Abstract
Background
At the start of the COVID-19 pandemic, HIV experts suggested that an increase in mental health diagnoses and substance use among people living with HIV (PLHIV) may be an unintended consequence of COVID-19 mitigation efforts (e.g., limiting social contact). We evaluated short-term trajectories in binge drinking, marijuana, and recreational drug use in a prospective cohort of PLHIV.
Methods
Data (N = 2121 PLHIV) consist of survey responses on substance use behaviors from two pre-COVID-19 (October 2018-September 2019) and one COVID-19-era (April 2020-September 2020) timepoints within the MACS/WIHS Combined Cohort Study (MWCCS). We conducted group-based trajectory models, triangulated with generalized linear mixed models, to assess changes in binge drinking, daily marijuana use, and recreational drug use at the start of the pandemic. Controlling for age and race/ethnicity, we tested whether trajectories differed by sex and early-pandemic depressive symptoms, loneliness, and social support.
Results
Group-based trajectory models yielded two trajectory groups for binge drinking (none vs. any), marijuana (none/infrequent vs. daily), and recreational drug use (none vs. any). Binge drinking and recreational drug use decreased at the beginning of the pandemic. Generalized linear mixed model supported these trends. Consistent with prior research, male sex and having depressive symptoms early pandemic were positively associated with each substance use outcomes. Social support was inversely associated with recreational drug use.
Conclusions
Contrary to hypotheses, problematic substance use behaviors decreased from pre-pandemic to the post-pandemic follow-up in our sample of PLHIV. Ongoing surveillance is needed to assess whether this pattern persists as the pandemic continues.
Keywords: HIV, Substance use, Longitudinal, COVID-19
1. Introduction
Problematic substance use trajectories among people living with HIV (PLHIV) during the COVID-19 pandemic are poorly understood (Starks et al., 2020, Wang et al., 2021). Addressing this omission is critical for several reasons. The onset of the COVID-19 pandemic in the United States (March 2020) drastically disrupted people’s daily routines, livelihoods, and outlets for social participation (Kumar, 2020). Recommended transmission mitigation strategies, like limiting social contact and physical distancing, required people to adapt their personal and professional lives to comply with support infection control mandates (Rauschenberg et al., 2021). Although critical for mitigating the spread of COVID-19, these strategies have also adversely affected the general public’s mental health (Diaz-Martinez et al., 2021). Researchers have suggested that the social circumstances arising from the COVID-19 pandemic may have contributed to increases in psychosocial stress (e.g., depressive symptoms, loneliness), problematic alcohol and recreational drug use, and limited access to mental health support, harm reduction, and substance use treatment (Carrico et al., 2020; Diaz-Martinez et al., 2021; Hochstatter et al., 2021; Karamouzian et al., 2020; Marziali et al., 2020; Núñez et al., 2021). These predictions warrant prioritized attention for PLHIV given this population’s disproportionate vulnerability to adverse outcomes, even in pre-pandemic times (Hochstatter et al., 2021, Wang et al., 2021).
Many studies have investigated problematic alcohol and recreational drug use trajectories in PLHIV, particularly in the contexts of stress, coping, and sexual risk-taking (Glynn et al., 2019, Mimiaga et al., 2013, Moitra et al., 2020, Pence et al., 2008, Rosen et al., 2017, Wardell et al., 2018). Prior studies have found that problematic alcohol consumption and recreational drug use commonly co-occur with other adverse psychosocial conditions, including depressive symptoms, loneliness, and inadequate social support (Javanbakht et al., 2020, Mannes et al., 2016, Rubtsova et al., 2017, Siconolfi et al., 2013, Valdes et al., 2021). Problematic alcohol, heavy marijuana, and recreational drug use are linked to suboptimal HIV outcomes and quality of life, including sexual risk-taking, impaired cognitive function, non-adherence to HIV care recommendations, weakened immunity, and premature mortality (American Addiction Centers, 2021, Bensley et al., 2018, Brown et al., 2019, Duko et al., 2019, Millar et al., 2017). Yet, few studies have investigated whether problematic alcohol, marijuana, and recreational drug use have changed since the COVID-19 pandemic in PLHIV.
Our study’s objective was to compare the short-term trajectories in binge drinking, marijuana, and recreational drug use spanning pre-COVID-19 pandemic to early pandemic timepoints among a prospective cohort of PLHIV. We hypothesized that substance use would increase during the pandemic. Additionally, we hypothesized that depressive symptoms and loneliness would be linked to increased substance use behaviors and social support would be associated with decreased substance use behaviors. We argue that psychosocial conditions and a lack of social support at the start of the pandemic may serve as proxies for prevalent adversity in participants’ lives and their potential to avoid negative trajectories.
2. Methods
2.1. MACS/WIHS Combined Cohort Study (MWCCS)
The MWCCS merged two prospective observational cohort studies, the Multicenter AIDS Cohort Study (MACS) and the Women’s Interagency HIV Study (WIHS) (D’Souza et al., 2021). Before they were combined, the MACS and the WIHS were the two longest-running epidemiologic HIV studies in the US designed to characterize the natural and treatment trajectories of the epidemic. Re-enrollment was ongoing at the time of this analysis, but together Combined, the two cohorts have 4016 active participants targeted for MWCCS enrollment. Most participants are between 40 and 69 years old (79%) and nearly two-thirds are people of color (65%).
Since the MACS (1984) and WIHS (1994) began, participants attend semi-annual clinic visits for a series of physiological examinations and behavioral health surveys (Adimora et al., 2018, Egan et al., 2021; Meanley et al., 2020). In early 2020, a phone-based interview was developed to collect data on COVID-related health and substance use (D’Souza et al., 2021), and was administered at both MACS (Baltimore, MD/Washington, DC, Chicago, IL, Los Angeles, CA, and Pittsburgh, PA/Columbus, OH) and WIHS sites (Atlanta, GA, the Birmingham, AL-Jackson, MS corridor, Chapel Hill, NC, Chicago, IL, Los Angeles, CA, Miami, FL, New York, NY, San Francisco, CA, and Washington, DC). All study procedures were approved by each site’s Institutional Review Boards. Data collection instruments are available at https://statepi.jhsph.edu/mwccs/data-collection-forms/.
2.2. Study sample
Active MACS and WIHS participants were recruited for the study, irrespective of whether they had HIV (D’Souza et al., 2021). However, the current analysis included 2121 (of 2492, 85.1%) PLWHIV who completed the phone-based COVID visit (April–September 2020) and were interviewed at the two most recent visits prior to the COVID-19 pandemic (October 2018–March 2019; April–September 2019).
2.3. Measures
Table 1 provides a timeline of when primary independent and dependent variables were collected.
Table 1.
