Abstract
c-Jun activation by mitogen-activated protein kinases has been implicated in various cellular signal responses. We investigated how JNK and c-Jun contribute to neuronal differentiation, cell survival, and apoptosis. In differentiated PC12 cells, JNK signaling can induce apoptosis and c-Jun mediates this response. In contrast, we show that in PC12 cells that are not yet differentiated, the AP-1 family member ATF-2 and not c-Jun acts as an executor of apoptosis. In this context c-Jun expression protects against apoptosis and triggers neurite formation. Thus, c-Jun has opposite functions before and after neuronal differentiation. These findings suggest a model in which the balance between ATF-2 and Jun activity in PC12 cells governs the choice between differentiation towards a neuronal fate and an apoptotic program. Further analysis of c-Jun mutants showed that the differentiation response requires functional dimerization and DNA-binding domains and that it is stimulated by phosphorylation in the transactivation domain. In contrast, c-Jun mutants incompetent for DNA binding or dimerization and also mutants lacking JNK binding and phosphorylation sites that cannot elicit neuronal differentiation efficiently protect PC12 cells from apoptosis. Hence, the protective role of c-Jun appears to be mediated by an unconventional mechanism that is separable from its function as a classical AP-1 transcription factor.
Jun NH2-terminal kinases (JNKs), a subfamily of the stress-activated mitogen-activated protein kinases (MAPKs), have complex functions in the control of programmed cell death, or apoptosis. Perhaps best understood is the role of JNK during neuronal cell death. Targeted mutagenesis experiments in the mouse have demonstrated the existence of an excitotoxin-induced signaling pathway that leads, via the activation of JNK-3 (a neuron-specific form of JNK) and the subsequent phosphorylation of the transcription factor c-Jun on serines 63 and 73, to the induction of cell death in hippocampal neurons (reviewed in references 4 and 20). The thus-triggered apoptotic program appears to involve de novo transcription, activated by phosphorylated c-Jun (1). The function of c-Jun phosphorylation by JNK as a trigger for neuronal apoptosis is further supported by a large body of experimental evidence, obtained in model systems such as PC12 cells or explanted primary neurons (see below).
The ability of JNKs to mediate cell death is not restricted to neurons. JNK-deficient (jnk1−/− and jnk2−/−) mouse embryonic fibroblasts are incompetent to undergo apoptosis in response to UV light (29). Unexpectedly, the primary effect of JNKs as mediators of UV-induced apoptosis in primary mouse embryonic fibroblasts appears to involve cytochrome c release from mitochondria and does not require gene transcription. In a different paradigm, however—the apoptotic response of 3T3 fibroblasts to DNA damage—JNK instructs cells to commit suicide via transcriptional activation of the Fas ligand CD95-L (16). Evidently, there are multiple mechanisms by which JNK stimulation can direct cells towards suicide. The complex role of JNK in the control of apoptosis is further illustrated by the phenotype displayed by JNK 1- and 2-deficient mice (17). As expected based on the experiments described above, certain apoptotic responses are abolished in these animals, leading for example to reduced cell degeneration during hindbrain and neural tube formation. In contrast, however, forebrain cells undergo apoptosis much more frequently in jnk1−/− jnk2−/− mice than in wild-type controls. This indicates that, depending on the biological context, loss of JNK function can lead both to excessive and to insufficient levels of cell death.
c-Jun, one of the principle mediators of the transcriptional response to JNK activation, plays an equally enigmatic role in the control of cell death. c-Jun is a member of the AP-1 family of leucine zipper transcription factors. It can form DNA-binding homodimers or heterodimers with other family members such as JunD, c-Fos, and ATF-2 (reviewed in reference 15). Strong evidence identifies c-Jun as an executor of death signals in neurons and neuronally differentiated PC12 cells as well as in fibroblasts (16, 29). However, in other biological situations c-Jun acts as an antiapoptotic factor. During mouse hepatogenesis (1, 6, 12), the presence of c-Jun prevents apoptosis. An antiapoptotic function has also been proposed based on studies with c-Jun-deficient fibroblasts, which became protected against stress-induced apoptosis upon reintroduction of c-Jun by a viral vector (36). The molecular basis for the dual role of c-Jun in apoptosis is not fully understood. It may depend on the target gene activated by c-Jun in the respective cell type. As pointed out above, c-Jun can serve as a transcriptional activator of the apoptosis-inducing Fas ligand (16, 19) but also as a repressor of the tumor suppressor and death agonist p53 (26), depending on the cell type.
To better understand the parameters that determine the specific response to JNK signaling in the context of cell death, we performed experiments with PC12 cell cultures, which provide a defined system for studying differential responses to MAPK signaling. In particular, the signaling events leading to apoptosis in PC12 cells can be dissected using various gene transfer and chemical approaches. In response to nerve growth factor (NGF) treatment, PC12 cells adopt a sympathetic neuronal phenotype (27). Once differentiated, the cells become NGF dependent, and removal of the neurotrophin triggers the JNK signaling cascade and results in apoptosis (37). Interestingly, c-Jun has been implicated both in neuronal differentiation in response to NGF and in death upon NGF withdrawal. In previously published studies, in which neuronally differentiated PC12 cells or sympathetic neurons were used, apoptosis induced by NGF withdrawal was prevented by dominant-negative mutants or by microinjected antibodies specific for c-Jun (7, 9, 37). In addition, expression of an activated form of MEKK1, an upstream activator of the JNK pathway, or of c-JunAsp, a pseudophosphorylated and, hence, constitutively active mutant of c-Jun, was sufficient to trigger apoptosis in cerebellar granule neurons (34). Reproducing these findings, we observed that microinjection of differentiated PC12 cells with a plasmid coding for c-JunAsp reduced PC12 cell survival in comparison to cells that received c-Junwt or c-JunAla, a mutant which cannot be phosphorylated by JNK (S. Leppä, unpublished observation). Collectively, these results indicate that activation of c-Jun causes apoptosis in differentiated PC12 and other neuronal cells. Undifferentiated cells, however, behave differently. Here, c-JunAsp leads to differentiation and, importantly, has no adverse effect on cell viability (21). MEKK1 activation, on the other hand, efficiently triggers apoptosis in both the differentiated and undifferentiated cells. These findings imply that the mechanisms regulating apoptosis and the response to c-Jun activation differ, depending on the differentiation state of the cell. Here we compare the functions of c-Jun as a mediator of differentiation, death, and survival. Our findings indicate that these different responses require different biochemical functions of c-Jun.
