this study, the primary endpoints were again strict and potentially subjective. Composite disease activity measures did not identify a potential superiority with rituximab. There were no significant, consistent differences over time in the ESSDAI or European League Against Rheumatism Sjogren’s Syndrome Patient Reported Index (ESSPRI) scores between the two treatment arms. This suggests that the choice of primary endpoints may not be the sole explanation behind the lack of success of rituximab in clinical trials. Indeed, the four rituximab RCTs have not shown consistent outcomes. This may relate to the relatively small number of patients involved, the heterogeneity of the study population as a whole and differences in trial recruitment criteria.
It is clear that treatment with rituximab depletes B-cells from the peripheral blood and salivary glands and leads to a decrease in proinflammatory cytokines, but the clinical effectiveness in routine care has not been established [28–30]. Methods to stratify patients who may respond to rituximab have been suggested including an assessment of baseline B-cell infiltration of parotid parenchyma but this form of stratification would be challenging in clinical practice [28]. Interestingly, a recent symptom-based stratification approach has identified a subset of SS patients who may be responsive to rituximab therapy [31].
Rituximab remains the most frequently used biologic treatment in SS and is endorsed by national guidelines for those with significant systemic manifestations of disease, despite a lack of clear success in trials [4, 32–34]. However, there is registry data to support its use in this setting, e.g. in patients with vasculitis or cryoglobulinaemia-associated peripheral nervous system involvement [35]. The autoimmunity and rituximab registry data has shown that, in patients with systemic involvement and a median ESSDAI of 11, 60% responded to a course of rituximab with a reduction in corticosteroid use and change in median ESSDAI from 11 to 7.5. Over half of the patients were retreated with rituximab [36].
The registry data demonstrates the clinical confidence that comes from the successful use of rituximab in other autoimmune conditions, rather than its trial evidence. The four rituximab RCTs have not set out to look at systemic manifestations of disease, or to identify longer-term treatment benefits. Its use in individual patients with systemically active disease can be defended given the lack of alternatives and the safety profile observed from the registry and trial data [37]. Further studies in B-cell depletion may benefit from patient stratification and focussing on the subgroup with systemic involvement in which there is strong evidence of B-cell dysfunction.
B-cell activating factor (BAFF)
Alternative strategies for targeting B-cells have been suggested including BAFF, a member of the TNF ligand family, which promotes B-cell survival, maturation and germinal centre maintenance [38, 39].
Belimumab is a fully human monoclonal antibody which targets BAFF. The Phase II study, BELISS, which enrolled 30 patients provided promising outcome data. At 28 weeks, 60% of patients had achieved the primary end point of an improvement in two of five areas: a 30% decrease in dryness, fatigue, musculoskeletal pain, physician assessed systemic activity VAS or >25% improvement in any B-cell activation markers. It was notable that the proportion of patients seeing improvements in fatigue and musculoskeletal pain was relatively lower than the other domains [18]. Targeting BAFF in isolation may not improve the features of SS that patients commonly report in clinic: fatigue and musculoskeletal pain.
In responders, long-term treatment with belimumab may be beneficial as continuation of treatment was shown to provide a stable, sustained clinical response and a continued decrease in B-cell activation markers at 52 weeks. Improvements in parotid swelling, lymphadenopathy and the articular domain were noted, but there were no significant changes in lacrimal or salivary function [40]. A review, 12 months after the trial had ended, found that, in this group of 13 patients, the ESSDAI had increased from 3.5 at the end of treatment to 7.0. Over the same time period, levels of BAFF, RF and IgM were also shown to increase significantly; providing further support for the efficacy of belimumab [41].
Additional B-cell strategies
Baseline serum BAFF inversely correlates with the duration of B-cell depletion with rituximab and serum BAFF levels rise after rituximab [29, 42]. It has been proposed that a combination of BAFF inhibition, with B-cell depletion, may improve B-cell depletion and postpone B-cell repopulation. Elevated levels of BAFF in the inflamed target tissue microenvironment may render B-cells resistant to depletion. Therefore, pre-treatment with belimumab may result in more successful depletion of pathogenic B-cells with rituximab. After rituximab, blocking the surge in BAFF with belimumab may reduce the emergence of a new pool of autoreactive cells. An RCT investigating the co-administration of belimumab and rituximab in one arm, compared with placebo, belimumab monotherapy and rituximab monotherapy has been completed and the results are awaited. In the co-administration arm, belimumab is given between weeks 0 and 24 with rituximab at weeks 8 and 10.