Constructs measured by data collection wave.
| Pre-COVID |
COVID |
|||
|---|---|---|---|---|
| Time 1 |
Time 2 |
Time 3 |
||
| Constructs | October 2018-March 2019 | April-September 2019 | April-June 2020 | July-September 2020 |
| Substance use outcome | ||||
| Binge Alcohol consumption | X | X | – | X |
| Daily Marijuana use | X | X | – | X |
| Non-prescription drug use | X | X | – | X |
| Primary independent variables | ||||
| Depressive symptoms | – | – | X | – |
| Loneliness | – | – | X | – |
| Functional social Support | – | – | X | – |
Note. X – Time when data were collected for corresponding variable; - Time when data were not collected for corresponding variable.
2.3.1. Substance use outcomes
2.3.1.1. Alcohol use
Participants indicated whether they had consumed any alcoholic beverage since their last study visit. Those with affirmative responses reported the frequency with which they consumed at least five (women) or six (men) alcoholic beverages in one sitting (never, less than monthly, monthly, weekly, daily, or almost daily). Aligned with prior research (Crane et al., 2021), we dichotomized responses to indicate 0 = no binge drinking and 1 = any binge drinking.
2.3.1.2. Drug use
Participants answered one question about the frequency with which they used pot, marijuana, or hash since their last visit (never, less often, monthly, weekly, or daily). In prior research, daily marijuana, irrespective of within-day frequency, was associated with missed clinical visits among PLHIV (Kipp et al., 2017). We dichotomized marijuana as 0 = non-user or non-daily user and 1 = daily user. Participants responded to multiple single-item questions on whether they had consumed crack or cocaine (i.e., smoked, snorted, swallowed, injected), taken speed, meth, or ice, and/or taken heroin since their last visit. We investigated these substances based on previously observed associations with poor HIV care-related outcomes (Baum et al., 2009, Fulcher et al., 2021, Gwadz et al., 2016). Items were summed and recoded as 0 = no recreational use and 1 = any recreational use.
2.3.2. Primary independent variables
Depressive symptoms were measured using the 10-item Center for Epidemiological Studies Depression Scale (CES-D 10; Cronbach’s alpha = 0.76) and dichotomized according to clinical cutoffs for significant symptomatology (0 = CES-D 10 < 10, 1 = CES-D 10 > 10; Zhang et al., 2012). Loneliness scores were calculated from the 3-item Loneliness Brief Form scale (e.g., “How often do you feel isolated from others?” Cronbach’s alpha = 0.78; Ballivian et al., 2020). Items were rated as 0 = Hardly ever, 1 = Some of the time, 2 = Often. Participants’ responses were summed: higher scores indicated higher levels of loneliness. Functional social support was measured using a single item that was previously assessed with the MACS cohort (e.g., “Is there someone you can talk to about things that are important to you – someone you can count on for understanding or support?” Friedman et al., 2017). Participants reported 0 = No one, 1 = One person, 2 = 2–3 people, 3 = 4–5 people, 4 = 6 or more people.
2.3.3. Covariates
Participants self-reported their age, race/ethnicity, and educational attainment. Race/ethnicity options included non-Hispanic White, Hispanic White, non-Hispanic Black, Hispanic Black, American Indian or Alaskan Native, Asian or Pacific Islander, non-Hispanic Other, and Hispanic Other. Because some cells were small, we collapsed the race variable into 4 groups: 0 = non-Hispanic White, 1 = non-Hispanic Black, 2 = Hispanic all races, 3 – non-Hispanic other. Educational attainment was dichotomized as 0 = High school degree or less and 1 = Any college. Lastly, assigned sex at birth was determined by participants’ ID numbers linked to the MACS (0 = male) or the WIHS (1 = female).
2.4. Statistical analyses
We developed group-based trajectory models in SAS 9.4 (SAS Institute Inc, 2013) to identify clusters of individuals who shared similar trajectories in binge drinking, daily marijuana use, and recreational drug use over time. We used the multivariable mixture modeling macro, PROC TRAJ, which employs maximum likelihood to estimate model parameters and handles missing values (Jones et al., 2001). We tested one- to five-group quadratic trajectory models to find a best-fitting model. Following these iterative processes, we evaluated improved model fit based on Bayesian Information Criterion (BIC), the size of each group, and the probability of membership in a specific group; specifically lower BIC values, > 5% of the sample in each group, and an average probability of ≥ 0.70 for participants in each trajectory group (Nagin, 2005). After finding the best-fit number of group trajectories, we adjusted each trajectory for the best shape (i.e., intercept only, linear, quadratic, or cubic). We iteratively compared models with increasing numbers of groups, omitting and adding parameters based on BIC values to assess improvements in goodness-of-fit.
Based on the group-based trajectory analyses, we derived a categorical variable representing trajectory group membership for each substance use behavior to test their associations with other co-factors. Fisher’s exact tests were used for categorical variables and student’s t-tests for continuous variables. We tested bivariate and multivariate logistic regression models to estimate the association between trajectory group membership and co-factors. Finally, we tested generalized linear mixed models (PROC GLIMMIX; Zhu, 2014) using repeated measures for each substance use outcome to further confirm our results. We did not assume any structured error correlations due to limited timepoints; therefore, we modeled our data using unstructured correlation matrices. We included time along with co-factors used in the group-based trajectory models as independent variables. We also tested an interaction of sex assigned at birth by time to account for potential cohort effects. We allowed only the intercept to vary between subjects and the regression slopes as fixed.
3. Results
3.1. Sample characteristics
Participant characteristics are described in Table 2. Participants had a mean age of 53.5 years (SD = 10.1), were predominantly people of color (73.6%), assigned female sex at birth (62.3%), and completed some college (50.8%). On average, participants reported moderate levels of COVID-era functional social support (m = 2.2, standard deviation [sd] = 1.1; range: 0 – 4) and low levels of loneliness (m = 1.6, sd = 1.8; range: 0 – 6); 40% reported significant depressive symptoms. Across all timepoints, substance use prevalence ranged from 14.0% to 20.4% for binge drinking, 10.5–11.9% for daily marijuana use, and 5.4–8.0% for recreational drug use. Two-group trajectory solutions allowing only the intercept for the first trajectory and the linear and quadratic terms for the second trajectory were selected for all three substance use behaviors (lowest BIC’s [BIC = −2322.3; −1368.1; −1143.84, respectively], with at least 9% of the sample in the smallest group, and the average probability of group membership exceeding 0.91). The results of the two-group solutions are shown in Supplemental Table 1 and Fig. 1.
Table 2.