MATERIALS AND METHODS
Plasmids.
Plasmids for cytomegalovirus (CMV) enhancer-driven expression of epitope-tagged c-Junwt, c-JunAla, c-JunAsp, c-JunΔ31–57, c-JunbZIP, c-Jun1–223NLS, and c-Fos mammalian cells and nuclear β-galactosidase and a reporter plasmid, −60/+63 col LUC (see below), have been described (21, 23, 30, 31). The Renilla luciferase control vector driven by the human ubiquitin promoter was a gift from Carsten Weiss. CMV enhancer-driven expression vectors for hemagglutinin (HA)-tagged mutant forms of c-Jun were constructed by replacing the PstI-HpaI fragment of c-Junwt with a mutated fragment derived from pHJ19 MUT series (2). The murine JunD and JunB cDNAs were provided by M. Yaniv; they were cloned into a CMV enhancer-driven expression vector, and an HA epitope tag was inserted C terminally. The constructs for mammalian expression for ATF-2 derivatives were provided by P. Angel, and the construct for constitutively active MEKK1 (35) was provided by R. J. Davies.
Cell culture, microinjection, and transfections.
Rat pheochromocytoma PC12 cells were routinely cultured in collagen-coated dishes in a humidified 7.5% CO2 atmosphere at 37°C in Dulbecco modified Eagle medium supplemented with 10% horse serum and 5% fetal calf serum. For microinjection, cells were seeded on laminin-coated plastic plates (20 μg of mouse laminin [Sigma] per ml) to provide better adhesion and to facilitate neurite outgrowth. Microinjections were performed using an automated injection system and a Zeiss inverted microscope. All plasmids were injected into the nucleus at a concentration of 50 μg of expression vector per ml unless otherwise stated. A total of 100 to 150 cells were injected per experiment. Human 293 cells were cultured in a humidified 5% CO2 atmosphere at 37°C in Dulbecco modified Eagle medium with 10% fetal calf serum. Transient transfections into PC12 cells and 293 cells were done with Fugene reagent according to the manufacturer's instructions (Roche).
Immunostaining.
Cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS), washed with PBS, and permeabilized with 0.1% Triton X-100 in PBS on ice. Blocking with 1% bovine serum albumin (BSA) in PBS for 30 min and incubations with primary antibodies in 1% BSA–PBS for 1 h were done at room temperature. Antibodies included a monoclonal antibody (MAb) against β-galactosidase (Promega), a MAb against the HA epitope (clone 12CA5), a MAb against the myc epitope (clone 9E10), and a polyclonal antibody against c-Jun (2). After several washes, bound antibodies were visualized using a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Jackson Laboratories) for 1 h at room temperature. The morphology of the cells was visualized using tetramethyl rhodamine isothiocyanate (TRITC)-labeled phalloidin (Sigma). Cells were further washed extensively with PBS, and Hoechst dye 33258 (Sigma) was included in the last wash to stain the nuclei. Finally, the cells were mounted under a coverslip using Mowiol. Samples were examined using a Zeiss LSM410 confocal imaging system. For quantification of neurite outgrowth, the cells forming neurites longer than twice the diameter of the cell body were scored as differentiated. TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining was performed according to the manufacturer's instructions (Roche). The statistical significance of differences seen in cell survival and neurite formation assays was analyzed using Student's t test. All P values were two tailed.
Western blot analysis.
293 cells were lysed directly in sodium dodecyl sulfate (SDS) sample buffer and sonicated with a microtipped Branson sonifier. Samples were separated on an SDS–10% polyacrylamide gel and transferred onto nitrocellulose membranes by electroblotting. Detection was performed using a MAb against the HA epitope. Horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson Laboratories. The blots were developed using an enhanced chemiluminescence protocol (Amersham).
Luciferase assays.
AP-1 activity was assayed using a reporter plasmid, −60/+63 col LUC (30). The plasmid carries a luciferase gene under the control of an AP-1-responsive element present within a collagenase gene promoter. The activity of collagenase reporter was normalized to the activity of the control reporter (Renilla). Dual luciferase assays were performed according to the manufacturer's instructions (Promega).
RESULTS
c-Jun protects undifferentiated PC12 cells from ΔMEKK1-induced apoptosis.
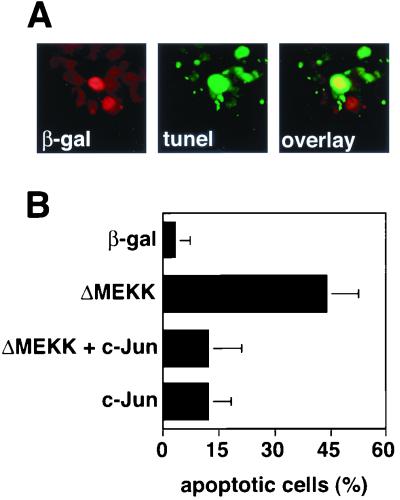
As pointed out in the introduction, the effect of c-Jun in the control of cell death may vary depending on the biological context. The differentiation state of the cell, for example, may influence whether c-Jun activation causes cell death. To directly examine if and how the consequences of c-Jun and JNK signaling on apoptosis differ between differentiated and undifferentiated PC12 cells, we undertook microinjection experiments. Consistent with previous studies (21, 37), deliberate activation of the JNK pathway by microinjection of a plasmid coding for the signal-independent catalytically active domain of MEKK1 (ΔMEKK1) induced prominent apoptosis in undifferentiated cells, as characterized by TUNEL staining (Fig. 1). This response was accompanied by morphological changes that are characteristic of apoptosis, including shrinkage of the cell bodies, membrane blebbing, and disruption of the nuclear membranes (Fig. 2A). At 36 h after injection with the ΔMEKK1 expression vector, only 5% of the cells were alive (Fig. 2B), whereas most of the control cells (91%) injected with an expression vector for a nuclear β-galactosidase survived (Fig. 2B).
