Abstract
Objectives:
Electroconvulsive therapy (ECT) is an effective treatment for depressive disorders and approved for use in adolescents and adults, but it is unclear whether efficacy or cognitive side effect burden differs with age or if effectiveness in usual clinical practice matches that in prospective studies. We examined the effects of ECT on depression and cognition in a large clinical cohort.
Methods:
A retrospective cohort study of patients ages 16 and older receiving ECT between 2011 and 2020 and who were evaluated with the Quick Inventory of Depressive Symptomatology (QIDS), the Behavior and Symptom Identification Scale-24 (BASIS-24), and the Montreal Cognitive Assessment (MoCA) at baseline and after treatment #10.
Results:
Among 1,698 patients, ECT was associated with a decrease in depression symptoms (QIDS reduction from 17.1±4.9. to 10.1±5.2) and improvement in self-reported mental health (BASIS-24 scores improved from 1.92±0.55 to 1.17±0.60). There was a reduction in MoCA scores from 25.8±3.1 to 25.4±3.1. In multivariate models, age was not associated with a differential QIDS or BASIS-24 response, but older age was associated with a lesser reduction in MoCA.
Conclusion:
Among 1,698 patients aged 16 and older, ECT was associated with improvement in depression and overall self-reported mental health, with a slight decrease in cognition. Age was not associated with changes in efficacy, but older age was associated with a lesser cognitive change as measured by the MoCA. These results provide normative data of real-world effectiveness of ECT, and add further support to its utility in patients with severe psychiatric illness.
Keywords: electroconvulsive therapy, cohort studies, treatment outcome
Introduction
Electroconvulsive therapy (ECT) is an effective,1 safe,2,3 and cost-effective4 treatment for affective disorders that is effective even in patients with multiple prior medication failures.5,6 In the United States ECT is used in elderly patients at higher rates than in younger individuals;7 however, existing evidence is inconsistent, variably suggesting that older age is associated with is a higher8 or equal9,10 response rate to ECT. This uncertainty about age dependency extends to the side effect profile of ECT which may differ by age of the patient.11 While less structured than trial settings, observational data from clinical practice can be analyzed to develop real world evidence applicable to neuropsychiatric disorders and treatment side effects which augments existing trail data.12-17
Aims of the study
This study presents a retrospective cohort of patients treated at a single referral center reporting the effects of age, sex, diagnosis, and treatment location at the time of first treatment (inpatient vs. outpatient) on the effectiveness, cognitive effects, and duration in treatment of ECT as a normative contribution to the evolving trial literature.
Methods
Population and setting
This was a single center retrospective cohort study of all patients aged 16 and older who received ECT during the study period of May 2011 through March 2020 at a freestanding psychiatric referral hospital. Inclusion criterion was the presence of patient-reported symptom measures prior to the first ECT treatment. All included patients were followed until discontinuation of ECT or through the tenth ECT treatment, whichever came first. For patients with multiple ECT courses during the study period only data from the initial series were included. This retrospective chart review study was approved by the Partners Healthcare Institutional Review Board with a waiver of informed consent.
ECT Treatment Procedure
All patients received ECT using a Mecta Spectrum 5000Q (Tualatin, OR). The first session consisted of an individualized seizure threshold determination, as previously reported.18,19 Subsequent treatments were at a default of 6x seizure threshold for right unilateral treatments, at a default schedule of three times weekly. Electrode placement and dose were then adjusted clinically by the treating psychiatrist based on response, and did not follow a prespecified dose escalation procedure.20,21 Methohexital was the default anesthetic agent (with weight-based dosing of 1 mg/kg), but etomidate, propofol, or ketamine could be used at the discretion of the treating psychiatrist or anesthesiologist. Succinylcholine was used as the muscle relaxant for all patients.