Characteristics of participants in the MWCCS living with HIV (n = 2121).
| Mean (SD) | N (%) | |
|---|---|---|
| Age in years (n = 2121) | 53.52 (10.13) | |
| Race/Ethnicity (n = 2002) | ||
| Non-Hispanic White | 528 (26.4%) | |
| Non-Hispanic Black | 1124 (56.1%) | |
| Hispanic All Races | 297 (14.8%) | |
| Non-Hispanic Other | 53 (2.7%) | |
| Sex assigned at birth (n = 2121) | ||
| Male | 800 (37.7%) | |
| Female | 1321 (62.3%) | |
| Education (n = 2002) | ||
| High School or less | 985 (49.2%) | |
| Any College | 1017 (50.8%) | |
| Binge alcohol use | ||
| Time 1 (n = 1950) | 397 (20.4%) | |
| Time 2 (n = 1932) | 402 (20.8%) | |
| Time 3 (n = 1803) | 252 (14.0%) | |
| Daily marijuana use | ||
| Time 1 (n = 1947) | 211 (10.8%) | |
| Time 2 (n = 1929) | 229 (11.9%) | |
| Time 3 (n = 1804) | 190 (10.5%) | |
| Non-prescription drug use | ||
| Time 1 (n = 1953) | 175 (8.0%) | |
| Time 2 (n = 1931) | 172 (8.9%) | |
| Time 3 (n = 1804) | 98 (5.4%) | |
| Depressive symptoms (n = 1971) | ||
| CESD 10 < 10 | 1198 (60.8%) | |
| CESD 10 ≥ 10 | 773 (39.2%) | |
| Functional social support (n = 1971), range: 0 – 4 | 2.22 (1.10) | |
| Loneliness (n = 1971), range: 0 – 6 | 1.61 (1.82) |
Note. Abbreviations: MWCCS: MACS/WIHS Combined Cohort Study; HIV = Human Immunodeficiency Virus; CESD: Center for Epidemiological Studies – Depression.
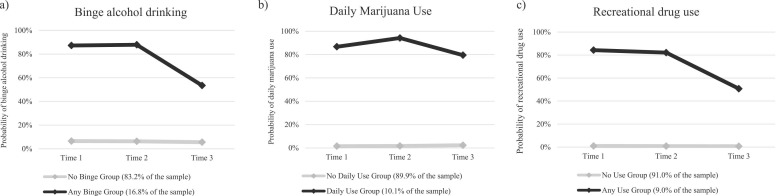
Fig. 1.
Probabilities of alcohol and substance use at two visits pre-COVID (Time 1 and Time 2) and during COVID (Time 3) by group trajectories.
3.2. Binge drinking
3.2.1. Group-based trajectory model
The first group accounted for most of the sample (83.2%) and was characterized as ‘no binge drinking.’ Across the three timepoints, there were no significant changes in the probability of being in the binge drinking group (range 5.5–6.5%) among this ‘no binge drinking’ group; therefore, only the intercept was included (b 0 [SE] = −2.73 [0.09]; p < 0.001) in the final model. The second group (16.8%) was characterized as ‘any binge drinking.’ In this group, the probability of binge drinking was high and similar between time 1 (87.3%) to time 2 (87.9%). The probability of binge drinking decreased sharply during the epidemic (from time 2 [87.9%] to time 3 [53.5%]).
3.2.2. Logistic regressions
In bivariate analyses ( Table 3), age was inversely associated with ‘any binge’ group membership. Male sex, Hispanic identity, depressive symptoms, and loneliness were positively associated with being in the ‘any binge drinking’ trajectory group. We observed non-significant associations by education and levels of functional social support. In the multivariable model, male sex (Adjusted Odds Ratio [AOR] = 3.91; CI = 2.88, 5.30; p < 0.001) and depressive symptoms (AOR = 1.45; CI = 1.07, 1.97; p = 0.016) remained associated with being in the ‘any binge drinking’ group after adjusting for age and race/ethnicity.
Table 3.
Logistic regression models characterizing short-term group-based substance use trajectories among people living with HIV in the MWCCS.
| Binge drinking trajectories 0: No binge; 1: Any binge |
Marijuana use trajectories 0: No daily use; 1: Daily use |
Non-prescription drug use trajectories 0: No use; 1: Any use |
||||
|---|---|---|---|---|---|---|
| Variable | OR | AOR | OR | AOR | OR | AOR |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Age (10-year increase) | 0.68*** (0.61, 0.76) |
0.63*** (0.55, 0.72) |
0.74*** (0.64, 0.85) |
0.73*** (0.62, 0.85) |
0.84* (0.72, 0.97) |
0.84* (0.70, 0.99) |
| Race/Ethnicity | ||||||
| Non-Hispanic Black | REF | REF | REF | REF | REF | REF |
| Non-Hispanic White | 1.45** (1.10, 1.92) |
1.01 (0.71, 1.45) |
1.02 (0.73, 1.43) |
0.93 (0.61, 1.42) |
0.92 (0.64, 1.33) |
0.56* (0.36, 0.88) |
| Hispanic All Races | 1.77*** (1.28, 2.45) |
1.20 (0.82, 1.74) |
0.81 (0.51, 1.27) |
0.64* (0.39, 1.03) |
0.83 (0.52, 1.33) |
0.54* (0.32, 0.91) |
| Non-Hispanic Other | 1.15 (0.53, 2.48) |
1.04 (0.46, 2.37) |
2.04* (1.00, 4.17) |
1.55 (0.73, 3.33) |
0.60 (0.18, 1.94) |
0.47 (0.14, 1.59) |
| Sex assigned at birth | ||||||
| Female | REF | REF | REF | REF | REF | REF |
| Male | 2.80*** (2.22, 3.54) |
3.91*** (2.88, 5.30) |
1.38* (1.04, 1.83) |
1.83** (1.28, 2.61) |
2.50*** (1.85, 3.38) |
3.62*** (2.46, 5.33) |
| Education | ||||||
| High School or less | REF | - | REF | - | REF | - |
| Any College | 1.24 (0.98, 1.58) |
- | 0.86 (0.64, 1.14) |
- | 2.12 (0.89, 1.66) |
- |
| Depressive symptoms | ||||||
| CES-D 10 scores < 10 | REF | REF | REF | REF | REF | REF |
| CES-D 10 scores > 10 | 1.46** (1.15, 1.87) |
1.45* (1.07, 1.97) |
1.56** (1.17, 2.09) |
1.47* (1.05, 2.08) |
1.77 *** (1.29, 2.42) |
1.60* (1.09, 2.35) |
| Functional social support† | 0.97 (0.88, 1.09) |
1.03 (0.91, 1.16) |
0.85* (0.74, 0.97) |
0.87ǂ (0.75, 1.00) |
0.81** (0.70, 0.93) |
0.84* (0.72, 0.99) |
| Loneliness† | 1.07* (1.00, 1.14) |
1.01 (0.93, 1.09) |
1.08ǂ (0.99, 1.16) |
0.99 (0.91, 1.09) |
1.17*** (1.08, 1.26) |
1.06 (0.96, 1.17) |
Note 1. Education was excluded in adjusted models.