FIG. 1.
c-Jun expression rescues PC12 cells from ΔMEKK-induced apoptosis. (A) Induction of apoptosis in PC12 cells expressing ΔMEKK. PC12 cells were transfected with expression vectors for ΔMEKK and nuclear β-galactosidase (β-gal). After 24 h, the cells were fixed and double stained with anti-β-galactosidase antibody to detect transfected cells and with TUNEL reagent to mark cells undergoing apoptosis. Nuclei of cells expressing ΔMEKK appear red (left panel), and apoptotic cells are visualized in green (middle panel). (B) Quantification of apoptosis. The percentage of TUNEL-positive cells among the transfected cells was determined. The data are the means ± standard errors of two separate experiments.
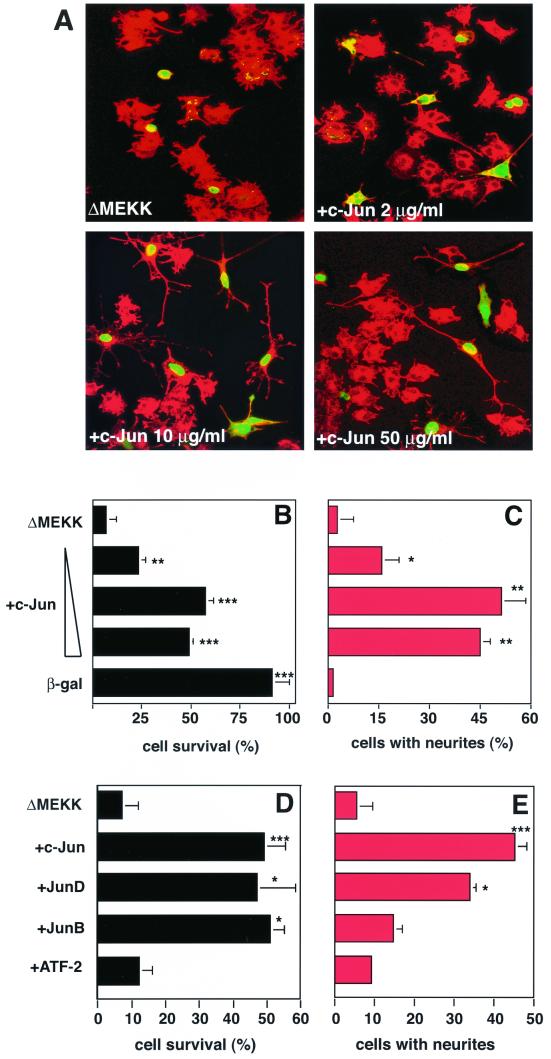
FIG. 2.
Jun proteins but not ATF-2 rescue PC12 cells from ΔMEKK-induced apoptosis. (A) Morphology of PC12 cells expressing ΔMEKK alone or ΔMEKK with increasing concentrations of c-Jun, as indicated. Nuclear β-galactosidase was coexpressed to mark the injected cells. After 36 h, the cells were fixed and stained with anti-β-galactosidase. Injected cells were detected using FITC-labeled secondary antibodies (green), and the morphology of the cells was visualized by actin staining (red). Cells were examined by confocal microscopy. (B and D) Quantification of cell survival. The percentage of viable cells was determined. In apoptotic cells, β-galactosidase staining is punctate, and the cell bodies have shrunk. (C and E) Quantitation of neurite outgrowth. The percentage of the cells with neurites exceeding twice the cell length among the microinjected (FITC positive) cells is shown. c-Jun, JunB, JunD, and ATF-2 were compared for their ability to counteract ΔMEKK-induced cell death (D) and to induce neuronal differentiation (E). The data are the means ± standard errors of two or three separate experiments. Statistically significant differences from values for ΔMEKK-expressing cells are indicated as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
c-Jun is expressed at very low levels in undifferentiated PC12 cells, even after activation of the JNK pathway (21), suggesting that it may not be involved in the observed apoptotic effect of ΔMEKK1. To examine whether c-Jun might, nevertheless, be able to contribute to ΔMEKK1-induced apoptosis of undifferentiated PC12 cells, as it does in differentiated ones, we coinjected expression plasmids for both c-Jun and ΔMEKK1. Surprisingly, c-Jun did not enhance cell death but prevented it in the undifferentiated PC12 cells (Fig. 1 and 2). This effect was concentration dependent; even when the amount of the c-Jun expression vector was decreased from the standard concentration of 50 μg/ml in the injection solution to 2 μg/ml, cell survival was still significantly increased (P = 0.005). Suppression of apoptosis by c-Jun expression was accompanied by the formation of long neurites from the cell bodies, as was previously reported (21) (Fig. 2A and C).
The findings described above indicate that c-Jun not only serves as a differentiation factor but in addition acts antiapoptotically in PC12 cells. To investigate whether one or both of these functions may be shared by related transcription factors, we tested several other members of the AP-1 family for their effects on undifferentiated PC12 cells (Fig. 2C and D). Among the Jun proteins, JunD acts very similarly to c-Jun in that it effectively promotes neurite outgrowth and prevents apoptosis when expressed along with ΔMEKK1. JunB, on the other hand, suppresses apoptosis to the same extent as c-Jun and JunD but is a very inefficient inducer of neuronal differentiation. The AP-1 family member ATF-2 can neither suppress apoptosis nor drive PC12 cells into neuronal differentiation. All AP-1 proteins were expressed at comparable levels, based on immunostaining. Two Jun family members, c-Jun and JunD, shared the ability to induce neuronal differentiation, an activity that JunB does not have. However, all three Jun proteins can protect against apoptosis. Thus, the capacities to promote differentiation and to prevent death are not coupled, suggesting that they might be mediated by separable molecular activities.