Scales and measurements
Patients receiving ECT were tracked using multiple self-reported scales. Depressive symptoms was measured using the Quick Inventory of Depressive Symptomatology Self Report 16 item scale (QIDS), with a score range of 0 to 27 with higher scores indicating worse depression.22 Overall self-reported mental health status was tracked with the Behavior and Symptom Identification Scale-24 (BASIS-24), with a score range of 0-4 with higher scores indicating worse mental health.23 Cognition was assessed using the Montreal Cognitive Assessment (MoCA), with a score range from 0 to 30 with higher scores indicating better cognition.24 Measurements were obtained at baseline before treatment and repeated following the fifth and tenth treatments for the BASIS-24 and QIDS and following the tenth treatment for the MoCA. Alternative versions of the MoCA were given for the initial and follow-up assessment to reduce practice effects that may be caused by repeated cognitive assessments.25 Demographic information is based on self-report from the baseline BASIS-24 assessment. Diagnosis is the clinical diagnoses recorded at the time of first ECT treatment. Patients diagnosed with bipolar disorder included those in any phase of illness (depressed, manic, or mixed). Patients with psychotic disorders, schizoaffective disorder, catatonia, or any other mental health conditions were classified as “other.”
Statistical Analysis
Patients were excluded from the primary analysis if they lacked follow-up QIDS results following treatment 10±2. This time point was chosen because it was the first point following baseline when data for all scales (QIDS, BASIS-24, and MoCA) was available and because it represents a typical acute course length.20,21 Comparisons between the included and excluded groups were made using two-sided t tests for continuous variables and chi-square tests for categorical variables. The change in QIDS, BASIS-24, or MoCA between baseline and treatment #10 was calculated using the Kruskal-Wallis rank sum test. For the primary analysis, the change in QIDS, BASIS-24, or MoCA between baseline and treatment #10 was analyzed using linear regression adjusting for age, sex, diagnosis (major depressive disorder, bipolar affective disorder, other), and location at the time of first treatment (inpatient vs. outpatient). The binary outcome of continuing ECT through follow-up assessment at treatment 10±2 was assessed using logistic regression, with age, sex, diagnosis (major depressive disorder, bipolar affective disorder, other), location at the time of first treatment (inpatient vs. outpatient), baseline QIDS, baseline MoCA, and baseline BASIS-24 as descriptors. Analysis were completed using R (v 4, Vienna, Austria).
Results
During the study period 2,659 patients began ECT and reported baseline symptom scales, including 286 patients who received more than one treatment series during the study period. A total of 1,698 patients (63.9%) met criteria for the primary analysis by having repeat QIDS results at treatment 10±2. Included and excluded patients were similar in age and sex, but a higher proportion of excluded patients began treatment as inpatients and excluded patients were more likely to be diagnosed with conditions other than MDD (Table S1). Among the 1,698 included patients, mean age was 45.7 ± 16.9 years, and 720 (42.4%) were male (Table 1). Demographically, 1,559 (91.8%) self-identified as White, while 50 (2.9%) identified as Asian and 30 (1.8%) as Black. Approximately two thirds of patients (1,109; 65.3%) began ECT as inpatients, while the rest started treatment as outpatients. Diagnostically, MDD (1,271; 74.9%) was the principle diagnosis, with BPAD (342; 20.1%) and other (85; 5%) for the remainder of patients. Initial ECT treatments utilized predominantly right unilateral ultrabrief pulse stimuli (RUL-UBP; 1,568; 92.3%), with right unilateral brief pulse (RUL-BP; 74; 4.4%) and bilateral placement (65; 3.3%) for the remainder. There was an association between the ECT parameters utilized and the age of the patient, with patients aged ≥ 50 utilizing RUL-UBP at a lower rate (667 of 735 patients; 90.7%) than patients younger than 50 (901 of 963 patients, 93.6%) (χ2(1, N=1,698)=4.67, p=0.031).
