Abstract
Objectives:
Electroconvulsive therapy (ECT) effectively treats depressive disorders, but many patients will have subsequent relapses. While some guidelines suggest prior response to ECT is an indication for ECT in a subsequent mood episode, it is unknown whether response to ECT is correlated between treatment courses. This study explores whether response to ECT at a first treatment correlates with response to treatment in a second independent ECT course.
Methods:
Single-center retrospective cohort of patients receiving two different ECT treatment courses between 2011 and 2020 and who self-reported depression symptoms using the Quick Inventory of Depressive Symptomatology (QIDS) at baseline and following treatment #5.
Results:
286 patients received two independent ECT series during the study period, of whom 153 received at least 5 treatments in both series. Patients had similar QIDS scores at the start of each treatment series (Pearson’s correlation, r = 0.58, p <0.001), but the change in QIDS following 5 ECT treatments was not correlated between series for individual patients (Pearson’s correlation, r = 0.083, p = 0.31). In multivariate analyses, change in QIDS was similar for both treatment series, but patients were less likely to receive 5 treatments in the second treatment series.
Limitations:
retrospective cohort cannot control for factors influencing access to repeat ECT treatment
Conclusions:
While on average final QIDS score was the same following two independent treatment courses, for individual patients the change in depression symptoms was not correlated between treatment series. Further research is needed to identify factors that may predict longitudinal ECT response.
Keywords: electroconvulsive therapy, cohort studies, affective disorders
Introduction:
Electroconvulsive therapy (ECT) is a highly effective treatment for affective disorders—especially for those with severe or treatment resistant symptomatology—with over 50% of patients with unipolar or bipolar depression achieving remission (Bahji et al., 2019; Dierckx et al., 2012). ECT has been shown to improve depressive symptoms, reduce rates of readmission within 30 days, (Slade et al., 2017) and to acutely decrease suicidal ideation and risk of suicide within12 months of treatment (Kellner, Li, et al., 2016; Rönnqvist et al., 2021). While ECT is efficacious as an acute treatment, patients are at risk of relapse after stopping ECT especially within the first 6 months, with reported rates of relapse within 6 months ranging from 50–84% (Hermida et al., 2018; Jelovac et al., 2013). Relapse rates have been shown to be consistent across major depressive disorder (MDD) and bipolar depression (BPAD) (Itagaki et al., 2017), with strategies including maintenance pharmacotherapy (Kellner et al., 2006; Kellner, Husain, et al., 2016) and maintenance ECT (Kellner et al., 2006; Kellner, Husain, et al., 2016; Luccarelli, McCoy, et al., 2020a) contributing to more durable remission. Despite maximal treatment, many patients will have subsequent mood episodes requiring additional treatment, and the optimal treatment of such patients remains uncertain. Especially for patients who benefited from an initial ECT course, additional ECT may be offered during subsequent mood episodes. Indeed, clinical guidelines include prior response to ECT as an indication for future treatment (Tess & Smetana, 2021). Additionally, clinical trials of ECT efficacy demonstrate wide variations in the number of participants that had undergone ECT treatment prior to the trial, with anywhere from 0 – 58% of patients reporting a previous series of ECT treatment (Anderson et al., 2017; Eranti et al., 2007; Prudic et al., 2004; van den Broek et al., 2006; Wijkstra et al., 2000; Yildiz et al., 2010). Most prospective trials have not stratified response rate on the basis of prior ECT, and none has reported outcomes of the same patient across multiple ECT treatment courses during distinct mood episodes. To address this important gap in the literature, this study sought to determine if clinical response to a first series of ECT predicted response to a second series of ECT, looking both at individual patient response and aggregate cohort response.
Methods:
Population, setting, and treatment procedures
The cohort and treatment methods have been described previously (Luccarelli, McCoy, Shannon, et al., 2021b, 2021a). To summarize, this was a retrospective cohort study of patients receiving ECT at a single freestanding psychiatric hospital between May 2011 and June 2020. Patients received ECT using a Mecta Sepctrum 5000Q (Tualatin, OR), and their individualized seizure threshold was determined at first treatment (Luccarelli, McCoy, et al., 2020b; Luccarelli, McCoy, Seiner, et al., 2021). Treatments were given 3 times weekly, with modifications to both dosing and electrode placement determined by the clinical judgement of the treating psychiatrist. Methohexital was the default anesthetic, and succinylcholine muscle relaxant was used for all patients. Due to the volume of the service, ECT was performed by a team of >10 psychiatrists, so all patients were treated by multiple different physicians.
The present study was limited to participants who received 2 or more series of ECT treatments within the study period. For individuals who received more than 2 series of ECT, only their first and second treatment series within the study period were included. To compare only acute courses of treatment, participants were excluded from analysis if their 5th treatment within a series was more than 30 days from their initial treatment. Additionally, participants were excluded from analysis if their second treatment series started within 90 days of the start of their first treatment series.
The Quick Inventory of Depressive Symptomatology – Self Report 16 item scale (QIDS) (Rush et al., 2003) was administered as part of routine clinical care prior to the first treatment and after treatment #5. The QIDS includes nine depressive symptom domains. Demographic data is from patient self-report, and diagnosis was extracted from the patient’s clinical record at the time of their first treatment. This study was reviewed by the Mass General Brigham Institutional Review Board and approved with a waiver of informed consent.