The protective effect of c-Jun is independent of JNK binding and phosphorylation.
JNK activates c-Jun by phosphorylating Ser 63 and 73 and Thr 91 and/or Thr 93 residues in the transactivation domain (1, 5, 11, 18, 23, 25). Homologous phosphorylation sites are found in JunD yet are absent in JunB (14). Thus, the presence of these sites correlates with the ability of Jun proteins to induce neuronal differentiation in the previous experiment but not with the antiapoptotic effect. To directly address the significance of c-Jun phosphorylation by JNK for the survival function, we expressed ΔMEKK1 along with c-JunAla, in which all the above-mentioned sites are replaced by alanine residues and which therefore cannot be phosphorylated by JNK (Fig. 3A). After 36 to 48 h, the numbers of cells which survived and/or differentiated were scored. We found that the apoptotic effect of ΔMEKK1 was significantly suppressed by c-JunAla and that this suppression was quantitatively similar to that obtained with c-Junwt or c-JunAsp. The latter is a gain-of-function mutant of c-Jun in which the MAPK phosphorylation sites are replaced by negatively charged phosphate-mimetic aspartic acid residues. Thus, the ability of c-Jun to antagonize MEKK-initiated apoptosis is independent of phosphorylation by JNK (Fig. 3C). In contrast, ΔMEKK1 expression increased the efficiency of neuronal differentiation elicited by c-Junwt expression. This was, presumably, due to phosphorylation of c-Jun by JNK, since the moderate neurite outgrowth observed after expression of c-JunAla was not increased when ΔMEKK1 was also introduced into the cells (compare Fig. 3B and D). We conclude that the function of c-Jun as a survival factor does not require MAPK phosphorylation, whereas this modification is important for its role in differentiation.
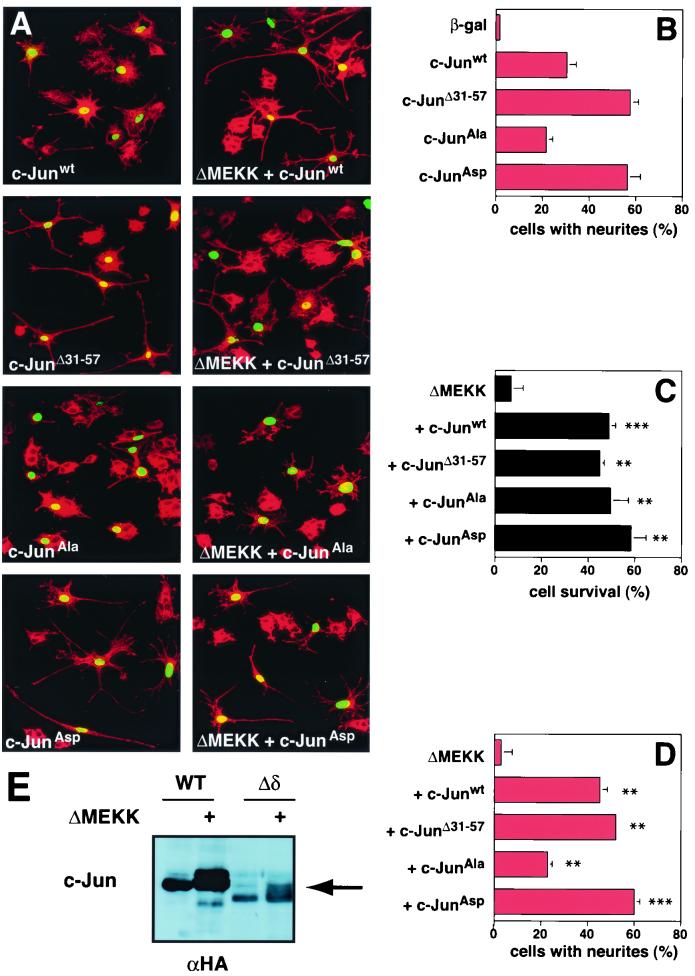
FIG. 3.
c-Jun-mediated protection from ΔMEKK-induced apoptosis is JNK independent. (A) Morphology of cells expressing c-Jun derivatives and ΔMEKK. PC12 cells were injected with c-Junwt, c-JunΔ31–57, c-JunAla, or c-JunAsp expression vectors in the presence or absence of ΔMEKK, as indicated. Nuclear β-galactosidase was coexpressed to mark the injected cells. The cells were fixed after 40 h, stained with anti-β-galactosidase (green) and TRITC-phalloidin (red), and examined by confocal microscopy. (B to D) Quantification of cell survival and neurite outgrowth was performed as for Fig. 1. The data are means ± standard errors of two or three separate experiments. Statistically significant differences from values for ΔMEKK-expressing cells are indicated as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (E) c-JunΔ31–57 is phosphorylated in response to ΔMEKK. HA-tagged c-Jun and c-JunΔ31–57 were expressed alone or with ΔMEKK in 293 cells. Cells were harvested 24 h posttransfection, and whole-cell extracts were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting using anti-HA antibody.