The monoclonal antibody ianalumab targets the BAFF receptor on B-cells, leading to the combination of cell lysis and blockade of BAFF-related signalling. This agent has provided promising results in a small group of patients with SS and an ESSDAI of ≥6. Ianalumab led to B-cell depletion, was well-tolerated and trends in clinical improvement were noted with marked improvements in fatigue. Further studies with this agent are underway [43].
Another B-cell target is CD22, a co-receptor of the B-cell receptor, which showed initial promise in Phase I/II trials. It has had renewed focus following the identification of positive patient outcomes in post hoc analyses for the EMBODY trial. In this study, patients with SLE and coexistent SS treated with epratuzumab had a decrease in autoantibodies, IgM and B-cell counts [44]. The clinical improvement was not seen in patients without associated SS and further RCTs are required to examine this further [45].
Co-stimulation blockade
T-cell co-stimulation is an additional potential target in the treatment of SS. Abatacept, which targets the CD28: CD80/86 pathway, has been examined in a small open label study, active Sjogren abatacept pilot (ASAP). Clinical disease activity, measured by ESSDAI, decreased alongside RF and IgG levels during treatment. Salivary and lacrimal gland function remained unchanged [17]. Interestingly, treatment with abatacept was shown to decrease germinal centres per mm2, but not focus score or area of lymphocytic infiltrate. This is likely to be related to the requirement for co-stimulation from activated follicular T helper cells for germinal centre formation [46]. Two recently published Phase III studies have not demonstrated a significant improvement in ESSDAI with abatacept over placebo in patients with an ESSDAI of ≥5 at enrolment [15, 16]. Changes in disease relevant biomarkers, including immunoglobulin levels, were seen but clinical efficacy for abatacept was not observed in this group of patients using predefined outcome criteria.
A second co-stimulation pathway focuses on CD40-CD40L interactions between T and B-cells, which contribute to aberrant lymphocyte activation in inflamed tissue. CD40 stimulation leads to B-cell activation with upregulation of MHCII, B-cell expansion and differentiation. It is central to the humoral immune response and critical to germinal centre formation and production of class switched antibodies [47]. In mouse models of SS, inhibition of this pathway blocks ELS formation and autoantibody generation [48].
Indeed, higher CD40 expression has been found on cultured salivary gland cells from SS patients over controls [49]. Early studies in autoimmune disease initially targeted CD40L but an increase in thromboembolic events, thought to be related to the action on CD40L on platelets, was identified and work has moved on to CD40 despite initially promising results in autoimmune disease [50].
Iscalimab (CFZ533), an anti-CD40 monoclonal antibody, has been studied in SS in a small multicentre, double blind, RCT of <50 patients. Patients received the drug by the subcutaneous or intravenous route or placebo. There was no increase in adverse events and, although no therapeutic efficacy was seen with the lower subcutaneous dose, the higher intravenous dose (10 mg/kg) did result in a significant reduction in the ESSDAI [19]. The preliminary efficacy and therapeutic potential of CD40 blockade is encouraging and additional agents targeting CD40 are also under evaluation.
IL-6
IL-6 is critical to plasma cell differentiation and B-cell activation, with IL-6 inhibition established as a safe mode of action in other rheumatic diseases. A recent RCT enrolled 110 patients with SS and medium/high disease activity. Patients were randomized to placebo or monthly infusions of tocilizumab and the primary end point was assessed at week 24. Response was defined using a composite outcome measure requiring a decrease of at least three points in ESSDAI, no new ESSDAI domains involved and no worsening on the physician’s global VAS of ≥1/10. The primary outcome was not reached in this study, nor were changes in secondary outcomes such as immunoglobulin levels; suggesting that IL-6 is not a primary driver of disease in SS. A high placebo effect was also observed with an improvement in the ESSDAI of ≥3 in 60% of patients in this group [51].
Biological therapies against additional immune targets have also been investigated but are beyond the scope of this review (see Supplementary Table S1, available at Rheumatology online).