Statistical Analysis
The primary outcome was change in QIDS score from baseline to treatment #5; treatment response was modelled both for individual patients and as a cohort average. First, to determine if on average baseline QIDS varied between treatment series, we constructed a linear model to predict baseline QIDS score and included age, sex, treatment series (1st or 2nd), diagnosis (MDD, BPAD, other), and treatment location (inpatient or outpatient) as covariates. Next, to determine if individual patients had comparable symptom severity at the beginning of each series, the baseline QIDS was compared between series using Pearson’s correlation. To assess individual symptom response (measured as the change in QIDS between baseline and treatment #5), the correlation between a patient’s response in series 1 and response in series 2 was assessed using Pearson’s correlation.
Then, to assess the effectiveness of ECT on aggregate, we constructed a linear model to predict change in QIDS score from baseline to treatment #5, using the following covariates: age, sex, treatment series, diagnosis, treatment location, and baseline QIDS. Next, to determine if improvement in individual symptom domains assessed on the QIDS was correlated across treatment series, we used a Spearman rank correlation to compare a patient’s change in symptom domain scores from baseline to treatment #5 between series 1 and series 2. Since this was an exploratory analysis, we did not correct for multiple comparisons. Finally, we assessed what factors influenced the odds of a patient reaching treatment #5 using logistic regression including the above covariates. All analyses were completed in R (version 3.6.0).
Results:
A total of 286 patients met inclusion criteria for analysis (Table S1), of whom 153 patients reached treatment #5 in both Series 1 and Series 2 and were included in the primary analysis (Figure S1). Demographic data for patients who received at least 5 treatments in both series are presented in Table 1 and described here. Patients had a mean age of 53.93 years (SD = 12.47) at the time of the first treatment series, and a mean age of 55.59 years (SD = 12.66) for the second treatment series. Patients were predominantly female and predominantly white. In series 1, 69.28% (n = 106) of patients had a diagnosis of MDD (series 2: 69.93%; n=107) and 26.14% (n = 40) had a diagnosis of BPAD (series 2: 26.80%; n=41). Treatment was initiated in the inpatient setting for 60.13% of patients in series 1 (n = 92) and 47.06% of patients in series 2 (n= 72). Right unilateral electrode placement was used for 92.16% (n = 141) of patients in series 1 and 86.27% (n = 132) of patients in series 2. Ultrabrief pulse stimuli was used for 86.27% of initial treatments in series 1 and 75.82% of treatments in series 2.
Table 1:
Baseline demographic data for each series among patients receiving at least 5 ECT treatments in each series.
| Series 1 (n, %) | Series 2 (n, %) | |
|---|---|---|
| N | 153 | 153 |
| Age (mean (SD)), years | 53.93 (12.47) | 55.59 (12.66) |
| Sex (Female) | 94 (61.44) | 92 (60.13) |
| Race | ||
| White | 146 (95.42) | 147 (96.08) |
| Native American | 1 (0.65) | 1 (0.65) |
| Asian | 1 (0.65) | 1 (0.65) |
| Black | 1 (0.65) | 1 (0.65) |
| Pacific Islander | 0 (0.00) | 0 (0.00) |
| Other | 4 (0.26) | 3 (0.20) |
| Ethnicity | ||
| Latino/Latina | 1 (0.65) | 3 (1.96) |
| Employment in past 30 days | ||
| Full-time | 4 (2.61) | 12 (7.84) |
| Part-time | 7 (4.58) | 14 (9.15) |
| None | 102 (66.67) | 107 (69.93) |
| Student (yes) | 2 (1.31) | 2 (1.31) |
| On disability (yes) | 55 (35.95) | 64 (41.83) |
| Education | ||
| 8th Grade or Less | 1 (0.65) | 1 (0.65) |
| Some high school | 1 (0.65) | 1 (0.65) |
| High school graduate/GED | 18 (11.76) | 17 (11.11) |
| Some college | 35 (22.88) | 38 (24.84) |
| 4-year college graduate | 39 (25.49) | 38 (24.84) |
| Post-college education | 57 (37.25) | 58 (37.91) |
| Subjective Physical Health | ||
| Very poor | 0 (0.00) | 2 (1.31) |
| Poor | 18 (11.76) | 22 (14.28) |
| Good | 86 (56.21) | 86 (56.21) |
| Very Good | 32 (20.92) | 35 (22.88) |
| Excellent | 15 (9.80) | 6 (3.92) |
| Ever been homeless (yes) | 14 (9.15) | 11 (7.19) |
| Initial Treatment Location | ||
| Inpatient | 92 (60.13) | 72 (47.06) |
| Outpatient | 60 (39.22) | 78 (50.98) |
| Clinical Diagnosis | ||
| Major depressive disorder | 106 (69.28) | 107 (69.93) |
| Bipolar affective disorder | 40 (26.14) | 41 (26.80) |
| Other | 7 (4.58) | 5 (3.27) |
| ECT electrode placement | ||
| Right unilateral | 141 (92.16) | 132 (86.27) |
| Bilateral | 12 (7.84) | 18 (11.74) |
| ECT pulse width | ||
| Ultrabrief pulse (<0.5 ms) | 132 (86.27) | 116 (75.82) |
| Brief pulse (0.5–2 ms) | 21 (13.73) | 37 (24.18) |
| Baseline QIDS - mean (SD) | 17.36 (4.76) | 16.53 (4.93) |
| Change in QIDS – mean (SD) | −6.67 (5.48) | −5.82 (5.15) |
Patients had a median of 520 days between their treatment series (IQR = 281 – 777; Figure S2). The average baseline QIDS score in series 1 was 17.36 (SD = 4.76) and 16.53 (SD = 4.93) in series 2, indicating severe depressive symptoms. On aggregate, baseline QIDS did not significantly differ between treatment series in a multivariate model controlling for age, sex, diagnosis, and treatment location (B = −0.38, p = 0.47; Table S2). The severity of baseline depressive symptoms for individual patients was likewise correlated between treatment series, meaning that individual patients began ECT with similar depression severity at the start of each treatment course (Figure S3; QIDS: r = 0.58, p <0.001).