With phosphorylation not involved, an alternative explanation for the protective function of exogenously expressed c-Jun would be that the protein titrates out activated JNK. If the escape from apoptosis caused by c-Jun expression was simply due to sequestration of JNK, the δ deletion mutant c-JunΔ31–57, which lacks the docking site for the kinase, would be expected not to rescue PC12 cells from ΔMEKK1-induced apoptosis. However, expression of this δ domain-less mutant, along with ΔMEKK1, prevented cell death as effectively as wild-type c-Jun (Fig. 3C). In addition, prominent neurite outgrowth was induced (Fig. 3D). In fact, the deletion mutant lacking the δ domain caused differentiation more efficiently than wild-type c-Jun (Fig. 3B). Consistently, the δ deletion mutant is a more potent inducer of AP-1 reporter activity than c-Junwt (data not shown) (2). The gain-of-function characteristics of c-JunΔ31–57 are surprising in light of the fact that this mutant, lacking the main JNK docking site, was not expected to be N-terminally phosphorylated. Interestingly, however, at least under the experimental conditions employed, the mutant appeared to be efficiently phosphorylated when cotransfected with ΔMEKK, as judged by SDS-gel mobility (Fig. 3E). The reason for this effect and the question of how c-Jun can be phosphorylated in the absence of a JNK docking site await experimental clarification. These results indicate that JNK binding is not required for the phosphorylation, antiapoptotis, and differentiation activities of c-Jun in undifferentiated PC12 cells.
c-Jun can antagonize MEKK-induced cell death independently of DNA binding and dimerization.
To further investigate the importance of the distinct functional domains of c-Jun for the antiapoptotic effect, we tested whether the deletion mutants c-JunbZIP and c-Jun1–223NLS, which lack transactivation and bZIP domains, respectively, could prevent ΔMEKK1-induced death of undifferentiated PC12 cells. The N-terminally truncated mutant, c-JunbZIP, acts as a dominant interfering mutant of c-Jun, presumably by sequestering endogenous AP-1 components or by occupying the AP-1 DNA-binding site. In contrast to the effect of dominant-negative c-Jun in differentiated PC12 cells, where it suppresses ΔMEKK1-induced apoptosis (21, 37), the expression of c-JunbZIP did not have this effect in undifferentiated PC12 cells (Fig. 4B). This illustrates further that AP-1 activation does not play a positive role in mediating apoptosis in the undifferentiated cells. c-Jun1–223NLS, which includes the transactivation and JNK-binding domains fused to a simian virus 40 nuclear localization signal in order to ensure correct localization in the nucleus, was equally inefficient in preventing apoptosis (Fig. 4B) and in inducing differentiation (Fig. 4C). These results imply that the general integrity of c-Jun is required for its ability to antagonize MEKK1-mediated death and to induce differentiation. However, some lesions, such as removal of the phosphorylation sites that impair transcriptional functions and the ability of c-Jun to initiate neuronal differentiation, do not affect the protective function observed in undifferentiated PC12 cells. Furthermore, the failure of c-Jun1–223NLS, which contains all the known transcriptional activation domains of c-Jun, to rescue cells from apoptosis indicates that the underlying mechanism is unlikely to result from “squelching,” the competition for transcription coactivators.
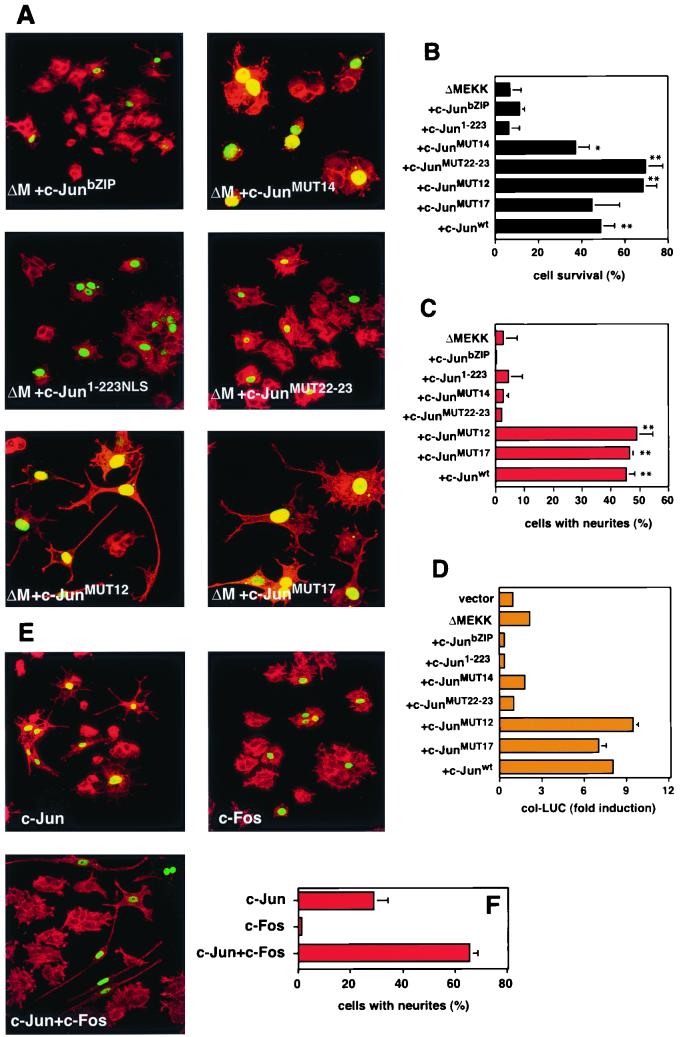
FIG. 4.