Table 1:
baseline demographics of the sample
| (n, %) | |
|---|---|
| N | 1,698 |
| Age (mean ± SD), years | 45.7±16.9 |
| Age distribution | |
| <18 | 23 (1.4) |
| 18-29 | 375 (22.1) |
| 30-49 | 549 (32.3) |
| 50-65 | 498 (29.3) |
| 65+ | 253 (14.9) |
| Sex (male) | 720 ( 42.4) |
| Race | |
| White | 1,559 (91.8) |
| Native American | 12 (0.7) |
| Asian | 50 (2.9) |
| Black | 30 (1.8) |
| Pacific Islander | 2 (0.1) |
| Other | 14 (0.8) |
| Unknown | 8 (0.5) |
| Ethnicity | |
| Latino/Latina | 31 (1.8) |
| Not Latino/Latina | 847 (49.9) |
| Unknown | 820 (48.3) |
| Employment in past 30 days | |
| Full-time | 222 (13.1) |
| Part-time | 104 (6.1) |
| None | 1,065 (62.7) |
| Unknown | 307 (18.1) |
| Student (yes) | 231 (13.6) |
| On disability (yes) | 451 (26.6) |
| Education | |
| 8th Grade or Less | 8 (0.5) |
| Some high school | 50 (2.9) |
| High school graduate/GED | 182 (10.7) |
| Some college | 482 (28.4) |
| 4 year college graduate | 440 (25.9) |
| Postcollege education | 522 (30.7) |
| Number missing | 14 (0.8) |
| Subjective Physical Health | |
| Very poor | 22 (1.3) |
| Poor | 213 (12.5) |
| Good | 939 (55.3) |
| Very Good | 398 (23.4) |
| Excellent | 112 (6.6) |
| Number missing | 14 (0.8) |
| Treatment setting for ECT #1 | |
| Inpatient | 1,109 (65.3) |
| Outpatient | 576 (33.9) |
| Number missing | 13 ( 0.8) |
| Clinical Diagnosis | |
| Major depressive disorder | 1,271 (74.9) |
| Bipolar affective disorder | 342 (20.1) |
| Other | 85 (5.0) |
| Initial ECT Parameters | |
| RUL-UBP | 1,568 (92.3) |
| RUL-BP | 74 (4.4) |
| Bilateral | 56 (3.3) |
| Baseline QIDS (mean ± SD) | 17.1±4.9 |
| Baseline BASIS-24 (mean ± SD) | 1.92±0.55 |
| Baseline MoCA (mean ± SD) | 25.8±3.1 |
Patients began ECT with severe-range depression, with baseline QIDS of 17.1±4.9. Over the course of treatment there was a reduction in QIDS to 11.4±5.4 following treatment #5 and 10.1±5.2 at treatment #10 (Kruskal-Wallis rank sum test; χ2 =1312.6, df=2, p < 0.0001) (Figure 1, top). In a linear model of the change in QIDS from baseline to treatment #10 with sex, diagnosis, and initial treatment location as descriptor variables, age was not significantly associated with change in QIDS. A diagnosis of MDD was associated with a greater improvement in QIDS (estimate 1.42; 95% CI 0.18 to 2.66; p=0.025), while beginning treatment as an outpatient was associated with less improvement (estimate −1.51; 95% CI −2.07 to −0.95; p<0.001) (Table 2). The rate of remission from depression (defined as QIDS < 6) following treatment #10 was 21.0% among those patients who had QIDS data at that point. If all those who exited treatment prior to #10 are assumed to have remitted prior to exit, the remission rate among all those initiating treatment was 49.9%. If, instead, all those who exited treatment prior to #10 are assumed to have exited without achieving remission the remission rate among all those initiating treatment was 13.3%. Treatments beyond #10 and any associated response were not considered.
Figure 1:
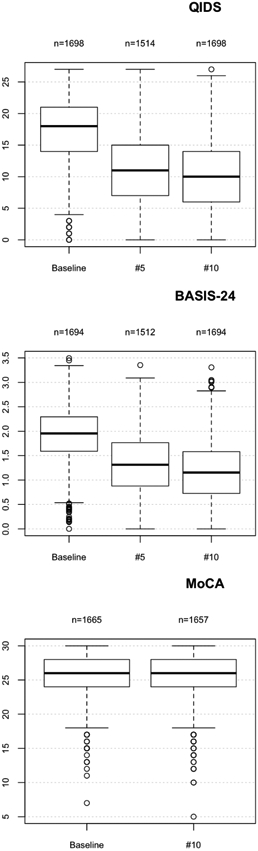
Box plot of QIDS (top), BASIS-24 (middle), and MoCA (bottom) scores between baseline and treatment #10.