Conclusion
The trial data for the use of biologic treatments in SS has been relatively disappointing and the reasons are likely to be multifactorial. The sample sizes of many of these studies are relatively small and, when testing a heterogeneous population, clinically relevant responses that may only be achieved in certain disease subgroups could be missed. In this regard, using the data from the UK Primary SS Registry (UKPSSR) cohort, the French ASSESS cohort and the Stavanger cohort, four clinical subtypes have been identified – low symptom burden, high symptom burden, dryness dominant with fatigue and pain dominant with fatigue – each with distinct molecular profiles, clinical manifestations and responses to immunomodulatory therapies [31]. Therefore, careful stratification of SS patients may facilitate future therapeutic development, for instance, by focussing on those with a particular clinical phenotype or pathological or molecular subgroup biomarkers [8, 52, 53].
There is marked variability in the inclusion criteria and outcome measures (Table 1) employed in the studies described. Over the last 5 years, objective measures and assessment strategies have become established enabling a more standardized approach. The 2016 ACR/EULAR classification criteria help standardize the patients recruited to studies and the adoption of ESSDAI improves and standardizes assessments of systemic disease activity [54].
A large EU industry-academia consortium, the NECESSITY project, recognizes that successful clinical trial design in this heterogeneous population requires patient stratification and sensitive outcome measures. The project, which started in 2019, aims to identify discriminative biomarkers for patient stratification and to develop sensitive clinical endpoints to improve the design of clinical trials in this complex disease [55–58]. In the future, it may be appropriate, given the laboratory improvements seen in a proportion of studies described here, to repeat trials of B-cell therapies with patient stratification and novel, standardized outcome measures.
Another consideration in therapeutic development is to clarify the overall aim of treatment. Is it the prevention of systemic involvement, in which case one could consider focussing on those with risk factors for this, or improving fatigue and pain that may be influenced by co-morbidities and may not be entirely driven by systemic inflammation [59–61]. In SS, pain, depression and fatigue are important predictors of health-related quality of life, work disability and physician consultations [62, 63]. Strategies focussing on fatigue and depression in SS are critical to developing patient-centred care and improving health outcomes.
Beyond trial design, there are additional challenges in the study of Sjogrens’ syndrome, namely: patients present late, there may not be residual glandular function to improve and the challenges of assessing disease activity rather than disease damage. Furthermore, the pathophysiology underlying the extra-glandular manifestations of SS is much less well understood than the glandular pathology and this hampers the development of treatment strategies for this aspect of disease.
There remains a large unmet need in this condition, which in contrast to other autoimmune conditions has yet to benefit from the significant advances in evidence-based systemic therapies, but promising strategies continue to emerge.
Funding: This paper is published as part of a supplement supported by a grant from UCB Pharma.
Disclosure statement: W.-F.N. has consulted for GlaxoSmithKline, Novartis, Abbvie, Sanofi, Bristol Myers Squibb, MedImmune, UCB Pharma and Janssen in the area of Sjogren’s syndrome and received research project funding from Resolve Therapeutics, electroCore and Abbvie. The other authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Mariette X, Criswell LA.. Primary Sjogren's Syndrome. N Engl J Med 2018;378:931–9. [DOI] [PubMed] [Google Scholar]
- 2. Theander E, Henriksson G, Ljungberg O. et al. Lymphoma and other malignancies in primary Sjogren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006;65:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meijer JM, Meiners PM, Huddleston Slater JJ. et al. Health-related quality of life, employment and disability in patients with Sjogren's syndrome. Rheumatology 2009;48:1077–82. [DOI] [PubMed] [Google Scholar]