c-Jun dimerization and DNA binding are not necessary for rescue from ΔMEKK-induced apoptosis. (A) Morphology of cells expressing c-Jun mutants and ΔMEKK. PC12 cells were injected with expression vectors for ΔMEKK together with c-Junwt or various c-Jun mutants as indicated. A plasmid coding for nuclear β-galactosidase was coexpressed to mark the injected cells. After 40 h, the cells were fixed, stained with anti-β-galactosidase and TRITC-phalloidin, and examined by confocal microscopy. MUT14 (K268I C269D) and MUT22-23 (L294P L308A) are defective in DNA binding and dimerization, respectively. MUT12 (K254I A255D) binds DNA poorly as a homodimer but well as a heterodimer with Fos. MUT17 (K288I A289D) forms slightly more stable homodimers than wild-type c-Jun. All mutants have been described previously (2). (B and C) Quantification of cell survival and neurite outgrowth was performed as for Fig. 1. The data are means ± standard errors of two or three separate experiments. Statistically significant differences from ΔMEKK-expressing cells are indicated as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (D) Transcriptional activation of collagenase reporter by ΔMEKK and c-Jun mutants. PC12 cells were transfected with AP-1-responsive collagenase luciferase (col-LUC) and a control reporter (Renilla luciferase vector driven by the human ubiquitin promoter) together with expression vectors for ΔMEKK and c-Jun mutants, as indicated. The cells were harvested after 36 h for a dual luciferase assay. The firefly luciferase activity was normalized against the Renilla luciferase readings from the cotransfected internal control reporter. The data are representative of three independent experiments done in duplicate (means ± standard errors). (E) Morphology of cells expressing c-Jun and c-Fos. PC12 cells were injected with expression vectors for c-Jun, c-Fos, or both, as indicated. A plasmid coding for nuclear β-galactosidase was coexpressed to mark the injected cells. After 40 h, the cells were fixed and stained with anti-β-galactosidase and TRITC-phalloidin and examined by confocal microscopy. (F) Quantification of neurite outgrowth was performed as for Fig. 1. The data are the means ± standard errors of two or three separate experiments.
The results described above indicate that c-Jun's ability to antagonize MEKK1-induced apoptosis in PC12 cells relies on an unconventional mechanism. To investigate whether other aspects of c-Jun function that are essential for its activity as a transcription factor are required for the antiapoptotic effect, we analyzed a panel of previously characterized c-Jun mutants in which dimerization and/or DNA binding functions are impaired (2). c-JunMUT14 homodimers and even heterodimers of this mutant with wild-type c-Fos cannot bind to DNA due to two amino acid substitutions in the basic domain. When we tested its transactivation potential in PC12 cells, we observed that unlike c-Junwt, c-JunMUT14 does not enhance MEKK-induced AP-1 responsive promoter activity (Fig. 4D). Consistently, c-JunMUT14 cannot drive PC12 cells towards neuronal differentiation (Fig. 4C). However, the same mutant still causes significant suppression of ΔMEKK1-induced apoptosis (Fig. 4B). Thus, DNA binding and 12-O-tetradecanoyl-phorbol-13-acetate-responsive-element-dependent transactivation are not required for the rescue effect. c-JunMUT12 carries a less severe mutation in the DNA-binding domain, which causes a significant decrease of c-Jun's ability to bind DNA as a homodimer. This mutant can, however, bind to AP-1 sites well when dimerized with c-Fos (D. Bohmann, unpublished data). Moreover, it can potentiate the MEKK1-induced reporter activity in PC12 cells (Fig. 4D). Interestingly, in the microinjection assay, c-JunMUT12 behaves in all respects like the wild-type protein: it not only blocks apoptosis but also efficiently induces neuronal differentiation. This result suggests that c-Jun acts as a heterodimer when it activates the neuronal differentiation. Consistent with this idea, coexpression of c-Jun and c-Fos results in more efficient neuronal differentiation than the expression of either c-Jun or c-Fos alone (Fig. 4E).
Next, we investigated whether dimerization and the integrity of the leucine zipper are important for protection from death. Two point mutations within the leucine zipper of c-JunMUT22–23 abolish its ability to homodimerize or heterodimerize with other AP-1 proteins (2, 24). Like c-JunMUT14, this form of c-Jun could rescue PC12 cells from apoptosis but had completely lost the ability to stimulate neurite formation (Fig. 4C) and AP-1 transcriptional activity (Fig. 4D). c-JunMUT17, which has a mildly enhancing effect on homodimerization but not on heterodimerization with Fos (2, 28), was also tested. This mutation did not, however, affect the activity of c-Jun in the differentiation or survival assay. From all the data together, it appears that the antiapoptotic function of c-Jun described here is not mediated by the conventional AP-1 activity of the protein, which involves dimerization, DNA binding, and transcriptional activation of target genes. Rather, in this context, c-Jun acts in a manner independent of its DNA-binding capacity, possibly by interacting with other proteins.
ATF-2 mediates MEKK-induced apoptosis in undifferentiated PC12 cells.
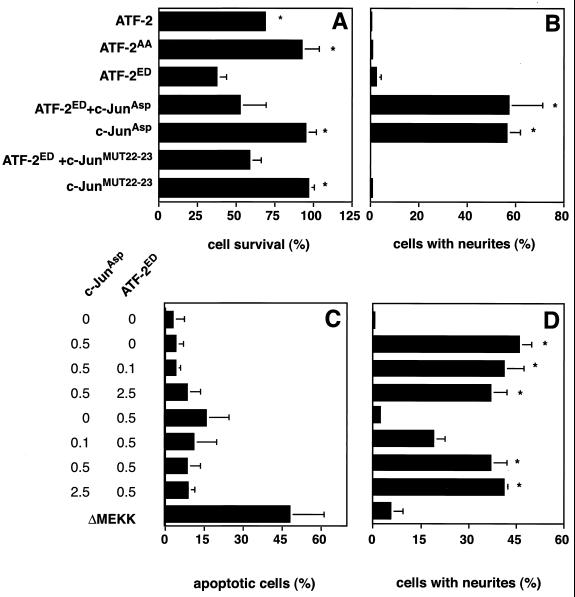
The results described above indicate that, in contrast to its previously described role in neuronally differentiated cells, where c-Jun mediates cell death in response to MEKK activity, it protects undifferentiated cells from such a fate. This raises the question of which effector, if not c-Jun, mediates the apoptotic response to MEKK activation in the undifferentiated state. A potential candidate is the bZIP protein ATF-2. This transcription factor is phosphorylated and activated by JNK and thus can mediate MEKK responses (8, 32). Phosphorylated ATF-2 has been detected in rat brain neurons and PC12 cells upon apoptosis-inducing insults, such as hypoxia or okadaic acid treatment (33). We tested a potential role of ATF-2 phosphorylation in death and differentiation of PC12 cells by the microinjection assay. ATF-2 derivatives, in which the JNK phosphorylation sites had been mutated, were introduced into PC12 cells (Fig. 5). After 48 h the numbers of cells which survived or differentiated were counted. When constitutively active ATF-2 (ATF-2ED) was expressed in PC12 cells, a decrease in surviving cells (Fig. 5A), and increased apoptosis (Fig. 5C) were observed. In comparison, expression of ATF-2WT caused a moderate decrease in cell survival, whereas ATF-2AA, which cannot be phosphorylated by JNK, failed to induce cell death. Unlike c-JunAsp, none of the ATF-2 derivatives could induce PC12 cell differentiation. Thus, ATF-2 phosphorylation by JNK in response to MEKK signaling triggers apoptosis of PC12 cells in their undifferentiated state. To investigate whether ATF-2 might represent the death-inducing principle that is antagonized by c-Jun, we coexpressed ATF-2ED along with c-Jun derivatives in undifferentiated PC12 cells. Indeed, c-JunAsp could partially counteract ATF-2-induced cell death and push the cells along the alternative path of neuronal differentiation.