Table 2:
linear model of the change in QIDS from baseline to treatment #10 with age, sex, diagnosis, and location at the time of first treatment (inpatient vs. outpatient) as descriptor variables. Bolded values are significant at the level of p < 0.05.
| Predictors | Estimates | CI | p |
|---|---|---|---|
| Age | 0.01 | −0.00 – 0.03 | 0.066 |
| Sex (male) | −0.48 | −1.02 – 0.06 | 0.079 |
| Diagnosis | |||
| MDD | 1.42 | 0.18 – 2.66 | 0.025 |
| BPAD | 0.77 | −0.56 – 2.11 | 0.255 |
| Location (outpatient) | −1.51 | −2.07 – −0.95 | <0.001 |
Overall self-reported mental health was assessed with the BASIS-24, with baseline scores of 1.92±0.55. There was improvement by treatment #5 (1.33±0.61) and treatment #10 (1.17±0.60) (Kruskal-Wallis rank sum test; χ2 =1222.7, df=2, p < 0.0001) (Figure 1, middle). In a linear model of the change in BASIS-24 from baseline to treatment #10 with sex, diagnosis, and initial treatment location as descriptor variables, age was not significantly associated with change in BASIS-24. In contrast, male sex (estimate −0.07; 95% CI −0.13 to −0.01; p=0.018) and beginning treatment as an outpatient (estimate −0.16; 95% CI −0.22 to −0.10; p<0.001) were associated with less improvement in BASIS-24 (Table 3).
Table 3:
linear model of the change in BASIS-24 from baseline to treatment #10 with age, sex, diagnosis, and location at the time of first treatment (inpatient vs. outpatient) as descriptor variables. Bolded values are significant at the level of p < 0.05.
| Predictors | Estimates | CI | p |
|---|---|---|---|
| Age | 0 | −0.00 – 0.00 | 0.16 |
| Sex (male) | −0.07 | −0.13 – −0.01 | 0.018 |
| Diagnosis | |||
| MDD | 0.09 | −0.04 – 0.23 | 0.179 |
| BPAD | 0.05 | −0.10 – 0.19 | 0.536 |
| Location (outpatient) | −0.16 | −0.22 – −0.10 | <0.001 |
Cognition was assessed using the MoCA. At baseline patients had normal-range cognition, with a mean of 25.8±3.1, which reduced to 25.4±3.1 after treatment #10 (Kruskal-Wallis rank sum test; χ2 =21.3, df=1, p < 0.0001) (Figure 1, bottom). In a linear model of the change in MoCA from baseline to treatment #10 with sex, diagnosis, and initial treatment location as descriptor variables, increasing age was associated with a smaller decline in MoCA (estimate −0.03; 95% CI −0.03 to −0.02; p<0.001), while other variables were not significantly associated (Table 4).
Table 4:
linear model of the change in MoCA from baseline to treatment #10 with sex, diagnosis, and location at the time of first treatment (inpatient vs. outpatient) as descriptor variables. Bolded values are significant at the level of p < 0.05.
| Predictors | Estimates | CI | p |
|---|---|---|---|
| Age | −0.03 | −0.03 – −0.02 | <0.001 |
| Sex (male) | −0.17 | −0.48 – 0.14 | 0.292 |
| Diagnosis | |||
| MDD | 0.43 | −0.30 – 1.15 | 0.25 |
| BPAD | 0.2 | −0.58 – 0.98 | 0.616 |
| Location (outpatient) | 0.15 | −0.17 – 0.48 | 0.349 |
In a logistic model of the binary outcome of continuing in ECT through treatment #10 vs. discontinuing prior to this point, a higher initial MoCA score, higher initial QIDS score, a diagnosis of MDD, and beginning treatment as an outpatient were all associated with a higher odds of continuing ECT for at least 10 treatments. Higher baseline BASIS-24 score was associated with a lower odds of continuing ECT for at least 10 treatments. (Table S2). Age was not associated with retention in ECT for at least 10 treatments.