Note 2. Abbreviations: MWCCS = MACS/WIHS Combined Cohort Study; OR = [unadjusted] odds ratio; AOR = adjusted odds ratio; CI = confidence interval; CES-D = Center for Epidemiological Study – Depression.
Note 3. *** p <.001; ** p <.01; * p <.05; ǂp ≈.05.
Note 4. † Higher scores indicating higher levels of functional social support and loneliness.
3.2.3. Generalized linear mixed model
The prevalence of binge drinking changed over time ( Table 4; p < 0.0001), where the prevalence was lower at time 3 (beta coefficient [b] = −0.30; standard error [SE] = 0.14; p = 0.029) compared to time 1. However, binge drinking at time 2 was not statistically different compared to time 1 (b = −0.10; SE = 0.13; p = 0.447) and at time 3 compared to time 2 (b = −0.20; SE = 0.14; p = 0.153). Like the trajectory group analysis, male sex (p < 0.0001) and depressive symptoms (p = 0.004) were associated with binge drinking after adjusting for age and race/ethnicity. We observed a significant interaction of sex assigned at birth by time (p = 0.0004); specifically, both men and women exhibited a significant decrease in binge drinking at time 3 compared to time 1 but the decrease was larger in men (b = −0.65; SE = 0.21; p = 0.002) than in women (b = −0.30; SE = 0.14; p = 0.029).
Table 4.
Generalized linear mixed models characterizing changes in substance use behaviors among people living with HIV in the MWCCS.
| Binge Drinking |
Daily marijuana use |
Non-prescription drug use |
||||
|---|---|---|---|---|---|---|
| b (SE) | p-value | b (SE) | p-value | b (SE) | p-value | |
| Timeǂ | < 0.0001 | 0.370 | < 0.0001 | |||
| Time 1 | REF | - | REF | - | REF | |
| Time 2 | -0.10 (0.13) | 0.447 | 0.14 (0.13) | 0.276 | -0.06 (0.14) | -0.673 |
| Time 3 | -0.30 (0.14) | 0.029 | -0.04 (0.13) | 0.793 | -0.67 (0.16) | < 0.0001 |
| Age (10-year increase) | -0.46 (0.06) | < 0.0001 | -0.39 (0.08) | < 0.0001 | -0.14 (0.08) | 0.110 |
| Race/Ethnicity | 0.674 | 0.195 | 0.003 | |||
| Non-Hispanic BlackNon-Hispanic WhiteHispanic All RacesNon-Hispanic Other | -0.08 (0.15)REF0.13 (0.16)-0.11 (0.34) | 0.602-0.4010.746 | -0.09 (0.20)REF-0.41 (0.23)0.39 (0.40) | 0.649-0.0710.326 | -0.69 (0.22)REF-0.66 (0.26)-0.96 (0.59) | 0.002-0.0100.104 |
| Sex assigned at birth⸹ | ||||||
| Female | REF | - | REF | - | REF | - |
| Male | 1.20 (0.17) | < 0.0001 | 0.56 (0.17) | 0.001 | 1.33 (0.19) | < 0.0001 |
| Depressive symptoms | ||||||
| CES-D 10 scores < 10 | REF | - | REF | - | REF | - |
| CES-D 10 scores > 10 | 0.36 (0.12) | 0.004 | 0.47 (0.17) | 0.005 | 0.55 (0.19) | 0.004 |
| Functional social support† | 0.01 (0.05) | 0.871 | -0.09 (0.07) | 0.196 | -0.20 (0.08) | 0.010 |
| Loneliness† | 0.001 (0.03) | 0.974 | -0.003 (0.05) | 0.949 | 0.07 (0.05) | 0.163 |
| Intercept | 0.22 (0.33) | 0.507 | -0.67 (0.43) | 0.122 | -2.27 (0.49) | < 0.0001 |
| Interaction⸭ | 0.0004 | |||||
| Sex (Male) * Time 1 | REF | - | - | - | - | - |
| Sex (Male) * Time 2 | 0.17 (0.19) | 0.370 | - | - | - | - |
| Sex (Male) * Time 3 | -0.65 (0.21) | 0.002 | - | - | - | - |
Note 1. As shown in table, the interaction term between sex and time was significant for binge drinking and included in that final model. Interaction term between sex and time was not significant in models of daily marijuana and of non-prescription drug use and was thus excluded in final model for those outcomes.
Note 2. The model with interaction term ( binge drinking model) needs different interpretation for beta coefficients. ǂ the effect of time when sex is female; ⸹ the effect of sex at time 1; ⸭ the additive effect of time when sex is male.
Note 3. For non- prescription drug use, there were statistically significant differences between Time 3 and Time 2.
Note 4. † Higher scores indicating higher levels of functional social support and loneliness.
3.3. Daily marijuana use
3.3.1. Group-based trajectory model
The first group (89.9%) was characterized as non- or infrequent marijuana users (i.e., ‘no daily use’). There were no significant changes in marijuana use across the three timepoints (range 1.5–2.3%). The second group (10.1%) was characterized as ‘daily marijuana users.’ We observed statistically significant linear and quadratic terms (Supplement Table 1), indicating a decreasing trend in marijuana use during the pandemic among the daily use group. Within this group, the probability of daily marijuana use increased pre-COVID-19 (time 1 [86.8%] to time 2 [94.24%]), then decreased at the beginning of the COVID-19 pandemic (time 3 [79.5%]).
3.3.2. Logistic regression models
Bivariate analyses (Table 3) exhibited older age to be inversely associated with being a daily marijuana user. Male sex, non-Hispanic other race, and depressive symptoms were positively associated with being a ‘daily user.’ Functional social support was negatively associated with being in the ‘daily marijuana user’ group. No statistically significant unadjusted associations were observed by educational attainment (p = 0.293). Loneliness approached but did not achieve statistical significance at the bivariate level (p = 0.054). In the multivariable model, male sex (AOR = 1.83; CI = 1.28, 2.61; p = 0.001) and depressive symptoms (AOR = 1.47; CI = 1.05, 2.08; p = 0.027) was positively associated with being in the ‘daily marijuana user’ group after adjusting for age and race/ethnicity. Functional social support closely approached (AOR = 0.87; CI = 0.75, 1.00; p = 0.051), but did not achieve statistical significance with marijuana use trajectories.
3.3.3. Generalized linear mixed models
There was no significant change in marijuana use over time (Table 4; p = 0.370). Like the group-based trajectory analysis, male sex (p = 0.001) and depressive symptoms (p = 0.005) were associated with daily marijuana use, adjusting for age and race/ethnicity. There was no statistically significant interaction between sex assigned at birth and time (p = 0.551).