FIG. 5.
Activated ATF-2 induces cell death in PC12 cells. (A and B) PC12 cells were injected with plasmids encoding ATF-2WT, ATF-2AA, or ATF-2ED alone or in the presence of c-JunAsp or MUT22-23, as indicated. Nuclear β-galactosidase was coexpressed to mark the injected cells. (C and D) PC12 cells were transfected with 0.5 μg of the expression vectors for c-JunAsp or ATF-2ED per 3-cm dish in the presence of increasing amounts of ATF-2ED or c-JunAsp vectors (0, 0.1, 0.5, and 2.5 μg), respectively. Nuclear β-galactosidase was coexpressed. The cells were fixed after 48 h, stained with anti-β-galactosidase and TRITC-phalloidin, and examined by confocal microscopy. Quantification of cell survival and neurite outgrowth was performed as for Fig. 1. For panel C, cells undergoing apoptosis were identified and quantitated by measuring nuclear fragmentation with Hoechst staining. The data are the mean vs ± standard errors of two separate experiments. Statistically significant differences relative to values for ATF-2ED-expressing cells are indicated with an asterisk (P < 0.05).
To corroborate these results with an alternative experimental approach, we used transient transfection to express different amounts of cJunAsp and ATF-2ED in undifferentiated PC12 cells (Fig. 5C and D). This titration experiment shows a dosage-dependent effect of ATF-2 in the induction of apoptosis and of c-Jun in antiapoptosis and the induction of neuronal differentiation. These results indicate that the balance between ATF-2 and c-Jun is important in determining the PC12 cell responses. The suppression of the ATF-2ED-induced apoptosis could also be observed when the nondimerizing c-Jun mutant MUT22-23 was employed. However, this c-Jun mutant could not trigger the differentiation program. Evidently, c-Jun can suppress the ATF-2-mediated apoptosis with same characteristics as when it antagonizes ΔMEKK.
DISCUSSION
The biological program that is initiated upon exposure of undifferentiated PC12 cells to NGF encompasses the cessation of proliferation, the suppression of apoptosis, and the triggering of neuronal differentiation. We have previously demonstrated that c-Jun can mediate the differentiation effects of NGF (21). Here we show that a further aspect of the NGF response, namely, survival, is supported by c-Jun (Fig. 6). In its capacity as a differentiation factor, c-Jun seems to work like a conventional AP-1 transcription factor, since functional DNA-binding, dimerization, and transactivation domains are all necessary for neurite formation. In addition, phosphorylation of the MAPK target residues, as induced by NGF treatment (21), enhances the differentiation response as well as transcriptional activation of an AP-1 reporter, whereas a dominant interfering mutant inhibits both of these functions.
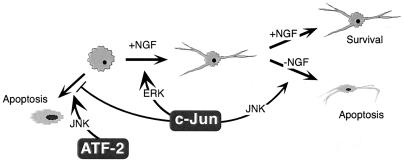
FIG. 6.
Multiple functions of c-Jun in the control of apoptosis and neuronal differentiation. c-Jun prevents undifferentiated PC12 cells from undergoing MEKK1- and ATF-2-mediated apoptosis and promotes their neuronal differentiation. The first function does not require dimerization, DNA binding, or MAPK phosphorylation, whereas the latter appears to be a conventional AP-1 effect stimulated by ERK. Once differentiated, PC12 cells react to AP-1 activation through the JNK pathway by initiating apoptosis.
It is worth noting that obliteration of the JNK-docking site by removal of the δ domain generates a gain-of-function mutant for neuronal differentiation. Interestingly, the δ deletion, as found in v-Jun, also increases the transforming potential of the protein (3). Perhaps in cell transformation, a process in which c-Jun has been found to cooperate with oncogenic Ras (13, 22), and in NGF-dependent differentiation of PC12 cells, which is also thought to be mediated by Ras, c-Jun acts in a comparable manner.
In its second guise, c-Jun acts antiapoptotically and interferes with MEKK-induced cell death when PC12 cells are not yet neuronally differentiated. For this effect, several canonical functions of c-Jun as an AP-1 transcription factor are not essential. A physical interaction with other leucine zipper proteins and with JNK and even a direct contact to DNA appear not to be required for c-Jun to help undifferentiated PC12 cells survive MEKK1 activation. Furthermore, dominant-negative forms of the protein cannot suppress the antiapoptotic function of c-Jun. This novel activity of c-Jun is shared by its close relatives JunD and JunB. In a paper on a similar topic, Le-Nicolescu and colleagues reported that activation of JNK in a PC12 cell line in which ΔMEKK1 can be inducibly expressed from a stably transfected vector causes apoptosis irrespective of the differentiation state (19), i.e., they did not observe the protective function of c-Jun described here. This may be due to the modified PC12 line used in this study. The basal levels of JNK activity present in these cells before induction might create a milieu resembling in some respect that of differentiated cells, where c-Jun activation leads to cell death. As the study presented here used de novo introduction of Jun and MEKK proteins in naïve PC12 cells, this situation was avoided.