The average change in QIDS score from baseline to treatment #5 for series 1 was −6.67 (SD = 5.48) and −5.82 (SD = 5.15) in series 2. Among individual patients, there was not a significant correlation between series for change in QIDS score over the course of 5 treatments (r = 0.083, p = 0.31, Figure 1). As a sensitivity analysis, to determine if there was a differential response by age, the cohort was stratified at median age (55.2 years), and the correlations were repeated among each group. There was not a significant correlation between series for change in QIDS score for either age group (Age < 55.2: r = 0.03, p = 0.8; Age > 55.2: r = 0.13, p = 0.26). After controlling for age, sex, diagnosis, treatment location, and baseline depressive severity, treatment series was not significantly associated with change in QIDS from baseline to treatment #5 (Table 2). Patients with MDD and BPAD had significantly smaller change in QIDS scores than those diagnosed with other psychiatric disorders (MDD: B = −3.61, p = 0.01 and BPAD: B = −4.04, p = 0.01). A greater baseline QIDS score was associated with a smaller change in QIDS score following Treatment #5 (B = −0.47, p = <0.001). The change in two of the nine QIDS symptom domains (Interest and Suicidal Ideation) were significantly correlated across treatment series (Figure 2).
Figure 1:
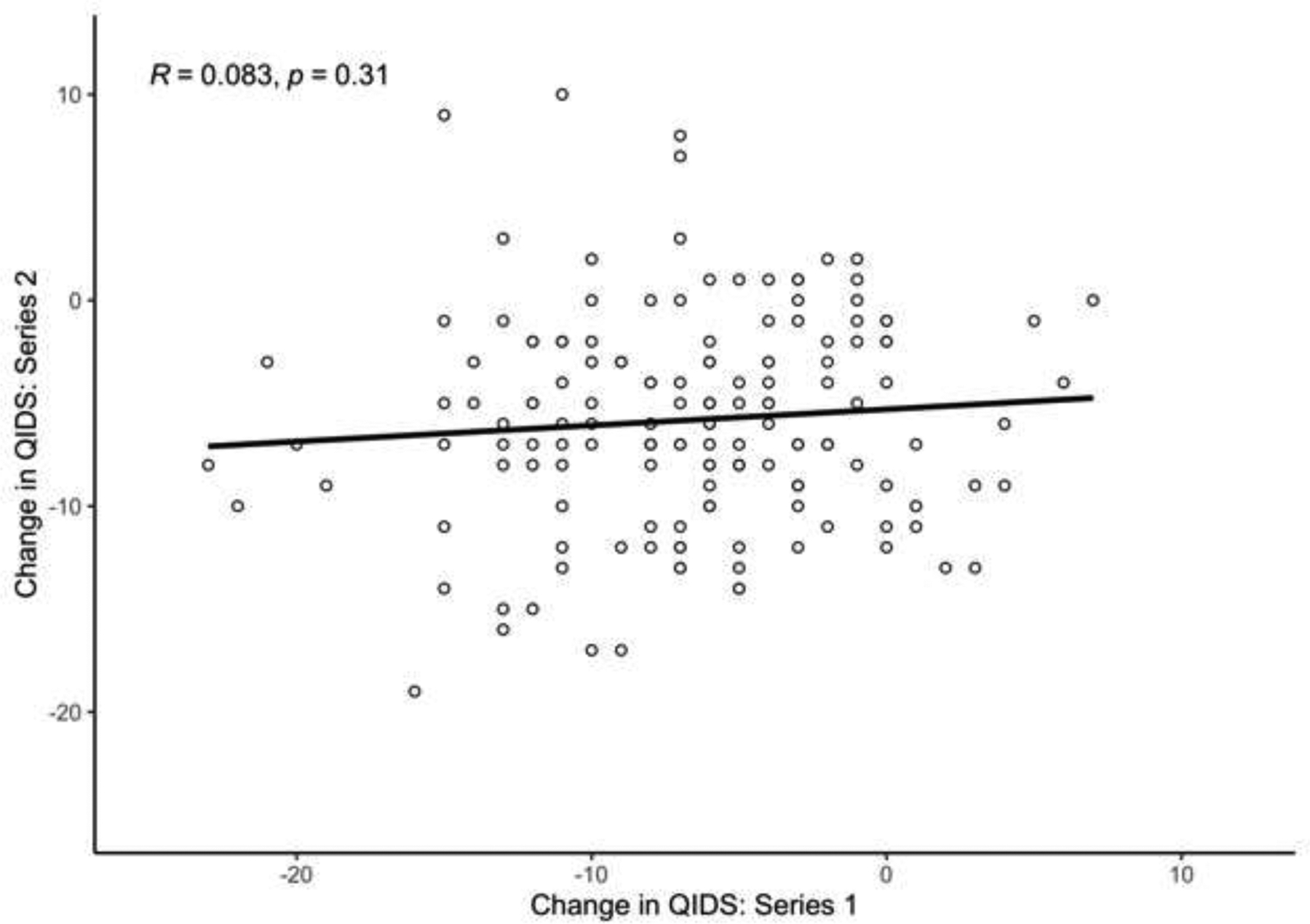
Change in QIDS between baseline and treatment #5 in a first ECT series (X axis) vs. change in QIDS between baseline and treatment #5 in a second ECT series (Y axis).