Discussion
In this single-center retrospective cohort study of 1,698 patients receiving acute course ECT with predominantly right unilateral ultrabrief stimuli, there was a significant improvement in depression and overall self-reported mental health status during treatment with a slight decline in cognition as measured by the MoCA. Patients on average began treatment with severe depression symptoms, as measured by the QIDS, and after 10 treatments had depressive symptoms in the mild to moderate range. This improvement is notable given the high rate of treatment-resistance seen in ECT patients. While prior psychiatric treatment history is not available in this cohort, in one US-based ECT trials patients had received a mean of 5 medication trials prior to ECT referral,26 and Level 4 of pharmacotherapy in the STAR-D treatment algorithm achieved response (defined as 50% symptom reduction) in only ~17% of patients.27 As many patients continued treatment beyond ECT #10, further effects may have realized over subsequent treatments, as was seen in a subset of 100 of these patients who continued for up to 100 treatments and who demonstrated continued QIDS improvement throughout maintenance ECT.28
In this sample, neither age nor sex was associated with a change in QIDS response during ECT, nor was age associated with differential response in overall self-reported mental health on the BASIS-24. The non-impact of age on these outcomes is concordant with the findings of Socci et al. who found that among 402 patients receiving ECT, response and remission rates did not significantly differ based on age buckets of young, middle age, or old patients.10 Likewise Birkenhäger et al. treating age as a continuous variable in multivariate models similar to ours did not find age to be a significant predictor of improvement in a sample of 141 patients.9 In contrast, based on the prospective Consortium for Research in ECT (CORE) trial of 253 patients, O’Connor et al found that age as a continuous variable positively influenced response to treatment.8 Additionally Güney et al. found that health related quality of life improved more in older ECT patients relative to younger ones based on 1066 patients from the Swedish ECT quality register.29 It is unclear what may be responsible for this discordance in findings, although notably there are differences in the types of ECT delivered in many of these samples (e.g. bilateral electrode placement in CORE vs predominantly right unilateral ultrabrief pulse in this sample) and it is unclear if this explains this inconsistency or whether other well recognized challenges of clinical trials might be at work.30,31 In our sample inpatients showed a greater degree of improvement in QIDS than outpatients, but also had higher baseline QIDS scores, and so this may reflect a greater potential improvement due to increased illness severity or additional effects independent of ECT treatment that may occur during psychiatric hospitalization.
There was a statistically significant reduction in MoCA during the treatment course, however the magnitude of this effect was small at 0.5 points. The MoCA has demonstrated sensitivity to detecting subtle cognitive changes during ECT,32 and a reduction of 0.5 points is less than the suggested minimal clinically important difference in MoCA of 1.22-2.15.33,34 Increasing age was associated with a smaller magnitude of cognitive decrease, with each decade of age calculated to result in a relative 0.3 point change in MoCA. As the range of ages in the sample was more than 70 years, even this small change may be clinically significant when comparing the oldest and youngest patients. Notably, cognitive testing in this sample was done prior to treatment #11, which on average occurred 2-3 days following the tenth treatment. Prior systematic studies suggesting most cognitive changes do not persist for more than 3 days,35,36 and it is unclear whether the cognitive changes observed in this sample would persist at a longer follow-up interval. It is unclear why older age may be relatively protective from adverse cognitive effects as measured by the MoCA, but not significantly affect outcomes for either depression or overall self-reported mental health. A previous large national cohort study found that the use of ECT in patients >70 years old was associated with a decreased rate of dementia relative to non-treated patients hospitalized for affective disorders.37 This may imply a neuroprotective effect of ECT in this older population, but the mechanism for this, if true, is unclear.
Strengths of this study include large sample size, real world cohort heterogeneity, and diversity of outcomes considered. Indeed, the 1,698 patients reported here are nearly as many as the pooled sample size (1,680 for MDD, 377 for BPD) of a recent meta-analysis of ECT effectiveness.1 This study explores treatment effectiveness for depression, overall self-reported mental health, and cognition, and therefore allows for assessment of both effects and cognitive side effects of treatment. Moreover, this study reports on a heterogeneous cohort of real-world patients presenting for and ultimately moving forward with treatment, with broad inclusion criteria.
Limitations
Whereas real world evidence presents unique strengths, the clinical data generating process on which this evidence is based and observational nature of these designs produce a range of important limitations to be considered.38-41 Due to inclusion criteria requiring baseline and follow-up data, approximately 36% of patients beginning ECT were not included in the primary analysis, which may hinder analysis of the most ill patients who may not have been physically or cognitively able to complete baseline or follow-up assessments (e.g. due to catatonia or severe neurovegetative symptoms). We are unable to systematically assess reasons for dropout in these patients, and so the effectiveness and tolerability in these patients is unclear. Furthermore our data was not structured to assess for overall remission rate as data was collected at a specific treatment number, rather than at the end of the treatment course. We are unable to assess how many patients who had not achieved remission by treatment #10 may have gone on to improve further with subsequent treatments. As the majority of our sample was treated with RUL-UBP ECT, which may require more treatments to achieve remission than other stimulus types,42 some patients continuing with additional treatment may have achieved greater response with time.28 Furthermore we are unable to assess the effects of baseline medications, medication changes, or psychotherapeutic changes which may have co-occurred with ECT treatment. Additionally, as an observational study we are unable to assess causality, and without a control group who did not receive ECT we are unable to assess how responsive this sample may have been to other treatments. As data were only recorded every 5th treatment we are unable to we are unable to consistently track changes in electrode placement or pulse width that may have occurred during treatment, and so we cannot assess how often and at what treatment patients may have transition among ECT parameters. Likewise, we are this unable to assess whether there are differences in the effectiveness or tolerability of different ECT parameters, nor if age modulates these effects.