- 4. Price EJ, Rauz S, Tappuni AR. et al. The British Society for Rheumatology guideline for the management of adults with primary Sjogren's Syndrome. Rheumatology 2017;56:1828. [DOI] [PubMed] [Google Scholar]
- 5. Groom J, Kalled SL, Cutler AH. et al. Association of BAFF/BLyS overexpression and altered B-cell differentiation with Sjogren's syndrome. J Clin Invest 2002;109:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jonsson R, Theander E, Sjostrom B, Brokstad K, Henriksson G.. Autoantibodies present before symptom onset in primary Sjogren syndrome. JAMA 2013;310:1854–5. [DOI] [PubMed] [Google Scholar]
- 7. Kroese FG, Abdulahad WH, Haacke E. et al. B-cell hyperactivity in primary Sjogren's syndrome. Expert Rev Clin Immunol 2014;10:483–99. [DOI] [PubMed] [Google Scholar]
- 8. James K, Chipeta C, Parker A. et al. B-cell activity markers are associated with different disease activity domains in primary Sjogren's syndrome. Rheumatology 2018;57:1222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen A, Odendahl M, Reiter K. et al. Diminished peripheral blood memory B-cells and accumulation of memory B-cells in the salivary glands of patients with Sjogren's syndrome. Arthritis Rheum 2002;46:2160–71. [DOI] [PubMed] [Google Scholar]
- 10. Hansen A, Gosemann M, Pruss A. et al. Abnormalities in peripheral B-cell memory of patients with primary Sjogren's syndrome. Arthritis Rheum 2004;50:1897–908. [DOI] [PubMed] [Google Scholar]
- 11. Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM.. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun 2010;34:400–7. [DOI] [PubMed] [Google Scholar]
- 12. Salomonsson S, Jonsson MV, Skarstein K. et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum 2003;48:3187–201. [DOI] [PubMed] [Google Scholar]
- 13. Gellrich S, Rutz S, Borkowski A. et al. Analysis of V(H)-D-J(H) gene transcripts in B-cells infiltrating the salivary glands and lymph node tissues of patients with Sjogren's syndrome. Arthritis Rheum 1999;42:240–7. [DOI] [PubMed] [Google Scholar]
- 14. Routsias JG, Goules JD, Charalampakis G. et al. Malignant lymphoma in primary Sjogren's syndrome: an update on the pathogenesis and treatment. Semin Arthritis Rheum 2013;43:178–86. [DOI] [PubMed] [Google Scholar]
- 15. Baer AN, Gottenberg JE, St Clair EW. et al. Efficacy and safety of abatacept in active primary Sjogren's syndrome: results of a phase III, randomised, placebo-controlled trial. Ann Rheum Dis 2021;80:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Nimwegen JF, Mossel E, van Zuiden GS. et al. Abatacept treatment for patients with early active primary Sjogren's syndrome: a single-centre, randomised, double-blind, placebo-controlled, phase 3 trial (ASAP-III study). Lancet Rheumatol 2020;2:E153–E63. [DOI] [PubMed] [Google Scholar]
- 17. Meiners PM, Vissink A, Kroese FG. et al. Abatacept treatment reduces disease activity in early primary Sjogren's syndrome (open-label proof of concept ASAP study). Ann Rheum Dis 2014;73:1393–6. [DOI] [PubMed] [Google Scholar]
- 18. Mariette X, Seror R, Quartuccio L. et al. Efficacy and safety of belimumab in primary Sjogren's syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis 2015;74:526–31. [DOI] [PubMed] [Google Scholar]
- 19. Fisher BA, Szanto A, Ng WF, Bombardieri M. et al. ssessment of the anti-CD40 antibody iscalimab in patients with primary Sjogren's syndrome: a multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study. Lancet Rheumatol 2020;2:E142–E52. [DOI] [PubMed] [Google Scholar]
- 20. Devauchelle-Pensec V, Mariette X, Jousse-Joulin S. et al. Treatment of primary Sjogren syndrome with rituximab: a randomized trial. Ann Intern Med 2014;160:233–242– 42. [DOI] [PubMed] [Google Scholar]
- 21. Bowman SJ, Everett CC, O'Dwyer JL. et al. Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjogren's syndrome. Arthritis Rheumatol 2017;69:1440–50. [DOI] [PubMed] [Google Scholar]
- 22. Pijpe J, van Imhoff GW, Spijkervet FK. et al. Rituximab treatment in patients with primary Sjogren's syndrome: an open-label phase II study. Arthritis Rheum 2005;52:2740–50. [DOI] [PubMed] [Google Scholar]
- 23. Devauchelle-Pensec V, Pennec Y, Morvan J. et al. Improvement of Sjogren's syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 2007;57:310–7. [DOI] [PubMed] [Google Scholar]