Table 2:
Linear regression examining effect of demographic/baseline variables on change in QIDS score from baseline to treatment #5
| Beta | t value | P value | |
|---|---|---|---|
| Age | −0.002 | −0.07 | 0.95 |
| Sex | 0.75 | 1.34 | 0.18 |
| Series 2 | 0.40 | 0.74 | 0.46 |
| Diagnosis: MDD | −3.61 | −2.59 | 0.01 * |
| Diagnosis: BPAD | −4.04 | −2.78 | 0.01 * |
| Location: Outpatient | 0.50 | 0.88 | 0.38 |
| Baseline QIDS score | −0.47 | −7.79 | <0.001 * |
p < 0.05
Figure 2:
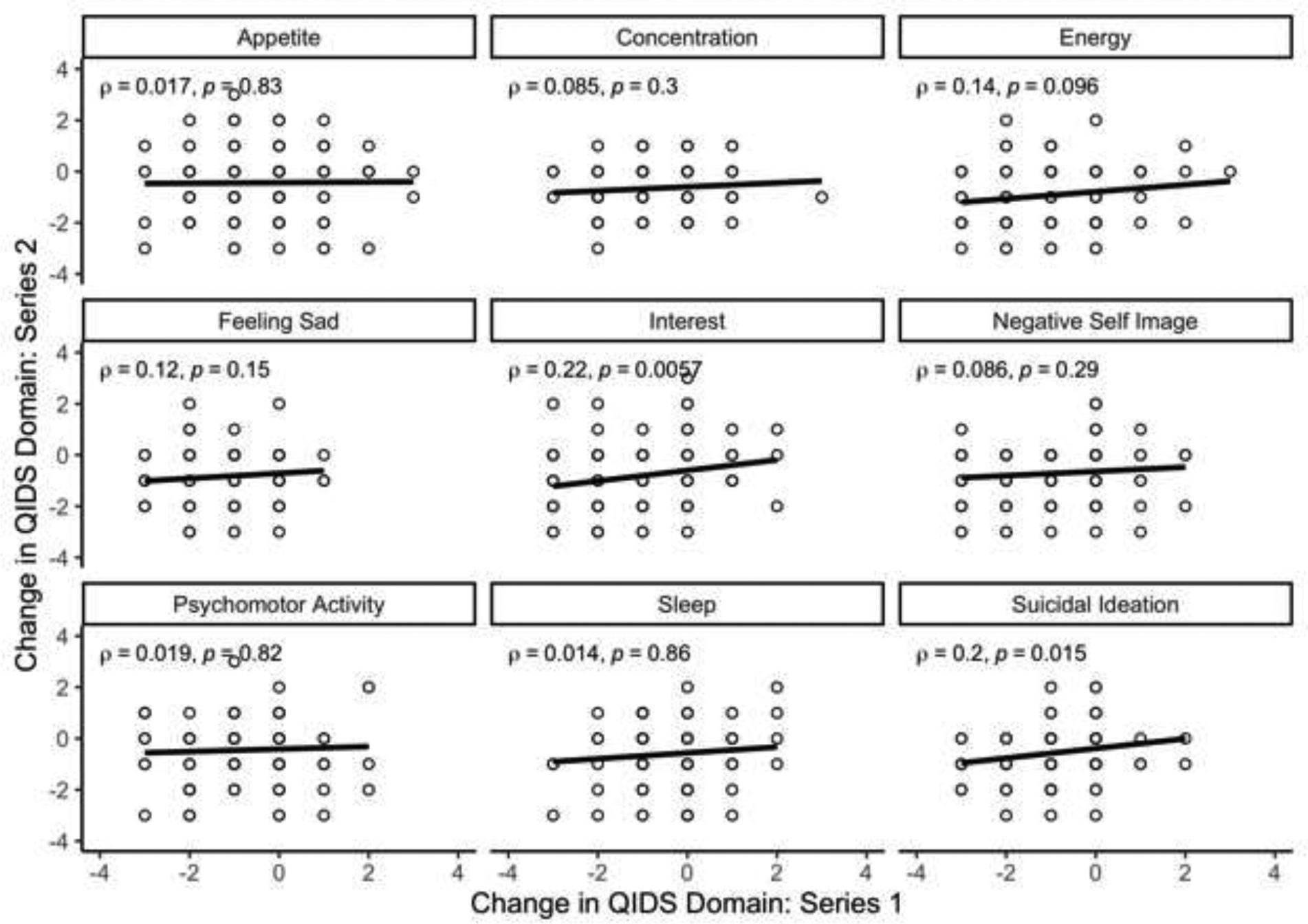
Change in QIDS domain scores between baseline and treatment #5 in a first ECT series (X axis) vs. a second ECT series (Y axis).