The nonconventional mechanism by which c-Jun, JunB, and JunD bring about the rescue function remains a matter of speculation at this point. Our experiments identify the AP-1 family member ATF-2 as an agonist of cell death in undifferentiated PC12 cells. A dominant activated form of ATF-2 can mimic the effect of deliberate JNK activation and induce apoptosis. As in the case of MEKK1-induced cell death, this effect can be suppressed by coexpression of c-Jun. Interestingly, c-Jun appears to counteract ATF-2-and ΔMEKK1-mediated death by the same unconventional mechanism that does not require dimerization or DNA binding. This makes a simple model where c-Jun would modulate ATF-2 function by direct contact unlikely. An indirect effect, such as competition for cofactors, may be an alternative explanation, even though the inability of the isolated transactivation region to inhibit apoptosis appears to argue against a classical squelching mechanism. We speculate that balance between ATF-2 and Jun activities in the differentiation-competent PC12 cells will be an important factor in the decision between the alternative fates of neuronal differentiation and death.
Our experiments implicate Jun proteins in the control of two opposing programs in neuronal cells as they can either cause cell differentiation and survival or initiate programmed cell death. Studies in primary neurons suffering axotomy yielded related results. Herdegen and colleagues described two alternative neuronal fates after axon transection, regeneration, or apoptosis (10). Interestingly, it was found that elevated c-Jun levels and c-Jun phosphorylation are involved in both of these responses. This indicates that the mechanism described here is not a peculiarity of the PC12 cell system but reflects a physiological regulatory phenomenon in the brain.
Neuronal differentiation and apoptosis are phenomena of major medical significance, and it is of central interest to control the underlying regulatory events by pharmacological intervention, for example, after brain injury or stroke or during neurodegenerative diseases. The finding that c-Jun can be an effector of several apparently opposing functions (death, survival, and differentiation) may sound disheartening at first, as it suggests that interference with c-Jun function may be have effects too pleiotropic to be therapeutically useful. However, if the survival function of c-Jun is mediated by a mechanism distinct from the apoptotic effect, selective interference with one or the other phenomenon may be possible and beneficial.
ACKNOWLEDGMENTS
We thank P. Angel, R. J. Davis, S. Gutkind, M. Treier, M. Yaniv, C. Weiss, and J. Woodgett for expression plasmids. J. Westermarck, C. Weiss, and C. Ovitt are acknowledged for helpful comments on the manuscript.
S.L. is supported by fellowships from the Academy of Finland, The Finnish Cancer Society, and The Helsinki Biocentrum.
REFERENCES
- 1.Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 2.Bohmann D, Tjian R. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell. 1989;59:709–717. doi: 10.1016/0092-8674(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 3.Bos T J, Monteclaro F S, Mitsunobu F, Ball A R, Chang C H W, Nishimura T, Vogt P K. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modifications in coding and noncoding sequences. Genes Dev. 1990;4:1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- 4.Davis R J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 5.Dérijard B, Hibi M, Wu I, Barrett T, Su B, Deng T, Karin M, Davis R. JNK1: a protein kinase stimulated by UV-light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 6.Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner E F, Zatloukal K. Functions of c-Jun in liver and heart development. J Cell Biol. 1999;145:1049–1061. doi: 10.1083/jcb.145.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Campbell D, Dérijard B, Davis R. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 9.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L M. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 10.Herdegen T, Skene P, Bahr M. The c-Jun transcription factor—bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 11.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 12.Hilberg F, Aguzzi A, Howells N, Wagner E F. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16:4504–4511. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–939. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- 15.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 16.Kolbus A, Herr I, Schreiber M, Debatin K M, Wagner E F, Angel P. c-Jun-dependent CD95-L expression is a rate-limiting step in the induction of apoptosis by alkylating agents. Mol Cell Biol. 2000;20:575–582. doi: 10.1128/mcb.20.2.575-582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuan C Y, Yang D D, Samanta Roy D R, Davis R J, Rakic P, Flavell R A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 19.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F-X, Green D R, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leppä S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 21.Leppä S, Saffrich R, Ansorge W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd A, Yancheva N, Wasylyk B. Transformation suppressor activity of a jun transcription factor lacking its activation domain. Nature. 1991;352:635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- 23.Papavassiliou A G, Treier M, Bohmann D. Intramolecular signal transduction in c-Jun. EMBO J. 1995;14:2014–2019. doi: 10.1002/j.1460-2075.1995.tb07193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papavassiliou A G, Treier M, Chavrier C, Bohmann D. Targeted degradation of c-Fos, but not v-Fos, by a phosphorylation-dependent signal on c-Jun. Science. 1992;258:1941–1944. doi: 10.1126/science.1470918. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner E F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng M, Greenberg M E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 28.Smith S E, Bohmann D. Functional dissection of AP-1 transcription factors using the Gal4 adaptor assay. Cell Growth Differ. 1992;3:523–529. [PubMed] [Google Scholar]
- 29.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 30.Treier M, Bohmann D, Mlodzik M. Jun cooperates with the Ets-domain protein Pointed to induce photoreceptor R7 fate in the Drosophila eye. Cell. 1995;83:753–760. doi: 10.1016/0092-8674(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 31.Treier M, Staszewski L M, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the δ domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 32.Van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich A, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walton M, Woodgate A M, Sirimanne E, Gluckman P, Dragunow M. ATF-2 phosphorylation in apoptotic neuronal death. Brain Res Mol Brain Res. 1998;63:198–204. doi: 10.1016/s0169-328x(98)00275-7. [DOI] [PubMed] [Google Scholar]
- 34.Watson A, Eilers A, Lallemand D, Kyriakis J, Rubin L L, Ham J. Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons. J Neurosci. 1998;18:751–762. doi: 10.1523/JNEUROSCI.18-02-00751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 36.Wisdom R, Johnson R S, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effect of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]