Moreover, our cognitive assessments were limited to a single metric, the MoCA, which does not address the issue of autobiographical memory loss following ECT that is among the most distressing complications noted from treatments.43 As a result we are unable to comment as to whether autobiographical memory loss or other cognitive domains not captured by the MoCA may or may not differ by age.
Conclusions
In conclusion, among 1,698 patients across a wide age range treated with predominantly right unilateral ultrabrief stimuli, ECT was associated with improvement in depression and overall self-reported mental health, with a negative change in cognition that is less than the minimum clinically important threshold. Age was not associated with efficacy as measured by the QIDS or BASIS-24, but older age was associated with a lesser cognitive change as measured by the MoCA. These results provide normative estimations of real-world effectiveness of ECT in patients of widely varying age with severe psychiatric illness.
Supplementary Material
Significant Outcomes:
Treatment with ECT was associated with improvements in depression (as measured by the QIDS) and overall self-reported mental health (as measured by the BASIS-24) with a slight reduction in cognition (as measured by the MoCA)
Age was not associated with a differential QIDS or BASIS-24 response, but older age was associated with a lesser reduction in MoCA
Limitations:
Although the sample size is large, all data is derived from a single study center from retrospective chart review
Effects of concomitant medication changes cannot be assessed
Patients excluded if unable to complete self-reported outcome measures, which may exclude the most ill patients
Funding
This work was supported by the National Institute of Mental Health (R25MH094612, JL; R01MH120991, THM; 5R01MH112737-03, MEH) The sponsors had no role in study design, writing of the report, or data collection, analysis, or interpretation.
Footnotes
Conflicts of Interest
THM receives research funding from the Stanley Center at the Broad Institute, the Brain and Behavior Research Foundation, National Institute of Mental Health, National Human Genome Research Institute Home, and Telefonica Alfa. The remaining authors have no disclosures to report.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Bibliography
- 1.Bahji A, Hawken ER, Sepehry AA, Cabrera CA, Vazquez G. ECT beyond unipolar major depression: systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand. 2019;139(3):214–226. doi: 10.1111/acps.12994 [DOI] [PubMed] [Google Scholar]
- 2.Luccarelli J, Henry ME, McCoy TH. Quantification of fracture rate during electroconvulsive therapy (ECT) using state-mandated reporting data. Brain Stimul Basic Transl Clin Res Neuromodulation. 2020;13(3):523–524. doi: 10.1016/j.brs.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tørring N, Sanghani SN, Petrides G, Kellner CH, Østergaard SD. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388–397. doi: 10.1111/acps.12721 [DOI] [PubMed] [Google Scholar]
- 4.Ross EL, Zivin K, Maixner DF. Cost-effectiveness of Electroconvulsive Therapy vs Pharmacotherapy/Psychotherapy for Treatment-Resistant Depression in the United States. JAMA Psychiatry. 2018;75(7):713–722. doi: 10.1001/jamapsychiatry.2018.0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. 2015;76(10):1374–1384. doi: 10.4088/JCP.14r09528 [DOI] [PubMed] [Google Scholar]
- 6.Heijnen WT, Birkenhäger TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616–619. doi: 10.1097/JCP.0b013e3181ee0f5f [DOI] [PubMed] [Google Scholar]
- 7.Luccarelli J, Henry ME, McCoy TH. Demographics of Patients Receiving Electroconvulsive Therapy Based on State-Mandated Reporting Data. J ECT. 2020;36(4):229–233. doi: 10.1097/YCT.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor MK, Knapp R, Husain M, et al. The Influence of Age on the Response of Major Depression to Electroconvulsive Therapy: A C.O.R.E. Report. Am J Geriatr Psychiatry. 2001;9(4):382–390. doi: 10.1097/00019442-200111000-00006 [DOI] [PubMed] [Google Scholar]