Finally, in a logistic model of the odds of continuing in ECT for at least 5 treatments, the adjusted odds of reaching treatment #5 were greater for patients with a higher baseline QIDS scores (OR = 1.05, 95% CI: 1.01 – 1.09; Table 3). The odds of reaching treatment #5 were decreased for patients during their second treatment series (OR = 0.27, 95% CI: 0.17 – 0.41).
Table 3:
Adjusted odds ratios for association between baseline and demographic variables and reaching treatment 5
| Factor | OR | 95% CI |
|---|---|---|
| Age | 1.01 | 1.00 – 1.03 |
| Sex | 1.12 | 0.74 – 1.70 |
| Series 2 | 0.27 | 0.17 – 0.41 |
| Diagnosis: MDD | 2.03 | 0.86 – 4.59 |
| Diagnosis: BPAD | 1.53 | 0.63 – 3.61 |
| Location: Outpatient | 1.40 | 0.91 – 2.15 |
| Baseline QIDS score | 1.05 | 1.01 – 1.09 |
Discussion:
Among 153 patients who had at least 5 ECT treatments in two distinct treatment series occurring a median of 520 days apart, change in depressive symptoms measured by the QIDS was not significantly correlated between ECT series. However, the change in two QIDS subdomains—suicidal ideation and interest—were significantly correlated between treatment series. Looking at aggregate trends, the average baseline QIDS score of the cohort was similar for each of the two series, and likewise in a multivariate model the average change in QIDS score did not vary between treatment series. This is consistent with an overall effectiveness of ECT that does not diminish with repeat treatment. These aggregate trends, however, masked more significant variability among individuals. This is consistent with recent analysis of the cognitive outcomes of ECT which has identified significant inter-individual variability in cognitive trajectories over time (Hebbrecht et al., 2020; Obbels et al., 2021), variability that was masked when looking at group averages.
As was true in aggregate, individual patients likewise entered their two ECT treatment series with similar depression severity, but unlike the group trend, the overall individual response to ECT was not correlated between treatment series. This means that an individual’s above average response to the first treatment series was not correlated with similarly robust response for that person in the subsequent treatment series, and likewise poor initial response to one series was not correlated with poor response in the subsequent series.
Given the well demonstrated efficacy of ECT treatment in a single treatment course, it is reassuring that average treatment response in a subsequent course is similarly high. On an individual level, the lack of predictive value for change in overall depressive symptoms across ECT series is surprising, especially since guidelines include prior treatment response to ECT as an indicator for future treatment with ECT (Tess & Smetana, 2021). This leads us to consider whether the effect of ECT is state or trait dependent. Traits are behavioral or biological markers that are the result of the underlying pathophysiology of the disease or genetic predisposition, while state markers refers to the more transient clinical manifestations of illness (Chen et al., 2006; Saccuzzo & Braff, 1986, p.). If ECT was addressing a trait issue, we would expect that an individual patient would demonstrate a consistent response across ECT series as the ECT works to correct for the underlying abnormality. However, the variable response to ECT demonstrated in this study may suggest that a patient’s response to ECT is more dependent on a patient’s state than on an underlying trait.
These results do, however, suggest that an improvement in the suicidal ideation and interest domains on the QIDS may be consistent across treatment series. This finding is consistent with prior work demonstrating an acute decrease in suicidal ideation after ECT treatment (Kellner, Li, et al., 2016; Rönnqvist et al., 2021). This finding also emphasizes the importance of assessing the response of individual depressive symptom domains in addition to overall change in depressive symptoms, as ECT may selectively improve certain domains. If replicated, these findings suggest the need for additional research on predicting which patients are most likely to benefit from an ECT course. This study suggests that individual patient characteristics that may be associated with a favorable or poor response to ECT in one disease episode may not necessarily persist in subsequent episodes. The mechanism of ECT’s antidepressant effects remains unknown, and patients who fail to respond to one type of ECT may nonetheless respond to further treatments during the same mood episode (Sackeim et al., 2020). Likewise, this study suggests that a poor response to ECT in a prior episode may not portend a subsequent poor response if attempted at a later date.