- 24. Meijer JM, Meiners PM, Vissink A. et al. Effectiveness of rituximab treatment in primary Sjogren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010;62:960–8. [DOI] [PubMed] [Google Scholar]
- 25. Dass S, Bowman SJ, Vital EM. et al. Reduction of fatigue in Sjogren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis 2008;67:1541–4. [DOI] [PubMed] [Google Scholar]
- 26. Carubbi F, Cipriani P, Marrelli A. et al. Efficacy and safety of rituximab treatment in early primary Sjogren's syndrome: a prospective, multi-center, follow-up study. Arthritis Res Ther 2013;15:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jousse-Joulin S, Devauchelle-Pensec V, Cornec D. et al. Brief report: ultrasonographic assessment of salivary gland response to rituximab in primary Sjogren's syndrome. Arthritis Rheumatol 2015;67: 1623–8. [DOI] [PubMed] [Google Scholar]
- 28. Delli K, Haacke EA, Kroese FG. et al. Towards personalised treatment in primary Sjogren's syndrome: baseline parotid histopathology predicts responsiveness to rituximab treatment. Ann Rheum Dis 2016;75:1933–8. [DOI] [PubMed] [Google Scholar]
- 29. Pers JO, Devauchelle V, Daridon C. et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjogren's syndrome. Arthritis Rheum 2007;56:1464–77. [DOI] [PubMed] [Google Scholar]
- 30. Pollard RP, Abdulahad WH, Bootsma H. et al. Predominantly proinflammatory cytokines decrease after B-cell depletion therapy in patients with primary Sjogren's syndrome. Ann Rheum Dis 2013;72:2048–50. [DOI] [PubMed] [Google Scholar]
- 31. Tarn J, Howard-Tripp N, Lendrem D. et al. Symptom-based stratification of patients with primary Sjögren’s syndrome: multi-dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatology 2019;1:e85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramos-Casals M, Brito-Zeron P, Bombardieri S, et al. EULAR recommendations for the management of Sjogren's syndrome with topical and systemic therapies. Ann Rheum Dis 2020;79:3–18. [DOI] [PubMed] [Google Scholar]
- 33. Carsons SE, Vivino FB, Parke A. et al. Treatment guidelines for rheumatologic manifestations of Sjogren's syndrome: use of biologic agents, management of fatigue, and inflammatory musculoskeletal pain. Arthritis Care Res 2017;69:517–27. [DOI] [PubMed] [Google Scholar]
- 34. Sumida T, Azuma N, Moriyama M. et al. Clinical practice guideline for Sjogren's syndrome 2017. Mod Rheumatol 2018;28:383–408. [DOI] [PubMed] [Google Scholar]
- 35. Mekinian A, Ravaud P, Hatron PY. et al. Efficacy of rituximab in primary Sjogren's syndrome with peripheral nervous system involvement: results from the AIR registry. Ann Rheum Dis 2012;71:84–7. [DOI] [PubMed] [Google Scholar]
- 36. Gottenberg JE, Cinquetti G, Larroche C. et al. Efficacy of rituximab in systemic manifestations of primary Sjogren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis 2013;72:1026–31. [DOI] [PubMed] [Google Scholar]
- 37. Souza FB, Porfirio GJ, Andriolo BN, Albuquerque JV, Trevisani VF.. Rituximab effectiveness and safety for treating primary Sjogren's syndrome (pSS): systematic review and meta-analysis. PLoS One 2016;11:e0150749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Batten M, Groom J, Cachero TG. et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med 2000;192:1453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol 2005;17:282–9. [DOI] [PubMed] [Google Scholar]
- 40. De Vita S, Quartuccio L, Seror R. et al. Efficacy and safety of belimumab given for 12 months in primary Sjogren's syndrome: the BELISS open-label phase II study. Rheumatology 2015;54:2249–56. [DOI] [PubMed] [Google Scholar]
- 41. Quartuccio L, Salvin S, Corazza L. et al. Efficacy of belimumab and targeting of rheumatoid factor-positive B-cell expansion in Sjogren's syndrome: follow-up after the end of the phase II open-label BELISS study. Clin Exp Rheumatol 2016;34:311–4. [PubMed] [Google Scholar]
- 42. Pollard RP, Abdulahad WH, Vissink A. et al. Serum levels of BAFF, but not APRIL, are increased after rituximab treatment in patients with primary Sjogren's syndrome: data from a placebo-controlled clinical trial. Ann Rheum Dis 2013;72:146–8. [DOI] [PubMed] [Google Scholar]
- 43. Dorner T, Posch MG, Li Y. et al. Treatment of primary Sjogren's syndrome with ianalumab (VAY736) targeting B-cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis 2019;78:641–7. [DOI] [PubMed] [Google Scholar]