- 9.Birkenhäger TK, Pluijms EM, Ju MR, Mulder PG, den Broek van WW. Influence of age on the efficacy of electroconvulsive therapy in major depression: A retrospective study. J Affect Disord. 2010;126(1-2):257–261. doi: 10.1016/j.jad.2010.02.131 [DOI] [PubMed] [Google Scholar]
- 10.Socci C, Medda P, Toni C, et al. Electroconvulsive therapy and age: Age-related clinical features and effectiveness in treatment resistant major depressive episode. J Affect Disord. 2018;227:627–632. doi: 10.1016/j.jad.2017.11.064 [DOI] [PubMed] [Google Scholar]
- 11.Damm J, Eser D, Schüle C, et al. Influence of Age on Effectiveness and Tolerability of Electroconvulsive Therapy. J ECT. 2010;26(4):282–288. doi: 10.1097/YCT.0b013e3181cadbf5 [DOI] [PubMed] [Google Scholar]
- 12.Pradier MF, Hughes MC, McCoy TH, Barroilhet SA, Doshi-Velez F, Perlis RH. Predicting change in diagnosis from major depression to bipolar disorder after antidepressant initiation. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2021;46(2):455–461. doi: 10.1038/s41386-020-00838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther. 2018;35(11):1763–1774. doi: 10.1007/s12325-018-0805-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna SP, Wilburn J. Patient value: its nature, measurement, and role in real world evidence studies and outcomes-based reimbursement. J Med Econ. 2018;21(5):474–480. doi: 10.1080/13696998.2018.1450260 [DOI] [PubMed] [Google Scholar]
- 15.Castro VM, Roberson AM, McCoy TH, et al. Stratifying Risk for Renal Insufficiency Among Lithium-Treated Patients: An Electronic Health Record Study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41(4):1138–1143. doi: 10.1038/npp.2015.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCoy TH, Castro VM, Hart KL, Perlis RH. Stratified delirium risk using prescription medication data in a state-wide cohort. Gen Hosp Psychiatry. 2021;71:114–120. doi: 10.1016/j.genhosppsych.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. doi: 10.1056/NEJMoa0806994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luccarelli J, McCoy TH, Seiner SJ, Henry ME. Charge required to induce a seizure during initial dose titration using right unilateral brief pulse electroconvulsive therapy. Brain Stimul Basic Transl Clin Res Neuromodulation. 2020;13(6):1504–1506. doi: 10.1016/j.brs.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luccarelli J, McCoy THJ, Seiner SJ, Henry ME. Total Charge Required to Induce a Seizure in a Retrospective Cohort of Patients Undergoing Dose Titration of Right Unilateral Ultrabrief Pulse Electroconvulsive Therapy. J ECT. 2021;37(1):40–45. doi: 10.1097/YCT.0000000000000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luccarelli J, McCoy TH, Shannon AP, Forester BP, Seiner SJ, Henry ME. Rate of continuing acute course treatment using right unilateral ultrabrief pulse electroconvulsive therapy at a large academic medical center. Eur Arch Psychiatry Clin Neurosci. 2021;271(1):191–197. doi: 10.1007/s00406-020-01202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luccarelli J, McCoy THJ, Shannon AP, Forester BP, Seiner SJ, Henry ME. Duration of Treatment in Electroconvulsive Therapy Among Patients Beginning With Acute Course Right Unilateral Brief Pulse Stimuli. J ECT. 2021;Publish Ahead of Print. doi: 10.1097/YCT.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 23.Eisen SV, Normand S-L, Belanger AJ, Spiro AI, Esch D. The Revised Behavior and Symptom Identification Scale (BASIS-R): Reliability and Validity. Med Care. 2004;42(12):1230–1241. [DOI] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 25.Costa AS, Fimm B, Friesen P, et al. Alternate-form reliability of the Montreal cognitive assessment screening test in a clinical setting. Dement Geriatr Cogn Disord. 2012;33(6):379–384. doi: 10.1159/000340006 [DOI] [PubMed] [Google Scholar]
- 26.Sackeim HA, Dillingham EM, Prudic J, et al. Effect of Concomitant Pharmacotherapy on Electroconvulsive Therapy Outcomes: Short-term Efficacy and Adverse Effects. Arch Gen Psychiatry. 2009;66(7):729. doi: 10.1001/archgenpsychiatry.2009.75 [DOI] [PubMed] [Google Scholar]