3.4. Recreational drug use
3.4.1. Group-based trajectory models
The first group accounted for 91.0% of the sample and was classified as ‘no use.’ There was a non-statistically significant linear trend (time 1 =1.5%; time 2 =1.7%; time 3 =2.3%) over time (Supplement Table 1). The second group (9.0%) was characterized as ‘any use.’ Linear and quadratic terms were statistically significant in the final model, indicating a decreased trend in recreational drug use during the pandemic among participants in the any use group.
3.4.2. Logistic regression models
Bivariate analyses (Table 3) indicated that age and functional social support were inversely associated with being in the ‘any user’ group. Male sex, depressive symptoms, and loneliness were positively associated with being in the any recreational drug use trajectory group. There were no statistically significant bivariate associations by race/ethnicity (p = 0.806) or education level (p = 0.228). In the adjusted model, only male sex (AOR = 3.62; CI = 2.46, 5.33; p < 0.001), depressive symptoms (AOR = 1.60; CI = 1.09, 2.35; p = 0.016), and functional social support (AOR = 0.84; CI = 0.72, 0.99; p = 0.033) remained statistically significant with the recreational drug use trajectory group membership after adjusting for age and race/ethnicity.
3.4.3. Generalized linear mixed models
The prevalence of recreational drug use changed over time (Table 4; p < 0.0001). Changes in the prevalence of recreational drug use approached a statistically significant decrease at time 3 compared to time 1 (b = −0.67; SE = 0.16; p < 0.0001) and at time 3 compared to time 2 (b = −0.61; SE = 0.16; p = 0.0002). Recreational drug use was not statistically lower at time 2 compared to time 1 (b = −0.06; SE = 0.14; p = 0.673). Male sex (p < 0.0001), depressive symptoms (p = 0.004), and lower levels of functional social support (p = 0.010) were associated with recreational drug use, adjusting for age and race/ethnicity. The interaction between sex assigned at birth and time was not statistically significant (p = 0.113).
4. Discussion
4.1. Main findings
The current study is among the first to examine short-term problematic alcohol, marijuana, and recreational drug use trajectories during the beginning of the COVID-19 pandemic in a large, prospective cohort of PLHIV in the United States. We observed two-level, short-term, group-based trajectories that distinguished participants as non-binge drinkers versus binge drinkers, non- or infrequent marijuana users versus daily marijuana users, and users versus non-users of recreational drugs. Contrary to expert predictions (Carrico et al., 2020; Hochstatter et al., 2021), binge alcohol drinking and recreational drug use declined from pre-pandemic data collection to early-pandemic follow-up. We observed non-significant decreases in daily marijuana use across timepoints. Though we cannot identify specific factors that inform these trends, findings from research with PLHIV suggest a potential association between perceived susceptibility to SARS-CoV-2 acquisition and symptoms and changes in recreational drug use behaviors (Wang et al., 2021). The pandemic ushered in widespread fear, anxiety, and uncertainty as no one knew how COVID-19 might increase the risk for SARS-CoV-2 acquisition among PLHIV (Lesko and Bengston, 2021). Despite these fears and perceptions, investigating the psychosocial and behavioral strengths that influenced the decline of alcohol and recreational drug use behaviors warrants further scrutiny.
Alternatively, alcohol and recreational drug use may have decreased because of structural COVID-19 mitigation practices implemented nationwide. Local mandates limited social gatherings and shuttered businesses (e.g., restaurants and bars), practices that may have stymied alcohol and recreational drug use, especially among persons who consume substances primarily within social and sexual contexts (Abouk and Heydari, 2021). PLHIV’s adherence levels to COVID-19 mitigation efforts, like social distancing, may better inform changes in substance use behaviors.
When observing between-group differences, depressive symptoms were consistently associated with each substance use trajectory. These findings are consistent with prior studies (Mannes et al., 2021, Kelso-Chichetto et al., 2018). Though researchers have previously linked social support and loneliness to alcohol and substance use behaviors among PLHIV (Mannes et al., 2017, Moitra et al., 2020), our analyses yielded inconsistent findings. In bivariate tests, loneliness levels early in the pandemic were positively associated with binge drinking and substance use trajectories. In multivariable models, functional social support was inversely associated with membership in the ‘any recreational drug use’ trajectory group, reinforcing the health-promotive qualities of social relationships in PLHIV’s lives (Friedman et al., 2017). Our non-significant findings may be attributed to the limited variability of psychosocial factors within our sample. Overall, PLHIV in the MWCCS reported low levels of loneliness and high levels of functional support when the pandemic began. Over 75% reported having two to three people that they can rely on for support. Consistent with prior studies, male sex was positively linked to substance use trajectories, which may reflect sex-related disparities in psychosocial and environmental risk factors (Becker et al., 2017).
4.2. Limitations
Although we examined group-based trajectories in alcohol, marijuana, and recreational drug use, we did not analyze other consequences of COVID-19, such as changes in livelihood, and the impact of the pandemic on loved ones. Therefore, we cannot pinpoint specific stressors or strengths that shaped our participants’ substance use trajectories. Our results may not apply to other samples of men and women living with HIV. MACS and WIHS participants are part of a longstanding HIV survivor cohort, who are predominantly middle-aged, although the mean age of our sample aligns with the statistic that over half of PLHIV in the United States are above the age of 50 (CDC, 2021). In prior studies, pre-COVID substance use trajectories in PLHIV have exhibited age-related differences on specific substances consumed (e.g., greater marijuana use in older cohorts, greater recreational drug use in younger cohorts; Mannes et al., 2019; Noor et al., 2021); therefore, cohorts of younger aged individuals may report different COVID-19 substance use patterns. Our sample also represents people who are largely linked to health care, and accustomed to the requirements involved in cohort study participation (Ware et al., 2019). Furthermore, the right skew distribution of some of our study’s psychosocial variables (e.g., social support and loneliness) may inform inconsistent or different findings observed with more psychosocially diverse samples. Our sample’s low rates of substance use, particularly with recreational drugs, limited our capacity to explore more nuanced ways of looking at group-based trajectories, such as the frequency and specific types of substances that participants used. Furthermore, we were unable to distinguish participants who use marijuana for medical versus recreational purposes; many study sites are in states where HIV seropositivity is an eligible indicator for medical cannabis use. Finally, our study is susceptible to the inherent challenges of conducting survey research, including limitations linked to self-report, subjectivity, social desirability, and the proclivity of participants to under-report behaviors on sensitive topics, like mental health and substance use.