Limitations:
While real world evidence, as presented in this study, offers complementary evidence to that provided by prospective research studies, the observational nature of these designs and the processes for gathering the clinical data on which they are based produce a range of important limitations (Eichler et al., 2021; Kim et al., 2018; Nazha et al., 2021; Sherman et al., 2016). In this study, for instance, diagnoses are clinical in nature and not based on structured interview, which reflects usual clinical practice but hinders comparisons to prospective studies. Furthermore, outcomes on the QIDS are self-reported. While clinician-rated and self-reported versions of the QIDS have been shown to track well in patients with depression (Bernstein et al., 2007; Rush et al., 2006), this has not been studied in the ECT population and so we are unable to assess whether outcomes would be similar using a different depression rating scale. Additionally, patients in the sample were treated with heterogeneous ECT parameters and doses, which were not standardized according to a prespecified formula, so we are unable to assess for a potential difference between ECT montages and dosing regimens. Likewise due to the method of data collection (with scales measured every 5th treatment) we are unable to report metrics including anesthetic, anesthetic does, ECT electrode placement, total dose, or seizure duration that are frequently measured in prospective studies.
Beyond these more general limitations of retrospective cohort studies, there are several limitations more specific to the current study. Definitionally patients are only included in this study if they accessed ECT on at least two occasions. There may be multiple socioeconomic factors that influence a patient’s ability to return for a second series of ECT treatments. Prior work has demonstrated geographic and racial disparities in access to and utilization of ECT within the United States and striking differences in ECT utilization rates around the world (Leiknes et al., 2012; Luccarelli, Henry, et al., 2020). In addition to geographic differences in the availability of ECT, prior work has also demonstrated variability in the societal / clinician beliefs about ECT as well as variations in the dosing and administration parameters of ECT treatments despite international guidelines (Leiknes et al., 2012). Future work is needed to examine how these factors may influence a patient’s access to or utilization of a second series of ECT treatments.
Additionally, since ECT treatment often is limited to those individuals that have not responded to other interventions, patients returning for a second course of ECT may represent a particularly refractory sub-population. Prior work indicates that ECT is generally underutilized but those individuals that do receive ECT have a higher number psychiatric comorbidities, substance use disorders, and increased care utilization (Wilkinson et al., 2018). While we are unable to compare our sample to other members of our cohort who did not return for a second series of ECT, our data does suggest that a higher baseline QIDS score was associated with a smaller improvement in QIDS score at treatment #5 and a greater likelihood of reaching treatment #5 within a series. This does suggest that our sample may be skewed towards those with more severe symptomatology. At the same time, patients who were too ill to complete baseline symptom severity scales were excluded, so results may not be generalizable to the most severely ill individuals.
Patients were also more likely to reach their 5th treatment in their first series of ECT than their second series of ECT. It may be that patients demonstrated an improvement in their first few treatments and dropped out before their 5th treatment. Alternatively, this may represent a population that found ECT to be intolerable or did not see an improvement in their symptoms and were unable or unwilling to continue with the series. Since this was a retrospective examination of data collected as part of clinical care, and symptom severity scores were only routinely collected after the 5th treatment, it is impossible to distinguish between these, or alternative, explanations for the increased rates of attrition within the second ECT series. Likewise, we are unable to assess whether individual response over a larger number of treatments may show greater correlation.
Finally, since the medication regimens for these patients were managed by their independent providers, we are unable to assess the impact of pharmacological dosing or changes to their treatment regimens on these results. Furthermore, we are unable to control for possible changes in psychotherapy provided during treatment courses.
Conclusion:
Among 153 patients who had at least 5 ECT treatments in two distinct treatment series, individual change in depressive symptoms during a first series of ECT treatments was not correlated with change in symptom severity during a second ECT series. On aggregate, however, ECT response was equal in both treatment series. Future work is needed to replicate this finding and to identify factors that predict longitudinal ECT response.
Supplementary Material
Highlights.
This study presents 286 patients each of who received two independent series of ECT treatments
On average, treatment response was equal in the two treatment courses
For individual patients, response to ECT was not correlated between treatment courses
Funding
This work was supported by the National Institute of Mental Health (R25MH094612, JL; R01MH120991, THM; 5R01MH112737-03, MEH) The sponsors had no role in study design, writing of the report, or data collection, analysis, or interpretation.