- 27.McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine Versus Venlafaxine Plus Mirtazapine Following Three Failed Antidepressant Medication Trials for Depression: A STAR*D Report. Am J Psychiatry. 2006;163(9):1531–1541. doi: 10.1176/ajp.2006.163.9.1531 [DOI] [PubMed] [Google Scholar]
- 28.Luccarelli J, McCoy TH, Seiner SJ, Henry ME. Maintenance ECT is associated with sustained improvement in depression symptoms without adverse cognitive effects in a retrospective cohort of 100 patients each receiving 50 or more ECT treatments. J Affect Disord. 2020;271:109–114. doi: 10.1016/j.jad.2020.03.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Güney P, Ekman CJ, Hammar Å, et al. Electroconvulsive Therapy in Depression: Improvement in Quality of Life Depending on Age and Sex. J Ect. 2020;36(4):242. doi: 10.1097/YCT.0000000000000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. doi: 10.1186/s13063-015-1023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Averitt AJ, Weng C, Ryan P, Perotte A. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med. 2020;3:67. doi: 10.1038/s41746-020-0277-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moirand R, Galvao F, Lecompte M, Poulet E, Haesebaert F, Brunelin J. Usefulness of the Montreal Cognitive Assessment (MoCA) to monitor cognitive impairments in depressed patients receiving electroconvulsive therapy. Psychiatry Res. 2018;259:476–481. doi: 10.1016/j.psychres.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 33.Wu C-Y, Hung S-J, Lin K, Chen K-H, Chen P, Tsay P-K. Responsiveness, Minimal Clinically Important Difference, and Validity of the MoCA in Stroke Rehabilitation. Occup Ther Int. 2019;2019. doi: 10.1155/2019/2517658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan HH, Xu J, Teoh HL, et al. Decline in changing Montreal Cognitive Assessment (MoCA) scores is associated with post-stroke cognitive decline determined by a formal neuropsychological evaluation. PLOS ONE. 2017;12(3):e0173291. doi: 10.1371/journal.pone.0173291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568–577. doi: 10.1016/j.biopsych.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 36.Landry M, Moreno A, Patry S, Potvin S, Lemasson M. Current Practices of Electroconvulsive Therapy in Mental Disorders: A Systematic Review and Meta-Analysis of Short and Long-Term Cognitive Effects. J ECT. Published online October 1, 2020. doi: 10.1097/YCT.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 37.Osler M, Rozing MP, Christensen GT, Andersen PK, Jørgensen MB. Electroconvulsive therapy and risk of dementia in patients with affective disorders: a cohort study. Lancet Psychiatry. 2018;5(4):348–356. doi: 10.1016/S2215-0366(18)30056-7 [DOI] [PubMed] [Google Scholar]
- 38.Eichler H-G, Pignatti F, Schwarzer-Daum B, et al. Randomized Controlled Trials Versus Real World Evidence: Neither Magic Nor Myth. Clin Pharmacol Ther. 2021;109(5):1212–1218. doi: 10.1002/cpt.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazha B, Yang JC-H, Owonikoko TK. Benefits and limitations of real-world evidence: lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2021;17(8):965–977. doi: 10.2217/fon-2020-0951 [DOI] [PubMed] [Google Scholar]
- 40.Kim H-S, Lee S, Kim JH. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J Korean Med Sci. 2018;33(34):e213. doi: 10.3346/jkms.2018.33.e213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence - What Is It and What Can It Tell Us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- 42.Tor P-C, Bautovich A, Wang M-J, Martin D, Harvey SB, Loo C. A Systematic Review and Meta-Analysis of Brief Versus Ultrabrief Right Unilateral Electroconvulsive Therapy for Depression. J Clin Psychiatry. 2015;76(9):e1092–1098. doi: 10.4088/JCP.14r09145 [DOI] [PubMed] [Google Scholar]
- 43.Sackeim HA. Autobiographical Memory and ECT: Don’t Throw Out the Baby. J ECT. 2014;30(3):177–186. doi: 10.1097/YCT.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