4.3. Future research
Future studies should investigate the ongoing impact of COVID-19 on the psychosocial well-being of PLHIV. This study examined substance use trajectories only in the short-term. Future MWCCS analyses may improve the precision in identifying substance use trajectories among PLHIV, and to assess the long-term psychosocial consequences of the pandemic beyond the three timepoints included in our analysis. Because depressive symptoms, loneliness, and social support variables were collected at the start of COVID-19 pandemic efforts (i.e., April-September 2020), future analyses should examine how changes in co-occurring psychosocial profiles alter PLHIV’s substance use trajectories. Additional studies may benefit from alternative conceptualizations of substance use to inform pandemic-related trajectories (e.g., binge drinking versus hazardous drinking; medical versus recreational marijuana use; stimulant versus opioid use). Furthermore, the Centers for Disease Control and Prevention reported that overdose deaths have accelerated during the COVID-19 pandemic (CDC, 2020). Though our study focuses on substance use frequency, future studies should evaluate how pandemic stressors have impacted the amount of alcohol and drugs PLHIV consumed at times of use. Finally, researchers should implement mixed-methods approaches to better identify health-promotive factors (e.g., health and socially supportive resources utilized throughout the COVID-19 pandemic) that interrupt problematic trajectories in substance use.
4.4. Conclusions
The COVID-19 pandemic has emphasized the importance of monitoring and evaluating alcohol and substance use in PLHIV. Since the start of the pandemic, health providers have adapted their service provision to maximize patients’ retention in healthcare (Lin et al., 2020, Molfenter et al., 2021). As mitigation recommendations continue to be updated, consistent evaluations of PLHIV’s substance use trajectories amidst the COVID-19 pandemic may better equip health systems to effectively respond to complex and co-occurring challenges as new public health emergencies arise. Furthermore, researchers predicted that mitigation strategies elevated peoples’ risks for adverse conditions, like depression and loneliness. These conditions already disproportionately effect PLHIV and are linked to decreased engagement in HIV care. Yet, we found that binge alcohol consumption and recreational drug use decreased at the beginning of the pandemic among substance-using PLHIV. Furthermore, depressive symptoms distinguished PLHIV with substance use trajectories from those with non-use trajectories. Ongoing surveillance must continue in order to substantiate our findings, elucidate the COVID-19 pandemic’s complex and co-occurring contributions to psychosocial conditions, and inform the supportive factors that interrupt negative behavioral health trajectories experienced by PLHIV.
Contributors
SM, MF, TW, AT, and JM conceived the original idea of the manuscript. SM, SC, MP, and AD contributed to the data analysis. SM and SC developed the first and revised drafts of the manuscript, overseen by MF, MP, and TW. All co-authors provided extensive feedback in preparation of the final draft.
Conflicts of Interest
All authors have no conflicts of interest to report.
Acknowledgements
Data in this manuscript were collected by the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS, United States (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS, United States (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS, United States (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS, United States (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center, United States (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS, United States (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS, United States (Steven Wolinsky), U01-HL146240; Northern California CRS, United States (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS, United States (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS, United States (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS, United States (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS, United States (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS, United States (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS, United States (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute, United States (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), United States; National Institute On Aging (NIA), United States; National Institute Of Dental & Craniofacial Research (NIDCR), United States; National Institute Of Allergy And Infectious Diseases (NIAID), United States; National Institute Of Neurological Disorders And Stroke (NINDS), United States; National Institute Of Mental Health (NIMH), United States; National Institute On Drug Abuse (NIDA), United States; National Institute Of Nursing Research (NINR), United States; National Cancer Institute (NCI), United States; National Institute on Alcohol Abuse and Alcoholism (NIAAA), United States; National Institute on Deafness and Other Communication Disorders (NIDCD), United States; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), United States; National Institute on Minority Health and Health Disparities (NIMHD), United States; and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR), United States. MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), United States; UL1-TR003098 (JHU ICTR), United States; UL1-TR001881 (UCLA CTSI), United States; P30-AI-050409 (Atlanta CFAR), United States; P30-AI-073961 (Miami CFAR), United States; P30-AI-050410 (UNC CFAR), United States; P30-AI-027767 (UAB CFAR), United States; and P30-MH-116867 (Miami CHARM), United States. This work was also supported by the National Institute on Minority Health and Health Disparities (United States) under grant 5R01MD010680-05 (Friedman and Plankey). The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2021.109233.
Appendix A. Supplementary material
Supplementary material
.
References
- Abouk R., Heydari B. The immediate effet of COVID-19 policies on social-distancing behavior in the United States. Public Health Rep. 2021;136(2):245–252. doi: 10.1177/0033354920976575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adimora A.A., Ramirez C., Benning L., Greenblatt R.M., Kempf M.C., Tien P.C., Kassaye S.G., Anastos K., Cohen M., Minkoff H., Wingood G., Ofotokun I., Fischl M.A., Gange S. Cohort profile: the Women’s Interagency HIV Study (WIHS) Int. J. Epidemiol. 2018;47(2):393–394i. doi: 10.1093/ije/dyy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Addiction Centers, 2021. Substance abuse & HIV/AIDS. Retrieved on June 12, 2021 at 〈https://americanaddictioncenters.org/health-complications-addiction/substance-abuse-hiv-aids〉.
- Ballivian J., Alcaide M.L., Cecchini D., Jones D.L., Abbamonte J.M., Cassetti I. Impact of COVID-19-related stress and lockdown on mental health among people living with HIV in Argentina. J. Acquir. Immune Defic. Syndr. 2020;85(4):475–482. doi: 10.1097/QAI.0000000000002493. [DOI] [PubMed] [Google Scholar]
- Baum M., Carlin R., Shenghan L., Sales S., Page B., Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J. Acquir. Immune Defic. Syndr. 2009;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Becker J.B., McClellan M.L., Reed B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017;95(1–2):136–174. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley K.M., McGinnis K.A., Fiellin D.A., Gordon A.J., Kraemer K.L., Bryant K.J., Edelman E.J., Crystal S., Gaither J.R., Korthuis P.T., Marshall B.D.L., Ornelas I.J., Gary Chan K.C., Dombrowski J.C., Fortney J., Justice A.C., Williams E.C. Racial/ethnic differences in the association between alcohol use and mortality among men living with HIV. Addict. Sci. Clin. Pract. 2018;13(1):2. doi: 10.1186/s13722-017-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.J., Serovich J.M., Laschober T.C., Kimberly J.A. Age and racial disparities in substance use and self-reported viral suppression among men who have sex with men with HIV. Int. J. STD AIDS. 2019;29(12):1174–1182. doi: 10.1177/0956462418779663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico, A.W., Horvath, K.J., Grov, C., Moskowitz, J.T., Pahwa, S., Pallikkuth, S., Hirshfield, S., 2020. Double jeopardy: methamphetamine use and HIV risk factors for COVID-19. AIDS Behav. 24, 30320–3023. 10.1007/s10461-020-02854-w. [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention, 2020. Overdose deaths accelerating during COVID-19. Accessed on September 18, 2021, at 〈https://www.cdc.gov/media/releases/2020/p1218-overdose- deaths-covid-19.html〉.