Conflicts of Interest
THM receives research funding from the Stanley Center at the Broad Institute, the Brain and Behavior Research Foundation, National Institute of Mental Health, National Human Genome Research Institute Home, and Telefonica Alfa. The remaining authors have no disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Anderson IM, Blamire A, Branton T, Clark R, Downey D, Dunn G, Easton A, Elliott R, Elwell C, Hayden K, Holland F, Karim S, Loo C, Lowe J, Nair R, Oakley T, Prakash A, Sharma PK, Williams SR, … Williamson A (2017). Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine-ECT): A multicentre, double-blind, randomised, parallel-group, superiority trial. The Lancet Psychiatry, 4(5), 365–377. 10.1016/S2215-0366(17)30077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Hawken ER, Sepehry AA, Cabrera CA, & Vazquez G (2019). ECT beyond unipolar major depression: Systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatrica Scandinavica, 139(3), 214–226. 10.1111/acps.12994 [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Rush AJ, Carmody TJ, Woo A, & Trivedi MH (2007). Clinical vs. Self-report versions of the quick inventory of depressive symptomatology in a public sector sample. Journal of Psychiatric Research, 41(3–4), 239–246. 10.1016/j.jpsychires.2006.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cinnamon Bidwell L, & Norton D (2006). Trait vs. State Markers for Schizophrenia: Identification and Characterization Through Visual Processes. Current Psychiatry Reviews, 2(4), 431–438. 10.2174/157340006778699729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx B, Heijnen WT, van den Broek WW, & Birkenhäger TK (2012). Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: A meta-analysis. Bipolar Disorders, 14(2), 146–150. 10.1111/j.1399-5618.2012.00997.x [DOI] [PubMed] [Google Scholar]
- Eichler H-G, Pignatti F, Schwarzer-Daum B, Hidalgo-Simon A, Eichler I, Arlett P, Humphreys A, Vamvakas S, Brun N, & Rasi G (2021). Randomized Controlled Trials Versus Real World Evidence: Neither Magic Nor Myth. Clinical Pharmacology & Therapeutics, 109(5), 1212–1218. 10.1002/cpt.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eranti S, Mogg A, Pluck G, Landau S, Purvis R, Brown RG, Howard R, Knapp M, Philpot M, Rabe-Hesketh S, Romeo R, Rothwell J, Edwards D, & McLoughlin DM (2007). A Randomized, Controlled Trial With 6-Month Follow-Up of Repetitive Transcranial Magnetic Stimulation and Electroconvulsive Therapy for Severe Depression. American Journal of Psychiatry, 164(1), 73–81. 10.1176/ajp.2007.164.1.73 [DOI] [PubMed] [Google Scholar]
- Hebbrecht K, Giltay EJ, Birkenhäger TK, Sabbe B, Verwijk E, Obbels J, Roelant E, Schrijvers D, & Van Diermen L (2020). Cognitive change after electroconvulsive therapy in mood disorders measured with the Montreal Cognitive Assessment. Acta Psychiatrica Scandinavica, 142(5), 413–422. 10.1111/acps.13231 [DOI] [PubMed] [Google Scholar]
- Hermida AP, Glass OM, Shafi H, & McDonald WM (2018). Electroconvulsive Therapy in Depression. Psychiatric Clinics of North America, 41(3), 341–353. 10.1016/j.psc.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Itagaki K, Takebayashi M, Shibasaki C, Kajitani N, Abe H, Okada-Tsuchioka M, & Yamawaki S (2017). Factors associated with relapse after a response to electroconvulsive therapy in unipolar versus bipolar depression. Journal of Affective Disorders, 208, 113–119. 10.1016/j.jad.2016.08.047 [DOI] [PubMed] [Google Scholar]
- Jelovac A, Kolshus E, & McLoughlin DM (2013). Relapse Following Successful Electroconvulsive Therapy for Major Depression: A Meta-Analysis. Neuropsychopharmacology, 38(12), 2467–2474. 10.1038/npp.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, Young RC, Sampson S, McClintock SM, Mueller M, Prudic J, Greenberg RM, Weiner RD, Bailine SH, Rosenquist PB, Raza A, Kaliora S, Latoussakis V, Tobias KG, … CORE/PRIDE Work Group. (2016). A Novel Strategy for Continuation ECT in Geriatric Depression: Phase 2 of the PRIDE Study. The American Journal of Psychiatry, 173(11), 1110–1118. 10.1176/appi.ajp.2016.16010118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, Mueller M, Bernstein HJ, O’Connor K, Smith G, Biggs M, Bailine SH, Malur C, Yim E, McClintock S, Sampson S, & Fink M (2006). Continuation Electroconvulsive Therapy vs Pharmacotherapy for Relapse Prevention in Major Depression: A Multisite Study From the Consortium for Research in Electroconvulsive Therapy (CORE). Archives of General Psychiatry, 63(12), 1337–1344. 10.1001/archpsyc.63.12.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Li EH, Farber KG, Geduldig ET, & Ahle GM (2016). Electroconvulsive Therapy (ECT) and Suicide Prevention. Current Treatment Options in Psychiatry, 3(1), 73–81. 10.1007/s40501-016-0067-8 [DOI] [Google Scholar]