- 44. Steinfeld SD, Tant L, Burmester GR. et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren's syndrome: an open-label phase I/II study. Arthritis Res Ther 2006;8:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gottenberg JE, Dorner T, Bootsma H. et al. Efficacy of epratuzumab, an anti-CD22 monoclonal IgG antibody, in systemic lupus erythematosus patients with associated Sjogren's syndrome: post hoc analyses from the EMBODY trials. Arthritis Rheumatol 2018;70:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haacke EA, van der Vegt B, Meiners PM. et al. Abatacept treatment of patients with primary Sjogren's syndrome results in a decrease of germinal centres in salivary gland tissue. Clin Exp Rheumatol 2017;35:317–20. [PubMed] [Google Scholar]
- 47. Kawabe T, Naka T, Yoshida K. et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1994;1:167–78. [DOI] [PubMed] [Google Scholar]
- 48. Wieczorek G, Bigaud M, Pfister S. et al. Blockade of CD40-CD154 pathway interactions suppresses ectopic lymphoid structures and inhibits pathology in the NOD/ShiLtJ mouse model of Sjogren's syndrome. Ann Rheum Dis 2019;78:974–8. [DOI] [PubMed] [Google Scholar]
- 49. Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN.. CD40 on salivary gland epithelial cells: high constitutive expression by cultured cells from Sjogren's syndrome patients indicating their intrinsic activation. Clin Exp Immunol 2002;127:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sidiropoulos PI, Boumpas DT.. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 2004;13:391–7. [DOI] [PubMed] [Google Scholar]
- 51. Felten R, Devauchelle-Pensec V, Seror R. et al. Interleukin 6 receptor inhibition in primary Sjogren syndrome: a multicentre double-blind randomised placebo-controlled trial. Ann Rheum Dis 2020; doi: annrheumdis-2020-218467. [DOI] [PubMed] [Google Scholar]
- 52. Gairy K, Knight C, Anthony P, Hoskin B.. Burden of illness among subgroups of patients with primary Sjogren's syndrome and systemic involvement. Rheumatology 2021;60:1871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bautista-Vargas M, Vivas AJ, Tobon GJ.. Minor salivary gland biopsy: its role in the classification and prognosis of Sjogren's syndrome. Autoimmun Rev 2020;19: 102690. [DOI] [PubMed] [Google Scholar]
- 54. Shiboski CH, Shiboski SC, Seror R. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.NECESSITYProject. www.necessity-h2020.eu (15 June 2021, date last accessed)
- 56. Hammenfors DS, Valim V, Bica B. et al. Juvenile Sjogren's syndrome: clinical characteristics with focus on salivary gland ultrasonography. Arthritis Care Res 2020;72:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riviere E, Pascaud J, Tchitchek N. et al. Salivary gland epithelial cells from patients with Sjogren's syndrome induce B-lymphocyte survival and activation. Ann Rheum Dis 2020;79:1468–77. [DOI] [PubMed] [Google Scholar]
- 58. Riviere E, Pascaud J, Virone A. et al. Interleukin-7/Interferon axis drives T-cell and salivary gland epithelial cell interactions in Sjogren's syndrome. Arthritis Rheumatol 2021;73:631–40. [DOI] [PubMed] [Google Scholar]
- 59. Davies K, Mirza K, Tarn J. et al. Fatigue in primary Sjogren's syndrome (pSS) is associated with lower levels of proinflammatory cytokines: a validation study. Rheumatol Int 2019;39:1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Howard Tripp N, Tarn J, Natasari A. et al. Fatigue in primary Sjogren's syndrome is associated with lower levels of proinflammatory cytokines. RMD Open 2016;2:e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. James K, Al-Ali S, Tarn J. et al. A transcriptional signature of fatigue derived from patients with primary Sjogren's syndrome. PLoS One 2015;10:e0143970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lendrem D, Mitchell S, McMeekin P. et al. Health-related utility values of patients with primary Sjogren's syndrome and its predictors. Ann Rheum Dis 2014;73:1362–8. [DOI] [PubMed] [Google Scholar]
- 63. Westhoff G, Dorner T, Zink A.. Fatigue and depression predict physician visits and work disability in women with primary Sjogren's syndrome: results from a cohort study. Rheumatology 2012;51:262–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.