- Centers for Disease Control and Prevention, 2021. HIV and Older Americans. Accessed on September 18, 2021, at 〈https://www.cdc.gov/hiv/group/age/olderamericans/index.html〉.
- Crane H.M., Nance R.M., Whitney B.M., Ruderman S., Tsui J.I., Chander G., McCaul M.E., Lau B., Mayer K.H., Batey D.S., Safren S.A., Moore R.D., Eron J.J., Napravnik S., Mathews W.C., Fredericksen R.J., Hahn A.W., Mugavero M.J., Lober W.B., Saag M.S., Kitahata M.M., Delaney J.A.C. Drug and alcohol use among people living with HIV in care in the United States by geographic region. AIDS Care. 2021 doi: 10.1080/09540121.2021.1874274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Martinez J., Tamargo J.A., Delgado-Enciso I., Liu Q., Acuña L., Laverde E., Barbieri M.A., Trepka M.J., Campa A., Siminski S., Gorbach P.M., Baum M.K. Resilience, anxiety, stress, and substance use patterns during COVID-19 pandemic in the Miami Adult Studies on HIV (MASH) Cohort. AIDS Behav. 2021 doi: 10.1007/s10461-021-03292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza G., Bhondoekhan F., Benning L., Margolick J.B., Adedimeji A.A., Adimora A.A., Alcaide M.L., Cohen M.H., Detels R., Friedman M.R., Holman S., Konkle-Parker D.J., Merenstein D., Ofotokun I., Palella F., Alekruse S., Brown T.T., Tien P.C. Characteristics of the MACS-WIHS combined cohort study: opportunities for research on aging with HIV in the longest U.S. observational study of HIV. Am. J. Epidemiol. 2021 doi: 10.1093/aje/kwab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duko B., Ayalew M., Ayano G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Addict. Sci. Clin. Pract. 2019;14:52. doi: 10.1186/s13011-019-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J.E., Haberlen S.A., Meanley S., Ware D., Brown A.L., Siconolfi D., Brennan-Ing M., Stall R., Plankey M.W., Friedman M.R. Understanding patterns of healthy aging among men who have sex with men: study methods. JMIR Res. Protoc. 2021;0(0) doi: 10.2196/25750. 〈https://www.researchprotocols.org/2021/0/e0/〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M.R., Coulter R.W.S., Silvestre A.J., Stall R., Teplin L., Shoptaw S., Surkan P.J., Plankey M.W. Someone to count on: social support as an effect modifier of viral load suppression in a prospective cohort study. AIDS Care. 2017;29(4):469–480. doi: 10.1080/09540121.2016.1211614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher J.A., Javanbakht M., Shover C.L., Ragsdale A., Brookmeyer R., Shoptaw S., Gorbach P.M. Comparative impact of methamphetamine and other drug use on viral suppression among sexual minority men on antiretroviral therapy. Drug Alcohol Depend. 2021;221(1) doi: 10.1016/j.drugalcdep.2021.108622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn T.R., Llabre M.M., Lee J.S., Bedoya C.A., Pinkston M.M., O’Cleirigh C., Safren S.A. Pathways to health: an examination of HIV-related stigma, life stressors, depression, and substance use. Int. J. Behav. Med. 2019;26:286–296. doi: 10.1007/s12529-019-09786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz M., de Guzman R., Freeman R., Kutnick A., Silverman E., Leonard N.R., Ritchie A.S., Muñoz-Plaza C., Salomon N., Wolfe H., Hilliard C., Cleland C.M., Honig S. Exploring how substance use impedes engagement along the HIV care continuum: a qualitative study. Front. Public Health. 2016;4(62):1–13. doi: 10.3389/fpubh.2016.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstatter K.R., Akhtar W.Z., Dietz S., Pe-Romashko K., Gustafson D.H., Shah D.V., Krechel S., Liebert C., Miller R., El-Bassel N., Westergaard R.P. Potential influences of the COVID-19 pandemic on drug use and HIV care among people living with HIV and substance use disorders: experience from a pilot mHealth intervention. AIDS Behav. 2021;25:354–359. doi: 10.1007/s10461-020-02976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht M., Shoptaw S., Ragsdale A., Brookmeyer R., Bolan R., Gorbach P.M. Depressive symptoms and substance use: changes overtime among a cohort of HIV-positive and HIV negative MSM. Drug Alcohol Depend. 2020;207 doi: 10.1016/j.ddrugalcdep.2019.107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.L., Nagin D.S., Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 2001;29:374–393. doi: 10.1177/0049124101029003005. [DOI] [Google Scholar]
- Karamouzian M., Johnson C., Kerr T. Public health messaging and harm reduction in the time of COVID-19. Lancet Psychiatry. 2020;7(5):390–391. doi: 10.1016/S2215-0366(20)30144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso-Chichetto N.E., Plankey M., Abraham A.G., Ennis N., Chen X., Bolan R., Cook R.L. Association between alcohol consumption trajectories and clinical profiles among women and men living with HIV. Am. J. Drug Alcohol Abus. 2018;44(1):85–94. doi: 10.1080/00952990.2017.1335317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp A.M., Rebeiro P.F., Shepherd B.E., Brinkley-Rubinstein L., Turner M., Bebawy S., Sterling T.R., Hulgan T. Daily marijuana use is associated with missed clinic appointments among HIV-infected persons in HIV care. AIDS Behav. 2017;21:1196–2004. doi: 10.1007/s10461-017-1716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. COVID 19 and its mental health consequences. J. Ment. Health. 2020;180(6):817–818. doi: 10.1080/09638237.2020.1757052. [DOI] [PubMed] [Google Scholar]
- Lesko C.R., Bengston A.M. HIV and COVID-19: intersecting epidemics with many unknowns. Am. J. Epidemiol. 2021;190(1):10–16. doi: 10.1093/aje/kwaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Fernandez A.C., Bonar E.E. Telehealth for substance-using populations in the age of coronavirus disease 2019. JAMA Psychiatry. 2020;77(2):1209–1210. doi: 10.1001/jamapsychiatry.2020.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannes Z.L., Bryant V.E., Burrell L.E., Lu H., Ferguson E.G., Zhou Z., Cook R.L., Ennis N. The prevalence and patterns of substance use by birth cohort among HIV-positive adults in Florida. Aging Ment. Health. 2019;23(4):515–523. doi: 10.1080/13607863.2018.1430740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannes Z.L., Burrell L.E., Bryant V.E., Dunne E.M., Hearn L.E., Whitehead N.E. Loneliness and substance use: the influence of gender among HIV+ Black/African American adults 50. AIDS Care. 2016;28(5):598–602. doi: 10.1080/09540121.2015.1120269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannes Z.L., Burrell L.E., Dunne E.M., Hearn L.E., Whitehead N.E. Contextualizing psychosocial determinants of alcohol use by age cohorts of adults living with HIV, ages 50 and older. J. Assoc. Nurses AIDS Care. 2017;28(2):279–288. doi: 10.1016/j.jana.