- Kim H-S, Lee S, & Kim JH (2018). Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. Journal of Korean Medical Science, 33(34), e213. 10.3346/jkms.2018.33.e213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiknes KA, Schweder LJ, & Høie B (2012). Contemporary use and practice of electroconvulsive therapy worldwide. Brain and Behavior, 2(3), 283–344. 10.1002/brb3.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, Henry ME, & McCoy TH (2020). Demographics of Patients Receiving Electroconvulsive Therapy Based on State-Mandated Reporting Data. The Journal of ECT, 36(4), 229–233. 10.1097/YCT.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy THJ, Seiner SJ, & Henry ME (2021). Total Charge Required to Induce a Seizure in a Retrospective Cohort of Patients Undergoing Dose Titration of Right Unilateral Ultrabrief Pulse Electroconvulsive Therapy. The Journal of ECT, 37(1), 40–45. 10.1097/YCT.0000000000000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy THJ, Shannon AP, Forester BP, Seiner SJ, & Henry ME (2021a). Duration of Treatment in Electroconvulsive Therapy Among Patients Beginning With Acute Course Right Unilateral Brief Pulse Stimuli. The Journal of ECT, Publish Ahead of Print. 10.1097/YCT.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Seiner SJ, & Henry ME (2020a). Maintenance ECT is associated with sustained improvement in depression symptoms without adverse cognitive effects in a retrospective cohort of 100 patients each receiving 50 or more ECT treatments. Journal of Affective Disorders, 271, 109–114. 10.1016/j.jad.2020.03.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Seiner SJ, & Henry ME (2020b). Charge required to induce a seizure during initial dose titration using right unilateral brief pulse electroconvulsive therapy. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 13(6), 1504–1506. 10.1016/j.brs.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Shannon AP, Forester BP, Seiner SJ, & Henry ME (2021b). Rate of continuing acute course treatment using right unilateral ultrabrief pulse electroconvulsive therapy at a large academic medical center. European Archives of Psychiatry and Clinical Neuroscience, 271(1), 191–197. 10.1007/s00406-020-01202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazha B, Yang JC-H, & Owonikoko TK (2021). Benefits and limitations of real-world evidence: Lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncology, 17(8), 965–977. 10.2217/fon-2020-0951 [DOI] [PubMed] [Google Scholar]
- Obbels J, Vansteelandt K, Bouckaert F, Dols A, Stek M, Verwijk E, & Sienaert P (2021). Neurocognitive functioning after electroconvulsive therapy in late-life depression: A 4-year prospective study: a four-year prospective study. Acta Psychiatrica Scandinavica, 143(2), 141–150. 10.1111/acps.13252 [DOI] [PubMed] [Google Scholar]
- Prudic J, Olfson M, Marcus SC, Fuller RB, & Sackeim HA (2004). Effectiveness of electroconvulsive therapy in community settings. Biological Psychiatry, 55(3), 301–312. 10.1016/j.biopsych.2003.09.015 [DOI] [PubMed] [Google Scholar]
- Rönnqvist I, Nilsson FK, & Nordenskjöld A (2021). Electroconvulsive Therapy and the Risk of Suicide in Hospitalized Patients With Major Depressive Disorder. JAMA Network Open, 4(7), e2116589. 10.1001/jamanetworkopen.2021.16589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Ibrahim HM, Trivedi MH, Biggs MM, Shores-Wilson K, Crismon ML, Toprac MG, & Kashner TM (2006). Comparison of self-report and clinician ratings on two inventories of depressive symptomatology. Psychiatric Services (Washington, D.C.), 57(6), 829–837. 10.1176/ps.2006.57.6.829 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, & Keller MB (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, & Braff DL (1986). Information-processing Abnormalities: Trait- and State-dependent Components. Schizophrenia Bulletin, 12(3), 447–459. 10.1093/schbul/12.3.447 [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Nobler MS, Haskett RF, Mulsant BH, Rosenquist PB, & McCall WV (2020). The benefits and costs of changing treatment technique in electroconvulsive therapy due to insufficient improvement of a major depressive episode. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 13(5), 1284–1295. 10.1016/j.brs.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, Shuren J, Temple R, Woodcock J, Yue LQ, & Califf RM (2016). Real-World Evidence—What Is It and What Can It Tell Us? The New England Journal of Medicine, 375(23), 2293–2297. 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- Slade EP, Jahn DR, Regenold WT, & Case BG (2017). Association of Electroconvulsive Therapy With Psychiatric Readmissions in US Hospitals. JAMA Psychiatry, 74(8), 798. 10.1001/jamapsychiatry.2017.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tess A, & Smetana GW (2021, April 2). Medical consultation for electroconvulsive therapy. UpToDate. https://www.uptodate.com/contents/medical-consultation-for-electroconvulsive-therapy [Google Scholar]
- van den Broek WW, Birkenhäger TK, Mulder PGH, Bruijn JA, & Moleman P (2006). Imipramine Is Effective in Preventing Relapse in Electroconvulsive Therapy-Responsive Depressed Inpatients With Prior Pharmacotherapy Treatment Failure: A Randomized, Placebo-Controlled Trial. The Journal of Clinical Psychiatry, 67(02), 263–268. 10.4088/JCP.v67n0213 [DOI] [PubMed] [Google Scholar]
- Wijkstra J, Nolen WA, Algra A, Van Vliet IM, & Kahn RS (2000). Relapse prevention in major depressive disorder after successful ECT: A literature review and a naturalistic case series: Relapse prevention in MDD after ECT. Acta Psychiatrica Scandinavica, 102(6), 454–460. 10.1034/j.1600-0447.2000.102006454.x [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Agbese E, Leslie DL, & Rosenheck RA (2018). Identifying Recipients of Electroconvulsive Therapy: Data From Privately Insured Americans. Psychiatric Services, 69(5), 542–548. 10.1176/appi.ps.201700364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz A, Mantar A, Simsek S, Onur E, Gökmen N, & Fidaner H (2010). Combination of Pharmacotherapy With Electroconvulsive Therapy in Prevention of Depressive Relapse: A Pilot Controlled Trial. The Journal of ECT, 26(2), 104–110. 10.1097/YCT.0b013e3181c189f7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