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannes Z.L., Dunne E.M., Ferguson E.G., Cook R.L., Ennis N. Symptoms of generalized anxiety disorder as a risk factor for substance use among adults living with HIV. AIDS Care. 2021;33(5):623–632. doi: 10.1080/09540121.2020.1808163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marziali M.E., Card K.G., McLinden T., Wang L., Trigg J., Hogg R.S. Physical distancing in COVID-19 may exacerbate experiences of social isolation among people living with HIV. AIDS Behav. 2020;24:2250–2252. doi: 10.1007/s10461-020-02872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanley S.P., Stall R.D., Dakwar O., Egan J.E., Friedman M.R., Haberlen S.A., Okafor C., Teplin L.A., Plankey M.W. Characterizing experiences of conversion therapy among middle-aged and older men who have sex with men from the Multicenter AIDS Cohort Study. Sex Res. Soc. Policy. 2020;17(2):334–342. doi: 10.1007/s13178-019-00396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar B.M., Starks T.J., Gurung S., Parsons J.T. The impact of comorbidities, depression, and substance use problems on quality of life among older adults living with HIV. AIDS Behav. 2017;21:1684–1690. doi: 10.1007/s10461-016-1613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga M.J., Reisner S.L., Grasso C., Crane H.M., Safren S.A., Kitahata M.M., Schumacher J.E., Mathews W.C., Mayer K.H. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am. J. Public Health. 2013;103(8):1456–1467. doi: 10.2105/ajph.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra E., Andersen B.J., Herman D.S., Hayaki J., Pinkston M.M., Kim H.N., Stein M.D. Examination of using alcohol to cope, depressive symptoms, and perceived social support in persons with HIV and Hepatitis C. AIDS Care. 2020;32(10):1238–1245. doi: 10.1080/09540121.2020.1734177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter T., Roger N., Chaple M., Behlman S., Cody O., Hartzler B., Johnson E., Nichols M., Stilen P., Becker S. Use of telehealth in substance use disorder services during and after COVID-19: online survey study. JMIR Ment. Health. 2021;8(2) doi: 10.2196/25835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D.S. Harvard University Press; Cambridge, MA: 2005. Group-based Modeling of Development. [Google Scholar]
- Noor S.W., Hart T.A., Okafor C.N., Ware D., Chew K.W., D’Souza G., Ho K., Friedman M.R., Plankey M. Staying or moving: results of a latent transition analysis examining intra- individual stability of recreational substance use among MS in the Multicenter AIDS Cohort Study from 2004 to 2016. Drug Alcohol Depend. 2021;220 doi: 10.1016/j.drugalcdep.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez A., Sreeganga S.D., Ramaprasad A. Access to healthcare during COVID-19. Int. J. Environ. Res. Public Health. 2021;18:2980. doi: 10.3390/ijerph18062980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence B.W., Theilman N.M., Whetten K., Ostermann J., Kumar V., Mugavero M.J. Coping strategies and patterns of alcohol and drug use among HIV-infected patients in the United States Southeast. AIDS Patient Care STDs. 2008;22(11):869–877. doi: 10.1089/apc.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschenberg C., Schick A., Hirjak D., Seidler A., Paetzold I., Apfelbacher C., Riedel-Heller S., Reininghaus U. Evidence synthesis of digital interventions to mitigate the negative impact of the COVID_19 pandemic on public mental health: rapid meta-review. J. Med. Internet Res. 2021;23(3) doi: 10.2196/23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen R.K., McGarrity L.A., Salmoirago-Blotcher E., Rich C., Rana A., Carey M.P. Telephone-delivered mindfulness training for people living with HIV: a qualitative 360o inquiry. AIDS Behav. 2017;21:3194–3201. doi: 10.1007/s10461-017-1857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova A.A., Kempf M.C., Taylor T.N., Konkle-Parker D., Wingood G.M., Holstad M.M. Healthy aging in older women living with HIV infection: a systematic review of psychosocial factors. Curr. HIV/AIDS Rep. 2017;14:17–30. doi: 10.1007/s11904-017-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS Institute Inc; Cary, NC: 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. [Google Scholar]
- Siconolfi D.E., Halkitis P.N., Barton S.C., Kingdon M.J., Perez-Figueroa R.E., Arias-Martinez V., Karpiak S., Brennan-Ing M. Psychosocial and demographic correlates of drug use in a sample of HIV-positive adults ages 50 and older. Prev. Sci. 2013;14:618–627. doi: 10.1007/s11121-012-0338-6. [DOI] [PubMed] [Google Scholar]
- Starks T.J., Jones S.S., Sauermilch D., Benedict M., Adebayo T., Cain D., Simpson K.N. Evaluating the impact of COVID-19: a cohort comparison study of drug use and risky sexual behavior among sexual minority men in the U.S.A. Drug Alcohol Depend. 2020;216 doi: 10.1016/j.drugalcdep.2020.108260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes B., Salani D., De Santis J.P. A comparison of psychosocial factors, substance use behaviors, and sexual behaviors by self-reported HIV status among middle-aged Hispanic men who have sex with men. Hisp. Health Care Int. 2021;19(1):38–46. doi: 10.1177/1540415320923568. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ibañez G.E., Vaddiparti K., Stetten N.E., Sajdea R., Porges E.C., Cohen R.A., Cook R.L. Change in marijuana use and its associated factors among persons living with HIV (PLWH) during the COVID-19 pandemic: findings from a prospective cohort. Drug Alcohol Depend. 2021;225 doi: 10.1016/j.drugalcdep.2021.108770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell J.D., Shuper P.A., Rourke S.B., Hendershot C.S. Stigma, coping, and alcohol use severity among people living with HIV: a prospective analysis of bidirectional and mediated associations. Ann. Behav. Med. 2018;42(9):762–772. doi: 10.1093/abm/kax050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware D., Palella F.J., Chew K.W., Friedman M.R., D’Souza G., Ho K., Plankey M. Examination of polypharmacy trajectories among HIV-positive and HIV-negative men in an ongoing longitudinal cohort from 2004 to 2016. AIDS Patient Care STDs. 2019;33(8):354–365. doi: 10.1089/apc.2019.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., O’Brien N., Forrest J.I., Salters K.A., Patterson T.L., Montaner J.S.G., Hogg R.S., Lima V.D. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. SAS Institute; Cary, NC: 2014. Analyzing Multilevel Models with the Glimmix Procedure. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material